Abstract
The Pax6 transcription factor plays a key role in ocular development of vertebrates and invertebrates. Homozygosity of the Pax6 null mutation in human and mice results in arrest of optic vesicle development and failure to initiate lens formation. This phenotype obscures the understanding of autonomous function of Pax6 in these tissue components and during later developmental stages. We employed the Cre/loxP approach to inactivate Pax6 specifically in the eye surface ectoderm concomitantly with lens induction. Although lens induction occurred in the mutant, as indicated by Sox2 up-regulation in the surface ectoderm, further development of the lens was arrested. Hence, Pax6 activity was found to be essential in the specified ectoderm for lens placode formation. Furthermore, this mutant model allowed us for the first time to address in vivo the development of a completely normal retina in the absence of early lens structures. Remarkably, several independent, fully differentiated neuroretinas developed in a single optic vesicle in the absence of a lens, demonstrating that the developing lens is not necessary to instruct the differentiation of the neuroretina but is, rather, required for the correct placement of a single retina in the eye.
Keywords: Pax6, lens induction, lens placode, optic vesicle, Cre/loxP, conditional knockout
The vertebrate eye develops from different tissue components including the facial surface ectoderm (SE), the optic vesicle (OV), a lateral evagination from the wall of the diencephalon, and the surrounding mesenchyme. Successive signals between the OV and the SE are thought to mediate the coordinate development of these two structures. The OV contacts the lens-competent ectoderm and induces a response that leads to the thickening of the ectoderm, termed lens placode. While the lens placode internalizes to form the lens vesicle, the distal part of the OV invaginates to form the optic cup with the inner layer developing into the neuroretina (NR) and the outer layer forming the retinal pigmented epithelium (RPE; Pei and Rhodin 1970). The signals directing the coordinate growth of these two tissue components have been the subject of extensive investigation by classical embryologists (Lopashov and Stroeva 1961; Grainger 1992). While the requirement of the OV for lens induction has been well documented and recently re-evaluated (Grainger 1992; Grainger et al. 1997) the influence of the early lens structure on the development of the retina is poorly defined.
Pax6 is a paired-domain and homeodomain-containing transcription factor necessary for normal development of the eye, nose, pancreas, and brain (Walther and Gruss 1991; Schmahl et al. 1993; St-Onge et al. 1997). In the embryonic eye, Pax6 expression from embryonic day 8 (E8) onward is before any morphological differentiation (Walther and Gruss 1991; Grindley et al. 1995). In the SE, expression of Pax6 starts before placode formation. Expression is maintained in the differentiating lens and persists in the adult lens and corneal epithelium. The expression of Pax6 in the anterior neural plate includes the optic pit from which the OV evaginates. In the OV, Pax6 expression is restricted distally and is excluded from the optic stalk and the RPE. As neuronal differentiation is initiated, Pax6 expression is down-regulated in most differentiating neurons but is maintained in the ganglion and amacrin cells. This dynamic and evolutionarily conserved expression pattern suggested that Pax6 plays different roles during eye development (Macdonald and Wilson 1997).
Eye development is extremely sensitive to the levels of Pax6. Reduction in the levels of Pax6 in heterozygotes for a Pax6 null allele results in ocular abnormalities including Aniridia in humans (Glaser et al. 1995) and Small eye in mice and rat (Hogan et al. 1986; Hill et al. 1991; Matsuo et al. 1993). Interestingly, overexpression of Pax6 in mice also results in microphthalmia and loss of photoreceptors, demonstrating a function of Pax6 in retinal specification (Schedl et al. 1996). Moreover, eye structures do not develop in mice homozygous for the Pax6 null allele. In the absence of Pax6 activity, the OV evaginates from the brain, but contact with the SE is not maintained, the NR and RPE do not differentiate, and finally, the OV degenerates. The SE-derived eye structures are completely absent, as lens induction does not occur in the mutants (Grindley et al. 1995). This complex phenotype obscured attempts to study the autonomous functions of Pax6 in these mutually interacting tissue components and to address the later roles of this gene (Grindley et al. 1995).
To study the in vivo functions of Pax6 specifically in the lens surface ectoderm after lens induction, and to reveal the role of the lens primordium in retina development, we employed the Cre/loxP approach. We generated a spatially and temporally defined somatic deletion of Pax6 in the SE (Le-mutant). We demonstrate that Pax6 in the specified lens ectoderm is required for lens placode formation, and we identify possible downstream targets of Pax6 in the ectoderm. Furthermore, we define for the first time the influence of the lens placode on the patterning and morphology of the retina. We show that the early lens structures are required for the correct placement of a single retina in the eye.
Results
Establishment of the Pax6floxline and the somatic mutation of Pax6 in the eye SE
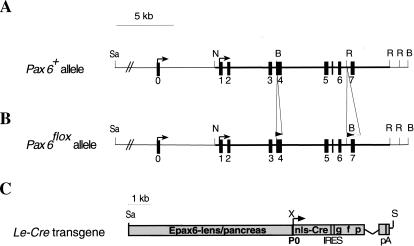
To address the functions of Pax6 in vivo in defined tissue components of the eye at a specific developmental stage, we employed the Cre/loxP recombination approach (Gu et al. 1994). A mouse line was established in which the region encoding the amino terminus of Pax6, including the initiator methionine and most of the paired domain, was flanked by two loxP sequences (Fig. 1A,B). Mice carrying the targeted allele with the neomycin selection cassette in the intron between exons 6 and 7 revealed a hypomorphic phenotype (R. Ashery-Padan and P. Gruss, unpubl.). After removal of the selection cassette the phenotype of Pax6flox/Pax6flox (Fig. 1B) mice was completely reversed to normal.
Figure 1.
Targeted insertion of loxP sites into the Pax6 gene and analysis of Cre activity in the Le-Cre transgenic line. Structure of wild-type (A) and targeted (B) Pax6 loci. One loxP site was introduced in exon 4 upstream of the first ATG. The second loxP was followed by the selection cassette flanked by FRT sequences inserted between exons 6 and 7. The selection cassette was removed to establish the Pax6flox allele (see Materials and Methods). (C) Schematic representation of Le-Cre transgene. A 6.5-kb SacII/XmnI genomic region including the upstream regulatory sequences and the first Pax6 promoter (P0) cloned upstream of sequences encoding the nls-Cre followed by internal ribosome binding sites (IRES) and green fluorescent protein (GFP). The recombination pattern was detected by enzymatic reaction (Lobe et al. 1999) on whole mount (D) or sections (E,F) of Z/AP;Le-Cre embryos at E9.5 (D,E) and E15.5 (F). (Arrows) Transcription start sites; (filled rectangles) exons; (open triangle) FRT; (filled triangles) loxP. (c) Cornea; (con) conjuctiva; (el) eyelid; (le) lens; (nls) nuclear localization signal; (nr) neuroretina; (ov) optic vesicle; (p) pancreas; (pA) poly A; (rpe) retinal pigmented epithelium; (se) surface ectoderm. (B) BamHI; (N) NotI; (R) EcoRV; (Sa) SacII; (S) Sfi; (X) XmnI.
A 6.5-kb genomic fragment from the mouse Pax6 gene (Fig. 1C) has been shown to activate Pax6 expression in the SE and, subsequently, in the developing lens, cornea, and pancreas in transgenic mice (Williams et al. 1998; Kammandel et al. 1999; Xu et al. 1999). We used this fragment to activate the expression of Cre and green fluorescence protein (GFP) from a bicistronic cassette in a transgenic mouse line termed Le-Cre (Fig. 1C). The expression of the transgene was followed by detection of GFP fluorescence, whereas the recombination pattern mediated by the Le-Cre line was characterized by analysis of the progeny obtained from the cross with the Z/AP reporter line (Lobe et al. 1999; Fig. 1D–F). In this reporter line, Cre-mediated excision removed the LacZ/neomycin fusion gene, allowing the expression of the human alkaline phosphatase (hAP) reporter.
The onset of Le-Cre transgene expression in the eye was evident from E9 onward. At E9.5, the Cre-mediated recombination was detected in most cells of the SE extending dorsally and caudally around the developing eye (Fig. 1D,E). At E15.5, recombination was restricted to the SE-derived eye structures including the developing lens, cornea, conjuctiva, and skin of the eyelids (Fig. 1F).
To achieve efficient and rapid somatic deletion of Pax6, we performed our experiments using only one functional allele of Pax6. Le-Cre mice were mated with mice carrying the knockout allele of Pax6 (Pax6lacZ; St-Onge et al. 1997). Offspring heterozygous for both Le-Cre and the Pax6lacZ alleles were mated with mice heterozygous for the floxed Pax6 allele (Pax6flox). This breeding generated at the expected Mendelian ratio the desired mutant mice (Pax6lacZ/Pax6floxLe-Cre) named here Le-mutant. The control littermates analyzed were the Pax6lacZ/Pax6flox mice, unless otherwise indicated.
Pax6 is eliminated from the SE of the Le-mutant concomitantly with lens induction
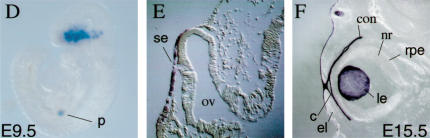
Establishment of contact between the SE and OV occurs at E9 (18–20 somites), followed by the response of the SE to inductive signals emanating from the OV. These inductive signals result in lens specification of the overlying SE (E9–E9.5). Subsequently, a thickening of the SE becomes evident at the site of the developing lens placode (25–27 somites E9.5). In our Le-mutant embryos, Pax6 protein was detected in the SE at E9 (Fig. 2A) in accordance with the observed recombination pattern (Fig. 1D–F). Pax6 protein was eliminated from the Le-mutant SE between E9 and E9.5 and was no longer detectable at E9.5 (cf. Le-mutant Fig. 2C,D with control Fig. 2B). Thus, removal of Pax6 protein from the SE was concomitant with lens induction. In the OV, Pax6 expression was maintained at normal levels (Fig. 2C,D; Fig. 3G), demonstrating that the somatic mutation was restricted to the SE.
Figure 2.
Elimination of Pax6 protein from the SE is completed at E9.5. (A) Pax6 protein is detected in transverse paraffin section of E9 Le-mutant OV and SE. At E9.5, Pax6 is not detected in the SE of the Le-mutant (C,D, arrowheads), but is present in the SE of Pax6+/Pax6+;Le-Cre control (B). (D) Magnification of Pax6 distribution detected in (C). In the SE, GFP fluorescence is detected in cryosections of E9.5 control (B) and Le-mutant (C) eyes. (E) From E10, GFP fluorescence is detected in the pancreas (open arrowhead) and lens (filled arrowhead) of Pax6+/Pax6+;Le-Cre embryos. (F) In the Le-mutant E10 embryos, GFP is only detected in the pancreas and not in the eyes. The whole embryo appears slightly green because of unspecific fluorescence observed also in completely wild-type littermates. (ov) optic vesicle; (se) surface ectoderm.
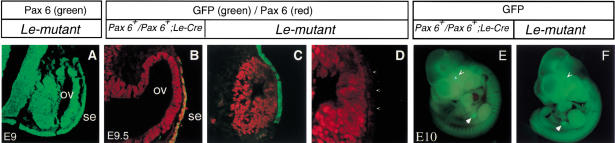
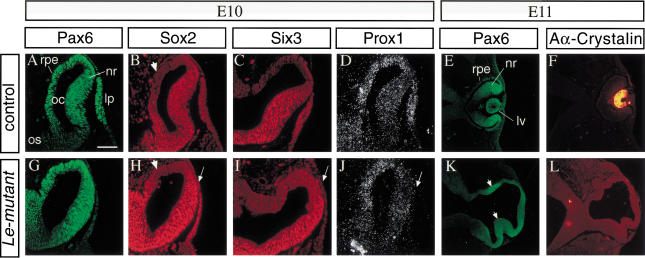
Figure 3.
The lens ectoderm is specified, but lens placode does not form in the Le-mutant eyes. Molecular analysis of the phenotype of E10 (A–D, G–J) and E11 (E,F,K,L) embryos by indirect immunofluorescence. Transverse paraffin sections, double labeled with specific antibodies to Pax6 (A,G) and Sox2 (B,H). Pax6 (G) is not detected above background levels in the SE of the Le-mutant embryos, while Sox2 is detected in the SE (H, arrow) similar to the up-regulation of Sox2 observed in control eyes (B). Adjacent section immunolabeled with antibodies to Six3 (C,I) show that Six3 is not detected above background level in the Le-mutant (I) ectoderm but is expressed in the lens placode of control littermate (C). (D,J) In situ hybridization with 35S labeled Prox1 RNA probe demonstrate that Prox1, which is normally expressed in the lens placode (D), is not detected above background levels in the Le-mutant SE (J). Double immunolabeling with specific antibodies to Pax6 (E,K) and αA-crystallin on E11 control (F) and mutant eyes (L). In the Le-mutant eye, two regions of the OV seem to invaginate, and Pax6 is enhanced at these regions (K, arrowheads). Arrows in H–J, point to the lens ectoderm. Arrowheads in B and H mark regions of presumptive RPE in which Sox2 is not detected above background level. (lp) Lens placode; (lv) lens vesicle; (nr) neuroretina; (oc) optic cup; (os) optic stalk; (rpe) retinal pigmented epithelium. Scale bar 50 μm in A–D, G–J and 100μm in E,F,K,L.
GFP fluorescence was detected in the SE of Pax6+/Pax6+,Le-Cre embryos at E9.5 (Fig. 2B) and was later maintained in the developing lens and pancreas (Fig. 2E). In the Le-mutant, GFP fluorescence was detected in the SE at E9.5 (Fig. 2C) and then disappeared from the SE (Fig. 2F), while expression of the transgene was maintained in the pancreas (Fig. 2F). The inactivation of transgene expression in the Le-mutant eye could be caused by changes in cell fate. Alternatively, the transcription activity of the Pax6 regulatory element in the Le-Cre transgene is dependent on Pax6 activity in the eye.
In the complete absence of Pax6, proper contact between SE and OV is not maintained (Grindley et al. 1995). However, when Pax6 was deleted only from the SE, the contact between the OV and the SE was maintained (cf. Fig. 2C,D with 2B), indicating that maintenance of the contact between the SE and OV is not dependent on the continued presence of Pax6 in the SE. This observation is in accordance with the recent results obtained from analysis of mouse aggregation chimeras between wild-type and Small eye mutant cells (Collinson et al. 2000). Taken together, these results show that the maintenance of the contact between the OV and the SE is exclusively dependent on Pax6 function in the OV. The Le-mutant phenotype therefore reveals the autonomous function of Pax6 in the SE when the contact and signals from the OV are not disrupted.
Lens ectoderm is specified in Le-mutant embryos
Previous attempts to address the functions of Pax6 in the developing eye utilized tissue recombination experiments of rSey mutant embryos (Fujiwara et al. 1994) or analyses of mouse aggregation chimeras (Quinn et al. 1996). These results suggested that Pax6 has a cell autonomous function in the SE, which is essential for establishing the competence of the ectoderm to respond to signals from the OV (Fujiwara et al. 1994; Quinn et al. 1996). The up-regulation of the transcription factor Sox2 is one of the earliest detectable responses in the presumptive lens ectoderm to the inductive signals of the OV (Furuta and Hogan 1998; Kamachi et al. 1998; Zygar et al. 1998; Wawersik et al. 1999). Up-regulation of Sox2 was not detectable in mice homozygous for the Pax6sey-1neu allele (Furuta and Hogan 1998; Wawersik et al. 1999). In our Le-mutant mice, Sox2 protein was detected in the SE at E10 (Fig. 3H), similar to Sox2 distribution seen in control littermates (Fig. 3B). This result demonstrated that the early expression of Pax6 in Le-mutant eyes, before and during lens induction, was sufficient for specifying the lens ectoderm. The Le-mutant therefore provided a unique opportunity to study the role of Pax6 in the SE after lens induction had occurred.
Pax6 in the specified ectoderm is essential for lens placode formation
During normal development, up-regulation of Sox2 precedes the morphological appearance of a lens placode. In Le-mutant embryos, the expression of Sox2 in the specified ectoderm was not followed by lens placode formation, as neither thickening nor invagination of the SE was observed (Fig. 3H). We also followed the distribution of Six3 protein and Prox1 transcript in control and Le-mutant eyes. Expression of both genes has been reported to be initiated around the time of placode formation (Oliver et al. 1993, 1995). Although the expression of both genes was detected in the lens placode of the control littermates (Fig. 3C,D), neither Six3 protein nor Prox1 transcripts were detected in the SE of Le-mutant embryos (Fig. 3I,J). In accordance with the apparent arrest in lens development, αA-crystalline, which is expressed in the lens pit stage (Oguni et al. 1994; Fig. 3F) was not detected in the Le-mutant eye (Fig. 3L).
We conclude that from E9.5 onward, the progression in lens formation including proliferation and differentiation of the specified ectoderm is strictly dependent on Pax6 activity in the SE (see Discussion).
The delay in cup formation does not disrupt the initial patterning of the retinal progenitors in the OV
The prevention of lens formation in Le-mutant allowed us for the first time to address directly in vivo the patterning and differentiation of the completely normal retina in the absence of all lens structures.
The first morphological difference between the Le-mutant and the control OV was evident at E10–E10.5, as no invagination of the distal wall of the OV was apparent (Fig. 3G), while in the control littermates, optic cup formation was initiated (Fig. 3A). Despite this morphological difference, the distribution of Pax6, Sox2, and Six3 proteins within the Le-mutant OV was similar to the distribution of these proteins in the OV of the control littermates. At E10, Pax6 was detected throughout the distal part of the OV in both control (Fig. 3A) and Le-mutant eyes (Fig. 3G). Sox2 and Six3 were detected in most of the OV, excluding a small dorsal region that corresponds to the future RPE domain (Le-mutant in Fig. 3H,I and control in Fig. 3B,C).
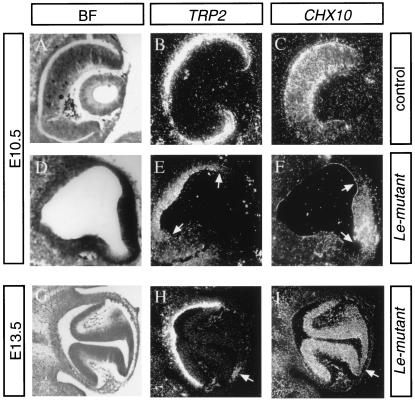
During normal development, two populations of progenitors (RPE and NR) separate from the bipotential cells of the OV. The RPE progenitors express tyrosinase-related protein-2 (Trp2; Steel et al. 1992), while in the earliest retinal neuroepithelial cells, Chx10 is expressed (Liu et al. 1994). We followed the distribution of these markers in E10.5 and E13.5 in Le-mutant and control eyes. At E10.5, the optic cup had already formed in the control eyes (Fig. 4A). Chx10 expression was detected in the NR layer (Fig. 4C), while Trp2 transcripts were detected in the outer RPE layer (Fig. 4B). In Le-mutant eyes, the optic cup did not form (Fig. 4D); however, the two separate populations of cells were detected. Chx10 was identified in the distal part of the OV (Fig. 4F), which corresponds to the NR layer. Trp2 was expressed in the proximal lateral domains (Fig. 4E), corresponding to the RPE domain in normal eyes. Thus, the formation and the separation of RPE and NR progenitors in the Le-mutant OV were properly initiated.
Figure 4.
The initial delineation of prospective NR and RPE dissolve to several NR and RPE domains. In situ hybridization with 35S-labeled TRP2 or Chx10 RNA probes on adjacent sections through the eye regions of E10.5 and E13.5 eyes. Bright field images (BF) shown in A, D, and G. In the eye of E10.5 control littermate (A–C), Trp2 is localized in the prospective RPE located in the outer layer of the cup (B), while Chx10 is expressed in the NR layer (C). Cup formation is delayed in the Le-mutant eyes (D). Trp2, however, is expressed in the proximal (E, between arrows) region, while Chx10 transcripts demarcate the prospective NR located distally (F, between arrows). At E13.5, invagination of the NR forms two folds in the Le-mutant eye (G). Trp2 is expressed not only in the proximal part of the Le-mutant eyecup but also in patches of cells located between the two retina folds (arrow in H). Chx10 is distributed in the two folds and is down-regulated in the region between them (arrow in I).
The several separated NR-folds in the Le-mutant eye develop to fully differentiated neuroretinas
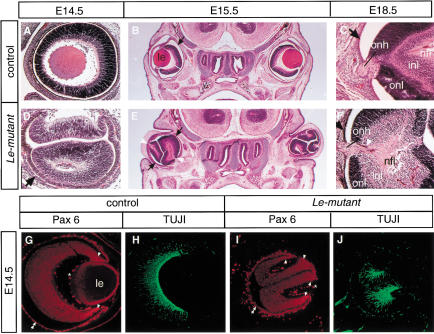
After a delay of ∼1 d (E11), as compared to control (E10), the thickening and invagination of two or three NR domains in the OV were morphologically apparent in the Le-mutant (Fig. 3K). Interestingly, some of the NR domains developed in regions that opposed the mesenchyme (Figs. 3K, 5E), and RPE was differentiated from distal regions of the OV (Fig. 4G). Pax6 expression was enhanced in these invaginating domains (Fig. 3K) and subsequently in the NR developing from them (Fig. 5I). At later stages of development, the morphology of the Le-mutant retina (Fig. 5D–F) suggested that each fold developed separately to form a retina domain. The separation between these folds was evident from histological analysis of the Le-mutant eyes performed both on sagittal and cross sections (Fig. 5D,E) and observed in serial sections (data not shown). Moreover, patches of RPE cells differentiated between the retina folds as these cells expressed Trp2 (Fig. 4H). Further differentiation of the RPE was evident in Le-mutant eyes as cells gave rise to the characteristic cuboidal epithelium that accumulated pigment (Fig. 5D), and Pax6 was down-regulated in these cells (Fig. 5I).
Figure 5.
Several neuroretinas form in the absence of all lens structures in the Le-mutant mice. Hematoxilin-eosin (HE) staining (A–F) of transverse (B,C,E,F) or sagittal sections (A,D) through the eye region of the Le-mutant (D–F) and control littermates; Paxflox/Pax6lacZ (A,C) or Pax6+/Pax6+ (B). Cell layers including nerve fiber layer (nfl), inner nuclear layer (inl), and outer nuclear layer (onl) are distinguished in the Le-mutant (F) and in the control eye (C) at E18.5. The black arrows point out the RPE (B–F). Note that the axons exit each retina fold not from the optic disc but, rather, from an opening between the folds, which in a normal eye would have been occupied by the lens (white arrowheads in E,F). Furthermore, an optic nerve is observed in Le-mutant eyes (F). Coimmunolabeling with antibodies to Pax6 (G,I) and to βIII tubulin (H,J) show that each retina fold develops autonomously in the Le-mutant eye. Enhanced Pax6 expression is marked with arrowheads, RPE with two arrowheads. (le) Lens; (onh) optic nerve head; (*) unspecific fluorescence from blood cells.
The autonomous development of each retina fold was further demonstrated by the apparent normal distribution of Pax6 and class III β-tubulin (TUJI) within each of the NR folds. Pax6 expression was higher at the borders of the folds (Fig. 5I), which is similar to the enhanced expression of Pax6 at the presumptive iris in wild-type eyes (Fig. 5G). Furthermore, TUJI staining at the E14.5 Le-mutant retina displayed the characteristic pattern of neuronal cell differentiation seen in normal development (Snow and Robson 1995; McCabe et al. 1999). Thus, in each of the Le-mutant NR-units (Fig. 5J) and in the NR of the control littermates (Fig. 5H), the neuronal cell differentiation started from the center and progressed towards the periphery.
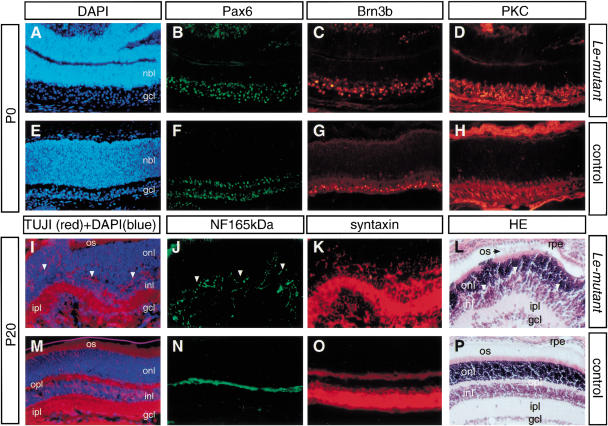
In the neonatal and adult Le-mutant retina, expression of specific neuronal cell markers was similar to the expression of these markers in the retina of control mice. These included Brn3b (ganglion cells; Fig. 6C,G), protein kinase C (bipolar cells; Fig. 6D,H), neurofilament 165kD (horizontal cells; Fig. 6J,N), and syntaxin (amacrine cells; Fig. 6K,O). The neuroretinal cells formed three layers that could be distinguished in the retina of adult Le-mutant mice (Fig. 6L). The lamination, however, was incomplete, as outer and inner plexiform layers did not separate in all regions. The extensive folding of the neuroretinas in the Le-mutant eye led to a loss of contact between the RPE and the NR at regions of contact between the NR folds (Fig. 5D,E,I). In the absence of RPE, a disruption of the laminar architecture has been reported (Raymond and Jackson 1995). A similar effect may possibly explain the lamination defects detected in the Le-mutant NR.
Figure 6.
Retinal lamination and the major classes of retinal neurons are present in the Le-mutant retina. The Le-mutant neuroretina displays restricted Pax6 distribution (B) and lamination into nbl and gcl (A) similar to the single retina of the control eye (E,F). Immunohistochemistry and histology on newborn (P0, A–H) and mature (P20, I–P) eyes in adjacent sections of retina. Note the presence of retinal ganglion cells (Brn3b, C,G), bipolar cells (protein kinase C, D,H), horizontal cells (neurofilament 165 kD, J,N), amacrine cells (syntaxin, K,O), photoreceptor cells (TUJI cell bodies and processes in the onl, I,M), and photoreceptor outer segments (HE staining, L,P) in the Le-mutant (A–D,I–L) and the control (E–H,M–P) retina. All laminae, onl, inl, gcl, are present in the mature Le-mutant retina, as revealed by HE (L,P) and DAPI+TUJ1 (I,M) staining. However, onl and inl are not separated by a pronounced opl (arrowheads in I,J). The absence of a continuous opl in the Le-mutant retina possibly accounts for the dispersed appearance of horizontal cell processes between inl and onl (J). (gcl) ganglion cell layer; (HE) hematoxilin-eosin; (inl) inner nuclear layer; (ipl) inner plexiform layer; (nbl) neuroblast layer; (onl) outer nuclear layer; (opl) outer plexiform layer; (os) outer segments of photoreceptors; (rpe) retinal pigment epithelium.
Taken together, these results demonstrate that in the absence of the early lens structures, several separated NR folds will form. These NR folds have an intrinsic capacity to form a multilayered structure in the absence of a developing lens. We may, therefore, deduce that the developing lens is not required for the differentiation of the NR but to define the position and restrict the number of retinas formed in the vertebrate eye.
Discussion
The current model for lens induction in vertebrates suggests that the prospective lens ectoderm is determined in a series of sequential steps (Grainger 1992; Saha et al. 1992). Initially, during the late gastrula stage of the amphibian embryo, the competence of the ectoderm to respond to the induction signals is acquired. Subsequently, planar signals from the anterior neural plate induce the lens-forming bias in the head ectoderm. Finally, the contact with the underlying OV triggers the specification of the lens ectoderm leading to changes in gene expression during the preplacode stage (Wawersik et al. 1999). These changes are followed by the formation of the lens placode.
In vertebrates, Otx2 (Zygar et al. 1998), BMP4 (Furuta and Hogan 1998), and Pax6 have been implicated to play parallel roles in maintaining the competence of the SE to respond to the signals from the OV. Several lines of evidence pointed to the importance of Pax6 in this process: First, early expression of Pax6 in the ectoderm coincides with the lens-competence stage (Walther et al. 1991; Grindley et al. 1995; Zygar et al. 1998). Second, tissue recombination experiment has demonstrated that Pax6−/− ectoderm rather than Pax6−/− OV is responsible for the lens defects in Pax6−/− mice (Fujiwara et al. 1994). Third, Pax6 chimera analysis has shown that Pax6 is required before formation of the lens placode (Collinson et al. 2000). Furthermore, markers indicative of lens specification such as Sox2 and Frissled-related protein (sFRP2) are not expressed in the SE of Pax6−/− embryos (Furuta and Hogan 1998; Wawersik et al. 1999). This apparent requirement for Pax6 for the initial response of the ectoderm hampered earlier studies aimed at understanding the role and identifying the downstream targets of Pax6 after lens specification. In the Le-mutant, however, Pax6 expression occurs early and is switched off, specifically in the SE, before placode formation. The analyses of Le-mutant revealed that Pax6 is required for the transition from lens specification to lens placode.
Up-regulation of Sox2 is one of the first responses of the specified ectoderm to the inductive signals; Sox2 expression precedes lens placode formation, and its expression is dependent on signals from the OV (Furuta and Hogan 1998; Kamachi et al. 1998; Zygar et al. 1998). Furthermore, Sox2 is not up-regulated in the SE of Pax6, BMP4, or BMP7 mutant embryos, in which lens induction is impaired (Furuta and Hogan 1998; Wawersik et al. 1999). In the Le-mutant embryos, the early, normal expression of Pax6 in the eye was sufficient for the up-regulation of Sox2. These results demonstrate that up-regulation of Sox2 is dependent on Pax6, while continuous Pax6 activity in the SE is not required for the maintenance of Sox2 expression. By employing Le-mutant, we can not define whether Pax6 in the responding SE or Pax6 in the inducing OV mediates the initial up-regulation of Sox2. The Le-mutant nevertheless allowed us, for the first time, to address directly the autonomous function of Pax6 in the SE following lens specification during the formation of the lens placode.
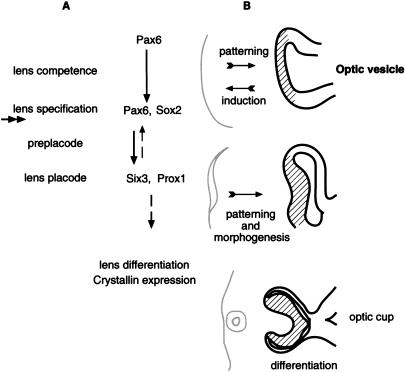
The preplacode stage precedes the appearance of the lens placode. During this stage, feedback signals are required to maintain the expression of Pax6 and other genes in the ectoderm (Grindley et al. 1995; Wawersik et al. 1999). BMP7 has been implicated in playing a role in this step (Wawersik et al. 1999). The expression of Six3 (homolog of the Drosophila sine-oculis gene; Oliver et al. 1995) and Prox1 (homolog of the Drosophila prospero gene; Oliver et al. 1993) have been reported to coincide with the lens placode stage. In this article, we demonstrate that Pax6 is essential in the preplacode for lens placode formation (Fig. 7). This conclusion is based on the following observations: In the Le-mutant, the lens placode did not form. Furthermore, the transcriptional activity of the Le-Cre transgene was not maintained. Finally, the expression of Six3 and Prox1 in the specified ectoderm is absent and, thus, dependent on Pax6.
Figure 7.
A scheme summarizing the possible roles of Pax6 in the SE leading to lens placode formation (A). Three steps precede the formation of the lens placode: lens competence, lens specification, and the preplacode (maintenance) stage. The competence of the ectoderm to respond to the inductive signals from the OV is mediated by Pax6. During lens induction, the upregulation of Sox2 is dependent on Pax6 activity. Inactivation of Pax6 in Le-mutant occurred concomitantly with lens induction and after the up-regulation of Sox2 (the approximate time of inactivation is marked with double arrow). The expression of lens specific genes, Six3 and Prox1, in the preplacode stage is dependent on Pax6 activity. Feedback signals possibly mediated by six3 are required to maintain Pax6 expression in the SE (dashed arrow). The regulatory genes promote lens placode formation and subsequent lens differentiation. (B) Schematic presentation of the influence of the early lens structures (gray) on the patterning and morphology of the OV (black) in mice. The initial patterning of the OV to NR (lines) and RPE progenitors is not dependent on Pax6 activity in the specified ectoderm or on lens placode formation. However, the interaction with the early lens structures is required to define the position of the optic cup and for maintaining the identity of the presumptive NR and RPE domains. Subsequent differentiation of the retina is not dependent on lens structures.
The dependence of several regulatory genes on Pax6 function after lens formation probably accounts for the complete arrest in lens development observed in Le-mutant. The role of Six3 is currently unknown; however, members of the vertebrate Six genes, HSIX1 and XOptx2, have been shown to affect cell proliferation (Ford et al. 1998; Zuber et al. 1999). It is possible that loss of Six3 in the SE causes a failure in the onset of proliferation, which is required for lens placode formation. Prox1 has been recently shown to play an important role in lens fiber elongation (Wigle et al. 1999), while Pax6 was inferred to play a direct role in regulation of crystalline expression (Cvekl and Piatigorsky 1996). Thus, probably both Prox1 and Pax6 are essential during later stages of lens development (Fig. 7).
Vertebrate gene families, homologous to the Drosophila eye-determination genes, have been identified recently, and the regulatory interactions between them seem to be conserved and redeployed during vertebrate somite development (Heanue et al. 1999; Relaix and Buckingham 1999). The complete dependence of Six3 expression on Pax6 activity in the specified ectoderm of the Le-mutant is similar to the reported dependence of so on eye expression described in Drosophila (Halder et al. 1998). This result implies that the regulatory hierarchy between Pax6 and Six3 in the SE during the preplacode stage is conserved in evolution. In the OV, however, these genes seem to function in parallel pathways as the expression of the vertebrate so homologs Six3 and Six6/optx2 is maintained in the Pax6−/− mice (Jean et al. 1999; Xu et al. 1999).
Existence of a positive feedback loop for maintaining Pax6 expression during the preplacode stage has been proposed based on the BMP7−/− phenotype (Wawersik et al. 1999). Furthermore, loss of ectodermal Pax6 expression during lens placode formation was observed (Grindley et al. 1995). In line with these results, we observed that the Le-Cre transgene was not transcriptionally active in the Le-mutant SE after E9.5. This indicates that the transcription activity of the Pax6 regulatory regions in the transgene depends on Pax6, and thus, confirms the requirement for an autoregulatory feedback loop. In Drosophila, so participates in a positive feedback loop to maintain eye expression in the eye disc. Therefore, it is possible that Six3 in the preplacode is part of a regulatory loop mediating the maintenance of Pax6 expression (Fig. 7). Indeed, up-regulation of Pax6 on misexpression of Six3 was demonstrated in transgenic mice, and Six3 binding sites have been identified on the Pax6 regulatory sequences employed in the Le-Cre transgene (G. Goudreau and P. Gruss, unpubl.).
The influence of the lens, after the lens vesicle stage, on the development of the retina was studied by mechanical ablation of the lens (Coulombre and Coulombre 1964) and targeted expression of toxins (Kaur et al. 1989; Harrington et al. 1991). In these studies, convolutions of the retina were observed. These folds were attributed to change in ocular pressure, and it was concluded that following the formation of the optic cup and the lens vesicle, retinal development is mostly independent from the lens. The roles of the early signals emanating from the lens primordium to the OV were addressed by early experiments of classical embryologists (Lopashov and Stroeva 1964) and in recent work in chicks (Hyer et al. 1998). Two main functions were attributed to the early signals: delineation of the prospective NR and the RPE domains from the bipotential population of the OV precursors, and mediation of the parallel invaginations of the two tissues to form the lens vesicle and the optic cup (Stroeva 1960; Barnstable 1987).
There are only few mouse mutants, including Pax6 (Grindley et al. 1995), BMP7 (Wawersik et al. 1999), and BMP4 (Furuta and Hogan 1998), in which the absence of the lens placode has been reported. In these mutants, as well as in the Pax6 chimera studies (Quinn et al. 1996; Collinson et al. 2000), the influence of the SE on the development of the OV could not be directly addressed, mainly because of direct requirement for these genes in both the inducting OV as well as in the responding SE. The absence of a lens placode in our conditional Le-mutant mice exposed the in vivo role of the earliest lens structures in retina patterning, morphology, and differentiation.
The delay in invagination of the OV in the E10 Le-mutant eye was the first obvious morphological phenotype of the retina. This phenotype reveals the essential role of the lens placode for optic cup formation. Despite this morphological change, the initial distribution of Trp2 and Chx10 transcripts in the OV suggests that the separation of the bipotential population of cells in the OV to the prospective RPE and NR domains occurred in the Le-mutant. This result supports the view that the progenitor domains in the OV are established independent from the lens placode and possibly before the placode is formed (Hyer et al. 1998; Fig. 7).
At subsequent stages of development (E11), several invaginations were observed in the Le-mutant OV. These retina folds seem to form separate retina subcompartments based on the histology of the Le-mutant eyes, the distribution of Pax6 and TUJI staining in each retina fold, and the expression of Trp2 between the folds. Moreover, some of the NR domains evolved in proximal regions that opposed the mesenchyme, while RPE differentiated from distal parts of the OV (Figs. 4,5). This suggests that a change in progenitor cell fate has occurred in these regions.
There are several possible explanations for the formation of the retina subcompartments in Le-mutant eyes: The signals from the lens might influence the pattern of cell proliferation or the timing of retina cell differentiation. Both alternations would result in a change in the distribution and/or an increase of overall cell number in the retina, leading to irregular folding of the retina within the limited space of the eye. Changes in ocular pressure might also contribute to the extensive folding of the retina, as suggested by the early works on lens ablation (Coulombre and Coulombre 1964). Such folds might result in subcompartments that give rise to multiple retinas. Change in retinal cell fate has been reported to occur in vertebrates before cup formation, either because of changes in ocular pressure (Stroeva 1960) or as result of disturbance of contact between the NR and RPE (Orts-Llorca and Genis-Galvez 1960). The delay in optic cup formation in Le-mutant eye might, therefore, result in subsequent changes in progenitor cell fate leading to apparent patches of NR and RPE domains.
Following the invagination, each retina fold seems to develop autonomously. In each fold, a central to peripheral pattern of neuronal cell differentiation was observed, similar to the pattern of neuronal cell differentiation in normal retina (Snow and Robson 1994). In postnatal Le-mutant eyes, the different retinal neuronal cell types were detected and the neuroretina was laminated. Hence, the capacity to form a multilayered retina is autonomous to each fold and is independent of any lens structures.
Our results demonstrate that signals from the early lens structures play a role in maintaining the fate of retina progenitors present in the OV. The signals from the early lens structures that influence the morphology of the underlying retina could be mechanical (such as pressure from the growing lens) and molecular. Various growth factors, signaling molecules and their corresponding receptors, were shown to be expressed during early eye development (McAvoy and Chamberlain 1990; Bao and Cepko 1997; Dudley and Robertson 1997; Jasoni et al. 1999; Wawersik et al. 1999; Enwright and Grainger 2000). The Le-mutant provides a unique mutant model to resolve and to identify, in future studies, the molecular mechanism underlying the influence of the lens placode on the developing retina.
The summary of our results presented schematically in Figure 7 shows that the spatially and temporally defined somatic mutation allowed us both to address the autonomous functions of Pax6 in the SE and to define the instructive role of Pax6 mutant cells on the development of adjacent wild-type retina. Studies employing this approach, therefore, allow the addressing of questions previously not available to in vivo investigations.
Materials and methods
Mouse lines
Pax6flox/Pax6+ mice were generated by homologous recombination in R1 embryonic stem cells according to standard procedures. Positive clones were used to produce chimeric animals by morula aggregation. Chimeras were mated to NMRI mice for germ-line transmission. Deletion of the selection cassette was achieved by crossing with the HACTB::Flp mice (Dymecki 1996), resulting in the complete reversion of the phenotype in Pax6flox/Pax6+ mice. The Paxflox/Pax6+ mice have been maintained on an outbred genetic background (NMRI). Le-Cre transgenic lines were generated by microinjections (Hogan et al. 1994) and maintained in an FVB inbred background. Analysis of Cre-mediated recombination pattern in Le-Cre line was performed by mating to the Z/AP reporter line as described (Lobe et al. 1999). The Le-Cre mice were crossed with Pax6lacZ/Pax6+ mice (St-Onge et al. 1997) to obtain F1 Pax6lacZ/Pax6+;Le-Cre mice (FVB), which were further crossed with Pax6flox/Pax6+ mice. The genotypes were determined by PCR analysis on DNA extracted from the tail or yolk sac. Noon of the day of vaginal plug observation was considered E0.5 of embryogenesis. Somites were counted for verification of the embryonic age.
Immunohistochemistry
Immunohistochemistry was performed on cryosections or dewaxed sections. The sections were blocked for 2 h in PBSTG (0.2% Tween 20, 0.2% gelatin in PBS), incubated overnight with primary antibodies, washed with PBSTG, incubated 2 h at room temperature with the secondary antibodies, washed with PBSTG, and mounted in moviol. Unspecific fluorescence from adjacent nonexpressing tissue on the same slide served to define the background levels. Primary antibodies were αA-crystallin (kindly provided by Dr. K. Kato, Institute for Developmental Research, Aichi Human Service Center, Kasugai, Japan), Brn3B (Santa Cruz Biotechnology), hAP (Sigma), Pax6 polyclonal (BAbCO), Synatxin (Sigma), PKC (Sigma), Six3 polyclonal antibodies (kindly provided by Dr. G. Oliver, St. Jude Children's Research Hospital, Memphis, Tennessee), Sox2 polyclonal antibodies (kindly provided by Dr. R. Lovell-Badge, National Institute for Medical Research, London, England), and TUJI (BAbCO). Monoclonal antibody to Pax6 was developed by A. Kawakami, monoclonal antibody to the NF165kDa by T. M. Jessell and J. Dodd; both antibodies were obtained from developmental studies hybridoma bank maintained by the Department of Biological Sciences, Iowa City, IA. Secondary antibodies were Alexa 488 or 594-conjugated goat antimouse or Goat antirabbit IgG (MoBiTec).
In situ hybridization
To detect Trp2 (Steel et al. 1992), Chx10 (Liu et al. 1994), Prox1 (Oliver et al. 1993), Six3 (Oliver et al. 1995), and mRNA single-stranded antisense RNA35S-UTP probes were produced by in vitro transcription of gene-specific fragments. The procedure of hybridization was as described (Stoykova and Gruss 1994).
Microscopy
Fluorescent images were taken with a confocal laser-scanning system consisting of a SLM 410 Zeiss confocal microscope with a 20× or 40× oil objective.
Acknowledgments
We thank Thomas Butterbrodt and Elke Wischmeyer for excellent technical assistance; B. Meyer, S. Mahsur, and T. Schulz for their contribution in establishing the targeted allele; U. Franke for the microinjection; C. Lobe and A. Nagy for Z/AP mice; S. Dymecki for the HACTB::Flp mice; L. St-Onge and B. Kammandal for Pax6 genomic fragments used in the constructs; E.N. Meyers and G.R. Martin, K. Fellenberg and K. Rajewsky, F. Buchholz and F.A. Stewart for reagents; L. Pevny and R. Lovell-Badge for Sox2 antibody; K. Kato for αA-crystallin antibody; and G. Oliver for Six3 antibodies. We also thank G. Goudreau for sharing unpublished data and A. Stoykova, M. Salminen, and M. Belaoussoff for critical comments on the manuscript. R.A.-P. was supported by a long-term EMBO fellowship. This research was supported by the Max Planck Society and the EU BIO4 CT 960042.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL pgruss@gwdg.de; FAX 49-551-201-1504
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.184000.
References
- Bao ZZ, Cepko CL. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ. A molecular view of vertebrate retinal development. Mol Neurobiol. 1987;1:9–46. doi: 10.1007/BF02935263. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Hill RE, West JD. Different roles for pax6 in the optic vesicle and facial epithelium mediate early morphogenesis of the murine eye. Development. 2000;127:945–956. doi: 10.1242/dev.127.5.945. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL. Lens development. I. Role of the lens in eye growth. J Exp Zool. 1964;156:39–47. doi: 10.1002/jez.1401560104. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: Many roles for Pax-6. Bioessays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Dymecki SM. Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. Proc Natl Acad Sci. 1996;93:6191–6196. doi: 10.1073/pnas.93.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwright JF, III, Grainger RM. Altered retinoid signaling in the heads of small eye mouse embryos. Dev Biol. 2000;221:10–22. doi: 10.1006/dbio.2000.9652. [DOI] [PubMed] [Google Scholar]
- Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: A possible mechanism of breast carcinogenesis. Proc Natl Acad Sci. 1998;95:12608–12613. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Uchida T, Osumi-Yamashita N, Eto K. Uchida rat (rSey): A new mutant rat with craniofacial abnormalities resembling those of the mouse Sey mutant. Differentiation. 1994;57:31–38. doi: 10.1046/j.1432-0436.1994.5710031.x. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes & Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser TD, Walton S, Cai J, Epstein J, Jepeal L, Mass RL. Pax6 gene mutations in aniridia. In: Wiggs J, editor. Molecular genetics of ocular diseases. New York: Wiley Liss; 1995. pp. 51–82. [Google Scholar]
- Grainger RM. Embryonic lens induction: Shedding light on vertebrate tissue determination. Trends Genet. 1992;8:349–355. doi: 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- Grainger RM, Mannion JE, Cook TL, Jr, Zygar CA. Defining intermediate stages in cell determination: Acquisition of a lens-forming bias in head ectoderm during lens determination. Dev Genet. 1997;20:246–257. doi: 10.1002/(SICI)1520-6408(1997)20:3<246::AID-DVG7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophilacompound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- Harrington L, Klintworth GK, Secor TE, Breitman ML. Developmental analysis of ocular morphogenesis in α A- crystallin/diphtheria toxin transgenic mice undergoing ablation of the lens. Dev Biol. 1991;148:508–516. doi: 10.1016/0012-1606(91)90269-9. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Reshef R, Davis RJ, Mardon G, Oliver G, Tomarev S, Lassar AB, Tabin CJ. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophilaeye formation. Genes & Dev. 1999;13:3231–3243. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): A homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development. 1998;125:869–877. doi: 10.1242/dev.125.5.869. [DOI] [PubMed] [Google Scholar]
- Jasoni C, Hendrickson A, Roelink H. Analysis of chicken Wnt-13 expression demonstrates coincidence with cell division in the developing eye and is consistent with a role in induction. Dev Dyn. 1999;215:215–224. doi: 10.1002/(SICI)1097-0177(199907)215:3<215::AID-AJA4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Jean D, Bernier G, Gruss P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/hypothalamic axis and the ventral optic stalk. Mech Dev. 1999;84:31–40. doi: 10.1016/s0925-4773(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development. 1998;125:2521–2532. doi: 10.1242/dev.125.13.2521. [DOI] [PubMed] [Google Scholar]
- Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- Kaur S, Key B, Stock J, McNeish JD, Akeson R, Potter SS. Targeted ablation of α-crystallin-synthesizing cells produces lens-deficient eyes in transgenic mice. Development. 1989;105:613–619. doi: 10.1242/dev.105.3.613. [DOI] [PubMed] [Google Scholar]
- Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, Kalnins VI, McInnes RR. Developmental expression of a novel murine homeobox gene (Chx10): Evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Lopashov GV, Stroeva OG. Advances in morphogenesis. New York: Academic Press; 1961. Morphogenesis of the vertebrate eye; pp. 331–377. [Google Scholar]
- ————— . Development of the eye. Moscow: Israel Program for Scientific Translations; 1964. [Google Scholar]
- Macdonald, R. and Wilson, S.W. 1997. Distribution of Pax6 protein during eye development suggests discrete roles in proliferative and differentiated visual cells. Dev. Genes Evol. 363–369. [DOI] [PubMed]
- Matsuo T, Osumi-Yamashita N, Noji S, Ohuchi H, Koyama E, Myokai F, Matsuo N, Taniguchi S, Doi H, Iseki S, et al. A mutation in the Pax-6gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet. 1993;3:299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Growth factors in the eye. Prog Growth Factor Res. 1990;2:29–43. doi: 10.1016/0955-2235(90)90008-8. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Gunther EC, Reh TA. The development of the pattern of retinal ganglion cells in the chick retina: Mechanisms that control differentiation. Development. 1999;126:5713–5724. doi: 10.1242/dev.126.24.5713. [DOI] [PubMed] [Google Scholar]
- Oguni M, Setogawa T, Hashimoto R, Tanaka O, Shinohara H, Kato K. Ontogeny of alpha-crystallin subunits in the lens of human and rat embryos. Cell Tissue Res. 1994;276:151–154. doi: 10.1007/BF00354794. [DOI] [PubMed] [Google Scholar]
- Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Orts-Llorca F, Genis-Galvez JM. Experimental production of retinal septa in the chick embryo: Differentiation of pigment epithelium into neural retina. Acta Anat. 1960;42:31–79. doi: 10.1159/000141635. [DOI] [PubMed] [Google Scholar]
- Pei YF, Rhodin JA. The prenatal development of the mouse eye. Anat Rec. 1970;168:105–125. doi: 10.1002/ar.1091680109. [DOI] [PubMed] [Google Scholar]
- Quinn JC, West JD, Hill RE. Multiple functions for Pax6 in mouse eye and nasal development. Genes & Dev. 1996;10:435–446. doi: 10.1101/gad.10.4.435. [DOI] [PubMed] [Google Scholar]
- Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol. 1995;5:1286–1295. doi: 10.1016/s0960-9822(95)00255-7. [DOI] [PubMed] [Google Scholar]
- Relaix F, Buckingham M. From insect eye to vertebrate muscle: Redeployment of a regulatory network. Genes & Dev. 1999;13:3171–3178. doi: 10.1101/gad.13.24.3171. [DOI] [PubMed] [Google Scholar]
- Saha MS, Servetnick M, Grainger RM. Vertebrate eye development. Curr Opin Genet Dev. 1992;2:582–588. doi: 10.1016/s0959-437x(05)80176-5. [DOI] [PubMed] [Google Scholar]
- Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND. Influence of PAX6 gene dosage on development: Overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Knoedlseder M, Favor J, Davidson D. Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6-locus. Acta Neuropathol. 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- Snow RL, Robson JA. Ganglion cell neurogenesis, migration and early differentiation in the chick retina. Neuroscience. 1994;58:399–409. doi: 10.1016/0306-4522(94)90046-9. [DOI] [PubMed] [Google Scholar]
- ————— Migration and differentiation of neurons in the retina and optic tectum of the chick. Exp Neurol. 1995;134:13–24. doi: 10.1006/exnr.1995.1032. [DOI] [PubMed] [Google Scholar]
- Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing α-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeva OG. Experimental analysis of the eye morphogenesis in mammals. J Embryol Exp Morph. 1960;8:349–368. [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Walther C, Guenet JL, Simon D, Deutsch U, Jostes B, Goulding MD, Plachov D, Balling R, Gruss P. Pax: A murine multigene family of paired box-containing genes. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Williams SC, Altmann CR, Chow RL, Hemmati-Brivanlou A, Lang RA. A highly conserved lens transcriptional control element from the Pax-6 gene. Mech Dev. 1998;73:225–229. doi: 10.1016/s0925-4773(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zhang X, Heaney S, Yoon A, Michelson AM, Maas RL. Regulation of Pax6 expression is conserved between mice and flies. Development. 1999;126:383–395. doi: 10.1242/dev.126.2.383. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Perron M, Philpott A, Bang A, Harris WA. Giant eyes in Xenopus laevisby overexpression of XOptx2. Cell. 1999;98:341–352. doi: 10.1016/s0092-8674(00)81963-7. [DOI] [PubMed] [Google Scholar]
- Zygar CA, Cook TL, Grainger RM., Jr Gene activation during early stages of lens induction in Xenopus. Development. 1998;125:3509–3519. doi: 10.1242/dev.125.17.3509. [DOI] [PubMed] [Google Scholar]