Abstract
We reported (Yarasheski KE, Zachwieja JJ, Gischler J, Crow-ley J, Horgan MM, and Powderly WG. Am J Physiol Endocrinol Metab 275: E577–E583, 1998) that AIDS muscle wasting was associated with an inappropriately low rate of muscle protein synthesis and an elevated glutamine rate of appearance (Ra Gln). We hypothesized that high plasma HIV RNA caused dysregulation of muscle amino acid metabolism. We determined whether a reduction in HIV RNA (≥1 log) increased muscle protein synthesis rate and reduced Ra Gln and muscle proteasome activity in 10 men and 1 woman (22–57 yr, 60–108 kg, 17–33 kg muscle) with advanced HIV (CD4 = 0–311 cells/μl; HIV RNA = 10–375 × 103 copies/ml). We utilized stable isotope tracer methodologies ([13C]Leu and [15N]Gln) to measure the fractional rate of mixed muscle protein synthesis and plasma Ra Gln in these subjects before and 4 mo after initiating their first or a salvage antiretroviral therapy regimen. After treatment, median CD4 increased (98 vs. 139 cells/μl, P = 0.009) and median HIV RNA was reduced (155,828 vs. 100 copies/ml, P = 0.003). Mixed muscle protein synthesis rate increased (0.062 ± 0.005 vs. 0.078 ± 0.006%/h, P = 0.01), Ra Gln decreased (387 ± 33 vs. 323 ± 15 μmol·kg fat-free mass−1·h−1, P = 0.04), and muscle proteasome chymotrypsin-like catalytic activity was reduced 14% (P = 0.03). Muscle mass was only modestly increased (1 kg, P = not significant). We estimated that, for each 10,000 copies/ml reduction in HIV RNA, ~3 g of additional muscle protein are synthesized per day. These findings suggest that reducing HIV RNA increases muscle protein synthesis and reduces muscle proteolysis, but muscle protein synthesis relative to whole body protein synthesis rate is not restored to normal, so muscle mass is not substantially increased.
Keywords: human immunodeficiency virus, metabolic complications, body composition, mass spectrometry, antiretroviral medications, cachexia, lentivirus
Before the availability of highly active antiretroviral therapy (HAART), human immunodeficiency virus (HIV)-associated muscle protein wasting and weight loss were pervasive problems associated with high rates of morbidity and mortality (for review see Ref. 11). In developing countries where HAART is not currently available, muscle protein wasting and weight loss persist as life-threatening problems.
In the current era of HAART, HIV-associated muscle protein wasting continues to be a clinical problem (11, 36, 38). Morbidity and mortality rates are low in effectively treated patients with undetectable plasma viremia (HIV-1 RNA), and patients are gaining weight. But the weight gain is primarily visceral adipose tissue, sometimes associated with peripheral lipoatrophy. Longitudinal and cross-sectional studies indicate that muscle or lean mass is not being restored to normal levels (22, 24). Failure to maintain/restore lean mass appears to be particularly problematic in HIV-infected people with initially low (<15%) body fat content (26).
The pathogenesis for HIV-associated muscle wasting is only partially understood. Nutritional, hormonal, biochemical, inflammatory, viral, and behavioral factors play a role (21, 25, 26, 33, 38). It appears that protein synthetic and proteolytic pathways are both involved. We have reported that, under controlled nutritional conditions, acquired immunodeficiency syndome (AIDS) muscle wasting is associated with a dysregulation of muscle amino acid metabolism compared with asymptomatic HIV-infected people and seronegative control subjects (44). In AIDS wasting, the portion of whole body protein synthesis that is devoted to the synthesis of muscle proteins is lower, and glutamine rate of appearance into the circulation (Ra Gln) is higher than in HIV-asymptomatic and control subjects. Llovera et al. (20) reported greater expression of the genes encoding for the ubiquitin-ATP-dependent proteolytic system in muscle samples obtained from AIDS-wasting patients than in seronegative control subjects. Transgenic mice expressing HIV-1 develop muscle wasting, leukocyte infiltration into muscle, and increased mRNA expression for ubiquitin and proteasome core proteins (13). Interestingly, when these mice were treated with a chemical inhibitor of IκB kinase 2, nuclear factor-κB-dependent gene expression was suppressed, muscle mass was preserved, and life span was increased. In humans, it is not clear whether HIV infection per se signals the dysregulation of muscle amino acid metabolism.
We hypothesized that HIV, or some viral factor associated with chronic HIV infection, is responsible for the observed dysregulation of muscle amino acid/protein metabolism. To test this, we determined whether a reduction in plasma HIV-1 RNA (≥1 log) increases muscle protein synthesis rate and reduces Ra Gln and muscle proteasome activity in people living with advanced HIV infection.
METHODS
Subjects
HIV-infected volunteers were recruited from the AIDS Clinical Trials Unit and the Infectious Disease Clinics at Washington University School of Medicine (Table 1). Ten HIV-positive men and one HIV-positive woman [22–57 yr, 19–36 kg/m2, median HIV RNA 155,828 (range 10–353 × 103) copies/ml, median CD4 98 (0–311) cells/μl] were enrolled. All subjects were weight stable (±5%) for 1 mo before enrollment. Two of these subjects had no history of weight loss, were initially naive to all anti-HIV medications, and were scheduled to initiate HAART (Table 2). Nine of the subjects had lost weight in the past and were experienced patients who were clinically stable on HAART for ≥1 mo before enrollment but were scheduled to change to a “salvage” HAART regimen because they were developing resistance to their current HAART regimen. Muscle amino acid metabolism was examined at baseline (High HIV RNA) and 4.2 ± 1.8 mo after the medication change when plasma HIV RNA was reduced (Low HIV RNA).
Table 1.
Subject characteristics
| Parameter | High HIV RNA | Low HIV RNA | P Value |
|---|---|---|---|
| Age, yr | 39±4 | 40±3 | NS |
| Height, cm | 176±3 | 176±3 | NS |
| Weight, kg | 79.5±5.5 | 82.6±6.0 | 0.23 |
| BMI, kg/m2 | 25.9±2.1 | 26.8±2.3 | 0.23 |
| FFM, kg | 58.2±3.2 | 59.4±3.2 | 0.33 |
| Legs FFM, kg | 17.5±1.2 | 18.4±1.1 | 0.32 |
| Arms FFM, kg | 6.7±0.6 | 6.9±0.6 | 0.42 |
| Fat, kg | 21.3±3.8 | 23.2±4.3 | 0.28 |
| TBW, kg | 40.6±3.8 | 42.3±3.0 | 0.29 |
| Muscle mass, kg | 25±2 | 26±2 | 0.67 |
| RMR, kcal/day | 2,000±105 | 2,069±132 | 0.53 |
| CD4, cells/μl | 145±38 | 217±52 | 0.009 |
| Plasma HIV RNA, copies/ml | 167,236±41,409 | 2,607±2,236 | 0.003 |
Values are means ± SE. High HIV RNA, baseline measurement of human immunodeficiency virus (HIV) RNA; Low HIV RNA, measurement 4.2 ± 1.8 mo after medication change. BMI, body mass index; FFM, fat-free mass; TBW, total body water; RMR, resting metabolic rate; NS, not significant.
Table 2.
Anti-HIV medication changes used to reduce plasma HIV RNA
| Subject | High HIV RNA | Low HIV RNA |
|---|---|---|
| 1 | D4T, 3TC, ABC, RTV | 3TC, ABC, NVP |
| 2 | AZT, D4T | DDI, 3TC, NFV |
| 3 | DDI, ABC, EFV, APV, RTV | DDI, TDF, LPV, RTV |
| 4 | D4T, 3TC, NFV | D4T, 3TC, ABC, RTV |
| 5 | EFV, APV | 3TC, ABC, LPV, RTV |
| 6 | AZT, 3TC, IDV, RTV | D4T, ABC, LPV, RTV |
| 7 | AZT, 3TC | D4T, ABC, IDV, RTV |
| 8 | Naive | AZT, 3TC, EFV, NFV |
| 9 | D4T, HU, IDV, APV | D4T, DDI, IDV, RTV |
| 10 | AZT, 3TC, NFV | AZT, 3TC, EFV |
| 11 | Naive | AZT, 3TC, NVP |
NRTI, nucleoside reverse transcriptase inhibitors: AZT, zidovudine; 3TC, lamivudine; D4T, stavudine; DDI, didanosine; ABC, abacavir; DDC, zalcitabine. NNRTI, nonnucleoside reverse transcriptase inhibitors: EFV, efavirenz; NVP, nevirapine. NTRTI, nucleotide reverse transcriptase inhibitor: TDF, tenofovir. RNTI, ribonucleotide reductase inhibitor: HU, hydroxyurea. PI, protease inhibitors: IDV, indinavir; RTV, ritonavir; NFV, nelfinavir; APV, amprenavir; LPV, lopinavir.
Before enrollment, volunteers received a physical examination, including a medical/medication history, a blood chemistry profile, serum endocrine profile [testosterone, IGF-I, insulin, C-peptide, glucagon, leptin, cortisol, TSH, and thyroxine], complete blood cell count (including CD4 count), and plasma HIV RNA quantitation (Roche Amplicor HIV-1 Monitor; Roche Diagnostics, Indianapolis, IN). Volunteers with plasma HIV-1 RNA >10,000 copies/ml were included. Volunteers were excluded if they were taking prescription medications that might affect muscle amino acid metabolism (β-adrenergic blockers, β-agonists, Ca2+ channel blockers, corticosteroids) or if they had a metabolic (e.g., diabetes), neuromuscular (moderate to severe peripheral neuropathy), or other disorder that might affect muscle amino acid metabolism. None of the subjects took anabolic agents or appetite stimulants during the course of the study and for ≥1 mo before the baseline study. None of the subjects was exercise trained or participated regularly in exercise activities that would constitute exercise training (as defined in Ref. 29). The Human Studies Committee at Washington University School of Medicine approved the study, and all subjects provided informed consent before participating.
Dietary control
Subjects were admitted to the General Clinical Research Center (GCRC) for a 48-h period. During that time, they consumed a flesh-free diet containing defined, adequate amounts of protein and calories. Flesh-free meals were employed to reduce the effects of dietary creatinine on 24-h urinary creatinine excretion measurements used to estimate whole body muscle mass (37). Before admission, a research dietician surveyed each participant’s typical eating habits and designed the 2-day meal plan to provide 1.5 g protein·kg−1·day−1 and 130–190 kJ (31–45 kcal)·kg−1·day−1. Of the total calories, ~20% was derived from protein, ~50% from carbohydrate, and ~30% from fat. The controlled intake did not differ in composition from the subject’s habitual intake. The meals were prepared in the GCRC Research Kitchen and served to the subjects in the GCRC. The subjects were instructed to eat all food provided and no other food/drink. Any small amount not consumed was weighed, and the daily intake record was corrected accordingly.
Body composition
After an overnight fast, whole body and regional fat and fat-free mass (FFM) were measured using a Hologic QDR-2000 enhanced-array whole body dual-energy X-ray absorptiometer (Waltham, MA). Whole body images were obtained at baseline and after plasma HIV RNA was reduced. Both images for each subject were processed by the same technologist using Hologic software (v. 5.64A).
Estimates of total body muscle mass were derived by averaging two 24-h urine creatinine excretion values and by assuming that 1 g of urinary creatinine excreted per day is equivalent to 20 kg muscle mass (37). Urine creatinine concentration was measured using a stable isotope dilution assay and gas chromatography-mass spectrometry [Agilent 6890N Gas Chromatograph and Agilent 5973N Mass Selective Detector (GC-MS), Palo Alto, CA] as described (41).
Total body water (TBW; intra- and extracellular) was measured by the dilution of orally administered 2H2O (0.25 g/kg; Cambridge Isotope Laboratories) into body fluids, as described previously (30, 43). Serum samples (2 ml) were obtained before and 3 and 4 h after administration of 2H2O. Two hundred microliters of an internal standard were added ([2H9]-tert-butanol), and 2H2O enrichment was measured using 1H magnetic resonance spectroscopy. TBW was calculated from the average of the 3- and 4-h samples (30).
Whole body glutamine and leucine rates of appearance and muscle protein synthesis rate
After the evening meal on day 2 (1800), whole body and skeletal muscle amino acid kinetics were determined during a 13-h overnight (1900–0800) intravenous infusion of [2-15N]glutamine (6.8 μmol·kg−1·h−1, ~99 atom%; Cambridge Isotope Laboratories, Andover, MA) (9, 44). A primed (7.58 μmol/kg) constant intravenous infusion (7.58 μmol·kg−1·h−1) of [1-13C]leucine (~99atom%, Cambridge Isotope Laboratories) was used to measure the rates of whole body proteolysis, nonoxidative leucine disposal, and leucine oxidation, using the reciprocal pool model (15, 21, 23, 32), and to measure the in vivo fractional rate of incorporation of [13C]leucine into skeletal muscle proteins (2, 40, 42, 44). Amino acid kinetics were measured after an overnight fast and expressed per kilogram of FFM.
In blood samples obtained before and at 30-min intervals during the last 2.5 h of the amino acid tracer infusions, plasma glutamine was isolated using a modification of a described anion-exchange chromatographic method (8). [U-13C5]glutamine (Cambridge Isotope Laboratories) was added to the plasma before isolation and used as an internal standard for the quantification of plasma glutamine concentration. Plasma glutamine was converted to the N-heptafluorobutyryl propyl ester derivative and analyzed using GC-MS in negative ion chemical ionization mode (7). 15N enrichment was determined by selected ion monitoring of ion intensity at mass-to-charge ratios (m/z) of 407 and 408, and glutamine concentration was determined by monitoring ions at m/z 407 and 412 at the appropriate retention time. Plasma Ra Gln was calculated as described (8, 9).
For quantitation of plasma leucine concentration, [U-13C6]leucine (Cambridge Isotope Laboratories) was added to the plasma before isolation and used as an internal standard. Plasma leucine was converted to the N-heptafluorobutyryl propyl ester derivative and analyzed using GC-MS in negative ion chemical ionization mode. Leucine concentration was determined by monitoring ions at m/z 349 and 355 at the appropriate retention time.
In the same blood samples, plasma α-ketoisocaproic acid (KIC) was isolated and derivatized, and 13C enrichment was measured (selected ion monitoring of m/z 232 and 233) using GC-electron ionization-MS (32, 44). The plasma [α-13C]KIC enrichment value was used to calculate the rate of whole body protein breakdown (23, 40, 44).
Exhaled breath samples were collected into 20-ml evacuated tubes (Becton-Dickinson Vacutainer; Franklin Lakes, NJ) before and at the end of the amino acid tracer infusion. Breath samples were analyzed for 13CO2 enrichment (m/z 45 and 44 ions) using a gas isotope ratio-MS (Finnigan Delta+ XL-IRMS, Bremen, Germany). The 13CO2 enrichment values were used in conjunction with 15-min measurements of CO2 production (ml/min) made at the same time points using a Delta-Trac indirect calorimeter (Sensormedics, Yorba Linda, CA) to determine the rate of whole body leucine oxidation (23, 44). The difference between the measured whole body protein breakdown rate and leucine oxidation rate is the nonoxidative disposal rate of leucine, which represents the whole body protein synthesis rate (23, 40, 44). Indirect calorimetry was also used to measure resting metabolic rate (RMR).
The fractional rate of mixed muscle protein synthesis was measured as the in vivo rate of incorporation of [13C]leucine into proteins isolated from two vastus lateralis muscle samples obtained ~12 h apart during the tracer infusion study. One muscle sample was removed 90 min after the tracer amino acid infusion began, and a second muscle sample was removed from the contralateral vastus lateralis at the end of the infusion. Tissue free amino acids were extracted from 10–20 mg of muscle by homogenization in 10% trichloroacetic acid (TCA). The N-heptafluorobutyryl propyl esters were formed (7), and the [13C]leucine abundance was measured by selected ion monitoring of ions at m/z 349 and 350 with GC-MS. The muscle free [13C]leucine enrichment was used to represent the precursor pool enrichment (2, 3, 39) for the calculation of mixed muscle protein synthesis rate (Ks, in %/day).
[13C]leucine enrichment in mixed muscle proteins (TCA precipitate) was measured using GC-combustion-IRMS (2, 42, 44). The N-acetyl-n-propyl esters were formed, leucine was separated on a DB-1 capillary GC column (P. J. Cobert, St. Louis, MO) and combusted to CO2 (960°C), and the 13CO2-to-12CO2 ratio was analyzed (m/z 44 and 45) using IRMS. Mixed muscle protein synthesis rate (%/h) was calculated as described (2, 42, 44). As before, we calculated the portion of whole body protein synthesis rate that was accounted for by the synthesis of mixed muscle proteins. This calculation required a conversion of the fractional rate of mixed muscle protein synthesis (%/h) to the absolute rate of mixed muscle protein synthesis (g protein/h), using the product of the fractional rate of muscle protein synthesis (measured in the vastus lateralis) and whole body muscle mass (from urine creatinine excretion). This requires the assumption that muscle is 19% protein (2, 44) and that leucine comprises 7% of all muscle proteins (23). It also assumes that the branched-chain amino acid leucine has only two intracellular metabolic fates: deamination and conversion to KIC for subsequent oxidation to CO2 or incorporation into proteins (nonoxidative leucine disposal).
Muscle proteasome catalytic activity
To assess functional activation of the ubiquitin-proteasome pathway in skeletal muscle extracts, we measured the three best characterized ATP-ubiquitin-independent peptidase activities of the 20S proteasome core: chymotrypsin-like (ChT-L), trypsin-like (T-L), and peptidyl glutamyl peptide hydrolase (PGPH) (35).
Peptidase activities were measured in crude muscle extracts by monitoring the release of 7-amido-4-methylcoumarin from synthetic peptide substrates, N-succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (N-suc-LLVY-AMC) for ChT-L activity, N-tert-butyloxycarbonyl-Leu-Ser-Thr-Arg-7-amido-4-methylcoumarin (N-t-BOC-LSTR-AMC) for T-L activity, and N-benzyloxycarbonyl-Leu-Leu-Glu-7-amido-4-methylcoumarin (N-CBZ-LLE-AMC) for PGPH activity.
Muscle samples obtained at the end of the tracer infusion were used for peptidase activity determination. Frozen tissue (10 mg wet wt) was homogenized in 1 ml of buffer containing 50 mM Tris, pH 8.0, 1 mM EDTA, 1 mM EGTA, 10% (vol/vol) glycerol, 2 mM pepstatin A, and 50 nM E-64 [trans-epoxysuccinyl-L-leucylamido(4-guanidino)butane] on ice in an all-glass Potter-Elvehjem tissue grinder. Homogenates were sonicated and centrifuged at 15,000 g for 10 min at 0–4°C. The supernatants were transferred to clean tubes and kept on ice.
The reaction mixture contained between 240 and 370 μg of extracted muscle protein, 50 mM Tris, pH 8.0, 1 mM EDTA, 1 mM EGTA, 2 mM DTT, and 40 μM N-suc-LLVY-AMC (for ChT-L), 40 μM N-t-BOC-LSTR-AMC (for T-L), and 100 μM N-CBZ-LLE-AMC (for PGPH) activity. Protein was determined by the Biuret method using bovine serum albumin as reference. AMC release was monitored fluorometrically (excitation 360 nm, emission 465 nm) at 37°C in 96-well plates. Slopes corresponding to maximal reaction rates, increase in fluorescence per minute, were compared with AMC standards to determine the amount of AMC released.
Proteasome peptidase activity was determined by the difference in rates of substrate hydrolysis in muscle extracts that had been preincubated in the presence of lactacystin (BIOMOL Research Laboratories, Plymouth Meeting, PA), a proteasome-specific inhibitor (10), and untreated samples. Peptidase activity was expressed as milliunits per gram protein, where one unit of activity is equivalent to the release of 1 μmol AMC/min under the specified reaction conditions. All reagents were obtained from Sigma Chemical (St. Louis, MO).
Statistics
A paired t-test was used to compare all parameters measured at baseline (High HIV RNA) and after plasma HIV RNA was reduced (Low HIV RNA). Pearson correlation was used to examine relationships between two variables. Significant differences were noted by P <0.05. Means ± SE are reported for all variables, except CD4 and plasma HIV RNA, where median ± SE are reported.
RESULTS
Virology
All 11 subjects reduced their plasma HIV RNA levels and increased their CD4 counts within 2–8 mo after starting or switching their antiviral medications. After treatment, median CD4 increased (98 vs. 139 cells/μl, P = 0.009) and median HIV RNA was reduced (155,828 vs. 100 copies/ml, P = 0.003; Tables 1 and 2).
Body composition
Body mass index, FFM, muscle mass, fat mass, TBW, and RMR were not changed after plasma HIV RNA was reduced (Table 1). However, a correlation was noted between the reduction in plasma HIV RNA and the changes in muscle mass (Pearson r2 = 0.51, P = 0.007). Muscle mass represented 43% of FFM in these HIV-infected subjects regardless of the amount of HIV RNA. This value was 53% in seronegative subjects studied previously (44).
Serum hormone concentrations
Circulating levels of anabolic and catabolic hormones were not significantly altered after plasma HIV RNA was reduced (Table 3). It is important to note that the statistical power of these observations was low (16–44%) because these were not primary outcome measures. As a result, it is possible that alterations in circulating levels of IGF-I, testosterone, insulin, cortisol, or thyroid hormone contributed to changes in muscle protein synthesis rate and plasma Ra Gln after plasma HIV RNA was reduced.
Table 3.
Serum endocrine parameters
| Parameter | High HIV RNA | Low HIV RNA | P Value |
|---|---|---|---|
| Testosterone, ng/dl | 604±135 | 722±178 | 0.28 |
| IGF-I, ng/ml | 204±18 | 235±22 | 0.08 |
| Insulin, μU/ml | 11.4±3.3 | 14.2±3.4 | 0.30 |
| C-peptide, ng/ml | 1.9±0.3 | 1.4±0.3 | 0.06 |
| Glucagon, pg/ml | 113±19 | 97±13 | 0.08 |
| Leptin, ng/ml | 8.9±3.6 | 11.6±3.6 | 0.08 |
| Cortisol, μg/dl | 12±1 | 14±2 | 0.57 |
| TSH, μIU/ml | 1.6±0.3 | 2.1±0.3 | 0.15 |
| T4, μg/dl | 6.7±1.1 | 5.7±1.1 | 0.08 |
Values are means ± SE. IGF-I, insulin-like growth factor I; TSH, thyroid stimulating hormone; T4, thyroxine.
Whole body and muscle amino acid kinetics
Plasma leucine and glutamine concentrations and the rates of protein breakdown, nonoxidative leucine disposal (i.e., protein synthesis), and leucine oxidation were not changed after plasma HIV RNA was reduced (Table 4; see Fig. 2). Whole body protein synthesis and proteolysis rates were similar to those reported previously for HIV-positive asymptomatic and AIDS-wasting subjects, and these were significantly greater than in seronegative subjects (44).
Table 4.
Whole body amino acid kinetics and concentrations
| Parameter | High HIV RNA | Low HIV RNA | P Value |
|---|---|---|---|
| Leucine, μM | 157±10 | 169±8 | 0.24 |
| Glutamine, μM | 682±43 | 674±42 | 0.81 |
| Glutamine Ra, μmol·kg FFM−1·h−1 | 387±33 | 323±15 | 0.04 |
| Protein breakdown, μmol·kg FFM−1·h−1 | 151±5 | 150±5 | 0.82 |
| Nonoxidative leucine disposal, μmol·kg FFM−1·h−1 | 128±4 | 128±4 | 0.98 |
| Leucine oxidation, μmol·kg FFM−1·h−1 | 23±2 | 22±2 | 0.57 |
Values are means ± SE. Ra, rate of appearance into the plasma.
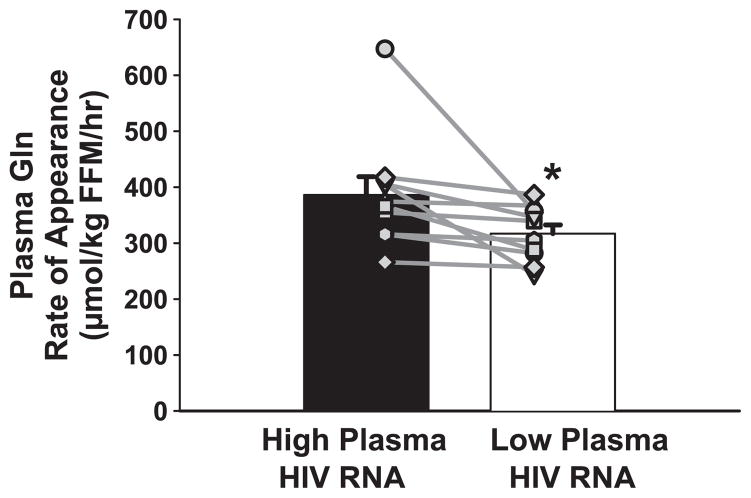
Fig. 2.
Plasma glutamine (Gln) rate of appearance was reduced when plasma HIV RNA was reduced (*P = 0.04). FFM, fat-free mass. Symbols represent individual subjects.
Plasma Ra Gln decreased (387 ± 33 vs. 323 ± 15 μmol·kg FFM−1·h−1, P = 0.04) after plasma HIV RNA was reduced. In the high HIV RNA condition, Ra Gln was similar to that measured previously in HIV-positive asymptomatic subjects (44) and within the normal range reported by others (8, 9, 27).
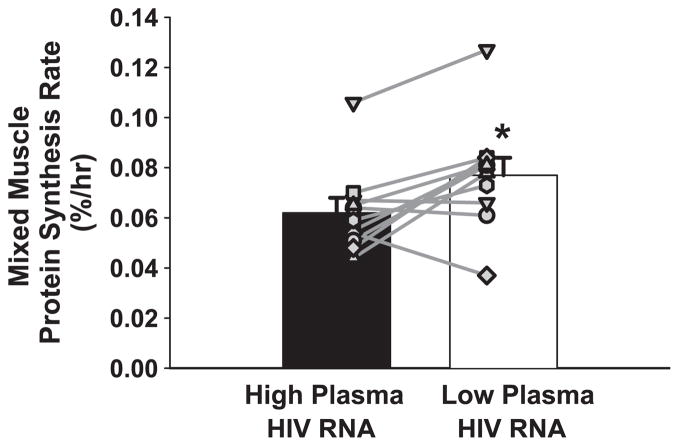
Mixed muscle protein synthesis rate increased (0.062 ± 0.005 vs. 0.078 ± 0.006%/h, P = 0.01) after plasma HIV RNA was reduced (Fig. 1). These synthesis rates were calculated using muscle tissue free [13C]leucine as the precursor pool enrichment, and our previous study used plasma [13C]KIC enrichment for this calculation. This explains why the current synthesis rates are slightly higher than the previous synthesis rates. In the present study, when we used [13C]KIC as the precursor pool enrichment, the findings were exactly the same: muscle protein synthesis rate was significantly increased when plasma HIV RNA was reduced (High HIV RNA 0.044 ± 0.005%/h vs. Low HIV RNA 0.055 ± 0.006%/h, P = 0.01). As expected, when [13C]KIC was used as the precursor pool enrichment, the mean muscle protein synthesis rate for High HIV RNA was in agreement and precisely between the mean values reported previously for HIV-positive asymptomatic (0.048 ± 0.003%/h) and AIDS-wasting subjects (0.035 ± 0.004%/h). Interestingly, the five subjects with the smallest increases or a slight decrease in muscle protein synthesis rate (0.03 ± 0.1%/day) all received abacavir (ABC) as a component of their anti-HIV regimen.
Fig. 1.
Mixed muscle protein synthesis rate was increased when plasma human immunodeficiency virus (HIV) RNA was reduced (*P = 0.01). Symbols represent individual subjects. Muscle protein synthesis expressed as a fraction of whole body protein synthesis (Table 4) was 12 ± 2% when HIV RNA was high and 15 ± 2% when HIV RNA was lower (P = 0.01), but still lower than 24–25% in seronegative control subjects (44).
At baseline, the rate of mixed muscle protein synthesis represented 12 ± 2% of the whole body protein synthesis rate (using [13C]KIC to represent the precursor pool enrichment). After plasma HIV RNA was reduced, mixed muscle protein synthesis represented 15 ± 2% of the whole body protein synthesis rate (P = 0.01 vs. High plasma HIV RNA). In our previous study (44), muscle protein synthesis represented 24–25% of whole body protein synthesis rate in seronegative and HIV-positive asymptomatic subjects. In AIDS-wasting subjects (44), and in the present study when plasma HIV RNA was highest, muscle protein synthesis rate represented only 12–17% of the whole body protein synthesis rate.
Muscle ChT-L, T-L, and PGPH activities
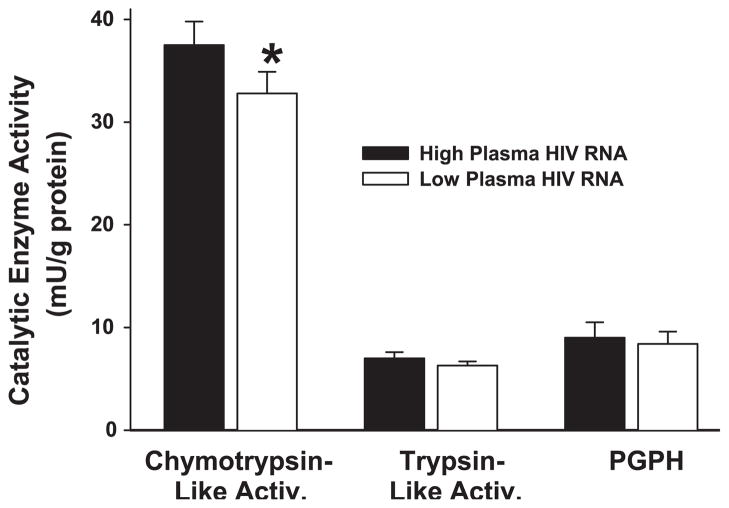
Lowering plasma HIV RNA reduced muscle proteasome ChT-L activity (14%, P = 0.03) but did not affect T-L or PGPH activities (P > 0.07; Fig. 3). The reductions in ChT-L activity were not correlated with the reductions in plasma HIV RNA or the changes in muscle mass [P = not significant (NS)]. If the increment in mixed muscle protein synthesis and the decrement in muscle ChT-L catalytic activity in each subject are extrapolated over the time they were treated, we estimate that the mean increase in muscle protein mass would be 1.3–1.4 kg. Urine creatinine excretion measures indicated that, on average, subjects gained 1 kg of muscle mass (Table 1).
Fig. 3.
Muscle chymotrypsin-like activity was reduced when plasma HIV RNA was reduced (*P = 0.03), whereas the catalytic activity of other enzymes present in the 20S proteasome core [trypsin-like, peptidyl glutamyl peptide hydrolase (PGPH)] was not affected.
DISCUSSION
These findings indicate that reducing plasma HIV RNA improves muscle amino acid and protein metabolism. Subjects with high plasma HIV RNA had low muscle mass-to-FFM ratios and high rates of whole body protein synthesis and proteolysis, but a smaller portion of the accelerated whole body protein turnover rate was utilized to synthesize muscle protein (12%). We suggest that this represents dysregulated muscle amino acid metabolism, partly because in HIV-seronegative subjects muscle protein accounted for 24–25% of the whole body protein synthesis rate (44).
HAART substantially reduced plasma HIV RNA, and muscle protein synthetic rate (%/h) increased to a normal value, but it was still only 15% of the whole body protein synthetic rate. HAART improved muscle amino acid kinetics but did not correct them. Muscle mass and muscle mass-to-FFM ratio were not significantly increased after plasma HIV RNA was reduced, despite the fact that muscle protein synthesis rate was increased and muscle proteolytic activity was reduced. This indicates that, even when plasma HIV RNA was reduced to therapeutically acceptable levels, the muscle protein synthesis rate relative to whole body protein synthesis was still lower than normal. This failure to completely restore muscle protein synthesis to normal may explain why muscle and body cell mass are not restored to normal in patients effectively treated with HAART (22, 26, 38). We suggest that some factor associated with a high HIV RNA adversely affects muscle amino acid metabolism and that lowering circulating HIV RNA to near-undetectable levels does not completely eliminate the suppressive effects of this factor on muscle protein accretion. For example, one candidate factor associated with HIV infection that possesses the potential to affect muscle amino acid metabolism is the HIV-1 accessory protein Vpr (virus protein-R) (18, 31).
Reducing plasma HIV RNA reduced muscle chymotrypsin-like catalytic activity. This implies that proteolytic activity for at least one enzyme in the 20S proteasome core was reduced. The mechanism for this inhibition may be the anti-HIV medications used to reduce plasma HIV RNA. In several cell types (T cell lymphocytes and lymphoma, hepatoma), protease inhibitors [indinavir (IDV), ritonavir (RTV), saquinavir (SQV)] and reverse transcriptase inhibitors alone [lamivudine (3TC), zidovudine (azidothymidine; AZT)], but especially in combination, inhibit chymotrypsin-like and trypsin-like catalytic activities, but not PGPH activity, in isolated proteasomes (1, 19, 28). The relevance of these in vitro observations has been questioned on the basis of the supraphysiological drug concentrations used (50–100 μM) (17). In each of the 11 subjects studied here, these medications (3TC, AZT, IDV, RTV, SQV) constituted at least one component of the anti-HIV regimen used to lower HIV RNA (Table 2). Our in vivo findings suggest that physiological concentrations of anti-HIV medications may inhibit muscle proteasome chymotrypsin-like catalytic activity. We suspect, and evidence supports the notion, that HIV infection activates the ubiquitin-proteasome proteolytic pathway (13, 20) and anti-HIV medications may reduce or restore this activation to lower or more normal levels. Effective HAART appeared to lower the rate of muscle proteolysis but suboptimally increased the rate of muscle protein synthesis relative to whole body protein synthesis, so muscle mass was not dramatically increased.
Plasma Ra Gln declined when plasma HIV RNA was reduced, and this indicates that muscle proteolysis or de novo synthesis of glutamine was reduced. About 65% of plasma Ra Gln derives from de novo synthesis in skeletal muscle, and ~35% of plasma Ra Gln derives from direct release of glutamine from muscle proteolysis (27). The gastrointestinal tract (enterocytes), kidneys, liver, and immune cells (T-lymphocytes, macrophages) all utilize glutamine as a source of energy. In other cachectic conditions (cancer, trauma, surgery, catabolic hormone excess), plasma Ra Gln and muscle glutamine synthetase expression are elevated (14). Our findings support the notion that, in the cachectic state characterized by high plasma HIV RNA, leucine incorporation into skeletal muscle proteins is reduced, perhaps because intramuscular leucine transamination for the synthesis of glutamine is activated, and less leucine is available for muscle protein synthetic pathways (14). The undesirable result of activated synthesis of glutamine (and alanine) from leucine (and other branched-chain amino acids) in severe illness is muscle protein wasting (14). In high plasma HIV RNA-induced cachexia, presumably there exists an increased utilization of nitrogen or glutamine in enterocytes, T-lymphocytes, macrophages, kidneys, or liver.
We have not identified the cell or tissue where glutamine uptake is increased in HIV infection, but T-lymphocytes are actively destroyed by HIV infection and turn over rapidly in HIV-infected people. It is interesting to note that, in two small randomized, double-blind, placebo-controlled studies, glutamine-containing nutritional supplements increased body weight, body cell mass, lean body mass, and intracellular water content in AIDS-wasting patients (6, 34). In another study, glutamine supplementation reduced the severity of nelfinavir-associated diarrhea in HIV-infected people (16). Taken together, these findings support the notion that glutamine is a conditionally essential amino acid in HIV-infected people with high plasma HIV RNA. A reduction in circulating levels of HIV RNA reduced the rate of muscle glutamine export, suggesting that either 1) the signal that activated muscle glutamine efflux was removed, or 2) the requirements for glutamine by other cells/tissues in the body were reduced.
It is reassuring that the amino acid kinetic measurements agreed with the static measurement of muscle mass and that the reduction in plasma HIV RNA correlated with the change in muscle mass. The changes in muscle protein synthesis and proteolysis rates predicted an increase in muscle mass (1.3–1.4 kg) that only minimally exceeded the indirect estimate for the increase in muscle mass (1 kg). The small difference may be accounted for because the increase in muscle protein synthesis and the reduction in catalytic activity are not instantaneous and simultaneous events that occur after switching/starting medications that reduce HIV RNA. Also, not all muscle chymotrypsin-like catalytic activity in the proteasome is devoted to myofibrillar protein breakdown, and other proteolytic pathways exist in muscle. On the basis of the average reduction in plasma HIV RNA, the average increase in muscle protein synthesis rate, the amount of time between the baseline and low HIV RNA measurements, and the assumption that muscle is 19% protein, we estimate that, for each 10,000 copies/ml reduction in plasma HIV RNA, ~3 g of additional muscle protein are synthesized per day.
It is possible that these findings can be attributed to a favorable effect of lowering plasma HIV RNA on circulating levels of testosterone, IGF-I, insulin, C-peptide, glucagon, leptin, cortisol, or thyroid hormone levels (anabolic or catabolic hormones). Although these serum hormone levels were not changed after HIV RNA was reduced, the sample size was limited, and we have not eliminated the possibility that several other endocrine/autocrine/paracrine factors or cytokines that were not measured changed in a manner that can explain these findings.
There are some limitations. The medication regimens used to lower plasma HIV RNA in these subjects were very different. Each HIV-infected subject was treated with very complex and variable medication regimens at baseline, and no standard, single medication regimen is safe and efficacious for lowering plasma HIV RNA in every subject. Many factors that were not under our control determined what regimen was used to lower HIV RNA. As a result, we cannot attribute the improvements in muscle amino acid metabolism to a single medication or drug class. Despite the variability in medication regimens, the changes in muscle amino acid metabolism were consistent, with one exception; subjects receiving ABC tended to have the smallest increments or a slight decrement in muscle protein synthesis rate (Fig. 1). The reasons for this are unknown. In human muscle cells, in vitro cytotoxicity and mitochondrial toxicity of ABC is less than for other nucleoside reverse transcriptase inhibitors (e.g., zalcitabine and stavudine) and nucleotide reverse transcriptase inhibitors (tenofovir) (4, 5). We conclude that lowering plasma HIV RNA is either directly or indirectly associated with improved muscle amino acid metabolism.
Despite the reduction in plasma HIV RNA to very low levels in this study, this does not mean that the HIV virus and its potential effects were completely eradicated from these subjects. Even with undetectable HIV RNA in the circulation, HIV replication continues in tissue sanctuaries for HIV (lymph nodes, macrophages, dendritic cells). Our working hypothesis is that this active, low-level HIV replication or a viral protein produced during HIV replication persists in the low plasma HIV RNA condition, and this explains the observation that the fraction of whole body protein synthesis that is attributable to muscle protein synthesis remains lower than in HIV-seronegative control subjects. We have not identified the component of HIV replication that affects muscle amino acid metabolism, but several candidate factors exist.
The measurement of mixed muscle protein synthesis does not specifically indicate which muscle protein(s) was affected by the presence (high or low) of plasma HIV RNA. The techniques used to isolate mixed muscle proteins primarily reflect the in vivo synthesis rate for myosin heavy chain (12). This suggests that the synthesis rate for a contractile protein that is responsible for muscle force production was affected by the presence of HIV RNA in the circulation.
In summary, muscle protein synthesis rate was reduced and muscle proteolysis was increased when the plasma HIV RNA level was high. Muscle amino acid metabolism was improved but not completely normalized when plasma HIV RNA was reduced, so muscle mass was only marginally affected. These findings support the existence of biological interactions between factors associated with chronic viral infection and muscle amino acid metabolism. The HIV-related factor that regulates muscle amino acid metabolism is under investigation. Glutamine may be conditionally essential in HIV-infected people with high plasma HIV RNA. Identifying anti-HIV medication regimens that effectively reduce plasma HIV RNA without adversely affecting amino acid, glucose, and lipid metabolism is a clinically important challenge.
Acknowledgments
We thank the subjects for time and altruism. Sherry Claxton, RD, Mary Hoffmann, Amanda Becker, Jennifer Chen, Erin Laciny, and Jill Schulte provided technical assistance.
GRANTS
Support, through National Institutes of Health grants, was provided by the nurses and staff at the AIDS Clinical Trials Unit (AI-25903), the General Clinical Research Center (RR-00036), and the Clinical Nutrition Research Unit (DK-56341). K. E. Yarasheski was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-49393, DK-54163, and DK-59531 and by the Mass Spectrometry Research Center (RR-00954).
References
- 1.Andre P, Groettrup M, Klenerman P, de Giuli R, Booth BL, Jr, Cerundolo V, Bonneville M, Jotereau F, Zinkernagel RM, Lotteau V. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci USA. 1998;95:13120–13124. doi: 10.1073/pnas.95.22.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balagopal P. In-vivo measurement of protein synthesis in humans. Curr Opin Clin Nutr Metab Care. 1998;1:467–473. doi: 10.1097/00075197-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Baumann PQ, Stirewalt WS, O’Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol Endocrinol Metab. 1994;267:E203–E209. doi: 10.1152/ajpendo.1994.267.2.E203. [DOI] [PubMed] [Google Scholar]
- 4.Birkus G, Hitchcock MJM, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:716–723. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cihlar T, Birkus G, Greenwalt DE, Hitchcock MJM. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antiviral Res. 2002;54:37–45. doi: 10.1016/s0166-3542(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 6.Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, Rathmacher JA. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. J Parenter Enteral Nutr. 2000;24:133–139. doi: 10.1177/0148607100024003133. [DOI] [PubMed] [Google Scholar]
- 7.Crowley JR, Yarasheski K, Leeuwenburgh C, Turk J, Heinecke JW. Isotope dilution mass spectrometric quantification of 3-nitrotyrosine in proteins and tissues is facilitated by reduction to 3-aminotyrosine. Anal Biochem. 1998;259:127–135. doi: 10.1006/abio.1998.2635. [DOI] [PubMed] [Google Scholar]
- 8.Darmaun D, Manary MJ, Matthews DE. A method for measuring both glutamine and glutamate levels and stable isotope enrichments. Anal Biochem. 1985;147:92–102. doi: 10.1016/0003-2697(85)90013-2. [DOI] [PubMed] [Google Scholar]
- 9.Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol Endocrinol Metab. 1986;251:E117–E126. doi: 10.1152/ajpendo.1986.251.1.E117. [DOI] [PubMed] [Google Scholar]
- 10.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 11.Grinspoon S, Mulligan K Department of Health and Human Services Working Group on the Prevention and Treatment of Wasting and Weight Loss. Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis. 2003;36:S69–S78. doi: 10.1086/367561. [DOI] [PubMed] [Google Scholar]
- 12.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 13.Heckmann A, Waltzinger C, Jolicoeur P, Dreano M, Kosco-Vilbois MH, Sagot Y. IKK2 inhibitor alleviates kidney and wasting diseases in a murine model of human AIDS. Am J Pathol. 2004;164:1253–1262. doi: 10.1016/S0002-9440(10)63213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holecek M. Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition. 2002;18:130–133. doi: 10.1016/s0899-9007(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 15.Horber FF, Horber-Feyder CM, Krayer S, Schwenk WF, Haymond MW. Plasma reciprocal pool specific activity predicts that of intracellular free leucine for protein synthesis. Am J Physiol Endocrinol Metab. 1989;257:E385–E399. doi: 10.1152/ajpendo.1989.257.3.E385. [DOI] [PubMed] [Google Scholar]
- 16.Huffman FG, Walgren ME. L-glutamine supplementation improves nelfinavir-associated diarrhea in HIV-infected individuals. HIV Clin Trials. 2003;4:324–329. doi: 10.1310/BFDT-J2GH-27L7-905G. [DOI] [PubMed] [Google Scholar]
- 17.Kelleher AD, Sewell AK, Price DA. Comment. Dyslipidemia due to retroviral protease inhibitors. Nat Med. 2002;8:308. doi: 10.1038/nm0402-308a. [DOI] [PubMed] [Google Scholar]
- 18.Kino T, Gragerov A, Slobodskaya O, Tsopanomichalou M, Chrousos GP, Pavlakis GN. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J Virol. 2002;76:9724–9734. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang JS, Distler O, Cooper DA, Jamil H, Deckelbaum RJ, Ginsberg HN, Sturley SL. HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med. 2001;7:1327–1331. doi: 10.1038/nm1201-1327. [DOI] [PubMed] [Google Scholar]
- 20.Llovera M, Garcia-Martinez C, Agell N, Lopez-Soriano FJ, Authier FJ, Gherardi RK, Argiles JM. Ubiquitin and proteasome gene expression is increased in skeletal muscle of slim AIDS patients. Int J Mol Med. 1998;2:69–73. [PubMed] [Google Scholar]
- 21.Macallan DC, McNurlan MA, Milne E, Calder AG, Garlick PJ, Griffin GE. Whole-body protein turnover from leucine kinetics and the response to nutrition in human immunodeficiency virus infection. Am J Clin Nutr. 1995;61:818–826. doi: 10.1093/ajcn/61.4.818. [DOI] [PubMed] [Google Scholar]
- 22.Mallon PWG, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[13C]leucine. Am J Physiol Endocrinol Metab. 1980;238:E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- 24.McDermott AY, Shevitz A, Knox T, Roubenoff R, Kehayias J, Gorbach S. Effect of highly active antiretroviral therapy on fat, lean, and bone mass in HIV-seropositive men and women. Am J Clin Nutr. 2001;74:679–686. doi: 10.1093/ajcn/74.5.679. [DOI] [PubMed] [Google Scholar]
- 25.McNurlan MA, Garlick PJ, Steigbigel RT, DeCristofaro KA, Frost RA, Lang CH, Johnson RW, Santasier AM, Cabahug CJ, Fuhrer J, Gelato MC. Responsiveness of muscle protein synthesis to growth hormone administration in HIV-infected individuals declines with severity of disease. J Clin Invest. 1997;100:2125–2132. doi: 10.1172/JCI119747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulligan K, Tai VW, Schambelan M. Cross-sectional and longitudinal evaluation of body composition in men with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:43–48. doi: 10.1097/00042560-199705010-00007. [DOI] [PubMed] [Google Scholar]
- 27.Nurjhan N, Bucci A, Perriello G, Stumvoll M, Dailey G, Bier DM, Toft I, Jenssen TG, Gerich JE. Glutamine: a major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J Clin Invest. 1995;95:272–277. doi: 10.1172/JCI117651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccinini M, Rinaudo MT, Chiapello N, Ricotti E, Baldovino S, Mostert M, Tovo PA. The human 26S proteasome is a target of antiretroviral agents. AIDS. 2002;16:693–700. doi: 10.1097/00002030-200203290-00004. [DOI] [PubMed] [Google Scholar]
- 29.Pollock ML, Gaesser GA, Butcher JD, Després JP, Dishman RK, Franklin BA, Ewing Garber C. ACSM position stand on the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 30.Rebouche CJ, Pearson GA, Serfass RE, Roth CW, Finley JW. Evaluation of nuclear magnetic resonance spectroscopy for determination of deuterium abundance in body fluids: application to measurement of total body water in human infants. Am J Clin Nutr. 1987;45:373–380. doi: 10.1093/ajcn/45.2.373. [DOI] [PubMed] [Google Scholar]
- 31.Refaeli Y, Levy DN, Weiner DB. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc Natl Acad Sci USA. 1995;92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz HP, Karl IE, Bier DM. The alpha-keto acids of branched-chain amino acids: simplified derivatization for physiological samples and complete separation as quinoxalinols by packed column gas chromatography. Anal Biochem. 1980;108:360–366. doi: 10.1016/0003-2697(80)90600-4. [DOI] [PubMed] [Google Scholar]
- 33.Selberg O, Suttmann U, Melzer A, Deicher H, Muller MJ, Henkel E, McMillan DC. Effect of increased protein intake and nutritional status on whole-body protein metabolism of AIDS patients with weight loss. Metabolism. 1995;44:1159–1165. doi: 10.1016/0026-0495(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 34.Shabert J, Winslow C, Lacey JM, Wilmore DW. Glutamine-antioxidant supplementation increases body cell mass in AIDS patients with weight loss: a randomized, double-blind controlled trial. Nutrition. 1999;15:860–864. doi: 10.1016/s0899-9007(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 35.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996;271:26690–26697. doi: 10.1074/jbc.271.43.26690. [DOI] [PubMed] [Google Scholar]
- 36.Tang AM. Weight loss, wasting, and survival in HIV-positive patients: current strategies. AIDS Read. 2003;13:S23–S27. [PubMed] [Google Scholar]
- 37.Wang ZM, Gallagher D, Nelson ME, Matthews DE, Heymsfield SB. Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr. 1996;63:863–869. doi: 10.1093/ajcn/63.6.863. [DOI] [PubMed] [Google Scholar]
- 38.Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL. Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;31:803–805. doi: 10.1086/314027. [DOI] [PubMed] [Google Scholar]
- 39.Watt PW, Lindsay Y, Scrimgeour CM, Chien PAF, Gibson JNA, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. 2. New York: Wiley-Liss; 1992. [Google Scholar]
- 41.Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥76 yr old. Am J Physiol Endocrinol Metab. 1999;277:E118–E125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- 42.Yarasheski KE, Smith K, Rennie MJ, Bier DM. Measurement of muscle protein fractional synthetic rate by capillary gas chromatography/combustion/ isotope ratio mass spectrometry. Biol Mass Spectrom. 1992;21:486–490. doi: 10.1002/bms.1200211004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol Endocrinol Metab. 1995;268:E268–E276. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- 44.Yarasheski KE, Zachwieja JJ, Gischler J, Crowley J, Horgan MM, Powderly WG. Increased plasma Gln and Leu Ra and inappropriately low muscle protein synthesis rate in AIDS wasting. Am J Physiol Endocrinol Metab. 1998;275:E577–E583. doi: 10.1152/ajpendo.1998.275.4.E577. [DOI] [PMC free article] [PubMed] [Google Scholar]