Abstract
Pharmacologic doses of growth hormone (GH) reduce HIV-associated fat accumulation but may worsen glucose metabolism. We investigated the effects of a low dose of GH (1 mg per day) in HIV-infected men with fat accumulation and found that such treatment reduced total fat and increased lean body mass without significant changes in glucose tolerance or insulin sensitivity. Visceral adipose tissue (VAT) levels did not change significantly for the group as a whole, although a reduction in the VAT level was seen in patients with a greater VAT level at baseline.
In the current era of HAART, various syndromes of altered fat distribution, including fat accumulation in the dorsocervical region and abdomen, have been reported in patients with HIV infection [1–3]. For many patients, these body composition changes have adversely affected quality of life [4]. In addition, there are rising concerns that they may be associated with insulin resistance and cardiovascular risk [3, 5, 6].
Previously, we reported that a 6-month course of treatment with a pharmacologic dose of growth hormone (GH) at 3 mg per day reduced total body fat and excess visceral adipose tissue (VAT) in patients with HIV-associated fat accumulation [7]. After an initial worsening in glucose homeostasis, presumably caused by GH-induced hepatic and peripheral insulin resistance, there was subsequent improvement toward pretreatment levels at the end of the study [7, 8]. Other published studies [9, 10], including recently reported data from a large randomized trial of GH therapy [11], confirm the efficacy of pharmacologic GH therapy in reducing total and visceral fat levels (GH dosage, 4–6 mg daily or on alternate days). These studies also suggest the possibility of a dose-response effect with GH [10–11]. Because many patients receiving HAART who experience excess fat accumulation are insulin resistant [3, 6] and thus predisposed to frank diabetes with GH treatment, we thought that it was important to investigate the effectiveness of a lower GH dose in this patient population. The current pilot study was undertaken to determine the metabolic and body composition effects of GH at a dosage of 1 mg per day.
Methods
Five HIV-positive men who had experienced abdominal girth increase and dorsocervical fat pad enlargement while receiving antiretroviral therapy were enrolled in the study. All 5 men had a waist-to-hip ratio >0.95 and a waist circumference >90 cm (table 1). Patients with overt diabetes, abnormal glucose tolerance (2 h–glucose level of >140 mg/dL after a 75-g oral glucose load), fasting triglyceride level ≫1000 mg/dL, or active malignancies were excluded. The average duration of HIV infection (±SD) was 11 ± 4 years. All patients were receiving a stable antiretroviral regimen that they continued to receive during the study (table 1). None had received systemic glucocorticoids or megestrol acetate in the previous 5 years. The study was approved by the Committee on Human Research at the University of California, San Francisco, and written consent was obtained from each subject before enrollment.
Table 1.
Baseline characteristics of subjects in a pilot study of the effects of low-dose growth hormone in HIV-infected men with fat accumulation.
| Patient | Age, years | BMI | Waist circumference, cm | Waist-to-hip ratio | CD4+ cell count, cells/μL | Antiretroviral regimena |
|---|---|---|---|---|---|---|
| 1 | 48 | 25.7 | 93.0 | 0.98 | 412 | D4T, 3TC, IDV, RTV |
| 2 | 49 | 23.8 | 92.5 | 0.98 | 449 | RTV, AMP, EFV |
| 3 | 46 | 28.0 | 94.5 | 1.00 | 527 | DDI, 3TC, NEV |
| 4 | 58 | 26.1 | 102.5 | 1.04 | 1433 | 3TC, IDV, EFV |
| 5 | 41 | 29.8 | 102.2 | 1.02 | 464 | AZT, 3TC, RTV/LPV |
NOTE. All patients had experienced both enlargement of the dorsocervical fat pad and an increase in abdominal girth; in addition, patients 1, 2, and 4 also complained of loss of fat in the face and extremities. AMP, amprenavir; AZT, zidovudine; BMI, body mass index (calculated as weight [in kg] divided by height [in m] squared); DDI, didanosine; D4T, stavudine; EFV, efavirenz; IDV, indinavir; LPV, lopinavir; NEV, nevirapine; RTV, ritonavir; 3TC, lamivudine.
All patients received the same antiretroviral regimen for ≥6 months prior to the study, with the exception of patient 2, who underwent a transient (<1 month) substitution of delavirdine for RTV 2 months before the study but then returned to his stable regimen of RTV, AMP, and EFV, which he had been receiving for the previous year. Patient 2 was also receiving human chorionic gonadotropin (for Kaposi sarcoma in remission) and atorvastatin, and patient 3 was receiving testosterone (replacement), gemfibrozil, and niacin during the study.
Subjects were admitted to the General Clinical Research Center at San Francisco General Hospital and were placed on a diet with fixed nutrient proportions, as described elsewhere [7]. Subjects underwent a 5-day inpatient metabolic study that included total and regional body composition studies, fasting lipid level measurements, an oral glucose tolerance test, and a euglycemic hyperinsulinemic clamp. Thereafter, subjects began treatment with GH (Serono Laboratories) at 1 mg per day (11–14 μg/kg per day) by subcutaneous injection. The same 5-day metabolic ward assessments were performed at month 1 and month 6 of GH therapy.
Weight, height, and anthropometric measurements were performed as described elsewhere [7], including buffalo hump size, which was measured as length times width along surface contours. Fat and lean body mass (LBM) were measured by dual-energy x-ray absorptiometry (Lunar model DPX), with manual analyses of regional fat [1, 7]. Abdominal adipose tissue was measured by CT using a HiSpeed CT/i Scanner (GE Medical Systems), and visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) area was determined at the level of the fourth to fifth lumbar vertebrae disc space, as described elsewhere [7].
Fasting lipid, lipoprotein, free fatty acid, glucose, glycosylated hemoglobin (HgA1C), and insulin-like growth factor type 1 (IGF-1) levels and CD4 T-lymphocyte cell count were measured as described previously [7]. A 75 g oral glucose tolerance test was performed after a 10-h overnight fast, and the area-under-curve (AUC) for glucose was calculated as described elsewhere [7]. Peripheral insulin sensitivity was measured by a 3-h euglycemic, hyperinsulinemic clamp according to the method of DeFronzo [12] and as used by us previously [7]. Peripheral insulin sensitivity (M value) was calculated on the basis of steady state glucose infusion rates during the final 60 min of the clamp and LBM (in kilograms), and it was adjusted for the steady-state insulin concentration (I) achieved (M/I) [7, 12].
Oxygen consumption and carbon dioxide production were measured under fasting conditions and during the clamp by indirect calorimetry (DeltaTrac metabolic cart; SensorMedics), and resting energy expenditure and substrate oxidation rates were calculated with use of stoichiometrically-derived equations [13].
Data are expressed as mean ± SD. Differences between data obtained at baseline and data obtained at 6 months of therapy were analyzed by Student’s paired t test for continuous outcomes. Measurements performed at baseline, 1 month, and 6 months were analyzed using analysis of variance for repeated measures. If the global comparison was statistically significant, pairwise comparisons were conducted using the Student-Newman-Keuls test for multiple comparisons. A 2-sided P value of <.05 was considered statistically significant. All analyses were performed using SigmaStat, software version 2.0 (SPSS).
Results
Overall, GH was well tolerated, with the most-common complaints being very mild arthralgias (in 2 patients) and nonpitting edema in the extremities (in 1 patient) that improved over the course of the study. However, patient 3 developed carpal tunnel syndrome; the dose of GH was reduced to 0.5 mg per day for 2 weeks and was then discontinued at month 4 of therapy. His final study measurements were performed just prior to discontinuation.
Total body fat and trunk fat decreased and lean body mass increased in all subjects (table 2). There were no significant changes in appendicular fat or body weight. Three patients experienced a reduction in VAT of 14%–54% (figure 1), whereas 2 patients experienced an increase in VAT of 7%–10%; for the group as a whole, there were no significant changes in VAT, abdominal SAT, waist circumference, or waist-to-hip ratio. All patients had a reduction in buffalo hump size ranging from 13% to 89% (median reduction, 56%).
Table 2.
Body composition during growth hormone treatment in a pilot study of the effects of low-dose growth hormone in HIV-infected men with fat accumulation.
| Method, measure | Baseline | Month 1 of therapy | Month 6 of therapy | Pa |
|---|---|---|---|---|
| Dual energy x-ray absorptiometry, mean kg ± SD | ||||
| Total body fat | 19.0 ± 3.9 | 17.5 ± 3.2 | 16.3 ± 4.8b | .04 |
| Trunk fat | 12.9 ± 2.0 | 11.8 ± 2.1 | 10.7 ± 2.9b | .04 |
| Appendicular fat | 5.2 ± 1.6 | 4.8 ± 0.9 | 4.8 ± 1.6 | .42 |
| Lean body mass | 59.8 ± 4.2 | 61.1 ± 4.8 | 63.6 ± 5.4b | .008 |
| Abdominal CT, mean cm2 ± SD | ||||
| Visceral fat | 197.7 ± 107.0 | … | 162.0 ± 83.7 | .20 |
| Subcutaneous fat | 185.0 ± 14.8 | … | 170.6 ± 44.1 | .38 |
P values were obtained using repeated measures analysis of variance for values (for fat and lean body mass) at baseline, month 1, and month 6 of therapy and paired t test for values (visceral and subcutaneous fat area) at baseline and month 6 of therapy.
P < .05, compared with baseline, by Student-Newman-Keuls test.
Figure 1.
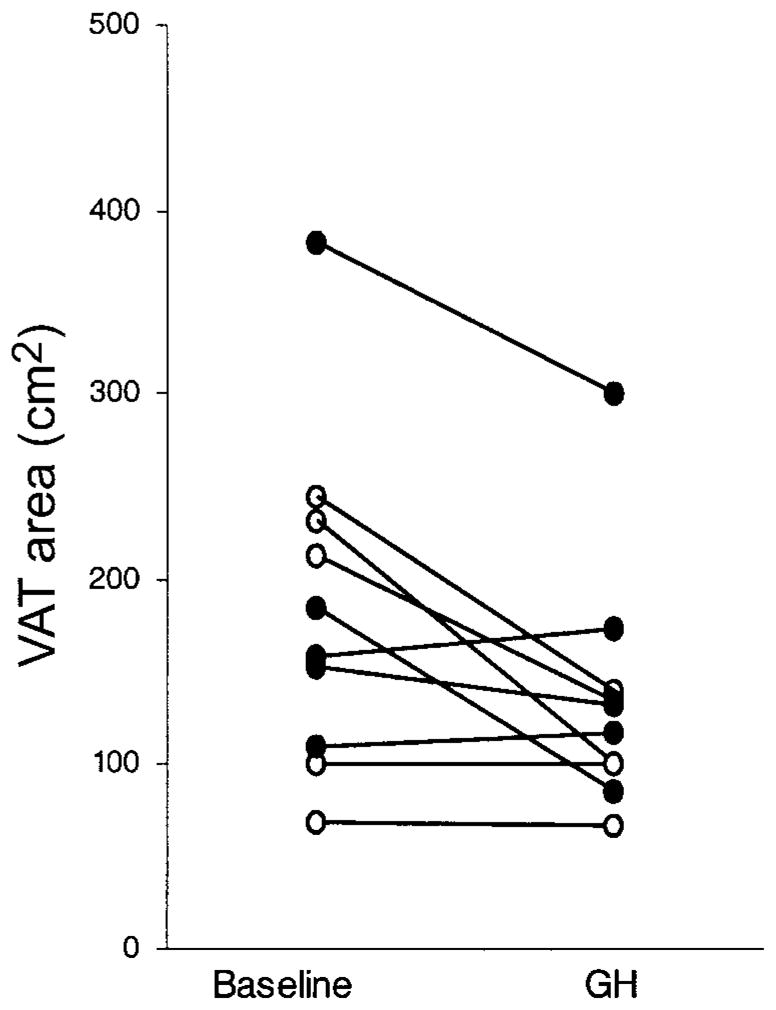
Changes in abdominal visceral adipose tissue (VAT) at the level of the fourth to fifth lumbar vertebrae disc space after treatment with growth hormone (GH) for 6 months in the present study and in a previously reported study [7]. ●, the 5 patients from the current study who received 1 mg of GH per day; ○, the 5 patients from our previous study [7] who received 3 mg of GH per day.
IGF-1 levels increased significantly at both month 1 (315 ± 73 ng/mL; P < .001 ) and month 6 of therapy (353 ± 133 ng/mL; P < .001 ), compared with baseline (142 ± 67 ng/mL). During the 6 months of treatment, there were no significant changes in fasting free fatty acid levels; triglyceride levels; total, high-density lipoprotein cholesterol levels; and calculated low-density lipoprotein cholesterol levels; nor were there significant changes in fasting glucose levels or HgA1C. Although a trend to transient worsening in glucose tolerance was noted in 4 patients (followed by improvement towards baseline in 3 patients), overall differences were not significant (glucose AUC, 431 ± 15, 464 ± 47, and 438 ± 47 mg × h/dL at baseline, month 1, and month 6, respectively; P = .30). There was also no significant change in insulin sensitivity, as measured by the euglycemic hyperinsulinemic clamp (M/I, 4.97 ± 3.35, 3.52 ± 2.84, and 4.43 ± 1.73 mg/kgLBM × min/μUINSULIN/mL [×100], respectively; P =.44).
Resting energy expenditure was unchanged during GH treatment (37.4 ± 1.0, 38.0 ± 2.3, and 37.3 ± 1.8 kcal/kgLBM per day at baseline, month 1, and month 6, respectively; P = .57). There were trends to increased fasting lipid oxidation (1.34 ± 0.23, 1.49 ± .20, and 1.56 ± 0.12 mg/kgLBM/min, respectively; P = .065 ) and decreased carbohydrate oxidation (2.34 ± 0.55, 2.17 ± 0.66, and 1.89 ± 0.31 mg/kgLBM/min, respectively; P = .09). Neither resting energy expenditure nor substrate oxidation rates measured during hyperinsulinemia changed with GH treatment.
Discussion
These results indicate that even at a dosage as low as 1 mg per day, GH reduces total body fat and trunk fat and increases LBM in HIV-infected men with fat accumulation. The average magnitude of fat loss was slightly more than one-half that seen in our previous study of GH at 3 mg per day [7], consistent with more modest effects of the lower dose on lipid oxidation. In the present study, lipid oxidation increased by only 16%, whereas it increased by 84% in patients treated with GH at 3 mg per day (P < .001) [8].
It is interesting that, in both this and our former study [7], patients who lost VAT during GH treatment had the largest amount of VAT at baseline (figure 1). Thus, it is possible that the level of baseline VAT is a factor in determining the amount of VAT reduction with GH. The broad range of intra-abdominal fat content, despite a waist-to-hip ratio >0.95 and a waist circumference >90 cm, may have contributed to the variable response of VAT to GH in these patients. Future studies should consider whether there are additional anthropometric criteria to better identify patients with increased VAT (or perhaps excess VAT should be used as an entry criterion), because these individuals may benefit most from GH therapy. In addition, the optimal dose and duration of treatment needs to be determined. For example, in HIV-seronegative men with abdominal obesity, an even lower dosage of GH (e.g., 9.5 μg/kg per day) over a longer treatment period was effective in reducing visceral adiposity and in improving glucose and lipid metabolism [14].
Changes in appendicular fat were not observed in this study, nor were they observed in our prior pilot study [7], although further loss of subcutaneous fat with GH treatment is a potential concern in patients with peripheral lipoatrophy. Other studies have shown that appendicular fat stores may be reduced with GH treatment [10, 15], and it is possible that a similar effect will be evident after a longer period of GH treatment, despite the lower dose.
In contrast to a dosage of 3 mg per day, the effects of GH at 1 mg per day on glucose metabolism, as measured by fasting glucose, oral glucose tolerance, and insulin-mediated glucose uptake, were more modest and not statistically significant. Although we observed a lower increment in IGF-1 with a 1-mg-per-day dosage of GH, compared with a 3-mg-per-day dosage, IGF-1 levels reached the supraphysiologic range for 3 patients, and 1 patient developed carpal tunnel syndrome, requiring treatment cessation. Thus, prolonged GH treatment, even at this lower dosage, should be considered cautiously, given the adverse consequences of long-term GH excess.
In summary, the results of this pilot study suggest that treatment with GH at a dose of 1 mg per day reduces total body fat and increases LBM without significant adverse effects on glucose metabolism. These results need to be confirmed in a larger randomized study. Additional studies are also needed to determine whether this dose of GH is effective in reducing VAT, particularly in patients with documented high visceral fat content.
Acknowledgments
We thank the San Francisco General Hospital (SFGH) General Clinic Research Center Nursing and Dietary staff, the SFGH Department of Radiology, B. Chang, J. Hirai, C. Yee-Hicaiji, J. Shigenaga, V. Tai, L. Alvarez, and G. Del Puerto, for their help in performing this study, and Serono Laboratories, for their provision of growth hormone.
Financial support. The National Institutes of Health (grants DK45833 and DK54615) and a Clinical Associate Physician Award from the National Center for Research Resources (grant RR00083-41S1, to J.C.L.). All studies were conducted in the General Clinical Research Center at SFGH with support by the National Center for Research Resources, National Institutes of Health (grant RR00083).
Footnotes
Conflict of interest. J.C.L., K.M., C.G., and M.S. have received research support and/or funding from Serono Laboratories. In addition, Serono Laboratories provided the growth hormone used in this and other studies performed by these investigators. All other authors: No conflict.
References
- 1.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998;351:867–70. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–5. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 3.Saint-Marc T, Partisani M, Poizot-Martin I, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lenert LA, Feddersen M, Sturley A, Lee D. Adverse effects of medications and trade-offs between length of life and quality of life in human immunodeficiency virus infection. Am J Med. 2002;113:229–32. doi: 10.1016/s0002-9343(02)01156-7. [DOI] [PubMed] [Google Scholar]
- 5.Hadigan C, Meigs JB, Wilson PW, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909–16. doi: 10.1086/368185. [DOI] [PubMed] [Google Scholar]
- 6.Addy CL, Gavrila A, Tsiodras S, Brodovicz K, Karchmer AW, Mantzoros CS. Hypoadiponectinemia is associated with insulin resistance, hyper-triglyceridemia, and fat redistribution in human immunodeficiency virus–infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab. 2003;88:627–36. doi: 10.1210/jc.2002-020795. [DOI] [PubMed] [Google Scholar]
- 7.Lo JC, Mulligan K, Noor MA, et al. The effects of recombinant human growth hormone on body composition and glucose metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2001;86:3480–7. doi: 10.1210/jcem.86.8.7785. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz JM, Mulligan K, Lee J, et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87:942. doi: 10.1210/jcem.87.2.8391. [DOI] [PubMed] [Google Scholar]
- 9.Wanke C, Gerrior J, Kantaros J, Coakley E, Albrecht M. Recombinant human growth hormone improves the fat redistribution syndrome (lipodystrophy) in patients with HIV. AIDS. 1999;13:2099–103. doi: 10.1097/00002030-199910220-00013. [DOI] [PubMed] [Google Scholar]
- 10.Engelson ES, Glesby MJ, Mendez D, et al. Effect of recombinant human growth hormone in the treatment of visceral fat accumulation in HIV infection. J Acquir Immune Defic Syndr. 2002;30:379–91. doi: 10.1097/00042560-200208010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kotler DP, Muurahainen N, Grunfeld C, et al. Effects of growth hormone on abnormal visceral adipose tissue accumulation and dyslipidemia in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35:239–52. doi: 10.1097/00126334-200403010-00004. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 14.Johannsson G, Marin P, Lonn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82:727–34. doi: 10.1210/jcem.82.3.3809. [DOI] [PubMed] [Google Scholar]
- 15.Tai VW, Schambelan M, Algren H, Shayevich C, Mulligan K. Effects of recombinant human growth hormone on fat distribution in patients with human immunodeficiency virus–associated wasting. Clin Infect Dis. 2002;35:1258–62. doi: 10.1086/343051. [DOI] [PubMed] [Google Scholar]


