Abstract
Ceftriaxone is a beta-lactam antibiotic which has been found to increase the expression and function of the major glutamate transporter, GLT-1. It has previously been shown that GLT-1 expression is decreased in the nucleus accumbens following cocaine self-administration and extinction training; ceftriaxone given in the days immediately prior to reinstatement testing attenuates both cue- and cocaine-primed reinstatement. Here we tested the ability of ceftriaxone pre-treatment (for 5 days prior to the first cocaine exposure) to prevent the induction of cocaine sensitization and the acquisition of cocaine self-administration. We also tested whether ceftriaxone administered only during self-administration attenuates the reinstatement of extinguished cocaine-seeking. We found that ceftriaxone did not affect the acquisition of cocaine self-administration but was able to attenuate reinstatement weeks after ceftriaxone administration ceased. This attenuation in reinstatement was accompanied by a restoration of GLT-1 expression in the nucleus accumbens. Ceftriaxone also attenuated locomotor behavior following the first cocaine injection and prevented the induction of cocaine but not caffeine sensitization. While ceftriaxone-treated animals did not sensitize to caffeine, they displayed reduced caffeine-induced locomotion following repeated caffeine treatment, indicating a possible dopaminergic effect of ceftriaxone. Taken together, these results indicate that ceftriaxone produces enduring changes in glutamate homeostasis in the nucleus accumbens which counteract addiction-related behaviors.
1. Introduction
Cocaine addiction is a chronic disease characterized by an inability to regulate drug-seeking behavior. Even when long periods of abstinence are achieved, humans report that drug craving persists into late withdrawal, resulting in a high rate of relapse [1]. Relapse can be modeled in animals with the reinstatement paradigm, where animals are trained to self-administer drug in an operant chamber until behavior becomes stable. The drug-seeking response is then extinguished and “reinstated” with one of the stimuli known to cause relapse in humans, namely stress, a cue associated with drug delivery, or the drug itself [2]. Dopamine (DA) release in the nucleus accumbens (NAcc) core is known to be essential for the reinforcing effects of cocaine [3]. However, it is the release of glutamate into this region that is most strongly implicated in the reinstatement of the cocaine seeking response [4].
The involvement of glutamate in the reinstatement response has led to the glutamate homeostasis theory of cocaine addiction [5]. This theory postulates that that in late withdrawal (2–3 weeks) from chronic cocaine, basal glutamate levels in the NAcc are reduced [6, 7] and cocaine-induced synaptic glutamate transmission in this region drives the reinstatement response [4]. Furthermore, the lower basal glutamate levels arise from compromised activity of the cystine-glutamate exchanger, or system xC−, which exchanges extracellular cystine for intracellular glutamate and is the source of the majority of extracellular glutamate in the NAcc [8]. The protein xCT is the catalytic subunit of system xC−, and levels of this protein are decreased in the NAcc core following 3 weeks withdrawal from cocaine self-administration [9]. Expression of the glutamate transporter-1 (GLT-1) is also decreased at this time [9]. GLT-1 is expressed predominantly by astrocytes [10] and expression is highest on portions of the glial membrane which face the neuropil [11]. This positioning allows GLT-1 to prevent almost all glutamate spillover from the synapse [12, 13]. Thus the reduction in GLT-1 expression following cocaine self-administration may partially account for the increased extra-synaptic glutamate measured during reinstatement [4, 6, 7].
The importance of altered glutamate homeostasis in cocaine addiction is highlighted by the finding that restoring homeostasis with the cysteine pro-drug, N-acetylcysteine (NAC) attenuates relapse to cocaine-seeking in both animal models [6, 7, 14] and in a human clinical pilot study [15]. NAC has been shown to stimulate system xC− and thus increase NAcc basal glutamate levels [6]. NAC also increases protein expression of both xCT and GLT-1 in the NAcc [9]. Similarly, the beta-lactam antibiotic ceftriaxone has also been shown to increase expression of xCT [9] and GLT-1 in the NAcc following withdrawal from cocaine [9, 16]. While the mechanism behind the ability of NAC to increase expression of both xCT and GLT-1 is currently unknown, ceftriaxone increases the transcription of the GLT-1 gene resulting in increased glutamate transport [17] and induces a known transcriptional regulator of xCT, Nrf2, and thereby increases system xC− activity [18]. It has previously been shown that chronic ceftriaxone treatment during extinction training attenuates both cue- and cocaine-primed reinstatement [9]. Here, we examined the ability of ceftriaxone to prevent both the acquisition of cocaine self-administration and the induction of cocaine-sensitized locomotion. While dopamine is the neurotransmitter which is primarily associated with the acquisition of cocaine self-administration (for review see [19]), glutamate transmission likely plays a role as well. It is known that acute cocaine increases extracellular glutamate levels in the nucleus accumbens [20, 21 but see also 22]. Blockade of mGluR5 receptors with MPEP prevents the acquisition of cocaine, but not food, self-administration in squirrel monkeys, as does antagonism of NMDA receptors with dizocilpine [23]. Additionally, mGluR5-null mice do not acquire cocaine self-administration; blockade of mGluR5 in wild-type mice produces the identical effect [24]. Moreover, the role of mGluR5 in acquisition is a dopamine-independent event, as dopamine levels during self-administration were normal in both mGluR5-null and wild-type animals [24]. Thus, there is evidence that glutamate signaling plays a role in the acquisition of cocaine self-administration, and it was hypothesized that decreasing glutamate spillover into the synapse by up-regulating GLT-1 expression with ceftriaxone would prevent the acquisition of cocaine self-administration.
Sensitization of the locomotor response to repeated injections of drugs of abuse is thought to be analogous to the sensitization of incentive salience attributed to addictive drugs and drug-paired cues in human addicts (see [25] for review). The induction of cocaine sensitization relies on glutamate transmission (see [26] for review) while caffeine sensitization relies on dopamine transmission [27]. Thus, it was hypothesized that ceftriaxone would prevent sensitization of the locomotor response to cocaine but not caffeine.
2. Methods
2.1 Subjects
Male Sprague-Dawley rats (Charles River Laboratories, Indianapolis, IN) weighing 250–275g were housed individually in a temperature controlled vivarium on a 12h/12h light-dark cycle (lights off at 0700h). All experiments were conducted during the dark phase of the light cycle. Animals were provided with ad libitum water and 20g of food/day. All experiments were conducted according to specifications of the National Institute of Health Guide for the Care and Use of Laboratory Animals. A total of 100 rats were used for this study.
2.2 Drugs
Cocaine hydrochloride was generously donated by the National Institute on Drug Abuse (Bethesda, MD) and was dissolved in 0.9% physiological saline for injection. For the purposes of self-administration, a 4 mg/ml solution was prepared and subjects received 0.25 mg/infusion. Doses of 15 and 30 mg/kg cocaine (IP) were administered for the sensitization study, and the dose of 15 mg/kg cocaine (IP) was administered to test for the occurrence of cocaine-primed reinstatement. Caffeine (Sigma-Aldrich, St. Louis, MO) was dissolved in saline vehicle and administered in a dose of 10 mg/kg. Ceftriaxone (Nova Plus, Irving, TX) was dissolved in saline and administered in a dose of 200 mg/kg. This dose was chosen since it has previously been shown to attenuate the reinstatement of cocaine seeking and up-regulate GLT-1 expression in the nucleus accumbens [9,16].
2.3 Self-administration Procedures
After one week of acclimatization to the colony room and handling procedures, animals underwent surgery for the implantation of chronic jugular catheters for the delivery of intravenous cocaine. One end of the catheter was inserted into the jugular vein and secured in place by suture. The other end was passed subdermally to an incision on the back where it was attached to a stainless steel guide cannula (Plastics One, Inc., Roanoke, VA) that protruded from the incision. The cannula was affixed to Mersilene Mesh (Ethicon, Somerville, NJ) that was laid flat and subcutaneously on the rats’ back so that tissue could grow through the mesh and secure the cannula in place. Catheter patency was maintained by daily injections of 0.1 mL heparin (100 IU/ml) prepared in 0.9% physiological saline. The antibiotic Timentin (0.3 in 0.1 mL, GlaxoSmithKline, Research Triangle Park, NC) was administered intravenously for one week post-surgery. Rats were allowed to recover for 7 days prior to behavioral training. One goal of the present study was to investigate the ability of ceftriaxone to prevent the acquisition of cocaine self-administration. To that end, a subset of animals (n=15) were treated with ceftriaxone (200 mg/k IP) for 5 days prior to the first day of cocaine self-administration; control animals (n=15) were treated with vehicle (saline; 0.3 mL). Ceftriaxone or vehicle treatment lasted the duration of the cocaine self-administration period and ceased when extinction training began. During the self-administration period, ceftriaxone was administered immediately after each daily operant session.
Animals self-administered cocaine in a two-lever operant chamber (Med Associates, St. Albans, VT). Cocaine was available on an FR-1 schedule of reinforcement in which each press on the active lever (always the right lever) resulted in the delivery of 0.25 mL cocaine, the illumination of the stimulus light above the active lever and the delivery of a tone (2900 Hz). The light signified the length of the time-out (TO) period (20 s) during which presses on the active lever were recorded but did not result in delivery of drug. Acquisition of cocaine self-administration was arbitrarily defined as achieving 3 consecutive days of earning at least 10 infusions/2 hr session. Many recent studies using this dose of cocaine use the criteria of 10/infusions/session [14,[28, 29]]. Animals were excluded from the study if they did not achieve this criteria within 8 days. Animals which were not excluded continued to self-administer cocaine for 7 additional sessions for a total of 10 self-administration sessions. Subsequent to the completion of the cocaine self-administration phase of the experiment, animals underwent extinction training during which time responses on the previously active lever no longer produced cocaine or the presentation of the drug-paired cues. Animals received one daily 2 hr extinction session for a minimum of two weeks. After pressing reached 20% of self-administration levels, animals were tested for cue-primed reinstatement. During this test, presses on the previously active lever did not deliver drug but did result in the presentation of the drug-paired cues. Animals were then subjected to extinction procedures for a minimum of 3 sessions or until the previous extinction criterion was met, and were tested for cocaine-primed reinstatement with 10 mg/kg cocaine (IP). Ceftriaxone (or vehicle) was not administered during extinction training or reinstatement testing.
For the purposes of conducting western blotting, a separate group of animals (n= 18) was permitted to self-administer cocaine as described above but did not undergo reinstatement testing. Instead, these animals underwent rapid decapitation following 2 weeks of extinction. Additionally, a third group of animals was included in this portion of the experiment and were yoked-saline controls which received an infusion of saline (0.05 ml over 2.7 s) when their yoked counterpart received a cocaine infusion.
2.4 Tissue Preparation
At the conclusion of the 2-week extinction training period, animals underwent rapid decapitation. The brains were removed and the nucleus accumbens was dissected and homogenized in 0.32M sucrose buffer containing protease inhibitors (cOmplete Mini Tablets, Roche, Indianapolis, IN). After homogenization and removal of cell debris and nuclei by low-speed centrifugation (900 g × 10 min), samples were centrifuged at 10,000 g. The resulting pellet was re-suspended in sucrose buffer and protease inhibitors, and spun at 10,000 g. The supernatant was discarded and the resulting pellet contained the membrane fraction (P2) and was suspended in 1% SDS in RIPA. Protein content was measured using the Bradford assay.
2.5 Western Blotting
Proteins were separated using 10% SDS-PAGE and transferred to PVDF membrane. The membranes were blocked in 3% milk and probed overnight at 4°C with primary antibody against GLT-1 (1:1000 Millipore, Billerica, MA) or xCT (1:100, generously donated by Peter Kalivas, MUSC). Membranes were washed with TBS-Tween-20 and incubated with secondary antibody at room temperature. After visualization (Pierce Western Mouse Pico Kit, ThermoScientific, Rockford, IL) band density was measured with NIH Image J software. Subsequently, the same membranes were blotted for the protein calnexin as a loading control (1:20,000, Stressgen, Victoria, B.C.). The band density of GLT-1 and xCT were normalized to the expression of the control protein calnexin on the same blot and statistical analyses were carried out on this normalized measure.
2.6 Locomotor Behavior
Behavioral activity was measured in a photocell apparatus (Omnitech Electronics Inc., Columbus, OH). On the day prior to the first locomotor test subjects were placed into the apparatus for a 60 min acclimation period and then received a saline injection (0.3 mL, IP) and behavioral activity was recorded for 120 minutes. The following day (Day 1), animals were placed in the apparatus for the 60 min acclimation period before receiving an injection of cocaine (15 mg/kg, IP). Behavior was recorded in 10 min increments for the 120 min following the cocaine injection as well as for the habituation period. Motor activity was quantified as total distance traveled (estimated by breaking of adjacent photobeams), total horizontal beam breaks, vertical movements, and stereotypy counts (estimated by repetitive breaking of the same photobeam). On Days 2–5, animals were treated with 30 mg/kg cocaine but behavior was not monitored. On Day 7, animals were administered with 15 mg/kg cocaine and behavior was again monitored in the same manner as on Day 1. To evaluate the effects of ceftriaxone on locomotor sensitization to cocaine, 4 groups were used: a group treated with vehicle prior to receiving the cocaine sensitization protocol (Veh-Coc), a group treated with ceftriaxone prior to receiving the cocaine sensitization protocol (Cef-Coc), a group which received vehicle injections and did not receive cocaine (Veh-Sal), and a group which received ceftriaxone injections and did not receive cocaine (Cef-Sal). A similar design was used to test for the presence of caffeine sensitization, however only two groups were used: a group treated with vehicle prior to receiving the caffeine sensitization protocol (Veh-Caff), and a group treated with ceftriaxone prior to receiving the caffeine sensitization protocol (Cef-Caff). Caffeine was administered on Days 1–7 at the dose of 10 mg/kg IP.
2.7 Data Analyses
The behavioral data were compared using Analyses of Variance (ANOVA) or, when comparing only two groups, a two-tailed Independent-sample t-test was used. When statistical significance (p< 0.05) was obtained with an ANOVA, the Least Significant Differences (LSD) post-hoc test was used to assess specific group differences.
3. Results
3.1 Ceftriaxone treatment and cocaine self-administration, extinction, and reinstatement
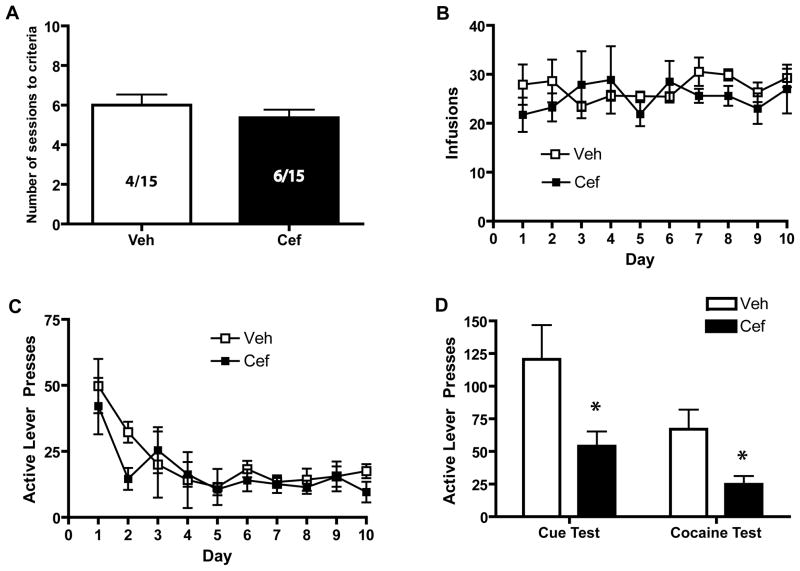
Ceftriaxone treatment failed to affect the mean number of days required for acquisition of the operant response which produced cocaine (Fig. 1B; F(1,18)=0.908, n.s.). Acquisition was arbitrarily defined as achieving 10 cocaine infusions/2 hr session for 3 consecutive days. Animals which did not acquire the self-administration response within 8 sessions were eliminated from the study. Both treatment groups began the experiment with 15 animals; 4 of the vehicle-treated animals were excluded and 6 of the ceftriaxone-treated animals were excluded for failing to acquire cocaine self-administration. Furthermore, once animals had acquired the self-administration response, there were no differences in self-administration behavior [Fig. 1B; Group: F(1,17) = 0.132, n.s.; Time × Group: F(1,17) =0.813, n.s.]. A two-way ANOVA was computed on the number of lever presses made during extinction training: there was no effect of Group [(F(1,13) = 0.742, n.s.] and no Time × Group interaction [F(1,13) = 0.736, n.s.], indicating that both ceftriaxone- and vehicle-treated animals extinguished the drug-seeking response in a similar manner [Fig. 1C; Time: F(9,117)= 3885, p<0.01]. Taken together, these data indicate that treatment with ceftriaxone prior to and during cocaine self-administration does not affect the acquisition, maintenance, or extinction of an operant response yielding cocaine. However, ceftriaxone treatment during self-administration did significantly affect later reinstatement behavior (Fig. 1D). Both cue- [t(1,13)=2.295, p<0.05] and cocaine-primed reinstatement [t(1,13)=2.569, p<0.05] were significantly attenuated in ceftriaxone-treated animals relative to those treated with vehicle.
Figure 1.
The effects of ceftriaxone on the acquisition (A), maintenance (B), extinction (C), and reinstatement (D) of the cocaine self-administration response (lever presses). Ceftriaxone administration occurred prior to and during cocaine self-administration and not during extinction training or reinstatement testing. Significant differences (p<0.05) are indicated by *.
3.2 Western Blotting
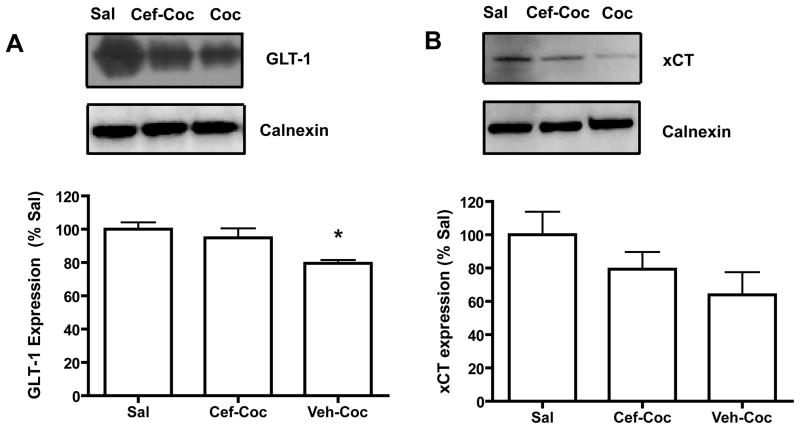
Western blotting was conducted on nucleus accumbens tissue obtained from cocaine self-administering animals which had either been treated with vehicle (Veh-Coc) or ceftriaxone (Cef-Coc) prior to and during self-administration as well as yoked-saline control animals (n=6; Sal). Of this cohort of animals, 3 of 9 vehicle-treated animals were excluded for a failure to acquire cocaine self-administration, while 3 of 9 ceftriaxone-treated animals were excluded for the same reason. Cocaine self-administering animals displayed significantly less GLT-1 and ceftriaxone restored GLT-1 levels to those of Sal controls (Fig. 2A). A one-way ANOVA was conducted on the normalized band densities of GLT-1 and revealed a significant effect of treatment group [F(2,15)=4.536, p<0.05]. Post-hoc tests revealed that this effect stemmed from significant differences between the Veh-Coc and Sal animals (p<0.05) as well as between the Veh-Coc and Cef-Coc animals (p<0.05). The expression of GLT-1 in Cef-Coc animals did not differ from Sal (p=.439). Cocaine self-administering rats did not display decreased xCT expression as previously reported, (Fig. 2B). A one-way ANOVA conducted on the density of xCT in the same three treatment groups revealed no differences in xCT expression [F(2,15) = 1.837, n.s.]. A Cef-Sal group was not included in this portion of the experiment, as ceftriaxone has previously been shown not to affect GLT-1 or xCT expression in naïve animals [9].
Figure 2.
The effects of ceftriaxone treatment prior to and during cocaine self-administration on the expression of xCT and GLT-1 two weeks post-cocaine. Significant differences (p<0.05) from Sal are indicated by *.
3.3 Locomotor Sensitization
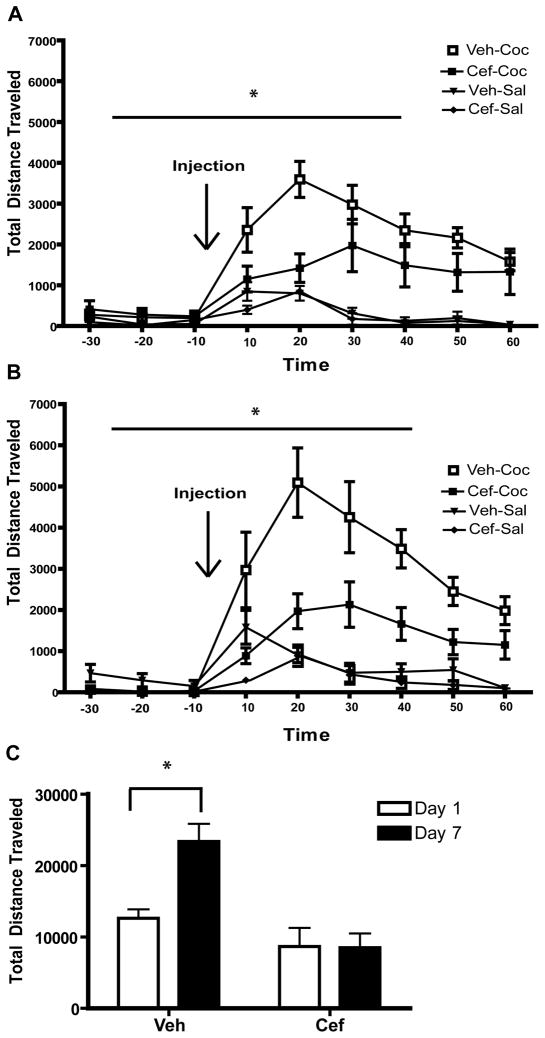
For cocaine- and caffeine-induced locomotor testing, behavior was recorded on Days 1 and 7. Fig. 3A shows the Day 1 data (30 minutes prior to injection through 60 minutes post-injection) from the cocaine protocol. A two-way ANOVA with repeated measures on one factor (Time) was conducted on this data revealed a significant effect of Group [F(3,28) = 15.596, p<0.001] and a significant Time × Group interaction [F(3,28) = 6.247, p <0.001] as well as a significant effect of Time [(F(8,224) = 24.430, p<0.001]. Post-hoc tests revealed that the significant effect of Group stemmed from differences between the Veh-Coc group and all other groups [Veh-Sal: p<0.01; Cef-Coc: p<0.05; Cef-Sal: p<0.01]. The Cef-Coc group also differed from both Sal groups [Veh-Sal: p<0.001; Cef-Sal: p<0.001], while the Veh-Sal and Cef-Sal groups did not differ (p=0.837). A two-way ANOVA conducted on the data from Day 7 (Fig. 3B) revealed similar main effects [Group: F(3,27) = 20.901, p <0.001; Time: F(8,216) = 25.107, p <0.001; Time × Group: F(3,27) = 7.320, p <0.001]. The Day 7 data from one subject in the Veh-Sal group was considered an outlier (> 2 standard deviations from the mean) and was excluded from statistical analysis. Post-hoc tests revealed a similar pattern of differences between groups on Day 7 as on Day 1, with one notable exception: the Cef-Coc group no longer differed from the Veh-Sal group (p=0.092). The Veh-Coc group again differed from all other groups (Veh-Sal: p<0.001; Cef-Coc: p<0.001; Cef-Sal: p<0.001). The Cef-Coc group also differed from the Cef-Sal group (p<0.05), while the Veh-Sal and Cef-Sal groups did not differ (p=0.355). Sensitization to cocaine is defined as an increase in locomotion following repeated cocaine administration. Thus, we compared cocaine-induced locomotor activity on Day 1 to Day 7 in both the vehicle- and ceftriaxone-treated groups (Fig. 3C) using paired-sample t-tests. The Veh-Coc group displayed a significant increase in locomotor activity from Day 1 to Day 7 [t(1,8) = 2.423, p<0.05] while the Cef-Coc group did not [t(1,8) = 0.104, n.s.].
Figure 3.
Ceftriaxone significantly attenuates cocaine-induced locomotion and the induction of cocaine sensitization. The locomotor response to acute (A) and repeated (B) cocaine is attenuated in animals treated with ceftriaxone. Cocaine sensitization is prevented by chronic treatment with ceftriaxone (C). Significant differences (p<0.05) are indicated by *.
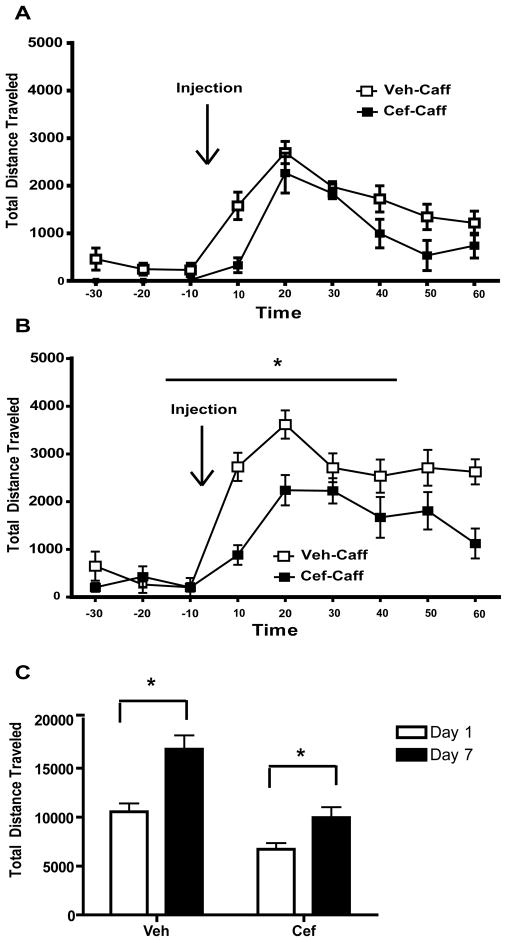
Similar analyses were used for the caffeine-induced locomotor behavior (Fig. 4). A two-way ANOVA with repeated measures on one factor (Time) conducted on the data from Day 1 (30 minutes prior to injection through 60 minutes post-injection) revealed a significant effect of Time [F(8,96) = 29.326, p<0.001] and Group [F(1,12) = 10.635, p <0.01] but no Time × Group interaction [F(1,12) = 1.380, n.s.]. A two-way ANOVA conducted on the data from Day 7 revealed significant effects of Time [F(8,96) = 36.691, p<0.001, Group F(1,12) = 8.937, p<0.05] and a significant Time × Group interaction [F(1,12) = 4.162, p <0.001]. We compared caffeine-induced locomotor activity on Day 1 to Day 7 in both the vehicle- and ceftriaxone-treated groups using paired-sample t-tests. Veh-Coc displayed a significant increase in locomotor activity from Day 1 to Day 7 [t(1,7) = 5.123, p<0.01] as did the Cef-Coc group [t(1,5) = 3.158, p<0.05], indicating that both groups sensitized to the locomotor-stimulating effects of caffeine.
Figure 4.
The effects of ceftriaxone on locomotor activity following acute and repeated caffeine. Ceftriaxone does not affect locomotion following an acute injection of caffeine (A). Ceftriaxone attenuates the locomotor response to caffeine following repeated caffeine pretreatment (B) but does not prevent caffeine sensitization (C). Significant differences (p<0.05) are indicated by *.
4. Discussion
Glutamate levels in the nucleus accumbens increase upon the first administration of cocaine [20, 21 but see also 22] and the stimulation of NMDA and mGluR5 receptors is required for the acquisition of cocaine self-administration in mice and squirrel monkeys [23, 24]. Thus, we predicted that by limiting glutamate spillover through increasing GLT-1 expression with the beta-lactam antibiotic, ceftriaxone, we could prevent the acquisition of cocaine self-administration in rats. However, pretreatment with ceftriaxone (200 mg/kg) did not affect the acquisition or maintenance of cocaine self-administration on a fixed-ratio 1 (FR-1) schedule of reinforcement (Fig. 1A, B). In contrast, the same dose of ceftriaxone used here has been shown to prevent the acquisition of cocaine self-administration (FR-1 schedule) in mice [30]. In support of the present results, N-acetylcysteine (like ceftriaxone) increases the expression of xCT and GLT-1 [9] and also does not affect the amount of cocaine self-administered on an FR-1 schedule in 2 hr daily sessions [7]. Thus, it can be concluded that increasing the expression of xCT and GLT-1 does not reduce the amount of cocaine self-administered on a fixed-ratio schedule of reinforcement in rats. However, it has been argued that a fixed ratio schedule of reinforcement is ineffective at assessing the ability of pharmaceutical or physical manipulations to affect self-administration [31]. Both ceftriaxone [30] and N-acetylcysteine [32] are capable of reducing cocaine-seeking when a progressive ratio [30] and second-order schedule of reinforcement [32] are used to assess more specifically the motivation to seek cocaine. It is also possible that ceftriaxone would be capable of preventing the self-administration of doses of intravenous cocaine other than the dose used here (0.75 mg/kg/infusion).
Treatment with ceftriaxone prior to and during self-administration was able to attenuate both cue- and cocaine-primed reinstatement of cocaine-seeking weeks after ceftriaxone administration ceased (Fig. 1D). This most likely arose from the ability of ceftriaxone to prevent the decrease in xCT and GLT-1 expression that has been observed following cocaine self-administration and 2–3 weeks of extinction training ([9]; Fig. 2A, B). While we did not observe a significant decrease in xCT expression in vehicle-treated animals as previously reported [9], ceftriaxone-treated animals did not display decreased xCT expression relative to controls (Figure 2B). The failure to detect a significant difference in xCT expression between controls and vehicle-treated cocaine animals likely stemmed from low statistical power in the three experimental groups (n=6/group). N-acetylcysteine attenuates the reinstatement of cocaine-seeking when administered acutely prior to testing [6, 7] and when a chronic treatment regimen is given during extinction training or abstinence [14]. When administered during cocaine self-administration, N-acetylcysteine also attenuates reinstatement weeks after its last administration [7]. Thus, it is likely that the ability of ceftriaxone and N-acetylcysteine to restore levels of xCT and GLT-1 in the nucleus accumbens underlies their ability to produce long-lasting reductions in cocaine-seeking behavior.
Acute cocaine injections increase striatal dopamine release [33] and DA receptor 1 (D1) antagonists decrease cocaine-induced locomotion [34]. Glutamate is also involved in the acute effects of cocaine on locomotion, as both AMPA [35] and NMDA antagonists [36] exert inhibitory effects on cocaine-induced locomotion. There is an extensive literature that demonstrates that glutamate and not dopamine transmission is involved in the induction of cocaine sensitization (for review see [26]). Here, we show that ceftriaxone inhibits locomotion following an acute injection of cocaine (Fig. 3A) and prevents the induction of cocaine sensitization (Fig. 3B, C). It is likely ceftriaxone-induced GLT-1 up-regulation dampens cocaine-induced glutamate release, thereby preventing the induction of sensitization and attenuating the locomotor response to acute cocaine. However, since dopamine is also involved in the locomotor response to acute cocaine, a ceftriaxone-dopamine interaction could also explain the ability of ceftriaxone to attenuate cocaine-induced locomotion (Fig. 3A).
To tease apart the potential effects of ceftriaxone on glutamate and dopamine, we investigated the effects of ceftriaxone on caffeine locomotion and sensitization. Acute caffeine administration increases locomotion but does not affect glutamate transmission in the NAcc [37]. Inhibiting glutamate transmission in the NAcc decreases the locomotor-stimulating effects of cocaine, but not caffeine [36]. The locomotor-stimulating effects of an acute caffeine injection are dependent on dopamine release [38] and repeated treatment with caffeine produces increased striatal dopamine levels [27]. Here we found that ceftriaxone does not prevent the induction of caffeine sensitization (Fig. 4C), however, ceftriaxone-treated animals displayed significantly less caffeine-induced locomotion (compared to vehicle-treated animals) following repeated caffeine injections (Fig. 4B). This data indicates that ceftriaxone may exert modulatory effects on the dopamine system.
Taken together, these results indicate that ceftriaxone can significantly impact two cocaine addiction-related behaviors: locomotor sensitization and the reinstatement of extinguished cocaine-seeking. Particularly notable is the ability of ceftriaxone to attenuate both cue- and cocaine-primed reinstatement weeks after ceftriaxone administration ceases. Although data presented here indicates a potential role for dopamine, ceftriaxone most likely impacts these cocaine behaviors by increasing GLT-1 and xCT expression, thereby producing a long-lasting restoration of glutamate homeostasis in the nucleus accumbens.
Research Highlights.
Ceftriaxone is an antibiotic which increases the expression and function of GLT-1
Ceftriaxone did not affect the acquisition of cocaine self-administration
Ceftriaxone attenuated cue- and coc-reinstatement weeks after administration ceased
Ceftriaxone attenuated acute and sensitized cocaine locomotion
Ceftriaxone did not prevent the induction of caffeine sensitization
Acknowledgments
This research was supported by National Institute on Drug Abuse grant DA026010 awarded to Lori Knackstedt. The authors would also like to thank David Cornish and Sara Jensen for their assistance in collecting the data presented here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Brien C. Drug addiction and drug abuse. In: Hardman JLL, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 621–42. [Google Scholar]
- 2.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–20. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 4.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 6.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 7.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–25. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 11.Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+, K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12:515–25. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- 12.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 13.Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J Neurophysiol. 2000;83:2835–43. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- 14.Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-Acetylcysteine during Abstinence or Extinction after Cocaine Self-Administration Produces Enduring Reductions in Drug Seeking. J Pharmacol Exp Ther. 2011;337:487–93. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–94. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 18.Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111:332–43. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- 19.Fibiger HC, Phillips AG, Brown EE. The neurobiology of cocaine-induced reinforcement. Ciba Found Symp. 1992;166:96–111. doi: 10.1002/9780470514245.ch7. discussion -24. [DOI] [PubMed] [Google Scholar]
- 20.Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–9. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- 21.Reid MS, Hsu K, Jr, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Robinson SE, Kunko PM, Smith JA, Wallace MJ, Mo Q, Maher JR. Extracellular aspartate concentration increases in nucleus accumbens after cocaine sensitization. Eur J Pharmacol. 1997;319:31–6. doi: 10.1016/s0014-2999(96)00923-5. [DOI] [PubMed] [Google Scholar]
- 23.Platt DM, Rowlett JK, Spealman RD. Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 2008;200:167–76. doi: 10.1007/s00213-008-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 26.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CW, Wang CS, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce sensitization and cross-sensitization behavior associated with increased striatal dopamine in mice. J Biomed Sci. 2010;17:4. doi: 10.1186/1423-0127-17-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–92. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacchioni AM, Gabriele A, See RE. Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res. 2011;218:296–300. doi: 10.1016/j.bbr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, et al. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011 doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 32.Murray JE, Everitt BJ, Belin D. N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00330.x. [DOI] [PubMed] [Google Scholar]
- 33.Broderick PA. Cocaine: on-line analysis of an accumbens amine neural basis for psychomotor behavior. Pharmacol Biochem Behav. 1991;40:959–68. doi: 10.1016/0091-3057(91)90112-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabib S, Castellano C, Cestari V, Filibeck U, Puglisi-Allegra S. D1 and D2 receptor antagonists differently affect cocaine-induced locomotor hyperactivity in the mouse. Psychopharmacology (Berl) 1991;105:335–9. doi: 10.1007/BF02244427. [DOI] [PubMed] [Google Scholar]
- 35.Witkin JM. Blockade of the locomotor stimulant effects of cocaine and methamphetamine by glutamate antagonists. Life Sci. 1993;53:PL405–10. doi: 10.1016/0024-3205(93)90496-p. [DOI] [PubMed] [Google Scholar]
- 36.Pulvirenti L, Swerdlow NR, Koob GF. Microinjection of a glutamate antagonist into the nucleus accumbens reduces psychostimulant locomotion in rats. Neurosci Lett. 1989;103:213–8. doi: 10.1016/0304-3940(89)90578-8. [DOI] [PubMed] [Google Scholar]
- 37.Dalia A, Uretsky NJ, Wallace LJ. Dopaminergic agonists administered into the nucleus accumbens: effects on extracellular glutamate and on locomotor activity. Brain Res. 1998;788:111–7. doi: 10.1016/s0006-8993(97)01518-7. [DOI] [PubMed] [Google Scholar]
- 38.Garrett BE, Holtzman SG. D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats. Pharmacol Biochem Behav. 1994;47:89–94. doi: 10.1016/0091-3057(94)90115-5. [DOI] [PubMed] [Google Scholar]