Abstract
The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM), initiated in 2000, investigates the prevalence and correlates of changes in fat distribution, insulin resistance, and dyslipidemia among human immunodeficiency virus (HIV)-infected men and women compared with a population-based group of control men and women. Between June 2000 and September 2002, 1,480 participants (1,183 HIV-infected persons and 297 controls) were enrolled in FRAM. Measurements taken included whole-body magnetic resonance imaging for quantification of regional fat, anthropometric measurements, central laboratory analysis of metabolites, and assessment of symptoms, sociodemographic factors, and lifestyle. Similar measurements were repeated among FRAM participants 4 years later (FRAM 2) for investigation of the progression of fat distribution changes, insulin resistance, and hyperlipidemia. In FRAM 2, which is ongoing, investigators are also determining the associations of subclinical cardiovascular disease, as measured by carotid intimal-medial wall thickness, with HIV infection, fat distribution changes, insulin resistance, and other proatherogenic changes in serum lipid levels. The demographic characteristics of HIV-infected FRAM men and women were comparable to those reported from a national random sampling of HIV-infected men and women receiving medical care in the United States. The representativeness of the FRAM sample increases its value as a resource for studies on fat distribution, metabolic changes, and atherosclerosis in HIV infection.
Keywords: body fat distribution, dyslipidemias, HIV infections, insulin resistance, lipodystrophy, metabolism
A human immunodeficiency virus (HIV)-associated syndrome of lipodystrophy attributed to the use of antiretroviral protease inhibitors was first described in 1998 (1). The syndrome was defined as fat loss at peripheral body sites (arms, legs, face, and buttocks) and fat gain at central body sites (dorsocervical area and abdomen), accompanied by insulin resistance and hyperlipidemia (1). In additional reports, the prevalence of the lipodystrophy syndrome varied widely, from 2 percent to 83 percent, because the reports were based on differing subjective criteria, including self-report, examination, or both (2). Furthermore, there was no consensus as to how lipodystrophy was defined (3). Investigators described lipodystrophy variously as involving the presence of 1) lipoatrophy alone, lipohypertrophy alone, or a combination of both (4–8); 2) the combination of both lipoatrophy and lipohypertrophy (9–12); 3) lipoatrophy alone (13, 14); or 4) lipohypertrophy alone (15–17). In most studies, investigators did not compare persons undergoing these clinical observations with HIV-uninfected persons in order to determine the excess prevalence above a control level.
Problems with defining the syndrome have led to difficulties in understanding associated factors, including the influence of antiretroviral drugs. Prior to the availability of protease inhibitors, metabolic changes were observed in patients with HIV, including hypertriglyceridemia and decreased levels of low density lipoprotein cholesterol and high density lipoprotein cholesterol (18, 19).
The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) was initiated in 2000 to examine the prevalence and correlates of changes in fat distribution, insulin resistance, and dyslipidemia in a geographically and ethnically diverse population of HIV-infected US men and women and a comparison group of controls. Unlike most other cross-sectional studies done at that time, FRAM used objective measures of fat distribution, including whole-body magnetic resonance imaging, for quantification of regional and total subcutaneous and visceral fat. These objective measures allow investigators to measure fat distribution as a continuous variable rather than analyze lipodystrophy as a dichotomous outcome based on clinical assessment, as was the standard in the field.
FRAM 2, which began in 2004 and is ongoing, investigates the progression of fat and metabolic changes with repeated measurements and also includes measurement of carotid intimal-medial wall thickness and more sensitive tests of disorders of glucose and fat metabolism. Plans for FRAM 2 are to elucidate the roles of HIV infection, fat distribution, and metabolic changes in subclinical cardiovascular disease or atherosclerosis.
Herein, we describe the methodology and design of the FRAM and FRAM 2 studies and the characteristics of the FRAM cohort in comparison with a known representative sample of HIV-infected adults from the HIV Cost and Services Utilization Study (HCSUS).
MATERIALS AND METHODS
The FRAM and FRAM 2 studies
Study objectives
The major objectives of FRAM were to: 1) separately study each component of the “lipodys-trophy syndrome;” 2) determine the associations among the body fat depots (unlike most other studies, FRAM does not presume a syndrome of peripheral fat loss and central fat gain but rather objectively studies the association for each body fat depot); 3) determine the factors associated with fat distribution in HIV infection by separately examining factors associated with the volume of each regional subcutaneous or visceral adipose tissue depot in comparison with controls, including use of antiretroviral drugs by class and individually (FRAM uses direct measurements of adipose tissue, unlike most other studies, which have used subjective outcomes of fat change, including participant report and clinical examination); 4) determine the HIV- and non-HIV-related factors associated with insulin resistance and dyslipidemias (among HIV studies, the ability to examine the association of regional subcutaneous and visceral adipose tissue and metabolic abnormalities is unique to FRAM); and 5) evaluate clinical methods of identifying lipodystrophy in relation to direct measures of fat. The major objectives of FRAM 2 are to: 1) assess the progression of fat and metabolic changes; 2) examine traditional and HIV-related risk factors that may lead to progression of fat and metabolic changes; and 3) define traditional and HIV-related risk factors associated with atherosclerosis.
Study designs
FRAM was funded by the National Institutes of Health as a cross-sectional study. FRAM 2 was later funded in order to restudy participants enrolled in the first FRAM study. FRAM targeted enrollment of 1,200 HIV-infected men and women and 300 control men and women. HIV-infected participants were recruited from 16 participating HIV or infectious disease clinics or cohorts (see Acknowledgments). Participating sites were selected on the basis of geographic diversity and the ability to follow the study protocol.
Control participants were enrolled from the Coronary Artery Risk Development in Young Adults (CARDIA) Study (20, 21). CARDIA participants were recruited in 1986 as a population-based sample of healthy 18- to 30 year-old Caucasian and African-American men and women from four US cities for a longitudinal study of cardiovascular disease risk factors. FRAM recruited CARDIA participants enrolled in an ancillary study, the Visceral Fat and Metabolic Rate in Young Adults (VIM) Study (22). The VIM Study recruited participants from two CARDIA sites in 1995–1996, enrolling 100 CARDIA participants from each of the CARDIA race-gender groups, with body mass indexes (weight (kg)/height (m)2) being distributed similarly above and below the race- and gender-specific medians of the CARDIA parent study. VIM participants were selected as a control group for FRAM because VIM participants 1) had previously participated in similar rigorous fat and metabolic tests; 2) had a body mass index distribution similar to that of the population-based CARDIA parent study; and 3) had a similar age range and racial makeup as the majority of HIV-infected persons in the United States.
Recruitment
Investigators at each of the 16 HIV sites were asked to enroll 75 patients. The recruitment schema was based on each site’s providing the Data Coordinating Center with a numerically coded list of all patients seen at its clinic in 1999. Each list was then randomly ordered, and names were grouped into blocks of five potential participants. The sites were given several sets of blocks for recruitment. Potential participants within consecutive blocks were contacted by telephone, letter, or e-mail, or in person during a regularly scheduled clinic visit. Initial contact was usually made by the primary care provider or study personnel after obtaining permission from the potential participant or his/her provider. A new block was provided only after at least three unsuccessful contact attempts using at least three available modes of communication (i.e., telephone, letter, clinic visit, or e-mail) had been made for each member of a block.
A planned interim assessment of recruitment by the study’s principal investigator and the National Institutes of Health was performed during the data collection phase and resulted in modifications to the recruitment strategy. The enrollment of HIV-infected women into the study was increased to better determine differences between HIV-infected women and control women. After approximately 800 HIV-infected men were enrolled, subsequent blocks sent to HIV sites consisted of African-American and Caucasian women. Recruitment and data collection were discontinued at two HIV sites because of difficulties in recruiting participants.
All VIM study participants who agreed to participate in the CARDIA Study in 2000–2001 were asked whether they would be willing to participate in the FRAM Study at the time they consented to VIM participation.
Prospective FRAM participants were excluded if they were: 1) under the age of 18 years; 2) pregnant (as determined by a urine pregnancy test at the time of consent); 3) planned to become pregnant within the next 3 months; or 4) had contraindications to magnetic resonance imaging or dual energy x-ray absorptiometry scanning (metal implants, claustrophobia, or weight greater than 136 kg and height greater than 6′5″ (77 inches or 19.6 cm), per the specifications of the scanner manufacturers).
Informed consent was obtained from all participants in accordance with guidelines for human experimentation of the US Department of Health and Human Services and the institutional review board of each participating institution.
Examination components
For each participant, data components were to be collected within a 3-month period. The final FRAM participant examination occurred in September 2002. Data collection for FRAM 2 began in October 2004. There will be an approximately 5-year interval between the FRAM and FRAM 2 examinations. Table 1 summarizes the components of the FRAM and FRAM 2 examinations.
TABLE 1.
Data collection components in the Study of Fat Redistribution and Metabolic Change in HIV* Infection (FRAM), United States, 2000–2002 (FRAM 1) and 2004–2006 (FRAM 2)
| Questionnaires | Physical examination | Imaging | Laboratory | Medical record abstraction |
|---|---|---|---|---|
Survey of body changes in the last 5 years
Survey of HIV risk factors Sociodemographic factors Medical and health history
Physical activity Family history |
Visual inspection of body sites
Anthropometric dat Blood pressure measurement |
Magnetic resonance imaging
|
Blood
|
All current medications Past medications
History (past 5 years)
HIV risk factors†, ‡ |
HIV, human immunodeficiency virus.
Performed at HIV sites only.
Performed at the first FRAM examination (2000–2002) only.
Performed at the FRAM 2 examination (2004–2006) only.
Obtained at both examinations, with additional time points from oral glucose tolerance testing performed at the FRAM 2 examination (2004–2006) only.
Quality assurance and standardization methods
FRAM research associates were centrally trained in standardized data collection and processing procedures. A detailed manual of operations was developed, distributed to each site, and regularly updated. Certification in interviewing, anthropometry, blood pressure measurement, and (at HIV sites) chart abstraction was required before participant enrollment. The Image Reading Center (located at St. Luke’s-Roosevelt Hospital Center (New York, New York)) trained magnetic resonance imaging and dual energy x-ray absorptiometry technologists on-site at the first FRAM examination. In both FRAM and FRAM 2, certification was based on images sent to the Image Reading Center. For assistance with standardization of the values obtained in dual energy x-ray absorptiometry scanning, a body phantom was scanned at all sites. In FRAM 2, the Ultrasound Reading Center (Department of Radiology, New England Medical Center (Boston, Massachusetts)) centrally trained ultrasonographers. Certification was based on images sent to the Ultrasound Reading Center. All data collection procedures in FRAM and FRAM 2 were observed at site visits.
Questionnaires
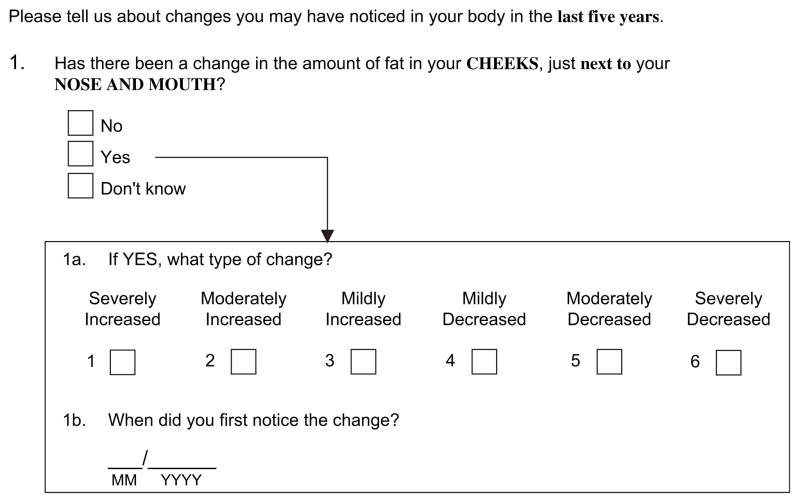
A self-administered questionnaire on body fat changes asked each HIV and control participant whether he or she had undergone any change in body fat in the last 5 years at several locations—the cheeks next to the nose, the lateral aspect of the face, or the legs, arms, buttocks (peripheral sites), back, chest, neck, or abdomen—and whether he/she had undergone a change in waist size (central sites) (figure 1).
FIGURE 1.
Example question from a self-administered survey of change in the amount of fat at a specific body site, Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM), United States, 2000–2002. HIV, human immunodeficiency virus.
Research associates obtained a detailed history of medication use, including starting and stopping dates. At HIV sites, an antiretroviral picture chart was used to assist participants in recalling antiretroviral medications taken, and questions were asked about adherence to antiretroviral medication regimens. Other information obtained included data on sociodemographic factors and medical history (e.g., history of diabetes, dyslipidemia, or cardiovascular disease; reproductive history in women; and weight history). At HIV sites, participants were asked about risk factors for HIV infection and HIV-related conditions. Physical activity, adequacy of food intake, and use of alcohol, tobacco, and illicit drugs were assessed using standardized instruments (21–24). Research associates administer these questionnaires again in FRAM 2.
Physical examination
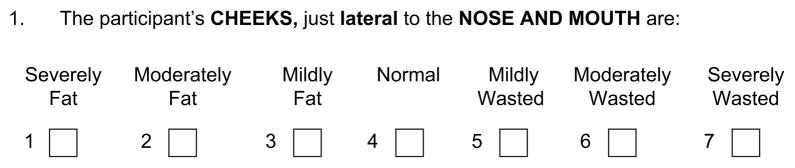
Using a standardized examination, research associates from both HIV sites and control sites determined whether participants had more or less fat than healthy people in each of the same body areas asked about in the self-administered questionnaire. Research associates were encouraged to perform the examination prior to the participant’s filling out the self-administered questionnaire on body fat changes. If the questionnaire had been filled out first, the research associates were required not to look at what the participant had reported on the questionnaire. During centralized training, research associates were shown slides of and given an atlas of photographs of body areas that are most affected in HIV-infected patients, including a range of abnormalities as well as normal findings. Research associates were asked to rate the amount of fat at each body site (figure 2). The same standardized examination is performed in FRAM 2.
FIGURE 2.
Example item from a physical examination survey of the appearance of fat at a specific body site, Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM), United States, 2000–2002. Criteria for rating the appearance of fat were based on those of the HIV Outpatient Study: mild =only seen if looked for; moderate =easily seen; severe =obvious immediately (33). HIV, human immunodeficiency virus.
In FRAM, research associates measured height and weight; used the Gulick II tape with tensiometer (Country Technology, Inc., Gaysmills, Wisconsin) to measure neck, waist, hip, and thigh circumferences; and used a Lange skinfold caliper (Beta Technologies, Inc., Santa Cruz, California) to measure skinfolds of the tricep, midthigh, and abdomen. Research associates measured resting blood pressure three times in seated participants using a mercury sphygmomanometer (W. A. Baum, Inc., Copiague, New York), according to a standardized protocol (25). In FRAM 2, research associates measure height and weight; obtain a chest circumference measurement in addition to neck, waist, hip, and thigh circumferences; and take a subscapular skinfold measurement. Blood pressure is measured with a Dinamap Pro100 automated oscillometric sphygmomanometer (General Electric Medical Systems, Waukesha, Wisconsin) using the same protocol as in FRAM.
Imaging
Whole-body magnetic resonance imaging was performed to quantify body composition, particularly regional subcutaneous and visceral adipose tissue volume, in all participants, using a standardized protocol (26). Participants were asked to lie supine with the arms extended over the head. Using the intervertebral space between the fourth and fifth lumbar vertebrae as the origin, transverse images (10-mm slice thickness) were obtained every 40 mm from the hands (above the head) to the feet. Magnetic resonance imaging scans were segmented using image analysis software (Tomovision, Inc., Montreal, Quebec, Canada). For each depot, volume was calculated via a mathematical algorithm for the space between two consecutive slices (27). The scans were centrally analyzed at the Image Reading Center. The same protocol is being used in FRAM 2, with minor technical modifications to additionally allow for the quantification of intermuscular adipose tissue volume.
Dual energy x-ray absorptiometry scans were performed in FRAM to measure regional body composition, including fat and lean mass. The scans were centrally analyzed at the Image Reading Center. In FRAM 2, bone mineral density at the spine and hip are measured, and the Image Reading Center also analyzes those scans.
Carotid ultrasonography using high-resolution B-mode ultrasound is performed in FRAM 2 to capture images of the right and left common carotid and internal carotid arteries (28). Six computer-generated lines drawn on the carotid study image by a certified reader identify the adventitia, media, and lumen in the near wall and far wall of the carotid artery and allow measurement of intimal-medial thickness. The Ultrasound Reading Center centrally analyzes these readings.
Laboratory procedures and specimen collection
Blood specimens collected for the study of lipids, lipoproteins, and glucose metabolism and for other chemical analyses, including liver function tests, were processed centrally at Covance Central Lab Service (Indianapolis, Indiana). CD4 T-lymphocyte count (cells/mm3) and percentage and HIV RNA level were measured in HIV-infected participants. A random urine sample was collected for determination of microalbumin and creatinine levels. Similar studies are being performed in FRAM 2, including a 2-hour oral glucose tolerance test and a fat tolerance test. For the fat tolerance test, blood is collected before the participant consumes a high-fat drink and again 3.5 hours later. Fresh plasma samples are then analyzed for lipid levels and lipoprotein density and fraction using nuclear magnetic resonance.
Aliquots of serum, plasma, and extracted DNA were stored in FRAM. Specialized research studies for markers of systemic inflammation, hormones related to body fat and glucose metabolism, and hepatitis B and C serologic testing and hepatitis C virus RNA testing on all participants were also performed. Serum and plasma are additionally being stored in FRAM 2.
Chart abstraction
In FRAM, research associates at HIV sites performed medical chart abstraction of data on medications, prior weights, CD4 cell counts, plasma HIV RNA levels, and medical conditions. The dose, frequency, and starting dates were recorded for all medications the participant was taking at the time of the FRAM examination. For all antiretroviral medications, as well as anabolic or other hormone medications taken in the past, the starting and stopping dates of use were recorded. For other medications taken in the past, including hypoglycemic agents, hypolipidemic agents, and antihypertensive agents, only starting dates were recorded. For FRAM control participants, current medications were assessed using the standard protocol from the CARDIA Study. Similar data are extracted in FRAM 2.
Data management
In the first FRAM examination, data were entered onto paper forms. Research associates at the HIV clinical sites faxed completed forms to the Data Coordinating Center. At control sites, completed forms were scanned and transmitted electronically. Data from the forms were then entered into the FRAM database. In FRAM 2, data are directly entered into a personal computer and uploaded to the Data Coordinating Center. In FRAM, the validity of data was checked through examination of outliers and double entry of data, and this is also being done in FRAM 2. The Data Coordinating Center regularly creates reports including information on the status of recruitment, retention, and data collection.
Analysis of participant characteristics
After stratification by sex, the demographic and clinical characteristics of HIV-infected FRAM participants were compared with those of HCSUS participants, a nationally representative sample of HIV-infected adults receiving medical care in the United States. HCSUS investigators used multistage random probability sampling to select a cohort of HIV-infected patients who were at least 18 years of age and had made at least one clinical visit for regular medical care in the United States between January 5 and February 29, 1996. HCSUS investigators estimated that 231,400 adults with known HIV infection received medical care during the first 2 months of 1996. The characteristics of FRAM and HCSUS participants were compared using the standard 95 percent confidence interval for the percentage difference. The HCSUS data set was downloaded from the public web-site of the Agency for Health Care Policy and Research (http://www.ahcpr.gov/data/hcsus.htm).
RESULTS
A total of 1,183 HIV-infected persons (825 men, 350 women, and eight transgendered persons) and 297 controls were enrolled in the FRAM Study between June 2000 and September 2002. Of 4,208 patients listed on clinic rosters whose names were randomly ordered and provided to the 16 HIV sites, a contact attempt was made for 3,164. No contact was attempted for 1,044 patients, for various reasons (e.g., the patient was known not to be HIV-infected or had been lost to follow-up, had moved away, or no longer had valid contact information). Figure 3 shows the outcome of the contact attempts for the 3,164 HIV-infected patients. Of the 1,828 persons who were reached and determined to be eligible, 404 (22 percent) declined to participate; 241 (13 percent) agreed to participate but did not appear for the examination; and 1,183 (65 percent) agreed to participate and were examined. Twenty-three participants were examined at the two HIV sites at which the study was discontinued. The numbers of participants examined at the remaining 14 sites ranged from 38 to 148.
FIGURE 3.
Schema of results of recruitment at human immunodeficiency virus (HIV) study sites, Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM), United States, 2000–2002.
The percentages of men and women who declined to participate were the same (22 percent); the percentages were similar among persons who agreed to participate but never appeared for the examination (12 percent of men vs. 16 percent of women) and persons who were examined (65 percent of men vs. 62 percent of women). The percentages of African Americans (n =764) and Caucasians (n =844) who declined to participate (21 percent vs. 24 percent) or did not appear for the examination after agreeing to participate (11 percent vs. 16 percent) were similar. The percentage of Caucasian participants examined was greater than the percentage of African Americans examined (67 percent vs. 60 percent; 95 percent confidence interval (CI) for difference in percentage: 1, 13).
Table 2 shows the baseline characteristics of the 1,183 HIV-infected men and women examined in comparison with a national probability sample of HIV-infected US men and women receiving regular medical care that was assembled in HCSUS (29). Because HCSUS was conducted during the first 2 months of 1996, the ages of FRAM participants on January 31, 1996, were determined and were used in the comparison of age ranges between FRAM and HCSUS. After adjustment for the age of the FRAM participants, the percentages in different age ranges were similar in FRAM and HCSUS for both men and women. Percentages in different racial groups and HIV risk categories appeared fairly similar for men and women in comparison with their corresponding groups in HCSUS. More FRAM men and women had a CD4 cell-count nadir under 200 cells/mm3 than did HCSUS men and women. The prevalence of a history of a clinical acquired immunodeficiency syndrome (AIDS) diagnosis was similar between FRAM men and HCSUS men but was greater in FRAM women than in HCSUS women.
TABLE 2.
Demographic and clinical characteristics of human immunodeficiency virus (HIV)-infected men and women from the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) as compared with men and women from the HIV Cost and Services Utilization Study (HCSUS), United States, 2000–2002 (FRAM) and 1996 (HCSUS)*
| Characteristic | Men
|
Women
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FRAM n =825 |
HCSUS n =177,408 |
% difference | 95% CI† for % difference | FRAM n =350 |
HCSUS n =52,200 |
% difference | 95% CI for % difference | |||||
| No. | % | No. | % | No. | % | No. | % | |||||
| Age (years)‡ | ||||||||||||
| 18–34 | 283 | 34 | 55,552 | 31 | 4 | −2, 9 | 159 | 46 | 22,968 | 44 | 2 | −6, 10 |
| 35–49 | 449 | 55 | 100,352 | 56 | −1 | −3, 6 | 167 | 48 | 25,056 | 48 | 0 | −8, 8 |
| ≥50 | 88 | 11 | 21,504 | 12 | −1 | −5, 8 | 22 | 6 | 4,176 | 8 | −2 | −9, 12 |
| Race/ethnicity | ||||||||||||
| White (non-Hispanic) | 454 | 55 | 100,352 | 56 | −1 | −4, 6 | 115 | 33 | 13,050 | 25 | 8 | −1, 17 |
| African-American | 269 | 33 | 48,384 | 27 | 6 | 0, 11 | 192 | 55 | 28,188 | 54 | 1 | −6, 8 |
| Hispanic | 83 | 10 | 25,088 | 14 | −4 | −3, 10 | 30 | 9 | 9,396 | 18 | −9 | −1, 20 |
| Other | 19 | 2 | 5,376 | 3 | −1 | −6, 8 | 13 | 4 | 1,044 | 2 | 2 | −9, 12 |
| Risk category§ | ||||||||||||
| Injection drug use | 141 | 18 | 41,216 | 23 | −5 | −1, 11 | 91 | 27 | 14,616 | 28 | −1 | −9, 10 |
| Men who have sex with men | 516 | 66 | 111,104 | 62 | 4 | 0, 8 | ||||||
| Heterosexual contact | 77 | 10 | 16,128 | 9 | 1 | −6, 8 | 187 | 56 | 26,622 | 51 | 5 | −2, 12 |
| Other | 48 | 6 | 10,752 | 6 | 0 | −7, 7 | 55 | 17 | 10,440 | 20 | −4 | −6, 13 |
| Nadir of CD4 cell count¶ (cells/mm3) | ||||||||||||
| 0–199 | 507 | 62 | 100,352 | 56 | 6 | 1, 10 | 204 | 59 | 22,446 | 43 | 16 | 9, 22 |
| 200–499 | 261 | 32 | 64,512 | 36 | −4 | −1, 10 | 123 | 35 | 22,446 | 43 | −8 | −1, 16 |
| ≥500 | 57 | 7 | 14,336 | 8 | −1 | −6, 8 | 22 | 6 | 7,308 | 14 | −8 | −3, 18 |
| Clinical stage of HIV disease | ||||||||||||
| AIDS† (CDC† category C) | 346 | 42 | 73,472 | 41 | 1 | −4, 6 | 156 | 45 | 15,138 | 29 | 16 | 8, 23 |
| Symptomatic/no AIDS (CDC category B) | 362 | 44 | 86,016 | 48 | −4 | −1, 9 | 146 | 42 | 32,886 | 63 | −21 | 13, 29 |
| No symptoms (CDC category A) | 117 | 14 | 19,712 | 11 | 3 | −3, 10 | 48 | 14 | 4,176 | 8 | 6 | −4, 16 |
Transgendered persons (n =8) were excluded from the comparison of men and women from FRAM and HCSUS. Missing values were not included in the denominator for FRAM, since missing values were not included in the denominator for HCSUS.
CI, confidence interval; AIDS, acquired immunodeficiency syndrome; CDC, Centers for Disease Control and Prevention.
Ages of FRAM participants were adjusted to the HCSUS date of January 31, 1996. Numbers do not add up to the total number of subjects for FRAM men and women, because with adjustment for age in 1996 some participants were no longer in the 18- to 34-year age range corresponding to HCSUS.
“Other” includes hemophiliac and blood product infections. Numbers do not add up to the total number of subjects for FRAM men and women because of missing data.
Numbers for FRAM women do not add up to the total number of subjects because one woman was missing data on CD4 cell count.
When the HIV-infected FRAM men were compared with the HIV-infected FRAM women, the women were more likely to be African-American (55 percent of women vs. 33 percent of men; 95 percent CI for difference: 13, 31), to have a lower educational status (58 percent vs. 37 percent; 95 percent CI: 12, 30), to be unemployed (60 percent vs. 39 percent; 95 percent CI: 12, 30), and to have a lower income (67 percent vs. 50 percent; 95 percent CI: 10, 25). These differences between HIV-infected men and women in FRAM were similar to the differences observed between HIV-infected men and women in HCSUS.
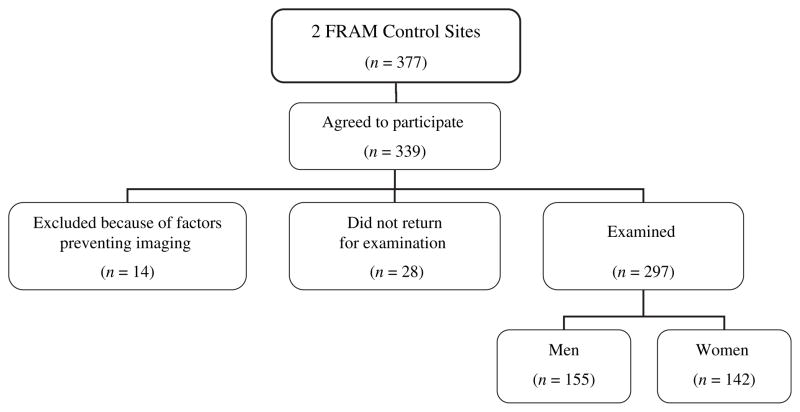
Figure 4 shows the recruitment outcome for the 377 VIM/ CARDIA participants who were contacted and reached regarding participation in both the 2000 VIM follow-up examination and FRAM. Because of the original VIM sampling strategy, there were similar proportions of African Americans and Caucasians. HIV testing was not done in the CARDIA cohort, but on the basis of previous medication data and demographic factors, the prevalence of HIV infection in CARDIA is estimated to be less than 1 percent (unpublished data), and none of the CARDIA participants included in FRAM were found to be taking antiretroviral medications.
FIGURE 4.
Schema of results of recruitment at control study sites, Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM), United States, 2000–2002. HIV, human immunodeficiency virus.
The anthropometric characteristics of the 297 FRAM controls (155 men and 142 women) were compared with those of similar-aged men and women in the Third National Health and Nutrition Examination Survey (NHANES III) (30). Among Caucasian men, average body mass indexes were similar in FRAM controls and NHANES III participants (27.3 vs. 27.5; 95 percent CI for difference: −0.9, 1.3). Among African-American men, body mass index was higher in FRAM controls than in NHANES III participants (28.2 vs. 26.7; 95 percent CI: 0.2, 2.8). Among Caucasian women, FRAM controls had a lower body mass index than NHANES III participants (25.4 vs. 27.6; 95 percent CI: 0.5, 3.9). Among African-American women, there was no difference in body mass index between FRAM controls and NHANES III participants (31.2 vs. 29.9; 95 percent CI: −0.6, 3.2).
DISCUSSION
The FRAM Study is unique for several reasons. To our knowledge, FRAM is one of the largest studies specifically dedicated to studying fat redistribution and metabolic changes in HIV-infected persons using standardized and rigorous data collection procedures. We believe it is the largest study to date to investigate fat changes in HIV using a randomly selected population of patients and to directly measure regional fat using magnetic resonance imaging. Furthermore, we have demonstrated that HIV-infected FRAM men and women are similar to a national probability sample of HIV-infected patients receiving medical care in the United States in 1996 (29) in terms of age, race/ethnicity, and exposure status. A higher percentage of HIV-infected FRAM men and women had a CD4 cell-count nadir under 200 cells/mm3 than participants in HCSUS. This may be explained by the nearly 5-year interval between HCSUS and FRAM and the potentially longer duration of time for which the FRAM participants were infected. However, prevalences of a history of clinical AIDS were similar between FRAM men and HCSUS men but greater in FRAM women than in HCSUS women. Therefore, findings from the FRAM Study may be generalizable to HIV-infected patients receiving medical care in the United States and do not represent a biased sample of HIV-infected patients preselected on the basis of their body fat distribution, as has been done in other studies. We do not feel that the discontinuation of the study at two HIV sites was a limitation in terms of the study’s representativeness.
Another strength of FRAM is that our comparison control group was taken from an already-established longitudinal study designed to be representative of healthy Caucasian and African-American US men and women aged 18–30 years in 1986. The body mass indexes of the Caucasian control men and the African-American control women were similar to those of their respective groups in NHANES III. Caucasian men and African-American women are the groups most affected by HIV infection in the United States. The use of a population-based control group has the advantage of providing normative data for controls and eliminating investigator selection bias. Given that FRAM is focused on changes in fat distribution among persons with HIV infection, the representative body mass index characteristics of such controls provide a well-suited population for comparison.
A limitation of our study is that FRAM controls were not recruited on the basis of their having similar risk behaviors as HIV-infected FRAM participants. Other large US HIV cohort studies, including the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study, include HIV-uninfected men and women, respectively, with risk characteristics similar to those of their HIV-infected participants. Although the HIV-uninfected persons from these cohorts had similar risk behaviors as the HIV-infected group, the mean body mass index of the HIV-uninfected group was higher than that of the HIV-infected group (31, 32). In FRAM, body mass indexes were similar between HIV-infected and control women, but HIV-infected men had a lower body mass index than control men. It is difficult to find controls who are identical to HIV-infected persons with regard to aspects other than HIV infection.
The FRAM Study is poised to make important contributions to our understanding of fat and metabolic changes in HIV infection and the impact of these changes on cardiovascular disease in this population. FRAM does not presume a syndrome of central lipohypertrophy and peripheral lipo-atrophy accompanied by insulin resistance and dyslipide-mia. Rather, FRAM separately studies the factors that are associated with each component of what has been defined as the “lipodystrophy syndrome” using direct measures of fat and metabolism, and determines the associations among the changes. The characteristics of the FRAM cohort are similar to those of a national probability sample of HIV-infected US adults receiving medical care (HCSUS), and this increases the generalizability of the FRAM findings. The wealth of data collected in the FRAM Study using a scientifically objective, quantitative approach and the representativeness of the FRAM sample increase its value as a resource for studies on fat distribution, metabolic changes, and atherosclerosis in HIV infection.
Acknowledgments
The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) is supported by grants from the National Institutes of Health (R01-DK-57508, R01-HL-74814, and R01-HL-53359). National Institutes of Health support for this project is also provided through grants to General Clinical Research Centers (M01-RR00036, M01-RR00051, M01-RR00052, M01-RR00054, M01-RR00083, M01-RR00636, and M01-RR00865).
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- FRAM
Fat Redistribution and Metabolic Change in HIV Infection
- HCSUS
HIV Cost and Services Utilization Study
- HIV
human immunodeficiency virus
- NHANES III
Third National Health and Nutrition Examination Survey
- VIM
Visceral Fat and Metabolic Rate in Young Adults
FRAM study sites and investigators
human immunodeficiency virus sites—University Hospitals of Cleveland, Cleveland, Ohio (Barbara Gripshover); Tufts University, Medford, Massachusetts (Abby Shevitz and Christine Wanke); Stanford University, Stanford, California (Andrew Zolopa and Lisa Gooze); University of Alabama at Birmingham, Birmingham, Alabama (Michael Saag and Barbara Smith); Johns Hopkins University, Baltimore, Maryland (Joseph Cofrancesco and Adrian Dobs); University of Colorado Health Sciences Center, Denver, Colorado (Constance Benson and Lisa Kosmiski); University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Charles van der Horst; David Wohl (FRAM 2)); University of California, San Diego (W. Christopher Matthews and Daniel Lee); Washington University, St. Louis, Missouri (William Powderly and Kevin Yarasheski; Erin Quirk and E. Turner Overton (FRAM 2)); Veterans Affairs Medical Center, Atlanta, Georgia (David Rimland); University of California, Los Angeles (Judith Currier and Matthew Leibowitz); Veterans Affairs Medical Center, New York, New York (Michael Simberkoff and Juan Bandres); Veterans Affairs Medical Center, Washington, DC (Cynthia Gibert and Fred Gordin); St. Luke’s-Roosevelt Hospital Center, New York, New York (Donald Kotler and Ellen Engelson); University of California, San Francisco (Morris Schambelan and Kathleen Mulligan); Indiana University, Bloomington, Indiana (Michael Dube); control sites—Kaiser Permanente, Oakland, California (Stephen Sidney); University of Alabama at Birmingham (Cora E. Lewis). Data Coordinating Center: University of Alabama at Birmingham (O. Dale Williams, Heather McCreath, Charles Katholi, George Howard, Tekeda Ferguson, and Anthony Goudie); University of Washington, Seattle, Washington (Richard Kronmal and Emily Larson (FRAM 2)). Image Reading Center: St. Luke’s-Roosevelt Hospital Center (Steven Heymsfield, Jack Wang, and Mark Punyanitya). Ultrasound Reading Center: New England Medical Center, Boston, Massachusetts (Daniel O’Leary, Joseph Polak, and Laurie Funk). Office of the Principal Investigator: University of California, San Francisco; San Francisco Veterans Affairs Medical Center; and the Northern California Institute for Research and Education, San Francisco, California (Carl Grunfeld, Phyllis Tien, Peter Bacchetti, Dennis Osmond, Andrew Avins, Sharon Safrin, Michael Shlipak, Mae Pang, and Heather Southwell).
Dr. Constance Benson has served as a scientific advisor, consultant, or study investigator for the following pharmaceutical companies: Abbott Laboratories; Merck; Boehringer Ingelheim GmbH; Gilead; GlaxoSmithKline; Tibotec; and Johnson and Johnson Research. Dr. Carl Grunfeld attended a Basic Science Advisory Board Core Meeting for Bristol-Myers Squibb.
References
- 1.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Safrin S, Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13:2493–505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]
- 3.Tien PC, Grunfeld C. What is HIV-associated lipodystrophy? Defining fat distribution changes in HIV infection. Curr Opin Infect Dis. 2004;17:27–32. doi: 10.1097/00001432-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Saint-Marc T, Partisani M, Poizot-Martin I, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO Study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Martinez E, Mocroft A, Garcia-Viejo MA, et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet. 2001;357:592–8. doi: 10.1016/S0140-6736(00)04056-3. [DOI] [PubMed] [Google Scholar]
- 6.Galli M, Cozzi-Lepri A, Ridolfo AL, et al. Incidence of adipose tissue alterations in first-line antiretroviral therapy: The LipoICoNa Study. Arch Intern Med. 2002;162:2621–8. doi: 10.1001/archinte.162.22.2621. [DOI] [PubMed] [Google Scholar]
- 7.Galli M, Veglia F, Angarano G, et al. Gender differences in antiretroviral drug-related adipose tissue alterations. J Acquir Immune Defic Syndr. 2003;34:58–61. doi: 10.1097/00126334-200309010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Saves M, Raffi F, Capeau J, et al. Factors related to lipo-dystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34:1397–405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–18. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 10.Madge S, Kinloch-de-Loes S, Mercey D, et al. Lipodystrophy in patients naive to HIV protease inhibitors. AIDS. 1999;13:735–7. doi: 10.1097/00002030-199904160-00020. [DOI] [PubMed] [Google Scholar]
- 11.Gervasoni C, Ridolfo AL, Trifiro G, et al. Redistribution of body fat in HIV-infected women undergoing combined anti-retroviral therapy. AIDS. 1999;13:465–71. doi: 10.1097/00002030-199903110-00004. [DOI] [PubMed] [Google Scholar]
- 12.Dong KL, Bausserman LL, Flynn MM, et al. Changes in body habitus and serum lipid abnormalities in HIV-positive women on highly active antiretroviral therapy (HAART) J Acquir Immune Defic Syndr. 1999;21:107–13. [PubMed] [Google Scholar]
- 13.Lichtenstein K, Delaney K, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1 infected patients. J Acquir Immune Defic Syndr. 2003;32:48–56. doi: 10.1097/00126334-200301010-00007. [DOI] [PubMed] [Google Scholar]
- 14.Mallal SA, John M, Moore CB, et al. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14:1309–16. doi: 10.1097/00002030-200007070-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lo JC, Mulligan K, Tai VW, et al. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998;351:867–70. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 16.Miller KK, Daly PA, Sentochnik D, et al. Pseudo-Cushing’s syndrome in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:68–72. doi: 10.1086/514638. [DOI] [PubMed] [Google Scholar]
- 17.Miller KD, Jones E, Yanovski JA, et al. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–5. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 18.Grunfeld C, Kotler DP, Hamadeh R, et al. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- 19.Grunfeld C, Pang M, Doerrler W, et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–52. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 20.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Control Clin Trials. 1987;8(suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 21.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 22.Hill JO, Sidney S, Lewis CE, et al. Racial differences in amounts of visceral adipose tissue in young adults: The CARDIA (Coronary Artery Risk Development in Young Adults) Study. Am J Clin Nutr. 1999;69:381–7. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 23.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–45. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 24.Hoegerman GS, Lewis CE, Flack J, et al. Lack of association of recreational cocaine and alcohol use with left ventricular mass in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Coll Cardiol. 1995;25:895–900. doi: 10.1016/0735-1097(94)00469-7. [DOI] [PubMed] [Google Scholar]
- 25.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–70. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–58. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 27.Shen W, Wang Z, Tang H, et al. Volume estimates by imaging methods: model comparisons with visible woman as the reference. Obes Res. 2003;11:217–25. doi: 10.1038/oby.2003.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 29.Bozzette SA, Berry SH, Duan N, et al. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998;339:1897–904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics. The Third National Health and Nutrition Examination Survey (NHANES III, 1988–94) reference manuals and reports—October 1996. (CD-ROM) Hyattsville, MD: National Center for Health Statistics; 1996. (Available from the National Technical Information Service, Springfield, Virginia) [Google Scholar]
- 31.Palella FJ, Jr, Cole SR, Chmiel JS, et al. Anthropometrics and examiner-reported body habitus abnormalities in the Multi-center AIDS Cohort Study. Clin Infect Dis. 2004;38:903–7. doi: 10.1086/381684. [DOI] [PubMed] [Google Scholar]
- 32.Tien PC, Cole SR, Williams CM, et al. Incidence of lipo-atrophy and lipohypertrophy in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2003;34:461–6. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–98. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]