Abstract
We aimed to investigate the relationship between dietary saturated fat on fasting triglyceride (TG) and cholesterol levels, and any mediation of this relationship by dietary carbohydrate intake. Men and women in the NHLBI Genetics of Lipid-Lowering Drugs and Diet Network (GOLDN) study (n = 1036, mean age ± SD = 49 ± 16 y) were included. Mixed linear models were run with saturated fat as a predictor variable and fasting TG, very low density lipoprotein cholesterol (VLDL-C), low density cholesterol (LDL-C) and high density cholesterol (HDL-C) as separate outcome variables. Subsequent models were run which included dietary carbohydrate as a predictor variable, and an interaction term between saturated fat and carbohydrate. All models controlled for age, sex, BMI, blood pressure and dietary covariates. In models that included only saturated fat as a predictor, saturated fat did not show significant associations with fasting lipids. When carbohydrate intake and an interaction term between carbohydrates and saturated fat intake was included, carbohydrate intake did not associate with lipids, but there was an inverse relationship between saturated fat intake and VLDL-C (P = 0.01) with a significant interaction (P = 0.01) between saturated fat and carbohydrate with regard to fasting VLDL-C concentrations. Similar results were observed for fasting TG levels. We conclude that, when controlling for carbohydrate intake, higher saturated fat was associated with lower VLDL-C and TGs. This was not the case at higher intakes of carbohydrate. This has important implications for dietary advice aimed at reducing TG and VLDL-C levels.
Keywords: carbohydrate, saturated fat, cholesterol, interaction, triglycerides, VLDL
Introduction
Cardiovascular disease (CVD) is associated with a dyslipidemic profile that includes increased LDL-C and decreased HDL-C and may include high TGs.1 Early animal models showed that a high saturated fat intake can lead to increased serum cholesterol and the development of atherosclerotic plaques.2 These were initially substantiated by epidemiological3–10 and clinical intervention11–14 studies in humans and reducing dietary fat, especially saturated fat, remains the main-stay of dietary advice aimed at reducing CVD risk.
However, more recent studies have started to challenge the idea that saturated fat intake is, in itself, a risk factor for dyslipidemia. Low carbohydrate diets, where a portion of energy from carbohydrates is exchanged for energy from saturated fat, are associated with a decrease in a number of CVD risk factors, including decreased blood pressure, increased HDL-C and TG,15 just as the exchange of fat, particularly saturated fat, for carbohydrate is associated with an increase in TG, VLDL-C and LDL-C in lipid profiles in hypercholesterolemic and hyperlipidemic (raised LDL-C and TG) patients.16 Given that there were differences between the hypercholesterolemic and hyperlipidemic groups, it is important to replicate this in healthy groups before the results can be generalized to the general population. The reasons underlying the beneficial effects of low carbohydrate diets are not clear, but may arise through de novo VLDL-TG lipogensis (shown mostly from the ingestion of simple sugars),17 or through reduced plasma VLDL-TG clearance, shown with both diets high in simple sugar, and high in solid-food high-fiber carbohydrate sources, but low in simple sugars.18 Without the increased secretion of VLDL-TG and hepatic uptake of free fatty acids,19 dietary fat intake may not increase dyslipidemia. Understanding the relationship between carbohydrate and saturated fat intake together, and their effects on serum lipids, is thus crucial for the dietary management of CVD risk.
This study examined the relationship between dietary fat, dietary carbohydrate and lipidemia (TG, VLDL-C, LDL-C and HDL-C) using data from a large cross-sectional study. In addition we examined whether any relationship between saturated fat intake and lipids was mediated by carbohydrate intake. We used linear mixed models to test whether, based on habitual dietary intake, there is an association between saturated fat intake and fasting plasma TG and cholesterol levels when controlling for other dietary covariates. We further ran mixed linear models that included an interaction term between carbohydrate and fat intake to examine whether the association between dietary fat on serum lipids varies across levels of dietary carbohydrate intake.
Results
Participant characteristics
Table 1 shows characteristics of men and women who participated in the Genetics of Lipid Lowering Drugs network (GOLDN) study. There were significant differences (P < 0.05) between men and women with regard to intake of macronutrients and lipid profiles; notably men had a significantly lower percentage of their total energy from carbohydrates and polyunsaturated fatty acids (PUFAs), but a higher percentage of their overall diet was composed of monounsaturated fatty acids (MUFAs) and trans fatty acids. Men had higher fasting TGs, VLDL-C, LDL-C and lower HDL-C than women. In addition, men had higher fasting insulin and glucose levels.
Table 1.
Sample characteristic means (standard deviations in brackets) for men and women.
| Men N = 497 |
Women N = 539 |
P-value | |
|---|---|---|---|
| Age, y | 49.2 (16.2) | 48.3 (16.2) | <0.001 |
| Current smokers, % | 6.8 | 7.8 | 0.56 |
| Current drinkers, % | 48.1 | 51.0 | 0.35 |
| BMI, kg/m2 | 28.6 (4.9) | 28.1 (6.3) | <0.001 |
| Systolic blood pressure, (mmHg) | 71.2 (9.6) | 66.2 (9.1) | <0.0001 |
| Diastolic blood pressure, (mmHg) | 119.5 (15.4) | 113.0 (17.2) | <0.0001 |
| Total food energy intake; kcal/day | 2364 (969) | 1780 (707) | <0.001 |
| Carbohydrates, % energy | 47.6 (8.4) | 50.2 (8.1) | <0.001 |
| Saturated fat, % energy | 12.2 (2.7) | 11.6 (2.7) | <0.001 |
| Protein, % energy | 15.9 (2.7) | 15.8 (2.9) | <0.001 |
| PUFA, % energy | 7.4 (2.0) | 7.9 (2.2) | <0.001 |
| MUFA, % energy | 13.7 (0.3) | 13.0 (2.8) | <0.001 |
| Trans fat, % energy | 2.2 (0.6) | 2.3 (2.0) | <0.001 |
| Glycemic load | 137 (60.4) | 107 (1.4) | <0.001 |
| Fasting TGs (mmol/L) | 1.7 (1.6) | 1.4 (0.9) | 0.30 |
| Fasting VLDL-C (mmol/L) | 3.2 (3.2) | 2.3 (2.0) | <0.0001 |
| Fasting LDL-C (mmol/L) | 3.2 (0.8) | 3.1 (0.8) | <0.001 |
| Fasting HDL-C (mmol/L) | 1.1 (0.3) | 1.4 (0.4) | <0.001 |
| Fasting glucose (mg/dL) | 105.2 (19.7) | 97.8 (15.92) | <0.001 |
| Fasting insulin (mU/L) | 14.2 (8.6) | 13.4 (7.8) | <0.001 |
Abbreviations: BMI, Body mass index; PUFA, Polyunsaturated fatty acids; MUFA, Monounsaturated fatty acids; TGs, Triglycerides; VLDL-C, Very low density lipoprotein cholesterol; LDL-C, Low density lipoprotein cholesterol; HDL-C, High density lipoprotein cholesterol.
Table 2 shows the distribution of potential confounders by tertiles of energy from carbohydrate. Individuals with lower proportion of energy from carbohydrate were significantly more likely to be male, current or past smokers and current drinkers; therefore models looking at the moderation of the association between saturated fat and TGs by carbohydrate intake controlled for these factors.
Table 2.
Participant characteristic by tertiles of percent energy from carbohydrate intake.
| Tertile 1 (N = 345) |
Tertile 2 (N = 345) |
Tertile 3 (N = 346) |
P-value | |
|---|---|---|---|---|
| Carbohydrates, % energy | 40 (5.3) | 49.11 (1.9) | 57.71 (4.7) | <0.001 |
| Gender, % male | 56.5 | 46.5 | 40.9 | <0.001 |
| Age, y | 48.1 (13.3) | 47.9 (15.6) | 50.2 (19.1) | 0.11 |
| Current smokers, % | 12.5 | 4.9 | 4.7 | <0.001 |
| Current drinkers, % | 60.9 | 48.0 | 40.0 | <0.001 |
| BMI, kg/m2 | 29.0 (6.0) | 27.8 (5.3) | 28.2 (5.6) | 0.02 |
| Systolic blood pressure, (mmHg) | 69.3 (9.7) | 68.5 (9.5) | 68.0 (9.8) | 0.10 |
| Diastolic blood pressure, (mmHg) | 115.5 (16.4) | 115.4 (16.0) | 117.47 (17.5) | 0.08 |
| Total energy intake, kcal/d | 2244 (956) | 2099 (867) | 1838 (801) | <0.001 |
| Saturated fat, % energy | 13.6 (2.6) | 12.1 (2.0) | 10.0 (2.0) | <0.001 |
| Protein; % energy | 17.0 (3.0) | 15.8 (2.2) | 14.7 (2.7) | <0.001 |
| PUFA, % energy | 8.9 (2.4) | 7.6 (1.7) | 6.4 (1.5) | <0.001 |
| MUFA, % energy | 15.6 (2.6) | 13.4 (1.6) | 11.0 (1.9) | <0.001 |
| Transfat, % energy | 2.3 (0.6) | 2.2 (0.5) | 1.9 (0.5) | <0.001 |
| Glycemic load of diet | 108 (49.0) | 127 (54.6) | 131 (60.6) | <0.001 |
| Fasting TGs (mmol/L) | 1.6 (1.3) | 1.6 (1.6) | 1.6 (1.0) | 0.42 |
| Fasting VLDL-C (mmol/L) | 2.9 (2.8) | 2.7 (3.0) | 2.7 (2.2) | 0.31 |
| Fasting LDL-C (mmol/L) | 3.2 (0.8) | 3.1 (0.8) | 3.1 (0.8) | 0.34 |
| Fasting HDL-C (mmol/L) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 0.96 |
| Fasting glucose (mg/dL) | 104.3 (21.7) | 99.9 (16.8) | 99.8 (15.1) | 0.001 |
| Fasting insulin (mu/L) | 14.3 (9.8) | 13.3 (7.7) | 13.7 (6.9) | 0.25 |
Abbreviations: BMI, Body mass index; PUFA, Polyunsaturated fatty acids; MUFA, Monounsaturated fatty acids acids; TGs, Triglycerides; VLDL-C, Very low density lipoprotein cholesterol; LDL-C, Low density lipoprotein cholesterol; HDL-C, High density lipoprotein cholesterol.
Main effects of saturated fat on lipids
Mixed models were fitted which looked for an association between saturated fat and lipids. From the fully adjusted model, saturated fat intake did not show a significant association with fasting TG (P = 0.56), VLDL-C (P = 0.32), LDL-C (P = 0.57) or HDL-C (P = 0.46).
Main effects of saturated fat on fasting lipids when controlling for carbohydrate Intake
In fully adjusted models that included a saturated fat -carbohydrate interaction term, carbohydrate intake showed no main effect on TG (P = 0.56; Table 3), VLDL-C (P = 0.25; Table 3), LDL-C (P = 0.41; Table 3) or HDL-C (P = 0.31; Table 3). Saturated fat showed a significant main effect on VLDL-C, with higher saturated fat being associated with lowered VLDL-C (P = 0.01; Table 3) but not on TG (P = 0.08; Table 3), LDL-C (P = 0.75; Table 3) nor HDL-C (P = 0.14; Table 3).
Table 3.
Parameter estimates from mixed linear regression models examining the associations between carbohydrate intake, saturated fat intake and an interaction between carbohydrate and saturated fat intake on plasma lipids.*
| Beta (β) | SE | P-value | |
|---|---|---|---|
| TGs | |||
| Carbohydrate intake | −0.005 | 0.01 | 0.56 |
| Saturated fat intake | −0.05 | 0.03 | 0.08 |
| Interaction term | 0.001 | 0.001 | 0.04 |
| VLDL-C | |||
| Carbohydrate intake | −0.01 | 0.01 | 0.25 |
| Saturated fat intake | −0.09 | 0.04 | 0.01 |
| Interaction term | 0.002 | 0.001 | 0.01 |
| LDL-C | |||
| Carbohydrate intake | −0.01 | 0.01 | 0.41 |
| Saturated fat intake | −0.01 | 0.04 | 0.75 |
| Interaction term | −0.0002 | 0.001 | 0.76 |
| HDL-C | |||
| Carbohydrate intake | −0.005 | 0.005 | 0.31 |
| Saturated fat intake | −0.02 | 0.01 | 0.14 |
| Interaction term | 0.001 | 0.0003 | 0.02 |
Notes: Models control for sex, age, smoking status, alcohol intake, study site, BMI, overall food energy intake, glycemic load of diet and percentage energy from transfats, polyunsaturated fatty acids, monounsaturated fatty acids and protein.
Abbreviations: TGs, Triglycerides; VLDL-C, Very low density lipoprotein cholesterol; LDL-C, Low density lipoprotein cholesterol; HDL-C, High density lipoprotein cholesterol.
Interactions between carbohydrate and saturated fat on fasting lipids
Although continuous dietary variables were used in the mixed models, graphs are presented using tertiles of carbohydrate intake (as above) and saturated fat divided into ‘high’ and ‘low’ categories. Saturated fat was divided into these categories by splitting the ordered distribution of fat intake into two equal-sized categories. This is done for easy interpretation.
Fully adjusted models revealed a significant interaction was observed between carbohydrate intake and saturated fat intake on fasting TG concentrations (P = 0.04; Table 3, Fig. 1). Over the whole sample, saturated fat did not significantly affect fasting TG concentrations. However, the effect of saturated fat intake on fasting TGs was mediated by carbohydrate intake; at low carbohydrate intakes, higher intake of saturated fat was associated with lower fasting TGs, but this effect had disappeared by the highest tertile of carbohydrate intake (Fig. 1). The interaction was similarly significant for VLDL-C concentrations (P = 0.01; Table 3; Fig. 2). Saturated fat was significantly protective against high VLDL-C levels over the whole sample, but this effect was only seen at lower carbohydrate intakes—the association between saturated fat and low VLDL-C was not significant in the highest tertile of carbohydrate intake.
Figure 1.
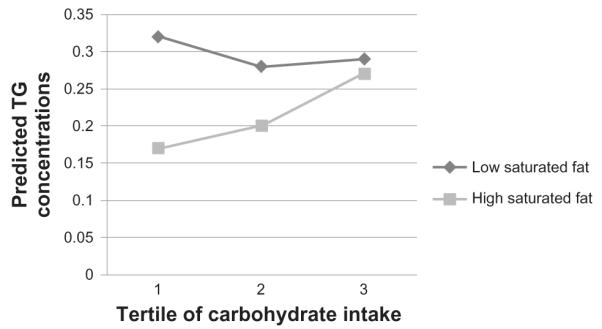
Triglyceride (TG) concentrations by carbohydrate intake tertiles, split into high and low saturated fat intake.
Note: TG concentrations are predicted values after log transformed TG values were regressed for the potential effects of sex, age, smoking status, alcohol intake, centre of data collection, BMI, blood pressure, overall food energy intake, glycemic load of diet and percentage intakes of trans- fats, PUFAs, MUFAs and protein. Exact P-values for each parameter can be found in Table 3.
Figure 2.
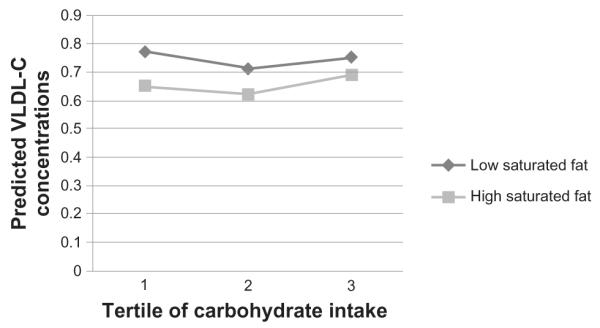
Very low density cholesterol (VLDL-C) concentrations by carbohydrate intake tertiles, split into high and low saturated fat intake.
Note: VLDL-C concentrations are predicted values after log transformed VLDL-C values were regressed for the potential effects of sex, age, smoking status, alcohol intake, centre of data collection, BMI, blood pressure, overall food energy intake, glycemic load of diet and percentage intakes of trans- fats, PUFAs, MUFAs and protein. Exact P-values for each parameter can be found in Table 3.
In addition, there was a significant interaction (P = 0.02; Table 3, Fig. 4) between carbohydrates and saturated fat on fasting HDL-C concentrations, although this showed a slightly different pattern of results. Although overall saturated fat intake did not show a main effect on HDL-C levels in the model (P = 0.14), higher saturated fat was associated with lower HDL-C in the highest tertile of carbohydrate intake (Fig. 4).
Figure 4.
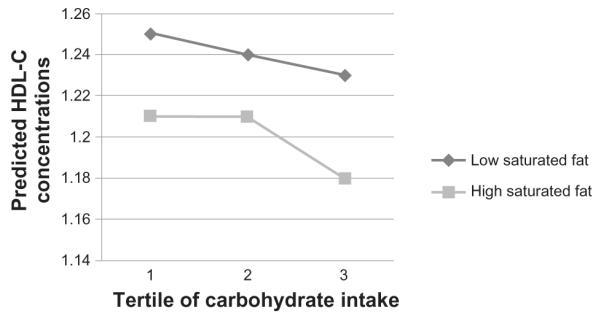
High density cholesterol (HDL-C) concentrations by carbohydrate intake tertiles, split into high and low saturated fat intake.
Note: LDL-C concentrations are predicted values after regression for the potential effects of sex, age, smoking status, alcohol intake, centre of data collection, BMI, blood pressure, overall food energy intake, glycemic load of diet and percentage intakes of trans- fats, PUFAs, MUFAs and protein. Exact P-values for each parameter can be found in Table 3.
We did not observe significant interactions between carbohydrate and saturated fat intake on LDL-C (P = 0.76; Table 3, Fig. 3).
Figure 3.
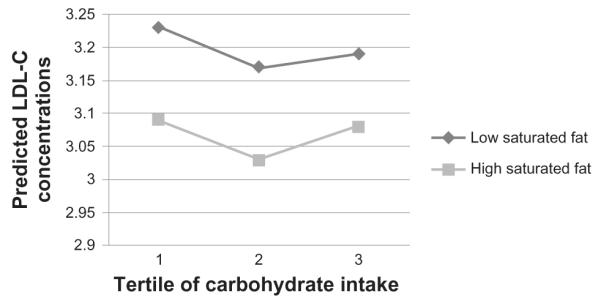
Low density cholesterol (LDL-C) concentrations by carbohydrate intake tertiles, split into high and low saturated fat intake.
Note: LDL-C concentrations are predicted values after regression for the potential effects of sex, age, smoking status, alcohol intake, centre of data collection, BMI, blood pressure, overall food energy intake, glycemic load of diet and percentage intakes of trans- fats, PUFAs, MUFAs and protein. Exact P-values for each parameter can be found in Table 3.
Discussion
We aimed to investigate the relationship between dietary saturated fat intake, dietary carbohydrate intake and fasting lipids. When not controlling for carbohydrate intake, there was no association between saturated fat intake and TG or any fraction of cholesterol. However, when models accounted for the mediation of saturated fat on lipids by carbohydrate intake, we then found a significant association between higher saturated fat intake and lower VLDL-C (P = 0.01), and a significant interaction (P = 0.01) between the two macronutrients. This interaction revealed that the overall association between higher saturated fat and lower VLDL-C disappeared at higher intakes of dietary carbohydrate. The associations between saturated fat intake and TGs were similar to those for VLDL-C, except the overall association between saturated fat and TG did not quite reach significance (P = 0.08). The potential benefit of a reduction in TG and VLDL-C concentrations associated with increased saturated fat intakes at lower carbohydrate levels, were off-set by high saturated fat being associated with lower HDL-C (the “good” cholesterol) at lower carbohydrate intakes.
Despite early epidemiological studies reporting increased saturated fat to be associated with increased TG and VLDL-C concentrations,3–10 data are emerging showing no association between saturated fat and CVD risk,20–24 with a recent meta-analysis on prospective data concluding there was no association between saturated fat intake and CVD risk across studies.25 Indeed a recent study on Japanese men showed that saturated fat was associated with a lowering of LDL-C, although this did not reach statistical significance.26 However, the issue of whether saturated fat intake is associated with dyslipidemia remains controversial; individual differences such as age or gender have been shown to affect the relationship between saturated fat and coronary heart disease7 and the nature and direction of the relationship was debated in a recent issue of the American Journal of Clinical Nutrition.27,28 Our data support the newer line of evidence showing association of saturated fat on lipids, when carbohydrate consumption is not accounted for.
It is frequently observed that diets where fat is substituted for carbohydrate, increase fasting TGs and their precursors.29,30 As this is not always the finding from studies28 the need to continue to investigate this relationship is emphasized. Our results support data that show higher dietary saturated fat to be associated with lower fasting VLDL-C and TG. However, we additionally show that this association is not seen at a high carbohydrate intake. This confirms in a large, epidemiological “free-living” design, the inference of results from exchange studies where exchanging fat for carbohydrate increases TG and VLDL-C, and exchanging carbohydrate for fat has the reverse effects.16,27,29–31 To our knowledge, no study has yet examined whether carbohydrates mediate the effects of saturated fat on fasting lipids using an epidemiological design, and we suggest that failing to account for an interaction between the two macronutrients may have mask the link between lower VLDL-C and TG and saturated fat.
A diet high in carbohydrates may interact with saturated fat through two possible pathways: (1) post prandial clearance mechanisms that arise from high fructose corn syrup (HFCS). HFCS is the most commonly used sweetener in the United States and may therefore account for a considerable amount of dietary carbohydrates. It has been shown that fructose, and HFCS leads to higher postprandial TG concentrations.32 This raise in postprandial TG is believed to occur enhanced de novo lipogensis via the activation of sterol regulatory element binding protein 1-c (SREBP-1c) and carbohydrate response element binding protein (ChREB), a nuclear transcription factor that has been suggested to mediate the effects of fructose.33 Alternatively, (2) decreased clearance of VLDL-TG. This occurs through the ingestion of carbohydrate,18 and may additionally be mediated through increased insulin resistance which arises from a higher carbohydrate intake.34 This hypothesis is supported by research on the effect of the glycemic load (GL) of the diet. Lower GL diets result in a slower, steadier release of insulin, with a lower overall peak. New research suggests that the effects of increasing carbohydrate intake in TG and VLDL-C levels may based on the type of carbohydrate that is increased; replacement of saturated fat with low GL carbohydrates may not show this effect,35 indicating that the effects of carbohydrates on lipoproteins may be insulin mediated. Given the potential role of insulin resistance in mediating the effects of saturated fat on lipoproteins, it would be of interest to repeat the current analysis in diabetic, or pre-diabetic patients, which is not possible in the current sample due to a lack of power. Although a fuller assessment of the role of insulin resistance in mediating the metabolism of carbohydrate and saturated fat is beyond the scope of the current analysis, we do report that homeostatic model assessment of insulin resistance (HOMA-IR) was significantly predicted by saturated fat intake (P = 0.11), carbohydrate intake (P = 0.99) nor was there an interaction between saturated fat and carbohydrate intake on HOMA-IR (P = 0.56).
Our results do entirely concur with previous data; some data have shown that that high carbohydrate intake is associated with higher TG and VLDL-C,16 which we do not see in our data. The reasons for this are not clear, but could reflect either the healthy study population, the effects of longer term dietary patterns, or the overall high carbohydrate intake of the participants (with the lowest tertile of carbohydrate intake averaging 40% of energy intake from carbohydrate), when compared to controlled exchange studies that can be more restrictive. In addition the average age of our participants was 49 years, and the relationship between saturated fat and lipids may be age dependent warranting replication in a younger cohort.7
Thus, the results from this study need to be viewed in light of a number of limitations. First, we used existing data from a cross-sectional study, so were unable to establish any causal association. Secondly, dietary intakes were assessed once with a food-frequency questionnaire which has some inherent errors. Although this instrument has been validated in other US populations, it has not been validated specifically for our study population. Finally, we did not have data on potential dietary mediators, nor did we analyze other health outcomes in relation to saturated fat intake, making us unable to make general public health recommendations. A potential dietary mediator of interest would be the type of carbohydrate intake (although overall GL of the diet was included as a covariate in the regression models). This would be a useful avenue for future research, given that whole grains may confer protective effects on TG and VLDL-C concentrations36,37 while simple sugars and highly refined grains confer more detrimental effects.38 The exchange of high GL carbohydrate sources, such as simple sugars, for lower GL sources, such as whole grains, confers lipid profile benefits in addition to lower fasting insulin levels39 and reduced insulin resistance,40 emphasizing the need to investigate the role of insulin in the interaction between carbohydrate and saturated fat intake that we report here. In terms of health outcomes other than lipid profile, although we controlled for BMI, we were unable to examine other correlates of saturated fat intake, such as liver function, which would be another important avenue for future research, However, the GOLDN study provided an opportunity to study the relation between macronutrients and lipids across a broad spectrum of weight and clinical characteristics, and focus on habitual dietary intake in a non controlled way.
We also note that it not clear whether carbohydrate induced TG and VLDL-C levels confer the same atherogenic risk as other elevations in TG,29,30,41–43 and this requires further investigation.
There is importance in understanding the pathways through which macronutrient intakes affect dyslipidemia and CVD. We recommend future studies replicate these findings and examine the differential roles of different types of carbohydrate in mediating saturated fat intake on dyslipidemia. Overall, our data suggest that the role of saturated fat in dyslipidemia is mediated through carbohydrate intake, which if validated of which has important implications for dietary advice.
Methods
Participants
1328 men and women were recruited and screened for the in the GOLDN study population. All participants were white men and women recruited from Minneapolis, Minnesota and Salt Lake City, Utah. The details of the GOLDN study have been published elsewhere.44 Subsequent to recruitment, we excluded data from participants who were unwilling to stop lipid lowering pharmacological interventions, those with a self-reported history of kidney or Grave’s disease and those in the top and lowest one percentile of total energy intake. In addition, those with missing data on potential confounders were excluded leaving a final sample for analysis of 1036 individuals across 187 families, consisting of 497 men and 539 women (mean ± SD = 48.8 ± 16.2 years of age). The primary aim of the GOLDN study was to characterize the role of genetic and dietary factors on an individual’s response to a high fat diet and fenofibrate. The protocol for this study was approved by the Institutional Review Boards at the University of Minnesota, University of Utah, Tufts University/New England Medical Center and the University of Alabama at Birmingham. Written informed consent was obtained from all participants in accordance with the ethical standards of both data collection sites.
Data collection
Clinical characteristics, including anthropometric and blood-pressure measurements, were taken at the study clinics where a fasting blood sample was also drawn as described previously.44 Questionnaires were administered to collect demographic data and information on lifestyle attributes and medical history.
Dietary intake
We used the Diet History Questionnaire (DHQ) developed by the National Cancer Institute to assess habitual dietary intake. This instrument is a Food Frequency Questionnaire that measures habitual dietary intake over the preceding six months. The DHQ was validated against four 24-hr dietary recalls for ability to assess dietary intake and showed an average correlation of 0.62.45
Biochemical measurements
All plasma samples were analyzed together at the end of the study. Measurements of plasma TG, VLDL-C, LDL-C and HDL-C were determined using enzymatic assays as previously described.46
Statistical analysis
Statistical analyses were carried out using SAS for Windows, version 9.2 (SAS Institute, Cary, NC).
Before running multivariate linear mixed models to examine main effects and potential interactions between carbohydrate and saturated fat intake on fasting lipids, levels of potential confounders across carbohydrate intake levels were examined. For this initial, exploratory analysis, carbohydrate intake was divided; the ordered distribution (ordered by percentage of the dietary intake as carbohydrate) of the study population was divided into tertiles. The significance of differences in the distribution of continuous variables, by carbohydrate tertile, was examined using linear mixed models that adjusted for correlations within pedigrees. The significance of categorical variables was tested using a 2-df chi-squared test of difference. Means and standard errors reported are for raw data.
To maximize power, carbohydrate and saturated fat intake were used in multilevel mixed models as continuous variables. Unlike HDL-C and LDL-C which were normally distributed, TG and VLDL-C had skewed distribution and were log-transformed. The association between saturated fat and plasma lipids and the interaction between carbohydrate and saturated fat on lipids, both expressed as percent of total energy intake, were tested using a mixed model implemented in the Mixed Procedure in SAS (SAS institute, Cary, NC).
Potential confounders were adjusted for in a step-wise fashion. The following separate models were run sequentially. The outcome variable was the lipid (or TG), and predictor variables were:
1. Saturated fat.
2. Saturated fat, age and sex. Both are expected to confound with lipids 47 and show significant differences across potential confounders in the current study (Table 1) and across tertiles of carbohydrate intake (Table 2);
3. Saturated fat, age, sex and smoking, alcohol and BMI. Alcohol and BMI showed significant differences across tertiles of carbohydrate intake (P < 0.05; Table 2);
4. Saturated fat, age, sex, smoking, alcohol, BMI and fat intake broken into trans-, PUFA and MUFA fat intake differed across tertiles of carbohydrate intake (P < 0.001; Table 2);
5. Saturated fat, age and sex, smoking, alcohol and BMI, fat intake and type of carbohydrate intake as measured by GL. GL may confer differential effects on fasting lipids across the distribution;39,40
6. Saturated fat, age, sex, smoking, alcohol and BMI, fat intake, type of carbohydrate intake and percentage of the diet as protein. Protein was added given recent findings on the thermogenic effect of protein;48
7. Saturated fat, age, sex, smoking, alcohol and BMI, fat intake, type of carbohydrate intake, percentage of the diet as protein and systolic and diastolic blood pressure, taken at the time of the blood draw,
After looking for a main effect of saturated fat on lipids, the models were rerun including carbohydrate as a predictor variable and an interaction term between carbohydrate and saturated fat. The direction and significance (with an alpha of P < 0.05) of results did not change between models 2–6 for both sets of analyses, so results from the “fully adjusted” models that control for age, sex, smoking, alcohol and BMI, fat intake, type of carbohydrate intake and percentage of the diet as protein as covariates are presented.
Acknowledgements
We are grateful to the staff of the GOLDN study for the assistance in data collection and management. This study was funded by NHLBI grant number U01HL072524-04.
Footnotes
Author Contribution ACW conceived the hypothesis, designed the analysis, conducted the analysis and wrote the manuscript. EKK designed the analysis and was involved in critical review of the manuscript. IBB, HKT, JMO were involved in critical review of the manuscript. DKA was involved in critical review of the manuscript and collected the data.
Disclosures Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Lloyd-Jones D, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Anitschkow NN. Charles C Thomas; Springfield, IL: 1967. pp. 21–44. [Google Scholar]
- 3.Ascherio A, et al. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ. 1996;313:84–90. doi: 10.1136/bmj.313.7049.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boniface DR, Tefft ME. Dietary fats and 16-year coronary heart disease mortality in a cohort of men and women in Great Britain. Eur J Clin Nutr. 2002;56:786–92. doi: 10.1038/sj.ejcn.1601509. [DOI] [PubMed] [Google Scholar]
- 5.Esrey KL, Joseph L, Grover SA. Relationship between dietary intake and coronary heart disease mortality: lipid research clinics prevalence follow-up study. J Clin Epidemiol. 1996;49:211–6. doi: 10.1016/0895-4356(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Palmieri MR, et al. Relationship of dietary intake to subsequent coronary heart disease incidence: The Puerto Rico Heart Health Program. Am J Clin Nutr. 1980;33:1818–27. doi: 10.1093/ajcn/33.8.1818. [DOI] [PubMed] [Google Scholar]
- 7.Jakobsen MU, Overvad K, Dyerberg J, Schroll M, Heitmann BL. Dietary fat and risk of coronary heart disease: possible effect modification by gender and age. Am J Epidemiol. 2004;160:141–9. doi: 10.1093/aje/kwh193. [DOI] [PubMed] [Google Scholar]
- 8.Robertson TL, et al. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California. Coronary heart disease risk factors in Japan and Hawaii. Am J Cardiol. 1977;39:244–9. doi: 10.1016/s0002-9149(77)80198-7. [DOI] [PubMed] [Google Scholar]
- 9.Robertson TL, et al. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California. Incidence of myocardial infarction and death from coronary heart disease. Am J Cardiol. 1977;39:239–43. doi: 10.1016/s0002-9149(77)80197-5. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, et al. Dietary fat intake and risk of coronary heart disease: the Strong Heart Study. Am J Clin Nutr. 2006;84:894–902. doi: 10.1093/ajcn/84.4.894. [DOI] [PubMed] [Google Scholar]
- 11.Dayton S, et al. A controlled clinical trial of a diet high in unsaturated fat. Preliminary observations. N Engl J Med. 1962;266:1017–23. doi: 10.1056/NEJM196205172662001. [DOI] [PubMed] [Google Scholar]
- 12.Dayton S, Pearce ML. Diet high in unsaturated fat. A controlled clinical trial. Minn Med. 1969;52:1237–42. doi: 10.1161/01.cir.40.1s2.ii-1. [DOI] [PubMed] [Google Scholar]
- 13.Leren P. The Oslo diet-heart study. Eleven-year report. Circulation. 1970;42:935–42. doi: 10.1161/01.cir.42.5.935. [DOI] [PubMed] [Google Scholar]
- 14.Turpeinen O, et al. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int J Epidemiol. 1979;8:99–118. doi: 10.1093/ije/8.2.99. [DOI] [PubMed] [Google Scholar]
- 15.Appel LJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 16.Knopp RH, et al. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med. 2000;225:191–9. doi: 10.1046/j.1525-1373.2000.22524.x. [DOI] [PubMed] [Google Scholar]
- 17.Park JY, et al. Prevalence of diabetes, impaired glucose tolerance, and impaired fasting glucose in a rural population of Korea, according to 1997 American Diabetes Association and 1985 World Health Organization criteria. Diabetes Care. 2000;23:707–8. doi: 10.2337/diacare.23.5.707. [DOI] [PubMed] [Google Scholar]
- 18.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–96. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topping DL, Mayes PA. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972;126:295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushi LH, et al. Diet and 20-year mortality from coronary heart disease. The Ireland-Boston Diet-Heart Study. N Engl J Med. 1985;312:811–8. doi: 10.1056/NEJM198503283121302. [DOI] [PubMed] [Google Scholar]
- 21.Leosdottir M, Nilsson PM, Nilsson JA, Berglund G. Cardiovascular event risk in relation to dietary fat intake in middle-aged individuals: data from The Malmo Diet and Cancer Study. Eur J Cardiovasc Prev Rehabil. 2007;14:701–6. doi: 10.1097/HJR.0b013e3282a56c45. [DOI] [PubMed] [Google Scholar]
- 22.Pietinen P, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol. 1997;145:876–87. doi: 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- 23.Posner BM, et al. Dietary lipid predictors of coronary heart disease in men. The Framingham Study. Arch Intern Med. 1991;151:1181–7. [PubMed] [Google Scholar]
- 24.Tucker KL, et al. The combination of high fruit and vegetable and low saturated fat intakes is more protective against mortality in aging men than is either alone: the Baltimore Longitudinal Study of Aging. J Nutr. 2005;135:556–61. doi: 10.1093/jn/135.3.556. [DOI] [PubMed] [Google Scholar]
- 25.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–9. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamagishi K, et al. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study? Am J Clin Nutr. 2010 doi: 10.3945/ajcn.2009.29146. [DOI] [PubMed] [Google Scholar]
- 27.Katan MB, Brouwer IA, Clarke R, Geleijnse JM, Mensink RP. Saturated fat and heart disease. Am J Clin Nutr. 2010;92:459–60. doi: 10.3945/ajcn.2010.29752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarborough P, Rayner M, van DI, Norum K. Meta-analysis of effect of saturated fat intake on cardiovascular disease: overadjustment obscures true associations. Am J Clin Nutr. 2010;92:458–9. doi: 10.3945/ajcn.2010.29504. [DOI] [PubMed] [Google Scholar]
- 29.Katan MB. High-oil compared with low-fat, high-carbohydrate diets in the prevention of ischemic heart disease. Am J Clin Nutr. 1997;66:974S–9. doi: 10.1093/ajcn/66.4.974S. [DOI] [PubMed] [Google Scholar]
- 30.Katan MB, Grundy SM, Willett WC. Should a low-fat, high-carbohydrate diet be recommended for everyone? Beyond low-fat diets. N Engl J Med. 1997;337:563–6. [PubMed] [Google Scholar]
- 31.Appel LJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–64. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 32.Teff KL, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 33.Spruss A, Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J Nutr Biochem. 2009;20:657–62. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403–11. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 35.Hu FB. Are refined carbohydrates worse than saturated fat? Am J Clin Nutr. 2010;91:1541–2. doi: 10.3945/ajcn.2010.29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson JW, Hanna TJ, Peng X, Kryscio RJ. Whole grain foods and heart disease risk. J Am Coll Nutr. 2000;19:291S–9. doi: 10.1080/07315724.2000.10718963. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, et al. Whole grain consumption and risk of ischemic stroke in women: A prospective study. JAMA. 2000;284:1534–40. doi: 10.1001/jama.284.12.1534. [DOI] [PubMed] [Google Scholar]
- 38.Liu S. Intake of refined carbohydrates and whole grain foods in relation to risk of type 2 diabetes mellitus and coronary heart disease. J Am Coll Nutr. 2002;21:298–306. doi: 10.1080/07315724.2002.10719227. [DOI] [PubMed] [Google Scholar]
- 39.Jang Y, Lee JH, Kim OY, Park HY, Lee SY. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arterioscler Thromb Vasc Biol. 2001;21:2065–71. doi: 10.1161/hq1201.100258. [DOI] [PubMed] [Google Scholar]
- 40.Steffen LM, et al. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am J Epidemiol. 2003;158:243–50. doi: 10.1093/aje/kwg146. [DOI] [PubMed] [Google Scholar]
- 41.Connor WE, Connor SL. Should a low-fat, high-carbohydrate diet be recommended for everyone? The case for a low-fat, high-carbohydrate diet. N Engl J Med. 1997;337:562–3. doi: 10.1056/NEJM199708213370811. [DOI] [PubMed] [Google Scholar]
- 42.Ornish D. Low-fat diets. N Engl J Med. 1998;338:127–9. doi: 10.1056/NEJM199801083380212. [DOI] [PubMed] [Google Scholar]
- 43.Rudel LL. Low-fat diets. N Engl J Med. 1998;338:128–9. [PubMed] [Google Scholar]
- 44.Kabagambe EK, et al. Smoking, inflammatory patterns and postprandial hypertriglyceridemia. Atherosclerosis. 2009;203:633–9. doi: 10.1016/j.atherosclerosis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millen AE, Midthune D, Thompson FE, Kipnis V, Subar AF. The National Cancer Institute diet history questionnaire: validation of pyramid food servings. Am J Epidemiol. 2006;163:279–88. doi: 10.1093/aje/kwj031. [DOI] [PubMed] [Google Scholar]
- 46.Lai CQ, et al. Fenofibrate effect on triglyceride and postprandial response of apolipoprotein A5 variants: the GOLDN study. Arterioscler Thromb Vasc Biol. 2007;27:1417–25. doi: 10.1161/ATVBAHA.107.140103. [DOI] [PubMed] [Google Scholar]
- 47.Jarvik GP, et al. Genetic influences on age-related change in total cholesterol, low density lipoprotein-cholesterol, and triglyceride levels: longitudinal apolipoprotein E genotype effects. Genet Epidemiol. 1994;11:375–84. doi: 10.1002/gepi.1370110407. [DOI] [PubMed] [Google Scholar]
- 48.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–85. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]


