Abstract
Inflammation, although first characterized by Cornelius Celsus, a physician in first Century Rome, it was Rudolf Virchow, a German physician in nineteenth century who suggested a link between inflammation and cancer, cardiovascular diseases, diabetes, pulmonary diseases, neurological diseases and other chronic diseases. Extensive research within last three decades has confirmed these observations and identified the molecular basis for most chronic diseases and for the associated inflammation. The transcription factor, Nuclear Factor-kappaB (NF-κB) that controls over 500 different gene products, has emerged as major mediator of inflammation. Thus agents that can inhibit NF-κB and diminish chronic inflammation have potential to prevent or delay the onset of the chronic diseases and further even treat them. In an attempt to identify novel anti-inflammatory agents which are safe and effective, in contrast to high throughput screen, we have turned to “reverse pharmacology” or “bed to benchside” approach. We found that Ayurveda, a science of long life, almost 6000 years old, can serve as a “goldmine” for novel anti-inflammatory agents used for centuries to treat chronic diseases. The current review is an attempt to provide description of various Ayurvedic plants currently used for treatment, their active chemical components, and the inflammatory pathways that they inhibit.
2. Introduction
Current estimates are that it may cost as much as over a billion dollar to develop a drug by a pharmaceutical company. Today’s Magic bullets or targeted therapies are expensive as cost of treating advanced colorectal cancer patient that was $500 in 1999 is $250,000 in 2007 as indicated by Leonard Saltz, from Memorial Sloan-Kettering cancer Center, New York. Despite billions that have been spent, the death rate from most cancers has barely budged. For instance glioblastoma, kills almost everyone who gets it, usually in a little over a year. Radiation and chemotherapy regimen has become the standard of care, which comes with a cost range from $100,000 to $500,000. It has been estimated that most population in the world can not afford these smart therapies. Besides cost, safety is a major concern. Similarly it is being asked if someone invented a pill to cut a cancer risk in half, would you take it? Although tamoxifen, raloxifen, celcoxib and finesteride have been approved, they are not very well accepted. The reason for this being is the side effects. For instance, among 1,000 women, 19 would be expected to develop breast cancer over the next five years but if those women all took tamoxifen, however, 9 of those women would avoid breast cancer. Tamoxifen is expected to cause 21 additional cases of endometrial cancer, a cancer of the uterine lining that is typically treatable when caught early. An additional 21 would develop blood clots, 31 would develop cataracts and 12 would develop sexual problems. More than half of the 1,000 women would naturally develop hormonal symptoms like hot flashes, changes in vaginal discharge or irregular periods, tamoxifen would cause those symptoms in about an additional 120 women. Raloxifene has been shown to significantly reduce breast cancer risk but with fewer side effects.
To identify a drug that is safe, affordable and effective is a challenge to modern medicine today. Why modern drugs are so unsafe? Why these drugs costs so much? Why are these drugs so ineffective? All these questions require serious thinking “out of the box”. For instance the realization that most chronic diseases are multigenic and thus multi-targeted approach, also called promiscuity in drug development, is needed. As many as 500 gene products or proteins or kinases or signaling intermediates have been linked with any given chronic disease. Thus inhibition of a single kinase or a pathway is unlikely to treat the disease. It has been shown that 74% of all drugs approved by the FDA within last decade for cancer are based on natural products. How to design a drug that is safe, multi-targeted and yet affordable, we have turned to traditional medicine such as Ayurveda which is almost six thousand years old. Ayurveda is a traditional healing system originated in India approximately 6,000 years ago. In Sanskrit, Ayu means “Life” and Veda means “knowledge or science.” Ayurveda can be interpreted as the Science of Life. This “Science of Life” is a holistic healing system, which is designed to promote good health and longevity rather than curing a disease (therapy). Three kinds of primary body constitutions or traits (“prakriti”) have been defined based on three “doshas”, viz, Vata, Pitta, and Kapha. Any imbalance in these dosha results into a disease. To restore the balance, the Ayurveda recommends a customized therapy based on the “prakriti” of an individual. Doshas can be influenced by the food one eats, the type of lifestyle one leads. The term “vata” comes from “vaayu” (in Sanskrit), which means air. The oxidative stress could be caused by insufficient air (vaayu) inhaled, and imbalance in metabolism (the two other tridoshas, the pitta and the kapha). According to ‘Charaka Samhita’, there are five categories of vata. The ‘Prana vata’ is related to inhalation of air, whereas, the ‘Udana vata’ is related to exhalation. ‘Vyana Vata’ regulates the heart and circulatory system; Samana Vata regulates digestive tract, and the ‘Apana Vata’ works in elimination of wastes [1]. Since reactive oxygen species produced ROS) in the body are composed of many species, such as, oxygen ions, peroxides, hydroxyl radicals, etc.; one would require a combination of antioxidants to quench them altogether. Plant polyphenolics though are good source of antioxidants, but they differ in their abilities to quench difference species of ROS [2–4]. Therefore, one may need to use a combination of phytochemicals.
Holistic treatment is the hallmark of treatment in Ayurveda. It demands that one herb or one drug would not cure the imbalance of “Dosha”. Therefore, traditionally, in most of the cases, a combination of herbs and plants (which are even part of staple food) are recommended for treatment [5]. This would probably the most ancient recommendation for a “Combinatorial and Mutlti-targted Therapy”. It is quite possible that a so called crude herbal formulation has a combination of compounds, where one compound either potentiates the effect other, or increases the bioavailibilty, or reduces the toxicity. A best example is the routine use of turmeric in combination with black pepper as a spice. It is now known that the bioavailibity of curcumin (active ingredient of turmeric) is increased by piperine (an active compound in black pepper) by preventing the glucuronidation of the curcumin [6]. Experimentation and documentation of more of such scientific information is highly desirable, and scientific researches to substantiate the use of mixtures of plants in Ayurveda (Table 1) are a worthwhile venture.
Table 1.
Ayurvedic formulations and their uses*
| Formulation | Plants used | Uses |
|---|---|---|
| Amrutanjan Balm | Cinnamomum camphora, C. zeylanicum, Cymbopogon citrates, Eucalyptus polybractea, Gaultheria sp., Mentha arvensis, M. piperita, Rosa sp., Thymus vulgaris | Cures pain, sprain, cold and sinus |
| Ayurslim | Commiphora wightii, Garcinia cambogia, G. sylvestre, Terminalia chebula, Trigonella foenum-graecum | Burns fat and reduces cholesterol |
| Dabur Chyawanprash | Phyllanthus emblica, Piper longum, Pistacia integerrima Pueraria tuberosa, Tinospora cordifolia | Protects from infections, coughs, cold and stress; adjuvant pulmonary tuberculosis therapy |
| Divya Arshkalpa Vati | Aloe vera, Azadirachta indica, Berberis aristata, C. camphora, Daemenorops draco, Sapindus sp., Solanum nigrum, T. chebula | Cures piles, burning sensation and colic pain |
| Divya Ashmarihara Kvath | Bergenia ligulata, Boerhavia diffusa, Crataeva nurvula Dolichos biflorus, Tribulus terrestris | Diuretic, anti-oedemous; dissolves kidney, urinary & gall bladder stones |
| Divya Ashmarihara Rasa | Hajarala yahuda, Hordeum vulgare, Raphanus sativus | Diuretic, dissolves calculi, relieves pains, anti-oedemous. |
| Divya Danta Manjana | Acacia arabica, Anacyclus pyrethrum, A. indica, C. camphora M. piperita, Mimusops elengi, P. longum, Quercus infectoria, Syzygium aromaticum, Zanthoxylum alatum | Strengthens the gums and stops bleeding |
| Divya Gaisahara Choorna | Citrus limon, Cuminum cymimum, Ferula foetida, Piper nigra, T. chebula, Trachyspermum ammi | Reduces gas, acidity, flatulence, colic pain & anorexia |
| Divya Kayakalpa Kvatha | Acacia catechu, A. indica, B. aristata, Cassia tora, Cedrus deodara, Curcuma longa, Leucas cephalotes, Picrorhiza kurroa, Pongamia pinnata, Psorlia corylifolia, Pterocarpus santalinus, Rubia cordifolia, Swertia chirata T. cordifolia | Cures eczema, leprosy, filariasis; helps in reducing obesity |
| Divya Kayakalp Vati | A. catechu, A. indica, B. aristata, C. tora, C. deodara, Citrullus colocynthis, C. longa, Emblica officinalis, Leucas cephalotes, Nigella sativa, P. kurroa, Pongamia pinnata P. santalinus, R. cordifolia, Sarsa parilla, Solanum xantho-carpum, S. chirata, Terminalia belerica, T. chebula, T. cordifolia | Purifies blood, removes acne, pimples; cures from ring-worms, itches, pruritus, eczema, leucoderma & psoriasis |
| Divya Madhu-kalpa Vati | Acacia arabica, Aconitum heterophilum, Aegle marmelos, Andrographis paniculata, A. indica, C. longa, C. zedoaria Emblica officinalis, Ficus bengalensis, G. sylvestre, Holarrhena antidysenterica, Momordica charantia, N. sativa, P. kurroa, S. chirata, Syzyium cumini, T. belerica, T. chebula, T. cordifolia, T. terrestris, T. foenum-graecum, Withania somnifera | Balance insulin secretion, strengthens immune system |
| Divya Medha Kwatha | Bacopa monnieri, Celatrus paniculatus, Convovulus pluricaulis, Foeniculum vulgare, Lavandula stoechas, Nardostachys jatamansi, Onosma bracteatum, W. somnifera | Cures chronic headache, migraine, sleeplessness and depression |
| Divya Mukta Vati | Acorus calamus, B. monnieri, C. paniculatus, C. pluricaulis, Inula racemosa, L. stoechas, N. jatamansi, Rauwolfia serpentina, T. arjuna, W. somnifera | Cures high blood pressure, insomnia, palpitation, chest pain |
| Divya Pidantaka Kvatha | Cyperus rotundus, Nyctanthes arbortristis, Piper chaba, P. longum, Pluchea lanceolata, Ricinus communis, T. ammi, Vitex negundo, W. somnifera, Z. officinale | Useful in joint pain, sciatica, osteo-arthritis, gout, rheumatoid arthritis, muscular and skeletal pains and oedema. |
| Divya Udaramrita Vati | A. marmelos, A. vera, B. diffusa, E. officinalis, Mangifera indica, Operculina turpethum, Phyllanthus niruri, P. kurroa, Plumbago zeylanica, S. nigrum, T. belerica, T. ammi | Cures jaundice, anaemia, chronic fever, diarrhoea and abdominal pain |
| Divya Yauvanamrita Vati | Anacyclus pyrethrum, Asparagus racemosus, Castorium, Crocus sativus, Mucuna pruriens, Myristica fragrans Sida cordifolia | Strengthens heart and brain, promotes luster and youthness and cures impotency. |
| Divya Udarakalpa Curna | Cassia angustifolia, F. vulgare, Glycyrrhiza glabra, Rosa centifolia, T. chebula | Stimulates digestion and removes constipation |
| Divya Kayakalpa Taila | A. indica, B. aristata, C. tora, C. deodara, C. longa, E. officinalis, Leucas cephalotes, N. sativa, P. kurroa, P. pinnata, Psorlia corylifolia, P. santalinus, R. cordifolia, Saphindus trifoliatus, Sarsa parilla, Sesamum indicum, Solanum indicum, S. chirata, T. chebula, T. cordifolia | Cures skin diseases like ring worm, itching, sun burning, eczema, leucoderma, psoriasis, utricaria and skin allergy |
| Divya Kesa Taila | Abrus precatorius, B. monnieri, B. aristata, Callicarpa macrophylla, C. rotundus, Eclipta alba, E. officinalis, Fagonia cretica, Indigofera tinctoria, Mesua ferrea, N. jatamansi, Nelumbo nucifera, Onosma ehioides, Pandanus tectorius, P. santalinus, S. cordifolia, Symplocos crataegoides | Cures untimely hair fall, dandruff, alopecia, premature graying of hair |
| Divya Churna | F. vulgare, Ipomoea nil, R. centifolia, T. chebula, Z. officinale | Cures abdominal pain, flatulence, heaviness & nausea |
| Divya Peya (Herbal Tea) | Adhatoda vasia, B. monnieri, B. diffusa, C. zeylanicum, C. | Controls cholesterol and protects from heart diseases; |
| pluricaulis, Cymbopogon martini, C. rotundus, Elettaria cardamomum, Ephedra gerardiana, F. vulgare, M. fragrans, Nelumbo nucifera, Ocimum sanctum, P. niruri, P. chaba, P. longum, P. nigrum, P. zeylanica, P. santalinus, R. centifolia, Santalum album, S. aromaticum, T. arjuna, T. cordifolia, Viola odorata, W. somnifera, Z. officinale | promotes immunity, stimulates digestion | |
| Divya Dhara | C. camphora, M. piperita, S. aromaticum, T. ammi | Cures asthma, cholera, ear-diseases, epitaxis, trauma, utricaria, cough, colic pain and flatulence |
| Divya Pidantaka Rasa | A. marmelos, Clerodendron phlomoides, Commiphora mukul, C. rotundus, Gmelina arborea, Moringa oleifera, Oroxylum indicum, P. lanceolata, Pseudarthria viscida, S. indicum, Stereospermum suaveolen, Strychnos nuxvomica T. cordifolia, T. ammi, T. terrestris, Uraria lagopoides, V. negundo, W. somnifera | Useful in joint pain, arthritis, lumbar pain, cervical spondylitis and sciatica |
| Divya Pidantaka Taila | Aconitum ferox, A. calamus, A. marmelos, Allium sativum, Anethum sowa, A. racemosus, B. aristata, Butea mono-sperma, Calotropis procera, C. paniculatus, C. zeylanicum, C. phlomoides, C. longa, Datura metel, E. alba, F. vulgare, G. glabra, G. arborea, Hebenaria intermedia, I. racemosa, Lilium polyphyllum, Malaxis acuminate, M. ferrea, N. jatamansi, Oroxylum indicum, Paderia foetida, P. chaba, P. longum, P. lanceolata, P. zeylanica, Polygonatum verticillatum, Pseudarthria viscida, R. communis, Roscoea alpina, R. cordifolia, Sesamum indicum, S. indicum, Stereospermum suaveolen, Strychnos nuxvomica, T. ammi, T. terrestris, Uraria lagopoides, Valeriana wallichii, V. negundo, Z. officinale | Relieves pain of lumbar region, knee-joints, cervical spondylitis, oedema & inflammation |
| Divya Madhunasini Vati | A. arabica, A. heterophilum, A. marmelos, A. paniculata, A. indica, C. longa, C. zedoaria, E. officinalis, F. bengalensis, G. sylvestre, Holarrhena antidysenterica, M. charantia, N. sativa, P. kurroa, S. chirata, Syzyium cumini, T. belerica, T. chebula, T. cordifolia, T. terrestris, T. foenum-graecum, W. somnifera | Balance insulin secretion, strengthens immune system |
| Divya Medha Vati | A. calamus, B. monnieri, C. paniculatus, C. pluricaulis, I. racemosa, Lavandula stoechas, N. jatamansi, W. somnifera | Cures mental disorders, depression and epileptic fits |
| Divya Amirta Rasayana | A. racemosus, B. monnieri, Bambusa arundinacea, C. zeylanicum, C. pluricaulis, C. sativus, E. cardamomum, E. officinalis, M. prurita, Prunus amygdalus | Rejuvenates and nourishes the brain and body |
| Divya Medohara Vati | B. diffusa, C. mukul, E. officinalis, E. ribes, Operculina turpethum, P. kurroa, T. belerica, T. chebula | Thyroid disorders, rheumatic arthritis, joint pains, pain to lumbar region and knee joints. |
| Divya Svasari Rasa | A. feroz, Anacyclus pyrethrum, Capparis moonii, C. zeylanicum, G. glabra, P. longum, P. nigrum, Pistacia integerrima, S. aromaticum, Z. officinale | Bronchitis, cough, coryza, cold, asthma and sinusitis |
| Divya Strirasayana Vati | A. racemosus, Bambusa arundinacea, B. aristata, Bryonia laciniosa, C. deodara, C. mukul, E. officinalis, G. glabra, M. ferrea, N. nucifera, Putranjiva roxburghii, S. album, Saraca asoca, S. cordifolia, T. belerica, T. chebula, W. somnifera | Leucorrhoea, menorrhagia, irregularity in menstruation |
| Divya Hridayamrita Vati | B. diffusa, C. mukul, C. rotundus, P. lanceolatus, P. zeylanica T. arjuna, T. cordifolia, V. negundo | Removes the arterial block, angina pain and palpitation |
| Divya Silajita Rasayana Vati | E. officinalis, P. niruri, T. belerica, T. chebula, W. somnifera | Diabetes & leucorrhoea |
| Divya Sarvakalpa Kvatha | B. diffusa, Cassia fistula, P. niruri, S. nigrum | Cures jaundice, oedema, oliguria, oedema |
| Divya Kanti Lepa | A. catechu, C. camphora, Curcuma amada, C. longa, M. fragrans, R. cordifolia, S. album, Valeriana wallichii | Cures skin disorders viz pimples, acne, wrinkles on face |
| Divya Vatari Churna | M. oleifera, P. kurroa, T. foenum-graecum, W. somnifera, Z. officinale | Cures rheumatoid arthritis, sciatica, pain in back and lumbar region |
| Himalaya Abana | A. calamus, A. racemosus, B. diffusa, C. copticum, Celastrus paniculatus, Centella asiatica, Cinnamomum cassia, C. wightii, C. pluricaulis, C. sativus, C. rotundus, E. alba, E. cardamomum, E. ribes, E. officinalis, F. vulgare, G. glabra, N. jatamansi, Nepeta hindostana, O. sanctum, P. longum, Rosa damascena, R. centifolia, S. album, S. aromaticum, T. arjuna, T. chebula, T. cordifolia, W. somnifera, Z. officinale | Hyperlipidemia and hypertension, adjuvant in angina therapy |
| Himalaya Cystone | Achyranthes aspera, Cyperus scariosus, Didymocarpus pedicellata, Onosma bracteatum, R. cordifolia, Saxifraga ligulata, Vernonia cinerea | Prevents urinary infections & stone formation |
| Himalaya Dental Cream | A. arabica, A. catechu, A. farnesiana, Pistacia, C. copticum E. ribes, E. officinalis, Mimusops elengi, Punica granatum Salvadora persica, T. bellerica, T. chebula, V. negundo, Zanthoxylum alatum | Stops gum bleeding, boils and sores |
| Himalaya Diabecon | Abutilon indicum, A. vera, A. racemosus, B. aristata, B. diffusa, Casearia esculenta, C. wightii, C. longa, Eugenia jambolana, G. glabra, G. arborea, Gossypium herbaceum G. sylvestre, M. charantia, O. sanctum, Phyllanthus amarus, P. nigrum, Pterocarpus marsupium, Rumex maritimus, Sphaeranthus indicus, S. chirata, T. cordifolia, T. terrestris, Trikatu, Triphala | Reduce blood sugar levels and diabetic complications |
| Himalyaya Geriforte | Achillea millefolium, Argyeria speciosa, Asparagus adscendens, A. racemosus, B. aristata, Caesalpinia digyna, Capparis spinosa, C. copticum, Cassia occidentalis, Centella asiatica, Cichorium intybus, C. sativus, C. longa, E. alba, E. cardamomum, G. glabra, M. pruriens, M. fragrans, P. longum, S. aromaticum, Tamarix gallica, T. arjuna, T. chebula | Slows down aging, reduces stress and enhances immunity |
| Himalaya Himplasia | A. catechu, A. racemosus, Caesalpinia bonducella, Tribulus terrestris | Inhibits prostatic stromal proliferation & improves fertility |
| Himalaya Liv52 | A. millefolium, C. spinosa, C. occidentalis, Cichorium intybus, S. nigrum, Tamarix gallica, T. arjuna | Pre- and early cirrhosis, viral hepatitis & alcoholic liver disease |
| Himalaya Menosan | A. racemosa, Centella asiatica, G. glabra, Saraca indica S. cordifolia, T. chebula | Regulates overall hormonal balance & urinary tract function |
| Himalaya Mentat | A. calamus, B. monnieri, C. paniculatus, C. asiatica, E. ribes, E. cardamomum, E. officinalis, Evolvulus alsinoides, F. vulgare, Ipomoea digitata, M. pruriens, M. fragrans, N. jatamansi, Orchis mascula, Oroxylum indicum, P. amygdalus, S. aromaticum, T. arjuna, T. bellirica, T. chebula, T. cordifolia, Valeriana sp. W. somnifera, Z. officinale | Adjuvant in Parkinson’s and Alzheimers diseases |
| Himalaya Pilex | Bauhinia variegata, B. aristata, C. fistula, C. wightii, E. officinalis, Melia azadirachta, M. ferrea, T. bellirica, T. chebula | Treats piles, cures hemorrhoids, treats varicose veins |
| Himalaya Purim | A. paniculata, Pistacia, C. fistula, Crataeva magna, C. longa, E. alba, E. ribes, E. officinalis, P. kurroa, Psoralea corylifolia, Saussurea lappa, T. bellerica, T. chebula, T. cordifolia | Regulates detoxification and cleansing |
| Himalaya Reosto | C. wightii, Sida cordifolia, T. arjuna, Vanda roxburghii, W. somnifera | Reverses hypogonadism and osteoporosis |
| Himalaya Rumalaya Forte | Alpinia galanga, Boswellia serrata, C. wightii, G. glabra T. cordifolia, Tribulus terrestris | Relieves pain from arthritis and traumatic inflammation |
| Rumalaya Gel | B. serrata, Cedrus deodara, Cinnamomum zeylanicum Gaultheria fragrantissima, M. arvensis, Pinus roxburghii V. negundo, Z. officinale | Analgesic, relieves pain, joint mobility |
| Himalaya Septilin | C. mukul, E. officinalis, G. glabra, Moringa pterygosperma R. cordifolia, T. cordifolia | Strengthens immune system & body’s defense mechanisms |
| Himalaya Triphala | E. officinalis, T. bellrica, T. chebula | Reduces high blood pressure and hyper tension; effective in irritable bowel syndrome, ulcerative colitis and tumor |
Boldface indicates anti-inflammatory activity shown in Table 4.
The information is derived from websites http://www.divyayoga.com, http://www.himalayahealthcare.com, and http://amrutanjan.com, to illustrate as examples of Ayurvedic formulations, with no connection or affiliations to them of the authors.
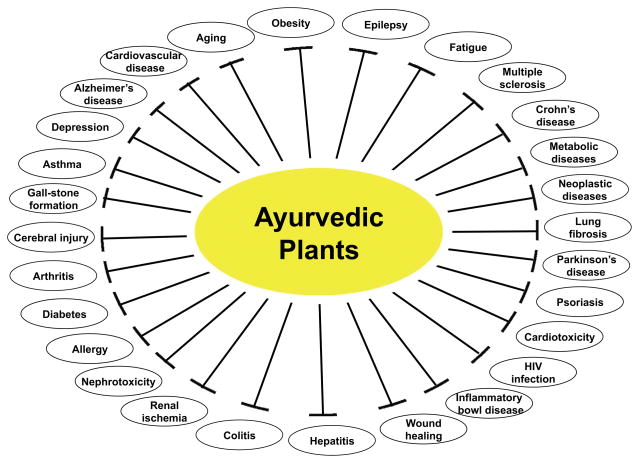
We describe here almost 200 different plants that have been used in Ayurveda to treat various chronic diseases (Table 2; Fig. 1). The active component from some of these plants that can modify the inflammatory pathways linked to chronic diseases, are also indicated. Some of these active components have been studied by us and others extensively at the preclinical level. This approach we describe as a “reverse pharmacology”[7] or “bed to benchside” approach to validate the knowledge that has been known for long time.
Table 2.
A list of Ayurvedic plants, their active components and their role in chronic diseases
| Plant | Active compounds | In-vitro | In-vivo | Review |
|---|---|---|---|---|
| Abrus precatorius | Abruquinones | [243] | [11] | |
| Abrin | [244] | [245] | ||
| Abrus agglutinin | [246] | [246] | ||
| ABP | [247] | |||
| Abutilon indicum | Extract | [12] | ||
| Acacia arabica | Gum | [248] | ||
| Acacia catechu | Flavocoxid | [14] | [249] | |
| Acacia farnesiana | Acasiane, Farnesirane | [15] | ||
| Achillea millefolium | Casticin | [250] | [251] | |
| Extract | [252] | |||
| Achyranthes aspera | Extract | [17, 18, 253] | ||
| Acorus calamus | Extract | [20] | [21, 254] | |
| Adhatoda vasica | Extract | [255, 256] | [257] | |
| Ambroxol | [22] | |||
| Aegle marmelos | Marmelin | [25] | [258] | |
| Extract | [259] | |||
| Allium sativum | Thiacremonone | [29] | ||
| 1,2-vinyldithiin | [30] | [260–263] | ||
| Diallyl sulfide/trisulfide | [264, 265] | |||
| Allicin | [266, 267] | |||
| Extract | [26, 27] | |||
| Aloe vera | Aloe-emodin | [268, 269] | [270] | |
| Extract | [271, 272] | [273, 274] | ||
| Alpinia galanga | Acetoxychavicol acetate | [275–277] | ||
| Andrographis paniculata | Andrographolide | [278–285] | [286–290] | |
| Neoandrographolide | [291] | |||
| Andrograpanin | [292, 293] | |||
| Extract | [294, 295] | |||
| Composition | [296, 297] | |||
| Areca catechu | Extract | [39, 298] | ||
| Argyeria speciosa | Extract | [17, 299–302] | ||
| Asparagus adscendens | Extract | [40] | [303] | |
| Asparagus racemosus | Extract | [304] | [41, 305, 306] | [307, 308] |
| Azadirachta indica | Azadirachtin/ | [43, 309] | [310–312] | |
| Nimbolide | [42, 313] | |||
| Extract | [314] | [312, 315–320] | ||
| Bacopa monnieri | Bacoside-A | [44, 45] | ||
| Triterpene saponins | [321] | |||
| Extract | [322] | [323, 324] | ||
| Bambusa arundinacea | Extract | [46] | ||
| Bauhinia variegata | Extract | [47, 48] | [325–327] | |
| Flavonoids | [328] | |||
| Berberis aristata | Berberine | [50] | [329, 330] | |
| Bergenia ligulata | Extract | [331] | ||
| Boerhaavia diffusa | Extract | [332–335] | [334, 336–339] | |
| Punarnavine | [340, 341] | |||
| Flavonoids | [342] | |||
| Boswellia serrata | AKBA | [61, 343–345] | [54–56, 58–60, 346–348] | |
| Bryonia laciniosa | Extract | [62] | ||
| Butea monosperma | Butrin/isobutrin/butein | [349] | ||
| Stigmosterol | [350] | |||
| Extract | [351] | [352–357] | ||
| Caesalpinia bonducella | Oil/Extract | [358–360] | ||
| Caesalpinia digyna | Extract | [64] | ||
| Calotropis procera | Latex extract | [361] | [362–365] | |
| Capparis spinosa | Extract | [68, 366] | ||
| Carum copticum | Extract | [367–369] | ||
| Casearia esculenta | 3-hydroxymethyl xylitol | [370] | ||
| Extract | [371] | |||
| Cassia angustifolia | Exract | [71] | ||
| Cassia fistula | Extract | [72, 372–374] | ||
| Cassia occidentalis | Extract | [375] | [73, 376, 377] | [378] |
| Cassia tora | Ononitol monohydrate | [379] | ||
| Extract | [380] | [381–383] | ||
| Cedrus deodara | Extract/oil | [384] | [75, 385] | |
| Celastrus paniculatus | Extract | [386] | [76, 387] | |
| Cichorium intybus | Magnolialide | [388] | ||
| Cichotyboside | [389] | |||
| Extract | [77, 390, 391] | |||
| Cinnamomum camphora | Extract | [79] | [247] | |
| Cinnamomum cassia | Extract | [392] | [80, 393, 394] | [395] |
| Cinnamaldehyde | [396–398] | |||
| Cinnnamomum zeylanicum | Extract | [399–401] | [402, 403] | |
| Oil | [81] | |||
| Citrullus colocynthis | Extract | [404–407] | ||
| Commiphora mukul | Guggulsterone | [408–410] | [411–416] | [417–419] |
| Gugulipid | [420, 421] | |||
| BHUx | [422] | |||
| Commiphora wightii | Extract | [423, 424] | ||
| Convolvulus pluricaulis | Extract | [425–427] | ||
| Crocus sativus | Safranal, Crocin | [428] | [429–432] | [433–435] |
| Extract | [436, 437] | [438, 439] | ||
| Cuminum cymimum | Extract/oil | [91, 440–444] | ||
| Curcuma amada | Extract | [92] | [445] | |
| Curcuma longa | Curcumin | [93–95] | [446–449] | [450–464] |
| Curcuma zedoaria | Curdione | [465, 466] | [96] | |
| Extract | [467–469] | [470, 471] | ||
| Cymbopogon citratus | Citral | [472, 473] | ||
| Extract/oil | [98, 474] | [97, 475–477] | ||
| Cyperus rotundus | Extract | [478, 479] | [480] | |
| Cyperus scariosus | Extract | [101] | ||
| Didymocarpus pedicellata | Extract | [104] | ||
| Dolichos biflorus | Extract | [481, 482] | ||
| Eclipta alba | Extract | [483–487] | ||
| Elettaria cardamomum | Extract | [110] | [109, 488–490] | |
| Embelia ribes | Embelin | [491] | [492–495] | |
| Extract | [496–498] | |||
| Emblica officinalis | Pyrogallol | [499] | [500] | |
| Extract | [20, 501–504] | |||
| Eugenia jambolana | Extract | [505] | [506–510] | |
| Evolvulus alsinoides | Extract | [511–513] | ||
| Fagonia cretica | Extract | [116] | ||
| Ferula assafoetida | Gum/Extract | [514] | [515–517] | |
| Ficus bengalensis | Leucodelphinidin | [518] | ||
| Extract | [118, 519–522] | |||
| Foeniculum vulgare | Anethole | [120] | [523] | |
| Extract | [524] | [523, 525–528] | ||
| Garcinia cambogia | Garcinol | [529–531] | [532] | [533] |
| Extract | [121, 534, 535] | |||
| Glycyrrhiza glabra | Isoliquiritigenin | [536] | ||
| Glycyrrhizin | [537] | |||
| Glabridin | [538] | [538] | ||
| Extract | [539–541] | [126, 540, 542] | ||
| Gymnema sylvestre | Extract | [543–545] | [546, 547] | |
| Hemidesmus indicus | HMBA | [548] | [549, 550] | |
| Extract | [551] | |||
| Holarrhena antidysenterica | Extract | [552] | ||
| Hordeum vulgare | Extract | [134, 553] | [554] | |
| Indigofera tinctoria | TCA | [555] | ||
| Indigtone | [556] | |||
| Extract | [557, 558] | |||
| Inula racemosa | Extract | [559] | [136, 543, 560] | |
| Ipomoea nil | Extract | [561] | ||
| Lavendula stoechas | Extract | [562, 563] | ||
| Leucas cephalotes | Extract | [140] | ||
| Mangifera indica | Extract | [564–567] | [565, 568–573] | |
| 3beta-taraxerol | [574] | |||
| Mangiferin | [575] | |||
| Mentha piperita | Extract | [576] | [577, 578] | [579] |
| Oil | [580, 581] | |||
| Flavonoid | [582] | |||
| Mimusops elengi | Extract | [147, 583] | ||
| Momordica charantia | Extract | [584–588] | [589–594] | [148, 595] |
| Moringa oleifera | Extract | [596–601] | [602] | |
| Mucuna pruriens | Extract | [603–607] | ||
| Nardostachys jatamansi | Extract | [608–611] | ||
| Nelumbo nucifera | (S)-armepavine | [154, 612, 613] | [614] | |
| Neferine | [615] | |||
| Kaempferol | [616] | |||
| Isoliensinine | [617] | |||
| Extract | [618–620] | |||
| Nigella sativa | Thymoquinone | [621] | [621–628] | [629–631] |
| Polyphenols | [632] | |||
| Extract | [633] | [624, 634–637] | ||
| Nyctanthes arbortristis | arbortristoside-A | [156] | ||
| Extract | [638, 639] | |||
| Ocimum sanctum | Eugenol | [640, 641] | [642, 643] | |
| Extract/oil | [644] | [320, 645–647] | ||
| Operculina turpethum | Extract | [161, 648] | ||
| Orchis mascula | Extract | [162] | ||
| Oroxylum indicum | Baicalein | [163] | ||
| Extract | [649, 650] | [651] | ||
| Phyllanthus amarus | Niranthin | [165] | ||
| Phyllanthin | [652] | |||
| Extract/lignan | [653–659] | |||
| Phyllanthus niruri | Arabinogalactan | [660] | [661] | |
| Extract/lignan | [662] | [662–666] | ||
| Picrorhiza kurroa | Picroliv | [667–670] | [671] | |
| Extract | [166, 672] | |||
| Piper chaba | Amides/Extracts | [673, 674] | ||
| Piper longum | Piperine | [675] | [675–677] | |
| Piperlongumine | [678] | |||
| Piperinic acid | [679] | [679, 680] | ||
| Extract/oil | [167] | [681–684] | ||
| Piper nigrum | Piperine | [685–687] | [688, 689] | |
| Extract | [110] | [690] | ||
| Pistacia integerrima | Extract | [169, 691] | ||
| Pluchea lanceolata | Extract | [692, 693] | ||
| Plumbago zeylanica | Plumbagin | [172, 694, 695] | [664, 696] | |
| Seselin | [697] | |||
| Suberosin | [698] | |||
| Pongamia pinnata | Pongamol, Karanjin | [699] | ||
| Extract | [700–704] | |||
| Psoralea corylifolia | Bavachin | [705] | ||
| Psoralen | [706] | |||
| Bakuchiol | [707, 708] | |||
| Furocoumarins | [709] | |||
| Extract | [710, 711] | [710, 712] | ||
| Pterocarpus marsupium | Pterostilbene | [179] | ||
| Flavanoids | [713] | |||
| Extract | [714] | |||
| Pterocarpus santalinus | Savinin | [715] | ||
| Extract | [180, 716] | |||
| Pueraria tuberaosa | Lupinoside | [717] | ||
| Extract | [718] | |||
| Punica granatum | Ellagitannins | [181, 719] | [720–722] | |
| Punicalagin | [723] | [181, 723] | ||
| Anthocyanin | [724] | |||
| Extract | [725, 726] | [727–729] | ||
| Putranjiva roxburghii | Extract | [182] | ||
| Quercus infectoria | Extract | [183, 730] | ||
| Raphanus sativus | Extract | [731] | [732, 733] | [734] |
| Ricinus communis | Ricinoleic acid | [188] | ||
| Extract | [735–737] | |||
| Rosa damascena | Flavonoids | [190] | ||
| Extract/oil | [738–740] | |||
| Rubia cordifolia | Rubiadin | [741] | ||
| Mollugin | [191] | |||
| Extract | [742–744] | |||
| Rumex maritimus | Extract | [745] | ||
| Salvadora persica | Extract | [193] | ||
| Santalum album | Oil | [746] | ||
| Lignan | [747] | |||
| Saphindus trifoliatus | Extract | [196, 748] | ||
| Saraca indica | Saracin | [198] | ||
| Saussurea lappa | Extract | [749] | [17] | |
| Lactone | [750] | |||
| Arctigenin | [751] | |||
| Cynaropicrin | [752, 753] | |||
| Costunolide | [754] | |||
| Sesamum indicum | Sesaminol | [755, 756] | ||
| Sesamin | [201] | |||
| Sesamol | [757] | |||
| Extract | [758–761] | |||
| Sida cordfolia | Extract | [762–766] | ||
| Solanum indicum | Extract | [767] | [203] | |
| Solanum nigrum | Extract | [768, 769] | [748, 755, 770–773] | |
| Solanum xanthocarpum | Extract | [206, 774] | ||
| Sphaeranthus indicus | 7-hydroxyfrullanolide | [775] | ||
| Extract | [207, 776] | |||
| Stereospermum suaveolen | Extract | [208, 777] | ||
| Strychnos nuxvomica | Brucine | [209] | [778] | |
| Extract | [779, 780] | |||
| Swertia chirata | Amarogentin | [210] | ||
| Extract | [781, 782] | |||
| Symplocos crataegoides | Triterpenes | [211] | ||
| Syzygium aromaticum | Acetyl eugenol | [783] | ||
| Extract | [212, 784, 785] | [786] | ||
| Syzyium cumini | ferulic acid | [787] [788] | ||
| Extract | [789–792] | |||
| Tamarix gallica | Extract | [214, 793] | ||
| Terminalia arjuna | Arjunic acid | [794] | ||
| Casuarinin | [795, 796] | |||
| Terminoside A | [797] | |||
| Extract | [215, 216, 798–800] | [801] | ||
| Terminalia belerica | Extract | [549, 802–806] | ||
| Terminalia chebula | Chebulagic acid | [807] | [808] | |
| Extract | [549, 803, 806, 809–812] | |||
| Thymus vulgaris | Thymol, Carvacrol | [813] | ||
| Extract | [220] | [814] | ||
| Tinospora cordifolia | Extract | [815] | [222, 506, 816–819] | |
| Trachyspermum ammi | Extract | [225, 820] | ||
| Tribulus terrestris | Tribulosin | [821] | ||
| Saponion | [822] | [823] | ||
| Extract | [226, 228, 824, 825] | |||
| Trigonella foenum-graecum | Diosgenin | [826] | [229, 826, 827] | |
| Galactomannan | [828] | |||
| Extract | [829–836] | |||
| Valeriana wallichii | Extract | [837, 838] | ||
| Vanda roxburghii | Extract | [235, 839] | ||
| Vernonia cinerea | Extract | [236] | [757, 840–843] | |
| Viola odorata | Cycloviolacin | [844] | ||
| Extract | [845] | |||
| Vitex negundo | Vitexins | [238] | ||
| Diterpenes | [846] | |||
| Extract | [847–850] | |||
| Withania somnifera | Withaferin-A | [851, 852] | [241] | [853] |
| Withanolide sulfoxide | [240] | [854] | ||
| Withanamides | [855] | |||
| Extract | [856] | [857–859] | ||
| Zingiber officinale | [6]-Gingerol | [860] | [860, 861] | |
| 6-Shogaol | [862–864] | |||
| Extract | [865, 866] | [867–871] | [872, 873] |
ABP, abrin-derived peptide; AKBA, Acetyl-11-keto-β-boswellic acid; TCA, trans-tetracos-15-enoic acid; HMBA, 2-hydroxy-4-methoxy benzoic acid
Fig. 1.
The use of Ayurvedic plants for treatment of various chronic diseases.
Inflammatory pathways & chronic diseases
Nuclear factor-κB (NF-κB), a nuclear transcription factor, was first identified in 1986 by Sen and Baltimore [8]. As its name implies, it is a nuclear factor bound to an enhancer element of the immunoglobulin kappa light chain gene in B cells. First considered a B-cell transcription factor, NF-κB is now known to comprise a family of ubiquitous proteins. NF-κB proteins contain a Rel homology domain (DNA-binding domain/dimerization domain) with a nuclear localization sequence; such sequences are conserved from Drosophila to man. Class I proteins include p50, p52, p100, and p105. Multiple copies of ankyrin repeats are present in p100 and p105; proteolytic cleavage of p100 forms p52 and that of p105 forms p50. These protein, in turn, form dimers with class II proteins (c-Rel, RelB, and RelA/p65), which exclusively contain C-terminal activation domains. Whereas RelB forms only heterodimers, all the other proteins can form both homo- and heterodimers. NF-κB is the most common heterodimer formed between Rel A and p50. Dimeric NF-κB transcription factors bind to the 10-base-pair consensus site GGGPuNNPyPyCC, where Pu is purine, Py is pyrimidine, and N is any base. The individual dimers have distinct DNA-binding specificities for a collection of related B sites.
The various inhibitors of NF-κB include IκBα, IκBβ, IκBγ (derived from the C-terminal of p100), IκBε, Bcl-3, pp40 (a chicken homologue), cactus (a Drosophila homologue), and avian swine fever virus protein p28, p105 and p100 can also function to retain NF-κB subunits in the cytoplasm. All of these proteins are characterized by the presence of multiple ankyrin repeats. Perhaps the most common and best-understood form of NF-κB consists of p50, p65, and IκBα. IκBα mediates transient gene expression, whereas IκBβ mediates persistent response.
The IκB proteins are expressed in a tissue-specific manner and have distinct affinities for individual Rel/NF-κB complexes. IκBs contain six or more ankyrin repeats, an N-terminal regulatory domain, and a C-terminal domain that contains a proline-glutamic acid-serine-threonine motif. IκBs bind to NF-κB dimers and sterically block the function of their nuclear localization sequences, thereby causing their cytoplasmic retention. Most agents that activate NF-κB mediate the phosphorylation-induced degradation of IκB. On receipt of a signal, phosphorylation of IκBα takes place on two conserved serine residues (S32 and S36) in the N-terminal regulatory domain. However, another member of the IκB family, Bcl-3, stimulates transcription after interacting with p50 and p52 subunits of NF-κB. Several of the IκB kinases (IKKs) have been characterized, namely, IKKα, IKKβ, and IKK. Mutation analysis revealed that IKKα and not IKKβ mediates proinflammatory signals. Once phosphorylated, the IκBs, which are still bound to NF-κB, almost immediately undergo a second posttranslational modification known as polyubiquitination. The major ubiquitin acceptor sites in human IκBα are lysines 21 and 22. Protein ubiquitination occurs through the E1 ubiquitin-activating enzyme, the E2 ubiquitin-conjugating enzyme, and the E3 ubiquitin protein ligases. After ubiquitination, IκBs are degraded in 26S proteasomes, leading to the release of NF-κB dimers, which translocate into the nucleus [9]. In contrast, the activation of NF-κB in response to ultraviolet (UV) radiation is accompanied by IκBα degradation but not phosphorylation on the N-terminus of IκBa. Hypoxia or pervanadate treatment stimulates the phosphorylation of IκBα at tyrosine 42, but other IκBs do not have a tyrosine at this position. Phosphorylation on Ser-276 by the catalytic subunit of protein kinase A contributes to the intrinsic transcriptional capacity of the p65 subunit of NF-κB. The catalytic subunit of protein kinase A was also found to be associated with NF-κB and IκB in the cytoplasm and was able to phosphorylate p65 only after IκB degradation [7]. In addition, a site-directed mutant of p65 (Ser-276 to Ala) is phosphorylated at Ser 529 in response to tumor necrosis factor (TNF), suggesting that multiple physiologic stimuli modulate p65 through distinct phosphorylation sites to control transcriptional activity. RelA (C-terminus) has been shown to interact with basal transcriptional apparatus proteins such as TATA-binding protein (TBP), transcription factor (TF) IIB and TBP-associated factor (TAF) 105 and with coactivators such as cAMP responsive element binding protein (CBP) and p300, although the actual role of these interactions is not clear [10]. This pathway is well conserved, both in structure and function, from Drosophila to humans.
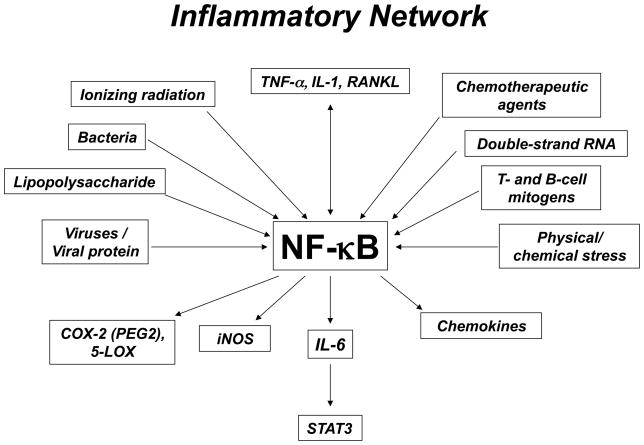
NF-κB is activated by many divergent stimuli, including proinflammatory cytokines (e.g., TNF-a, interleukin-1 [IL-1]), T- and B-cell mitogens, bacteria, lipopolysaccharide (LPS), viruses, viral proteins, double-stranded RNA, and physical and chemical stresses. Cellular stresses, including ionizing radiation and chemotherapeutic agents, also activate NF-κB (Fig. 2).
Fig. 2.
Activation of inflammatory network by various agents.
Although much has been learned since the discovery of NF-κB, the precise mechanism of its activation is still not fully understood. Depending on the stimulus, this mechanism involves overlapping and nonoverlapping steps. For example, TNF, one of the most potent activators of NF-κB, interacts with the TNF receptor (TNFR) and then recruits a protein called TNFR-associated death domain. This protein binds to TNFR-associated factor (TRAF) 2, which recruits NF-κB–inducing kinase (NIK), which in turn activates IKK. IKK phosphorylates IκBa at serines 32 and 36, which leads to ubiquitination at lysines 21 and 22, and this leads to the degradation of IκBa by the 26S proteasome. This degradation results in translocation of NF-κB to the nucleus, where it binds to its consensus sequence (5′-GGGACTTTC-3′) and activates gene expression. Thus, NF-κB can be monitored by the IκBa degradation seen on Western blotting, by the NF-κB binding to DNA seen on electrophoretic mobility shift assay, or by the NF-κB–dependent reporter gene expression seen on transient transfection.
Besides the previously described canonical NF-κB activation pathway, a noncanonical NF-κB activation pathway is activated by CD40L, lymphotoxin (LT)-β, receptor activator of NF-κB ligand (RANKL), and B-cell-activating factor of the TNF family (BAFF), all members of the TNF family. This pathway does not involve IκBa but instead involves direct phosphorylation and ubiquitin-dependent degradation of p100. Current research indicates that NF-κB activation is highly complex and may involve dozens of different protein kinases. Besides NIK, IKK-α, and IKK-β, NF-κB activation may also require the involvement of other kinases, such as atypical protein kinase C, protein kinase C-z, pp90rsk, double-stranded RNA-dependent protein kinase, cot kinase (also called TPL2), mitogen-activated protein kinase kinase kinase 1, 2, and 3), phosphatidylinositol 3 protein kinase, Akt, mixed lineage kinase 3, hematopoietic progenitor kinase-1, transforming growth factor β–activated kinase 1, and c-raf kinase. These kinases may form a cascade, and different cascades may form depending on the NF-κB activator. For instance, IKK can be phosphorylated by NIK, mitogen-activated protein kinase kinase kinase, or Akt. Although IKK is required for NF-κB activation by most agents, a few (such as human epithelil receptor type 2, H2O2, pervanadate, x-rays, and -radiation) activate NF-κB through IKK-independent pathways. Although several signaling proteins and protein kinases have been identified recently that mediate NF-κB activation, more kinases and protein phosphatases remain to be identified. Besides the ubiquitin-dependent 26S proteasome, which has a role in IκBα degradation, other proteases have also been implicated in NF-κB activation.
The genetic deletions of different NF-κB proteins produce numerous phenotypic changes. For instance, deletion of the rel a gene induced embryonic lethality in mice, probably due to massive apoptosis in the liver. In addition, the mouse embryo fibroblasts (MEFs) from rel a-deletion mice were found to be hypersensitive to TNF-induced apoptosis. These results indicate a negative role for NF-κB in TNF-induced apoptosis. Furthermore, mice lacking the RelA subunit were brought to term only in aTNFR1-deficient background. These mice lacked lymph nodes, Peyer’s patches, and an organized splenic microarchitecture, and they had a profound defect in their T-cell–dependent antigen responses. Analyses of TNFR1/RelA-deficient embryonic tissues and of radiation chimeras suggest that the dependence on RelA is manifested not in hematopoietic cells but rather in radioresistant stromal cells, which are needed for the development of secondary lymphoid organs. In contrast to the deletion of Rel A, the deletion of the IκBa gene leads to early neonatal lethality caused by inflammatory dermatitis and granulocytosis that are most likely induced by constitutive activation of NF-κB, leading to expression of the granulocyte colony-stimulating factor. Once NF-kB is activated, it causes the expression of almost 500 different gene products that includes enzymes, cytokines, adhesion molecules and other signaling intermediates closely linked with inflammation (Table 3).
Table 3.
List of inflammatory gene products regulated by NF-κB*
| Enzymes | Stress response genes | Gro b | CD21 | Lox-1 | Clone 330 |
|---|---|---|---|---|---|
| 11bHSD2 | 12-LOX | Gro g | CD38 | Ly49 | Clone 68 |
| 17bHSD | 5-LOX | Gro-1 | CD3g | Mdr1 | Connexin32 |
| ABC Transporters | COX-2 | ICOS | CD40 | mGlu2 | Cyclin D1 |
| ADH | Cu/Zn SOD | IFN-g | CD48 | Mu-OR | Cyclin D2 |
| AID | CYP2C11 | IiGp1 | CD83 | NMDA-RS 2A | Cyclin D3 |
| alpha 1ACT | CYP2E1 | IL-1 RA | CD86 | NP Y-Y1 R | DIF2 |
| AMACR | CYP7b | IL-10 | CD98 | NR-1 | DMT1 |
| ARFRP1 | FHC | IL-11 | CXCR | Oxytocin R | Elafin |
| ASS | GCL | IL-12A | CXCR2 | PAF-R1 | Endothelin 1 |
| Aromatase | GCLM | IL-12B | F11-R | P-gp | Ephrin-A1 |
| ART2.1 | HSP90-a | IL-13 | Fc e R II | RAGE | epsilon-Globin |
| BACE-1 | iNOS | IL-15 | FcRn | Transcription factors | Factor VIII |
| Btk | MAP4K1 | IL-17 | HLA-G | /Regulators | FHC |
| Cathepsin B | Mx1 | IL-1a | ICOS | A20 | Gadd45b |
| Cathepsin L | DTD | IL-1b | Ig C g1 | ABIN-3 | Galectin 3 |
| cdk6 | cPLA2 | IL-2 | Ig e heavy chain | AR | Galpha i2 |
| CGT | SENP2 | IL-23A | Ig g1 | Bcl-3 | GBP-1 |
| CHI3L1 | SEPS1 | IL-27 | Ig g4 | BMI-1 | GIF |
| CRAD1 | SOD1 | IL-6 | Ig k light chain | C/EBPd | Gro-1 |
| CRAD2 | SOD2 | IL-8 | IL-2 R a-chain | CDX1 | GS3686 |
| Collagenase 1 | Early response genes | IL-9 | Invariant Chain II | c-fos | HK protein |
| DDH | B94 | IP-10 | Kinin B1 Receptor | c-myb | HCCS1 |
| DNASIL2 | Egr-1 | KC | MHC-I (H-2Kb) | c-myc | HMG14 |
| DYPD | p22/PRG1 | LIX | MHC-I HLA-B7 | c-rel | IBABP |
| EL | p62 | Lymphotoxin a | Nod2 | DC-SCRIPT | IMP2 |
| ENO2 | TIEG | Lymphotoxin b | PGRP-S | Dmp1 | K15 Keratin |
| gamma-GCS | Viruses | MCP-1/JE | Polymeric Ig R | E2F3a | K3 Keratin |
| GAD67 | AV | MIG | T-CR b chain | Elf3 | K6b Keratin |
| GCL | AVV | MIP-1a,b | T-CR/CD3g | ELYS | Lactoferrin |
| GCLM | BLV | MIP-2 | TLR-2 | ETR101 | Laminin-B2 |
| GCLC | CMV | MIP-3a/CCL20 | TLR9 | Gata-3 | Lipocalin-2 |
| GD3-synthase | EBV | mob-1 | TNF-Receptor | GCR | MCT1 |
| Gelatinase B | HBV | NAP-78 | TREM-1 | HIF-1a | Mir125b |
| G6PD | HIV-1 | RANTES | b-2 Microglobulin | HOXA9 | Mir146a, b |
| Glc-6-Pase | HPV-16 | T-CA gene 3 | Growth factors/ligands | IkB-a | Mir155 |
| GnRH II | HSV | TNFalpha | Activin A | IkB-e | MNE1 |
| gp91 phox | JCV | TNFbeta | Angiopoietin | IRF-1 | Mts1 |
| Granzyme B | SIV | TRAIL | BCAP | IRF-2 | Mucin |
| GSTP1-1 | SV-40 | Treefoil factor-3 | BDNF | IRF-4 | MBP |
| H+-K+ATPase a2 | Apoptosis Regulators | VEGI | BLNK | IRF-7 | Naf1 |
| Heparanase | ASC | Cell adhesion molecules | BLyS | jmjD3 | NGL |
| HO-1 | B7-H1 | CD44 | BMP-2 | junB | NLF1 |
| Has | Bax | DC-SIGN | BMP-4 | Lef1 | p11 |
| IDO | Bcl-2 | ELAM-1 | CGRP | LZIP | p21-CIP1 |
| iNOS | Bcl-xL | Endoglin | EPO | PA28 a | |
| ITD-2 | Bfl1/A1 | Fibronectin | FGF8 | nfkb1 | PA28 b |
| L-PGDS | Bim | ICAM-1 | FLRG | nfkb2 | PAI-1 |
| Lysozyme | BNIP3 | MadCAM-1 | G-CSF | NLRP2 | Pax8 |
| MMP-3 | Caspase-11 | NCAM | GM-CSF | NURR1 | PCBD |
| MMp-9 | CD95 (Fas) | P-selectin | HGF/SF | Osterix | Perforin |
| MKP-1 | c-FLIP | Tenascin-C | IGFBP-1 | p53 | PGK1 |
| MLCK | CIDEA | VCAM-1 | IGFBP-2 | PR | POMC |
| Mthfr | FAPase-1 | Acute phase proteins | M-CSF | PU.1 | PSG |
| GnT-I | Fas-Ligand | Angiotensinogen | Midkine | relb | PDYN |
| n-NOS | IAPs | b-defensin-2 | NGF | Snail | PSA |
| PDE7A1 | IEX-1L | C4b BP | NK-1R | Sox9 | PTEN |
| PGES | Nr13 | CF-κB | NK4 | Stat5a | RAG-1 |
| PIK3CA | TRAF-1 | CF-C4 | Nrg1 | Tfec | RAG-2 |
| PIM-1 | TRAF-2 | C-RP | OPN | Twist | RbAp48 |
| PKAalpha | TRAF-2 BP | Hepcidin | PDGF B chain | WT1 | RICK |
| PKCdelta | XIAP | LPS BP | PlGF | YY1 | S100A6 |
| PLCdelta | Cytokines/Chemokines | Pentraxin-3 | Proenkephalin | Miscellaneous | SerpinE2 |
| Plk3 | aka (LAG-1) | SAA1 | Prolactin | AGP | SH3BGRL |
| PP5 | BAFF | SAA2 | SCF | a1-AT | SK2 channels |
| PTGIS | b-Interferon | SAA3 | THBS1 | a2(I) collagen | Skp2 |
| PTHrP | BLIMP-1 | Tissue factor-1 | THBS2 | ABCG5 | Spergen-1 |
| PTP1B | CCL15 | UPA | VEGF C | ABCG8 | SWS1 |
| RACK1 | CCL17 | Antigen presentation | WNT10B | AbetaH-J-J | Syncytin-1 |
| REV3 | CCL19 | Complement B | Cell-surface receptors | AFP | Syndecan-4 |
| Serpin 2A | CCL20 | Complement component 3 | A1 AR | AMH | TASK-2 |
| SIAT1 | CCL22 | Complement Receptor 2 | A2A | APOBEC2 | TAUT |
| Slfn-2 | CCL23 | Peptide Transporter TAP1 | a2B-AR | Apo CIII | TFPI-2 |
| SNARK | CCL28 | Proteasome Subunit LMP2 | ABCA1 | Apo D | Transferrin |
| sGC-1 | CCL5 | Tapasin | ABCC6 | Apol E | TRIF |
| SSAT | CINC-1 | Immunoreceptors | ADAM19 | AQP4 | TRPC1 |
| SUV3 | CXCL 11 | B7.1 (CD80) | AS Na-channel | b-amyloid | UBE2M |
| TERT | CXCL5 | BRL-1 | Bradykinin B1-R | Biglycan | UCP-2 |
| TG | CXCL6 | CCR5 | CD23 | BRCA2 | Uroplakin Ib |
| TTG | EBI3/IL-27B | CCR7 | CD69 | Calsarcin-1 | Vimentin |
| type II-sPLA(2) | Eotaxin | CD134 | DOR | Caveolin-1 | zeta-Globin |
| CD137 | EGFR | Claudin-2 | |||
| UPase | Fractalkine | CD154 | ErbB2 | Clone 156 | |
| XDH | Gro a | Gal1 R |
Adopted from www.nf-kb.org
NF-κ B and Chronic diseases
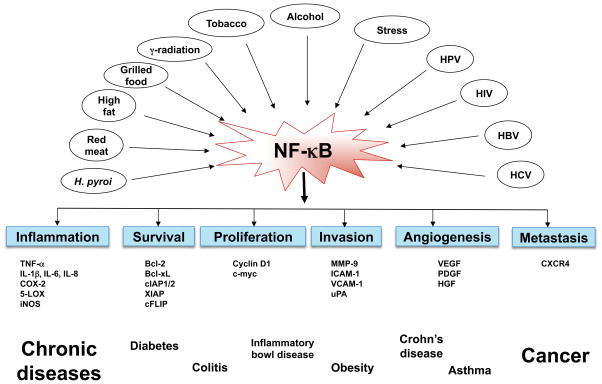
NF-κB activation has been implicated in a wide variety of diseases, including cancers, diabetes mellitus, cardiovascular diseases, autoimmune diseases, viral replication, septic shock, neurodegenerative disorders, ataxia telangiectasia (AT), arthritis, asthma, inflammatory bowel disease, and several other inflammatory conditions (Fig. 3). For example, activation of NF-κB by LPS may contribute to the development of septic shock because NF-κB activates transcription of the inducible nitric oxide synthase (iNOS) genes known to be involved in septic shock. Similarly, autoimmune diseases such as systemic lupus erythematosus may also involve activation of NF-κB. Additionally, in chronic Alzheimer’s disease, the amyloid β peptide causes production of reactive oxygen intermediates and indirectly activates gene expression through B sites. The influenza virus protein hemagglutinin also activates NF-κB, and this activation may contribute to viral induction of cytokines and to some of the symptoms associated with influenza. Furthermore, the oxidized lipids from the low density lipoproteins associated with atherosclerosis activate NF-κB, which then activates other genes, and mice that are susceptible to atherosclerosis exhibit NF-κB activation when fed an atherogenic diet. Another important contributor to atherosclerosis is thrombin, which stimulates the proliferation of vascular smooth muscle cells through the activation of NF-κB. Finally, a truncated form of IκBa was shown to protect AT cells, which express constitutive levels of an NF-κB–like activity, from ionizing radiation. In light of all these findings, the abnormal activation or expression of NF-κB is clearly associated with a wide variety of pathologic conditions.
Fig. 3.
Activation of inflammatory network by life style factors and its contribution to chronic diseases.
Ayurvedic plants, their active components and their molecular targets
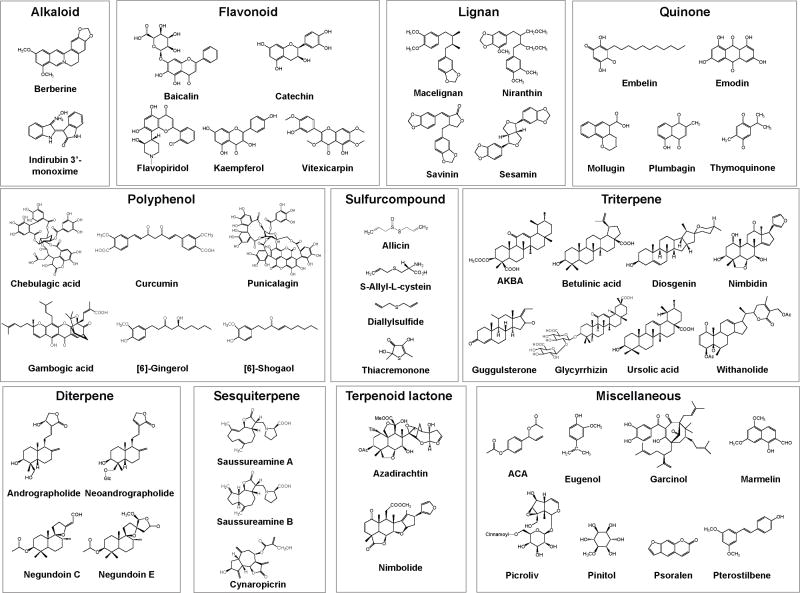
Almost 200 Ayurvedic plants have been identified that exhibit anti-inflammatory activities. The active component from some of these plants is shown in Fig. 4. The molecular targets of these compounds are shown in Table 4. More specific description of these plants, active components and molecular targets are described below:
Fig. 4.
Structures of active phytochemicals derived from Ayurvedic plants.
Table 4.
Selected Ayurvedic Plants, Their Phytochemicals and Their Molecular Targets
| Ayurvedic Plants Alkaloid | Active Compounds | Molecular Targets | References |
|---|---|---|---|
| Berberis aristata | Berberine | NF-κB, COX-2 | [50] |
| Indigofera tinctoria | Indirubin | NF-κB, COX-2 | [135] |
| Flavonoid | |||
| Acacia catechu | Baicalin, catechin | COX-2, 5-LOX, iNOS | [14] |
| Dysoxylum binectariferum | Flavopiridol | NF-κB, COX-2 | [107] |
| Nelumbo nucifera | Kaempferol | NF-κB, COX-2, iNOS | [155] |
| Lignan | |||
| Myristica fragrans | Macelignan | NF-κB, COX-2 | [874, 875] |
| Phyllanthus amarus | Niranthin | PAFR | [165] |
| Pterocarpus santalinus | Savinin | TNF-α | [715] |
| Quinone | |||
| Embelia ribes | Embelin | NF-κB, COX-2 | [112] |
| Aloe vera | Emodin, lectin | NF-κB, TNF-α | [32, 876] |
| Rubia cordifolia | Mollugin | NF-κB | [191] |
| Plumbago zeylanica | Plumbagin | NF-κB, COX-2, STAT-3 | [172, 173] |
| Nigella sativa | Thymoquinone | NF-κB, TNF-α, IL-1β, COX-2 | [8, 624] |
| Polyphenol | |||
| Terminalia chebula | Chebulagic acid | COX-1, COX-2, 5-LOX | [807] |
| Curcuma longa | Curcumin | NF-κB, COX-2, STAT-3 | [94, 95, 877] |
| Punica granatum | Punicalagin | NF-κB, COX-2 | [181] |
| Garcinia cambogia | Garcinol, gambogic acid | NF-κB, COX-2, 5-LOX, iNOS | [123, 878, 879] |
| Zingiber officinale | [6]-Gingerol, shogaol | NF-κB, COX-2 | [862, 880] |
| Sulfur Compound | |||
| Allium sativum | S-Allyl-L-cysteine, allicin, diallyl sulfide, thiacremonone | NF-κB, TNF-α, iNOS, COX-2, IL-6, MCP-1, IL-8, IL-10, MIG, IL-1β | [29, 264, 265, 267, 881] |
| Triterpine | |||
| Boswellia serrata | Boswellic acid | NF-κB, COX-2, STAT-3, 5-LOX | [61, 882] |
| Callicarpa macrophylla | Betulinic acid | NF-κB, COX-2, STAT-3 | [65, 66] |
| Trigonella foenum-graecum | Diosgenin | NF-κB, COX-2, STAT-3 | [229, 230] |
| Commiphora mukul | Guggulsterone | NF-κB, STAT-3 | [83, 85] |
| Commiphora wightii | Guggulsterone | NF-κB, STAT-3 | [83, 85] |
| Glycyrrhiza glabra | Glycyrrhizin | iNOS, NF-κB, IL-4, IL-5, IFN-γ | [883, 884] |
| Ocimum sanctum | Ursolic acid | NF-κB, COX-2, STAT-3 | [159, 160] |
| Withania somnifera | Withanolides | NF-κB, COX-2 | [239, 240] |
| Diterpine | |||
| Andrographis paniculata | Andrographolide, neoandrographolide | NF-κB, TNF-α, IL-6, iNOS, IFN-γ, IL-12p70, COX-2 | [278, 279, 283, 291, 293, 294, 885] |
| Vitex negundo | Negundoin C, E | iNOS, COX-2 | [846] |
| Sesquiterpine | |||
| Saussurea lappa | Cynaropicrin, saussureamines A, B | TNF-α, NF-κB, iNOS | [886–888] |
| Terpenoid Lactone | |||
| Azadirachta indica | Azadirachtin | NF-κB, STAT-3 | [42] |
| Nimbidin, nimbolide | PGE2, IL-1, NF-κB | [43, 183] | |
| Miscellaneous | |||
| Abies pindrow | Pinitol | NF-κB, COX-2 | [10] |
| Aegle marmelos | Marmelin | COX-2, IL-8, TNF-α | [889] |
| Alpinia galanga | ACA | NF-κB, COX-2, iNOS | [34, 890] |
| Boerhaavia diffusa | Punarnavine | NF-κB | [53] |
| Foeniculum vulgare | Anethole | NF-κB, COX-2 | [119] |
| Picrorhiza kurroa | Picroliv | NF-κB, COX-2 | [166] |
| Psoralea corylifolia | Psoralen | IL-10 | [178] |
| Pterocarpus marsupium | Pterostilbene | COX-2 | [179] |
| Solanum nigrum | Phytoglycoprotein | NF-κB, iNOS, COX-2 | [891] |
| Syzygium aromaticum | Eugenol | NF-κB, COX-2 | [892] |
| Tinospora cordifolia | (1,4)-alpha-D-glucan | NF-κB | [893] |
5-LOX, 5-lipooxygenase; COX-2, cyclooxygenase-2; IL-1, interleukin (IL)-1; iNOS, inducible nitrogen oxide synthase; IP-10, IFN-γ-induced protein-10; MCP-1, monocyte chemotactic protein-1; MIG, monokine induced by IFN-γ; MIP-α, macrophage inflammatory protein-1 alpha; NF-κB, nuclear factor-kappaB; PAFR, platelet-activating factor-receptor; PGE2, prostaglandin E2; STAT-3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor-alpha
1. Abies pindrow
A. pindrow, known as the ‘talisapatra’ tree in Sanskrit and ‘morinda’ in Hindi, is found in abundance in the deciduous forests of Himalayas. Its leaves have been used as Ayurvedic remedy for fever, respiratory and inflammatory ailments. Anti-diabetic, anti-inflammatory, analgesic, hypnotic and anti-ulcerogenic activities in rats, hypotensive effect in dogs, and endurance enhancing in swimm stress in mice have been reported for extracts and fractions from A. pindrow leaves [11]. Pinitol (3-O-methyl-chiroinositol), a component of A. pindrow was reported to suppress NF-κB activation both induced by inflammatory stimuli and carcinogens and constitutive NF-κB activation noted in most tumor cells. The suppression of NF-κB activation by pinitol occurred through inhibition of the activation of IκBα kinase, leading to sequential suppression of IκBα phosphorylation and degradation, p65 phosphorylation and nuclear translocation, and NF-κB-dependent reporter gene expression. The inhibition of NF-κB activation thereby led to down-regulation of gene products involved in inflammation (cyclooxygenase [COX]-2), proliferation (cyclin D1 and c-myc), invasion (matrix metalloproteinase [MMP]-9), angiogenesis (vascular endothelial growth factor; VEGF), and cell survival (cIAP1, cIAP2, X-linked inhibitor apoptosis protein [XIAP], Bcl-2, and Bcl-xL). Suppression of these gene products by pinitol enhanced the apoptosis induced by TNF and chemotherapeutic agents and suppressed TNF-induced cellular invasion [12].
2. Abrus precatorius
Leaves, roots and seeds of Abrus precatorius, known commonly as Jequirity, Crab’s Eye, Rosary Pea, or Indian licorice are used for medicinal purposes. A tea is made from the leaves and used to treat fevers, coughs and colds. Abruquinones, the isoflavanquinones isolated from the roots have strong anti-inflammatory and antiallergic effects. Wang et al. [13] suggests that the anti-inflammatory effect of abruquinone is mediated partly by suppressing the release of chemical mediators from mast cells and partly by preventing vascular permeability changes caused by mediators.
3. Abutilon indicum
In traditional medicine, A. indicum is used as a demulcent, aphrodisiac, laxative, diuretic, pulmonary and sedative. The aqueous extract of the plant has antidiabetic properties, which inhibited glucose absorption and stimulated insulin secretion [14].
4. Acacia arabica
The gum of Acacia Arabica is the source of useful medicaments and used for treating gingivitis and for reducing plaque. The hypoglycemic effect was indicated that the powdered seeds of Acacia by initiating the release of insulin from pancreatic beta cells of normal rabbits [15].
5. Acacia catechu
Altavilla et al. [16] studied the anti-inflammatory activity of Flavocoxid, a mixed extract containing baicalin and catechin from Acacia catechu that acts as a dual inhibitor of cyclooxygenase (COX) and 5-lipoxygenase (LOX) enzymes and showed that Flavocoxid significantly inhibited COX-2, 5-LOX and inducible nitric oxide (NO) synthase (iNOS) expression in LPS-stimulated peritoneal rat macrophages.
6. Acacia farnesiana
The bark and the flowers of Acacia farnesiana are the parts most used in traditional medicine. Among all the isolated compounds viz., acasiane A & B, farnesirane A and farnesirane B, three diterpenes, two triterpenes, eight flavonoids, and betulinic acid showed moderate anti-inflammatory activities against five human cancer cell lines [17].
7. Achillea millefolium
It has seen historical use as a medicine for treatment of inflammatory diseases. It has been used to treat complaints such as inflammation, pain, wounds, hemorrhages, hepato-biliary disorders and gastrointestinal disturbances such as ulcer, liver cirrosis, chronic hepatitis and diabetes. Anti-tumor activity was studied by Tozyo et al. [18] and showed that achimillic acids A, B and C from A. millefolium were found to be active against mouse P-388 leukemia cells in vivo.
8. Achyranthes aspera
Achyranthes aspera is used in the indigenous systems of medicine for the treatment of inflammatory conditions and had hypoglycaemic effect. Its extracts are also showed anti-inflammatory effects in carrageenin-induced paw oedema in rat [19] and exerted anti-carcinogenic effects in vivo two-stage mouse skin carcinogenesis [20].
9. Acorus calamus
Acorus calamus L., sweet flag, is widely employed in modern herbal medicine as an aromatic stimulant and mild tonic. In Ayurveda, it is highly valued as a rejuvenator for the brain and nervous system and as a remedy for digestive disorders. This plant also exerts antidiabetic, anti-adipogenic, and hypolipidemic activities [21]. A. calamus also showed anti-inflammatory effects, and it might be mediated by suppression of NF-κB and interferon regulatory factor 3 (IRF3) [22]. Also, several reports indicated the neuroprotective effects of A. calamus in cortex of rat brain [23].
10. Adhatoda vasica
The extracts of Adhatoda vasica have been used to treat bronchitis, asthma, ulcer and rheumatism. Gibb [24] showed ambroxol, a natural alkaloid found in A. vasica, inhibited IgE-dependent basophil mediator release.
11. Aegle marmelos
The compounds, 6-methyl-4-chromanone, isolated from Aegle marmelos by Nicolis et al [25] showed inhibition of IL-8 in the IB3-1 CF cells in vitro. Cardenolide, periplogenin, isolated from the leaves of Aegle marmelos protected the doxorubicin induced cardiotoxicity and lipid peroxidation in rats by reversing the increase in serum creatine kinase-MB, glutamate-pyruvate transaminase, and tissue LPO [26]. Subramaniam et al. [27] reported that marmelin, an ethyl acetate fraction of Aegle marmelos extracts suppressed TNF-alpha-mediated activation and translocation of NF-kappaB, inhibited AKT and ERK phosphorylation both in-vitro and in tumor xenografts.
12. Allium Sativum
The possible therapeutic effects of garlic extract in the treatment of IBD patients showed that it reduced the inflammation by inhibiting cell-mediated T- helper-1 and inflammatory cytokines (TNF-alpha, IL-1alpha, IL-6, IL-8, T-cell IFN-gamma and IL-2) while upregulating IL-10 production [28]. Zare A et al [29] showed significant decrease in allergic airway inflammation levels in murine models. The water-soluble allyl sulfur-containing compound, S-Allyl-L-cysteine Sulfoxide (ACSO), have antioxidant and anti-inflammatory activities and Hui et al [30]showed it could inhibit proinflammatory cytokine-induced adhesion of monocytes to endothelial cells by inhibiting the MAPK signaling and related ICAM-1 expression. Ban JO [31]found another sulfur compound, thiacremonone inhibiting the NF-kappaB activation via interaction with the sulfhydryl group of NF-kappaB molecules, thus could be a used for the treatment of inflammatory and arthritic diseases. Keophiphath M et al [32] used 1,2-vinyldithiin on human preadipocytes to reduce Obesity, a state of chronic low-grade inflammation and found to be a novel, antiobesity nutraceutical.
13. Aloe vera
It has been used in the treatment of a variety of disorders including wounds and burns. In addition to its wound healing property Aloe vera, has also been shown to have antidiabetic and hypoglycemic properties [33]. Emodin is an active component from A. vera exerts anti-inflammatory effects. Emodin suppressed the activation of NF-κB in human umbelical vein endothelial cells (EC) in a dose- and time-dependent manner. Emodin inhibited degradation of IκB, an inhibitory subunit of NF-κB. Thus, emodin also downmodulated adhesion molecules like ICAM-1, VCAM-1, and ELAM-1 contain NF-κB binding sites in their promoter region in EC [34].
14. Alpinia galanga
Alpinia galanga, a plant in the ginger family, is an herb used in cooking. Grzanna et al. [35] documented that Alpinia galanga extract (GE) can inhibit the activation of human monocytic THP-1 cells by different proinflammatory stimuli and reduce the expression of a wide range of inflammation-related genes such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, COX-2, macrophage inflammatory proteins (MIP)-α, monocyte chemotactic protein (MCP)-1, and chemokine ligand-10 (IP-10), in microglial-like cells in the central nervous system. The active component from this ginger, 1′-acetoxychavicol acetate (ACA), has been shown to inhibit phorbol ester-induced skin tumor promotion, azoxymethane-induced colonic aberrant crypt foci, estrogen-related endometrial carcinogenesis, hepatic focal lesions, rat oral carcinogenesis, and N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Ichikawa et al. [36] reported that ACA suppressed NF-κB activation induced by a wide variety of inflammatory and carcinogenic agents, doxorubicin, and cigarette smoke condensate. Suppression was not cell type specific, because both inducible and constitutive NF-κB activations were blocked by ACA. ACA did not interfere with the binding of NF-κB to the DNA, but, rather, inhibited IκB kinase activation, IκBα phosphorylation, IκBα degradation, p65 phosphorylation, and subsequent p65 nuclear translocation. ACA also inhibited NF-κB-dependent reporter gene expression activated by TNF, TNF-receptor (TNFR)-1, TNFR-associated death domain protein (TRADD), TNFR-associated factor-2 (TARF-2), and IκB kinase, but not that activated by p65. Consequently, ACA suppressed the expression of TNF-induced NF-κB-regulated proliferative (e.g., cyclin D1 and c-Myc), antiapoptotic (survivin, IAP1, IAP2, XIAP, Bcl-2, Bcl-xL, Bfl-1/A1, and FLIP), and metastatic (COX-2-2, ICAM-1, VEGF and MMP-9) gene products. ACA also enhanced the apoptosis induced by TNF and chemotherapeutic agents and suppressed invasion. Thus, ACA suppressed RANKL signaling had a potential to suppress bone loss. ACA inhibited RANKL signaling and consequent osteoclastogenesis in RAW 264.7 cells, a murine monocytic cell line through suppression of RANKL-induced NF-κB signaling pathway. ACA also inhibited the osteoclastogenesis induced by human cancer cell lines such as breast cancer, multiple myeloma, and head and neck squamous cell carcinoma [37].
15. Anacyclus pyrethrum
The root of Anacyclus pyrethrum or Mount Atlas daisy is mostly used in Siddha medicine. The fractions from A. pyrethrum showed a marked stimulating effect on the reticulo-endothelial system and increased the number of peritoneal exudate cells, and spleen cells of mice [38].
16. Andrographis paniculata
A. paniculata, literally ‘king of bitters’ is used in traditional Siddha and Ayurvedic systems of medicine as well as in tribal medicine in India and some other countries for multiple clinical applications, such as rheumatoid arthritis and inflammatory symptoms of sinusitis. Andrographolide, a diterpenoid lactone, and the major active principle isolated from the plant A. paniculata, has been shown to possess a strong anti-inflammatory activity through suppression of inflammatory mediators such as NF-κB, TNF-α, IL-6, MIP-2, iNOS and COX-2 [39]. The anti-diabetic potential of the plant extract was shown by evoked insulin secretion [40].
17. Areca catechu
Betel nut, a partial muscarinic agonist, is one of the mostly widely used substances across Asia has been hypothesized to have beneficial effects on both positive and negative symptoms of schizophrenia. The extracts from this plant has shown to hypotensive properties through its ability to inhibit the pressor responses to both angiotensin I and II [41].
18. Argyeria speciosa
Argyreia speciosa is an important ‘rasayana’ herb in Indian System of medicine that possessed a strong antioxidant, anti-inflammatory and anti-arthritic activity. The ethanolic extract significantly inhibited paw edema induced by carrageenan and Freund’s complete adjuvant and prevented accumulation of inflammatory cells in carrageenan-induced peritonitis [19].
19. Asparagus adscendens
The plant is a rich source of potential anti-diabetic agents. It has been reported to stimulate insulin secretion, enhance insulin action and to inhibit starch digestion [42].
20. Asparagus racemosus
Commonly mentioned as a rasayana in the ayurveda, the plant is considered to be of medicinal importance because of the presence of steroidal saponins and sapogenins in various parts of the plant. It has also been used for nervous disorders, inflammation, liver diseases and certain infectious diseases. The immunomodulating property of the plant has been shown to protect the rat and mice against abdominal sepsis. A recent study showed that potent phytochemicals present in the roots of the plant viz., phytosterols, saponins, polyphenols, flavonoids and ascorbic acid has the ability to regulate cholesterol metabolism and to improve antioxidant status in hypercholesteremic rats [43].
21. Azadirachta indica
The plant is known for its medicinal properties since ancient time. A number of phytochemical isolated chiefly from the leaves of the plant has been shown to possess immunomodulatory, anti-inflammatory, antihyperglycaemic, antiulcer, antimalarial, antifungal, antibacterial, antiviral, antioxidant, antimutagenic and anticarcinogenic properties. A recent report indicated that azadirachtin obtained from the plant possess anti-tumor property and has the potential to target NF-κB [44]. Nimbolide, a limonoid derived from the leaves and flowers of the plant has been shown to exhibit numerous biological activities including anti-cancer [45].
22. Bacopa monnieri
In the Indian system of medicine the plant is known as Brahmi. The administration of extract from the plant has been reported to significantly improve short-term and long-term memory. Bacoside-A has also been reported to prevent the occurrence of seizures and to reduce impaired peripheral nervous system in epileptic rats [46]. The methanolic extract as well as Bacoside-A isolated from the plant has been reported to possess wound-healing activity in Swiss albino rats [47].
23. Bambusa arundinacea
The leaves of the plant have been shown useful in inflammatory conditions, have the ability to heal the wound and have also been shown to check diarrhea in cattle. Manna, a crystalline substance obtained from the plant has been shown useful in ayurvedic medicine for ptosis and paralytic complaints. The methanol extract from the plant has been shown to possess antiinflammatory effect on carrgeenin-induced oedema in rats [48].
24. Bauhinia variegata
The powdered bark from the plant has been traditionally used in ayurvedic medicines as a tonic to the liver. The ethanolic extract and the roseoside (major constituent) from the plant have been reported to enhance insulin release in insulin secreting cell line [49]. The extract from the plant has been reported to exert anticarcinogenic and antimutagenic activity in swiss albino mice. The ethanol extract from the plant has also shown potential to possess chemopreventive property against N-nitrosodiethylamine induced liver tumor and human cancer cell lines [50].
25. Berberis aristata
Berberis aristata DC (Berberidaceae) known, as ‘daruharidra’ is an evergreen, spinescent shrub with known antichlamydial, antiplatelet, antimicrobial and hepatoprotective activity. Its root mainly contains berberine chloride, palmatine chloride. Root bark extract of the plant is taken twice a day for 1–2 weeks by the tribal people in Sikkim (a north-east state of India) and Darjeeling Himalayan regin to treat diabetes. Both the herbs are well known for their anti-inflammatory activity. Berberine has also been found to be effective in experimental herpetic uveitis [51]. Berberine was also shown to abolish NF-κB activation induced by various inflammatory agents and carcinogens. This alkaloid also suppressed constitutive NF-κB activation found in certain tumor cells. Suppression of NF-κB activation occurred through the inhibition of phosphorylation and degradation of IκBαalpha by the inhibition of IκB kinase (IKK) activation, leading to suppression of phosphorylation and nuclear translocation of p65, and finally to inhibition of NF-κB reporter activity. Inhibition of IKK by berbeine was direct and could be reversed by reducing agents. Site-specific mutagenesis suggested the involvement of cysteine residue 179 in IKK. Berberine also suppressed the expression of NF-κB-regulated gene products involved in antiapoptosis (Bcl-xL, Survivin, IAP1, IAP2, and cFLIP), proliferation (cyclin D1), inflammation (COX-2), and invasion (MMP-9). Suppression of antiapoptotic gene products correlated with enhancement of apoptosis induced by TNF and chemotherapeutic agents and with inhibition of TNF-induced cellular invasion [52]. Thus this indicates that this medicinal plant exhibits activities against inflammation linked to most chronic diseases
26. Bergenia ligulata
Bergenia ligulata are popularly known in India as Pashanbheda. Bergenia ligulata (family, Saxifragaceae) has been used for centuries in South Asia, mainly India and Pakistan, for a wide range of ailments. The roots of B. ligulata have been used for the therapy of urinary stones, chronic ulcers, viral hepatitis, and benign prostatic hypertrophy. In addition, B. ligulata has anti-inflammatory and cytoprotective properties. However, the most important activities are its diuretic and lithotriptic effects.
27. Boerhaavia diffusa
Boerhaavia diffusa L. is commonly known as ‘Punarnava’ and its various parts are used in the treatment of cancer, jaundice, dyspepsia, inflammation, enlargement of spleen, abdominal pain and as an anti-stress agent [53, 54]. Administration of aqueous methanol extract of Boerhaavia diffusa was found to be effective in reducing the metastases formation by B167-10 melanoma cells and Punarnavine, an alkaloid from Boerhaavia diffusa enhanced the immune response against metastatic progression of B16F-10 melanoma cells in mice ([55]
28. Boswellia serrata
Extracts from Indian Ayurvedic medicinal plant Boswellia serrata (BE) contains beta boswellic acid, a pentacyclic triterpene and the active component of the gum resin (also called frankincense in European pharmacopeia) secreted by the bark of the tree. BE has been used for centuries in traditional Ayurvedic medicine for a wide variety of inflammatory diseases including inflammatory bowel disease [56] and rheumatoid arthritis [57]. BE has been shown to inhibit leukotriene biosynthesis from endogenous arachidonic acid in intact peripheral mononuclear neutrophils through the inhibition of 5-lipooxygenase (LOX), with IC50 as low as 1.5 μM[58]. Other pentacyclic triterpenes (amyrin and ursolic acid) lack this activity. Further studies revealed that the pentacyclic triterpene ring structure, hydrophilic group on C4 ring A, and 11-keto function are all essential for 5-LOX inhibitory activity [59]. By photoaffinity labeling, it was shown that BE binds to 5-LOX at a site distinct from substrate binding site [60]. BE has also been shown to inhibit leukocyte elastase with an IC50 of 15 μM [61], topoisomerase (topo) I and II α53 with affinity constant (KD) of 70.6 nM and 7.6 nM, respectively [62]. BE has been shown to inhibit the growth of a wide variety of tumor cells including glioma [63], colon cancer [64, 65], leukemia cells [66–70], human melanoma [71], hepatoma [72] and prostate cancer cells [73]. The apoptotic effects of BE are mediated through various mechanisms including inhibition of topoisomerase I and II without inhibiting DNA fragmentation [63, 69] and downregulation of cyclin D1, bcl-2, and bcl-xl. Apoptotic effects of BE in hepatoma and colon cancer cells were found to be mediated through caspase-8 activation[64, 72]. Recently BE was reported to induce death receptor (DR)-5 but not DR-4 or Fas through increased expression levels of CAAT/enhancer binding protein homologous protein (CHOP), which led to the activation of caspase-8 in prostate cancer cells [74]. BE also downregulated the expression of androgen receptor through modulation of Sp1 binding activity in prostate cancer cells [75].
The secretion and activity of matrix metalloproteinases (MMPs) from human fibrosarcoma HT-1080 cells was also found to be suppressed by BE [71]. The anti-inflammatory effects of this agent are further demonstrated by studies that showed that LPS-induced TNF production is blocked by BE [76]. Anti-proliferative and anti-inflammatory effects of BE are also mediated through the suppression of the NF-κB pathway2, [77] and STAT3 pathway [78]. Microarray analysis revealed that BE modulated 113 of 552 genes induced by TNF-a in human endothelial cells including MMP-3, MMP-10 and MMP-12 [79], and protected animals against experimental arthritis [80].
Numerous animal studies have been performed with BE. In guinea pigs, BE modulated the biosynthesis of leukotrienes and the course of experimental autoimmune encephalomyelitis (EAE)[81]. Topical application of a methanolic extract of Boswellia serrata (BE) to the backs of mice markedly inhibited TPA-induced increase in skin inflammation, epidermal proliferation, the number of epidermal cell layers, and tumor promotion in 7,12-dimethylbenz[a]anthracene (DMBA)-initiated mice [66]. BE potently attenuated experimental ileitis (inflammation of the ileum) in rats [82], an experimental model of inflammatory bowel disease (IBD). In another study, Anthoni et al [83] examined the mechanisms by which BE mediated its effects in experimental colitis. They showed that BE conferred protection in experimental murine colitis induced by dextran sodium sulfate (DSS). Clinical measurements of disease activity and histology were used to assess disease progression, and intravital microscopy was employed to monitor the adhesion of leukocytes and platelets in postcapillary venules of the inflamed colon. BE treatment significantly blunted disease activity as assessed both grossly and by histology. By using in vivo MatrigelTM plug assay, it was shown that BE inhibited bFGF-induced angiogenesis [84]. Also, Wistar rats treated with BE 14 days after inoculation of C6 tumor cells into their right caudate nuclei survived more than twice as long as untreated mice. Furthermore, when treatment was started immediately after implantation and stopped after 14 days, a higher dose of BE produced significantly smaller tumors with greater apoptotic fractions than untreated mice, suggesting that it might have both therapeutic and chemopreventive effects [85]. Toxicity studies with BE in rats and primates showed no pathological changes in hematological, biochemical, or histological parameters at doses up to 1000 mg/kg. The LD50 has been established at >2 g/kg [86].
29. Bryonia laciniosa
Bryonia laciniosa leaves extract have been used in traditional folk medicine to treat numerous diseases. The methanol extract of B. laciniosa exhibited analgesic and antipyretic activity in the tested experimental animal models. The extract showed inhibition on the hind paw oedema in rats caused by histamine and serotonin respectively [87].
30. Butea monosperma
Butea monosperma (Lam.) (family: Fabaceae) also known as flame of the forest or Palasa in Sanskrit, and in the traditional system of medicine known as ‘Ayurveda’, Butea monosperma has been used in the treatment of a variety of ailments including liver disorders. Nearly every part of Butea monosperma has been used as tonic, astringent, aphrodisiac and diuretic. The main constituent of its flower is butrin (7,3′,4′-trihydroxyflavanone-7,3′-diglucoside) and isobutrin (3,4,2′,4′-tetrahydroxychalcone-3,4′-diglucoside) have been shown to be hepatoprotective [88].
31. Caesalpinia bonducella
C. bonducella FLEMING (Caesalpiniaceae) plant is well known for its medicinal and therapeutic values in Indian Ayurveda. It possesses the anti-inflammatory, analgesic activities against ascites carcinoma and prevention of autoimmune diseases. It has also potent antipyretic and antinociceptive activities [89].
32. Caesalpinia digyna
Several members of the species of genus Caesalpinia are used traditionally for a wide variety of ethnomedical properties such as anti-inflammatory, antidiabetic, antioxidant and hepatoprotective [89]. The plant is one of the ingredients of an indigenous drug preparation “Geriforte”, which has been used for curing senile prurites with excellent results. The methanol extract of Caesalpinia digyna root exhibited strong scavenging effect on free radical and inhibition of lipid peroxidation [89].
33. Callicarpa macrophylla
C. macrophylla is used for treatment of rheumatic joints. Betulinic acid (BA), a pure compound from C. macrophylla, has been reported to be a selective inducer of apoptosis in tumor cells. It also exhibits anti-inflammatory and immunomodulatory properties. BA has been reported to suppress the activation of NF-κB activation through suppression of IκB kinase, thus abrogate the phosphorylation and degradation of IκBa. Treatment of cells with this triterpinoid also suppressed NF-κB-dependent reporter gene expression and the production of NF-κB-regulated gene products such as COX-2 and MMP-9 induced by inflammatory stimuli. Furthermore, BA enhanced TNF-induced apoptosis [90]. It also inhibits constitutive activation of STAT3 and STAT3-regulated gene products such as Bcl-xL, Bcl-2, cyclin D1 and survivin [91].
34. Calotropis procera
The latex of the plant Calotropis procera has been reported to exhibit potent anti-inflammatory activity against carrageenin and formalin that are known to release various mediators. Its anti-inflammatory effect is caused by inhibiting PGE2 [92].
35. Capparis Spinosa
Capparis spinosa has been employed as a flavoring in cooking and as a diuretic, hypertensive, and tonic (e.g., as a poultice) since ancient times. The ethanol extract from fruits of C. spinosa (ECS) significantly reduced the production of O2-, H2O2, and ROS. ECS exhibits a notable activity in protecting against oxidative stress and interrupting of ROS-ERK1/2-Ha-Ras signal loop, suggesting its potential protective effects against skin sclerosis [93].
36. Carum copticum
Carum copticum. Linn. (Family: Umbelliferae) is popularly known as Ajowan. As a traditional medicine, the seeds of this plant are made into a decoction and used for curing diarrhoeas, amoebiasis, febrile conditions and stomach disorders. It is much valued for its antispasmodic, antiseptic properties and effects against curing dyspepsia and disorders of inflammation [94]. In the Unani system, ajowan is used as an enhancer of body’s resistance. And Antiinflammatory effects of the total alcoholic extract (TAE) and total aqueous extract (TAQ) in 100 mg/kg doses from the seeds of Carum copticum. Linn were significantly had in acute rat model (carrageenan induced rat paw oedema) and a sub acute rat model (cotton pellet induced granuloma).
37. Casearia esculenta
Casearia esculenta Roxb. (Flacourtiaceae) has been a popular remedy for the treatment of diabetes mellitus and is one of the major ingredients of D-400, the largest selling antidiabetic drug in India (Himalaya Drug Company, Bangalore). The root extract from C. esculenta has been reported to reduce blood sugar level in animal model, and show antihyperglycemic property in a STZ-induced diabetic rats [95].
38. Cassia angustifolia
Cassia angustifolia are widely used against skin disorders in traditional Chinese medicine. And also has anti-inflammatory activity [96].
39. Cassia fistula
Cassia fistula linn (Caesalpinaceae) tree is one of the most widespread in the forests of India. The whole plant possesses medicinal properties useful in the treatment of skin diseases, inflammatory diseases, rheumatism, anorexia and jaundice. The heaptoprotective activity and the hypoglycaemic activity have been reported [97]. And anti-inflammatory and antioxidant activities of the aqueous and methanolic extracts of the Cassia fistula Linn. bark were confirmed in Wistar albino rats (both acute and chronic models).
40. Cassia occidentalis
Cassia occidentalis L. is used to cure various diseases. This weed has been known to exert antimicrobial (antibacterial, antifungal, laxative, analgesic, chloretic and diuretic properties), hepatoprotective, anti-inflammatory, antimutagenic and anticarcinogenic activity. Anti-inflammatory effects of these extracts to lower the lipid peroxide content, γ-glutamyl transpeptidase and phospholipase A2 activity in the exudates of cotton pellet granuloma, resulting in the reduced availability of arachidonic acid, a precursor of prostaglandin biosynthesis, and/or by stabilization of the lysosomal membrane system. And target for anticarcinogenic activity is Lck (p56lck) protein tyrosine kinase [98].
41. Cassia tora
Cassia tora L. has been also prescribed in oriental herb medicine to treat night blindness, hypertension, hypercholesterolemia, constipation, hypoglycemic, hypolipidemic, antimutagenic}, anticlastogenicity, and antihepatotoxic activities. Several polyherbal formulations including C. tora seeds are available at Chinese markets for preventing the formation of atherosclerosis plaques. Recently, it was reported that emodin and obtusifolin in Cassia tora L. might be the components having antidiabetic functions since they exhibited a significant inhibitory activity on advanced glycation end products formation [99].
42. Cedrus deodara
The wood of C. deodara has been used since ancient days in Ayurvedic medical practice for the treatment of inflammations and rheumatoid arthritis, anti-cancer activity, potent disinfectant, anti-fungal properties, and analgesic activity [100].
43. Celastrus paniculatus
The oil obtained from the seeds of Celastrus paniculatus Willd. (Celastraceae) is largely used in Ayurvedic medicine for sedative action, as an anti-rheumatic agent, alleviation of intestinal spasms, analgesic and anti-inflammatory activities and anti-diarrhoea [101].
44. Cichorium intybus
Chicory roots have been used as a digestive aid, diuretic, laxative, and mild sedative. Additionally, hepatoprotective agents have been described in the seeds. Its aqueous, ethanolic, and methanolic extracts have been shown to affect cholesterol uptake and tumor development in mice [102], prevent immunotoxicity induced by ethanol, and have anti-inflammatory properties in vitro and in vivo [103].
45. Cinnamomum camphora
Cinnamomum camphora Sieb (Lauraceae) has long been prescribed in traditional medicine for the treatment of inflammation-related diseases such as rheumatism, sprains, bronchitis and muscle pains. C. camphora has anti-inflammatory mechanisms blocked the production of IL-1, IL-6 and the TNF-α from RAW264.7 cells and NO, PGE2 production in lipopolysaccharaide (LPS)/interferon (IFN)-γ-activated macrophages [104].
46. Cinnamomum cassia
Cinnamomum cassia is used to cure various diseases. This weed has been known to has antimicrobial, laxative, analgesic, chloretic, diuretic and and antidiabetic activity [105].
47. Cinnamomum zeylanicum
C. zeylanicum is described as having stimulant, antiflatulent, antiemetic and antidiarrhoeal properties. The principle constituent of cinnamomum bark is the volatile oil which contains cinnamic aldehyde, eugenol and terpenes. The cinnamon oil from C. zeylanicum ameliorated early stage diabetic nephropathy in alloxan-induced diabetic nephropathy [106].
48. Citrullus colocynthis
This cucurbitaceae is widely used in Tunisian folk medicine and it possesses therapeutic activities against a wide range of ailments including inflammatory disorders, arthritis and gout [107].
49. Commiphora wightii
Guggulsterone [4,17(20)-pregnadiene-3,16-dione] is a plant sterol derived from the gum resin (guggulu) of the tree Commiphora mukul. The resin of the C mukul tree has been used in Ayurvedic medicine for centuries to treat such ailments as obesity, bone fractures, arthritis, inflammation, cardiovascular disease, and lipid disorders [108, 109]. This steroid has been shown to bind to the farnesoid × receptor [110] and modulate expression of proteins with antiapoptotic, cell survival, cell proliferation angiogenic, and metastatic activities in tumor cells. Guggulsterone mediates gene expression through regulation of various transcription factors, including NF-κB [111], STAT-3 [112] and various steroid receptors such as androgen receptor and glucocorticoid receptors [113].
Gujral et al demonstrated the anti-arthritic and anti-inflammatory activity of gum guggul [114]. Sharma et al showed its activity in experimental arthritis induced by mycobacterial adjuvant [115]. The effectiveness of guggul for treating osteoarthritis of the knee has also been demonstrated [116]. Recent studies have shown that guggulsterone is an antagonist for bile acid receptor farnesoid × receptor (FXR) [110, 117]. Other studies have shown that guggulsterone enhances transcription of the bile salt export pump [118]Thus guggulsterone is an important regulator of cholesterol homeostasis. Meselhy et al showed that guggulsterone can suppress inducible nitric oxide synthetase (iNOS) expression induced by LPS in macrophages [119]. Because NF-κB has been implicated in obesity, inflammation, hyperlipidemia, atherosclerosis, and osteoarthritis and in the LPS-induced expression of iNOS, we speculated that guggulsterone mediates its effects, at least in part, through suppression of NF-κB activation. We have shown that guggulsterone will downregulate NF-kB activation and potentiate apoptosis induced by TNF, taxol and doxorubicin in human myeloid tumor cells [111]. Our laboratory recently showed that guggulsterone suppressed DNA binding of NF-κB induced by TNF, phorbol ester, okadaic acid, cigarette smoke condensate, hydrogen peroxide, and interleukin 1 (14). Guggulsterone also suppressed constitutive NF-κB activation expressed in many tumor cells. Through inhibition of IκBa kinase activation, this steroid blocked IκBa phosphorylation and degradation, thus suppressing p65 phosphorylation and nuclear translocation [111]. Guggulsterone downregulates expression of cyclooxygenase (COX)-2, matrix metalloprotease (MMP)-9), cyclin D1 expression, VEGF and of antiapoptotic gene products (IAP1, XIAP, Bfl-1/A1, Bcl-2, cFLIP, and survivin through the downregulation of NF-κB activation (14). Others have shown that guggulsterone alone will induce apoptosis in acute myeloid leukemia [120] and in prostate cancer cells [121] through the activation of caspases.
Guggulsterone inhibits osteoclastogenesis induced by NF-κB ligand (RANKL), and by breast tumor cells. Because guggulsterone can suppress the NF-κB activation induced by various carcinogens, our laboratory investigated whether guggulsterone could modulate RANKL signaling and osteoclastogenesis induced by RANKL or tumor cells (see appendix; [122]). We found that treatment of monocytes with guggulsterone suppressed RANKL–activated NF-κB activation (as indicated by gel-shift assay) and that this suppression correlated with inhibition of IκBα kinase and phosphorylation and degradation of IκBα, an inhibitor of NF-κB. Guggulsterone also suppressed the differentiation of monocytes to osteoclasts in a dose- and time-dependent manner. Finally, differentiation to osteoclasts induced by coincubating human breast tumor cells (MDA-MB-468) or human multiple myeloma (U266) cells with monocytes was also completely suppressed by guggulsterone. Collectively, our results indicate that guggulsterone suppresses RANKL and tumor cell–induced osteoclastogenesis by suppressing the activation of NF-κB.
50. Convolvulus pluricaulis
Convolvulus pluricaulis (CP) is known as Shankhpushpi (or shankapushpi), an herb that has been used in India for hundreds of years for nervous disorders such as stress, anxiety and insomnia. CP has an antiulcerogenic effect due to augmentation of mucosal defensive factors such as mucin secretion, lifespan of mucosal cells and glycoproteins rather than on the offensive factors such as acid-pepsin [123].
51. Crataeva nurvula
Crataeva nurvula has an antioxidant potential. SOD mimetic activity was found to be in Crataeva nurvula. Lipid peroxidation inhibitory potential was found to be in Crataeva nurvala and also showed a comparatively high NO quenching capacity [124].
52. Crocus sativus
Crocus sativus L. (saffron) is used in folk medicine, for example as an antiedematogenic agent. Aqueous and ethanolic extracts of saffron stigma and petal have an antinociceptive effect, as well as acute and/or chronic anti-inflammatory activity [125].
53. Cuminum cyminum
Cumin seeds (Cuminum cyminum L.) are largely used as a condiment or spice in Indian food. They are also medicinally useful to correct hoarseness of voice, gonorrhea, dyspepsia, and chronic diarrhea. Cumin supplementation significantly reduced the incidence and number of tumors in the colon. Cumin prevented the accumulation of lipids in tissues and optimized the excretion of fecal sterols and bile acids [126].
54. Curcuma amada
C. amada belonging to the family of Zingiberaceae, popularly known as mango ginger, has been known for its potent antioxidant activity. Mango ginger (C. amada) contains significant amounts of phenolics as both free and bound forms. Both free and bound phenolic fractions of mango ginger were found to be antioxidant and effective in inhibiting H+,K+-ATPase activity and H. pylori growth [127].
55. Curcuma longa
Curcuma longa (turmeric) has a long history of use in Ayurvedic medicine as a treatment for inflammatory conditions. Turmeric constituents include the three curcuminoids: curcumin (diferuloylmethane; the primary constituent and the one responsible for its vibrant yellow color), demethoxycurcumin, and bisdemethoxycurcumin. Curcumin (diferuloylmethane), an anti-inflammatory agent used in traditional medicine, has been shown to suppress cellular transformation, proliferation, invasion, angiogenesis, and metastasis. Curcumin suppressed TNF-induced NF-κB activation and NF-κB-dependent reporter gene expression. Such TNF-induced NF-κB-regulated gene products involved in cellular proliferation (COX-2, cyclin D1, and c-myc), antiapoptosis (IAP1, IAP2, XIAP, Bcl-2, Bcl-xL, Bfl-1/A1, TRAF1, and cellular cFLIP), and metastasis (VEGF, MMP-9, ICAM-1) were also downregulated by curcumin. COX-2 promoter activity induced by TNF was abrogated by curcumin [128, 129]. Bharti et al [130] reported that curcumin inhibited IL-6-induced STAT3 phosphorylation and consequent STAT3 nuclear translocation. Various activities of curcumin against different chronic diseases has been extensively reviewed by us and others [131–144].
56. Curcuma zedoaria
Curcuma zedoaria Rosc is a perennial herb found in tropical countries, such as India, Japan and Thailand. Various parts of this plant are used in Ayurveda and other folk medicines for the treatment of different ailments such as diarrhea, cancer. C. zedoaria has been used for phytochemical and pharmacological medicine [145].
57. Cymbopogon citratus
Cymbopogon citratus, commonly called as lemongrass, is a natural herb that contains citral and is a widely used herb as a food flavoring, as a perfume, and for its analgesic and anti-inflammatory purposes. Lemon grass intake ameliorated ileitis through decreasing lymphocyte migration by inhibiting beta7-expression in SAMP1/Yit mice [146]The extract also showed reduction in the release of pro-inflammatory mediators TNF-alpha and NO significantly indicating an anti-inflammatory effect [147].
58. Cymbopogon martini
Antifungal efficacy of essential oils (EO) of Cymbopogon martini has been well documented. EOs displayed strong antifungal effects. EOs has been used for treatment of dermatophyte infections and may be recommended as an alternative to synthetic drug for topical application [148].
59. Cyperus rotundus
Cyperus rotundus (Family Cyperaceae) is used both as a functional food and as a drug. The extract exhibited high reduction capability and powerful free radical scavenging, especially against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and superoxide anions as well as a moderate effect on NO [149].
60. Cyperus scariosus
Cyperus scariosus, Br. (Syn: C. pertenuis, Roxb.;family: Cyperaceae) is a delicate grass, growing luxuriously in damp. The brown coloured plant rhizomes have a folkloric reputation as cordial, tonic, emmenagogue, vermifuge, diuretic, diaphoretic and desiccant. It remains an important component of several prescriptions used in the native system of medicine to treat a variety of diseases including diarrhoea, epilepsy, gonorrhoea, syphilis and liver damage. The hepatoprotective activity of aqueous-methanolic extract of C. scariosus was investigated against acetaminophen and CCl4-induced hepatic damage [150].
61. Daemonorops draco
Dragon’s blood is a non-specific name for red resinous exudations from quite different plant species endemic to various regions around the globe that belong to the genera Dracaena (Africa) and Daemonorops (South-East Asia), more rarely also to the genera Pterocarpus and Croton (both South America). Dragon’s blood is used for medicinal purposes where it is endemic [151].
62. Datura metel
Datura metel L. of Solanaceae family is a sub-glabrous shrubby herb found to exist throughout the world. It is frequently used in traditional systems of medicines as narcotic, anodyne and antispasmodic. The leaves of D. metel are reported to have anticholinergic activity and are used to relieve the spasm of bronchioles in asthma [152].
63. Didymocarpus pedicellata
Didymocarpus pedicellata R. Br. (Gesneriaceae) is widely used in traditional Indian medicines against renal afflictions. D. pedicellata extract was found to possess a high content of total polyphenolics, exhibit potent reducing power and significantly scavenge free radicals including several reactive oxygen species (ROS) and reactive nitrogen species (RNS). The extract also significantly and dose-dependently protected against Fe-NTA plus H2O2-mediated damage to lipids and DNA [153].
64. Dolichos biflorus
Dolichos biflorus has traditionally been used to dissolve existing renal calculi, provide symptom relief, and prevent recurrence. D. biflorus has been demonstrated that it inhibits calcium phosphate crystallization. D. biflorus causes the urinary magnesium increase that considered an inhibitor of stone formation [154].
65. Dysoxylum binectariferum
The fruit of this plant has anti-inflammatory, diuretic, and CNS depressant activities. The stem bark contains an alkaloid, rohitukine, which exhibited anti-inflammatory and immunomodulatory property. Flavopiridol, synthetic flavone derived from rohitukine, is known as potent inhibitor of several cyclin-dependent kinases (CDK) and undergoes Phase III clinical trial, currently. Flavopilidol has been reported that it suppressed NF-κB in a dose- and time-dependent manner in several cell types. This effect was mediated through inhibition of NF-κB signaling pathway. Flavopiridol also inhibited the expression of the TNF-induced NF-κB-regulated gene products cyclin D1, COX-2, and MMP-9 [155]. Therefore, flavopiridol suppressed TNF-induced activation of activator protein-1 (AP-1) through suppression of various mitogen-activated protein kinases, including c-Jun NH(2)-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and p44/p42 MAPK. It is noteworthy that this flavone also suppressed the expression of various antiapoptotic proteins, such as IAP-1, IAP-2, XIAP, Bcl-2, Bcl-xL, and TRAF-1.Flavopiridol also inhibited the TNF-induced induction of intercellular adhesion molecule-1, c-Myc, and c-Fos, all known to mediate tumorigenesis [156].
66. Eclipta alba
The hepatoprotective effect of the ethanol/water (1:1) extract of Eclipta alba (Ea) has been studied at subcellular levels in rats against CCl4-induced hepatotoxicity. Ea significantly counteracted CCl4-induced inhibition of the hepatic microsomal drug metabolising enzyme amidopyrine N-demethylase and membrane bound glucose 6-phosphatase. The loss of hepatic lysosomal acid phosphatase and alkaline phosphatase by CCl4 was significantly restored by Ea. The study shows that hepatoprotective activity of Ea is by regulating the levels of hepatic microsomal drug metabolising enzymes [157].
67. Elettaria cardamomum
Elettaria cardamomum (Cardamom) was shown to play a wide range of health-promoting roles against various conditions such as constipation, colic, diarrhea, dyspepsia, vomiting, headache, epilepsy, and cardiovascular diseases. Cardamom was reported to exhibit spasmogenic, spasmolytic, blood pressure-lowering, vasodilator, diuretic, and sedative activities [158]. Recently, experimental evidence suggests that cardamom extracts display anti-cancer activities [159]. It has been reported that aqueous suspensions of cardamom have protective effects on experimentally induced colon carcinogenesis by virtue of their anti-inflammatory, anti-proliferative and pro-apoptotic activity.
68. Embelia ribes
The fruit of the Embelia ribes Burm. plant (Myrsinaceae) (called false black pepper in English, Vidanda in Sanskrit, and Babrang in Hindi languages) has been used to treat fever, inflammatory diseases, and a variety of gastrointestinal ailments for thousands of years. Embelin from E. ribes has been shown to have antitumor, anti-inflammatory, and analgesic properties. More recently, active principle, embelin was also identified as a cell-permeable, small molecular weight inhibitor of the X chromosome-linked inhibitor-of-apoptosis protein (XIAP), an antiapoptotic protein, through structure-based computational screening of a traditional herbal medicine three-dimensional structure database of 8221 individual traditional herbal products [160]. Embelin also inhibited activity through modulation of NF-κB activation. Embelin inhibited both inducible and constitutive NF-κB activation was abrogated by embelin. Thus, embelin inhibited sequentially the TNF-induced activation of the IκB kinase, IκBa phosphorylation, IκBa degradation, and p65 phosphorylation and nuclear translocation. Furthermore, embelin down-regulated gene products involved in cell survival, proliferation, invasion, and metastasis of the tumor. This down-regulation was associated with enhanced apoptosis by cytokine and chemotherapeutic agents [161].
69. Emblica officinalis
Emblica officinalis (Family: Euphorbiaceae) indigenous to India, is valued for its unique tannins and flavanoids, which contain very powerful antioxidant properties and used for the treatment of a number of diseases, such as dyslipidemia and atherosclerosis, as hepatoprotective, radioprotective, antibacterial, antitumor, and anti-inflammatory agents [162].
70. Eugenia jambolana
Eugenia jambolana Lam. (Myrtaceae), popularly known as Jamun, is being widely used to treat liver dysfunctions and diabetes by the traditional practitioners for over many centuries. This plant has been reported to have both antidiabetic as well as ulcer protective effects. The extract of jamun pulp showed hypoplycemic activity by stimulating insulin secretion [163]. The chemical constituents of the seed of E. jambolana Lam. are gallic acid, ellagic acid, corilagin, ellagitannins, isoquercetin, quercetin, caffeic acid, ferulic acid, guaiacol, resorcinaldimethyl ether, lignaglucoside, veratrole, β-sitosterol, palmitic acid etc.
71. Evolvulus alsinoides
Evolvulus alsinoides (shankhpushpi which is considered as Medhya Rasayana) is an Ayurvedic drug used for its action on the central nervous system, especially for boosting memory and improving intellect. In the Ayurvedic system of medicine, the whole herb of ‘Shankhpushpi’ has been employed clinically for centuries for its memory potentiating, anxiolytic and tranquilizing properties. The crude extracts of E. alsinoides showed a marked reduction in inflammation and edema in adjuvant induced arthritic rat model [164].
72. Fagonia cretica
Fagonia cretica linn is tropical herbs and have been extensively used in the treatment of various types of haematological, hepatic, neurological and inflammatory conditions. The antioxidant and antibacterial properties of Fagonia cretica have also been well documented. F. cretica also overcome the oxidative stress mediated injury during ischemic neuronal injury via modulating the antioxidant pool of the cells [165].
73. Ferula assafoetida
Ferula assafoetida is used as a food spice in many Asian countries and for the treatment of asthma, bronchitis, ulcer, kidney stone, pain, and cancer in traditional herbal medicine. F. assafoetida L. was reported to have antitumor, antimutagenic and antiviral activities. Farnesiferol C is one of the sesquiterpene coumarin compounds isolated from the resin of Ferula assafoetida L., which have antitumor and antiangiogenic activity [166]. Farnesiferol C inhibits proliferation and angiogenesis by decreasing expression of CD31 and VEGF. It decreased the binding of VEGF to VEGFR1/Flt-1, but not to VEGFR2/KDR/Flk-1.It also decreased the phosphorylation of most of the kinases downstream of VEGFR2: focal adhesion kinase, Src, extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase, and c-jun-NH2-kinase without affecting AKT.
74. Ficus bengalensis
Ficus bengalensis Linn. Family: (Moraceae) is a very large tree distributed throughout India. It is commonly known as ‘Bargad’ in Hindi or ‘Indian Banyan tree’ and considered as holy tree of India. Information based on ethnomedicinal survey reveals that the herbal preparations of different parts of Ficus bengalensis had been considered as effective economical and safe treatments for curing various diseases, such as diarrhoea, respiratory disorders, certain skin diseases and diabetes. Extracts of Ficus bengalensis bark was also found to reduce allergy and stress in asthmatic condition in milk-induced leucocytosis and milk-induced eosinophilia [167].
75. Foeniculum vulgare
Foeniculum vulgare Mill (Fennel) belonging to the Family Apiaceae (Umbelliferae) is a perennial herb native to the Mediterranean region. Dried fruits of Fennel possess a fragrant odour and a pleasant aromatic taste. They are used for flavouring soups, meat dishes, sauces and confectionary items. The fruits are aromatic, stimulant, carminative and are considered to be useful in diseases of the chest, spleen and kidney. Anethole, a chief constituent fennel, has been shown to block both inflammation and carcinogenesis, but just how these effects are mediated is not known. One possibility is TNF-mediated signaling, which has also been associated with both inflammation and carcinogenesis. Anethole suppressed TNF-induced NF-κB activation through inhibition of IκBα phosphorylation and degradation. Anethole also blocked the NF-κB activation induced by a variety of other inflammatory agents. Besides NF-κB, anethole also suppressed TNF-induced activation of the transcription factor AP-1, c-jun N-terminal kinase and MAPK-kinase. In addition, anethole abrogated TNF-induced apoptosis as measured by both caspase activation and cell viability [168]. The antitumor activity of anethole against Ehrlich ascites carcinoma has been reported [169].
76. Garcinia cambogia
Garcinia cambogia, an edible fruit native to southeastern Asia, contains large quantities of hydroxy citric acid (HCA), which has been shown to inhibit ATP citrate, suppress de novo fatty acid synthesis and food intake, and consequently decrease body weight gain. G. cambogia also acts as an antiulcerogenic agent by decreasing the acidity and to increasing the mucosal defence in the gastric areas [170]. Gambogic acid (GA) its active component has been shown to possess anticancer activity through targeting to histone acetyltransferases [171]. Pandey et al [172] reported that GA enhanced apoptosis induced by TNF and chemotherapeutic agents, inhibited the expression of gene products involved in antiapoptosis (IAP1 and IAP2, Bcl-2, Bcl-xL, and TRAF1), proliferation (cyclin D1 and c-Myc), invasion (COX-2 and MMP-9), and angiogenesis (VEGF), all of which are known to be regulated by NF-κB. GA suppressed NF-κB activation induced by various inflammatory agents and carcinogens and this, accompanied by the inhibition of TAK1/TAB1-mediated IKK activation, inhibited IκBα phosphorylation and degradation, suppressed p65 phosphorylation and nuclear translocation, and finally abrogated NF-κB-dependent reporter gene expression. GA also significantly inhibited HUVEC proliferation, migration, invasion, tube formation, and microvessel growth. In a xenograft prostate tumor model, GA effectively inhibited tumor angiogenesis and suppressed tumor growth with low side effects using metronomic chemotherapy with GA. Therefore, GA inhibited the activations of VEGFR2 and its downstream protein kinases, such as c-Src, focal adhesion kinase, and AKT[173].
77. Gaultheria yunnanensis
Among various species of Gaultheria, Gaultheria yunnanensis are used widely in the south of China to treat rheumatoid arthritis. G. yunnanensis displays considerable effects against Freund’s complete adjuvant induced arthritis in rats, which is in concordance with clinical practice. n-Butanol extracts and both of the eluants with water and 30% ethanol produce a significant decrease in the paw edema. 30% ethanol eluants show a stronger activity than others. It also possesses analgesic and anti-inflammatory activities, which may be mediated, at least partly, through the suppression of inflammatory mediators [174].
78. Glycyrrhiza glabra
Glycyrrhiza glabra L. is one such medicinal plant whose dried roots and stolons form an important component of various Ayurvedic formulations. There are number of reports of G. glabra with anti-inflammatory, anticancer, antihepatotoxic, antimicrobial, antioxidant, anti-genotoxic, hepatoprotective, cytoprotective and cytotoxic activities. Other than these, licorice extract also has antidepressant properties. This antidepressant-like effect of liquorice extract is mediated by increase of brain norepinephrine and dopamine, but not by increase of serotonin [175].
79. Gmelina arborea
Gmelina arborea’s decoction is used as a diuretic for loosening phlegm, as an appetite stimulant and in the treatment of various stomach disorders, fevers, skin problems and liver disorders. G. arborea is an important ingredient of generic Ayurvedic formulation “Dashamularishta” prescribed for several gynaecological disorders and used in several commercial ayurvedic preparations. In vitro studies on bark and fruit extracts showed antioxidant activity and protected liver slice culture cells by alleviating oxidative stress-induced damage to liver cells. Ex vivo studies of the extract on perfused isolated rabbit jejunum and in vivo studies based on castor oil-induced model proved to have activity against diarrhea in mice but at low doses [176].
80. Gossypium herbaceum
The extracts of Gossypium herbaceum have antimicrobial, antimutagenic and hepatoprotective properties. Gossypol is a yellow pigment, present in Gossypium herbaceum plants, which has drawn the attention of many scientist because of its wide biological activities such as contraceptive [177] and anticancer [178].
81. Gymnema sylvestre
Gymnema sylvestre is a plant used in India and parts of Asia as a natural treatment for diabetes or “sweet urine.” The hypoglycemic action of Gymnema leaves was first documented in the late 1920s. Gymnema is reported to increase glucose uptake and utilization and improve the function of pancreatic beta cells. Gymnema may also decrease glucose absorption in the gastrointestinal tract. Phytochemically the plant has been reported to contain gymnemagenin the sapogenin, gymnemic acid-III, -IV, -V, -VIII, and -IX, were isolated in pure states from the hot water extract of leaves of G. sylvestre [179]. Glycoside of gymnemic acid may block the absorption of sugar from the intestine and sweet taste of sugars. Plant extract also increases the number of insulin producing cells in pancreas and balance insulin level.
82. Hajarala yahuda
It is one of the main ingredients used in the ayurvedic preparation ashmarihar ras. This preparation contains other ingredients like Yava-kshara, Muli-kshara, and Shveta-parpati, and it is sold as a diuretic (http://divyaproducts.com/index.php?dispatch=products.view&product_id=9).
83. Hebenaria intermedia
It is an endangered medicinal orchid known as ‘Ruddhi’ in ayurveda and distributed in grassy slopes between 2000–3000 m in Himalayan region. It is one among the constituents of “Chyawanprash” an anti aging supplements, which is purely herbal in nature.
84. Hemidesmus indicus
It has been widely used in treatment of various diseases including leprosy, leucoderma, leucorrhoea, syphilis, chronic rheumatism, asthma, bronchitis, gravel and other urinary diseases. The active principles of Hemidesmus indicus, 2-hydroxy 4-methoxy benzoic acid and pregnane glycoside, showed antihyperlipidaemic and antidyslipidemic effects [180, 181].
85. Holarrhena antidysenterica
Most of the study on this plant shows its animicrobial effects and there are no literatures available on chronic diseases. Arseculeratne et al. [182] showed that rats treated with extracts of this plant produced liver lesions, disruption of the centrilobular veins, haemorrhage in the centrilobular sinusoids, focal hepatocellular necrosis and histopathology in the lungs and kidneys. These symptoms were compatible with the action of pyrrolizidine alkaloids.
86. Hordeum vulgare
Germinated barley foodstuffs (GBF), which are derived from brewer’s spent grain are a highly safe food substance. In an acute experimental colitis model, GBF increases butyrate production in the lower intestine and prevents mucosal damage and bloody diarrhoea. Phenolic extracts from whole barley kernel possess high antioxidant, antiradical, and antiproliferative potentials [183].
87. Indigofera tinctoria
True indigo the common name for Indigofera tinctoria is the original sources of indigo dye and is obtained from its leaves. Indirubin, an active principle from indigo has been demonstrated that it had anti-inflammatory and anti-cancer activity through suppression of transcription factor NF-κB [184]. Sethi et al. [184] reported that indirubin suppressed NF-κB activation induced by various inflammatory agents and carcinogens. Indirubin also blocked the phosphorylation and degradation of IκBα through the inhibition of activation of IκB kinase and phosphorylation and nuclear translocation of p65. NF-κB reporter activity induced by TNFR1, TNF receptor-associated death domain, TRAF2, TAK1, NF-κB-inducing kinase, and IKKb was inhibited by indirubin but not that induced by p65 transfection. Thus, indirubin inhibited the expression of NF-κB-regulated gene products involved in antiapoptosis (IAP1, IAP2, Bcl-2, Bcl-xL, and TRAF1), proliferation (cyclin D1 and c-Myc), and invasion (COX-2 and MMP-9). This correlated with enhancement of the apoptosis induced by TNF and the chemotherapeutic agent taxol in human leukemic KBM-5 cells. Indirubin also suppressed cytokine-induced cellular invasion.
88. Inula racemosa
It is an ornamental plant of the Asteraceae family that is used both internally, as well as externally in ayurveda. Externally it is used to dress the wounds and ulcers as the herb possesses antiseptic, antibacterial and antifungal activity and internally it is used in anorexia (loss of appetite) and dyspepsia (indigestion), cough, hiccup, bronchial asthma, reducing the excessive body fats, wound healing, amenorrhea as well as dysmenorrheal. In vivo study the extracts of Inula racemosa decreased total cholesterol, triglycerides, low-density lipoprotein cholesterol and the atherogenic index, and increased high-density lipoprotein cholesterol compared with the positive control by scavenging thiobarbituric acid reactive substances and modulating levels of reduced glutathione in liver, and superoxide dismutase and glutathione peroxidase in heart [185].
89. Ipomoea digitata
Vidhari Kand (Ipomoea digitata) comes in the plant family of sweet potato. In Ayurveda it is used as a general tonic. The leaves contain organic acids, isobutyric, (S)-2-methylbutyric, tiglic, n-decanoic, n-dodecanoic, cinnamic acids, and glycosidic acids, quamoclinic acid A and operculinic acid A [186].
90. Ipomoea nil
Ipomoea nil is a species of morning glory known as white-edge morning glory, ivy morning glory, and Japanese morning glory. Much of the medicinal uses of this plant remain unknown. The plant is much grown for its beautiful flowers and their fruits are consumed. There are many species of ipomoea, which has laxative or purgative properties. The seeds contains diterpene glycosides, and they posses moderate to mild anti cancer property against various cancer cell lines [187].
91. Lavandula stoechas
L. stoechas occurs naturally in the Mediterranean region. It has been used for a long time in traditional medicine as an anticonvulsant and antispasmodic. There are very limited literatures available about its medicinal properties and most of them describe about the isolation and constituents of essential oils from the lavender and its antimicrobial property. The roots contains triterpenes, 18-hydroxy-27-norolean-12,14-dien-30-al-28-oic acid and 3 beta-hydroxy-1-oxo-olean-12-ene-30-al-28-oic acid which has been evaluated for the anticancer property [188]. Apart from that there are no literatures available about its use in preventing chronic diseases.
92. Leucas cephalotes
Leucas cephalotes, the flowering annual herb, is a common weed, which is used in ayurveda to treat several ailments including diabetes. The ethanolic extract of leaves regulates carbohydrate and lipid metabolism and improves antioxidant status in insulin-dependent and non-insulin-dependent diabetes mellitus rats through modulating hepatic glycogen, blood urea and creatinine contents, and hexokinase and glucose-6-phosphatase activities [189].
93. Malaxis acuminate
There are no literatures available about the medicinal uses of this plant. This plant is otherwise called as Jeevak and found in India, China, and South-East Asia, at elevations up to to 1400 m. It is a small to medium sized, hot to warm growing terrestrial or lithophytic orchid. Its pseudobulbs are sweet, refrigerant, aphrodisiac, febrifuge and tonic. They are useful in haematemesis, fever, seminal weakness, burning sensations, dipsia, emaciation, tuberculosis and general debility (http://www.flowersofindia.net/catalog/slides/Jeevak.html).
94. Mangifera indica
Mango is rich in a variety of phytochemicals and nutrients such as vitamins A, C, B6, K and E, polyphenols, omega-3 and -6 poly unsaturated fatty acids and provitamin A carotenoids. The mango contains a triterpene called lupeol which shows strong anti-cancer activity by disrupting survivin/cFLIP activation, modulation of expression levels of cyclins-A, -B1, -D1, -D2, -E2, cyclin-dependent kinase (cdk)-2, CDK-inhibitor p21 and finally inducing G2/M cell cycle arrest [190]. Mangiferin, a xanthone glucoside, isolated from the leaves of Mangifera indica possesses significant antidiabetic, antihyperlipidemic and antiatherogenic properties [191].
95. Mentha piperita
Peppermint has a long tradition of medicinal use, with archaeological evidence placing its use as far back as ten thousand years ago. Many literatures show its anticancer and radioprotective potentials in vivo [192, 193]. It also posses anti-nociceptive effect against acetic acid-induced writhing and hot plate-induced thermal stimulation and also anti-inflammatory effect against xylene-induced ear oedema and cotton-pellet granuloma [194].
96. Mesua ferrea
The xanthones, mesuaxantbone-A, mesuaxanthone-B and euxanthone, that have been isolated from mesua ferrea exhibits anti-inflammatory activity in normal and adrenalectomised rats as tested by carrageenin induced hind paw oedema, cotton pellet granuloma and granuloma pouch techniques. M. ferrea also posses antioxidant [195], but the molecular mechanisms for its activities are yet to be elucidated.
97. Mimusops elengi
The bark, flowers, fruits and seeds of Mimusops elengi are generally used as astringent, cooling, anthelmintic, tonic, and febrifuge. It is mainly used in dental ailments like bleeding gums, pyorrhea, dental caries and loose teeth. Ethyl acetate extract possesses anti-ulcer activity against experimental gastric ulcers by decreasing the gastric acid secretory activity along with strengthening of mucosal defensive mechanisms [196].
98. Momordica charantia
M. charantia is depicted that it is helpful in treating wound, ulcer, dysmenorrhea, eczema, gout, jaundice, kidney stone, leprosy, leucorrhea, piles, pneumonia, psoriasis, rheumatism and scabies. Earlier studies performed with MC extract have demontostrated its antidiabetic, antiviral, antitumor, antileukemic, antibacterial, antihelminthic, antimutagenic, antimycobacterial, antioxidant, antiulcer, anti-inflammatory, hypocholesterolemic, hypotriglyceridemic, hypotensive, immunostimulant and insecticidal properties [197, 198].
99. Moringa oleifera
Various parts of this plant such as the leaves, roots, seed, bark, fruit, flowers and immature pods act as cardiac and circulatory stimulants, possess antitumor, antipyretic, antiepileptic, antiinflammatory, antiulcer, antispasmodic, diuretic, antihypertensive, cholesterol lowering, antioxidant, antidiabetic, hepatoprotective, antibacterial and antifungal activities. Ethanolic extract of seeds showed protection against acetylcholine-induced broncho-constriction and airway inflammation [199].
100. Mucuna pruriens
Mucuna pruriens seeds contain high concentrations of levodopa, a direct precursor of the neurotransmitter dopamine. It has long been used in traditional Ayurvedic Indian medicine for diseases including Parkinson’s disease. This plant also showed the hypoglycaemic activitys in alloxan diabetic rats [200].
101. Nigella sativa
N. sativa has been used in the treatment of a variety of illnesses, including bronchial asthma, headache, dysentery, infections, obesity, back pain, hypertension, gastrointestinal problems, and eczema. Several components of black cumin have been identified, including thymoquione, thymol, thymohydroquinone, and dithymquinone. Thymoquinone (TQ), the most abundant component of black seed oil, has been reported to exhibit antioxidant, antiinflammatory, and chemopreventive effects. For instance, TQ has been shown to suppress the proliferation of various tumor cells, including colorectal carcinoma, breast adenocarcinoma, osteosarcoma, ovarian carcinoma, myeloblastic leukemia, and pancreatic carcinoma, although it is minimally toxic to normal cells. TQ, the active principle of N. sativa, has been reported that it suppressed NF-κB activation induced by various carcinogens and inflammatory stimuli. The suppression of NF-κB signaling pathway led down-regulated the expression of NF-κB-regulated antiapoptotic (IAP1, IAP2, XIAP Bcl-2, Bcl-xL, and survivin), proliferative (cyclin D1, cyclooxygenase-2, and c-Myc), and angiogenic (matrix metalloproteinase-9 and vascular endothelial growth factor) gene products [10]. This led to potentiation of apoptosis induced by tumor necrosis factor and chemotherapeutic agents. Indeed, Yi et al [201] reported that TQ effectively inhibited human umbilical vein endothelial cell migration, invasion, and tube formation. TQ inhibited cell proliferation and suppressed the activation of AKT and extracellular signal-regulated kinase. Therefore, TQ blocked angiogenesis in both in vitro and in vivo, prevented tumor angiogenesis in a xenograft human prostate cancer (PC3) model in mouse, and inhibited human prostate tumor growth at low dosage with almost no chemotoxic side effects. Thymoquinone inhibited vascular endothelial growth factor-induced extracellular signal-regulated kinase activation but showed no inhibitory effects on vascular endothelial growth factor receptor 2 activation. Overall, these results indicate that TQ could be used as a potential drug candidate for cancer therapy.
102. Nardostachys jatamansi
Nardostachys jatamansi Jones DC (commonly named as jatamansi) is used for treatment of mental disorders, insomnia, hyperlipidemia, hypertension and heart diseases. It has protective effect in parkinsonism, epilepsy, cerebral ischemia, liver damage. Various sesquiterpenes (such as Jatamansic acid and Jatamansone) have been reported to be present in the rhizomes of the plant [202].
103. Nelumbo nucifera
Lotus has been known for a long time in Ayurvedic literature as an antipyretic, diuretic, as an astringent remedy in diarrhoea and as an aphrodisiac. The dry powder prepared from its flowers has recently been used in the treatment of Diabetes mellitus by Ayurvedic physicians. Armepavine (Arm), an active compound from N. nucifera, has been shown to exert immunosuppressive effects both in vitro and in vivo through inhibition of NF-κB activation pathways [203]. In vitro, Arm suppressed NF-κB activation and MAPK (p38, ERK1/2, and JNK) phosphorylations and in vivo, Arm attenuated the mRNA expression levels of col1α2, TGF-β1, TIMP-1, ICAM-1, iNOS, and IL-6 genes. Kim et al [204] has also shown the downregulation of iNOS and TNF alpha expression via NF-κB modulation another active compound, kaempferol.
104. Nyctanthes arbortristis
Traditionally, the flowers of Nyctanthes arbortristis are known to be effective as stomachic, carminative, astringent, antibilious, expectorant, hair tonic and are used in the treatment of piles and various skin diseases. The bark is used for the treatment of bronchitis and snakebite. N. arbortristis exhibits potential anti-inflammatory and antinociceptive activity by inhibiting histamine and serotonin induced edema formation and its analgesic may be due to inhibition of the action of prostaglandins [205]. Oral administration of leaf and fruit extracts from N. arbortritis reduced the expression of various cytokines, such as TNF-α, IL-1β and IL-6, in arthritic mice [205].
105. Ocimum sanctum
Ocimum sanctum commonly known as tulsi in Ayurvedic medicine has demonstrated various medicinal values predominantly by its antioxidant property. Different parts of the plant are traditionally used in Ayurveda and Siddha systems for the treatment of diverse ailments like infections, skin diseases, hepatic disorders and as an antidote for snake bite and scorpion sting. The plant has also demonstrated antidiabetic [206] and hypolipidemic effect [207]. Ursolic acid (UA), a pentacyclic triterpene acid from O. sanctum has been reported to suppress NF-κB activation induced by various carcinogens including TNF, phorbol ester, okadaic acid, H2O2, and cigarette smoke. Ursolic acid inhibited degradation and phosphorylation of IκBα, IκB kinase activation, p65 phosphorylation, p65 nuclear translocation, and NF-κB-dependent reporter gene expression. The inhibition of NF-κB activation correlated with suppression of NF-κB-dependent cyclin D1, COX-2, and MMP-9 expression [208]. UA also inhibited both constitutive and IL-6-inducible STAT3 activation in a dose- and time-dependent manner in multiple myeloma cells. The suppression was mediated through the inhibition of activation of upstream kinases c-Src, JAK-1, JAK-2, and ERK1/2. Ursolic acid down-regulated the expression of STAT3-regulated gene products such as cyclin D1, Bcl-2, Bcl-xL, survivin, Mcl-1, and vascular endothelial growth factor. Finally, ursolic acid inhibited proliferation and induced apoptosis and the accumulation of cells in G1-G0 phase of cell cycle. This triterpenoid also significantly potentiated the apoptotic effects of thalidomide and bortezomib in multiple myeloma cells [209].
106. Operculina turpethum
O. turpethum (Family: Convolvulaceae), commonly known as trivrit or nishot in the western part of India and adjoining Pakistan, is a plant with immense ethno-medicinal value. O. turpethum extract is used to treat wide range of ailments. For instance, it is used to relieve periodic fevers, constipation, flatulence and colic obesity, to treat anaemia, splenomegaly, raised lipid levels and obesity. Turpethinic acids (A, B, C, D, and E) are isolated from the resin of the plant and lupeol, betulin, and β-sitosterol are isolated from the stem. Recently, their structurally related compounds like lupeol, betulin and sitosterol have been identified to posses a variety of pharmacological activities such as hepatoprotective, anticancer, and anti-inflammatory effects [210].
107. Orchis mascula
O. mascula has bean proved to have antihypertensive, antidyslipidemic and endothelial modulating activities [211]. These effects are mediated through multiple pathways that include direct vasodilation by calcium channel blockade and reduction of plasma lipids by inhibition of biosynthesis, absorption and secretion.
108. Oroxylum indicum
In Indian traditional medicine, the roots as well as stem bark of Oroxylum indicum (Family: Bignoniaceae), commonly known as ‘Syonaka’, has been used for centuries for the treatment of various gastric disorders. Oroxylum indicum used in Bangladeshi folk medicine has bean studied for its anticancer potential. Baicalein from O. indicum showed anti-cancer potential in leukemia cells through inducing cell cycle arrest and apoptosis [212].
109. Pandanus tectorius
Pandanus tectorius is a species of Pandanus (screwpine) that is native to Malesia, eastern Australia, and the Pacific Islands. The fruit can be eaten raw or cooked and is a major source of food in Micronesia, especially in the atolls. In Kiribati, pandanus leaves are used in treatments for cold/flu, hepatitis, dysuria, asthma, boils, and cancer, while the roots are used in a decoction to treat hemorrhoids. In Hawai’i the main parts used in making traditional medicines are the fruits, male flowers, and aerial roots [213]. These are used individually or in combination with other ingredients to treat a wide range of illnesses, including digestive and respiratory disorders.
110. Phyllanthus amarus
Phyllanthus amarus is traditionally used to treat flu, dropsy, diabetes, and jaundice, but it has also been reported to inhibit hepatocellular carcinoma development. P. amarus has potent free radical scavenging activity and could scavenge superoxides and hydroxyl radicals and inhibit lipid peroxides. Moreover, Kassuya et al., have shown that the extract from P. amarus or some of the purified lignans such as phyltetralin, nirtetralin and niranthin exhibit in vivo and in vitro anti-inflammatory properties. These anti-inflammatory properties are probably mediated through its direct ability to interact with platelet activating factor receptor binding sites [214].
111. Phyllanthus niruri
Phyllanthus niruri is a widespread tropical plant commonly found in coastal areas, including South East Asia, Southern India and China. Extracts of this herb have shown promise in treating a wide range of human diseases, such as dysentery, influenza, vaginitis, tumours, diabetes, diuretics, jaundice, kidney stones and dyspepsia. The plant is also useful for treating hepatotoxicity, hepatitis B, hyperglycaemia and viral and bacterial diseases. P. niruri has been used in Ayurvedic medicine for over 2000 years.
112. Picrorhiza kurroa
The extracts from roots and rhizomes of this plant (commonly called as katuka, kutki, or kutaki) are used to treat a variety of ailments, including fever, hepatitis, allergies, asthma, and other inflammatory diseases. Picroliv, an iridoid glycoside derived from P. kurroa, interfered the activation of NF-κB signal cascade. Picroliv abrogated TNF-induced activation of NF-κB thorough inhibition of IκB kinase, leading to inhibition of phosphorylation and degradation of IκBα. It also inhibited phosphorylation and nuclear translocation of p65. Further, picroliv directly inhibits the binding of p65 to DNA, which was reversed by the treatment with reducing agents, suggesting a role for a cysteine residue in interaction with picroliv. Mutation of Cys(38) in p65 to serine abolished this effect of picroliv. NF-κB inhibition by picroliv leads to suppression of NF-κB-regulated proteins, including those linked with cell survival (inhibitor of apoptosis protein 1, Bcl-2, Bcl-xL, survivin, and TNF receptor-associated factor 2), proliferation (cyclin D1 and cyclooxygenase-2), angiogenesis (vascular endothelial growth factor), and invasion (intercellular adhesion molecule-1 and matrix metalloproteinase-9). Suppression of these proteins enhanced apoptosis induced by TNF [215].
113. Pinus roxburghii
Pinus roxburghii (Chir Pine) named after William Roxburgh, is a pine native to the Himalaya. The turpentine obtained from the resin is antiseptic, diuretic, rubefacient and vermifuge. It is a valuable remedy used internally in the treatment of kidney and bladder complaints and is used both internally and as a rub and steam bath in the treatment of rheumatic affections. It is also very beneficial to the respiratory system and so is useful in treating diseases of the mucous membranes and respiratory complaints such as coughs, colds, influenza and TB. Externally it is a very beneficial treatment for a variety of skin complaints, wounds, sores, burns, boils etc and is used in the form of liniment plasters, poultices, herbal steam baths and inhalers. The wood is diaphoretic and stimulant. It is useful in treating burning of the body, cough, fainting and ulcers.
114. Piper chaba
The plant Piper chaba is a climbing, glabrous shrub available in various parts of India and Malay Islands. Furthermore, P. chaba is commonly used as pepper in the southern part of Bangladesh. Various parts of this plant have been extensively used in different traditional formulations including ayurveda. The root is useful against asthma, bronchitis, and consumption. The fruit is thermogenic, anthelmintic, expectorant, carminative and improves appetite and taste and is also used against asthma, bronchitis, fever, inflammation, piles, pain in the abdomen and at the anus. The fruit has stimulant and carminative properties, and is used in haemorrhoidal affections. Stem is used to alley post-delivery pain in mothers and also useful in rheumatic pains and diarrhea.
115. Piper longum
Piper longum (Long pepper) is a flowering vine in the family Piperaceae, cultivated for its fruit, which is usually dried and used as a spice and seasoning. The fruits contain the alkaloid piperine. P. longum is a component of Indian traditional medicine reported to be used as a remedy for treating gonorrhea, menstrual pain, tuberculosis, sleeping problems, respiratory tract, infection, chronic gut-related pain and arthritic conditions. Piper extracts and piperine possess inhibitory activities on prostaglandin and leukotrienes COX-l inhibitory effect, as well as on NF-κB activation, and thus exhibit anti-inflammatory activity [216, 217].
116. Piper nigrum
Black pepper (Piper nigrum) is commonly used as a spice in human diets, but it is also used as a medicine, a preservative, and a perfume in many Asian countries. An extract of the active phenolic component, piperine, is well known to provide beneficial physiological effects. It stimulates the digestive enzymes of pancreas, protects against oxidative damage, lowers lipid peroxidation, and enhances the bioavailability of a number of therapeutic drugs. In addition, its anti-inflammatory activities have been demonstrated in rat models of carrageenan- induced rat paw edema, cotton pellet-induced granuloma, and a croton oil-induced granuloma pouch. Constituents of the piper species have shown in vitro inhibitory activity against the enzymes responsible for leukotriene and prostaglandin biosynthesis, 5-lipoxygenase and COX-1, respectively [217]. These effects of piperine seem to be beneficial for inflammatory diseases that are accompanied by severe pain; for example, rheumatoid arthritis.
117. Pistacia integerrima
Pistacia integerrima (Anacardiaceae) is a moderate size deciduous tree with a short stout bole widely distributed in the sub-alpine regions of Himalaya ranging from: Indus to Kumaun. The plant has been used in traditional medicine for rheumatic pain, analgesic and antipyretic effects, and analgesic and anti-inflammatory activities [218].
118. Pluchea lanceolata
Pluchea lanceolata is widely used for rheumatism and allied disorders, diseases of the abdomen, dyspepsia, bronchitis and inflammation. The decoction of P. lanceolata has been used traditionally for the treatment of arthritis. Flavanols isolated from this plant, such as quercetin and rutin are known for their antihistamine, anti-inflammatory and antiviral activities. Quercetin has been shown to mediate down-regulation of mutant p53 in human breast cancer cell lines [219] and to inhibit chemically induced carcinogenesis.
119. Plumbago zeylanica
The root of Plumbago zeylanica (also called Chitrak), a major source of plumbagin, has been used in the Indian medicine since the period of Charaka, from 750 BC, as an antiatherogenic, cardiotonic, hepatoprotective, and neuroprotective agent [220]. Plumbagin has been shown to exert anticancer and antiproliferative activities in animal models as well as in cells in culture. Sandur et al., suggest that plumbagin may be effective against cancer not only by suppressing invasion but also by inhibiting angiogenesis and inflammation through inhibition of the NF-κB signaling pathway [221]. Recently, plumbagin shown that it inhibited both constitutive and interleukin 6-inducible STAT3 phosphorylation in human multiple myeloma cells and this correlated with the inhibition of c-Src, Janus-activated kinase (JAK)1, and JAK2 activation. Thus, the inhibition of STAT3 singling pathway by plumbagin leading the chemosensitization of cancer cells [222].
120. Polygonatum verticillatum
P. verticillatum is a perennial rhizomatous herb with an extensive range through the northern Hemisiphere from Europe to the Himalayas to Siberia. The syrup of the fresh rhizome of this plant is used in the treatment of pain, pyrexia, burning sensation and for phthisis. Other ethnobotanical uses of the plant include as emollient, aphrodisiac, vitiated condition of pitta and vata, appetizer and tonic, galactagogue (increases milk release) and weakness. Recently, this plant also reported its antinociceptive activity [223].
121. Pongamia pinnata
Pongamia pinnata is a medium sized glabrous tree, found throughout India and further distributed eastwards, mainly in the littoral regions of South Eastern Asia and Australia. The seed and seed oil of this plant have been used for treating various inflammatory and infectious diseases such as leucoderma, leprosy, lumbago, muscular and articular rheumatism. The leaves are hot, digestive, laxative, anthelmintic and cure piles, wounds and other inflammations [224].
122. Prunus amygdalus
Prunus amygdalus is a species of tree native to the Middle East. Claimed health benefits of this plant include improved complexion, improved movement of food through the colon (feces) and the prevention of cancer. In Ayurveda, P.amygdalus is considered a nutritive for the brain and nervous system. Recent studies have shown that the constituents of this tree have anti-inflammatory, immunity boosting, and anti-hepatotoxicity effects [225].
123. Pseudarthria viscida
In Ayurvedic medicines, this plant is namely, ‘Dashamoola’ ‘Mahanarayana Taila’ and ‘Dhantara Taila’. It is used to treat vitiated conditions of pita and vata, asthma, tuberculosis, helminthiasis, dyspepsia, diarrheas, neurasthenia, diabetes, cardiopathy, hyperthermia and general debility and as a nasal drop in headache. The ethanol-induced gastric ulceration in mice was significantly reduced by oral administration of P. viscida extract [226].
124. Psoralea corylifolia
The seeds of Psoralea corylifolia (Babchi) contain a variety of coumarins including psoralen with medicinal uses. Psoralen plus UVA (PUVA) is used as a very effective treatment modality for various diseases, including psoriasis, eczema, vitiligo and cutaneous T-cell lymphoma. PUVA-induced immune suppression and/or apoptosis are proposed to be responsible for the therapeutic potential [227].
125. Pterocarpus marsupium
Parts of this plant (heart wood, leaves, flowers) have long been used for their medicinal properties in Ayurveda. The heartwood is used as an astringent and in the treatment of inflammation and diabetes. A multicentric study has shown that a preparation from the plant is effective in reducing levels of blood glucose and glycosylated haemoglobin in patients with non-insulin-dependent diabetes mellitus. Extract from the plant has been reported to selectively inhibit COX-2 [228].
126. Pterocarpus santalinus
It is indigenous to the Indian Peninsula and is chiefly of importance from its yielding the red dye-wood known as red saunders. The heartwood obtained from the plant has various medicinal properties, as a cooling agent, antipyretic, anti-inflammatory, anthelmintic, tonic, hemorrhage, dysentery, aphrodisiac, to treat eye diseases, mental aberrations and ulcers. In a recent study, ethanolic extract obtained from the bark of plant was found to decrease hyperglycemia by increasing glycolysis and decreasing gluconeogenesis in streptozotocin-induced diabetic rats [229].
127. Pueraria tuberosa
It is a perennial climber, growing throughout tropical parts of India. In the Ayurvedic system of medicine, the plant is used as a drug of choice to manage pain, inflammation and other related diseases. The Chayawanprash, one of the popular ayurvedic formulations with powerful anti-oxidant potential contains the plant extract as active component.
128. Punica granatum (Pomegranate)
The tree represents a phytochemical reservoir of heuristic medicinal value. The seed, juice, peel, leaf, flower, bark, and roots of the plant have pharmacologic activity. The juice and peel possess anticancer activities, including interference with tumor cell proliferation, cell cycle, invasion and angiogenesis. Some of the known compounds with anti-cancer and anti-inflammatory activity obtained from the plant include -tocopherol, ursolic acid, punicic acid, hydroxycinnamic acids, quercetin, ellagitannins, flavonols, flavones, apigenin, maslinic acid and asiatic acid. Ellagitannins, and punicalagin from pomegranate have been reported to inhibit cancer cell proliferation and apoptosis through the modulation of NF-κB signaling pathway and suppression of NF-κB-regulated gene expression. Juice (PJ), total pomegranate tannin extract (TPT) and punicalagin from pomegranate significantly suppressed TNFα-induced COX-2 protein expression. Additionally, PJ reduced phosphorylation of the p65 subunit and inhibited NF-κB DNA-binding. TPT and punicalagin, also, suppressed NF-κB DNA-binding, whereas ellagic acid was ineffective. PJ also abolished TNFα-induced AKT activation, needed for NF-κB activity. Therefore, the polyphenolic phytochemicals in the pomegranate can play an important role in the modulation of inflammatory cell signaling in colon cancer cells [230].
129. Putranjiva roxburghii
It is a moderate-sized, evergreen tree, growing up to 12 m in height. The pollen from the plant has been found an important aeroallergen for type I hypersensitivity. The leaf extract from the plant has shown potential as antinociceptive, antipyretic, and anti-inflammatory activities in mice [231].
130. Quercus infectoria
It is a small tree, most abundant in Asia Minor, and extends up to middle Asia. The galls of the plant possess pleiotropic therapeutic activities, with particular efficacy against inflammatory diseases. Oral administration of gall extract from the plant has been reported to significantly inhibit carrageenan, histamine, serotonin and prostaglandin E2 (PGE2) induced paw oedemas, while topical application of gall extract has the ability to inhibit phorbol-12-myristate-13-acetate (PMA) induced ear inflammation in various in vivo and in vitro experimental models [232].
131. Raphanus sativus
It is a cruciferous plant, rich on flavonoids, isothiocyanates, and phenolic acids and has potential as anti-inflammatory and immunomodulatory agent both in vitro and in vivo. The granules from the plant have shown potential to act as anti-inflammatory agent in a high fat diet fed rat model [233].
132. Rauwolfia serpentina
R. serpentina is a tropical woody plant of the Apocyanaceae family ingenious to Asia, South America and Africa. Extracts of different parts of the plant had been used in Hindu medicine for snakebite, insomnia, insanity and many other diseases. It is one of the fundamental herbs used in traditional Chinese medicine. It is also one of the main ingredients in the ayurvedic formulation ‘Divya Mukta Vati’ used to cure high blood pressure. The plant contains a number of bioactive components, including ajmaline, deserpidine, rescinnamine, serpentinine and reserpine. Reserpine, an alkaloid from Rauwolfia serpentina, was widely used for its antihypertensive action and single dose of R. serpentina formulation is effective, showed by Leary et al [234] in a double-blind placebo-controlled investigation. In animal models reserpine has been reported to significantly reduce inflammation [235, 236].
133. Ricinus communis
Commonly known as castor oil plant, it is indigenous to the southeastern Mediterranean Basin, Eastern Africa, and India. The seed from the plant is a rich source of triglycerides (mainly ricinolein) and ricin. The oil obtained from the seed of the plant has been used as a laxative, purgative, and cathartic in Unani, Ayurvedic and other ethnomedical systems. Traditional Ayurvedic medicine considers castor oil the king of medicines for curing arthritic diseases. In a study carried out in guinea-pig eyelid, ricinolein was found to possess both pro-inflammatory and anti-inflammatory properties that were observed upon acute and repeated application of the compound, respectively [237].
134. Rosa centifolia
The plant is particular to the French city of Grasse. It is widely cultivated for its singular fragrance - clear and sweet, with light notes of honey and green earth. The plant is one of the ingredients in ayurvedic formulations ‘Divya udarakalpa curna’, ‘Divya Curna’, ‘Divya peya’, ‘Himalaya abana’. The flowers are commercially harvested for the production of rose oil, which is commonly used in perfumery. In a study, the plant was found effective in decreasing the severity of menstrual cramps in women[238].
135. Rosa damascena
The plant is a rose hybrid, derived from Rosa gallica and Rosa moschata and grows as deciduous shrub. R. damascena has been reported to exert antidiabetic, antiolytic and to use for treating ophthalmic disorders. The roxyloside A (flavonoid) obtained from the plant was shown potential of inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase system and was proposed to be effective in improving the cardiovascular system [239]. The oil from the plant has been quoted extensively in ancient literature as being an anxiolytic treatment.
136. Roscoea alpina
It is a common Himalayan wildflower and occurs most frequently in open woodlands and on rocky slopes. It is one of the main ingredients in the ayurvedic formulation ‘Divya pidantaka taila’ used for relieving joint pain, cervical spondylitis, trauma, oedema and inflammation.
137. Rubia cordifolia
It is a flowering plant belonging to the family Rubiaceae. It is a common medicinal plant used in the preparation of different formulations in Ayurveda. The root of the plant is commonly known as Manjistha and its dried samples are sold in the market under the name Manjith. The roots of the plant are used as anti-inflammatory, haemostatic, antidysentric, antipyretic, analgesic and the anthelmentic agent. It is also used in the cure of leucoderma, ulcers, urinary discharges, jaundice, and piles. Mollugin, one of the active compounds obtained from the plant was recently shown to inhibit TNF--induced expression of inflammatory molecules by inhibiting NF-κB activation in colon cancer cells [240].
138. Rumex maritimus
It is one of the ingredients in the ayurvedic formulation ‘Himalayan Diabecon’ that has potential to lower blood sugar levels and minimizing long-term diabetic complications. Leaves are applied to burns; seeds are tonic and remove pain from the back. The methanol extract from the root of the plant has shown antidiarrhoeal activity in mice [241].
139. Salvadora persica
The plant has been used for centuries as a natural toothbrush and contains a number of medically beneficial properties including abrasives, antiseptics and astringent. The plant has been shown to contain trimethylamine salvadorine, chloride, fluoride, silica, sulphur, vitamin C, resins and traces of tannins, saponins, flavonids and sterol. The plant has also been shown to possess antiulcer activity in an experimental rat model [242].
140. Santalum album
It is a small tropical tree of the Santalaceae family and has been utilised, cultivated and traded for many years, some cultures placing great significance on its fragrant and medicinal qualities. Plant has been the primary source of sandalwood. The wood from the plant has been shown effective against kidney and urinary disorders. The sandalwood oil obtained from the plant has been used as aromatherapy agent to relieve anxiety, stress, and depression [243].
141. Sapindus trifoliatus
A thick aqueous solution of the pericarp from the plant is used for the treatment of hemicrania, hysteria or epilepsy in folklore medicine. The aqueous extract of the plant has shown antihyperalgesic activity by antagonising dopamine D2 activity in mice [244]. The ethanolic extract from the seeds of the plant has shown anti-inflammatory activity in wistar rats [245].
142. Saraca asoca
Saraca asoca (local names: Ashok, Anganapriya, etc.) is a medicinal plant whose bark is astringent and used in menorrhagia, bleeding haemorrhoids, haemorrhagic dysentery, bone fractires, strangury, vesical calculi, hyperdypsia, and inflammation. This plant is known to contain tannins, flavonoids, proanthocyanidins and leucoanthocyanidins in the bark. Two procyanidin dimers from S. asoca have shown the inhibitory effect of prostaglandin H2 (PGH2) synthetase [246].
143. Saraca indica
It is known to be useful for uterine disease, internal piles, diabetes, dyspepsia, indigestion, burning sensation, blood disorders, fractures, tumors, bites, ulcerations, and skin discoloration. The lectin saracin, purified from Saraca indica seed integument, has been found to agglutinate human lymphocytes and erythrocytes [247].
144. Saussurea lappa
S. lappa is considered as antiseptic, astringent, diuretic, aphrodisiac, antispasmodic, antihelmentic, and sedative and are used for the treatment of asthma, dyspepsia, rheumatism, cough, throat infections, tuberculosis, leprosy, malaria, convulsions, fever, helminth infestation and many other diseases. It is also used as a part of preparation for the treatment of various liver disorders. Recently, this plant is reported that it had antiulcer, anti-inflammatory and antiarthritic activates [19, 248].
145. Saxifraga ligulata
This plant is reported to be helpful in dissolving kidney stones and urinary antiseptic in action. In lower doses, the extract is mildly diuretic.
146. Sesamum indicum
The seeds of S. indicum (sesame) are used as a demulcent in respiratory affections, infantile cholera, diarrhea, dysentery and other bowel affections and bladder diseases. The seed powder is known to be benefit in amenorrhea, dysmenorrhea, ulcers and bleeding piles. The active component sesaminol is reported to its antioxidative, neuroprotective effects. Other lignan from sesamne, sesamol, has been offered protection against increased blood pressure, hyperlipidaemia [249]. Recently, Harikumar et al [250] found that sesamin, lignan from S. indicum, inhibited the proliferation of a wide variety of tumor cells including leukemia, multiple myeloma, and cancers of the colon, prostate, breast, pancreas, and lung. Sesamin also potentiated TNF-induced apoptosis and this correlated with the suppression of gene products linked to cell survival (Bcl-2 and survivin), proliferation (cyclin D1), inflammation (COX-2), invasion (MMP-9, ICAM-1), and angiogenesis (VEGF). Sesamin downregulated constitutive and inducible NF-κB activation induced by various inflammatory stimuli and carcinogens, and inhibited the degradation of IκBα, through the suppression of phosphorylation of IκBα and inhibition of activation of IκB kinase, thus resulting in the suppression of p65 phosphorylation and nuclear translocation.
147. Sida cordifolia
It is reported to possess analgesic, anti-inflammatory, anticancer, diuretic, laxative, hypoglycemic and hepatoprotective activities. Further, studies showed that aqueous fraction of hydroalcoholic extract of leaves induce vasorelaxation, hypotension and bradycardia. This plant also used for treatment of Parkinson’s disease and as an anti-rheumatic agent and CNS depressant. The plant alkaloid cryptolepine from S. cordifolia has been reported to induces cell cycle arrest in a human osteosarcoma cell line [251]
148. Solanum indicum (syn. Solanum anguivi)
S. indicum has been used on treatment of hypertension and diabetics. The steroidal glycosides from fruits have been claimed in folk medicine to have an antihypertensive effect [252] and anticancer effect [253].
149. Solanum nigrum
This plant is known as ‘Black nightshade’ that have been extensively used to cure liver disorders, chronic skin ailments (psoriasis and ringworm), inflammatory conditions, painful periods, fevers, diarrhoea, eye diseases, hydrophobia, etc. The plant contains glycoalkaloids (solanine, solamargine, solanigrine and solasodine), steroidal glycosides (β-solamargine, solasonine and α,β-solansodamine), diosgenin, gitogenin, tannin and polyphenolic compounds. The fruits are commonly used as hepatoprotective agents [254], which also afford protection against free radical mediated damage.
150. Solanum xanthocarpum
S. xanthocarpum are known for several medicinal uses like anthelmintic, antipyretic, laxative, antiinflammatory, antiasthmatic and aphrodisiac activities. The stem, flowers and fruits are prescribed for relief in burning sensation in the feet accompanied by vesicular eruptions. The hot aqueous extract of dried fruits is used for treating cough, fever and heart diseases. Results from pilot study with patient having allergy and asthma showed that S. xanthocarpum relived the bronchial asthma by bronchodilating effect, reducing the bronchial mucosal edema, and/or reducing in the secretions within the airway lumen [255].
151. Sphaeranthus indicus
S. indicus, commonly called Gorakhmundi (in Hindi), is plant is known to possess various medicinal properties. It is reported to use in epileptic convulsions, mental illnesses and hemicranias. It is also used to treat vitiated conditions of jaundice, diabetes, leprosy, fever, pectoralgia, cough, gastropathy, hernia, haemorrhoids, helminthiasis, dyspepsia, skin diseases and as a nerve tonic. The oil from the plant root has been used to treat scrofula and as an aphrodisiac, while the external application of the herb paste has been reported to use treatment for pruritus, oedema, arthritis, filariasis, gout and cervical adenopathy [256].
152. Stereospermum suaveolens
Stereospermum suaveolens is a medicinal tree species and various parts of the plants are used by traditional healers, rural communities and pharmaceutical companies as a remedy for vomiting, eructation, piles, acidity, diarrhoea, gonorrhoea, loss of taste, malaria and other fevers. Balasubramanian et al [257] showed that ethanol extract of Stereospermum suaveolens possesses maximum anti-inflammatory activity in various rat models of carrageenan-, dextran-, and histamine-induced hind paw edema, and cotton pellet-induced granuloma formation in a dose-dependent manner.
153. Strychnos nuxvomica
The dried seeds of S. nuxvomica have been claimed to improve blood circulation and relieve rheumatic pain. Historically, this plant has been widely used in treating diseases, such as tumor and rheumatic arthritis. It also has been used in gastro-hepatic disease and as analgesic, stimulant. Indeed, it also used to treatment of paralysis, diabetes, gonorrhea, anemia and bronchitis etc. Brucine and brucine N-oxide from this plant are reported to exert anti-inflammatory effects through reduction of prostaglandin E2 release [258].
154. Swertia chirata
This plant is known as ‘Chirata’, and multifarious therapeutic value. It is used as an antimalarial, a bitter stomachic, anthelmintic, and as a remedy for scanty urine, epilepsy, ulcer, bronchial asthma and certain type of mental disorder. Studies on the biological activities of S. chirata extract reveal that this bitter plant possesses antioxidant, antidiabetic, antimicrobial, anticholinergic and chemopreventive activity. It contains mangiferin along with various secoiridoid glycosides (i.e. amarogentin, amaroswerin, sweroside and swertiamarin). The secoiridoid glycoside, amarogentin, is reported suppressing COX-2 [259]and also possesses various biological activities such as chemopreventive, antibacterial, anticholinergic and antihepatitis activity.
155. Symplocos crataegoides (syn. Symplocos paniculata)
S. crataegoides has been used for treatment of menorrhagia, eye diseases, bowel complaints, dysentery, inflammations, vaginal discharges, leprosy and ulcers, as a gargle for giving firmness to spongy and bleeding gums. And its bark juice is also used to sprains and muscular swellings. The ursane-type triterpenes from this plant showed that the inhibitory effect on protein tyrosine phosphatase 1B (PTP1B) has been proposed as a therapy for treatment of type 2 diabetes and obesity [260].
156. Syzygium aromaticum
Traditionally, it has been used to treat respiratory and digestive ailments. Cloves are used as a carminative, to increase hydrochloric acid in the stomach and to improve peristalsis. Cloves are also said to be a natural anthelmintic. The aqueous clove infusion was showed antiseptic and antibiotic properties, clove is used to treat toothache and as an ingredient in a popular toothpaste and mouthwash in India. An anti-herpes virus compound eugenin was purified from clove, which could inhibit viral DNA synthesis. In addition water extracts of clove have shown the inhibitory effect on hepatitis C virus protease. Eugenol, the principle component of clove has been shown to oxygen radical scavenging activity and antitumor potentials targeting COX-2, cMyc, H-ras [261]
157. Syzygium cumini
The bark of the plant is astringent to the bowels, sweet, refrigerant, carminative, diuretic, digestive, antihelminthic, febrifuge, constipating, stomachic and antibacterial. The fruits and seeds are used to treat diabetes, pharyngitis, spleenopathy, urethrorrhea and ringworm infection. The leaves have been extensively used to treat diabetes, constipation, leucorrhoea, stomachalgia, fever, gastropathy, strangury, and dermopathy and to inhibit blood discharges in the faeces. This plant has been also reported to poses acetyl oleanolic acid, triterpenoids, ellagic acid, isoquercitin, quercetin, kaempferol and myricetin in different concentrations. Recently, S. cumini also shown that the protective effect against radiation-induced DNA damage in human peripheral blood lymphocytes [262].
158. Tamarix gallica
This plant is commonly kwon as “Jhau”, s shrub of the family Tamaricaceae. The fruits and leaves have been employed in traditional medicine as an astringent for dysentery and chronic diarrhea, aperitif, stimulant of perspiration, diuretic, active against leucoderma, spleen trouble and eye diseases. Indeed, methanolic extract of this halophyte was investigated for its anti-carcinogenic and chemopreventive activities by evaluating the levels of hepatic antioxidant defense [263].
159. Terminalia arjuna
Arjuna bark (Terminalia arjuna) is a medicinal plant of the genus Terminalia, widely used in functional heart problems including angina, hypertension and deposits in arteries. It has been also useful in treatment of angina pectoris, heart failure, coronary artery disease and hypercholesterolemia. In vivo study showed its anti-inflammatory, immunomodulatory and antinociceptive activity in mice and rats [264]. In clinical study, terminalia had shown decreases platelet activation which ultimate leads in antithrombotic properties [265].
160. Terminalia belerica
Terminalia belerica has been used for its lowering serum glucose level and antioxidant activity by reducing lipid peroxidation, scavenge hydroxyl radical and superoxide radicals. This plant has been used for treatment of digestive and liver disorders. It can significantly reduce the total cholesterol, low density lipoprotein (LDL), very low density lipoprotein (VLDL), high density lipoprotein (HDL) and free fatty acid in experimentally induced hypercholesteremic rats [180, 266].
161. Terminalia chebula Retz
Terminalia chebula Retz. (Combretaceae) have been known from ancient times and were described by Charaka in his text “Charaka Samhita”. It contains the active ingredient chebulagic acid. Aqueous extract of Terminalia chebula had shown numbers of activity such as digestive, allergic and infectious diseases like cough and skin disorders, antimicrobial activity anti-diabetic and antioxidant properties [267]. It also reported to have strong anti-anaphylactic actions, anti-inflammatory and analgesic properties [268].
162. Thymus vulgaris
Thymus vulgaris (Lamiaceae) is aromatic plants, which is distributed in subtropical countries. It contains acetophenone glycosides, phenols, thymol (40%) and carvacrol (15%). It is mainly used in smooth muscle relaxing effect, asthma, bronchitis and other respiratory diseases [269]. Thymol significantly reduced the level of DNA damage induced in K562 cells by the strong oxidant H2O2 [270].
163. Tinospora cordifolia
Tinospora cordifolia mainly contain different type of aporphine alkaloids and clerodane diterpenes. It is also used as an adjuvant for the prevention and/or management of insulin resistance and disorders related to it. Epoxy clerodane diterpene which obtained from it, also used against diethylnitrosamine-induced hepatocellular carcinoma [271]. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting matrix metalloproteinases and nuclear translocation of NF-κB and its DNA binding activity [272]. This plant is also used as antidiabetic in streptozotocin-induced diabetes mellitus mouse model [273].
164. Trachyspermum ammi
T. ammi is used for relieving kidney stone pains and urolithiasis. So far, its diuretic properties have been reported widely in literature and it is actively used in various drug formulations of kidney stone treatments. Trachyspermum ammi shown prevention in DMBA- and B(a)-P induced skin and forestomach papillomagenesis [274].
165. Tribulus terrestris
Tribulus terrestris had shown chemopreventive effect against 7,12- dimethylbenz (a) anthracene induced skin papillomagenesis in mice by oral gavage for 7 days [275]. It also showed protective effects in diabetes mellitus [276]. In vivo study suggested that methanolic and aqueous extracts of T. terrestris having antihypertensive and vasodilator effects [277].
166. Trigonella foenum-graecum
Diosgenin, a steroidal saponin present in fenugreek (Trigonella foenum graecum) and other plants, has been shown to suppress inflammation, inhibit proliferation, and induce apoptosis in a variety of tumor cells. It down-regulate TNF-induced expression of NF-κB-regulated gene products involved in cell proliferation (cyclin D1, COX-2, c-myc), antiapoptosis (IAP1, Bcl-2, Bcl-xL, Bfl-1/A1, TRAF1 and cFLIP), and invasion (MMP-9) [278]. It also inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells [279]. 4-hydroxyisoleucine, an unusual amino acid isolated from Trigonella foenum-graecum seeds was characterized in type II diabetes [280].
167. Uraria lagopoides
It exhibits as antiviral compound and completely inhibits the growth of Reo virus [281]. Alcohol and aqueous extract of aerial parts of Uraria lagopoides showed anti-inflammatory activity in the rat paw edema test.
168. Valeriana officinalis
Valeriana officinalis (Valerianaceae) has been used for treating mild nervous tension and temporary sleeping problems. In traditional European medicine it has been also reported as an antiinflammatory remedy. Ethanolic extract of the underground parts of V. officinalis showed inhibitory activity against NF-κB on HeLa cells [282].
169. Valeriana wallichii
V. wallichii shown antidepressant effect, and used in gastrointestinal and cardiovascular disorders, as antispasmodic and hypotensive. This action is through K(ATP) channel activation [283]. It is also used in treating anxiety, tremors, inflammations of the joints, chorea, neurosis, and dysmenorrheal in Indian ayurveda.
170. Vanda roxburghii
Extract of V. roxburghii has wound-healing potential in rats. It augments the uterine contractions and is a bronchodilator, digestant and blood purifier. It is used in diseases like gout, rheumatic disorders, asthma, abdominal pain, fever and edema [284].
171. Vernonia cinerea
V. cinerea exhibits anti-cancer and anti-helmintic, anti-diuretic, anti-inflammatory, analgesic, anti-pyretic and anti-bacterial activities. Treatment of V. cinerea methanolic extract also showed an anti-inflammatory effect though enhancing phagocytic activity of peritoneal macrophages. Moreover the extract downregulated inflammatory mediators such as iNOS and COX-2, and decreased secretion of TNFa, IL-1β and IL-6 in LPS-treated macrophages [285].
172. Viola odorata
The leaf of this plant is used for treatment of tumor. Cyclotide from V. odorata showed cytotoxic effects against various drug-resistant tumor cells [286].
173. Vitex negundo
This plant showed anti-asthma, expectorant, antisepsis, hypoxia tolerance enhancement and also shown to could decrease blood glucose level. Vitexins have cytotoxic effect on various types of cancer cell lines and also have antitumor activity on tumor xenograft models including breast, prostate, liver, and cervical cancers [287].
174. Withania somnifera
The plant Withania somnifera Dunal (Ashwagandha), also known as Indian ginseng, is widely used in the Ayurvedic system of medicine to treat tumors, inflammation, arthritis, asthma, and hypertension. Chemical investigation of the roots and leaves of this plant has yielded bioactive withanolides. Withanolides suppressed NF-κB activation induced by a variety of inflammatory and carcinogenic agents; including TNF-α, IL-1β, doxorubicin, and cigarette smoke condensate. It also suppressed both inducible and constitutive NF-κB activation. The suppression occurred through the inhibition of inhibitory subunit of IκBα kinase activation, IκBα phosphorylation, IκBα degradation, p65 phosphorylation, and subsequent p65 nuclear translocation. Consequently, withanolide suppressed the expression of TNF-induced NF-κB-regulated gene products such as IAP-1, Bfl-1/A1, and FADD-like IL-1β-converting enzyme-inhibitory protein, COX-2 and ICAM-1, enhanced the apoptosis induced by TNF and chemotherapeutic agents, and suppressed cellular TNF-induced invasion and receptor activator of NF-κB ligand-induced osteoclastogenesis [288]. Withanolide sulfoxide is another active compound of this plant inhibits COX-2 expression [289]. Withaferin-A (WA) is a bioactive compound derived from W. somnifera, which showed ant-tumor activity through inhibition of Notch-1 signaling and down regulates prosurvival pathways, such as Akt/NF-κB/Bcl-2 [290].
175. Zingiber officinale
Traditionally, ginger has been used to treat a wide range of ailments including gastrointestinal disorders, such as stomachaches, abdominal spasm, nausea, and vomiting, as well as in arthritis and motion sickness. Phytochemical studies showed that the plant is rich in a large number of substances, including gingerols and shogaols. These compounds display diverse biological activities such as antioxidant, anti-inflammatory, and anticarcinogenic properties. They also exhibit a spasmolytic activity, which is mediated via blocking Ca2+ channels. A number of recent studies have renewed interest in ginger for the treatment of chronic inflammatory conditions [291].
Conclusions
Overall from this description, it is clear that reverse pharmacology approach to examine the plants for drug development is a viable approach. To fully validate this approach, further clinical trials are needed to examine their potential. It is anticipated that this approach will not be as expensive as currently used and the compounds/drugs isolated will be safe. Also one must question why using a single chemical compound is preferred as a drug as compare to extracts from the whole plants. Benefits of a single chemical entity may be in convenience to understand its molecular mechanism. However, it may not be beneficial to the patient when examined, in part, due to the possibility of development of resistance to a single chemical entity. It is possible that when whole plant extract or combination of plant extracts are used, it may exhibit improved bioavailability and lower toxicity, as compared to single chemical entity.
References
- 1.Valiathan MS. Legacy of Charaka. Chennai, India: Orient Longman; 2003. [Google Scholar]
- 2.Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review) J Nutr Biochem. 2003;14:64–73. doi: 10.1016/s0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 3.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson DE, Hurst RD. Polyphenolic phytochemicals--just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–16. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 6.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 7.Vaidya A. Reverse pharmacological correlates of ayurvedic drug actions. Indian J Pharmacol. 2006;38:311–5. [Google Scholar]
- 8.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Ahn KS, Sethi G, Aggarwal BB. Nuclear factor-kappa B: from clone to clinic. Curr Mol Med. 2007;7:619–37. doi: 10.2174/156652407782564363. [DOI] [PubMed] [Google Scholar]
- 10.Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–70. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 11.Singh RK, Pandey BL, Tripathi M, Pandey VB. Anti-inflammatory effect of (+)-pinitol. Fitoterapia. 2001;72:168–70. doi: 10.1016/s0367-326x(00)00267-7. [DOI] [PubMed] [Google Scholar]
- 12.Sethi G, Ahn KS, Sung B, Aggarwal BB. Pinitol targets nuclear factor-kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol Cancer Ther. 2008;7:1604–14. doi: 10.1158/1535-7163.MCT-07-2424. [DOI] [PubMed] [Google Scholar]
- 13.Wang JP, Hsu MF, Chang LC, Kuo JS, Kuo SC. Inhibition of plasma extravasation by abruquinone A, a natural isoflavanquinone isolated from Abrus precatorius. Eur J Pharmacol. 1995;273:73–81. doi: 10.1016/0014-2999(94)00673-u. [DOI] [PubMed] [Google Scholar]
- 14.Krisanapun C, Peungvicha P, Temsiririrkkul R, Wongkrajang Y. Aqueous extract of Abutilon indicum Sweet inhibits glucose absorption and stimulates insulin secretion in rodents. Nutr Res. 2009;29:579–87. doi: 10.1016/j.nutres.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Wadood A, Wadood N, Shah SA. Effects of Acacia arabica and Caralluma edulis on blood glucose levels of normal and alloxan diabetic rabbits. J Pak Med Assoc. 1989;39:208–12. [PubMed] [Google Scholar]
- 16.Altavilla D, Squadrito F, Bitto A, Polito F, Burnett BP, Di Stefano V, et al. Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophages. Br J Pharmacol. 2009;157:1410–8. doi: 10.1111/j.1476-5381.2009.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin AS, Lin CR, Du YC, Lubken T, Chiang MY, Chen IH, et al. Acasiane A and B and farnesirane A and B, diterpene derivatives from the roots of Acacia farnesiana. Planta Med. 2009;75:256–61. doi: 10.1055/s-0028-1112201. [DOI] [PubMed] [Google Scholar]
- 18.Tozyo T, Yoshimura Y, Sakurai K, Uchida N, Takeda Y, Nakai H, et al. Novel antitumor sesquiterpenoids in Achillea millefolium. Chem Pharm Bull (Tokyo) 1994;42:1096–100. doi: 10.1248/cpb.42.1096. [DOI] [PubMed] [Google Scholar]
- 19.Gokhale AB, Damre AS, Kulkami KR, Saraf MN. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine. 2002;9:433–7. doi: 10.1078/09447110260571689. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty A, Brantner A, Mukainaka T, Nobukuni Y, Kuchide M, Konoshima T, et al. Cancer chemopreventive activity of Achyranthes aspera leaves on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2002;177:1–5. doi: 10.1016/s0304-3835(01)00766-2. [DOI] [PubMed] [Google Scholar]
- 21.Wu HS, Zhu DF, Zhou CX, Feng CR, Lou YJ, Yang B, et al. Insulin sensitizing activity of ethyl acetate fraction of Acorus calamus L. in vitro and in vivo. J Ethnopharmacol. 2009;123:288–92. doi: 10.1016/j.jep.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Han TH, Lee SG. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J Ethnopharmacol. 2009;122:149–56. doi: 10.1016/j.jep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Hazra R, Ray K, Guha D. Inhibitory role of Acorus calamus in ferric chloride-induced epileptogenesis in rat. Hum Exp Toxicol. 2007;26:947–53. doi: 10.1177/0960327107087791. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs BF. Differential modulation of IgE-dependent activation of human basophils by ambroxol and related secretolytic analogues. Int J Immunopathol Pharmacol. 2009;22:919–27. doi: 10.1177/039463200902200407. [DOI] [PubMed] [Google Scholar]
- 25.Nicolis E, Lampronti I, Dechecchi MC, Borgatti M, Tamanini A, Bezzerri V, et al. Modulation of expression of IL-8 gene in bronchial epithelial cells by 5-methoxypsoralen. Int Immunopharmacol. 2009;9:1411–22. doi: 10.1016/j.intimp.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Panda S, Kar A. Periplogenin-3-O- -D-glucopyranosyl -(1-->6)- -D-glucopyaranosyl- -(1-->4) -D-cymaropyranoside, isolated from Aegle marmelos protects doxorubicin induced cardiovascular problems and hepatotoxicity in rats. Cardiovasc Ther. 2009;27:108–16. doi: 10.1111/j.1755-5922.2009.00078.x. [DOI] [PubMed] [Google Scholar]
- 27.Subramaniam D, Giridharan P, Murmu N, Shankaranarayanan NP, May R, Houchen CW, et al. Activation of apoptosis by 1-hydroxy-5,7-dimethoxy-2-naphthalene-carboxaldehyde, a novel compound from Aegle marmelos. Cancer Res. 2008;68:8573–81. doi: 10.1158/0008-5472.CAN-08-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–15. doi: 10.1002/cyto.10133. [DOI] [PubMed] [Google Scholar]
- 29.Zare A, Farzaneh P, Pourpak Z, Zahedi F, Moin M, Shahabi S, et al. Purified aged garlic extract modulates allergic airway inflammation in BALB/c mice. Iran J Allergy Asthma Immunol. 2008;7:133–41. [PubMed] [Google Scholar]
- 30.Hui C, Like W, Yan F, Tian X, Qiuyan W, Lifeng H. S-allyl-L-cysteine sulfoxide inhibits tumor necrosis factor-alpha induced monocyte adhesion and intercellular cell adhesion molecule-1 expression in human umbilical vein endothelial cells. Anat Rec (Hoboken) 293:421–30. doi: 10.1002/ar.21070. [DOI] [PubMed] [Google Scholar]
- 31.Ban JO, Oh JH, Kim TM, Kim DJ, Jeong HS, Han SB, et al. Anti-inflammatory and arthritic effects of thiacremonone, a novel sulfur compound isolated from garlic via inhibition of NF-kappaB. Arthritis Res Ther. 2009;11:R145. doi: 10.1186/ar2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keophiphath M, Priem F, Jacquemond-Collet I, Clement K, Lacasa D. 1,2-vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J Nutr. 2009;139:2055–60. doi: 10.3945/jn.109.105452. [DOI] [PubMed] [Google Scholar]
- 33.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol. 1998;59:179–86. doi: 10.1016/s0378-8741(97)00112-8. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Dhawan S, Aggarwal BB. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. 1998;17:913–8. doi: 10.1038/sj.onc.1201998. [DOI] [PubMed] [Google Scholar]
- 35.Grzanna R, Phan P, Polotsky A, Lindmark L, Frondoza CG. Ginger extract inhibits beta-amyloid peptide-induced cytokine and chemokine expression in cultured THP-1 monocytes. J Altern Complement Med. 2004;10:1009–13. doi: 10.1089/acm.2004.10.1009. [DOI] [PubMed] [Google Scholar]
- 36.Ichikawa H, Takada Y, Murakami A, Aggarwal BB. Identification of a novel blocker of I kappa B alpha kinase that enhances cellular apoptosis and inhibits cellular invasion through suppression of NF-kappa B-regulated gene products. J Immunol. 2005;174:7383–92. doi: 10.4049/jimmunol.174.11.7383. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa H, Murakami A, Aggarwal BB. 1′-Acetoxychavicol acetate inhibits RANKL-induced osteoclastic differentiation of RAW 264. 7 monocytic cells by suppressing nuclear factor-kappaB activation. Mol Cancer Res. 2006;4:275–81. doi: 10.1158/1541-7786.MCR-05-0227. [DOI] [PubMed] [Google Scholar]
- 38.Bendjeddou D, Lalaoui K, Satta D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J Ethnopharmacol. 2003;88:155–60. doi: 10.1016/s0378-8741(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 39.Chao WW, Kuo YH, Lin BF. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappaB transactivation inhibition. J Agric Food Chem. 58:2505–12. doi: 10.1021/jf903629j. [DOI] [PubMed] [Google Scholar]
- 40.Wibudi A, Kiranadi B, Manalu W, winarto A, Suyono S. The traditional plant, Andrographis paniculata (Sambiloto), exhibits insulin-releasing actions in vitro. Acta Med Indones. 2008;40:63–8. [PubMed] [Google Scholar]
- 41.Inokuchi J, Okabe H, Yamauchi T, Nagamatsu A, Nonaka G, Nishioka I. Antihypertensive substance in seeds of Areca catechu L. Life Sci. 1986;38:1375–82. doi: 10.1016/0024-3205(86)90470-4. [DOI] [PubMed] [Google Scholar]
- 42.Mathews JN, Flatt PR, Abdel-Wahab YH. Asparagus adscendens (Shweta musali) stimulates insulin secretion, insulin action and inhibits starch digestion. Br J Nutr. 2006;95:576–81. doi: 10.1079/bjn20051650. [DOI] [PubMed] [Google Scholar]
- 43.Visavadiya NP, Narasimhacharya AV. Asparagus root regulates cholesterol metabolism and improves antioxidant status in hypercholesteremic rats. Evid Based Complement Alternat Med. 2009;6:219–26. doi: 10.1093/ecam/nem091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thoh M, Kumar P, Nagarajaram HA, Manna SK. Azadirachtin interacts with the tumor necrosis factor (TNF) binding domain of its receptors and inhibits TNF-induced biological responses. J Biol Chem. 285:5888–95. doi: 10.1074/jbc.M109.065847. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Harish Kumar G, Vidya Priyadarsini R, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9263-3. [DOI] [PubMed] [Google Scholar]
- 46.Mathew J, Paul J, Nandhu MS, Paulose CS. Increased excitability and metabolism in pilocarpine induced epileptic rats: Effect of Bacopa monnieri. Fitoterapia. doi: 10.1016/j.fitote.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Sharath R, Harish BG, Krishna V, Sathyanarayana BN, Swamy HM. Wound healing and protease inhibition activity of Bacoside-A, isolated from Bacopa monnieri wettest. Phytother Res. doi: 10.1002/ptr.3115. [DOI] [PubMed] [Google Scholar]
- 48.Muniappan M, Sundararaj T. Antiinflammatory and antiulcer activities of Bambusa arundinacea. J Ethnopharmacol. 2003;88:161–7. doi: 10.1016/s0378-8741(03)00183-1. [DOI] [PubMed] [Google Scholar]
- 49.Frankish N, de Sousa Menezes F, Mills C, Sheridan H. Enhancement of Insulin Release from the beta-Cell Line INS-1 by an Ethanolic Extract of Bauhinia variegata and Its Major Constituent Roseoside. Planta Med. doi: 10.1055/s-0029-1240868. [DOI] [PubMed] [Google Scholar]
- 50.Rajkapoor B, Jayakar B, Murugesh N, Sakthisekaran D. Chemoprevention and cytotoxic effect of Bauhinia variegata against N-nitrosodiethylamine induced liver tumors and human cancer cell lines. J Ethnopharmacol. 2006;104:407–9. doi: 10.1016/j.jep.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 51.Mohan M, Sekhar GC, Mahajan VM. Berberine: an indigenous drug in experimental herpetic uveitis. Indian J Ophthalmol. 1983;31:65–8. [PubMed] [Google Scholar]
- 52.Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370–9. doi: 10.1158/0008-5472.CAN-08-0511. [DOI] [PubMed] [Google Scholar]
- 53.Kirtikar KRa, Basu BD. Boerhaavia diffusa. Indian Medicinal Plants. Allahabad, India: Lalit Mohan Basu Publications; 1956. [Google Scholar]
- 54.Leslie Taylor ND. Boerhaavia diffusa. The Healing Power of Rainforest Herbs. New York, USA: Square one Publishers; 2005. [Google Scholar]
- 55.Manu KA, Kuttan G. Effect of Punarnavine, an alkaloid from Boerhaavia diffusa, on cell-mediated immune responses and TIMP-1 in B16F-10 metastatic melanoma-bearing mice. Immunopharmacol Immunotoxicol. 2007;29:569–86. doi: 10.1080/08923970701692676. [DOI] [PubMed] [Google Scholar]
- 56.Gupta I, Parihar A, Malhotra P, Singh GB, Ludtke R, Safayhi H, et al. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur J Med Res. 1997;2:37–43. [PubMed] [Google Scholar]
- 57.Ammon HP. Salai Guggal - Boswellia serrata: from a herbal medicine to a non-redox inhibitor of leukotriene biosynthesis. Eur J Med Res. 1996;1:369–70. [PubMed] [Google Scholar]
- 58.Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995;47:1212–6. [PubMed] [Google Scholar]
- 59.Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HP, Safayhi H. Acetyl-11-keto-beta-boswellic acid (AKBA): structure requirements for binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol. 1996;117:615–8. doi: 10.1111/j.1476-5381.1996.tb15235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sailer ER, Schweizer S, Boden SE, Ammon HP, Safayhi H. Characterization of an acetyl-11-keto-beta-boswellic acid and arachidonate-binding regulatory site of 5-lipoxygenase using photoaffinity labeling. Eur J Biochem. 1998;256:364–8. doi: 10.1046/j.1432-1327.1998.2560364.x. [DOI] [PubMed] [Google Scholar]
- 61.Safayhi H, Rall B, Sailer ER, Ammon HP. Inhibition by boswellic acids of human leukocyte elastase. J Pharmacol Exp Ther. 1997;281:460–3. [PubMed] [Google Scholar]
- 62.Syrovets T, Buchele B, Gedig E, Slupsky JR, Simmet T. Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and IIalpha. Mol Pharmacol. 2000;58:71–81. doi: 10.1124/mol.58.1.71. [DOI] [PubMed] [Google Scholar]
- 63.Glaser T, Winter S, Groscurth P, Safayhi H, Sailer ER, Ammon HP, et al. Boswellic acids and malignant glioma: induction of apoptosis but no modulation of drug sensitivity. Br J Cancer. 1999;80:756–65. doi: 10.1038/sj.bjc.6690419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu JJ, Nilsson A, Oredsson S, Badmaev V, Zhao WZ, Duan RD. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002;23:2087–93. doi: 10.1093/carcin/23.12.2087. [DOI] [PubMed] [Google Scholar]
- 65.Liu JJ, Huang B, Hooi SC. Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br J Pharmacol. 2006;148:1099–107. doi: 10.1038/sj.bjp.0706817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang MT, Badmaev V, Ding Y, Liu Y, Xie JG, Ho CT. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225–30. doi: 10.1002/biof.5520130135. [DOI] [PubMed] [Google Scholar]
- 67.Shao Y, Ho CT, Chin CK, Badmaev V, Ma W, Huang MT. Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta Med. 1998;64:328–31. doi: 10.1055/s-2006-957444. [DOI] [PubMed] [Google Scholar]
- 68.Xia L, Chen D, Han R, Fang Q, Waxman S, Jing Y. Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol Cancer Ther. 2005;4:381–8. doi: 10.1158/1535-7163.MCT-03-0266. [DOI] [PubMed] [Google Scholar]
- 69.Hoernlein RF, Orlikowsky T, Zehrer C, Niethammer D, Sailer ER, Simmet T, et al. Acetyl-11-keto-beta-boswellic acid induces apoptosis in HL-60 and CCRF-CEM cells and inhibits topoisomerase I. J Pharmacol Exp Ther. 1999;288:613–9. [PubMed] [Google Scholar]
- 70.Bhushan S, Kumar A, Malik F, Andotra SS, Sethi VK, Kaur IP, et al. A triterpenediol from Boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in human leukemia HL-60 cells. Apoptosis. 2007;12:1911–26. doi: 10.1007/s10495-007-0105-5. [DOI] [PubMed] [Google Scholar]
- 71.Zhao W, Entschladen F, Liu H, Niggemann B, Fang Q, Zaenker KS, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cells. Cancer Detect Prev. 2003;27:67–75. doi: 10.1016/s0361-090x(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 72.Liu JJ, Nilsson A, Oredsson S, Badmaev V, Duan RD. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int J Mol Med. 2002;10:501–5. [PubMed] [Google Scholar]
- 73.Syrovets T, Gschwend JE, Buchele B, Laumonnier Y, Zugmaier W, Genze F, et al. Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J Biol Chem. 2005;280:6170–80. doi: 10.1074/jbc.M409477200. [DOI] [PubMed] [Google Scholar]
- 74.Lu M, Xia L, Hua H, Jing Y. Acetyl-keto-beta-boswellic acid induces apoptosis through a death receptor 5-mediated pathway in prostate cancer cells. Cancer Res. 2008;68:1180–6. doi: 10.1158/0008-5472.CAN-07-2978. [DOI] [PubMed] [Google Scholar]
- 75.Yuan HQ, Kong F, Wang XL, Young CY, Hu XY, Lou HX. Inhibitory effect of acetyl-11-keto-beta-boswellic acid on androgen receptor by interference of Sp1 binding activity in prostate cancer cells. Biochem Pharmacol. 2008;75:2112–21. doi: 10.1016/j.bcp.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Syrovets T, Buchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J Immunol. 2005;174:498–506. doi: 10.4049/jimmunol.174.1.498. [DOI] [PubMed] [Google Scholar]
- 77.Takada Y, Ichikawa H, Badmaev V, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J Immunol. 2006;176:3127–40. doi: 10.4049/jimmunol.176.5.3127. [DOI] [PubMed] [Google Scholar]
- 78.Kunnumakkara AB, Nair AS, Sung B, Pandey MK, Aggarwal BB. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol Cancer Res. 2009;7:118–28. doi: 10.1158/1541-7786.MCR-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Roy S, Khanna S, Shah H, Rink C, Phillips C, Preuss H, et al. Human genome screen to identify the genetic basis of the anti-inflammatory effects of Boswellia in microvascular endothelial cells. DNA Cell Biol. 2005;24:244–55. doi: 10.1089/dna.2005.24.244. [DOI] [PubMed] [Google Scholar]
- 80.Roy S, Khanna S, Krishnaraju AV, Subbaraju GV, Yasmin T, Bagchi D, et al. Regulation of vascular responses to inflammation: inducible matrix metalloproteinase-3 expression in human microvascular endothelial cells is sensitive to antiinflammatory Boswellia. Antioxid Redox Signal. 2006;8:653–60. doi: 10.1089/ars.2006.8.653. [DOI] [PubMed] [Google Scholar]
- 81.Wildfeuer A, Neu IS, Safayhi H, Metzger G, Wehrmann M, Vogel U, et al. Effects of boswellic acids extracted from a herbal medicine on the biosynthesis of leukotrienes and the course of experimental autoimmune encephalomyelitis. Arzneimittelforschung. 1998;48:668–74. [PubMed] [Google Scholar]
- 82.Krieglstein CF, Anthoni C, Rijcken EJ, Laukotter M, Spiegel HU, Boden SE, et al. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int J Colorectal Dis. 2001;16:88–95. doi: 10.1007/s003840100292. [DOI] [PubMed] [Google Scholar]
- 83.Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Muller S, et al. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1131–7. doi: 10.1152/ajpgi.00562.2005. [DOI] [PubMed] [Google Scholar]
- 84.Singh SK, Bhusari S, Singh R, Saxena A, Mondhe D, Qazi GN. Effect of acetyl 11-keto beta-boswellic acid on metastatic growth factor responsible for angiogenesis. Vascul Pharmacol. 2007;46:333–7. doi: 10.1016/j.vph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Winking M, Sarikaya S, Rahmanian A, Jodicke A, Boker DK. Boswellic acids inhibit glioma growth: a new treatment option? J Neurooncol. 2000;46:97–103. doi: 10.1023/a:1006387010528. [DOI] [PubMed] [Google Scholar]
- 86.Singh GB, Atal CK. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986;18:407–12. doi: 10.1007/BF01965005. [DOI] [PubMed] [Google Scholar]
- 87.Gupta M, Mazumdar UK, Sivakumar T, Vamsi ML, Karki SS, Sambathkumar R, et al. Evaluation of anti-inflammatory activity of chloroform extract of Bryonia laciniosa in experimental animal models. Biol Pharm Bull. 2003;26:1342–4. doi: 10.1248/bpb.26.1342. [DOI] [PubMed] [Google Scholar]
- 88.Wagner H, Geyer B, Fiebig M, Kiso Y, Hikino H. Isobutrin and butrin, the antihepatotoxic principles of Butea monosperma flowers. Planta Med. 1986:77–9. [PubMed] [Google Scholar]
- 89.Srinivasan R, Chandrasekar MJ, Nanjan MJ, Suresh B. Antioxidant activity of Caesalpinia digyna root. J Ethnopharmacol. 2007;113:284–91. doi: 10.1016/j.jep.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 90.Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-kappa B activation through inhibition of I kappa B alpha kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol. 2003;171:3278–86. doi: 10.4049/jimmunol.171.6.3278. [DOI] [PubMed] [Google Scholar]
- 91.Pandey MK, Sung B, Aggarwal BB. Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int J Cancer. 2009 doi: 10.1002/ijc.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arya S, Kumar VL. Antiinflammatory efficacy of extracts of latex of Calotropis procera against different mediators of inflammation. Mediators Inflamm. 2005;2005:228–32. doi: 10.1155/MI.2005.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao YL, Li X, Zheng M. Capparis spinosa protects against oxidative stress in systemic sclerosis dermal fibroblasts. Arch Dermatol Res. 2009 doi: 10.1007/s00403-009-0998-7. [DOI] [PubMed] [Google Scholar]
- 94.Zahin M, Ahmad I, Aqil F. Antioxidant and antimutagenic activity of Carum copticum fruit extracts. Toxicol In Vitro. doi: 10.1016/j.tiv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Prakasam A, Sethupathy S, Pugalendi KV. Influence of Casearia esculenta root extract on protein metabolism and marker enzymes in streptozotocin-induced diabetic rats. Pol J Pharmacol. 2004;56:587–93. [PubMed] [Google Scholar]
- 96.Cuellar MJ, Giner RM, Recio MC, Manez S, Rios JL. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72:221–9. doi: 10.1016/s0367-326x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 97.Bhakta T, Banerjee S, Mandal SC, Maity TK, Saha BP, Pal M. Hepatoprotective activity of Cassia fistula leaf extract. Phytomedicine. 2001;8:220–4. doi: 10.1078/0944-7113-00029. [DOI] [PubMed] [Google Scholar]
- 98.Sadique J, Chandra T, Thenmozhi V, Elango V. Biochemical modes of action of Cassia occidentalis and Cardiospermum halicacabum in inflammation. J Ethnopharmacol. 1987;19:201–12. doi: 10.1016/0378-8741(87)90042-0. [DOI] [PubMed] [Google Scholar]
- 99.Jang DS, Lee GY, Kim YS, Lee YM, Kim CS, Yoo JL, et al. Anthraquinones from the seeds of Cassia tora with inhibitory activity on protein glycation and aldose reductase. Biol Pharm Bull. 2007;30:2207–10. doi: 10.1248/bpb.30.2207. [DOI] [PubMed] [Google Scholar]
- 100.Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN. Studies on the anti-inflammatory and analgesic activity of Cedrus deodara (Roxb.) Loud. wood oil. J Ethnopharmacol. 1999;65:21–7. doi: 10.1016/s0378-8741(98)00150-0. [DOI] [PubMed] [Google Scholar]
- 101.Ahmad F, Khan RA, Rasheed S. Preliminary screening of methanolic extracts of Celastrus paniculatus and Tecomella undulata for analgesic and anti-inflammatory activities. J Ethnopharmacol. 1994;42:193–8. doi: 10.1016/0378-8741(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 102.Hazra B, Sarkar R, Bhattacharyya S, Roy P. Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia. 2002;73:730–3. doi: 10.1016/s0367-326x(02)00232-0. [DOI] [PubMed] [Google Scholar]
- 103.Cavin C, Delannoy M, Malnoe A, Debefve E, Touche A, Courtois D, et al. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem Biophys Res Commun. 2005;327:742–9. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 104.Lee HJ, Hyun EA, Yoon WJ, Kim BH, Rhee MH, Kang HK, et al. In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J Ethnopharmacol. 2006;103:208–16. doi: 10.1016/j.jep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 105.Verspohl EJ, Bauer K, Neddermann E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother Res. 2005;19:203–6. doi: 10.1002/ptr.1643. [DOI] [PubMed] [Google Scholar]
- 106.Mishra A, Bhatti R, Singh A, Singh Ishar MP. Ameliorative effect of the cinnamon oil from Cinnamomum zeylanicum upon early stage diabetic nephropathy. Planta Med. 76:412–7. doi: 10.1055/s-0029-1186237. [DOI] [PubMed] [Google Scholar]
- 107.Marzouk B, Marzouk Z, Decor R, Edziri H, Haloui E, Fenina N, et al. Antibacterial and anticandidal screening of Tunisian Citrullus colocynthis Schrad. from Medenine. J Ethnopharmacol. 2009;125:344–9. doi: 10.1016/j.jep.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 108.Urizar NL, Moore DD. GUGULIPID: a natural cholesterol-lowering agent. Annu Rev Nutr. 2003;23:303–13. doi: 10.1146/annurev.nutr.23.011702.073102. [DOI] [PubMed] [Google Scholar]
- 109.Sinal CJ, Gonzalez FJ. Guggulsterone: an old approach to a new problem. Trends Endocrinol Metab. 2002;13:275–6. doi: 10.1016/s1043-2760(02)00640-9. [DOI] [PubMed] [Google Scholar]
- 110.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–6. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- 111.Shishodia S, Aggarwal BB. Guggulsterone inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J Biol Chem. 2004;279:47148–58. doi: 10.1074/jbc.M408093200. [DOI] [PubMed] [Google Scholar]
- 112.Ahn KS, Sethi G, Sung B, Goel A, Ralhan R, Aggarwal BB. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–15. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 113.Burris TP, Montrose C, Houck KA, Osborne HE, Bocchinfuso WP, Yaden BC, et al. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol Pharmacol. 2005;67:948–54. doi: 10.1124/mol.104.007054. [DOI] [PubMed] [Google Scholar]
- 114.Gujral ML, Sareen K, Tangri KK, Amma MK, Roy AK. Antiarthritic and anti-inflammatory activity of gum guggul (Balsamodendron mukul Hook) Indian J Physiol Pharmacol. 1960;4:267–73. [PubMed] [Google Scholar]
- 115.Sharma JN. Comparison of the anti-inflammatory activity of Commiphora mukul (an indigenous drug) with those of phenylbutazone and ibuprofen in experimental arthritis induced by mycobacterial adjuvant. Arzneimittelforschung. 1977;27:1455–7. [PubMed] [Google Scholar]
- 116.Singh BB, Mishra LC, Vinjamury SP, Aquilina N, Singh VJ, Shepard N. The effectiveness of Commiphora mukul for osteoarthritis of the knee: an outcomes study. Altern Ther Health Med. 2003;9:74–9. [PubMed] [Google Scholar]
- 117.Cui J, Huang L, Zhao A, Lew JL, Yu J, Sahoo S, et al. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem. 2003;278:10214–20. doi: 10.1074/jbc.M209323200. [DOI] [PubMed] [Google Scholar]
- 118.Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002;16:1590–7. doi: 10.1210/mend.16.7.0894. [DOI] [PubMed] [Google Scholar]
- 119.Meselhy MR. Inhibition of LPS-induced NO production by the oleogum resin of Commiphora wightii and its constituents. Phytochemistry. 2003;62:213–8. doi: 10.1016/s0031-9422(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 120.Samudio I, Konopleva M, Safe S, McQueen T, Andreeff M. Guggulsterones induce apoptosis and differentiation in acute myeloid leukemia: identification of isomer-specific antileukemic activities of the pregnadienedione structure. Mol Cancer Ther. 2005;4:1982–92. doi: 10.1158/1535-7163.MCT-05-0247. [DOI] [PubMed] [Google Scholar]
- 121.Singh SV, Zeng Y, Xiao D, Vogel VG, Nelson JB, Dhir R, et al. Caspase-dependent apoptosis induction by guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, in PC-3 human prostate cancer cells is mediated by Bax and Bak. Mol Cancer Ther. 2005;4:1747–54. doi: 10.1158/1535-7163.MCT-05-0223. [DOI] [PubMed] [Google Scholar]
- 122.Ichikawa H, Aggarwal BB. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand and by tumor cells by suppressing nuclear factor-kappaB activation. Clin Cancer Res. 2006;12:662–8. doi: 10.1158/1078-0432.CCR-05-1749. [DOI] [PubMed] [Google Scholar]
- 123.Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006;20:1023–35. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- 124.Kumari A, Kakkar P. Screening of antioxidant potential of selected barks of Indian medicinal plants by multiple in vitro assays. Biomed Environ Sci. 2008;21:24–9. doi: 10.1016/S0895-3988(08)60003-3. [DOI] [PubMed] [Google Scholar]
- 125.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nalini N, Manju V, Menon VP. Effect of spices on lipid metabolism in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J Med Food. 2006;9:237–45. doi: 10.1089/jmf.2006.9.237. [DOI] [PubMed] [Google Scholar]
- 127.Siddaraju MN, Dharmesh SM. Inhibition of gastric H(+), K(+)-ATPase and Helicobacter pylori growth by phenolic antioxidants of Curcuma amada. J Agric Food Chem. 2007;55:7377–86. doi: 10.1021/jf070719r. [DOI] [PubMed] [Google Scholar]
- 128.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 129.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 130.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 131.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 133.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 134.Bright JJ. Curcumin and autoimmune disease. Adv Exp Med Biol. 2007;595:425–51. doi: 10.1007/978-0-387-46401-5_19. [DOI] [PubMed] [Google Scholar]
- 135.Das T, Sa G, Saha B, Das K. Multifocal signal modulation therapy of cancer: ancient weapon, modern targets. Mol Cell Biochem. 336:85–95. doi: 10.1007/s11010-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 136.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 137.Hanai H, Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr Pharm Des. 2009;15:2087–94. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]
- 138.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–53. [PubMed] [Google Scholar]
- 140.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–7. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 141.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–25. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 142.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Srivastava G, Mehta JL. Currying the heart: curcumin and cardioprotection. J Cardiovasc Pharmacol Ther. 2009;14:22–7. doi: 10.1177/1074248408329608. [DOI] [PubMed] [Google Scholar]
- 144.Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149–72. doi: 10.1007/978-0-387-46401-5_5. [DOI] [PubMed] [Google Scholar]
- 145.Lobo R, Prabhu KS, Shirwaikar A. Curcuma zedoaria Rosc. (white turmeric): a review of its chemical, pharmacological and ethnomedicinal properties. J Pharm Pharmacol. 2009;61:13–21. doi: 10.1211/jpp/61.01.0003. [DOI] [PubMed] [Google Scholar]
- 146.Watanabe C, Hokari R, Komoto S, Kurihara C, Okada Y, Matsunaga H, et al. Lemon grass (Cymbopogon citratus) ameliorates murine spontaneous ileitis by decreasing lymphocyte recruitment to the inflamed intestine. Microcirculation. 17:321–32. doi: 10.1111/j.1549-8719.2010.00032.x. [DOI] [PubMed] [Google Scholar]
- 147.Tiwari M, Dwivedi U, Kakkar P. Suppression of oxidative stress and pro-inflammatory mediators by Cymbopogon citratus D Stapf extract in lipopolysaccharide stimulated murine alveolar macrophages. Food Chem Toxicol. doi: 10.1016/j.fct.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 148.Prasad CS, Shukla R, Kumar A, Dubey NK. In vitro and in vivo antifungal activity of essential oils of Cymbopogon martini and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses. 2009 doi: 10.1111/j.1439-0507.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 149.Natarajan KS, Narasimhan M, Shanmugasundaram KR, Shanmugasundaram ER. Antioxidant activity of a salt-spice-herbal mixture against free radical induction. J Ethnopharmacol. 2006;105:76–83. doi: 10.1016/j.jep.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 150.Gilani AU, Janbaz KH. Studies on protective effect of Cyperus scariosus extract on acetaminophen and CCl4-induced hepatotoxicity. Gen Pharmacol. 1995;26:627–31. doi: 10.1016/0306-3623(94)00200-7. [DOI] [PubMed] [Google Scholar]
- 151.Baumer U, Dietemann P. Identification and differentiation of dragon’s blood in works of art using gas chromatography/mass spectrometry. Anal Bioanal Chem. doi: 10.1007/s00216-010-3620-0. [DOI] [PubMed] [Google Scholar]
- 152.Rajesh, Sharma GL. Studies on antimycotic properties of Datura metel. J Ethnopharmacol. 2002;80:193–7. doi: 10.1016/s0378-8741(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 153.Kaur G, Lone IA, Athar M, Alam MS. Protective effect of Didymocarpus pedicellata on ferric nitrilotriacetate (Fe-NTA) induced renal oxidative stress and hyperproliferative response. Chem Biol Interact. 2007;165:33–44. doi: 10.1016/j.cbi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 154.Kieley S, Dwivedi R, Monga M. Ayurvedic medicine and renal calculi. J Endourol. 2008;22:1613–6. doi: 10.1089/end.2008.0020. [DOI] [PubMed] [Google Scholar]
- 155.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-kappaB activation induced by various carcinogens and inflammatory agents through inhibition of IkappaBalpha kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279:4750–9. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 156.Takada Y, Sethi G, Sung B, Aggarwal BB. Flavopiridol suppresses tumor necrosis factor-induced activation of activator protein-1, c-Jun N-terminal kinase, p38 mitogen-activated protein kinase (MAPK), p44/p42 MAPK, and Akt, inhibits expression of antiapoptotic gene products, and enhances apoptosis through cytochrome c release and caspase activation in human myeloid cells. Mol Pharmacol. 2008;73:1549–57. doi: 10.1124/mol.107.041350. [DOI] [PubMed] [Google Scholar]
- 157.Saxena AK, Singh B, Anand KK. Hepatoprotective effects of Eclipta alba on subcellular levels in rats. J Ethnopharmacol. 1993;40:155–61. doi: 10.1016/0378-8741(93)90063-b. [DOI] [PubMed] [Google Scholar]
- 158.Jamal A, Javed K, Aslam M, Jafri MA. Gastroprotective effect of cardamom, Elettaria cardamomum Maton. fruits in rats. J Ethnopharmacol. 2006;103:149–53. doi: 10.1016/j.jep.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 159.Majdalawieh AF, Carr RI. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum) J Med Food. 13:371–81. doi: 10.1089/jmf.2009.1131. [DOI] [PubMed] [Google Scholar]
- 160.Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, Roller PP, et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47:2430–40. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 161.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71:209–19. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 162.Jindal A, Soyal D, Sharma A, Goyal PK. Protective effect of an extract of Emblica officinalis against radiation-induced damage in mice. Integr Cancer Ther. 2009;8:98–105. doi: 10.1177/1534735409331455. [DOI] [PubMed] [Google Scholar]
- 163.Achrekar S, Kaklij GS, Pote MS, Kelkar SM. Hypoglycemic activity of Eugenia jambolana and Ficus bengalensis: mechanism of action. In Vivo. 1991;5:143–7. [PubMed] [Google Scholar]
- 164.Ganju L, Karan D, Chanda S, Srivastava KK, Sawhney RC, Selvamurthy W. Immunomodulatory effects of agents of plant origin. Biomed Pharmacother. 2003;57:296–300. doi: 10.1016/s0753-3322(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 165.Rawal AK, Muddeshwar MG, Biswas SK. Rubia cordifolia, Fagonia cretica linn and Tinospora cordifolia exert neuroprotection by modulating the antioxidant system in rat hippocampal slices subjected to oxygen glucose deprivation. BMC Complement Altern Med. 2004;4:11. doi: 10.1186/1472-6882-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee JH, Choi S, Lee Y, Lee HJ, Kim KH, Ahn KS, et al. Herbal compound farnesiferol C exerts antiangiogenic and antitumor activity and targets multiple aspects of VEGFR1 (Flt1) or VEGFR2 (Flk1) signaling cascades. Mol Cancer Ther. 9:389–99. doi: 10.1158/1535-7163.MCT-09-0775. [DOI] [PubMed] [Google Scholar]
- 167.Taur DJ, Nirmal SA, Patil RY, Kharya MD. Antistress and antiallergic effects of Ficus bengalensis bark in asthma. Nat Prod Res. 2007;21:1266–70. doi: 10.1080/14786410701757330. [DOI] [PubMed] [Google Scholar]
- 168.Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–50. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 169.al-Harbi MM, Qureshi S, Raza M, Ahmed MM, Giangreco AB, Shah AH. Influence of anethole treatment on the tumour induced by Ehrlich ascites carcinoma cells in paw of Swiss albino mice. Eur J Cancer Prev. 1995;4:307–18. doi: 10.1097/00008469-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 170.Mahendran P, Vanisree AJ, Shyamala Devi CS. The antiulcer activity of Garcinia cambogia extract against indomethacin-induced gastric ulcer in rats. Phytother Res. 2002;16:80–3. doi: 10.1002/ptr.946. [DOI] [PubMed] [Google Scholar]
- 171.Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, et al. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004;279:33716–26. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 172.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood. 2007;110:3517–25. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–50. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang B, Li JB, Zhang DM, Ding Y, Du GH. Analgesic and anti-inflammatory activities of a fraction rich in gaultherin isolated from Gaultheria yunnanensis (FRANCH) REHDER. Biol Pharm Bull. 2007;30:465–9. doi: 10.1248/bpb.30.465. [DOI] [PubMed] [Google Scholar]
- 175.Dhingra D, Sharma A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests Prog Neuropsychopharmacol. Biol Psychiatry. 2006;30:449–54. doi: 10.1016/j.pnpbp.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 176.Agunu A, Yusuf S, Andrew GO, Zezi AU, Abdurahman EM. Evaluation of five medicinal plants used in diarrhoea treatment in Nigeria. J Ethnopharmacol. 2005;101:27–30. doi: 10.1016/j.jep.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 177.Ojha P, Dhar JD, Dwivedi AK, Singh RL, Gupta G. Effect of antispermatogenic agents on cell marker enzymes of rat Sertoli cells in vitro. Contraception. 2006;73:102–6. doi: 10.1016/j.contraception.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 178.Mi JX, Wang GF, Wang HB, Sun XQ, Ni XY, Zhang XW, et al. Synergistic antitumoral activity and induction of apoptosis by novel pan Bcl-2 proteins inhibitor apogossypolone with adriamycin in human hepatocellular carcinoma. Acta Pharmacol Sin. 2008;29:1467–77. doi: 10.1111/j.1745-7254.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 179.Liu HM, Kiuchi F, Tsuda Y. Isolation and structure elucidation of gymnemic acids, antisweet principles of Gymnema sylvestre. Chem Pharm Bull (Tokyo) 1992;40:1366–75. doi: 10.1248/cpb.40.1366. [DOI] [PubMed] [Google Scholar]
- 180.Saravanan N, Nalini N. Effect of 2-hydroxy 4-methoxy benzoic acid on an experimental model of hyperlipidaemia, induced by chronic ethanol treatment. J Pharm Pharmacol. 2007;59:1537–42. doi: 10.1211/jpp.59.11.0011. [DOI] [PubMed] [Google Scholar]
- 181.Sethi A, Bhatia A, Srivastava S, Bhatia G, Khan MM, Khanna AK, et al. Pregnane glycoside from Hemidesmus indicus as a potential anti-oxidant and anti-dyslipidemic agent. Nat Prod Res. :1–7. doi: 10.1080/14786410802265084. [DOI] [PubMed] [Google Scholar]
- 182.Arseculeratne SN, Gunatilaka AA, Panabokke RG. Studies on medicinal plants of Sri Lanka: occurrence of pyrrolizidine alkaloids and hepatotoxic properties in some traditional medicinal herbs. J Ethnopharmacol. 1981;4:159–77. doi: 10.1016/0378-8741(81)90033-7. [DOI] [PubMed] [Google Scholar]
- 183.Madhujith T, Shahidi F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgarae L) cultivars and their potential for inhibition of low-density lipoprotein (LDL) cholesterol oxidation. J Agric Food Chem. 2007;55:5018–24. doi: 10.1021/jf070072a. [DOI] [PubMed] [Google Scholar]
- 184.Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem. 2006;281:23425–35. doi: 10.1074/jbc.M602627200. [DOI] [PubMed] [Google Scholar]
- 185.Mangathayaru K, Kuruvilla S, Balakrishna K, Venkhatesh J. Modulatory effect of Inula racemosa Hook. f. (Asteraceae) on experimental atherosclerosis in guinea-pigs. J Pharm Pharmacol. 2009;61:1111–8. doi: 10.1211/jpp/61.08.0016. [DOI] [PubMed] [Google Scholar]
- 186.Ono M, Fukuda H, Murata H, Miyahara K. Resin glycosides from the leaves and stems of Ipomoea digitata. J Nat Med. 2009;63:176–80. doi: 10.1007/s11418-008-0309-1. [DOI] [PubMed] [Google Scholar]
- 187.Kim KH, Choi SU, Lee KR. Diterpene glycosides from the seeds of Pharbitis nil. J Nat Prod. 2009;72:1121–7. doi: 10.1021/np900101t. [DOI] [PubMed] [Google Scholar]
- 188.Topcu G, Ayral MN, Aydin A, Goren AC, Chai HB, Pezzuto JM. Triterpenoids of the roots of Lavandula stoechas ssp. stoechas. Pharmazie. 2001;56:892–5. [PubMed] [Google Scholar]
- 189.Bavarva JH, Narasimhacharya AV. Leucas cephalotes regulates carbohydrate and lipid metabolism and improves antioxidant status in IDDM and NIDDM rats. J Ethnopharmacol. 127:98–102. doi: 10.1016/j.jep.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 190.Saleem M, Murtaza I, Witkowsky O, Kohl AM, Maddodi N. Lupeol triterpene, a novel diet-based microtubule targeting agent: disrupts survivin/cFLIP activation in prostate cancer cells. Biochem Biophys Res Commun. 2009;388:576–82. doi: 10.1016/j.bbrc.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol. 2005;97:497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 192.Samarth RM, Panwar M, Kumar M, Kumar A. Radioprotective influence of Mentha piperita (Linn) against gamma irradiation in mice: Antioxidant and radical scavenging activity. Int J Radiat Biol. 2006;82:331–7. doi: 10.1080/09553000600771523. [DOI] [PubMed] [Google Scholar]
- 193.Samarth RM, Goyal PK, Kumar A. Protection of swiss albino mice against whole-body gamma irradiation by Mentha piperita (Linn. ) Phytother Res. 2004;18:546–50. doi: 10.1002/ptr.1483. [DOI] [PubMed] [Google Scholar]
- 194.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117–24. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 195.Yadav AS, Bhatnagar D. Inhibition of iron induced lipid peroxidation and antioxidant activity of Indian spices and Acacia in vitro. Plant Foods Hum Nutr. 65:18–24. doi: 10.1007/s11130-009-0150-z. [DOI] [PubMed] [Google Scholar]
- 196.Shah PJ, Gandhi MS, Shah MB, Goswami SS, Santani D. Study of Mimusops elengi bark in experimental gastric ulcers. J Ethnopharmacol. 2003;89:305–11. doi: 10.1016/j.jep.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 197.Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. J Ethnopharmacol. 2004;93:123–32. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 198.Schmourlo G, Mendonca-Filho RR, Alviano CS, Costa SS. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J Ethnopharmacol. 2005;96:563–8. doi: 10.1016/j.jep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 199.Mahajan SG, Mehta AA. Effect of Moringa oleifera Lam. seed extract on ovalbumin-induced airway inflammation in guinea pigs. Inhal Toxicol. 2008;20:897–909. doi: 10.1080/08958370802027443. [DOI] [PubMed] [Google Scholar]
- 200.Kar A, Choudhary BK, Bandyopadhyay NG. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol. 2003;84:105–8. doi: 10.1016/s0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- 201.Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7:1789–96. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chatterjee A, Basak B, Saha M, Dutta U, Mukhopadhyay C, Banerji J, et al. Structure and stereochemistry of nardostachysin, a new terpenoid ester constituent of the rhizomes of Nardostachys jatamansi. J Nat Prod. 2000;63:1531–3. doi: 10.1021/np990503m. [DOI] [PubMed] [Google Scholar]
- 203.Weng TC, Shen CC, Chiu YT, Lin YL, Kuo CD, Huang YT. Inhibitory effects of armepavine against hepatic fibrosis in rats. J Biomed Sci. 2009;16:78. doi: 10.1186/1423-0127-16-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim HK, Park HR, Lee JS, Chung TS, Chung HY, Chung J. Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology. 2007;8:399–408. doi: 10.1007/s10522-007-9083-9. [DOI] [PubMed] [Google Scholar]
- 205.Das S, Sasmal D, Basu SP. Anti-inflammatory and antinociceptive activity of arbortristoside-A. J Ethnopharmacol. 2008;116:198–203. doi: 10.1016/j.jep.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 206.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 207.Sarkar A, Lavania SC, Pandey DN, Pant MC. Changes in the blood lipid profile after administration of Ocimum sanctum (Tulsi) leaves in the normal albino rabbits. Indian J Physiol Pharmacol. 1994;38:311–2. [PubMed] [Google Scholar]
- 208.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–83. [PubMed] [Google Scholar]
- 209.Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 210.Ahmad R, Ahmed S, Khan NU, Hasnain AU. Operculina turpethum attenuates N-nitrosodimethylamine induced toxic liver injury and clastogenicity in rats. Chem Biol Interact. 2009;181:145–53. doi: 10.1016/j.cbi.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 211.Aziz N, Mehmood MH, Siddiqi HS, Mandukhail SU, Sadiq F, Maan W, et al. Antihypertensive, antidyslipidemic and endothelial modulating activities of Orchis mascula. Hypertens Res. 2009;32:997–1003. doi: 10.1038/hr.2009.148. [DOI] [PubMed] [Google Scholar]
- 212.Roy MK, Nakahara K, Na TV, Trakoontivakorn G, Takenaka M, Isobe S, et al. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Pharmazie. 2007;62:149–53. [PubMed] [Google Scholar]
- 213.Tan MA, Takayama H, Aimi N, Kitajima M, Franzblau SG, Nonato MG. Antitubercular triterpenes and phytosterols from Pandanus tectorius Soland. var. laevis. J Nat Med. 2008;62:232–5. doi: 10.1007/s11418-007-0218-8. [DOI] [PubMed] [Google Scholar]
- 214.Kassuya CA, Silvestre A, Menezes-de-Lima O, Jr, Marotta DM, Rehder VL, Calixto JB. Antiinflammatory and antiallodynic actions of the lignan niranthin isolated from Phyllanthus amarus. Evidence for interaction with platelet activating factor receptor. Eur J Pharmacol. 2006;546:182–8. doi: 10.1016/j.ejphar.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 215.Anand P, Kunnumakkara AB, Harikumar KB, Ahn KS, Badmaev V, Aggarwal BB. Modification of cysteine residue in p65 subunit of nuclear factor-kappaB (NF-kappaB) by picroliv suppresses NF-kappaB-regulated gene products and potentiates apoptosis. Cancer Res. 2008;68:8861–70. doi: 10.1158/0008-5472.CAN-08-1902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 216.Singh N, Kumar S, Singh P, Raj HG, Prasad AK, Parmar VS, et al. Piper longum Linn. Extract inhibits TNF-alpha-induced expression of cell adhesion molecules by inhibiting NF-kappaB activation and microsomal lipid peroxidation. Phytomedicine. 2008;15:284–91. doi: 10.1016/j.phymed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 217.Stohr JR, Xiao PG, Bauer R. Constituents of Chinese Piper species and their inhibitory activity on prostaglandin and leukotriene biosynthesis in vitro. J Ethnopharmacol. 2001;75:133–9. doi: 10.1016/s0378-8741(00)00397-4. [DOI] [PubMed] [Google Scholar]
- 218.Ahmad NS, Waheed A, Farman M, Qayyum A. Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J Ethnopharmacol. doi: 10.1016/j.jep.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 219.Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994;54:2424–8. [PubMed] [Google Scholar]
- 220.Tilak JC, Adhikari S, Devasagayam TP. Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox Rep. 2004;9:219–27. doi: 10.1179/135100004225005976. [DOI] [PubMed] [Google Scholar]
- 221.Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 222.Sandur SK, Pandey MK, Sung B, Aggarwal BB. 5-hydroxy-2-methyl-1,4-naphthoquinone, a vitamin K3 analogue, suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase, SHP-1: potential role in chemosensitization. Mol Cancer Res. 8:107–18. doi: 10.1158/1541-7786.MCR-09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Khan H, Saeed M, Gilani AU, Khan MA, Dar A, Khan I. The antinociceptive activity of Polygonatumverticillatum rhizomes in pain models. J Ethnopharmacol. 127:521–7. doi: 10.1016/j.jep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 224.Badole SL, Bodhankar SL. Investigation of antihyperglycaemic activity of aqueous and petroleum ether extract of stem bark of Pongamia pinnata on serum glucose level in diabetic mice. J Ethnopharmacol. 2009;123:115–20. doi: 10.1016/j.jep.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 225.Sang S, Kikuzaki H, Lapsley K, Rosen RT, Nakatani N, Ho CT. Sphingolipid and other constituents from almond nuts (Prunus amygdalus Batsch) J Agric Food Chem. 2002;50:4709–12. doi: 10.1021/jf020262f. [DOI] [PubMed] [Google Scholar]
- 226.Babu TD, Sasidharan N, Vijayan FP, Padikkala J. Comparative phytochemical and biological analysis to detect the genuineness of substitutes of the plant Moovila in drug preparations. J Basic Clin Physiol Pharmacol. 2008;19:119–30. doi: 10.1515/jbcpp.2008.19.2.119. [DOI] [PubMed] [Google Scholar]
- 227.Wolf P, Nghiem DX, Walterscheid JP, Byrne S, Matsumura Y, Bucana C, et al. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am J Pathol. 2006;169:795–805. doi: 10.2353/ajpath.2006.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hougee S, Faber J, Sanders A, de Jong RB, van den Berg WB, Garssen J, et al. Selective COX-2 inhibition by a Pterocarpus marsupium extract characterized by pterostilbene, and its activity in healthy human volunteers. Planta Med. 2005;71:387–92. doi: 10.1055/s-2005-864130. [DOI] [PubMed] [Google Scholar]
- 229.Kondeti VK, Badri KR, Maddirala DR, Thur SK, Fatima SS, Kasetti RB, et al. Effect of Pterocarpus santalinus bark, on blood glucose, serum lipids, plasma insulin and hepatic carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 48:1281–7. doi: 10.1016/j.fct.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 230.Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–5. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 231.Reanmongkol W, Noppapan T, Subhadhirasakul S. Antinociceptive, antipyretic, and anti-inflammatory activities of Putranjiva roxburghii Wall. leaf extract in experimental animals. J Nat Med. 2009;63:290–6. doi: 10.1007/s11418-009-0336-6. [DOI] [PubMed] [Google Scholar]
- 232.Kaur G, Hamid H, Ali A, Alam MS, Athar M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol. 2004;90:285–92. doi: 10.1016/j.jep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 233.Sipos P, Hagymasi K, Lugasi A, Feher E, Blazovics A. Effects of black radish root (Raphanus sativus L. var niger) on the colon mucosa in rats fed a fat rich diet. Phytother Res. 2002;16:677–9. doi: 10.1002/ptr.950. [DOI] [PubMed] [Google Scholar]
- 234.Leary WP, Reyes AJ, van der Byl K. Effects of single doses of two antihypertensive rauwolfia-diuretic combinations on urinary excretion. S Afr Med J. 1986;70:95–8. [PubMed] [Google Scholar]
- 235.Lam FY, Ferrell WR. Neurogenic component of different models of acute inflammation in the rat knee joint. Ann Rheum Dis. 1991;50:747–51. doi: 10.1136/ard.50.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mokhtarian F, Griffin DE. The role of mast cells in virus-induced inflammation in the murine central nervous system. Cell Immunol. 1984;86:491–500. doi: 10.1016/0008-8749(84)90404-0. [DOI] [PubMed] [Google Scholar]
- 237.Vieira C, Fetzer S, Sauer SK, Evangelista S, Averbeck B, Kress M, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87–95. doi: 10.1007/s002100100427. [DOI] [PubMed] [Google Scholar]
- 238.Han SH, Hur MH, Buckle J, Choi J, Lee MS. Effect of aromatherapy on symptoms of dysmenorrhea in college students: A randomized placebo-controlled clinical trial. J Altern Complement Med. 2006;12:535–41. doi: 10.1089/acm.2006.12.535. [DOI] [PubMed] [Google Scholar]
- 239.Kwon EK, Lee DY, Lee H, Kim DO, Baek NI, Kim YE, et al. Flavonoids from the buds of Rosa damascena inhibit the activity of 3-hydroxy-3-methylglutaryl-coenzyme a reductase and angiotensin I-converting enzyme. J Agric Food Chem. 58:882–6. doi: 10.1021/jf903515f. [DOI] [PubMed] [Google Scholar]
- 240.Kim KJ, Lee JS, Kwak MK, Choi HG, Yong CS, Kim JA, et al. Anti-inflammatory action of mollugin and its synthetic derivatives in HT-29 human colonic epithelial cells is mediated through inhibition of NF-kappaB activation. Eur J Pharmacol. 2009;622:52–7. doi: 10.1016/j.ejphar.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 241.Rouf AS, Islam MS, Rahman MT. Evaluation of antidiarrhoeal activity Rumex maritimus root. J Ethnopharmacol. 2003;84:307–10. doi: 10.1016/s0378-8741(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 242.Sanogo R, Monforte MT, Daquino A, Rossitto A, Maur DD, Galati EM. Antiulcer activity of Salvadora persica L: structural modifications. Phytomedicine. 1999;6:363–6. doi: 10.1016/s0944-7113(99)80060-9. [DOI] [PubMed] [Google Scholar]
- 243.Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4:1305–16. [PubMed] [Google Scholar]
- 244.Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL, Arora SK. Effect of Sapindus trifoliatus on hyperalgesic in vivo migraine models. Braz J Med Biol Res. 2005;38:469–75. doi: 10.1590/s0100-879x2005000300019. [DOI] [PubMed] [Google Scholar]
- 245.Arul B, Kothai R, Jacob P, Sangameswaran B, Sureshkumar K. Anti-inflammatory activity of Sapindus trifoliatus Linn. J Herb Pharmacother. 2004;4:43–50. [PubMed] [Google Scholar]
- 246.Middelkoop TB, Labadie RP. The action of Saraca asoca Roxb. de Wilde bark on the PGH2 synthetase enzyme complex of the sheep vesicular gland. Z Naturforsch C. 1985;40:523–6. doi: 10.1515/znc-1985-7-812. [DOI] [PubMed] [Google Scholar]
- 247.Ghosh S, Majumder M, Majumder S, Ganguly NK, Chatterjee BP. Saracin: A lectin from Saraca indica seed integument induces apoptosis in human T-lymphocytes. Arch Biochem Biophys. 1999;371:163–8. doi: 10.1006/abbi.1999.1418. [DOI] [PubMed] [Google Scholar]
- 248.Li Y, Xu C, Zhang Q, Liu JY, Tan RX. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol. 2005;98:329–33. doi: 10.1016/j.jep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 249.Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355:97–104. doi: 10.1016/j.cccn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 250.Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, Guha S, et al. Sesamin Manifests Chemopreventive Effects through the Suppression of NF-{kappa}B-Regulated Cell Survival, Proliferation, Invasion, and Angiogenic Gene Products. Mol Cancer Res. doi: 10.1158/1541-7786.MCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Matsui TA, Sowa Y, Murata H, Takagi K, Nakanishi R, Aoki S, et al. The plant alkaloid cryptolepine induces p21WAF1/CIP1 and cell cycle arrest in a human osteosarcoma cell line. Int J Oncol. 2007;31:915–22. [PubMed] [Google Scholar]
- 252.Bahgat A, Abdel-Aziz H, Raafat M, Mahdy A, El-Khatib AS, Ismail A, et al. Solanum indicum ssp. distichum extract is effective against L-NAME-induced hypertension in rats. Fundam Clin Pharmacol. 2008;22:693–9. doi: 10.1111/j.1472-8206.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 253.Ma P, Cao TT, Gu GF, Zhao X, Du YG, Zhang Y. Inducement effect of synthetic indiosides from Solanum indicum L. on apoptosis of human hepatocarcinoma cell line Bel-7402 and its mechanism. Ai Zheng. 2006;25:438–42. [PubMed] [Google Scholar]
- 254.Raju K, Anbuganapathi G, Gokulakrishnan V, Rajkapoor B, Jayakar B, Manian S. Effect of dried fruits of Solanum nigrum LINN against CCl4-induced hepatic damage in rats. Biol Pharm Bull. 2003;26:1618–9. doi: 10.1248/bpb.26.1618. [DOI] [PubMed] [Google Scholar]
- 255.Govindan S, Viswanathan S, Vijayasekaran V, Alagappan R. A pilot study on the clinical efficacy of Solanum xanthocarpum and Solanum trilobatum in bronchial asthma. J Ethnopharmacol. 1999;66:205–10. doi: 10.1016/s0378-8741(98)00160-3. [DOI] [PubMed] [Google Scholar]
- 256.Prabhu KS, Lobo R, Shirwaikar A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide diabetic rats. J Pharm Pharmacol. 2008;60:909–16. doi: 10.1211/jpp.60.7.0013. [DOI] [PubMed] [Google Scholar]
- 257.Balasubramanian T, Chatterjee TK, Sarkar M, Meena SL. Anti-inflammatory effect of Stereospermum suaveolens ethanol extract in rats. Pharm Biol. 48:318–23. doi: 10.3109/13880200903127383. [DOI] [PubMed] [Google Scholar]
- 258.Yin W, Deng XK, Yin FZ, Zhang XC, Cai BC. The cytotoxicity induced by brucine from the seed of Strychnos nux-vomica proceeds via apoptosis and is mediated by cyclooxygenase 2 and caspase 3 in SMMC 7221 cells. Food Chem Toxicol. 2007;45:1700–8. doi: 10.1016/j.fct.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 259.Saha P, Mandal S, Das A, Das S. Amarogentin can reduce hyperproliferation by downregulation of Cox-II and upregulation of apoptosis in mouse skin carcinogenesis model. Cancer Lett. 2006;244:252–9. doi: 10.1016/j.canlet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 260.Na M, Yang S, He L, Oh H, Kim BS, Oh WK, et al. Inhibition of protein tyrosine phosphatase 1B by ursane-type triterpenes isolated from Symplocos paniculata. Planta Med. 2006;72:261–3. doi: 10.1055/s-2005-873194. [DOI] [PubMed] [Google Scholar]
- 261.Banerjee S, Panda CK, Das S. Clove (Syzygium aromaticum L), a potential chemopreventive agent for lung cancer. Carcinogenesis. 2006;27:1645–54. doi: 10.1093/carcin/bgi372. [DOI] [PubMed] [Google Scholar]
- 262.Jagetia GC, Baliga MS. Syzygium cumini (Jamun) reduces the radiation-induced DNA damage in the cultured human peripheral blood lymphocytes: a preliminary study. Toxicol Lett. 2002;132:19–25. doi: 10.1016/s0378-4274(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 263.Sehrawat A, Sultana S. Evaluation of possible mechanisms of protective role of Tamarix gallica against DEN initiated and 2-AAF promoted hepatocarcinogenesis in male Wistar rats. Life Sci. 2006;79:1456–65. doi: 10.1016/j.lfs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 264.Halder S, Bharal N, Mediratta PK, Kaur I, Sharma KK. Anti-inflammatory, immunomodulatory and antinociceptive activity of Terminalia arjuna Roxb bark powder in mice and rats. Indian J Exp Biol. 2009;47:577–83. [PubMed] [Google Scholar]
- 265.Malik N, Dhawan V, Bahl A, Kaul D. Inhibitory effects of Terminalia arjuna on platelet activation in vitro in healthy subjects and patients with coronary artery disease. Platelets. 2009;20:183–90. doi: 10.1080/09537100902809004. [DOI] [PubMed] [Google Scholar]
- 266.Shaila HP, Udupa AL, Udupa SL. Preventive actions of Terminalia belerica in experimentally induced atherosclerosis. Int J Cardiol. 1995;49:101–6. doi: 10.1016/0167-5273(95)02285-5. [DOI] [PubMed] [Google Scholar]
- 267.Shinde SL, Junne SB, Wadje SS, Baig MM. The diversity of antibacterial compounds of Terminalia species (Combretaceae) Pak J Biol Sci. 2009;12:1483–6. doi: 10.3923/pjbs.2009.1483.1486. [DOI] [PubMed] [Google Scholar]
- 268.Shin TY, Jeong HJ, Kim DK, Kim SH, Lee JK, Chae BS, et al. Inhibitory action of water soluble fraction of Terminalia chebula on systemic and local anaphylaxis. J Ethnopharmacol. 2001;74:133–40. doi: 10.1016/s0378-8741(00)00360-3. [DOI] [PubMed] [Google Scholar]
- 269.Vigo E, Cepeda A, Gualillo O, Perez-Fernandez R. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A. 1 murine macrophages. J Pharm Pharmacol. 2004;56:257–63. doi: 10.1211/0022357022665. [DOI] [PubMed] [Google Scholar]
- 270.Horvathova E, Turcaniova V, Slamenova D. Comparative study of DNA-damaging and DNA-protective effects of selected components of essential plant oils in human leukemic cells K562. Neoplasma. 2007;54:478–83. [PubMed] [Google Scholar]
- 271.Dhanasekaran M, Baskar AA, Ignacimuthu S, Agastian P, Duraipandiyan V. Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest New Drugs. 2009;27:347–55. doi: 10.1007/s10637-008-9181-9. [DOI] [PubMed] [Google Scholar]
- 272.Thippeswamy G, Sheela ML, Salimath BP. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF-<kappa>B and its DNA binding activity. Eur J Pharmacol. 2008;588:141–50. doi: 10.1016/j.ejphar.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 273.Umamaheswari S, Mainzen Prince PS. Antihyperglycaemic effect of ‘Ilogen-Excel’, an ayurvedic herbal formulation in streptozotocin-induced diabetes mellitus. Acta Pol Pharm. 2007;64:53–61. [PubMed] [Google Scholar]
- 274.Singh B, Kale RK. Chemomodulatory effect of Trachyspermum ammi on murine skin and forestomach papillomagenesis. Nutr Cancer. 62:74–84. doi: 10.1080/01635580903191478. [DOI] [PubMed] [Google Scholar]
- 275.Kumar M, Soni AK, Shukla S, Kumar A. Chemopreventive potential of Tribulus terrestris against 7,12- dimethylbenz (a) anthracene induced skin papillomagenesis in mice. Asian Pac J Cancer Prev. 2006;7:289–94. [PubMed] [Google Scholar]
- 276.Amin A, Lotfy M, Shafiullah M, Adeghate E. The protective effect of Tribulus terrestris in diabetes. Ann N Y Acad Sci. 2006;1084:391–401. doi: 10.1196/annals.1372.005. [DOI] [PubMed] [Google Scholar]
- 277.Phillips OA, Mathew KT, Oriowo MA. Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris in rats. J Ethnopharmacol. 2006;104:351–5. doi: 10.1016/j.jep.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 278.Shishodia S, Aggarwal BB. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, I kappa B kinase activation and NF-kappa B-regulated gene expression. Oncogene. 2006;25:1463–73. doi: 10.1038/sj.onc.1209194. [DOI] [PubMed] [Google Scholar]
- 279.Li F, Fernandez PP, Rajendran P, Hui KM, Sethi G. Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett. 292:197–207. doi: 10.1016/j.canlet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 280.Singh AB, Tamarkar AK, Narender T, Srivastava AK. Antihyperglycaemic effect of an unusual amino acid (4-hydroxyisoleucine) in C57BL/KsJ-db/db mice. Nat Prod Res. 24:258–65. doi: 10.1080/14786410902836693. [DOI] [PubMed] [Google Scholar]
- 281.Jabbar S, Khan MT, Choudhuri MS, Sil BK. Bioactivity studies of the individual ingredients of the Dashamularishta. Pak J Pharm Sci. 2004;17:9–17. [PubMed] [Google Scholar]
- 282.Jacobo-Herrera NJ, Vartiainen N, Bremner P, Gibbons S, Koistinaho J, Heinrich M. NF-kappaB modulators from Valeriana officinalis. Phytother Res. 2006;20:917–9. doi: 10.1002/ptr.1972. [DOI] [PubMed] [Google Scholar]
- 283.Gilani AH, Khan AU, Jabeen Q, Subhan F, Ghafar R. Antispasmodic and blood pressure lowering effects of Valeriana wallichii are mediated through K+ channel activation. J Ethnopharmacol. 2005;100:347–52. doi: 10.1016/j.jep.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 284.Nayak BS, Suresh R, Rao AV, Pillai GK, Davis EM, Ramkissoon V, et al. Evaluation of wound healing activity of Vanda roxburghii R. Br(Orchidacea): a preclinical study in a rat model. Int J Low Extrem Wounds. 2005;4:200–4. doi: 10.1177/1534734605282994. [DOI] [PubMed] [Google Scholar]
- 285.Pratheeshkumar P, Kuttan G. Modulation of immune response by Vernonia cinerea L. inhibits the proinflammatory cytokine profile, iNOS, and COX-2 expression in LPS-stimulated macrophages. Immunopharmacol Immunotoxicol. doi: 10.3109/08923971003745977. [DOI] [PubMed] [Google Scholar]
- 286.Lindholm P, Goransson U, Johansson S, Claeson P, Gullbo J, Larsson R, et al. Cyclotides: a novel type of cytotoxic agents. Mol Cancer Ther. 2002;1:365–9. [PubMed] [Google Scholar]
- 287.Zhou Y, Liu YE, Cao J, Zeng G, Shen C, Li Y, et al. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin Cancer Res. 2009;15:5161–9. doi: 10.1158/1078-0432.CCR-09-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006;5:1434–45. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 289.Mulabagal V, Subbaraju GV, Rao CV, Sivaramakrishna C, Dewitt DL, Holmes D, et al. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother Res. 2009;23:987–92. doi: 10.1002/ptr.2736. [DOI] [PubMed] [Google Scholar]
- 290.Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther. 9:202–10. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.El-Abhar HS, Hammad LN, Gawad HS. Modulating effect of ginger extract on rats with ulcerative colitis. J Ethnopharmacol. 2008;118:367–72. doi: 10.1016/j.jep.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 292.Kuo SC, Chen SC, Chen LH, Wu JB, Wang JP, Teng CM. Potent antiplatelet, anti-inflammatory and antiallergic isoflavanquinones from the roots of Abrus precatorius. Planta Med. 1995;61:307–12. doi: 10.1055/s-2006-958089. [DOI] [PubMed] [Google Scholar]
- 293.Ramnath V, Rekha PS, Kuttan G, Kuttan R. Regulation of Caspase-3 and Bcl-2 Expression in Dalton’s Lymphoma Ascites Cells by Abrin. Evid Based Complement Alternat Med. 2009;6:233–8. doi: 10.1093/ecam/nem099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ramnath V, Kuttan G, Kuttan R. Antitumour effect of abrin on transplanted tumours in mice. Indian J Physiol Pharmacol. 2002;46:69–77. [PubMed] [Google Scholar]
- 295.Ghosh D, Maiti TK. Immunomodulatory and anti-tumor activities of native and heat denatured Abrus agglutinin. Immunobiology. 2007;212:589–99. doi: 10.1016/j.imbio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 296.Bhutia SK, Mallick SK, Maiti S, Maiti TK. Inhibitory effect of Abrus abrin-derived peptide fraction against Dalton’s lymphoma ascites model. Phytomedicine. 2009;16:377–85. doi: 10.1016/j.phymed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 297.Clark DT, Gazi MI, Cox SW, Eley BM, Tinsley GF. The effects of Acacia arabica gum on the in vitro growth and protease activities of periodontopathic bacteria. J Clin Periodontol. 1993;20:238–43. doi: 10.1111/j.1600-051x.1993.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 298.Morgan SL, Baggott JE, Moreland L, Desmond R, Kendrach AC. The safety of flavocoxid, a medical food, in the dietary management of knee osteoarthritis. J Med Food. 2009;12:1143–8. doi: 10.1089/jmf.2008.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Haidara K, Zamir L, Shi QW, Batist G. The flavonoid Casticin has multiple mechanisms of tumor cytotoxicity action. Cancer Lett. 2006;242:180–90. doi: 10.1016/j.canlet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 300.Benedek B, Kopp B. Achillea millefolium L. s.l. revisited: recent findings confirm the traditional use. Wien Med Wochenschr. 2007;157:312–4. doi: 10.1007/s10354-007-0431-9. [DOI] [PubMed] [Google Scholar]
- 301.Cavalcanti AM, Baggio CH, Freitas CS, Rieck L, de Sousa RS, Da Silva-Santos JE, et al. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharmacol. 2006;107:277–84. doi: 10.1016/j.jep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 302.Vetrichelvan T, Jegadeesan M. Effect of alcohol extract of Achyranthes aspera Linn. on acute and subacute inflammation. Phytother Res. 2003;17:77–9. doi: 10.1002/ptr.1070. [DOI] [PubMed] [Google Scholar]
- 303.Shukla PK, Khanna VK, Ali MM, Maurya R, Khan MY, Srimal RC. Neuroprotective effect of Acorus calamus against middle cerebral artery occlusion-induced ischaemia in rat. Hum Exp Toxicol. 2006;25:187–94. doi: 10.1191/0960327106ht613oa. [DOI] [PubMed] [Google Scholar]
- 304.Shrivastava N, Srivastava A, Banerjee A, Nivsarkar M. Anti-ulcer activity of Adhatoda vasica Nees. J Herb Pharmacother. 2006;6:43–9. [PubMed] [Google Scholar]
- 305.Jahangir T, Sultana S. Tumor Promotion and Oxidative Stress in Ferric Nitrilotriacetate-Mediated Renal Carcinogenesis: Protection by Adhatoda vasica. Toxicol Mech Methods. 2007;17:421–30. doi: 10.1080/15376510601131297. [DOI] [PubMed] [Google Scholar]
- 306.Claeson UP, Malmfors T, Wikman G, Bruhn JG. Adhatoda vasica: a critical review of ethnopharmacological and toxicological data. J Ethnopharmacol. 2000;72:1–20. doi: 10.1016/s0378-8741(00)00225-7. [DOI] [PubMed] [Google Scholar]
- 307.Maity P, Hansda D, Bandyopadhyay U, Mishra DK. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelos (L) Corr Indian J Exp Biol. 2009;47:849–61. [PubMed] [Google Scholar]
- 308.Khan TH, Sultana S. Antioxidant and hepatoprotective potential of Aegle marmelos Correa. against CCl4-induced oxidative stress and early tumor events. J Enzyme Inhib Med Chem. 2009;24:320–7. doi: 10.1080/14756360802167754. [DOI] [PubMed] [Google Scholar]
- 309.Aviello G, Abenavoli L, Borrelli F, Capasso R, Izzo AA, Lembo F, et al. Garlic: empiricism or science? Nat Prod Commun. 2009;4:1785–96. [PubMed] [Google Scholar]
- 310.Butt MS, Sultan MT, Iqbal J. Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr. 2009;49:538–51. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 311.Iciek M, Kwiecien I, Wlodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50:247–65. doi: 10.1002/em.20474. [DOI] [PubMed] [Google Scholar]
- 312.Pittler MH, Ernst E. Clinical effectiveness of garlic (Allium sativum) Mol Nutr Food Res. 2007;51:1382–5. doi: 10.1002/mnfr.200700073. [DOI] [PubMed] [Google Scholar]
- 313.Lei YP, Chen HW, Sheen LY, Lii CK. Diallyl disulfide and diallyl trisulfide suppress oxidized LDL-induced vascular cell adhesion molecule and E-selectin expression through protein kinase A- and B-dependent signaling pathways. J Nutr. 2008;138:996–1003. doi: 10.1093/jn/138.6.996. [DOI] [PubMed] [Google Scholar]
- 314.Lee HS, Lee CH, Tsai HC, Salter DM. Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1beta. Osteoarthritis Cartilage. 2009;17:91–9. doi: 10.1016/j.joca.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 315.Lang A, Lahav M, Sakhnini E, Barshack I, Fidder HH, Avidan B, et al. Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin Nutr. 2004;23:1199–208. doi: 10.1016/j.clnu.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 316.Son EW, Mo SJ, Rhee DK, Pyo S. Inhibition of ICAM-1 expression by garlic component, allicin, in gamma-irradiated human vascular endothelial cells via downregulation of the JNK signaling pathway. Int Immunopharmacol. 2006;6:1788–95. doi: 10.1016/j.intimp.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 317.Guo J, Xiao B, Liu Q, Gong Z, Le Y. Suppression of C-myc expression associates with anti-proliferation of aloe-emodin on gastric cancer cells. Cancer Invest. 2008;26:369–74. doi: 10.1080/07357900701788130. [DOI] [PubMed] [Google Scholar]
- 318.Lin JG, Chen GW, Li TM, Chouh ST, Tan TW, Chung JG. Aloe-emodin induces apoptosis in T24 human bladder cancer cells through the p53 dependent apoptotic pathway. J Urol. 2006;175:343–7. doi: 10.1016/S0022-5347(05)00005-4. [DOI] [PubMed] [Google Scholar]
- 319.Feily A, Namazi MR. Aloe vera in dermatology: a brief review. G Ital Dermatol Venereol. 2009;144:85–91. [PubMed] [Google Scholar]
- 320.Eamlamnam K, Patumraj S, Visedopas N, Thong-Ngam D. Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats. World J Gastroenterol. 2006;12:2034–9. doi: 10.3748/wjg.v12.i13.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Akev N, Turkay G, Can A, Gurel A, Yildiz F, Yardibi H, et al. Effect of Aloe vera leaf pulp extract on Ehrlich ascites tumours in mice. Eur J Cancer Prev. 2007;16:151–7. doi: 10.1097/01.cej.0000220642.54094.16. [DOI] [PubMed] [Google Scholar]
- 322.Eshun K, He Q. Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries--a review. Crit Rev Food Sci Nutr. 2004;44:91–6. doi: 10.1080/10408690490424694. [DOI] [PubMed] [Google Scholar]
- 323.Vogler BK, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract. 1999;49:823–8. [PMC free article] [PubMed] [Google Scholar]
- 324.Ye Y, Li B. 1′S-1′-acetoxychavicol acetate isolated from Alpinia galanga inhibits human immunodeficiency virus type 1 replication by blocking Rev transport. J Gen Virol. 2006;87:2047–53. doi: 10.1099/vir.0.81685-0. [DOI] [PubMed] [Google Scholar]
- 325.Matsuda H, Morikawa T, Managi H, Yoshikawa M. Antiallergic principles from Alpinia galanga: structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem Lett. 2003;13:3197–202. doi: 10.1016/s0960-894x(03)00710-8. [DOI] [PubMed] [Google Scholar]
- 326.Morikawa T, Ando S, Matsuda H, Kataoka S, Muraoka O, Yoshikawa M. Inhibitors of nitric oxide production from the rhizomes of Alpinia galanga: structures of new 8–9′ linked neolignans and sesquineolignan. Chem Pharm Bull (Tokyo) 2005;53:625–30. doi: 10.1248/cpb.53.625. [DOI] [PubMed] [Google Scholar]
- 327.Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Respir Crit Care Med. 2009;179:657–65. doi: 10.1164/rccm.200809-1516OC. [DOI] [PubMed] [Google Scholar]
- 328.Burgos RA, Seguel K, Perez M, Meneses A, Ortega M, Guarda MI, et al. Andrographolide inhibits IFN-gamma and IL-2 cytokine production and protects against cell apoptosis. Planta Med. 2005;71:429–34. doi: 10.1055/s-2005-864138. [DOI] [PubMed] [Google Scholar]
- 329.Yang L, Wu D, Luo K, Wu S, Wu P. Andrographolide enhances 5-fluorouracil-induced apoptosis via caspase-8-dependent mitochondrial pathway involving p53 participation in hepatocellular carcinoma (SMMC-7721) cells. Cancer Lett. 2009;276:180–8. doi: 10.1016/j.canlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 330.Shi MD, Lin HH, Lee YC, Chao JK, Lin RA, Chen JH. Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem Biol Interact. 2008;174:201–10. doi: 10.1016/j.cbi.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 331.Zhou J, Lu GD, Ong CS, Ong CN, Shen HM. Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis via p53-mediated death receptor 4 up-regulation. Mol Cancer Ther. 2008;7:2170–80. doi: 10.1158/1535-7163.MCT-08-0071. [DOI] [PubMed] [Google Scholar]
- 332.Zhao F, He EQ, Wang L, Liu K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J Asian Nat Prod Res. 2008;10:467–73. doi: 10.1080/10286020801948334. [DOI] [PubMed] [Google Scholar]
- 333.Harjotaruno S, Widyawaruyanti A, Sismindari, Zaini NC. Apoptosis Inducing Effect of Andrographolide on TD-47 Human Breast Cancer Cell Line. Afr J Tradit Complement Altern Med. 2007;4:345–51. doi: 10.4314/ajtcam.v4i3.31228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sheeja K, Guruvayoorappan C, Kuttan G. Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int Immunopharmacol. 2007;7:211–21. doi: 10.1016/j.intimp.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 335.Trivedi NP, Rawal UM, Patel BP. Potency of andrographolide as an antitumor compound in BHC-induced liver damage. Integr Cancer Ther. 2009;8:177–89. doi: 10.1177/1534735409335606. [DOI] [PubMed] [Google Scholar]
- 336.Abu-Ghefreh AA, Canatan H, Ezeamuzie CI. In vitro and in vivo anti-inflammatory effects of andrographolide. Int Immunopharmacol. 2009;9:313–8. doi: 10.1016/j.intimp.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 337.Iruretagoyena MI, Tobar JA, Gonzalez PA, Sepulveda SE, Figueroa CA, Burgos RA, et al. Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J Pharmacol Exp Ther. 2005;312:366–72. doi: 10.1124/jpet.104.072512. [DOI] [PubMed] [Google Scholar]
- 338.Sheeja K, Kuttan G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and andrographolide. Immunopharmacol Immunotoxicol. 2007;29:81–93. doi: 10.1080/08923970701282726. [DOI] [PubMed] [Google Scholar]
- 339.Sheeja K, Kuttan G. Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma-bearing mice. Integr Cancer Ther. 2007;6:66–73. doi: 10.1177/1534735406298975. [DOI] [PubMed] [Google Scholar]
- 340.Liu J, Wang ZT, Ji LL, Ge BX. Inhibitory effects of neoandrographolide on nitric oxide and prostaglandin E2 production in LPS-stimulated murine macrophage. Mol Cell Biochem. 2007;298:49–57. doi: 10.1007/s11010-006-9349-6. [DOI] [PubMed] [Google Scholar]
- 341.Ji LL, Wang Z, Dong F, Zhang WB, Wang ZT. Andrograpanin, a compound isolated from anti-inflammatory traditional Chinese medicine Andrographis paniculata, enhances chemokine SDF-1alpha-induced leukocytes chemotaxis. J Cell Biochem. 2005;95:970–8. doi: 10.1002/jcb.20464. [DOI] [PubMed] [Google Scholar]
- 342.Liu J, Wang ZT, Ge BX. Andrograpanin, isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways. Int Immunopharmacol. 2008;8:951–8. doi: 10.1016/j.intimp.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 343.Chao WW, Kuo YH, Hsieh SL, Lin BF. Inhibitory effects of ethyl acetate extract of Andrographis paniculata on NF-{kappa}B trans-activation activity and LPS-induced acute inflammation in mice. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nep120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Chandrasekaran CV, Gupta A, Agarwal A. Effect of an extract of Andrographis paniculata leaves on inflammatory and allergic mediators in vitro. J Ethnopharmacol. 129:203–7. doi: 10.1016/j.jep.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 345.Burgos RA, Hancke JL, Bertoglio JC, Aguirre V, Arriagada S, Calvo M, et al. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: a prospective randomized placebo-controlled trial. Clin Rheumatol. 2009;28:931–46. doi: 10.1007/s10067-009-1180-5. [DOI] [PubMed] [Google Scholar]
- 346.Gabrielian ES, Shukarian AK, Goukasova GI, Chandanian GL, Panossian AG, Wikman G, et al. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine. 2002;9:589–97. doi: 10.1078/094471102321616391. [DOI] [PubMed] [Google Scholar]
- 347.Lee KK, Choi JD. The effects of areca catechu L extract on anti-inflammation and anti-melanogenesis. Int J Cosmet Sci. 1999;21:275–84. doi: 10.1046/j.1467-2494.1999.196590.x. [DOI] [PubMed] [Google Scholar]
- 348.Habbu P, Shastry R, Mahadevan KM, Joshi H, Das S. Hepatoprotective and antioxidant effects of argyreia speciosa in rats. Afr J Tradit Complement Altern Med. 2008;5:158–64. doi: 10.4314/ajtcam.v5i2.31268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Gokhale AB, Damre AS, Saraf MN. Investigations into the immunomodulatory activity of Argyreia speciosa. J Ethnopharmacol. 2003;84:109–14. doi: 10.1016/s0378-8741(02)00168-x. [DOI] [PubMed] [Google Scholar]
- 350.Bachhav RS, Gulecha VS, Upasani CD. Analgesic and anti-inflammatory activity of Argyreia speciosa root. Indian J Pharmacol. 2009;41:158–61. doi: 10.4103/0253-7613.56066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Habbu PV, Mahadevan KM, Kulkarni PV, Daulatsingh C, Veerapur VP, Shastry RA. Adaptogenic and in vitro antioxidant activity of flavanoids and other fractions of Argyreia speciosa (Burm.f) Boj. in acute and chronic stress paradigms in rodents. Indian J Exp Biol. 48:53–60. [PubMed] [Google Scholar]
- 352.Kanwar AS, Bhutani KK. Effects of Chlorophytum arundinaceum, Asparagus adscendens and Asparagus racemosus on pro-inflammatory cytokine and corticosterone levels produced by stress. Phytother Res. doi: 10.1002/ptr.3218. [DOI] [PubMed] [Google Scholar]
- 353.Visavadiya NP, Soni B, Madamwar D. Suppression of reactive oxygen species and nitric oxide by Asparagus racemosus root extract using in vitro studies. Cell Mol Biol (Noisy-le-grand) 2009;55(Suppl):OL1083–95. [PubMed] [Google Scholar]
- 354.Singh GK, Garabadu D, Muruganandam AV, Joshi VK, Krishnamurthy S. Antidepressant activity of Asparagus racemosus in rodent models. Pharmacol Biochem Behav. 2009;91:283–90. doi: 10.1016/j.pbb.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 355.Agrawal A, Sharma M, Rai SK, Singh B, Tiwari M, Chandra R. The effect of the aqueous extract of the roots of Asparagus racemosus on hepatocarcinogenesis initiated by diethylnitrosamine. Phytother Res. 2008;22:1175–82. doi: 10.1002/ptr.2391. [DOI] [PubMed] [Google Scholar]
- 356.Goyal RK, Singh J, Lal H. Asparagus racemosus--an update. Indian J Med Sci. 2003;57:408–14. [PubMed] [Google Scholar]
- 357.Bopana N, Saxena S. Asparagus racemosus--ethnopharmacological evaluation and conservation needs. J Ethnopharmacol. 2007;110:1–15. doi: 10.1016/j.jep.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 358.Priyadarsini RV, Manikandan P, Kumar GH, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic Res. 2009;43:492–504. doi: 10.1080/10715760902870637. [DOI] [PubMed] [Google Scholar]
- 359.Gangar SC, Sandhir R, Koul A. Anti-clastogenic activity of Azadirachta indica against benzo(a)pyrene in murine forestomach tumorigenesis bioassay. Acta Pol Pharm. 67:381–90. [PubMed] [Google Scholar]
- 360.Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149–6. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 361.Maity P, Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. The use of neem for controlling gastric hyperacidity and ulcer. Phytother Res. 2009;23:747–55. doi: 10.1002/ptr.2721. [DOI] [PubMed] [Google Scholar]
- 362.Akihisa T, Noto T, Takahashi A, Fujita Y, Banno N, Tokuda H, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indicia A. Juss. (neem) J Oleo Sci. 2009;58:581–94. doi: 10.5650/jos.58.581. [DOI] [PubMed] [Google Scholar]
- 363.Bose A, Chakraborty K, Sarkar K, Goswami S, Chakraborty T, Pal S, et al. Neem leaf glycoprotein induces perforin-mediated tumor cell killing by T and NK cells through differential regulation of IFNgamma signaling. J Immunother. 2009;32:42–53. doi: 10.1097/CJI.0b013e31818e997d. [DOI] [PubMed] [Google Scholar]
- 364.Ramzanighara A, Ezzatighadi F, Rai DV, Dhawan DK. Effect of Neem (Azadirchta indica) on serum glycoprotein contents of rats administered 1,2 dimethylhydrazine. Toxicol Mech Methods. 2009;19:298–301. doi: 10.1080/15376510802646523. [DOI] [PubMed] [Google Scholar]
- 365.Vinothini G, Manikandan P, Anandan R, Nagini S. Chemoprevention of rat mammary carcinogenesis by Azadirachta indica leaf fractions: modulation of hormone status, xenobiotic-metabolizing enzymes, oxidative stress, cell proliferation and apoptosis. Food Chem Toxicol. 2009;47:1852–63. doi: 10.1016/j.fct.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 366.Ghosh D, Bose A, Haque E, Baral R. Neem (Azadirachta indica) leaf preparation prevents leukocyte apoptosis mediated by cisplatin plus 5-fluorouracil treatment in Swiss mice. Chemotherapy. 2009;55:137–44. doi: 10.1159/000211558. [DOI] [PubMed] [Google Scholar]
- 367.Bhat M, Kothiwale SK, Tirmale AR, Bhargava SY, Joshi BN. Antidiabetic Properties of Azardiracta indica and Bougainvillea spectabilis: In Vivo Studies in Murine Diabetes Model. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nep033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Koul A, Bharrhan S, Singh B, Rishi P. Potential of Azadirachta indica against Salmonella typhimurium-induced inflammation in BALB/c mice. Inflammopharmacology. 2009;17:29–36. doi: 10.1007/s10787-008-8032-9. [DOI] [PubMed] [Google Scholar]
- 369.Manikandan P, Vidjaya Letchoumy P, Prathiba D, Nagini S. Combinatorial chemopreventive effect of Azadirachta indica and Ocimum sanctum on oxidant-antioxidant status, cell proliferation, apoptosis and angiogenesis in a rat forestomach carcinogenesis model. Singapore Med J. 2008;49:814–22. [PubMed] [Google Scholar]
- 370.Zhou Y, Shen YH, Zhang C, Su J, Liu RH, Zhang WD. Triterpene saponins from Bacopa monnieri and their antidepressant effects in two mice models. J Nat Prod. 2007;70:652–5. doi: 10.1021/np060470s. [DOI] [PubMed] [Google Scholar]
- 371.Limpeanchob N, Jaipan S, Rattanakaruna S, Phrompittayarat W, Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;120:112–7. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 372.Uabundit N, Wattanathorn J, Mucimapura S, Ingkaninan K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J Ethnopharmacol. 127:26–31. doi: 10.1016/j.jep.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 373.Krishnakumar A, Abraham PM, Paul J, Paulose CS. Down-regulation of cerebellar 5-HT(2C) receptors in pilocarpine-induced epilepsy in rats: therapeutic role of Bacopa monnieri extract. J Neurol Sci. 2009;284:124–8. doi: 10.1016/j.jns.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 374.Agrawal RC, Pandey S. Evaluation of anticarcinogenic and antimutagenic potential of Bauhinia variegata extract in Swiss albino mice. Asian Pac J Cancer Prev. 2009;10:913–6. [PubMed] [Google Scholar]
- 375.Bodakhe SH, Ram A. Hepatoprotective properties of Bauhinia variegata bark extract. Yakugaku Zasshi. 2007;127:1503–7. doi: 10.1248/yakushi.127.1503. [DOI] [PubMed] [Google Scholar]
- 376.Rajkapoor B, Jayakar B, Murugesh N. Antitumour activity of Bauhinia variegata on Dalton’s ascitic lymphoma. J Ethnopharmacol. 2003;89:107–9. doi: 10.1016/s0378-8741(03)00264-2. [DOI] [PubMed] [Google Scholar]
- 377.Rao Y, Fang SH, Tzeng YM. Antiinflammatory activities of flavonoids and a triterpene caffeate isolated from Bauhinia variegata. Phytother Res. 2008;22:957–62. doi: 10.1002/ptr.2448. [DOI] [PubMed] [Google Scholar]
- 378.Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol. 2008;589:163–72. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 379.Gupta SK, Agarwal R, Srivastava S, Agarwal P, Agrawal SS, Saxena R, et al. The anti-inflammatory effects of Curcuma longa and Berberis aristata in endotoxin-induced uveitis in rabbits. Invest Ophthalmol Vis Sci. 2008;49:4036–40. doi: 10.1167/iovs.07-1186. [DOI] [PubMed] [Google Scholar]
- 380.Rajbhandari M, Wegner U, Schopke T, Lindequist U, Mentel R. Inhibitory effect of Bergenia ligulata on influenza virus A. Pharmazie. 2003;58:268–71. [PubMed] [Google Scholar]
- 381.Olaleye MT, Akinmoladun AC, Ogunboye AA, Akindahunsi AA. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem Toxicol. 48:2200–5. doi: 10.1016/j.fct.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 382.Sreeja S, Sreeja S. An in vitro study on antiproliferative and antiestrogenic effects of Boerhaavia diffusa L. extracts J Ethnopharmacol. 2009;126:221–5. doi: 10.1016/j.jep.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 383.Manu KA, Kuttan G. Boerhaavia diffusa stimulates cell-mediated immune response by upregulating IL-2 and downregulating the pro-inflammatory cytokines and GM-CSF in B16F-10 metastatic melanoma bearing mice. J Exp Ther Oncol. 2008;7:17–29. [PubMed] [Google Scholar]
- 384.Kaur M, Goel RK. Anti-convulsant Activity of Boerhaavia diffusa: Plausible Role of Calcium Channel Antagonism. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nep192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Pari L, Amarnath Satheesh M. Antidiabetic effect of Boerhavia diffusa: effect on serum and tissue lipids in experimental diabetes. J Med Food. 2004;7:472–6. doi: 10.1089/jmf.2004.7.472. [DOI] [PubMed] [Google Scholar]
- 386.Pari L, Amarnath Satheesh M. Antidiabetic activity of Boerhaavia diffusa L: effect on hepatic key enzymes in experimental diabetes. J Ethnopharmacol. 2004;91:109–13. doi: 10.1016/j.jep.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 387.Leyon PV, Lini CC, Kuttan G. Inhibitory effect of Boerhaavia diffusa on experimental metastasis by B16F10 melanoma in C57BL/6 mice. Life Sci. 2005;76:1339–49. doi: 10.1016/j.lfs.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 388.Bharali R, Azad MR, Tabassum J. Chemopreventive action of Boerhaavia diffusa on DMBA-induced skin carcinogenesis in mice. Indian J Physiol Pharmacol. 2003;47:459–64. [PubMed] [Google Scholar]
- 389.Manu KA, Kuttan G. Punarnavine induces apoptosis in B16F-10 melanoma cells by inhibiting NF-kappaB signaling. Asian Pac J Cancer Prev. 2009;10:1031–7. [PubMed] [Google Scholar]
- 390.Manu KA, Kuttan G. Anti-metastatic potential of Punarnavine, an alkaloid from Boerhaavia diffusa Linn. Immunobiology. 2009;214:245–55. doi: 10.1016/j.imbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 391.Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S. Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int Immunopharmacol. 2005;5:541–53. doi: 10.1016/j.intimp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 392.Gayathri B, Manjula N, Vinaykumar KS, Lakshmi BS, Balakrishnan A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFalpha, IL-1beta, NO and MAP kinases. Int Immunopharmacol. 2007;7:473–82. doi: 10.1016/j.intimp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 393.Pang X, Yi Z, Zhang X, Sung B, Qu W, Lian X, et al. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009;69:5893–900. doi: 10.1158/0008-5472.CAN-09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sharma ML, Bani S, Singh GB. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int J Immunopharmacol. 1989;11:647–52. doi: 10.1016/0192-0561(89)90150-1. [DOI] [PubMed] [Google Scholar]
- 395.Madisch A, Miehlke S, Eichele O, Mrwa J, Bethke B, Kuhlisch E, et al. Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial. Int J Colorectal Dis. 2007;22:1445–51. doi: 10.1007/s00384-007-0364-1. [DOI] [PubMed] [Google Scholar]
- 396.Cuaz-Perolin C, Billiet L, Bauge E, Copin C, Scott-Algara D, Genze F, et al. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2008;28:272–7. doi: 10.1161/ATVBAHA.107.155606. [DOI] [PubMed] [Google Scholar]
- 397.Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Johri RK, et al. The gastric ulcer protective effect of boswellic acids, a leukotriene inhibitor from Boswellia serrata, in rats. Phytomedicine. 2008;15:408–15. doi: 10.1016/j.phymed.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 398.Sharma R, Singh S, Singh GD, Khajuria A, Sidiq T, Singh SK, et al. In vivo genotoxicity evaluation of a plant based antiarthritic and anticancer therapeutic agent Boswelic acids in rodents. Phytomedicine. 2009;16:1112–8. doi: 10.1016/j.phymed.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 399.Rasheed Z, Akhtar N, Khan A, Khan KA, Haqqi TM. Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells: suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8. J Pharmacol Exp Ther. 333:354–63. doi: 10.1124/jpet.109.165209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Panda S, Jafri M, Kar A, Meheta BK. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia. 2009;80:123–6. doi: 10.1016/j.fitote.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 401.Choedon T, Shukla SK, Kumar V. Chemopreventive and anti-cancer properties of the aqueous extract of flowers of Butea monosperma. J Ethnopharmacol. 129:208–13. doi: 10.1016/j.jep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 402.Sharma N, Garg V. Antidiabetic and antioxidant potential of ethanolic extract of Butea monosperma leaves in alloxan-induced diabetic mice. Indian J Biochem Biophys. 2009;46:99–105. [PubMed] [Google Scholar]
- 403.Sharma N, Shukla S. Hepatoprotective potential of aqueous extract of Butea monosperma against CCl(4) induced damage in rats. Exp Toxicol Pathol. doi: 10.1016/j.etp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 404.Shahavi VM, Desai SK. Anti-inflammatory activity of Butea monosperma flowers. Fitoterapia. 2008;79:82–5. doi: 10.1016/j.fitote.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 405.Sehrawat A, Sultana S. Chemoprevention by Butea monosperma of hepatic carcinogenesis and oxidative damage in male wistar rats. Asian Pac J Cancer Prev. 2006;7:140–8. [PubMed] [Google Scholar]
- 406.Sumitra M, Manikandan P, Suguna L. Efficacy of Butea monosperma on dermal wound healing in rats. Int J Biochem Cell Biol. 2005;37:566–73. doi: 10.1016/j.biocel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 407.Kasture VS, Kasture SB, Chopde CT. Anticonvulsive activity of Butea monosperma flowers in laboratory animals. Pharmacol Biochem Behav. 2002;72:965–72. doi: 10.1016/s0091-3057(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 408.Shukla S, Mehta A, Mehta P, Vyas SP, Shukla S, Bajpai VK. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem Toxicol. 48:61–4. doi: 10.1016/j.fct.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 409.Chakrabarti S, Biswas TK, Seal T, Rokeya B, Ali L, Azad Khan AK, et al. Antidiabetic activity of Caesalpinia bonducella F. in chronic type 2 diabetic model in Long-Evans rats and evaluation of insulin secretagogue property of its fractions on isolated islets. J Ethnopharmacol. 2005;97:117–22. doi: 10.1016/j.jep.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 410.Gupta M, Mazumder UK, Kumar RS, Sivakumar T, Vamsi ML. Antitumor activity and antioxidant status of Caesalpinia bonducella against Ehrlich ascites carcinoma in Swiss albino mice. J Pharmacol Sci. 2004;94:177–84. doi: 10.1254/jphs.94.177. [DOI] [PubMed] [Google Scholar]
- 411.Mathur R, Gupta SK, Mathur SR, Velpandian T. Anti-tumor studies with extracts of Calotropis procera (Ait.) R.Br. root employing Hep2 cells and their possible mechanism of action. Indian J Exp Biol. 2009;47:343–8. [PubMed] [Google Scholar]
- 412.Bharti S, Wahane VD, Kumar VL. Protective effect of Calotropis procera latex extracts on experimentally induced gastric ulcers in rat. J Ethnopharmacol. 127:440–4. doi: 10.1016/j.jep.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 413.Ramachandra Setty S, Quereshi AA, Viswanath Swamy AH, Patil T, Prakash T, Prabhu K, et al. Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia. 2007;78:451–4. doi: 10.1016/j.fitote.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 414.Kumar VL, Roy S. Calotropis procera latex extract affords protection against inflammation and oxidative stress in Freund’s complete adjuvant-induced monoarthritis in rats. Mediators Inflamm. 2007;2007:47523. doi: 10.1155/2007/47523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Kumar VL, Sehgal R. Calotropis procera latex-induced inflammatory hyperalgesia - effect of bradyzide and morphine. Auton Autacoid Pharmacol. 2007;27:143–9. doi: 10.1111/j.1474-8673.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- 416.Lam SK, Ng TB. A protein with antiproliferative, antifungal and HIV-1 reverse transcriptase inhibitory activities from caper (Capparis spinosa) seeds. Phytomedicine. 2009;16:444–50. doi: 10.1016/j.phymed.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 417.Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS. Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol. 2005;98:127–35. doi: 10.1016/j.jep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 418.Deeptha K, Kamaleeswari M, Sengottuvelan M, Nalini N. Dose dependent inhibitory effect of dietary caraway on 1,2-dimethylhydrazine induced colonic aberrant crypt foci and bacterial enzyme activity in rats. Invest New Drugs. 2006;24:479–88. doi: 10.1007/s10637-006-6801-0. [DOI] [PubMed] [Google Scholar]
- 419.Dashti-Rahmatabadi MH, Hejazian SH, Morshedi A, Rafati A. The analgesic effect of Carum copticum extract and morphine on phasic pain in mice. J Ethnopharmacol. 2007;109:226–8. doi: 10.1016/j.jep.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 420.Chandramohan G, Al-Numair KS, Sridevi M, Pugalendi KV. Antihyperlipidemic activity of 3-hydroxymethyl xylitol, a novel antidiabetic compound isolated from Casearia esculenta (Roxb.) root, in streptozotocin-diabetic rats. J Biochem Mol Toxicol. 24:95–101. doi: 10.1002/jbt.20317. [DOI] [PubMed] [Google Scholar]
- 421.Prakasam A, Sethupathy S, Pugalendi KV. Antiperoxidative and antioxidant effects of Casearia esculenta root extract in streptozotocin-induced diabetic rats. Yale J Biol Med. 2005;78:15–23. [PMC free article] [PubMed] [Google Scholar]
- 422.Gupta M, Mazumder UK, Rath N, Mukhopadhyay DK. Antitumor activity of methanolic extract of Cassia fistula L. seed against Ehrlich ascites carcinoma. J Ethnopharmacol. 2000;72:151–6. doi: 10.1016/s0378-8741(00)00227-0. [DOI] [PubMed] [Google Scholar]
- 423.el-Saadany SS, el-Massry RA, Labib SM, Sitohy MZ. The biochemical role and hypocholesterolaemic potential of the legume Cassia fistula in hypercholesterolaemic rats. Nahrung. 1991;35:807–15. doi: 10.1002/food.19910350804. [DOI] [PubMed] [Google Scholar]
- 424.Pradeep K, Mohan CV, Gobianand K, Karthikeyan S. Effect of Cassia fistula Linn. leaf extract on diethylnitrosamine induced hepatic injury in rats. Chem Biol Interact. 2007;167:12–8. doi: 10.1016/j.cbi.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 425.Sharma N, Trikha P, Athar M, Raisuddin S. In vitro inhibition of carcinogen-induced mutagenicity by Cassia occidentalis and Emblica officinalis. Drug Chem Toxicol. 2000;23:477–84. doi: 10.1081/dct-100100129. [DOI] [PubMed] [Google Scholar]
- 426.Jafri MA, Jalis Subhani M, Javed K, Singh S. Hepatoprotective activity of leaves of Cassia occidentalis against paracetamol and ethyl alcohol intoxication in rats. J Ethnopharmacol. 1999;66:355–61. doi: 10.1016/s0378-8741(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 427.Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13–8. doi: 10.1016/s0378-8741(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 428.Yadav JP, Arya V, Yadav S, Panghal M, Kumar S, Dhankhar S. Cassia occidentalis L.: a review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia. 81:223–30. doi: 10.1016/j.fitote.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 429.Dhanasekaran M, Ignacimuthu S, Agastian P. Potential hepatoprotective activity of ononitol monohydrate isolated from Cassia tora L. on carbon tetrachloride induced hepatotoxicity in wistar rats. Phytomedicine. 2009;16:891–5. doi: 10.1016/j.phymed.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 430.Rejiya CS, Cibin TR, Abraham A. Leaves of Cassia tora as a novel cancer therapeutic--an in vitro study. Toxicol In Vitro. 2009;23:1034–8. doi: 10.1016/j.tiv.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 431.Lim SHHK. Hypoglycemic effect of fractions of Cassia tora extract in Streptozotocin-induced diabetic Rats. Journal of the Korean Society of Food Science and Nutrition. 1997;13:23–9. [Google Scholar]
- 432.Cho IJ, Lee C, Ha TY. Hypolipidemic effect of soluble fiber isolated from seeds of Cassia tora Linn. in rats fed a high-cholesterol diet. J Agric Food Chem. 2007;55:1592–6. doi: 10.1021/jf0622127. [DOI] [PubMed] [Google Scholar]
- 433.Nam J, Choi H. Effect of butanol fraction from Cassia tora L. seeds on glycemic control and insulin secretion in diabetic rats. Nutr Res Pract. 2008;2:240–6. doi: 10.4162/nrp.2008.2.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Singh SK, Shanmugavel M, Kampasi H, Singh R, Mondhe DM, Rao JM, et al. Chemically standardized isolates from Cedrus deodara stem wood having anticancer activity. Planta Med. 2007;73:519–26. doi: 10.1055/s-2007-967185. [DOI] [PubMed] [Google Scholar]
- 435.Shinde UA, Kulkarni KR, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, et al. Mast cell stabilizing and lipoxygenase inhibitory activity of Cedrus deodara (Roxb.) Loud. wood oil. Indian J Exp Biol. 1999;37:258–61. [PubMed] [Google Scholar]
- 436.Godkar PB, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed oil and organic extracts attenuate hydrogen peroxide- and glutamate-induced injury in embryonic rat forebrain neuronal cells. Phytomedicine. 2006;13:29–36. doi: 10.1016/j.phymed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 437.Kumar MH, Gupta YK. Antioxidant property of Celastrus paniculatus willd : a possible mechanism in enhancing cognition. Phytomedicine. 2002;9:302–11. doi: 10.1078/0944-7113-00136. [DOI] [PubMed] [Google Scholar]
- 438.Lee KT, Kim JI, Park HJ, Yoo KO, Han YN, Miyamoto K. Differentiation-inducing effect of magnolialide, a 1 beta-hydroxyeudesmanolide isolated from Cichorium intybus, on human leukemia cells. Biol Pharm Bull. 2000;23:1005–7. doi: 10.1248/bpb.23.1005. [DOI] [PubMed] [Google Scholar]
- 439.Ahmed B, Khan S, Masood MH, Siddique AH. Anti-hepatotoxic activity of cichotyboside, a sesquiterpene glycoside from the seeds of Cichorium intybus. J Asian Nat Prod Res. 2008;10:223–31. doi: 10.1080/10286020701590764. [DOI] [PubMed] [Google Scholar]
- 440.Kim M. The water-soluble extract of chicory reduces cholesterol uptake in gut-perfused rats. Nutrition Research. 2000;20:1017–26. [Google Scholar]
- 441.Pushparaj PN, Low HK, Manikandan J, Tan BK, Tan CH. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111:430–4. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 442.Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul-Ghanekar R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 10:210. doi: 10.1186/1471-2407-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Lim CS, Kim EY, Lee HS, Soh Y, Sohn Y, Kim SY, et al. Protective effects of Cinnamomum cassia Blume in the fibrogenesis of activated HSC-T6 cells and dimethylnitrosamine-induced acute liver injury in SD rats. Biosci Biotechnol Biochem. 74:477–83. doi: 10.1271/bbb.90435. [DOI] [PubMed] [Google Scholar]
- 444.Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11:1100–13. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 445.Dugoua JJ, Seely D, Perri D, Cooley K, Forelli T, Mills E, et al. From type 2 diabetes to antioxidant activity: a systematic review of the safety and efficacy of common and cassia cinnamon bark. Can J Physiol Pharmacol. 2007;85:837–47. doi: 10.1139/Y07-080. [DOI] [PubMed] [Google Scholar]
- 446.Lee CW, Lee SH, Lee JW, Ban JO, Lee SY, Yoo HS, et al. 2-hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth through AP-1 inactivation. J Pharmacol Sci. 2007;104:19–28. doi: 10.1254/jphs.fp0061204. [DOI] [PubMed] [Google Scholar]
- 447.Ng LT, Wu SJ. Antiproliferative Activity of Cinnamomum cassia Constituents and Effects of Pifithrin-Alpha on Their Apoptotic Signaling Pathways in Hep G2 Cells. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nep220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Ka H, Park HJ, Jung HJ, Choi JW, Cho KS, Ha J, et al. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003;196:143–52. doi: 10.1016/s0304-3835(03)00238-6. [DOI] [PubMed] [Google Scholar]
- 449.Cao H, Urban JF, Jr, Anderson RA. Cinnamon polyphenol extract affects immune responses by regulating anti- and proinflammatory and glucose transporter gene expression in mouse macrophages. J Nutr. 2008;138:833–40. doi: 10.1093/jn/138.5.833. [DOI] [PubMed] [Google Scholar]
- 450.Kwon HK, Jeon WK, Hwang JS, Lee CG, So JS, Park JA, et al. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett. 2009;278:174–82. doi: 10.1016/j.canlet.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 451.Qin B, Dawson H, Polansky MM, Anderson RA. Cinnamon extract attenuates TNF-alpha-induced intestinal lipoprotein ApoB48 overproduction by regulating inflammatory, insulin, and lipoprotein pathways in enterocytes. Horm Metab Res. 2009;41:516–22. doi: 10.1055/s-0029-1202813. [DOI] [PubMed] [Google Scholar]
- 452.Kanuri G, Weber S, Volynets V, Spruss A, Bischoff SC, Bergheim I. Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. J Nutr. 2009;139:482–7. doi: 10.3945/jn.108.100495. [DOI] [PubMed] [Google Scholar]
- 453.Moselhy SS, Ali HK. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res. 2009;42:93–8. [PubMed] [Google Scholar]
- 454.Abdel-Hassan IA, Abdel-Barry JA, Tariq Mohammeda S. The hypoglycaemic and antihyperglycaemic effect of citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J Ethnopharmacol. 2000;71:325–30. doi: 10.1016/s0378-8741(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 455.Daradka H, Almasad MM, Qazan W, El-Banna NM, Samara OH. Hypolipidaemic effects of Citrullus colocynthis L. in rabbits. Pak J Biol Sci. 2007;10:2768–71. doi: 10.3923/pjbs.2007.2768.2771. [DOI] [PubMed] [Google Scholar]
- 456.Huseini HF, Darvishzadeh F, Heshmat R, Jafariazar Z, Raza M, Larijani B. The clinical investigation of Citrullus colocynthis (L) schrad fruit in treatment of Type II diabetic patients: a randomized, double blind, placebo-controlled clinical trial. Phytother Res. 2009;23:1186–9. doi: 10.1002/ptr.2754. [DOI] [PubMed] [Google Scholar]
- 457.Marzouk B, Marzouk Z, Haloui E, Fenina N, Bouraoui A, Aouni M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J Ethnopharmacol. 128:15–9. doi: 10.1016/j.jep.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 458.Manjula N, Gayathri B, Vinaykumar KS, Shankernarayanan NP, Vishwakarma RA, Balakrishnan A. Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down regulation of TNF-alpha, IL-1beta and IL-2. Int Immunopharmacol. 2006;6:122–32. doi: 10.1016/j.intimp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 459.Shishodia S, Sethi G, Ahn KS, Aggarwal BB. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem Pharmacol. 2007;74:118–30. doi: 10.1016/j.bcp.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Mencarelli A, Renga B, Palladino G, Distrutti E, Fiorucci S. The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem Pharmacol. 2009;78:1214–23. doi: 10.1016/j.bcp.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 461.Sharma B, Salunke R, Srivastava S, Majumder C, Roy P. Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem Toxicol. 2009;47:2631–9. doi: 10.1016/j.fct.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 462.Nohr LA, Rasmussen LB, Straand J. Resin from the mukul myrrh tree, guggul, can it be used for treating hypercholesterolemia? A randomized, controlled study. Complement Ther Med. 2009;17:16–22. doi: 10.1016/j.ctim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 463.Yu BZ, Kaimal R, Bai S, El Sayed KA, Tatulian SA, Apitz RJ, et al. Effect of guggulsterone and cembranoids of Commiphora mukul on pancreatic phospholipase A(2): role in hypocholesterolemia. J Nat Prod. 2009;72:24–8. doi: 10.1021/np8004453. [DOI] [PubMed] [Google Scholar]
- 464.Panda S, Kar A. Guggulu (Commiphora mukul) potentially ameliorates hypothyroidism in female mice. Phytother Res. 2005;19:78–80. doi: 10.1002/ptr.1602. [DOI] [PubMed] [Google Scholar]
- 465.Deng R. Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. Cardiovasc Drug Rev. 2007;25:375–90. doi: 10.1111/j.1527-3466.2007.00023.x. [DOI] [PubMed] [Google Scholar]
- 466.Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB. The guggul for chronic diseases: ancient medicine, modern targets. Anticancer Res. 2008;28:3647–64. [PubMed] [Google Scholar]
- 467.Agarwal RC, Singh SP, Saran RK, Das SK, Sinha N, Asthana OP, et al. Clinical trial of gugulipid--a new hypolipidemic agent of plant origin in primary hyperlipidemia. Indian J Med Res. 1986;84:626–34. [PubMed] [Google Scholar]
- 468.Szapary PO, Wolfe ML, Bloedon LT, Cucchiara AJ, DerMarderosian AH, Cirigliano MD, et al. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA. 2003;290:765–72. doi: 10.1001/jama.290.6.765. [DOI] [PubMed] [Google Scholar]
- 469.Tripathi YB, Reddy MM, Pandey RS, Subhashini J, Tiwari OP, Singh BK, et al. Anti-inflammatory properties of BHUx, a polyherbal formulation to prevent atherosclerosis. Inflammopharmacology. 2004;12:131–52. doi: 10.1163/1568560041352301. [DOI] [PubMed] [Google Scholar]
- 470.Chauhan CK, Joshi MJ, Vaidya AD. Growth inhibition of struvite crystals in the presence of herbal extract Commiphora wightii. J Mater Sci Mater Med. 2009;20 (Suppl 1):S85–92. doi: 10.1007/s10856-008-3489-z. [DOI] [PubMed] [Google Scholar]
- 471.Bihaqi SW, Sharma M, Singh AP, Tiwari M. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J Ethnopharmacol. 2009;124:409–15. doi: 10.1016/j.jep.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 472.Dhingra D, Valecha R. Evaluation of the antidepressant-like activity of Convolvulus pluricaulis choisy in the mouse forced swim and tail suspension tests. Med Sci Monit. 2007;13:BR155–61. [PubMed] [Google Scholar]
- 473.Sairam K, Rao CV, Goel RK. Effect of Convolvulus pluricaulis Chois on gastric ulceration and secretion in rats. Indian J Exp Biol. 2001;39:350–4. [PubMed] [Google Scholar]
- 474.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29:175–80. [PMC free article] [PubMed] [Google Scholar]
- 475.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 24:990–4. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 476.Asdaq SM, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. 162:358–72. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 477.Xu GL, Li G, Ma HP, Zhong H, Liu F, Ao GZ. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264. 7 cells. J Agric Food Chem. 2009;57:8325–30. doi: 10.1021/jf901752f. [DOI] [PubMed] [Google Scholar]
- 478.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–50. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr. 2007;157:315–9. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- 480.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 481.Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: a review. Cancer Biother. 1995;10:257–64. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 482.Tavakkol-Afshari J, Brook A, Mousavi SH. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol. 2008;46:3443–7. doi: 10.1016/j.fct.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 483.Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, et al. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770:578–84. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 484.Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology (Berl) 207:637–43. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 485.Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, et al. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med. 64:24–30. doi: 10.1007/s11418-009-0360-6. [DOI] [PubMed] [Google Scholar]
- 486.Sambaiah K, Srinivasan K. Effect of cumin, cinnamon, ginger, mustard and tamarind in induced hypercholesterolemic rats. Nahrung. 1991;35:47–51. doi: 10.1002/food.19910350112. [DOI] [PubMed] [Google Scholar]
- 487.Gagandeep, Dhanalakshmi S, Mendiz E, Rao AR, Kale RK. Chemopreventive effects of Cuminum cyminum in chemically induced forestomach and uterine cervix tumors in murine model systems. Nutr Cancer. 2003;47:171–80. doi: 10.1207/s15327914nc4702_10. [DOI] [PubMed] [Google Scholar]
- 488.Janahmadi M, Niazi F, Danyali S, Kamalinejad M. Effects of the fruit essential oil of Cuminum cyminum Linn. (Apiaceae) on pentylenetetrazol-induced epileptiform activity in F1 neurones of Helix aspersa. J Ethnopharmacol. 2006;104:278–82. doi: 10.1016/j.jep.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 489.Shirke SS, Jagtap AG. Effects of methanolic extract of Cuminum cyminum on total serum cholesterol in ovariectomized rats. Indian J Pharmacol. 2009;41:92–3. doi: 10.4103/0253-7613.51353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Jagtap AG, Patil PB. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food Chem Toxicol. 48:2030–6. doi: 10.1016/j.fct.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 491.Jatoi SA, Kikuchi A, Gilani SA, Watanabe KN. Phytochemical, pharmacological and ethnobotanical studies in mango ginger (Curcuma amada Roxb; Zingiberaceae) Phytother Res. 2007;21:507–16. doi: 10.1002/ptr.2137. [DOI] [PubMed] [Google Scholar]
- 492.Arafa HM. Uroprotective effects of curcumin in cyclophosphamide-induced haemorrhagic cystitis paradigm. Basic Clin Pharmacol Toxicol. 2009;104:393–9. doi: 10.1111/j.1742-7843.2009.00379.x. [DOI] [PubMed] [Google Scholar]
- 493.Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, et al. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–9. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Martelli L, Ragazzi E, di Mario F, Martelli M, Castagliuolo I, Dal Maschio M, et al. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol Motil. 2007;19:668–74. doi: 10.1111/j.1365-2982.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 495.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–9. [PubMed] [Google Scholar]
- 496.Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Arch Physiol Biochem. 2008;114:127–49. doi: 10.1080/13813450802033958. [DOI] [PubMed] [Google Scholar]
- 497.Yoshioka T, Fujii E, Endo M, Wada K, Tokunaga Y, Shiba N, et al. Antiinflammatory potency of dehydrocurdione, a zedoary-derived sesquiterpene. Inflamm Res. 1998;47:476–81. doi: 10.1007/s000110050361. [DOI] [PubMed] [Google Scholar]
- 498.Oh OJ, Min HY, Lee SK. Inhibition of inducible prostaglandin E2 production and cyclooxygenase-2 expression by curdione from Curcuma zedoaria. Archives of pharmacal research. 2007;30:1236–9. doi: 10.1007/BF02980264. [DOI] [PubMed] [Google Scholar]
- 499.Seo WG, Hwang JC, Kang SK, Jin UH, Suh SJ, Moon SK, et al. Suppressive effect of Zedoariae rhizoma on pulmonary metastasis of B16 melanoma cells. Journal of ethnopharmacology. 2005;101:249–57. doi: 10.1016/j.jep.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 500.Makabe H, Maru N, Kuwabara A, Kamo T, Hirota M. Anti-inflammatory sesquiterpenes from Curcuma zedoaria. Natural product research. 2006;20:680–5. doi: 10.1080/14786410500462900. [DOI] [PubMed] [Google Scholar]
- 501.Matsuda H, Tewtrakul S, Morikawa T, Nakamura A, Yoshikawa M. Anti-allergic principles from Thai zedoary: structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorganic & medicinal chemistry. 2004;12:5891–8. doi: 10.1016/j.bmc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 502.Matsuda H, Ninomiya K, Morikawa T, Yoshikawa M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/lipopolysaccharide-induced liver injury. Bioorganic & medicinal chemistry letters. 1998;8:339–44. doi: 10.1016/s0960-894x(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 503.Kim KI, Kim JW, Hong BS, Shin DH, Cho HY, Kim HK, et al. Antitumor, genotoxicity and anticlastogenic activities of polysaccharide from Curcuma zedoaria. Molecules and cells. 2000;10:392–8. [PubMed] [Google Scholar]
- 504.Katsukawa M, Nakata R, Takizawa Y, Hori K, Takahashi S, Inoue H. Citral, a component of lemongrass oil, activates PPARalpha and gamma and suppresses COX-2 expression. Biochim Biophys Acta. doi: 10.1016/j.bbalip.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 505.Lee HJ, Jeong HS, Kim DJ, Noh YH, Yuk DY, Hong JT. Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-kappa B activation in RAW264. 7 cells. Arch Pharm Res. 2008;31:342–9. doi: 10.1007/s12272-001-1162-0. [DOI] [PubMed] [Google Scholar]
- 506.Figueirinha A, Cruz MT, Francisco V, Lopes MC, Batista MT. Anti-inflammatory activity of Cymbopogon citratus leaf infusion in lipopolysaccharide-stimulated dendritic cells: contribution of the polyphenols. J Med Food. 13:681–90. doi: 10.1089/jmf.2009.0115. [DOI] [PubMed] [Google Scholar]
- 507.Carbajal D, Casaco A, Arruzazabala L, Gonzalez R, Tolon Z. Pharmacological study of Cymbopogon citratus leaves. J Ethnopharmacol. 1989;25:103–7. doi: 10.1016/0378-8741(89)90049-4. [DOI] [PubMed] [Google Scholar]
- 508.Sforcin JM, Amaral JT, Fernandes A, Jr, Sousa JP, Bastos JK. Lemongrass effects on IL-1beta and IL-6 production by macrophages. Nat Prod Res. 2009;23:1151–9. doi: 10.1080/14786410902800681. [DOI] [PubMed] [Google Scholar]
- 509.Silva MR, Ximenes RM, da Costa JG, Leal LK, de Lopes AA, Viana GS. Comparative anticonvulsant activities of the essential oils (EOs) from Cymbopogon winterianus Jowitt and Cymbopogon citratus (DC) Stapf. in mice. Naunyn Schmiedebergs Arch Pharmacol. 381:415–26. doi: 10.1007/s00210-010-0494-9. [DOI] [PubMed] [Google Scholar]
- 510.Kilani S, Ben Sghaier M, Limem I, Bouhlel I, Boubaker J, Bhouri W, et al. In vitro evaluation of antibacterial, antioxidant, cytotoxic and apoptotic activities of the tubers infusion and extracts of Cyperus rotundus. Bioresource technology. 2008;99:9004–8. doi: 10.1016/j.biortech.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 511.Kilani-Jaziri S, Neffati A, Limem I, Boubaker J, Skandrani I, Sghair MB, et al. Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chemico-biological interactions. 2009;181:85–94. doi: 10.1016/j.cbi.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 512.Raut NA, Gaikwad NJ. Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia. 2006;77:585–8. doi: 10.1016/j.fitote.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 513.Muthu AK, Sethupathy S, Manavalan R, Karar PK. Hypolipidemic effect of methanolic extract of Dolichos biflorus Linn. in high fat diet fed rats. Indian journal of experimental biology. 2005;43:522–5. [PubMed] [Google Scholar]
- 514.Bijarnia RK, Kaur T, Singla SK, Tandon C. A novel calcium oxalate crystal growth inhibitory protein from the seeds of Dolichos biflorus (L) The protein journal. 2009;28:161–8. doi: 10.1007/s10930-009-9179-y. [DOI] [PubMed] [Google Scholar]
- 515.Singh B, Saxena AK, Chandan BK, Agarwal SG, Anand KK. In vivo hepatoprotective activity of active fraction from ethanolic extract of Eclipta alba leaves. Indian journal of physiology and pharmacology. 2001;45:435–41. [PubMed] [Google Scholar]
- 516.Jayathirtha MG, Mishra SH. Preliminary immunomodulatory activities of methanol extracts of Eclipta alba and Centella asiatica. Phytomedicine. 2004;11:361–5. doi: 10.1078/0944711041495236. [DOI] [PubMed] [Google Scholar]
- 517.Ananthi J, Prakasam A, Pugalendi KV. Antihyperglycemic activity of Eclipta alba leaf on alloxan-induced diabetic rats. The Yale journal of biology and medicine. 2003;76:97–102. [PMC free article] [PubMed] [Google Scholar]
- 518.Thakur VD, Mengi SA. Neuropharmacological profile of Eclipta alba (Linn) Hassk Journal of ethnopharmacology. 2005;102:23–31. doi: 10.1016/j.jep.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 519.Rangineni V, Sharada D, Saxena S. Diuretic, hypotensive, and hypocholesterolemic effects of Eclipta alba in mild hypertensive subjects: a pilot study. Journal of medicinal food. 2007;10:143–8. doi: 10.1089/jmf.2006.0000. [DOI] [PubMed] [Google Scholar]
- 520.Sengupta A, Ghosh S, Bhattacharjee S. Dietary cardamom inhibits the formation of azoxymethane-induced aberrant crypt foci in mice and reduces COX-2 and iNOS expression in the colon. Asian Pac J Cancer Prev. 2005;6:118–22. [PubMed] [Google Scholar]
- 521.Bhattacharjee S, Rana T, Sengupta A. Inhibition of lipid peroxidation and enhancement of GST activity by cardamom and cinnamon during chemically induced colon carcinogenesis in Swiss albino mice. Asian Pac J Cancer Prev. 2007;8:578–82. [PubMed] [Google Scholar]
- 522.Gilani AH, Jabeen Q, Khan AU, Shah AJ. Gut modulatory, blood pressure lowering, diuretic and sedative activities of cardamom. Journal of ethnopharmacology. 2008;115:463–72. doi: 10.1016/j.jep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 523.Xu M, Cui J, Fu H, Proksch P, Lin W, Li M. Embelin derivatives and their anticancer activity through microtubule disassembly. Planta medica. 2005;71:944–8. doi: 10.1055/s-2005-871250. [DOI] [PubMed] [Google Scholar]
- 524.Chitra M, Sukumar E, Suja V, Devi CS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1994;40:109–13. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 525.Sreepriya M, Bali G. Chemopreventive effects of embelin and curcumin against N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Fitoterapia. 2005;76:549–55. doi: 10.1016/j.fitote.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 526.Singh D, Singh R, Singh P, Gupta RS. Effects of Embelin on Lipid Peroxidation and Free Radical Scavenging Activity against Liver Damage in Rats. Basic & clinical pharmacology & toxicology. 2009 doi: 10.1111/j.1742-7843.2009.00429.x. [DOI] [PubMed] [Google Scholar]
- 527.Mahendran S, Thippeswamy BS, Veerapur VP, Badami S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine. doi: 10.1016/j.phymed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 528.Bhandari U, Ansari MN. Antihyperglycaemic activity of aqueous extract of Embelia ribes Burm in streptozotocin-induced diabetic rats. Indian journal of experimental biology. 2008;46:607–13. [PubMed] [Google Scholar]
- 529.Bhandari U, Ansari MN, Islam F. Cardioprotective effect of aqueous extract of Embelia ribes Burm fruits against isoproterenol-induced myocardial infarction in albino rats. Indian journal of experimental biology. 2008;46:35–40. [PubMed] [Google Scholar]
- 530.Nazam Ansari M, Bhandari U, Islam F, Tripathi CD. Evaluation of antioxidant and neuroprotective effect of ethanolic extract of Embelia ribes Burm in focal cerebral ischemia/reperfusion-induced oxidative stress in rats. Fundamental & clinical pharmacology. 2008;22:305–14. doi: 10.1111/j.1472-8206.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 531.Nicolis E, Lampronti I, Dechecchi MC, Borgatti M, Tamanini A, Bianchi N, et al. Pyrogallol, an active compound from the medicinal plant Emblica officinalis, regulates expression of pro-inflammatory genes in bronchial epithelial cells. International immunopharmacology. 2008;8:1672–80. doi: 10.1016/j.intimp.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 532.Yang CJ, Wang CS, Hung JY, Huang HW, Chia YC, Wang PH, et al. Pyrogallol induces G2-M arrest in human lung cancer cells and inhibits tumor growth in an animal model. Lung cancer (Amsterdam, Netherlands) 2009;66:162–8. doi: 10.1016/j.lungcan.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 533.Sultana S, Ahmed S, Jahangir T. Emblica officinalis and hepatocarcinogenesis: a chemopreventive study in Wistar rats. Journal of ethnopharmacology. 2008;118:1–6. doi: 10.1016/j.jep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 534.Saini A, Sharma S, Chhibber S. Protective efficacy of Emblica officinalis against Klebsiella pneumoniae induced pneumonia in mice. The Indian journal of medical research. 2008;128:188–93. [PubMed] [Google Scholar]
- 535.Kumar NP, Annamalai AR, Thakur RS. Antinociceptive property of Emblica officinalis Gaertn (Amla) in high fat diet-fed/low dose streptozotocin induced diabetic neuropathy in rats. Indian journal of experimental biology. 2009;47:737–42. [PubMed] [Google Scholar]
- 536.Muthuraman A, Sood S, Singla SK. The antiinflammatory potential of phenolic compounds from Emblica officinalis L. in rat. Inflammopharmacology. doi: 10.1007/s10787-010-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Li L, Adams LS, Chen S, Killian C, Ahmed A, Seeram NP. Eugenia jambolana Lam. berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. Journal of agricultural and food chemistry. 2009;57:826–31. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Grover JK, Rathi SS, Vats V. Amelioration of experimental diabetic neuropathy and gastropathy in rats following oral administration of plant (Eugenia jambolana, Mucuna pruriens and Tinospora cordifolia) extracts. Indian J Exp Biol. 2002;40:273–6. [PubMed] [Google Scholar]
- 539.Sharma SB, Nasir A, Prabhu KM, Murthy PS. Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. Journal of ethnopharmacology. 2006;104:367–73. doi: 10.1016/j.jep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 540.Sharma B, Balomajumder C, Roy P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol. 2008;46:2376–83. doi: 10.1016/j.fct.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 541.Chaturvedi A, Bhawani G, Agarwal PK, Goel S, Singh A, Goel RK. Antidiabetic and antiulcer effects of extract of Eugenia jambolana seed in mild diabetic rats: study on gastric mucosal offensive acid-pepsin secretion. Indian journal of physiology and pharmacology. 2009;53:137–46. [PubMed] [Google Scholar]
- 542.Tanwar RS, Sharma SB, Singh UR, Prabhu KM. Attenuation of renal dysfunction by anti-hyperglycemic compound isolated from fruit pulp of Eugenia jambolana in streptozotocin-induced diabetic rats. Indian journal of biochemistry & biophysics. 47:83–9. [PubMed] [Google Scholar]
- 543.Siripurapu KB, Gupta P, Bhatia G, Maurya R, Nath C, Palit G. Adaptogenic and anti-amnesic properties of Evolvulus alsinoides in rodents. Pharmacology, biochemistry, and behavior. 2005;81:424–32. doi: 10.1016/j.pbb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 544.Gupta P, Akanksha, Siripurapu KB, Ahmad A, Palit G, Arora A, et al. Anti-stress constituents of Evolvulus alsinoides: an ayurvedic crude drug. Chemical & pharmaceutical bulletin. 2007;55:771–5. doi: 10.1248/cpb.55.771. [DOI] [PubMed] [Google Scholar]
- 545.Nahata A, Patil UK, Dixit VK. Effect of Evolvulus alsinoides Linn. on learning behavior and memory enhancement activity in rodents. Phytother Res. 24:486–93. doi: 10.1002/ptr.2932. [DOI] [PubMed] [Google Scholar]
- 546.Lee CL, Chiang LC, Cheng LH, Liaw CC, Abd El-Razek MH, Chang FR, et al. Influenza A (H(1)N(1)) Antiviral and Cytotoxic Agents from Ferula assa-foetida. Journal of natural products. 2009;72:1568–72. doi: 10.1021/np900158f. [DOI] [PubMed] [Google Scholar]
- 547.Mallikarjuna GU, Dhanalakshmi S, Raisuddin S, Rao AR. Chemomodulatory influence of Ferula asafoetida on mammary epithelial differentiation, hepatic drug metabolizing enzymes, antioxidant profiles and N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Breast cancer research and treatment. 2003;81:1–10. doi: 10.1023/a:1025448620558. [DOI] [PubMed] [Google Scholar]
- 548.Fatehi M, Farifteh F, Fatehi-Hassanabad Z. Antispasmodic and hypotensive effects of Ferula asafoetida gum extract. Journal of ethnopharmacology. 2004;91:321–4. doi: 10.1016/j.jep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 549.Abu-Zaiton AS. Anti-diabetic activity of Ferula assafoetida extract in normal and alloxan-induced diabetic rats. Pakistan journal of biological sciences: PJBS. 13:97–100. doi: 10.3923/pjbs.2010.97.100. [DOI] [PubMed] [Google Scholar]
- 550.Geetha BS, Mathew BC, Augusti KT. Hypoglycemic effects of leucodelphinidin derivative isolated from Ficus bengalensis (Linn) Indian journal of physiology and pharmacology. 1994;38:220–2. [PubMed] [Google Scholar]
- 551.Daniel RS, Devi KS, Augusti KT, Sudhakaran Nair CR. Mechanism of action of antiatherogenic and related effects of Ficus bengalensis Linn. flavonoids in experimental animals. Indian journal of experimental biology. 2003;41:296–303. [PubMed] [Google Scholar]
- 552.Shukla R, Gupta S, Gambhir JK, Prabhu KM, Murthy PS. Antioxidant effect of aqueous extract of the bark of Ficus bengalensis in hypercholesterolaemic rabbits. Journal of ethnopharmacology. 2004;92:47–51. doi: 10.1016/j.jep.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 553.Singh RK, Mehta S, Jaiswal D, Rai PK, Watal G. Antidiabetic effect of Ficus bengalensis aerial roots in experimental animals. Journal of ethnopharmacology. 2009;123:110–4. doi: 10.1016/j.jep.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 554.Thakare VN, Suralkar AA, Deshpande AD, Naik SR. Stem bark extraction of Ficus bengalensis Linn for anti-inflammatory and analgesic activity in animal models. Indian journal of experimental biology. 48:39–45. [PubMed] [Google Scholar]
- 555.Tognolini M, Ballabeni V, Bertoni S, Bruni R, Impicciatore M, Barocelli E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol Res. 2007;56:254–60. doi: 10.1016/j.phrs.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 556.Miguel MG, Cruz C, Faleiro L, Simoes MT, Figueiredo AC, Barroso JG, et al. Foeniculum vulgare essential oils: chemical composition, antioxidant and antimicrobial activities. Natural product communications. 5:319–28. [PubMed] [Google Scholar]
- 557.Choi EM, Hwang JK. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004;75:557–65. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 558.Joshi H, Parle M. Cholinergic basis of memory-strengthening effect of Foeniculum vulgare Linn. Journal of medicinal food. 2006;9:413–7. doi: 10.1089/jmf.2006.9.413. [DOI] [PubMed] [Google Scholar]
- 559.Agarwal R, Gupta SK, Agrawal SS, Srivastava S, Saxena R. Oculohypotensive effects of foeniculum vulgare in experimental models of glaucoma. Indian journal of physiology and pharmacology. 2008;52:77–83. [PubMed] [Google Scholar]
- 560.Singh B, Kale RK. Chemomodulatory action of Foeniculum vulgare (Fennel) on skin and forestomach papillomagenesis, enzymes associated with xenobiotic metabolism and antioxidant status in murine model system. Food Chem Toxicol. 2008;46:3842–50. doi: 10.1016/j.fct.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 561.Pan MH, Chang WL, Lin-Shiau SY, Ho CT, Lin JK. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. Journal of agricultural and food chemistry. 2001;49:1464–74. doi: 10.1021/jf001129v. [DOI] [PubMed] [Google Scholar]
- 562.Prasad S, Ravindran J, Sung B, Pandey MK, Aggarwal BB. Garcinol potentiates TRAIL-induced apoptosis through modulation of death receptors and antiapoptotic proteins. Molecular cancer therapeutics. 9:856–68. doi: 10.1158/1535-7163.MCT-09-1113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 563.Ahmad A, Wang Z, Ali R, Maitah MY, Kong D, Banerjee S, et al. Apoptosis-inducing effect of garcinol is mediated by NF-kappaB signaling in breast cancer cells. Journal of cellular biochemistry. 109:1134–41. doi: 10.1002/jcb.22492. [DOI] [PubMed] [Google Scholar]
- 564.Yoshida K, Tanaka T, Hirose Y, Yamaguchi F, Kohno H, Toida M, et al. Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Cancer letters. 2005;221:29–39. doi: 10.1016/j.canlet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 565.Padhye S, Ahmad A, Oswal N, Sarkar FH. Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. Journal of hematology & oncology. 2009;2:38. doi: 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. Jama. 1998;280:1596–600. doi: 10.1001/jama.280.18.1596. [DOI] [PubMed] [Google Scholar]
- 567.Kolodziejczyk J, Masullo M, Olas B, Piacente S, Wachowicz B. Effects of garcinol and guttiferone K isolated from Garcinia cambogia on oxidative/nitrative modifications in blood platelets and plasma. Platelets. 2009;20:487–92. doi: 10.3109/09537100903165182. [DOI] [PubMed] [Google Scholar]
- 568.Lau GT, Ye L, Leung LK. The licorice flavonoid isoliquiritigenin suppresses phorbol ester-induced cyclooxygenase-2 expression in the non-tumorigenic MCF-10A breast cell line. Planta medica. 76:780–5. doi: 10.1055/s-0029-1240699. [DOI] [PubMed] [Google Scholar]
- 569.Wan XY, Luo M, Li XD, He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chemico-biological interactions. 2009;181:15–9. doi: 10.1016/j.cbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 570.Yu XQ, Xue CC, Zhou ZW, Li CG, Du YM, Liang J, et al. In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice) Life sciences. 2008;82:68–78. doi: 10.1016/j.lfs.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 571.Visavadiya NP, Soni B, Dalwadi N. Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. International journal of food sciences and nutrition. 2009;60 (Suppl 2):135–49. doi: 10.1080/09637480902877998. [DOI] [PubMed] [Google Scholar]
- 572.Shin YW, Bae EA, Lee B, Lee SH, Kim JA, Kim YS, et al. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta medica. 2007;73:257–61. doi: 10.1055/s-2007-967126. [DOI] [PubMed] [Google Scholar]
- 573.Sheela ML, Ramakrishna MK, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. International immunopharmacology. 2006;6:494–8. doi: 10.1016/j.intimp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 574.Visavadiya NP, Narasimhacharya AV. Hypocholesterolaemic and antioxidant effects of Glycyrrhiza glabra (Linn) in rats. Molecular nutrition & food research. 2006;50:1080–6. doi: 10.1002/mnfr.200600063. [DOI] [PubMed] [Google Scholar]
- 575.Gholap S, Kar A. Effects of Inula racemosa root and Gymnema sylvestre leaf extracts in the regulation of corticosteroid induced diabetes mellitus: involvement of thyroid hormones. Pharmazie. 2003;58:413–5. [PubMed] [Google Scholar]
- 576.Daisy P, Eliza J, Mohamed Farook KA. A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. Journal of ethnopharmacology. 2009;126:339–44. doi: 10.1016/j.jep.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 577.Yadav M, Lavania A, Tomar R, Prasad GB, Jain S, Yadav H. Complementary and comparative study on hypoglycemic and antihyperglycemic activity of various extracts of Eugenia jambolana seed, Momordica charantia fruits, Gymnema sylvestre, and Trigonella foenum graecum seeds in rats. Applied biochemistry and biotechnology. 160:2388–400. doi: 10.1007/s12010-009-8799-1. [DOI] [PubMed] [Google Scholar]
- 578.Kanetkar P, Singhal R, Kamat M. Gymnema sylvestre: A Memoir. Journal of clinical biochemistry and nutrition. 2007;41:77–81. doi: 10.3164/jcbn.2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Leach MJ. Gymnema sylvestre for diabetes mellitus: a systematic review. Journal of alternative and complementary medicine (New York, NY. 2007;13:977–83. doi: 10.1089/acm.2006.6387. [DOI] [PubMed] [Google Scholar]
- 580.Alam MI, Gomes A. Viper venom-induced inflammation and inhibition of free radical formation by pure compound (2-hydroxy-4-methoxy benzoic acid) isolated and purified from anantamul (Hemidesmus indicus R. BR) root extract. Toxicon. 1998;36:207–15. doi: 10.1016/s0041-0101(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 581.Saravanan S, Srikumar R, Manikandan S, Jeya Parthasarathy N, Sheela Devi R. Hypolipidemic effect of triphala in experimentally induced hypercholesteremic rats. Yakugaku Zasshi. 2007;127:385–8. doi: 10.1248/yakushi.127.385. [DOI] [PubMed] [Google Scholar]
- 582.Gayathri M, Kannabiran K. 2-hydroxy 4-methoxy benzoic acid isolated from roots of Hemidesmus indicus ameliorates liver, kidney and pancreas injury due to streptozotocin-induced diabetes in rats. Indian J Exp Biol. 48:159–64. [PubMed] [Google Scholar]
- 583.Sultana S, Alam A, Khan N, Sharma S. Inhibition of cutaneous oxidative stress and two-stage skin carcinogenesis by Hemidesmus indicus (L) in Swiss albino mice. Indian J Exp Biol. 2003;41:1416–23. [PubMed] [Google Scholar]
- 584.Kavitha D, Shilpa PN, Devaraj SN. Antibacterial and antidiarrhoeal effects of alkaloids of Holarrhena antidysenterica WALL. Indian journal of experimental biology. 2004;42:589–94. [PubMed] [Google Scholar]
- 585.Jeong JB, Hong SC, Jeong HJ. 3,4-dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity. Phytomedicine. 2009;16:85–94. doi: 10.1016/j.phymed.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 586.Akramiene D, Grazeliene G, Didziapetriene J, Kevelaitis E. Treatment of Lewis lung carcinoma by photodynamic therapy and glucan from barley. Medicina (Kaunas) 2009;45:480–5. [PubMed] [Google Scholar]
- 587.Singh B, Chandan BK, Sharma N, Bhardwaj V, Satti NK, Gupta VN, et al. Isolation, structure elucidation and in vivo hepatoprotective potential of trans-tetracos-15-enoic acid from Indigofera tinctoria Linn. Phytother Res. 2006;20:831–9. doi: 10.1002/ptr.1856. [DOI] [PubMed] [Google Scholar]
- 588.Singh B, Saxena AK, Chandan BK, Bhardwaj V, Gupta VN, Suri OP, et al. Hepatoprotective activity of indigtone--a bioactive fraction from Indigofera tinctoria Linn. Phytother Res. 2001;15:294–7. doi: 10.1002/ptr.760. [DOI] [PubMed] [Google Scholar]
- 589.Puri A, Khaliq T, Rajendran SM, Bhatia G, Chandra R, Narender T. Antidyslipidemic activity of Indigofera tinctoria. J Herb Pharmacother. 2007;7:59–64. doi: 10.1300/j157v07n01_05. [DOI] [PubMed] [Google Scholar]
- 590.Sreepriya M, Devaki T, Balakrishna K, Apparanantham T. Effect of Indigofera tinctoria Linn on liver antioxidant defense system during D-galactosamine/endotoxin-induced acute hepatitis in rodents. Indian J Exp Biol. 2001;39:181–4. [PubMed] [Google Scholar]
- 591.Pal HC, Sehar I, Bhushan S, Gupta BD, Saxena AK. Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol In Vitro. doi: 10.1016/j.tiv.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 592.Srivastava S, Gupta PP, Prasad R, Dixit KS, Palit G, Ali B, et al. Evaluation of antiallergic activity (type I hypersensitivity) of Inula racemosa in rats. Indian J Physiol Pharmacol. 1999;43:235–41. [PubMed] [Google Scholar]
- 593.Ko SG, Koh SH, Jun CY, Nam CG, Bae HS, Shin MK. Induction of apoptosis by Saussurea lappa and Pharbitis nil on AGS gastric cancer cells. Biol Pharm Bull. 2004;27:1604–10. doi: 10.1248/bpb.27.1604. [DOI] [PubMed] [Google Scholar]
- 594.Gamez MJ, Jimenez J, Risco S, Zarzuelo A. Hypoglycemic activity in various species of the genus Lavandula. Part 1: Lavandula stoechas L and Lavandula multifida L. Pharmazie. 1987;42:706–7. [PubMed] [Google Scholar]
- 595.Gilani AH, Aziz N, Khan MA, Shaheen F, Jabeen Q, Siddiqui BS, et al. Ethnopharmacological evaluation of the anticonvulsant, sedative and antispasmodic activities of Lavandula stoechas L. J Ethnopharmacol. 2000;71:161–7. doi: 10.1016/s0378-8741(99)00198-1. [DOI] [PubMed] [Google Scholar]
- 596.Lemus-Molina Y, Sanchez-Gomez MV, Delgado-Hernandez R, Matute C. Mangifera indica L. extract attenuates glutamate-induced neurotoxicity on rat cortical neurons. Neurotoxicology. 2009;30:1053–8. doi: 10.1016/j.neuro.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 597.Garrido G, Gonzalez D, Lemus Y, Garcia D, Lodeiro L, Quintero G, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG) Pharmacol Res. 2004;50:143–9. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 598.Noratto GD, Bertoldi MC, Krenek K, Talcott ST, Stringheta PC, Mertens-Talcott SU. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J Agric Food Chem. 58:4104–12. doi: 10.1021/jf903161g. [DOI] [PubMed] [Google Scholar]
- 599.Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of fruit extract of Mangifera indica L. against oxidative stress cytotoxicity. Plant Foods Hum Nutr. 65:83–9. doi: 10.1007/s11130-010-0161-9. [DOI] [PubMed] [Google Scholar]
- 600.Kumar S, Maheshwari KK, Singh V. Effects of Mangifera indica fruit extract on cognitive deficits in mice. J Environ Biol. 2009;30:563–6. [PubMed] [Google Scholar]
- 601.Severi JA, Lima ZP, Kushima H, Brito AR, Santos LC, Vilegas W, et al. Polyphenols with antiulcerogenic action from aqueous decoction of mango leaves (Mangifera indica L) Molecules. 2009;14:1098–110. doi: 10.3390/molecules14031098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Akila M, Devaraj H. Synergistic effect of tincture of Crataegus and Mangifera indica L. extract on hyperlipidemic and antioxidant status in atherogenic rats. Vascul Pharmacol. 2008;49:173–7. doi: 10.1016/j.vph.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 603.Parmar HS, Kar A. Possible amelioration of atherogenic diet induced dyslipidemia, hypothyroidism and hyperglycemia by the peel extracts of Mangifera indica, Cucumis melo and Citrullus vulgaris fruits in rats. Biofactors. 2008;33:13–24. doi: 10.1002/biof.5520330102. [DOI] [PubMed] [Google Scholar]
- 604.Pardo-Andreu GL, Philip SJ, Riano A, Sanchez C, Viada C, Nunez-Selles AJ, et al. Mangifera indica L. (Vimang) protection against serum oxidative stress in elderly humans. Arch Med Res. 2006;37:158–64. doi: 10.1016/j.arcmed.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 605.Ojewole JA. Antiinflammatory, analgesic and hypoglycemic effects of Mangifera indica Linn. (Anacardiaceae) stem-bark aqueous extract. Methods Find Exp Clin Pharmacol. 2005;27:547–54. doi: 10.1358/mf.2005.27.8.928308. [DOI] [PubMed] [Google Scholar]
- 606.Sangeetha KN, Sujatha S, Muthusamy VS, Anand S, Nithya N, Velmurugan D, et al. 3beta-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim Biophys Acta. 1800:359–66. doi: 10.1016/j.bbagen.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 607.Carvalho AC, Guedes MM, de Souza AL, Trevisan MT, Lima AF, Santos FA, et al. Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med. 2007;73:1372–6. doi: 10.1055/s-2007-990231. [DOI] [PubMed] [Google Scholar]
- 608.Kumar A, Samarth RM, Yasmeen S, Sharma A, Sugahara T, Terado T, et al. Anticancer and radioprotective potentials of Mentha piperita. Biofactors. 2004;22:87–91. doi: 10.1002/biof.5520220117. [DOI] [PubMed] [Google Scholar]
- 609.Sharma A, Sharma MK, Kumar M. Protective effect of Mentha piperita against arsenic-induced toxicity in liver of Swiss albino mice. Basic Clin Pharmacol Toxicol. 2007;100:249–57. doi: 10.1111/j.1742-7843.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- 610.Samarth RM. Protection against radiation induced hematopoietic damage in bone marrow of Swiss albino mice by Mentha piperita (Linn) J Radiat Res (Tokyo) 2007;48:523–8. doi: 10.1269/jrr.07052. [DOI] [PubMed] [Google Scholar]
- 611.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L) Phytother Res. 2006;20:619–33. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 612.Shkurupii VA, Odintsova OA, Kazarinova NV, Tkrachenko KG. [Use of essential oil of peppermint (Mentha piperita) in the complex treatment of patients with infiltrative pulmonary tuberculosis] Probl Tuberk Bolezn Legk. 2006:43–5. [PubMed] [Google Scholar]
- 613.de Sousa AA, Soares PM, de Almeida AN, Maia AR, de Souza EP, Assreuy AM. Antispasmodic effect of Mentha piperita essential oil on tracheal smooth muscle of rats. J Ethnopharmacol. 130:433–6. doi: 10.1016/j.jep.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 614.Inoue T, Sugimoto Y, Masuda H, Kamei C. Antiallergic effect of flavonoid glycosides obtained from Mentha piperita L. Biol Pharm Bull. 2002;25:256–9. doi: 10.1248/bpb.25.256. [DOI] [PubMed] [Google Scholar]
- 615.Dar A, Behbahanian S, Malik A, Jahan N. Hypotensive effect of the methanolic extract of Mimusops elengi in normotensive rats. Phytomedicine. 1999;6:373–8. doi: 10.1016/S0944-7113(99)80062-2. [DOI] [PubMed] [Google Scholar]
- 616.Kobori M, Nakayama H, Fukushima K, Ohnishi-Kameyama M, Ono H, Fukushima T, et al. Bitter gourd suppresses lipopolysaccharide-induced inflammatory responses. J Agric Food Chem. 2008;56:4004–11. doi: 10.1021/jf800052y. [DOI] [PubMed] [Google Scholar]
- 617.Akihisa T, Higo N, Tokuda H, Ukiya M, Akazawa H, Tochigi Y, et al. Cucurbitane-type triterpenoids from the fruits of Momordica charantia and their cancer chemopreventive effects. J Nat Prod. 2007;70:1233–9. doi: 10.1021/np068075p. [DOI] [PubMed] [Google Scholar]
- 618.Fan JM, Zhang Q, Xu J, Zhu S, Ke T, Gao de F, et al. Inhibition on Hepatitis B virus in vitro of recombinant MAP30 from bitter melon. Mol Biol Rep. 2009;36:381–8. doi: 10.1007/s11033-007-9191-2. [DOI] [PubMed] [Google Scholar]
- 619.Lii CK, Chen HW, Yun WT, Liu KL. Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviata ser.) fruit extracts on inflammatory responses in RAW264. 7 macrophages. J Ethnopharmacol. 2009;122:227–33. doi: 10.1016/j.jep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 620.Ray RB, Raychoudhuri A, Steele R, Nerurkar P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res. 70:1925–31. doi: 10.1158/0008-5472.CAN-09-3438. [DOI] [PubMed] [Google Scholar]
- 621.Uebanso T, Arai H, Taketani Y, Fukaya M, Yamamoto H, Mizuno A, et al. Extracts of Momordica charantia suppress postprandial hyperglycemia in rats. J Nutr Sci Vitaminol (Tokyo) 2007;53:482–8. doi: 10.3177/jnsv.53.482. [DOI] [PubMed] [Google Scholar]
- 622.Shih CC, Lin CH, Lin WL. Effects of Momordica charantia on insulin resistance and visceral obesity in mice on high-fat diet. Diabetes Res Clin Pract. 2008;81:134–43. doi: 10.1016/j.diabres.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 623.Teoh SL, Latiff AA, Das S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin Exp Dermatol. 2009;34:815–22. doi: 10.1111/j.1365-2230.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 624.Alam S, Asad M, Asdaq SM, Prasad VS. Antiulcer activity of methanolic extract of Momordica charantia L. in rats. J Ethnopharmacol. 2009;123:464–9. doi: 10.1016/j.jep.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 625.Nerurkar P, Ray RB. Bitter melon: antagonist to cancer. Pharm Res. 27:1049–53. doi: 10.1007/s11095-010-0057-2. [DOI] [PubMed] [Google Scholar]
- 626.Klomann SD, Mueller AS, Pallauf J, Krawinkel MB. Antidiabetic effects of bitter gourd extracts in insulin-resistant db/db mice. Br J Nutr. :1–8. doi: 10.1017/S0007114510002680. [DOI] [PubMed] [Google Scholar]
- 627.Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): a mini review. Br J Nutr. 2009;102:1703–8. doi: 10.1017/S0007114509992054. [DOI] [PubMed] [Google Scholar]
- 628.Mahajan SG, Mali RG, Mehta AA. Protective Effect of Ethanolic Extract of Seeds of Moringa oleifera Lam. Against Inflammation Associated with Development of Arthritis in Rats. J Immunotoxicol. 2007;4:39–47. doi: 10.1080/15476910601115184. [DOI] [PubMed] [Google Scholar]
- 629.Mahajan SG, Banerjee A, Chauhan BF, Padh H, Nivsarkar M, Mehta AA. Inhibitory effect of n-butanol fraction of Moringa oleifera Lam. seeds on ovalbumin-induced airway inflammation in a guinea pig model of asthma. Int J Toxicol. 2009;28:519–27. doi: 10.1177/1091581809345165. [DOI] [PubMed] [Google Scholar]
- 630.Mahajan SG, Mehta AA. Immunosuppressive activity of ethanolic extract of seeds of Moringa oleifera Lam. in experimental immune inflammation. J Ethnopharmacol. 130:183–6. doi: 10.1016/j.jep.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 631.Nandave M, Ojha SK, Joshi S, Kumari S, Arya DS. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J Med Food. 2009;12:47–55. doi: 10.1089/jmf.2007.0563. [DOI] [PubMed] [Google Scholar]
- 632.Mishra D, Gupta R, Pant SC, Kushwah P, Satish HT, Flora SJ. Co-administration of monoisoamyl dimercaptosuccinic acid and Moringa oleifera seed powder protects arsenic-induced oxidative stress and metal distribution in mice. Toxicol Mech Methods. 2009;19:169–82. doi: 10.1080/15376510701795751. [DOI] [PubMed] [Google Scholar]
- 633.Hamza AA. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem Toxicol. 48:345–55. doi: 10.1016/j.fct.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 634.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 635.Rathi SS, Grover JK, Vats V. The effect of Momordica charantia and Mucuna pruriens in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. Phytother Res. 2002;16:236–43. doi: 10.1002/ptr.842. [DOI] [PubMed] [Google Scholar]
- 636.Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, Watt H, et al. Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75:1672–7. doi: 10.1136/jnnp.2003.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Bhaskar A, Vidhya VG, Ramya M. Hypoglycemic effect of Mucuna pruriens seed extract on normal and streptozotocin-diabetic rats. Fitoterapia. 2008;79:539–43. doi: 10.1016/j.fitote.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 638.Kasture S, Pontis S, Pinna A, Schintu N, Spina L, Longoni R, et al. Assessment of symptomatic and neuroprotective efficacy of Mucuna pruriens seed extract in rodent model of Parkinson’s disease. Neurotox Res. 2009;15:111–22. doi: 10.1007/s12640-009-9011-7. [DOI] [PubMed] [Google Scholar]
- 639.Lieu CA, Kunselman AR, Manyam BV, Venkiteswaran K, Subramanian T. A water extract of Mucuna pruriens provides long-term amelioration of parkinsonism with reduced risk for dyskinesias. Parkinsonism Relat Disord. 16:458–65. doi: 10.1016/j.parkreldis.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Ahmad M, Yousuf S, Khan MB, Hoda MN, Ahmad AS, Ansari MA, et al. Attenuation by Nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: behavioral, neurochemical, and immunohistochemical studies. Pharmacol Biochem Behav. 2006;83:150–60. doi: 10.1016/j.pbb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 641.Dhingra D, Goyal PK. Inhibition of MAO and GABA: probable mechanisms for antidepressant-like activity of Nardostachys jatamansi DC. in mice. Indian J Exp Biol. 2008;46:212–8. [PubMed] [Google Scholar]
- 642.Bae GS, Park HJ, Kim DY, Song JM, Kim TH, Oh HJ, et al. Nardostachys jatamansi protects against cerulein-induced acute pancreatitis. Pancreas. 39:520–9. doi: 10.1097/MPA.0b013e3181bd93ce. [DOI] [PubMed] [Google Scholar]
- 643.Song MY, Bae UJ, Lee BH, Kwon KB, Seo EA, Park SJ, et al. Nardostachys jatamansi extract protects against cytokine-induced beta-cell damage and streptozotocin-induced diabetes. World J Gastroenterol. 16:3249–57. doi: 10.3748/wjg.v16.i26.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Liu CP, Tsai WJ, Shen CC, Lin YL, Liao JF, Chen CF, et al. Inhibition of (S)-armepavine from Nelumbo nucifera on autoimmune disease of MRL/MpJ-lpr/lpr mice. Eur J Pharmacol. 2006;531:270–9. doi: 10.1016/j.ejphar.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 645.Ka SM, Kuo YC, Ho PJ, Tsai PY, Hsu YJ, Tsai WJ, et al. (S)-armepavine from Chinese medicine improves experimental autoimmune crescentic glomerulonephritis. Rheumatology (Oxford) doi: 10.1093/rheumatology/keq164. [DOI] [PubMed] [Google Scholar]
- 646.Mukherjee PK, Mukherjee D, Maji AK, Rai S, Heinrich M. The sacred lotus (Nelumbo nucifera) - phytochemical and therapeutic profile. J Pharm Pharmacol. 2009;61:407–22. doi: 10.1211/jpp/61.04.0001. [DOI] [PubMed] [Google Scholar]
- 647.Sugimoto Y, Furutani S, Nishimura K, Itoh A, Tanahashi T, Nakajima H, et al. Antidepressant-like effects of neferine in the forced swimming test involve the serotonin1A (5-HT1A) receptor in mice. Eur J Pharmacol. 634:62–7. doi: 10.1016/j.ejphar.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 648.Shim SY, Choi JS, Byun DS. Kaempferol isolated from Nelumbo nucifera stamens negatively regulates FcepsilonRI expression in human basophilic KU812F cells. J Microbiol Biotechnol. 2009;19:155–60. doi: 10.4014/jmb.0804.259. [DOI] [PubMed] [Google Scholar]
- 649.Xiao JH, Zhang JH, Chen HL, Feng XL, Wang JL. Inhibitory effects of isoliensinine on bleomycin-induced pulmonary fibrosis in mice. Planta Med. 2005;71:225–30. doi: 10.1055/s-2005-837821. [DOI] [PubMed] [Google Scholar]
- 650.Sohn DH, Kim YC, Oh SH, Park EJ, Li X, Lee BH. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine. 2003;10:165–9. doi: 10.1078/094471103321659889. [DOI] [PubMed] [Google Scholar]
- 651.Mani SS, Subramanian IP, Pillai SS, Muthusamy K. Evaluation of Hypoglycemic Activity of Inorganic Constituents in Nelumbo nucifera Seeds on Streptozotocin-Induced Diabetes in Rats. Biol Trace Elem Res. doi: 10.1007/s12011-010-8614-4. [DOI] [PubMed] [Google Scholar]
- 652.Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 29:87. doi: 10.1186/1756-9966-29-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Nader MA, el-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 33:637–43. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- 654.Helal GK. Thymoquinone supplementation ameliorates acute endotoxemia-induced liver dysfunction in rats. Pak J Pharm Sci. 23:131–7. [PubMed] [Google Scholar]
- 655.Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009;11:373–81. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Mohamed A, Waris HM, Ramadan H, Quereshi M, Kalra J. Amelioration of chronic relapsing experimental autoimmune encephalomyelitis (cr-eae) using thymoquinone - biomed 2009. Biomed Sci Instrum. 2009;45:274–9. [PubMed] [Google Scholar]
- 657.Juhas S, Cikos S, Czikkova S, Vesela J, Il’kova G, Hajek T, et al. Effects of borneol and thymoquinone on TNBS-induced colitis in mice. Folia Biol (Praha) 2008;54:1–7. [PubMed] [Google Scholar]
- 658.El Mezayen R, El Gazzar M, Nicolls MR, Marecki JC, Dreskin SC, Nomiyama H. Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol Lett. 2006;106:72–81. doi: 10.1016/j.imlet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 659.Tekeoglu I, Dogan A, Demiralp L. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2006;20:869–71. doi: 10.1002/ptr.1964. [DOI] [PubMed] [Google Scholar]
- 660.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 661.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 662.Ragheb A, Attia A, Eldin WS, Elbarbry F, Gazarin S, Shoker A. The protective effect of thymoquinone, an anti-oxidant and anti-inflammatory agent, against renal injury: a review. Saudi J Kidney Dis Transpl. 2009;20:741–52. [PubMed] [Google Scholar]
- 663.Ghannadi A, Hajhashemi V, Jafarabadi H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food. 2005;8:488–93. doi: 10.1089/jmf.2005.8.488. [DOI] [PubMed] [Google Scholar]
- 664.Majdalawieh AF, Hmaidan R, Carr RI. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J Ethnopharmacol. doi: 10.1016/j.jep.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 665.Bashir MU, Qureshi HJ. Analgesic Effect of Nigella sativa Seeds Extract on Experimentally Induced Pain in Albino Mice. J Coll Physicians Surg Pak. 20:464–7. [PubMed] [Google Scholar]
- 666.Isik H, Cevikbas A, Gurer US, Kiran B, Uresin Y, Rayaman P, et al. Potential adjuvant effects of Nigella sativa seeds to improve specific immunotherapy in allergic rhinitis patients. Med Princ Pract. 19:206–11. doi: 10.1159/000285289. [DOI] [PubMed] [Google Scholar]
- 667.Boskabady MH, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 17:707–13. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 668.Akhondian J, Parsa A, Rakhshande H. The effect of Nigella sativa L. (black cumin seed) on intractable pediatric seizures. Med Sci Monit. 2007;13:CR555–9. [PubMed] [Google Scholar]
- 669.Rathore B, Paul B, Chaudhury BP, Saxena AK, Sahu AP, Gupta YK. Comparative studies of different organs of Nyctanthes arbortristis in modulation of cytokines in murine model of arthritis. Biomed Environ Sci. 2007;20:154–9. [PubMed] [Google Scholar]
- 670.Gupta P, Bajpai SK, Chandra K, Singh KL, Tandon JS. Antiviral profile of Nyctanthes arbortristis L. against encephalitis causing viruses. Indian J Exp Biol. 2005;43:1156–60. [PubMed] [Google Scholar]
- 671.Sen P, Maiti PC, Puri S, Ray A, Audulov NA, Valdman AV. Mechanism of anti-stress activity of Ocimum sanctum Linn, eugenol and Tinospora malabarica in experimental animals. Indian J Exp Biol. 1992;30:592–6. [PubMed] [Google Scholar]
- 672.Kath RK, Gupta RK. Antioxidant activity of hydroalcoholic leaf extract of ocimum sanctum in animal models of peptic ulcer. Indian J Physiol Pharmacol. 2006;50:391–6. [PubMed] [Google Scholar]
- 673.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol. 2005;49:125–31. [PubMed] [Google Scholar]
- 674.Mondal S, Mirdha BR, Mahapatra SC. The science behind sacredness of Tulsi (Ocimum sanctum Linn) Indian J Physiol Pharmacol. 2009;53:291–306. [PubMed] [Google Scholar]
- 675.Magesh V, Lee JC, Ahn KS, Lee HJ, Lee HJ, Lee EO, et al. Ocimum sanctum induces apoptosis in A549 lung cancer cells and suppresses the in vivo growth of Lewis lung carcinoma cells. Phytother Res. 2009;23:1385–91. doi: 10.1002/ptr.2784. [DOI] [PubMed] [Google Scholar]
- 676.Kaur G, Jaggi AS, Singh N. Exploring the potential effect of Ocimum sanctum in vincristine-induced neuropathic pain in rats. J Brachial Plex Peripher Nerve Inj. 5:3. doi: 10.1186/1749-7221-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Suanarunsawat T, Devakul Na Ayutthaya W, Songsak T, Thirawarapan S, Poungshompoo S. Antioxidant Activity and Lipid-Lowering Effect of Essential Oils Extracted from Ocimum sanctum L. Leaves in Rats Fed with a High Cholesterol Diet. J Clin Biochem Nutr. 46:52–9. doi: 10.3164/jcbn.09-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Jyoti S, Satendra S, Sushma S, Anjana T, Shashi S. Antistressor activity of Ocimum sanctum (Tulsi) against experimentally induced oxidative stress in rabbits. Methods Find Exp Clin Pharmacol. 2007;29:411–6. doi: 10.1358/mf.2007.29.6.1118135. [DOI] [PubMed] [Google Scholar]
- 679.Anbuselvam C, Vijayavel K, Balasubramanian MP. Protective effect of Operculina turpethum against 7,12-dimethyl benz(a)anthracene induced oxidative stress with reference to breast cancer in experimental rats. Chem Biol Interact. 2007;168:229–36. doi: 10.1016/j.cbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 680.Jiwajinda S, Santisopasri V, Murakami A, Kim OK, Kim HW, Ohigashi H. Suppressive Effects of Edible Thai Plants on Superoxide and Nitric Oxide Generation. Asian Pac J Cancer Prev. 2002;3:215–23. [PubMed] [Google Scholar]
- 681.Siriwatanametanon N, Fiebich BL, Efferth T, Prieto JM, Heinrich M. Traditionally used Thai medicinal plants: in vitro anti-inflammatory, anticancer and antioxidant activities. J Ethnopharmacol. 130:196–207. doi: 10.1016/j.jep.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 682.Laupattarakasem P, Houghton PJ, Hoult JR, Itharat A. An evaluation of the activity related to inflammation of four plants used in Thailand to treat arthritis. J Ethnopharmacol. 2003;85:207–15. doi: 10.1016/s0378-8741(02)00367-7. [DOI] [PubMed] [Google Scholar]
- 683.Chirdchupunseree H, Pramyothin P. Protective activity of phyllanthin in ethanol-treated primary culture of rat hepatocytes. J Ethnopharmacol. 128:172–6. doi: 10.1016/j.jep.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 684.Rajeshkumar NV, Kuttan R. Phyllanthus amarus extract administration increases the life span of rats with hepatocellular carcinoma. J Ethnopharmacol. 2000;73:215–9. doi: 10.1016/s0378-8741(00)00311-1. [DOI] [PubMed] [Google Scholar]
- 685.Kiemer AK, Hartung T, Huber C, Vollmar AM. Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-kappaB pathway. J Hepatol. 2003;38:289–97. doi: 10.1016/s0168-8278(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 686.Kassuya CA, Leite DF, de Melo LV, Rehder VL, Calixto JB. Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Med. 2005;71:721–6. doi: 10.1055/s-2005-871258. [DOI] [PubMed] [Google Scholar]
- 687.Kassuya CA, Silvestre AA, Rehder VL, Calixto JB. Anti-allodynic and anti-oedematogenic properties of the extract and lignans from Phyllanthus amarus in models of persistent inflammatory and neuropathic pain. Eur J Pharmacol. 2003;478:145–53. doi: 10.1016/j.ejphar.2003.08.079. [DOI] [PubMed] [Google Scholar]
- 688.Xin-Hua W, Chang-Qing L, Xing-Bo G, Lin-Chun F. A comparative study of Phyllanthus amarus compound and interferon in the treatment of chronic viral hepatitis B. Southeast Asian J Trop Med Public Health. 2001;32:140–2. [PubMed] [Google Scholar]
- 689.Adeneye AA, Amole OO, Adeneye AK. Hypoglycemic and hypocholesterolemic activities of the aqueous leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia. 2006;77:511–4. doi: 10.1016/j.fitote.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 690.Raphael KR, Sabu M, Kumar KH, Kuttan R. Inhibition of N-Methyl N′-nitro-N-nitrosoguanidine (MNNG) induced gastric carcinogenesis by Phyllanthus amarus extract. Asian Pac J Cancer Prev. 2006;7:299–302. [PubMed] [Google Scholar]
- 691.Mellinger CG, Carbonero ER, Noleto GR, Cipriani TR, Oliveira MB, Gorin PA, et al. Chemical and biological properties of an arabinogalactan from Phyllanthusniruri. J Nat Prod. 2005;68:1479–83. doi: 10.1021/np050129s. [DOI] [PubMed] [Google Scholar]
- 692.Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58:1559–70. doi: 10.1211/jpp.58.12.0001. [DOI] [PubMed] [Google Scholar]
- 693.Venkateswaran PS, Millman I, Blumberg BS. Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: in vitro and in vivo studies. Proc Natl Acad Sci U S A. 1987;84:274–8. doi: 10.1073/pnas.84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Chatterjee M, Sil PC. Hepatoprotective effect of aqueous extract of Phyllanthus niruri on nimesulide-induced oxidative stress in vivo. Indian J Biochem Biophys. 2006;43:299–305. [PubMed] [Google Scholar]
- 695.Sharma I, Gusain D, Dixit VP. Hypolipidaemic and antiatherosclerotic effects of plumbagin in rabbits. Indian J Physiol Pharmacol. 1991;35:10–4. [PubMed] [Google Scholar]
- 696.Murugaiyah V, Chan KL. Mechanisms of antihyperuricemic effect of Phyllanthus niruri and its lignan constituents. J Ethnopharmacol. 2009;124:233–9. doi: 10.1016/j.jep.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 697.Nworu CS, Akah PA, Okoye FB, Proksch P, Esimone CO. The effects of Phyllanthus niruri aqueous extract on the activation of murine lymphocytes and bone marrow-derived macrophages. Immunol Invest. 39:245–67. doi: 10.3109/08820131003599585. [DOI] [PubMed] [Google Scholar]
- 698.Dhuley JN. Effect of picroliv administration on hepatic microsomal mixed function oxidases and glutathione-conjugating enzyme system in cholestatic rats. Hindustan Antibiot Bull. 2005;47–48:13–9. [PubMed] [Google Scholar]
- 699.Vivekanandan P, Gobianand K, Priya S, Vijayalakshmi P, Karthikeyan S. Protective effect of picroliv against hydrazine-induced hyperlipidemia and hepatic steatosis in rats. Drug Chem Toxicol. 2007;30:241–52. doi: 10.1080/01480540701375216. [DOI] [PubMed] [Google Scholar]
- 700.Gupta A, Khajuria A, Singh J, Bedi KL, Satti NK, Dutt P, et al. Immunomodulatory activity of biopolymeric fraction RLJ-NE-205 from Picrorhiza kurroa. Int Immunopharmacol. 2006;6:1543–9. doi: 10.1016/j.intimp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 701.Rastogi R, Saksena S, Garg NK, Kapoor NK, Agarwal DP, Dhawan BN. Picroliv protects against alcohol-induced chronic hepatotoxicity in rats. Planta Med. 1996;62:283–5. doi: 10.1055/s-2006-957882. [DOI] [PubMed] [Google Scholar]
- 702.Verma PC, Basu V, Gupta V, Saxena G, Rahman LU. Pharmacology and chemistry of a potent hepatoprotective compound Picroliv isolated from the roots and rhizomes of Picrorhiza kurroa royle ex benth (kutki) Curr Pharm Biotechnol. 2009;10:641–9. doi: 10.2174/138920109789069314. [DOI] [PubMed] [Google Scholar]
- 703.Zhang Y, DeWitt DL, Murugesan S, Nair MG. Novel lipid-peroxidation- and cyclooxygenase-inhibitory tannins from Picrorhiza kurroa seeds. Chem Biodivers. 2004;1:426–41. doi: 10.1002/cbdv.200490036. [DOI] [PubMed] [Google Scholar]
- 704.Morikawa T, Matsuda H, Yamaguchi I, Pongpiriyadacha Y, Yoshikawa M. New amides and gastroprotective constituents from the fruit of Piper chaba. Planta Med. 2004;70:152–9. doi: 10.1055/s-2004-815493. [DOI] [PubMed] [Google Scholar]
- 705.Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I, Yoshikawa M. Hepatoprotective amide constituents from the fruit of Piper chaba: Structural requirements, mode of action, and new amides. Bioorg Med Chem. 2009;17:7313–23. doi: 10.1016/j.bmc.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 706.Pradeep CR, Kuttan G. Effect of piperine on the inhibition of nitric oxide (NO) and TNF-alpha production. Immunopharmacol Immunotoxicol. 2003;25:337–46. doi: 10.1081/iph-120024502. [DOI] [PubMed] [Google Scholar]
- 707.Bae GS, Kim MS, Jung WS, Seo SW, Yun SW, Kim SG, et al. Inhibition of lipopolysaccharide-induced inflammatory responses by piperine. Eur J Pharmacol. 642:154–62. doi: 10.1016/j.ejphar.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 708.Lee SA, Hong SS, Han XH, Hwang JS, Oh GJ, Lee KS, et al. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem Pharm Bull (Tokyo) 2005;53:832–5. doi: 10.1248/cpb.53.832. [DOI] [PubMed] [Google Scholar]
- 709.Iwashita M, Oka N, Ohkubo S, Saito M, Nakahata N. Piperlongumine, a constituent of Piper longum L. inhibits rabbit platelet aggregation as a thromboxane A(2) receptor antagonist. Eur J Pharmacol. 2007;570:38–42. doi: 10.1016/j.ejphar.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 710.Devan P, Bani S, Suri KA, Satti NK, Qazi GN. Immunomodulation exhibited by piperinic acid through suppression of proinflammatory cytokines. Int Immunopharmacol. 2007;7:889–99. doi: 10.1016/j.intimp.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 711.Sunila ES, Kuttan G. Piper longum inhibits VEGF and proinflammatory cytokines and tumor-induced angiogenesis in C57BL/6 mice. Int Immunopharmacol. 2006;6:733–41. doi: 10.1016/j.intimp.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 712.Kumar A, Panghal S, Mallapur SS, Kumar M, Ram V, Singh BK. Antiinflammatory Activity of Piper longum Fruit Oil. Indian J Pharm Sci. 2009;71:454–6. doi: 10.4103/0250-474X.57300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Subramanian U, Poongavanam S, Vanisree AJ. Studies on the neuroprotective role of Piper longum in C6 glioma induced rats. Invest New Drugs. 28:615–23. doi: 10.1007/s10637-009-9301-1. [DOI] [PubMed] [Google Scholar]
- 714.Wakade AS, Shah AS, Kulkarni MP, Juvekar AR. Protective effect of Piper longum L. on oxidative stress induced injury and cellular abnormality in adriamycin induced cardiotoxicity in rats. Indian J Exp Biol. 2008;46:528–33. [PubMed] [Google Scholar]
- 715.Agrawal AK, Rao CV, Sairam K, Joshi VK, Goel RK. Effect of Piper longum Linn, Zingiber officianalis Linn and Ferula species on gastric ulceration and secretion in rats. Indian J Exp Biol. 2000;38:994–8. [PubMed] [Google Scholar]
- 716.Chonpathompikunlert P, Wattanathorn J, Muchimapura S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem Toxicol. 48:798–802. doi: 10.1016/j.fct.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 717.Vijayakumar RS, Nalini N. Piperine, an active principle from Piper nigrum, modulates hormonal and apo lipoprotein profiles in hyperlipidemic rats. J Basic Clin Physiol Pharmacol. 2006;17:71–86. doi: 10.1515/jbcpp.2006.17.2.71. [DOI] [PubMed] [Google Scholar]
- 718.Pattanaik S, Hota D, Prabhakar S, Kharbanda P, Pandhi P. Effect of piperine on the steady-state pharmacokinetics of phenytoin in patients with epilepsy. Phytother Res. 2006;20:683–6. doi: 10.1002/ptr.1937. [DOI] [PubMed] [Google Scholar]
- 719.Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47:735–48. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- 720.Wei K, Dou DQ, Pei YP, Chen YJ. Comparison of the chemical constituents and pharmacological action of Piper nigrum Linn. with P. methysticum forst. Zhongguo Zhong Yao Za Zhi. 2002;27:328–33. [PubMed] [Google Scholar]
- 721.Hirata N, Naruto S, Inaba K, Itoh K, Tokunaga M, Iinuma M, et al. Histamine release inhibitory activity of Piper nigrum leaf. Biol Pharm Bull. 2008;31:1973–6. doi: 10.1248/bpb.31.1973. [DOI] [PubMed] [Google Scholar]
- 722.Ahmad NS, Farman M, Najmi MH, Mian KB, Hasan A. Pharmacological basis for use of Pistacia integerrima leaves in hyperuricemia and gout. J Ethnopharmacol. 2008;117:478–82. doi: 10.1016/j.jep.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 723.Jahangir T, Sultana S. Modulatory effects of Pluchea lanceolata against chemically induced oxidative damage, hyperproliferation and two-stage renal carcinogenesis in Wistar rats. Mol Cell Biochem. 2006;291:175–85. doi: 10.1007/s11010-006-9213-8. [DOI] [PubMed] [Google Scholar]
- 724.Bhagwat DP, Kharya MD, Bani S, Kaul A, Kour K, Chauhan PS, et al. Immunosuppressive properties of Pluchea lanceolata leaves. Indian J Pharmacol. 42:21–6. doi: 10.4103/0253-7613.62405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Yang SJ, Chang SC, Wen HC, Chen CY, Liao JF, Chang CH. Plumbagin activates ERK1/2 and Akt via superoxide, Src and PI3-kinase in 3T3-L1 cells. Eur J Pharmacol. 638:21–8. doi: 10.1016/j.ejphar.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 726.Checker R, Sharma D, Sandur SK, Khanam S, Poduval TB. Anti-inflammatory effects of plumbagin are mediated by inhibition of NF-kappaB activation in lymphocytes. Int Immunopharmacol. 2009;9:949–58. doi: 10.1016/j.intimp.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 727.Parimala R, Sachdanandam P. Effect of Plumbagin on some glucose metabolising enzymes studied in rats in experimental hepatoma. Mol Cell Biochem. 1993;125:59–63. doi: 10.1007/BF00926835. [DOI] [PubMed] [Google Scholar]
- 728.Tsai WJ, Chen YC, Wu MH, Lin LC, Chuang KA, Chang SC, et al. Seselin from Plumbago zeylanica inhibits phytohemagglutinin (PHA)-stimulated cell proliferation in human peripheral blood mononuclear cells. J Ethnopharmacol. 2008;119:67–73. doi: 10.1016/j.jep.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 729.Chen YC, Tsai WJ, Wu MH, Lin LC, Kuo YC. Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB. Br J Pharmacol. 2007;150:298–312. doi: 10.1038/sj.bjp.0706987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Tamrakar AK, Yadav PP, Tiwari P, Maurya R, Srivastava AK. Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits. J Ethnopharmacol. 2008;118:435–9. doi: 10.1016/j.jep.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 731.Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Prakash VR. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol. 2001;78:151–7. doi: 10.1016/s0378-8741(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 732.Punitha R, Manoharan S. Antihyperglycemic and antilipidperoxidative effects of Pongamia pinnata (Linn) Pierre flowers in alloxan induced diabetic rats. J Ethnopharmacol. 2006;105:39–46. doi: 10.1016/j.jep.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 733.Raghavendra M, Trigunayat A, Singh RK, Mitra S, Goel RK, Acharya SB. Effect of ethanolic extract of root of Pongamia pinnata (L) pierre on oxidative stress, behavioral and histopathological alterations induced by cerebral ischemia--reperfusion and long-term hypoperfusion in rats. Indian J Exp Biol. 2007;45:868–76. [PubMed] [Google Scholar]
- 734.Prabha T, Dorababu M, Goel S, Agarwal PK, Singh A, Joshi VK, et al. Effect of methanolic extract of Pongamia pinnata Linn seed on gastro-duodenal ulceration and mucosal offensive and defensive factors in rats. Indian J Exp Biol. 2009;47:649–59. [PubMed] [Google Scholar]
- 735.Badole SL, Bodhankar SL. Antidiabetic activity of cycloart-23-ene-3beta, 25-diol (B2) isolated from Pongamia pinnata (L. Pierre) in streptozotocin-nicotinamide induced diabetic mice. Eur J Pharmacol. 632:103–9. doi: 10.1016/j.ejphar.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 736.Choi JH, Rho MC, Lee SW, Choi JN, Kim K, Song GY, et al. Bavachin and isobavachalcone, acyl-coenzyme A: cholesterol acyltransferase inhibitors from Psoralea corylifolia. Arch Pharm Res. 2008;31:1419–23. doi: 10.1007/s12272-001-2126-x. [DOI] [PubMed] [Google Scholar]
- 737.Xu Q, Pan Y, Yi LT, Li YC, Mo SF, Jiang FX, et al. Antidepressant-like effects of psoralen isolated from the seeds of Psoralea corylifolia in the mouse forced swimming test. Biol Pharm Bull. 2008;31:1109–14. doi: 10.1248/bpb.31.1109. [DOI] [PubMed] [Google Scholar]
- 738.Cho H, Jun JY, Song EK, Kang KH, Baek HY, Ko YS, et al. Bakuchiol: a hepatoprotective compound of Psoralea corylifolia on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med. 2001;67:750–1. doi: 10.1055/s-2001-18347. [DOI] [PubMed] [Google Scholar]
- 739.Wu CZ, Hong SS, Cai XF, Dat NT, Nan JX, Hwang BY, et al. Hypoxia-inducible factor-1 and nuclear factor-kappaB inhibitory meroterpene analogues of bakuchiol, a constituent of the seeds of Psoralea corylifolia. Bioorg Med Chem Lett. 2008;18:2619–23. doi: 10.1016/j.bmcl.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 740.Chen Y, Wang HD, Xia X, Kung HF, Pan Y, Kong LD. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the chronic mild stress model of depression in mice. Phytomedicine. 2007;14:523–9. doi: 10.1016/j.phymed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 741.Zhao G, Zheng XW, Qin GW, Gai Y, Jiang ZH, Guo LH. In vitro dopaminergic neuroprotective and in vivo antiparkinsonian-like effects of Delta 3,2-hydroxybakuchiol isolated from Psoralea corylifolia (L) Cell Mol Life Sci. 2009;66:1617–29. doi: 10.1007/s00018-009-9030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.An HJ, Seo MJ, Choi IY, Park RK, Jeong S, Lee JY, et al. Induction of nitric oxide & tumour necrosis factor-alpha by Psoralea corylifolia. Indian J Med Res. 2008;128:752–8. [PubMed] [Google Scholar]
- 743.Latha PG, Evans DA, Panikkar KR, Jayavardhanan KK. Immunomodulatory and antitumour properties of Psoralea corylifolia seeds. Fitoterapia. 2000;71:223–31. doi: 10.1016/s0367-326x(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 744.Jahromi MA, Ray AB. Antihyperlipidemic effect of flavonoids from Pterocarpus marsupium. J Nat Prod. 1993;56:989–94. doi: 10.1021/np50097a001. [DOI] [PubMed] [Google Scholar]
- 745.Dhanabal SP, Kokate CK, Ramanathan M, Kumar EP, Suresh B. Hypoglycaemic activity of Pterocarpus marsupium Roxb. Phytother Res. 2006;20:4–8. doi: 10.1002/ptr.1819. [DOI] [PubMed] [Google Scholar]
- 746.Cho JY, Park J, Kim PS, Yoo ES, Baik KU, Park MH. Savinin, a lignan from Pterocarpus santalinus inhibits tumor necrosis factor-alpha production and T cell proliferation. Biol Pharm Bull. 2001;24:167–71. doi: 10.1248/bpb.24.167. [DOI] [PubMed] [Google Scholar]
- 747.Narayan S, Devi RS, Srinivasan P, Shyamala Devi CS. Pterocarpus santalinus: a traditional herbal drug as a protectant against ibuprofen induced gastric ulcers. Phytother Res. 2005;19:958–62. doi: 10.1002/ptr.1764. [DOI] [PubMed] [Google Scholar]
- 748.Dey D, Pal BC, Biswas T, Roy SS, Bandyopadhyay A, Mandal SK, et al. A Lupinoside prevented fatty acid induced inhibition of insulin sensitivity in 3T3 L1 adipocytes. Mol Cell Biochem. 2007;300:149–57. doi: 10.1007/s11010-006-9378-1. [DOI] [PubMed] [Google Scholar]
- 749.Santosh N, Mohan K, Royana S, Yamini TB. Hepatotoxicity of tubers of Indian Kudzu (Pueraria tuberosa) in rats. Food Chem Toxicol. 48:1066–71. doi: 10.1016/j.fct.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 750.Dell’agli M, Galli GV, Bulgari M, Basilico N, Romeo S, Bhattacharya D, et al. Ellagitannins of the fruit rind of pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanisms involved in the onset of malaria. Malar J. 9:208. doi: 10.1186/1475-2875-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Katz SR, Newman RA, Lansky EP. Punica granatum: heuristic treatment for diabetes mellitus. J Med Food. 2007;10:213–7. doi: 10.1089/jmf.2006.290. [DOI] [PubMed] [Google Scholar]
- 752.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 753.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L): a review. Altern Med Rev. 2008;13:128–44. [PubMed] [Google Scholar]
- 754.Lee SI, Kim BS, Kim KS, Lee S, Shin KS, Lim JS. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem Biophys Res Commun. 2008;371:799–803. doi: 10.1016/j.bbrc.2008.04.150. [DOI] [PubMed] [Google Scholar]
- 755.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 756.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes in vitro. J Inflamm (Lond) 2008;5:9. doi: 10.1186/1476-9255-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr. 2005;135:2096–102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Toklu HZ, Sehirli O, Ozyurt H, Mayadagli AA, Eksioglu-Demiralp E, Cetinel S, et al. Punica granatum peel extract protects against ionizing radiation-induced enteritis and leukocyte apoptosis in rats. J Radiat Res (Tokyo) 2009;50:345–53. doi: 10.1269/jrr.08126. [DOI] [PubMed] [Google Scholar]
- 759.Jung KH, Kim MJ, Ha E, Uhm YK, Kim HK, Chung JH, et al. Suppressive effect of Punica granatum on the production of tumor necrosis factor (Tnf) in BV2 microglial cells. Biol Pharm Bull. 2006;29:1258–61. doi: 10.1248/bpb.29.1258. [DOI] [PubMed] [Google Scholar]
- 760.Huang TH, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, et al. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharmacol. 2005;207:160–9. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 761.Pithayanukul P, Nithitanakool S, Bavovada R. Hepatoprotective potential of extracts from seeds of Areca catechu and nutgalls of Quercus infectoria. Molecules. 2009;14:4987–5000. doi: 10.3390/molecules14124987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Wang LS, Sun XD, Cao Y, Wang L, Li FJ, Wang YF. Antioxidant and pro-oxidant properties of acylated pelargonidin derivatives extracted from red radish (Raphanus sativus var. niger, Brassicaceae) Food Chem Toxicol. doi: 10.1016/j.fct.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 763.Chaturvedi P. Inhibitory Response of Raphanus sativus on Lipid Peroxidation in Albino Rats. Evid Based Complement Alternat Med. 2008;5:55–9. doi: 10.1093/ecam/nel077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Salah-Abbes JB, Abbes S, Ouanes Z, Houas Z, Abdel-Wahhab MA, Bacha H, et al. Tunisian radish extract (Raphanus sativus) enhances the antioxidant status and protects against oxidative stress induced by zearalenone in Balb/c mice. J Appl Toxicol. 2008;28:6–14. doi: 10.1002/jat.1240. [DOI] [PubMed] [Google Scholar]
- 765.Gutierrez RM, Perez RL. Raphanus sativus (Radish): their chemistry and biology. ScientificWorldJournal. 2004;4:811–37. doi: 10.1100/tsw.2004.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Ilavarasan R, Mallika M, Venkataraman S. Anti-inflammatory and free radical scavenging activity of Ricinus communis root extract. J Ethnopharmacol. 2006;103:478–80. doi: 10.1016/j.jep.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 767.Shokeen P, Anand P, Murali YK, Tandon V. Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol. 2008;46:3458–66. doi: 10.1016/j.fct.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 768.Lomash V, Parihar SK, Jain NK, Katiyar AK. Effect of Solanum nigrum and Ricinus communis extracts on histamine and carrageenan-induced inflammation in the chicken skin. Cell Mol Biol (Noisy-le-grand) 56(Suppl):OL1239–51. [PubMed] [Google Scholar]
- 769.Mahmood N, Piacente S, Pizza C, Burke A, Khan AI, Hay AJ. The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem Biophys Res Commun. 1996;229:73–9. doi: 10.1006/bbrc.1996.1759. [DOI] [PubMed] [Google Scholar]
- 770.Ramezani R, Moghimi A, Rakhshandeh H, Ejtehadi H, Kheirabadi M. The effect of Rosa damascena essential oil on the amygdala electrical kindling seizures in rat. Pak J Biol Sci. 2008;11:746–51. doi: 10.3923/pjbs.2008.746.751. [DOI] [PubMed] [Google Scholar]
- 771.Gholamhoseinian A, Fallah H, Sharifi far F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on alpha-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009;16:935–41. doi: 10.1016/j.phymed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 772.Rao GM, Rao CV, Pushpangadan P, Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J Ethnopharmacol. 2006;103:484–90. doi: 10.1016/j.jep.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 773.Jain A, Basal E. Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine. 2003;10:34–8. doi: 10.1078/094471103321648638. [DOI] [PubMed] [Google Scholar]
- 774.Patil RA, Jagdale SC, Kasture SB. Antihyperglycemic, antistress and nootropic activity of roots of Rubia cordifolia Linn. Indian J Exp Biol. 2006;44:987–92. [PubMed] [Google Scholar]
- 775.Joy J, Nair CK. Amelioration of cisplatin induced nephrotoxicity in Swiss albino mice by Rubia cordifolia extract. J Cancer Res Ther. 2008;4:111–5. doi: 10.4103/0973-1482.43139. [DOI] [PubMed] [Google Scholar]
- 776.Islam MS, Rahman MT, Rouf AS, Rahman F. Evaluation of neuropharmacological effects of Rumex maritimus Linn. (Polygonaceae) root extracts. Pharmazie. 2003;58:738–41. [PubMed] [Google Scholar]
- 777.Dwivedi C, Abu-Ghazaleh A. Chemopreventive effects of sandalwood oil on skin papillomas in mice. Eur J Cancer Prev. 1997;6:399–401. doi: 10.1097/00008469-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 778.Matsuo Y, Mimaki Y. Lignans from Santalum album and their cytotoxic activities. Chem Pharm Bull (Tokyo) 58:587–90. doi: 10.1248/cpb.58.587. [DOI] [PubMed] [Google Scholar]
- 779.Arulmozhi V, Krishnaveni M, Karthishwaran K, Dhamodharan G, Mirunalini S. Antioxidant and antihyperlipidemic effect of Solanum nigrum fruit extract on the experimental model against chronic ethanol toxicity. Pharmacogn Mag. 6:42–50. doi: 10.4103/0973-1296.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Kim EJ, Lim SS, Park SY, Shin HK, Kim JS, Park JH. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem Toxicol. 2008;46:3651–8. doi: 10.1016/j.fct.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 781.Cho MK, Jang YP, Kim YC, Kim SG. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: the role in TNF-alpha inhibition. Int Immunopharmacol. 2004;4:1419–29. doi: 10.1016/j.intimp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 782.Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 783.Cho JY, Kim AR, Jung JH, Chun T, Rhee MH, Yoo ES. Cytotoxic and pro-apoptotic activities of cynaropicrin, a sesquiterpene lactone, on the viability of leukocyte cancer cell lines. Eur J Pharmacol. 2004;492:85–94. doi: 10.1016/j.ejphar.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 784.Kang JS, Yoon YD, Lee KH, Park SK, Kim HM. Costunolide inhibits interleukin-1beta expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264. 7 cells. Biochem Biophys Res Commun. 2004;313:171–7. doi: 10.1016/j.bbrc.2003.11.109. [DOI] [PubMed] [Google Scholar]
- 785.Jainu M, Devi CS. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L) on experimental ulcer models: possible mechanism for the inhibition of acid formation. J Ethnopharmacol. 2006;104:156–63. doi: 10.1016/j.jep.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 786.Kumar P, Kalonia H, Kumar A. Protective effect of sesamol against 3-nitropropionic acid-induced cognitive dysfunction and altered glutathione redox balance in rats. Basic Clin Pharmacol Toxicol. 107:577–82. doi: 10.1111/j.1742-7843.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- 787.Kumar PP, Kuttan G. Vernonia cinerea L. scavenges free radicals and regulates nitric oxide and proinflammatory cytokines profile in carrageenan induced paw edema model. Immunopharmacol Immunotoxicol. 2009;31:94–102. doi: 10.1080/08923970802438391. [DOI] [PubMed] [Google Scholar]
- 788.Visavadiya NP, Narasimhacharya AV. Sesame as a hypocholesteraemic and antioxidant dietary component. Food Chem Toxicol. 2008;46:1889–95. doi: 10.1016/j.fct.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 789.Visavadiya NP, Soni B, Dalwadi N. Free radical scavenging and antiatherogenic activities of Sesamum indicum seed extracts in chemical and biological model systems. Food Chem Toxicol. 2009;47:2507–15. doi: 10.1016/j.fct.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 790.Xu H, Yang X, Yang J, Qi W, Liu C, Yang Y. Antitumor effect of alcohol extract from Sesamum indicum flower on S180 and H22 experimental tumor. Zhong Yao Cai. 2003;26:272–3. [PubMed] [Google Scholar]
- 791.Takeuchi H, Mooi LY, Inagaki Y, He P. Hypoglycemic effect of a hot-water extract from defatted sesame (Sesamum indicum L) seed on the blood glucose level in genetically diabetic KK-Ay mice. Biosci Biotechnol Biochem. 2001;65:2318–21. doi: 10.1271/bbb.65.2318. [DOI] [PubMed] [Google Scholar]
- 792.Franzotti EM, Santos CV, Rodrigues HM, Mourao RH, Andrade MR, Antoniolli AR. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca) J Ethnopharmacol. 2000;72:273–7. doi: 10.1016/s0378-8741(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 793.Medeiros IA, Santos MR, Nascimento NM, Duarte JC. Cardiovascular effects of Sida cordifolia leaves extract in rats. Fitoterapia. 2006;77:19–27. doi: 10.1016/j.fitote.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 794.Philip BK, Muralidharan A, Natarajan B, Varadamurthy S, Venkataraman S. Preliminary evaluation of anti-pyretic and anti-ulcerogenic activities of Sida cordifolia methanolic extract. Fitoterapia. 2008;79:229–31. doi: 10.1016/j.fitote.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 795.Sumanth M, Mustafa SS. Antistress, Adoptogenic Activity of Sida cordifolia Roots in Mice. Indian J Pharm Sci. 2009;71:323–4. doi: 10.4103/0250-474X.56027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Swathy SS, Panicker S, Nithya RS, Anuja MM, Rejitha S, Indira M. Antiperoxidative and Antiinflammatory Effect of Sida Cordifolia Linn. on Quinolinic Acid Induced Neurotoxicity. Neurochem Res. doi: 10.1007/s11064-010-0192-5. [DOI] [PubMed] [Google Scholar]
- 797.Reyes BA, Bautista ND, Tanquilut NC, Anunciado RV, Leung AB, Sanchez GC, et al. Anti-diabetic potentials of Momordica charantia and Andrographis paniculata and their effects on estrous cyclicity of alloxan-induced diabetic rats. J Ethnopharmacol. 2006;105:196–200. doi: 10.1016/j.jep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 798.Heo KS, Lee SJ, Ko JH, Lim K, Lim KT. Glycoprotein isolated from Solanum nigrum L. inhibits the DNA-binding activities of NF-kappaB and AP-1, and increases the production of nitric oxide in TPA-stimulated MCF-7 cells. Toxicol In Vitro. 2004;18:755–63. doi: 10.1016/j.tiv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 799.Yang MY, Hsu LS, Peng CH, Shi YS, Wu CH, Wang CJ. Polyphenol-rich extracts from Solanum nigrum attenuated PKC alpha-mediated migration and invasion of hepatocellular carcinoma cells. J Agric Food Chem. 58:5806–14. doi: 10.1021/jf100718b. [DOI] [PubMed] [Google Scholar]
- 800.Hsieh CC, Fang HL, Lina WC. Inhibitory effect of Solanum nigrum on thioacetamide-induced liver fibrosis in mice. J Ethnopharmacol. 2008;119:117–21. doi: 10.1016/j.jep.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 801.Wannang NN, Anuka JA, Kwanashie HO, Gyang SS, Auta A. Anti-seizure activity of the aqueous leaf extract of Solanum nigrum linn (solanaceae) in experimental animals. Afr Health Sci. 2008;8:74–9. [PMC free article] [PubMed] [Google Scholar]
- 802.Li J, Li QW, Gao DW, Han ZS, Lu WZ. Antitumor and immunomodulating effects of polysaccharides isolated from Solanum nigrum Linne. Phytother Res. 2009;23:1524–30. doi: 10.1002/ptr.2769. [DOI] [PubMed] [Google Scholar]
- 803.Zakaria ZA, Sulaiman MR, Morsid NA, Aris A, Zainal H, Pojan NH, et al. Antinociceptive, anti-inflammatory and antipyretic effects of Solanum nigrum aqueous extract in animal models. Methods Find Exp Clin Pharmacol. 2009;31:81–8. doi: 10.1358/mf.2009.31.2.1353876. [DOI] [PubMed] [Google Scholar]
- 804.Kar DM, Maharana L, Pattnaik S, Dash GK. Studies on hypoglycaemic activity of Solanum xanthocarpum Schrad. & Wendl. fruit extract in rats. J Ethnopharmacol. 2006;108:251–6. doi: 10.1016/j.jep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 805.Fonseca LC, Dadarkar SS, Lobo AS, Suthar AC, Chauhan VS, Chandrababu S, et al. 7-hydroxyfrullanolide, a sesquiterpene lactone, inhibits pro-inflammatory cytokine production from immune cells and is orally efficacious in animal models of inflammation. Eur J Pharmacol. doi: 10.1016/j.ejphar.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 806.Bafna AR, Mishra SH. Protective effect of bioactive fraction of Sphaeranthus indicus Linn. against cyclophosphamide induced suppression of humoral immunity in mice. J Ethnopharmacol. 2006;104:426–9. doi: 10.1016/j.jep.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 807.Chandrashekhar VM, Muchandi AA, Sudi SV, Ganapty S. Hepatoprotective activity of Stereospermum suaveolens against CCl4-induced liver damage in albino rats. Pharm Biol. 48:524–8. doi: 10.3109/13880200903173601. [DOI] [PubMed] [Google Scholar]
- 808.Yin W, Wang TS, Yin FZ, Cai BC. Analgesic and anti-inflammatory properties of brucine and brucine N-oxide extracted from seeds of Strychnos nux-vomica. J Ethnopharmacol. 2003;88:205–14. doi: 10.1016/s0378-8741(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 809.Deng XK, Yin W, Li WD, Yin FZ, Lu XY, Zhang XC, et al. The anti-tumor effects of alkaloids from the seeds of Strychnos nux-vomica on HepG2 cells and its possible mechanism. J Ethnopharmacol. 2006;106:179–86. doi: 10.1016/j.jep.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 810.Rao PS, Ramanadham M, Prasad MN. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line - RPMI 8226. Food Chem Toxicol. 2009;47:283–8. doi: 10.1016/j.fct.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 811.Saha P, Mandal S, Das A, Das PC, Das S. Evaluation of the anticarcinogenic activity of Swertia chirata Buch. Ham, an Indian medicinal plant, on DMBA-induced mouse skin carcinogenesis model. Phytother Res. 2004;18:373–8. doi: 10.1002/ptr.1436. [DOI] [PubMed] [Google Scholar]
- 812.Rafatullah S, Tariq M, Mossa JS, al-Yahya MA, al-Said MS, Ageel AM. Protective effect of Swertia chirata against indomethacin and other ulcerogenic agent-induced gastric ulcers. Drugs Exp Clin Res. 1993;19:69–73. [PubMed] [Google Scholar]
- 813.Srivastava KC, Malhotra N. Acetyl eugenol, a component of oil of cloves (Syzygium aromaticum L) inhibits aggregation and alters arachidonic acid metabolism in human blood platelets. Prostaglandins Leukot Essent Fatty Acids. 1991;42:73–81. doi: 10.1016/0952-3278(91)90070-l. [DOI] [PubMed] [Google Scholar]
- 814.Banerjee S, Das S. Anticarcinogenic effects of an aqueous infusion of cloves on skin carcinogenesis. Asian Pac J Cancer Prev. 2005;6:304–8. [PubMed] [Google Scholar]
- 815.Tanko Y, Mohammed A, Okasha MA, Umar AH, Magaji RA. Anti-nociceptive and anti-inflammatory activities of ethanol extract of syzygium aromaticum flower bud in Wistar rats and mice. Afr J Tradit Complement Altern Med. 2008;5:209–12. doi: 10.4314/ajtcam.v5i2.31275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, et al. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res. 2007;21:501–6. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- 817.Mandal S, Barik B, Mallick C, De D, Ghosh D. Therapeutic effect of ferulic acid, an ethereal fraction of ethanolic extract of seed of Syzygium cumini against streptozotocin-induced diabetes in male rat. Methods Find Exp Clin Pharmacol. 2008;30:121–8. doi: 10.1358/mf.2008.30.2.1143090. [DOI] [PubMed] [Google Scholar]
- 818.Helmstadter A. Syzygium cumini (L) SKEELS (Myrtaceae) against diabetes--125 years of research. Pharmazie. 2008;63:91–101. [PubMed] [Google Scholar]
- 819.Muruganandan S, Pant S, Srinivasan K, Chandra S, Tandan SK, Lal J, et al. Inhibitory role of Syzygium cumini on autacoid-induced inflammation in rats. Indian J Physiol Pharmacol. 2002;46:482–6. [PubMed] [Google Scholar]
- 820.Muruganandan S, Srinivasan K, Chandra S, Tandan SK, Lal J, Raviprakash V. Anti-inflammatory activity of Syzygium cumini bark. Fitoterapia. 2001;72:369–75. doi: 10.1016/s0367-326x(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 821.Brito FA, Lima LA, Ramos MF, Nakamura MJ, Cavalher-Machado SC, Siani AC, et al. Pharmacological study of anti-allergic activity of Syzygium cumini (L) Skeels. Braz J Med Biol Res. 2007;40:105–15. doi: 10.1590/s0100-879x2007000100014. [DOI] [PubMed] [Google Scholar]
- 822.Parmar J, Sharma P, Verma P, Goyal PK. Chemopreventive action of Syzygium cumini on DMBA-induced skin papillomagenesis in mice. Asian Pac J Cancer Prev. 11:261–5. [PubMed] [Google Scholar]
- 823.Sehrawat A, Sultana S. Tamarix gallica ameliorates thioacetamide-induced hepatic oxidative stress and hyperproliferative response in Wistar rats. J Enzyme Inhib Med Chem. 2006;21:215–23. doi: 10.1080/14756360500480673. [DOI] [PubMed] [Google Scholar]
- 824.Sun FY, Chen XP, Wang JH, Qin HL, Yang SR, Du GH. Arjunic acid, a strong free radical scavenger from Terminalia arjuna. Am J Chin Med. 2008;36:197–207. doi: 10.1142/S0192415X08005709. [DOI] [PubMed] [Google Scholar]
- 825.Kuo PL, Hsu YL, Lin TC, Chang JK, Lin CC. Induction of cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells by casuarinin from the bark of Terminalia arjuna Linn. Anticancer Drugs. 2005;16:409–15. doi: 10.1097/00001813-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 826.Chen CH, Liu TZ, Kuo TC, Lu FJ, Chen YC, Chang-Chien YW, et al. Casuarinin protects cultured MDCK cells from hydrogen peroxide-induced oxidative stress and DNA oxidative damage. Planta Med. 2004;70:1022–6. doi: 10.1055/s-2004-832641. [DOI] [PubMed] [Google Scholar]
- 827.Ali A, Kaur G, Hamid H, Abdullah T, Ali M, Niwa M, et al. Terminoside A, a new triterpene glycoside from the bark of Terminalia arjuna inhibits nitric oxide production in murine macrophages. J Asian Nat Prod Res. 2003;5:137–42. doi: 10.1080/1028602031000066834. [DOI] [PubMed] [Google Scholar]
- 828.Gupta R, Singhal S, Goyle A, Sharma VN. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree-bark powder: a randomised placebo-controlled trial. J Assoc Physicians India. 2001;49:231–5. [PubMed] [Google Scholar]
- 829.Devi RS, Kist M, Vani G, Devi CS. Effect of methanolic extract of Terminalia arjuna against Helicobacter pylori 26695 lipopolysaccharide-induced gastric ulcer in rats. J Pharm Pharmacol. 2008;60:505–14. doi: 10.1211/jpp.60.4.0014. [DOI] [PubMed] [Google Scholar]
- 830.Kumar S, Enjamoori R, Jaiswal A, Ray R, Seth S, Maulik SK. Catecholamine-induced myocardial fibrosis and oxidative stress is attenuated by Terminalia arjuna (Roxb. ) J Pharm Pharmacol. 2009;61:1529–36. doi: 10.1211/jpp/61.11.0013. [DOI] [PubMed] [Google Scholar]
- 831.Dwivedi S. Terminalia arjuna Wight & Arn--a useful drug for cardiovascular disorders. J Ethnopharmacol. 2007;114:114–29. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 832.Tariq M, Hussain SJ, Asif M, Jahan M. Protective effect of fruit extracts of Emblica officinalis (Gaertn). & Terminalia belerica (Roxb) in experimental myocardial necrosis in rats. Indian J Exp Biol. 1977;15:485–6. [PubMed] [Google Scholar]
- 833.Shaila HP, Udupa SL, Udupa AL. Hypolipidemic activity of three indigenous drugs in experimentally induced atherosclerosis. Int J Cardiol. 1998;67:119–24. doi: 10.1016/s0167-5273(98)00281-2. [DOI] [PubMed] [Google Scholar]
- 834.Srikumar R, Parthasarathy NJ, Manikandan S, Narayanan GS, Sheeladevi R. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol Cell Biochem. 2006;283:67–74. doi: 10.1007/s11010-006-2271-0. [DOI] [PubMed] [Google Scholar]
- 835.Sabu MC, Kuttan R. Antidiabetic and antioxidant activity of Terminalia belerica. Roxb Indian J Exp Biol. 2009;47:270–5. [PubMed] [Google Scholar]
- 836.Hazra B, Sarkar R, Biswas S, Mandal N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement Altern Med. 10:20. doi: 10.1186/1472-6882-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Reddy DB, Reddy TC, Jyotsna G, Sharan S, Priya N, Lakshmipathi V, et al. Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz. induces apoptosis in COLO-205 cell line. J Ethnopharmacol. 2009;124:506–12. doi: 10.1016/j.jep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 838.Lee SI, Hyun PM, Kim SH, Kim KS, Lee SK, Kim BS, et al. Suppression of the onset and progression of collagen-induced arthritis by chebulagic acid screened from a natural product library. Arthritis Rheum. 2005;52:345–53. doi: 10.1002/art.20715. [DOI] [PubMed] [Google Scholar]
- 839.Sabu MC, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81:155–60. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 840.Amit A, Saxena VS, Pratibha N, D’Souza P, Bagchi M, Bagchi D, et al. Mast cell stabilization, lipoxygenase inhibition, hyaluronidase inhibition, antihistaminic and antispasmodic activities of Aller-7, a novel botanical formulation for allergic rhinitis. Drugs Exp Clin Res. 2003;29:107–15. [PubMed] [Google Scholar]
- 841.Prasad L, Husain Khan T, Jahangir T, Sultana S. Chemomodulatory effects of Terminalia chebula against nickel chloride induced oxidative stress and tumor promotion response in male Wistar rats. J Trace Elem Med Biol. 2006;20:233–9. doi: 10.1016/j.jtemb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 842.Patel CD, Modi VD, Chakraborty BS, Mathuria N, Dadhaniya P, Borade PA, et al. Effect of a novel antiinflammatory polyherbal preparation (Sudarshanam oil) on hematological parameters in Wistar rats. Acta Pol Pharm. 67:277–81. [PubMed] [Google Scholar]
- 843.Jukic M, Politeo O, Maksimovic M, Milos M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother Res. 2007;21:259–61. doi: 10.1002/ptr.2063. [DOI] [PubMed] [Google Scholar]
- 844.Okazaki K, Kawazoe K, Takaishi Y. Human platelet aggregation inhibitors from thyme (Thymus vulgaris L) Phytother Res. 2002;16:398–9. doi: 10.1002/ptr.979. [DOI] [PubMed] [Google Scholar]
- 845.Singh N, Singh SM, Shrivastava P. Effect of Tinospora cordifolia on the antitumor activity of tumor-associated macrophages-derived dendritic cells. Immunopharmacol Immunotoxicol. 2005;27:1–14. doi: 10.1081/iph-51287. [DOI] [PubMed] [Google Scholar]
- 846.Bafna PA, Balaraman R. Anti-ulcer and anti-oxidant activity of pepticare, a herbomineral formulation. Phytomedicine. 2005;12:264–70. doi: 10.1016/j.phymed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 847.Desai VR, Ramkrishnan R, Chintalwar GJ, Sainis KB. G1–4A, an immunomodulatory polysaccharide from Tinospora cordifolia, modulates macrophage responses and protects mice against lipopolysaccharide induced endotoxic shock. Int Immunopharmacol. 2007;7:1375–86. doi: 10.1016/j.intimp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 848.Patel SS, Shah RS, Goyal RK. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J Exp Biol. 2009;47:564–70. [PubMed] [Google Scholar]
- 849.Akhtar S. Use of Tinospora cordifolia in HIV infection. Indian J Pharmacol. 42:57. doi: 10.4103/0253-7613.62402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Kaur T, Bijarnia RK, Singla SK, Tandon C. In vivo efficacy of Trachyspermum ammi anticalcifying protein in urolithiatic rat model. J Ethnopharmacol. 2009;126:459–62. doi: 10.1016/j.jep.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 851.Zhang S, Li H, Yang SJ. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacol Sin. 31:671–8. doi: 10.1038/aps.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Sun B, Qu W, Bai Z. The inhibitory effect of saponins from Tribulus terrestris on Bcap-37 breast cancer cell line in vitro. Zhong Yao Cai. 2003;26:104–6. [PubMed] [Google Scholar]
- 853.Liu XM, Huang QF, Zhang YL, Lou JL, Liu HS, Zheng H. Effects of Tribulus terrestris L. saponion on apoptosis of cortical neurons induced by hypoxia-reoxygenation in rats. Zhong Xi Yi Jie He Xue Bao. 2008;6:45–50. doi: 10.3736/jcim20080110. [DOI] [PubMed] [Google Scholar]
- 854.Heidari MR, Mehrabani M, Pardakhty A, Khazaeli P, Zahedi MJ, Yakhchali M, et al. The analgesic effect of Tribulus terrestris extract and comparison of gastric ulcerogenicity of the extract with indomethacine in animal experiments. Ann N Y Acad Sci. 2007;1095:418–27. doi: 10.1196/annals.1397.045. [DOI] [PubMed] [Google Scholar]
- 855.Tuncer MA, Yaymaci B, Sati L, Cayli S, Acar G, Altug T, et al. Influence of Tribulus terrestris extract on lipid profile and endothelial structure in developing atherosclerotic lesions in the aorta of rabbits on a high-cholesterol diet. Acta Histochem. 2009;111:488–500. doi: 10.1016/j.acthis.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 856.Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1392–8. [PubMed] [Google Scholar]
- 857.Uemura T, Hirai S, Mizoguchi N, Goto T, Lee JY, Taketani K, et al. Diosgenin present in fenugreek improves glucose metabolism by promoting adipocyte differentiation and inhibiting inflammation in adipose tissues. Mol Nutr Food Res. doi: 10.1002/mnfr.200900609. [DOI] [PubMed] [Google Scholar]
- 858.Srichamroen A, Thomson AB, Field CJ, Basu TK. In vitro intestinal glucose uptake is inhibited by galactomannan from Canadian fenugreek seed (Trigonella foenum graecum L) in genetically lean and obese rats. Nutr Res. 2009;29:49–54. doi: 10.1016/j.nutres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 859.Pandian RS, Anuradha CV, Viswanathan P. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Ethnopharmacol. 2002;81:393–7. doi: 10.1016/s0378-8741(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 860.Tahiliani P, Kar A. The combined effects of Trigonella and Allium extracts in the regulation of hyperthyroidism in rats. Phytomedicine. 2003;10:665–8. doi: 10.1078/0944-7113-00277. [DOI] [PubMed] [Google Scholar]
- 861.Raju J, Bird RP. Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-alpha levels by Trigonella foenum graecum (fenugreek) seeds in Zucker obese (fa/fa) rats. Int J Obes (Lond) 2006;30:1298–307. doi: 10.1038/sj.ijo.0803254. [DOI] [PubMed] [Google Scholar]
- 862.Hannan JM, Ali L, Rokeya B, Khaleque J, Akhter M, Flatt PR, et al. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br J Nutr. 2007;97:514–21. doi: 10.1017/S0007114507657869. [DOI] [PubMed] [Google Scholar]
- 863.Vyas S, Agrawal RP, Solanki P, Trivedi P. Analgesic and anti-inflammatory activities of Trigonella foenum-graecum (seed) extract. Acta Pol Pharm. 2008;65:473–6. [PubMed] [Google Scholar]
- 864.Gupta SK, Kalaiselvan V, Srivastava S, Saxena R, Agrawal SS. Trigonella foenum-graecum (Fenugreek) Protects Against Selenite-Induced Oxidative Stress in Experimental Cataractogenesis. Biol Trace Elem Res. 2009 doi: 10.1007/s12011-009-8540-5. [DOI] [PubMed] [Google Scholar]
- 865.Reddy RL, Srinivasan K. Fenugreek seeds reduce atherogenic diet-induced cholesterol gallstone formation in experimental mice. Can J Physiol Pharmacol. 2009;87:933–43. doi: 10.1139/y09-084. [DOI] [PubMed] [Google Scholar]
- 866.Varjas T, Nowrasteh G, Budan F, Horvath G, Cseh J, Gyongyi Z, et al. The effect of fenugreek on the gene expression of arachidonic acid metabolizing enzymes. Phytother Res. doi: 10.1002/ptr.3231. [DOI] [PubMed] [Google Scholar]
- 867.Rehni AK, Pantlya HS, Shri R, Singh M. Effect of chlorophyll and aqueous extracts of Bacopa monniera and Valeriana wallichii on ischaemia and reperfusion-induced cerebral injury in mice. Indian J Exp Biol. 2007;45:764–9. [PubMed] [Google Scholar]
- 868.Subhan F, Karim N, Gilani AH, Sewell RD. Terpenoid content of Valeriana wallichii extracts and antidepressant-like response profiles. Phytother Res. 24:686–91. doi: 10.1002/ptr.2980. [DOI] [PubMed] [Google Scholar]
- 869.Prasad DN, Achari G. A study of anti-arthritic action of Vanda roxburghii in albino rats. J Indian Med Assoc. 1966;46:234–7. [PubMed] [Google Scholar]
- 870.Latha RM, Geetha T, Varalakshmi P. Effect of Vernonia cinerea Less flower extract in adjuvant-induced arthritis. Gen Pharmacol. 1998;31:601–6. doi: 10.1016/s0306-3623(98)00049-4. [DOI] [PubMed] [Google Scholar]
- 871.Mazumder UK, Gupta M, Manikandan L, Bhattacharya S, Haldar PK, Roy S. Evaluation of anti-inflammatory activity of Vernonia cinerea Less. extract in rats. Phytomedicine. 2003;10:185–8. doi: 10.1078/094471103321659915. [DOI] [PubMed] [Google Scholar]
- 872.Iwalewa EO, Iwalewa OJ, Adeboye JO. Analgesic, antipyretic, anti-inflammatory effects of methanol, chloroform and ether extracts of Vernonia cinerea less leaf. J Ethnopharmacol. 2003;86:229–34. doi: 10.1016/s0378-8741(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 873.Leelarungrayub D, Pratanaphon S, Pothongsunun P, Sriboonreung T, Yankai A, Bloomer RJ. Vernonia cinerea Less. supplementation and strenuous exercise reduce smoking rate: relation to oxidative stress status and beta-endorphin release in active smokers. J Int Soc Sports Nutr. 7:21. doi: 10.1186/1550-2783-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 874.Gerlach SL, Rathinakumar R, Chakravarty G, Goransson U, Wimley WC, Darwin SP, et al. Anticancer and chemosensitizing abilities of cycloviolacin O2 from Viola odorata and psyle cyclotides from psychotria leptothyrsa. Biopolymers. doi: 10.1002/bip.21435. [DOI] [PubMed] [Google Scholar]
- 875.Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak J Pharm Sci. 23:29–34. [PubMed] [Google Scholar]
- 876.Zheng CJ, Huang BK, Wang Y, Ye Q, Han T, Zhang QY, et al. Anti-inflammatory diterpenes from the seeds of Vitex negundo. Bioorg Med Chem. 18:175–81. doi: 10.1016/j.bmc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 877.Chawla AS, Sharma AK, Handa SS, Dhar KL. Chemical investigation and anti-inflammatory activity of Vitex negundo seeds. J Nat Prod. 1992;55:163–7. doi: 10.1021/np50080a002. [DOI] [PubMed] [Google Scholar]
- 878.Dharmasiri MG, Jayakody JR, Galhena G, Liyanage SS, Ratnasooriya WD. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87:199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 879.Tandon VR, Gupta RK. Vitex negundo Linn (VN) leaf extract as an adjuvant therapy to standard anti-inflammatory drugs. Indian J Med Res. 2006;124:447–50. [PubMed] [Google Scholar]
- 880.Rooban BN, Lija Y, Biju PG, Sasikala V, Sahasranamam V, Abraham A. Vitex negundo attenuates calpain activation and cataractogenesis in selenite models. Exp Eye Res. 2009;88:575–82. doi: 10.1016/j.exer.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 881.Maitra R, Porter MA, Huang S, Gilmour BP. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J Inflamm (Lond) 2009;6:15. doi: 10.1186/1476-9255-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Park HJ, Rayalam S, Della-Fera MA, Ambati S, Yang JY, Baile CA. Withaferin A induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Biofactors. 2008;33:137–48. doi: 10.1002/biof.5520330206. [DOI] [PubMed] [Google Scholar]
- 883.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–46. [PubMed] [Google Scholar]
- 884.Kour K, Pandey A, Suri KA, Satti NK, Gupta KK, Bani S. Restoration of stress-induced altered T cell function and corresponding cytokines patterns by Withanolide A. Int Immunopharmacol. 2009;9:1137–44. doi: 10.1016/j.intimp.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 885.Jayaprakasam B, Padmanabhan K, Nair MG. Withanamides in Withania somnifera fruit protect PC-12 cells from beta-amyloid responsible for Alzheimer’s disease. Phytother Res. 24:859–63. doi: 10.1002/ptr.3033. [DOI] [PubMed] [Google Scholar]
- 886.Sumantran VN, Chandwaskar R, Joshi AK, Boddul S, Patwardhan B, Chopra A, et al. The relationship between chondroprotective and antiinflammatory effects of Withania somnifera root and glucosamine sulphate on human osteoarthritic cartilage in vitro. Phytother Res. 2008;22:1342–8. doi: 10.1002/ptr.2498. [DOI] [PubMed] [Google Scholar]
- 887.Bhatnagar M, Sisodia SS, Bhatnagar R. Antiulcer and antioxidant activity of Asparagus racemosus Willd and Withania somnifera Dunal in rats. Ann N Y Acad Sci. 2005;1056:261–78. doi: 10.1196/annals.1352.027. [DOI] [PubMed] [Google Scholar]
- 888.Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, et al. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci. 2009;10:2367–82. doi: 10.3390/ijms10052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.Jeyanthi T, Subramanian P. Protective effect of Withania somnifera root powder on lipid peroxidation and antioxidant status in gentamicin-induced nephrotoxic rats. J Basic Clin Physiol Pharmacol. 21:61–78. doi: 10.1515/jbcpp.2010.21.1.61. [DOI] [PubMed] [Google Scholar]
- 890.Kim JK, Kim Y, Na KM, Surh YJ, Kim TY. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic Res. 2007;41:603–14. doi: 10.1080/10715760701209896. [DOI] [PubMed] [Google Scholar]
- 891.Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 127:515–20. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 892.Pan MH, Hsieh MC, Hsu PC, Ho SY, Lai CS, Wu H, et al. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol Nutr Food Res. 2008;52:1467–77. doi: 10.1002/mnfr.200700515. [DOI] [PubMed] [Google Scholar]
- 893.Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, et al. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res. 2008;52:527–37. doi: 10.1002/mnfr.200700157. [DOI] [PubMed] [Google Scholar]
- 894.Sang S, Hong J, Wu H, Liu J, Yang CS, Pan MH, et al. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 2009;57:10645–50. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Brown AC, Shah C, Liu J, Pham JT, Zhang JG, Jadus MR. Ginger’s (Zingiber officinale Roscoe) inhibition of rat colonic adenocarcinoma cells proliferation and angiogenesis in vitro. Phytother Res. 2009;23:640–5. doi: 10.1002/ptr.2677. [DOI] [PubMed] [Google Scholar]
- 896.Guahk GH, Ha SK, Jung HS, Kang C, Kim CH, Kim YB, et al. Zingiber officinale protects HaCaT cells and C57BL/6 mice from ultraviolet B-induced inflammation. J Med Food. 13:673–80. doi: 10.1089/jmf.2009.1239. [DOI] [PubMed] [Google Scholar]
- 897.Lantz RC, Chen GJ, Sarihan M, Solyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–8. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 898.Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96:660–6. doi: 10.1079/bjn20061849. [DOI] [PubMed] [Google Scholar]
- 899.Ahui ML, Champy P, Ramadan A, Pham Van L, Araujo L, Brou Andre K, et al. Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation. Int Immunopharmacol. 2008;8:1626–32. doi: 10.1016/j.intimp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 900.Habib SH, Makpol S, Abdul Hamid NA, Das S, Ngah WZ, Yusof YA. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo) 2008;63:807–13. doi: 10.1590/S1807-59322008000600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Lakshmi BV, Sudhakar M. Attenuation of acute and chronic restraint stress-induced perturbations in experimental animals by Zingiber officinale Roscoe. Food Chem Toxicol. 48:530–5. doi: 10.1016/j.fct.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 902.Grzanna R, Lindmark L, Frondoza CG. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–32. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 903.Kundu JK, Na HK, Surh YJ. Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum Nutr. 2009;61:182–92. doi: 10.1159/000212750. [DOI] [PubMed] [Google Scholar]
- 904.Anggakusuma Yanti, Hwang JK. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. J Dermatol Sci. 57:114–22. doi: 10.1016/j.jdermsci.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 905.Ma J, Hwang YK, Cho WH, Han SH, Hwang JK, Han JS. Macelignan attenuates activations of mitogen-activated protein kinases and nuclear factor kappa B induced by lipopolysaccharide in microglial cells. Biol Pharm Bull. 2009;32:1085–90. doi: 10.1248/bpb.32.1085. [DOI] [PubMed] [Google Scholar]
- 906.Akev N, Turkay G, Can A, Gurel A, Yildiz F, Yardibi H, et al. Tumour preventive effect of Aloe vera leaf pulp lectin (Aloctin I) on Ehrlich ascites tumours in mice. Phytother Res. 2007;21:1070–5. doi: 10.1002/ptr.2215. [DOI] [PubMed] [Google Scholar]
- 907.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–17. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 908.Hong J, Sang S, Park HJ, Kwon SJ, Suh N, Huang MT, et al. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis. 2006;27:278–86. doi: 10.1093/carcin/bgi208. [DOI] [PubMed] [Google Scholar]
- 909.Koeberle A, Northoff H, Werz O. Identification of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol. Biochem Pharmacol. 2009;77:1513–21. doi: 10.1016/j.bcp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 910.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24:2558–67. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 911.Liu KL, Chen HW, Wang RY, Lei YP, Sheen LY, Lii CK. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW 264. 7 macrophages. J Agric Food Chem. 2006;54:3472–8. doi: 10.1021/jf060043k. [DOI] [PubMed] [Google Scholar]
- 912.Genovese T, Menegazzi M, Mazzon E, Crisafulli C, Di Paola R, Dal Bosco M, et al. Glycyrrhizin reduces secondary inflammatory process after spinal cord compression injury in mice. Shock. 2009;31:367–75. doi: 10.1097/SHK.0b013e3181833b08. [DOI] [PubMed] [Google Scholar]
- 913.Ram A, Mabalirajan U, Das M, Bhattacharya I, Dinda AK, Gangal SV, et al. Glycyrrhizin alleviates experimental allergic asthma in mice. Int Immunopharmacol. 2006;6:1468–77. doi: 10.1016/j.intimp.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 914.Chiou WF, Chen CF, Lin JJ. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264. 7 cells by andrographolide. Br J Pharmacol. 2000;129:1553–60. doi: 10.1038/sj.bjp.0703191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Cho JY, Park J, Yoo ES, Baik KU, Jung JH, Lee J, et al. Inhibitory effect of sesquiterpene lactones from Saussurea lappa on tumor necrosis factor-alpha production in murine macrophage-like cells. Planta Med. 1998;64:594–7. doi: 10.1055/s-2006-957528. [DOI] [PubMed] [Google Scholar]
- 916.Oh GS, Pae HO, Chung HT, Kwon JW, Lee JH, Kwon TO, et al. Dehydrocostus lactone enhances tumor necrosis factor-alpha-induced apoptosis of human leukemia HL-60 cells. Immunopharmacol Immunotoxicol. 2004;26:163–75. doi: 10.1081/iph-120037712. [DOI] [PubMed] [Google Scholar]
- 917.Jin M, Lee HJ, Ryu JH, Chung KS. Inhibition of LPS-induced NO production and NF-kappaB activation by a sesquiterpene from Saussurea lappa. Arch Pharm Res. 2000;23:54–8. doi: 10.1007/BF02976467. [DOI] [PubMed] [Google Scholar]
- 918.Lampronti I, Khan MT, Bianchi N, Ather A, Borgatti M, Vizziello L, et al. Bangladeshi medicinal plant extracts inhibiting molecular interactions between nuclear factors and target DNA sequences mimicking NF-kappaB binding sites. Med Chem. 2005;1:327–33. doi: 10.2174/1573406054368684. [DOI] [PubMed] [Google Scholar]
- 919.Murakami A, Ohigashi H. Cancer-preventive anti-oxidants that attenuate free radical generation by inflammatory cells. Biol Chem. 2006;387:387–92. doi: 10.1515/BC.2006.052. [DOI] [PubMed] [Google Scholar]
- 920.Joo HY, Lim K, Lim KT. Phytoglycoprotein (150 kDa) isolated from Solanum nigrum Linne has a preventive effect on dextran sodium sulfate-induced colitis in A/J mouse. J Appl Toxicol. 2009;29:207–13. doi: 10.1002/jat.1396. [DOI] [PubMed] [Google Scholar]
- 921.Lee YY, Yang SF, Ho WH, Lee YH, Hung SL. Eugenol modulates cyclooxygenase-2 expression through the activation of nuclear factor kappa B in human osteoblasts. J Endod. 2007;33:1177–82. doi: 10.1016/j.joen.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 922.Nair PK, Melnick SJ, Ramachandran R, Escalon E, Ramachandran C. Mechanism of macrophage activation by (1,4)-alpha-D-glucan isolated from Tinospora cordifolia. Int Immunopharmacol. 2006;6:1815–24. doi: 10.1016/j.intimp.2006.07.028. [DOI] [PubMed] [Google Scholar]