Abstract
In infants, respiratory infection elicits tachypnea. To begin to evaluate the role of brainstem cytokine expression in modulation of breathing pattern changes, we compared the pattern generated after endotracheal instillation of lipopolysaccharide (LPS) in in vivo rat pups to local pro-inflammatory cytokine injection in the nucleus tractus solitarius (nTS) in an in vitro en bloc brainstem spinal cord preparation. We hypothesized that both challenges would elicit similar changes in patterning of respiration. In anesthetized, spontaneously breathing rat pups, lipopolysaccharide (LPS) or saline was instilled in the airway of urethane-anesthetized rats (postnatal day 10–11). We recorded diaphragm EMG over the subsequent 2 hours and saw a 20–30% decrease in interburst interval (Te) at 20–80 min post-injection in LPS-instilled animals with no significant change in Ti. In contrast, IL-1β injections into the nTS of en bloc in vitro brainstem-spinal cord preparations from 0 to 5 day-old pups maintained Ti and caused an increase in Te as early as 20 min later, decreasing frequency for 80 to 120 minutes after injection. Our results suggest that the neonatal respiratory response to the cytokine IL-1β mediated inflammatory response depends on the site of the inflammatory stimulus and that the direct effect of IL-1β in the nTS is to slow rather than increase rate.
Keywords: cytokines, modulation, control of breathing, nTS, in vivo, in vitro
1. Introduction
Perinatal inflammation in response to infection during pregnancy has been implicated in morbidity associated with preterm birth. Premature infants born under these conditions are often exposed to bacterial pathogens introduced via the respiratory system and the subsequent immune response results in a cascade of pro-inflammatory cytokine production (Shalak et al., 2002). Maternal infection and this resulting inflammatory response are significant risk factors for central nervous system damage in premature newborns (Grether and Nelson, 1997; Wu and Colford, 2000; Grether et al., 2003; Nelson 2009) as well as full-term infants (Wu et al., 2003). Breathing pattern abnormalities ranging from tachypnea to apnea of prematurity are prominent initial manifestations of sepsis—which is whole body, systemic inflammation in response to infection—and may contribute to morbidity in this high risk population (Darnall et al., 1997; Fanaroff et al., 1998; Navar-Boggan et al., 2010).
Very little is known about the mechanisms by which inflammation alters control of breathing. In both newborn rats and mice, interleukin-1-beta (IL-1β) and prostaglandins have been implicated in the modulation of breathing pattern (Olsson et al., 2003; Hofstetter et al., 2007). Prostaglandin E2, produced in response to IL–1β, can alter synaptic transmission in the nucleus tractus solitarius (nTS), the first central synapse for cardio-respiratory afferents (Marty et al. 2008). Systemic administration of IL-1β and tumor necrosis factor-alpha (TNF–α) has been shown to increase message for cytokine expression in the nTS (Churchill et al., 2006) suggesting that either local humoral access, via the area postrema or, perhaps, through vagal afferents, results in a positive feedback loop for increased cytokine expression locally and, eventually, prolonged inflammatory changes in the nervous system.
Here we report results from our experiments examining the role that endotoxin exposure and the subsequent cytokine response play in modulating breathing activity in early postnatal life. As we have reported elsewhere in this issue (Balan et al., 2011), LPS instillation in the airway induces cytokine expression in the brainstem of rat pups. To further explore the role that peripheral and central expression of pro-inflammatory cytokines plays in modulating respiratory control circuitry, we have performed in vivo experiments to evaluate changes in breathing pattern continuously after instillation of LPS into the airway in anesthetized animals. Our working hypothesis is that LPS-induced cytokine release alters processing of afferent sensory information and thus alters rhythm-generation in the brainstem. However, first we evaluated the direct effect of IL-1β in the nTS in the absence of chemo- and mechano-afferent inputs to the nTS. So, to determine if IL-1β can evoke changes in fictive inspiratory drive, we used focal microinjection of IL-1β into the caudal commissural nTS in an in vitro en bloc preparation and quantified changes in inspiratory output from cranial and spinal rootlets. This work provides us with an initial understanding of the early, acute, inflammatory response and how this alters respiratory control in the time period immediately following induction of inflammation.
2. Materials and Methods
2.1 In vivo physiology experiments
All procedures were performed in accordance with protocols approved by the Case Western Reserve University Institutional Animal Care and Use Committee (IACUC). Sprague-Dawley rats (Charles River, Wilmington, MA) ages 10 to 11 postnatal days were used for these studies. Rat pups were anesthetized with urethane (ethyl carbamate, 4g/kg i.p.; Sigma, St. Louis, MO). After a surgical plane of anesthesia was achieved, an incision was made in the neck and the muscles retracted to expose the trachea. A small incision was then made in the trachea just caudal to the larynx, and a tracheal cannula of polyethylene tubing (ID = 0.76 mm OD = 1.22 mm) was inserted into the trachea and tied in place using 4-0 suture thread. A fine, stainless steel, bipolar hook electrode was then placed in the diaphragm to record electromyographic diaphragm (EMG) activity as an index of inspiratory drive. All rat pups breathed spontaneously throughout the entire experimental procedure. Lipopolysaccharide (LPS; 0.1μg/g; Sigma), the outer coating of gram-negative bacteria, in a volume of 10μl was administered directly into the trachea using a Hamilton syringe inserted through the tracheal cannula (N = 10). An identical volume of vehicle (0.9% NaCl) was administered into the tracheas of control animals (N = 11).
2.2 In vitro en bloc experiments
For our in vitro en bloc brainstem-spinal cord preparations, we used brainstems taken from neonatal rat pups (ages P0–5). All en bloc preparations were prepared using previously published methods (Smith and Feldman, 1987). The rats were deeply anesthetized with isoflurane and the brainstem and spinal cord was rapidly removed in chilled (4–6°C) artificial cerebrospinal fluid (aCSF), equilibrated with 95%O2-5% CO2. The aCSF contained (in mM) NaCl (124), KCl (3), CaCl2-2H2O (1.5), MgSO4-7H2O (1.0), NaH2PO4-H2O (0.5), NaHCO3 (25), D-Glucose (30)). The brainstem-spinal cord preparations were transferred to a recording dish continuously perfused with 95%O2–5%CO2 equilibrated aCSF and heated to 27°C. Fictive motor output was recorded from ventral nerve rootlets using boro-silicate glass suction electrodes pulled on a horizontal puller (P97, Flaming/Brown Micropipette puller, Sutter Instruments, Novato, CA). Pressure injections of IL-1β (Sigma, St. Louis, MO) into the commissural nTS were made using a glass pipette (5–10 μm tip diameter) attached to a picospritzer (General Valve Picospritzer II, Parker-Hannifin, Cleveland, OH). IL-1β was dissolved in distilled H2O to a final concentration of 5 μg/μL, and then a 2 μL volume was injected while monitoring the displacement of the fluid meniscus using a microscope equipped with an eyepiece reticle.
2.3 Data collection and statistics
For both in vivo diaphragmatic EMG activity and in vitro fictive motor output (neurogram), Grass P511 amplifiers and headstages were used (Grass Technologies, West Warwick, RI). EMG and neurogram were recorded using PowerLab hardware and Chart software (ADInstruments, Colorado Springs, CO) in real-time at 4–10 kilosamples/s and stored in a laboratory microcomputer for later off-line analysis. Data were analyzed on a breath-by-breath basis using MATLAB software (Mathworks, Natick, MA) for frequency, inspiratory time (Ti), expiratory time (Te), burst area, and histogram entropy measures. The data was then normalized to baseline activity for subsequent comparison and expressed as mean ± standard deviation (SD) or standard error of the mean (SE). Baseline values for EMG data were taken during the 10 minutes prior to injection of LPS or vehicle. The signal bandpass for all EMG recordings was 0.3 to 1 kHz with both the raw and averaged, rectified signals (50 ms averaging window) recorded continuously over 2 to 3 hours. A 15-min baseline was recorded for the in vitro en bloc prep after stable output and the set temperature were reached (typically within 15 minutes from placing the preparation in the recording chamber) and before IL-1β injection. The signal was recorded continuously after injection, and a 10-burst interval was examined for each time point. All data was normalized to individual baseline and is reported as a % of baseline ± S.E.M. We used normalized Te to compensate for the wide range of individual variability seen in both in vivo and in vitro preparations. Ten rat pups were injected with vehicle and 12 with IL-1β. Mean data for the initial time points represent an n of 10 and 12, respectively, exceptions are: 40 to 60 min (control n = 9), and 60 to 80 min (control: n = 9, IL-1β: n = 11). The signal bandpass for all in vitro en bloc recordings was either 0.3 or 0.1 to 1 or 3 kHz with both the raw and full wave rectified, averaged signals (200 ms window) recorded. Data were compared using two-way ANOVA for time and saline vs. LPS or IL-1β with Tukey, HSD post-hoc comparisons. P values ≤ 0.05 were considered significant.
3. Results
3.1 LPS instillation into the airway in vivo
In 10–11 day-old rats, LPS instillation resulted in acute changes in breathing pattern. We recorded diaphragmatic electromyogram (EMG) continuously from shortly after induction of anesthesia to the termination of the experiment (2 h after instillation). After obtaining a baseline EMG recording, LPS was injected into the trachea using a Hamilton syringe (Fig. 1A). Representative examples of saline-injected and LPS-injected (both raw and integrated EMG neurogram) are shown in Figure 1B. We observed a significantly shorter (p < 0.05) normalized Te for 20 to 80 minutes after the injection in LPS-instilled animals (Fig. 2B). The decrease in Te ranged from 79 to 71% of baseline, or a reduction of approximately 20 – 30% (see example in Fig. 1 and summary data in Figure 2A, B; n =10 for LPS and 11 for saline). Note that the burst duration (Ti) did not change significantly over the time course of the experiment in either in vivo group. Figure 2, panel C, shows summary data for integrated burst area, which represents the inspiratory motor drive. LPS instilled animals had a significantly greater area at 80 – 100 and 100 – 120 minutes from injection than saline injected animals (asterisks in Figure 2, panel C) and LPS animals showed a time-dependent increase in area that was significant at 80 – 120 minutes post-injection.
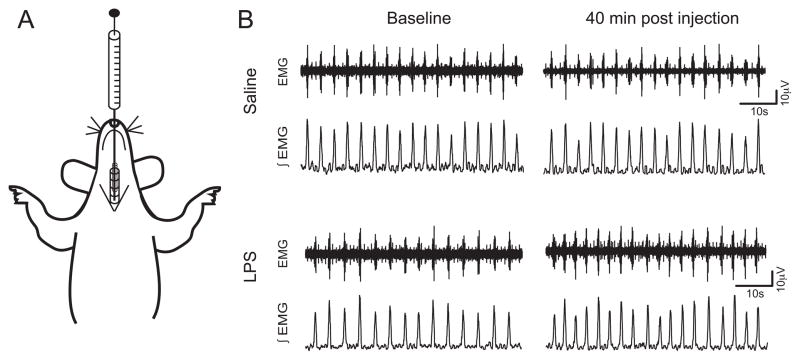
Figure 1.
Diaphragm electromyogram (EMG) was used to measure respiratory changes after LPS injection in the trachea. (A) An illustration representing our in vivo preparation, with a syringe positioned to inject LPS into the upper airway of a spontaneously breathing rat pup. (B) Sample traces from a control and an LPS-injected animal. Raw EMG data (top trace) were integrated (bottom traces) and analyzed to quantify changes in respiratory pattern. The first pair of traces for each animal represents the baseline while the second pair was taken 40 minutes after the injection.
Figure 2.
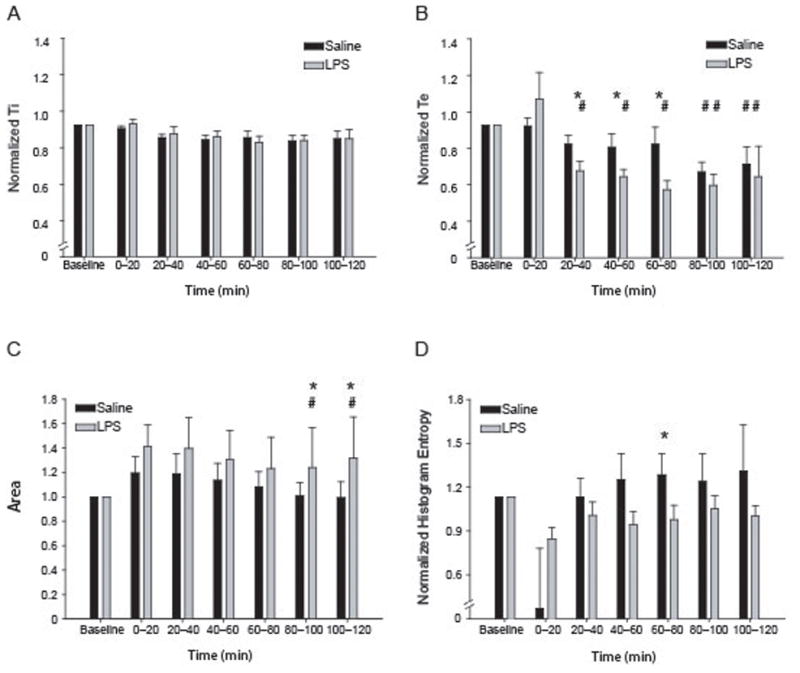
Injection of LPS into the trachea alters respiratory rhythm. (A–B) Summary data for LPS and control animals. (A) Ti shows no significant changes after LPS injection when compared to control. (B) LPS decreases the Te 20–80 minutes post injection (79.4 ± 4.2, 76.7 ± 3.3, 70.9 ± 4.1% of baseline) when compared to control (91.5 ± 4.0, 89.9 ± 6.2, 91.6 ± 7.7%). (C) Burst area is not significantly different in these preparations though the LPS-injected animals have a slightly larger burst area throughout the recording period. (D) LPS injection alters the normalized histogram entropy for interburst interval. LPS lowers the mean histogram entropy, with 60 – 80 min post-injection significantly lower than control. * indicates significant differences from control group (p<.05); # indicates significant difference across time (indicated over the control or IL-1β bars).
In addition to the example in panel C, Figure 2D shows the normalized histogram entropy for saline and LPS animals over all time periods in the experiment. We have previously used information theory-based measures of variability and regularity to quantify changes in respiratory activity (Erickson et al., 2007; Yu et al., 2008; Foglyano et al., 2009) and here we compared the interval between inspiratory EMG efforts (Figure 2D) using histogram entropy. We normalized the resulting histogram entropy values to compare across all animals in both groups; this indicates the relative magnitude of change in variability of Te’s for saline vs. LPS animals. We only saw a significant reduction in complexity at the 60 to 80 min interval for both groups. Of note, the EMG of LPS animals showed a decrease in the histogram entropy (an index of variability) for Te.
3.2 IL-1β injection into nTS
We have provided data suggesting that, in the absence of afferent signaling, the cytokine response in the nTS was present but attenuated (Balan et al. 2011). However, the time course and magnitude of cytokine-induced changes in brainstem circuitry is still unclear. We tested direct injection of IL-1β into the nTS in a preparation devoid of vagal afferent input to evaluate the direct role, if any, that this pro-inflammatory cytokine plays in altering bursting output from the brainstem.
In Fig. 3A, we have diagrammed the in vitro en bloc (brainstem-spinal cord) preparation with a suction electrode and the microinjection pipette used to administer IL-1β. In rat pups at postnatal 0 – 5 days, direct injection of IL-1β (10μg total) into the nTS significantly reduced the rate of fictive respiratory motor output (n=12 for IL-1β and 10 for control). The raw and integrated neurogram from the first ventral cervical rootlet (C1) had a robust signal in these preparations over 2 to 3 hours though we also recorded from C3–5 rootlets in some preparations. The tracings shown in Fig. 3B are from an individual animal (P2, 8g) in which IL-1β microinjection slowed fictive inspiratory output recorded at the C1 ventral spinal rootlet. Mean data are shown for the vehicle-injected (control) and IL-1β groups in Figure 4. In contrast to the in vivo response to LPS, which showed no change in Ti, Ti had a tendency to shorten in the saline-injected brainstems whereas the brainstems with IL-1β injections had Ti values that remained close to the initial baseline (attenuated by approximately 10%). This is consistent with a previous report that respiratory output deteriorates over time in the in vitro en bloc preparation (Suzue 1984). The mean Ti changes can be seen in Figure 4, panel A. Ti was longer after IL-1β injection (88–92% of baseline) than after saline injection (81 – 86% of baseline) at 20 – 120 min after the injection. Interburst interval (Te) (Fig 4, panel B) increased significantly at 80 to 120 min post IL-1β injection in caudal-commissural nTS (approximately 150% of baseline) compared to control injections (approximately 122% of baseline) additionally, the IL-1β injected preparations also showed a significant decrease in activity over 20 to 80 min post injection. Both control and IL-1β preparations showed a significant time-dependent decrease in Te at 80 – 120 min. Figure 4C shows the summary data for integrated burst area. IL-1β decreases burst area immediately after injection and sustains the decrease up to 100 minutes post injection (56–70% of baseline, see figure) compared to control (67–90% of baseline, p<0.05) over 0 to 120 min after injection. Frequency was significantly reduced (75 – 78.2% of baseline) at 80 to 120 min after IL-1β injection when compared to control injection (93 – 94 %) at 80 to 120 minutes post injection. The time course for changes in Te and burst frequency match make sense in light of the diffusion limitations that make the rhythm-generating circuitry hypoxic over the course of the experiment, however the immediate effects of IL-1β injection on Ti and burst area was not expected and suggests the need for further investigation (Marty et al 2008).
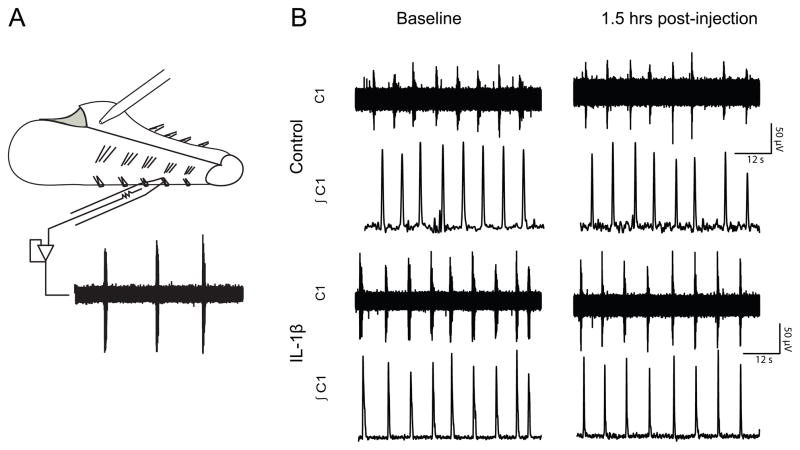
Figure 3.
Fictive motor output was recorded from cranial nerve rootlets. (A.) An illustration representing the in vitro en bloc brainstem-spinal cord preparation. Injections (2μL) were made in the caudal, commisural NTS and motor output was recorded from cranial nerve rootlets using glass suction electrodes. (B) A sample trace from a control preparation receiving saline and a preparation receiving an IL-1β (10μg) injection. Raw data (top traces) were integrated (bottom traces) and analyzed to determine changes in breathing patterns. The traces are 1 min segments.
Figure 4.
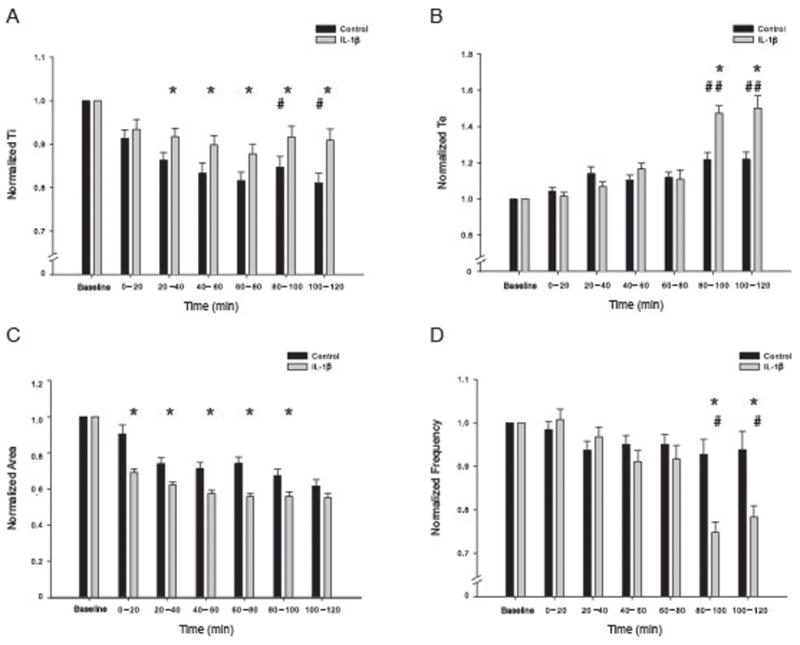
Injection of IL-1β into the caudal/commissural region of the nTS alters fictive inspiratory output from the in vitro en bloc brainstem-spinal cord. (A) Ti remained close to baseline levels after IL-1β injection while control animals saw a time-dependent decrease in activity. (B) Te was significantly higher than control for both time-points after 80 minutes (147.3 ± 4.3, 150.1 ± 6.9%; 121.8 ± 3.9, 121.9 ± 3.8% of baseline respectively). (C) Burst area is significantly smaller after IL-1β injection when compared to control. The burst area is decreased immediately after injection and continues until 100 minutes post injection (see results). The burst area is still smaller after 100 minutes but the difference is no longer statistically significant. (D) Summary data for IL-1β and control burst frequency. Frequency was significantly higher than control for both time-points after 80 minutes (74.7 ± 2.4, 78.2 ± 2.7%; 92.7 ± 3.5, 93.8 ± 4.2 % of baseline respectfully). * indicates significant difference from control group at each time point (p<.05); # indicates significant difference across time (indicated over the control or IL-1β bars).
4. Discussion
Here we report results from experiments designed to elucidate how respiratory drive is altered by LPS-induced inflammation and the subsequent increase in local pro-inflammatory cytokine release in response to inflammation. Our work is unique in that we have focused on early developmental animal models and we have attempted to compartmentalize inflammation to the airway in our in vivo experiments and followed the changes in breathing pattern continuously over the next two hours to allow detection and quantification of acute changes in respiratory pattern as they evolve. We have also used focal microinjection of IL-1β in the nTS of the en bloc in vitro brainstem-spinal cord preparation to quantify centrally-mediated changes in inspiratory drive. We found that instillation of LPS directly into the trachea of rat pups decreased Te persisting for more than an hour, whereas with direct injection of IL-1β into the caudal-commissural nTS of in vitro en bloc preparations, Te increased and the decrease in Ti was attenuated.
With pulmonary tract infection, cytokines—including IL-1β, IL-6, and TNF-α—can be released into the circulation and, depending on the severity of infection, can induce a systemic response (Simi et al., 2007). Typically, after the infectious agent is removed, cytokine production is reduced and the CNS also plays a role in attenuating cytokine production, with increased activity inhibiting cytokine production (Tracey, 2002). However, under certain pathological conditions cytokine production remains elevated and can lead to severe consequences. Cytokines are involved in eliciting sickness behavior, including fever, weight loss, and depression (scored by decreased social interaction) in rats (Konsman et al., 2008). Here we have focused on initiating the cascade of pro-inflammatory cytokines in a relatively compartmentalized model of infection by injecting LPS into the trachea and maintaining the animal over two hours post the LPS instillation and recording changes in respiratory drive continuously. In our in vitro preparations presented herein, we have used IL-1β injections as a surrogate for the broader inflammatory response and localized our injections to the nTS regions that receive afferent input from the lungs and airways.
4.1 Breathing pattern changes of in vivo anesthetized rat to instilled LPS
We used P10 – 11 anesthetized rat pups to evaluate breathing changes in response to the LPS-induced acute inflammatory response in our developing rat model. We are specifically interested in the manner in which the immune system of immature animals reacts to septic challenge and this anesthetized in vivo model serves to further our understanding of the pathophysiology of inflammation in the developing autonomic nervous system. In our model, instillation of LPS in the trachea mimics an acute, local infection of the upper airway in neonates. In response to this immune challenge, we observed a significant decrease in interburst interval in LPS-injected rat pups but no difference in burst duration. The changes in Te may result from increased activation of vagal c or A-δ fibers but below that required to elicit an apnea that can occur with activation of these fiber types (Wilson and Bonham, 1997).
4.2 Fictive inspiratory drive of in vitro en bloc brainstem-spinal cord after IL-1β injection
We injected IL-1β into the nTS of in vitro en bloc preparations—a model devoid of vagal afferent inputs—and quantified changes in inspiratory-like drive for two to three hours following injection. We observed significant changes in fictive bursting rhythm which may be due to altered local signaling within the nTS and/or a decrease in traffic to the preBötzinger Complex (pBC). The differential effects on burst duration and inter-burst interval suggests two pathways by which inspiratory drive is altered. IL-1β prevented the attenuation of Ti that we observed in the control preparations and we do not yet have an explanation for this obersvation. The Ti in IL-1β was significantly greater than saline-instilled animals early after injection (within 20 – 40 minutes), while the increase in Te occurs later (80 to 120 min). The disruption of nTS input to the pBC may alter inspiratory timing after an upregulation of PGE2 release while Ti may be changed due to increased input to the premotor neurons, perhaps disinhibited by removal of the vagal afferent input to the brainstem. Modulation of the local nTS network by IL-1β and PGE2’s action within the local nTS network has been suggested by Marty and colleagues (2008) to have a biphasic effect on synaptic transmission of both vagal afferent inputs and local interneurons projecting on nTS projection neurons. The differences in activity seen at 80 to 120 minutes in both control and IL-1β are likely due to deterioration of the preparation and hypoxic damage to the core rhythm-generating circuitry and this kind of deterioration has previously been reported in en bloc in vitro preparations (Suzue 1984).
4.3 Involvement of the nTS in inflammatory responses
Marty et al. (2008) showed that, in brainstem slices incubated with IL-1β (for one hour), nTS neurons showed an increase in spontaneous activity that was not due to IL-1β directly but rather due to the activation of the COX-2 pathway (see Figure 5) and increased local PGE2 release which modulate nTS neurons via two separate presynaptic pathways. One pathway is hypothesized to be the vagal afferent inputs which are attenuated by PGE2 while the other pathway is believed to be the local interneuronal network within the nTS and increases the excitability of nTS neurons (Marty et al. 2008). In our in vivo experiments we saw an increase in the frequency of ventilation with LPS instillation and this may be a result of the facilitation that occurs as the COX-2 pathway is upregulated. Interestingly, our in vitro preparations showed a decrease in frequency of inspiratory bursting and this is difficult to explain in light of these two pathways but is most likely due to direct action locally. Hofstetter et al. (2007) recently showed that PGE2 can alter breathing patterns both in vivo and in vitro en bloc preparations. The fact that the inflammatory response is mediated by a change in protein expression may explain why significant differences were observed for the en bloc IL-1β injections after 80 minutes. IL-1β does not seem to be acting via the same mechanism in vitro as in vivo.
Figure 5.
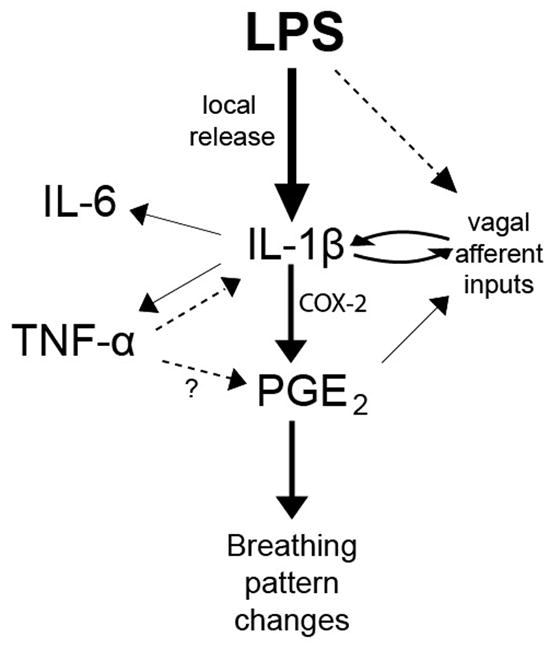
A representation of how infection is translated into altered autonomic control. The invading bacteria are sensed by the immune system and IL-1β release is increased. IL-1β leads to the production of the other pro-inflammatory cytokines, IL-6 and TNF-α, as well as the downstream molecule PGE2. IL-1β also increases vagal afferent signaling to the NTS. TNF-α and vagal stimulation can positively feedback and increase the production of IL-1β, possibly leading to a runaway immune response which disrupts respiration.
The nTS plays a crucial role in integrating afferent signaling from the lungs, heart, kidney, and other viscera as well as propagating autonomic afferent input to regulatory centers of the brainstem (Bonham and McCrimmon, 1990; Sun, 1995; Ito and Sved, 1997; Travagli et al., 2006). Because the nTS is a convergence point for these afferents (Kalia and Mesulam, 1980a,b), altered local excitability in the nTS might occur in response to systemic or visceral inflammation which is sensed via these vagal afferents (Konsman et al., 2008). In addition to LPS injection, cytokines have been injected peripherally and can induce autonomic changes as well as increase mRNA message for IL-1β, IL-6, and TNF-α mRNA in the nTS (Graff and Gozal, 1999; Churchill et al., 2006) probably through increased afferent traffic (Ek et al., 1998).
Increased vagal afferent signaling elicits an increase in cytokine production in the nTS; however, this might not account for the entire increase in production (Balan et a., 2011). Cytokines are also believed to act upon receptors at the blood-brain barrier and activate downstream messengers that act locally, as peripheral administration of cytokines increases cytokine levels in the nTS. In response to infection, a dramatic increase in cytokine production is observed in the nTS (Churchill et al., 2006; Balan et al., 2011). Several different cell types, including microglia, neurons, and astrocytes, can produce cytokines in the CNS, but which cell type is responsible for the increase in production observed during neuroinflammation and the time course of acute and chronic inflammatory changes in the brainstem unknown.
4.4 The role of cytokines in neural development
A cascade of events occurs during infection and they depend upon an inflammatory response that results in the release of a host of cytokines, both pro- and anti-inflammatory (Dinarello, 1991). Cytokines evoke changes in neural activity that we are only now beginning to understand. Autonomic changes in response to infection have widely been reported but we have little mechanistic detail regarding the way in which humoral agents such as cytokines can provoke changes in neural activity (Olsson et al., 2003; Hofstetter et al., 2007; Marty et al., 2008). Moreover, developmental changes in the autonomic nervous system may result in a very different inflammatory response when compared to that seen in adults (Walker et al., 2011) where they play a role in development and may serve as trophic factors that promote circuit organization in the nervous system (Gadient and Otten, 1994). Early exposure to elevated cytokines may have long-term neurodevelopmental consequences, particularly in autonomic control. Figure 5 provides an overview of the sequence of events we believe to be occurring in the brainstem when LPS initiates a cascade of cytokine release and alters control of breathing. With LPS exposure, IL-1β release occurs and this, in turn, stimulates production and release of IL-6 and TNF-α. The main result of this upregulation and release of cytokines is induction of prostaglandin release and altered synaptic traffic to affect downstream autonomic circuits controlling breathing.
Previous work has suggested that pro-inflammatory cytokines can play a fundamental role in neurodevelopment, and that altering the normal balance of cytokines in the brain after infection can cause abnormal development. Pregnant mice infected with the influenza virus gave birth to pups that displayed abnormal behavioral and social interactions (Shi et al., 2003; Churchill et al., 2006). Pregnant mice injected with IL-6 give birth to pups that also exhibit developmental differences from non-infected controls (Smith et al., 2007). IL-1β and TNF-α activate a downstream transcription factor, nuclear factor κb (NF-κb), that results in altered synaptic transmission and plays a role in development (O’Neill and Kaltschmidt, 1997). Other investigators have reported that infection can increase cytokine levels in the CNS (Qin et al., 2007). So, altering cytokine levels during crucial developmental periods has the potential to lead to long-term changes in CNS function.
4.5 Interaction of cytokines and the COX- pathway
Figure 5 summarizes our hypothesized scheme of the interaction between systemic infection (LPS in the periphery) and central autonomic processing. IL-1β is central to the changes in breathing pattern evoked by infection and this is most likely due to its precedence in the COX-2 pathway. Once PGE2 is produced, local excitability changes in nTS alter the gain of projections to the rhythm-generating and pattern transmission network in the ventral brainstem. The results that we have presented here provide a first attempt to differentiate the role that compartmentalized infection plays in modulating the central control of respiration via gating in the nTS. We have restricted our focus to IL-1β and LPS in this set of experiments and the role that other pro-inflammatory cytokines (IL-6 and TNF-α for example) play in altered control of breathing in response to infection has yet to be explored. Additionally, the role that COX-2 pathway plays in altering neural processing in the brainstem in response sepsis and inflammatory state will need further exploration.
4.6 Critique of Methods
We have attempted to provide a comparison of two animal models of inflammation. The in vitro en bloc preparation was obtained from young rat pups to promote long-lasting fictive inspiratory drive while we used older animals for our in vivo experiments. The developmental changes from P0 to P11 include a host of changes in neurotransmitter profile (Liu et al., 2001; Herlenius and Lagercrantz, 2001) and we realize that developmental changes in the underlying neural circuitry that processes sensory inputs and generates inspiratory rhythm may be radically different in these preparations. Additionally, both preparations showed time-dependent deterioration. We chose not to monitor blood gases in our in vivo experiments (due to the limited volume and access considerations in these small animals) and the en bloc preparation runs down over time. These limitations may be addressed by more extensive in vivo work or moving to a working heart-brainstem preparation in the future. Further experiments to characterize the underlying developmental changes in breathing circuitry as well as development of the immune response in the period immediately following birth are critically necessary. We present these experiments as proof of concept that these models can facilitate our understanding of how the immune response is transduced into altered neural control of autonomic function.
Conclusions and Clinical Relevance
Endotoxin (LPS) and IL-1β are the first two agents in a cascade of cellular signaling that is crucial to our immune response to infection. In neonates, with a developing but immature system, inflammation can exert a devastating influence upon neural development and autonomic control. We have shown that changes in inspiratory drive occur at the level of the brainstem very soon after exposure to endotoxin or local microinjection of a pro-inflammatory cytokine. Because inflammation has such a broad impact upon autonomic function—depending upon the severity of infection—development of animal models that allow dissection of the mechanisms by which dysregulation of autonomic control occurs is key to progress in our understanding of neural network changes and the time course of immune responses to sepsis.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants RO1HL056470 (to C. G. Wilson) and R21HL098628 (to R. J. Martin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balan KV, Kc P, Hoxha Z, Mayer CA, Wilson CG, Martin RM. Vagal afferents modulate cytokine-mediated respiratory control at the neonatal brainstem. Respir Physiol Neurobiol. 2011 doi: 10.1016/j.resp.2011.03.003. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol. 1990;427(1):261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Ryan RM, Ball HJ, MacDonald CJ. Prostaglandin E(2) inhibits calcium current in two sub-populations of acutely isolated mouse trigeminal sensory neurons. J Physiol. 2002;539(Pt 2):433–444. doi: 10.1113/jphysiol.2001.013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;93(2):929–941. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- Churchill L, Taishi P, Wang M, Brandt J, Cearley C, Rehman A, Krueger JM. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1 [beta] or tumor necrosis factor [alpha] Brain Res. 2006;1120(1):64–73. doi: 10.1016/j.brainres.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Kattwinkel J, Nattie C, Robinson M. Margin of safety for discharge after apnea in preterm infants. Pediatrics. 1997;100(5):795–801. doi: 10.1542/peds.100.5.795. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77(8):1627–1652. [PubMed] [Google Scholar]
- Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci. 1998;18(22):9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159(1):85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer CR, Tyson JE, Philips JB, 3rd, Edwards W, Lucey JF, Catz CS, Shankaran S, Oh W. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Ped, Infect Dis J. 1998;17(7):593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- Foglyano R, Kaffashi F, Dick TE, Loparo K, Wilson CG. Quantifying the complexity of neural network output using entropy measures. BMC Neurosci. 2009;10(Suppl 1):322. [Google Scholar]
- Gadient R, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Res. 1994;637(1–2):10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Graff GR, Gozal D. Cardiorespiratory responses to interleukin-1beta in adult rats: role of nitric oxide, eicosanoids and glucocorticoids. Archives Physiol Biochem. 1999;107(2):97–112. doi: 10.1076/apab.107.2.97.4344. [DOI] [PubMed] [Google Scholar]
- Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278(3):207–211. [PubMed] [Google Scholar]
- Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Archives Ped Adolescent Med. 2003;157(1):26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23(3):144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Hofstetter AO, Saha S, Siljehav V, Jakobsson PJ, Herlenius E. The induced prostaglandin E2 pathway is a key regulator of the respiratory response to infection and hypoxia in neonates. Proc Natl Acad Sci USA. 2007;104(23):9894–9. doi: 10.1073/pnas.0611468104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Human Devel. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- Ito S, Sved AF. Influence of GABA in the nucleus of the solitary tract on blood pressure in baroreceptor-denervated rats. Am J Physiol. 1997;273(5 Pt 2):R1657–1662. doi: 10.1152/ajpregu.1997.273.5.R1657. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhang YH, Clark JD, Tempel BL, Nicol GD. Prostaglandin E2 inhibits the potassium current in sensory neurons from hyperalgesic Kv1.1 knockout mice. Neurosci. 2003;119(1):65–72. doi: 10.1016/s0306-4522(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Jin Y, Wang Z, Zhang Y, Yang B, Wang WH. PGE2 inhibits apical K channels in the CCD through activation of the MAPK pathway. Am J Physiol. 2007;293(4):F1299–1307. doi: 10.1152/ajprenal.00293.2007. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980a;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980b;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Euro J Neurosci. 2008;28(12):2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Kwong K, Lee L. Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol. 2005;564(Pt 2):437–450. doi: 10.1113/jphysiol.2004.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Schild JH. Persistent tetrodotoxin-resistant Na+ currents are activated by prostaglandin E2 via cyclic AMP-dependent pathway in C-type nodose neurons of adult rats. Biochem Biophys Res Comm. 2007;355(4):1064–1068. doi: 10.1016/j.bbrc.2007.02.086. [DOI] [PubMed] [Google Scholar]
- Liu C, Qian L, Su Y, Bao L. Prostaglandin E2 promotes Na1.8 trafficking via its intracellular RRR motif through the protein kinase A pathway. Traffic. 2010;11(3):405–417. doi: 10.1111/j.1600-0854.2009.01027.x. [DOI] [PubMed] [Google Scholar]
- Liu YY, Ju G, Wong-Riley MT. Distribution and colocalization of neurotransmitters and receptors in the pre-Bötzinger complex of rats. J App Physiol. 2001;91(3):1387–1395. doi: 10.1152/jappl.2001.91.3.1387. [DOI] [PubMed] [Google Scholar]
- Marty V, El Hachmane M, Amédée T. Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta. Euro J Neurosci. 2008;27(12):3132–3150. doi: 10.1111/j.1460-9568.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- Navar-Boggan AM, Halsey NA, Golden WC, Escobar GJ, Massolo M, Klein NP. Risk of fever and sepsis evaluations after routine immunizations in the neonatal intensive care unit. J Perinatol. 2010;30(9):604–609. doi: 10.1038/jp.2010.8. [DOI] [PubMed] [Google Scholar]
- Nelson KB. Infection in pregnancy and cerebral palsy. Developmental Medicine and Child Neurology. 2009;51(4):253–254. doi: 10.1111/j.1469-8749.2008.03256.x. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends in Neurosciences. 1997;20(6):252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Olsson A, Kayhan G, Lagercrantz H, Herlenius E. IL-1 beta depresses respiration and anoxic survival via a prostaglandin-dependent pathway in neonatal rats. Pediatr Res. 2003;54(3):326–31. doi: 10.1203/01.PDR.0000076665.62641.A2. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. PNAS, USA. 1991;88(6):2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Waxman SG. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Research. 2004;1023(2):264–271. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics. 2002;110(4):673–680. doi: 10.1542/peds.110.4.673. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal Influenza Infection Causes Marked Behavioral and Pharmacological Changes in the Offspring. J Neurosci. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans. 2007;35(Pt 5):1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. Journal of Neuroscience Methods. 1987;21(2–4):321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Progress in neurobiology. 1995;47(3):157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Physiol. 2006;68(1):279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalonga N, David M, Bielanska J, Vicente R, Comes N, Valenzuela C, Felipe A. Immunomodulation of voltage-dependent K+ channels in macrophages: molecular and biophysical consequences. J Gen Physiol. 2010;135(2):135–147. doi: 10.1085/jgp.200910334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Smolders MA, Gemen EF, Antonius TA, Leuvenink J, de Vries E. Development of lymphocyte subpopulations in preterm infants. Scand J Immunol. 2011;73(1):53–58. doi: 10.1111/j.1365-3083.2010.02473.x. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Bonham AC. Effect of cardiopulmonary C fibre activation on the firing activity of ventral respiratory group neurones in the rat. J Physiol. 1997;504(pt2):453–66. doi: 10.1111/j.1469-7793.1997.453be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Colford JM. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284(11):1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290(20):2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Chen X, Foglyano RM, Wilson CG, Solomon IC. Respiratory network complexity in neonatal rat in vivo and in vitro. Adv Exper Med Biol. 2008;605:393–398. doi: 10.1007/978-0-387-73693-8_69. [DOI] [PubMed] [Google Scholar]
- Zhang L, Karpinski E, Benishin CG. Prostaglandin E2 modulates a non-inactivating potassium current in rat neurohypophyseal nerve terminals. Neurochem Intern. 1999;35(5):345–355. doi: 10.1016/s0197-0186(99)00073-x. [DOI] [PubMed] [Google Scholar]
- Zheng T, Kakimura J, Matsutomi T, Nakamoto C, Ogata N. Prostaglandin E2 has no effect on two components of tetrodotoxin-resistant Na+ current in mouse dorsal root ganglion. J Pharm Sci. 2007;103(1):93–102. doi: 10.1254/jphs.fp0061402. [DOI] [PubMed] [Google Scholar]