Abstract
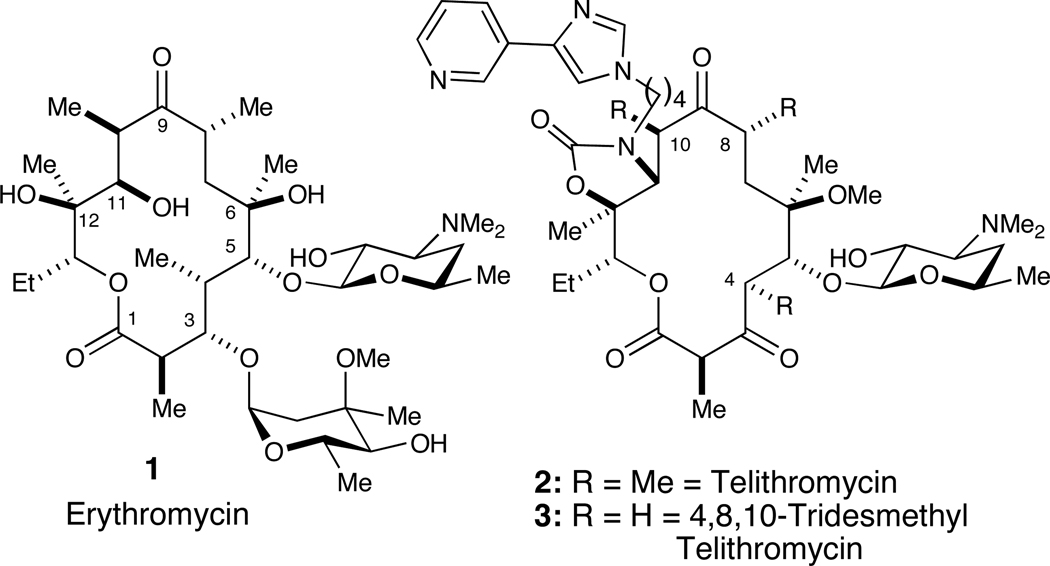
Novel sources of antibiotics are required to address the serious problem of antibiotic resistance. Telithromycin (2) is a third-generation macrolide antibiotic prepared from erythromycin (1) and used clinically since 2004. Herein we report the details of our efforts that ultimately led to the total synthesis of (−)-4,8,10-tridesmethyl telithromycin (3) wherein methyl groups have been replaced with hydrogens. The synthesis of desmethyl macrolides has emerged as a novel strategy for preparing bioactive antibiotics.
Keywords: total synthesis, ketolide antibiotics, antibiotic resistance, telithromycin, desmethyl analogues
1. Introduction
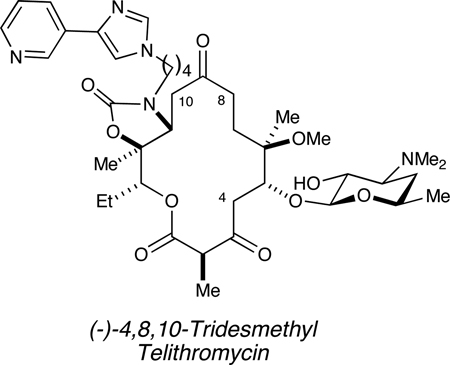
The incessant rise in antibiotic-resistant bacteria is a serious public health issue.1,2 To address this need, we have initiated a structure-based drug design program wherein desmethyl analogues (i.e., CH3→H) of the 3rd-generation macrolide antibiotic telithromycin (2) are prepared via chemical synthesis (Figure 1). Inspiration for desmethylation came from the Steitz’s analysis of x-ray crystal structures of 1 and 2 bound to the 50S ribosomal subunits of H. marismortui, which explains how ribosomal point mutations at critical residues (i.e., A2058G, E. coli numbering) confer antibiotic resistance.3
Figure 1.
Structures of erythromycin (1), telithromycin (2) and novel analogue (−)-4,8,10-tridesmethyl telithromycin (3).
Most modern macrolide antibiotics are semisynthetic analogs of erythromycin A, of which clarithromycin, azithromycin and telithromycin are examples.4 As a part of our desmethylation approach to address antibiotic resistance, herein we report the details of our synthetic efforts culminating in the total synthesis of (−)-4,8,10-tridesmethyl telithromycin (3).5 We have employed a de novo synthetic strategy to access this simplified ketolide scaffold.
2. Results and Discussion
2.1 Retrosynthesis
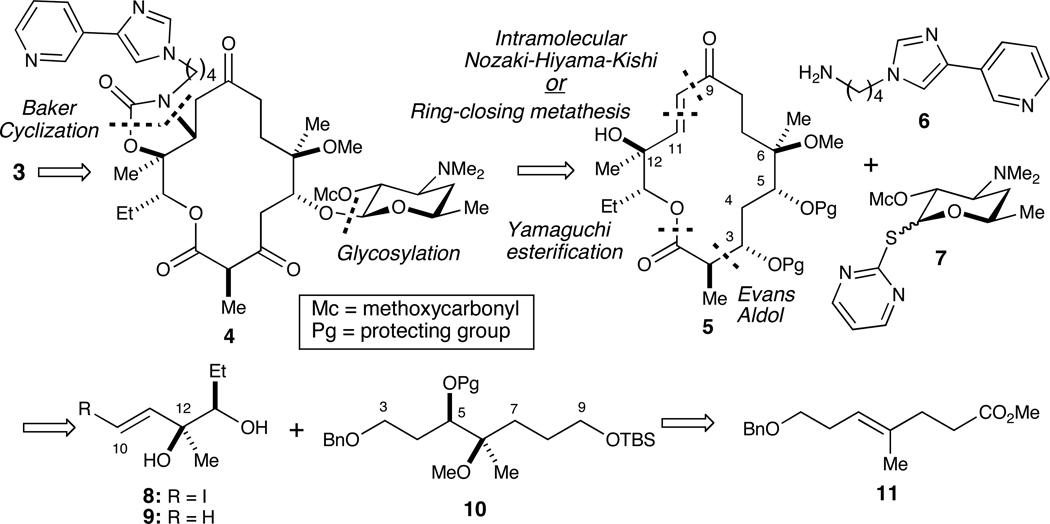
Our approach toward the synthesis of (−)-4,8,10-tridesmethyl telithromycin (3) is shown in Scheme 1. The retrosynthetic strategy employed was guided by prior art in the field. Specifically, we envisioned the use of a sequential one-pot carbamoylation/intramolecular aza-Michael method developed by Baker and co-workers at Abbott to install the C11–C12 oxazolidinone with pendant butylarene to access 4 from 5.6 In fact, this tactic was employed by Hoechst Marion Roussel (HMR) with 6 in their synthesis of HMR-3647 (i.e., telithromycin).7 Glycosylation of a C5 hydroxyl acceptor derived from 5 would be accomplished with known desosamine donor 7.8 To prepare macroketolactone 5, we utilized two tactics employed in the synthesis of the related ketolide, narbonolide: (1) the intramolecular Nozaki-Hiyama-Kishi (NHK) reaction employed by Sherman and Fecik9 and (2) the intramolecular ring-closing metathesis (RCM) strategy employed by Kang and co-workers.10 The NHK and RCM substrates could be accessed by an intermolecular Yamaguchi esterification,11 which then leads to fragments 8, 9 and 10, the latter of which will be subjected to the Evans aldol reaction to set stereocenters at C2 and C3.12 Finally, the Sharpless asymmetric dihydroxylation (AD) reaction13 of olefin 11, which is prepared via a Johnson-Claisen orthoester rearrangement, would establish the requisite configurations and oxidation states at both C5 and C6.
Scheme 1.
Retrosynthesis of (−)-4,8,10-tridesmethyl telithromycin (3).
2.2 Synthesis
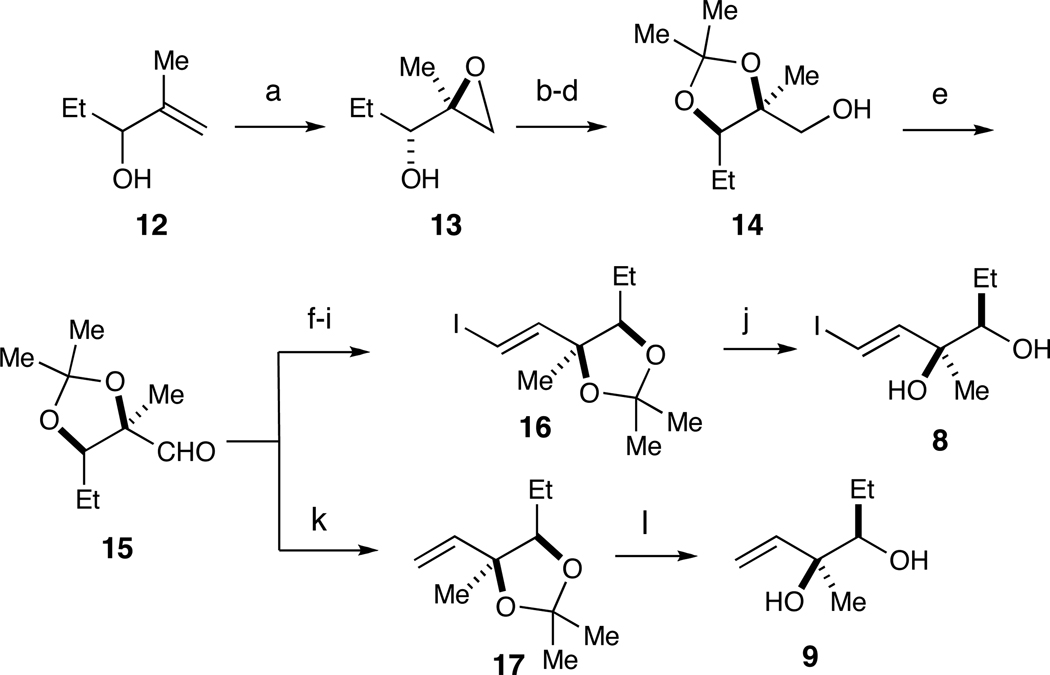
The preparation of fragments 8 and 9 was accomplished from aldehyde 15, which was synthesized from 12 (Scheme 2). Kinetic resolution of rac-12 via the Sharpless asymmetric epoxidation protocol provided enantioenriched alcohol 13 in 32% yield (92% ee).14 Regioselective ring-opening with pivalic acid and Ti(O-iPr)4, acetonide formation and treatment with MeLi afforded alcohol 14 (59% over three steps).15 Swern oxidation of 14 to aldehyde 1516 proceeded without incident. At this point, 15 was subjected to the Corey-Fuchs alkynylation protocol (59% yield from 14).17 Hydrostannation of the intermediary alkyne with Bu3SnH and catalytic AIBN proceeded with excellent stereocontrol to afford an (E)-vinyl stannane,18 which was converted to vinyl iodide 16 with I2 in 80% over two steps. Removal of the acetonide in 16 with 1 M HCl (aq.) furnished fragment 8 (72% yield). Access to fragment 9 was accomplished by (1) Wittig methylenation of 15 and (2) removal of the acetonide in 17 under acidic conditions in 53% yield from 14.16
Scheme 2.
Synthesis of fragments 8 and 9 from aldehyde 15.a
a Reagents and conditions: (a) (−)-DIPT, Ti(Oi-Pr)4, t-BuOOH, 32% (92% ee); (b) PivOH, Ti(Oi-Pr)4; (c) Me2C(OMe)2, PPTS; (d) MeLi, 59% over three steps; (e) (COCl)2, DMSO, Et3N; (f) CBr4, Ph3P, CH2Cl2, 65% over two steps; (g) n-BuLi, THF, 90%; (h) cat. AIBN, Bu3SnH, C6H6; (i) I2, CH2Cl2, 80% over two steps; (j) 1N HCl (aq.), 72%; (k) Ph3P=CH2; (l) 1 N HCl (aq.), 53% over three steps from 14.
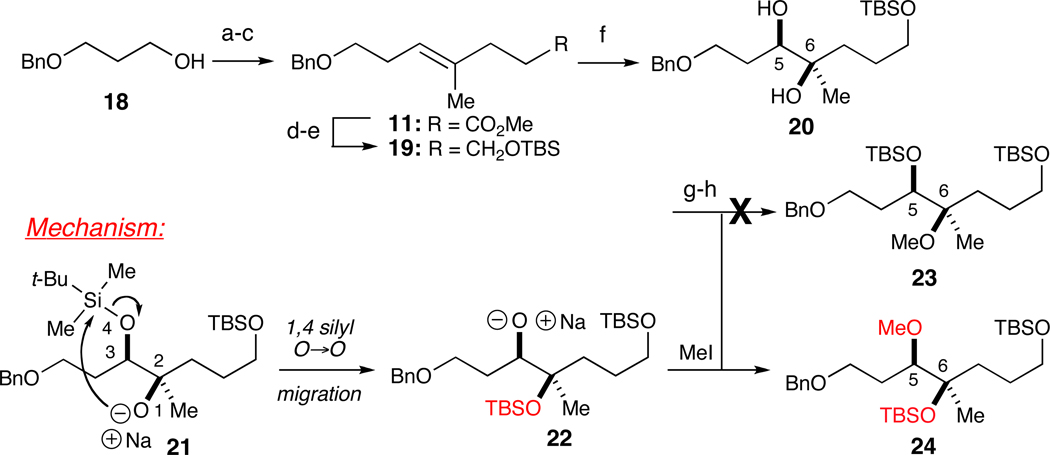
With fragments 8 and 9 in hand, attention was turned to the preparation of fragment 10 (Scheme 1). To this end, commercially available 3-benzyloxy-propanol (18) was oxidized using the Swern protocol (Scheme 3). Addition of 2-propenyl MgBr and subsequent Johnson-Claisen orthoester rearrangement afforded enoate 11 in 48% overall yield. Reduction of the ester and protection of the newly formed alcohol as its tert-butyldimethylsilyl (TBS) ether provided 19 (78% yield over two steps).19 Sharpless dihydroxylation (AD mix-β) established the requisite stereochemistry of the hydroxyls at C5 and C6 in 91% yield (er>20:1).13 Selective protection of the secondary C5 alcohol with TBSOTf and 2,6-lutidine followed by treatment with NaH and MeI resulted in the formation of 24 as opposed to the desired regioisomer 23 in 70% over two steps via a 1,4 silyl O→O migration (Scheme 3).20 This migration came to light when we succeeded in obtaining a single crystal x-ray structure of cyclized product 35 (see Scheme 5). A survey of the literature revealed this to be a common undesired pathway that attends the generation of a nucleophilic alkoxide bearing a tethered trialkylsilyl ether.21 With the undesired regioisomer in hand, we pressed forward.
Scheme 3.
Unexpected synthesis of regioisomeric fragment 24.a
a Reagents and conditions: (a) (COCl)2, DMSO, Et3N; (b) 2-propenyl MgBr, THF; (c) (MeO)3CCH3, EtCO2H, 48% over three steps; (d) LiAlH4, Et2O; (e) TBSCl, imidazole, 78% over two steps; (f) AD mix-β, 91% (er>20:1); (g) TBSOTf, 2,6-lutidine; (h) NaH, MeI, 70% over two steps.
Scheme 5.
Macrocyclization of 33 and x-ray structure of macroketolactone 35.a
a Reagents and conditions: (a) CSA, MeOH, 85%; (b) DMP, NaHCO3, 80%; (c) CrCl2, cat. NiCl2, DMSO, 50%; (d) DMP, Pyridine, 82%.
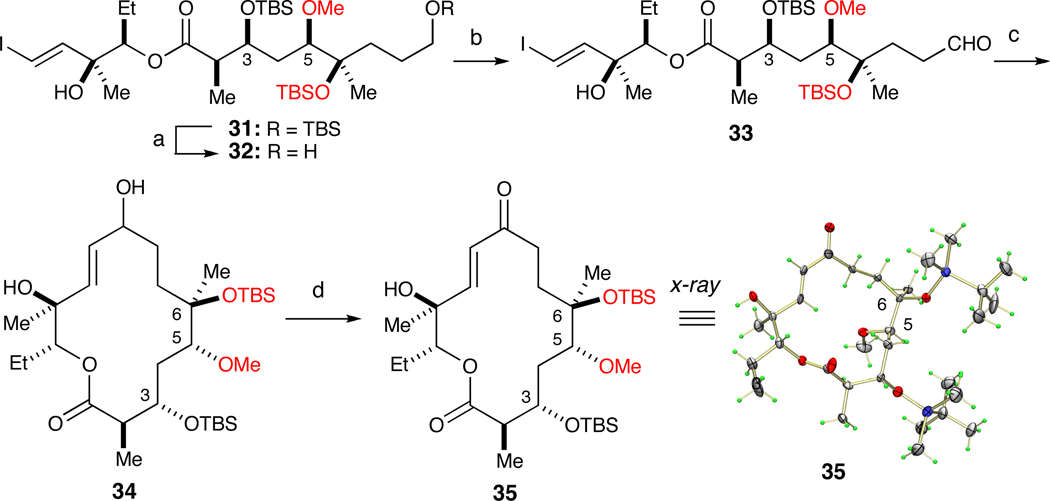
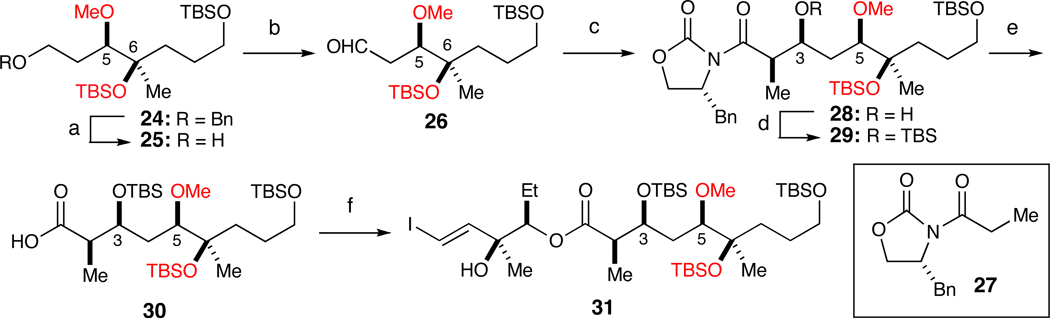
Hydrogenolysis of benzyl ether 24 afforded alcohol 25 in 80% yield (Scheme 4). Swern oxidation of 25 to aldehyde 26 was followed by the Evans aldol reaction with propionimide 27, which set the stereochemistry at C2 and C3 positions in 78% yield (dr>20:1) over two steps. Protection of the C3 hydroxyl in 28 with TBSOTf and removal of the auxiliary with LiOOH delivered acid 30 in 85% yield over two steps. Chemo-selective Yamaguchi esterification of 30 and diol 8 afforded ester 31 in 74% yield.
Scheme 4.
Elaboration of 24 into macrocyclization precursor 31.a
a Reagents and conditions: (a) H2, Pd/C, 80%; (b) (COCl)2, DMSO, Et3N; (c) 27, Bu2BOTf, Et3N, 78% (dr>20:1) over two steps; (d) TBSOTf, 2,6-lutidine; (e) LiOOH, THF, H2O, 85% over two steps. (f) Cl3PhCOCl, Et3N, DMAP, 8, 74%.
To prepare the intramolecular NHK substrate 33, the primary TBS ether of 31 was selectively removed under acidic conditions in 85% yield (Scheme 5). Oxidation of alcohol 32 with the Dess-Martin Periodinane (DMP)22 and NaHCO3 proceeded smoothly to furnish 33 in 80% yield. The key step was thus effected with by adding excess CrCl2 and catalytic NiCl2 to 33 in degassed DMSO (0.0025 M) at rt for 16 h, affording a 50% yield of 34 as a 1:1 mixture of diastereomeric allylic alcohols at C9. Subsequent oxidation of 34 with DMP and pyridine furnished macroketolactone 35, which fortunately was a solid. Recrystallization of 35 from Et2O by slow evaporation resulted in crystals suitable for x-ray analysis. To our surprise, the groups on C5 and C6 positions in regioisomeric 33 had been transposed.
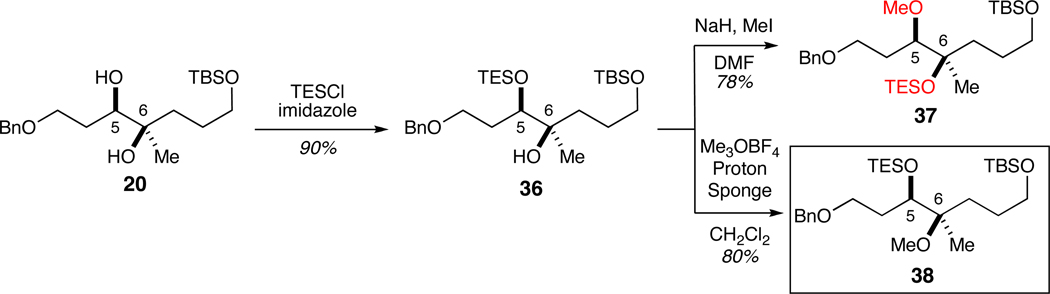
To rectify this issue, we revisited the methylation reaction responsible for the attendant transposition. We reasoned that employing a more reactive methylating agent (e.g., Meerwein’s salt) would avoid the unwanted 1,4 O→O silyl migration enabled by reactive metal alkoxide 21 (Scheme 3). In addition, we recruited the TES protecting group to establish an orthogonal set at C3 and C5 and offer recourse in the event a regioselective glycosylation was unsuccessful (Scheme 6). To prepare substrate 36, we protected the secondary C5 hydroxyl as a TES ether under standard conditions (90% yield). Methylation of 36 with Me3OBF4 (i.e., Meerwein’s salt) and Proton Sponge in CH2Cl2 afforded desired regioisomer 38.23 Moreover, subjection to rearrangement conditions (i.e., NaH and MeI) furnished the expected, undesired regioisomer 37.
Scheme 6.
Synthesis of both regioisomeric fragments 37 and 38 (desired).
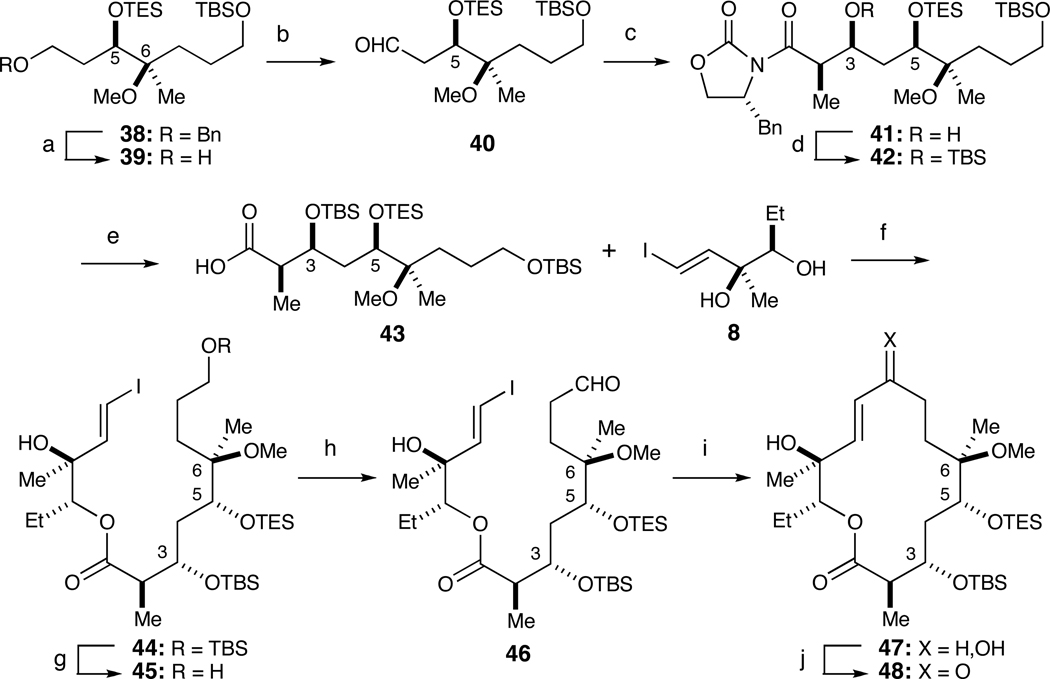
With correct regioisomer 38 in hand, we reiterated the sequence employed in Schemes 4 and 5. Thus, removal of the benzyl ether in 38 afforded alcohol 39 in 85% yield (Scheme 7). Oxidation of 39 with the Swern protocol followed by the Evans aldol reaction furnished 41 in 78% yield (dr>20:1). Protection of the C3 hydroxyl with TBSOTf and removal of the auxiliary with LiOOH resulted in acid 43 in 85% yield over two steps. Yamaguchi esterification of 43 and 8 delivered ester 44 in 75% yield. Removal of the primary TBS group with TBAF/AcOH (78% borsm) and subsequent DMP oxidation (78% yield) set the stage for the intramolecular NHK on 46. In the event, a 50% yield of 47 was obtained. Oxidation of the ~1:1 diastereomeric mixture of allylic alcohols at C9 furnished desired macroketolactone 48 in 80% yield.
Scheme 7.
Intramolecular Nozaki-Hiyama-Kishi (NHK) route to macroketolactone 48.a
a Reagents and conditions: (a) H2, Pd/C, 85%; (b) (COCl)2, DMSO, Et3N; (c) 27, Bu2BOTf, Et3N, dr>20:1, 78% over 2 steps; (d) TBSOTf, 2,6-lutidine; (e) LiOOH, THF, H2O, 85% over two steps; (f) Cl3PhCOCl, Et3N, DMAP, 75%; (g) TBAF, AcOH, 60%; (h) DMP, NaHCO3, 78%; (i) CrCl2, cat. NiCl2, DMSO, 50%; (j) DMP, 80%
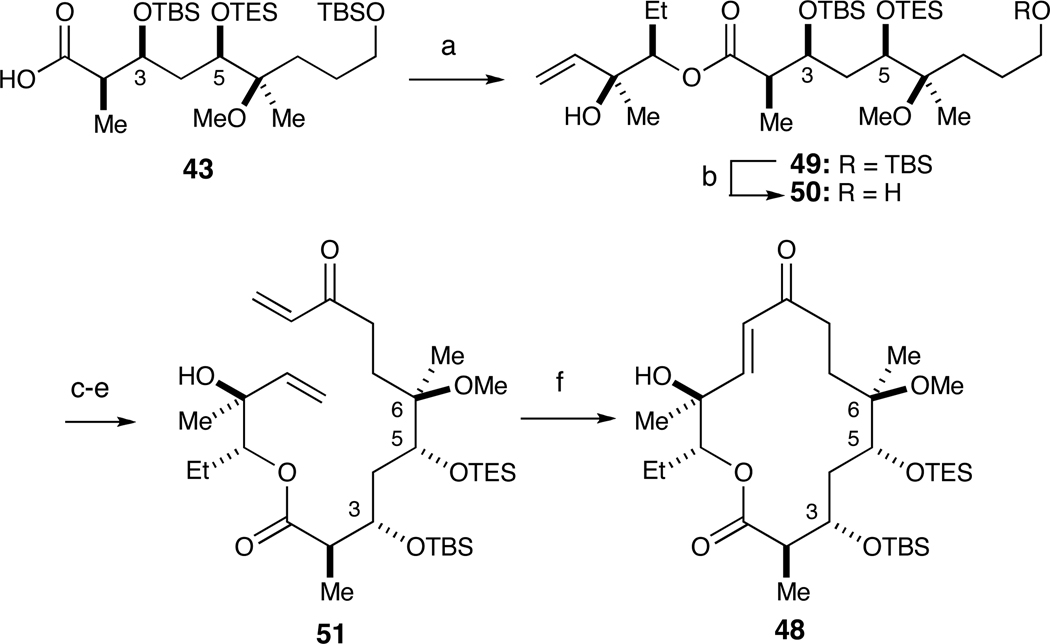
In parallel, we developed a scalable RCM route inspired by Kang’s synthesis of narbonolide (Scheme 8).24 To this end, we employed Yamaguchi’s method to couple acid 43 and diol 9 to access ester 49 in 75% yield. Selective removal of the primary TBS ether with TBAF/AcOH afforded alcohol 50 (65% yield). Oxidation of 50 with DMP, treatment with vinyl MgBr and subsequent oxidation with DMP furnished RCM substrate 51 in 60% over three steps. The RCM reaction proceeded smoothly in CH2Cl2 (0.01 M) at rt for 20 h with 20 mol% Grubbs 2nd Generation catalyst to provide 48 in 60% yield.25
Scheme 8.
Alternate ring-closing metathesis (RCM) route to macroketolactone 48.a
a Reagents and conditions: (a) Cl3PhCOCl, Et3N, DMAP, 9, 78%; (b) TBAF, AcOH, 65%; (c) Dess-Martin Periodinane, NaHCO3; (d) Vinyl MgBr, THF; (e) Dess-Martin Periodinane, CH2Cl2, 60% over three steps; (f) 20 mol% Grubbs II cat., 60%.
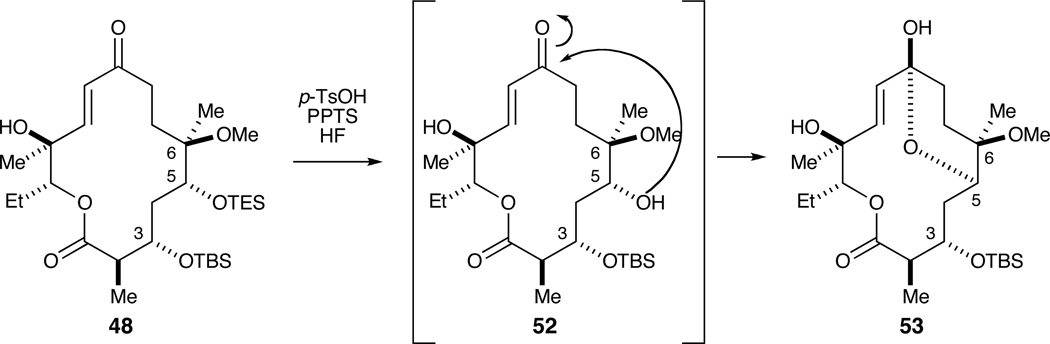
With macroketolactone 48 in hand, attention was directed at the installation of the desosamine moiety onto the C5 hydroxyl. To this end, the C5 TES ether was removed under various conditions (e.g., p-TsOH, PPTS, HF). Unfortunately, this was accompanied by ketalization at the C9 position (Scheme 9) as a consequence of the acidic conditions employed to remove the TES group
Scheme 9.
Selective removal of C5 TES group and attendant ketalization.
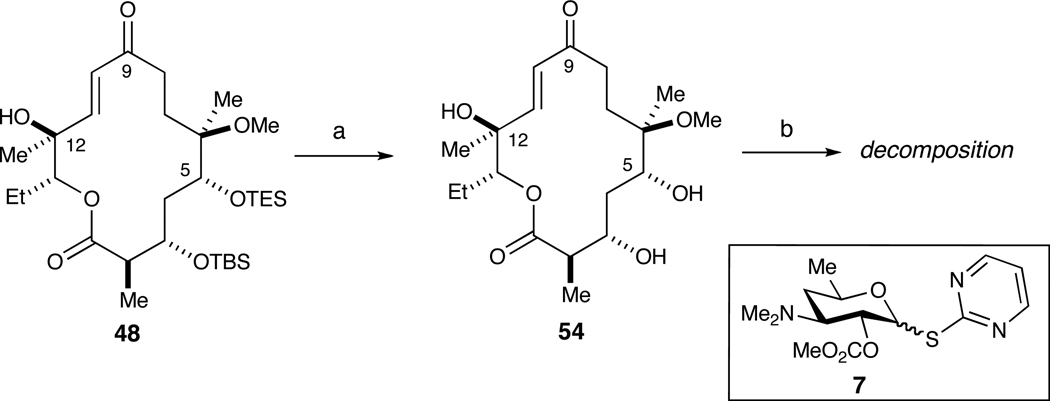
Treatment of 48 with TBAF (i.e., basic conditions) resulted in the removal of both silyl groups, affording triol 54 in 70% yield (Scheme 10). Studies by Martin26 and Toshima and Tatsuta27 both demonstrated the viability of regioselective glycosylations in closely related erythronolide systems. Attempted regioselective glycosylation of 54 at C5 in the presence of C3 with donor 7 under the agency of AgOTf and 2,6-di-t-Bu-4-Me-pyridine (DTBMP) led exclusively to the decomposition of starting material.
Scheme 10.
Unsuccessful regioselective glycosylation of diol 54 with donor 7.a
a Reagents and conditions: (a) TBAF, 4 Å MS, THF, 70%; (b) 7, AgOTf, DTBMP.
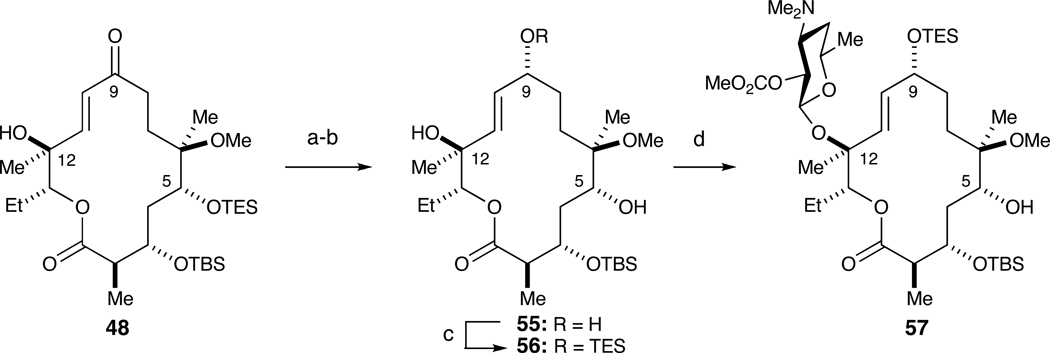
Recourse at this point was made to a more conservative strategy. Specifically, we chose to install desosamine with the C3 position blocked assuming the secondary C5 hydroxyl would be more reactive than the hindered tertiary C12 hydroxyl. To test this plan, the C9 ketone of 48 was first subjected to a Luche reduction to give an inseparable 7:1 mixture of diastereomers that was carried throughout the synthesis (Scheme 11).28 Treatment with p-TsOH in methanol selectively removed the C5 TES ether in the presence of the C3 TBS ether to afford triol 55. Regioselective protection of the more reactive allylic C9 hydroxyl with TESCl and imidazole furnished diol 56. Glycosylation of 56 with donor 7, AgOTf and DTBMP resulted in the desosamine at the C12 position, as opposed to the desired C5. This was confirmed by (1) 2D NMR experiments, particularly an HMBC correlation between the anomeric proton in desosamine with the C12 carbon and (2) removal of C9 TES and oxidation of product with DMP resulted in a triketo species (structure not shown).
Scheme 11.
Unexpected glycosylation of tertiary C12 hydroxyl in the presence of secondary C5 hydroxyl.a
a Reagents and conditions: (a) NaBH4, CeCl3•7H2O, dr=7:1; (b) p-TsOH, MeOH, 75%; (c) TESCl, imidazole; (d) 7, AgOTf, DTBMP, 42% over two steps.
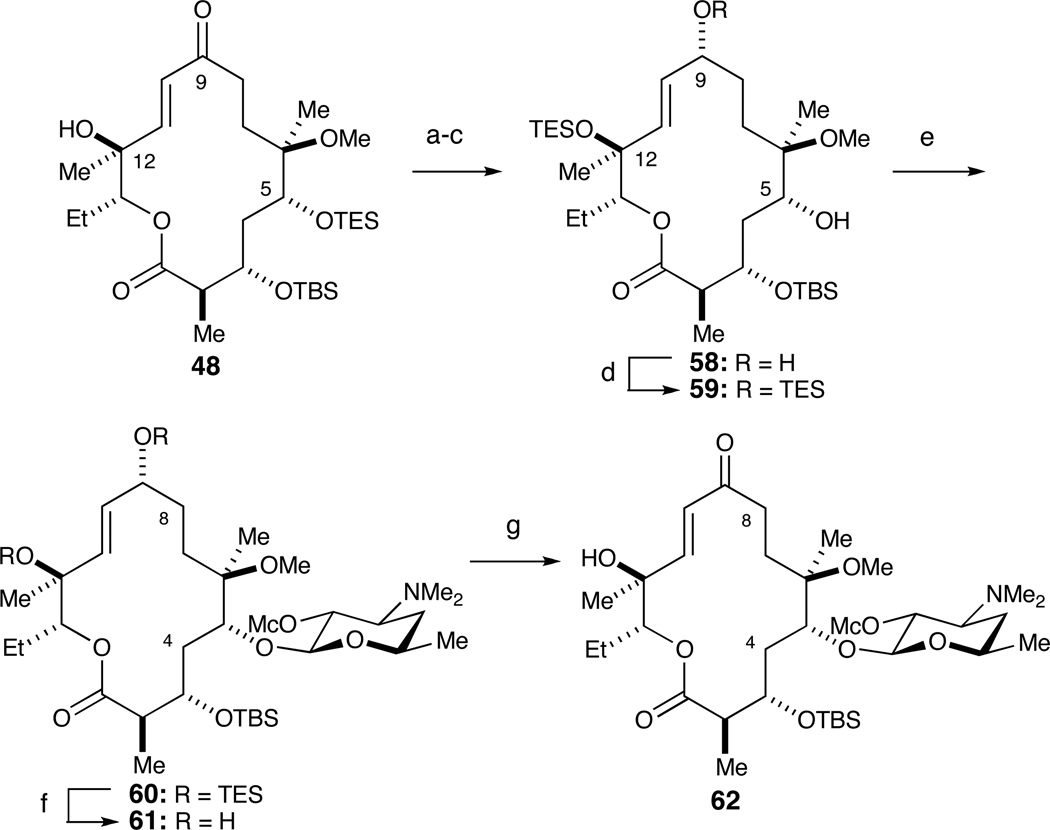
We speculate that glycosylation at the more hindered C-12 hydroxyl is due to two factors. First, the C-12 hydroxyl in 56 is allylic and therefore more nucleophilic (and less sterically demanding) than Tatsuta’s C-12 substrate, which was flanked by a 9,11-isopropylidene ketal.27 Second, conformational properties of macrolactone acceptor 56 may render C-5 more sterically shielded. The unexpected glycosylation at the more hindered position necessitated protection at this position as well (Scheme 12). Accordingly, macroketolactone 48 was first reduced under Luche conditions. Silylation of both C9 and C12 hydroxyls with TESOTf and 2,6-lutidine and subsequent treatment with p-TsOH selectively afforded 58 wherein only the tertiary C12 hydroxyl remained protected (52% yield over three steps). Regioselective silylation of the allylic C9 alcohol with TESCl furnished 59. Glycosylation at the C5 position with donor 7 was accomplished in 50% yield over two steps. Fluoride-mediated cleavage of silyl ethers at C9, C12 with TBAF followed by DMP oxidation at C9 afforded glycosylated macroketolactone 62 (84% yield over two steps). The structure of 62 was rigorously confirmed by 2D NMR experiments (see supporting information).
Scheme 12.
Synthesis of macroketolactone 62.a
a Reagents and conditions: (a) NaBH4, CeCl3•7H2O, dr=7:1; (b) TESOTf, 2,6-lutidine; (c) p-TsOH, 52% over three steps; (d) TESCl, imidazole; (e) 7, AgOTf, DTBMP, 50% over two steps; (f) 1M TBAF, THF, 95%; (g) DMP, CH2Cl2, 88%.
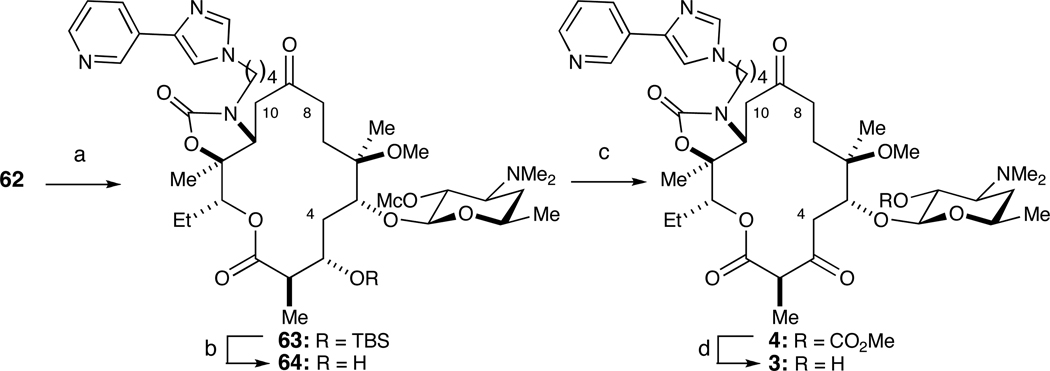
The endgame for (−)-4,8,10-tridesmethyl telithromycin (3) began with a sequence employed by HMR to prepare C11–C12 oxazolidinones, originally developed by Baker and co-workers at Abbott.6 Activation of the C12 alcohol in 62 with NaH and carbonyldiimidazole (CDI) followed by treatment with butylamine 67 effected a tandem carbamoylation/intramolecular aza-Michael sequence to stereoselectively afford oxazolidinone 63 in 35% overall yield. Removal of the C3 TBS ether with tris(dimethylamino)sulfonium difluorotrimethylsilicate (TAS-F) proceeded in 70% yield.29 Alternative fluoride-mediated methods (e.g., TBAF, HF•pyridine, HF) were all inferior. Corey-Kim oxidation furnished the C3 ketone 4.30 Finally, methanolysis removed the methyl carbonate from the C2’ position of desosamine to deliver (−)-4,8,10-tridesmethyl telithromycin (3) in 45% overall yield.
3. Conclusion
In conclusion, we have prepared (−)-4,8,10-tridesmethyl telithromycin (3), a desmethyl analogue of FDA-approved ketolide antibiotic telithromycin (2), by total synthesis. Both the intramolecular NHK and RCM reactions were used to prepare the 14-membered ring. Glycosylation using known desosamine donor 7 and Baker’s sequential one-pot carbamoylation/intramolecular aza-Michael method with amine 6 were representative key steps. Ultimately, we were able to prepare a total of 12.1 mg of analogue 3 in 42 steps (31 steps in the longest linear sequence), which was active against several wild type and resistant bacterial strains.5
4. Experimental section
(R)-5-((R)-3-(benzyloxy)-1-methoxypropyl)-2,2,3,3,5,10,10,11,11-nonamethyl-4,9-dioxa-3,10-disiladodecane (24). To a solution of diol 20 (2.5 g, 18.29 mmol) in CH2Cl2 (30 mL) at −20 °C was added 2,6-lutidine (2.10 g, 19.59 mmol) and TBSOTf (2.07 g, 7.83 mmol). After 2 h, the reaction was quenched by adding sat’d aq. NaHCO3 (20 mL). The aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic layers were washed with brine (20 mL), dried (Na2SO4) and filtered. The solvent was evaporated under reduced pressure, azeotroped with toluene (20 mL) and dried under high vacuum. The residue was dissolved in THF (130 mL), MeI (2.01 g, 32.65 mmol) was added, and the solution was cooled to 0 °C. Sodium hydride (0.78 g, 32.65 mmol) was added slowly in portions. The reaction mixture was warmed to rt and stirred for 16 h. The reaction mixture was quenched by adding MeOH (30 mL) followed by H2O (50 mL) at 0 °C. The reaction mixture was diluted with Et2O (150 mL), and the aqueous layer was extracted with Et2O (2 × 50 mL). The combined organics were washed with brine (50 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (0.4:1) to afford 2.9 g (70 %) of 24 as colorless oil. [α]23D +9.4° (c 1.8, CH2Cl2); IR (film) 2880, 1475, 1422, 1361, 1055, 733, 696 cm−1; 1H NMR (400 MHz) δ 7.27-7.17 (m, 5H), 4.45 (d, J = 11.6 Hz, 1H), 4.41 (d, J = 12.0 Hz, 1H), 3.53-3.47 (m, 4H), 3.33 (s, 3H), 3.08 (dd, J = 10.0, 2.2 Hz, 1H), 1.90-1.87 (m, 1H), 1.55-1.34 (m, 5H), 1.14 (s, 3H), 0.82 (s, 9H), 0.80 (s, 9H), 0.02 (s, 6H), −0.04 (s, 6H); 13C NMR (100 MHz) δ 138.6, 128.3 (2), 127.5 (2), 127.4, 84.9, 78.4, 72.8, 67.8, 63.7, 60.7, 35.1, 30.8, 27.0, 26.1 (3C), 26.0 (3C), 24.0, 18.4, 18.3, −1.8, −1.9, −5.3 (2); HRMS (FAB) calc’d for C28H54O4Si2+Na = 533.3458, found 533.3442.
(3R,4R)-4,7-bis((tert-butyldimethylsilyl)oxy)-3-methoxy-4-methylheptan-1-ol (25). To a solution of ether 24 (2.9 g, 5.67 mmol) in EtOH (56 mL) was added 10% Pd/C (0.6 g, 0.56 mmol) under an atmosphere of H2. The reaction mixture was followed 4–8 h (TLC control). The reaction mixture was filtered through a Celite plug, which had been previously washed with EtOAc. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (0.8:1) to afford 1.9 g (80%) of 25 as a colorless oil. [α]23D +6.0° (c 6.8, CH2Cl2); IR (film) 3306, 2953, 2928, 2885, 1471, 1462, 1253, 1098, 1055, 831, 811, 770 cm−1; 1H NMR (400 MHz) δ 3.78-3.72 (m, 2H), 3.57-3.52 (m, 2H), 3.48 (s, 3H), 3.20 (dd, J = 10.0, 2.8 Hz, 1H), 2.40 (bs, 1H), 1.82-1.76 (m, 1H), 1.65-1.39 (m, 5H), 1.21 (s, 3H), 0.87 (m, 9H), 0.84 (s, 9H), 0.95 (m, 6H), 0.02 (s, 6H); 13C NMR (100 MHz) δ 86.6, 78.6, 63.6, 61.0, 60.8, 35.4, 33.0, 26.9, 26.0 (3C), 25.9 (3C), 23.9, 18.3, 18.2, −1.8, −1.9, −5.4 (2C); HRMS (FAB) calc’d for C21H48O4Si2+Na = 443.2989, found 443.2973.
(R)-4-benzyl-3-((2R,3S,5R,6R)-6,9-bis((tert-butyldimethylsilyl)oxy)-3-hydroxy-5-methoxy-2,6-dimethylnonanoyl)oxazolidin-2-one (28). To a solution of oxalyl chloride (0.61 g, 4.86 mmol) in CH2Cl2 (30 mL) at −78 °C was added DMSO (0.79 g, 10.12 mmol) dropwise. After stirring for 10 min, alcohol 25 (1.70 g, 4.05 mmol) in CH2Cl2 (10 mL) was added via cannula, and the reaction mixture was stirred at −78 °C for 45 min. Triethylamine (1.02 g, 10.12 mmol) was added dropwise by cannula, and the reaction mixture was slowly warmed to rt. After 2 h, the reaction was quenched with dropwise addition of H2O (10 mL). The organic layer was separated and washed with brine (10 mL), dried (Na2SO4) and concentrated under reduced pressure. The crude product was dissolved in Et2O (50 mL), filtered through a plug of silica gel using ether, concentrated and dried under high vacuum. This material was used directly without further purification. To a solution of (R)-4-benzyl-3-propionyl-2-oxazolidinone (27) (0.94 g, 4.05 mmol) in CH2Cl2 (20 mL) was added dibutylborontriflate (5.30 mL of a 1.0 M solution in CH2Cl2, 5.26 mmol) and triethylamine (0.61 g, 6.07 mmol) dropwise at 0 °C. The solution was cooled to −78 °C and to this was added the aldehyde (1.70 g, 4.05 mmol) in CH2Cl2 (10 mL) at −78 °C. The resulting solution was stirred for 20 min at −78 °C. After warming the solution to 0 °C, the reaction mixture was stirred an additional hour. The reaction was terminated by adding a pH 7 aq. phosphate buffer solution (0.2 M aq. sodium hydrogen phosphate/0.1 M aq. citric acid, 82:18, 8.0 mL) and MeOH (24.2 mL). To this cloudy solution was added a solution of MeOH and 30% H2O2 (2:1, 24.2 mL), and the resulting solution was stirred for 1 h at 0 °C. The solution was concentrated and extracted with EtOAc (3 × 60 mL). The organic layer was washed with sat’d aq. NaHCO3 (50 mL), brine (50 mL), dried (Na2SO4) and filtered. The solvent was evaporated under reduced pressure and the residue purified by flash chromatography eluting with EtOAc/hexanes (2:1) to afford 1.50 g (78%) of 28 as colorless oil. [α]23D −39.2° (c 3.75, CH2Cl2); IR (film) 3500, 2953, 2885, 2856, 1781, 1696, 1382, 1359, 1252, 1208, 1195, 1097, 1051, 1004, 832, 771, 700 cm−1; 1H NMR (400 MHz) δ 7.30-7.14 (m, 5H), 4.65-4.60 (m, 1H), 4.17-4.10 (m, 2H), 4.07-4.04 (m, 1H), 3.82-3.79 (m, 2H), 3.63 (d, J = 1.2 Hz, 1H), 3.52 (t, J = 5.8 Hz, 2H), 3.46 (s, 3H), 3.25-3.20 (m, 2H), 2.71 (dd, J = 13.4, 9.4 Hz, 1H), 1.74-1.34 (m, 6H), 1.22 (d, J = 7.2 Hz, 3H), 1.19 (s, 3H), 0.83 (s, 9H), 0.80 (s, 9H), 0.05 (s, 6H), −0.01 (s, 6H); 13C NMR (100 MHz) δ 175.8, 153.1, 135.2, 129.4 (2C), 128.9 (2C), 127.3, 88.7, 78.7, 71.8, 66.0, 63.6, 60.7, 55.3, 43.0, 37.7, 36.3, 35.1, 34.2, 26.9, 26.0 (3C), 25.9 (3C), 24.1, 18.3, 11.4, −1.8, −1.8, −5.3; HRMS (FAB) calc’d for C34H61NO7Si2 +Na = 674.3884, found 674.3868.
(2R,3S,5R,6R)-3,6,9-tris((tert-butyldimethylsilyl)oxy)-5-methoxy-2,6-dimethylnonanoic acid (30). To a stirred solution of aldol 28 (1.41 g, 2.16 mmol) in CH2Cl2 (15 mL) at 0 °C was added 2,6-lutidine (0.41 g, 3.89 mmol) and TBSOTf (0.85 g, 3.24 mmol). The reaction mixture was stirred for 15 min at 0 °C and quenched with sat’d aq. NaHCO3 (10 mL). The organic layer was separated, washed with brine (10 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the crude product dissolved in THF/H2O (4:1, 31 mL). To this was added 30% aq. H2O2 (1.14 mL, 10.07 mmol) and 0.8 M aq. LiOH (4.6 mL, 3.65 mmol) at 0 °C. The reaction was warmed to rt and stirred for 16 h. The reaction was quenched by adding aq. Na2SO3 (1.33 M, 10 mL) and aq. NH4Cl solution (10 mL). The reaction mixture was then diluted with EtOAc (40 mL). The organic layer was separated and the aqueous layer was back-extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with brine (20 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure and the residue purified by flash chromatography eluting with EtOAc/hexanes (0.4/1) to yield 1.11 g (85%) of 30 as a colorless oil. [α]23D +5.4° (c 2.5, CH2Cl2); IR (film) 3538, 2953, 2928, 2886, 2856, 1709, 1471, 1462, 1252, 1097, 1053, 1003, 832, 803 cm−1; 1H NMR (400 MHz) δ 4.35-4.32 (m, 1H), 3.60-3.52 (m, 2H), 3.50 (s, 3H), 2.98 (d, J = 9.6 Hz, 1H), 2.69-2.64 (m, 1H), 1.82-1.76 (m, 1H), 1.67-1.40 (m, 5H), 1.21 (s, 3H), 1.14 (d, J = 7.2 Hz, 3H), 0.88 (s, 9H), 0.87 (s, 18H), 0.10 (s, 3H), 0.09 (s, 3H), 0.07 (s, 3H), 0.03 (s, 9H); 13C NMR (100 MHz) δ 180.1, 84.7, 78.8, 70.7, 63.5, 60.5, 43.4, 35.8, 35.8, 27.1, 26.1 (3C), 25.9 (3C), 25.8 (3C), 24.1, 18.3, 17.9, 9.5, −1.7, −1.8, −2.0, −4.2, −5.1, −5.3 (2C); HRMS (FAB) calc’d for C30H66O6Si3+Na = 629.4065, found 629.4047.
(2R,3S,5R,6R)-(3R,4S,E)-4-hydroxy-6-iodo-4-methylhex-5-en-3-yl 3,6,9-tris((tert-butyldimethylsilyl)oxy)-5-methoxy-2,6-dimethylnonanoate (31). To a solution of acid 30 (0.22 g, 0.36 mmol) in THF (4 mL) at rt was added Et3N (0.04 g, 0.38 mmol) and 2,4,6-trichlorobenzoyl chloride (0.01 g, 0.40 mmol). The reaction mixture was stirred for 3 h at rt, and the solids were filtered and washed with hexanes (10 mL). The combined filtrates were concentrated under reduced pressure, dried under vacuum, and dissolved in toluene (5 mL). To this solution was added iododiol 8 (0.11 g, 0.43 mmol) in toluene (2 mL) and DMAP (0.06 g, 0.49 mmol). After being stirred for 16 h at rt, the reaction mixture was diluted with EtOAc (20 mL), washed with sat’d aq. NaHCO3 (10 mL), dried (Na2SO4) and filtered. The solvent was evaporated under reduced pressure and the residue purified by flash chromatography eluting with EtOAc/hexanes (0.4/1) to afford 0.24 g (74%) of 31 as a colorless oil. [α]23D +11.2° (c 1.2, CH2Cl2); IR (film) 3445, 2951, 2936, 2909, 2877, 2857, 1729, 1461, 1251, 1192, 1095, 1004, 950, 836, 775 cm−1; 1H NMR (400 MHz) δ 6.68 (d, J = 14.6 Hz, 1H), 6.43 (d, J = 14.6, 1H), 4.81 (dd, J = 10.4, 2.8 Hz, 1H), 4.14-4.10 (m, 1H), 3.58-3.50 (m, 2H), 3.50 (s, 3H), 3.25 (dd, J = 9.6, 3.2 Hz, 1H), 3.18 (bs, 1H), 2.74-2.61 (m,1H), 1.68-1.35 (m, 8H), 1.25 (s, 3H), 1.21 (d, J = 4.8 Hz, 3H), 1.17 (s, 3H), 0.90 (s, 9H), 0.89-0.86 (m, 21H), 0.11 (s, 6H), 0.07 (s, 3H), 0.06 (s, 3H), 0.02 (s, 6H); 13C NMR (100 MHz) δ 175.8, 148.0, 83.9, 80.2, 79.3, 77.7, 71.2, 63.4, 60.7, 44.3, 36.5, 35.2, 26.7, 26.2 (3C), 26.1, 25.9 (6C), 24.8, 23.5, 23.2, 18.4, 18.3, 18.0, 14.4, 10.7, −1.6, −1.7, −3.8, −4.5, −5.4 (2C); HRMS (FAB) calc’d for C37H77IO7Si3+Na = 867.3920, found 867.3934.
(2R,3S,5R,6R)-(3R,4S,E)-4-hydroxy-6-iodo-4-methylhex-5-en-3-yl 3,6-bis((tert-butyldimethylsilyl)oxy)-9-hydroxy-5-methoxy-2,6-dimethylnonanoate (32). To a solution of alcohol 31 (0.27 g, 0.32 mmol) in MeOH (7 mL) was added CSA (0.015 g, 0.065 mmol) at 0 °C. After stirring for 1 h, the reaction was quenched with NaHCO3 (0.040 g). The mixture was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (1/5) to afford 0.20 g (85%) of 32 as a colorless oil. [α]23D +14.5° (c 0.9, CH2Cl2); IR (film) 3458, 2952, 2936, 2908, 2877, 1724, 1461, 1251, 1194, 1094, 1045, 1005, 836, 775, 735 cm−1; 1H NMR (400 MHz) δ 6.67 (d, J = 11.6 Hz, 1H), 6.42 (d, J = 11.6, 1H), 4.80 (dd, J = 8.4, 2.0 Hz, 1H), 4.80 (dt, J = 5.6, 2.0 Hz, 1H), 3.59 (t, J = 4.4 Hz, 2H), 3.47 (s, 3H), 3.32-3.27 (m, 2H), 2.74-2.70 (m, 1H), 1.75-1.40 (m, 8H), 1.24 (s, 3H), 1.21 (d, J = 4.8 Hz, 3H), 1.21 (s, 3H), 0.90 (s, 9H), 0.88-0.85 (m, 12H), 0.11 (s, 3H), 0.10 (s, 3H), 0.08 (s,3H), 0.06 (s, 3H); 13C NMR (100 MHz) δ 175.8, 147.8, 84.3, 80.2, 78.9, 77.7, 77.4, 77.3, 63.3, 60.4, 44.6, 35.8, 34.9, 29.6, 26.2 (3C), 25.9 (3C), 24.5, 24.1, 23.2, 18.4, 18.0, 15.0, 10.6, −1.6, −1.7, −3.9, −4.5; HRMS (FAB) calc’d for C31H63IO7Si2+Na = 753.3055, found 753.3053.
(2R,3S,5R,6R)-(3R,4S,E)-4-hydroxy-6-iodo-4-methylhex-5-en-3-yl 3,6-bis((tert-butyldimethylsilyl)oxy)-5-methoxy-2,6-dimethyl-9-oxononanoate (33). Dess-Martin periodinane (0.53 g, 0.31 mmol) and NaHCO3 (0.13 g, 1.57 mmol) were suspended in CH2Cl2 (3 mL). Alcohol 32 (0.23 g, 0.31 mmol) in CH2Cl2 (3 mL) was added dropwise via cannula into the reaction mixture. After 1 h at rt, the reaction mixture was added to a mixture of sat’d aq. NaHCO3 (5 mL), sat’d aq. Na2SO3 (5 mL) and H2O (10 mL). The mixture was extracted with Et2O (2 × 20 mL). The combined organic layers were washed with brine (10 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (0.4/1) to afford 0.18 g (80%) of 33 as a foam. [α]23D +14.9° (c 0.75, CH2Cl2); IR (film) 2962, 2886, 2853, 1736, 1695, 1461, 1401, 1250, 1190, 1094, 1050 cm−1; 1H NMR (400 MHz) δ 9.8 (s, 1H), 6.67 (d, J = 14.4 Hz, 1H), 6.43 (d, J = 14.4 Hz, 1H), 4.81 (dd, J = 10.4, 2.8 Hz, 1H), 4.11 (dt, J = 6.8, 3.2 Hz, 1H), 3.47 (s, 3H), 3.21 (d, J = 9.6 Hz, 2.4, 1H), 3.11 (bs, 1H), 1.85-1.50 (m, 8H), 1.25 (s, 3H), 1.21 (d, J = 7.2 Hz, 3H), 1.21 (s, 3H), 0.90 (s, 9H), 0.89-0.85 (m, 12H), 0.12 (s, 3H), 0.11 (s, 3H), 0.08 (s, 3H), 0.04 (s, 3H); 13C NMR (100 MHz) δ 202.1, 175.7, 148.0, 84.1, 80.2, 78.4, 77.7, 71.1, 60.7, 44.5, 38.7, 35.2, 31.4, 26.1 (3C), 25.9 (3C), 24.6, 23.5, 23.1, 18.4, 18.0, 14.6, 10.7, −1.6, −1.8, −3.9, −4.6; HRMS (FAB) calc’d for C31H61IO7Si2+Na = 751.2898, found 751.2892.
(3R,4S,6R,7R,13S,14R,E)-4,7-bis((tert-butyldimethylsilyl)oxy)-14-ethyl-10,13-dihydroxy-6-methoxy-3,7,13-trimethyloxacyclotetradec-11-en-2-one (34). To a solution of aldehyde 33 (0.20 g, 0.27 mmol) in DMSO (125 mL) at rt was added CrCl2 (0.33g, 2.75 mmol) and NiCl2 (0.004 mg, 0.027 mmol). The reaction was stirred for 16 h and quenched by the addition of H2O (60 mL). The mixture was diluted with EtOAc (500 mL), and the layers were separated. The organic layer was washed with H2O (3 × 50 mL). The combined aqueous layers were back-extracted with EtOAc (3 × 200 mL). The combined organic layers were washed with brine (200 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (1/2) to afford 0.06 g (dr = 1:1 at C9) (50%) of 34 as a foam. IR (film) 3465, 2953, 2909, 1731, 1461, 1251, 1095, 1252, 1118, 1093, 1067 cm−1; 1H NMR (400 MHz) δ 6.85 (d, J = 16.4 Hz, 1H), 6.19 (d, J = 16.4 Hz, 1H), 4.83 (dd, J = 10.8, 1.2 Hz, 1H), 4.33 (t, J = 8.4 Hz, 1H), 3.76 (dd, J = 9.6, 3.2 Hz, 1H), 3.23 (dt, J = 12.8, 3.5 Hz, 1H), 3.12 (s, 3H), 2.83 (bs, 1H), 2.46-2.38 (m, 1H), 1.99-1.90 (m, 2H), 1.81-1.52 (m, 5H), 1.33 (s, 3H), 1.19 (d, J = 8.0 Hz, 3H), 1.18 (s, 3H), 0.94 (t, J = 8.2 Hz, 9H), 0.89 (s, 9H), 0.86 (t, J = 7.2 Hz, 3H), 0.60 (q, J = 7.8 Hz, 6H), 0.13 (s, 3H), 0.04 (s, 3H); 13C NMR (100 MHz) δ 204.1, 175.7, 151.8, 127.7, 80.1, 78.9, 74.1, 73.2, 71.2, 49.0, 48.3, 44.7, 34.6, 31.1, 26.2, 21.9, 21.0, 19.7, 18.4, 16.1, 10.3, 6.7, 5.1, −3.0, −4.8; HRMS (FAB) calc’d for C31H62O7Si2+Na = 625.3932, found 625.3925
(3R,4S,6R,7R,13S,14R,E)-4,7-bis((tert-butyldimethylsilyl)oxy)-14-ethyl-13-hydroxy-6-methoxy-3,7,13-trimethyloxacyclotetradec-11-ene-2,10-dione (35). Dess-Martin periodinane (0.07 g, 0.15 mmol) was added to alcohol 34 (0.03 g, 0.05 mmol) in CH2Cl2 (5 mL) and pyridine (0.15 mL). After 1 h at rt, the reaction mixture was added to a mixture of sat’d aq. NaHCO3 (5 mL), sat’d aq. Na2SO3 (5 mL) and H2O (10 mL). The mixture was extracted with Et2O (2 × 20 mL). The combined organic layers were washed with brine (10 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure and the residue was purified by flash chromatography eluting with EtOAc/hexanes (1.2/1) to afford 0.024 g (82%) of 35 as a white solid (mp = 192–195 °C); [α]23D +6.7° (c 2.7, CH2Cl2); IR (film) 3443, 2956, 2930, 2893, 2883, 2857, 1733, 1658, 1461, 1262, 1126, 1095, 1066, 1053, 1001, 835 cm−1; 1H NMR (400 MHz) δ 7.08 (d, J = 16.4 Hz, 1H), 6.14 (d, J = 16.4 Hz, 1H), 4.77 (dd, J = 10.0, 2.8 Hz, 1H), 4.05 (dd, J = 10.0, 6.8 Hz, 1H), 3.46 (s, 3H), 3.40-3.31 (m, 2H), 3.02-2.95 (m, 1H), 2.52 (bs, 1H), 2.09-1.52 (m, 10H), 1.34 (s, 3H), 1.30 (s, 3H), 1.25 (d, J = 6.8 Hz, 3H), 0.95-0.89 (m, 12H), 0.80 (s, 3H), 0.07 (s, 3H), 0.07 (m, 6H), 0.05 (s, 3H); 13C NMR (100 MHz) δ 203.7, 176.2, 152.5, 127.5, 86.6, 77.3, 76.6, 73.7, 71.7, 59.8, 45.4, 44.7, 34.8, 33.7, 32.7, 26.0 (6C), 25.5, 22.4, 21.5, 18.1, 17.4, 10.6, −1.7, −1.8, −3.9, −4.6; HRMS (FAB) calc’d for C31H60O7Si2+Na = 623.3775 found 623.3736.
(5R,6R)-5-(2-(benzyloxy)ethyl)-3,3-diethyl-6,11,11,12,12-pentamethyl-4,10-dioxa-3,11-disilatridecan-6-ol (36). To a solution of diol 20 (1.0 g, 2.61 mmol) in DMF (26 mL) at 0 °C was added imidazole (0.25 g, 3.65 mmol) and TESCl (0.47 g, 3.13 mmol). The reaction mixture was warmed to rt. After 1.5 h, the reaction was quenched by with H2O (40 mL). The aqueous layer was extracted with ether (4 × 50 mL). The combined organic layers were washed with H2O (50 mL) brine (50 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (1/5) to afford 1.16 g (90%) of 36 as a colorless oil. [α]23D +7.7° (c 8.3, CH2Cl2); IR (film) 3443, 2956, 2930, 2893, 2883, 2857, 1733, 1658, 1461, 1262, 1126, 1095, 1066, 1053, 1001, 835 cm−1; 1H NMR (400 MHz) δ 7.29-7.18 (m, 5H), 4.43 (s, 2H), 3.61 (dd, J = 7.2, 3.6 Hz, 1H), 3.54 (t, J = 6.4 Hz, 2H), 3.50-3.47 (m, 1H), 2.47(s, 1H), 1.88-1.80 (m, 1H), 1.65-1.36 (m, 5H), 1.02(s, 3H), 0.88 (t, J = 6.4 Hz, 9H), 0.82 (s, 9H), 0.55 (q, J = 7.6 Hz, 6H), −0.02 (s, 6H); 13C NMR (100 MHz) δ 138.3, 128.3 (2), 127.6 (2), 127.5, 76.0, 74.1, 72.9, 67.3, 63.8, 34.9, 33.1, 26.8, 25.9 (3C), 21.8, 18.2, 6.9 (3C), 5.2 (3C), −5.3 (2C); HRMS (FAB) calc’d for C27H52O4Si2+Na = 519.3302 found 519.3286.
(R)-8-((R)-3-(benzyloxy)-1-methoxypropyl)-10,10-diethyl-2,2,3,3,8-pentamethyl-4,9-dioxa-3,10-disiladodecane (37). To a solution of 36 (0.50 g, 1.00 mmol) in THF (10 mL) was added MeI (2.01 g, 32.65 mmol), and the reaction mixture was cooled to 0 °C. Sodium hydride (0.78 g, 32.65 mmol) was then added in small portions. The reaction mixture was allowed to warm to rt and stirred for 16 h. The reaction mixture was quenched by adding MeOH (3 mL) followed by H2O (5 mL) at 0 °C. The reaction mixture was diluted with Et2O (15 mL), and the aqueous layer was back-extracted with Et2O (2 × 5 mL). The combined organic layers were washed with brine (5 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (0.4:1) to afford 0.4 g (78 %) of 37 as a colorless oil. [α]23D +8.4° (c 3.8, CH2Cl2); IR (film) 2880, 1475, 1422, 1361, 1055, 733, 696 cm−1; 1H NMR (400 MHz) δ 7.35-7.26 (m, 5H), 4.54 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 12.0 Hz, 1H), 3.61-3.55 (m, 4H), 3.42 (s, 3H), 3.17 (dd, J = 10.4, 2.4 Hz, 1H), 1.93-1.89 (m, 1H), 1.65-1.39 (m, 5H), 1.20 (s, 3H), 0.95 (t, J = 8 Hz, 9H), 0.89 (s, 9H), 0.59 (q, J = 8 Hz, 6H), 0.04 (s, 6H); 13C NMR (100 MHz) δ 138.6, 128.3 (2C), 127.6 (2C), 127.4, 84.6, 78.2, 72.8, 67.8, 63.7, 60.8, 35.4, 30.8, 27.0, 26.0 (3C), 24.2, 18.3, 7.2 (3C), 6.9 (3C), −5.3 (2C); HRMS (FAB) calc’d for C28H54O4Si2+Na = 533.3458, found 533.3455.
(8R,9R)-9-(2-(benzyloxy)ethyl)-11,11-diethyl-8-methoxy-2,2,3,3,8-pentamethyl-4,10-dioxa-3,11-disilatridecane (38). To a solution of 36 (9.08 g, 18.29 mmol) in CH2Cl2 (90 mL) at 0 °C was added Proton Sponge (11.74 g, 54.87 mmol) and Me3OBF4 (8.11 g, 54.87 mmol). The reaction mixture was warmed to rt and stirred for 16 h. The reaction mixture was concentrated and quenched with sat’d aq. NH4Cl (50 mL). The reaction mixture was diluted with Et2O (100 mL), and the aqueous layer was back-extracted with Et2O (2 × 50 mL). The combined organic layers were washed with 1N aq. HCl solution (60 mL), sat’d aq. NaHCO3 (80 mL), H2O (80 mL), brine (50 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (1/25) to afford 7.4 g (80 %) of 38 as an oil. [α]23D = +11.5° (c 2.6,CH2Cl2); IR (film) 1255, 1361, 1471, 2857, 2953, 3398, 1096, 899, 733, 696 cm−1; 1H NMR (400 MHz) δ 7.27-7.20 (m, 5H), 4.45 (d, J = 12.0 Hz, 1H), 4.41 (d, J = 12.0 Hz, 1H), 3.74 (dd, J = 9.8, 2.2 Hz, 2H), 3.54-3.48 (m, 4H), 3.07 (s, 3H), 1.84-1.76 (m, 1H), 1.60-1.28 (m, 4H), 0.98 (s, 3H), 0.88-0.84 (m, 9H), 0.83 (s, 9H), 0.55-0.49 (m, 6H), −0.02 (s, 6H); 13C NMR (100 MHz) δ 138.6, 128.3 (2C), 127.6 (2C), 127.4, 78.8, 73.7, 72.9, 67.9, 63.5, 48.7, 32.7, 29.9, 26.0, 25.9 (3C), 18.2, 18.0, 7.1 (3C), 5.3 (3C), −5.3 (2C); HRMS (FAB) calc’d for C28H54O4Si2+Na = 533.3458, found 533.3445.
(2R,3S,5R,6R)-(3R,4S,E)-4-hydroxy-6-iodo-4-methylhex-5-en-3-yl 3,9-bis((tert-butyldimethylsilyl)oxy)-6-methoxy-2,6-dimethyl-5-((triethylsilyl)oxy)nonanoate (44). To a solution of acid 43 (0.60 g, 0.98 mmol) in THF (10 mL) at rt were added Et3N (0.1 g, 1.03 mmol) and 2,4,6-trichlorobenzoyl chloride (0.26 g, 1.08 mmol). The reaction mixture was stirred for 3 h at rt, and the solids were filtered and washed with hexanes (20 mL). The combined filtrates were concentrated under reduced pressure, dried under vacuum, and dissolved in toluene (15 mL). To this solution was added diol 8 (0.26 g, 0.98 mmol) in toluene (5 mL) and DMAP (0.16 g, 1.33 mmol). After stirring for 16 h at rt, the reaction mixture was diluted with EtOAc (50 mL), washed with sat’d aq. NaHCO3 (20 mL), dried (Na2SO4) and filtered. The solvent was evaporated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (0.4/1) to afford 0.68 g (75%) of 44 as a colorless oil. [α]23D +27.4° (c 0.7, CH2Cl2); IR (film) 3460, 2951, 2933, 2878, 1732, 1471, 1462, 1381, 1254, 1199, 1095, 1073, 1044, 1005, 949, 834, 774, 736 cm−1; 1H NMR (400 MHz) δ 6.54 (d, J = 14.4 Hz, 1H), 6.41 (d, J = 14.4, 1H), 4.78 (dd, J = 9.6, 3.2 Hz, 1H), 4.48 (dt, J = 9.6, 3.2 Hz, 1H), 3.60-3.55 (m, 3H), 3.10 (s, 3H), 2.67-2.62 (m, 1H), 2.33 (bs, 1H), 1.79-1.34 (m, 8H), 1.23 (s, 3H), 1.17 (d, J = 6.8 Hz, 3H), 1.02 (s, 3H), 0.94 (t, J = 8 Hz, 9H), 0.88-0.86 (m, 12H), 0.84 (s, 9H), 0.66-0.55 (m, 6H), 0.06 (s, 3H), 0.03 (s, 6H); 13C NMR (100 MHz) δ 175.0, 147.6, 80.0, 79.0, 77.6, 77.2, 73.6, 69.6, 63.2, 48.3, 42.7, 37.3, 29.4, 26.0, 25.9 (6C), 25.2, 22.6, 18.2, 18.0, 17.4, 10.6, 9.6, 7.2 (3C), 5.4 (3C), −3.8, −4.6, −5.4 (2C); HRMS (FAB) calc’d for C37H77IO7Si3+Na = 867.3920 found 867.3900.
(2R,3S,5R,6R)-(3R,4S,E)-4-hydroxy-6-iodo-4-methylhex-5-en-3-yl 3-((tert-butyldimethylsilyl)oxy)-9-hydroxy-6-methoxy-2,6-dimethyl-5-((triethylsilyl)oxy)nonanoate (45). To a solution of TBAF•3H2O (0.90 g, 2.86 mmol) in DMF (28 mL) was added AcOH (0.70 g, 2.01 mmol). After stirring for 30 min, the solution was added to 44 (0.68 g, 0.95 mmol), and the reaction mixture was stirred for 20 h at rt. The reaction was quenched with H2O (20 mL), and the aqueous layer was back-extracted with Et2O (4 × 60 mL). The combined organic layers were washed with sat’d aq. NaHCO3 (50 mL), H2O (50 mL), brine (50 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (1/5) to afford 0.35 g (60%) of 45 as a colorless oil. [α]23D +30.8° (c 0.6, CH2Cl2); IR (film) 3458, 2951, 2936, 2909, 2877, 1729, 1461, 1251, 1192, 1095, 1069, 1044, 1004, 950, 940, 836, 775 cm−1; 1H NMR (400 MHz) δ 6.55 (d, J = 14.4 Hz, 1H), 6.41 (d, J = 14.4, 1H), 4.77 (dd, J = 10.0, 3.2 Hz, 1H), 4.45 (dt, J = 9.6, 3.2 Hz, 1H), 3.66 (dd, J = 10.4, 2.0 Hz, 1H), 3.62-3.58 (m, 2H), 3.14 (s, 3H), 2.67 (dq, J = 4.8, 2.4 Hz, 1H), 2.55 (bs, 1H), 2.03 (m, 1H), 1.82-1.42 (m, 8H), 1.23 (s, 3H), 1.18 (d, J = 7.2 Hz, 3H), 1.05 (s, 3H), 0.94 (t, J = 8.0 Hz, 9H), 0.86-0.84 (m, 12H), 0.66-0.55 (m, 6H), 0.77 (s, 3H), 0.05 (s, 3H); 13C NMR (100 MHz) δ 175.0, 147.6, 80.1, 79.3, 77.5, 77.3,73.5, 69.8, 63.2, 48.6, 43.4, 37.7, 30.2, 26.2, 25.9 (3C), 24.9, 22.7, 18.1, 17.4, 10.6, 10.3, 7.1 (3C), 5.4 (3C), −3.9, −4.7; HRMS (FAB) calc’d for C31H63IO7Si2+Na = 753.3055 found 753.3032.
(2R,3S,5R,6R)-(3R,4S,E)-4-hydroxy-6-iodo-4-methylhex-5-en-3-yl 3-((tert-butyldimethylsilyl)oxy)-6-methoxy-2,6-dimethyl-9-oxo-5-((triethylsilyl)oxy)nonanoate (46). Dess-Martin periodinane (0.23 g, 0.54 mmol) and NaHCO3 (0.19 g, 2.25 mmol) were suspended in CH2Cl2 (3 mL). Alcohol 45 (0.33 g, 0.45 mmol) in CH2Cl2 (4 mL) was added dropwise via cannula into the reaction mixture. After 1 h at rt, the reaction mixture was added to a mixture of sat’d aq. NaHCO3 (5 mL), sat’d aq. Na2SO3 (5 mL) and H2O (10 mL). The mixture was extracted with Et2O (2 × 20 mL). The combined organic layers were washed with brine (10 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (1/5) to afford 0.28 g (78% yield) of 46 as a colorless oil. [α]23D +32.8° (c 0.7, CH2Cl2); IR (film) 2952, 2879, 2877, 1733, 1684, 1463, 1411, 1379, 1250, 1190, 1094, 1050 cm−1; 1H NMR (400 MHz) δ 9.76 (m, 1H), 6.55 (d, J = 14.4 Hz, 1H), 6.41 (d, J = 14.4 Hz, 1H), 4.78 (dd, J = 10.0, 3.2 Hz, 1H), 4.44 (dt, J = 8.8, 3.6 Hz, 1H), 3.59 (dd, J = 9.6, 2.4 Hz, 1H), 3.10 (s, 3H), 2.67 (dq, J = 4.8, 2.4 Hz, 1H), 2.53-2.35 (m, 3H), 1.99-1.91 (m, 1H), 1.77-1.47 (m, 6H), 1.24 (s, 3H), 1.17 (d, J = 7.2 Hz, 3H), 1.08 (s, 3H), 0.95 (t, J = 8.4 Hz, 9H), 0.87-0.85 (m, 12H), 0.66-0.55 (m, 6H), 0.07 (s, 3H), 0.05 (s, 3H); 13C NMR (100 MHz) δ 202.1, 174.8, 147.6, 80.1, 78.6, 77.5, 77.2, 73.4, 69.8, 48.8, 43.3, 38.0, 37.6, 25.8 (3C), 25.6, 24.9, 22.7, 18.1, 17.7, 10.6, 10.2, 7.1 (3C), 5.4 (3C), −3.9, −4.6; HRMS (FAB) calc’d for C31H61IO7Si2+Na = 751.2898 found 751.2892.
(3R,4S,6R,7R,13S,14R,E)-4-((tert-butyldimethylsilyl)oxy)-14-ethyl-10,13-dihydroxy-7-methoxy-3,7,13-trimethyl-6-((triethylsilyl)oxy)oxacyclotetradec-11-en-2-one (47). To a solution of aldehyde 46 (0.28 g, 0.27 mmol) in DMSO (158 mL) at rt was added CrCl2 (0.47g, 3.84 mmol) and NiCl2 (0.005 mg, 0.038 mmol). The reaction mixture was stirred for 16 h then quenched by the addition of H2O (90 mL). The mixture was diluted with EtOAc (600 mL), and the layers were separated. The organic layer was washed with H2O (3 × 100 mL). The combined aqueous layers were back-extracted with EtOAc (3 × 200 mL). The combined organic layers were washed with brine (200 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (2/5) to afford 0.12 g (dr = 1:1 at C9) (50%) of 47 as a foam. IR (film) 3443, 2953, 2936, 2909, 2878, 1731, 1461, 1251, 1095, 1057, 1005, 972, 854, 831, 815, 774 cm−1; 1H NMR (400 MHz) δ 5.75-5.60 (m, 2H), 4.81 (td, J = 10.6, 2.0 Hz, 1H), 4.30-4.12 (m, 1H), 4.07-3.98 (m, 1H), 3.14 (s, 3H), 2.69-2.46 (m, 2H), 2.19-1.73 (m, 2H), 1.53-133 (m, 4H), 1.29-1.26 (m, 3H), 1.21-1.16 (m, 3H), 1.12-1.03 (m, 3H), 0.97-0.89 (m, 12H), 0.87-0.84 (s, 9H), 0.64-0.53 (m, 6H), 0.10-0.01 (m, 6H); 13C NMR (100 MHz) δ 175.7, 175.3, 136.2, 133.7, 133.2, 132.4, 80.3, 79.6, 79.4, 79.3, 74.4, 73.9, 73.7, 73.4, 72.9, 72.6, 70.5, 70.3, 48.7, 48.3, 42.5, 40.4, 30.6, 30.5, 29.6, 27.5, 26.1, 25.8, 23.3, 22.1, 20.6, 19.1, 18.3, 18.0, 17.4, 15.5, 15.0, 10.6, 10.6, 7.1, 7.0, 5.3, 5.1, −3.4, −4.1, −4.8, −5.0; HRMS (FAB) calc’d for C31H62O7Si2+Na = 625.3932, found 625.3911.
(1R,3S,4R,7R,8S,14R,E)-3-((tert-butyldimethylsilyl)oxy)-7-ethyl-8,11-dihydroxy-14-methoxy-4,8,14-trimethyl-6,15-dioxabicyclo[9.3.1]pentadec-9-en-5-one (53). To a solution of 48 (0.030 g, 0.05 mmol) in MeOH (7 mL) was added p-TsOH (2 mg, 0.01 mmol) at 0 °C. After 3 h at this temperature, the reaction was quenched by adding sat’d aq. NaHCO3 (5 mL). The reaction mixture was diluted with EtOAc (20 mL) and the aqueous layer was back-extracted with EtOAc (2 × 5 mL). The combined organic layers were washed with brine (10 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure and purified directly by flash chromatography eluting with hexanes/EtOAc (4:1) to afford 12 mg (52%) of 53 as a foam. [α]23D +10.7° (c 0.8, CH2Cl2); IR (film) 3441, 2954, 2934, 2882, 1729, 1461, 1374, 1264, 1178, 1145, 1072, 1006, 961, 834, 775, 702 cm−1; 1H NMR (400 MHz) δ 6.07 (d, J = 16.4 Hz, 1H), 5.95 (d, J = 16.4 Hz, 1H), 4.85 (t, J = 3.6 Hz, 1H), 4.54 (dd, J = 11.2, 2.4 Hz, 1H), 4.29 (d, J = 9.6 Hz, 1H), 3.94-3.90 (m, 1H), 3.21 (s, 3H), 2.90-2.82 (m, 1H), 2.06-1.95 (m, 3H), 1.85-1.71 (m, 2H), 1.57-1.46 (m, 2H), 2.59 (bs, 2H), 1.30 (s, 3H), 1.27 (d, J = 6.8 Hz, 3H), 1.27 (s, 3H), 0.88 (t, J = 7.2 Hz, 3H), 0.90 (s, 9H), 0.08 (s, 3H), 0.05 (s, 3H); 13C NMR (100 MHz) δ 175.4, 146.1, 136.3, 122.8, 102.9, 83.2, 74.7, 73.6, 71.7, 70.9, 49.1, 44.4, 31.3, 29.3, 25.9 (3C), 21.6, 21.2, 18.0, 16.4, 10.5, −4.1, −4.7; HRMS (FAB) calc’d for C25H46O7Si+K = 525.2650, found 525.2667.
(3R,4S,6R,7R,13S,14R,E)-14-ethyl-4,6,13-trihydroxy-7-methoxy-3,7,13-trimethyloxacyclotetradec-11-ene-2,10-dione (54). To a solution of 48 (34 mg, 0.05 mmol) in THF (2.2 mL) was added TBAF•3H2O (54 mg, 0.17mmol) at 0 °C and allowed to slowly warm to 10 °C and stirred for 6h. The reaction mixture was quenched by slowly adding sat’d aq. NaHCO3 (3 mL). The mixture was diluted with EtOAc (5 mL), and the aqueous layer was extracted with EtOAc (2 × 5 mL). The combined organic layers were washed with brine (10 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was purified by flash chromatography eluting with EtOAc/hexanes (3:2) to afford 13 mg of 54 (70%) as a foam. [α]23D +3.5° (c 0.9, CH2Cl2); IR (film) 3441, 2956, 2878, 1727, 1462, 1372, 1265, 1178, 1093 cm−1; 1H NMR (400 MHz) δ 6.74 (d, J = 16.0 Hz, 1H), 6.19 (d, J = 16.0 Hz, 1H), 4.87 (dd, J = 10.8, 2.4 Hz, 1H), 4.00 (t, J = 8.8 Hz, 1H), 3.86 (dd, J = 10.8, 2.4 Hz, 1H), 3.72 (bs, 1H), 3.20 (s, 3H), 2.88 (bs, 1H), 2.65-2.53 (m, 3H), 2.31-2.23 (m, 1H), 12.95-1.84 (m, 2H), 1.74-1.46 (m, 5H), 1.33 (s, 3H), 1.30 (d, J = 6.8 Hz, 3H), 1.01 (s, 3H), 0.91 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz) δ 202.2, 175.9, 150.4, 128.3, 79.9, 78.7, 73.8, 73.2, 48.9, 46.8, 37.1, 33.5, 30.6, 22.0, 21.8, 20.8, 16.2, 14.1, 10.4; HRMS (FAB) calc’d for C19H32O7+Na = 395.2046 found 395.2024.
(3R,4S,6R,7R,10R,13S,14R,E)-4-((tert-butyldimethylsilyl)oxy)-14-ethyl-6,10,13-trihydroxy-7-methoxy-3,7,13-trimethyloxacyclotetradec-11-en-2-one (55). To a solution of 48 (0.075 g, 0.12 mmol) in MeOH (3 mL) at rt was added CeCl3•7H2O (53 mg, 0.14 mmol). The reaction mixture was cooled to −60 °C after which NaBH4 (5.3 mg, 0.14 mmol) was added. After 30 min at this temperature, the reaction was quenched by adding sat’d aq. NH4Cl (2 mL). Ethyl acetate (10 mL) was added, and the aqueous layer was back-extracted with EtOAc (2 × 5 mL). The combined organic layers were washed with 1N HCl solution (5 mL), sat’d aq. NaHCO3 (5 mL), H2O (5 mL), brine (5 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, and the residue was dried under vacuum. The residue was dissolved in MeOH (17 mL) and cooled to 0 °C after which p-TsOH (4.5 mg, 0.024 mmol) was added. The reaction mixture was stirred at 0 °C for 3 h after which the reaction was quenched by adding NaHCO3 (10 mg). The mixture was concentrated and purified directly by flash chromatography eluting with EtOAc/hexanes (2/5) to afford 44 mg of 55 (75%) as a foam. (dr = 7:1 dr at C9 by 1H NMR). Major 9-(R) isomer: IR (film) 3441, 2956, 2878, 1727, 1462, 1372, 1265, 1178, 1093 cm−1; 1H NMR (400 MHz) δ 5.90-5.84 (m, 1H), 5.71 (m, 1H), 4.87-4.84 (m, 1H), 4.13-4.04 (m, 2H), 3.75-3.73 (m, 1H), 3.20 (s, 3H), 2.80-2.70 (m, 2H), 2.52 (bs, 1H), 2.13 (bs, 1H), 2.02-1.96 (m, 1H), 1.83-1.42 (m, 5H), 1.30 (s, 3H), 1.22 (m, 1H), 1.26 (s, 3H), 1.22 (d, J = 6.4 Hz, 3H), 1.07 (s, 3H), 0.93 (t, J = 7.2 Hz, 3H), 0.87 (s, 9H), 0.10 (s, 6H); 13C NMR (100 MHz) δ 175.4, 133.7, 133.2, 81.5, 78.6, 73.9, 72.6, 72.1, 71.7, 49.4, 48.0, 37.2, 31.0, 29.6, 27.6, 25.8 (3C), 24.5, 23.5, 17.5, 15.1, 10.8, −4.6, −4.7; HRMS (FAB) calc’d for C25H48O7Si+Na = 511.3067, found 511.3042.
(2S,3R,4R,6S)-2-(((2R,3S,6R,9R,10R,12S,13R,E)-12-((tert-butyldimethylsilyl)oxy)-2-ethyl-10-hydroxy-9-methoxy-3,9,13-trimethyl-14-oxo-6-((triethylsilyl)oxy)oxacyclotetradec-4-en-3-yl)oxy)-4-(dimethylamino)-6-methyltetrahydro-2H-pyran-3-yl methyl carbonate (57). To a solution of 55 (70 mg, 0.14 mmol) in DMF (1.5 mL) and CH2Cl2 (1.5 mL) at rt were added imidazole (12 mg, 0.16 mmol) and DMAP (2 mg). The reaction mixture was cooled to −78 °C, and TESCl (25 mg, 0.15 mmol) was added. After stirring for 3 h at −78 °C the reaction was quenched by adding sat’d aq. NH4Cl (3 mL). The mixture was diluted with EtOAc (10 mL), and the aqueous layer was back-extracted with EtOAc (2 × 5 mL). The combined organic layers were washed with H2O (5 mL), dried (Na2SO4) and filtered. The solvent was concentrated under reduced pressure, azeotroped with toluene (5 mL), and the crude alcohol was taken to the next step. To a suspension of freshly activated 4 Å molecular sieves (1.55 g) and AgOTf (0.72 g, 2.82 mmol) in CH2Cl2/toluene (5 mL, 1:1) was added dropwise by cannula a mixture of C5 alcohol (0.14 mmol), desosamine donor 7 (0.28 g, 0.85 mmol) and 2,6-di-tert-butyl-4-methylpyridine (0.18 g, 0.85 mmol) in CH2Cl2 (2.5 mL) at 0 °C. The reaction flask was wrapped with aluminum foil, warmed to rt and stirred for an additional 20 h. The reaction was quenched with Et3N (4.0 mL), filtered through Celite, and eluted with EtOAc (50 mL). The filtrate was washed with sat’d aq. NaHCO3 (20 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography eluting with hexanes/EtOAc (3:2) to afford 42% of 57 as a foam (dr = 7:1 dr at C9 by 1H NMR). Major 9-(R) isomer: IR (film) 3441, 2956, 2878, 1727, 1462, 1372, 1265, 1178, 1093 cm−1; 1H NMR (400 MHz) δ 5.80 (m, 1H), 5.48 (d, J = 16.4 Hz, 1H), 5.15 (dd, J = 10.0, 1.6 Hz, 1H), 4.55 (dd, J = 10.4, 7.6 Hz, 1H), 4.48 (d, J = 7.6 Hz, 1H), 3.96-3.93 (m, 2H), 3.77 (s, 3H), 3.72-3.68 (m, 1H), 3.47-3.43(m, 1H), 3.18 (s, 3H), 2.75-2.56 (m, 2H), 2.27 (m, 9H), 2.33-2.30 (m, 1H), 1.26 (s, 3H), 1.21 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.0 Hz, 3H), 1.04 (s, 3H), 0.95 (t, J = 8.0 Hz, 9H), 0.88 (s, 9H), 0.85 (t, J = 11.4 Hz, 3H), 0.62-0.52 (m, 6H), 0.12 (s, 3H), 0.10 (s, 3H); 13C NMR (100 MHz) δ 174.3, 153.3, 135.8, 131.2, 96.6, 79.7, 78.7, 77.8, 75.1, 73.9, 73.6, 73.0, 68.8, 63.0, 54.6, 49.1, 48.6, 40.6 (2C), 37.7, 31.7, 30.8, 29.6, 28.1, 25.8(3C), 22.9, 21.2, 20.6, 18.0, 15.6, 10.6, 6.9 (3C), 5.0 (3C), −4.2, −4.4; HRMS (FAB) calc’d for C41H79NO11Si2+Na = 840.5089, found 840.5081.
Supplementary Material
Scheme 13.
Endgame for (−)-4,8,10-tridesmethyl telithromycin (3).a
a Reagents and conditions: (a) NaH, CDI, THF/DMF then 6, 35%; (b) TASF, DMF/H2O, 70%; (c) NCS, DMS, Et3N; (d) MeOH, 45% over two steps.
Acknowledgements
We thank Dr. Richard Pederson (Materia, Inc.) for catalyst support. Finally, this work was supported by the NIH (AI080968).
Footnotes
Supporting Information Available
General experimental procedures and NMR spectra (1H and 13C) for 24–25, 28, 30–38, 44–47, 53–55, 57. Crystallographic details of 35. Complete structural assignments (2D NMR analysis) of 62. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Doern GV, Heilmann KP, Huynh HK, Rhomberg PR, Coffman SL, Brueggemann AB. Antimicrob. Agents Chemother. 2001;45:1721. doi: 10.1128/AAC.45.6.1721-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Fox JL. Nat. Biotechnol. 2006;24:1521. doi: 10.1038/nbt1206-1521. [DOI] [PubMed] [Google Scholar]; (b) Walsh CT. Nat. Rev. Microbiol. 2003;1:65. doi: 10.1038/nrmicro727. [DOI] [PubMed] [Google Scholar]
- 3.Tu D, Blaha G, Moore PB, Steitz TA. Cell. 2005;121:257. doi: 10.1016/j.cell.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Ma XD, Ma ST. Curr. Med. Chem. 2011;18:1993. doi: 10.2174/092986711795590075. [DOI] [PubMed] [Google Scholar]
- 5.Velvadapu V, Paul T, Wagh B, Klepacki D, Guvench O, MacKerell A, Andrade RB. ACS Med. Chem. Lett. 2010;2:68. doi: 10.1021/ml1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker WR, Clark JD, Stephens RL, Kim KH. J. Org. Chem. 1988;53:2340. [Google Scholar]
- 7.Denis A, Agouridas C, Auger J-M, Benedeti Y, Bonnefoy A, Bretin F, Chantot F, Dussarat A, Fromentin C, D’Ambrieres SG, Lachaud S, Laurin P, Le Martret O, Loyau V, Tessot N, Pejac J-M, Perron S. Bioorg. Med. Chem. Lett. 1999;9:3075. doi: 10.1016/s0960-894x(99)00534-x. [DOI] [PubMed] [Google Scholar]
- 8.(a) Woodward RB, Logusch E, Nambiar KP, Sakan K, Ward DE, Auyeung BW, Balaram P, Browne LJ, Card PJ, Chen CH, Chenevert RB, Fliri A, Frobel K, Gais HJ, Garratt DG, Hayakawa K, Heggie W, Hesson DP, Hoppe D, Hoppe I, Hyatt JA, Ikeda D, Jacobi PA, Kim KS, Kobuke Y, Kojima K, Krowicki K, Lee VJ, Leutert T, Malchenko S, Martens J, Mathews RS, Ong BS, Press JB, RajanBabu TV, Rousseau G, Sauter HM, Suzuki M, Tatsuta K, Tolbert LM, Truesdale EA, Uchida I, Ueda Y, Uyehara T, Vasella AT, Vladuchick WC, Wade PA, Williams RM, Wong HNC. J. Am. Chem. Soc. 1981;103:3215. [Google Scholar]; (b) Velvadapu V, Andrade RB. Carb. Res. 2008;343:145. doi: 10.1016/j.carres.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Venkatraman L, Salomon CE, Sherman DE, Fecik RA. J. Org. Chem. 2006;71:9853. doi: 10.1021/jo062047u. [DOI] [PubMed] [Google Scholar]
- 10.Xuan R, Oh H-S, Lee Y, Kang H-Y. J. Org. Chem. 2008;73:1456. doi: 10.1021/jo702384d. [DOI] [PubMed] [Google Scholar]
- 11.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989. [Google Scholar]
- 12.Evans DA, Bartroli J, Shih TL. J. Am. Chem. Soc. 1981;103:2127. [Google Scholar]
- 13.Kolb HC, Van Nieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483. [Google Scholar]
- 14.Sharpless KB, Hanson RM. J. Org. Chem. 1986;51:1922. [Google Scholar]
- 15.Martin SF, Lee W-C, Pacofsky GJ, Gist RP, Mulhern TA. J. Am. Chem. Soc. 1994;116:4674. [Google Scholar]
- 16.Oh HS, Xuan R, Kang H-Y. Org. Biomol. Chem. 2009;7:4458. doi: 10.1039/b911200f. [DOI] [PubMed] [Google Scholar]
- 17.Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;13:3769. [Google Scholar]
- 18.Yadav JS, Pratap TV, Rajender V. J. Org. Chem. 2007;72:5882. doi: 10.1021/jo0704762. [DOI] [PubMed] [Google Scholar]
- 19.Johnson WS, Werthemann L, Bartlett WR, Brocksom TJ, Li T-T, Faulkner DJ, Petersen MR. J. Am. Chem. Soc. 1970;92:741. [Google Scholar]
- 20.Rucker C. Chem. Rev. 1995;95:1009. [Google Scholar]
- 21.Hillier MC, Meyers AI. Tetrahedron Lett. 2001;42:5145–5147. and references cited therein. [Google Scholar]
- 22.Dess DB, Martin JC. J. Org. Chem. 1983;48:4155. [Google Scholar]
- 23.2D NMR experiments were used to confirm this structure.
- 24.Xuan R, Oh H-S, Lee Y, Kang H-Y. J. Org. Chem. 2008;73:1456–1461. doi: 10.1021/jo702384d. [DOI] [PubMed] [Google Scholar]
- 25.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 26.Hergenrother PJ, Hodgson A, Judd AS, Lee W-C, Martin SF. Angew. Chem. Int. Ed. 2003;42:3278. doi: 10.1002/anie.200351136. [DOI] [PubMed] [Google Scholar]
- 27.Toshima K, Nozaki Y, Mukaiyama S, Tamai T, Nakata M, Tatsuta K, Kinoshita M. J. Am. Chem. Soc. 1995;117:3717. [Google Scholar]
- 28.The absolute stereochemistry of the C9 carbinol was not rigorously established as it is ultimately inconsequential vis-à-vis 3. We assigned the C9 configuration based on studies of stereoselective hydride reductions of ketones Dauben WG, Fonken GH, Noyce DS. J. Am. Chem. Soc. 1956;78:2579.
- 29.Scheidt KA, Chen H, Follows BC, Chemler SR, Coffey DS, Roush WR. J. Org. Chem. 1998;63:6436. [Google Scholar]
- 30.Corey EJ, Kim CU. J. Am. Chem. Soc. 1972;94:7586. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.