Abstract
Alcohol use, which typically begins during adolescence and differs between males and females, is influenced by both the rewarding and aversive properties of the drug. One way adolescent alcohol use may modulate later consumption is by reducing alcohol s aversive properties. Here, we used a conditioned taste aversion (CTA) paradigm to determine if pre-exposure to alcohol (ethanol) during adolescence would attenuate ethanol-induced CTA assessed in adulthood in a sex-dependent manner. Male and female Long-Evans rats were given intraperitoneal (i.p.) injections of saline or 3.0 g/kg ethanol in a binge-like pattern during postnatal days (PD) 35–45. In adulthood (> PD 100), rats were given access to 0.1% saccharin, followed by saline or ethanol (1.0 or 1.5 g/kg, i.p.), over four conditioning sessions. We found sex differences in ethanol-induced CTA, with males developing a more robust aversion earlier in conditioning. Sex differences in the effects of pre-exposure were also evident: males, but not females, showed an attenuated CTA in adulthood following ethanol pre-exposure, which occurred approximately nine weeks earlier. Taken together, these findings indicate that males are more sensitive to the aversive properties of ethanol than females. In addition, the ability of pre-exposure to the ethanol US to attenuate CTA is enhanced in males compared to females.
Keywords: ethanol, adolescence, conditioned taste aversion, US pre-exposure effect, sex differences
1. Introduction
In the United States, the majority of individuals begin consuming alcohol (ethanol) during adolescence [1]. Ethanol use during this time period is associated with increased drinking and vulnerability to developing dependence later in life [2]. Sex differences in ethanol intake, with males consuming more and having higher rates of dependence, are most notable in adulthood [3], but the divergence may originate in late adolescence. For example, boys and girls who reach puberty early have higher rates of ethanol use [4, 5] and early menarche is associated with a higher incidence of drinking and smoking in adolescent girls [6, 7]. Similarly, as rodents progress through adolescence (postnatal day PD 28–60; [8, 9]), sex differences in ethanol-induced behavior emerge. Males and females show similar ethanol consumption and withdrawal in early adolescence [10, 11], yet by adulthood, females typically drink more ethanol [10–12] and display less severe withdrawal symptoms [13–16]. We recently demonstrated that sex-specific drinking patterns of adult rats are influenced by exposure to ethanol during adolescence and can be altered by gonadectomy performed prior to the onset of puberty [17]. Thus, sex differences in ethanol-induced behavior may be due at least in part to the developmental changes in neurobiology and hormones taking place during adolescence [18].
Interplay between the reinforcing and aversive properties of drugs is thought to motivate drug-taking behavior [19–22]. As such, ethanol exposure during adolescence may influence subsequent consumption by altering the reinforcing value of ethanol [23–25] or by modulating the drug s aversive properties [26, 27]. One method that has proven particularly useful for studying the aversive properties of drugs is the conditioned taste aversion procedure (CTA, [28]). In the traditional form of this Pavlovian conditioning paradigm [29, 30], animals’ are given access to a novel tastant (the conditioned stimulus, or CS), followed by exposure to a drug compound (the unconditioned stimulus, or US). The conditioned response (CR) is reflected as reduced consumption (or avoidance) of the CS when it is presented at a later time. Notably, the development of CTA is influenced by pre-exposure to the US [31]. This “US pre-exposure effect”, which has been demonstrated with a variety of CTA-inducing agents including LiCl, cocaine, amphetamine, morphine, and ethanol [32–37], manifests as reduction in the avoidance of the CS. That is, animals’ pre-exposed to the US tend to consume greater quantities of the CS than those not pre-exposed to the US.
In most cases, the US pre-exposure effect has been investigated following relatively short intervals (i.e. 1–14 days) between pre-exposure and conditioning and the effect tends to diminish considerably as this interval increases (e.g., [36, 38]). Nonetheless, previous studies indicate that in male rodents pre-exposure to ethanol, nicotine, or diazepam attenuates CTA assessed approximately 4–6 weeks later [27, 39–42]. However, it remains unclear if the effect persists longer than these intervals and whether females are also sensitive to pre-exposure, as previous studies have only assessed the effect in male rodents.
The purpose of the present study was to investigate whether adult males and females display disparate ethanol-induced CTA and to determine if there are sex differences in the expression of CTA in rats that were exposed to ethanol in adolescence. Rats were administered ethanol using a binge-like pattern that has been shown to alter cognition, motor behavior, and ethanol consumption in adulthood [17, 43, 44].
Assessment of CTA to ethanol was performed approximately 9 weeks following the last pre-exposure treatment.
2. Materials and Methods
2.1 Subjects
A total of 118 male and female Long-Evans rats, which were the offspring of stock rats that were purchased from Simonson Labs (Gilroy, CA) or Taconic Farms (Albany, NY) and bred in our animal facility, were used in these experiments. For the duration of the experiment, they were housed in same-sex groups of 2–3 littermates/cage on a 12 hr light/dark cycle (lights on at 0800 h) and maintained in a temperature controlled room. Food and water were available ad libitum, except as noted below. All procedures were consistent with the ‘Principles of Laboratory Animal Care’ (NIH Publication no. 85–23) and were approved by the IACUC at the University of Illinois, Urbana-Champaign, USA.
2.2 Binge-like exposure to saline or ethanol
Male (n = 67) and female (n = 51) rats were administered ethanol or saline in a binge-like pattern of exposure beginning on PD 35. The binge treatment included i.p. injections of saline or 3.0 g/kg ethanol (25% w/v; Ricca Chemical, Arlington, TX), once per day for two consecutive days, followed by one day with no injections. The final exposure occurred on PD 45, for a total of 8 injections across 11 days. This pattern of exposure has been shown previously to produce enduring behavioral and neurophysiological changes that persist into adulthood [17, 43, 44].
2.3 Effects of US pre-exposure on ethanol-induced CTA
Rats that were pre-exposed to saline or ethanol began water restriction on PD 100. Water bottles were removed from their home cages and rats were placed individually into drinking chambers (35 × 25 × 31 cm acrylic tub containing beta chip bedding) for 30-min/day. During this time, they had access to water presented via a 100 ml sipper tube that contained a double ball-bearing stopper to minimize leakage (Ancare, Bellmore, NY). After the rats’ daily water intake stabilized (approx. 7–10 days), a conditioning procedure was implemented that has previously been shown to produce robust ethanol-induced CTA [45]. Conditioning occurred across four cycles, each of which lasted four days. On the first day of each cycle, rats were given 30-min access to a single sipper tube that contained 0.1% saccharin. Approximately 20-min later, they received an i.p. injection of saline, 1.0 or 1.5 g/kg ethanol (w/v) and were immediately placed back into their homecages. The next three days were ‘water recovery days’, during which rats were allowed 30-min access to water. In cases where a rat s weight fell below 85% of its weight on the day prior to the start of water restriction (~10% of the rats in this study), an additional 30-min period of access to water was granted ≥ 2 hrs after the rat was returned to its homecage. After the fourth conditioning cycle, the strength of CTA was assessed in all rats during an aversion test in which they had access to the 0.1% saccharin solution for a final 30-min drinking session.
2.4 Data analysis
Fluid intake (ml) for each daily drinking session was calculated by weighing the drinking tubes before and after each drinking session. Baseline water intake was calculated as the mean intake over the last three baseline drinking sessions for each group. Due to group differences in baseline saccharin consumption, each group’s mean consumption on conditioning day 1 served as the 100% value for the analyses, and intake on each subsequent session was taken as a percentage of this value. A three-way ANOVA (sex × pre-exposure × age) was used to assess the animals’ weights during adolescence and adulthood. Separate two-way ANOVAs (sex × pre-exposure) were used to assess sex differences in baseline water and saccharin intake. A four-way mixed factorial ANOVA was used to assess sex differences in saccharin intake during the conditioning phase with sex (male or female), pre-exposure (saline or 3.0 g/kg ethanol) and US (saline, 1.0, or 1.5 g/kg ethanol) as the between subjects factors and conditioning session (1–4) as the repeated factor. Additionally, three-way and two-way ANOVAs were used to assess the expression of CTA during the aversion test in males and females. Significant main effects and interactions were further assessed by Holm-Sidak post-hoc tests.
3. Results
Pre-exposure to ethanol had a transient influence on the animals’ body weights during adolescence. As reported previously with this treatment procedure (Sherrill et al., 2011), males and females given repeated ethanol weighed on average less on the last day of pre-exposure (PD 45) compared to those given saline. Three-way ANOVA revealed a significant sex × age × pre-exposure interaction (F2,224 = 3.82, p < 0.05). Males given ethanol weighed ~16% less than saline controls after binge-like exposure (155 ± 2.8 vs. 184 ± 2.9 g), whereas ethanol pre-exposed females weighed ~7% less than saline-treated controls (128 ± 1.6 vs. 138 ± 1.5 g). However, by PD 100 there were no significant differences in body weight between ethanol and saline pre-exposed males (380 ± 6.2 vs. 379 ± 6.4 g) or females (228 ± 3.4 and 233 ± 2.7 g).
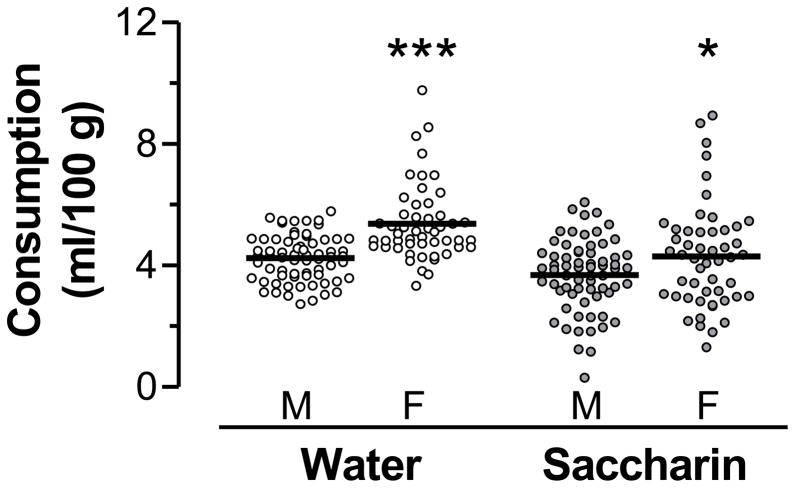
Before conditioning began in adulthood, rats were given 30-min daily access to water to acclimate them to the drinking schedule. Separate two-way ANOVAs (sex × pre-exposure) revealed a significant sex difference in baseline water intake and saccharin consumption (Fig. 1), with females drinking more water per body weight prior to the start of conditioning (main effect of sex: F1,114 = 37.6, p < 0.001) and more saccharin per body weight during the first conditioning session (main effect of sex: F1,114 = 5.40, p < 0.05). The main effects of pre-exposure and the interactions were not significant (p-values > 0.05) for water or saccharin intake. Thus, the data in Fig. 1 are shown collapsed across pre-exposure condition.
Figure 1.
Baseline water and saccharin consumption per body weight for males (M) and females (F). Data for individual rats are shown with the horizontal line indicating the mean value for the group. ***p < 0.001 and *p < 0.05, compared to males.
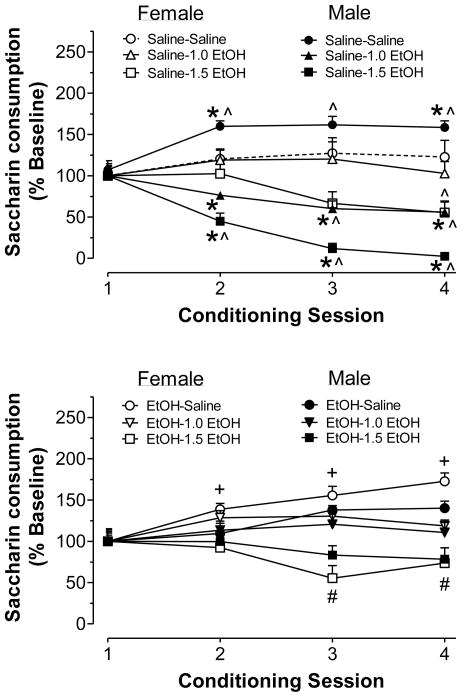
To facilitate within- and between-group comparisons of saccharin consumption across conditioning days, data for individual rats were normalized to their baseline level of saccharin intake. An omnibus ANOVA of these data revealed a 4-way interaction between sex, pre-exposure, US, and conditioning session (F6,315 = 3.32, p < 0.01). Among saline pre-exposed rats (Fig. 2a), males drank significantly more saccharin than females during sessions 2 and 4. In addition, saccharin consumption escalated across conditioning sessions in the group of males given saline as the US. However, after one saccharin-ethanol pairing, males drank significantly less saccharin than females, and showed reduced saccharin consumption across conditioning (i.e. sessions 2–4). Thus, males displayed CTA after just one pairing of saccharin and ethanol; meanwhile, saccharin consumption increased across sessions in males given saline as the US. Saccharin consumption in saline pre-exposed females remained relatively similar across conditioning sessions, with the only exception occurring after three pairings of saccharin and 1.5 g/kg ethanol. Relative to their baseline intake on session 1, saline pre-exposed females consumed significantly less saccharin on the fourth conditioning session. Thus, females acquired CTA more slowly than males, and only demonstrated CTA following conditioning with 1.5 g/kg ethanol.
Figure 2.
Saccharin consumption during conditioning sessions in males compared to females. Rats were pre-exposed to saline (top) or 3.0 g/kg ethanol (bottom) in a binge-like pattern to from PD 35–45. CTA was assessed after all the rats reached PD 100. During conditioning, rats were given 30-min access to 0.1% saccharin (CS) followed by an i.p. injection of saline, 1.0 g/kg or 1.5 g/kg EtOH (US). Shown is saccharin intake during each conditioning session as a percentage of consumption during the initial session. Groups (n = 8–12 each) are represented as pre-exposure (Saline or EtOH)-US (Saline, 1.0 EtOH, or 1.5 EtOH). *p < 0.05, compared to saline pre-exposed females within session and US; ^p < 0.05, compared to session 1, within US and sex; +p < 0.05, compared to session 1, within the saline US and collapsed across sex; #p < 0.05, compared to session 1, within 1.5 g/kg ethanol US and collapsed across sex.
Sex differences in CTA acquisition were abolished in animals’ pre-exposed to ethanol (Fig 2b.). Both males and females demonstrated a similar degree of CTA, as indicated by a reduction of saccharin intake after two pairings of saccharin and 1.5 g/kg ethanol. However, lack of sex differences in ethanol pre-exposed rats is most likely due to an attenuation of CTA in males following conditioning to both doses of ethanol (1.0 or 1.5 g/kg). There was no significant effect of ethanol pre-exposure on CTA acquisition in females.
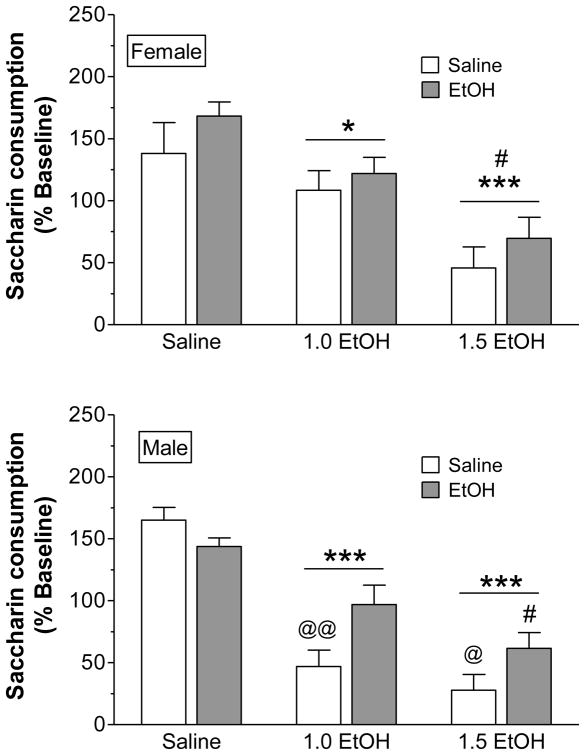
Analysis of saccharin intake during the aversion test provided additional evidence for sex differences in CTA and the US pre-exposure effect (Fig. 3). Three-way ANOVA revealed significant main effects of sex (F1,106 = 4.93, p < 0.05), pre-exposure (F1,106 = 6.87, p < 0.05), and US (F2,106 = 53.6, p < 0.001). Males consumed less saccharin than females during the aversion test, suggesting CTA was more robust in male rats. In addition, CTA was dose dependent, with the strongest CTA arising in rats given the 1.5 g/kg ethanol dose during conditioning. In order to investigate the effects of ethanol pre-exposure on saccharin intake during the aversion test, separate two-way ANOVAs were performed for males and females. ANOVA revealed a significant pre-exposure x US interaction in males (F2,61 = 4.76, p < 0.05), but not in females (p > 0.05). Similar to CTA acquisition, males showed reduced saccharin consumption at 1.0 and 1.5 g/kg ethanol, and an attenuation of CTA was evident in ethanol pre-exposed rats. Despite a significant reduction in saccharin intake following conditioning with both ethanol doses (main effect of US: F1,45 = 16.4, p < 0.001), CTA was not significantly influenced by ethanol pre-exposure in females.
Figure 3.
Saccharin consumption in females (top) and males (bottom) during the aversion test (n = 8–12/group). The test was 30 min in duration and occurred three days following the last conditioning session with saline, 1.0 or 1.5 g/kg EtOH. *p < 0.05 and ***p < 0.001, compared to saline US and collapsed across pre-exposure; #p < 0.01, compared to 1.0 EtOH US and collapsed across pre-exposure; @p < 0.05 and @@p < 0.01, compared to EtOH pre-exposed group, within US dose.
4. Discussion
In the present study, we investigated potential sex differences in ethanol-induced CTA and in the ability of ethanol pre-exposure during adolescence to modulate the aversive properties of ethanol in adulthood. In adult rats pre-exposed to saline during adolescence, we found that ethanol-induced CTA was enhanced in males compared to females. Specifically, males showed evidence of a CTA (i.e., reduced saccharin consumption compared to saline US controls) after only a single CS-US pairing and at both doses of the ethanol US. The CTA in females was not evident until at least the third pairing and only at the highest dose of the ethanol US. In addition, the results of the aversion test revealed that the magnitude of CTA was greater in males compared to females, particularly at the 1.0 g/kg dose of ethanol. Sex differences in the effects of ethanol pre-exposure were also evident. In males, ethanol pre-exposure ~9 weeks prior to the first saccharin-ethanol pairing led to a reduction in the CTA that developed to either 1.0 or 1.5 g/kg ethanol. In females, however, this pre-exposure had no effect on the CTA with a 1.0 g/kg ethanol US and a modest, though statistically non-significant, effect on the CTA with a 1.5 g/kg US. Thus, the long lasting effects of US pre-exposure are more prominent in males compared to females.
The sex differences in CTA we observed in our saline pre-exposed groups suggest that males and females respond differently to the aversive properties of ethanol, with males being more sensitive. We found that males developed CTA after fewer conditioning sessions and demonstrated more robust aversion when 1.0 and 1.5 g/kg served as the US (i.e. males show a leftward shift in the curve). After three pairings of saccharin and 1.5 g/kg ethanol, saline pre-exposed males were consuming 98% less saccharin than they were at baseline; females were consuming 45% less. Greater sensitivity to ethanol’s aversive properties in males has been reported previously. For example, males experience more severe ethanol withdrawal symptoms than females, including increased hypothermia and anxiety behavior following ethanol exposure [15, 46, 47]. Importantly, the enhanced sensitivity of male rodents to ethanol-induced aversion may contribute to their reduced ethanol self-administration behavior [11, 12, 17]. Consistent with this notion, previous studies have demonstrated an inverse relationship between ethanol self-administration and ethanol-induced CTA. In these reports, rodents that consume lower doses of ethanol also show more robust CTA [48, 49], though this may be strain dependent [50–52]. Another potential contributing factor for the enhanced sensitivity of males to ethanol-induced CTA is that aversive learning is enhanced in males, compared to females, and therefore they are quicker to learn the CS-US association between saccharin and ethanol. Since CTA was first described nearly a half a century ago, numerous reports have documented sex differences in CTA learning. Indeed, they have been observed when classic aversive stimuli such as lithium chloride (LiCl) served as the US [53–55], and also with certain drugs of abuse [52, 56–58]. Other reports, however, indicate no sex differences in CTA [54, 55, 59]. In previous studies with ethanol, sex differences are not always consistent and are influenced by social context, age, and strain [52, 57, 59]. Furthermore, sex differences typically manifest as differences in rate of extinction between males and females, rather than as differences in CTA acquisition [53]. One additional factor that seems to dictate the direction of the sex difference is the nature of the US. For example, females tend to develop more robust CTA to amphetamine [58], while showing less aversion to cocaine and LiCl [54–56]. Overall, the bulk of the literature on sex differences in CTA suggests no consistent evidence of a sex difference in aversive learning, per se. Moreover, studies using other models of aversive learning, such as conditioned place aversion and conditioned fear, have shown enhanced aversive learning in females compared to males [60, 61], enhanced learning in males compared to females [62], and no sex differences [63].
Given the differences in ethanol metabolism between adult male and female rats [47, 64], the relatively modest CTA displayed by females could be due to more rapid drug clearance. Hence, because females metabolize ethanol more quickly than males, they might experience the ethanol US to be less aversive. Given that CTA is influenced by the dose and duration of the US [45, 65], sex differences in peak blood ethanol concentration (BEC) or rate of ethanol metabolism might therefore underlie differences in ethanol-induced CTA between the sexes. However, BEC has not proven to be a reliable measure of ethanol’s aversive properties. Some studies suggest a positive relationship between BEC and the aversive qualities of ethanol [46, 47, 66], whereas others have found no association [45, 67], or a negative relationship [49, 68]. Furthermore, the aversive properties of ethanol have been reported to occur within 1–5 min following exposure [69]; thus, any differences in rate of ethanol clearance (occurring over minutes or hours) are not likely to contribute to the immediate aversion associated with the drug. Although we did not measure BEC in the present study, and therefore cannot definitively determine the role of metabolism in the current findings, it is unlikely that the rate of ethanol metabolism is primarily responsible for sex differences in ethanol-induced CTA.
In addition to their enhanced sensitivity to ethanol-induced CTA, males were also more sensitive to the ethanol pre-exposure effect. Previous studies in adult male rodents have also demonstrated a US pre-exposure effect for ethanol [35, 38, 70]. The attenuation of CTA has been shown to persist for up to 6 weeks when the exposure occurs in adolescence and is not likely due to disruptions in associative learning [27]. Here, we found that the pre-exposure effect persists in males for up to 9 weeks when ethanol is administered during adolescence. Although a number of potential mechanisms have been suggested to mediate the US pre-exposure effect [31], it seems unlikely that our results were influenced by pre-exposure effects on ethanol metabolism. A recent study using a similar binge-like pattern of ethanol exposure in adolescence showed no effects of pre-exposure on BECs measured after re-exposure during adulthood [71]. As such, a more likely explanation for the long-lasting effect of ethanol pre-exposure shown here is that exposure to ethanol during adolescence induced changes in neurophysiology that altered rats’ responsiveness to ethanol in adulthood. In support of this hypothesis, binge-like exposure to ethanol during adolescence has been shown previously to produce long-lasting changes in the expression of dopaminergic and glutamatergic receptors in the prefrontal cortex and nucleus accumbens [25]. In addition, microdialysis studies indicate that nucleus accumbens dopamine levels are elevated in adult rats pre-exposed to ethanol during adolescence [72]. Together, these neuroadaptations may contribute to altered sensitivity to the aversive, as well as the rewarding, properties of ethanol.
The sex differences in the effects of ethanol pre-exposure on ethanol-induced CTA may be influence by differences in gonadal hormones between males and females. Recently, our lab demonstrated that gonadectomy before puberty abolished sex differences in ethanol consumption during adulthood [17]. Based on this and other studies showing that sex differences in ethanol-induced behavior predominantly emerge post-puberty [18, 73], we hypothesized that rising hormones during adolescence contribute to the observed sexual dimorphism. Further support for this hypothesis was provided recently by an elegant study from Vetter-O’ Hagen and Spear (2011) showing that sex differences in adult drinking behavior were influenced by pre-pubertal gonadectomy [74]. Specifically, pre-pubertal castration led males to increase their consumption of ethanol in adulthood. Castration in adulthood also increased consumption, however, suggesting that gonadal hormones during adolescence may not solely account for male-like drinking behavior. Another intriguing finding from that study was that rats in the sham-surgery control groups drank more ethanol than controls that did not undergo surgery, suggesting that a single exposure to surgical anesthesia during adolescence can alter behavior in adulthood. Thus, in future studies of the role of gonadal hormones in sex differences in the US pre-exposure effect, it will be important to control for surgery stress and exposure to anesthesia.
In summary, we found that males exhibit a more rapid and robust ethanol-induced CTA than females. Furthermore, pre-exposure to ethanol during adolescence led to a long-lasting attenuation of the aversive properties of ethanol in males, but not females. Further studies are warranted to elucidate the mechanisms by which ethanol pre-exposure influences CTA across protracted time spans. Exposure to ethanol during adolescence produces neuroadaptations in brain regions well known to mediate the rewarding and aversive properties of drugs, although the relationship between these changes and aversive learning is unknown. Nonetheless, the effects of ethanol pre-exposure on the aversive properties of the drug are interesting in light of previous reports showing that animals’ pre-exposed to ethanol during adolescence tend to consume greater quantities of ethanol in adulthood [17, 23, 44, 75]. Thus, this change in ethanol self-administration may be due, in part, to a reduction of ethanol’s aversive properties. From the current study it is not possible to determine the extent to which gonadal hormones have an impact on sex differences in ethanol-induced CTA and the US pre-exposure effect. Given the pivotal role puberty plays in adult male and female ethanol-induced behavior, future studies should address whether rising gonadal hormones during adolescence influence sensitivity to ethanol’s aversive qualities later in life.
Acknowledgments
This study was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21 AA017354). We thank Diana Lone, Meghan Skotnicki, Ashley Guerrero, and Jenna Wellington for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 3.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 4.Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114:e300–6. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tschann JM, Adler NE, Irwin CE, Jr, Millstein SG, Turner RA, Kegeles SM. Initiation of substance use in early adolescence: the roles of pubertal timing and emotional distress. Health Psychol. 1994;13:326–333. doi: 10.1037//0278-6133.13.4.326. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DM, Killen JD, Hayward C, Robinson TN, Hammer LD, Kraemer HC, Varady A, Taylor CB. Timing and rate of sexual maturation and the onset of cigarette and alcohol use among teenage girls. Arch Pediatr Adolesc Med. 1994;148:789–795. doi: 10.1001/archpedi.1994.02170080019004. [DOI] [PubMed] [Google Scholar]
- 7.Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: associations between and within families across late adolescence. Dev Psychol. 2000;36:180–189. [PubMed] [Google Scholar]
- 8.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 9.Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- 10.Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 11.Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 12.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 13.Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- 14.Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–1696. [PubMed] [Google Scholar]
- 15.Wiren KM, Hashimoto JG, Alele PE, Devaud LL, Price KL, Middaugh LD, Grant KA, Finn DA. Impact of sex: determination of alcohol neuroadaptation and reinforcement. Alcohol Clin Exp Res. 2006;30:233–242. doi: 10.1111/j.1530-0277.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- 16.Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Res. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- 17.Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav Brain Res. 2011;216:569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Brockwell NT, Eikelboom R, Beninger RJ. Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacol Biochem Behav. 1991;38:513–517. doi: 10.1016/0091-3057(91)90006-n. [DOI] [PubMed] [Google Scholar]
- 20.Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–479. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang YC, Huang AC, Hsiao S. Paradoxical simultaneous occurrence of amphetamine-induced conditioned taste aversion and conditioned place preference with the same single drug injection: a new “pre- and post-association” experimental paradigm. Pharmacol Biochem Behav. 2010;95:80–87. doi: 10.1016/j.pbb.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191:1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- 23.Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav. 2010;96:476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 26.Chung CS, Wang J, Wehman M, Rhoads DE. Severity of alcohol withdrawal symptoms depends on developmental stage of Long-Evans rats. Pharmacol Biochem Behav. 2008;89:137–144. doi: 10.1016/j.pbb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 28.Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Conditioned Taste Aversion: An Annotated Bibliography. http://www.ctalearning.com.
- 30.Riley AL, Freeman KB. Conditioned flavor aversions: assessment of drug-induced suppression of food intake. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8.6E) doi: 10.1002/0471142301.ns0806es29. [DOI] [PubMed] [Google Scholar]
- 31.Riley AL, Simpson GR. The Attenuating Effects of Drug Preexposure on Taste Aversion Conditioning: Generality, Experimental Parameters, Underlying Mechanisms, and Implications for Drug use and Abuse. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2001. p. 505. [Google Scholar]
- 32.Riley AL, Simpson GR. Cocaine preexposure fails to sensitize the acquisition of cocaine-induced taste aversions. Pharmacol Biochem Behav. 1999;63:193–199. doi: 10.1016/s0091-3057(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 33.Rabin BM, Hunt WA, Lee J. Attenuation and cross-attenuation in taste aversion learning in the rat: studies with ionizing radiation, lithium chloride and ethanol. Pharmacol Biochem Behav. 1988;31:909–918. doi: 10.1016/0091-3057(88)90404-2. [DOI] [PubMed] [Google Scholar]
- 34.Holman EW. The effect of drug habituation before and after taste aversion learning in rats. Anim Learn Behav. 1976;4:329–332. [Google Scholar]
- 35.Berman RF, Cannon DS. The effect of prior ethanol experience on ethanol-induced saccharin aversions. Physiol Behav. 1974;12:1041–1044. doi: 10.1016/0031-9384(74)90152-8. [DOI] [PubMed] [Google Scholar]
- 36.Cappell H, Le Blanc AE. Conditioned aversion by amphetamine: rates of acquisition and loss of the attenuating effects of prior exposure. Psychopharmacologia. 1975;43:157–162. doi: 10.1007/BF00421018. [DOI] [PubMed] [Google Scholar]
- 37.Cappell H, LeBlanc AE. Parametric investigations of the effects of prior exposure to amphetamine and morphine on conditioned gustatory aversion. Psychopharmacology (Berl) 1977;51:265–271. doi: 10.1007/BF00431634. [DOI] [PubMed] [Google Scholar]
- 38.Barker LM, Johns T. Effect of ethanol preexposure on ethanol-induced conditioned taste aversion. J Stud Alcohol. 1978;39:39–46. doi: 10.15288/jsa.1978.39.39. [DOI] [PubMed] [Google Scholar]
- 39.Graham DL, Diaz-Granados JL. Periadolescent exposure to ethanol and diazepam alters the aversive properties of ethanol in adult mice. Pharmacol Biochem Behav. 2006;84:406–414. doi: 10.1016/j.pbb.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Hutchison MA, Albaugh DL, Riley AL. Exposure to alcohol during adolescence alters the aversive and locomotor-activating effects of cocaine in adult rats. Pharmacol Biochem Behav. 2010;97:370–376. doi: 10.1016/j.pbb.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Hutchison MA, Riley AL. Adolescent exposure to nicotine alters the aversive effects of cocaine in adult rats. Neurotoxicol Teratol. 2008;30:404–411. doi: 10.1016/j.ntt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Rinker JA, Hutchison MA, Chen SA, Thorsell A, Heilig M, Riley AL. Exposure to nicotine during periadolescence or early adulthood alters aversive and physiological effects induced by ethanol. Pharmacol Biochem Behav. 2011;99:7–16. doi: 10.1016/j.pbb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- 44.Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471–4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 45.Rinker JA, Busse GD, Roma PG, Chen SA, Barr CS, Riley AL. The effects of nicotine on ethanol-induced conditioned taste aversions in Long-Evans rats. Psychopharmacology (Berl) 2008;197:409–419. doi: 10.1007/s00213-007-1050-2. [DOI] [PubMed] [Google Scholar]
- 46.Crabbe JC, Feller DJ, Dorow JS. Sensitivity and tolerance to ethanol-induced hypothermia in genetically selected mice. J Pharmacol Exp Ther. 1989;249:456–461. [PubMed] [Google Scholar]
- 47.Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- 48.Orr TE, Whitford-Stoddard JL, Elkins RL. Taste-aversion-prone (TAP) rats and taste-aversion-resistant (TAR) rats differ in ethanol self-administration, but not in ethanol clearance or general consumption. Alcohol. 2004;33:1–7. doi: 10.1016/j.alcohol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Roma PG, Flint WW, Higley JD, Riley AL. Assessment of the aversive and rewarding effects of alcohol in Fischer and Lewis rats. Psychopharmacology (Berl) 2006;189:187–199. doi: 10.1007/s00213-006-0553-6. [DOI] [PubMed] [Google Scholar]
- 50.Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- 51.Cannon DS, Leeka JK, Block AK. Ethanol self-administration patterns and taste aversion learning across inbred rat strains. Pharmacol Biochem Behav. 1994;47:795–802. doi: 10.1016/0091-3057(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 52.Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63:91–99. [PubMed] [Google Scholar]
- 53.Chambers KC, Yuan D, Brownson EA, Wang Y. Sexual dimorphisms in conditioned taste aversions: Mechanism and function. In: Bouton ME, Fanselow MS, editors. Learning, motivation, and cognition: The functional behaviorism of Robert C Bolles. Washington, DC, US: American Psychological Association; 1997. pp. 195–224. [Google Scholar]
- 54.Chambers KC, Sengstake CB, Yoder RL, Thornton JE. Sexually dimorphic acquisition of a conditioned taste aversion in rats: effects of gonadectomy, testosterone replacement and water deprivation. Physiol Behav. 1981;27:83–88. doi: 10.1016/0031-9384(81)90303-6. [DOI] [PubMed] [Google Scholar]
- 55.Randall-Thompson JF, Riley AL. Morphine-induced conditioned taste aversions: assessment of sexual dimorphism. Pharmacol Biochem Behav. 2003;76:373–381. doi: 10.1016/j.pbb.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Busse GD, Freeman KB, Riley AL. The interaction of sex and route of drug administration in cocaine-induced conditioned taste aversions. Pharmacol Biochem Behav. 2005;81:814–820. doi: 10.1016/j.pbb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roma PG, Davis CM, Kohut SJ, Huntsberry ME, Riley AL. Early maternal separation and sex differences in the aversive effects of amphetamine in adult rats. Physiol Behav. 2008;93:897–904. doi: 10.1016/j.physbeh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Jones JD, Busse GD, Riley AL. Strain-dependent sex differences in the effects of alcohol on cocaine-induced taste aversions. Pharmacol Biochem Behav. 2006;83:554–560. doi: 10.1016/j.pbb.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Stock HS, Caldarone B, Abrahamsen G, Mongeluzi D, Wilson MA, Rosellini RA. Sex differences in relation to conditioned fear-induced enhancement of morphine analgesia. Physiol Behav. 2001;72:439–447. doi: 10.1016/s0031-9384(00)00426-1. [DOI] [PubMed] [Google Scholar]
- 61.Beatty WW, Beatty PA. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol. 1970;73:446–455. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- 62.Pryce CR, Lehmann J, Feldon J. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav. 1999;64:753–759. doi: 10.1016/s0091-3057(99)00147-1. [DOI] [PubMed] [Google Scholar]
- 63.Itzhak Y, Roger-Sanchez C, Anderson KL. Role of the nNOS gene in ethanol-induced conditioned place preference in mice. Alcohol. 2009;43:285–291. doi: 10.1016/j.alcohol.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crippens D, White ML, George MA, Jaworski JN, Brunner LJ, Lancaster FE, Gonzales RA. Gender differences in blood levels, but not brain levels, of ethanol in rats. Alcohol Clin Exp Res. 1999;23:414–420. [PubMed] [Google Scholar]
- 65.Goudie AJ, Dickins DW. Nitrous oxide-induced conditioned taste aversions in rats: the role of duration of drug exposure and its relation to the taste aversion-self-administration “paradox”. Pharmacol Biochem Behav. 1978;9:587–592. doi: 10.1016/0091-3057(78)90207-1. [DOI] [PubMed] [Google Scholar]
- 66.Eckardt MJ. The role of orosensory stimuli from ethanol and blood-alcohol levels in producing conditioned taste aversion in the rat. Psychopharmacologia. 1975;44:267–271. doi: 10.1007/BF00428905. [DOI] [PubMed] [Google Scholar]
- 67.Roma PG, Chen SA, Barr CS, Riley AL. Dissociation between the aversive and pharmacokinetic effects of ethanol in female Fischer and Lewis rats. Behav Brain Res. 2007;182:51–56. doi: 10.1016/j.bbr.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orr TE, Whitford-Stoddard JL, Elkins RL. Taste-aversion-prone (TAP) rats and taste-aversion-resistant (TAR) rats differ in ethanol self-administration, but not in ethanol clearance or general consumption. Alcohol. 2004;33:1–7. doi: 10.1016/j.alcohol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham CL, Clemans JM, Fidler TL. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol Biochem Behav. 2002;72:659–668. doi: 10.1016/s0091-3057(02)00734-7. [DOI] [PubMed] [Google Scholar]
- 70.Cannon DS, Baker TB, Berman RF. Taste aversion disruption by drug pretreatment: dissociative and drug-specific effects. Pharmacol Biochem Behav. 1977;6:93–100. doi: 10.1016/0091-3057(77)90164-2. [DOI] [PubMed] [Google Scholar]
- 71.Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badanich KA, Maldonado AM, Kirstein CL. Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res. 2007;31:895–900. doi: 10.1111/j.1530-0277.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 73.Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29:535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vetter-O’Hagen CS, Spear LP. The Effects of Gonadectomy on Age- and Sex-Typical Patterns of Ethanol Consumption in Sprague-Dawley. Rats Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01555.x; 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]