Abstract
Cancer-immune (CI) equilibrium constitutes an important component of the cancer immunoediting theory. It is defined as a period during which our immune system and cancer live in harmony in the body. The immune system, though not able to completely eliminate the cancer, doesn’t allow it to progress or metastasize further. Mechanisms of this phase are poorly understood because this phase is difficult to identify even by the most modern detection methods. Till now, the work done on the equilibrium phase of cancer, suggests promising improvements in cancer therapy if the disease could be withheld in this phase. However, there are many queries which remain to be addressed about this interesting yet unresolved phase of cancer immunity.
Keywords: Immunoediting, Elimination, Immune equilibrium, Escape, Mechanisms
Introduction
The three theories of immunosurveillance, immunoevasion and immunoediting have ruled the field of cancer immunity since long. However, in recent times, the former two have given way to the modern concept of cancer immunoediting with its three E’s namely Elimination, Equilibrium and Escape. The “elimination phase” corresponds to the original concept of cancer immunosurveillance whereby cancer cells are successfully recognized and destroyed by the body’s immune system thus returning the tissues to their normal state. If the tumor cells are not completely eliminated they may proceed onto an “equilibrium phase” in which immune system is able to control the tumor growth but does not completely eliminate it. Over the time however, cancer may overcome the entire immune defense and enter in to the third phase of “escape” during which it progresses and metastasizes. It is thought that the emergence of clinical symptoms of cancer generally correlates with this stage [1].
Of the three phases of tumor-immune interaction much work has been focused on the escape phase. The evidence for “equilibrium phase” also called cancer-immune (CI) equilibrium is however least developed. Time and again, questions have been raised against its very existence. Recent experimental and clinical studies however have confirmed the occurrence of this dormant phase in cancers [2]. Exploration of CI equilibrium is believed to be important as this may help in stopping the progression and spread of the cancer thus converting it from the deadly into a chronic disease. However, at present, it is not clear whether the equilibrium phase is beneficial to us or a harbinger of the drug resistant culprits that enable the cancer to survive in the darker corners of the body. Although, many articles have been published on immunoediting in general, the mechanisms related to CI equilibrium are still unclear. This review makes an effort to address some of the queries related to CI equilibrium and also brings forward few relevant questions the answers to which need to be searched further.
First and Foremost Question - Does the Equilibrium Phase Really Exist?
The concept of the CI equilibrium first came into being to explain the period of immune dormancy experienced in several tumors. The phenomenon of relapse seen in many cancers after one to two decades of remission was attributed to the fact that some of the tumor cells remained in the body despite the treatment [2]. These cells were believed to be at peace with our immune system wherein both could survive simultaneously. An insult, physical, chemical or genetic, abolished this equilibrium, thereby, giving rise to a progressive tumor. The occurrence of minimal residual disease (MRD) in leukemia and some solid malignancies lends further support to the equilibrium hypothesis. In MRD, a small number of malignant cells remain in the body below the threshold of conventional morphologic or cytogenetic recognition. Tumors in organ transplant recipients without its presence in donors in overt form also suggest that these could have been present in donors in a dormant state at the same or a distant site [3, 4]. The immunosuppression in the recipient however, provided the stimulus needed for the immune escape and formation of a full-blown cancer [5]. Understanding of preneoplastic conditions like monoclonal gammopathy of undetermined significance (MGUS) has helped further to strengthen the belief in equilibrium phase of cancer. The existence of an immune response to premalignant MGUS cells that eventually progress to multiple myeloma (MM) is consistent with the CI equilibrium, with the immune system controlling, but not eliminating, MGUS cells that eventually evolve and progress to malignancy [6, 7].
The existence of non-immune mechanisms of cancer dormancy in humans including the cellular and angiogenic dormancy provide additional support to the existence of immune dormancy and CI equilibrium in the malignancies. In the former case the tumor cells enter a state of quiescence or senescence due to unknown mechanisms, whereas in the later they undergo enhanced apoptosis owing to poor vascularisation and hypoxia [2, 7].
A major recent study by Koebel et al served an important milestone in proving the concept of CI equilibrium. They injected mice with small doses of a carcinogen methylcholantherene. Mice developing outright tumors were set aside. The remaining mice were found to have small, stable masses at the injection site which grew into full-blown cancers when certain components of the immune system were disabled, thus suggesting that the immunity had previously been holding the tumors in check [8]. However, whether these results can be fully extrapolated to humans or not remains to be seen.
Some of the experimental studies have pointed towards the existence of two types of equilibrium phase. In one, the solitary tumor cells may remain quiescent, undergoing neither cell division nor apoptosis [9] and in the second, the proliferation is balanced by apoptosis, resulting in no increase in size [10]. In the former, the immune system may eventually eliminate all tumor cells, leading to an outcome similar to elimination. In the later scenario, the constant interaction of the immune system with tumors over a long period of time may ultimately sculpt the phenotype of developing tumors.
What are the Mechanisms Involved in CI Equilibrium?
The concept that immune system can protect the host from tumors was initially proposed by Ehrlich in 1909 [11]. Since then many animal and experimental studies have been carried out on tumor-immune interaction. However, the mechanisms involved in CI equilibrium are still not well characterized. These mechanisms could be better understood by considering the events pre and post equilibrium.
What Drives CI Equilibrium?
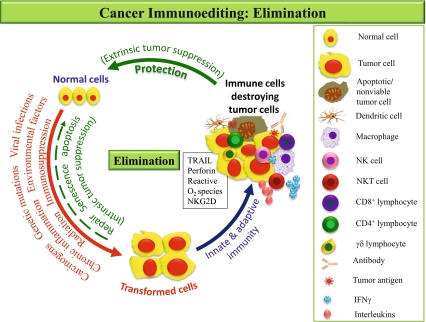
Before CI equilibrium there is a phase of elimination and if it successfully eradicates the growing tumor the process of immunoediting is completed without any progression to subsequent phases. The elimination phase is more or less similar to that seen in host defense to microbial pathogens and involves both innate and adaptive arms of the immune system. This phase may be activated once the normal cells are transformed secondary to various stimuli (Fig. 1). The transformed cells, developing after failure of intrinsic tumor suppressor mechanism, initiate stromal remodeling which results in local tissue disruption. This is recognized as a danger signal by the cells of innate immune system i.e. natural killer cells (NK cells)/natural killer T cells (NKT cells)/γδ T cells and macrophages. The above interaction triggers extrinsic tumor suppressive mechanism with the generation of Interferon-γ (IFN-γ) and other interleukins (ILs) by the NK cells and macrophages [12]. The INF-γ activates antiproliferative, proapoptotic and angiostatic processes. Together they lead to death of a significant number of tumor cells. In addition, the macrophages by producing reactive oxygen (ROS) and reactive nitrogen intermediates and the NK cell via TRAIL (TNF related apoptosis inducing ligand) or perforin dependent mechanisms together kill the residual tumor cells [13]. This killing generates tumor antigens which activate the dendritic cells (DC) recruited at the tumor site. These DC capture tumor antigens and migrate to the draining lymph node where they activate naïve Th1 CD4+ T cells and hence the CD8+ cytotoxic lymphocytes (CTL). The tumor specific CD4+ and CD8+ T cells migrate to tumor site to kill viable antigen positive tumor cells. CD4+ T cells produce IL-2 which keeps tumor specific CD8+ T cells activated. CD8+ T cells recognize and kill the tumor cells directly, by IFN-γ dependent mechanisms of cell cycle inhibition, apoptosis, and angiostasis or by induction of macrophage tumoricidal activity. These processes may occur until the all the tumor cells are killed and process of elimination is completed. In case, any tumor cell variant is resistant to elimination and survives it is considered to have entered in to the equilibrium phase [14].
Fig. 1.
Elimination: Begins after transformation of normal cells as a result of one or more tumorigenic stimuli i.e. carcinogens. The transformation is normally prevented by intrinsic tumor suppressing mechanisms (i.e. repair, senescence or apoptosis). If these mechanisms fail to remove the transformed cells, extrinsic factors are induced involving both innate and adaptive immune response. Macrophages and NK cells play important role and eliminate tumor cells either by phagocytosis or direct cytotoxicity. Cells of adaptive immunity are also activated once the tumor antigens are presented to them by the antigen presenting cells i.e. DC leading to complete elimination
What Maintains the CI Equilibrium?
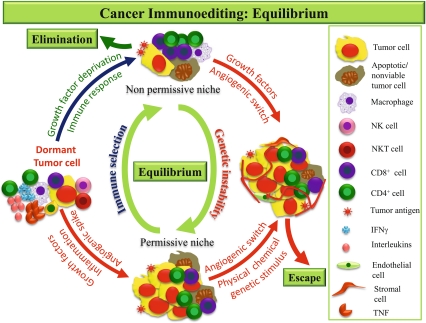
The equilibrium phase involves the continuous elimination of tumor cells and the production of resistant variants (Fig. 2). It is believed to be the longest of the three phases of cancer immunoediting and may be carried over a period of many years [1].
Fig. 2.
Equilibrium: Some of the cancer cells may resist elimination and undergo dormancy. These cells, being immunogenic, attract strong inflammatory response and various molecules i.e. INFγ, ILs and TNF etc are released by adaptive cells which keep a check on tumor multiplication. Therefore, during early stage of equilibrium the cells are quiescent and rate of apoptosis is low with no increase in tumor size. Persistent immune response, deprivation of growth promoting factors and hypoxia in the absence of vascular supply all create an unsuitable microenvironment (nonpermissive niche) for the tumor and eventually all tumor cells are eliminated leading to an outcome similar to elimination. Over the time or in later stage the tumor cells acquire genetic abnormities and angiogenic phenotype; they start proliferating rapidly and outgrow apoptosis resulting in a favorable microenvironment (permissive niche). The tumor is now ready to evade the CI equilibrium
Immunologically, the exact sequence of events in the equilibrium phase is not known. Nevertheless, it has been suggested that CI equilibrium is solely maintained by adaptive immunity in contrast to the other two phases of cancer immunity which require elements from innate as well as adaptive immune response [15]. The adaptive T cells are the key players in the maintenance of this dormant status [6]. Studies on different cancers have shown a significant reduction in tumor progression in case of infiltration of the tumor by the T lymphocytes [16, 17]. Mouse lymphoma studies have shown that malignant cells are kept at low numbers in the bone marrow owing to persistent antigen and memory T cells that are able to coordinate a CD4+ and CD8+ T cell-mediated response [18]. Loss of MHC class I and other components of class I processing pathway in cancer cells point towards the importance of CD8+ T cells, in particular, in preventing the disease progression [19].The extent of involvement of other T cell subtypes like the T regulatory cells (CD4+CD25+Foxp3+ T cells or Treg)) in maintenance of CI equilibrium however remains to be investigated.
The role of cytokines like IFN-γ and TNF has also been suggested by some studies. In a study by Hermelink et al the adaptive tumor antigen specific T cells were found to arrest the growth of experimentally induced pancreatic tumors in mice in the presence of a coordinated interaction between IFN-γ and TNF. In the absence of this interaction the same T cells promote angiogenesis and multistage carcinogenesis [20]. In other studies also the IFNs have been suggested to provide a selective pressure that may lead to progression from elimination to equilibrium phase of the cancer immunoediting process [21].
The immunogenicity of the cancer cells also varies during the three stages of cancer immunoediting. It has been found that, in equilibrium state, the tumor cells are more immunogenic compared to those spontaneously escaping the equilibrium and becoming growing tumors [22]. Moreover, the tumor antigens inducing the T cell response may also play an important part in deciding whether the immune response will promote or inhibit its progression. Ziegler et al in their study on colon cancer demonstrated strong promotion of Th2 type of response by tumor associated antigen EpCAM both invitro and invivo and hence tumor immune evasion [23]. The antigens which may be important for maintenance of CI equilibrium however remain to be identified. In addition to the above, the location of the T cells in the tumor may be important. Whereas the intratumoral T lymphocytes or tumor infiltrating lymphocytes (TIL) are more important in antitumor responses, those recruited around the tumor site (peritumoral) may not always contribute to the antitumor immune response [24–26]. These may, therefore, be investigated for their role in maintenance of the CI equilibrium.
Role of stroma in modulating the immune response to tumors has also been recognized. It has been found that stromal cells especially the myeloid stromal cells in solid tumors acquire immunosuppressive, proangiogenic, and phagocytic function and that the equilibrium between host and cancer can be caused by effector T cells killing the tumor stroma [27].
In addition, the nonimmune factors like genetic instability of cancer cells resulting from mutations in DNA stability genes, microsatellite & nucleotide excision repair instability or more commonly, from structural or numerical changes in whole chromosomes also help the tumor cells to resist the host’s immunological onslaught. Fluctuations in oncogene expression or alteration of relationship between tumor suppressor and oncogenes have been suggested as the other mechanisms involved in establishing the equilibrium [28].
Where do the Tumor Cells Hide Themselves During the Equilibrium Phase?
The dormant tumor cells are believed to reside in the “niches” made up of specialized vascular bed of endothelial cells, associated stromal cells of mesenchymal origin and extracellular matrix components. The endothelial cells lining the blood vessels and the lymphatics in the niches may help to regulate the anti-tumor immune responses [29]. Two types of niches have been proposed to exist—the tumor friendly or “permissive niche” and the tumor unfriendly or the nonpermissive niche”. Whereas the former may support the tumor growth by secretion of trophic angiogenic factors, the later are believed to facilitate its removal by immune cells or apoptosis due to lack of a supportive microenvironment. Though some of these trophic factors like vascular endothelial growth factor (VEGF) have been described the others remain to be identified. Under more favorable conditions the tumor cells can recirculate from these “niches” to different organs. These “niches” have been identified in the bone marrow however, their existence in other organs cannot be ruled out in the absence of a systematic search in these organs [28, 30].
In the later stages of equilibrium, despite the continuing immune response new variants of tumor cells having different types of genetic and molecular abnormalities arise which ultimately render them less immunogenic. The altered milieu characterized by the dysregulated cytokines, enhanced proteolysis, hypoxia, phagocytosis of apoptotic tumor cells and Fc-receptor cross-linking all result in ensuing immunosuppression [9, 31–34] which sets the stage for escape.
How does Tumor Evade Equilibrium?
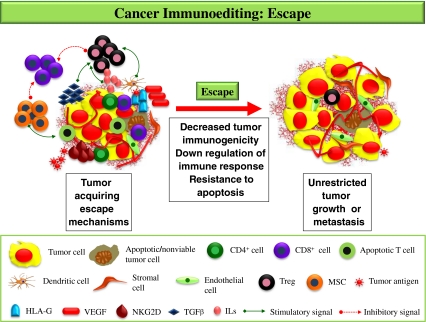
The evasion is brought about by the complex interplay of cancer cells, the cytokines and the immune cells (Fig. 3). Firstly, the immunosuppressive state is accentuated by secretion of cytokines like TGF-β and IL-10 by the tumor cells which in turn induce CTLA-4 (cytotoxic T lymphocyte associated antigen-4) or immunosuppressive Treg. Once generated, the Treg down regulate and interfere with functions of antitumor cytotoxic lymphocytes [35, 36].
Fig. 3.
Escape: Once in a favorable environment, the tumor sheds all its antigens and other molecules which immune cells use for their recognition i.e. MHC, NKG2D and therefore, become less immunogenic. Further with appearance of Treg, MSC and molecules like HLA-G there is down regulation of the adaptive immunity. Finally with rich vascular supply due to growth factors i.e. VEGF and resistance to apoptosis the tumor escape immune response, grow to a clinically detectable size or may even metastasize
Further, the loss of antigen expression & MHC on tumor cells, shedding of NKG2D ligands and development of INF-γ insensitivity may mask the tumor recognition by immune cells. The tumor antigens inducing T cell response may divert the immune response to Th2 type thereby facilitating immune evasion. Certain aberrant antigens expressed on tumor cells or macrophages e.g. HLA-G may inhibit the proliferative response of effector cells belonging to innate and adaptive immunity (NK and CD4+ and CD8+ T cells respectively). In addition, soluble HLA-G induces apoptosis of activated CD8+ T cells by binding to CD8+ and by triggering a Fas/FasL dependent pathway [37]. Production of myeloid suppressor cells (MSCs) with potent immunosuppressive function may also contribute to functional inactivation of CD8+ cells mediated via l-arginine depletion. Also, they enhance the activity of iNOS which produces nitric oxide (NO). The NO has been shown to interfere with IL-2 receptor signaling such that JAK-1 and −3, STAT-5, and ERK are not activated, leading to cell cycle arrest and T cell apoptosis [30, 38]. The membrane-bound HLA-G also down regulates IFN-γ secretion by T cells, whereas the soluble HLA-G stimulates the release of IL-3, IL-4, and IL-10 [37].
As the tumor grows it increases its vascularity by the secretion of VEGF from tumor cells which are responsible for switching of endothelial cells from a resting to rapid growth phase [39]. Failure of neovascularization or reorganization of the existing vasculature results in enhanced apoptosis or a non-angiogenic and non-progressing stable tumor owing to poor vascularisation [2, 40].
Identification of the Equilibrium Phase - the Real Challenge!
Till now there is no single biomarker that can clearly identify the equilibrium phase. One of the important reasons for this is the presence of tumor cells in low numbers during this phase. To detect hidden tumor cells in the tissues like bone marrow various biomarkers like Ki67, p120 or mutations in Kras, p53 and other specific markers have been tried but with limited success [48]. A panel of angiogenic switch-related biomarkers is also under development. These biomarkers include circulating endothelial progenitor cells and proteins in blood platelets, as well as matrix metalloproteinases detected in the urine [39].
Various techniques have been used to detect the dormant tumor cells. Immunocytochemistry (ICC) following Ficoll‑Hypaque density centrifugation has been used in certain epithelial tumors [41, 42]. However drawback of this technique is that it can detect only circulating tumor cells which may not always be found in the mononuclear cell fraction after Ficoll‑Hypaque density centrifugation.
Flowcytometry using monoclonal antibodies has also been tried with limited success [43]. Different types of enrichment methods have been used to overcome this problem such as use of immunomagnetic beads for separation of tumor cells from peripheral blood, bone marrow, ascetic fluid or pleural effusions. The beads, coated with monoclonal antibody, bind to the cells of interest and by the use of a magnet the cells can be selected [44–46]. When compared to ICC alone, immunomagnetic bead enrichment can provide a multi-fold increase in tumor cell detection. However, the major limitation of this newer technology is that the monoclonal antibodies used for selection generally are tumor associated and not tumor specific.
Recently established cellular magnetic resonance imaging (MRI) has shown great promise for experimental in vivo cell tracking. It combines ultra high resolution MRI with the use of sensitive and specific cell labeling agents (i.e. magnetic nanoparticles) [47].
Quantitative RT-PCR used in monitoring of circulating tumor cells in certain malignancies i.e. minimal residual disease may be of great value since it can detect as low as one malignant cell/105 to 106 normal cells. This, however, means that patients with no residual disease detectable by RT-PCR may still harbor up to a million malignant cells that could go unnoticed.
In animal models, the detection of single tumor cell can be achieved using these techniques and biomarkers [49–51]. However, as far as human malignancies are concerned there are several hurdles in development of a successful biomarker. The major problem is that the equilibrium phase is symptomless; therefore identification of the patients who need screening may be a problem. Besides this, it is not known whether equilibrium is more a property of cancer cells or of our immune system. Therefore before designing a specific biomarker or a detection method it is important to understand the mechanisms underlying a state of CI equilibrium and identification of the factors (environmental, immunological or molecular) involved in its maintenance.
Are There Any Therapeutic Implications of CI Equilibrium?
Despite so much emphasis on the host immunity in cancer, the results of immunotherapeutic interventions have proved to be disappointing. The real importance of recognizing and understanding the CI equilibrium lies in its immense potential as a therapeutic target. Holding the disease in this phase means stopping the progression, preventing the metastasis and thus converting the cancer from the deadly into a chronic disease. So far, the observations in patients of MRD suggest that the disease is more resistant to elimination during this phase as the cells are in a nondividing state [52–55]. As the immune system is not working it’s full, boosting up of our immune machinery during this phase itself may help in eliminating the tumor or at least preventing its subsequent escape. A DC vaccine derived from patients loaded tumor antigens can be an efficient method for stimulating anticancer immunity. A vaccination strategy may be used in combination with other approaches like enhancement of T cell costimulation by administering agonistic antibodies or by antagonistic antibodies specific for coinhibitory receptors such as CTLA-4 [34, 56, 57]. Equally important would be to develop interventions that counter the propensity of tumors to evade immune elimination, such as removing Treg or immunizing against the tumor stroma. Identifying the important immune cells involved in halting the cancer progression may help in designing the immune cell fractionation therapies aimed at preventing the metastasis.
Recognition of the factors crucial in maintaining the CI equilibrium has paved the way for development of therapies specifically targeting tumor promoting molecules e.g. angiogenic or tyrosine kinase inhibitors [58]. The properties and expression of various tumor antigens during this phase and studying the alterations leading to escape may aid in designing and development of immunotherapy targeting the tumor antigens. Last, but not the least, the “niches” which are the hiding places for tumor cells may be specifically targeted for rooting out the malignancy in its entirety [25].
Is There Any Prognostic Significance of CI Equilibrium?
Can we predict which cancer will go into the equilibrium phase? Are all prognostically favorable cancers characterized by presence of a well-defined equilibrium phase? Is there any effect of duration of equilibrium phase on the behavior of malignancy? Many studies have tried to link the immune cells in the cancer with the outcome. Presence of high numbers of TIL, especially with cytotoxic CD8+ T cells and memory phenotypes, has been reported to be an indicator of good prognosis in many cancers such as melanoma, breast cancer, ovarian cancer, non-Hodgkin’s lymphoma, head and neck cancer, non-small-cell lung cancer, esophageal cancer and urothelial carcinoma [59–61]. A similar positive correlation has also been observed between NK cell infiltration and the survival for gastric cancer, colorectal cancer and squamous cell lung cancer. A high density of helper T cells and memory cells has been shown to be associated with absence of tumor dissemination or metastasis. Similarly the density of mature DCs, which home exclusively in the T cell areas of tertiary lymphoid structures has also been shown to be associated with a favorable clinical outcome [62]. Besides the density, organization of these cells in and around the tumor is also important as immune compartments in each tumor region may control different tumor events. Tumor associated macrophages are supposed to have role in angiogenesis and both these are correlated with worse disease free survival prognosis [63]. The prognostic value of Treg in human cancers however is still debatable. Curiel et al in their pioneer study showed a positive correlation between number of Treg and advanced tumor stage [64]. However, data also suggest an increase in numbers of intratumoral Treg is not always associated with poor prognosis but may in fact correlate with good prognosis. Such findings have been reported for follicular lymphoma and Hodgkin’s lymphoma [65, 66]. Whether the above findings, applicable to cancer in general can be extended to equilibrium phase as well or not remains to be investigated.
Therefore, in conclusion, it can be said that the CI equilibrium is an important phase in cancer immunity. This phase depends upon the complex interplay of characteristics of transformed cells, the immune cells and the surrounding stroma. At present there are a number of queries regarding CI equilibrium but very few answers. Although several interesting deductions can be made from our past and present experience, the existence and events of the equilibrium phase need a certificate of long term, well planned human studies before the knowledge can actually be utilized to benefit the mankind.
Acknowledgments
Conflict of Interest The author(s) declare that they have no conflict of interest
Disclosure of Funding No funding has been received from any agency for this work
Authors’ Contributions AB conceptualized, designed and drafted the manuscript. YK has drafted the manuscript and has revised it critically for important intellectual content. Both the authors have read and approved the final manuscript.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Teng MWL, Swann JB, Koebel MC, Schreiber RD, Smyth JM. Immune-mediated dormancy: an equilibrium with cancer. J Leukocyte Biol. 2008;84:988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 3.Kauffman HM, McBride MA, Delmonico FL. First report of the United Network for Organ Sharing Transplant Tumor Registry: donors with a history of cancer. Transplantation. 2000;70:1747–1751. doi: 10.1097/00007890-200012270-00014. [DOI] [PubMed] [Google Scholar]
- 4.Myron KH, McBride MA, Cherikh WS, Spain PC, Marks WH, Roza AM. Transplant tumor registry: donor related malignancies. Transplantation. 2002;74:358–362. doi: 10.1097/00007890-200208150-00011. [DOI] [PubMed] [Google Scholar]
- 5.MacKie MR, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N Eng J Med. 2003;348:567–568. doi: 10.1056/NEJM200302063480620. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Eng J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 7.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198:1753–1757. doi: 10.1084/jem.20031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 9.Naumov GN, MacDonald IC, Chambers AF, Groom AC. Solitary cancer cells as a possible source of tumour dormancy? Semin Cancer Biol. 2001;11:271–276. doi: 10.1006/scbi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 10.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich P. Ueber den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5(Part 1):273–290. [Google Scholar]
- 12.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2008;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardone IS. Role of NK cells and adaptive immunity in “immunoediting”: recent developments. Immunologia. 2008;27:141–146. [Google Scholar]
- 14.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 15.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Eng J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 17.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 18.Mahnke YD, Schwendemann J, Beckhove P, Schirrmacher V. Maintenance of long term tumor-specific T-cell memory by residual dormant tumor cells. Immunology. 2005;115:325–336. doi: 10.1111/j.1365-2567.2005.02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrar JD, Katz KH, Windsor J, Thrush G, Scheuermann RH, Uhr JW, et al. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN—in establishing and maintaining the tumor-dormant state. J Immunol. 1999;162:2842–2849. [PubMed] [Google Scholar]
- 20.Hermelink MN, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, et al. Tnfr1 signaling and IFN-G signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 22.Baguley BC. Tumor stem cell niches: a new functional framework for the action of anticancer drugs. Recent patents on anti-cancer drug discovery. 2006;1:121–127. doi: 10.2174/157489206775246494. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler A, Heidenreich R, Braumüller H, Wolburg H, Weidemann S, Mocikat R, et al. EpCAM, a human tumor-associated antigen promotes Th2 development and tumor immune evasion. Blood. 2009;113:3494–3502. doi: 10.1182/blood-2008-08-175109. [DOI] [PubMed] [Google Scholar]
- 24.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 25.Kondo E, Koda K, Takiguchi N, Oda K, Seike K, Ishizuka M, et al. Preoperative natural killer cell activity as a prognostic factor for distant metastasis following surgery for colon cancer. Dig Surg. 2003;20:445–451. doi: 10.1159/000072714. [DOI] [PubMed] [Google Scholar]
- 26.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/S0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Zhang Y, Bowerman NA, Schietinger A, Fu YX, Kranz DM, et al. Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res. 2008;68:1563–1571. doi: 10.1158/0008-5472.CAN-07-5324. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 29.Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 30.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumor dormancy. APMIS. 2008;116:754–770. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 31.Felsher DW. Tumor dormancy: death and resurrection of cancer as seen through transgenic mouse models. Cell Cycle. 2006;5:1808–1811. doi: 10.4161/cc.5.16.3111. [DOI] [PubMed] [Google Scholar]
- 32.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 33.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakansson L. The capacity of the immune system to control cancer. Eur J Cancer. 2009;45:2068–2070. doi: 10.1016/j.ejca.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Vence LA, Palucka K, Fay JW, Ito T, Liu Y, Banchereau J, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz-Merino LDL, Grande-Pulido E, Albero-Tamarit A, Villenaa MEC. Cancer and immune response: old and new evidence for future challenges. The Oncologist. 2008;13:000–000. doi: 10.1634/theoncologist.2008-0166. [DOI] [PubMed] [Google Scholar]
- 37.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res. 2005;65(22):10139–10144. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 38.Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest. 2006;116:2587–2590. doi: 10.1172/JCI29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folkman J. Tumor angiogenesis: therapeutic implications. N Eng J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- 40.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beitsch JF, Leitch PD, Hoover M, Euhus S, Haley D, Morrison B, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 42.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Eng J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 43.Vredenburgh JJ, Silva O, Tyer C, DeSombre K, Bou-Ghalia A, Cook M, et al. A comparison of immunohistochemistry, two‑color immunofluorescence, and flow cytometry with cell sorting for the detection of micrometastatic breast cancer in the bone marrow. J Hematother. 1996;5:57–62. doi: 10.1089/scd.1.1996.5.57. [DOI] [PubMed] [Google Scholar]
- 44.Naume B, Borgen E, Tossvik S, Pavlak N, Oates D, Nesland JM. Detection of isolated tumor cells in peripheral blood and in BM: evaluation of a new enrichment method. Cytotherapy. 2004;6:244–252. doi: 10.1080/14653240410006086. [DOI] [PubMed] [Google Scholar]
- 45.Fodstad O, Hoifodt HK, Rye PD, Trones GE, Beiske K. New immunobead techniques for sensitive detection of malignant cells in blood and bone marrow. Proc Am Assoc Cancer Res. 1996;37:214. [Google Scholar]
- 46.Griwatz C, Brandt B, Assmann G, Zanker KS. An immunological enrichment method for epithelial cells from peripheral blood. J Immunol Methods. 1995;183:251–265. doi: 10.1016/0022-1759(95)00063-G. [DOI] [PubMed] [Google Scholar]
- 47.Arbab AS, Liu W, Frank JA. Cellular magnetic resonance imaging: current status and future prospects. Expert Rev Med Devices. 2006;3:427–439. doi: 10.1586/17434440.3.4.427. [DOI] [PubMed] [Google Scholar]
- 48.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 49.Harper J, Naumov GN, Exarhopoulos A, Bender E, Louis G, Folkman J, et al (2006) Predicting the switch to the angiogenic phenotype in a human tumor model. In: Proceedings of the American Association for Cancer Research 837
- 50.Klement G, Kikuchi L, Kieran M, Almog N, Yip TT, Folkman J. Early tumor detection using platelet uptake of angiogenesis regulators. Blood. 2004;104:239a. [Google Scholar]
- 51.Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi, et al. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood. 2008;111:1201–1207. doi: 10.1182/blood-2007-04-084798. [DOI] [PubMed] [Google Scholar]
- 52.Felsher DW. Tumor dormancy: death and resurrection of cancer as seen through transgenic mouse models. Cell Cycle. 2006;5:1808–1811. doi: 10.4161/cc.5.16.3111. [DOI] [PubMed] [Google Scholar]
- 53.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 54.Demicheli R. Tumor dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol. 2001;11:297–306. doi: 10.1006/scbi.2001.0385. [DOI] [PubMed] [Google Scholar]
- 55.Barrett AJ, Savani BN. Does chemotherapy modify the immune surveillance of hematological malignancies? Leukemia. 2009;23:53–58. doi: 10.1038/leu.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Favaro E, Amadori A, Indraccolo S. Cellular interactions in the vascular niche: implications in the regulation of tumor dormancy. APMIS. 2008;116:648–659. doi: 10.1111/j.1600-0463.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 59.Clemente CG, Mihm JMC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 60.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8 (+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 61.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Eng J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 62.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 63.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14(2):425–431. [PubMed] [Google Scholar]
- 64.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 65.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 66.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3 + regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]