Abstract
Objectives
The aims of this pilot study were to assess (1) the safety and tolerability of etanercept in dermatomyositis (DM), (2) the feasibility and safety of a forced prednisone taper, and (3) outcome measures, including those recommended by the International Myositis Assessment Clinical Study Group (IMACS).
Methods
We conducted a randomized, double-blind, placebo-controlled trial of etanercept (50 mg SQ weekly) for 52 weeks in DM subjects. Subjects were tapered off prednisone in a standardized schedule as tolerated over the initial 24 weeks of the study. Principal outcomes included adverse events, time from randomization to treatment failure (inability to wean off prednisone on schedule), and average prednisone dosage after Week 24.
Results
Sixteen subjects were randomized, 11 to etanercept and 5 to placebo. There were no significant differences in adverse event rates between the treatment groups, although 5 etanercept-treated and 1 placebo-treated subjects developed worsening rash. All 5 subjects receiving placebo were treatment failures (median time to treatment failure 148 days). In contrast, 5/11 subjects in the etanercept arm were successfully weaned off prednisone; the median time to treatment failure in this group was 358 days (p = 0.0002). The median of the average prednisone dosage after Week 24 was 29.2 mg/day in the placebo group and 1.2 mg/day in the etanercept group (p = 0.02). IMACS and other outcome measures demonstrated excellent test-retest reliability (intraclass correlation coefficients 0.79 - 0.99). There was no significant treatment effect on functional outcome.
Interpretation
The observation of no major safety concerns and a steroid-sparing effect in our study suggest that further investigation of etanercept as a treatment for DM is warranted.
Dermatomyositis (DM) is a subtype of inflammatory myopathy; prednisone is the initial treatment of choice [1]. Because many patients have disabling weakness despite treatment and the long-term side effects of prednisone and of second-line immunosuppressive agents better treatment options are needed. TNF-α may play a role in the pathogenesis of DM [2-8]. Etanercept is a soluble TNF-α receptor fusion protein that inactivates TNF-α and is effective in rheumatoid arthritis (RA) [9], ankylosing spondylitis [10], and psoriatic arthritis [11]. Small uncontrolled series of DM patients treated with various TNF-α blockers have had mixed results; some suggested a possible benefit [12-17], while others found no improvement or worsening of the myositis [18-21]. Side effects of etanercept include infection, malignancies, and induction of systemic lupus erythematosus (SLE), the risks of which might be increased in patients with DM [22-26].
An obstacle in designing therapeutic studies is the need to establish reliable and responsive outcome measures. IMACS proposed a core set of measures for disease outcome assessment and preliminary definitions of improvement (DOI) to be used in clinical trials in myositis [27-29].
The objectives of this pilot study were: (1) obtain preliminary data regarding the safety and tolerability of etanercept in DM; (2) assess the feasibility and safety of a forced prednisone withdrawal study design; and (3) evaluate different outcome measures.
METHODS
Study Design
This was a randomized, double-blind, placebo-controlled trial of etanercept (50 mg SQ weekly) in subjects with DM. The study was approved by the Ethics Committees at each site.
Subjects
Eligible subjects were 18 to 65 years old and had active DM defined by (a) symmetric proximal weakness, (b) characteristic rash, and (c) laboratory evidence of active DM with an elevated serum creatine kinase (CK), electromyography demonstrating myopathic features, abnormal skeletal muscle MRI, or a muscle biopsy demonstrating perifascicular atrophy and perivascular inflammation. Exclusion criteria included juvenile DM, uncontrolled diabetes mellitus, symptomatic cardiovascular or pulmonary disease, SLE, cancer, tuberculosis, active infection, chronic hepatitis B or C, other autoimmune neurological disorders, any prior or concurrent cyclophosphamide therapy or concurrent use of azathioprine or mycophenolate, or recent use of a live vaccine.
We initially planned to enroll 40 newly diagnosed DM subjects who were either treatment naïve or treated only with prednisone for less than two months. Due to slow recruitment, the protocol was modified in the last year to allow enrollment of refractory subjects on prednisone for greater than two months, a stable dosage of methotrexate for at least 1 month or intravenous immunoglobulin for at least 3 months. Recruitment began in March 2006 and ended in May 2009 after enrollment of the 16th subject.
Screening
The screening period could be up to 2 months in order to perform the necessary studies prior to initiating study drug. The following studies were be obtained: complete blood count, basic metabolic profile, CK, aldolase, thyroid function tests, 25-hydroxyvitamin D levels, urinalysis, antinuclear antibodies (ANAs), anti-double stranded DNA, SSA, SSB, U1RNP, PM-Scl, Scl-70, and Jo-1 antibodies (see Supplemental Methods), tuberculosis skin test (PPD), electrocardiogram (EKG), pulmonary function tests (PFTs), and dual energy x-ray absorptiometry (DEXA) for bone density. A work-up for underlying cancer was done, if not performed already within the past 6 months; this included a chest X-ray, mammogram, abdominal CT scan, pelvic CT scan or ultrasound in women, and a colonoscopy in patients over the age of 50 years.
During screening, newly diagnosed or treatment naïve subjects (new DM) were placed on prednisone 60 mg/day. If they were already on prednisone for less than 2 months or on a different dosage, their prednisone dosage was adjusted to 60 mg/day. Subjects with refractory DM, who were on prednisone for more than 2 months, stayed on their current dosage or had the dosage increased to up to 60 mg/day at the discretion of the treating physician. All subjects were treated with vitamin D 800 IU daily and calcium 1500 mg daily. Unless contraindicated, alendronate was started.
Randomization and Treatment Protocol
After treatment with prednisone for two months and completion of screening evaluations, subjects underwent baseline evaluation over two consecutive days in order to ascertain test-retest reliability of outcome measures. Afterwards, subjects were randomly assigned (3:1 allocation; stratified by DM status: new vs. refractory) to receive either etanercept 50 mg or placebo once weekly by subcutaneous injection (see Supplementary Methods). The dosage formulation for the placebo pre-filled liquid syringes consisted of 25 mM Na phosphate, 25 mM L-arginine-HCI, 100 mM NaCI, 1% Sucrose per syringe, pH 6.3.
After starting the study drug, the prednisone dosage was tapered over the next 24 weeks in newly diagnosed DM subjects by 5 mg/day every 2 weeks (e.g., dosage reduced to 55 mg/day starting Week 3, 50 mg/day starting Week 5 etc.). Refractory DM subjects remained on their baseline dosage of prednisone in keeping with the taper schedule for new DM subjects. For example, if they were on prednisone 60 mg daily or higher, they would take prednisone 60 mg/day for 2 weeks before initiating taper. If refractory DM subjects were on prednisone 40 mg/day, they would remain on this dosage for 10 weeks before initiating taper. Thus, by Week 25 all subjects (new DM and refractory DM) would be off of prednisone if they were able to tolerate the prednisone taper. Subjects were seen every 4 weeks to obtain study drug and assess clinical response, adverse events, and compliance (counting syringes and review of medication diaries).
Outcome Measures
The principal outcome measures were adverse events, time from randomization to treatment failure, and average prednisone dosage after Week 24. Adverse events and compliance with study medication and prednisone dosing were ascertained at each visit. Muscle strength was assessed by manual muscle testing (MMT) and quantitative myometry utilizing maximum voluntary isometric contraction testing (MVICT) [30,31]. MMT was performed on 26 muscle groups: neck flexors and extensors, bilateral shoulder abductors, elbow flexors and extensors, wrist flexors and extensors, hip flexors, extensors, and abductors, knee flexors and extensors, and ankle dorsiflexors and plantar flexors. Muscle strength of each muscle group was graded utilizing a modified MRC score that was converted to a 13-point scale: (Grade 0 = 0, 1=1, 2− = 1.67, 2 = 2, 2+ = 2.33, 3− = 2.67, 3 = 3, 3+ = 3.33, 4− = 3.67, 4 = 4, 4+ = 4.37, 5− = 4.67, 5 = 5) and the cumulative and average MMT scores were calculated. MVICT was performed on 5 muscle groups bilaterally: shoulder abductors, elbow flexors and extensors, knee flexors and extensors). Composite MVICT scores were derived by averaging the standardized (normalized) scores across the individual muscles. Other outcome assessments included measurements of disease activity by the Myositis Intention to Treat Activity Index (MITAX) and Myositis Disease Activity Assessment Visual Analogue Scales (MYOACT) (see Supplementary Methods), subject and physician assessments of global disease activity utilizing Likert and visual analog scales (VAS), objective functional testing (time to arise from a chair and time to walk 30 feet), and subjective functional abilities utilizing the Health Assessment Questionnaire (HAQ) [27,28]. Dermatological manifestations were graded using a modified cutaneous disease activity score index (CDASI) [32], patient VAS for pruritis, and relevant components of the MYOACT and MITAX. Quality of life was assessed utilizing the SF-36 health status survey and the Individualized Neuromuscular Quality of Life (INQoL) questionnaire [33].
We assessed these outcome measures at baseline, one day apart, in order to assess their intra-rater reliability. Laboratory assessment included measurements of muscle enzymes, antinuclear antibodies, complete blood counts, complete metabolic profile, DEXA, EKG, and PFTs. We also evaluated IMACS-recommended DOI [29].
Treatment failure was defined as one of the following: (1) worsening of the Physician Global Disease Activity Assessment by ≥ 2cm on a VAS; (2) worsening of the manual muscle testing composite score by ≥ 20%; (3) progression of weakness of orophayrngeal muscles sufficient to impair nutrition or pose a risk of aspiration; (4) 20% worsening of forced vital capacity or diffusion capacity; or (5) no improvement in muscle strength after 12 weeks. The study physicians also had latitude to slow the taper or increase the dosage of prednisone or add an alternative agent if they deemed it necessary at any point during the study even if subjects did not meet one of the above criteria.
In treatment failures, the prednisone dosage was doubled (up to 60 mg/d). If the subject was not on prednisone at the time, the subject was placed on a dosage that previously provided optimal control of the disease in the judgment of the treating physician. The subject remained on this dosage until improvement was demonstrated and then the taper was resumed but at a slower rate (every 4 weeks). If the subject had no improvement, the subject could be started on a second-line agent (e.g., IVIG, methotrexate) and prednisone dosage adjusted per the treating physician.
Statistical Analysis
Descriptive statistics were used to summarize subject disposition, adverse events and abnormal laboratory values by treatment group. Kaplan-Meier curves were used to describe the distribution of the time from randomization to treatment failure by treatment group, and a log-rank test was used to compare these distributions between the groups. The distributions of prednisone dosage after Week 24 were compared between the treatment groups using a Wilcoxon rank-sum test. Repeated measures analysis of covariance models were used to compare the treatment groups over time with respect to mean responses in continuous outcome variables. The models included treatment group, Week (categorical), the interaction between treatment group and Week, and the baseline value of the outcome variable as a covariate. A heterogeneous autoregressive (AR(1)) structure was assumed for the covariance matrix of the repeated measurements [34]. The model parameters were estimated using restricted maximum likelihood and a “missing at random” assumption was used for the missing data mechanism. All analyses were performed according to the intention-to-treat principle and included data from all randomized subjects.
Intra-rater reliability of the outcomes measured was assessed using intra-class correlation coefficients (ICCs), calculated using one-way random effects analysis of variance models. Ninety-five percent lower confidence bounds were computed for the ICCs. Responsiveness of outcome variables was quantified at the Week 24 and Week 52 visits using the effect size (mean change divided by the baseline standard deviation) (35) and the standardized response mean (mean change divided by the standard deviation of the change) [36]. No imputation was performed for missing data in the computation of these responsiveness statistics.
RESULTS
Characteristics of Subjects
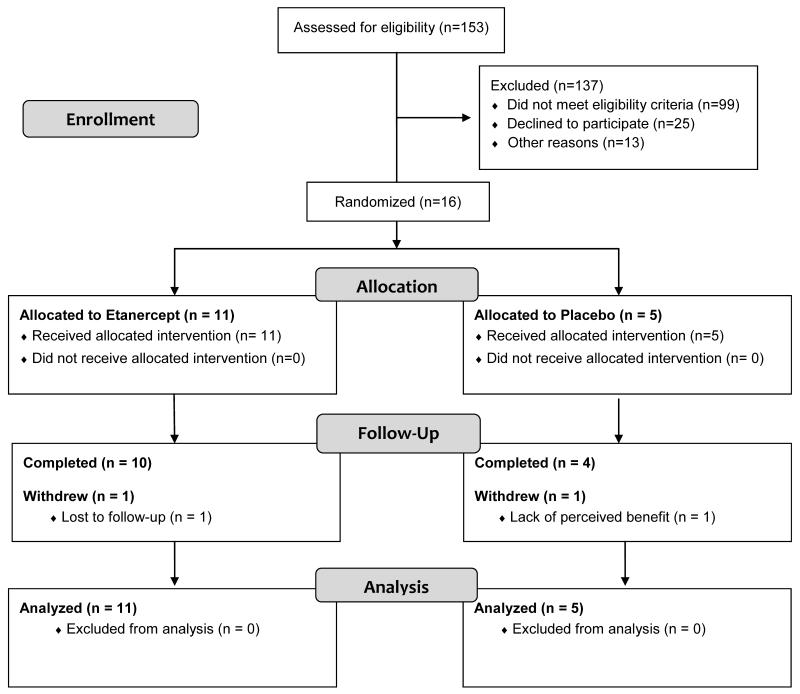
We pre-screened 153 patients and randomized 16 subjects (10 women and 6 men) (Faigure 1). Eleven subjects were randomized to receive etanercept (new 3, refractory 8) and 5 subjects to placebo (new 2, refractory 3). Subjects ranged in age from 21 to 61 years (mean 43.64 years). Mean baseline prednisone dosage was 45 mg/d in the etanercept group and 39 mg/d in the placebo group. Other key features were similar between the two groups at baseline (Table 1 and Supplementary Table 1).
Figure 1.
Participant flow is shown. Sixteen subjects were randomized; 5 were new and 11 were refractory DM patients. Most of the pre-screen failures were patients with well-controlled DM (8 patients) or refractory patients on prednisone or other second line agents prior to our modifying the protocol (71 patients). The main reason eligible patients did not participate was that they or their referring physicians wanted them to first try other treatments.
Table 1.
Baseline characteristics
| Etanercept (n = 11) |
Placebo (n = 5) |
|
|---|---|---|
| Women/Men (n) | 6/5 | 4/1 |
| New/Refractory DM (n) |
3/8 | 2/3 |
| Age (years) | 43.4 +/− 14.8 | 44.2 +/− 11.1 |
| Duration (years) | 1.1 +/− 0.8 | 2.2 +/− 3.4 |
| Baseline dosage of Prednisone (mg/day) |
45.0 +/− 18.0 | 39.0 +/− 16.7 |
| Physician Global Activity Assessment |
4.0 +/− 1.8 | 4.2 +/− 2.3 |
| Patient Global Activity Assessment |
5.4 +/− 2.3 | 6.1 +/− 2.2 |
| Average MMT Score |
4.5 +/−0.5 | 4.3 +/− 0.5 |
| Average standardized MVICT score |
−4.2+/− 2.1 | −4.5 +/− 1.6 |
| Average Percent of Predicted Normal MVICT score |
50.7 +/− 19.5 | 45.1 +/− 12.5 |
| MYOACT (total) | 0.13 +/− 0.07 | 0.11 +/− 0.03 |
| MYOACT- Muscle Disease Activity |
3.2 +/− 1.8 | 2.9 +/− 1.2 |
| MYOACT- Cutaneous Disease Activity |
4.0 +/− 2.2 | 2.4 +/− 1.9 |
| MYOACT- Extramuscular Global Assessment |
2.9 +/− 2.2 | 1.9 +/− 1.8 |
| CDASI | 11.9 +/− 6.1 | 8.4 +/− 7.7 |
| HAQ | 1.2 +/− 1.0 | 1.2 +/− 1.0 |
| INQoL –Overall Quality of Life score |
58.1 +/− 23.9 | 62.3 +/− 13.5 |
| INQoL -Weakness Score |
64.6 +/− 24.4 | 65.3 +/− 37.1 |
| SF-36 - Physical Component Summary |
32.0 +/− 8.6 | 29.7 +/− 12.0 |
| SF-36 - Mental Component Summary |
42.2 +/− 10.5 | 47.0 +/− 15.0 |
| Creatine Kinase (U/L) |
821 +/− 2069 | 1098 +/− 929 |
| Aldolase (U/L) | 16.1 +/− 22.3 | 18.4 +/− 10.0 |
| Bone Density - lumbar spine Z- score |
−0.3 +/− 1.2 | −0.2 +/− 1.5 |
Abbreviations: MMT = Manual Muscle Testing; MVICT = Maximum Voluntary Isometric Contraction testing; MYOACT = Myositis Disease Activity Visual Analogue Scales; HAQ = Health Assessment Questionnaire; CDASI = Cutaneous Disease Activity Score Index; INQoL = Individualized Neuromuscular Quality of Life; DEXA = Dual Energy Xray Absorptiometry
Values are mean +/− standard deviation unless otherwise indicated
Safety
There were no significant differences in adverse event rates between the treatment groups (Supplementary Table 2). In the etanercept group, 6 serious adverse events (SAEs) were reported. One subject’s girlfriend became pregnant and miscarried; another subject was hospitalized twice (once for a urinary tract infection and once for fever of unknown origin). A third subject was hospitalized 3 times (once for post-herpetic neuralgia and twice for psychosis). In the placebo group, 3 SAEs were reported. One subject was hospitalized for gastroenteritis, one for IVIG treatment of a DM flare because she could not receive it as an outpatient, and one for ovarian cancer discovered at the end of the study.
No placebo-treated subjects had an ANA at baseline, but one developed an ANA at the end of the study (the subject with the ovarian malignancy). In the etanercept group, 2 had ANAs at both baseline and Week 52, while 2 developed ANAs at Week 52. None developed antibodies to double stranded DNA or a SLE-like illness.
Treatment Failures and Prednisone Dosage
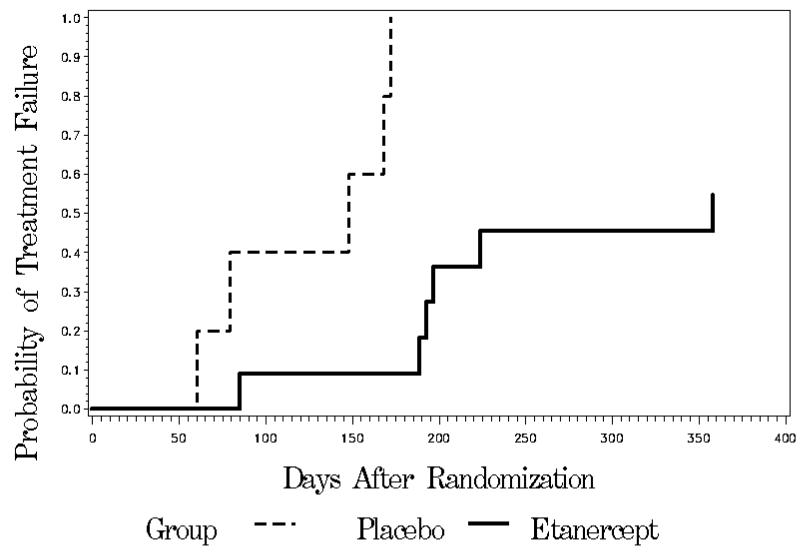
All 5 subjects receiving placebo were treatment failures (median time to treatment failure was 148 days). In contrast, 5 of 11 subjects in the etanercept arm were successfully weaned off prednisone (median time to treatment failure was 358 days, p = 0.0002) (Figure 2). The average time to treatment failure in the placebo group was 125 days, while the average time to failure in the 6 etanercept subjects that failed was 208 days. Three of the 6 treatment failures in the etanercept group and 1 of the 5 failures in the placebo group did not meet our research criteria for treatment failure, but the study physician felt the subjects needed to adjust treatment.
Figure 2.
Kaplan-Meier curves displaying the time from randomization to the first occurrence of treatment failure. There was a significant difference between the treatment groups favoring etanercept (p = 0.0002, log-rank test)
In the etanercept group, 5 of the 6 (83%) treatment failures were in previously refractory subjects. Two required an increase in prednisone dosage and the subsequent addition of second-line agents (methotrexate in one and IVIG and methotrexate in the other) and 1 had a worse skin rash treated with topical medication. In the placebo group, 3 of the 5 (60%) treatment failures were previously refractory subjects. Two of the 5 were treated by increasing the prednisone dosage. Three subjects also required the addition of another agent (one received IVIG; two were treated with IVIG and methotrexate).
Prednisone dosage from Weeks 25 to 52 in the placebo group (median 29.2 mg/day, range 9.9-62.6 mg/day) was significantly higher than that in the etanercept group (median 1.2 mg/day, range 0.0-31.1 mg/day) (p = 0.02). Compliance with study medication was excellent in both treatment groups (mean 99.7%, range 96-100% in the etanercept group; mean 96.7%, range 87-100% in the placebo group), as was compliance with prednisone (mean 92.6%, range 58-100% in the etanercept group; mean 97.6%, range 88-100% in the placebo group).
Clinical Outcome Measures
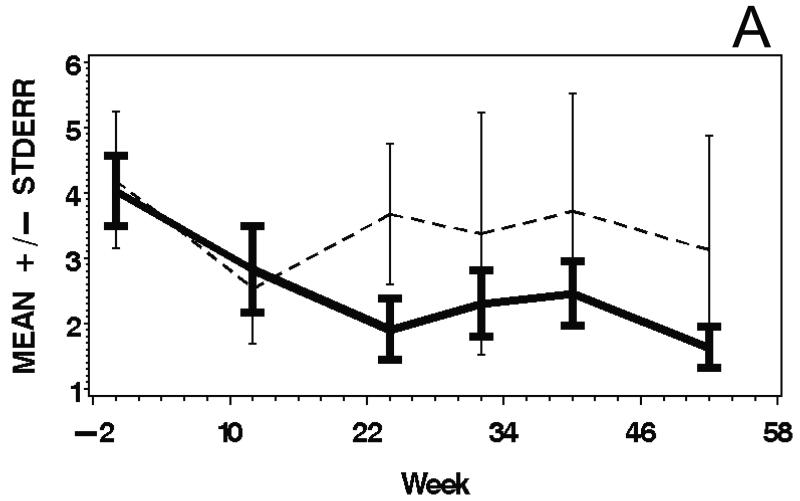
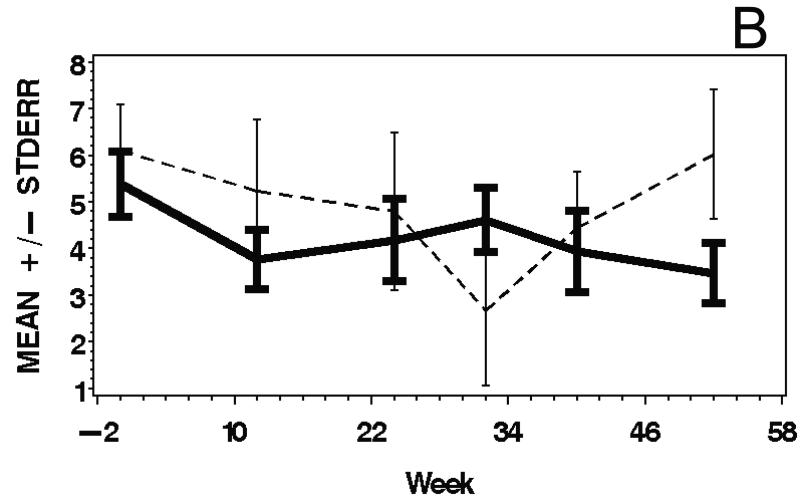
IMACS and other outcome measures demonstrated excellent test-retest reliability at the baseline visit (intraclass correlation coefficients 0.79 to 0.99) (Supplementary Table 3). There were significant improvements during the study, and moderate to large responsiveness was observed for various outcome measures with all subjects combined (Table 2). There were no significant treatment group differences in mean response during the course of the study. Those outcome measures with consistently moderate to large responsiveness included the Physician Global Activity, Patient Global Activity, average MMT, average MVICT, and SF-36-Physical Component Summary scores. Mean responses over time for selected outcome measures are shown in Figure 3.
Table 2.
Mean changes from baseline to Weeks 24 and 52 and responsiveness statistics
| (a) Week 24 | ||||||
|---|---|---|---|---|---|---|
| Outcome Variable |
Etanercept | Placebo | Overall | P-value† | ES | SRM |
| Average MMT score |
0.22 (0.05) | 0.27 (0.09) | 0.24 (0.05) | < 0.0001 | 0.46 | 0.87 |
| Average standardized MVICT score |
1.58 (0.40) | 0.59 (0.62) | 1.09 (0.37) | 0.006 | 0.67 | 0.80 |
| Average percent of predicted normal MVICT score |
12.1 (3.7) | 4.4 (5.7) | 8.2 (3.4) | 0.02 | 0.58 | 0.84 |
| Time to walk 30 feet (sec) |
−3.1 (1.4) | −1.9 (2.3) | −2.5 (1.4) | 0.07 | −0.17 | −0.42 |
| Physician global activity assessment |
−2.0 (0.7) | −1.0 (1.1) | −1.5 (0.7) | 0.03 | −0.90 | −0.80 |
| Patient global activity assessment |
−1.7 (0.9) | −2.1 (1.4) | −1.9 (0.9) | 0.03 | −0.73 | −0.53 |
| MYOACT overall score |
−0.029 (0.023) | −0.009 (0.036) | −0.019 (0.021) | 0.37 | −0.35 | −0.26 |
| MYOACT muscle disease activity score |
−1.14 (0.68) | −0.59 (1.04) | −0.87 (0.62) | 0.17 | −0.56 | −0.43 |
| MYOACT cutaneous disease activity score |
−0.84 (0.50) | 0.07 (0.77) | −0.39 (0.45) | 0.40 | −0.29 | −0.27 |
| CDASI score | −4.9 (1.5) | 1.5 (2.4) | −1.7 (1.4) | 0.23 | −0.41 | −0.40 |
| HAQ score | −0.44 (0.19) | −0.34 (0.29) | −0.39 (0.18) | 0.03 | −0.42 | −0.66 |
| SF-36 Physical Component Summary score |
7.0 (2.9) | 5.7 (4.6) | 6.3 (2.7) | 0.02 | 0.65 | 0.66 |
| SF-36 Mental Component Summary score |
−7.6 (4.6) | −1.5 (7.2) | −4.5 (4.3) | 0.30 | −0.42 | −0.31 |
| INQoL overall quality of life score |
0.5 (4.9) | 0.4 (7.3) | 0.4 (4.4) | 0.92 | −0.01 | −0.01 |
| Log (Creatine Kinase) (U/L) |
−0.10 (0.28) | 0.16 (0.43) | 0.03 (0.26) | 0.91 | −0.05 | −0.11 |
| (b) Week 52 | ||||||
|---|---|---|---|---|---|---|
| Outcome Variable |
Etanercept | Placebo | Overall | P- value† | ES | SRM |
| Average MMT score |
0.27 (0.06) | 0.21 (0.09) | 0.24 (0.06) | < 0.0001 | 0.52 | 0.97 |
| Average standardized MVICT score |
1.71 (0.41) | 0.47 (0.67) | 1.09 (0.39) | 0.009 | 0.70 | 0.68 |
| Average percent of predicted normal MVICT score |
13.0 (4.2) | 5.3 (7.0) | 9.1 (4.0) | 0.03 | 0.62 | 0.70 |
| Time to walk 30 feet (sec) |
−1.2 (2.1) | −2.3 (3.3) | −1.7 (2.0) | 0.37 | −0.10 | −0.43 |
| Physician global activity assessment |
−2.4 (0.6) | −1.3 (0.9) | −1.8 (0.5) | 0.0008 | −1.09 | −0.95 |
| Patient global activity assessment |
−2.4 (0.8) | −0.2 (1.3) | −1.3 (0.8) | 0.09 | −0.80 | −0.60 |
| MYOACT overall score |
−0.054 (0.025) | −0.003 (0.039) | −0.029 (0.023) | 0.22 | −0.63 | −0.41 |
| MYOACT muscle disease activity score |
−2.20 (0.58) | −0.79 (0.89) | −1.50 (0.53) | 0.007 | −1.07 | −0.76 |
| MYOACT cutaneous disease activity score |
−1.15 (0.68) | −0.71 (1.07) | −0.93 (0.63) | 0.15 | −0.48 | −0.39 |
| CDASI score | −3.1 (1.4) | −0.5 (2.2) | −1.8 (1.3) | 0.17 | −0.34 | −0.47 |
| HAQ score | −0.34 (0.14) | −0.32 (0.23) | −0.33 (0.14) | 0.02 | −0.36 | −0.67 |
| SF-36 Physical Component Summary score |
7.5 (2.6) | 1.1 (4.2) | 4.3 (2.5) | 0.09 | 0.59 | 0.67 |
| SF-36 Mental Component Summary score |
0.5 (3.0) | −0.8 (4.7) | −0.2 (2.8) | 0.95 | 0.02 | 0.02 |
| INQoL overall quality of life score |
−4.0 (5.9) | 1.4 (9.2) | −1.3 (5.5) | 0.81 | −0.08 | −0.10 |
| Log (Creatine Kinase) (U/L) |
−0.11 (0.32) | −0.95 (0.51) | −0.53 (0.30) | 0.08 | −0.21 | −0.30 |
Values for etanercept, placebo, and overall are mean (standard error) of changes from baseline to Week 24 or Week 52, estimated using a repeated measures analysis of covariance model (see text for details).
P-value is for a test of the null hypothesis of zero mean change overall.
Abbreviations: ES = Effect Size (mean change from baseline divided by the baseline standard deviation); SRM = Standardized Response Mean (mean change from baseline divided by the standard deviation of the change); MMT = Manual Muscle Testing; MVICT = Maximum Voluntary Isometric Contraction Testing; MYOACT = Myositis Disease Activity Assessment Visual Analogue Scales; CDASI = Cutaneous Disease Activity Score Index; HAQ = Health Assessment Questionnaire; INQoL = Individualized Neuromuscular Quality of Life
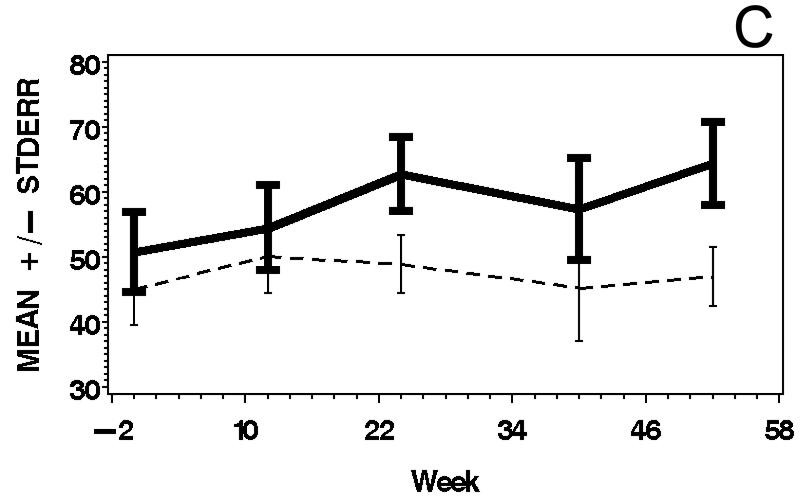
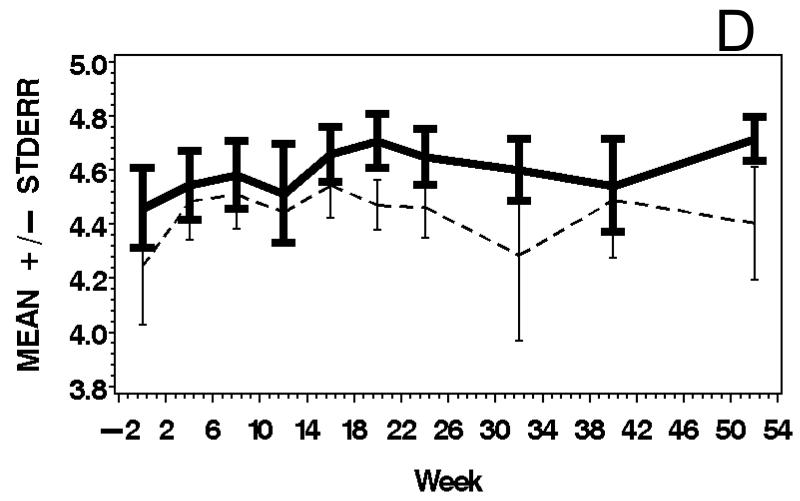
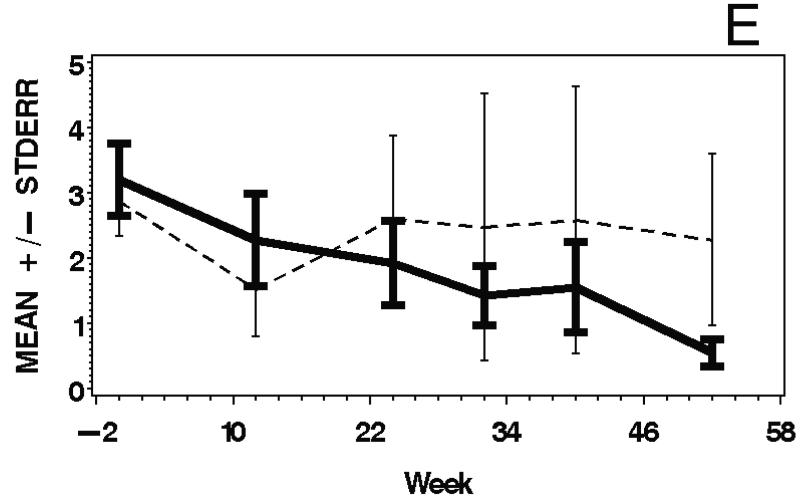
Figure 3.
Means and standard errors of Physician Global Activity Assessment score (A), Patient Global Activity Assessment score (B), average Percent of Predicted Normal (C), average Manual Muscle Testing (MMT) score (D), and MYOACT-Muscle Disease Activity score (E), are plotted over time by treatment group. There were statistically significant improvements from baseline in each of these outcomes (see Table 2), but no significant differences between the treatment groups.
IMACS Definitions of Improvement (DOI)
The top scoring IMACS DOI by consensus criteria was A1 (improvement in 3 of 6 core set measures by 20% or more, with no more than 2 worsening by at least 25%) leading to the recommendation that this be assessed in any clinical trial (28,29). We looked at all 6 suggested DOI (Supplementary Table 4). At Week 24, 9 subjects (82%) in the etanercept group met A1 criteria. Eight of 9 were successfully weaned off prednisone; the remaining subject required IVIG at Week 12. In contrast, only 2 of 5 (40%) placebo-treated subjects met A1 criteria and only one was able to be weaned off prednisone during this time.
At the end of the study, 6 subjects in the etanercept group met A1 criteria. Of these, 4 remained off prednisone and had no additional treatment. One subject was weaned off prednisone and received no other treatment, but he did not meet any DOI. The 5 etanercept subjects who were successfully weaned off prednisone had the following improvements in core set outcome measures: Physician Global Activity (mean 54.3%), Patient Global Activity (mean 55.3%), average MMT score (mean 5.2%), percent of predicted normal MVICT (mean 39.2%), MYO-ACT Extramuscular (mean 56.9%), and HAQ (mean 40.3%). Three placebo-treated subjects met A1 criteria at Week 52, but each required increased prednisone and 2 also needed the addition of second line agents (IVIG in one and IVIG and methotrexate in the other). In summary, at Week 52 four of 11 etanercept-treated subjects were receiving only the study drug and met IMACS DOI criteria in contrast to none of the placebo-treated subjects.
DISCUSSION
Etanercept was safe and well-tolerated over one year in our study. Two of the 11 etanercept-treated subjects developed newly elevated ANAs in the course of the study. None developed SLE, although rash worsened in 5 etanercept-treated subjects. The rash was initially attributed to worsening DM, although in one subject the rash improved at the end of the study upon discontinuing etanercept while he was on no other medications. This could have represented a lupus-like reaction related to etanercept. The one subject who developed cancer was on placebo.
There were valuable lessons learned from this study, especially in regard to the unanticipated difficulty recruiting subjects. Although the investigators felt that there was equipoise regarding the risk-benefit ratio of starting a second-line immunosuppressive agent along with corticosteroids at the time of initial diagnosis and treatment, many clinicians in our referral areas disagreed and felt that patients should be given both prednisone and methotrexate initially. These clinicians referred patients only after they failed both treatments. Many potential subjects also wanted to try at least prednisone before enrolling in the study. This hampered recruitment of newly diagnosed, treatment-naïve patients. The study design utilizing a forced prednisone taper may be useful in subsequent studies of myositis and other immune-mediated disorders, since we found a significant steroid-sparing effect of etanercept despite the small sample size.
Another objective of this study was to assess various outcome measures for myositis trials. We assessed IMACS-recommended core set of measures and other measures; most demonstrated excellent intra-rater reliability and responsiveness in our hands, particularly strength and global disease activity outcomes. Patients’ global assessments of their disease activity were usually worse than those of the physicians’ assessments. We looked at the performance of IMACS preliminary DOI, although our study was not designed to validate these definitions. It is important to validate the efficacy outcomes used here and the IMACS DOI and “definitions of worsening” prior to embarking on larger efficacy trials. The small sample size of our pilot trial and the allowance of rescue treatment limited our ability to detect effects of etanercept on efficacy outcomes not related to steroid-sparing and also limits the conclusions that can be drawn regarding the safety of etanercept. The observation of no major safety concerns and a steroid-sparing effect in our study suggest that further investigation of etanercept as a treatment for DM is warranted.
Supplementary Material
Acknowledgments
Study supported by: NIH R01 NS049639 (PI: Anthony A. Amato, MD); Amgen provided start up funding, etanercept, and matching placebo.
APPENDIX
Muscle Study Group Participants:
Principal Investigator: Anthony A. Amato, M.D.
Steering Committee: Rabi Tawil, M.D., John Kissel, M.D., Richard Barohn, M.D., Michael P. McDermott, Ph.D., Shree Pandya, RPT, Wendy King, RPT
Study Coordinators: Alexis Smirnow, Christine Annis, Kristen Roe
MSG Coordination and Biostatistics Center: Director: Rabi Tawil, M.D.; Biostatistician: Michael P. McDermott, Ph.D; Programmer/Analyst: Joanne Janciuras; Data Managers: Nuran Dilek, M.S. and William B. Martens; Information Analyst: Eileen Eastwood
Individual Study Site Participants:
Brigham and Women’s Hospital (3 subjects): (Investigators: Anthony Amato, M.D., Thomas Cochrane, MD; Clinical Evaluators: Merideth Donlan, Samantha Chused; Study Coordinator: Kristen Roe)
University of Kansas Medical Center (3 subjects): (Investigators: Richard Barohn, M.D.; Mazen Dimachkie, M.D., Daniel J. Aires, M.D., Kevin M. Latinis, M.D., Ph.D.; Clinical Evaluator: Laura Herbelin; Study Coordinator: Hiwot Michaels)
Oregon Health and Science Center (3 subjects): (Investigators: Edward Cupler, M.D., Atul Deodhar, M.D., Eric Simpson, M.D., Prinyarat Burusnukul, M.D., Eric Edgar, M.D.; Clinical Evaluator: Andrea Serdar; Study Coordinators: Thomas Brennan and Kathryn Gance, MA)
The Ohio State University (2 subjects): (Investigators: John Kissel, M.D, Miriam L. Freimer, M.D., Kevin V. Hackshaw, M.D.. Victoria Lawson, M.S., M.D.; Clinical Evaluator: Wendy M. King; Study Coordinator: Amy Bartlett)
University of Texas Southwestern Medical Center (2 subjects): (Investigators: Gil Wolfe M.D., Sharon Nations, M.D.; Clinical Evaluator: Rhonda McLin; Study Coordinator: Nina Gorham)
University of British Columbia (1 subject): (Investigators: Hannah Briemberg, M.D, Kristine M. Chapman, M.D., Jan P. Dutz, M.D.; Study Coordinator: Judy Wilson, RN; Clinical Evaluator: Franca Varelas)
Johns Hopkins Medical Center (1 subject): (Investigators: Kathryn Wagner, M.D., Lisa Christopher Stine, M.D., M.P.H.; Grant James Anhalt, Jon H. Meyerle, M.D.; Clinical Evaluator: Jennifer O. Swain, MS; Study Coordinator: Regina Brock-Simmons)
University of Washington (1 subject): (Investigators and evaluators: Michael Weiss, M.D., Ph.D, B. Jane Distad, M.D.; Study coordinators: John Lin, Joanna A. Haug, M.S., M.P.H, Sharon Downing, RN )
The authors would also like to thank Robert Griggs, M.D. and the members of the MSG Executive Committee, the Medical Monitor for the study (Jim Russell, D.O.), members of the Data and Safety Monitoring Board (Jeff Dawson, Sc.D., Petra Kaufmann, M.D., Joan M. Bathon, M.D.), and NINDS staff (Scott Janis, Ph.D., Robin Conwit, M.D.)
Footnotes
clinicaltrials.gov registry #: NCT00282880
References
- 1.Amato AA, Barohn RJ. Evaluation and treatment of inflammatory myopathies. J Neurol Neurosurg Psychiatry. 2009;80:1060–1068. doi: 10.1136/jnnp.2008.169375. [DOI] [PubMed] [Google Scholar]
- 2.DeBleeker JL, Meire VI, Declercq W, Van Aken EH. Immunolocalization of tumor necrosis factor-alpha and its receptors in inflammatory myopathies. Neuromusc Dis. 1999;9:239–246. doi: 10.1016/s0960-8966(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 3.DeBleeker JL, Engel AG. Expression of cell adhesion molecules in inflammatory myopathies and Duchenne dystrophy. J Neuropathol Exp Neurol. 1994;53:369–376. doi: 10.1097/00005072-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Fedcyyna TO, Lutz J, Pachman LM. Expression of TNFα by muscle fibers in biopsies from children with untreated juvenile dermatomyositis: association with the TNF-α allele. Clin Immunol. 2001;100:236–239. doi: 10.1006/clim.2001.5063. [DOI] [PubMed] [Google Scholar]
- 5.Kuru S, Inukai A, Liang Y, Doyu M, Takano A, Sobue G. Tumor necrosis factor-α expression in muscles of polymyositis and dermatomyositis. Acta Neuropathol (Berl) 2000;99:585–588. doi: 10.1007/s004010051165. [DOI] [PubMed] [Google Scholar]
- 6.Pachman LM, Liotta-Davis MR, Hong DK, et al. TNF alpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43:2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.De Bleeker JL, De Paepe B, Vanwalleghem IE, Schroder JM. Differential expression of chemokines in inflammatory myopathies. Neurology. 2002;58:1779–1785. doi: 10.1212/wnl.58.12.1779. [DOI] [PubMed] [Google Scholar]
- 8.Tezak Z, Hoffman EP, Pachman LM. Expression profiling in untreated juvenile dermatomyositis. J Immunol. 2002;168:4145–4163. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 9.Keystone EC, Schiff MH, Kremer JM, et al. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:353–356. doi: 10.1002/art.20019. [DOI] [PubMed] [Google Scholar]
- 10.Gorman JD, Sack KE, Davis JC. Treatment of ankylosising spondylitis by inhibition of tumor necrosis factor α. N Engl J Med. 2002;346:1349–56. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- 11.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. New Engl J Med. 2003:2014–22. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 12.Hengstman GJ, van den Hoogen FH, Barrera P, et al. Successful treatment of dermatomyositis and polymyositis with anti-tumor-necrosis-factor-alpha: preliminary observations. Eur Neurol. 2003:50–15. doi: 10.1159/000070852. [DOI] [PubMed] [Google Scholar]
- 13.Hengstman GJD, van den Hoogen FHJ, van Engelen BGM. Treatment of dDermatomyositis and polymyositis with anti-tumor necrosis factor-α: long-term follow-up. Eur Neurol. 2004;52:61–63. doi: 10.1159/000079547. [DOI] [PubMed] [Google Scholar]
- 14.Sprott H, Glatzel M, Michel BA. Treatment of myositis with etanercept (Enbrel), a recombinant human soluble fusion protein of TNF-alpha type II receptor and IgG1. Rheumatol. 2004;43:524–526. doi: 10.1093/rheumatology/keh062. [DOI] [PubMed] [Google Scholar]
- 15.Korkmaz C, Temiz G, Cetinibas F, Bukukkidan B. Case report: successful treatment of alveolar hypoventilation due to DM with anti-tumor necrosis factor-alpha. Rheumatol. 2004;43:937–938. doi: 10.1093/rheumatology/keh226. [DOI] [PubMed] [Google Scholar]
- 16.Labioche I, Liozon E, Weschler B, Loustaud-Ratti V, Soria P, Vidal E. Refractory polymyositis responding to infliximab: extended follow-up. Rheumatol. 2004;3:531–532. doi: 10.1093/rheumatology/keh079. [DOI] [PubMed] [Google Scholar]
- 17.Constanine K, Saadeh A. Etanercept is effective in the treatment of polymyositis/DM which is refractor to conventional therapy including steroids and other disease modifying agents [Abstract] Arthritis Rheum. 2000;43:S193. [Google Scholar]
- 18.Hengstman GJ, De Bleecker JL, Feist E, Vissing J, Denton CP, Manoussakis MN, Jensen H Slott, van Engelen BG, van den Hoogen FH. Open-label trial of anti-TNF-alpha in dermato- and polymyositis treated concomitantly with methotrexate. Eur Neurol. 2008;59:159–63. doi: 10.1159/000114036. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Rosenbach M, Kim EJ, Kim B, Werth VP, Dunham J. Tumor necrosis factor inhibitor-associated dermatomyositis. Arch Dermatol. 2010;146:780–4. doi: 10.1001/archdermatol.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iannone F, Scioscia C, Falappone PC, Covelli M, Lapadula G. Use of etanercept in the treatment of dermatomyositis: a case series. J Rheumatol. 2006;33:1802–1804. [PubMed] [Google Scholar]
- 21.Dastmalchi M, Grundtman C, Alexanderson H, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis. 2008;67:1670–1677. doi: 10.1136/ard.2007.077974. [DOI] [PubMed] [Google Scholar]
- 22.Fleischmann R, Iqbal I, Naneswar P, Quiceno A. Safety and efficacy of disease – modifying antirheumatic agents: focus on the benefits and risks of etanercept. Drug Safety. 2002;25:173–197. doi: 10.2165/00002018-200225030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Debrandt M, Vittecoq O, Descamps V, Le Loet X, Meyer O. Anti-TNF-alpha-induced systemic lupus syndrome. Clin Rheumatol. 2003;22:56–61. doi: 10.1007/s10067-002-0654-5. [DOI] [PubMed] [Google Scholar]
- 24.Carlson E, Rothfield N. Etanercept-induced lupus-like syndrome in a patient with rheumatoid arthritis. Arthritis Rheum. 2003;48:1165–1166. doi: 10.1002/art.11033. [DOI] [PubMed] [Google Scholar]
- 25.Cairns AP, Duncan MK, Hinder AE, Taggart AJ. New onset systemic lupus erythematosus in a patient receiving etanercept for rheumatoid arthritis. Ann Rheum Dis. 2002;61:1031. doi: 10.1136/ard.61.11.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakoor N, Michalska M, Harris CA, Block JA. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet. 2002;359:579–580. doi: 10.1016/S0140-6736(02)07714-0. [DOI] [PubMed] [Google Scholar]
- 27.Miller FW, Rider LG, Chung Y-L, et al. Proposed preliminary core measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology. 2001;40:1262–1273. doi: 10.1093/rheumatology/40.11.1262. [DOI] [PubMed] [Google Scholar]
- 28.Oddis CV, Rider LG, Reed AM, et al. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis Rheum. 2005;52:2607–15. doi: 10.1002/art.21291. [DOI] [PubMed] [Google Scholar]
- 29.Rider LG, Giannini EH, Brunner HI, Ruperto N, James-Newton L, Reed AM, Lachenbruch PA, Miller FW, International Myositis Assessment and Clinical Studies Group International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum. 2004 Jul;50(7):2281–90. doi: 10.1002/art.20349. [DOI] [PubMed] [Google Scholar]
- 30.Muscle Study Group A randomized trial of βINF1a (Avonex) in patients with inclusion body myositis (IBM) Neurology. 2001;57:1566–1570. doi: 10.1212/wnl.57.9.1566. [DOI] [PubMed] [Google Scholar]
- 31.Muscle Study Group Randomized pilot trial of high dose βINF1a in patients with inclusion body myositis. Neurology. 2004;63:718–720. doi: 10.1212/01.wnl.0000134675.98525.79. [DOI] [PubMed] [Google Scholar]
- 32.Klein RQ, Bangert CA, Costner M, et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol. 2008 Sep;159(4):887–94. doi: 10.1111/j.1365-2133.2008.08711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL) Neurology. 2007;68:1051–7. doi: 10.1212/01.wnl.0000257819.47628.41. [DOI] [PubMed] [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Second Edition John Wiley and Sons; Hoboken, NJ: 2002. [Google Scholar]
- 35.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(Suppl 3):S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 36.Liang MH, Larson MG, Cullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis Rheum. 1985;28:542–547. doi: 10.1002/art.1780280513. [DOI] [PubMed] [Google Scholar]
- 37.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53:459–68. doi: 10.1016/s0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.