Abstract
This is a longitudinal cohort study investigating the association between maternal HIV status and the reported onset of lactation. The Research to Improve Infant Nutrition and Growth project recruited 442 mothers from 3 antenatal clinics in the eastern region of Ghana, based on positive, negative, and unknown HIV status. Onset of lactation was assessed by maternal perception and validated with 2 subsamples: measurement of infant breast milk intake (n = 40) and daily infant weight measurement for 2 weeks (n = 150). Multivariate logistic regression was used to identify predictors of very early onset of lactation (onset of lactation < 6 hours). Predictors of very early onset of lactation include HIV-negative status (odds ratio = 2.68; P = .014), multiparity (odds ratio = 2.93; P = .009), vaginal delivery (odds ratio = 2.55; P = .035), and having a male child (odds ratio = 1.86; P = .032). The findings indicate an association between maternal HIV status and very early onset of lactation.
Keywords: exclusive breastfeeding, onset of lactation, HIV, Ghana
Breastfeeding is a complex process influenced by many factors: physiological, biological, psychological, geographical, economic, social, cultural, and even genetic. No one factor can explain the variations in breastfeeding outcomes. Successful breastfeeding requires the early initiation of breastfeeding with proper positioning and latching of the infant to the breast, effective suckling, and the transfer of adequate amounts of breast milk to the infant as well as skilled lactation consultants to provide advice and support to the mothers,1,2 following a timely onset of lactation (OL). OL has been defined as the sudden onset of milk production that occurs approximately 2 to 3 days postpartum, identified physiologically by dramatic changes in milk sugars, proteins, lipids, and salts.3 OL is said to be delayed if it occurs after 72 hours.4–6 Studies have identified several factors associated with delayed OL. These include (a) stress before, during, and after delivery, usually associated with longer duration of stage II labor and emergency cesarean section4,7–9; (b) nipple problems, including flat or inverted nipples5; (c) primiparity4,5,9– 11; and (d) maternal obesity.4,5,11–13
HIV may increase psychological and biological stress, which may result in delayed OL, negatively affecting exclusive breastfeeding success. A study in Zambia examined factors associated with exclusive breastfeeding (EBF) duration among 177 HIV-positive (HIV+) and 177 HIV-negative (HIV−) mothers.14 The authors found that 19.8% of the women reported OL on day 1, 89.8% by day 2, and only 0.6% at greater than 72 hours. This study did not find any association between the timing of OL and EBF duration. The study also reported no significant differences in EBF duration between HIV+ and HIV− mothers.
Other researchers have reported that delayed OL is associated with an increased risk of ending full breast-feeding,6,15,16 defined as either the infant receiving only breast milk with the exception of vitamins and mineral (EBF) or receiving breast milk as the predominant source of nourishment. Higher infant weight loss in the first few days postpartum is associated with lower incidence of EBF, and this increased infant weight loss has been associated with delayed OL.5 Delayed OL can have important implications on maternal infant-feeding choices, including EBF.17 As such, the timing of OL is an important predictor of breastfeeding success.16,18 In HIV-affected communities in sub-Saharan Africa, EBF has been found to have the advantage of reducing the risk of mother-to-child transmission (MTCT) of HIV over mixed feeding and increasing the likelihood of infant HIV-free survival over formula feeding in some settings.19–21 This makes EBF for first 6 months the practical infant-feeding option in these communities.22 Thus, it is essential to investigate the link between maternal HIV status and OL.
OL assessment methods include infant test weighing, which is considered the gold standard for estimating the amount of breast milk ingested by the infant23–25; measurements of changes in biochemical indicators such as casein, lactose, glucose, citrate, and phosphate26,27; and maternal perception of OL, which has been validated in a predominantly Caucasian population.28 Of these 3 methods, maternal perception of OL is easily assessed and is appropriate for use in field research studies, especially in developing countries, where infant test weighing and assessment biochemical markers are difficult and expensive.6,28 In cultures where this method has not been used, its validation is essential. The current research was conducted to validate the reported maternal perception of OL and to determine the influence of HIV on OL, breast health during the first month postpartum, and EBF during the first 3 months postpartum among Ghanaian women in the eastern region.
Methods
Subjects
Participants in this study were part of the longitudinal Research to Improve Infant Nutrition and Growth (RIING) project aimed at improving child health and nutrition in HIV-affected communities. Study selection criteria were age at least 18 years, pregnant and seeking antenatal care, and residing in the Manya and Yilo Krobo districts of the eastern region. Eligible participants were recruited from the antenatal clinics of 3 Baby-Friendly hospitals in the Manya Krobo district, where pregnant women had been offered voluntary counseling and testing (VCT) for HIV. The HIV status of the participants in this study was determined prenatally by the hospitals as part of their prevention of mother-to-child transmission (PMTCT) services sponsored by the Government of Ghana. The VCT starts with pretest counseling, where the mothers are told of the services available, followed by HIV testing using rapid diagnostic tests, the result of which is available on the day of testing. During posttest counseling, HIV+ women are informed about the available infant-feeding options and, if they opt to breastfeed, about the importance of EBF for the first 6 months. The antiretroviral nevirapine is available free of charge to all HIV+ mothers who deliver at the hospitals. The women receive a single oral dose of nevirapine at the onset of labor and the infant receives nevirapine syrup within 72 hours of life to reduce the risk of mother-to-child transmission. Pregnant women who test positive are also expected to undergo CD4+ cell count testing, and those with cell counts less than 350 are given access to antiretroviral therapy.
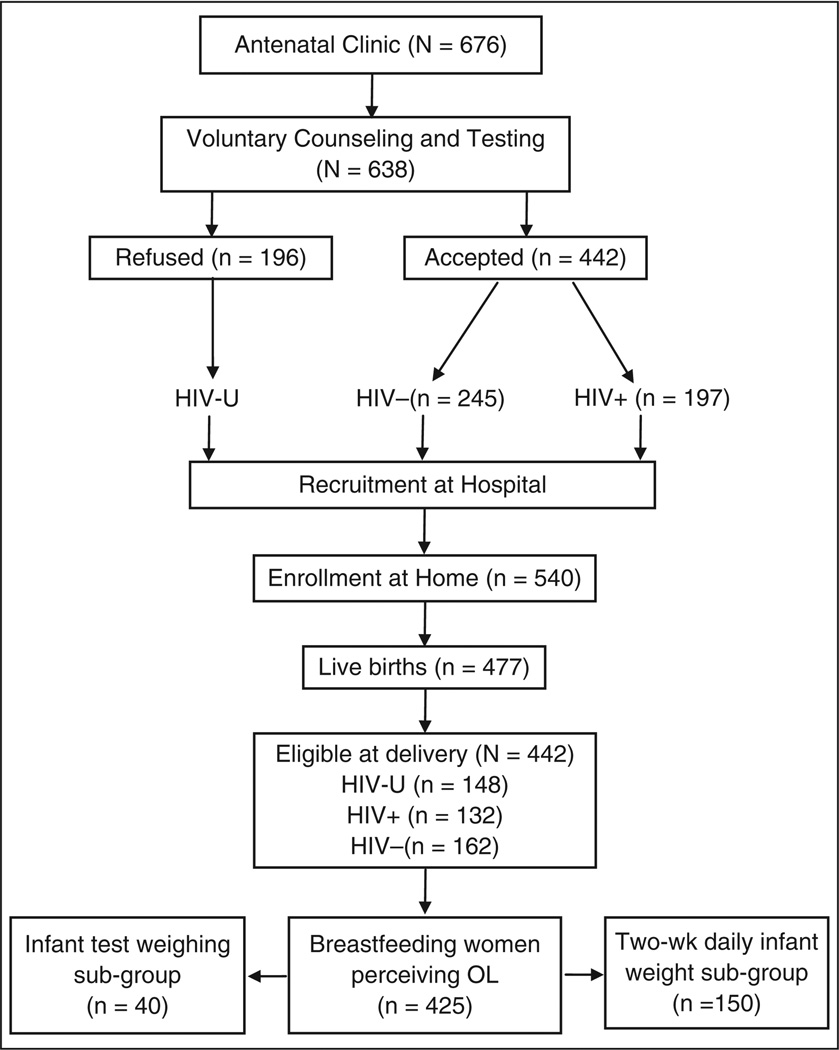
For the women who accepted VCT, another criterion for inclusion into the study was consenting to the disclosure of the HIV test result to the study supervisor only. Interested pregnant women completed a short enrollment and contact information form at the hospital. This was followed with home visits where the women were formally enrolled into the study after providing informed consent. Follow-up began immediately after a woman gave birth. Enrollment for this OL study began in January 2004 and by the end of June 2007, 676 pregnant women had been approached by the VCT nurses and showed interest in participating in the study. Of these, 638 met with the RIING supervisor at the hospital, filled out enrollment forms, and agreed to be contacted at home, and 540 of them completed the home visit and signed the informed consent form. Of these, 477 had live births and 425 had complete OL data (Figure 1). There were 10 sets of twins in the sample: 3 (2.5%) in the HIV+ group, 5 (3.1%) in the HIV− group, and 2 (1.4%) in the HIV-unknown group. Four HIV+ women chose not to breastfeed and were excluded from the study. Sample size calculations were made using estimates of effect size from preliminary data from the RIING project, based on an average OL of 33.3 ± 24.9 hours. A sample size of 134 per group was estimated based on a statistical power of 80% to detect a between-group OL difference of 9 hours. The calculations were based on a 2-sided test, with type I error (α) = .05.
Figure 1.
Flow diagram of study participation. HIV-U, women of unknown HIV status; HIV−, HIV-negative women; HIV+, HIV-positive women; OL, onset of lactation.
Ethical approval for the study protocols was provided by the Institutional Review Boards of Iowa State University, the University of Connecticut, the University of Ghana, and McGill University.
Study Variables
Structured questionnaires were used to collect data on participants’ socioeconomic and demographic characteristics such as maternal educational level, employment, completed age, parity, ethnicity, and marital status as well as whether the household had a telephone, TV, radio, bike, car, and refrigerator. Housing characteristics included home ownership and access to basic utilities such as water, electricity, and waste disposal. Psychosocial variables included maternal stress, postnatal depression, and measures of social capital such as social support, belonging to social groups, and changes in the number of friends and quality of friendships over the past year. Maternal stress was assessed by the 4-item perceived stress scale29 at enrollment. The Edinburg Postnatal Depression Scale was used to assess postnatal depression using a cutoff score of 12 or greater for depression, assessed at delivery.30 Maternal breast health, specifically clinical mastitis and nipple problems, was assessed twice weekly using a self-reported structured questionnaire. Nipple problems included sore, cracked, and bleeding nipples, and mothers who reported redness or hard, hot, and painful areas on the breast were classified as having mastitis.31 At these times, infant morbidity data, which included diarrhea and fever incidences, were also collected. Maternal and infant weights were measured using electronic digital scales (Tanita BWB-800s, Tanita Corporation of America, Arlington Heights, Illinois) accurate to ± 100 g, using the WHO Multicentre Growth Reference Study methodology.32 Maternal height was assessed at 6 months with a height/length measuring board (Shorr Productions, Olney, Maryland) to the nearest 0.1 cm. Infant-feeding practices were assessed twice weekly. Mothers were asked whether their infants were receiving only breast milk or had been given any of the following: nonmilk liquids, infant formula, other nonhuman milks, and solid or semi-solid foods. Infants who had been given only breast milk at 1, 2, and 3 months were identified as being exclusively breastfed.
For all mothers on the RIING project, OL data were collected by means of a structured questionnaire adapted from Chapman and Pérez-Escamilla.16 The women were asked, “Has your milk started to flow?” Which was better understood than “Has your milk come in?” This had been previously validated in Ghana33,34 and confirmed by our qualitative research. The questionnaire was administered to each new mother daily until she reported that the milk had started to flow.
To validate the maternal report of OL, we first investigated whether this report was consistent with infant growth and weight changes. For this substudy, a total of 150 RIING participants who gave birth between October 2005 and November 2006 were included. During this time, eligible mother–infant dyads were included until a sample of 50 babies born to HIV+ women, 50 to HIV− women, and 50 to women of unknown HIV status (HIV-U) had been measured. The infants were weighed daily from birth until 2 weeks of age with the digital scales. For each infant an average of 2 consecutive weights not exceeding 0.1 kg in difference were taken.
We used a convenience sample of 40 RIING participants who had uncomplicated singletons births, during the period December 2006 to April 2007, for infant test weighing. These participants were not part of the substudy of 150 participants. Most of the newborns were breastfed during their first hours. The attending nurses provided the mothers with instruction on lactation and recommended breastfeeding on demand. The test weighing was done within 24 hours of birth. All before and after breastfeeding measurements were taken in triplicate, using electronic precision scales fitted with infant seats (EA/B 15 DC E-1, Sartorius Inc., Goettingen, Germany) to the nearest 0.5 g. During these sessions, the babies were dressed in cotton cloth diapers covered with rubber pants to prevent losses from urine and feces. Insensible water losses (mean of 5.0 g/kg·h−1) were assessed by weighing the infant before and after a 10-minute period of not breastfeeding. Following each test weighing measurement, we collected data on OL. To prevent bias, the women were not told the test weighing results. All weighing scales were calibrated weekly using standard weights.
Statistical Analyses
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 15.0. All results were interpreted using P < .05 (2-sided) as the criterion for statistical significance.
For the 150 mother–infant dyads involved in the 2 weeks of daily infant measurements, birth weights obtained from the hospital records and those measured by the research assistants in the early postnatal period (within 24 hours of birth) were highly correlated (r = 0.883, P < .001). The Bland-Altman plot for examining the extent of agreement between the birth weights measured at the hospital and by one of our field workers showed that 95% confidence interval of agreement, based on the differences in birth weight measured between the 2 groups, was −0.28 kg to +0.27 kg. These findings indicated a very good agreement between the measurements from the 2 sources. Therefore, hospital birth weights for mothers delivering at hospitals were used for the analyses. The birth weights were missing for mothers who delivered vaginally at home (12.7%); therefore, the mean birth weights for infants delivered vaginally by HIV status were used to estimate these missing data. The group-specific means differences for the specified day were then used to impute all other missing data (19.5%). Multiple analyses of variance applied at different time points were used to compare mean weight differences and weight change differences by HIV status, adjusted for differences in birth weight. Least significant difference post hoc tests were run where appropriate to identify significant between-group differences.
The 40 mothers involved in the test weighing validation substudy were categorized based on whether they reported OL at the first postpartum visit. Baseline between-group characteristics were compared using Student’s t test. Chi-square analyses were used to assess group differences for categorical variables.
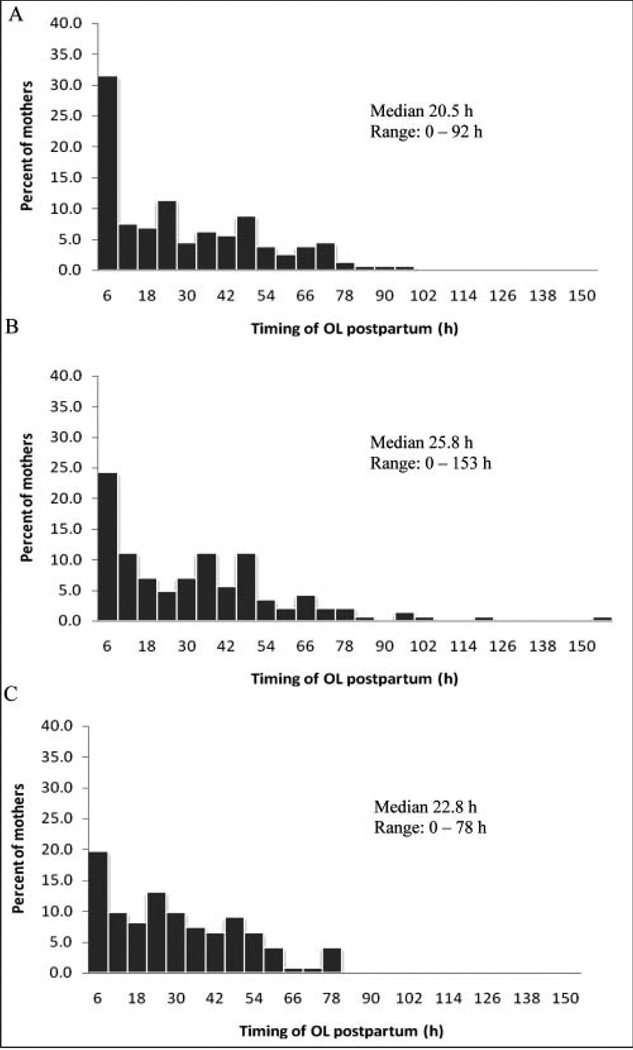
The association between OL, EBF, and HIV was investigated using 425 participants. The key dependent variable investigated was very early onset of lactation (VEOL) based on maternal perception (OL < 6 hours and OL ≥ 6 hours). Initially, we considered using the previously reported cutoff for delayed OL (ie, > 72 hours postpartum).4 However, an early inspection of the data showed that only 4.5% had OL greater than 72 hours. The frequency distributions of the timing of OL by HIV status clearly showed a shift to the left with nearly twice as many HIV− women reporting OL within the first 6 hours compared with HIV+ women (Figure 2). Six hours also corresponds to the 25th percentile for the timing of OL for the entire sample. EBF was defined as the infant receiving only breast milk over the previous month or since birth as measured at 1, 2, and 3 months. Clinical mastitis and nipple problems were reported at 1 month. The key independent variable was maternal HIV status, a categorical variable with 3 levels: HIV+, HIV−, and HIV-U. Other independent variables included key sociodemographic and anthropometric variables such maternal weight, height, and body mass index (BMI) and infant birth weight; stress, scored by summing across all 4 items after reversing the codes on 2 positively stated items; and postnatal depression, scored by summing across all items. Baseline between-group characteristics were compared using ANOVA for continuous variables and chi-square analyses for the categorical variables.
Figure 2.
Distributions of the maternal reported onset of lactation (OL) for (A) HIV-negative women (n = 159), (B) women with unknown HIV status women (n = 144), and (C) HIV-positive women (n = 122) showing the median and range.
A multivariate logistic regression model was used to examine the independent association of maternal HIV status with VEOL after adjustment for potential confounders. Covariates included in the final model were selected based on theoretical considerations and available empirical evidence. Besides the key independent variable (ie, maternal HIV status), covariates included were maternal age, education, ethnicity, occupation, marital status, parity, sex of index child, types of toilet facilities, house ownership, primary water source, type of delivery, postnatal depression and stress, and a composite variable that represented a combination of the values of 6 socioeconomic status indicators (television, telephone, radio, bike, car, and refrigerator).
Results
Validation of the Onset of Lactation
Infant weight changes across time
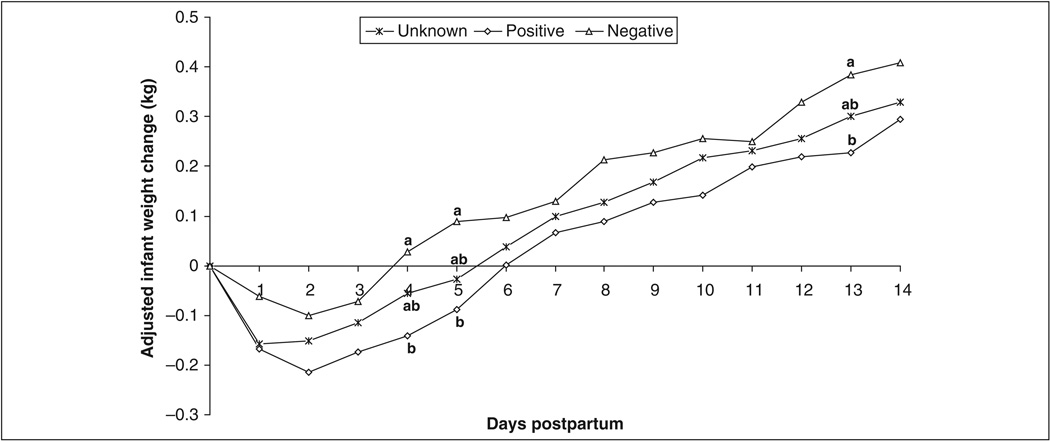
For the subsample of 150 HIV+, HIV−, and HIV-U women and their infants involved in the 2 weeks of daily infant weighing, significant group differences were found only for maternal education and weight (Table 1). HIV− mothers had significantly more years of formal education than their HIV+ counterparts and weighed significantly more than either HIV+ or HIV-U women. Difference between the groups with respect to postnatal depression and stress at birth and enrollment did not reach statistical significance. The average birth weight of all 150 infants was 3.12 ± 0.46 kg. Although infants of HIV-U women appeared to have lower birth weights, the difference did not reach statistical significance. The mean infant weight changes from 0 to 14 days, after adjustment for birth weight, is presented in Figure 3. Children of HIV+ women lost 5.4% of their birth weight on the first day, losing the greatest weight (7% total weight loss) by day 2. The children started to gain weight on the third day, regaining the birth weight after 5 days. A similar trend was observed for children born to HIV− women. However, these children lost less than 2% of the birth weight on the first day, increasing to 3.2% total weight loss on the second day and starting to gain weight by the third day. Children born to HIV-U women lost 5.0% of their birth weight on day 1 and began to gain weight by the second day, regaining the birth weight after 5 days. The differences in infant weight changes between children of HIV− and HIV+ women reached statistical significance at days 4, 5, and 13.
Table 1.
Demographic Characteristics of Subsample of 150 Mothers and Infantsa
| HIV-U (n = 50) |
HIV+ (n = 50) |
HIV− (n = 50) |
P Valueb |
|
|---|---|---|---|---|
| Marital status, n (%) | ||||
| Married | 15 (30.0) | 9 (18.0) | 20 (40.0) | .168 |
| Cohabitant | 25 (50.0) | 31 (62.0) | 24 (48.0) | |
| No partnerc | 10 (20.0) | 10 (20.0) | 6 (12.0) | |
| Primary maternal occupation, n (%) | ||||
| Trader | 26 (52.0) | 28 (56.0) | 19 (38.0) | .250 |
| Otherd | 21 (42.0) | 17 (34.0) | 28 (56.0) | |
| Unemployed | 3 (6.0) | 5 (10.0) | 3 (6.0) | |
| Ethnicity, n (%) | ||||
| Ga/Adangbe | 35 (70.0) | 39(78.0) | 28 (56.0) | .133 |
| Ewe | 8 (16.0) | 6 (12.0) | 16 (32.0) | |
| Akan | 5 (10.0) | 2 (4.0) | 3 (6.0) | |
| Northerner | 1 (2.0) | 0 (0.0) | 2 (4.0) | |
| Other | 1 (2.0) | 3 (6.0) | 1 (2.0) | |
| Primiparity, n (%) | ||||
| Yes | 12 (24.0) | 11 (22.0) | 14 (28.0) | .778 |
| No | 38 (76.0) | 39 (78.0) | 36 (72.0) | |
| Type of delivery, n (%) | ||||
| Cesarean | 6 (12.0) | 9 (18.0) | 9 (18.0) | .640 |
| Vaginal | 44 (88.0) | 41 (82.0) | 41 (82.0) | |
| Sex of child, n (%) | ||||
| Male | 27 (54.0) | 24 (48.0) | 30 (60.0) | .485 |
| Female | 23 (46.0) | 26 (52.0) | 20 (40.0) | |
| Maternal age, y, x̅ ± SD | 28.0 ± 6.2 | 28.0 ± 5.3 | 29.4 ± 6.4 | .398 |
| Education, y, x̅ ± SDe | 7.3 ± 3.31,2 | 6.3 ± 3.71 | 8.2 ± 3.12 | .022 |
| Parity, x̅ ± SD | 2.7 ± 1.7 | 2.5 ± 1.2 | 2.8 ± 1.8 | .771 |
| Maternal BMIf, kg/m2, x̅ ± SD | 24.5 ± 4.2 | 24.2 ± 3.4 | 25.7 ± 4.1 | .209 |
| Maternal weight, kg, x̅ ± SDe | 61.5 ± 11.41 | 58.6 ± 9.01 | 67.0 ± 12.62 | .001 |
| Infant birth weight, kg, x̅ ± SD | 3.03 ± 0.41 | 3.21 ± 0.46 | 3.20 ± 0.53 | .139 |
HIV-U, HIV status unknown; HIV+, HIV positive; HIV−, HIV negative; SD, standard deviation; BMI, body mass index.
Except where indicated chi-square analyses were used to determine differences between HIV groups.
Includes participants who identified themselves as single (15.3%), separated (1.3%), and divorced (0.7%).
Included seamstress, caterer, private secretary, hairdresser, teacher, baker, farmer, cleaner, army officer, glass cutter, pottery maker, and student.
Analysis of variance was used to determine group differences, with least significant difference post hoc analyses to determine between-group differences. Same superscripts indicate no significant between-group differences.
Maternal BMI was available for 116 participants; height was assessed at 6 months and weight at 1 month.
Figure 3.
Trends in mean infant weight changes by maternal HIV status adjusted for birth weight. Analysis of variance was used to determine group differences, with least significant difference post hoc analyses to determine between-group differences. Same superscripts indicate no significant between-group differences.
Infant test weighing
The subsample of women involved in the infant test weighing study was also drawn from the HIV+ (n = 11), HIV− (n = 17), and HIV-U (n = 12) groups. There were no significant differences among mothers who reported OL versus those who did not at the first postpartum visit (approximately 16.6 hours), except that mothers who reported OL were significantly older (P = .049). Although not significantly different between the 2 groups, the timing of the initiation of breastfeeding reported by the mothers was higher than recommended: 4.7 hours for mothers not reporting OL and 2.8 hours for mother who reported OL at the first visit. The average timing of the perception of the onset of milk flow reported by the mothers was 19.7 hours (Table 2). Infants of mothers who reported that their milk was flowing at the time of the first test weighing (which occurred on average at 16.6 ± 12.1 hours) had significantly higher breast milk intakes per breastfeeding episode, 15.5 ± 14.3 g vs. 6.2 ± 2.6 g (P = .006). Differences in the average initiation of breastfeeding, the duration of breastfeeding, and the timing of the first postpartum visit did not reach statistical significance among the groups. At the time when the women reported that their milk had come in, 87.5% of them reported a full or heavy feeling in the breast. More than half of the women reported other symptoms associated with OL, such as milk leaking from the breast (60.0%) and tingling when the baby was nursing (57.5%). Significantly more women who reported experiencing OL also reported a full or heavy feeling in the breast compared with those who did not, 85.7% versus 52.6%, respectively (P = .023). Milk leaking was reported only by mothers who had experienced OL at the first visit.
Table 2.
Comparison of Breast Milk Intakes by Onset of Lactation Status at First Postpartum Visit
| Mothers Reporting OL at First Postpartum Visit | ||||
|---|---|---|---|---|
| Total (N = 40) |
Yes (n = 21) |
No (n = 19) |
P Value |
|
| Initiation of breastfeeding, h | 3.7 ± 4.3 | 2.8 ± 3.4 | 4.7 ± 4.9 | .165 |
| Timing of first visit, h | 16.6 ± 12.1 | 17.1 ± 12.4 | 16.0 ± 12.1 | .789 |
| Breastfeeding duration per episode, min | 10.2 ± 4.1 | 11.1 ± 3.4 | 9.2 ± 4.4 | .144 |
| Breast milk intake per episode, g | 11.1 ± 11.1 | 15.5 ± 14.3 | 6.2 ± 2.6 | .006 |
| Timing of OL, h | 19.7 ± 18.6 | 8.1 ± 13.2 | 32.4 ± 15.1 | <.001 |
OL, onset of lactation. Values are x̅ ± SD.
Onset of Lactation and Maternal HIV Status
The final sample consisted of 122 HIV+, 159 HIV−, and 144 HIV-U mothers and their infants (Table 3). Significant group differences were found in socioeconomic, demographic, and anthropometric characteristics. Marital status and ethnicity were statistically different among the groups. HIV+ women had significantly fewer years of education than HIV− women (P = .001). HIV+ women had lower weight at 1 month (P < .001) and were more likely to have lower BMI (P = .005) than both HIV− and HIV-U women. Although infants born to HIV+ women had lower birth weight than those born to either HIV− and HIV-U women; the difference was not significantly different. In all, 7.8% of the infants had birth weights 2.5 kg or less: HIV− (4.2%), HIV+ (13.0%), HIV-U (7.0%) (P = .05). Access to tap water sources, flush toilet facilities in the home, and ownership of TV, radio, telephone, bike, and refrigerator were significantly different among the groups, with HIV+ women being less likely to have these belongings or access to basic utilities, indicative of lower socioeconomic status (Table 4). There were no significant between-group differences with regard to social capital. However, HIV+ and HIV-U women tended to be more likely not to belong to any social group. About 8% of the women showed signs of postnatal depression, with HIV− mothers being significantly less likely to show signs of this condition (P = .02). However, no significant group differences were observed for maternal stress.
Table 3.
Maternal and Infant Characteristics by Maternal HIV Statusa
| HIV-U (n = 144) |
HIV+ (n = 122) |
HIV− (n = 159) |
P Value |
|
|---|---|---|---|---|
| Marital status, n (%) | ||||
| Married | 32.6 | 16.4 | 45.9 | <.001 |
| Cohabitant | 50.7 | 58.2 | 41.5 | |
| No partnerb | 16.7 | 25.4 | 12.6 | |
| Occupation, n (%) | ||||
| Trader | 53.5 | 50.0 | 49.1 | .699 |
| Otherc | 41.7 | 41.0 | 42.8 | |
| Unemployed | 4.9 | 9.0 | 8.2 | |
| Ethnicity, n (%) | ||||
| Ga/Adangbe | 61.8 | 84.4 | 64.8 | .003 |
| Ewe | 25.7 | 9.8 | 22.0 | |
| Akan | 8.3 | 2.5 | 9.4 | |
| Northerner | 2.8 | 0.8 | 3.1 | |
| Other | 1.4 | 2.5 | 0.6 | |
| Primiparity, n (%) | 31.3 | 28.7 | 25.2 | .496 |
| Cesarean delivery, n (%) | 14.9 | 16.0 | 17.8 | .805 |
| Male child, n (%) | 49.3 | 52.1 | 52.2 | .858 |
| Maternal age, y, x̅ ± SD | 28.3 ± 5.9 | 27.7 ± 5.5 | 29.3 ± 6.0 | .064 |
| Education, y, x̅ ± SDd | 7.7 ± 3.41,2 | 6.6 ± 3.82 | 8.4 ± 3.51 | .001 |
| Parity, x̅ ± SD | 2.4 ± 1.5 | 2.4 ± 1.4 | 2.6 ± 1.5 | .328 |
| Maternal weight, kg, x̅ ± SDd,e | 63.6 ± 11.01 | 58.0 ± 10.42 | 64.6 ± 12.11 | <.001 |
| Maternal height, m, x̅ ± SDe | 1.59 ± 0.05 | 1.58 ± 0.05 | 1.59 ± 0.07 | .860 |
| Maternal BMI, kg/m2, x̅ ± SDd,f | 25.0 ± 4.31 | 23.3 ± 3.62 | 25.6 ± 4.71 | .005 |
| Infant birth weight, kg, x̅ ± SD | 3.13 ± 0.47 | 3.02 ± 0.47 | 3.13 ± 0.46 | .196 |
HIV-U, HIV status unknown; HIV+, HIV positive; HIV−, HIV negative; SD, standard deviation; BMI, body mass index. Results presented on 425 women for whom onset-of-lactation data were available.
Participants identified themselves as single, separated, and divorced.
Included seamstress, caterer, private secretary, hairdresser, teacher, baker, farmer, cleaner, army officer, glass maker, pottery maker, candy maker, student, and others.
Analysis of variance was run to determine group differences, with least significant difference post hoc analyses to determine between-group differences. Same superscripts indicate no significant between-group differences.
Maternal weight and height were available for 334 and 247 participants, respectively.
Maternal BMI was available for 240 participants.
Table 4.
Socioeconomic Characteristics by Maternal HIV Statusa
| HIV-U (n = 140) |
HIV+ (n = 114) |
HIV− (n = 155) |
P Value |
|
|---|---|---|---|---|
| House ownership | ||||
| Self | 20.0 | 19.3 | 20.0 | .276 |
| Extended family | 36.4 | 43.0 | 30.3 | |
| Nonfamily | 43.6 | 37.7 | 49.7 | |
| Primary water source | ||||
| Tap in house | 35.7 | 14.9 | 31.6 | <.001 |
| Public tap | 62.9 | 71.9 | 64.5 | |
| Otherb | 1.4 | 13.2 | 3.9 | |
| Toilet facilities | ||||
| Flush | 19.3 | 7.0 | 19.4 | .003 |
| KVIP | 57.1 | 57.9 | 44.5 | |
| Pit | 17.1 | 30.7 | 25.2 | |
| Otherc | 6.4 | 4.3 | 11.0 | |
| Have telephone | 52.5 | 28.1 | 50.3 | <.001 |
| Have electricity | 82.9 | 76.3 | 85.2 | .165 |
| Have TV | 59.3 | 38.6 | 63.9 | <.001 |
| Have radio | 80.0 | 65.8 | 81.9 | .004 |
| Have bike | 27.1 | 13.2 | 20.6 | .024 |
| Have car | 10.7 | 2.6 | 11.0 | .029 |
| Have refrigerator | 35.7 | 22.8 | 45.2 | .001 |
HIV-U, HIV status unknown; HIV+, HIV positive; HIV−, HIV negative; KVIP, Kumasi Ventilated Improved Pit latrine. Result presented on 425 women for whom onset-of-lactation data were available. Values are percentages.
Included the following: spring, river or stream, public well, borehole, well, bottled water, and dugout.
Included bucket and bush.
The distribution of the timing of OL for the 425 mothers showed approximately 52% of them experiencing OL within the first 24 hours postpartum. By the second day, the majority of the women had experienced OL (81.9%). In our sample, only 4.5% experienced OL after 72 hours. The 3 breast symptoms frequently reported to confirm OL were full/heavy feeling in the breast (85.7%), milk leaking (61.3%), and tingling (50.2%) when nursing. The median OL was 22.8, 20.5, and 25.8 hours for HIV+, HIV−, and HIV-U women, respectively (Figure 2). The distribution of the timing of OL shows a clear shift to the left for HIV− women and to a lesser extent for HIV-U women.
Factors Associated With Very Early Onset of Lactation
HIV− women were 2.7 times more likely to report VEOL (OL < 6 hours) compared with their HIV+ counterparts (P = .014). Likewise, HIV-U women were 2.2 times more likely than their HIV+ counterparts to report VEOL. Other significant predictors of VEOL included multiparity, vaginal delivery, and having a male child. Although single mothers in our sample were 2.4 times more likely to report VEOL than their married counterparts, this was not statistically significant. Primary water source, primary maternal occupation, ethnicity, house ownership, household possessions, postnatal stress and depression, and maternal age were not significant predictors for VEOL (Table 5).
Table 5.
Predictors of Very Early Onset of Lactationa
| Factors | n | % VEOL |
OR | 95% CI |
P Value |
|---|---|---|---|---|---|
| HIV status | |||||
| Unknown | 112 | 23.6 | 2.21 | 1.01–4.85 | .047 |
| Negative | 119 | 19.7 | 2.68 | 1.22–5.89 | .014 |
| Positive | 88 | 31.4 | 1.00 | Ref | |
| Parity | |||||
| Multiparity | 238 | 26.2 | 2.93 | 1.31–6.57 | .009 |
| Primiparity | 81 | 23.3 | 1.00 | Ref | |
| Type of delivery | |||||
| Vaginal | 264 | 15.9 | 2.55 | 1.07–6.08 | .035 |
| Cesarean | 55 | 28.5 | 1.00 | Ref | |
| Sex of index child | |||||
| Male | 164 | 28.1 | 1.86 | 1.06–3.29 | .032 |
| Female | 155 | 22.7 | 1.00 | Ref | |
| Marital status | |||||
| Cohabitant | 155 | 23.8 | 1.41 | 0.71–2.79 | .327 |
| No partner | 50 | 26.7 | 2.36 | 0.97–5.76 | .058 |
| Married | 114 | 27.1 | 1.00 | Ref | |
| Primary water source | |||||
| Public tap | 207 | 25.6 | 0.79 | 0.39–1.61 | .511 |
| Other | 20 | 30.4 | 2.70 | 0.73–9.99 | .138 |
| Tap in house | 92 | 25.9 | 1.00 | Ref | |
| Primary maternal occupation | |||||
| Other | 131 | 29.8 | 1.58 | 0.85–2.94 | .148 |
| Unemployed | 27 | 32.3 | 1.84 | 0.64–5.28 | .259 |
| Trader | 161 | 20.8 | 1.00 | Ref | |
| Ethnicity | |||||
| Other | .104 | 27.8 | 0.62 | 0.33–1.17 | .141 |
| Ga/Adangbe | 215 | 20.0 | 1.00 | Ref | |
| House ownership | |||||
| Extended family | 109 | 25.9 | 0.93 | 0.43–2.01 | .853 |
| Nonfamily | 144 | 26.0 | 1.07 | 0.51–2.26 | .863 |
| Self | 66 | 25.9 | 1.00 | Ref | |
| SES items | |||||
| One or 2 | 134 | 24.5 | 0.84 | 0.34–2.07 | .697 |
| More than 2 | 144 | 27.5 | 1.05 | 0.41–2.69 | .925 |
| None | 41 | 25.0 | 1.00 | Ref | |
| Postnatal depression present | |||||
| No | 296 | 25.1 | 2.74 | 0.58–13.03 | .205 |
| Yes | 23 | 10.3 | 1.00 | Ref | |
| Maternal stress | |||||
| Below median | 195 | 27.0 | 1.16 | 0.65–2.08 | .615 |
| Above median | 124 | 24.2 | 1.00 | Ref | |
| Maternal age, y | 319 | — | 0.97 | 0.91–1.03 | .295 |
| Education, y | 319 | — | 1.09 | 0.99–1.19 | .078 |
VEOL, very early onset of lactation; OR, odds ratio; CI, confidence interval; Ref, reference category; OL, onset of lactation; SES, socioeconomic status. Result presented on 425 women for whom onset-of-lactation data were available. Hosmer-Lemeshow goodness of fit parameters: χ28 = 6.31, P = .613.
HIV and Breast Health
Clinical mastitis was reported by 8 participants during the first 30 days postpartum, with 75% of these participants being HIV+. In all, 7.3% of the women reported nipple problems during the first month. Nipple problems were lowest among HIV+ women (4.3%) compared with 7.3% and 9.3% for HIV-U and HIV− women, respectively. Between-group differences in nipple problems were not statistically significant.
Exclusive Breastfeeding Rates and the Onset of Lactation
For the 358 women with breastfeeding data collected through 3 months, EBF rates were 94.1%, 92.5%, and 90.9% at 1, 2, and 3 months, respectively, using the previous-month definition. The corresponding since-birth EBF prevalence rates were 94.1%, 88.4%, and 82.3%, respectively. Chi-square analysis of the association between EBF and VEOL revealed that women reporting VEOL had significantly higher EBF rates but only at 2 months and only when using the previous-month definition (Table 6).
Table 6.
Exclusive Breastfeeding (EBF) Rates Over the Previous Month and Since Birth by Onset-of-Lactation (OL) Status
| Previous Month’s EBF Rates | EBF Rates Since Birth | |||||
|---|---|---|---|---|---|---|
| Month | OL < 6 h, n (%) |
OL ≥ 6 h, n (%) |
P Value |
OL < 6 h, n (%) |
OL ≥ 6 h, n (%) |
P Value |
| 1 | 89 (94.7) | 261 (93.9) | 1.000 | 89 (94.7) | 261 (93.9) | 1.000 |
| 2 | 93 (98.9) | 251 (90.3) | .003 | 88 (93.6) | 241 (86.7) | .092 |
| 3 | 88 (93.6) | 250 (89.9) | .407 | 82 (87.2) | 224 (80.6) | .162 |
Discussion
To our knowledge, our study is the first to show that HIV− status is a predictor for VEOL in sub-Saharan Africa. In our sample, HIV− women were almost 3 times as likely as their HIV+ counterparts to report VEOL. Our study confirms the findings of other studies that have reported associations between multiparity, vaginal deliveries, and earlier OL.4,5,7,8 OL in our study occurred much earlier than has been reported in the United States. In our study, only 4.5% reported OL greater than 72 hours. Chapman and Pérez-Escamilla reported delayed OL greater than 72 hours among 31% of their participants in Hartford, Connecticut.4 In a study in California, 22% of the study participants experienced delayed OL.5 Although the women in our study were slightly younger and had comparable rates of cesarean delivery to the California study, only 28.5% were primiparous. The percentages of primiparous women in the US studies were twice this and could explain some of the disparities in the results. Primiparity is a known risk factor for delayed OL and excessive infant weight loss. Our findings are, however, consistent with those reported in the literature outside the United States. Maternal reports of delayed OL in Zambia,14 Honduras,35 Guatemala,15 and Australia36 have ranged from 0.6% to 11.7%.
Our 2 substudies strongly suggest that the maternal report of OL is valid. Breast symptoms associated with OL, such as full or heavy feeling, milk leaking, and tingling, were commonly reported as OL cues. A comparison of neonatal weight loss during the first week postpartum between HIV subgroups also corroborated the validity of the maternal report of OL. Our findings show that on average, among all infants and within the HIV groups, there was maximum weight loss at day 2, and by day 3 the children had started to gain weight. In Italy, maximum weight loss among exclusively breastfed infant occurred between 3 and 5 days.37 Dewey et al5 reported that infant weight loss of 10% of birth weight or more is associated with delayed OL as well as suboptimal infant breastfeeding behaviors.5 In these studies, the babies lost most weight at 4 to 5 days and regained it in 10 or more days. In our subsample, 10% of the children lost 10% of their birth weight or more by the second day. Maximum weight loss occurred on the second day and birth weight was regained at around 6 days, confirming the earlier OL report in our sample. Finally, our test weighing results provided further confirmation of the validity of maternal report of OL; women in our study who reported that their milk had started flowing transferred significantly more breast milk to their infants.
Breast health results showed that both mastitis and nipple problems were present in our sample. The majority of mothers who reported clinical mastitis were HIV-positive. This is a public health concern, as subclinical mastitis has been identified as a risk factor for MTCT of HIV.38–42 Although the present study did not investigate subclinical mastitis, it is likely that there were relatively high rates of subclinical mastitis in the population. A recently completed study among mothers 3 to 4 months postpartum in the study area reported a prevalence of subclinical mastitis of 45.3%.43 Subclinical mastitis has been identified as one of the risk factors for poor growth in infants born to HIV+ women.44 However, Bland et al45 reported that breast health problems, which are usually caused by poor positioning and attachment, are rare in both HIV-infected and noninfected women receiving adequate lactation support.
EBF rates in our study were high and fully consistent with previous studies in Ghana.34,46,47 At 3 months, more than 80% of the women were still exclusively breastfeeding using either the since-birth or previous-month definition. EBF rates tended to be higher among women reporting VEOL. Given the difference by HIV status in OL and the inverse relationship between OL and EBF rates, HIV-infected women may require additional support to exclusively breastfeed. The association between HIV and nutritional status is well known, particularly in sub-Saharan Africa, where household food insecurity and poverty are endemic and almost 25 million people are living with HIV/AIDS.48 In our study, we found that HIV+ women were more likely to be of lower socioeconomic status and to have significantly fewer years of formal education.
Differences in infant morbidity according to maternal HIV status are unlikely to explain our results. Very little illness was reported within the first 2 weeks postpartum. During this time period, fever and diarrhea were reported in 9 and 1 of the children, respectively. There were no group differences regarding infant morbidity.
Several limitations of the research regarding the influence of HIV on OL exist. First, HIV testing was done prenatally, and the study makes the assumption that all HIV− women remained uninfected throughout the study. The clinical stage of HIV infection, CD4+ cell count, and antiretroviral drug use for the HIV+ mothers were unknown and could have affected the results. However, none of our participants showed clinical signs of full-blown AIDS, which is the end stage of the disease. Because the majority of the women learned of their HIV status during pregnancy, we believe that the knowledge of HIV+ status and the associated psychological, physiological, and biological stress could be largely responsible for the differences in OL and breastfeeding outcomes observed. In the present study, it was unknown how many of the HIV-exposed children were infected. Second, a convenience sampling method was used, thus limiting the generalization of the results to the rest of Ghana, although the findings are likely to be applicable to women who have access to antenatal care and PMTCT services. Third, the late notification of deliveries and home deliveries led to missing data in our sample, requiring the imputation of estimated values for the infant weight data.
Although stress as measured by the 4-item scale was not significantly associated with the maternal HIV status, there was some evidence that postnatal depression was, and the relationship between these factors and OL needs further investigation. Most of the outcomes of HIV-U women and their infants fell between those of the HIV+ and HIV− participants, confirming our expectation that the HIV-U participants were likely a mixture of both infected and uninfected women.
Our findings provide important insights on the association between HIV, VEOL, and early infant growth. Whereas the implications of delayed OL have been examined before,6 the full extent of the implications of not having VEOL for maternal and child health remains unknown, which may be particularly important in Ghana or in other countries near the equator. Likewise, the substantial difference in OL timing among women in the United States and elsewhere requires further exploration.
Acknowledgments
This project was funded by the RIING project through NIH/NICHD grant HD43620. We thank all the mothers and children who participated in this study. We are also grateful for the entire RIING project staff who made the implementation of this study possible.
Footnotes
No reported competing interests.
Contributor Information
Gloria E. Otoo, Department of Nutritional Sciences at the University of Connecticut and a lecturer at the Department of Nutrition and Food Science, University of Ghana.
Grace S. Marquis, Associate professor at the School of Dietetics and Human Nutrition at McGill University, Canada.
Daniel W. Sellen, Professor of anthropology nutritional sciences and public health, and Canada Research Chair in Human Ecology and Public Nutrition at the University of Toronto.
Donna J. Chapman, Assistant professor-in-residence in the Department of Nutritional Sciences at the University of Connecticut and assistant director of CEHDL.
Rafael Pérez-Escamilla, Professor of epidemiology and public health at the Yale University School of Public Health and the director of the University of Connecticut and the director of NIH EXPORT Center for Eliminating Health Disparities among Latinos (CEHDL).
References
- 1.Neifert MR. Breastmilk transfer: positioning, latch-on, and screening for problems in milk transfer. Clin Obstet Gynecol. 2004;47:656–675. doi: 10.1097/01.grf.0000136183.12304.96. [DOI] [PubMed] [Google Scholar]
- 2.Hall RT, Mercer AM, Teasley SL, et al. A breast-feeding assessment score to evaluate the risk for cessation of breast-feeding by 7 to 10 days of age. J Pediatr. 2002;141:659–664. doi: 10.1067/mpd.2002.129081. [DOI] [PubMed] [Google Scholar]
- 3.Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr. 2001;131:3005S–3008S. doi: 10.1093/jn/131.11.3005S. [DOI] [PubMed] [Google Scholar]
- 4.Chapman DJ, Perez-Escamilla R. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999;99:450–454;. doi: 10.1016/S0002-8223(99)00109-1. quiz 55–56. [DOI] [PubMed] [Google Scholar]
- 5.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3 pt 1):607–619. doi: 10.1542/peds.112.3.607. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Escamilla R, Chapman DJ. Validity and public health implications of maternal perception of the onset of lactation: an international analytical overview. J Nutr. 2001;131:3021S–3024S. doi: 10.1093/jn/131.11.3021S. [DOI] [PubMed] [Google Scholar]
- 7.Chen DC, Nommsen-Rivers L, Dewey KG, Lonnerdal B. Stress during labor and delivery and early lactation performance. Am J Clin Nutr. 1998;68:335–344. doi: 10.1093/ajcn/68.2.335. [DOI] [PubMed] [Google Scholar]
- 8.Grajeda R, Perez-Escamilla R. Stress during labor and delivery is associated with delayed onset of lactation among urban Guatemalan women. J Nutr. 2002;132:3055–3060. doi: 10.1093/jn/131.10.3055. [DOI] [PubMed] [Google Scholar]
- 9.Sievers E, Haase S, Oldigs HD, Schaub J. The impact of peripartum factors on the onset and duration of lactation. Biol Neonate. 2003;83:246–252. doi: 10.1159/000069485. [DOI] [PubMed] [Google Scholar]
- 10.Zuppa AA, Tornesello A, Papacci P, et al. Relationship between maternal parity, basal prolactin levels and neonatal breast milk intake. Biol Neonate. 1988;53:144–147. doi: 10.1159/000242775. [DOI] [PubMed] [Google Scholar]
- 11.Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. J Hum Lact. 2004;20:18–29. doi: 10.1177/0890334403261345. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr. 2007;27:103–121. doi: 10.1146/annurev.nutr.27.061406.093738. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113:e465–e471. doi: 10.1542/peds.113.5.e465. [DOI] [PubMed] [Google Scholar]
- 14.Chisenga M, Kasonka L, Makasa M, et al. Factors affecting the duration of exclusive breastfeeding among HIV-infected and -uninfected women in Lusaka, Zambia. J Hum Lact. 2005;21:266–275. doi: 10.1177/0890334405279251. [DOI] [PubMed] [Google Scholar]
- 15.Hruschka DJ, Sellen DW, Stein AD, Martorell R. Delayed onset of lactation and risk of ending full breast-feeding early in rural Guatemala. J Nutr. 2003;133:2592–2599. doi: 10.1093/jn/133.8.2592. [DOI] [PubMed] [Google Scholar]
- 16.Chapman DJ, Pérez-Escamilla R. Does delayed perception of the onset of lactation shorten breastfeeding duration? J Hum Lact. 1999;15:107–111. doi: 10.1177/089033449901500207. [DOI] [PubMed] [Google Scholar]
- 17.Segura-Millan S, Dewey KG, Perez-Escamilla R. Factors associated with perceived insufficient milk in a low-income urban population in Mexico. J Nutr. 1994;124:202–212. doi: 10.1093/jn/124.2.202. [DOI] [PubMed] [Google Scholar]
- 18.McCann MF, Bender DE. Perceived insufficient milk as a barrier to optimal infant feeding: examples from Bolivia. J Biosoc Sci. 2006;38:341–364. doi: 10.1017/S0021932005007170. [DOI] [PubMed] [Google Scholar]
- 19.Coutsoudis A, Pillay K, Kuhn L, et al. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 20.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 21.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 22.Consensus Statement. Geneva: World Health Organization; 2006. WHO HIV infant feeding technical consultation held on behalf of the Inter-agency Task Team (IATT) on prevention of HIV infection in pregnant women, mothers, and their infants. [Google Scholar]
- 23.Neville MC, Keller R, Seacat J, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48:1375–1386. doi: 10.1093/ajcn/48.6.1375. [DOI] [PubMed] [Google Scholar]
- 24.Scanlon KS, Alexander MP, Serdula MK, Davis MK, Bowman BA. Assessment of infant feeding: the validity of measuring milk intake. Nutr Rev. 2002;60:235–251. doi: 10.1301/002966402320289368. [DOI] [PubMed] [Google Scholar]
- 25.Neville MC. Measurement of milk transfer from mother to breast-fed infant. J Pediatr Gastroenterol Nutr. 1987;6:659–662. [PubMed] [Google Scholar]
- 26.Cregan MD, Hartmann PE. Computerized breast measurement from conception to weaning: clinical implications. J Hum Lact. 1999;15:89–96. doi: 10.1177/089033449901500202. [DOI] [PubMed] [Google Scholar]
- 27.Riordan J. Anatomy and physiology of lactation. In: Riordan J, editor. Breastfeeding and Human Lactation. 3rd ed. Sudbury, Mass: Jones & Bartlett; 2005. pp. 67–95. [Google Scholar]
- 28.Chapman DJ, Perez-Escamilla R. Maternal perception of the onset of lactation is a valid, public health indicator of lactogenesis stage II. J Nutr. 2000;130:2972–2980. doi: 10.1093/jn/130.12.2972. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 30.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession. 6th ed. Philadelphia, Pa: Elsevier Mosby; 2005. [Google Scholar]
- 32.de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(1 suppl):S27–S36. doi: 10.1177/15648265040251S104. [DOI] [PubMed] [Google Scholar]
- 33.Aidam BA, Perez-Escamilla R, Lartey A. Lactation counseling increases exclusive breast-feeding rates in Ghana. J Nutr. 2005;135:1691–1695. doi: 10.1093/jn/135.7.1691. [DOI] [PubMed] [Google Scholar]
- 34.Timpo OM. Programs and Policies Associated With Improved Exclusive Breastfeeding Rates in Ghana: 1989–2003. Storrs, Conn: University of Connecticut; 2007. [Google Scholar]
- 35.Perez-Escamilla R, Segura-Millan S, Canahuati J, Allen H. Prelacteal feeds are negatively associated with breast-feeding outcomes in Honduras. J Nutr. 1996;126:2765–2773. doi: 10.1093/jn/126.11.2765. [DOI] [PubMed] [Google Scholar]
- 36.Scott JA, Binns CW, Oddy WH. Predictors of delayed onset of lactation. Matern Child Nutr. 2007;3:186–193. doi: 10.1111/j.1740-8709.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manganaro R, Mami C, Marrone T, Marseglia L, Gemelli M. Incidence of dehydration and hypernatremia in exclusively breast-fed infants. J Pediatr. 2001;139:673–675. doi: 10.1067/mpd.2001.118880. [DOI] [PubMed] [Google Scholar]
- 38.Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci. 2000;918:156–162. doi: 10.1111/j.1749-6632.2000.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 39.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 40.Semba RD, Kumwenda N, Taha TE, et al. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol. 1999;6:671–674. doi: 10.1128/cdli.6.5.671-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John GC, Nduati RW, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 42.Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell ML, Tomkins AM. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol. 2000;478:211–223. doi: 10.1007/0-306-46830-1_19. [DOI] [PubMed] [Google Scholar]
- 43.Aryeetey RNO, Marquis GS, Timms L, Lartey A, Brakohiapa L. Subclinical mastitis is common among Ghanaian lactating women, 3 to 4 months postpartum. J Hum Lact. 2008;24:263–267. doi: 10.1177/0890334408316077. [DOI] [PubMed] [Google Scholar]
- 44.Makasa M, Kasonka L, Chisenga M, et al. Early growth of infants of HIV-infected and uninfected Zambian women. Trop Med Int Health. 2007;12:594–602. doi: 10.1111/j.1365-3156.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 45.Bland RM, Becquet R, Rollins NC, Coutsoudis A, Coovadia HM, Newell ML. Breast health problems are rare in both HIV-infected and HIV-uninfected women who receive counseling and support for breast-feeding in South Africa. Clin Infect Dis. 2007;45:1502–1510. doi: 10.1086/523320. [DOI] [PubMed] [Google Scholar]
- 46.Quinn VJ, Guyon AB, Schubert JW, Stone-Jimenez M, Hainsworth MD, Martin LH. Improving breastfeeding practices on a broad scale at the community level: success stories from Africa and Latin America. J Hum Lact. 2005;21:345–354. doi: 10.1177/0890334405278383. [DOI] [PubMed] [Google Scholar]
- 47.Measure DHS. Demographic and Health Survey (DHS) [Accessed April 2007]; http://www.measuredhs.com/
- 48.AIDS Epidemic Update: Special Report on HIV/AIDS. Geneva: World Health Organization; 2006. Dec, UNAIDS/WHO: Joint United Nations Program on HIV/AIDS and World Health Organization. [Google Scholar]