Abstract
Several studies indicate that Western dietary and lifestyle factors are responsible for the high incidence of colorectal cancer in industrialized countries. Diets rich in red and processed meat, refined starches, sugar, and saturated and trans-fatty acids but poor in fruits, vegetables, fiber, omega-3 fatty acids and whole grains are closely associated with an increased risk of colorectal cancer. Other main features of the western lifestyle, such as excess body mass and sedentary behaviours, are also strongly associated with higher risk of developing this cancer. Modifications of the western diet, notably increasing consumption of foods from plant origin and reducing that of red meat intake, and maintenance of physical activity and appropriate body mass could substantially reduce colorectal cancer incidence and mortality.
Keywords: Colorectal cancer, Prevention, Obesity, Inflammation, Phytochemicals, Physical activity
Colorectal Cancer, A Preventable Disease
Cancers of the colon and rectum, collectively refered to as colorectal cancers (CRC), constitutes a significant proportion of the global burden of cancer morbidity and mortality: approximately 1 million new cases of colorectal cancer are diagnosed each year and more than half a million people die from this disease, equivalent to approximately 8% of all cancer-related deaths worldwide [1]. Colorectal cancers, and their common precursors adenomatous colon polyps, are complex and heterogeneous diseases that arise from both somatic and germline mutations. In familial adenomatous polyposis (FAP), individuals with a germline mutation in one allele of the tumor suppressor gene adenomatous polyposis coli (APC) have generally a 100% chance of developing CRC by the age of 40 years if untreated [2]. In hereditary nonpolyposis colorectal cancer (HNPCC), also called Lynch syndrome, inherited mutations in genes involved in DNA mismatch-repair (primarily MLH1 and MSH2) confer a lifetime risk of CRC of about 80%, with cancers evident by the age of 45 years [3]. However, the Mendelian mode of inheritance of these two forms of CRC account for no more than 5% of all CRC cases, the large majority of patients rather developing a “sporadic” form of this disease [4]. This suggests that a large proportion of CRC are related to environmental factors and that this disease may thus be, in most cases, preventable.
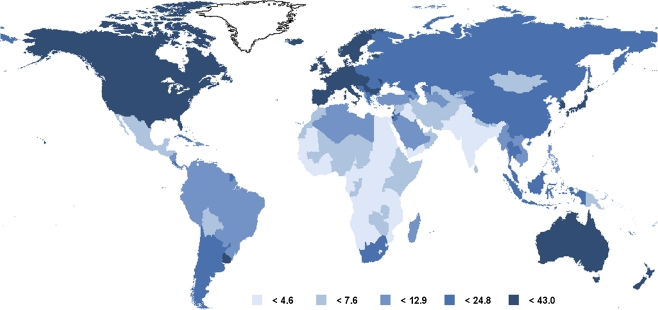
The influence of environmental factors in the development of CRC is exemplified by the large variations in rates of this cancer among countries (Fig. 1). The occurrence of CRC varies at least 25-fold worldwide, the highest incidence rates being observed in industrialized countries such as in the United States, Canada and New Zealand [5]. Importantly, the migration of individuals from low incidence areas to high incidence areas results in spectacular changes in the incidence rates of CRC, the risk of disease approaching that of the adopted country within one generation [6]. Moreover, although the majority of colorectal cancers continue to occur in industrialized countries, their incidence rates are rapidly rising in economically transitioning countries in many parts of the world, and these increases are thought to reflect the adoption of several features of the Western lifestyle [7]. In Japan for example, CRC incidence has dramatically risen following westernization, increasing as much as 90% over the past several decades in some registries (Miyagi and Yamagata) [8, 9]. Between 1983 and 2002, the incidence of CRC has also been shown to increase in 27 of 51 countries worldwide, particularly in Eastern Europe, most parts of Asia, as well as in some countries of South America, these increases also occurring concomitantly with the adoption of western diets and lifestyles [7]. Overall, these observations highlight the importance of environmental influences on colorectal carcinogenesis and suggest that lifestyle risk factors characteristics of industrialized countries such as diet, obesity and physical inactivity may all play a pivotal role in the etiology of the disease [10].
Fig. 1.
Age-standardized incidence of colorectal cancer in the world (per 100,000 individuals). From the International Agency for Research on Cancer, Lyon, France
Lifestyle Factors Associated with Colorectal Cancer Risk
Dietary Factors
Fiber-Rich Foods: Vegetables, Fruits and Grains The concept that diet influences the risk of CRC has been proposed more than 40 years ago, following the observation of the low incidence of these cancers in African populations that consume a high-fiber diet [11]. This «fiber hypothesis» postulates that dietary fiber (non-starch polysaccharides) from a number of plant-based foods (vegetables, fruits, grains) lower the risk of colorectal cancer by reducing transit time, diluting colonic contents and stimulating bacterial anaerobic fermentation to increase the production of short-chain fatty acids (acetate, propionate, and butyrate) [12]. This hypothesis was initially strengthened by several case–control studies showing an approximately 50% lower risk of colorectal cancer associated with higher intake of dietary fiber [13]. However, results from subsequent large prospective cohort studies failed to detect such a protective effect of either total dietary fiber [14, 15] or fruit and vegetable [16]. A pooled analysis of fruit and vegetable intake also failed to detect an association with overall colorectal cancer risk [17], raising doubts about the chemopreventive effect of these foods. The factors responsible for these discrepancies remain unclear but may reflect the fact that case–control studies are often more prone to bias since dietary information is collected after the diagnosis of cancer and patients are more likely to recall perceived unhealthy dietary behaviors [4]. Alternatively, foods supplying fiber also contribute many other nutrients and phytochemicals that possess several anticancer properties, including a variety of polyphenols, carotenoids, terpenes and sulfur-containing molecules such as thioethers and glucosinolates [18]. The absence of protection observed in cohort studies may thus rather reflect the poor intake of foods with the highest amounts of these anticancer phytochemicals. In this respect, it is noteworthy that the largely null findings of prospective studies were primarily obtained from cohorts located in the United States, where the most consumed vegetables (potatoes, iceberg lettuce and canned tomatoes) [19] are essentially devoided of chemopreventive molecules. By contrast, consumption of vegetables with the highest amounts of these molecules, such as cruciferous and green leafy vegetables, is very low in this country [20]. It is also noteworthy that in European countries with a more diversified intake of whole grains and fruits and vegetables, a recent prospective study showed an approximately 40% reduced risk of colorectal cancer among individuals with the highest intake of fiber [21]. Similarly, in this population, high intake of fruit and vegetables was associated with a 25% reduction in colon cancer risk [22]. A number of studies on the effect of certain fruits or vegetables with high content in anticancer phytochemicals, such as citrus fruits [23], cruciferous vegetables [24], dark-green vegetables and onions and garlic [25] suggest that these foods are strongly protective against colorectal adenoma, the precursors of most colorectal carcinomas. The combination of curcumin, the bioactive molecule of the Indian spice turmeric, and quercetin, a major dietary flavonoid, appears to reduce the number and size of ileal and rectal adenomas in patients with FAP [26], further suggesting that foods containing high amounts of anticancer phytochemicals can be endowed with significant chemopreventive properties against CRC. In this respect, it is noteworthy that regular drinking of green tea, an exceptional source of anticancer polyphenols [27] was recently associated with a large reduction (40%) in CRC risk in a cohort of 69,710 Chinese women [28]. Overall, these studies suggest that overall intake of fruits, vegetables, and fiber may not confer a «blanket» protection against colorectal cancer risk, with the possible exception of individuals with extremely low baseline levels of intake of these foods [4]. However, the inclusion of plant-based foods with the highest content in anticancer phytochemicals, such as cruciferous and dark-green vegetables, onion and garlic, citrus fruits as well as beverage such as green tea or spices such as turmeric is likely to be beneficial in terms of CRC prevention.
B Vitamins In addition to fiber and anticancer phytochemicals, foods of plant origin also contain a number of vitamins that may also participate to the prevention of colon carcinogenesis. Among these, B vitamins have received considerable attention over the last decades given their essential roles in a variety of processes involved in DNA synthesis, repair, and methylation [29]. Accordingly, studies indicate that higher intake of folate (vitamin B9), found in high amounts in dark-green vegetables, is associated with reduced risk of colorectal cancer or adenoma [30]. This association is only found with dietary folate but not with folate from supplements. In fact, supplementation with folic acid may even be harmful, especially for patients with a previous history of colon cancer, since a recent randomized secondary prevention trial found that these supplements increased the risk of recurrent advanced adenoma or recurrent adenomas [31]. This dual effect of folate on colon carcinogenesis possibly reflects a protective role of physiological levels of the vitamin on normal mucosa while aggressive supplementation may enhance the progression of already present microscopic lesions [31]. In addition to folate, higher intake of vitamin B6 or blood levels of pyridoxal 5′-phosphate (PLP, the active form of vitamin B6) are also associated with a significant decrease in risk of colorectal cancer. For example, a recent meta-analysis of prospective studies has shown that the risk of colorectal cancer decreased by 49% for every 100-pmol/mL increase in blood PLP levels [32].
Red and Processed Meat Intake There is a large body of evidence from ecological, case–control, and cohort studies that high consumption of red meat (beef, pork, or lamb) increases the risk of colorectal cancer in both men and women [14, 33–36]. Consumption of processed meats is also associated with such an increase in CRC risk [37]. The heme iron content of red and processed meats has long been suspected to explain this association [38] but more recent evidence suggest that the cooking process is likely to play a crucial role in this increased CRC risk associated with meat consumption. Cooking meat at high temperature by either frying, grilling, or broiling induces the interaction of muscle creatinine with amino acids, resulting in the formation of at least several 17 distinct highly mutagenic and carcinogenic heterocyclic amines [39]. Several studies have found that risk of colon cancer is specifically increased among individuals who consume charred meat or meat that has been prepared at high temperatures at prolonged durations [40, 41]. Overall, these studies indicate that substituting red meat with other sources of proteins (fish, poultry, legumes) could represent a valuable approach to reducing risk of colorectal cancer [4, 14].
Body Mass, Inflammation and Physical Activity
Obesity and CRC Risk Excess body weight, expressed as a body mass index (BMI) exceeding 25 (BMI is calculated as weight in kilograms [kg] divided by the square of height in meters [kg/m2]) is strongly associated with the risk of developing several types of cancers [42]. In the case of CRC several epidemiological studies have consistently associated excess body weight with an increased risk of colon cancer and adenoma, particularly for men [43, 44]. Compared with those with a BMI <23.0, the increased risk of CRC was 14% for individuals with a BMI of 23.0–24.9; 19% for a BMI of 25.0–27.4; 24% for BMI of 27.5–29.9; and 41% for BMI of ≥30.0 [4, 43].The mechanisms linking excess body weight to colorectal neoplasia remain to be understood. Overloaded adipocytes release excessive amounts of triglycerides into the blood, thereby promoting insulin resistance [45]. In addition, adipose tissue is increasingly recognized as an active endocrine organ that releases a wide variety of biologically functional molecules, collectively referred to as adipokines, that also participate to the resistance to insulin signals [46]. This obesity-related insulin resistance and associated hyperinsulinemia may be involved in colon cancer pathogenesis since high levels of insulin (as well as increased levels of insulin-like growth factor-1 triggered by hyperinsulinemia) may enhance cell proliferation and increase the risk of tumorigenesis [47]. In this context, it is noteworthy that abdominal obesity is more closely associated with insulin resistance and metabolic syndrome, especially when combined with other features of the western lifestyle that increase hyperinsulinemia, such as consumption of refined grains, simple sugars and physical inactivity. Accordingly, recent studies indicate that abdominal visceral adipose tissue volume represents a better predictor for risk of colorectal adenomas than body mass index or waist circumference [48]. Since central adiposity is found more often in men (android obesity), its effect on insulin levels may thus explain, at least in part, the stronger association of obesity with colorectal cancer in men compared to women.
Chronic Inflammation There is increasing evidence that increased lipid and adipokine levels associated with obesity triggered the development of chronic inflammation conditions that may participate to the development of cancer [49, 50]. Several epidemiological studies indicate that deregulated expression of inflammatory cytokines during chronic inflammatory conditions foster the development of several types of cancer including colorectal, gastric, bladder, liver, lung, pancreatic and cervical cancers [49] For example, individuals suffering from ulcerative colitis (UC) and Crohn disease of the colon, the major forms of idiopathic inflammatory bowel disease, have a 19-fold increase in risk for colon cancer [51]. The importance of inflammation for the development of CRC is well illustrated by the results of several studies showing that aspirin, NSAIDs and COX-2 selective inhibitors are associated with a lower risk of colorectal cancer and adenoma [4]. The mechanisms involved in this protective effect remain to be fully understood but likely involve the inhibition of COX-2 by these molecules [52]. Although the potential severe cardiovascular and gastrointestinal ulceration and bleeding side-effects associated with these drugs [53, 54] preclude their use as prophylactic agents, these results nevertheless indicate that the reduction of inflammation represents a promising approach for the prevention of colorectal cancer.In this context, there is now considerable evidence that Western diets rich in refined starches, sugar, and saturated and trans-fatty acids and poor in fruits, vegetables, fiber, omega-3 fatty acids and whole grains promote inflammation [55]. Inflammatory and immune cells from individuals consuming a typical Western diet contain a high proportion of the pro-inflammatory omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid and low proportions of anti-inflammatory omega-3 PUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [56]. Since arachidonic acid is the precursor of 2-series prostaglandins and 4-series leukotrienes, which are highly-active mediators of inflammation, a high dietary omega-6/omega-3 fatty acid ratio thus results in the generation of a proinflammatory state that may sustain the progression of several pathologies, including CRC [57]. A key aspect of the chemopreventive effects of diets containing higher amounts of foods from plant origin and less meat and refined starches could thus be through a reduction of inflammation. These findings highlight the tight interactions that exist between diet and the colon cancer microenvironment.
Physical Activity The reduction in physical activity associated with industrialization has driven a dramatic increase in the incidence of many chronic diseases, including cardiovascular diseases, hypertension, type 2 diabetes, pulmonary diseases, immune dysfunction, musculoskeletal diseases, neurodegenerative disorders as well as some types of cancers [58]. In this respect, there is consistent evidence that greater levels of physical activity are associated with an approximatively 40% reduction in the risk for colorectal cancer [59, 60]. Even moderate levels of physical activity (eg, brisk walking for 30 min per day) are associated with substantial benefits [4]. Although it was initially proposed that an increase in colonic mobility could be responsible for the preventive effect of physical activity on CRC risk, recent data strongly suggest that this reduction rather involve more systemic effects such as increased insulin sensitivity (and concomitant reduction in insulin levels) [61] as well as reduction of chronic inflammation [62]. In this latter case, elegant studies have shown that exercise is associated with a massive induction of the transcriptional coactivator peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and that this event controls muscle plasticity and suppresses a broad inflammatory response [62].
Alcohol and Smoking
Alcohol Consumption A large number of prospective cohort and case–control studies have suggested an association between alcohol intake and colon adenoma and both colon and rectal cancer risk [4, 63, 64]. These studies show that consumption of ≥30 g per day of alcohol is associated with a multivariate risk of colorectal cancer of 1.24 compared to low intake. Whether this increased risk is due to the effect of alcohol on folate levels, defective DNA methylation, impairment of the immune system or in the metabolism of carcinogens by the cytochrome P450 enzymes remain to be established. However, there is little doubt that minimizing alcohol intake should be promoted as a means of preventing colorectal cancer, especially among individuals with high levels of intake [4].
Cigarette Smoking Although smoking-associated cancers occur predominantly in organs that are directly in contact with cigarette smoke (e.g. lung, pharynx, oesophagus), carcinogens from tobacco can also reach the colorectal mucosa through the circulatory system and thereby cause genetic damages that ultimately lead to cancer development. Accordingly, tobacco use has been consistently associated with an increased risk of colorectal adenoma, well-established precursor lesions for CRC [65]. Studies performed to date indicate that a long induction period is required, a significant increased risk for CRC being observed only with long-term cigarette smoking (over three to four decades) [66]. For both incidence and mortality, the association was stronger for cancer of the rectum than of the colon [67].
Potential for Primary Prevention of Colorectal Cancer
Taken together, these studies clearly indicate that substantial reduction in CRC incidence could be achieved through modest modification of the western diet and lifestyle factors. For dietary factors, replacing the high intake of red and processed meats, high-fat dairy products, highly refined grains and starches, and sugars with poultry, fish, and plant foods as the primary sources of protein; monounsaturated, and polyunsaturated fats as the primary sources of fat and unrefined grains, legumes, and fruits as the primary sources of carbohydrates is likely to lower risk of colorectal cancer [4]. For lifestyle factors, in addition to avoiding smoking and excessive alcohol drinking, control of body weight and regular physical activity represent two essential aspects of CRC prevention, especially in individuals that have a familial predisposition to this cancer. In approximatively 30% of sporadic CRC cases, clustering of the disease in families suggest an autosomal dominant inheritance of susceptibility to colorectal neoplasia [68, 69]. However, inheritance determines individual susceptibility to colonic neoplasm while dietary and lifestyle factors determine which susceptible individuals will develop cancer [70]. In other words, the combination of a familial predisposition and an unhealthy lifestyle considerably increases risk of CRC.
The preventive potential of these modest changes in dietary and lifestyle factors is enormous; it was estimated that as much as 70% of colon cancers in the US population could be prevented with their application [71]. Moreover, because several diet and lifestyle factors that are known to increase risk for colorectal cancer also foster the development of other chronic diseases, changes to these factors would lead to significant reductions in their incidence and thus have significant benefits on the overall health status of the population.
Acknowledgments
This study was funded by a grant from NSERC and by the Research Chair in Cancer Treatment and Prevention.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000;18:1967–1979. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–2043. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 6.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 7.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomark Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 8.Minami Y, Nishino Y, Tsubono Y, Tsuji I, Hisamichi S. Increase of colon and rectal cancer incidence rates in Japan: trends in incidence rates in Miyagi Prefecture, 1959–1997. J Epidemiol. 2006;16:240–248. doi: 10.2188/jea.16.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yiu HY, Whittemore AS, Shibata A. Increasing colorectal cancer incidence rates in Japan. Int J Cancer. 2004;109:777–781. doi: 10.1002/ijc.20030. [DOI] [PubMed] [Google Scholar]
- 10.Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007. [Google Scholar]
- 11.Burkitt DP. Related disease—related cause? Lancet. 1969;2:1229–1231. doi: 10.1016/S0140-6736(69)90757-0. [DOI] [PubMed] [Google Scholar]
- 12.Scharlau D, Borowicki A, Habermann N, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39–53. doi: 10.1016/j.mrrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Howe GR, Benito E, Castelleto R, et al. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst. 1992;84:1887–1896. doi: 10.1093/jnci/84.24.1887. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Stampfer MJ, Colditz GA, et al. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs CS, Giovannucci EL, Colditz GA, et al. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med. 1999;340:169–176. doi: 10.1056/NEJM199901213400301. [DOI] [PubMed] [Google Scholar]
- 16.Michels KB, Giovannucci EL, Joshipura KJ, et al. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740–1752. doi: 10.1093/jnci/92.21.1740. [DOI] [PubMed] [Google Scholar]
- 17.Koushik A, Hunter DJ, Spiegelman D, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. 2007;99:1471–1483. doi: 10.1093/jnci/djm155. [DOI] [PubMed] [Google Scholar]
- 18.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 19.Krebs-Smith SM, Kantor LS. Choose a variety of fruits and vegetables daily: understanding the complexities. J Nutr. 2001;131:487S–501S. doi: 10.1093/jn/131.2.487S. [DOI] [PubMed] [Google Scholar]
- 20.Johnston CS, Taylor CA, Hampl JS. More Americans are eating «5 a day» but intakes of dark green and cruciferous vegetables remain low. J Nutr. 2000;130:3063–3067. doi: 10.1093/jn/130.12.3063. [DOI] [PubMed] [Google Scholar]
- 21.Bingham SA, Day NE, Luben R, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/S0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- 22.Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 23.Michels KB, Giovannucci E, Chan AT, Singhania R, Fuchs CS, Willett WC. Fruit and vegetable consumption and colorectal adenomas in the Nurses’ Health Study. Cancer Res. 2006;66:3942–3953. doi: 10.1158/0008-5472.CAN-05-3637. [DOI] [PubMed] [Google Scholar]
- 24.Witte JS, Longnecker MP, Bird CL, Lee ER, Frankl HD, Haile RW. Relation of vegetable, fruit, and grain consumption to colorectal adenomatous polyps. Am J Epidemiol. 1996;144:1015–1025. doi: 10.1093/oxfordjournals.aje.a008872. [DOI] [PubMed] [Google Scholar]
- 25.Millen AE, Subar AF, Graubard BI, et al. Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. Am J Clin Nutr. 2007;86:1754–1764. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Demeule M, Michaud-Levesque J, Annabi B, et al. Green tea catechins as novel antitumor and antiangiogenic compounds. Curr Med Chem Anticancer Agents. 2002;2:441–463. doi: 10.2174/1568011023353930. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, Shu XO, Li H, et al. Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol Biomark Prev. 2007;16:1219–1223. doi: 10.1158/1055-9965.EPI-07-0097. [DOI] [PubMed] [Google Scholar]
- 29.Powers HJ. Interaction among folate, riboflavin, genotype, and cancer, with reference to colorectal and cervical cancer. J Nutr. 2005;135:2960S–2966S. doi: 10.1093/jn/135.12.2960S. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132:2350S–2355S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 31.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 32.Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer. A meta-analysis of prospective studies. JAMA. 2010;303:1077–1083. doi: 10.1001/jama.2010.263. [DOI] [PubMed] [Google Scholar]
- 33.Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci E, Stampfer MJ, Colditz G, et al. Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst. 1992;84:91–98. doi: 10.1093/jnci/84.2.91. [DOI] [PubMed] [Google Scholar]
- 35.Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97:906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldbohm RA, Brandt PA, van ’t Veer P, et al. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res. 1994;54:718–723. [PubMed] [Google Scholar]
- 37.Sandhu MS, White IR, McPherson K. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol Biomark Prev. 2001;10:439–446. [PubMed] [Google Scholar]
- 38.Nelson RL, Davis FG, Sutter E, et al. Body iron stores and risk of colonic neoplasia. J Natl Cancer Inst. 1994;86:455–460. doi: 10.1093/jnci/86.6.455. [DOI] [PubMed] [Google Scholar]
- 39.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez ME, Jacobs ET, Ashbeck EL, et al. Meat intake, preparation methods, mutagens and colorectal adenoma recurrence. Carcinogenesis. 2007;28:2019–2027. doi: 10.1093/carcin/bgm179. [DOI] [PubMed] [Google Scholar]
- 41.Sinha R, Chow WH, Kulldorff M, et al. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res. 1999;59:4320–4324. [PubMed] [Google Scholar]
- 42.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 43.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11:19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 44.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70, 000 events. Cancer Epidemiol Biomark Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 45.Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta. 2010;1801:252–265. doi: 10.1016/j.bbalip.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46:1594–1603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 47.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 48.Nam SY, Kim BC, Han KS, et al. Abdominal visceral adipose tissue predicts risk of colorectal adenoma in both sexes. Clin Gastroenterol Hepatol. 2010;8:443–450. doi: 10.1016/j.cgh.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 53.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 54.Rostom A, Dube C, Lewin G, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the US Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 55.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 56.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–358. doi: 10.1079/PNS2002166. [DOI] [PubMed] [Google Scholar]
- 57.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 58.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 59.Colditz G, Cannuscio C, Frazier A. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control. 1997;8:649–667. doi: 10.1023/A:1018458700185. [DOI] [PubMed] [Google Scholar]
- 60.Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Regensteiner JG, Mayer EJ, Shetterly SM, et al. Relationship between habitual physical activity and insulin levels among nondiabetic men and women: San Luis Valley Diabetes Study. Diab Care. 1991;14:1066–1074. doi: 10.2337/diacare.14.11.1066. [DOI] [PubMed] [Google Scholar]
- 62.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 64.Ferrari P, Jenab M, Norat T, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC) Int J Cancer. 2007;121:2065–2072. doi: 10.1002/ijc.22966. [DOI] [PubMed] [Google Scholar]
- 65.Jacobson JS, Neugut AI, Murray T, Garbowski GC, Forde KA, Treat MR, Waye JD, Santos J, Ahsan H. Cigarette smoking and other behavioral risk factors for recurrence of colorectal adenomatous polyps (New York City, NY, USA) Cancer Causes Control. 1994;5:215–220. doi: 10.1007/BF01830239. [DOI] [PubMed] [Google Scholar]
- 66.Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomark Prev. 2001;10:725–731. [PubMed] [Google Scholar]
- 67.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 68.Burt RW, DiSario JA, Cannon-Albright L. Genetics of colon cancer: impact of inheritance on colon cancer risk. Annu Rev Med. 1995;46:371–379. doi: 10.1146/annurev.med.46.1.371. [DOI] [PubMed] [Google Scholar]
- 69.Marchand L, Zhao LP, Quiaoit F, Wilkens LR, Kolonel LN. Family history of colorectal cancer in the multiethnic population of Hawaii. Am J Epidemiol. 1996;144:1122–1128. doi: 10.1093/oxfordjournals.aje.a008890. [DOI] [PubMed] [Google Scholar]
- 70.Marchand L, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC. Independent and joint effects of family history and lifestyle on colorectal cancer risk: implications for prevention. Cancer Epidemiol Biomark Prev. 1999;8:45–51. [PubMed] [Google Scholar]
- 71.Platz EA, Willett WC, Colditz GA, et al. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11:579–588. doi: 10.1023/A:1008999232442. [DOI] [PubMed] [Google Scholar]