Abstract
Serum cytokine profiling is a powerful tool to link host immune defense with disease pathogenesis. Although several multiplex assays are commercially available, none has been rigorously validated in the context of chronic infectious disease (such as HIV infection). Here we compared the measurement of proinflammatory cytokines by two multiplex platforms: the Meso Scale Discovery (MSD) electrochemiluminscence assay and the Becton Dickinson Cytometric Bead Array (CBA) flow cytometric assay, using serum samples from HIV-infected and -uninfected donors. We evaluated the ability of these assays to: a) quantify circulating levels of native cytokines (IL-6, IL-8, IL-10, TNF-α, IL-12p70, IL-1β), and b) accurately recover known amounts of recombinant cytokines added to serum samples. Based on the standard curves, the sensitivity of the MSD system was only slightly better than the CBA. However, in serum the MSD platform consistently quantified levels of endogenous IL-12p70, TNF-α, and IL-10 that were undetectable by the CBA assay. The MSD assay was also more accurate as determined by an enhanced capacity to recover known concentrations of recombinant cytokines added to serum. Both assays performed equally well in quantifying IL-6 and IL-8, while neither assay quantified IL-1β with accuracy and precision. Interestingly, HIV infection did not affect the performance of either assay. Overall, the MSD assay provided a more reliable assessment of the proinflammatory cytokines tested in the serum of healthy and HIV-infected individuals.
Keywords: Multiplex assay, Cytokine, Biomarker, Serum, HIV
1. INTRODUCTION
Cytokines play a key role in orchestrating immune responses, including inflammation and defense against infectious pathogens. Due to their potent biological activity and localized effects in lymphoid tissues, circulating cytokine levels are generally very low, often below the limits of detection of traditional immunoassays. Because many cytokines have closely related or overlapping biological effects, quantitation of single cytokines may be of limited value. For these reasons, multiplex methods have been developed to measure numerous cytokines simultaneously in individual specimens (Breen et al., 2011; Leng et al., 2008; Young, 2009). These methods also reduce cost and time for analysis, and use samples more efficiently than single-cytokine measurements.
Two main formats exist for multiplex cytokine analysis: bead-based (liquid-phase) assays and plate-based (solid-phase) assays. The first relies on different populations of microbeads with discrete fluorescence characteristics, which can be distinguished using flow cytometry (Varro et al., 2007; Fu et al., 2010; Leng et al., 2008; Young, 2009). Each microbead is pre-coated with capture antibodies specific for different cytokines that are recognized by specific labeled secondary antibodies. Examples of bead-based assays are Luminex-based assays (Lumininex®), FlowCytomix™ (eBioscience), and the BD™ Cytometric Bead Array (CBA, BD Biosciences). The second format is in principle like an ELISA, with multiple capture antibodies spotted at defined positions in each well of a 96-well microplate (Chowdhury et al., 2009; Fichorova et al., 2008; Fu et al., 2010; Leng et al., 2008; Toedter et al., 2008; Young, 2009). Examples of plate-based assays are chromogenic multiplex ELISA (Q-Plex™, Quansys Bioscience), fluorescence-based assay (A2, Beckman Coulter), and chemiluminescence assays (Mosaic ELISA by R&D systems and SearchLight by Aushon Biosystems). Recently, another plate-based assay, which utilizes electrochemiluminescence, has been developed by Meso Scale Discovery (MSD).
Experience with multiplex assays remains limited, and cross-comparisons between different multiplex formats are needed to help investigators in selecting the optimal assay for cytokine profiling in biological specimens. Furthermore, it is important to validate assays in specimens from ill individuals because disease may alter serum composition and thus influence assay performance. For example, in HIV infection and other disease states elevated levels of circulating carrier proteins and soluble cytokine receptors have been reported to influence detection of cytokines in serum and plasma (Aziz et al., 1999; Engelberts et al., 1991; James et al., 1994; Svenson et al., 1993).
To our knowledge, there has been no direct comparison between the MSD electrochemiluminescece assay and the Cytometric Bead Array in the measurement of cytokines in healthy or diseased human serum. Therefore, we evaluated the relative performance of these assays in detecting endogenous and exogenous proinflammatory cytokines in serum from a small sample of healthy donors and from well-characterized HIV-infected people.
2. MATERIALS AND METHODS
2.1 Study participants
We used serum from the Baltimore, MD center of the Multicenter AIDS Cohort Study (MACS) repository. The MACS is a longitudinal study of the natural and treated history of HIV infection in adult men who have sex with men, which has followed cohorts of men semi-annually since 1984 (Dudley et al., 1995). Twelve specimens representing HIV-uninfected (n=3) and HIV-infected donors (n=9) were assayed. The latter were selected to cover a spectrum of HIV disease progression characteristics: (a): HIV-positive, AIDS-free, not receiving highly active antiretroviral therapy (HAART) (n=3); (b): HIV-positive, AIDS-free, receiving HAART (n=3); and (c) HIV-positive, with AIDS but not receiving HAART (n=3). AIDS was defined using the 1993 Centers for Disease Control (CDC) case definition (Castro et al., 1993), except that cases defined as AIDS solely by low CD4 T-cell count were not included. HAART was defined according to the Department of Health and Human Services (DHHS) guidelines available at http://aidsinfo.nih.gov.
HIV infection status was determined by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot analysis. Plasma HIV RNA concentration (viral load) was measured using the Roche Amplicor 1.5 Ultrasensitive kit (Roche Molecular Systems, Branchburg, NJ) with a lower limit of detection of 50 RNA copies/mL. CD4 T cell counts were determined using standardized flow cytometry (Schenker et al., 1993). The characteristics of the donors studied are summarized in Table 1. All samples were stored at −70°C, thawed once for aliquoting, and then stored at −70°C until testing. The study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, and all participants provided informed consent.
Table 1.
Clinical characteristics of study participants.
| Donor ID | Age in Years | HIV-1 Status | History of an AIDS- defining illnessa | Use of HAARTb | Log10 viral load (copies/mL) | CD4 T cell count (cells/μL) |
|---|---|---|---|---|---|---|
| 1 | 41 | + | − | − | 4.0 | 845 |
| 2 | 48 | + | − | − | 3.4 | 493 |
| 3 | 62 | + | − | − | <1.7 | 679 |
| 4 | 76 | + | − | + | <1.7 | 1112 |
| 5 | 62 | + | − | + | <1.7 | 408 |
| 6 | 51 | + | − | + | <1.7 | 301 |
| 7 | 55 | + | + | − | 5.5 | 140 |
| 8 | 47 | + | + | − | 5.2 | 154 |
| 9 | 48 | + | + | − | 3.0 | 16 |
| 10 | 74 | − | NAc | NA | NA | 971 |
| 11 | 68 | − | NA | NA | NA | 911 |
| 12 | 55 | − | NA | NA | NA | 756 |
1993 CDC definition, excluding cases that were defined only by low CD4 T-cell count
Highly active antiretroviral therapy
Not applicable because donors are HIV-negative
2.2 Multiplex cytokine assays
For the electrochemiluminescence studies, the Human ProInflammatory-9 Ultra-Sensitive Kit from MSD (Gaithersburg, MD), which measures IL-6, IL-8, IL-10, TNF-α, IL-12p70, IL-1β, GM-CSF, IL-2, and IFN-γ, was used. MSD plates were analyzed on the MS2400 imager (MSD). For the cytokine bead array measurements, the Human Inflammatory Cytokines CBA Kit (BD Biosciences, San Jose, CA) was used, which measures all of the above cytokines except GM-CSF, IL-2, and IFN-γ; data were acquired on a BD™ FACS Calibur flow cytometer. Both assays were performed according to the manufacturer’s instructions except for the use of modified calibration curves as described below. All standards and samples were measured in duplicate.
2.3. Performance parameters
Our validation parameters included: sensitivity, recovery, and precision (as defined below).
2.3.1. Sensitivity of calibration curve
The lower limit of quantification (LLOQ) and detection (LLOD) for each kit were determined from the respective standard curves for each cytokine. The LLOQ was defined as the lowest concentration on the standard curve which yielded a) a measured concentration within 25% of the nominal value, and b) a coefficient of variation (%CV) less than 25% (Chowdhury et al., 2009). The LLOD was defined as the lowest concentration on the standard curve whose readout was greater than 2.5 standard deviations above that of the blank. The LLOD is less stringent than the LLOQ as it is determined exclusively from the absolute value of the blank. The criteria for LLOQ and LLOD were suggested by the MSD package insert and for uniformity were applied to both the MSD and CBA assays.
2.3.2. Recovery and Precision
Recovery was evaluated as the ability to detect known concentrations of recombinant cytokine standards added to serum. Dilutions of the cytokine standard mix provided by each manufacturer were added to serum from the twelve donors studied to achieve final concentrations of 100, 50, 25, 12.5, and 6.25 pg/ml. The percent recovery was defined as 100 × [(concentration detected − endogenous concentration)/concentration added]. Recovery of 100 ± 25% of the nominal amount of added cytokine was considered acceptable (Chowdhury et al., 2009; Fu et al., 2010b).
Precision was assessed by computing the coefficient of variation (%CV) between duplicate measurements of the spiked samples. A %CV lower than 25% was considered acceptable, as previously described (Chowdhury et al., 2009).
2.4. Statistical analyses
Analyses were performed using Prism Software version 5.0 for Windows (GraphPad, San Diego, CA). Associations between MSD- and CBA-determined IL-6 and IL-8 concentrations were assessed by Spearman’s rank correlation test, and the agreement of these two results was assessed by Bland-Altman analysis. Two-tailed P values less than 0.05 were considered statistically significant.
3. RESULTS
3.1 Assay sensitivity
The MSD and CBA manufacturers recommend eight- and ten-point calibration curves ranging from 2500 – 0.6 pg/ml and 5,000 – 20 pg/ml, respectively (Table 2, vendor columns). We tested whether adding points at the lower portions of the curves (resulting in 12-point curves) would increase assay sensitivity. Using the MSD kit, the two types of calibration curves yielded virtually identical measurements of cytokines in native serum samples (regression line of y= 1.017× − 0.060; r= 0.997), but the modified curve gave more accurate LLOQ values. Therefore, the 12-point standard curve was used for the CBA assay, and all results presented here are based on the 12-point curves.
Table 2.
Comparison of standard curves used in this study.
| Standard Concentration | ||||
|---|---|---|---|---|
| Calibration Tube | MSD | CBA | ||
| Vendor | Modified | Vendor | Modified | |
| 1 | 2500a | 2500 | 5000 | 2500 |
| 2 | 625 | 625 | 2500 | 625 |
| 3 | 156 | 156 | 1250 | 156 |
| 4 | 39 | 39 | 625 | 39 |
| 5 | 9.8 | 9.8 | 312.5 | 9.8 |
| 6 | - | 4.9b | 156 | 4.9 |
| 7 | 2.4 | 2.4 | 80 | 2.4 |
| 8 | - | 1.2 | 40 | 1.2 |
| 9 | 0.6 | 0.6 | 20 | 0.6 |
| 10 | - | 0.3 | - | 0.3 |
| 11 | - | 0.15 | - | 0.15 |
| 12 | 0 | 0 | 0 | 0 |
Approximate concentration of standards in pg/ml in each tube
Bold = added in the modified standard curve
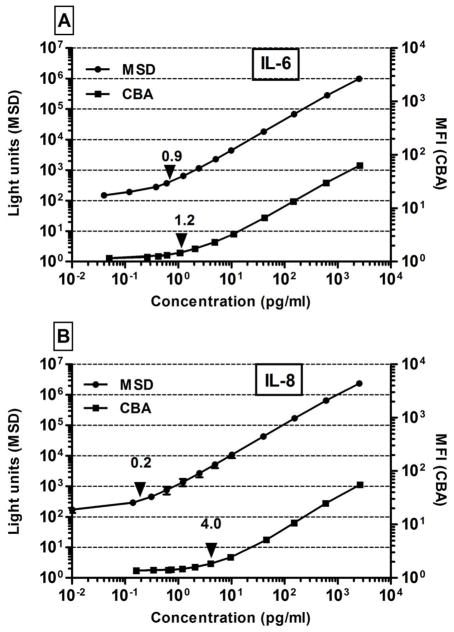
Standard curves were similar for all analytes tested in both the MSD and CBA assays (illustrated for IL-6) (Figure 1A). IL-8 appeared to be the exception, in that the MSD standard curve was more sensitive than that of the CBA (Figure 1B). LLOQs for the two assays are shown in Table 3. With the exception of IL-1β, MSD had lower LLOQs for all cytokines common to both kits, the differences being greatest for IL-12p70, IL-6, and IL-8. For IL-8, the LLOQ was 20-fold lower for MSD than for CBA, which is likely to represent a meaningful difference for samples with low levels of IL-8. LLOD values, however, were similar for all cytokines tested except IL-8, whose LLOD was 10-fold lower in the MSD assay. These data demonstrate that the LLOQ was as good or better with MSD as with CBA for the cytokines common to both kits. Thus, the MSD assay was more sensitive than the CBA for the majority of cytokine standards analyzed in this study.
Figure 1. Comparison of MSD and CBA standard curves.
Standard curves for IL-6 (A) and IL-8 (B) obtained using the MSD (●) and CBA (■) assays. Concentration for each standard is shown on the X-axis. The output signal is indicated as Light Units for MSD (left Y-axis) and Mean Fluorescence Intensity (MFI, right Y-axis) for CBA. Arrows on each curve indicate the lower limit of quantification. Error bars indicate ± 1 SD of the mean values of three separate experiments. Error bars are not shown where they were too small to be observable.
Table 3.
Sensitivity of multiplex assays according to standard curves.
| Cytokine | LLODa | LLOQb | ||
|---|---|---|---|---|
| MSD | CBA | MSD | CBA | |
| IL-6 | 0.3 ± 0.1c | 0.5 ± 0.3 | 0.7 ± 0.2 | 1.2 ± 0.7 |
| IL-8 | 0.1 ± 0.0 | 1.5 ± 0.5 | 0.2 ± 0.0 | 4.0 ± 4.8 |
| IL-10 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.8 ± 0.3 | 1.2 ± 0.1 |
| TNF-α | 0.5 ± 0.4 | 0.5 ± 0.4 | 1.0 ± 0.5 | 1.4 ± 0.5 |
| IL-12p70 | 0.9 ± 0.3 | 1.2 ± 0.2 | 1.7 ± 1.0 | 2.8 ± 1.4 |
| IL-1β | 1.0 ± 1.3 | 1.2 ± 0.2 | 2.4 ± 2.3 | 2.4 ± 1.3 |
Lower limit of detection
Lower limit of quantification
Mean ± 1 SD in pg/ml based on 3 replicates
3.2 Recovery of cytokines
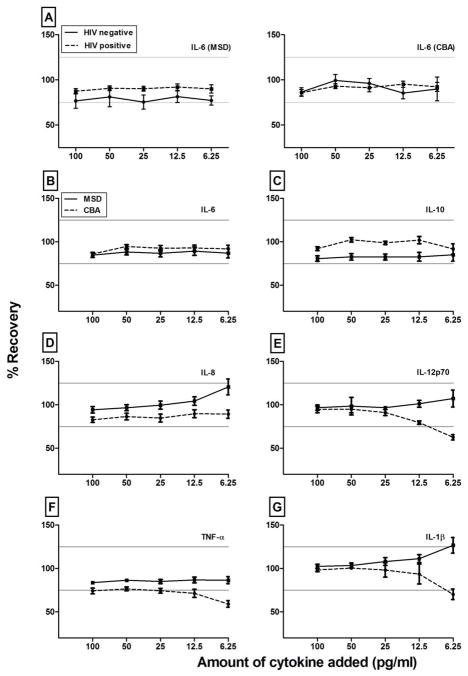
For all cytokines, recovery of added cytokines was equivalent for both HIV-uninfected and HIV-infected donors (illustrated for IL-6 in Figure 2A). Therefore, we combined the data from all donors for ease of analysis (Figure 2B-2G). Recoveries were similar and acceptable for IL-6 and IL-10 by both assays for all tested concentrations (Figure 2B and 2C), but MSD recovered added IL-12p70 and TNF-α more accurately than CBA at the lower concentrations (Figure 2E and 2F). These data suggest a matrix effect which disproportionally influenced the CBA assay. Thus, the sensitivity based on the standard curve (consisting of recombinant cytokine in assay diluent) did not necessarily reflect the sensitivity of the assay for endogenous cytokines in serum. In both platforms the intra-assay precision (%CV) was within the predefined limit of variability (<25%) for all cytokines except IL-1β (data not shown), which is consistent with the poor recovery of this cytokine. Neither assay quantified IL-1β accurately at 6.25 pg/ml. Overall, the ability of both the MSD and CBA assays to detect known amounts of added cytokines depended more on the cytokine and the amount added than on the HIV status of the donor.
Figure 2. Recovery of spiked cytokines from subject samples by MSD and CBA.
Figure A shows mean percent recovery of IL-6 added at final concentrations of 100, 50, 25, 12.5, and 6.25 pg/ml to serum samples of three HIV-negative (solid line) and nine HIV-positive donors (dashed line), as measured by the MSD (left) and CBA (right) kits. Figures B to F show the mean recovery of IL-6, IL-10, IL-8, IL-12p70, TNF-α, and IL-1β among all study participants obtained with the MSD (solid line) and CBA (dashed line) kits, regardless of HIV infection, AIDS, or therapy status (N=12). Horizontal gray lines show the 100 ± 25% acceptance criteria. Error bars show ± 1 standard error of the mean (SEM) of the mean values.
3.3 Detection of cytokines in HIV-positive and -negative human sera
The levels of cytokines detected in native sera from each donor are shown in Table 4. Using the MSD assay, all cytokines were quantifiable in at least 9 of the 12 specimens, except IL-1β which was quantified only in one sample. In contrast, the CBA assay did not quantify levels of TNF-α, and IL-12p70 in any of the specimens, and IL-10 was quantifiable in only 25%. Of note, for many samples endogenous cytokine levels were below 6.25 pg/ml, the lowest concentration tested in the recovery experiment. This finding may explain differences in assay performance between recovery of added cytokine standards and quantification of cytokines in serum.
Table 4.
Endogenous concentrations of cytokines in serum by multiplex assays.
| Donor ID | IL-6 | IL-8 | IL-10 | TNF-α | IL-12p70 | IL-1β | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSD | CBA | MSD | CBA | MSD | CBA | MSD | CBA | MSD | CBA | MSD | CBA | |
| 1 | 1.0a | 1.9 | 11.1 | 10.4 | 4.3 | -b | 10.4 | - | 7.1 | - | 2.0 | - |
| 2 | 1.9 | 3.3 | 9.6 | 21.1 | 1.6 | - | 16.5 | - | 1.2 | - | - | - |
| 3 | 1.0 | 2.2 | 6.9 | 7.8 | 1.3 | - | 10.2 | - | 0.9 | - | - | - |
| 4 | 1.9 | 3.6 | 15.7 | 20.2 | 1.3 | - | 12.2 | - | - | - | - | - |
| 5 | 0.8 | 1.7 | 13.7 | 13.9 | 2.9 | 1.3 | 13.1 | - | - | - | - | - |
| 6 | 2.8 | 3.6 | 20.6 | 28.5 | 2.5 | - | 18.5 | - | 2.1 | - | - | - |
| 7 | 2.2 | 3.9 | 21.8 | 27.1 | 7.1 | 3.8 | 28.4 | - | - | - | - | - |
| 8 | 5.2 | 9.3 | 28.3 | 40.7 | 7.2 | 4.8 | 30.4 | - | 2.4 | - | - | - |
| 9 | 4.7 | 3.4 | 21.5 | 22.9 | 5.0 | - | 21.6 | - | 6.2 | - | - | - |
| 10 | 1.7 | 2.4 | 13.5 | 22.2 | 2.1 | - | 12.4 | - | 6.2 | - | - | - |
| 11 | 2.0 | 2.0 | 17.9 | 18.3 | 2.4 | - | 18.9 | - | 2.3 | - | - | - |
| 12 | 1.0 | 3.8 | 7.9 | 14 | 1.4 | - | 11.3 | - | 0.9 | - | - | - |
| Quantifiablec | 12 (100) | 12 (100) | 12 (100) | 12 (100) | 12 (100) | 3 (25) | 12 (100) | 0 (0) | 9 (75) | 0 (0) | 1 (8.3) | 0 (0) |
Concentration in pg/ml
Cytokine concentration below the lower limit of quantification
Number (Percentage) of specimens with quantifiable amount of cytokine
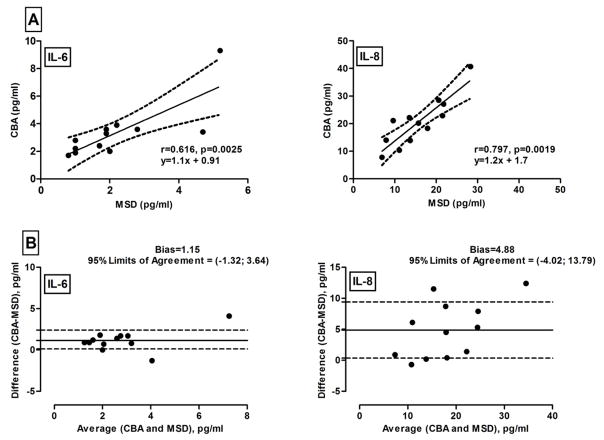
Since both assays quantified IL-6 and IL-8 in all of the samples tested, we examined the relationship between the measurements yielded by each assay. For both cytokines, the measurements were highly and significantly correlated (Figure 3A) and the CBA-determined values were slightly higher, although these biases were not statistically significant (Figure 3B).
Figure 3. Relationships between concentrations of IL-6 and IL-8 measured by MSD and CBA.
Cytokines were measured in twelve samples. Data pairs were cross-compared using the Spearman correlation test (panel A) and Bland-Altman plot (panel B). Solid lines are the regression line (A) and the mean bias (B) between MSD and CBA-derived concentrations. Dashed lines represent the confidence bands (A) and one standard deviation above and below the mean bias (B).
4. DISCUSSION
This was the first study to directly compare the performance of the Mesoscale Discovery (MSD) and the Cytometric Bead Array (CBA) multiplex cytokine assay platforms in analysis of human serum. Our results also expand on previous studies by providing information about MSD and CBA sensitivity, recovery, and precision in HIV-infected and -uninfected serum. Although MSD and CBA had similar sensitivities on standard curves for the majority of cytokines tested, MSD exhibited better recovery of exogenous recombinant cytokines (standards) added to serum, and better quantification of endogenous cytokines in serum, in both HIV-infected and -uninfected specimens. MSD detected IL-12p70, TNF-α, and IL-10 in serum more frequently than CBA (Table 4), while IL-6 and IL-8 were detectable in all samples with both assays, with both platforms yielding similar absolute values (Table 4).
In healthy donors, MSD has been reported to have a wider dynamic range, lower LLOQs, and higher frequency of quantifiable endogenous cytokines than other multiplex assays, indicating higher sensitivity (Chowdhury et al., 2009; Fu et al., 2010). The reasons for the overall better performance of the MSD assay in comparison to other technologies are not clear but could include the avidity of antibody pairs, the epitopes bound by the antibodies, and the detection method. Specifically, electrochemilumiscence, as used in the MSD assay, may be more sensitive than fluorescence and may also have lower background due to the narrow physical range of the electrical field used to induce electrochemilumiscence (Chowdhury et al., 2009; Leng et al., 2008; Young, 2009).
Some limitations of this study should be acknowledged. Our sample size was small and consisted only of adult men. Thus, gender or age effects might have been missed. Diseases other than HIV infection may yield different results. In addition, recovery experiments may not have covered the full physiological ranges for the cytokines tested, and only one type of multiplex kit from each manufacturer was examined. Finally, the performance characteristics of MSD and CBA assays in other matrices such as human plasma could be different from those observed in serum.
It is worth noting that modification of the MSD-recommended 8-point standard curve to a modified 12-point curve (Table 2) had essentially no effect on the calculated cytokine concentrations of unknown samples (data not shown). In other words, calculated values below the lowest standard in the 8-point standard curve were identical to values obtained from the 12-point curve which included standards that were lower than the cytokine levels in all samples.
Although financial considerations regarding multiplex cytokine assays have been reviewed elsewhere (Leng et al., 2008), it is important to discuss those that pertain to MSD and CBA. The kits used in this study had similar costs. However, GM-CSF, IL-2, and IFN-γ were not included in the CBA. On the other hand, the MSD requires a dedicated electrochemiluminescence analyzer while the CBA can be run most flow cytometers. Although such cytometers and electrochemiluminescence readers are comparable in price, many laboratories already have suitable cytometers. Thus, in addition to the data reported here, access to instrumentation would influence assay choice.
In summary, both MSD and CBA multiplex assays performed well in analyzing proinflammatory cytokines in small volumes of human serum. Nevertheless, the MSD assay was slightly better in a number of respects, including quantification of cytokines in serum, and may be more suitable for samples with low endogenous levels of the cytokines tested in this study.
Highlights.
In this study we compare the performance of two muliplex cytokine assays
We examined the CBA and MSD platforms using serum from HIV+ donors
We find serum matrix effects influence assay performance independent of HIV status
Overall, the MSD assay performed better detecting endogenous cytokines in serum
Acknowledgments
This work was supported by (1) the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Child Health and Human Development (NICH/HD), National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the NIH, DHHS (1U01AI068613), (2) UL1-RR025005 (GCRC), and (3) U01AI35042 (SHARE). We thank Stacey Meyerer and Akila Hadji for specimen management, and Jacquett R. Johnson and Lisette M. Johnson-Hill for data management. We also thank the Becton Dickinson Immune Function Laboratory in the Johns Hopkins Bloomberg School of Public Health for access to instrumentation and software.
The authors thank the Division of Clinical Research (DCR) of the NIAID, NIH, DHHS and the NIAID/University of Bamako HIV and Tuberculosis Research and Training Center (SEREFO Project) in Mali for their financial support to Djeneba Dabitao.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999;6:89. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, Dibben O, Margolick JB, Bream JH, Sambrano E, Martinez-Maza O, Sinclair E, Borrow P, Landay AL, Rinaldo CR, Norris PJ. A multi-site comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol. 2011 doi: 10.1128/CVI.05032-11. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro KG, Ward JW, Slutsker L, Buehler JW, Jaffe HW, Berkelman RL, Curran JW. 1993 Revised Classification-System for Hiv-Infection and Expanded Surveillance Case-Definition for Aids Among Adolescents and Adults (Reprinted from Mmwr, Vol 41, Pg Rr 17, 1992) Clinical Infectious Diseases. 1993;17:802. [PubMed] [Google Scholar]
- 4.Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods. 2009;340:55. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 6.Engelberts I, Stephens S, Francot GJ, van der Linden CJ, Buurman WA. Evidence for different effects of soluble TNF-receptors on various TNF measurements in human biological fluids. Lancet. 1991;338:515. doi: 10.1016/0140-6736(91)90591-c. [DOI] [PubMed] [Google Scholar]
- 7.Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel JC, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L, Mayer KH, Pasicznyk JM, Pallansch-Cokonis M, Poli G, Reichelderfer P, Roberts P, Rodriguez I, Saidi H, Sassi RR, Shattock R, Cummins JE., Jr Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. 2008;80:4741. doi: 10.1021/ac702628q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Q, Zhu J, van Eyk JE. Comparison of multiplex immunoassay platforms. Clin Chem. 2010;56:314. doi: 10.1373/clinchem.2009.135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James K, Milne I, Cunningham A, Elliott SF. The effect of alpha 2 macroglobulin in commercial cytokine assays. J Immunol Methods. 1994;168:33. doi: 10.1016/0022-1759(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 10.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenker EL, Hultin LE, Bauer KD, Ferbas J, Margolick JB, Giorgi JV. Evaluation of a dual-color flow cytometry immunophenotyping panel in a multicenter quality assurance program. Cytometry. 1993;14:307. doi: 10.1002/cyto.990140311. [DOI] [PubMed] [Google Scholar]
- 12.Svenson M, Hansen MB, Heegaard P, Abell K, Bendtzen K. Specific binding of interleukin 1 (IL-1) beta and IL-1 receptor antagonist (IL-1ra) to human serum. High-affinity binding of IL-1ra to soluble IL-1 receptor type I. Cytokine. 1993;5:427. doi: 10.1016/1043-4666(93)90032-z. [DOI] [PubMed] [Google Scholar]
- 13.Toedter G, Hayden K, Wagner C, Brodmerkel C. Simultaneous detection of eight analytes in human serum by two commercially available platforms for multiplex cytokine analysis. Clin Vaccine Immunol. 2008;15:42. doi: 10.1128/CVI.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varro R, Chen R, Sepulveda H, Apgar J. Bead-based multianalyte flow immunoassays: the cytometric bead array system. Methods Mol Biol. 2007;378:125. doi: 10.1007/978-1-59745-323-3_9. [DOI] [PubMed] [Google Scholar]
- 15.Young HA. Cytokine Multiplex Analysis. In: Kozlov SV, editor. Inflammation and Cancer. Humana Press, a part of Springer Science + Business Media, LLC; 2009. [Google Scholar]