Abstract
The total synthesis of (+)-vigulariol and (−)-sclerophytin A are reported in 15 steps and 16 steps, respectively, from a known compound. The flexible, readily scalable synthetic strategy allows for rapid construction of a critical tricyclic intermediate and is demonstrated via the synthesis of these two marine natural products. A key reaction in this synthetic protocol is a combination Wittig/intramolecular Diels-Alder cycloaddition.
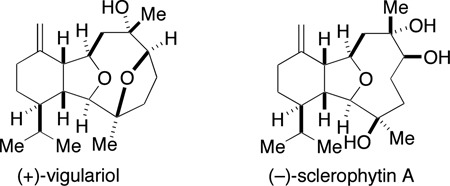
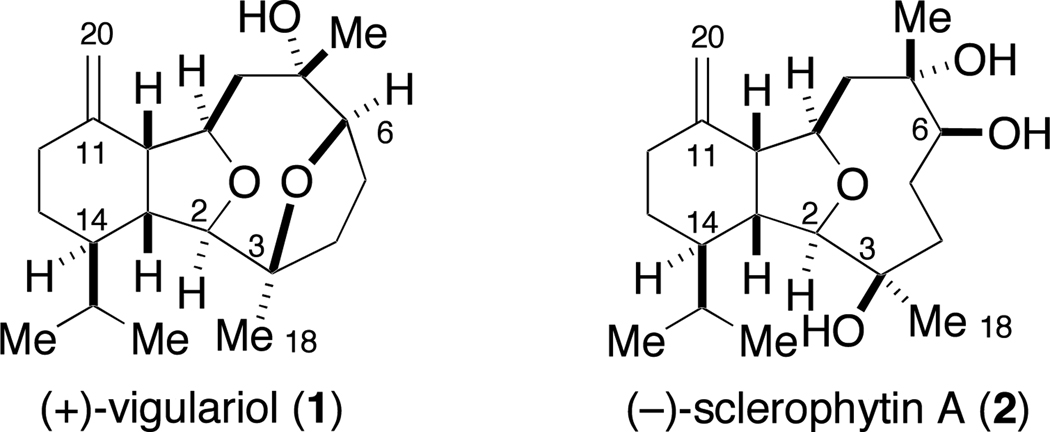
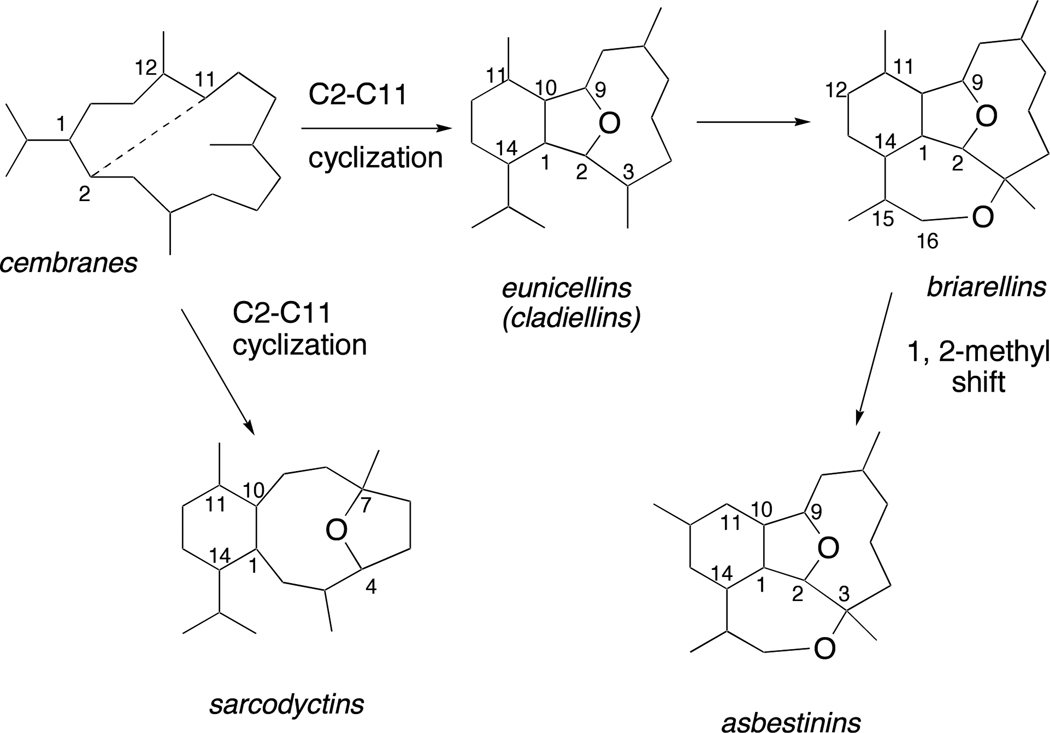
The marine environment is a proven source of structurally novel and biologically active natural products. In this context, (+)-vigulariol (1),1 first isolated off the coast of Taiwan from the sea pen octocoral Vigularia juncea and (−)-sclerophytin A (2)2 (Figure 1), isolated from the soft coral Sclerophytum capitalis collected in waters near Micronesia, are two diterpenoids belonging to the eunicellin (or cladiellin) family of C2–C11 cyclized cembranoid marine natural products. The C2–C11-cyclized cembranoids are related subclasses of secondary metabolites isolated from corals in the Caribbean and West Pacific and include the eunicellins, briarellins, asbestinins, and sarcodyctins.3 A proposed biosynthesis of the C2–C11 cyclized cembranoids is shown in Figure 2.4 Multiple members of this family of natural products have succumbed to total synthesis over the past several years.3d
Figure 1.
(+)-Vigulariol and (−)-sclerophytin A, two members of the eunicellin family of C2–C11 cyclized cembranoid natural products.
Figure 2.
Proposed biosynthesis of the C2–C11 cyclized cembranoids.
Based upon fish and mollusk lethality assays, the natural role of these metabolites is suggested to involve predator deterrence.5 Initial investigation into the biological activity of these compounds has shown that many of these natural products exhibit insect growth inhibition and in vitro cytotoxic activity against multiple cancer cell lines.1,2a,6 Vigulariol has promising pharmacological activity, demonstrating cytotoxic activity against the A 549 human lung adenocarcinoma cell culture line with an IC50 of 18.33 µg/mL.1 Sclerophytin A possesses even more potent cytotoxic activity: 1 ng/mL against the L1210 cell line.2a Due to the small amounts of material obtained from natural sources, additional biological data, such as mechanism of action and biological target, is limited for these compounds. A synthetic strategy that is amenable to future structure-activity relationship studies and is easily scalable will enable further investigation into the promising biological activity of these cembranoid natural products.
Vigulariol and sclerophytin A possess unique molecular architectures and their dense functionalization provides an intriguing challenge for chemical synthesis. The oxatricyclic framework of sclerophytin A contains eight stereocenters, six of them contiguous, a hexahydroisobenzofuran core, and an oxacyclononane ring unit. The tetracyclic framework of vigulariol contains the traditional tricyclic core seen in the eunicellins, plus an additional tetrahydrofuran ring system with the ring oxygen bridging C3 and C6 within the oxacylononane ring. These distinctive structural features have also drawn the interest of other research groups. There are three reported total syntheses of sclerophytin A,7 where vigulariol was an intermediate in two of these syntheses. Two additional total syntheses of vigulariol have been published8 – one of them racemic.8a Several groups have reported progress towards fragments or analogs of these eunicellins.9
The molecular complexity and cytotoxic activity of these marine metabolites continue to make them attractive targets for total synthesis. In designing an efficient synthetic route towards vigulariol and sclerophytin A, a practical method for formation of the nine-membered ring system must be considered. We have previously disclosed an asymmetric aldol−ring-closing metathesis strategy for the enantioselective construction of oxygen heterocycles10 and demonstrated its utility in the total synthesis of multiple medium ring ether-containing natural products.11
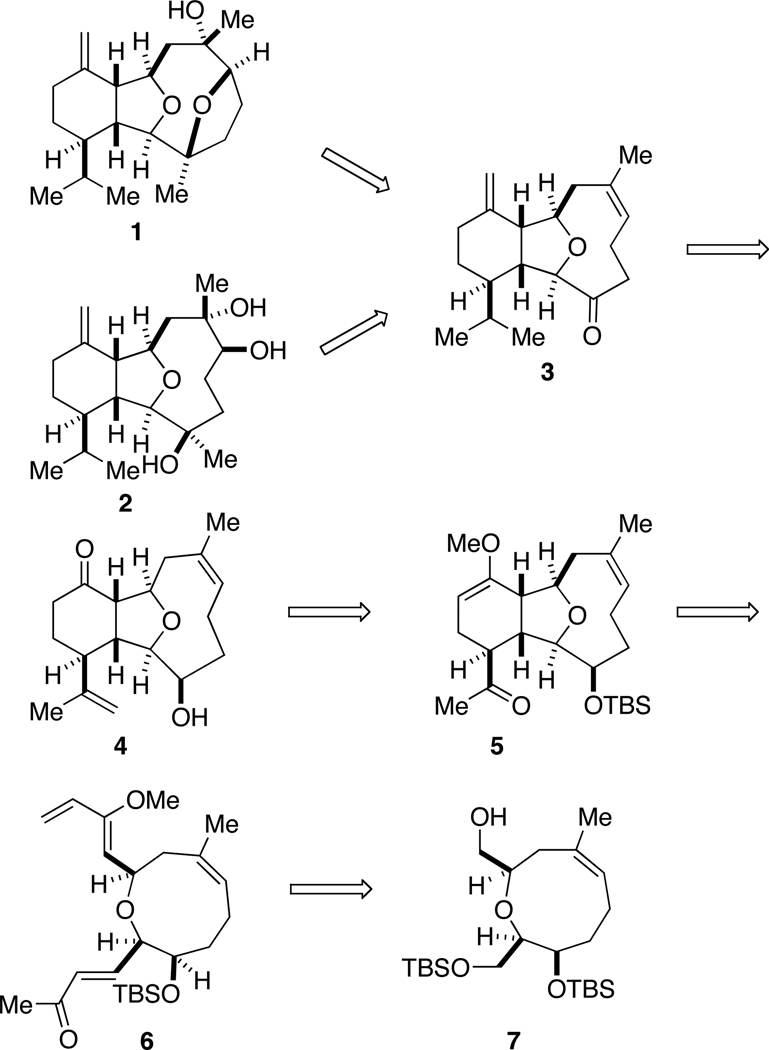
The retrosynthetic strategy for (+)-vigulariol and (−)-sclerophytin A is shown in Scheme 1. Vigulariol would be fashioned from the functionalized oxatricyclic core 3, following a nucleophilic ring-opening of an epoxide to form the bridged ether moiety. Ketone 3 could be elaborated into sclerophytin A following a recently reported three-step procedure.7g Terminal alkene 4 could be constructed via functional group manipulation from ketone 5, whose tricyclic core could be established via a stereoselective intramolecular Diels-Alder cycloaddition of enone 6. Finally, enone 6 would be derived from reported oxonene 7, using two key Wittig reactions to establish the required Diels-Alder diene.
Scheme 1.
Retrosynthetic analysis of (+)-vigulariol and (−)-sclerophytin A
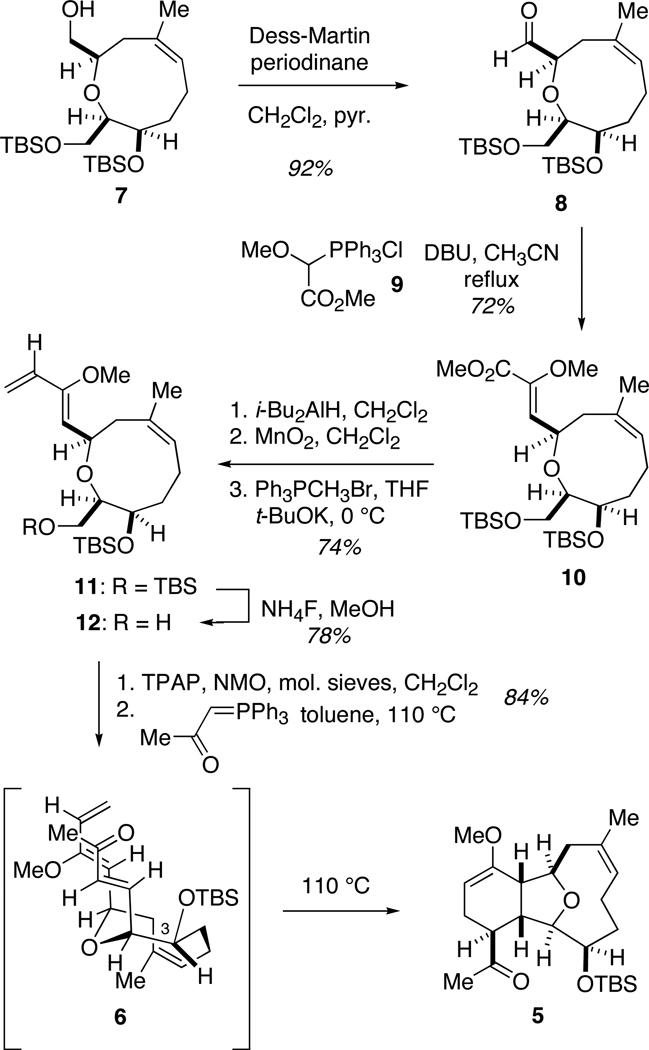
The synthesis of these two marine metabolites begins with oxonene alcohol 7, previously prepared in our laboratory in eight steps from (R)-benzylglycidyl ether utilizing the aformentioned asymmetric glycolate aldolring closing metathesis protocol.11k (Scheme 2) As shown in the retrosynthetic scheme, an initial objective in the synthesis is to install the required functionalities for the intramolecular Diels-Alder cylcoaddition. Toward this goal, alcohol 7 was oxidized to the corresponding aldehyde with Dess-Martin periodinane.12 Aldehyde 8 was treated with the Wittig reagent derived from salt 913 to form the corresponding enoate (Z:E = 11:1).
Scheme 2.
Syntheis of tricyclic ketone 5
Initial attempts to directly incorporate the requisite diene functionality employing addition of α-methoxyallyl phosphorus ylides14 to aldehyde 8 were not successful, so installation of the diene was instead achieved following a three step sequence. Upon treatment with DIBAL-H, ester 10 was reduced to the corresponding alcohol, followed by MnO2-mediated allylic oxidation to the aldehyde and a methylene Wittig olefination to produce diene 11. The primary TBS ether was selectively removed with ammonium fluoride15 in the presence of the acid labile enol ether to yield alcohol 12. After exploring numerous oxidation conditions, the acid-sensitive alcohol 12 was exposed to tetrapropylammonium perruthenate16 to afford an aldehyde, which was taken onto the next step without further purification.
One of the critical synthetic challenges in completing a total synthesis of either sclerophytin A or vigulariol is constructing the fully substituted tetrahydrofuran core. It was believed that this could be accomplished using a substrate-directed intramolecular Diels-Alder reaction, where the diastereoselectivity would be controlled by the oxonene ring. An additional benefit to this approach is that the cyclohexane ring is established concomitantly. We had previously validated this IMDA approach to the tetrasubstituted tetrahydrofuran core of the cembranoids.11j–m A fortuitous result noted during these studies was that upon formation of the desired (E)-enoate for the Diels-Alder reaction, the cycloaddition proceeded rapidly, often spontaneously at room temperature. Gratifyingly, upon treatment with (acetylmethylene)triphenylphosphorane,17 the crude aldehyde underwent an exo-selective Diels-Alder reaction to result in a single diastereomer (5). The orientation of the C3 hydroxyl group, as well as the size of the protecting group, is critical in influencing the diastereoselectivity of this key cyclization reaction.
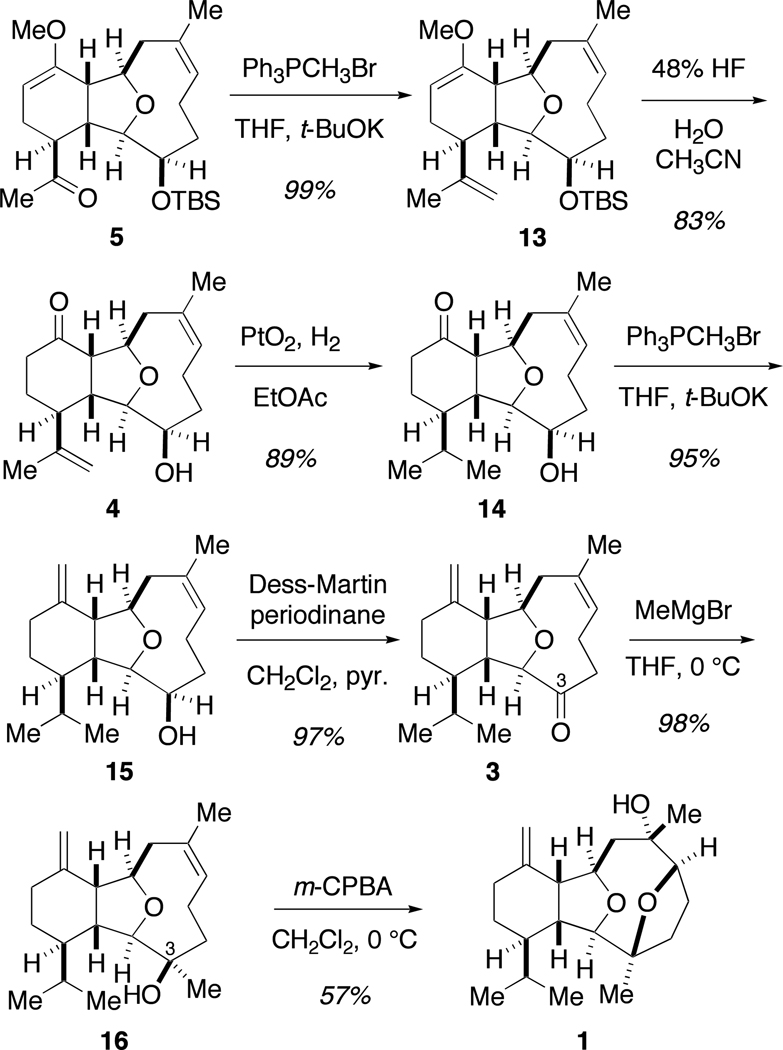
With the tricyclic core now constructed, attention turned to completion of the remaining functional group transformations on this skeleton (Scheme 3). From ketone 5, an exocyclic alkene was installed via a methylene Wittig olefination. Simultaneous cleavage of both the enol ether and the TBS protecting group of enol 13 occured after treatment with aqueous hydrogen fluoride to yield ketone 4.18 The more reactive exocyclic alkene at C15 was then carefully hydrogenated using platinum oxide,8a followed by a methylene Wittig olefination at the C11 ketone to form diene 15. Subsequent Dess-Martin oxidation14 of the C3 secondary alcohol, followed by exposure of the ketone to an excess of methylmagnesium bromide yielded tertiary alcohol 16 as a single diastereomer.
Scheme 3.
Completion of (+)-vigulariol (1)
With interest now focused on the completion of sclerophytin A, efforts were fixed on installing the required hydroxyl groups at C6 and C7. It was proposed that tertiary alcohol 16 could be converted into sclerophytin A via an epoxidation-ring-opening event or dihydroxylation strategy. Numerous attempts to prepare 2 via 16 were unsuccessful, even after protection of the tertiary alcohol. However, when diene 16 was treated with m-CPBA, regioselective epoxidation resulted to form a mixture of α- and β-epoxides, where only the α-epoxide was preferentially attacked by the C3 tertiary alcohol to form (+)-vigulariol (1). The experimental data of synthetic vigulariol were identical to those reported for the natural product ([α]25D = +3.1 (c 0.24, CHCl3); [α]27D lit. = +3.6 (c 0.24, CHCl3).1
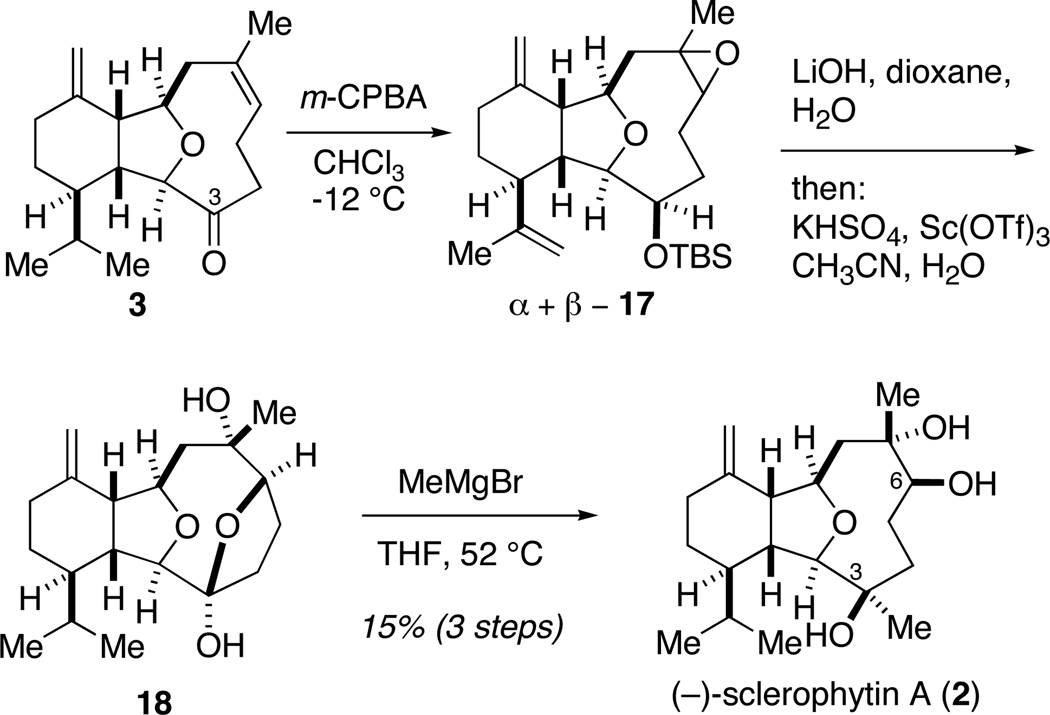
Turning attention back to sclerophytin A, we took advantage of the fact that our route intercepted a common intermediate (ketone 3) in a recently published total synthesis of sclerophytin A by Morken and co-workers.7g Ketone 3 was treated with m-CPBA and underwent regioselective epoxidation to form a mixture of epoxides (α- and β-17) (Scheme 4). Upon exposure of the epoxide mixture to aqueous LiOH, only the α-17 epoxide hydrolyzed to afford hemiketal 18. Conversion of the β-17 epoxide to hemiketal 18 was also achieved in the same reaction vessel after acidification with KHSO4 and treatment with Sc(OTf)3. The total synthesis of (−)-sclerophytin A was completed with subjection of 18 to methylmagnesium bromide to install the tertiary carbinol at C3. The characterization data was in agreement with those reported for the isolated natural product ([α]20D = −3.0 (c 0.10, CHCl3); [α]20D lit. = − 6.9 (c 0.087, CHCl3).7f
Scheme 4.
Completion of (−)-sclerophytin A
In summary, we have completed an enantioselective total syntheses of (+)-vigulariol in 15 steps and (−)-sclerophytin A in 16 steps from a reported oxonene intermediate. A key step in our synthetic strategy was the utilization of a Wittig/intramolecular Diels-Alder cycloaddition to establish the hydroisobenzofuran core of these eunicellin natural products.
Supplementary Material
Acknowledgment
This work was supported by a research grant from The National Institutes of Health (GM60567). We acknowledge a generous gift of (R)-benzylglycidyl ether from Diaso, Inc. The assistance of Dr. Sohrab Habibi (Director, Mass Spectrometry Facility, The University of North Carolina at Chapel Hill) is also acknowledged.
Footnotes
Supporting Information Available: Experimental procedures, as well as 1H and 13C NMR spectra for all new compounds, and synthetic (+)-vigulariol and (−)-sclerophytin A. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Su J-H, Huang H-C, Chao C-H, Yan L-Y, Wu Y-C, Wu C-C, Sheu J-H. Bull. Chem. Soc. Jpn. 2005;78:877–879. [Google Scholar]
- 2.(a) Sharma P, Alam M. J. Chem. Soc., Perkin Trans. 1. 1988:2537–2540. [Google Scholar]; (b) Alam M, Sharma P, Zektzer AS, Martin GE, Ji X, Van der Helm D. J. Org. Chem. 1989;54:1896–1900. [Google Scholar]
- 3.For reviews, see: Rodríguez AD. Tetrahedron. 1995;51:4571–4618. doi: 10.1016/0040-4020(95)00216-U. Bernardelli P, Paquette LA. Heterocycles. 1998;49:531–556. Sung P-J, Chen M-C. Heterocycles. 2002;57:1705–1715. Ellis JM, Crimmins MT. Chem. Rev. 2008;108:5278–5298. doi: 10.1021/cr078117u.
- 4.Stierle DB, Carte B, Faulkner DJ, Tagle B, Clardy J. J. Am. Chem. Soc. 1980;102:5088–5092. [Google Scholar]
- 5.(a) Maia LF, De Epifanio R, Eve T, Fenical W. J. Nat. Prod. 1999;62:1322–1324. doi: 10.1021/np990138z. [DOI] [PubMed] [Google Scholar]; (b) Harvell CD, West JM, Griggs C. Invertebr. Reprod. Dev. 1996;30:239–247. [Google Scholar]; (c) Harvell CD, Fenical W, Roussis V, Ruesink JL, Griggs CC, Greene CH. Mar. Ecol. Prog. Ser. 1993;93:165–173. [Google Scholar]
- 6.(a) Rodríguez AD, Cobar OM. Tetrahedron. 1995;51:6869–6880. [Google Scholar]; (b) Ortega MJ, Zubia E, Salva J. J. Nat. Prod. 1994;57:1584–1586. doi: 10.1021/np50113a021. [DOI] [PubMed] [Google Scholar]; (c) Rodríguez AD, Cobar OM. Tetrahedron. 1992;49:319–328. [Google Scholar]; (d) Morales JJ, Lorenzo D, Rodríguez AD. J. Nat. Prod. 1991;54:1368–1382. doi: 10.1021/np50077a021. [DOI] [PubMed] [Google Scholar]; (e) Selover SJ, Crews P, Tagle B, Clardy J. J. Org. Chem. 1981;46:964–970. [Google Scholar]
- 7.(a) Paquette LA, Moradei OM, Bernardelli P, Lange T. Org. Lett. 2000;2:1875–1878. doi: 10.1021/ol000083o. [DOI] [PubMed] [Google Scholar]; (b) Friedrich D, Doskotch RW, Paquette LA. Org. Lett. 2000;2:1879–1882. doi: 10.1021/ol000084g. [DOI] [PubMed] [Google Scholar]; (c) Bernardelli P, Moradei OM, Friedrich D, Yang J, Gallou F, Dyck BP, Doskotch RW, Lange T, Paquette LA. J. Am. Chem. Soc. 2001;123:9021–9032. doi: 10.1021/ja011285y. [DOI] [PubMed] [Google Scholar]; (d) Overman LE, Pennington LD. Org. Lett. 2000;2:2683–2686. doi: 10.1021/ol006236p. [DOI] [PubMed] [Google Scholar]; (e) MacMillan DWC, Overman LE, Pennington LD. J. Am. Chem. Soc. 2001;123:9033–9044. doi: 10.1021/ja016351a. [DOI] [PubMed] [Google Scholar]; (f) Gallou F, MacMillan DWC, Overman LE, Paquette LA, Pennington LD, Yang J. Org. Lett. 2001;3:135–137. doi: 10.1021/ol000345m. [DOI] [PubMed] [Google Scholar]; (g) Wang B, Ramirez AP, Slade JJ, Morken JP. J. Am. Chem. Soc. 2010;132:16380–16382. doi: 10.1021/ja108185z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Clark JS, Hayes ST, Wilson C, Gobbi L. Angew. Chem., Int. Ed. 2007;46:437–440. doi: 10.1002/anie.200603880. [DOI] [PubMed] [Google Scholar]; (b) Becker J, Bergander K, Froehlich R, Hoppe D. Angew. Chem., Int. Ed. 2008;47:1654–1657. doi: 10.1002/anie.200704678. [DOI] [PubMed] [Google Scholar]
- 9.(a) Jung ME, Pontillo J. J. Org. Chem. 2002;67:6848–6851. doi: 10.1021/jo016246j. [DOI] [PubMed] [Google Scholar]; (b) Molander GA, Jeffrey SC. Tetrahedron Lett. 2002;43:359–362. [Google Scholar]; (c) Jung ME, Pontillo J. Tetrahedron. 2003;59:2729–2736. [Google Scholar]; (d) Davidson JEP, Gilmour R, Ducki S, Davies JE, Green R, Burton JW, Holmes AB. Synlett. 2004:1434–1436. [Google Scholar]; (e) Bateman TD, Joshi AL, Moon K, Galitovskaya EN, Upreti M, Chambers TC, McIntosh MC. Bioorg. Med. Chem. Lett. 2009;19:6898–6901. doi: 10.1016/j.bmcl.2009.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crimmins MT, Choy AL. J. Org. Chem. 1997;62:7548–7549. doi: 10.1021/jo9716688. [DOI] [PubMed] [Google Scholar]
- 11.(a) Crimmins MT, Emmitte KA. Org. Lett. 1999;1:2029–2032. doi: 10.1021/ol991201e. [DOI] [PubMed] [Google Scholar]; (b) Crimmins MT, Choy AL. J. Am. Chem. Soc. 1999;121:5653–5660. [Google Scholar]; (c) Crimmins MT, Tabet EA. J. Am. Chem. Soc. 2000;122:5473–5476. [Google Scholar]; (d) Crimmins MT, Emmitte KA. Synthesis. 2000;6:899–903. [Google Scholar]; (e) Crimmins MT, Emmitte KA. J. Am. Chem. Soc. 2001;123:1533–1534. doi: 10.1021/ja005892h. [DOI] [PubMed] [Google Scholar]; (f) Crimmins MT, Emmitte KA, Choy AL. Tetrahedron. 2002;58:1817–1834. [Google Scholar]; (g) Crimmins MT, DeBaillie AC. Org. Lett. 2003;5:3009–3011. doi: 10.1021/ol034923l. [DOI] [PubMed] [Google Scholar]; (h) Crimmins MT, Powell MT. J. Am. Chem. Soc. 2003;125:7592–7595. doi: 10.1021/ja029956v. [DOI] [PubMed] [Google Scholar]; (i) Crimmins MT, Cleary PA. Heterocycles. 2003;61:87–92. [Google Scholar]; (j) Crimmins MT, Brown BH. J. Am. Chem. Soc. 2004;126:10264–10266. doi: 10.1021/ja046574b. [DOI] [PubMed] [Google Scholar]; (k) Crimmins MT, Ellis JM. J. Am. Chem. Soc. 2005;127:17200–17201. doi: 10.1021/ja056921x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Crimmins MT, Brown BH, Plake HR. J. Am. Chem. Soc. 2006;128:1371–1378. doi: 10.1021/ja056334b. [DOI] [PubMed] [Google Scholar]; (m) Crimmins MT, Ellis JM. J. Org. Chem. 2008;73:1649–1660. doi: 10.1021/jo0712695. [DOI] [PubMed] [Google Scholar]
- 12.Dess DM, Martin JC. J. Am. Chem. Soc. 1991;113:7277–7287. [Google Scholar]
- 13.(a) Quéron E, Lett R. Tetrahedron Lett. 2004;45:4527–4531. [Google Scholar]; (b) Seneci P, Leger I, Souchet M, Nadler G. Tetrahedron. 1997;53:17097–17114. [Google Scholar]
- 14.Kim S, Kim YC. Tetrahedron Lett. 1990;31:2901–2904. [Google Scholar]
- 15.Schinzer D, Bohm OM, Altmann K-H, Wartmann M. Synlett. 2004:1375–1378. [Google Scholar]
- 16.Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994:639–666. [Google Scholar]
- 17.Kuroda H, Hanaki E, Izawa H, Kano M, Itahashi H. Tetrahedron. 2004;60:1913–1920. [Google Scholar]
- 18.For a recent example illustrating the flexibility of this general synthetic strategy to the C2 C11 cembranoids, see: Crimmins MT, Mans MC, Rodríguez AD. Org. Lett. 2010;12:5028–5031. doi: 10.1021/ol102169w.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.