Abstract
It is important to know the contribution of specific allergens to a complex allergenic extract and to have a dependable method to assess the effector activity of an extract specifically depleted of that allergen. We have previously shown that removal of the major peanut allergen, Ara h 2, from a crude peanut extract (CPE) minimally altered the effector activity of the extract. Here we describe in detail the methodology used to generate specific rabbit anti-peptide antibodies to remove a related peanut allergen, Ara h 6, from CPE and describe an improvement in the RBL SX-38 cell assay used to assess the effector activity of treated extracts. Our results show that although Ara h 2 and Ara h 6 can be selectively removed from a CPE, removal of each alone from a CPE had no significant effect on the effector activity. However, removal of Ara h 2 and Ara h 6 together significantly reduced the effector activity of CPE.
Keywords: IgE, allergen, Ara h 2, Ara h 6, peanuts, peanut allergy
1. Introduction
Allergens are antigens that elicit an IgE response in susceptible individuals. The term “allergenic” has been used to describe both the ability of an antigen to elicit an IgE response and the ability of an allergen to cross-link IgE leading to cell activation. It is important to identify the most “allergenic” allergens that are clinically the most important.
The words “major allergen” suggested by the WHO/IUIS Allergen Nomenclature subcommittee in 1994 includes 1) a prominent band seen with sera from most patients on IgE immunoblots, 2) activity in basophil histamine release (BHR) assays, 3) activity in competitive ELISA assays, and 4) activity in animal models (King et al., 1995). Although there has been general agreement that the contribution of the allergen to the total potency of the extract for activation of IgE-sensitized mast cells or basophils (effector activity) should be demonstrated by absorption studies, this has rarely been done (King et al., 1995; Chapman, 2004).
De Groot et al. depleted an extract of cat dander of Fel d I (by 95%) with monoclonal and polyclonal antibodies and demonstrated that fel d I is a major allergen in cat dander (de Groot et al., 1988). Lombardero et al. depleted an extract of olive pollen of the allergen Ole e I using monoclonal antibodies against two non-overlapping epitopes. The removal of Ole e I resulted in a large reduction in the allergenic activity as measured by skin tests and BHR (Lombardero et al., 1992). However, these authors did not demonstrate that the allergen of interest (fel d1 or Ole e 1) were the only allergens removed. McDermott et al. successfully removed a major peanut allergen, Ara h 2, from a crude peanut extract and found that there was a statistically significant but very small effect on the ability of the CPE to activate sensitized RBL SX-38 cells (McDermott et al., 2007).
The effect of removing a specific allergen on the effector activity of an extract can be measured using a number of in vitro model systems such as the humanized RBL cell assay and ex vivo models such as basophil histamine release (BHR), the basophil activation test (BAT), or release of leukotrienes (LT) (Ocmant et al., 2009). These ex vivo assays require fresh cells for each experiment and basophils from some donors are non-responders (Ocmant et al., 2009). We have worked to refine assays based on the RBL SX-38 cell line for which frozen sera can be thawed when needed and day-to-day variability in cell function is less of a concern.
RBL SX-38 cells are rat basophilic leukemia cells that stably express approximately 70,000 copies per cell of the human high affinity receptor for IgE, FcεRI (Wiegand et al., 1996). The human receptor gives these cells the important property that they can bind IgE from the sera of allergic individuals and can be activated in an allergen-specific manner (Wiegand et al., 1996; Dibbern et al., 2003). However, these cells have been difficult to use due to serum-induced cell activation and cytotoxicity (Dibbern et al., 2003). These adverse effects seen with some human sera could be moderated by removal of IgG by using protein G under conditions that minimally affected IgE levels but this is relatively expensive and time consuming (Palmer et al., 2005). This report provides details of our approach to immunodeplete major peanut allergens from CPE and further efforts to optimize the RBL SX-38 cell assay for assessment of effector activity of allergens.
2. Materials and Methods
2.1. Subjects and sera
This study was approved by the Institutional Review Boards of the University of Colorado Denver. All subjects or their guardians signed informed consent and, for minors, assent. Individuals were selected on the basis of having a strong history of systemic reactions to peanuts and high concentrations of peanut-specific IgE. For the serum pool, 10 individual patient sera with peanut-specific IgE of ≥21 IU/ml (range 21 – 848 IU/ml) in the Pharmacia ImmunoCap® assay were combined proportionally based on their concentration of anti-peanut IgE. This gave a pool with 285 IU/ml of total IgE and 65 U/ml of anti-peanut IgE; values similar to what we have previously described (McDermott et al., 2007; Porterfield et al., 2009a). Only one of these 10 subjects donated serum to the previous studies (McDermott et al., 2007; Porterfield et al., 2009).
2.2. Rabbit antibodies to Ara h 1 and Ara h 2
Rabbit antibodies to Ara h 1 and to Ara h 2 used for immunoblots have been previously described (McDermott et al., 2007). A separate rabbit antibody to Ara h 2 (peptide, DLEVESGGRDRY) capable of immunodepleting both Ara h 2.01 and 2.02 from CPE has been previously described (McDermott et al., 2007).
2.3. Choice of peptides for production of rabbit antibodies to Ara h 6
Candidate peptides for production of antibodies to Ara h 6 were chosen for 1) uniqueness and 2) hydrophilicity and antigenicity using a commercial algorithm program (MacVector; Cary, NC). Peptides were obtained commercially (YenZym Antibodies, South San Francisco, CA) at 95% purity based on Mass Spectroscopy. Rabbit antibodies to a variety of peptides were raised by immunizing rabbits with either a peptide-KLH or peptide-thyroglobulin conjugate followed by affinity purification on a peptide-specific column and elution with 0.1 M glycine pH 2.5 (YenZym Antibodies, South San Francisco, CA). All antibodies were stored in 50% of glycerol at −20°C at 1 mg/ml. Pre-immune IgG was isolated from serum by binding to protein G and elution with glycine as above (Pierce, Rockland, IL).
2.4. Crude peanut extracts
Crude peanut extracts (CPE) were prepared and characterized as previously described (Porterfield et al., 2009). Our extracts contained approximately (by weight) 4% Ara h 2 and 6% Ara h 6 (data not shown).
2.5. Immunodepletion of antigens
Each affinity purified antibody was dialyzed into 100 mM PBS (pH 7.4) and 5 mg of antibody was covalently linked to 1 ml of packed AminoLink® beads (Pierce, Rockland, IL), according to the manufacturer’s instructions. Eighty to ninety percent of the IgG was bound. These columns were stored in 100 mM PBS (Pierce, Rockland, IL) with 0.05% azide for up to 3 months without loss of activity. One and one-half ml of CPE (at ε 3 mg/ml) was added to 1 ml packed beads with covalently linked antibody (~4mg), rocked overnight at 4°C and the unbound material was collected. The columns were then regenerated by treatment with four bed volumes of 0.1 M glycine-HCl, pH 2.3, followed by two bed volumes of 100 mM PBS. The material collected from the first pass was placed back on the column. Eluted CPE was then concentrated using a 3K cutoff Centricon column (Millipore; Billerica, MA) and stored at -70°C at a concentration of ε1mg/ml. A control column was constructed with purified pre-immune rabbit IgG to produce sham-treated CPE.
2.6. RBL SX-38 cell growth and assay
RBL SX-38 cell release assay was performed as previously described (McDermott et al., 2007). Briefly, 2 × 105 cells/ml were labeled with 1µCi/ml of tritiated 5-hydroxytryptamine (5-HT, serotonin; PerkinElmer Life Sciences, Covina, California) and passively sensitized overnight with 7.5% pooled serum. The sensitized cells were then challenged with allergens as noted and release of 3H-serotonin into the supernatant and the 3H–serotonin content of the residual cells were measured. Antigen-specific release was calculated. In these experiments, we modified RBL SX-38 cell culture conditions such that cells were slowly transitioned over 2 weeks into a culture medium containing a mixture of 3% human AB serum (Invitrogen, Carlsbad, CA) and 7% fetal calf serum (Dibbern et al., 2003; McDermott et al., 2007). These cells are now called RBL-SX38-AB cells. We found that the cells needed to be cultured in these conditions for ~1 month before the background was dependably <15% and the net signal was >25% release of the total cellular serotonin. By culturing RBL SX-38 cells in 3% human AB serum and 7% FBS they became less sensitive to the deleterious effects of human serum. These new culture conditions obviated the need for a step with protein G to remove IgG that was previously necessary to avoid cell activation seen with some sera and to avoid cell death seen with other sera (data not shown). Of note, we determined that a typical lot of human AB serum contained about 20 IU/ml of total IgE and no detectable anti-peanut IgE (data not shown). Thus, a 3% solution contained negligible levels of IgE. This entire assay has also been performed without radioactivity and with nearly identical results using the β-hexaminodase and β-glucuronidase assays (data not shown). For the enzyme assays, the minor alterations included extending the degranulation period to 60 minutes and resuspending the residual cells with 0.1% Triton X-100 in PBS instead of trypsin EDTA (Blanc et al., 2009).
2.7. Evaluation of immunodepleted CPE
We monitored depletion of Ara h 2 and Ara h 6 with immunoblots. Typically, 1 µg of control CPE and potentially allergen-depleted CPE were run on a 15% reducing gel and immunoblots were probed with affinity purified rabbit antibodies to anti-Ara h 1, Ara h 2 and Ara h 6 (1:10,000–1:100,000) and developed with HRP-labeled goat anti-rabbit IgG (Invitrogen, Carlsbad, CA). For assessment of IgE binding, 2 µg of each preparation was run on the gel and probed with the human serum pool (1:20) and developed with biotinylated murine anti-Human IgE (Invitrogen, Carlsbad, CA) and HRP-strep-Avdin (Sigma, St. Louis, MO). Of note, the developing antibodies for Ara h 2 and 6 were directed against peptides that were different from the antibodies used for immunodepletion.
Results and discussion
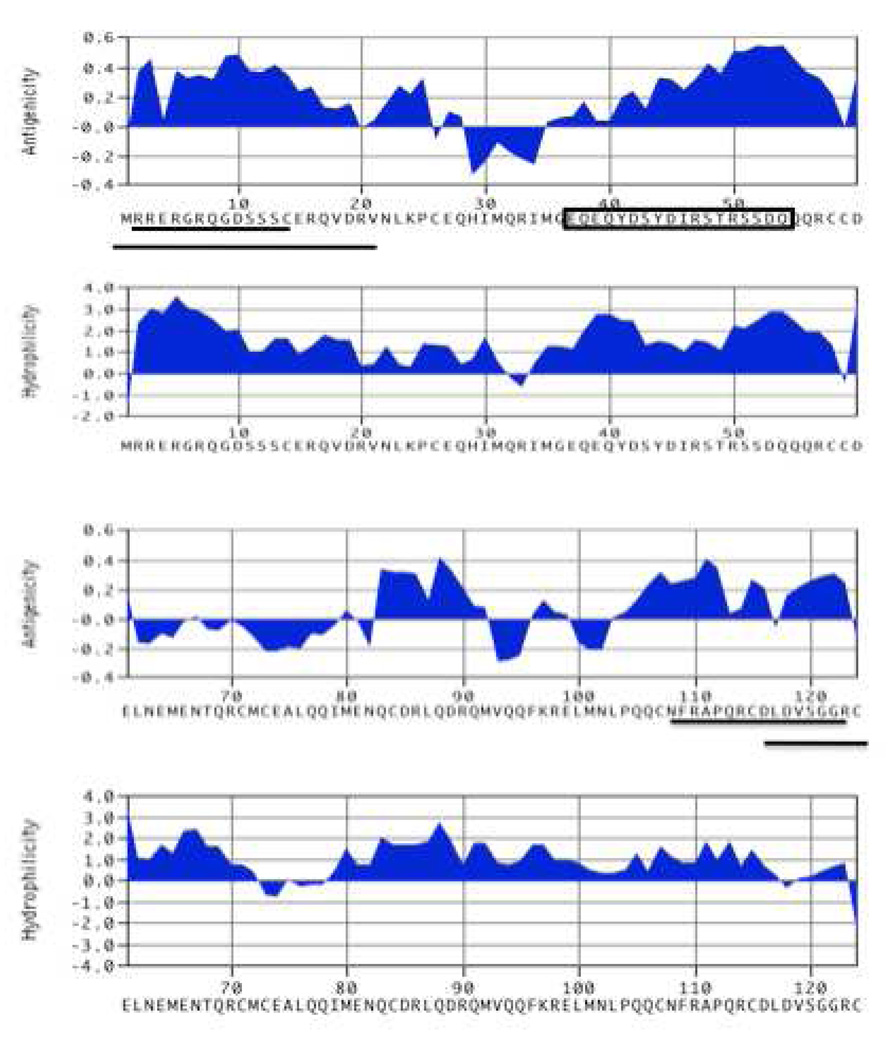
3.1. Hydrophilicity plots for Ara h 6
Figure 1 shows the Kyte-Doolittle plots for Ara h 6. We focused on those peptides that demonstrated the most hydrophilicity, antigenic index surface probability, and uniqueness of the sequence within the peanut proteome as discerned by protein BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (Figure 1).
Figure 1. Kyte-Doolittle plot of Ara h 6 showing the hydrophilicity, surface probability and antigenic index over the length of the Ara h 6 amino acid sequence.
The candidate regions for which we made anti-peptide antibodies that were not able to immunodeplete Ara h 6 are underlined and the peptide that was used to produce the antibody that specifically depleted of Ara h 6 is enclosed in a box.
3.2. Production of rabbit anti-peptide antibodies that can immunodeplete Ara h 6 from CPE
For reasons that we do not understand, generation of rabbit antibodies capable of immunodepleting Ara h 6 from CPE required more effort than did generation of antibody that could specifically deplete Ara h 2. Whereas Ara h 2 was easily depleted with an antibody to the C-terminus (McDermott et al., 2007), this was not the case with Ara h 6. For Ara h 6, we tested antibodies to 5 different peptides and two different conjugates before finding that an antibody to a loop (aa 37–55) near the N-terminus, when conjugtated to thyroglobulin, was effective (row 5, Table 1). Affinity purified antibodies that were raised against the unique Ara h 6 peptide, EQEQYDSYDIRSTRSSDQ (amino acids 37–55) linked to thyroglobulin (THY) were consistently capable of removing Ara h 6 without removing Ara h 2. Antibodies raised to the same peptide conjugated to keyhole limpet hemocyanin (KLH) were not successful at immunodepletion of Ara h 6 (Table 1)
Table 1.
Rabbit anti-peptide, anti-Ara h 6 antibodies
| Peptide | Original AA # | Carrier* | Effective | |
|---|---|---|---|---|
| 1 | RRERGRQGDSSSC-amide | 2–14 | KLH | No |
| 2 | MERRERGRQQGDSSSCERQVDR-amide | 1–20 | KLH | Weak |
| 3 | MERRERGRQQGDSSSCERQVDR-amide | 1–20 | THY | Weak |
| 4 | EQEQYDSYDIRSTRSSDQ-amide | 37–55 | KLH | No |
| 5 | EQEQYDSYDIRSTRSSDQ-amide | 37–55 | THY | Yes |
| 6 | NFRAPQRCDLDVSGGR-amide | 108–123 | KLH | No |
| 7 | DLDVSGGRCS-amide | 116–124 | KLH | No |
KLH = keyhole limpet hemocyanin; THY = thyroglobulin
3.3. Removal of Ara h 6 from CPE
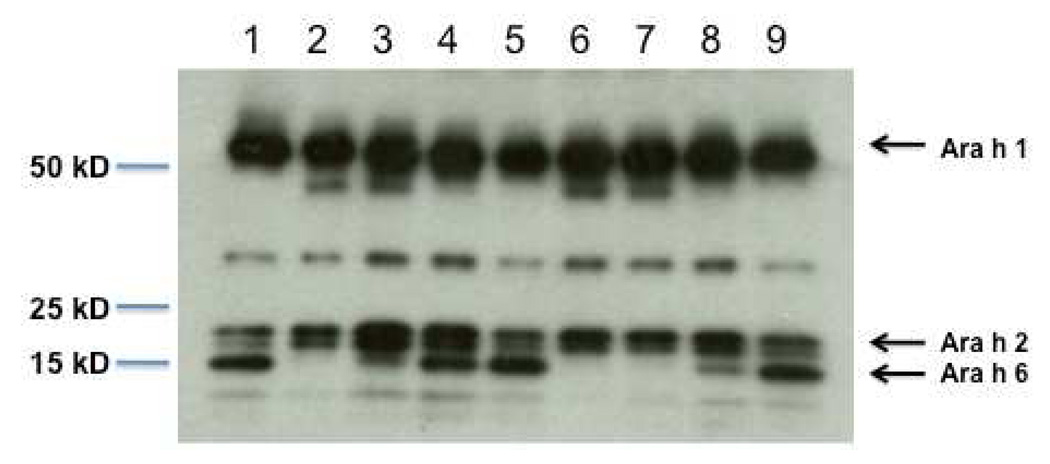
One ml of packed beads with anti-Ara h 6 (aa 37–55) covalently attached was able to deplete Ara h 6 from 1.5 ml of CPE at 3 mg/ml (4.5 mg of CPE) with one pass through the column and from 1.5 ml of CPE at 6 mg/ml (9 mg of CPE) with two cycles (see material and methods section and Figure 2). Since our CPE consists of approximately 4% Ara h 2 and 6% Ara h 6 as measured by a competitive ELISA (data not shown), we estimate that approximately 4 mg of this anti-Ara h 6 antibody linked to 1 ml of packed beads was, with two passes, able to remove approximately 540 µg of Ara h 6 from our CPE.
Figure 2. Immunoblot of CPE following addition of increasing amounts of CPE over a column with affinity purified rabbit anti-Ara h 6 (peptide, EQEQYDSYDIRSTRSSDQ).
Lane 1, untreated CPE (not passed over a column). Lanes 2–8, 1.5 ml of CPE at various concentrations as shown were passed over 1 ml of packed beads containing 4 – 4.5 mg of antibody. Beads were linked to the rabbit anti-Ara h 6 antibody for lanes 2, 3, 4, 6, 7, and 8. For lanes 5 and 9 the beads were linked to pre-immune IgG. CPE at 3 mg/ml (4.5 mg total) passed over an anti-Ara h 6 columns is shown in lane 2 (one pass) and lane 6 (two passes). CPE at 6 mg/ml (9 mg) passed over an anti-Ara h 6 column is shown in lane 3 (one pass) and lane 7 (two passes). CPE at 12 mg/ml (18 mg) passed over an anti-Ara h 6 column is shown in lane 4 (one pass) and lane 8 (two passes). The immunoblot was probed with a mixture of antibodies to Ara h 1, 2, and 6 as described in the materials and methods section.
3.4. Removal of both Ara h 2 and Ara h 6 reduced the effector activity whereas removal of either separately did not
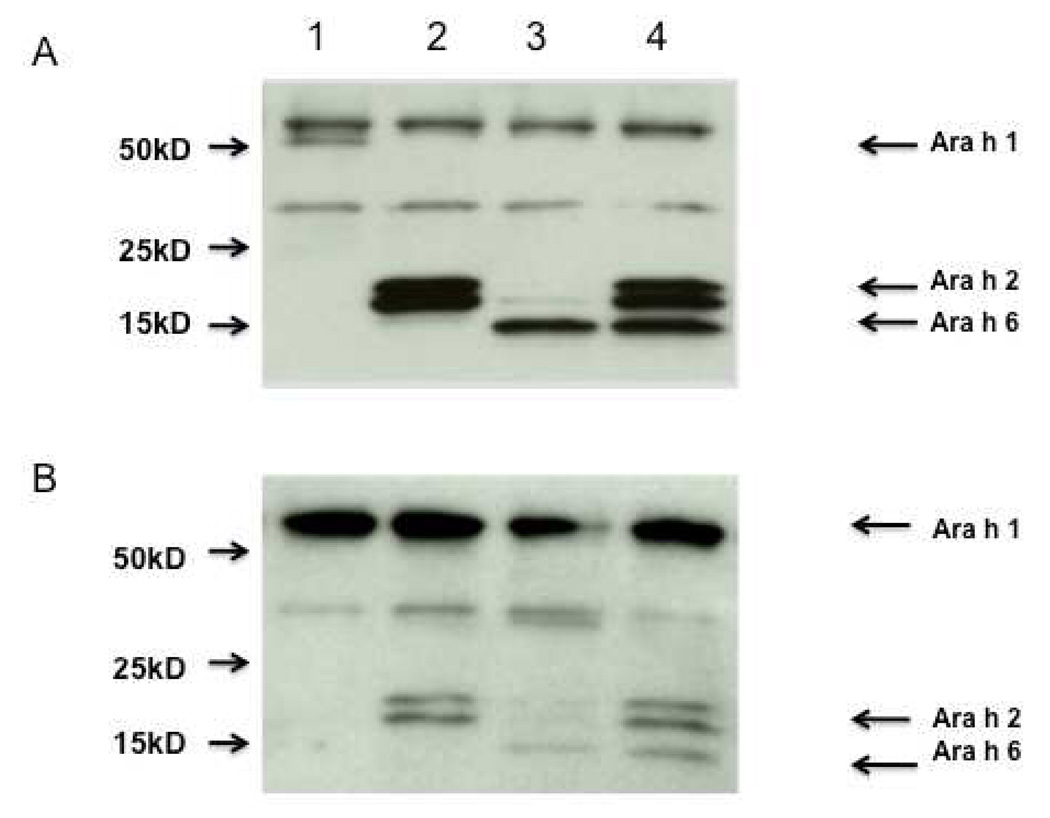
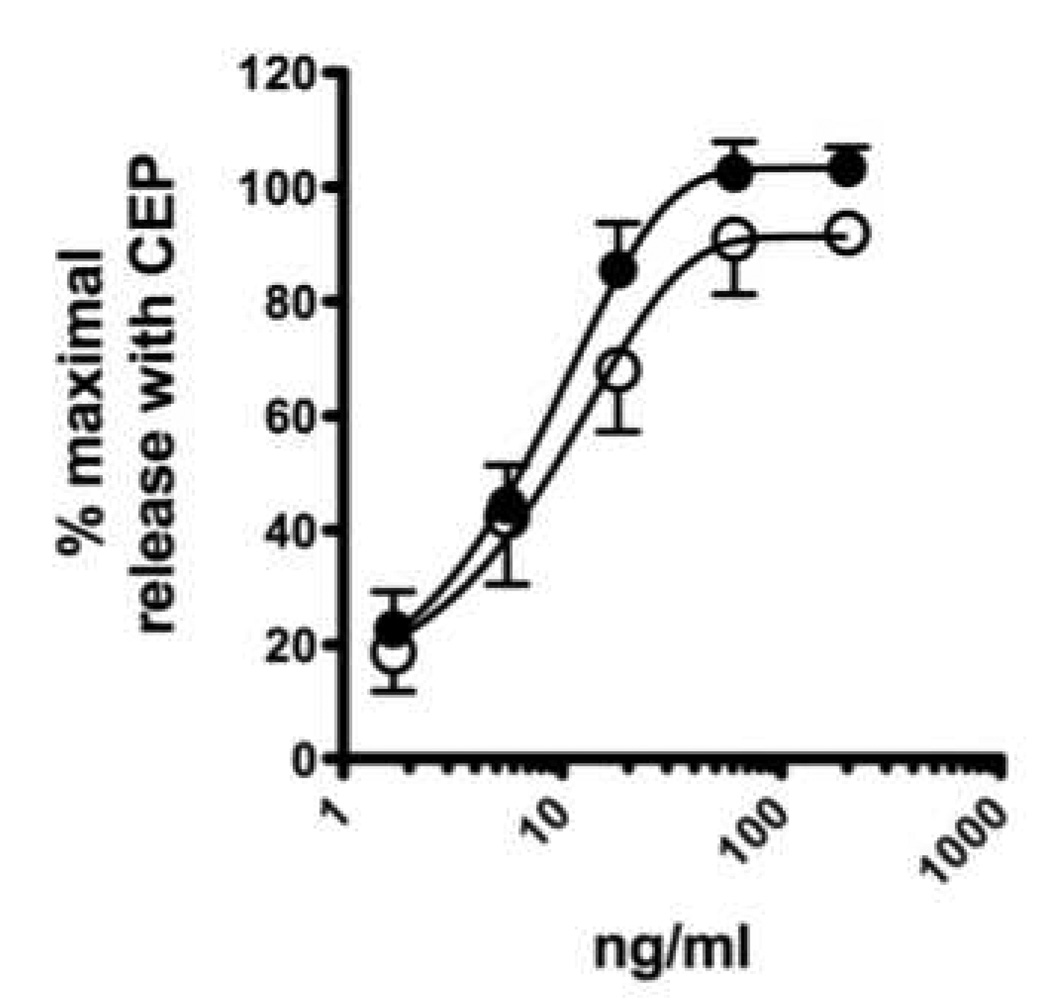
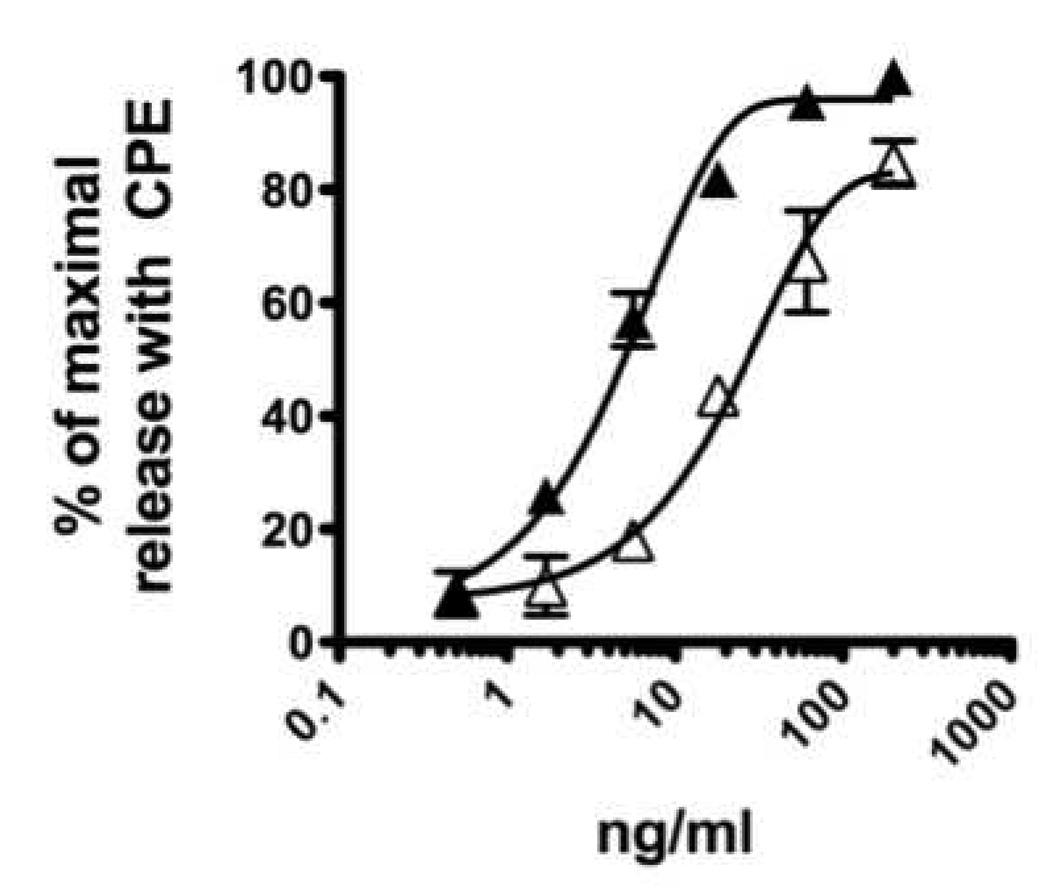
CPE (3 mg/ml) was incubated with 1 ml of packed beads linked to anti-Ara h 2 (see materials and methods section) and anti-Ara h 6 (aa 37–55) either separately or together. The effluent was collected and passed over the column (after the column was recycled) a second time. CPE passed over a column with pre-immune IgG is referred to as control CPE. Immunoblot analysis with both rabbit anti-peptide antibodies and with IgE from the serum of a pool of peanut allergic subjects confirmed that each protein was specifically depleted by ~99% when assessed either with rabbit anti-peptide antibodies (Figure 3A) or with human anti-peanut IgE (Figure 3B). Of note, IgE from the pooled human binds Ara h 2 and Ara h 6 with similar intensity. The depletion was allergen specific because the levels of Ara h 1 were not diminished. Unexpectedly, contrary to what is seen with the rabbit anti-peptide antibodies, the IgE immunoblots showed some removal of Ara h 6 with the anti-Ara h 2 antibody. RBL SX-38 cells were sensitized with IgE from our pool of peanut allergic sera and were stimulated with various doses of either untreated CPE, control CPE or CPE depleted of Ara h 2, Ara h 6 or both Ara h 2 and Ara h 6. As shown in Figure 4A and 4B, removal of Ara h 2 alone or Ara h 6 alone has no significant impact on the effector activity of our CPE whereas immunodepletion of both Ara h 2 and Ara h 6 together significantly reduced the effector activity of peanut extract (Fig. 4C). Control CPE gave a dose response indistinguishable from the original CPE (data not shown). These data are similar to what was seen when Ara h 2 and Ara h 6 were separated from other proteins in CPE by gel filtration chromatography, a method less precise than what is described here (Porterfield et al., 2009b). The residual activity of CPE depleted of both Ara h 2 and Ara h 6 may be due to the other peanut allergens such as Ara h 1 and Ara h 3. However we cannot exclude the possibility that trace amount of Ara h 2 and Ara h 6 or their fragments may be responsible for part of the residual effector activity.
Figure 3. Immunodepletion of Ara h 2, Ara h 6, and both.
Immunoblots are shown probed with a combination of rabbit anti-Ara h 1, 2, and 6 antibodies and developed with anti-rabbit IgG (A) and probed with the human pooled serum developed with anti-human IgE (B). Each lane contained 1µg (A) or 2 µg (B) of CPE following passage over columns containing both anti-Ara h 2 and anti-Ara h 6 (lane 1), anti-Ara h 6 alone (lane 2), anti-Ara h 2 alone (lane 3), or pre-immune IgG (lane 4). For this figure, the ratio of CPE to packed beads was ~4.5 mg of CPE to 1 ml of packed beads containing ~4 mg of affinity purified antibody.
Figure 4. Effector activity of CPE.
RBL-AB cells were sensitized with IgE from a pool of sera from highly peanut-allergic subjects as described in the materials and methods section and stimulated with CPE depleted of Ara h 2 alone (A), depleted of Ara h 6 alone (B) and depleted of both Ara h 2 plus Ara h 6 (C) as shown in Figure 3. Solid symbols are the CPE treated with pre-immune serum and open symbols are allergen depleted CPE. In each experiment (A, n=3; B, n=3; C, n=7), cells were stimulated in triplicate with a maximal amount of CPE (200 mg/ml). In order to pool the experimental results, the data are expressed as a percent of the maximal net (total minus background) degranulation in each assay. Maximal net degranulation with CPE (mean±SEM): A, 41±5%; B, 42±4%; C, 40±2%. If the standard error bars are not visible, they were smaller than the symbol. Best-fit lines were generated using a one-phase decay model (Prism 5.0c for MacIntosh; GraphPad Software, Inc. La Jolla, CA).
4. Conclusions
Removal of a specific protein from an allergenic extract requires a) identification of the most antigenic peptides, b) choice of the best carrier protein, and c) some trial and error. We successfully used this approach to generate rabbit peptide-specific antibodies that could specifically remove the important peanut allergens Ara h 2 and Ara h 6 from crude peanut extracts. When we assayed these extracts with an improved RBL cell assay (RBL-SX38-AB cells) to assess functional activity, we found that removal of both proteins was necessary to diminish the effector activity of CPE suggesting that both of these proteins are important peanut allergens. Approaches such as this help us understand the contributions of potentially important peanut allergens and should be applicable to other allergenic extracts.
Acknowledgments
Funding: RO1-AI052164 from National Institute of Allergy and Infectious Diseases to Dr. Dreskin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009;39:1277–1285. doi: 10.1111/j.1365-2222.2009.03294.x. [DOI] [PubMed] [Google Scholar]
- Chapman MD. Allergen Nomenclature. In: Lockey RF, Bukantz SC, Bousquet J, editors. Allergens and Allergen Immunotherapy. Marcel Dekker, Inc.; 2004. pp. 51–64. [Google Scholar]
- de Groot H, van Swieten P, van Leeuwen J, Lind P, Aalberse RC. Monoclonal antibodies to the major feline allergen Fel d I. I. Serologic and biologic activity of affinity-purified Fel d I and of Fel d I-depleted extract. J Allergy Clin Immunol. 1988;82:778–786. doi: 10.1016/0091-6749(88)90079-6. [DOI] [PubMed] [Google Scholar]
- Dibbern DJ, Palmer G, Williams P, Bock S, Dreskin S. RBL cells expressiing Human FceRI are a sensitive tool for exploring Functional IgE-Allergen Interactions. J. Immunol. Methods. 2003;274:37–45. doi: 10.1016/s0022-1759(02)00369-1. [DOI] [PubMed] [Google Scholar]
- King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills TA, Thomas W. Allergen nomenclature. Allergy. 1995;50:765–774. doi: 10.1111/j.1398-9995.1995.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Lombardero M, Quirce S, Duffort O, Barber D, Carpizo J, Chamorro MJ, Lezaun A, Carreira J. Monoclonal antibodies against Olea europaea major allergen: allergenic activity of affinity-purified allergen and depleted extract and development of a radioimmunoassay for the quantitation of the allergen. J Allergy Clin Immunol. 1992;89:884–894. doi: 10.1016/0091-6749(92)90445-8. [DOI] [PubMed] [Google Scholar]
- McDermott RA, Porterfield HS, El-Mezayan R, Schlichting D, Hansen KC, Duncan MW, Solomon B, Redzic J, Simpson M, Dreskin SC. Contribution of Ara h 2 to peanut-specific immunoglobulin E-mediated, cell activation. Clinical & Experimental Allergy. 2007;37:752–763. doi: 10.1111/j.1365-2222.2007.02701.x. [DOI] [PubMed] [Google Scholar]
- Ocmant A, Mulier S, Hanssens L, Goldman M, Casimir G, Mascart F, Schandene L. Basophil activation tests for the diagnosis of food allergy in children. Clin Exp Allergy. 2009;39:1234–1245. doi: 10.1111/j.1365-2222.2009.03292.x. [DOI] [PubMed] [Google Scholar]
- Palmer GW, Dibbern DA, Burks AW, Bannon GA, Bock SA, Porterfield HS, McDermott RA, Dreskin SC. Comparative Potency of Ara h 1 and Ara h 2 in Immunochemical and Functional Assays of Allergenicity. Clinical Immunology. 2005;115:301–312. doi: 10.1016/j.clim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variantsx. Clin Exp Allergy. 2009a;39:1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector Activity of Peanut Allergens: A critical role for Ara h 2, Ara h 6, and their variants. Clinical and Experimental Allergy. 2009b doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand TW, Williams PB, Dreskin SC, Jouvin MH, Kinet JP, Tasset D. High-affinity oligonucleotide ligands to human IgE inhibit binding to Fc epsilon receptor I. J Immunol. 1996;157:221–230. [PubMed] [Google Scholar]