Abstract
Background
The association between the use of highly active antiretroviral therapy (HAART) and an increased risk of metabolic syndrome and cardiovascular disease remains unclear.
Methods
We conducted a prospective, cross-sectional study of the risk factors associated with metabolic syndrome and cardiovascular disease among patients from an urban outpatient human immunodeficiency virus (HIV) clinic. Evaluation included laboratory data that were obtained after an overnight fast and a health survey that assessed traditional risk factors associated with cardiovascular disease, HIV-related factors, and comorbidities. Data collected were compared with data files from a cohort from the National Health and Nutrition Examination Survey (NHANES; 2001–2002) of persons who were seronegative for HIV infection who were matched for age, sex, race, and tobacco use.
Results
Four hundred seventy-one HIV-infected subjects provided complete data. The overall prevalence of metabolic syndrome was similar between the group HIV-infected patients and the group of persons who were seronegative for HIV infection (25.5% vs. 26.5%, respectively), although the HIV-infected patients had a significantly smaller waist circumference, lower body mass index, lower high-density lipoprotein cholesterol levels, higher triglyceride levels, and lower glucose levels, compared with the subjects from the NHANES cohort. Framingham 10-year risk scores were also similar between the 2 groups. HIV-infected patients with metabolic syndrome were more likely to be diabetic, older, and white and have a high CD4 cell count and body mass index, compared with patients without metabolic syndrome (P < .05 for all). The type or duration of antiretroviral therapy was not an independent risk factor for metabolic syndrome.
Conclusions
The prevalence of metabolic syndrome is high among HIV-infected persons, but not higher than the prevalence among HIV-uninfected persons. Traditional risk factors play a more significant role in the development of metabolic syndrome than do HIV treatment–associated factors.
HAART has resulted in sustained reductions of morbidity and mortality associated with HIV infection [1, 2]. Unfortunately, HAART has also been associated with metabolic complications that may increase patients’ risk of cardiovascular disease [3–5]. Specific antiretroviral therapies have been shown to increase proatherogenic lipid levels and contribute to the development of insulin resistance and visceral fat accumulation [5–7]. These characteristics are components of metabolic syndrome and are risk factors for diabetes and cardiovascular disease in the general population [8–10]. Protease inhibitors (PIs) in particular have been associated with an increased risk of cardiovascular disease [11–13], possibly in part because of their negative effect on lipids [14].
Recent studies of HIV-infected persons have revealed a high prevalence of metabolic syndrome among patients receiving HAART [15, 16]. Reported prevalence rates have been higher than rates reported for matched HIV-infected populations, yet they are similar to or even less than the 22%–24% rate of prevalence reported recently for the general US population [9]. Other data suggest that the increased prevalence of metabolic syndrome among HIV-infected persons may be more reflective of the burgeoning epidemic of obesity than a predominant effect of HAART [17]. Currently, data are lacking on risk for development of metabolic syndrome in HIV-infected persons, particularly in HIV-infected women and minorities, who represent an increasing proportion of the US and global HIV-infected population. HIV treatment guidelines recommend screening patients for metabolic complications and providing therapeutic interventions [18, 19]. Such measures may include intensified efforts to assess patients for risk factors for metabolic syndrome and cardiovascular disease as part of standard-of-care. For this study, the medical records of HIV-infected patients were reviewed to obtain risk factors for cardiovascular disease and laboratory data to (1) determine the prevalence of metabolic syndrome among a diverse HIV-infected outpatient population; (2) compare the prevalence of metabolic syndrome and diabetes with a contemporary US cohort of HIV-uninfected individuals who were matched for age, sex, race, and tobacco use; and (3) determine the risk factors for the development of metabolic syndrome that are unique to HIV-infected patients.
METHODS
Design
This was a prospective, cross-sectional evaluation of patients’ risk for the development of metabolic syndrome and cardiovascular disease. As part of standard-of-care, all HIV-infected patients who attended the Washington University HIV Clinic (St. Louis, MO) during January–July 2005 completed a cardiovascular risk survey with their providers. The survey included evaluations of traditional cardiac risk factors, HIV-related factors (duration of HIV infection and HAART, current medications, and nadir CD4 cell count), comorbid conditions, and use of medications for hypertension and dyslipidemia. Providers reviewed all information with patients, verified fasting status, and obtained lipid and glucose measurements, blood pressure, height, weight, and waist circumference. Providers obtained patients’ waist circumference in accordance with the National Institutes of Health (NIH) standard protocol [20]. The most recent HIV RNA level and CD4 cell count (from within 3 months of survey completion) were obtained from the medical chart and used for analysis. Pregnant women were excluded from the analysis. Traditional risk factors for metabolic syndrome and cardiovascular disease were compared with those obtained from the National Health and Nutrition Examination Survey (NHANES; 2001–2002) data files [21]. A Framingham 10-year risk score was calculated for all patients [22]. The study was reviewed and approved by the Washington University Institutional Review Board.
Definitions
According to National Cholesterol Education Program (NCEP) Adult Treatment Panel III guidelines [10], metabolic syndrome was defined as having ≥3 of the following criteria: abdominal obesity (waist circumference ≥102 cm for men and ≥88 cm for women), fasting triglyceride levels ≥150 mg/dL, high-density lipoprotein (HDL) cholesterol level <40 mg/dL for men and <50 mg/dL for women, fasting glucose levels of 100–125 mg/dL, or hypertension (blood pressure ≥130/85 mm Hg or current receipt of medication for hypertension). For both the NHANES cohort and the cohort of HIV-infected patients, use of a lipid-lowering agent was defined as use of a statin, fibrate, or niacin agent. An antihypertensive was any drug used to treat high blood pressure. Obesity was defined as a body mass index (BMI; calculated as the weight in kilograms divided by the square of height in meters) ≥30. HAART was defined as the use of 2 nucleosides (NRTIs) and a non-nucleoside reverse-transcriptase inhibitor (NNRTI), 2 NRTIs and a PI, or an NNRTI and a PI. Patients receiving triplenucleoside therapy only were not included in this analysis.
Analysis
Data were analyzed using χ2 or Fisher’s exact tests for categorical variables. Continuous variables were compared using Student’s t test or the Mann-Whitney U test for normally and nonnormally distributed variables. Partial correlations testing was used to test the strength of association between the diagnostic criteria for metabolic syndrome and other clinical variables for the cohort of HIV-infected patients. Significant variables were entered stepwise into a multiple logistic regression model to determine the best multivariate model. NCEP criteria for metabolic syndrome were not included in multivariate analyses. Instead, they were analyzed separately in a subanalysis focused on determining the risk (OR) of individual NCEP criterion for metabolic syndrome diagnosis. HIV RNA levels were log-transformed. All P values were 2-tailed.
For comparisons between the cohorts of patients infected with HIV and patients seronegative for HIV infection, HIV-infected patients were randomly matched to subjects from the NHANES (2001–2002) data files (Centers for Disease Control and Prevention Web site [21]). Subjects from the NHANES cohort were randomly chosen from those who matched our patients according to age (within 3 years), sex, race, and smoking status. HIV testing was only performed for subjects in the NHANES cohort who were <50 years of age; thus, we could only be certain that subjects in the NHANES cohort who were <50 years of age were not infected with HIV. However, the number of patients in the cohort of HIV-infected patients who were ≥50 years of age was generally low (16%), and the number of HIV-infected persons in the NHANES cohort who were ≥50 years of age was likely quite low. For predictors that were significantly different between the 2 cohorts, additional multiple regression analyses were performed controlling for age, race, sex, and BMI to determine predictors of individual NCEP criterion. Data were analyzed with use of the SPSS software package, version 12.0 (SPSS).
RESULTS
A total of 601 patients (394 men [66%] and 207 women [34%]) completed the metabolic survey. Sixty-one percent were African American, and 69% were currently receiving HAART; 10% were HAART naive. The proportion of women and African Americans in our clinic population was similar to that of the HIV-infected population in the United States [23]. The respective waist circumference and BMI for the entire cohort were 90.1 cm and 25.8 for men and 93.3 cm and 29.9 for women (P < .01). The mean current CD4 cell count was 449 cells/mm3. Seventy-seven percent of patients receiving HAART had an HIV RNA level <400 copies/mL.
Of the patients who completed the survey, 471 had complete laboratory data that were obtained during a period of fasting and were thus evaluated for diagnosis of metabolic syndrome (table 1). Among this subgroup, 120 patients (26%) had metabolic syndrome, and 381 patients (81%) met ≥1 of the criteria for risk of metabolic syndrome. During a univariate analysis that excluded the 5 NCEP criteria for metabolic syndrome, HIV-infected patients with metabolic syndrome were more likely to have a family history of cardiovascular disease or diabetes, be of white race and older age, have had a longer duration of HIV infection, and have a higher low-density lipoprotein (LDL) cholesterol level, a higher BMI, and a higher CD4 cell count, compared with HIV-infected persons without metabolic syndrome (P ≤ .05 for all characteristics). Using multivariate analyses excluding the 5 NCEP criteria, significant predictors of metabolic syndrome among HIV-infected patients were older age, a higher CD4 cell count, white race, and a higher BMI (P < .05 for all predictors). The comparison of patients with and without metabolic syndrome revealed no significant differences with respect to the use of HAART or the type of HAART (figure 1).
Table 1.
Patient characteristics according to metabolic syndrome diagnosis criteria.
| Characteristic | Patients with metabolic syndrome (n = 120) |
Patients without metabolic syndrome (n = 351) |
P |
|---|---|---|---|
| Age, mean years ± SEM | 43.4 ± 1.0 | 39.0 ± 0.5 | <.001 |
| Sex | |||
| Men | 80 (66.7) | 224 (63.8) | |
| Women | 40 (33.3) | 127 (36.2) | |
| Ethinicity | .02 | ||
| White | 54 (46.6) | 117 (34.1) | |
| African American | 62 (53.4) | 226 (65.9) | |
| Duration of HIV infection, mean years ± SEM | 8.6 ± 0.5 | 7.4 ± 0.3 | .05 |
| Current tobacco smoker | 54 (45.0) | 146 (41.6) | .52 |
| Receiving HAART | 88 (73.3) | 254 (72.4) | .91 |
| HIV RNA level <400 copies | 69 (79.3) | 203 (80.2) | .88 |
| Type of HAART | |||
| Ritonavir-boosted PI | 38 (31.7) | 97 (27.6) | .20 |
| Ritonavir and lopinavir | 15 (12.5) | 44 (12.5) | .89 |
| Ritonavir and atazanavir | 18 (15.0) | 49 (14.0) | .85 |
| Ritonavir and other PI | 5 (4.2) | 4 (1.1) | .20 |
| Unboosted PI | 5 (4.2) | 25 (7.1) | .20 |
| NNRTI | 49 (40.8) | 144 (41.0) | .97 |
| Duration of HAART, mean years ± SEM | 3.9 ± 0.4 | 3.2 ± 0.2 | .41 |
| Family history of diabetes | 60 (52.6) | 140 (41.5) | .05 |
| Family history of coronary heart disease | 69 (59.5) | 159 (48.3) | .04 |
| Current CD4 cell count, mean cells/mm3 ± SEM | 542 ± 27 | 417 ± 14 | <.001 |
| Mean BMI ± SEM | 31.3 ± 0.9 | 25.8 ± 0.3 | <.001 |
| Waist circumference, mean cm ± SEM | 104.6 ± 1.8 | 86.5 ± 0.8 | <.001 |
| Fasting LDL cholesterol level, mean mg/dL ± SEM | 107.7 ± 3.4 | 98.7 ± 1.9 | 0.03 |
| Fasting HDL cholesterol level, mean mg/dL ± SEM | 38.8 ± 1.0 | 51.9 ± 1.1 | <.001 |
| Fasting triglyceride level, mean mg/dL ± SEM | 252.4 ± 13.4 | 156.8 ± 8.9 | <.001 |
| Current use of lipid-lowering therapy | 24 (20.0) | 20 (5.7) | <.001 |
| Fasting glucose level, mean mg/dL ± SEM | 107.7 ± 3.0 | 87.9 ± 1.1 | <.001 |
| Diagnosis of hypertension | 82 (67.8) | 66 (18.8) | <.001 |
| Current use of antihypertensive therapy | 69(57.5) | 51(14.5) | <.001 |
| History of myocardial infarction | 4 (3.3) | 5 (1.4) | .24 |
| Diagnosis of diabetes | 28 (23.1) | 13 (3.7) | <.001 |
NOTE. Data are no. (%) of patients, unless otherwise indicated.
BMI, body mass index (defined as the weight in kilograms divided by the square of the height in meters); HDL, high-density lipoprotein; LDL, low-density lipoprotein; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Figure 1.
Type of HAART given to persons with and without metabolic syndrome. There were no significant differences between persons with or without metabolic syndrome for each of the antiretroviral therapy groups. PI, protease inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor.
A separate analysis was performed for the patients in the HIV-infected cohort to identify the contribution of each individual NCEP criterion towards diagnosis of metabolic syndrome. Despite a high prevalence of a low HDL level and high triglyceride levels, the presence of either an elevated fasting glucose level or hypertension were the strongest contributors to metabolic syndrome (OR, 11.4 [95% CI, 6.9–18.9] and 9.3 [95% CI, 5.8–14.9], respectively).
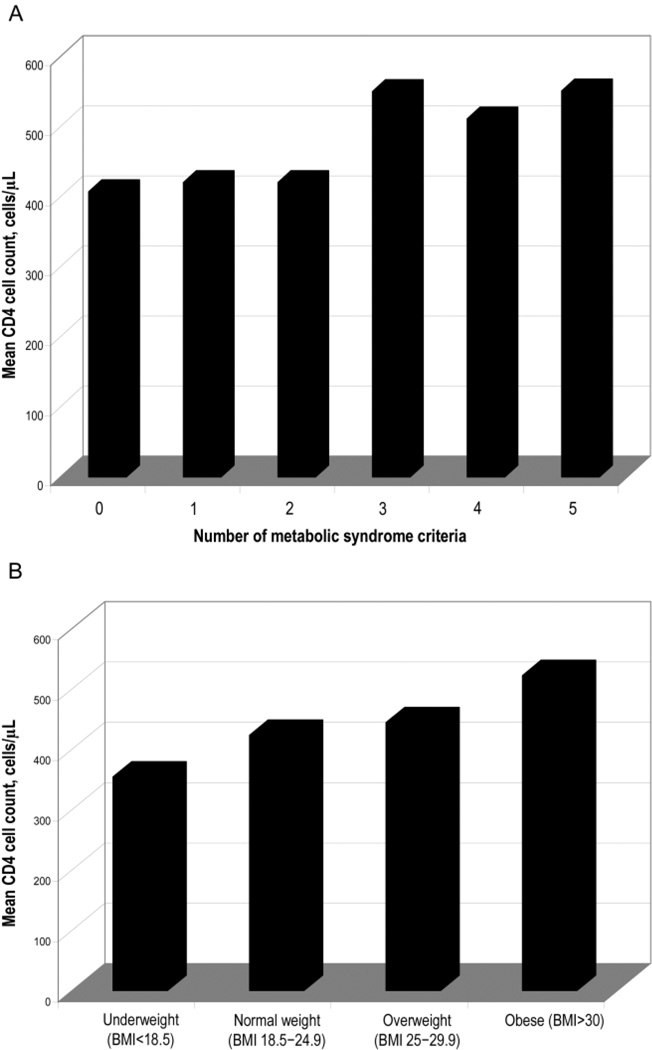
We also explored the finding of an increased CD4 cell count as an independent predictor of the occurrence of metabolic syndrome in HIV-infected patients. An increased CD4 cell count was a strong predictor of 2 NCEP criteria (elevated triglyceride and glucose levels; P < .01) after controlling for age, sex, race, and BMI. Likewise, an increased CD4 cell count correlated with the presence of an increased number of metabolic syndrome criteria and an increased BMI (P < .01 for both; figure 2A and 2B). The change in CD4 cell count (from nadir to current value) also tended to correlate with an increased number of metabolic syndrome criteria (P = .06).
Figure 2.
A, CD4 cell count in relation to the number of National Cholesterol Education Program (NCEP) criteria for metabolic syndrome. Patients received a diagnosis of metabolic syndrome if they met ≥3 NCEP criteria. B, CD4 cell count in relation to body mass index (BMI; defined as the weight in kilograms divided by the square of the height in meters). Underweight is defined as BMI <18.5, normal weight is defined as a BMI of 18.5–24.9, overweight is defined as a BMI of 25–29.9, and obese is defined as a BMI ≥30.
Sex and HIV-infected patients
Among HIV-infected patients with metabolic syndrome, men were significantly older, more likely to be white, have a family history of diabetes, and be receiving HAART, compared with women (table 2). Among the persons in this subgroup, men also had higher Framingham scores and triglyceride levels and lower BMI and waist circumference, compared with women (P < .01 for all).
Table 2.
Characteristics of HIV-infected patients with metabolic syndrome, according to sex.
| Characteristic | HIV-infected men with metabolic syndrome (n = 80) |
HIV-infected women with metabolic syndrome (n = 40) |
P |
|---|---|---|---|
| Age, mean years ± SEM | 45.1 ± 1.3 | 39.9 ± 1.4 | .01 |
| Ethnicity | <.001 | ||
| White | 45 (58.4) | 9 (23.1) | |
| African American | 32 (41.6) | 30 (76.9) | |
| Duration of HIV infection, mean years ± SEM | 8.5 ± 0.6 | 8.6 ± 0.8 | .95 |
| Current tobacco smoker | 37 (46.3) | 17 (42.5) | .85 |
| Receiving HAART | 67 (83.8) | 21 (52.5) | <.001 |
| HIV RNA level <400 copies | 56 (83.6) | 13 (61.9) | .03 |
| Family history of diabetes | 60 (52.6) | 140 (41.5) | .05 |
| Family history of coronary heart disease | 36 (47.4) | 24 (63.2) | .16 |
| CD4 cell count, mean cells/mm3 ± SEM | 522 ± 33 | 581 ± 50 | .32 |
| Mean BMI ± SEM | 28.5 ± 0.6 | 37.2 ± 2.0 | <.001 |
| Waist circumference, mean cm ± SEM | 100.0 ± 1.7 | 113.7 ± 3.6 | .001 |
| Fasting LDL cholesterol level, mean mg/dL ± SEM | 108.4 ± 1.3 | 108.5 ± 1.8 | .77 |
| Fasting HDL cholesterol level, mean mg/dL ± SEM | 38.0 ± 1.0 | 40.5 ± 1.1 | .26 |
| Fasting triglyceride level, mean mg/dL ± SEM | 295.2 ± 17.4 | 165.7 ± 11.2 | <.001 |
| Fasting glucose level, mean mg/dL ± SEM | 106.3 ± 3.0 | 110.4 ± 7.0 | .60 |
| Diagnosis of hypertension | 54 (67.5) | 28 (70.0) | .84 |
| History of myocardial infarction | 3 (3.8) | 1 (2.5) | 1.00 |
| Diagnosis of diabetes | 16 (20.0) | 12 (30.0) | .26 |
| Average 10-year coronary heart disease risk (based on Framingham score) | 8 | 3 | <.001 |
NOTE. Data are no. (%) of patients, unless otherwise indicated.
BMI, body mass index (defined as the weight in kilograms divided by the square of the height in meters); HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Race/ethnicity and HIV-infected patients
Among the general US population, the prevalence of metabolic syndrome appears to differ among African American and white men and women [9]; therefore, we examined this difference among the patients in the HIV-infected cohort. Among African Americans, waist circumference and BMI were significantly higher for women, compared with men. However, African American men tended to have higher levels of triglycerides and glucose and a lower HDL cholesterol level, compared with African American women. Overall, the prevalence of metabolic syndrome in the patients in the HIV-infected cohort was not significantly different between men and women when stratified by race (22% women vs. 21% men among African Americans subjects and 29% women vs. 32% men among white subjects).
The HIV-infected cohort vs. the NHANES cohort
The overall prevalence of metabolic syndrome and the Framingham scores were similar between the HIV-infected and NHANES cohorts. However, individual metabolic parameters differed significantly (table 3). HIV-infected patients with metabolic syndrome had smaller waist circumferences, lower BMIs, lower HDL cholesterol levels, higher triglyceride levels, and lower glucose levels, compared with patients in the NHANES cohort with metabolic syndrome (P < .01 for all). Despite the higher prevalence of dyslipidemia in the HIV-infected patients, compared with the patients in the NHANES cohort, nearly twice as many HIV-infected patients were already receiving lipid-lowering therapy (9.3% vs. 4.9%; P = .01).
Table 3.
Comparison between HIV-infected and NHANES (HIV-uninfected) cohorts.
| Characteristic | HIV-infected cohort (n = 471) |
NHANES cohort (n = 471) |
OR for HIV-infected vs. HIV-uninfected individuals |
P |
|---|---|---|---|---|
| Diagnosis of metabolic syndrome | 120 (25.5) | 125 (26.5) | NA | .77 |
| Age, mean years ± SEM | 40.2 ± 00.0 | 40.1 ± 0.00 | NA | .87 |
| Sex | 1.00 | |||
| Men | 304 (64.5) | 304 (64.5) | NA | |
| Women | 167 (34.5) | 167 (34.5) | NA | |
| Ethnicity | 1.00 | |||
| White | 171 (36.3) | 171 (36.3) | NA | |
| African American | 288 (61.1) | 288 (61.1) | NA | |
| Hispanic | 10 (2.1) | 10 (2.1) | NA | |
| Asian | 2 (0.5) | 2 (0.5) | NA | |
| Current tobacco smoker | 200 (42.5) | 200 (42.5) | NA | 1.00 |
| Mean BMI ± SEM | 27.3 ± 00.0 | 28.5 ± 00.0 | NA | .01 |
| Elevated waist circumferencea | 128 (30.7) | 207 (43.9) | 0.47 | <.001 |
| Low HDL cholesterol levela | 205 (43.5) | 141 (29.9) | 1.79 | <.001 |
| Elevated glucose levela | 102 (21.7) | 144 (30.6) | 0.60 | .001 |
| Elevated triglyceride levela | 208 (44.2) | 119 (25.3) | 2.37 | <.001 |
| Current use of lipid-lowering therapy | 44 (9.3) | 23 (4.9) | 2.01 | .01 |
| Diagnosis of HTNa | 148 (31.4) | 174 (36.9) | NA | .09 |
| Current use of antihypertensive therapya | 120 (25.5) | 84 (17.9) | 1.57 | .01 |
| Diagnosis of diabetes | 41 (8.7) | 45 (9.6) | NA | .70 |
| Mean Framingham score ± SEM | 0.5 ± 0.3 | 0.6 ± 0.3 | NA | .85 |
NOTE. Data are no. (%) of persons, unless otherwise indicated.
BMI, body mass index (defined as the weight in kilograms divided by the square of the height in meters); HDL, high-density lipoprotein; HTN, hypertension; NA, not applicable for these variables.
Part of criteria for metabolic syndrome as defined in the methods section of the text [10].
The effects of HAART
During multivariate analyses that controlled for age, sex, race, and BMI, current PI use was an independent predictor of an increased level of triglycerides only (P = .03). Patients who had a history of stavudine use were also more likely to have higher triglyceride levels than those who had no history of stavudine use (P = .05). Stavudine use was not associated with changes in BMI or waist circumference. Current NNRTI use and lower HIV RNA levels were found to be significant predictors of higher HDL cholesterol (P < .01 for both), whereas an increased duration of HIV infection was significantly associated with lower HDL cholesterol levels (P = .05). Other HIV-related predictors, such as nadir CD4 cell count, current use or duration of HAART, or current use of other specific antiretroviral drugs were not found to be significantly associated with dyslipidemia or other risk factors for metabolic syndrome during multivariate analyses. Our observations of the HIV-infected and NHANES cohorts combined revealed that HIV infection was a significant independent predictor of both elevated triglyceride levels and low HDL cholesterol level (P < .01 for both), after controlling for demographic and other traditional cardiac risk factors.
DISCUSSION
Among the patients of the urban, HIV-infected population, we found a high prevalence rate of metabolic syndrome and its associated risk factors. Nonetheless, the prevalence rate was similar to a well-matched, current population of HIV-uninfected individuals. For the patients in the HIV-infected cohort and excluding NCEP criteria, traditional factors (e.g., older age and higher BMI), higher CD4 cell count, and white race were the strongest predictors of the presence of metabolic syndrome. Unexpectedly, the duration and type of HAART were not strong predictors. Among the 5 NCEP criteria for metabolic syndrome, the presence of elevated fasting glucose or hypertension in association with HIV infection carried the highest risk of development of metabolic syndrome. Most HIV-infected patients with either of these diagnoses also tended to meet additional NCEP criteria for metabolic syndrome. In summary, among HIV-infected persons, traditional risk factors were more important predictors of metabolic syndrome than therapy-related factors.
Among HIV-related factors, although a higher CD4 cell count was an independent predictor of the development of metabolic syndrome, a higher BMI accounted for a substantial part of the CD4-attributable risk. Other studies have found a lower CD4 cell count to be associated with metabolic syndrome or increased cardiovascular risk [16, 24–26]. However, the current findings are consistent with the expected increase of weight, improved nutritional and immunologic status that occurs with effective anti-HIV therapy, and prolonged survival. The meaning of this finding is not clear, but it may reflect the physiological changes (unknown mechanisms) that accompany immune reconstitution or viral suppression. Better adherence to HAART was an unlikely factor, because similar percentages of patients with and without metabolic syndrome had an undetectable viral load. These potential links need to be examined further.
In the general US population, epidemic rates of obesity, insulin resistance, hypertension, and associated complications [9, 27] have been noted during recent years. The current findings suggest that HIV-infected persons treated with HAART are not spared from this emerging epidemic, as demonstrated by their high rates of obesity, hyperglycemia, hypertension, and dyslipidemia. These findings, along with a lack of independent effects of HAART on metabolic syndrome, support the notion that these traditional risk factors (i.e., hypertension, hyperglycemia, and obesity) may be more important than HAART-related factors for the prediction of metabolic syndrome and cardiovascular disease risk. The prevalence of diabetes was similar between the cohort of HIV-infected patients and the NHANES cohort, and mean fasting glucose levels were significantly lower in HIV-infected persons than in HIV-uninfected individuals. A recent study reported a high prevalence of diabetes in men receiving HAART [28], but these findings may also reflect the high prevalence of diabetes among US adults (estimated to be 9.6% for 2005) [29], which is comparable to the prevalence of diabetes among our clinic population. The high prevalence of hypertension among the patients in the HIV-infected cohort was also similar to the NHANES sample population and was comparable to the reported prevalence rates of hypertension for the general populations of North America and Europe [30].
An important finding was the higher prevalence of dyslipidemia, despite the greater use of lipid-lowering therapy for HIV-infected patients than for HIV-uninfected persons, although these cohorts had similar prevalences of metabolic syndrome. HIV infection is associated with an increase in triglyceride levels and a decrease in HDL level [31, 32]. Stavudine and PI use may also contribute to hypertriglyceridemia [33], which was consistent with our findings. Although dyslipidemia was predominant in the patients in our HIV-infected cohort, we found the presence of either hypertension or hyperglycemia carried a significantly more likely association with metabolic syndrome than the presence of high triglyceride levels, a low HDL level, or an elevated waist circumference. These results suggest that HIV and HAART-related dyslipidemia often occur as lone phenomena, whereas other traditional risk factors (i.e., hypertension and obesity) may occur as a cluster and, thus, carry higher risk for metabolic syndrome. Because use of lipid-lowering therapy itself was not a criterion for having metabolic syndrome, it is possible that our study underestimated the prevalence of metabolic syndrome. However, more HIV-infected patients than subjects of the NHANES cohort still had dyslipidemia that met NCEP criteria. Standard pharmacologic treatments for dyslipidemia in this HIV-infected cohort [19] were likely suboptimal because of the concurrent adverse effects of HIV infection and HAART.
Although numerous studies have implicated PIs as important risk factors for cardiovascular disease [3–7, 11–14, 24, 25], the use of PIs was not found to be an independent risk factor for metabolic syndrome when controlling for other demographic and traditional risk factors. Furthermore, when we compared the effects of specific PIs on lipid parameters and metabolic syndrome risk, we were unable to find any significant differences. However, nearly all of our patients who were receiving a PI-based regimen were using pharmacokinetic boosting with low-dose ritonavir therapy. Recent data have shown that even the “lipid-friendly” PI atazanavir may cause a small but significant elevation in triglyceride and total cholesterol levels when boosted with low-dose ritonavir therapy [34]. Although stavudine use has been associated with lipoatrophy, dyslipidemia, and insulin resistance [33, 35–37], only 24 patients who were observed at our clinic were currently receiving this drug; therefore, we likely did not have adequate power to detect any significant effect of current stavudine use on the risk for metabolic syndrome.
Surprisingly, race and sex were not independent predictors of metabolic syndrome in the patients in our HIV-infected cohort. Among African Americans in the general US population, women have a ~57% higher prevalence of metabolic syndrome, compared with men [9]. Also, recent evidence indicates that the prevalence of obesity is high (30%) for African American women [17]. We found an even higher prevalence of obesity among the African American women (45%, compared with 16% of African American men) in our cohort. Among African Americans, however, men had higher levels of triglycerides and lower levels of HDL cholesterol than did women. These findings help to explain the lack of difference in the prevalence of metabolic syndrome between men and women when stratified by race. Additionally, these findings support the notion that HIV-infected men and women may have different sex-specific risk factors for the metabolic syndrome.
Our study had several limitations. First, the use of specific antiretrovirals over a long period of time may have had long-lasting, irreversible metabolic effects that were not captured in this analysis. Given the large number of patients in our cohort, it was difficult to obtain reliable, complete antiretroviral histories with regard to the duration of the use of each antiretroviral drug. Also, the use of the fasting glucose level alone to diagnose impaired glucose tolerance likely underestimated the prevalence of insulin resistance, not only among the patients of the HIV-infected cohort, but also among the subjects of the NHANES cohort. Currently, oral glucose tolerance testing is not routine, standard-of-care for HIV infection and can be difficult to obtain, even from patients at risk for diabetes. Finally, an assumption has been made that HIV-infected persons with metabolic syndrome are at similar cardiovascular risk, compared with HIV-uninfected persons with metabolic syndrome, but the phenotype often differs between these 2 populations. In particular, HIV-infected persons may have significant peripheral lipoatrophy that contributes to insulin resistance and an elevated waist-to-hip ratio. Recent studies suggest that HIV-infected persons receiving HAART have a higher risk of acquiring diabetes [28] and, therefore, are likely to have a higher risk for cardiovascular disease [11]. These observations support the diagnosis of metabolic syndrome in HIV-infected persons as a likely predictor of higher risk for diabetes and cardiovascular disease, and it may serve as a simple, useful screening tool for this patient population.
In conclusion, although we found a high prevalence of metabolic syndrome among HIV-infected patients, this diagnosis was strongly associated with traditional cardiovascular risk factors rather than HAART-related effects. Additional longitudinal studies are needed to determine whether metabolic syndrome is equally predictive of future diabetes and heart disease in HIV-infected persons compared with HIV-uninfected persons.
Acknowledgments
Financial support. National Institutes of Health (K23 AI06533601 [to K.M.]; R01 DK049393, R01 DK059531, R21 DK074345, and R21 AT003083 [to K.Y.]; and P30 DK056341 [to Washington University]).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Sterne JAC, MA Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 3.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 4.Yarasheski KE, Tebas P, Sigmund C, et al. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr. 1999;21:209–216. doi: 10.1097/00126334-199907010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–875. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan AK, Nelson MR, Feher MD, Nelson MR, Gazzard BG. Marked hyperlipidaemia on ritonavir therapy. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199811000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Gilles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Assoc. Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Friis-Moller N, Sabin CA, Weber R, et al. Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg SD, Tong TC, Ward DJ, et al. HIV Outpatient Study (HOPS) Investigators. Protease inhibitor drug use and adverse cardiovascular outcomes in ambulatory HIV-infected persons. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 13.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Clinical Epidemiology Group from the French Hospital Database. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 14.Friis-Moller N, Reiss P, El-Sadr WM, et al. Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. Exposure to PI and NNRTI and risk of myocardial infarction: results from the D:A:D study [abstract 144]. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections (Denver); Alexandria, VA: Foundation for Retrovirology and Human Health; 2006. [Google Scholar]
- 15.Jerico C, Knobel H, Montero M, et al. Metabolic syndrome among HIV-infected patients: prevalence, characteristics, and related factors. Diabetes Care. 2005;28:144–149. doi: 10.2337/diacare.28.1.132. [DOI] [PubMed] [Google Scholar]
- 16.Palella F, Wang Z, Chu H, et al. Correlates and prevalence of the metabolic syndrome over time in the Multicenter AIDS Cohort Study (MACS) [abstract TuPe2.2B18]. Program and abstracts of the 3rd IAS Conference on HIV Pathogenesis and Treatment; Rio de Janeiro, Brazil. 2005. [Google Scholar]
- 17.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39:557–561. [PubMed] [Google Scholar]
- 18.Aberg JA, Gallant JE, Anderson J, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Disease Society of America. Clin Infect Dis. 2004;39:609–629. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- 19.Dubé MP, Stein J, Aberg J, et al. Guidelines for the evaluation and management of dyslipidemia in HIV-infected adults receiving antiretroviral therapy: recommendations of the HIV Medicine Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 20.National, Heart, Lung, and Blood Institute (NHLBI) Obesity Education Initiative Expert Panel. National Institutes of Health (NIH) Bethesda, MD: NIH, NHLBI; 1998. Clinical guidelines on identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. publication no. 98–4083. [PubMed] [Google Scholar]
- 21.The NHANES 2001–2002 datafiles. [Accessed 6 February 2006]; Available at: http://www.cdc.gov/nchs/about/major/nhanes/nhanes01-02.htm#Demographics.
- 22.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Vol 16. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. HIV/AIDS Surveillance Report, 2004; pp. 17–21. [Google Scholar]
- 24.Hsue PY, Joan CL, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima media thickness in patients with HIV infections. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 25.Maggi P, Serio G, Epifani G, et al. Premature lesions of the carotid vessels in HIV-1-infected patients treated with protease inhibitors. AIDS. 2000;14:123–128. doi: 10.1097/00002030-200011100-00001. [DOI] [PubMed] [Google Scholar]
- 26.David MH, Hornung R, Fichtenbaum JC. Ischemic cardiovascular disease in persons with human immunodeficiency virus infections. Clin Infect Dis. 2002;34:98–102. doi: 10.1086/324745. [DOI] [PubMed] [Google Scholar]
- 27.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 28.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 29.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) National diabetes statistics. [Accessed 7 June 2006]; Available at: http://diabetes.niddk.nih.gov/dm/pubs/statistics.
- 30.Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 31.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 32.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 33.Gallant JE, Staszewski S, Pozniak A, et al. Efficacy and safety of tenofovir df vs stavudine in combination therapy in antiretroviral-naïve patients. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 34.Malan N, Krantz E, David N, et al. Efficacy and safety of atazanavir-based therapy in antiretroviral naive HIV-1 infected subjects, both with and without ritonavir: 48-week results from A1429-089 [abstract 107LB]. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections (Denver); Alexandria, VA: Foundation for Retrovirology and Human Health; 2006. [Google Scholar]
- 35.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 36.Dubé M, Zackin R, Tebas P, et al. Prospective study of regional body composition in antiretroviral-naive subjects randomized to receive zidovudine -infected lamivudine or didanosine -infected stavudine combined with nelfinavir, efavirenz, or both: A5005s, a study of ACTG 384. Antiviral Ther. 2002;7:L18. [Google Scholar]
- 37.Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–1383. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]