Abstract
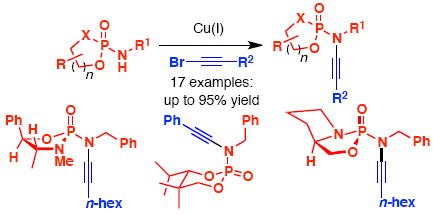
We describe here the first synthesis of N-phosphoryl ynamides featuring C- and P-chirality via copper(I)-catalyzed amidative cross-couplings between phosphoramidates and phosphordiamidates with alkynyl bromides. Also featured is a tandem aza-Claisen–hetero-[2+2] cycloaddition for the synthesis of N-phosphoryl azetidin-2-imines.
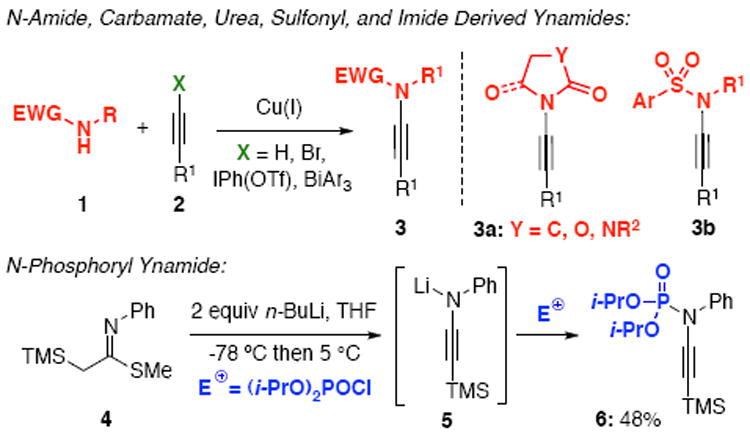
The chemistry of ynamides1 has continued to blossom, with the number of publications increasing at an astonishing rate.2 This surge of new and exciting chemistry is largely a result of the ease of ynamide synthesis using copper-catalyzed cross-coupling reactions3 [Scheme 1]. However, with the exception of Masson’s4 single example of trapping a lithiated ynamine 5 with diethyl chlorophosphate, all examples of ynamides to date utilize amide, carbamate, urea, sulfonyl, and, only recently, imide5 derived electron-withdrawing groups [EWG] to harness the ynamide’s reactivity. To the best of our knowledge, the amidative cross-coupling6 between an sp-C and a phosphoramidate7-8 has not been reported. We describe herein the first general synthesis of a novel class of N-phosphoryl ynamides capable of containing both C- and P-chirality. The highly Lewis-basic nature of the phosphoryl moiety as well as the ability to tune its electronegativity make the phosphoryl EWG ideal to further expand the already vast scope of reactions one can accomplish using ynamides. To this effect, we disclose an application towards highly functionalized N-phosphoryl azetidin-2-imines through a Staudinger-type ketenimine-imine [2+2] cycloaddition not possible with pre-existing ynamides.
Scheme 1.
Current Ynamide Electron-Withdrawing Groups.
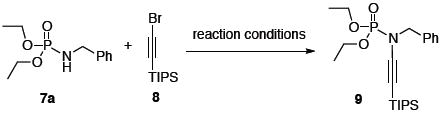
Our initial efforts were in optimizing the copper-catalyzed cross-coupling between phosphoramidate 7a and alkynyl bromide 8 [Table 1]. It was immediately clear that the strength of the base played a significant role in the reaction, with K3PO4 giving the best yield of ynamide 9 [entries 1–3]. Unfortunately, the stronger KOt-Bu base led to complete decomposition [entry 4]. Also, the combination of CuSO4·5H2O and 1,10-phenanthroline was far more efficient than both CuI and CuCN with DMEDA as the ligand [entry 3 vs. 5 – 6].
Table 1.
Optimization of Coupling Conditions.a
 | ||||
|---|---|---|---|---|
| entry | catalyst | ligand | base | yield [%]b |
| 1 | CuSO4·5H2O | 1,10-phenanthroline | K2CO3 | 38 |
| 2 | CuSO4·5H2O | 1,10-phenanthroline | Cs2CO3 | 59 |
| 3 | CuSO4·5H2O | 1,10-phenanthroline | K3PO4 | 68 |
| 4 | CuSO4·5H2O | 1,10-phenanthroline | KOt-Bu | – |
| 5 | CuI | DMEDA | K3PO4 | 25c |
| 6 | CuCN | DMEDA | K3PO4 | 27c |
Conditions: 15 mol % Cu, 30 mol % ligand, 2 equiv base, toluene (concn = 0.4 M), 95 °C, 24 h.
Isolated yields.
Reactions run at 110 °C.
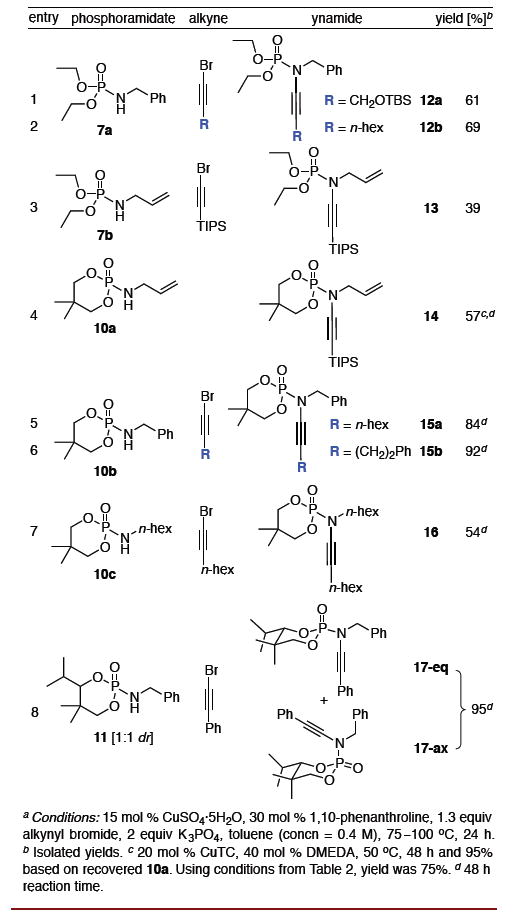
With an optimized protocol in hand, our next goal was to access the tolerance of the reaction to functionality on both the phosphoramidates and alkynyl bromides [Table 2]. Phosphoramidate 7a led smoothly to ynamides 12a and 12b in moderate yield [entries 1 and 2]. However with the N-allyl phosphoramidate 7b, a thermal aza-Claisen9 rearrangement of the ynamide product and ensuing hydrolysis10 occurred readily at 75 °C leading to a low yield of the desired ynamide 13 [entry 3]. This issue could be overcome using anhydrous coupling conditions [CuTC/DMEDA] and a lower reaction temperature to give N-allyl ynamide 14 in 57% yield, with almost complete recovery the unreacted 10a. In general, cyclic diol-derived phosphoramidates 10b and 10e gave ynamides 15 and 17 with higher yields [entries 5–7], likely due to the increased hydrolytic stability11 of both the amides and the ynamide products. Interestingly, these cyclic N-phosphoryl ynamides adopt a chair configuration with the N–C≡C in an axial orientation such that the nitrogen lone pair is delocalized into the σ*[P=O] [see X-ray of 15b in Figure 1].
Table 2.
Synthesis of N-Phosphoryl Ynamides from Diols.a
|
Figure 1.
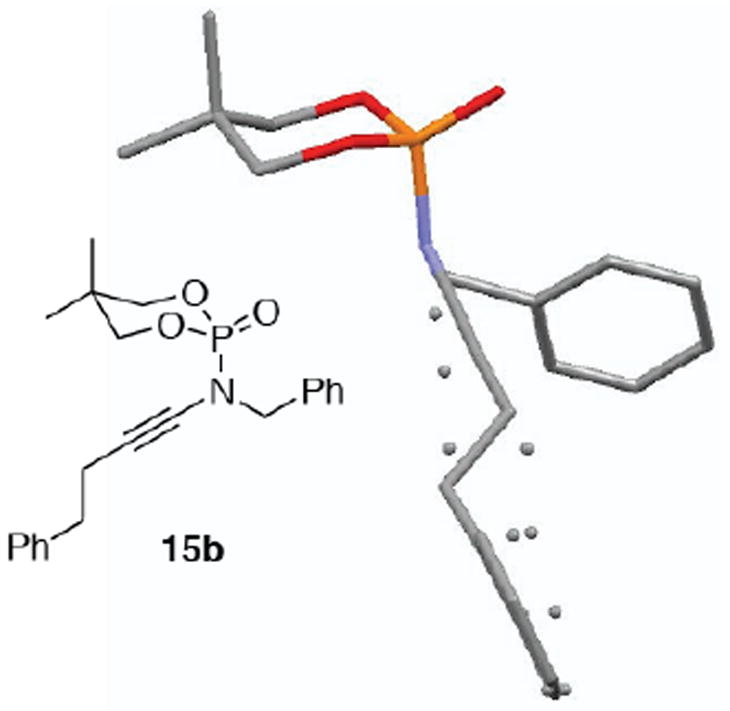
X-ray of Ynamide 15b.
By using a 1:1 diastereomeric mixture of phosphoramidates 11, ynamides 17-eq and 17-ax differing only in the stereochemistry at phosphorous could be obtained in excellent yield and separated by column chromatography [Table 2, entry 8]. X-ray crystallography and nOe analysis was used to determine the conformation of the two diastereomeric ynamides, revealing that 17-ax adopted a similar configuration to that of 15b, with the nitrogen atom axial and delocalized into the σ*[P=O] while the two oxygen lone pairs were delocalized into the σ*[P-N] [Figure 2]. Unfortunately, the X-ray of 17-eq was convoluted by the co-crystallization of both enantiomers in a 90:10 ratio as an example of whole molecule disorder. Regardless, it was evident that 17-eq adopted a chair configuration with the nitrogen atom equatorial and delocalized into the π*[P=O] with the two oxygen lone pairs delocalized into the σ*[P=O].
Figure 2.
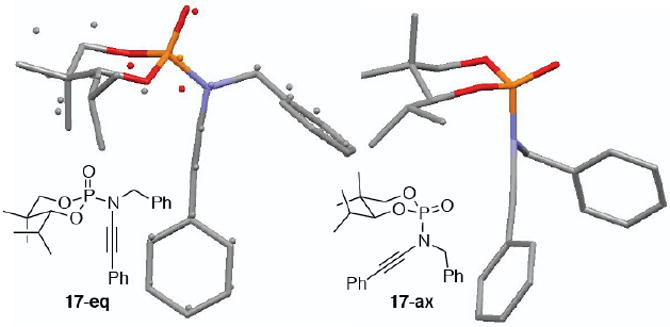
X-rays of Diastereomeric Ynamides 17-eq and 17-ax.
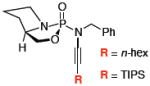
Next, we turned our attention to preparing ynamides bearing a chiral phosphoryl group, which is especially exciting as the phosphorous atom directly attached to the nitrogen of the ynamide can itself be chiral. Amino alcohols represent an obvious choice for preparing such chiral auxiliaries. Unfortunately, from the onset it was immediately apparent that the reactivity of 1,2-amino alcohol derived phosphordiamidates was very different from their 1,3-diol counterparts [Scheme 2]. To illustrate this point, the synthesis of ynamide 19a from L-proline derived phosphordiamidate 18 proceeded in a meager 13% yield even after 48 hrs at 110 °C using the previously optimized conditions. Though some starting material could be recovered after the reaction, the majority had decomposed. Unfortunately, anhydrous conditions using CuTC and DMEDA for 1R,2R-(–)-pseudoephedrine derived amide 20 also resulted in no formation of the desired ynamide 21, though 70% of the starting material could be recovered.
Scheme 2.
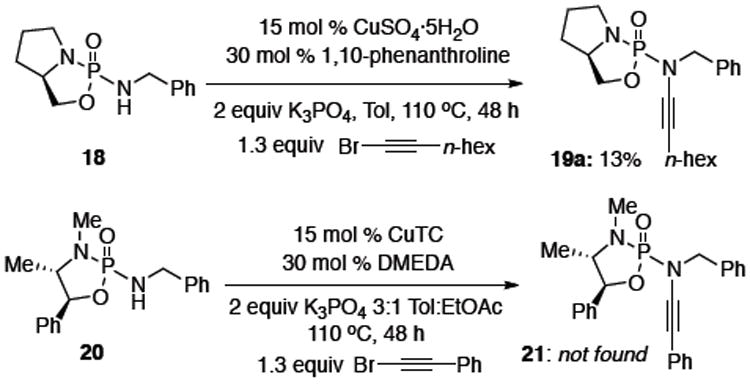
Attempts at Ynamide Synthesis from Amino Alcohols.
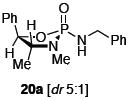
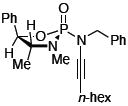
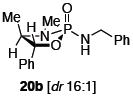
After much struggle, it was discovered that the addition of 10 equiv of NEt3 to the reaction mixture allowed for isolation of the desired ynamides 19a and 19b in low, but synthetically useful yields under anhydrous conditions [Table 3]. Furthermore, ynamide formation from the two separable diastereomers of 20 led to 23 and 24 in moderate yields. The stereochemistry of phosphordiamidates 18, 20a, and 20b was determined by nOe analysis [see supporting information]. The racemic 1,3-amino alcohol derived phosphoramidate 22 led to ynamides 25a and 25b resembling a phosphoryl version of Evan’s chiral auxiliary in moderate yields without the addition of NEt3.
Table 3.
Ynamides from Amino Alcohols.a
| entry | phosphor(di)amidate | alkyne | ynamide | yieldb | |
|---|---|---|---|---|---|
| 1 |
|
|
|
19a | 38 |
| 2 | 19b | 28 | |||
| 3 |
|
|
|
23 | 59 |
| 4 |
|
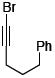
|
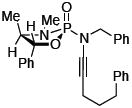
|
24 | 41 |
| 5 |
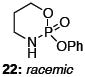
|
|
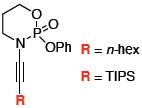
|
25a | 62c |
| 6 | 25b | 81c | |||
Conditions: 1.3 equiv alkynyl bromide, 20 mol % CuTC, 40 mol % DMEDA, 3 equiv Cs 2CO3, 10 equiv NEt 3, dioxane (concn = 0.4 M), 95 °C, 6 –24 h.
Isolated yields.
Same catalyst, but with 3:1 toluene:EtOAc (concn = 0.4 M), 2 equiv K3PO4, 95 °C, 24 h.
We had set out to investigate this new class of N-phosphoryl ynamides to solve a problem we had previously encountered while developing a thermal aza-Claisen rearrangement of N-sulfonyl, N-allyl ynamides, allowing for their use as precursors to ketenimines.9 Despite our best efforts, the nucleophilic trapping of the N-sulfonyl ketenimines was severely limited due to a very rapid intramolecular 1,3-sulfonyl shift yielding nitriles. Since amide, carbamate, and urea-derived ynamides failed to undergo such an aza-Claisen in our hands, we turned our attention to phosphoryl-based electron-withdrawing groups. So, we were delighted to see that during the coupling of N-allyl phosphoramidate 7b, some ketenimine hydrolysis product was isolated.10 Furthermore, heating of N-allyl ynamide 26b resulted in no formation of nitrile 28b, meaning the aza-Claisen is operational, but a 1,3-phosphoryl shift is not [Scheme 3]. This afforded us the opportunity to use the in situ generated ketenimine derived from 26b in a Staudinger-type ketenimine-imine [2+2] cycloaddition12 to give azetidin-2-imine13 30 bearing a quaternary carbon center with 4:1 diastereoselectivity, which was confirmed by nOe. While these types of tandem reactions initiated by an aza-Claisen rearrangement are the reason we initially undertook this project, they are by no means the only types of transformations possible with these N-phosphoryl ynamides.
Scheme 3.
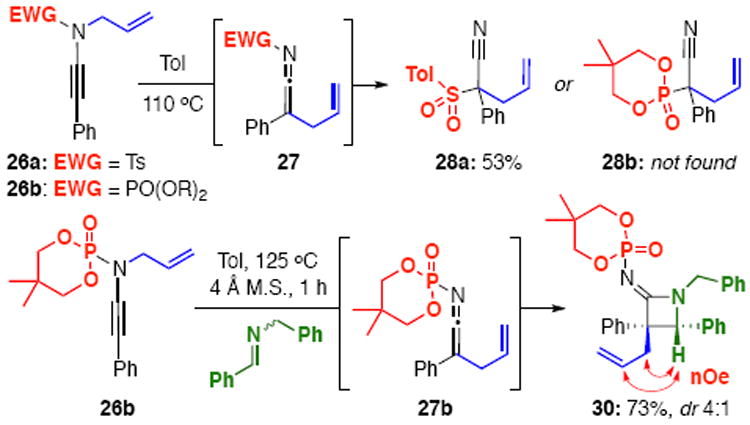
Towards the Synthesis of N-Phosphoryl Azetidin-2-imines.
We have described here the first practical synthesis of a new class of ynamides utilizing a variety of chiral and achiral diol and amino alcohol-derived phosphates as the required electron-withdrawing group. As one example of the type of chemistry one may accomplish with such ynamides, we have demonstrated a diastereoselective Staudinger-type ketenimine-imine [2+2] cycloaddition using N-phosphoryl ynamides as the synthetic precursor to give highly functionalized N-phosphoryl azetidin-2-imines. Investigations are currently underway to further develop these tandem sequences as well as to explore the use of the N-phosphoryl as a chiral auxiliary14-15 and a temporary tether.16
Supplementary Material
Acknowledgments
We thank NIH [GM066055] for funding. KAD thanks the American Chemical Society for a Division of Medical Chemistry Predoctoral Fellowship. We thank Dr. Vic Young and Mr. Gregory T. Rohde of the University of Minnesota for providing X-ray structural analysis.
Footnotes
Supporting Information Available: Experimental procedures as well as NMR spectra, and characterizations are available for all new compounds and free of charge via Internet http://pubs.acs.org.
Contributor Information
Kyle A. DeKorver, Email: dekorver@wisc.edu.
Richard P. Hsung, Email: rhsung@wisc.edu.
References
- 1.For current leading reviews on ynamides, see Evano G, Coste A, Jouvin K. Angew Chem Int Ed. 2010;49:2840. doi: 10.1002/anie.200905817. DeKorver KA, Li H, Lohse AG, Hayashi R, Lu Z, Zhang Y, Hsung RP. Chem Rev. 2010;110:5064. doi: 10.1021/cr100003s.
- 2.For ynamide papers published in 2011 alone, see: Balieu S, Toutah K, Carro L, Chamoreau L-M, Rouselière H, Courillon C. Tetrahedron Lett. 2011;52:2876. Fadel A, Legrand F, Evano G, Rabasso N. Adv Synth Catal. 2011;353:263. Schotes C, Mezzetti A. Angew Chem. 2011;123:3128. doi: 10.1002/anie.201007753. Barbazanges M, Meyer C, Cossy J, Turner P. Chem–Eur J. 2011;17:4480. doi: 10.1002/chem.201003265. Pizzetti M, Russo A, Petricci E. Chem–Eur J. 2011;17:4523. doi: 10.1002/chem.201100447. Garcia P, Evanno Y, George P, Sevrin M, Ricci G, Malacria M, Aubert C, Gandon V. Org Lett. 2011;13:2030. doi: 10.1021/ol200417p. Kramer S, Friis SD, Xin Z, Odabachian Y, Skrydstrup T. Org Lett. 2011;13:1750. doi: 10.1021/ol200269h. Li C, Zhang L. Org Lett. 2011;13:1738. doi: 10.1021/ol2002607. Wang YP, Danheiser RL. Tetrahedron Lett. 2011;52:2111. doi: 10.1016/j.tetlet.2010.11.002. Mak XY, Crombie AL, Danheiser RL. J Org Chem. 2011;76:1852. doi: 10.1021/jo2000308. Davies PW, Cremonesi A, Martin N. Chem Commun. 2011:379. doi: 10.1039/c0cc02736g. Xu C-F, Mei Xu M, Jia YX, Li C-Y. Org Lett. 2011;13:1556. doi: 10.1021/ol200270t. Chen Z, Zheng D, Wu J. Org Lett. 2011;13:848. doi: 10.1021/ol102775s. Shindoh N, Takemoto Y, Takasu K. Heterocycles. 2011;82:1133. Saito N, Katayama T, Saito K. Heterocycles. 2011;82:1181. Kramer S, Odabachian Y, Overgaard J, Rottländer M, Gagosz F, Skrydstrup T. Angew Chemie. 2011;123:5196. doi: 10.1002/anie.201100327. Nissen F, Richard V, Alayrac C, Witulski B. Chem Commun. 2011;47:6656. doi: 10.1039/c1cc11298h. Hashmi ASK, Schuster AM, Zimmer M, Rominger F. Chem–Eur J. 2011;17:5511. doi: 10.1002/chem.201100423. Gilboa N, Wang H, Houk KN, Marek I. Chem–Eur J. 2011;17:8000. doi: 10.1002/chem.201101049. Chemla F, Dulong F, Ferreira F, Nüllen MP, Pérez-Luna A. Synthesis. 2011;9:1347. Saito N, Saito K, Shiro M, Sato Y. Org Lett. 2011;13:2718. doi: 10.1021/ol200812y.
- 3.For leading reviews on the synthesis of ynamides, see: Tracey MR, Hsung RP, Antoline JA, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb Steve M., editor. Chapter 21.4. Georg Thieme Verlag KG; Stuttgart, Germany: 2005. Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379.
- 4.Fromont C, Masson S. Tetrahedron. 1999;55:5405. [Google Scholar]
- 5.Sueda T, Oshima A, Teno N. Org Lett. 2011;13:3996. doi: 10.1021/ol2014973. [DOI] [PubMed] [Google Scholar]
- 6.For an intramolecular Cu-catalyzed cross-coupling between an sp2-C and phosphoramidate, see: Yang T, Lin C, Fu H, Jiang Y, Zhao Y. Organic Lett. 2005;7:4781. doi: 10.1021/ol052126c.
- 7.For examples of phosphoramidates and phosphordiamidates as pharmaceuticals, see: Jeng AY, De Lombaert S. Curr Pharm Des. 1997;3:597. Colvin OM. Curr Pharm Des. 1999;5:555–8.
- 8.For examples as insectides, see: Milzner K, Reisser F. CH 554376. 1974 Anderson J, Homeyer B, Kuehle E, Scheinpflug H, Zeck WM, Simonet DE. US 4603214. 1986
- 9.(a) Zhang Y, DeKorver KA, Lohse AG, Zhang Y-S, Huang J, Hsung RP. Org Lett. 2009;11:899. doi: 10.1021/ol802844z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DeKorver KA, Hsung RP, Lohse AG, Zhang Y. Org Lett. 2010;12:1840. doi: 10.1021/ol100446p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) DeKorver KA, North TD, Hsung RP. Synlett. 2010:2397. doi: 10.1055/s-0030-1258544. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) DeKorver KA, Johnson WL, Zhang Y, Hsung RP, Dai H, Deng J, Lohse AG, Zhang Y-S. J Org Chem. 2011;76:5092. doi: 10.1021/jo200780x. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
10.During the formation of ynamide i bearing an N-allyl moity, an ensuing thermal aza-Claisen occurred followed by hydrolysis of the resulting ketenimine to give ii.
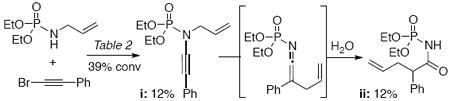
- 11.For leading references, see: Garrison AW, Boozer CE. J Am Chem Soc. 1968;90:3486. Núñez A, Berroterán D, Núñez O. Org Biomol Chem. 2003;1:2283. doi: 10.1039/b300916e.
- 12.For a review of ketenimine cycloadditions, see: Alajarin M, Vidal A, Tovar F. Targets Heterocycl Syst. 2000;4:293.
- 13.(a) Arnold B, Regitz M. Angew Chem Int Ed. 1979;4:320. [Google Scholar]; (b) Van Camp A, Goosens D, Moya-Portuguez M, Marchand-Brynaert J, Ghosez L. Tetrahedron Lett. 1980;21:3081. [Google Scholar]; (c) Alajarin M, Molina P, Vidal A. Tetrahedron Lett. 1996;37:8945. [Google Scholar]; (d) Whiting M, Fokin VV. Angew Chem Int Ed. 2006;45:3157. doi: 10.1002/anie.200503936. [DOI] [PubMed] [Google Scholar]
- 14.Molt O, Schrader T. Synthesis. 2002:2633. [Google Scholar]
- 15.Preliminary efforts at utilizing chiral amino-alcohol derived N-phosphoryl ynamides in Lewis acid-catalyzed reactions have been problematic thus far due to instability of the ynamides to acidic conditions. We are currently exploring their use in thermally driven pathways.
- 16.For leading reviews on utilizing phosphorous as a temporary tether, see: McReynolds MD, Dougherty JM, Hanson PR. Chem Rev. 2004;104:2239. doi: 10.1021/cr020109k. Thomas CD, McParland JP, Hanson PR. Eur J Org Chem. 2009;32:5487. doi: 10.1002/ejoc.200900560.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


