Abstract
Experimental and epidemiological studies indicate a strong link between chronic inflammation and tumor progression. Human colorectal cancer (CRC), a major cause of cancer-related death in Western countries, represents a paradigm for this link. Key features of cancer-related inflammation in CRC are the activation of transcription factors (e.g. NF-κB, STAT3), the expression of inflammatory cytokines and chemokines (e.g. TNFα, IL-6, CCL2, CXCL8) as well as a prominent leukocyte infiltrate. While considerable evidence indicates that the presence of lymphocytes of adaptive immunity may positively influence patient survival and clinical outcome in CRC, the role of tumor-associated macrophages (TAM) and of other lymphoid populations (e.g. Th17, Treg) is still unclear. In this review we will summarize the different and controversial effects that TAM play in CRC-related inflammation and progression of disease. The characterization of the most relevant inflammatory pathways in CRC is instrumental for the identification of new target molecules that could lead to improved diagnosis and treatment.
Keywords: Colorectal cancer, Tumor-associated macrophages, Inflammation, Cytokines, Chemokines
Introduction
Colorectal cancer is one of the most frequent human neoplasia and the third cause of cancer death in industrialized countries [1].
The pathogenesis of colorectal cancer (CRC) is a very complex process that involves interactions among environmental influences, germ-line factors dictating individual cancer susceptibility and accumulated somatic changes in the colorectal epithelium [1]. The majority of colorectal cancers are sporadic, arising from dysplastic adenomatous polyps. A multi-step process leads to the accumulation of genetic alterations that confer a selective growth advantage to the colonic epithelial cells and drive the transformation from normal epithelium to adenomatous polyp and finally to invasive colorectal cancer. These alterations are the consequence of mutations in genes involved in cell growth regulation (gatekeepers), such as tumor-suppressor genes (e.g. APC, Smad4 and p53) or oncogenes (e.g. K-Ras, c-myc, c-neu, c-src) [2]. About 10% of CRCs develops in the setting of well-defined hereditary syndromes. The two main forms are hereditary non-polyposis colorectal cancer (HNPCC) and familial adenomatous polyposis (FAP) [3, 4]. FAP is an autosomal-dominant disease, due in about 80% of cases, to a germ-line mutation in the adenomatous polyposis coli gene (APC) [1, 4]. HNPCC is an autosomal-dominant disease, caused by the alteration or epigenetic changes of genes that maintain genetic stability, such as DNA nucleotide mismatch repair genes (hMSH2, hMLH1, PMS1, PMS2, hMSH3) [5–7]. This abnormality results in extensive instability in repeated nucleotide sequences called microsatellites, hence the term microsatellite instability (MSI), in opposition to cancers that show microsatellite stability (MSS) [5]. MSI colorectal carcinomas are usually associated with a more favourable prognosis, less lymph node involvement and reduced occurrence of metastasis [8–12].
Besides the occurrence of genetic or epigenetic abnormalities, also the formation of an inflammatory microenvironment plays a pivotal role in colorectal cancer development. Hallmarks of the reactive tumor stroma are the presence of a prominent leukocyte infiltrate, a florid network of blood vessels, matrix proteins, as well as an abundance of cytokines and chemokines [13–15].
In this review we will discuss the pivotal role that inflammatory cells and mediators play in the constitution of the tumor microenvironment in colorectal cancer. In particular, we will focus our attention on the controversial role of tumor-associated macrophages in the progression and clinical outcome of colorectal cancer.
Inflammation and Cancer
Cancer-associated inflammation affects many aspects of malignancy, including the proliferation and survival of malignant cells, angiogenesis and tumor metastasis [16–21]. The connection between inflammation and cancer can be schematically viewed as consisting of two pathways: an intrinsic pathway, driven by genetic alterations that cause inflammation and neoplasia (such as oncogenes); and an extrinsic pathway, driven by inflammatory leukocytes in the context of chronic infectious or persistent inflammatory condition that increase cancer risk. Epidemiological studies have shown that a number of chronic infections predispose to various tumor types. For example, infection by Helicobacter pylori is associated with gastric cancer and mucosal lymphoma; viral infections are related to cervical and liver cancer. Other non-pathogen triggers of chronic inflammation are autoimmune diseases (e.g. inflammatory bowel disease), chemical irritants (e.g. asbestos, cigarette smoke) and inflammatory conditions of unknown origin (e.g. prostatitis associated with prostate cancer). Accordingly, treatment with non-steroidal anti-inflammatory agents decreases the incidence and the mortality of several tumors [22, 23].
A number of recent studies connected the activation of oncogenes to inflammation (intrinsic pathway). In addition to promoting cell autonomous proliferation, several oncogenes activate down-stream a cascade of inflammatory mediators. For example, components of the RAS-RAF signalling pathway induce the activation of the transcription factor NF-κB and the production of several inflammatory cytokines and chemokines [24, 25]. The oncogene MYC encodes a transcription factor that is over-expressed in many human tumors and promotes cell proliferation; in addition, MYC is involved in neo-angiogenesis and in remodelling of the extracellular microenvironment, with inflammatory cells, IL-1 and chemokines having important roles in this process [26]. A further example is offered by the tyrosine kinase RET, a prototypic transforming oncogene in human papillary carcinoma of the thyroid (PTC). Borrello et al. [27] demonstrated that RET/PTC activates, in primary human thyrocytes, an inflammatory programme leading to the build up of reactive microenvironment, including the expression of colony-stimulating factors (CSFs), interleukin-1 (IL-1), cyclooxygenase 2 (COX2) and chemokines attracting monocytes and dendritic cells (CCL2 and CCL20). Recently, we provided evidence that the oncogenic fusion transcript FUS-CHOP also activates an inflammatory programme in human myxoid lyposarcoma [28]. Co-operation between oncogene-derived transformation and exogenous inflammation has also been reported. In a mouse model of pancreatic cancer, cerulein-mediated chronic pancreatitis is required in concert with K-Ras mutation to induce pancreatic intraepithelial neoplasia and invasive ductal carcinoma [29].
Also the inactivation of tumor-suppressor genes may results in the production of inflammatory mediators. In a mouse model of breast carcinoma, inactivation of the gene encoding the type II TGFβ receptor stimulates the production of the inflammatory chemokine CXCL5 and of CXCL12 [30]. The von Hippel-Lindau tumor suppressor (VHL) is a component of the molecular complex that targets the transcription factor of hypoxia-inducible factor 1α (HIF-1α) for degradation. HIF1α interacts with the transcription factor NF-κB, resulting in the production of TNFα and of the chemokine receptor CXCR4 in renal-cell carcinoma cells, as well as in other malignancies [31, 32].
The Inflammatory Microenvironment in Colorectal Cancer
Colorectal cancer represents a paradigm of the cancer-related inflammation. Patients affected by inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) and Crohn’s disease (CD), are at increased risk of developing neoplasia [33, 34], with an extended incidence rate of 2.75 and 2.64 of CRC in patients with UC and CD, respectively [35]. Interestingly, in Helicobacter pylori-induced gastric tumors, pro-inflammatory signalling by TNF-α can induce β-catenin nuclear accumulation even without mutations in the APC gene. To the same end, activation of NF-κB and Akt pathways by pro-inflammatory signalling can promote β-catenin activation and favours colorectal cancer progression (extrinsic pathway) [36].
During colorectal carcinogenesis, colonic epithelial cells accumulate genetic mutations that confer a selective growth advantage to the neoplastic epithelial cells, leading to the transformation from normal epithelium to adenomatous polyp and finally to invasive colorectal cancer. This progression includes activation of the oncogenes K-ras and B-Raf, as well as inactivation of tumor suppressors, as TGF-β receptor (R)II, activin receptors, p53, and the pro-apoptotic protein Bax. Transformed epithelial cells are also able to secrete several inflammatory mediators that act on various types of pro-inflammatory leukocytes, endothelial cells and fibroblasts to establish a tumor promoting reactive microenvironment (intrinsic pathway): among these are cytokines such as TNFα, IL-1, IL-6, cyclooxygenase-2 (COX-2), innate immunity receptors and signalling molecules (Toll-like receptors (TLR)-4, MyD88, and the transcription factor NFκB.
Chemokines
Since their discovery, the chemokine system has been strongly connected with cancer biology: tumor infiltration by macrophages has served as a paradigm of the chemokine-mediated recruitment of leukocytes at tissue peripheral [37–41]. In the last decade our knowledge in chemokine functions has dramatically expanded and now includes the promotion of the angiogenic switch and direct effects on tumor cells survival, proliferation and dissemination [13, 42]. Among several chemokines, CCL2 plays a pivotal role in CRC. Popivanova et al. [43] demonstrated that the blocking of TNFα/TNFR axis resulted in reduced CCL2 mRNA expression, decreased macrophage infiltration and slower CRC progression. In addition, CCL2 antagonists inhibited COX-2 expression, attenuated neovascularization, and eventually decreased the number and size of colon tumors. We have recently analyzed the expression of a large panel of chemokines and their receptor in human CRC [44]. Several chemokines, such as CCL7, CCL20, CCL25, CXCL1 and CCL26, and chemokine receptors, such as CCR8, CCR6, CXCR2 are strongly up-regulated in tumor tissues. In particular, CCL3 and CCL4, both chemotactic for monocytes/macrophages and T-cells, are significantly over-expressed in tumors in comparison to normal colonic mucosa, as well as CXCL8, a chemokine involved in neutrophil recruitment and in neo-angiogenesis processes. Intriguingly, CXCL8 mRNA levels correlate with osteopontin (OPN) mRNA expression: these two mediators share some important functions such as cell mobility and cell survival via integrin activation. Chemokines and chemokine receptors are suggested to play a role also in tumor metastasis: clinical studies indicate that the expression of CXCR3, CXCR4 and CCR7 in primary CRC significantly correlates with tumor recurrence, patient survival and lymph node or liver metastasis [45–48]. Studies using cell culture systems indicate that CXCL10, CXCL12 as well as CXCL1, CXCL2 and CXCL8 stimulate colon carcinoma cell migration and invasion [46, 49, 50]. A further demonstration of the importance of chemokines in colorectal cancer comes from a recent study on the chemokine receptor D6. This is a promiscuous decoy receptor that scavanges several inflammatory CC chemokines. D6-deficient mice showed an increase in tumor burden in a model of colitis-associated cancer (CAC) [51].
TNFα
TNFα is a member of the TNF cytokine superfamily and is a key molecule regulating inflammation and host defense. Activation of TNF receptors (TNFRs) can trigger NF-κB and downstream survival pathways, or can activate caspase 8 and the associated apopotic signal [52]. TNFα induces the expression of chemokines from different cell types, such as epithelial cells, fibroblast, endothelial cells and leukocytes. This cytokine has been shown to have controversial roles in cancer, serving as a tumor-promoting or tumor-destructive factor. The contribution of TNFα in the development of CRC has been recently investigated in a genetic mouse model lacking the type 1 TNFR-p55 [53]. In this study, the abrogation of TNFα signalling in mice resulted in a significantly reduced colitis after treatment with azoxymethane (AOM) and dextran sodium sulfate (DSS), with decreased tissue damage, inflammatory cell infiltrates and cytokine production in the mucosa. In addition, a strongly reduced tumor formation was observed. Further, the administration of a specific TNFα antagonist in AOM/DSS-treated mice obtained similar results, underlying the potential of an anti-TNF therapeutic approach.
NF-κB
NF-κB is a key regulator of innate immunity and inflammation. While in normal conditions NF-κB is kept in an inactive form, in various types of cancer, including colorectal carcinoma is constitutively activated. Greten et al. [54] demonstrated that the functional abrogation of NF-κB in intestinal epithelial cells in mice did not affect the extent of inflammation, but resulted in a dramatic reduction in tumor numbers as a consequence of enhanced epithelial cell apotosis during early tumor development. In contrast, the conditional inactivation of NF-κB in myeloid cells strongly reduced the expression of many genes involved in the inflammatory response, such as IL-1β, TNFα, macrophage inflammatory protein 2 (MIP-2), COX-2 and intercellular adhesion molecule (ICAM). Also these mice exhibited a significant reduction in tumor size, although tumor number was similar or slightly reduced [54, 55]. Thus, NF-κB activation controls both the survival of transformed cells (intrinsic pathway) as well as the leukocyte-driven inflammation (extrinsic pathway) that provides signalling molecules that sustain tumor growth.
Toll-like Receptors
TLRs play a major role in sensing gut microbiota and activation of these receptors is required to maintain intestinal homeostasis. Genetic or functional disregulation of TLRs may be linked to chronic inflammation and tumor development. For example, polymorphisms in TLR4, a receptor required for innate immunity to gram-negative bacteria, have been associated with UC and CD [56]. In addition to their involvement in IBD, evidence is mounting to support a role for TLRs in carcinogenesis. Aberrant TLR signalling may contribute to the tumor-promoting activity of NFκB. Recently it was shown that a deficiency in MyD88, the TLR adaptor protein, significantly reduces tumor number and size in the APCmin mouse model of intestinal tumorigenesis [57]. Moreover, mice inoculated with colon cancer cells in which TLR4 was silenced showed an increased survival and tumors of significant smaller size in comparison to control mice [58].
TIR8
TIR8, also known as single immunoglobulin IL-1R-related molecule (SIGIRR), has been identified for its TIR domain, which is structurally conserved and shared with other member of the IL-1 receptor/TLRs family [59]. TIR8 inhibits signalling from TLR/IL-1R complex, possibly by trapping IRAK-1 and TRAF-6 [60–62]. Epithelial cells of the digestive tract express TIR8 and there is evidence for its non-redundant role in the gastrointestinal mucosa inflammation [63]. In a mouse model of colitis-associated cancer, TIR8-/- mice showed a strong increase in inflammation-related cancer susceptibility in comparison to wild-type mice [64]. These results unequivocally demonstrate the important role of inflammation in CRC progression.
COX-2
COX-2, the enzyme involved in the synthesis of prostaglandins and prostacyclins from arachidonic acid, is strongly related to colorectal carcinogenesis. COX-2 is not constitutively expressed in the colon mucosa; several studies have shown that it is already up-regulated in most adenomas and in virtually all colon carcinoma [65]. Indeed, overexpression of COX-2 in mice tumor xenografts enhanced tumor growth due to a proangiogenic effects. On the other hand, its absence inhibits the development of colorectal poyps in mice, and the use of selective COX-2-inhibitors in clinical trial showed a reduction in the number and size of colorectal polyps [66, 67].
Polymorphism in genes regulating inflammatory processes may alter the risk for neoplasia. Several polymorphisms in the flanking regions of COX-2 have been described, but their association with the risk of CRC remains unclear. However, one COX-2 variant (c.3618A/G polymorphism) possibly affecting RNA stability was associated with the presence of clinical features of good prognosis and higher survival rate of patients. [65, 68, 69]. Further studies, with higher number of patients, are needed to clarify whether genetic polymorphisms of COX-2 may affect colon cancer risk and if specific variants can really have prognostic value.
IL-6
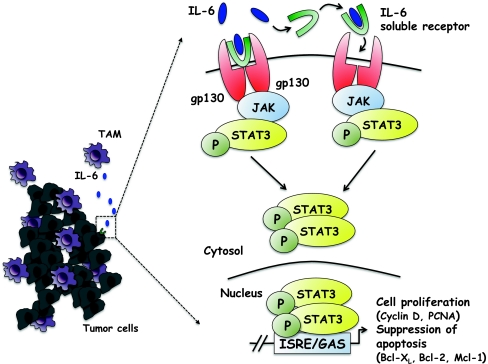
A special consideration should be recognized to the role of IL-6 in inflammation-related colorectal cancer. IL-6 is considered a major key player in the transition between acute and chronic inflammation as well as innate and acquired immunity [70]. IL-6 binds to soluble or membrane-bound IL-6 receptors (IL-6Rα) that interact with the membrane-associated gp130 subunit, and trigger the activation of Janus Kinases (JAKs) and downstream effectors STAT3, Shp2-Ras and phosphatidylinositol 3-kinase (PI3K)-Akt [71] (Fig. 1). IL-6 modulates the expression of chemokines and adhesion molecules thus suppressing neutrophil infiltration, promoting the accumulation of mononuclear leukocytes and leading to the resolution of acute inflammation and the activation of acquired immunity [72, 73]. Evidence of the role of IL-6 in intestinal inflammation comes from the demonstration that the inhibition of IL-6 signalling affects chemotaxis and apoptosis of lamina propria mononuclear cells, improving disease outcome in animal models of colitis [74].
Fig. 1.
TAM-derived IL-6 promotes tumor cell survival and proliferation. IL-6 binds both its membrane-bound and soluble-form receptor, leading to the dimerization of gp130 expressed by tumor cells and the activation of the JAK tyrosine kinase, which phosphorylates STAT3. Translocation of STAT3 into the nucleus induces gene transcription. In colorectal cancer, STAT3 induces the expression of genes important for the proliferation (cyclin D and PCNA) and the suppression of apoptosis (Bcl-XL, Bcl-2 and Mcl-1) strongly promoting neoplastic cell cycle-progression
In the last years, the involvement of IL-6 in colorectal cancer has been deeply investigated. Several studies indicate that IL-6 stimulates the growth of colon cancer cells in vitro [75], increases invasiveness of colon cancer cells and likely promotes secondary tumor formation [76, 77]. Moreover, serum levels of IL-6 in colorectal cancer patients are higher than in healthy controls, and significantly correlate with tumor staging and poorer survival rate [78]. Michael Karin’s group drew attention to the elevated levels of IL-6 in murine models of colitis-associated cancer (CAC) [54] and later reported that the gender bias in liver cancer susceptibility could be addressed to the higher IL-6 serum levels in male mice [79]. In more recent years, the same group [80] clearly demonstrated that IL-6, produced in an NF-κB-dependent manner in response to intestinal injury by innate immune cells within the lamina propria, regulates the proliferation of intestinal epithelial cells (IECs) and their preneoplastic derivatives during acute inflammation and CAC. In addition, exogenous administration of IL-6 in mice during tumor initiation resulted in an increased number of tumor foci, while IL-6 administration during the late stages of CAC growth increased tumor burden. Therefore, IL-6, mainly produced by myeloid cells, is considered an important player in the leukocyte-driven inflammation that promotes colon carcinogenesis (extrinsic pathway).
Formal evidence has been provided that IL-6-activated JAK-STAT3 pathway is also of major importance. STAT3 induces the expression of genes important for cell cycle progression (such as cyclin D and PCNA), and suppression of apoptosis (Bcl-XL, Bcl-2 and Mcl-1), eventually promoting cell survival and proliferation during colitis-associated tumorigenesis (intrinsic pathway) (Fig. 1). Specific ablation of STAT3 in intestinal epithelial cells suppresses cell proliferation and reduced tumor incidence in both a DSS-induced colitis model and CAC model [80, 81].
Further, Bollrath et al. [81] used gp130Y757F/Y757F mice, which express a mutant gp130 receptor molecule with enhanced STAT3 activity, to demonstrate a role for increased STAT3 activation in the acceleration of colorectal cancer. All together, the capacity of STAT3 to support intestinal cell proliferation not only facilitates healing after colitis-induced tissue injury, but also promotes mutagen-triggered transformation. An important aspect is that in IL-6-deficient mice, as well as in mice lacking STAT3, a considerably higher acute mucosal inflammation was observed [82]. This finding may be explained with higher tissue damage induced by DSS, in the absence of the STAT3-mediated ability to resist to apoptosis. Alternatively, as in the absence of STAT3 the IL-10 signalling is also impaired, it is likely that this increased inflammation is due to the lack of the well established anti-inflammatory and protective role of IL-10 on the colonic mucosa [83]. Intriguingly, this inflammatory burst did not increase tumorigenesis but rather was linked to a lower cancer incidence and tumor load. It may be possible that this acute inflammation, at variance from the smouldering inflammation of chronic conditions, may restrain tumor proliferation.
STAT3 may also influence the type of inflammatory leukocytes: in Grivennikov’s study, the number of regulatory T-cells increased and Th17 cells decreased in an IL-6 dependent manner. This observation is in accordance with several recent studies that linked IL-6 to Th17 differentiation.
Tumor Infiltrating Leukocytes in CRCs
A leukocyte infiltrate is already present in benign adenoma and is markedly increased in CRC tissues. Immune cells are localized both at the periphery and in the tumor stroma, occasionally invading cancer cell nests. Most represented leukocytes are T lymphocytes and macrophages, although some eosinophils, mast cells, NK cells and rare DC can be found [84–88].
Tumor Infiltrating Lymphocytes (TIL)
As for other neoplastic tissues [15], an abundance of CD3+ T cells in colorectal cancer is usually associated with more favourable prognosis. Indeed, the most convincing results of a protective anti-tumor effect of CD3+ lymphocytes have been provided in human CRC.
Several studies confirmed that high rate of tumor-infiltrating lymphocytes (TILs), in particular located intra-epithelially, is beneficial for patient outcome, being associated with earlier tumor stage, decreased local recurrence rate after surgery and improved overall and disease-free survival both in metastatic and non-metastatic patients [89–91]. Galon et al. [92] analysed, by gene expression profiling and immunohistochemistry, the type, density and localization (invasive margin or tumor center) of TILs in a large number of CRC cases. They identified a dominant cluster of genes involved in Th1 immune response that inversely correlated with tumor recurrence. Moreover they evaluated the levels of CD3+, CD8+, granzyme B and memory CD45RO+ T and demonstrated that adaptive immunity promotes patient survival, prevents tumor recurrence and that this beneficial effect may persist throughout tumor progression (stage II and III). Pages et al. [93] analysed TILs focusing on early metastatic invasion. They found an increase in mRNA levels for products and markers of Th1 effector T cells (CD8, T-BET, interferon regulatory factor 1, interferon-γ, granulosin and granzyme-B), and this increase is associated with prolonged survival and the absence of pathological signs of early metastatic invasion (vascular emboli, lymphatic invasion and perineural invasion, termed VELIPI). Moreover, the presence of effector memory T cells within the tumor, defined by the presence of CD3, CD8, CD45RO, CCR7, CD28 and CD27 markers was associated with VELIPI-negative tumors. In a recent study, our group investigated the levels of CD3+ T cells at the invasive margin of tumor at stage II and III colorectal cancer [94]. In line with the above studies, it was clearly demonstrated that CD3+ cells at the invasive front are associated with a lower risk of metachronous metastasis and a consequently survival advantage. However, this holds true only in node-negative patients (stage II) and loses statistical significance in stage III patients [94].
Tumor-associated Macrophages (TAM)
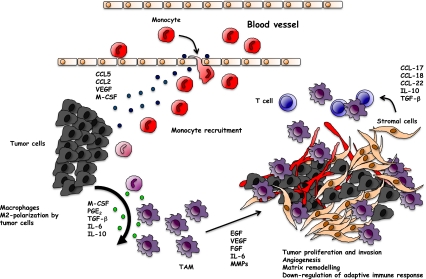
Macrophages are usually the most abundant immune population in the tumor-microevironment [95–97]. Although early studies demonstrated that appropriately stimulated macrophages are able to kill tumor cells in vitro, it is now generally accepted that tumor-associated macrophages (TAM), conditioned by the tumor-microenvironment, have no cytotoxic activity [97–99]. Indeed, most studies have shown that TAM exert several pro-tumor functions [19, 100, 101]. TAM derive from monocytic precursors circulating in blood and are recruited to tumor site by several molecules, such as the chemokines CCL2 and CCL5, vascular endothelial growth factor (VEGF), TGF-β and colony stimulating factors (GM-CSF and M-CSF) [39, 96, 102–104]. Recruited monocytes differentiate into mature macrophages within the tumor-microenvironment. The capability to express distinct functional programmes in response to different micro-environmental signals is a typical biological feature of macrophages [105–107]. Factors such as M-CSF, PGE2, TGF-β, IL-6 and IL-10 have the potential to modulate and polarize monocytes mainly into M2 macrophages (Fig. 2). Along a current concept, M2-polarized myeloid cells promote tissue remodelling and angiogenesis and secrete several growth factors [99, 108].
Fig. 2.
M2-macrophage polarization in the tumor microenvironment. Blood monocytes recruited by chemoattractants secreted by tumor cells (CCL2, CCL5, VEGF, M-CSF) differentiate in the tumor microenvironment. Tumor-derived IL-6, IL-10, TGF-β and PGE2 promote the polarization into M2-like macrophages with pro-tumor functions. By producing growth factors (e.g. EGF, FGF, VEGF, IL-6) and matrix-degrading enzymes (MMPs), TAM favour the neo-angiogenesis switch, tumor cell proliferation and invasion of surrounding tissues. By secreting chemokines (e.g CCL17, CCL18 and CCL22) TAM recruit naïve and Th2 lymphocytes, ineffective in mounting a protective anti-tumor immune response
In the tumor context, TAM resemble M2-polarized macrophages and have been shown to influence fundamental aspects of tumor biology [99]. Among the well documented pro-tumor functions of TAM is the production of a large array of growth factors for tumor cells and for the nascent blood vessels, which are essential for tumor proliferation and the neo-angiogenesis switch. These include for instance epidermal growth factors (EGF), TGF-β, VEGF. Further, TAM produce several proteolytic enzymes such as MMPs and cathepsins that incessantly degrade ECM proteins, thus favouring tumor expansion, motility and invasion [95, 97, 109–112]. The role of TAM in promoting tumor cell invasion and vessel intravasation has been documented by imaging techniques [113]. In addition, myeloid cells have been shown to play a key role in the construction of a pro-metastatic niche by favouring the new environment for seeding and growth of tumor cells [114, 115]. Another important pro-tumor function of TAM is repression of adaptive immune responses, which ultimately have an important impact on disease outcome [116]. Other myeloid cells potently contributing to immune suppression are myeloid-derived suppressor cells (MDSC). These heterogeneous myeloid cells are characterized by the phenotype CD11b+Gr1+ which includes CD11b+F4/80+ (likely macrophages) and CD11b+F4/80− cells. MDSC are increased in tumor tissues and in the spleen of tumor-bearing mice and potently suppress the proliferation and cytotoxic activity of T cells via the production of reactive oxygen species (ROS) and NO [117–119]. In the last years, MDSC are raising interest, since several studies have reported their presence in tumors and in inflammatory diseases. Recently, MDSCs have been involved in the progression of dysplasia in a mouse model of intestinal neoplasia [120].
In line with the above evidence, high density of TAM has been significantly associated with poor prognosis in the majority of tumors [17, 97, 101, 121]. For instance, an important study in Hodgkin lymphoma patients reported that a high CD68 macrophage count is strongly correlated with resistance to treatment and decreased survival [122].
TAM in Colorectal Cancer
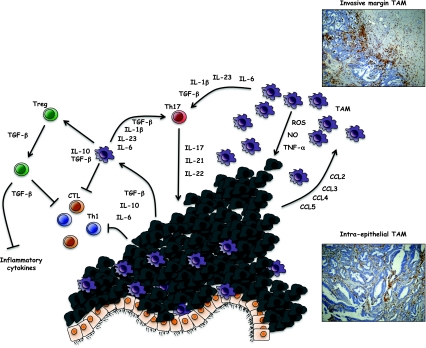
In spite of an abundant literature on the many pro-tumor functions of TAM in several tumor types, their role in colorectal cancer is controversial (Fig. 3). Some studies indicated that macrophages in CRC appear to have anti-tumor activity and are associated with improved disease-free survival [123, 124]. On the contrary, in other studies there is evidence that a massive macrophage infiltration is correlated with tumor progression, growth and disease aggressiveness.
Fig. 3.
TAM-regulate immune network in colorectal cancer. The role of TAM in colorectal cancer is controversial as both anti-tumor and pro-tumor effects have been reported. TAM accumulation at the tumor margin has been most frequently associated with longer patient survival. Although not formally demonstrated, TAM at invasive margin are likely to be less susceptible to the suppressive tumor microenvironment and may produce cytotoxic molecules (ROS, NO and TNF-α). TAM secrete key factors that affect lymphocyte differentiation into Th17 cells (IL-23, IL-6, IL-1β, TGF-β) or Treg (TGF-β, IL-10). While Treg inhibit anti-tumor adaptive immune responses, they may also have beneficial effects by decreasing the production of inflammatory cytokines. The role of Th17 cells in CRC and more in general in human tumors is still an open issue
TAM Pro-tumoral Activity in CRCs
TAM of colorectal cancer have been shown to secrete VEGF, thereby promoting angiogenesis and metastasis [125]. In an in vitro study, cytokines produced by TAM (IL-1, IL6, TNF-α) induced NFκB activation in colon cancer cells and production of VEGF [126]. Monotherapy with the anti-VEGF antibody bevacizumab was shown to induce neuropilin 1 (NRP1) expression in macrophages. Although the function of NRP1 in TAM is currently unclear, this molecule is considered an M2 marker [127]. A recent study showed that TAM promote removal of apoptotic colon cancer cells that express the sulfoglycolipids SM4s. During the process, the phenotype of TAM is modified, with an increased expression of TGFβ and IL-6, putatively contributing to further activate the angiogenic process [128]. As discussed above, IL-6 has a crucial role in colon tumorigenesis and TAM are the major producers of this cytokines. In addition, TAM-derived IL-6 induces STAT3-mediated IL-10 production in tumor cells, which has been correlated with poor prognosis [129]. Moreover, TGF-β, which is produced by both tumor cells and macrophages, plays a key role in the epithelial-to-mesenchymal transition (EMT), an event usually associated with tumor progression and metastasis [130, 131]. Colon cancer cells can also stimulate the production by macrophages of MMP2 and MMP9, promoting cell invasion by disrupting extra-cellular matrix and cleaving cell-adhesion molecules such as E-cadherin [132–134].
Another indication of the putative pro-tumoral action of TAM in CRC has been provided by Kaler et al. [135]: this study reported that TAM, through IL-1β, promote Wnt signalling in colon cancer cells, supporting tumor growth. The role of Wnt-β-catenin signalling in colorectal cancer progression has been extensively demonstrated [136, 137]. Pancione et al. [138] reported that reduction or loss of β-catenin and peroxisome proliferator-activated receptor-γ (PPAR-γ) expression was strongly correlated with massive TAM infiltration, increased COX-2 and tumor aggressiveness. Bollrath et al. also suggested that STAT3 may enhance nuclear localization of β-catenin, and it would be of interest to further investigate the crosstalk between the Wnt/β-catenin and the IL-6/gp130/STAT3 pathway [81].
TAM Anti-tumoral Activity in CRCs
Other studies have correlated the presence of infiltrating macrophages with good prognosis in colorectal cancer patients. Considering the effect of TAM in colon cancer, their localization appears of primary importance (Fig. 3). Ohtani et al. demonstrated that the expression of co-stimulatory molecules (CD80 and CD86) and ICAM-1 were increased in peritumoral macrophages while TAM from cancer stroma had significantly reduced expression [139]. Sugita et al. [140] supported this concept, showing that macrophages along the tumor margin were able to induce apotosis in cancer cells by a Fas ligand-dependent manner. The number of macrophages correlated with the number of apoptotic cancer cells; further, the degree of cancer cell apoptosis was inversely correlated with hematogenous metastasis, underlining the protective role of TAM. This anti-tumor effect of TAM was confirmed by other studies correlating macrophages infiltration and prognosis. Khorana et al. [141] analysed the presence of VEGF-expressing TAM, finding a significant association with favourable outcome in a multivariate analysis. Funada et al. [142] demonstrated that high levels of macrophage infiltration at the invasive margin correlated with an increased overall survival rate. Patients with low levels of macrophages infiltration had more advanced disease, higher rate of vascular invasion and lymph node metastasis.
More recently, Forssell et al. [123] demonstrated that a dense macrophage infiltration at the tumor front positively influenced prognosis in colon cancer and that direct macrophage-to-tumor cell contact was required to manifest the anti-tumorigenic activity. In agreement with these data, Zhou et al. [143] showed that high density of TAM at the invasive front was associated with lower occurrence of hepatic metastasis and improved prognosis of colorectal cancer patients.
In contrast with the studies mentioned above, Bailey et al. [144] found that, counting macrophages not only at the tumor margin but in all areas within the tumor, including necrotic areas, macrophage accumulation was not a good prognostic indicator. Macrophage counts significantly increases in malignant tissues of all stages compared with normal tissues and there is a trend for greater accumulation with advancing stage.
Overall, these data highlight a controversial role of macrophages in the progression of colorectal cancer, and suggest that the anti-tumor or pro-tumor activity of TAM may depend on their localization within cancer tissue. Peritumoral macrophages are likely to have less exposure to tumor-derived cytokines and are located in less hypoxic area: thereby they may differentiate into a tumoricidal rather than pro-tumoral phenotype. The mechanism behind a potential anti-tumor effect of TAM is not clarified and could potentially be due to the presence of a significant number of M1-polarized macrophages, able to mediate killing of tumor cells. In addition, it is reasonable to figure out that the macrophage balance may have different effects at different stages of tumor progression [145]. At early stages, the innate response, including macrophages, may be effective in the elimination of tumor cells and in the activation of adaptive immunity; at advanced stages, when tumor cell have escaped immuno-editing and adaptive immunity is ablated [146], newly recruited macrophages are likely to shift toward M2-polarized cells with pro-tumor function.
TAM and Modulation of Immune Responses
Activation of innate immunity is indispensable for the stimulation and orientation of adaptive immune responses, and macropages are key players in this crosstalk. In the tumor context, TAM and related myeloid cells have been mainly characterized as inhibitors of T-cell activation, via secretion of different suppressive mediators, such as IL-10, TGF-β and indoleamine 2,3-dioxygenase (IDO) [147].
In the last years, several studies focused on the interaction between macrophages and two CD4+ T cells subsets in CRCs: Th17 and regulatory T cells (Treg).
IL-23 and Th17
TAM produce IL-23, althought at lower levels than M1-macrophages and DC. IL-23 is a crucial cytokine for the polarization of Th17 lymphocytes, in concert with IL-1β, IL-6 and TGF-β [148–153]. Earlier studies identified IL-23 as anti-tumor cytokine: the inoculation of murine colorectal cancer cell lines transfected with IL-23 resulted in reduced tumor growth [154]. More recently, Langowski and colleagues [155] instead demonstrated that IL-23 expression is strongly increased in different types of cancer, including colon adenocarcinoma; IL-23 promoted tumor incidence and growth by stimulating inflammatory responses, up-regulation of MMP9, increased angiogenesis and reduced CD8 T-cell infiltration. In support of the hypothesis that IL23 may promote tumor development, human colorectal cancer expressed higher IL-23 mRNA levels compared to the corresponding cancer-free mucosa [156].
As mentioned above, IL-23 plays a pivotal role in the development and survival of Th17 cells. Th17 lymphocytes produce IL-17 and related cytokines (e.g. IL-21 and IL-22), which are important mediators to maintain mucosal homeostasis [157]. The role of Th17 cells in tumor pathogenesis is still not well defined. In a subcutaneous model of colon cancer, tumor growth and lung metastasis were enhanced in IL-17-deficient mice and accompanied by reduced IFNγ+ NK cells and IFNγ+ tumor-specific T cells, detected in tumor-draining lymph nodes. These results suggest that IL-17 and/or Th17 cells may promote protective tumor immunity [158]. In a different set of in vitro experiments, Lee et al. [159] showed that IL-17 inhibited the expression of Th1-recruiting chemokines (CXCL10, CXCL11 and CCL5) in a colon cancer cell line and simultaneously increased the expression of CCL20, a chemokine acting on Th17 cells. These latter results suggest that expression of IL-17 at tumor sites may amplify the recruitment of Th17 cells and inhibit or delay the recruitment of Th1 effector cells.
TAM and Regulatory T Cells (Treg)
Th17 cells are reciprocally related to Treg cells. The early differentiation of Treg and Th17 cells from naïve CD4+ T cells shares a requirement for TGF-β, indicating substantial plasticity in the development of these lymphocyte subsets [160, 161]. TAM are a major source of TGF-β, as well as cancer cells, and can directly induce Treg by cell-cell interaction via membrane-bound TGF-β [162]. Treg can suppress the cytotoxicity of CD8+ anti-tumor effectors and, indeed, are considered to importantly contribute to tumor immune evasion [163]. Different studies have analyzed the role of Treg in colorectal cancer, highlighting—once more—contrasting results.
Loddenkemper et al. [164] investigated infiltrating Treg in human CRC and found significantly higher numbers in tumor tissues compared to normal mucosa. However, no association was found between Treg density and patient survival. In contrast, Salama et colleagues [165] demonstrated that Treg were associated with better survival and showed even stronger prognostic significance than tumor infiltrating CD8+ and CD45RO+ T cells. As a confirmation of their potential protective effect, adoptive transfer of Treg into APCmin mice resulted in prevention of intestinal adenoma, as well as regression of some established tumors [166]. Notably, the French group that reported on the protective role of memory CD8+ lymphocytes in CRC [93] also looked at FoxP3+ cells and found no association with patient survival.
Very recently, a new regulatory T cell population characterized by the expression of CD8+ CD25+ and FoxP3+ (T8reg) was identified in blood and tissues of CRC patients [167]. Interestingly, T8reg cells were significantly elevated and were able to suppress the proliferation and cytokine production of conventional CD4+CD25− T cells ex vivo. T8reg numbers correlated with levels of IL-6 and TGF-β, as well as with tumor stage and micro-invasive status [167].
Taken together, despite evidence that Treg inhibit adaptive immunity and promote tumor progression in several neoplasia [168], distinct results are found in CRC. A possible explanation of these opposite effects is that Treg, by contrasting the production of inflammatory cytokines, may limit inflammation-dependent cancers, as are most CRC. On the other hand, in spontaneous and inflammation-independent cancers the predominant effects of tumor-induced Treg may result in suppression of protective adaptive immunity.
Conclusions
The connection between inflammation and cancer is now generally accepted, and colon cancer represents a paradigm of this connection, as especially in this tumor type there is clear evidence that persistent inflammation is linked to higher cancer risk and tumor development. In this review, we have extensively discussed the pivotal role that the inflammatory microenvironment plays in disease progression. Although genetic and epigenetic alterations drive the key initial transformation of normal enterocytes, cells of the innate immunity—and macrophages in particular—are strongly involved in several processes that eventually lead to tumor development, such as enhanced cancer cell survival and proliferation, neo-angiogenesis, matrix remodeling, as well as tumor cell invasion and distant metastasis. Cytokines, chemokines and growth factors produced by both transformed epithelial cells and TAM act on various types of pro-inflammatory leukocytes, on endothelial cells and fibroblasts, to establish a tumor promoting inflammatory microenvironment. With this evidence, it is clear that anti-inflammatory treatments may be beneficial both for tumor prevention and in therapeutic settings. Over the past decade, a number of compounds or antibodies inhibiting inflammatory mediators have been developed. Initial clinical trials have been performed in human chronic diseases (e.g. TNF in rheumatoid arthritis). Now that the relevance of inflammation in neoplasia is recognized, biological drugs are being and will be tested in oncological patients. Prolonged usage of non-steoidal anti-inflammatory drugs, and in particular the new generation of anti-COX2 compounds, has already clearly demonstrated a protective effect on the risk of developing colon cancer [22]. Although limited by considerable toxicity, these compounds have fulfilled the role to serve as proof of concept that reducing inflammation is indeed beneficial for cancer prevention. Given the central role of IL-6 in colorectal cancer, monoclonal antibodies directed to IL-6 or its receptor, or inhibitors of IL-6 signaling should be considered for clinical efficacy.
Leukocyte infiltration is a key point in the process of colorectal carcinogenesis. There is strong evidence that high T-cell infiltration is a favourable prognostic element in colon cancer. On the other hand, the role of myeloid cells is quite controversial. Unlike what happens in most solid malignancies, where TAM usually have a pro-tumor phenotype, TAM from CRC where reported to be associated either with a more favourable prognosis or with disease progression and metastasis. The different localization of TAM within the tumor (invasive margin vs tumor stroma) and consequent influence of the tumor microenvironment may in part explain these different effects. Therapies targeting TAM (e.g anti-CCL2 antibodies) are under way, but their use in colorectal cancer must wait a more precise definition of the functional role of these myeloid cells.
Acknowledgments
This work was supported by Associazione Italiana Ricerca Cancro (AIRC) Italy to PA and AM; grants from the European Community FP6 Project ATTACK-018914; Ministry of Health and Istituto Superiore Sanità Italy (Project oncology 2006 and Alleanza Contro il Cancro).
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Calvert PM, Frucht H. The genetics of colorectal cancer. Ann Intern Med. 2002;137:603–612. doi: 10.7326/0003-4819-137-7-200210010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 4.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525–2538. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 5.Soreide K, Janssen EA, Soiland H, Korner H, Baak JP. Microsatellite instability in colorectal cancer. Br J Surg. 2006;93:395–406. doi: 10.1002/bjs.5328. [DOI] [PubMed] [Google Scholar]
- 6.Laghi L, Bianchi P, Malesci A. Gender difference for promoter methylation pattern of hMLH1 and p16 in sporadic MSI colorectal cancer. Gastroenterology. 2003;124:1165–1166. doi: 10.1053/gast.2003.50199. [DOI] [PubMed] [Google Scholar]
- 7.Westra JL, Plukker JT, Buys CH, Hofstra RM. Genetic alterations in locally advanced stage II/III colon cancer: a search for prognostic markers. Clin Colorectal Cancer. 2004;4:252–259. doi: 10.3816/ccc.2004.n.024. [DOI] [PubMed] [Google Scholar]
- 8.Malesci A, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13:3831–3839. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 9.Prall F, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–816. doi: 10.1016/j.humpath.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Dolcetti R, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjea A, et al. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer. 2004;3:21. doi: 10.1186/1476-4598-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller A, Fishel R. Mismatch repair and the hereditary non-polyposis colorectal cancer syndrome (HNPCC) Cancer Invest. 2002;20:102–109. doi: 10.1081/cnv-120000371. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Dubois RN, Richmond A. The role of chemokines in intestinal inflammation and cancer. Curr Opin Pharmacol. 2009;9:688–696. doi: 10.1016/j.coph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meira LB, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 16.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 17.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 18.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 20.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 21.Witz IP. The tumor microenvironment: the making of a paradigm. Cancer Microenviron. 2009;2(Suppl 1):9–17. doi: 10.1007/s12307-009-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 23.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 24.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 2006;20:2527–2538. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borrello MG, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102:14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germano G, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70:2235–2244. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 29.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 32.Schioppa T, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta RB, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 37.Bottazzi B, et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 38.Rollins BJ, Sunday ME. Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol Cell Biol. 1991;11:3125–3131. doi: 10.1128/mcb.11.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negus RP, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95:2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opdenakker G, Damme J. Chemotactic factors, passive invasion and metastasis of cancer cells. Immunol Today. 1992;13:463–464. doi: 10.1016/0167-5699(92)90079-M. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 42.McConnell BB, Yang VW. The role of inflammation in the pathogenesis of colorectal cancer. Curr Colorectal Cancer Rep. 2009;5:69–74. doi: 10.1007/s11888-009-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popivanova BK, et al. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 44.Erreni M, et al. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009;460:105–121. doi: 10.1016/S0076-6879(09)05205-7. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 46.Schimanski CC, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 47.Gunther K, et al. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 48.Kawada K, et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26:4679–4688. doi: 10.1038/sj.onc.1210267. [DOI] [PubMed] [Google Scholar]
- 49.Sturm A, Baumgart DC, d’Heureuse JH, Hotz A, Wiedenmann B, Dignass AU. CXCL8 modulates human intestinal epithelial cells through a CXCR1 dependent pathway. Cytokine. 2005;29:42–48. doi: 10.1016/j.cyto.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Zipin-Roitman A, et al. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007;67:3396–3405. doi: 10.1158/0008-5472.CAN-06-3087. [DOI] [PubMed] [Google Scholar]
- 51.Vetrano S, et al. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut. 2010;59:197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- 52.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 53.Popivanova BK, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Pikarsky E, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 56.Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–243. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 58.Huang B, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 59.Thomassen E, Renshaw BR, Sims JE. Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily. Cytokine. 1999;11:389–399. doi: 10.1006/cyto.1998.0452. [DOI] [PubMed] [Google Scholar]
- 60.Mantovani A, Locati M, Polentarutti N, Vecchi A, Garlanda C. Extracellular and intracellular decoys in the tuning of inflammatory cytokines and Toll-like receptors: the new entry TIR8/SIGIRR. J Leukoc Biol. 2004;75:738–742. doi: 10.1189/jlb.1003473. [DOI] [PubMed] [Google Scholar]
- 61.Polentarutti N, et al. Unique pattern of expression and inhibition of IL-1 signaling by the IL-1 receptor family member TIR8/SIGIRR. Eur Cytokine Netw. 2003;14:211–218. [PubMed] [Google Scholar]
- 62.Wald D, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 63.Garlanda C, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garlanda C, et al. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- 65.Iglesias D, Nejda N, Azcoita MM, Schwartz SJ, Gonzalez-Aguilera JJ, Fernandez-Peralta AM. Effect of COX2-765G>C and c.3618A>G polymorphisms on the risk and survival of sporadic colorectal cancer. Cancer Causes Control. 2009;20:1421–1429. doi: 10.1007/s10552-009-9368-1. [DOI] [PubMed] [Google Scholar]
- 66.Arber N, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 67.Benelli R. Aspirin, COX-2, and the risk of colorectal cancer. N Engl J Med. 2007;357:824–825. doi: 10.1056/NEJMc071797. [DOI] [PubMed] [Google Scholar]
- 68.Pereira C, Medeiros RM, Dinis-Ribeiro MJ. Cyclooxygenase polymorphisms in gastric and colorectal carcinogenesis: are conclusive results available? Eur J Gastroenterol Hepatol. 2009;21:76–91. doi: 10.1097/MEG.0b013e32830ce7ba. [DOI] [PubMed] [Google Scholar]
- 69.Cross JT, Poole EM, Ulrich CM. A review of gene-drug interactions for nonsteroidal anti-inflammatory drug use in preventing colorectal neoplasia. Pharmacogenomics J. 2008;8:237–247. doi: 10.1038/sj.tpj.6500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 71.Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 72.McLoughlin RM, et al. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598–607. doi: 10.1172/JCI17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Atreya R, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 75.Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, Lahm H. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett. 2000;151:31–38. doi: 10.1016/s0304-3835(99)00401-2. [DOI] [PubMed] [Google Scholar]
- 76.Hsu CP, Chung YC. Influence of interleukin-6 on the invasiveness of human colorectal carcinoma. Anticancer Res. 2006;26:4607–4614. [PubMed] [Google Scholar]
- 77.Brozek W, Bises G, Girsch T, Cross HS, Kaiser HE, Peterlik M. Differentiation-dependent expression and mitogenic action of interleukin-6 in human colon carcinoma cells: relevance for tumour progression. Eur J Cancer. 2005;41:2347–2354. doi: 10.1016/j.ejca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int J Colorectal Dis. 2010;25:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 79.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 80.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bollrath J, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 84.Sandel MH, et al. Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol. 2005;42:541–546. doi: 10.1016/j.molimm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 85.Coca S, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544–1548. [PubMed] [Google Scholar]
- 87.Nagtegaal ID, et al. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect—a histopathological and immunohistochemical study. BMC Cancer. 2001;1:7. doi: 10.1186/1471-2407-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gounaris E, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Canna K, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiba T, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naito Y, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 92.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 93.Pages F, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 94.Laghi L, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 95.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 96.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 98.Dinapoli MR, Calderon CL, Lopez DM. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996;183:1323–1329. doi: 10.1084/jem.183.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 100.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 101.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Fu YX, Cai JP, Chin YH, Watson GA, Lopez DM. Regulation of leukocyte binding to endothelial tissues by tumor-derived GM-CSF. Int J Cancer. 1992;50:585–588. doi: 10.1002/ijc.2910500416. [DOI] [PubMed] [Google Scholar]
- 103.Yaal-Hahoshen N, et al. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res. 2006;12:4474–4480. doi: 10.1158/1078-0432.CCR-06-0074. [DOI] [PubMed] [Google Scholar]
- 104.Scholl SM, Crocker P, Tang R, Pouillart P, Pollard JW. Is colony-stimulating factor-1 a key mediator of breast cancer invasion and metastasis? Mol Carcinog. 1993;7:207–211. doi: 10.1002/mc.2940070402. [DOI] [PubMed] [Google Scholar]
- 105.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 106.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 107.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 108.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 109.O’Sullivan C, Lewis CE. Tumour-associated leucocytes: friends or foes in breast carcinoma. J Pathol. 1994;172:229–235. doi: 10.1002/path.1711720302. [DOI] [PubMed] [Google Scholar]
- 110.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 111.Mantovani A. Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest. 1994;71:5–16. [PubMed] [Google Scholar]
- 112.Konur A, Kreutz M, Knuchel R, Krause SW, Andreesen R. Cytokine repertoire during maturation of monocytes to macrophages within spheroids of malignant and non-malignant urothelial cell lines. Int J Cancer. 1998;78:648–653. doi: 10.1002/(sici)1097-0215(19981123)78:5<648::aid-ijc20>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 113.Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 114.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 117.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 118.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 120.Gounaris E, et al. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS One. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 122.Steidl C, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 124.Ohno S, et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res. 2003;23:5015–5022. [PubMed] [Google Scholar]
- 125.Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002;62:7042–7049. [PubMed] [Google Scholar]
- 126.Jedinak A, Dudhgaonkar S, Sliva D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology. 2010;215:242–249. doi: 10.1016/j.imbio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Xu L, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Popovic ZV, et al. Sulfated glycosphingolipid as mediator of phagocytosis: SM4s enhances apoptotic cell clearance and modulates macrophage activity. J Immunol. 2007;179:6770–6782. doi: 10.4049/jimmunol.179.10.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol. 2004;172:4630–4636. doi: 10.4049/jimmunol.172.7.4630. [DOI] [PubMed] [Google Scholar]
- 130.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 131.Massague J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reinacher-Schick A, et al. Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol. 2004;202:412–420. doi: 10.1002/path.1516. [DOI] [PubMed] [Google Scholar]
- 133.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Illemann M, et al. MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res. 2006;4:293–302. doi: 10.1158/1541-7786.MCR-06-0003. [DOI] [PubMed] [Google Scholar]
- 135.Kaler P, Godasi BN, Augenlicht L, Klampfer L (2009) The NF-kappaB/AKT-dependent induction of Wnt signaling in colon cancer cells by macrophages and IL-1beta. Cancer Microenviron [DOI] [PMC free article] [PubMed]
- 136.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 137.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 138.Pancione M, et al. Reduced beta-catenin and peroxisome proliferator-activated receptor-gamma expression levels are associated with colorectal cancer metastatic progression: correlation with tumor-associated macrophages, cyclooxygenase 2, and patient outcome. Hum Pathol. 2009;40:714–725. doi: 10.1016/j.humpath.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 139.Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible antitumor immunity? Lab Invest. 1997;77:231–241. [PubMed] [Google Scholar]
- 140.Sugita J, et al. Close association between Fas ligand (FasL; CD95L)-positive tumor-associated macrophages and apoptotic cancer cells along invasive margin of colorectal carcinoma: a proposal on tumor-host interactions. Jpn J Cancer Res. 2002;93:320–328. doi: 10.1111/j.1349-7006.2002.tb02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer. 2003;97:960–968. doi: 10.1002/cncr.11152. [DOI] [PubMed] [Google Scholar]
- 142.Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10:309–313. [PubMed] [Google Scholar]
- 143.Zhou Q, et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2010;8:13. doi: 10.1186/1479-5876-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bailey C, et al. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis. 2007;24:121–130. doi: 10.1007/s10585-007-9060-3. [DOI] [PubMed] [Google Scholar]
- 145.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 146.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 147.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 149.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santarlasci V, et al. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39:207–215. doi: 10.1002/eji.200838748. [DOI] [PubMed] [Google Scholar]
- 151.Das J, et al. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang YQ, et al. Induction of systemic immunity by expression of interleukin-23 in murine colon carcinoma cells. Int J Cancer. 2003;105:820–824. doi: 10.1002/ijc.11160. [DOI] [PubMed] [Google Scholar]
- 155.Langowski JL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 156.Stanilov N, Miteva L, Mintchev N, Stanilova S. High expression of Foxp3, IL-23p19 and survivin mRNA in colorectal carcinoma. Int J Colorectal Dis. 2009;24:151–157. doi: 10.1007/s00384-008-0588-8. [DOI] [PubMed] [Google Scholar]
- 157.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 158.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee JW, et al. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J Immunol. 2008;181:6536–6545. doi: 10.4049/jimmunol.181.9.6536. [DOI] [PubMed] [Google Scholar]
- 160.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 161.Veldhoen M, Stockinger B. TGFbeta1, a “Jack of all trades”: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–361. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 162.Savage ND, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181:2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 163.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 164.Loddenkemper C, Schernus M, Noutsias M, Stein H, Thiel E, Nagorsen D. In situ analysis of FOXP3+ regulatory T cells in human colorectal cancer. J Transl Med. 2006;4:52. doi: 10.1186/1479-5876-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Salama P, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 166.Erdman SE, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 167.Chaput N, et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 168.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]