SUMMARY
Compared to the developing visual system, where neuronal plasticity has been well characterized at multiple levels, little is known about plasticity in the adult, particularly within subcortical structures. We made intraocular injections of 2-amino-4-phosphonobutyric acid (APB) in adult cats to block visual responses in On-center retinal ganglion cells and examined the consequences on visual responses in the lateral geniculate nucleus (LGN) of the thalamus. In contrast to current views of retinogeniculate organization, which hold that On-center LGN neurons should become silent with APB, we find that ~50% of On-center neurons rapidly develop Off-center responses. The time course of these emergent responses and the actions of APB in the retina indicate the plasticity occurs within the LGN. These results suggest there is greater divergence of retinogeniculate connections than previously recognized and that functionally silent, non-specific retinal inputs can serve as a substrate for rapid plasticity in the adult.
Keywords: thalamus, activity, LGN, retina, APB
INTRODUCTION
A remarkable feature of neurons is their ability to modify their function and organization in response to changes in the activity of their inputs. This ability for neuronal plasticity is particularly robust and widespread during development, but can extend into adulthood under certain circumstances and to a more limited extent. For instance, in the visual system, where developmental plasticity has been demonstrated from retina to extrastriate cortex, adult plasticity is largely believed to be restricted to the cortex with subcortical structures losing their capacity for change after a critical period of development (Gilbert and Wiesel, 1992; Darian-Smith and Gilbert, 1995; Buonomono and Merzenich, 1998; Calford et al., 2002, 2003; Fox et al., 2002; Gilbert et al., 2009). Here, we challenge this view and present evidence for a novel form of adult plasticity measured in the lateral geniculate nucleus (LGN) of the thalamus.
A defining property of the adult visual system is the immediate segregation of On and Off channels used for signaling increases and decreases in light levels. These channels are established at the very first retinal synapse between the photoreceptor and bipolar cell and are thought to remain segregated through the LGN until converging in the primary visual cortex. Overwhelming evidence indicates On-center and Off-center LGN neurons receive stream-specific input, yet the possibility exists that these neurons may have access to information traveling in the other stream. For instance, if neurons in one stream received silent or masked input from neurons in the other stream, then this input could serve as a substrate for rapid plasticity in the adult. Consistent with this view, a small number of studies describe very weak “mistakes” in the connections made between retinal ganglion cells (RGCs) and LGN neurons (Hamos et al., 1987; Mastronarde, 1992; Usrey et al., 1999).
In this study, we silenced On-center RGCs with intraocular injections of the glutamate receptor agonist 2-amino-4-phosphonobutyric acid (APB; Slaughter and Miller, 1981; Bolz et al., 1984) and examined the consequence on visual responses in the adult LGN. Our results demonstrate that On-center neurons in the adult LGN are capable of undergoing a rapid transformation in their visual physiology, whereby On-center neurons experience a striking flip in their stimulus preference and develop an emergent Off-center response. Because this flip does not occur in the retina, nor is it accompanied by an increased latency indicative of polysynaptic mechanisms, our results support the hypothesis that functionally silent, non-specific connections in the retinogeniculate pathway serve as a substrate for adult plasticity in the early visual system.
RESULTS
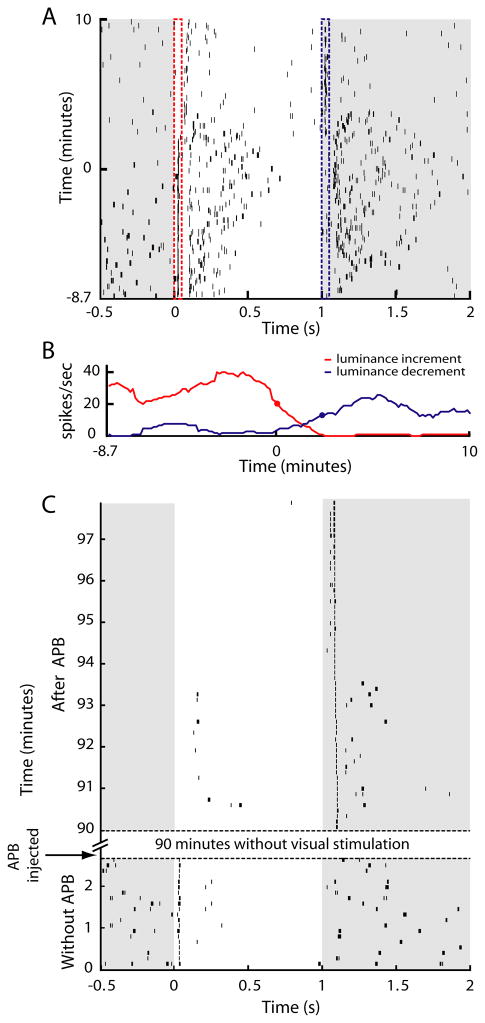
To study the consequences of silencing the On pathway on LGN physiology, we used a 7-channel multielectrode array (Thomas Recording, Giessen, Germany) to record the spiking activity of isolated LGN neurons in the anesthetized cat before and after silencing the On pathway with intraocular injections of APB. Figure 1A shows the spiking activity of a representative On-center LGN neuron to a repeating, spatially-uniform stimulus that alternated between grey (38 cd/m2) and white (76 cd/m2). As expected for On-center neurons, this neuron responded faithfully to stimulus transitions from grey to white prior to the onset of APB action (time 0) and became unresponsive to similar transitions following APB onset (Schiller, 1982; Schiller, 1984; Knapp and Mistler, 1983; Horton and Sherk, 1984). However, contrary to current views of retinogeniculate organization, which predict the LGN neuron should remain unresponsive to visual stimuli during APB action, the neuron rapidly developed an emergent Off response and, consequently, faithfully followed stimulus transitions from white to grey. The interval between time points marking a 50% reduction in On activity and a 50% of maximum increase in Off activity was 145 seconds (Figure 1B). Using this spatially-uniform stimulus, emergent Off responses were observed among ~50% of On-center neurons examined (15/34) with the remaining neurons becoming visually unresponsive during APB treatment.
Figure 1.
Time course of On to Off plasticity in the LGN. (A) Raster plot showing spiking activity of a representative On-center LGN neuron to a full-field visual stimulus that alternated between grey (38 candelas/m2) and white (76 candelas/m2). The vertical red and blues lines mark the windows used to quantify visual responses in panel B. (B) Quantification of responses to luminance increases and decreases. Red and blue traces show the neuron’s firing rate calculated from a sliding 20-trial window during the first 50 msec following stimulus transition. Red and blue circles indicate when On responses first decreased to 50% of maximum and Off responses first increased to 50% of maximum, respectively. Time zero in panel A (y axis) corresponds to the time when On response dropped to 50% of maximum. (C) Raster plot showing responses from an On-center LGN neuron that received no visual stimulation for 90 minutes following intraocular APB injection. Off responses are evident in the first trial following the hiatus from visual stimulation.
To determine whether the emergence of Off responses from On-center LGN neurons requires visually-evoked activity from the retina, we covered the eyes for 90 minutes following APB injection and compared neuronal responses before APB injection and immediately following the 90-minute period of darkness. As shown in Figure 1C, Off responses were clearly present immediately following the reintroduction of visual stimulation. Interestingly, the latencies of the emergent Off responses decreased progressively over the first 5–6 minutes following the reintroduction of visual stimulation. A long latency and sporadic On response was also evident transiently during this early period of visual stimulation, possibly reflecting the effects of APB on the OFF pathway (Sugihara et al., 1997; Rentería et al., 2006). A similar pattern for the emergence of Off responses occurred in 4 of 7 On-center LGN neurons examined with this paradigm; the remaining neurons were visually unresponsive. These findings indicate that while visual stimulation may contribute to the On to Off plasticity, changes in spontaneous activity are sufficient to initiate the effect. Along these lines, previous studies have shown APB has a much greater effect on reducing spontaneous activity among On-center RGCs compared to Off-center RGCs (Knapp and Mistler, 1983; Horton and Sherk, 1984).
Given the unexpected finding that intraocular APB can induce a switch in the response signature of On-center LGN neurons, we wished to confirm that the LGN, rather than the retina, is the site of this rapid plasticity. We therefore stimulated the retina with the same visual stimulus shown in Figure 1 and recorded electroretinograms (ERGs) in vivo (n=4) and single-unit responses from On-center RGCs in vitro (n=32) before and after APB application. As shown in Figure 2, APB silenced On responses in the retina without any indication of emergent Off responses. More importantly, every On-center cell became visually unresponsive with APB, indicating APB and our injection protocol blocked visual responses in On center RGCs and the On to Off plasticity measured in the LGN did not simply follow a similar transition in the eye.
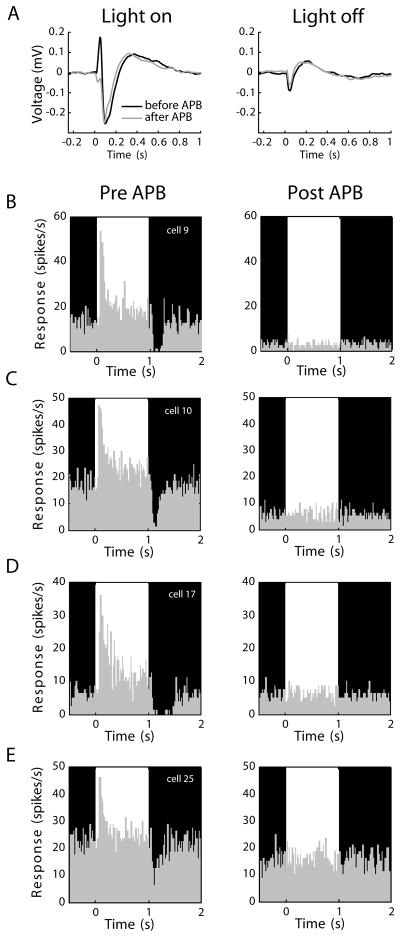
Figure 2.
APB effects in the eye. (A) Electroretinograms (ERGs) showing responses to a repeating full-field stimulus that alternated between a 1-second bright phase and a 1-second dark phase (76 and <1 cd/m2, respectively). Black traces show values before APB, grey traces show values after APB injection. In the light-on condition, the sharp upward deflection before APB injection (black trace) represents the coordinated On-bipolar cell depolarization. This deflection was absent following APB injection (grey trace), confirming APB silenced the On-pathway. In the light-off condition, the ERG was unaffected by APB injection, supporting the view that APB does not cause an enhancement of Off responses in the retina. Axis conventions as in Slaughter and Miller (1981). (B-E) Spiking responses of 4 representative On-center RGCs before and during APB perfusion, in vitro. Recordings were made from excised patches of retina using a 60-channel multielectrode array. In each panel, the bright and dark phases of an alternating stimulus are indicated in the background shading. Each of the On cells shows a clear elevation in spiking activity in response to increases in stimulus luminance. The same cells were unresponsive to visual stimulation during 300 μM APB treatment. Every On-center RGC in our sample (n=32) became visually unresponsive with APB.
Spatial specificity of emergent receptive fields
Having observed a striking, APB-induced flip in the response signature of On-center LGN neurons using a spatially-uniform stimulus, we next examined the effects of APB on the fine structure of LGN receptive fields by using a white-noise stimulus and reverse-correlation analysis (Figure 3, see Experimental Procedures). As expected and exemplified with the receptive field map of a representative Off-center neuron in Figure 3A, all Off-center neurons in our sample remained Off-center in the presence of APB (n=28 cells). In contrast, more than 50% of On-center neurons (n=35/52) underwent the rapid transformation in receptive field structure from On-center to Off-center, as in Figures 3C-E. The remaining On-center neurons were non-responsive to visual stimuli following APB treatment (n=17). Using receptive field size and response latency to classify cells as either X or Y (Usrey et al., 1999), we did not see a significant difference in the relative proportion of X and Y cells in the group of On-center cells that lost visual responsiveness following APB application versus those that developed Off-center responses (p=0.9, Wilcoxon rank sum test).
Figure 3.
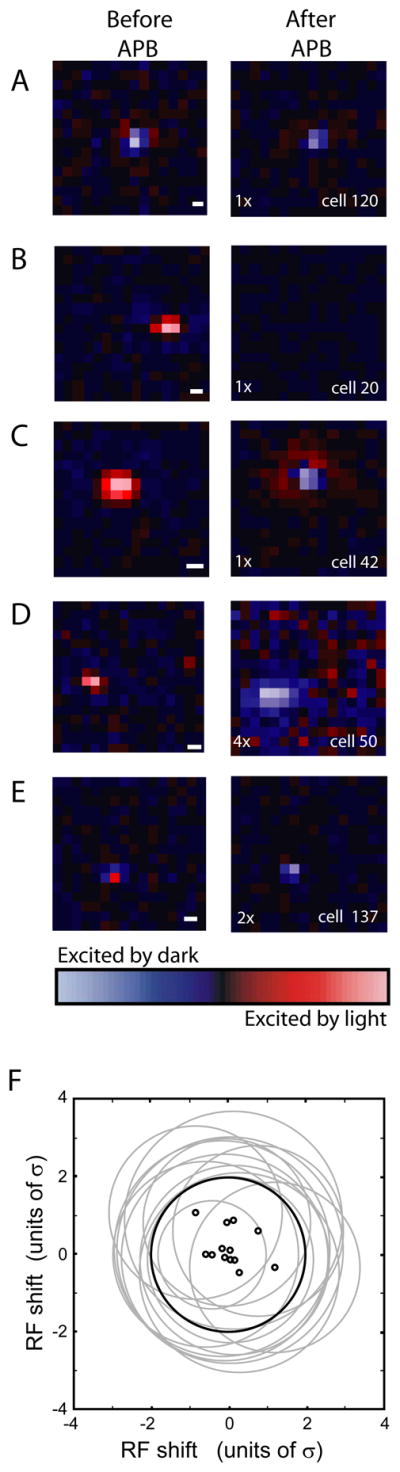
LGN RFs before and after intraocular injections of APB. (A-E) RF maps of 5 LGN neurons before and after intraocular injection of APB. RFs were mapped using a white-noise stimulus and reverse-correlation analysis. On responses shown in red, Off responses shown in blue. Scalebars indicate 1° of visual angle. Pixel brightness indicates strength of response. The strength of each post-APB RF is shown normalized to the pre-APB RF with a scaling factor indicated in the lower left of the panel. (A) A typical Off-center cell shows little difference before (left) and after (right) APB injection. (B) A typical On-center cell that became unresponsive following APB application. (C-E) Examples of On-center cells with emergent Off-center RFs following APB application. (F) Summary of the relative size and location of emergent Off-center RFs. The thick black circle corresponds to the initial RF center of 13 On-center neurons fitted with a Gaussian equation and shown with a radius of two space constants (2σ). The overlapping grey circles show the relative size and location of the emergent Off-center RFs with the centers indicated with small black circles. Emergent Off centers were 1.19 +/− 0.06 σ larger, on average, than initial the On centers. Emergent Off RFs were shifted by 0.6 +/− 0.12σ, on average, from the initial On centers.
We next compared the size and location of the emergent Off-center receptive fields to the original On-center receptive fields. To do so, we fit the original and emergent receptive field centers to a Gaussian equation and normalized coordinate distances by the size of each neuron’s original receptive field center (in space constants, see Experimental Procedures). Because intraocular injections can alter eye position and therefore the location of receptive fields, this analysis was only performed on cells simultaneously recorded with an Off cell whose receptive field served as a fiduciary marker (n=13 cells). As shown in Figure 3F, the Off-centers of the emergent receptive fields always overlapped the On-centers of the original receptive fields, consistent with spatial organization of On- and Off-center RGCs in the cat (Wässle et al., 1983). The mean displacement of emergent receptive field centers was just 0.60 +/− 0.12σ (sem), indicating a high degree of retinotopic specificity among the emergent receptive fields. An examination of receptive field size revealed a small, but significant increase in the size of emergent receptive field centers compared to their pre-APB counterparts (mean size increase = 0.19 +/− 0.06σ (sem); p<0.05, ANOVA), suggesting a decrease in the relative weight of the antagonistic surround and/or an increase in the spatial distribution of inputs during APB action.
Timing and strength of visual responses
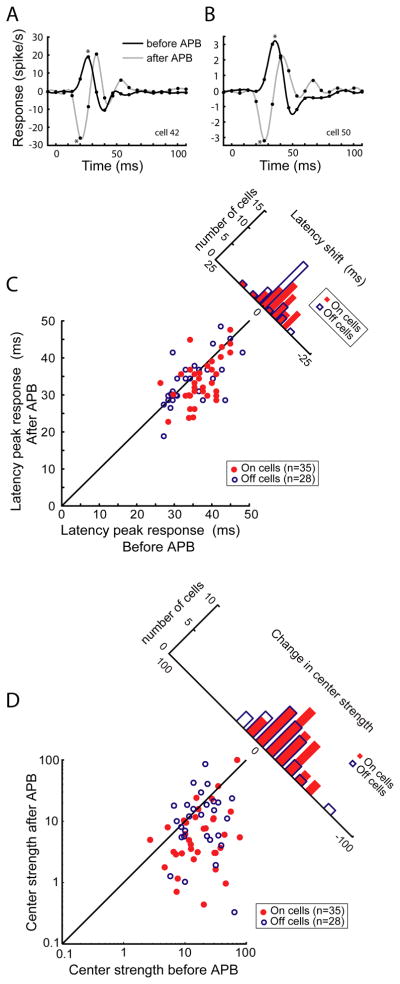
To determine whether polysynaptic circuit mechanisms might underlie the On-to-OFF plasticity of LGN responses, we calculated and compared impulse responses to the white-noise stimulus before and during APB action (see Experimental Procedures). Impulse responses from two On-center LGN neurons, generated before and during APB action (black and grey traces, respectively), are shown in Figures 4A and B. In these figures, the direction of the initial peak indicates whether the receptive field center is On or Off, as a positive peak corresponds to an increase in firing rate (above the mean) to a white stimulus (presented at time = 0) and a negative peak corresponds to an increase in firing rate (above the mean) to a black stimulus. From this initial peak, response latency was quantified as the time to reach maximum response, and response strength was quantified as the integral of the peak.
Figure 4.
Visual response latency and strength of responses before and after APB application. (A, B) Impulse responses from 2 On-center LGN neurons calculated before and after APB application. Impulse responses calculated from pixels in the RF maps corresponding to the RF center. Data points fitted with a cubic spline. Asterisks indicate the primary peak for each response. Both neurons show an emergent Off response after APB application. (C) Latency to peak center response before and after APB application. On cells with emergent Off responses shown in red, Off cells shown in blue. Following APB treatment, emergent Off responses were significantly faster than initial On responses; Off cell latencies were not affected by APB. (D) Center strength before and after APB application. Center strength was quantified as the integral of the primary impulse response peak. On cells with emergent Off responses shown in red, Off cells shown in blue. Both groups of LGN neurons show a significant decrease in center strength with APB.
Across our sample of LGN neurons (n=80 cells), visual response latency was slightly shorter for Off cells compared to On cells (33.7 +/− 1.1 ms vs. 36.2 +/− 0.8 ms, respectively; p=0.06, Wilcoxon Rank-Sum Test). While APB injection did not significantly influence visual response latency of the Off-center cells (Figure 4C, 32.7+/−1.1 ms, p= 0.56, Wilcoxon Rank-Sum Test), it did lead to a significant decrease in response latency of the On-center cells with emergent Off responses (Figure 4C, mean latency = 32.6 +/− 1.0 ms, p=0.006, ANOVA. This decrease in response latency provides useful information about the mechanism(s) underlying emergent Off responses. In particular, the decrease in latency for emergent Off responses indicates these emergent responses are not the result of polysynaptic inputs, such as corticogeniculate feedback, projections from the reticular nucleus, or collaterals of neighboring relay neurons (Sherman and Cox, 2003; Bickford et al., 2008), as the number of additional synapses involved with these circuits should increase response latency following APB.
Prior to APB injection, On-center and Off-center neurons did not differ significantly in the strength of their impulse response (Figure 4D; p=0.73, Wilcoxon Rank-Sum Test). Following APB injection, center strength decreased significantly for both populations of LGN neurons with the greatest reduction for cells initially defined as On-center. Response strength decreased on average by 30.5% for Off-center neurons (p<0.02, ANOVA; see also Rentería et al., 2006) and by 55.6% for On-center cells with emergent Off responses (p<0.01, Wilcoxon Rank-Sum Test). Thus, APB reduces the responsiveness of both On-center and Off-center LGN neurons. This result has important implications for the circuits underlying the visual responses of both On and Off-center LGN neurons. For On-center neurons, this result supports the proposal that APB can unmask weak or silent Off inputs from the retina. For Off-center neurons, this result is consistent with the view that APB can interfere with disynaptic inhibition provided by On-center interneurons that normally provide a “pull” to increase Off-center responses (Hirsch, 2003; Wang et al., 2011).
DISCUSSION
The goal of this study was to determine the consequences of selectively silencing stream-specific input from the eye on neuronal responses in the adult LGN. To do so, we made intraocular injections of APB to block visual responses in On-center RGCs and measured visual responses in the LGN. Approximately 50% of On-center LGN neurons became unresponsive to visual stimuli during APB treatment, a cellular response consistent with previous views of APB action and retinogeniculate organization (Slaughter and Miller, 1981; Knapp and Mistler, 1983; Bolz et al., 1984; Horton and Sherk, 1984; Schiller, 1984). The remaining On-center LGN neurons underwent a remarkable transformation in receptive field structure and rapidly acquired Off-center responses. These results not only support the hypothesis that functionally silent input from the retina can undergo rapid strengthening in the adult LGN, they also force a re-examination of current views on the specificity of neuronal circuits in the early visual system.
Given the high frequency of emergent Off-center receptive fields reported here, it is reasonable to ask why past studies did not identify such an effect. Previous studies using cats and monkeys clearly demonstrate an APB-induced loss of On-center responses among neurons in the LGN (Horton and Sherk, 1984; Schiller, 1984). However, because APB is non-reversible during the time course of an in vivo experiment and single electrodes were used to record neuronal responses, it was not practical to record continuously from large numbers of individual neurons before and after APB treatment. Instead, data was collected primarily from separate samples of neurons before and after APB application and, following APB application, only cells with Off-center receptive fields were visually active. Key to the success of the current study was the use of a multielectrode array, allowing simultaneous recording of several LGN neurons while APB took effect. This allowed us to observe directly the On-center to Off-center plasticity in receptive field structure.
APB blocks visual responses in the On pathway by selectively binding metabotropic glutamate receptors located in the synapses between photoreceptors and On-center bipolar cells (Slaughter and Miller, 1981; Bolz et al., 1984; Horton and Sherk, 1984). If the actions of APB were selective to the mechanisms that establish the receptive field center of bipolar cells and RGCs without affecting the receptive field surround, then our finding of an emergent Off response in the LGN could simply reflect a selective loss of the receptive field center. Our results and those of past studies, however, do not support such a possibility. In particular, visual response latency is known to be longer for the receptive field surround compared the center (Enroth-Cugell et al., 1983; Dawis et al., 1984; Cai et al., 1997; Usrey et al., 1999; Allen and Freeman, 2006). Consequently, if emergent Off responses were simply the result of silencing the On-center response, then the time course of the emergent Off response should be longer than the initial center response, not the same or shorter, as reported here. Moreover and consistent with previous reports, none of the On-center RGCs in this study showed Off responses following APB application (Slaughter and Miller, 1981; Massey et al., 1983).
Both the time course for emergent Off responses and the timing of those responses suggest APB leads to a rapid change in the synaptic strength of functionally silent, mismatched input from Off-center RGCs onto On-center LGN neurons. Specifically, the emergence of Off responses following APB application is too quick for an anatomical reorganization of inputs. Emergent Off responses are more likely the result of changes in the synaptic strength of mismatched retinal inputs or changes in the contributions made by polysynaptic sources. Because emergent Off responses show no evidence of an increase in visual response latency, it seems unlikely that polysynaptic circuits play a major role, as these circuits should increase response latency. Moreover, extrinsic sources of polysynaptic input lack the center/surround organization seen for emergent receptive fields. Finally, current understanding of the push/pull organization of LGN receptive fields holds that local GABAergic input onto On-center LGN neurons comes from Off-center cells that provide a “pull” to reinforce, not reverse, the On response (Hirsch, 2003; Wang et al., 2011).
Although the idea of mismatched projections from RGCs to LGN neurons contradicts current models of retinogeniculate circuitry, there is evidence for the existence of these connections in the literature. In particular, studies using cross-correlation analysis to examine the response properties of synaptically-connected RGCs and LGN neurons describe a small percentage of weakly connected cell pairs mismatched in their On/Off or X/Y signature (Mastronarde, 1992; Usrey et al., 1999; also see Hamos et al., 1987). These connections were likely the result of incomplete pruning of retinal afferents during development, as LGN neurons initially receive weak input from more than ten RGCs of mixed sign, but eventually receive only one or two dominant inputs once the pathway matures (Lui and Chen, 2008).
Blocking On activity in the retina removes a major source of excitatory drive to On-center LGN neurons. This decrease in excitatory drive likely leads to numerous changes in the intrinsic membrane properties of LGN neurons and the composition of their postsynaptic receptors. Past work in the peripheral nervous system has shown that decoupling skeletal muscle cells from their afferent input leads to an overall increase in input resistance, an increase in the number of acetylcholine receptors, and a general increase in excitability (Berg and Hall, 1975). Likewise, blocking retinal activity in rat pups results in a scaling up of excitatory synaptic currents in visual cortex (Desai et al., 2002). In addition to these possible mechanisms, silent synapses may also play a role in the emergence of Off responses from On-center LGN neurons (Liao et al., 2001). Evidence indicates that adult retinogeniculate synapses typically contain both AMPA and NMDA receptors (Esguerra et al., 1992). If synapses from mismatched Off ganglion cells are instead silent and express only NMDA receptors, then these synapses could become rapidly activated with the insertion of AMPA receptors. In support of this possibility, Chen et al. (2002) demonstrated that sustained afferent activity can lead to rapid short-term plasticity in the LGN through a process involving regulation of both AMPA and NMDA receptors and an overall desensitization of synapses.
In conclusion, we have identified a novel form of plasticity in the adult LGN whereby intraocular injections of APB lead to a rapid emergence of Off-center responses from On-center neurons. Our results suggest this plasticity likely relies on a rapid strengthening of weak or silent inputs from the retina. Moreover, these results indicate that visual neurons in the adult thalamus are capable of providing visual information to the cerebral cortex in the absence of their primary afferent drive. For the On to Off plasticity identified here, cortical reorganization would likely follow thalamic plasticity for this information to prove useful for vision. Given the challenges the visual system encounters during its lifetime—challenges including injury, stroke and disease—it is critical that we increase our understanding of the circuits capable of plasticity in the adult brain.
Experimental Procedures
Surgical Preparation
Fifteen adult cats (>6 months old, both sexes) were used in this study. All surgical and experimental procedures were performed in accordance with guidelines from the National Institutes of Health and were approved by the Animal Care and Use Committee at the University of California, Davis. Surgical anesthesia was induced with ketamine (10 mg/kg, IM) followed with thiopental sodium (20 mg/kg, IV, supplemented as needed). Animals were placed in a stereotaxic apparatus where body temperature was maintained at 37°C with a thermostatically-controlled heating blanket, and they were mechanically ventilated. A craniotomy was made above the LGN, and the dura was reflected. All wound margins were infused with lidocaine. A small metal ring was glued to the sclera of each eye to minimize eye movement and to secure the eye for intraocular injections of APB. The pupils were dilated with 1% atropine sulfate and the nictitating membranes were retracted with 10% phenylephrine. The eyes were fitted with contact lenses and focused on a screen located 76 cm in front the animal. Once surgical procedures were complete, anesthesia was maintained with thiopental sodium (2–3 mg/kg/hr, IV). Animals were then paralyzed with vecuronium bromide (0.2 mg/kg/hr, IV). Proper depth of anesthesia was ensured throughout the experiment by continuously monitoring the electroencephalogram, the electrocardiogram, and expired CO2. Animals were euthanized at the end of the experiment with an overdose of Euthasol (Virbac Corporation, Ft. Worth, TX).
Electrophysiological recordings
Single-unit recordings were made from LGN neurons in layers A and A1, in vivo, using a 7-channel multielectrode array (Thomas Recording Systems, Marburg, Germany). Neuronal responses were amplified and recorded to a PC equipped with a Power 1401 data acquisition interface and the Spike 2 software package (Cambridge Electronic Design, Cambridge, England). Spike isolation was based on waveform analysis and the presence of a refractory period, as indicated by the autocorrelogram.
Single-unit recordings were made from RGCs, in vitro, using a 60-channel multielectrode array (MultiChannel Systems, Reutlingen, Germany). Individual electrodes were 30μm in diameter and arranged on an 8×8 rectilinear grid with 200μm interelectrode spacing. Tissue preparation and recording procedures were similar those previously described (Sun et al., 2008). Briefly, the retinas were isolated and stored in buffered and oxygenated Minimum Essential Medium Eagle (MEME, M7278; Sigma-Aldrich) at room temperature. The retinas were cut into 5–8 mm2 rectangles, placed ganglion cell layer down on the multielecrode array, held in place with a piece of dialysis membrane, and superfused with buffered MEME (2 ml/min) at 37°C.
Electroretinograms (ERGs) were recorded using custom-made electrodes. The ERG signal was amplified and low-pass filtered at 100 Hz. 100–200 trials were averaged to yield the final ERG waveforms.
Visual stimuli
Visual stimuli were produced with a VSG2/5 or a ViSaGe visual stimulus generator for the in vivo and in vitro experiments, respectively (Cambridge Research Systems, Rochester, England). Stimuli were presented on a gamma-calibrated Sony Monitor (Sony Corporation, Tokyo, Japan) with a mean luminance of 38 candelas/m2 and a refresh rate of 140 Hz. Two types of visual stimuli were used to characterize visual responses, (1) a full-field, spatially uniform stimulus that stepped between two luminance levels (<1 and 76 cd/m2, or 38 and 76 cd/m2) and (2) a pseudorandom binary white-noise stimulus. The white-noise stimulus consisted of a 16 × 16 grid of squares (pixels) that were white or black one half of the time, as determined by an m-sequence of length 215−1.
APB injections and application
Intraocular injections of DL-2-amino-4-phosphonobutyric acid (APB; 0.14 mg in 20 μl saline; Sigma-Aldrich) were made through the sclera into the posterior chamber of the eye using a Hamilton syringe (Hamilton Company, Reno, NV) to achieve an estimated intraocular concentration of 300 μM (Horton and Sherk, 1984). The Hamilton syringe was inserted through a metal ring that secured the sclera to the stereotaxic frame and injections were guided using an ophthalmoscope. In some experiments, excised patches of retina were used for in vitro recordings. For these recordings, retinal tissue was perfused with 300 μM APB.
Data analysis
Spatiotemporal receptive field maps (kernels) were calculated from responses to the white-noise stimulus using reverse-correlation analysis. For each delay between stimulus and response and for each of the 16 × 16 pixels, we calculated the average stimulus that preceded a spike. For each of the pixels, the kernel can also be thought of as the average firing rate of the neuron, above or below the mean (the impulse response). When normalized by the product of the bin width and the total duration of the stimulus, the result is expressed in units of spikes/sec. Impulse responses were calculated from responses to pixels overlapping the receptive field center and were interpolated with a cubic spline (Matlab function spline; MathWorks, Natick, MA) to determine subregion strength and latency to peak response. Receptive field sizes were assessed from Gaussian fits of the receptive field centers and are reported as the size of the space constant, which is equal to the σ value.
Acknowledgments
This work was supported by EY13588, EY16182, and EY12576. Katie Neverkovec, Kelly Henning, and Daniel Sperka provided expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen EA, Freeman RD. Dynamic spatial processing originates in early visual pathways. J Neurosci. 2006;26:11763–11774. doi: 10.1523/JNEUROSCI.3297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DK, Hall ZW. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975;244:659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Wei H, Eisenback MA, Chomsung RD, Slusarczyk AS, Dankowsi AB. Synaptic organization of thalamocortical axon collaterals in the perigeniculate nucleus and dorsal lateral geniculate nucleus. J Comp Neurol. 2008;508:264–285. doi: 10.1002/cne.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J, Wässle H, Their P. Pharmacological modulation of on and off ganglion cells in the cat retina. Neuroscience. 1984;12:875–885. doi: 10.1016/0306-4522(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Cai D, DeAngelis GC, Freeman RD. Spatiotemporal receptive field organization in the lateral geniculate nucleus of cats and kittens. J Neurophysiol. 1997;78:1045–1061. doi: 10.1152/jn.1997.78.2.1045. [DOI] [PubMed] [Google Scholar]
- Calford MB, Wang C, Taglianetti V, Waleszczyk WJ, Burke W, Dreher B. Plasticity in adult cat visual cortex (area 17) following circumscribed monocular lesions of all retinal layers. J Physiol. 2000;524(Pt 2):587–602. doi: 10.1111/j.1469-7793.2000.t01-1-00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Wright LL, Metha AB, Taglianetti V. Topographic plasticity in primary visual cortex is mediated by local corticocortical connections. J Neurosci. 2003;23:6434–6442. doi: 10.1523/JNEUROSCI.23-16-06434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Blitz DM, Regehr WG. Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron. 2002;33:779–788. doi: 10.1016/s0896-6273(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971;231:191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cox CL, Reichova I, Sherman SM. Functional synaptic contacts by intranuclear axon collaterals of thalamic relay neurons. J Neurosci. 2003;23:7642–7646. doi: 10.1523/JNEUROSCI.23-20-07642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawis S, Shapley R, Kaplan E, Tranchina D. The receptive field organization of X-cells in the cat: spatiotemporal coupling and asymmetry. Vision Res. 1984;24:549–564. doi: 10.1016/0042-6989(84)90109-3. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–589. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Dubin MW, Cleland BG. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977;40:410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG, Schweitzer-Tong DE, Watson AB. Spatio-temporal interactions in cat retinal ganglion cells showing linear spatial summation. J Physiol (Lond) 1983;341:279–307. doi: 10.1113/jphysiol.1983.sp014806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra M, Kwon YH, Sur M. Retinogeniculate EPSPs recorded intracellularly in the ferret lateral geniculate nucleus in vitro: role of NMDA receptors. Vis Neurosci. 1992;8:545–555. doi: 10.1017/s0952523800005642. [DOI] [PubMed] [Google Scholar]
- Fox K, Wallace H, Glazewski S. Is there a thalamic component to experience-dependent cortical plasticity? Philos. Trans R Soc Lond B Biol Sci. 2002;357:1709–1715. doi: 10.1098/rstb.2002.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W, Piech V. Perceptual learning and adult cortical plasticity. J Physiol. 2009;587:2743–2751. doi: 10.1113/jphysiol.2009.171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Sherman SM. Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol. 1987;259:165–192. doi: 10.1002/cne.902590202. [DOI] [PubMed] [Google Scholar]
- Hirsch JA. Synaptic physiology and receptive field structure in the early visual pathway of the cat. Cereb Cortex. 2003;13:63–69. doi: 10.1093/cercor/13.1.63. [DOI] [PubMed] [Google Scholar]
- Horton JC, Sherk H. Receptive field properties in the cat’s lateral geniculate nucleus in the absence of on-center retinal input. J Neurosci. 1984;4:374–380. doi: 10.1523/JNEUROSCI.04-02-00374.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AG, Mistler LA. Response properties of cells in rabbit’s lateral geniculate nucleus during reversible blockade of retinal on-center channel. J Neurophysiol. 1983;50:1236–1245. doi: 10.1152/jn.1983.50.5.1236. [DOI] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen C. Different roles for AMPA and NMDA receptors in transmission at the immature retinogeniculate synapse. J Neurophysiol. 2008;99:629–643. doi: 10.1152/jn.01171.2007. [DOI] [PubMed] [Google Scholar]
- Massey SC, Redburn DA, Crawford ML. The effects of 2-amino-4-phosphonobutyric acid (APB) on the ERG and ganglion cell discharge of rabbit retina. Vision Res. 1983;23:1607–1613. doi: 10.1016/0042-6989(83)90174-8. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Nonlagged relay cells and interneurons in the cat lateral geniculate nucleus: receptive-field properties and retinal inputs. Vis Neurosci. 1992;8:407–441. doi: 10.1017/s0952523800004934. [DOI] [PubMed] [Google Scholar]
- Miller RF, Dacheux RF. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. II. Chloride-dependent ganglion cell mechanisms. J Gen Physiol. 1976;67:661–678. doi: 10.1085/jgp.67.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentería RC, Tian N, Cang J, Nakanishi S, Stryker MP, Copenhagen DR. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci. 2006;26:11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH. Central connections of the retinal ON and OFF pathways. Nature. 1982;297:580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Schiller PH. The connections of the retinal On and Off pathways to the lateral geniculate nucleus of the monkey. Vision Research. 1984;24:923–932. doi: 10.1016/0042-6989(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Sherk H, Horton JC. Receptive field properties in the cat’s area 17 in the absence of on-center geniculate input. J Neurosci. 1984;4:381–393. doi: 10.1523/JNEUROSCI.04-02-00381.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Slaughter MM. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara H, Inoue T, Nakanishi S, Fukuda Y. A late ON response remains in visual response of the mGluR6-deficient mouse. Neurosci Lett. 1997;233:137–140. doi: 10.1016/s0304-3940(97)00656-3. [DOI] [PubMed] [Google Scholar]
- Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 2008;105:13638–13643. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Specificity and strength of retinogeniculate connections. J Neurophysiol. 1999;82:3527–3540. doi: 10.1152/jn.1999.82.6.3527. [DOI] [PubMed] [Google Scholar]
- Wang X, Vaingankar V, Sanchez CS, Sommer FT, Hirsch JA. Thalamic interneurons and relay cells use complementary synaptic mechanisms for visual processing. Nat Neurosci. 2011;14:224–231. doi: 10.1038/nn.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Peichl L, Boycott BB. A spatial analysis of on- and off-ganglion cells in the cat retina. Vision Res. 1983;23:1151–1160. doi: 10.1016/0042-6989(83)90029-9. [DOI] [PubMed] [Google Scholar]