Abstract
Accumulating evidence suggests that gender-related differences are prominent in gastric motility functions in both health and disease. Women are more susceptible to gastroparesis than men. Though the mechanism(s) involved are not fully understood, impairment of the nitrergic system is one of the main factors responsible for the disease. Uncoupling of neuronal nitric oxide synthase (nNOS) causes a decreased synthesis of NO leading to a reduction in smooth muscle relaxation. Tetrahydrobiopterin (BH4) (an essential cofactor for nNOS) is a key regulator of nNOS activity for stomach dysfunction and gastroparesis. In addition, BH4 has been shown to be a potent antioxidant and anti-inflammatory agent. Well established by results from our laboratory, a diminished intracellular (BH4:total biopterin) ratio in diabetic female rats significantly impairs nNOS activity and function. Recent research has been focused on BH4 biosynthesis and gastroparesis because reduced BH4 cofactor levels can alter the production of NO by nNOS. Researchers are now paying more attention to the possibility of using BH4 as a therapeutic strategy in gastroparesis. The purpose of this review is to provide an overview of the regulation and function of nNOS by sex hormones and BH4 and its potential role in the treatment of gastroparesis.
Keywords: Gastric motility, Gastroparesis, Nitric oxide, Tetrahydrobiopterin, Oxidative stress, Sex hormones
Introduction
The effect of gender in a healthy population on gastric emptying remains controversial though it appears that women may have a slower solid and liquid emptying [1–4]. Ambulatory antroduodenal manometry has shown shorter migrating motor complex periods in women compared with men [2]. The mechanisms responsible for these differences are not completely understood and conflicting reports exist in the literature. As an example, in a study of duode-nojejunal motility, women in the follicular phase were found to exhibit motor activity similar to that of men [3]. In contrast, Knight et al. [4] demonstrated an attenuated postprandial antral contractile activity in the follicular phase of women compared with men. Postmenopausal women received sex hormone therapy display delayed gastric emptying compared to age-matched men or untreated women. Animal studies have shown that the solid gastric emptying rate is slower in ovary-intact compared to ovariectomized (depletion of ovarian hormones; estrogen and progesterone) female rats [5]. Studies by Chen et al. [5] have demonstrated that progesterone accelerates and a mixture of estradiol-17β (E2) + progesterone (P4) or E2 alone inhibits liquid gastric emptying in rats. Data from our laboratory demonstrated that gastric emptying for solids is slower during the estrus cycle while circulatory estrogen levels are elevated (Fig. 1a, b). Furthermore, Shah et al. [6] demonstrated that E2, but not P4, may be responsible for increased gastric nNOS expression and nitrergic relaxation. In addition, P4 treatment decreases the resting tension of fundus and inhibits the mean contractile amplitude of gastric antrum as well as the motility index of pylorus in rats [7]. It is clear that diabetes induction decreases both circulating E2 and P4 levels in females [8]. Collectively, the above studies suggest that in general gastric motility is slower (delayed gastric emptying but not gastroparesis) in women compared to men and this was perhaps due to elevated levels of sex steroid hormones and NO. A reduction in sex steroid hormones may downregulate NO-mediated gastric motility and thus leading to delayed gastric emptying/gastroparesis in diabetic women.
Fig. 1.
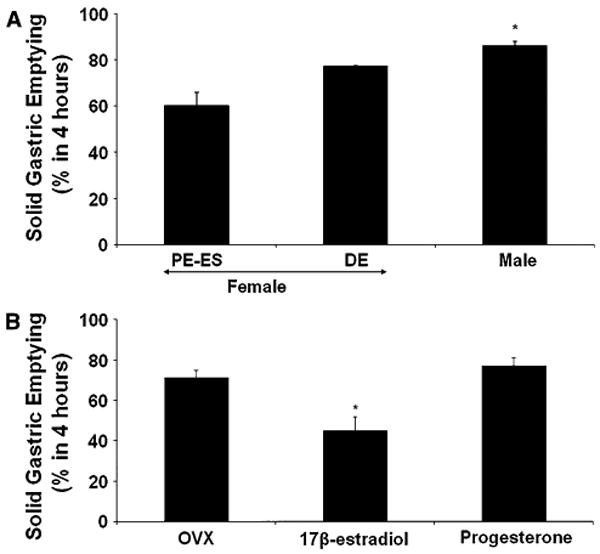
Effect of gender and hormone dependent changes on solid gastric emptying in female Sprague-Dawley rats. a Groups of female rats were used during proestrus (PE)/estrus (ES) (when circulatory sex hormone levels are elevated) or diestrus (DE) phase of a 4-day estrus cycle (when circulatory sex hormone levels are lower). The solid gastric emptying rate is slower during PE–ES compared to either DE female or male rats. The values are mean ± SE for 4–6 animals. Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with PE–ES group. b Effects of estradiol-17β (E2) or progesterone (P4) on solid gastric emptying in female rats. After 1 week of ovariectomy (OVX), Sprague-Dawley rats were implanted subcutaneously with a 21-day biodegradable release pellet of either (per kilogram body weight) E2 (2 mg), P4 (20 mg) or placebo (OVX). Gastric emptying for solids were assessed 21 days after hormone supplementations. The values are mean ± SE for 4–6 animals. Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with OVX group
Diabetic Gastroparesis
The most common forms of gastroparesis include disease secondary to diabetes or post-surgical complications, and idiopathic [1, 9]. Diabetics are at a high risk of developing gastroparesis. Symptoms of diabetic gastropathy can range from mild dyspepsia, nausea, early or easy satiety, bloating, and weight loss due to recurrent vomiting and abdominal pain. Delayed gastric emptying is often seen in type I diabetics (insulin-dependent), ranging from 27 to 58% of adult cases [9–11]. Likewise, gastroparesis is present in up to 30% of patients with type 2 diabetes (non-insulin-dependent) [9–11]. Gastroparesis affects the dietary state and, in diabetics, it also impairs glycemic control; secondary effects on organs can lead to increased mortality [10, 11]. The range of treatments includes dietary modifications and medications to stimulate gastric emptying (combination of prokinetic and antiemetic agents). In severe cases, treatment options include surgery (venting gastrostomy or jejunostomy) and gastric electrical stimulation [11].
Although gastroparesis is a significant health problem [12], the pathogenesis of gastric dysfunction and the mechanism for its gender bias are not well understood. Gastric motility requires integration of motor activities of all areas of the stomach and is largely regulated by: (1) excitatory (acetylcholine) and inhibitory (nitric oxide (NO) and vasoactive intestinal peptide) neurotransmitters working directly on smooth muscles, or (2) electrical signals that originate from the interstitial cells of Cajal (ICCs) [13–15]. Central modulation of enteric neuronal function (both inhibitory and excitatory) through the vagus nerve also plays an important role in gastric physiology and is predominantly cholinergic in character [16]. Nitrergic signaling, the principal nonadrenergic noncholinergic (NANC) inhibitory mechanism in the gastrointestinal tract, plays a critical role in the control of gastric accommodation and pyloric relaxation in response to a meal. In diabetics, enteric neuropathy may be particularly important [17]. Several studies have shown disturbances in enteric nerves, particularly involving nitrergic nerves in diabetes [13, 14, 18, 19]. Impairment in nitrergic relaxation resulting from either neuronal loss or dysfunction may contribute to the gastropathy seen in both streptozotocin (STZ)-induced diabetes as well as in spontaneously diabetic male rats and mice [20].
nNOS and Gastroparesis
In the absence of effective nitrergic output to muscle, gastric accommodation is impaired, resulting in early satiety and discomfort. Further, a functional obstruction at the gastric outlet due to a non-relaxing pylorus leads to delayed emptying [2, 21, 22]. Diabetic rats and mice show early defects in nitrergic relaxation and nNOS expression before neuronal degeneration in the pyloric sphincter is observed; these effects are reversed by insulin treatment [21, 23]. nNOS activity is a critical signaling node for regulating gastric motor function (reviewed in Vittal et al. [24]). nNOS catalyzes the formation of NO, which initiates smooth muscle relaxation. nNOS activity in turn is regulated by tetrahydrobiopterin (BH4), which couples electron flow to NO generation. Numerous studies have identified suppression or loss of nNOS activity as a major cause of development of gastroparesis [12, 14]. The importance of NO in gastric function has been established by the findings of pyloric hypertrophy and gastric dilation in mice with a targeted genomic deletion of nNOS (nNOS−/− mice) [25, 26].
Our laboratory previously demonstrated that gastric antrum nitrergic relaxation, nNOS alpha dimerization (but not total nNOS protein expression), and NO levels (Fig. 2) are higher in healthy female rats compared to age-matched males [13]. We showed that diabetes induction significantly impairs all of these parameters including NO levels (Fig. 2) in female but not in male rats [14]. In addition, our data provided evidence for the first time that STZ-induced diabetes (in female but not male rats) affects nitrergic relaxation and decreases nNOS alpha (α) dimerization, which in turn are ultimately associated with delayed gastric emptying [14]. Collectively, these data suggest that NO generating from nNOS is critical for gastric motility and subsequent gastric emptying and a change in this system may lead to gastroparesis in diabetic women.
Fig. 2.
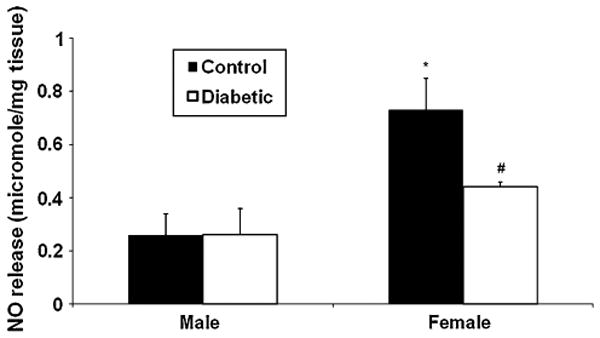
Effect of diabetes on nitric oxide (NO) levels in male and female Sprague-Dawley rat gastric antrum muscular tissues in vivo. Diabetes was induced with single injection of STZ (55 mg kg−1 body wt ip) as reported previously [48]. Control group was injected with vehicle (9 mmol citrate buffer) only. Twelve weeks after diabetes induction, groups of animals (control vs. diabetes) were euthanized; gastric antrum muscular strips were incubated for 24 h and assayed for NO release in the medium as reported earlier [48]. Gastric antrum NO levels are higher in adult healthy female compared to age matched male rats. In addition, NO levels are reduced only in female but not in male gastric antrum muscular tissues at the onset of diabetes. Values are mean ± SE (n = 4–6). Statistical significance was determined by Tukey test after one-way ANOVA. *P < 0.05 compared with male diabetic group; #P < 0.05 compared with male control group
NO and Smooth Muscle Function
In NANC neurons, NO is synthesized from l-arginine by Nnos [27] and then diffuses to gastrointestinal smooth muscle cells. The downstream effects of NO on gastrointestinal smooth muscle cells includes the activation of soluble guanylate cyclase (sGC), the production of cGMP (cyclic guanosine mono phosphate), and the stimulation of PKG (protein kinase G) which results in smooth muscle relaxation due to a decrease in intracellular calcium concentrations [27]. One of the mechanisms involved in the inactivation of the NO/cGMP/PKG pathway is oxidative stress, which affects NO bioavailability as well as NO's ability to activate sGC [27, 28]. Low BH4 levels impair the production of NO, and lead to increased superoxide radical production, due to nNOS uncoupling [29]. Our recent studies demonstrate that a diminished intracellular BH4:BH2 ratio is the molecular trigger responsible for NO insufficiency in diabetes [14]. Recently, activators of sGC have been introduced as a new approach for the modulation of NO-cGMP signaling pathways. It has been reported that ICCs express PKG and the sGCs, necessary to activate inhibitory pathways and thus are primary targets for NO released from neurons and other cells in the GI tract [30]. In smooth muscle cells, the NO/cGMP/PKG pathway is also regulated through phosphodiesterases (PDEs), enzymes that catalyze the hydrolysis of 3′,5′-cyclic nucleotides to inactive nucleotide 5′-monophosphates (Fig. 3) [27, 28]. PDE5 is the main PDE involved in cGMP catabolism in smooth muscle [27, 28]. In fact, a PDE5 inhibitor, sildenafil, has been shown to affect the impaired gastric accommodation by increasing postprandial gastric volume in healthy subjects [31]. In addition, these studies report that sildenafil has a minimal effect on solid gastric emptying whereas liquid emptying is delayed significantly suggesting a role for NO down-stream signaling pathway in gastric accommodation [31]. Alternatively, in the central nervous system (CNS), it is now increasingly appreciated that NO bioactivity is often actively transduced via S-nitrosothiol (SNO) signals rather than via activation of guanylyl cyclase (reviewed by Savidge Tor [32]).
Fig. 3.
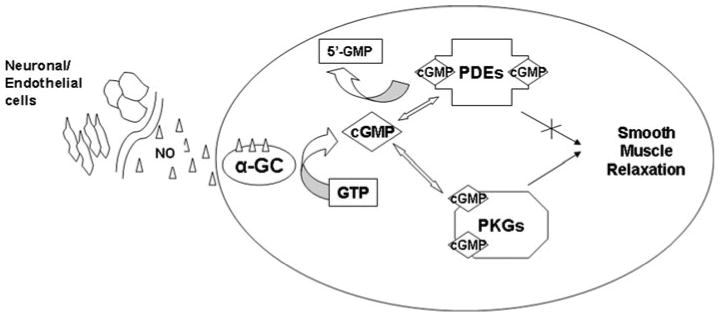
Nitric oxide signaling and cGMP targets. NO generated from nitric oxide synthases diffuses into cellular membranes and activates guanylate cyclases (α-GC). This activation converts GTP to cGMP. Increased levels of cGMP binds to and activates PKG which phosphorylates several proteins and lowers Ca2+ levels causing smooth muscle relaxation. cGMP binding to the catalytic site of PDE degrades cGMP to 5′-GMP
BH4 and nNOS Activity
NO is synthesized in cells by the family of NOS oxidoreductase enzymes that catalyze the transformation of l-arginine to l-citrulline with the formation of NO [27]. Several co-factors are known to be important for all NOS isoform activities [endothelial (e), neuronal (n), and inducible (i)] including NADPH (requires niacin), flavin adenine dinucleotide (requires riboflavin), calcium and BH4 [33].
The catalytic activity of NOS depends on dimerization of two NOS polypeptides. Dimerization results in the creation of high-affinity binding sites for BH4 and arginine in the oxygenase domain and enables electron transfer between flavin and heme groups [34]. Incubation with saturating concentrations of BH4 increases blood flow which may allow the oxygen-dependent isoform, nNOS, to produce more NO. This is because it induces a substantial conformational change in the homodimeric structure of nNOS, yielding a stabilized nNOS dimer with maximal NO-producing activity [34].
The activity of calcium-activated nNOS also depends on glutamate N-methyl-d-aspartate (NMDA) receptor channels, a process facilitated by postsynaptic density-95 (PSD-95) scaffolding protein. PSD-95 brings nNOS into the vicinity of calcium influx through NMDA receptors, increasing its activity. In response to an increased intra-cellular Ca2+, nNOS interacts with calmodulin (CaM). This Ca2+–CaM complex, in combination with BH4, binds to nNOS and induces its translocation from the plasma membrane into the cytoplasm. Phosphorylation of the nNOS and eNOS isoforms is also important for NOS activity. Studies showed that Ser1179 of eNOS is phosphorylated by protein kinase Akt, which increases electron flux through the reductase domain and NO production. In contrast, phosphorylation of nNOS at [Ser847] by CaM-dependent kinases leads to a decrease in NOS activity. The dephosphorylation of nNOS by calcineurin initiates the production NO. Heat shock protein-90 (Hsp90) is thought to facilitate signal transduction by bringing NOS into close proximity to its upstream activators, Ca2+/CaM. Hsp90 directly enhances CaM binding and thereby increases nNOS catalytic function. Protein interactions with nitric oxide synthases control the right time, the right place, and the production of the right amount of nitric oxide [34].
Selective proteolytic degradation of NOS is a mechanism for regulation of the enzyme [35]. The rapid proteolytic degradation by calpain has been suggested as a reason for the absence of nNOS in skeletal muscle sarcolemma in muscular dystrophy patients [35]. Moreover, nNOS is found in ubiquitin conjugates in lactacystin-treated HEK 293 cells or rat brain homogenates [36], strongly suggesting that the ubiquitin–proteasome pathway regulates the degradation of nNOS in vivo. Studies with the use of reticulocyte lysates and purified NOS indicate that the monomeric form of nNOS is preferentially ubiquitinated [36].
Clinical Importance of BH4 Therapy
Selectively augmenting endogenous BH4 levels by targeting overexpression of GTPCH (GTP cyclohydrolase) in vivo preserves eNOS dimerization and oxidative stress in STZ-induced diabetes mice [37, 38]. The beneficial effects of BH4 supplementation in reversing impaired endothelium dependent relaxation have also been demonstrated in human patients. BH4 therapy has been shown to be useful in improving endothelium-dependent relaxation in patients with hypercholesteromia, [39] in venous conduits used for coronary artery bypass graft surgery, [40] in patients with type II diabetes, [41] in normal epicardial arteries [42] and in smokers [43].
Our studies in rats demonstrate significant gender differences in gastric emptying, gastric antral nitrergic function and nNOS expression and dimerization in both health and disease [13]. Nitrergic relaxation is more pronounced in healthy females accounted for, perhaps, by an increased expression of the active dimeric form of nNOS alpha, accompanied by an increased gastric BH4 content [14]. On the other hand, our findings for the first time in GI literature demonstrate that chronic hyperglycemia causes a greater reduction in both gastric pyloric BH4 content and active forms of nNOS in females, leading to a significantly greater impairment of nitrergic relaxation as well as delayed gastric emptying. Supplementation of BH4 significantly restores delayed gastric emptying by attenuating a reduced nitrergic relaxation, nNOS activity and NO synthesis [14]. These findings may provide a biological explanation for the greater vulnerability of females of developing diabetic gastric dysmotility problems.
Sex Steroids and BH4
Estrogen treatment elevates both the expression of GTPCH1 and BH4 levels in rat brain neurons via estrogen receptor-mediated events [44, 45]. In vitro hyperglycemia decreases both BH4 biosynthesis as well as NO, and estrogen supplementation restores this effect via estrogen receptor alpha (ERα) in bovine aortic endothelial cell cultures [46].
We and others have reported that circulating estrogen levels are higher in female compared to male rats [47] and that diabetes induction decreases circulating estrogen and progesterone and their receptors in various tissues in both women and in female rats [8]. Our findings [48] correlate with those of Shah et al. [6] in which they demonstrated that E2, but not P4, may be responsible for increased gastric nNOS expression and nitrergic relaxation. In addition, we noticed that estrogen receptor alpha (ERα) mRNA (real-time PCR) and protein expression are elevated in healthy female compared to age-matched male gastric pylorus muscular tissue. Diabetes induction reduced gastric ERα protein expression in female but not in male rats [48]. However, recent studies demonstrated that gastroparesis symptoms in particular nausea and early satiety were elevated in the luteal phase of the menstrual cycle suggesting that endogenous sex hormones may be somewhat harmful rather than beneficial [49]. In addition, these studies further suggest that a variation in the symptoms was not seen in gastroparesis female patients on hormonal contraception [49]. However, the underlying mechanisms for this discrepancies are currently unknown. In general, pregnant women may have symptoms like nausea and vomiting associated with slower gastric motility perhaps due to elevated sex hormonal levels compared to non-pregnant women. Shah et al. [50] studies in pregnant rats concluded that decreased stomach motility (delayed gastric emptying) observed in pregnant women may be mediated by elevated sex steroid hormones and nitric oxide. Studies have shown that hormone therapy delays gastric emptying for solids in postmenopausal women compared to age-matched men or untreated women or premenopausal women [51]. Collectively, the above studies suggest that delayed gastric emptying (not gastroparesis-related delayed gastric emptying) is common in healthy women due to elevated sex hormones and nitric oxide. Despite the above findings, the beneficial role of E2 treatment on both GTPCH1 expression and eNOS expression in the diabetic vasculature has been well demonstrated. Therefore, we hypothesize that impairment in sex steroid hormones and their receptors by chronic hyperglycemia causes alterations in BH4 biosynthesis and the NO system causing delayed gastric emptying and/or gastroparesis. We propose that supplementation with sex steroid hormones elevate both BH4 and NO synthesis and maintain normal gastric emptying in diabetic rodents (Fig. 4). More in depth investigations are warranted to understand the molecular mechanisms on how sex hormones regulate BH4 synthesis and nNOS function.
Fig. 4.
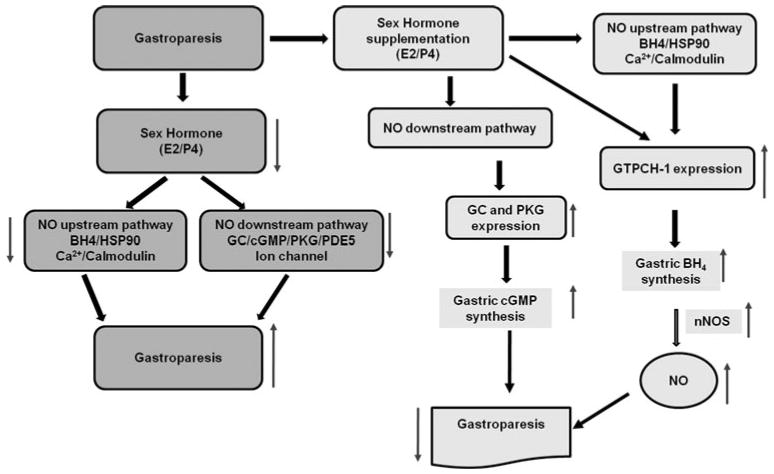
Schematic diagram for proposed mechanism of action in diabetic gastroparesis. Nitric oxide (NO) released in response to nerve stimulation from neuronal nitric oxide synthase (nNOS) causes relaxation of the smooth muscle by activating NO down stream signaling including guanylate cyclase (GC), cyclic guanosine monophosphate (cGMP)/phosphokinase G (PKG) and ion channels. We propose that in healthy females, nitric oxide-mediated normal gastric motility is regulated by increased levels of circulatory sex hormones and their gastric receptors. Diabetes causes a reduction in circulatory sex hormones and their gastric receptors and thus nitrergic-mediated gastric motility is impaired. Supplementation of sex hormones improves nNOS/NO synthesis and function by stimulating GTPCH/BH4 pathway and thus normalizes gastric motility in female diabetic rodents. In addition, supplementation of sex hormones may also activate (including but not limited to) calcium/CaM and HSP-90 (heat shock protein-90) known to regulate/activate nNOS/NO function
Discussion
Tetrahydrobiopterin deficiency, either by the de novo or salvage pathway, results in impairment of critical NOS enzymes, and impairment of NO generation and gastric motility function. Recently, we reported that dietary supplementation with sepiapterin, a precursor for BH4 biosynthesis via the salvage pathway, restored impaired nNOS dimerization and nitrergic relaxation as well as delayed gastric emptying [52]. This data together with our other reports suggest that the synthesis of BH4 by both pathways plays a role in regulating gastric motility functions in female rodents [14, 52]. NO has both a direct and indirect influence on neurotransmission. NO also increases blood flow in stomach and may restore gastric dysmotility. Increases in blood flow may allow the oxygen-dependent isoform, nNOS, to produce more NO and thus normalize impaired gastric motility in both diabetic and idiopathic gastroparesis patients. Recent studies have shown a significant gender difference in idiopathic gastroparesis patients, a finding accompanied by excess weight in those subjects [1]. Hyperlipidemia associated with obesity (overweight) and/or diabetes (obesity independent and/or dependent) may lead to elevated oxidative stress, which impairs the nitrergic system. Grover et al. [53] demonstrated that in addition to defects noticed with ICC, nNOS expression was decreased in more patients with idiopathic gastroparesis (40%) compared to diabetic (20%) gastroparesis. Indeed, recent findings from our laboratory using a low-density lipoprotein receptor knock-model (LDLR-KO; a model for hyperlipidemia and moderate oxidative stress) revealed that hyperlipidemia causes a reduced: (1) BH4:BH2 + B ratio, (2) NF-E2-related factor 2 level [Nrf2; transcriptional factor that regulates Phase II antioxidant enzymes, including hemoxygenase 1 (HO-1) as well as glutathione biosynthesis enzymes], (3) nNOS dimerization, (4) nitrergic relaxation, and (5) a delayed gastric emptying. Sepiapterin treatment restored all the above [54]. The above data provide a rationale for the use of BH4 as an antioxidant and to normalize impaired nNOS function in gastroparesis patients. Studies have shown that LDLR-KO mice fed a high fat diet display hyperglycemia, severe hypertriglyceridemia and elevated oxidative stress levels. The molecular mechanisms responsible for decreased nNOS dimerization and/or how BH4 suppresses LDL-and/or oxidative stress induced gastric dysmotility functions are not currently understood. Loss of ICC and/or reduced nNOS containing neurons associated with oxidative stress in the onset of diabetes may also account for the impairment of stomach motility and delay in gastric emptying [55]. Additional experiments are warranted to address in detail the mechanisms of nNOS regulation as well as the potential role of NO on gastric smooth muscle function in diabetogenic LDLR-KO mice and in other diabetic rodent models.
It also would be of interest to determine if BH4 deficiency is the leading cause for the development of gastroparesis in subjects and, if so, whether there is also a racial/ethnicity-dependent response. Although the experimental data are encouraging, a clinical trial will hopefully test for this in both idiopathic and diabetic gastroparesis.
Conclusion
Based on available data together with our recent findings, we suggest that the pathogenetic mechanisms of diabetic gastroparesis are common to both men and women; however, women appear to be disproportionately symptomatic because the motility of their stomachs is slower to begin with, perhaps due to elevated levels of circulating female sex hormones and nitric oxide. Sex steroid hormones, in particular estradiol-17β and/or soy isoflavones (estrogenic compounds), regulate eNOS- or nNOS-mediated neuroendocrine and vascular smooth muscle function through NMDA receptor channels, calcium/calmodulin and HSP-90 signaling. Although there is a controversy regarding the overall safety of using hormone therapy to protect against cardiovascular diseases in women, recent studies could not rule out the possibility that other formulations of oral estrogens and progesterones and/or transdermal estradiol with progesterone at physiological doses may provide a improved benefit–risk profile. Most importantly, estradiol-17β has been shown to regulate lipid metabolism as well as suppresses elevated oxidative stress and/or increases antioxidants; therefore, it is postulated that the longevity is greater in women than in men.
Idiopathic gastroparesis is a disorder that primarily affects young women, beginning acutely in 50% of cases; unexpectedly, many of these patients are overweight. The etiological connection of this gender bias is completely unknown. We speculate that changes in sex hormones and/or their receptors in myenteric neurons may alter gastric motility through a number of mechanisms including nitric oxide, lipid metabolism and oxidative stress. Therefore, it is important to investigate if these signaling mechanisms are altered with the exposure to oxidative stress and/or hyperlipidemia and, if so, whether sex steroid hormone treatment restores impaired nitrergic-mediated gastric motility functions in both diabetic and non-diabetic gastroparesis models.
Acknowledgments
This research was supported in part by P60DK020593 pilot project funds (PG), NIH-NIDDK R21DKO76704 (PG), RCMI G12RR03032 provided to PG as start-up funds at Meharry Medical College, Nashville, TN, USA.
Footnotes
Conflict of interest Dr. Gangula (through the University of Texas Medical Branch, Galveston, TX) has filed a patent application for the use of BH4 in gastroparesis subjects. Drs. Gangula, Sekhar and Mukhopadhyay prepared this manuscript.
Contributor Information
P. R. R. Gangula, Email: pgangula@mmc.edu, Department of Physiology, Center for Women's Health Research, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA.
K. R. Sekhar, Department of Radiation Oncology/Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN, USA
S. Mukhopadhyay, Department of Physiology, Center for Women's Health Research, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA
References
- 1.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aytug N, Giral A, Imeryuz N, et al. Gender influence on jejunal migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2001;280:G255–G263. doi: 10.1152/ajpgi.2001.280.2.G255. [DOI] [PubMed] [Google Scholar]
- 3.Soffer EE, Thongsawat S, Ellerbroek S. Prolonged ambulatory duodeno-jejunal manometry in humans: normal values and gender effect. Am J Gastroenterol. 1998;93:1318–1323. doi: 10.1111/j.1572-0241.1998.441_k.x. [DOI] [PubMed] [Google Scholar]
- 4.Knight LC, Parkman HP, Brown KL, et al. Delayed gastric emptying and decreased antral contractility in normal premeno-pausal women compared with men. Am J Gastroenterol. 1997;92:968–975. [PubMed] [Google Scholar]
- 5.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol. 1995;268:G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 6.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1546–R1554. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Zheng TZ, Li W, Qu SY, He DY. Action of progesterone on contractile activity of isolated gastric strips in rats. World J Gastroenterol. 2003;9:775–778. doi: 10.3748/wjg.v9.i4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim NN. Sex steroid hormones in diabetes-induced sexual dysfunction: focus on the female gender. J Sex Med. 2009;3:239–246. doi: 10.1111/j.1743-6109.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 9.Waseem S, Moshiree B, Draganov PV. Gastroparesis: symptoms, evaluation, and treatment. World J Gastroenterol. 2009;15:25–37. doi: 10.3748/wjg.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis: diagnosis and management. Drugs. 2009;69:971–986. doi: 10.2165/00003495-200969080-00003. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59:1716–1726. doi: 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–G733. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangula PR, Mukhopadhyay S, Ravella K, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G692–G699. doi: 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Owyang C. Musings on the wanderer: what's new in our understanding of vago-vagal reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G461–G469. doi: 10.1152/ajpgi.00119.2003. [DOI] [PubMed] [Google Scholar]
- 17.Belai A, Lincoln J, Milner P, Burnstock G. Progressive changes in adrenergic, serotonergic, and peptidergic nerves in proximal colon of streptozotocin-diabetic rats. Gastroenterology. 1988;95:1234–1241. doi: 10.1016/0016-5085(88)90356-3. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421–430. doi: 10.1007/s00535-003-1094-y. [DOI] [PubMed] [Google Scholar]
- 19.Cellek S. Point of NO return for nitrergic nerves in diabetes: a new insight into diabetic complications. Curr Pharm Des. 2004;10:3683–3695. doi: 10.2174/1381612043382792. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–1544. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 21.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 22.Ishiguchi T, Tada H, Nakagawa K, Yamamura T, Takahashi T. Hyperglycemia impairs antro-pyloric coordination and delays gastric emptying in conscious rats. Auton Neurosci. 2002;95:112–120. doi: 10.1016/s1566-0702(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 23.Belai A, Calcutt NA, Carrington AL, Diemel LT, Tomlinson DR, Burnstock G. Enteric neuropeptides in streptozotocin-diabetic rats; effects of insulin and aldose reductase inhibition. J Auton Nerv Syst. 1996;58:163–169. doi: 10.1016/0165-1838(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 24.Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis—a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–346. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 25.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 26.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766–773. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 27.Sharron H, Francis JLB, Cobin JD. cGMP dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Münzel T, Daiber A, Ullrich V, Mülsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylate cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 29.Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO− cycle. Med Hypotheses. 2007;69:821–825. doi: 10.1016/j.mehy.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 30.Iino S, Horiguchi K, Nojyo Y, Ward SM, Sanders KM. Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenterol Motil. 2009;21:542–550. doi: 10.1111/j.1365-2982.2008.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarnelli G, Sifrim D, Janssens J, Tack J. Influence of sildenafil on gastric sensorimotor function in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G988–G992. doi: 10.1152/ajpgi.00419.2003. [DOI] [PubMed] [Google Scholar]
- 32.Savidge TC. S-nitrosothiol signals in the enteric nervous system: lessons learnt from big brother. Front Neurosci. 2011;5:31. doi: 10.3389/fnins.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones RJ, Gao YT, Simone TM, Salerno JC, Smith SM. NADPH analog binding to constitutive nitric oxide activates electron transfer and NO synthesis. Nitric Oxide. 2006;14:228–237. doi: 10.1016/j.niox.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laine R, Ortiz de Montellano PR. Neuronal nitric oxide synthase isoforms and μ are closely related calpain-sensitive proteins. Mol Pharmacol. 1998;54:305–312. doi: 10.1124/mol.54.2.305. [DOI] [PubMed] [Google Scholar]
- 36.Bender AT, Demady DR, Osawa Y. Ubiquitination of neuronal nitric oxide synthase in vitro and in vivo. J Biol Chem. 2000;275:17407–17411. doi: 10.1074/jbc.M000155200. [DOI] [PubMed] [Google Scholar]
- 37.Cai S, Khoo J, Alp NG, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 38.Alp NJ, Mussa S, Khoo J, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I over expression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroes E, Kastelein J, Cosentino F, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maier W, Cosentino F, Lutolf RB, et al. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 42.Setoguchi S, Mohri M, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves endothelial dysfunction in coronary microcirculation in patients without epicardial coronary artery disease. J Am Coll Cardiol. 2001;38:493–498. doi: 10.1016/s0735-1097(01)01382-1. [DOI] [PubMed] [Google Scholar]
- 43.Heitzer T, Brockhoff C, Mayer B, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 44.Serova LI, Filipenko M, Schilt N, Veerasirikul M, Sabban EL. Estrogen-triggered activation of GTP cyclohydrolase 1 gene expression: role of estrogen receptor subtypes and interaction with cyclic AMP. Neuroscience. 2006;140:1253–1263. doi: 10.1016/j.neuroscience.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Lam KK, Lee YM, Hsiao G, Chen SY, Yen MH. Estrogen therapy replenishes vascular tetrahydrobiopterin and reduces oxidative stress in ovariectomized rats. Menopause. 2006;13:294–302. doi: 10.1097/01.gme.0000182806.99137.5e. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki-Akita A, Hayashi T, Ding QF, et al. 17beta-estradiol antagonizes the down-regulation of endothelial nitric-oxide synthase and GTP cyclohydrolase I by high glucose: relevance to postmenopausal diabetic cardiovascular disease. J Pharmacol Exp Ther. 2007;320:591–598. doi: 10.1124/jpet.106.111641. [DOI] [PubMed] [Google Scholar]
- 47.Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol. 2005;192:952–960. doi: 10.1016/j.ajog.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Gangula PR, Garfield RE, Pasricha PJ. 17-beta estradiol attenuates delayed gastric emptying and decreased neuronal nitric oxide synthase alpha (nNOS) expression in female diabetic rats. Gastroenterology. 2008;134:A-247. [Google Scholar]
- 49.Verrengia M, Sachdeva P, Gaughan J, Fisher RS, Parkman HP. Variation of symptoms during the menstrual cycle in female patients with gastroparesis. Neurogastroenterol Motil. doi: 10.1111/j.1365-2982.2011.01681.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Shah S, Hobbs A, Singh R, Cuevas J, Ignarro L, Chaudhuri G. Gastrointestinal motility during pregnancy: role of nitrergic component of NANC nerves. Am J Physiol Int Comp Physiol. 2000;279:R1478–R1485. doi: 10.1152/ajpregu.2000.279.4.R1478. [DOI] [PubMed] [Google Scholar]
- 51.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 52.Gangula PRR, Mukhopadhyay S, Pasricha PJ, Ravella P. Sepiapterin reverses the changes in gastric nNOS dimerization and function in diabetic gastroparesis. Neurogastroenterol Motil. 2010;22:1325–1331. doi: 10.1111/j.1365-2982.2010.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gangula PR, Chinnathambi V, Hale A, Mukhopadhyay S, Channon K, Ravella K. Impairment of nitrergic system and delayed gastric emptying in low-density lipoprotein receptor deficient (LDLR-KO) female mice. Neurogastroenterol Motil. doi: 10.1111/j.1365-2982.2011.01695.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashyap P, Farrugia G. Oxidative stress: key player of gastrointestinal complications of diabetes. Neurogastroenterol Motil. 2011:111–114. doi: 10.1111/j.1365-2982.2010.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


