Abstract
CART peptides are peptide neurotransmitters and hormones that are involved in many different physiological responses. While much is known about the peptides regarding their structure, processing and gene regulation, less is known about their postsynaptic actions and receptors. Using 125I-CART 61-102 as a ligand and unlabeled CART 61-102 or CART 55-102 as displacers, high-affinity specific binding was detected in PC12 cells. Differentiation of the PC12 cells increased specific binding several-fold. The increase in specific binding found after differentiation was inhibited by actinomycin D and cycloheximide, suggesting that the increase in specific binding was dependent on RNA and protein synthesis. CART 1-27, a peptide that has never been shown to elicit responses, did not displace 125I-CART61-102 binding, nor did more than 20 other peptides that were examined. Surprisingly, however, PACAP 1-38 and PACAP 6-38 were found to be low-affinity inhibitors of CART binding. CART treatment increased binding of 35S-GTPgamma-S to PC12 cell membranes. Moreover, CART treatment of intact PC12 cells elicited robust increases in phospho-ERK in a manner that was increased with differentiation, blocked by pertussis toxin and antagonized by PACAP 6-38. These findings extend previous research and suggest that the CART binding site in PC12 cells reflects a G protein-coupled receptor linked with Gi/o, and also demonstrate that PACAP 6-38 may be useful as a CART receptor antagonist.
Keywords: CART, CART peptide, CART receptor, CART binding, GPCR, PC12 cells, PACAP6-38
Introduction
CART peptides (CART 61-102 and CART 55-102) are major neuropeptides involved in a variety of functions including the actions of psychostimulants, feeding and body weight, neuroendocrine control, stress, depression and anxiety, and sleep and arousal (Rogge et al., 2008). In addition to a large number of studies on the behavioral and physiological effects of CART peptides, several key studies of the CART peptide system have been directed at defining the receptor(s) for the peptides. The earliest studies showed effects of the application of CART peptide on intracellular signaling. C-Fos levels were increased in several brain regions after peptide injection (Vrang et al., 1999; Aja et al., 2006; Pirnik et al., 2009), and adding CART peptide to hippocampal cells in culture resulted in an inhibition of voltage dependant Ca++ signaling (Yermolaieva et al., 2001). The latter effect was blocked by pertussis toxin, suggesting the involvement of Gi/o proteins. Also, icv administration of CART peptide caused an increase in CREB phosphorylation in CRF-containing neuron in the hypothalamic PVN (Sarkar et al., 2004), and CART peptide induced a phosphorylation of NMDA receptors by PKA and PKC signaling pathways (Chiu et al., 2009). Application of CART peptide to cultured AtT20 cells activated ERK signaling which was also reduced by pertussis toxin (Lakatos et al., 2005), and studies of CART-induced signaling in bovine granulosa cells also suggested the involvement of Gi/o proteins (Sen et al., 2007). Taken together, these studies suggest that CART peptides may exert many effects through a G protein-coupled receptor (GPCR) linked to Gi/o.
CART peptide binding studies have also been undertaken to identify a receptor binding site. Initial attempts aimed at finding specific receptor binding in brain tissue failed, mainly due to the presence of large amounts of nonspecific binding. However, binding studies in cultured cells have been more successful. Following the discovery of CART peptide stimulation of ERK signaling in AtT20 cells, specific binding was found in these same cells that was of high affinity and specific for CART peptides (Vicentic et al., 2005). CART binding has also been found in HepG2 cells and dissociated hypothalamic cells (Keller et al., 2006), and high-affinity specific binding was found in primary cell cultures of nucleus accumbens neurons (Jones and Kuhar, 2008). The latter study showed that GTP reduced the binding of CART peptide, but ATP did not, again suggesting a GPCR. Finally, a high-affinity and specific binding of CART peptide was found in PC12 cells (Maixnerova et al., 2007; Maletinska et al., 2007); an interesting aspect of the CART binding in PC12 cells was that it increased after differentiation into a more neuronal phenotype by application of NGF. It is not known, however, if this differentiation-dependent increase in CART binding to PC12 cells correlates with increases in functional CART receptors. Moreover, the pharmacological profile of the CART binding sites in PC12 cells has not been rigorously evaluated.
In this investigation, we explored the correlation between increased CART binding and increased CART-activated signaling in PC12 cells following differentiation. Furthermore, we characterized the pharmacological profile of the CART binding sites in PC12 cells, and surprisingly found that PACAP 6-38 can both displace CART binding and block CART-mediated functional responses. Thus, PACAP 6-38 represents a novel CART receptor antagonist.
Materials and Methods
Materials
CART61-102 was purchased from Bachem ( Bubendorf, Switzerland). Other peptides, including: CART 1-27, CART 1-39, CART 55-76, CART 62-102, CART 55-102, all Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) fragments, Neuropeptide Y (NPY), Angiotensin II (AngII ), Corticotropin Release Factor (CRF), Glucagon-like Peptide I (GLP-1), Glucagon-like Peptide II (GLP-2), Vasopressin, Neurotensin, Vasoactive Intestinal Peptides (VIP), Galanin, Ghrelin, Orexin A and B, were purchased from American Peptide Company (Sunnyvale, CA). Nerve Growth Factor 2.5S (NGF) was purchased from BD Biosciences (Bedfold, MA). Pertussin Toxin (PTX) was purchased from Calbiochem (Darmstadt, Germany). Polyethylene Imine, r-Amynobutyric acid (GABA), Complement C5a, Cycloheximide (CHX), Actinomycin D (ActiD), Cytochalasin D (CytoD), Gpp(NH)p and App(NH)p were purchased from Sigma (St. Louis, MO). Maxidilan and maxidilan antagonist were gifts from Dr Moro. Mouse anti-pERK, rabbit anti-ERK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). 35S-GTPgamma-S (1250 Ci/mmol) was obtained from Perkin Elmer (Waltham MA).
Culture of PC12 cells
Rat pheochromocytoma cell line PC12 was purchased from ATCC (Manassas, VA, USA). The cells were grown in RPMI 1640 medium (Invitrogen, Carlsbad, California) supplemented with 10% horse serum, 5% fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin. For binding studies, cells were seeded on Polyethylene Imine-coated 24-well plates (Corning, NY, USA). Cells were plated at 1.5 to 2 × 104 in each well and cultured for one day, then the medium was changed to RPMI 1640 supplemented with 1% horse serum, 0.5% fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin for one more day. Differentiation of cells was performed by the addition of NGF (50 ng/mL); PACAP (100 nM); or both of NGF and PACAP and confirmed by microscopy. The cell number was determined with Guava ViaCount Reagent (Guava Technologies, Hayward, CA).
Iodination of CART 61-102
CART61-102 (Bachem, Bubendorf, Switzerland) was iodinated at Tyr62 with 125I-NaI (using Iodo-Gen by PerkinElmer (Waltham, MA). Only mono-iodinated 125I-CART61-102 (Purified by HPLC) was used. The specific activity of 125I-CART61-102 was about 2200 Ci/mmol. 125I-CART61-102 was kept in aliquots at − 20 °C and used for saturation and competition binding studies within 1 month.
Binding procedures
125I-CART61-102 binding experiments were performed in intact, plated PC12 cells (Maletinska et al., 2007). Plated cells were incubated with 0.03 – 3 nM 125I-CART61-102 in saturation experiments, or with 1 nM 125I-CART61-102 and various peptides in competitive binding experiments. Binding was typically carried out in a total volume of 0.15 ml of binding buffer (20 mM HEPES buffer pH 7.4, 118 mM NaCl, 4.7 mM KCl and 5 mM MgCl2, 5.5 mM glucose, 1 mg/ml BSA, and 0.1 mg/ml basic pancreatic trypsin inhibitor) at 4 °C for 40 min, then the incubation was continued by transferring the plates to room temperature for 30 min. After incubation, cells were washed twice using cold phosphate buffered saline, and then solubilized in 0.5 mL of 0.25 N NaOH. Bound radioactivity was determined by γ-counting (COBRA II, AUTO-GAMMA, Perkin Elmer, Wellesley, MA, USA). Non-specific binding was determined using 1 uM CART61-102. As usual, specific binding was determined by subtracting nonspecific from total binding. Experiments were carried out in triplicates at least three times. Kd and Bmax values were calculated by standard procedures using GraphPad Prizm 4 Software (San Diego, CA). Ki values were calculated using the Cheng-Prusoff equation (radioligand concentration was 1 nM). In some experiments, Cycloheximide (CHX; 10 ug/ml), Actinomycin D (ActiD; 2 mM) and Cytochalasin D (CytoD; 4 mM) were added to PC12 cells for 30 min, and then treated or not by both NGF and PACAP for 6 hrs. After that, binding experiments were performed with 5 nM 125I-CART61-102.
For 125I-CART61-102 binding on cell membranes, cells were homogenized in 5 mM Tris–HCl, pH 7.5, 5 mM EDTA, 5 mM EGTA at 4°C and the lysate was centrifuged at 30 000 × g for 10 min. The membrane pellet (containing about 8 µg protein) was resuspended and incubated at room temperature for 1 hour in the binding buffer and 1 nM 125I-CART61-102 in the presence or absence of different reagents such as CART 61-102, Gpp(NH)p and App(NH)p), in a total volume of 100 µl. Gpp(NH)p and App(NH)p were pre-incubated with membranes at room temperature for 30 minutes. The reaction was terminated by adding 4 ml cold phosphate-buffered saline and then immediately centrifuged at 4°C. The surfaces of the pellets were washed twice and radioactivity was measured.
ERK activation and Western Blotting
Cells were treated using serum-free medium for at least 16 hrs, and some samples were treated with PTX (200ng/m) at the same time. NGF and/or PACAP was not present. CART 55-102 or 61-102 (0.3 µM) were added for 5 min and the cells were washed two times with cold PBS and lysed in solubilization buffer (M-PER Mammalian Protein Extraction Reagent (Thermo Scientific, Rockford, IL) containing 2.5 mM sodium pyrophosphate; 1 mM sodium vanadate; 10 µg/mL each of aprotinin and leupeptin; 2 mM phenylmethylsulfonyl fluoride). Total cell lysate for each sample (20 µg) was subjected to SDS-polyacrylamide gel electrophoresis and electrotransferred to a PVDF membrane (Invitrogen, Carlsbad, CA). After blocking with non-fat dry milk (20 mM Tris·HCl, pH 7.4, containing 150 mM NaCl, 0.1% Tween 20, and 5% (wt/vol)), the blots were incubated with specific antibodies (anti-p-ERK and anti-ERK) overnight at 4°C. The blots were then incubated with horseradish peroxidase-conjugated secondary antibody. The immunoactivity was visualized through an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ. The same amount of protein was loaded onto each lane. Densities were determined using Scion Image Software (NIH, Bethesda MD). “Relative” p-ERK expression was determined by taking the ratio of p-ERK to ERK band densities and setting that to 1 for controls. Positive control experiments demonstrated that NGF and PACAP 1-38 increased ERK phosphorylation as expected (data not shown).
[35S]-GTPgammaS binding assay
The assay was carried out as described previously (Zhao et al., 1998). Cells were lysed in 5 mM Tris–HCl, pH 7.5, 5 mM EDTA, 5 mM EGTA at 4°C and the lysate was centrifuged at 30 000 × g for 10 min. The membrane pellet (containing 5 µg protein) was resuspended and incubated at 30°C for 1 h in 20 mM HEPES, pH 7.4, 10 mM MgCl2, 100 mM NaCl, 10 µM GDP, 0.001% saponin and 0.05 nM 35S-GTP[gamma]S in the presence or absence of different reagents (CART 61-102, CART 5-102 and PACAP) in a total volume of 100 µl. The reaction was terminated by adding 4 ml phosphate-buffered saline and then immediately centrifuged at 4°C. The pellet was washed twice and radioactivity was measured by liquid scintillation spectrophotometry. Data were means of triplicate samples. Basal binding was determined in the absence of CART peptide and PACAP and non-specific binding was obtained in the presence of 10 µM GTPgammaS. The percentage stimulation of [35S]GTPgammaS was calculated as 100 × (c.p.m.sample-c.p.m.non-specific)/(c.p.m.basal-c.p.m.non-specific).
Statistical Analysis
Each experiment was repeated a minimum of three times. Statistical analyses were performed using analysis of variance or t tests as needed. Data are presented as means ± SE, and the value of P<0.05 was considered significant. GraphPad Prism 4 software (San Diego CA) was used for statistical analyses.
Results
Differentiation of PC12 cells by NGF and PACAP, and its effects on specific 125I-CART 61-102 binding
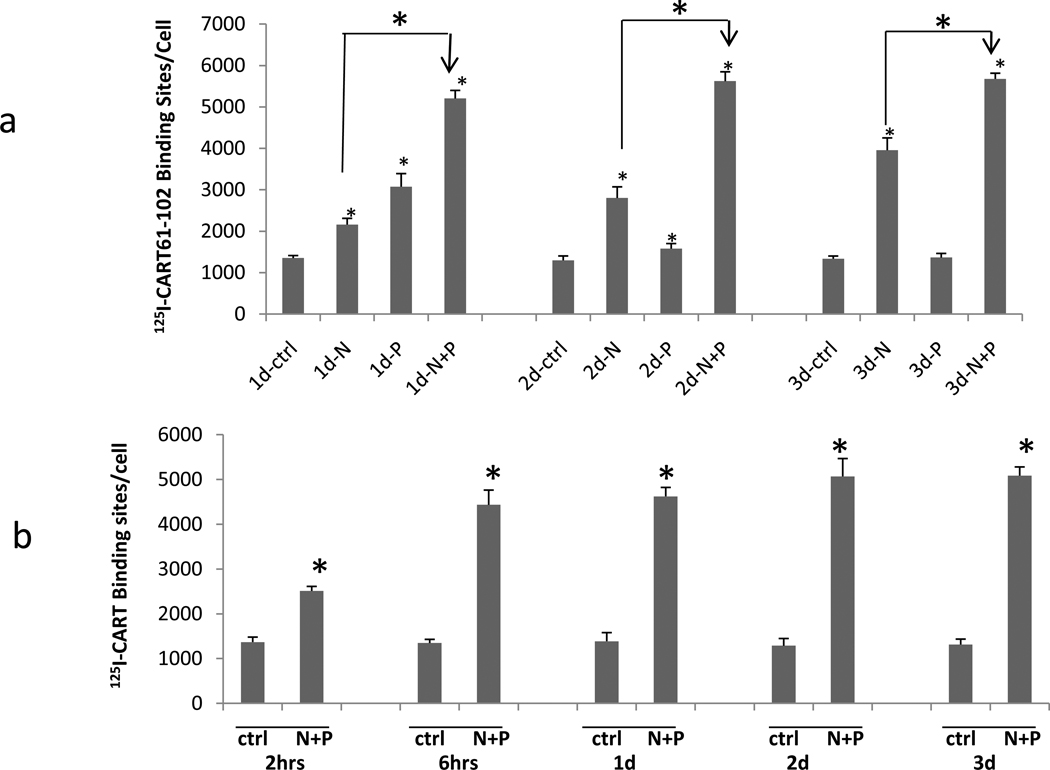
PC12 cells were incubated with NGF (N), PACAP (P), or both, as described in Methods. The time course of differentiation was examined and cellular morphology changed over a 3-day time course as expected (not shown). Also, the effect of differentiation by N, P, and N + P on specific CART peptide binding was examined at 1, 2 and 3 days. Without any N or P (no differentiation), CART binding did not change after 3 days. Addition of N alone resulted in a steady increase of binding over the 3-day period. Addition of P alone resulted in an increase in binding at 1 day that did not increase further. Addition of both N and P resulted in the largest increase in binding (Figure 1 a) which was maximal at one day and did not change over the 3 days. Binding at earlier time points were also examined after differentiation by both N and P. A significant increase in binding was found after 2 hours, and the increase was almost maximal by 6 hours (Figure 1 b). In subsequent studies, both N and P together were used for differentiation unless stated otherwise.
Figure 1. Differentiation of PC12 cells by NGF and PACAP, and its effects on 125 I-CART 61-102 Binding.
a. Time course of 125 I-CART 61-102 specific binding in NGF 50ng/mL (N), PACAP 100nM (P) or NGF+PACAP (N+P)-Differentiated PC12 cells at 1, 2 or 3 days. b. Time course from 2 hours to 3 days for 125I-CART 61-102 specific binding in N+P (NGF+PACAP)-differentiated PC12 cells. The concentration of radioligand was 5 nM. Data are mean +/− SEM cpms. N = 3 – 5 experiments. (* indicates P at least less than 0.05 compared to control (ctrl), or for the indicated comparison).
Saturation of specific binding of 125I-CART 61-102 to undifferentiated and differentiated cells
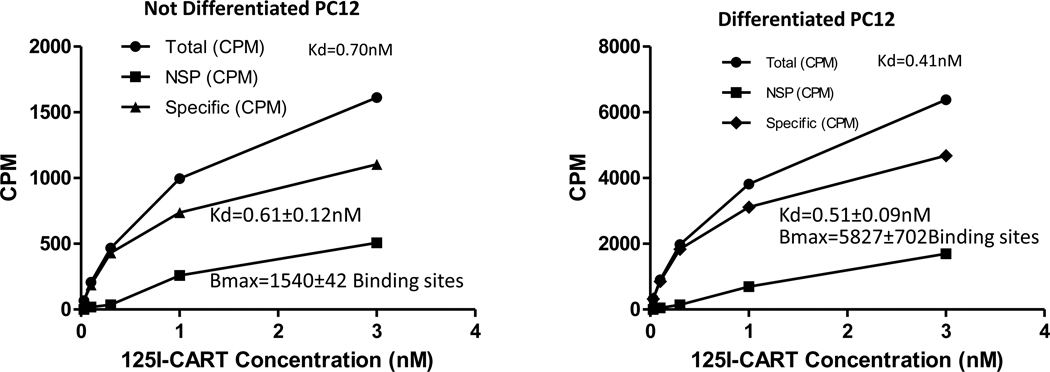
PC12 cells were either left undifferentiated or differentiated for 3 days, and binding was carried out as described in Methods. The 3-day time point was chosen because preliminary experiments showed that maximal binding was stable at that time. Kd values for specific binding were determined for both kinds of cells and were not significantly different from each other; the average Kd values were 0.61 and 0.51 (nM) as described in figure 2. In agreement with data shown in figure 1, specific binding increased about 4 fold after differentiation.
Figure 2. Saturation of specific binding of CART peptide to undifferentiated and differentiated cells.
Saturation of specific 125I-CART 61-102 binding to either undifferentiated or differentiated (N+P treated) PC12 cells was determined as described in Methods. After differentiation, the binding increased as previously shown. The Kd values for the specific binding for the individual curves shown are given near the top of the graph, and the average Kd values are shown as Mean +/− SEM cpms (n = 3).
Specificity of 125I-CART 61-102 binding
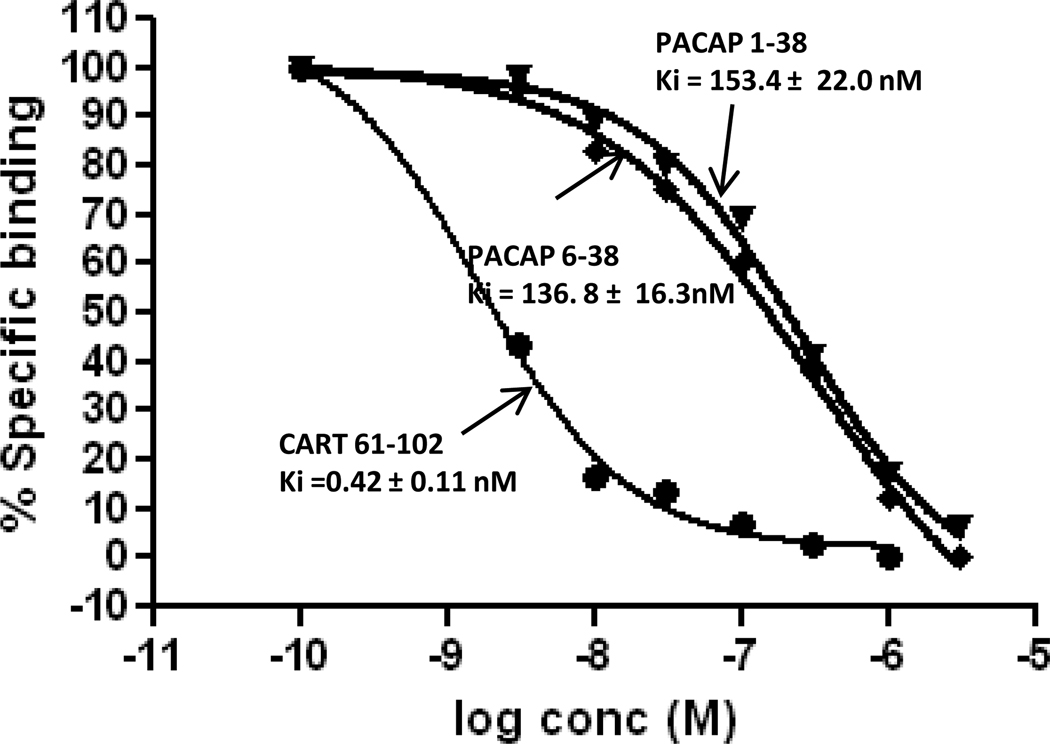
The Kd and Ki values of CART 62-102, CART 61-102, CART 55-102, and a large number of unrelated peptides were determined in both undifferentiated and differentiated PC12 cells (Table 1). The Kd and Ki values of the three CART peptides were not significantly different and were all in the 0.5 nM range. Surprisingly, PACAP 1-38 (P) and PACAP 6-38 inhibited binding, although with low affinity (130 – 150 nM) (Figure 3). The P that was added for differentiation (Figure 1) was not a factor in the binding experiments because the P was washed from the cells before binding was carried out (see Methods). All of the more than 20 other peptides examined had Ki values greater than 1 uM (Table 1).
Table 1.
| Peptide | Ki (nM) Differentiated Cells | Ki (nM) Nondifferentiated Cells |
|---|---|---|
| CART 61-102 | 0.42 ± 0.11 (Kd) | 0.53 ± 0.06 (Kd) |
| CART 62-102 | 0.50 ± 0.08 | |
| CART 55-102 | 0.49 ± 0.07 | |
| PACAP 1-38 | 153.4 ± 22.0 | |
| PACAP 6-38 | 136.8 ± 16.3 | |
| PACAP 16-38 | >1000 | >1000 |
| PACAP 28-38 | >1000 | >1000 |
| PACAP 31-38 | >1000 | >1000 |
| PACAP 1-27 | >1000 | >1000 |
| PACAP 6-27 | >1000 | >1000 |
| Maxidilan | >1000 | |
| Maxidilan Antagonist | >1000 | |
| CART 1-27 | >1000 | >1000 |
| CART 1-39 | >1000 | >1000 |
| CART 55-76 | >1000 | >1000 |
| NPY | >1000 | >1000 |
| Angiotensin II | >1000 | >1000 |
| CRF | >1000 | >1000 |
| GLP-1 | >1000 | >1000 |
| GLP-2 | >1000 | >1000 |
| vasopressin | >1000 | >1000 |
| Neurotensin | >1000 | >1000 |
| VIP | >1000 | >1000 |
| GABA | >1000 | >1000 |
| Galanin | >1000 | >1000 |
| Ghrelin | >1000 | >1000 |
| Orexin A | >1000 | >1000 |
| Orexin B | >1000 | >1000 |
| Complement 5a | >1000 | >1000 |
Specificity of 125I-CART 61-102 binding to PC12 cells. Experiments were carried out as described in Methods. Data are mean ± SEM nM (n=3 – 5). The blank spaces in the table indicate that those experiments were not done. See text for additional details.
Figure 3. Displacement curves for PACAP 1-38 and PACAP 6-38.
Binding of 1 nM 125ICART 61-102 was carried out in PC12 cells and various concentrations of competitors were added. The displacement of specific binding in a typical experiment is shown for CART 61-102, PACAP 1-38 and PACAP 6-38. Averages of results from 3-5 experiments are shown.
Effects of Gpp(NH)p or App(NH)p on 125I-CART peptide binding
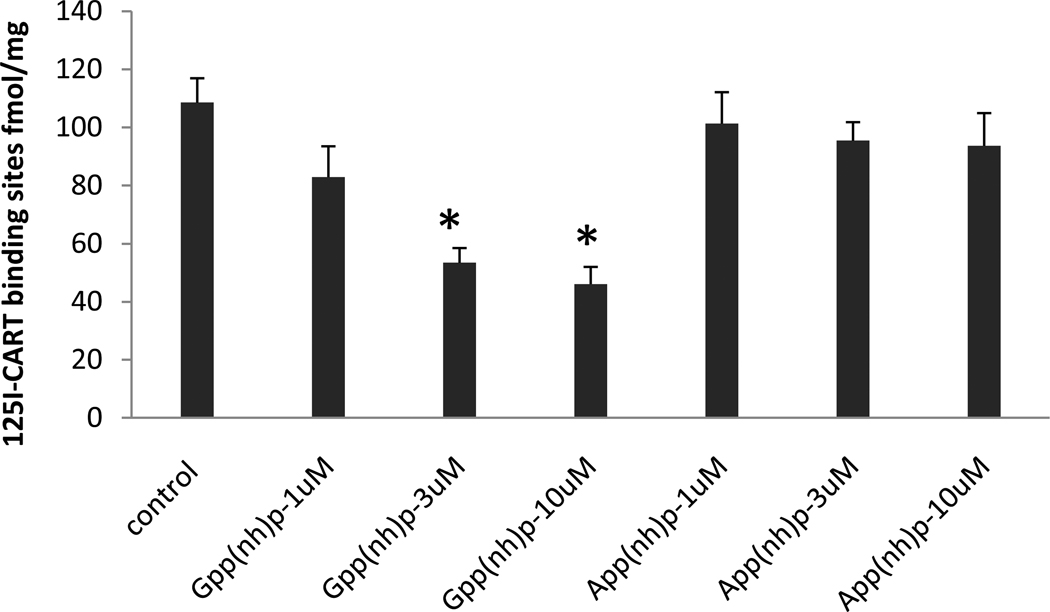
Because of the evidence in the literature that the CART peptide receptor is a GPCR coupled to Gi/o, at least in some cells, we examined the effects of Gpp(NH)p and App(NH)p on 125I-CART 61-102 binding to membranes of the differentiated PC12 cells. Membranes were used instead of whole cells to avoid problems with penetration of the reagents into the cells. In these assays, the Kd values were 0.68 +/− 0.06 nM, and the Bmax values were 108.5 +/− 8.4 fmol/mg protein. The concentrations of the GTP and ATP analogues were 1, 3, and 10 uM. Binding was more sensitive to Gpp(NH)p than to App(NH)p. Neither analogue reduced binding at 1 uM, but Gpp(NH)p reduced binding at 3 uM and 10uM while App(NH)p did not (figure 4).
Figure 4. Effects of Gpp(NH)p and App(NH)p on specific 125I-CART 61-102 binding.
The binding was sensitive to Gpp(NH)p; 3 and 10 uM reduced CART binding whereas the same concentrations of App(NH)p had no effect. Radioligand concentration was 1 nM. Data are mean +/− SEM, (n= 3 – 6). Asterisks indicate P<0.01. Analysis was by one way ANOVA with Tukey’s post hoc test. See Text for additional details.
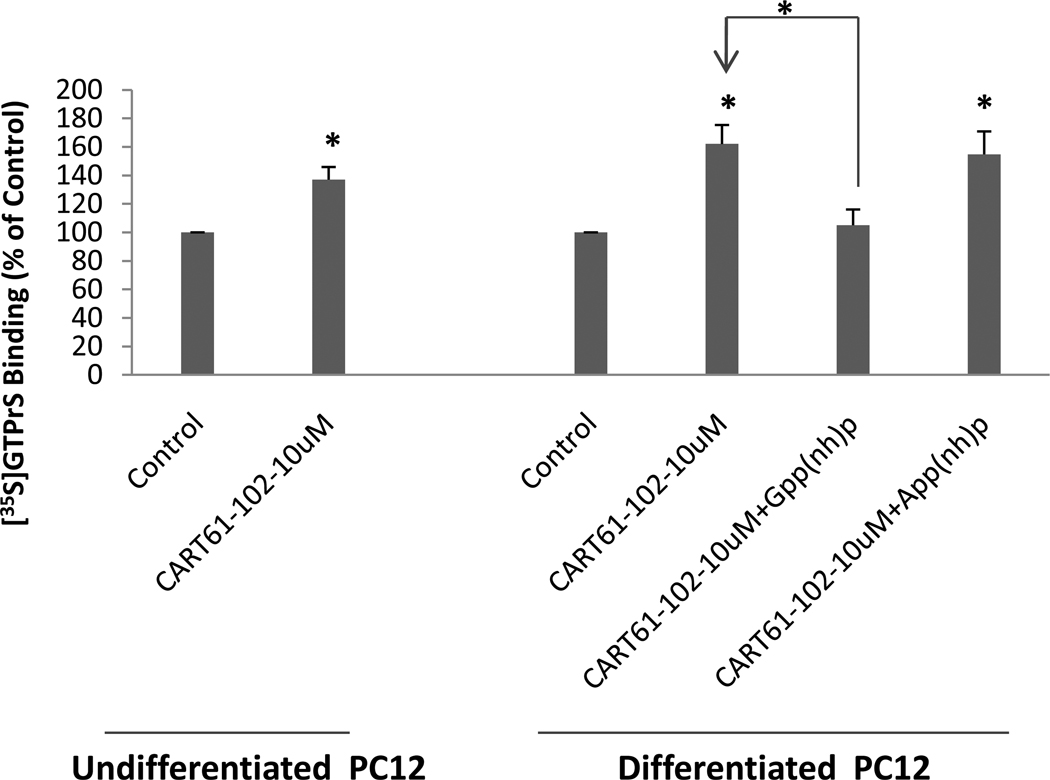
Effects of CART 61 -102 on 35S-GTPgammaS binding to PC12 membranes
To test further if the CART binding sites in PC12 cells might correlate with functional CART receptors, the effect of CART 61-102 on the binding of 35S-GTPgamma-S to PC12 cell membranes was carried out. The binding of 35S-GTPgamma-S increased significantly upon addition of 10 µM CART61-102 (Fig. 5). The binding of 35S-GTPgamma-S was blocked by 10 µM Gpp(NH)p but not by 10 µM App(NH)p, consistent with the idea that CART peptide interacts with a GPCR in PC12 cells (Figure 5).
Figure 5. Stimulation of 35S-GTPgammaS binding by CART 61-102 in differentiated PC12 cell membranes.
Addition of 10uM of CART61-102 stimulated binding. Gpp(NH)p was an effective competitor while App(NH)p was not, as expected. Data are mean +/− SEM (n=3–5), and * indicates a P values at least less than 0.05 using one way ANOVA with Tukey’s post hoc test.. See text for additional details.
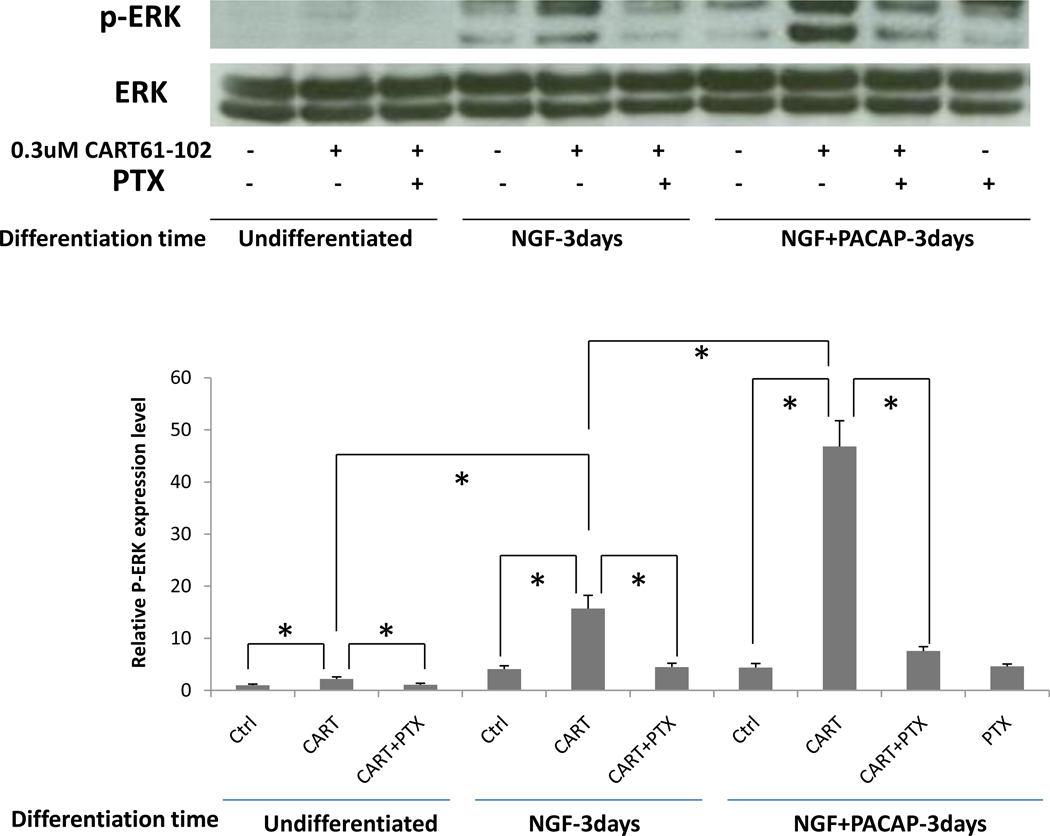
Effects of CART 61-102 on Phosphorylation of ERK in PC12 cells
We next determined whether CART peptide could increase phospho-ERK levels in PC12 cells as has been found in other cell types (Lakatos et al., 2005). In undifferentiated cells, the effect of peptide addition was small but significant; CART peptide slightly increased phospho-ERK levels, and this was inhibited by pertussis toxin (PTX). In cells treated with N for 3 days, addition of CART 61-102 produced a much more substantial increase in phospho-ERK, which was prevented by PTX. In cells differentiated with both N + P for 3 days, CART 61-102 stimulation yielded an even larger increase in phospho-ERK, which again was inhibited by PTX. PTX alone had no inhibitory effect compared to control (ctrl) (Figure 6). CART 55-102 had approximately the same effect as CART 61-102 (data not shown). In accompanying positive control experiments, we confirmed the previously-reported abilities of NGF and PACAP (acute treatments) to stimulate phospho-ERK in both undifferentiated and differentiated PC12 cells (data not shown). These results reveal that CART peptide can activate intracellular signaling through a GPCR coupled to Gi/o in PC12 cells and that CART-activated signaling increases with differentiation in a manner very similar to the increase in CART binding sites observed with differentiation.
Figure 6. Effects of CART 61-102 on production of p-ERK in PC 12 cells.
As described in the text, Western blotting (shown at the top) revealed that p-ERK was increased by adding CART 61-102 and CART 55-102 (data not shown) in N- and N+P-differentiated PC12 cells, compared to undifferentiated cells. This activation was reduced by pertussis toxin (PTX), a Gi/o inhibitor. The y-axis in the lower part of the figure gives relative densities of the film. Data were analyzed by 2 way ANOVA with * indicating that P<0.05. Data are mean +/− SEM (n = 3 – 5).
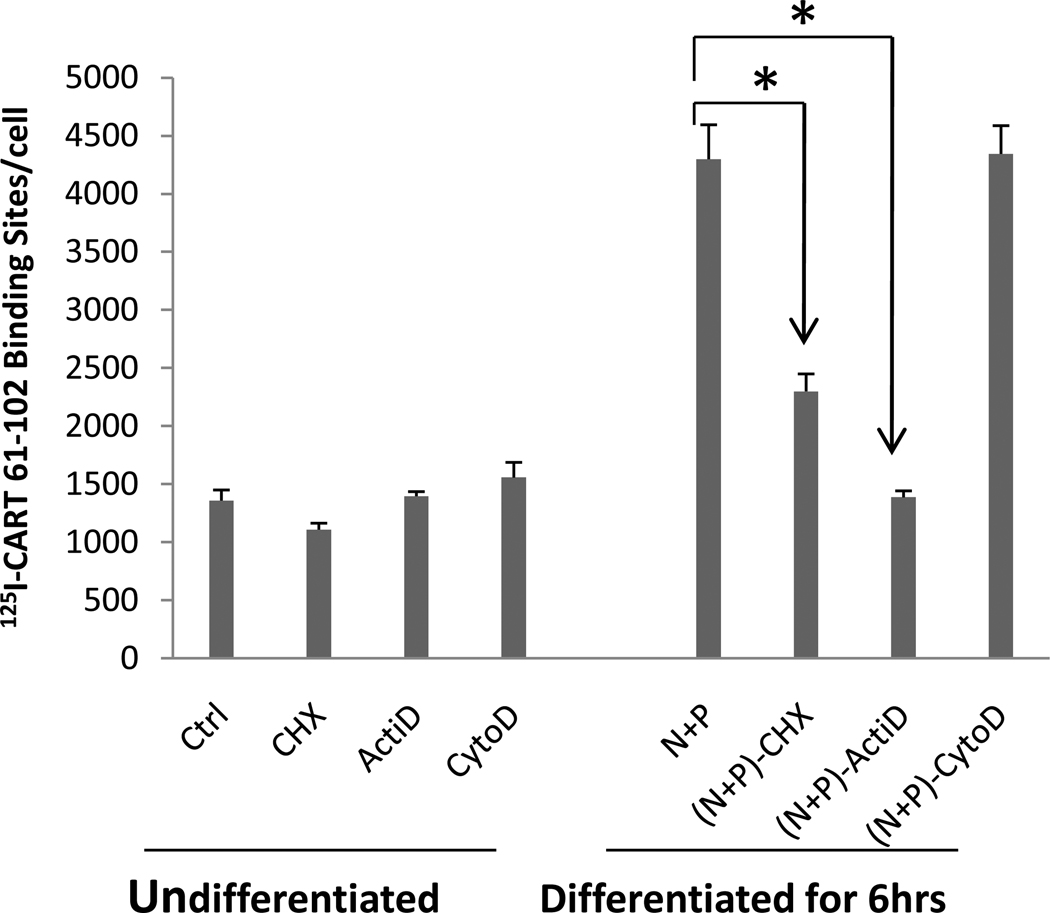
Effects of cycloheximide (CHX), actinomycin D (Acti-D) and cytochalasin D (CytoD) on specific 125I-CART 61-102 binding after differentiation of PC 12 cells
In order to test if protein synthesis, RNA synthesis, and/or a functional cell cytoskeleton were needed for the increase in CART binding induced by differentiation, various inhibitors were added before and maintained during the differentiation process (see Methods). In undifferentiated cells, none of the inhibitors had any effects on CART peptide specific binding after 6 hours. After 6 hours of differentiation with N + P, CART binding increased as expected, and both CHX and ActiD inhibited specific binding, while CytoD was without effect (Figure 7). This suggests that transcription and translation are needed for differentiation to produce the increase in binding.
Figure 7. Effects of cycloheximide (CHX), actinomycin D (Acti-D) and cytochalasin D (CytoD) on specific 125I-CART binding after differentiation of PC12 cells.
The effect of a protein synthesis inhibitor (cycloheximide, CHX), an RNA synthesis inhibitor (Actinomycin-D, ActiD) and a cytoskeleton inhibitor (Cytochalasin D, CytoD) on I125 -CART 61-102 specific binding was determined in both undifferentiated PC12 cells and in differentiated (by N+P for 6 hrs) cells as described in Methods. See text for discussion. The concentration of radioligand in the binding experiments was 5 nM. Data are mean +/− SEM cpms (n=3 – 5) and were analyzed with a 2 way ANOVA. * indicates P values which are at least less than 0.05.
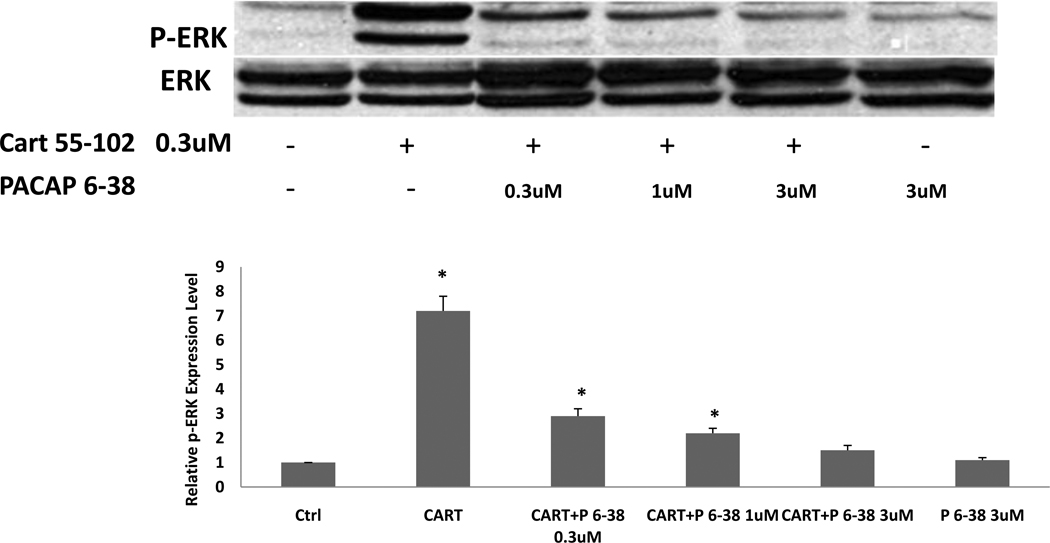
PACAP 6-38 blocks CART peptide activation of p-ERK
Because PACAP 1-38 and 6-38 were found to inhibit CART binding, and because PACAP 6-38 is a PAC1 receptor antagonist in many systems, we tested if PACAP 6-38 might be an antagonist at the CART peptide receptor as well. Accordingly, we tested if PACAP 6-38 (1 µM) would antagonize the activation of ERK induced by CART 55-102 or CART 61-102 (0.3 µM). Differentiated PC12 cells were prepared and exposed to CART peptides in the absence and presence of PACAP 6-38. Treatment with PACAP 6-38 alone had no effect on phospho-ERK levels. Strikingly, however, the activation of ERK by CART 55-102 (Figure 8) and CART 61-102 (not shown) was completely blocked by micromolar concentrations of PACAP 6-38. (Figure 8). In contrast, PACAP 16-38 had no effect on CART peptide –induced rises in phospho-ERK (data not shown), which is consistent with observations that this peptide also did not displace 125I-CART 61-102 binding (Table 1). These data reveal that PACAP 6-38, but not the shorter fragments of PACAP, can antagonize both 125I-CART 61-102 binding and CART peptide-induced functional responses in PC12 cells.
Figure 8. Antagonism of CART peptide activation of ERK by PACAP 6-38.
In differentiated PC12 cells, ERK activation was measured in Western blots (top of figure), and a significant activation was observed as noted above. Moreover, PACAP 6-38 (P6-38) inhibited the activation induced by CART 55-102 but had no effect on basal levels of p-ERK (quantification on bottom). The experiment was repeated 3 times with the same result. A one way ANOVA was used. *indicates P<0.05, compared to control (ctrl).
Discussion
In this study, specific binding of 125I-CART 61-102 was detected in the PC12 cells and increased upon differentiation with NGF, or PACAP or a combination of both NGF and PACAP. It has been established that NGF and PACAP cause differentiation of these cells (Grumolato et al., 2003; Sakai et al., 2004). The specific binding was saturable with a Kd in the subnanomolar range, and was highly specific for active CART peptides. The specific binding and CART signaling in the PC12 cells is more robust than in other cells ( Maletinska et al., 2007, Vicentic et al., 2006) in that between 50 and 80 % of the total binding is specific in differentiated PC12 cells, but is much less in other cell types. This is a useful and practical technical advantage. These findings confirm and build on those of Maletinska et al (Maletinska et al., 2007) and Maixnerova et al (Maixnerova et al., 2007) in PC12 cells, as we extend studies on the properties of CART binding and also assess CART-stimulated signaling in these cells.
We found that CART peptides increased the binding of 35S-GTPgamma-S in differentiated PC12 cells in a manner that was sensitive to Gpp(NH)p but not App(NH)p, indicating that 125I-CART 61-102 interacts with a G protein-coupled receptor in PC12 cells. Also, we tested if CART peptides would increase phospho-ERK levels in the differentiated PC12 cells. Interestingly, we found that the ability of CART to stimulate ERK activation increased with cell differentiation, similar to the increase in CART binding sites observed upon differentiation of the PC12 cells. Moreover, the increase in phospho-ERK levels was blocked by pertussis toxin, which provides additional evidence that the CART receptor couples to G proteins.
We found that CART binding to PC12 cells was specific and could not be displaced by inactive fragments of CART. These data are consistent with results from studies on other cell types. In AtT20 cells, for example, radiolabeled CART peptide binding could not be inhibited by a variety of peptides and small molecules at concentrations up to 1 µM (Vicentic et al., 2005). Moreover, non-radioactive CART 55-102 did not block radioligand ligand binding to any of 30 different peptide and additional receptors (Vicentic et al., 2006). Earlier studies on PC12 cells showed that various fragments of active CART peptides without the three disulfide bridges were only very weak inhibitors of CART peptide binding (Maixnerova et al., 2007, see also Dylag et al., 2006). Taken together, these findings indicate that the CART peptide receptor is very specific for active CART peptides. There have been reports of some smaller CART fragments, such as 62-76 and 85-102, having some functional activity in vivo (Maixnerova et al., 2007), but the effects are modest and require very high peptide concentrations, as we have shown with CART 62-76 (Lambert et al., 1998).
Surprisingly, we found that PACAP 1-38 and PACAP 6-38 were low-affinity inhibitors of CART peptide binding in PC12 cells. More than 20 other peptides were also examined, including other PACAP fragments, but only PACAP 1-38 and 6-38 were found to possess the ability to displace CART binding at sub-micromolar levels. Maxadilan and maxadilan antagonist, peptides known to interact with PACAP receptors (Moro et al., 1999; Tatsuno et al., 2001; Pereira et al., 2002; Reddy et al., 2008) did not block CART peptide binding. Thus, given the low affinity of PACAP 1-38 and 6-38 for displacing CART binding, and the lack of maxadilan displacement, it seems very likely that the CART binding sites in PC12 cells do not represent PACAP receptors. Moreover, earlier studies have shown that PACAP 6-38 is a weaker displacer of PACAP binding than PACAP 1-38 whereas the opposite was found in our studies for displacement of CART peptide binding (Robberecht et al., 1992; Gourlet et al., 1995). Because PACAP 6-38 is an antagonist of PACAP 1-38 at PAC1 receptors, at least in certain tissues (Robberecht et al., 1992; Vandermeers et al., 1992; Tornoe et al., 1997; Leyton et al., 1998) we tested if PACAP 6-38 might act as a CART receptor antagonist. Interestingly, PACAP 6-38 did antagonize the ability of CART to stimulate ERK activation. These data are significant because there are no currently accepted antagonists for the CART receptor, and this has been a major hindrance to many studies on CART actions. Although PACAP 6-38 exhibits relatively low affinity for the CART receptor, and even though it is not specific for the CART peptide receptor, it could be used as a lead compound in a search for more potent analogues.
In summary, these studies confirm the presence of saturable, specific binding of 125ICART 61-102 in PC12 cells, which increased upon differentiation of the cells. We have also significantly expanded upon previous studies in PC12 cells by providing a detailed pharmacological characterization of the CART binding sites and demonstrating that the increase in binding sites upon differentiation correlates with a striking increase in CART-activated signaling. We found that CART peptides enhanced 35S-GTPgammaS binding and increased phospho-ERK levels in a manner that was blocked by pertussis toxin. These data are consistent with the idea that the CART binding sites in PC12 cells represent GPCRs coupled to Gi/o. Importantly, the present studies identified PACAP 6-38 as a low-affinity CART receptor antagonist. These studies provide novel information about the properties of the CART receptor and set the stage for the potential targeting of this receptor by therapeutics.
Acknowledgments
The authors acknowledge the support of a contract from Pfizer and, of grants RR00165 and DA15162.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aja S, Ewing C, Lin J, Hyun J, Moran TH. Blockade of central GLP-1 receptors prevents CART-induced hypophagia and brain c-Fos expression. Peptides. 2006;27:157–164. doi: 10.1016/j.peptides.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Chiu HY, Lin HH, Lai CC. Potentiation of spinal NMDA-mediated nociception by cocaine- and amphetamine-regulated transcript peptide via PKA and PKC signaling pathways in rats. Regul Pept. 2009;158:77–85. doi: 10.1016/j.regpep.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Dylag T, Kotlinska J, Rafalski P, Pachuta A, Silberring J. The activity of CART peptide fragments. Peptides. 2006;27:1926–1933. doi: 10.1016/j.peptides.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Vandermeers-Piret MC, Rathe J, De Neef P, Robberecht P. Fragments of pituitary adenylate cyclase activating polypeptide discriminate between type I and II recombinant receptors. Eur J Pharmacol. 1995;287:7–11. doi: 10.1016/0014-2999(95)00467-5. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Louiset E, Alexandre D, Ait-Ali D, Turquier V, Fournier A, Fasolo A, Vaudry H, Anouar Y. PACAP and NGF regulate common and distinct traits of the sympathoadrenal lineage: effects on electrical properties, gene markers and transcription factors in differentiating PC12 cells. Eur J Neurosci. 2003;17:71–82. doi: 10.1046/j.1460-9568.2003.02426.x. [DOI] [PubMed] [Google Scholar]
- Jones DC, Kuhar MJ. CART receptor binding in primary cell cultures of the rat nucleus accumbens. Synapse. 2008;62:122–127. doi: 10.1002/syn.20476. [DOI] [PubMed] [Google Scholar]
- Keller PA, Compan V, Bockaert J, Giacobino JP, Charnay Y, Bouras C, Assimacopoulos-Jeannet F. Characterization and localization of cocaine- and amphetamine-regulated transcript (CART) binding sites. Peptides. 2006;27:1328–1334. doi: 10.1016/j.peptides.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384:198–202. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–298. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Leyton J, Coelho T, Coy DH, Jakowlew S, Birrer MJ, Moody TW. PACAP(6–38) inhibits the growth of prostate cancer cells. Cancer Lett. 1998;125:131–139. doi: 10.1016/s0304-3835(97)00525-9. [DOI] [PubMed] [Google Scholar]
- Maixnerova J, Hlavacek J, Blokesova D, Kowalczyk W, Elbert T, Sanda M, Blechova M, Zelezna B, Slaninova J, Maletinska L. Structure-activity relationship of CART (cocaine- and amphetamine-regulated transcript) peptide fragments. Peptides. 2007;28:1945–1953. doi: 10.1016/j.peptides.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Sloncova E, Elbert T, Slaninova J, Zelezna B. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur J Pharmacol. 2007;559:109–114. doi: 10.1016/j.ejphar.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Moro O, Wakita K, Ohnuma M, Denda S, Lerner EA, Tajima M. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem. 1999;274:23103–23110. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- Pereira P, Reddy VB, Kounga K, Bello Y, Lerner E. Maxadilan activates PAC1 receptors expressed in Xenopus laevis xelanophores. Pigment Cell Res. 2002;15:461–466. doi: 10.1034/j.1600-0749.2002.02077.x. [DOI] [PubMed] [Google Scholar]
- Pirnik Z, Maixnerova J, Matyskova R, Koutova D, Zelezna B, Maletinska L, Kiss A. Effect of anorexinergic peptides, cholecystokinin (CCK) and cocaine and amphetamine regulated transcript (CART) peptide, on the activity of neurons in hypothalamic structures of C57Bl/6 mice involved in the food intake regulation. Peptides. 2009;31:139–144. doi: 10.1016/j.peptides.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Reddy VB, Li Y, Lerner EA. Maxadilan, the PAC1 receptor, and leishmaniasis. J Mol Neurosci. 2008;36:241–244. doi: 10.1007/s12031-008-9079-1. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Gourlet P, De Neef P, Woussen-Colle MC, Vandermeers-Piret MC, Vandermeers A, Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6–38) as a potent antagonist. Eur J Biochem. 1992;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Hashimoto H, Shintani N, Katoh H, Negishi M, Kawaguchi C, Kasai A, Baba A. PACAP activates Rac1 and synergizes with NGF to activate ERK1/2, thereby inducing neurite outgrowth in PC12 cells. Brain Res Mol Brain Res. 2004;123:18–26. doi: 10.1016/j.molbrainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Wittmann G, Fekete C, Lechan RM. Central administration of cocaine- and amphetamine-regulated transcript increases phosphorylation of cAMP response element binding protein in corticotropin-releasing hormone-producing neurons but not in prothyrotropin-releasing hormone-producing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;999:181–192. doi: 10.1016/j.brainres.2003.11.062. [DOI] [PubMed] [Google Scholar]
- Sen A, Bettegowda A, Jimenez-Krassel F, Ireland JJ, Smith GW. Cocaine- and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells. Endocrinology. 2007;148:4400–4410. doi: 10.1210/en.2007-0332. [DOI] [PubMed] [Google Scholar]
- Tatsuno I, Uchida D, Tanaka T, Saeki N, Hirai A, Saito Y, Moro O, Tajima M. Maxadilan specifically interacts with PAC1 receptor, which is a dominant form of PACAP/VIP family receptors in cultured rat cortical neurons. Brain Res. 2001;889:138–148. doi: 10.1016/s0006-8993(00)03126-7. [DOI] [PubMed] [Google Scholar]
- Tornoe K, Hannibal J, Fahrenkrug J, Holst JJ. PACAP-(1–38) as neurotransmitter in pig pancreas: receptor activation revealed by the antagonist PACAP-(6–38) Am J Physiol. 1997;273:G436–G446. doi: 10.1152/ajpgi.1997.273.2.G436. [DOI] [PubMed] [Google Scholar]
- Vandermeers A, Vandenborre S, Hou X, de Neef P, Robberecht P, Vandermeers-Piret MC, Christophe J. Antagonistic properties are shifted back to agonistic properties by further N-terminal shortening of pituitary adenylate-cyclase-activating peptides in human neuroblastoma NB-OK-1 cell membranes. Eur J Biochem. 1992;208:815–819. doi: 10.1111/j.1432-1033.1992.tb17252.x. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Lakatos A, Jones D. The CART receptors: background and recent advances. Peptides. 2006;27:1934–1937. doi: 10.1016/j.peptides.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Lakatos A, Kuhar MJ. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: specific binding in AtT20 cells. Eur J Pharmacol. 2005;528:188–189. doi: 10.1016/j.ejphar.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res. 1999;818:499–509. doi: 10.1016/s0006-8993(98)01349-3. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci. 2001;21:7474–7480. doi: 10.1523/JNEUROSCI.21-19-07474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang Y, Xin SM, Ma L, Pei G. Attenuation of nociceptin/orphanin FQ-induced signaling by N-methyl-D-aspartate in neuronal cells. Neuroreport. 1998;9:631–636. doi: 10.1097/00001756-199803090-00013. [DOI] [PubMed] [Google Scholar]