Abstract
More than 161,000 lung cancer deaths are projected to occur in the U.S. in 2008. Of these, an estimated 10–15% will be caused by factors other than active smoking, corresponding to 16,000–24,000 deaths annually. Thus lung cancer in never smokers would rank among the most common causes of cancer mortality in the U.S. if considered to be a separate category. Slightly more than half of the lung cancers caused by factors other than active smoking occur in never smokers. As summarized in the accompanying article, lung cancers that occur in never smokers differ from those that occur in smokers in their molecular profile and response to targeted therapy. These recent laboratory and clinical observations highlight the importance of defining the genetic and environmental factors responsible for the development of lung cancer in never-smokers. This article summarizes available data on the clinical epidemiology of lung cancer in never smokers, and the several environmental risk factors that population-based research has implicated in the etiology of these cancers. Primary factors closely tied to lung cancer in never smokers include exposure to known and suspected carcinogens including radon, second-hand tobacco smoke, and other indoor air pollutants. Several other exposures have been implicated. However, a large fraction of lung cancers occurring in never-smokers cannot be definitively associated with established environmental risk factors, highlighting the need for additional epidemiologic research in this area.
LUNG CANCER OCCURRENCE IN NEVER SMOKERS
Approximately 10 – 15% of all lung cancers arise in never smokers, making lung cancer in never smokers one of the leading causes of cancer-related mortality (1–3). Given the impact of this disease, there is surprisingly little information available on the descriptive epidemiology of lung cancer in never smokers. General population statistics are largely uninformative because neither cancer registries nor routinely collected death certificates provide reliable information on lifetime smoking histories. In addition, reports on smoking from next-of-kin or in medical records are incomplete and often unreliable (4, 5). Only large-scale cohort studies can measure age-and sex-specific lung cancer rates in never smokers with reasonable precision, and these have generally studied mortality rather than incidence. Consequently, limited data have been available to resolve controversies such as whether women are more susceptible than men to develop lung cancer in the absence of smoking, whether the risk is higher in African Americans and Asians than in Caucasians, and whether the background risk has changed over time.
The other papers within this issue of CCR Focus present an overview, and a description of the implications of recent molecular insights (6, 7). This article reviews current information on the clinical epidemiology of and environmental risk factors for lung cancer in never smokers. It describes the sources of data, including historical records that preceded the widespread introduction of manufactured cigarettes; examines incidence and mortality rates in relation to age, gender, race/ethnicity, geographic location, and temporal trends; and identifies research needs.
Historical records indicate that lung cancer was rarely diagnosed in North America and Europe before the introduction and promotion of manufactured cigarettes. In 1912, it was described as “one of the rarest forms of cancer” (8). In 1914, the U.S. Census Office systematically surveyed death certificate information on 52,420 cancer deaths and identified only 371 attributed to cancer of the lung and pleura, representing 0.7% of the total (9). In Britain, the increase in lung cancer was seen earlier than in the U.S. because officers learned to smoke hand-rolled cigarettes in the Crimean war (1854–1856) (10). Whereas lung cancer comprised only two-tenths of one percent of all hospitalizations for cancer at the Manchester Royal Infirmary during the period 1868–1885, this percentage had increased ten-fold in men by 1901–1905 (11).
Population-based data on lung cancer incidence or death rates among people who never smoked are available for women in the U.S. during the 1930s and for women in other countries during time periods when few women smoked. In contrast, the lung cancer rates among men in Western countries were dominated by the effects of active cigarette smoking during most of the 20th century. In the U.S. for example, the lung cancer mortality rate among men was already increasing exponentially by the early 1930s when national mortality statistics first became available (12). In contrast, regular smoking was uncommon among women in the U.S. before World War II. National data on mortality and regional statistics on lung cancer incidence compiled during the 1930s largely reflect the background rates among women who never smoked actively. Similarly, smoking remains uncommon even today among women in many countries of Africa and Asia where strong cultural norms discourage women from smoking.
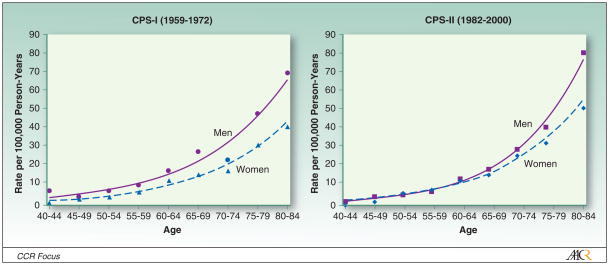
Also informative are large cohort studies that measure incidence or death rates prospectively in people who report their smoking history and various other risk factors at enrollment. Only the largest cohort studies provide stable age- and sex-specific rates. Detailed mortality data have been published on lung cancer death rates among never smokers enrolled in two large American Cancer Society cohorts, the Cancer Prevention Studies I (CPS-I) and II (CPS-II), which were initiated in the late 1950s and early 1980s, respectively, to characterize the risks of smoking. Approximately 1 million men and women in 25 states were enrolled in CPS-I in 1959 (3) and nearly 1.2 million men and women were enrolled nationwide in CPS-II in 1982. Researchers have followed their vital status through the present and have published detailed information on age-, sex-, and race-specific lung cancer death rates in never smokers for the entire 12-year follow-up of CPS-I (1959–1972) and 18-year follow-up of CPS-II (1982–2000) (3).
DESCRIPTIVE EPIDEMIOLOGY
Age
Lung cancer risk increases with age in both smokers and never smokers. Figure 1 shows the age-related increase in lung cancer death rates among white men and women, age 40–84 years, who reported no history of regular smoking in either of the large ACS cohorts, CPS-I and CPS-II (Figure 1) (3). Similar age patterns among never smokers have been reported previously for whites (13–16), Japanese (17) and African Americans (women only) (3). However, even the largest cohort studies cannot measure lung cancer rates reliably at younger ages. Lung cancer is sufficiently rare in people under age 40, especially among never smokers, that cohort studies would need to be prohibitively large and hence costly to accurately estimate the incidence or death rates in young adults. The diagnosis of lung cancer across all age cohorts depends strongly on access to minimally invasive diagnostic technologies that have become increasingly available over time, particularly in economically developed countries. These include the introduction of chest x-rays beginning in the 1930s (12), flexible bronchoscopy since the late 1960s (18), thin needle aspiration and computerized scans during the 1980s (19–21); and helical CT scans since the late 1990s (22, 23).
Figure 1.
Age- and sex-specific lung cancer death rates among white never smokers in the American Cancer Society Cancer Prevention Study cohorts, stratified by study.
Gender
Clinicians have observed that women outnumber men among lung cancer patients who report never having smoked regularly. This has been misinterpreted as evidence that “women who have never smoked are more likely to develop lung cancer than men who have never smoked” (24). However, risk represents the probability that an individual will develop the disease, not the number of affected people. There are more than twice as many women as men age 60 years and older who have never smoked, and this female predominance increases with age (3).
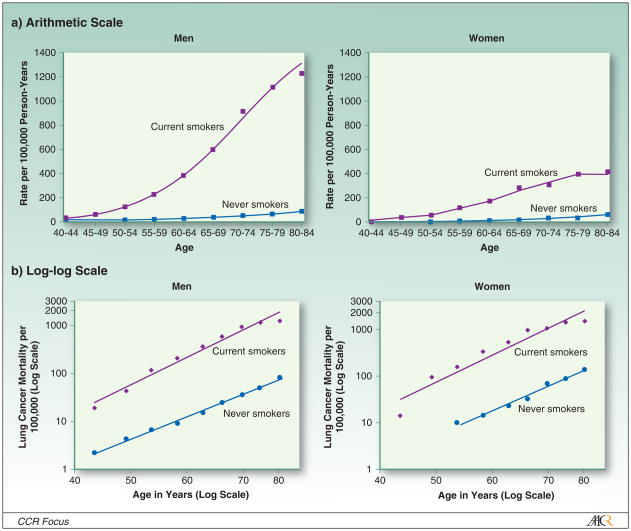
Prospective cohort studies have consistently found that the death rate from lung cancer is higher in men than women, both in the absence (Figure 1) (3) and presence (Figure 2) (25–27) of active smoking. However, the relationship with sex is less clear for incidence than for mortality (28). Wakelee et al. have noted that lung cancer incidence rates were slightly, although not significantly, higher in women than men among never smokers, age 40–79 in six cohort studies (29). Henschke et al. have observed that women are more likely to be diagnosed with lung cancer than men when screened using spiral computerized tomography (30, 31). Based on this observation, Henschke has hypothesized that lung cancer incidence may be higher in women than in men who smoke, even though the opposite is true for mortality. However, screening tests such as helical CT detect prevalent rather than incident cases, and therefore may be detecting undiagnosed lung cancers that progress more slowly in women than in men rather than representing higher occurrence (incidence) rates in women.
Figure 2.
Age-specific lung cancer death rates among white current smokers and never smokers in CPS-II, 1982–1988, stratified by sex.
Race/ethnicity
Lung cancer risk among never smokers has also been hypothesized to be higher in African Americans (3) and Asians (32) than in whites. The age-standardized death rate from lung cancer was approximately 40% higher among African American than white women who reported never smoking in CPS-II (3), but a statistically significant racial difference was not seen for never smoking women in CPS-I; there were insufficient data to measure the risk in black men. A case-control study found no evidence that lung cancer incidence was higher in African American than white never smokers in men or women, except among men aged 40–54 years (33).
Geographic variability
The incidence of lung cancer in women varies by as much as 30-fold, even among countries reported to have low prevalence of female smoking (34), and even in the age range 40–69 years where ascertainment is most comparable. The lowest female lung cancer incidence rates are reported in Africa (Algeria and Mali) and India (Ahmedabad, Bangalore, Madras and Mumbai), while the highest rates are in Pacific Rim countries (Philippines, Hong Kong, Japan, and the Chinese population of Singapore) and in China. Even within China, lung cancer risk in women varies widely (35). Li et al. reported a 20-fold difference in the lung cancer death rate between Chinese women living in counties at the 10th versus 90th percentiles of lung cancer death rates, as estimated from a retrospective mortality survey conducted from 1973 to 1975 (36). Some of this variation reflects historical patterns of high active smoking by older women in northeastern China (Tianjin and Harbin) and northern Thailand (Chiang Mai and Lampang). Other factors thought to contribute to the increased risk among some groups of Chinese women include indoor air pollution from coal smoke generated by unventilated coal-fueled fire pits and stoves (37, 38), volatilization of oils from cooking at high temperatures in open woks (39–42), and secondhand smoke (39, 43–45).
Temporal trends
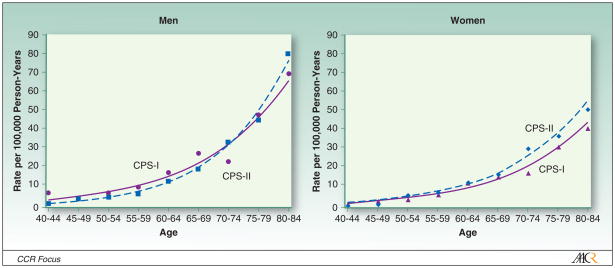
Several researchers have suggested that lung cancer risk is increasing in the general population due to factors other than tobacco smoking (46–50). However, there is little evidence to support this claim and considerable evidence against it. Vital statistics data for women aged 40–69 years in the U.S. in 1935–1940 show that female lung cancer death rates before the advent of female smoking were similar to those of women of the same age who have reported no history of active smoking in cohort studies carried out since 1960. In addition, changes have not been observed in the lung cancer death rates among men and women who reported never smoking status in comparisons of CPS-I (1959–1972) and CPS-II (1982–2004) (Figure 3) (3). The death rate was slightly lower in CPS-II than in CPS-I for white men ages 40 and above (HR=0.83, 95% CI=0.66–1.05) but slightly higher for white (RR=1.11, CI=0.98–1.25) and African American women (RR=1.15, 95% CI=0.62–2.13). The lung cancer death rates among never smokers in the two studies appear to be converging with longer follow-up of CPS-II, even at ages 80 years and above.
Figure 3.
Age- and sex-specific lung cancer death rates among white never smokers in CPS-I (1959–1972) and CPS-II (1982–2000), stratified by sex.
The analyses supporting a possible increase in lung cancer among never smokers have been based either on statistical modeling (47–50), or on a comparison of rates up to 1968 with the 1914 U.S. census survey (46). The modeling studies used epidemiological principles related to rates in populations to estimate the rate in never smokers; they did not have direct estimates as with the CPS-I and CPS-II data. Additionally, these modeling studies did not take into account the large and progressive increase in lung cancer risk associated with cigarette smoking that took place as the average duration of smoking has increased over time in the population. The 1914 survey is limited as a basis of comparison as it was conducted before pathologists began to look systematically for lung cancer. Neither respiratory cancer nor cancers of the lung and pleura were included in the International Classification of Diseases (ICD) until 1929 and 1938, respectively (51). It is plausible that some of the deaths attributed to tuberculosis in the early 20th century may have involved misdiagnosis of lung cancer. The death rate from tuberculosis decreased by two-thirds between 1915 and 1935 (52), a period when lung cancer mortality was rising, especially in men (9, 53).
To summarize, the best evidence on time trends of lung cancer in never smokers comes from CPS-I and CPS-II. At least for the time period covered by these two studies, lung cancer mortality does not appear to be increasing in never smokers.
Putting risks into perspective
It is not surprising that lung cancer risk is substantially lower among lifelong nonsmokers than cigarette smokers. Figure 2 illustrates the magnitude of the difference in lung cancer death rates between the CPS-II participants who reported either current or never smoking at the time of enrollment in the study. The data are presented graphically on both an arithmetic (above) and log-log scale (below) to illustrate that the age-related increase is almost linear in both groups when the data are transformed logarithmically, indicating approximately exponential relationships (25). The absolute rates are 20–25 times higher for the male current than never smokers, and 10–12 times higher for female current smokers than never smokers. Figure 2 is based on six-year follow-up of CPS-II (1982–1988) to minimize misclassification of current smokers who quit during follow-up (25).
The lung cancer death rate among never smokers, although “rare” by conventional definitions (<40,000 US deaths per year), is similar to the death rates from leukemia and endometrial cancer in women and cancers of the esophagus, kidney and liver in men in the United States, and may be even more important in other populations, including Chinese women (25, 54).
Research Needs
Better data are needed to answer several basic questions about the descriptive epidemiology of lung cancer in lifelong nonsmokers. Additional information on lung cancer incidence and death rates in relation to age, sex, and race/ethnicity will soon become available from a collaborative effort to pool data from large cohort studies. This will be a valuable resource, but will not resolve limitations in the data for African Americans (particularly men), and Asians (particularly for incidence) and will provide no data on lung cancer risk in never smoking Hispanics. It is unlikely that cohort studies alone can provide reliable estimates of lung cancer risk in never smokers under age 40 years, and so different approaches will be needed if this is to be resolved. Finally, further studies are needed to understand variations in lung cancer incidence and etiology among never smoking women in populations outside Europe and North America, in particular in East Asia.
HISTOPATHOLOGY
Beginning in the late 1960’s, changes have been observed in many countries in the frequency of different histological subtypes of lung cancer, with a declining proportion of squamous cell carcinomas and an increasing proportion of adenocarcinomas (55). These changes have been observed to vary by both gender and smoking status. At the start of the epidemic, the most frequently observed histological type of lung cancer among smokers was squamous cell carcinoma, especially in males. However, adenocarcinomas were more frequently observed in females regardless of smoking status. Among never smokers included in the past studies, largely women, adenocarcinomas were more frequent than squamous cell carcinomas and comprised the majority of tumors (56).
Many studies that have examined changes in the trends of histological subtypes, primarily using cancer registry data, have shown that adenocarcinomas have been rising among both men and women while rates of squamous cell carcinoma have been decreasing among men (56). Although most studies have not separated histological subtypes by smoking status, a few studies have examined the trends among never smokers (Supplemental Table 1). One study in Poland of 20,567 lung cancer cases found that squamous cell carcinoma was most common among male never smokers and current smokers (57). However, female never and current smokers were more likely to be diagnosed with adenocarcinomas. Data from the Cancer Surveillance Program of Orange County in the U.S. demonstrated that, although squamous cell carcinoma was the most common histological subtype observed among male never and current smokers, among women, adenocarcinoma was the most frequent type (58). This study was based on 1984 data.
Time trend studies have shown that regardless of smoking status, the rate of adenocarcinoma is increasing, while that of squamous cell carcinoma is decreasing among men. In several studies, the frequency of adenocarcinoma has surpassed that of squamous cell carcinoma. For example, a study of 437,976 Korean men in whom 1357 new lung cancer cases were indentified found that adenocarcinoma was the most frequent histological type among never smokers, former smokers, and current smokers (59). A study in Malaysia similarly showed that squamous cell carcinoma was the most frequent histological subtype among both male and female smokers from 1967–1976, but by 1991–1999 adenocarcinoma became the most frequent histological subtype for both sexes (60). However, among never smokers adenocarcinoma was the most frequent histological subtype in 1967–1976 and 1991–1999.
The available evidence demonstrates a bias toward adenocarcinoma among never smokers relative to smokers. There appear to be gender differences as well, with female never smokers and smokers tending to have adenocarcinomas more commonly than parallel male cohorts. Although adenocarcinoma is the most common histologic type among male never smokers, male smokers tend to have more squamous cell carcinomas.
Hypotheses concerning the shift in histopathology among smokers have focused on the potential role of the substantial changes in the characteristics of cigarettes and the associated changes in the dosages of carcinogens inhaled and the pattern of deposition in the lung (61). Puff volume has likely increased in the past few decades with the possibility that patterns of deposition in the lung have changed, tending toward enhanced deposition of tobacco smoke in the peripheral airways and alveoli (61). Nitrate levels in tobacco smoke have also increased, which enhances the combustion of tobacco smoke. Although more complete combustion decreases the concentrations of polycyclic aromatic hydrocarbons, the increased production of nitrogen oxides contributes to increased formation of tobacco specific nitrosamines (TSNAs). An increase in dosage of the potent TSNA 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNAL) has been postulated as one factor leading to the increase in adenocarcinomas (61, 62). NNAL induces lung carcinomas, predominantly adenomas and adenocarcinomas, in mice, regardless of route of administration (62, 63). These hypotheses about the shift in histopathology among smokers are also relevant for never smokers. Non-smokers inhale a mixture of sidestream smoke and exhaled mainstream smoke that is generally referred to as secondhand smoke (SHS). Never smokers exposed to SHS have experienced changes in the SHS mixture in the past few decades since increases in puff volume and enhancement of the combustion of tobacco smoke have increased the formation of TSNAs (49). These diverse changes in the inhaled doses of carcinogens delivered to smokers and nonsmokers are likely to have contributed to the time trend of changing histopathology. To date there has been limited research on differences in lung cancer histology subtypes among never smokers, pointing to a need to develop evidence in this area.
RISK FACTORS
Secondhand smoke
In 1981 published reports from Japan (64) and Greece (65) indicated increased lung cancer risk in never smoking women married to cigarette smokers. Subsequently this association has been examined in over 50 investigations conducted in the United States and other countries. Over the past 20 years, review groups have repeatedly and consistently concluded that exposure to SHS causes lung cancer in never smokers.
A causal association of involuntary smoking with lung cancer derives biological plausibility from the presence of carcinogens in sidestream smoke and the lack of a documented threshold dose for respiratory carcinogens in active smokers (66–69). Genotoxic activity had been demonstrated for many components of SHS (70–73). Experimental and real-world exposures of non-smokers to SHS lead to excretion of NNAL, a tobacco-specific carcinogen, in the urine (74, 75). Non-smokers exposed to SHS also have increased concentrations of adducts of tobacco-related carcinogens (76, 77). Additionally, Mauderly and colleagues, using an animal model, found that whole-body exposure in rats to cigarette smoke increases the risk of neoplastic proliferative lung lesions and induces lung cancer (78). In an autopsy study in Greece, Trichopoulos and colleagues (79) examined lung specimens from 400 persons 35 years of age and older and found that airway epithelial lesions were more common in nonsmokers married to smokers than in nonsmokers married to nonsmokers.
Epidemiologists have tested the association between lung cancer and involuntary smoking using case-control and cohort designs have consistently found that SHS exposure is associated with lung cancer risk in never smokers. For decades, the tobacco industry and its consultants attributed the association to bias (80). The potential for bias and the related methodologic issues are addressed at length in the 2006 Report of the U.S. Surgeon General (81) and elsewhere. Specific methodologic concerns have been misclassification of ever smokers as never smokers and inaccuracy in the classification of SHS exposure status in the different places where exposure occurs, particularly the home and workplaces. Quantitative and qualitative assessments of these sources of misclassification have led to the conclusion that bias from misclassification does not account for the observed association (81–85).
Use of spouse smoking alone to represent exposure to SHS does not cover exposures outside of the home (86) or necessarily all exposure inside the home, particularly during the time period relevant to the epidemiological studies. Klepeis and colleagues used data from the National Human Activity Pattern Survey to assess the contribution of the home and other indoor environments to SHS exposures (87). Overall, the data show that 43% of the time spent with a smoker is in a residence, while 7% is in the workplace, 9% in a vehicle, and 15% in a bar or restaurant. This survey may help to explain the results of a European study which found that the number of cigarettes smoked per day by the husband is only moderately correlated with “actual” exposure of women married to smokers (88). A pooled analysis of large-scale studies to assess the risk of lung cancer of never smokers exposed to spouse and workplace sources of SHS found an excess risk of 23% from exposure to spousal smoking and 27% from exposure to workplace sources of SHS (89).
In considering alternatives to a causal association, confounding has also been proposed as contributing to the association of SHS with lung cancer. Critics of these findings on SHS and lung cancer have argued that uncontrolled confounding by lifestyle, occupation, or other factors may explain the association (90, 91). In some countries, including the United States, smoking prevalence varies markedly with indicators of income and education, more recently tending to rise sharply with decreasing educational level and income (66, 92). In general, exposure to SHS follows a similar trend, and critics of the findings on SHS and lung cancer have argued that uncontrolled confounding by lifestyle, occupation, or other factors may explain the association. In fact, current data for the United States do indicate a generally less healthy lifestyle in those with greater SHS exposure (93). However, other than a few occupational exposures at high levels, as well as indoor radon, risk factors for lung cancer in never smokers that might confound the SHS association cannot be proffered and the relevance to past studies of these current associations of potential confounders with SHS exposure is uncertain.
The first major studies on SHS and lung cancer were reported in 1981, including Hirayama’s prospective cohort study of 91,540 never smoking women in Japan (64), and the case-control study in Athens, Greece, carried out by Trichopoulos and colleagues (65). By 1986, the evidence had mounted and three reports published in that year concluded that SHS was a cause of lung cancer. The International Agency for Research on Cancer (IARC) (69) concluded that exposure to SHS “gives rise to some risk of cancer.” In its monograph on tobacco smoking, the agency supported this conclusion on the basis of the characteristics of sidestream and mainstream smoke, the absorption of tobacco smoke materials during involuntary smoking, and the nature of dose response relationships for carcinogenesis. The National Research Council (94) and the U.S. Surgeon General (68) also concluded that involuntary smoking increases the incidence of lung cancer in never smokers. In reaching this conclusion, the National Research Council (94) cited the biological plausibility of the association between exposure to SHS and lung cancer and the supporting epidemiological evidence. Based on a pooled analysis of the epidemiological data adjusted for bias, the report concluded that the best estimate for the excess risk of lung cancer in never smokers married to smokers was 25%. The 1986 report of the Surgeon General (68) characterized involuntary smoking as a cause of lung cancer in never smokers. This conclusion was based on the extensive information already available on the carcinogenicity of active smoking, on the qualitative similarities between SHS and mainstream smoke, and on the epidemiological data on involuntary smoking.
In 1992 the U.S. Environmental Protection Agency (84) published its risk assessment of SHS as a Group A carcinogen. The agency’s evaluation drew on the toxicologic evidence on SHS and the extensive literature on active smoking. A meta-analysis of the 31 studies published to that time was central in the decision to classify SHS as a Group A carcinogen - namely a known human carcinogen. The meta-analysis considered the data from the epidemiologic studies by tiers of study quality and location and used an adjustment method for misclassification of smokers as never smokers. Overall, the analysis found a significantly increased risk of lung cancer in never smoking women married to smoking men; for the studies conducted in the United States, the estimated relative risk was 1.19 (90% CI: 1.04, 1.35).
Subsequent to the 1992 risk assessment, over 20 additional studies and several major reports have been published that further contribute to the evidence supporting a causal association between SHS and the risk of lung cancer (81, 95, 96). Among the additional studies, the multicenter study of Fontham and colleagues is the largest published to date (97), with 651 cases and 1,253 controls. It shows a significant increase in overall relative risk (OR = 1.26, 95% CI = 1.04, 1.54).
Hackshaw and colleagues (98) carried out a comprehensive meta-analysis in 1997, which included 39 published studies, and estimated an excess risk of lung cancer for never smokers married to smokers as 23% (95% CI: 13%, 34%). Adjustment for potential bias and confounding by diet did not alter the estimate. A subsequent IARC meta-analysis (96) including 46 studies and 6,257 cases yielded similar results: 24% (95% CI: 14%, 34%); incorporating the results from a cohort study with null results overall, but only 177 cases (99), did not change the findings (100). The most recent summaries from the 2006 Surgeon General’s Report are provided in Table 1.
Table 1.
Results of studies on exposure to secondhand smoke and risk of lung cancer among never smokers
| Study | Design/population | Exposure Group | Reference Group | Risk Estimate (95% CI) | Adjustment Variables | |
|---|---|---|---|---|---|---|
| Hackshaw et al. (1997) (98) | Meta-analysis: 39 studies (5 cohort, 34 case- control) of “lifelong non-smokers” 5005 total lung cancer cases Study locations: USA, Europe, Asia Publication dates: 1981–1997 |
Spousal Exposure: | Age only, when possible | |||
| Women (37 studies, 4626 cases): | ||||||
| Husband currently smoked | vs. | Husband never smoked | 1.24 (1.13–1.36) | |||
| Men (9 studies, 274 cases): | ||||||
| Wife currently smoked | vs. | Wife never smoked | 1.34 (0.97–1.84) | |||
| Men and Women (39 studies, 5005 cases): | ||||||
| Spouse currently smoked | vs. | Spouse never smoked | 1.23 (1.13–1.34) | |||
|
| ||||||
| Zhong et al. (2000) (41) | Meta-analysis: 40 studies (5 cohort, 35 case- control) of “lifetime non smoking” subjects 5140 total lung cancer cases Study locations: USA, Europe, Asia Publication dates: 1981–1999 |
Spousal Exposure: | Age and demographic characteristics, when possible | |||
| Women (40 studies, No. cases not stated): | ||||||
| Husband ever smoked | vs. | Husband never smoked | 1.20 (1.12–1.29) | |||
| Men (11 studies, 443 cases): | ||||||
| Wife ever smoked | vs. | Wife never smoked | 1.48 (1.13–1.92) | |||
| Workplace Exposure: | ||||||
| Women (14 studies, 2594 cases): | ||||||
| Workplace SHS exposure | vs. | No workplace SHS exposure | 1.15 (1.04–1.28) | |||
| Men (5 studies, 254 cases): | ||||||
| Workplace SHS exposure | vs. | No workplace SHS exposure | 1.29 (0.93–1.78) | |||
| Men and Women (19 studies, 2848 cases): | ||||||
| Workplace SHS exposure | vs. | No workplace SHS exposure | 1.16 (1.05–1.28) | |||
| Childhood Exposure: | ||||||
| Women (18 studies, 3584 cases): | ||||||
| Ever exposed to SHS during childhood | vs. | Never exposed to SHS during childhood | 0.91 (0.83–1.00) | |||
|
| ||||||
| Surgeon General Report (2006) (81) | Meta-analysis: 52 studies (8 cohort, 44 case- control) of “lifetime nonsmokers” Total number of cases not stated Study locations: North America, Europe, Asia Publication dates: 1981–2002 |
Spousal Exposure: | not stated | |||
| Women (No. studies and cases not stated): | ||||||
| Smoking husband | vs. | Nonsmoking husband | 1.22 (1.10–1.35) | |||
| Men (No. studies and cases not stated): | ||||||
| Smoking wife | vs. | Nonsmoking wife | 1.37 (1.05–1.79) | |||
| Workplace Exposure: | ||||||
| Women (25 studies, No. cases not stated): | ||||||
| Workplace SHS exposure | vs. | No workplace SHS exposure | 1.22 (1.10–1.35) | |||
| Men (11 studies, No. cases not stated): | ||||||
| Workplace SHS exposure | vs. | No workplace SHS exposure | 1.12 (0.86–1.50) | |||
| Men and Women (25 studies, No. cases not stated): | ||||||
| Workplace SHS exposure | vs. | No workplace SHS exposure | 1.22 (1.13–1.33) | |||
| Childhood Exposure: | ||||||
| Women (No. studies and cases not stated): | ||||||
| Mother smoked during childhood | vs. | Mother did not smoke during childhood | 1.28 (0.93–1.78) | |||
| Father smoked during childhood | vs. | Father did not smoke during childhood | 1.17 (0.91–1.50) | |||
| Men and Women (No. studies and cases not stated): | ||||||
| Mother smoked during childhood | vs. | Mother did not smoke during childhood | 1.15 (0.86–1.52) | |||
| Father smoked during childhood | vs. | Father did not smoke during childhood | 1.10 (0.89–1.36) | |||
| Either parent smoked during childhood | vs. | Neither parent smoked during childhood | 1.11 (0.94–1.31) | |||
Abbreviations:
CI: confidence interval
No.: number of
SHS: secondhand smoke
The extent of the lung cancer hazard associated with involuntary smoking in the United States and in other countries remains subject to some uncertainty, however, although estimates have been made that are useful indications of the magnitude of the disease risk (68, 101). In 1990 Repace and Lowrey (102) reviewed the risk assessments of lung cancer and exposure to SHS and estimated the numbers of lung cancer cases in U.S. nonsmokers attributable to exposure to SHS based on a mean of nine different risk estimates, suggesting an overall incidence of 4,500 to 5,000 cases. Similarly the 1992 estimate of the Environmental Protection Agency, based on the epidemiologic data, was about 3,000 cases annually (84). The California EPA estimates that at least 3,423, and perhaps as many as 8,866, lung cancer deaths were caused by SHS in the US in 2003. Of those 3,423 deaths, 967 were attributed to non-spousal exposures to SHS and 2,456 to spousal exposure (95). In summary, most comprehensive analyses suggest that in the US, SHS exposure is responsible for approximately 3000 – 5000 lung cancer deaths annually.
These calculations illustrate that exposure to SHS must be considered an important cause of lung cancer death from a public health perspective; exposure is involuntary and not subject to control. The Biological Effects of Ionizing Radiation (BEIR) VI Committee estimated that from 5% to 10% of lung cancer deaths in the United States in 1995 were in never smokers. The corresponding range of 8,000 to 16,000 deaths, as the total from lung cancer in never smokers, implies that from about 20% to 50% of the deaths are attributable to SHS (103). The specific risk assessments require assumptions concerning the extent and degree of exposure to SHS, exposure-response relationships, and the lifetime expression of the excess risk associated with exposure to SHS at different ages. Moreover the calculations do not consider the potential contributions of other exposures, such as occupational agents and indoor radon. The current decline in the prevalence of active smoking and the implementation of strong clean indoor air policies will reduce the relevance of estimates based on past patterns of smoking behavior.
Radon
Radon, long established as a respiratory carcinogen, is not only of concern for underground miners but for the population generally, as a ubiquitous contaminant of indoor air. Radon is an inert gas, produced naturally from radium in the decay series of uranium. Radon decays with a half-life of 3.82 days into a series of solid, short-lived radioisotopes that collectively are referred to as radon daughters, progeny, or decay products. As the biologic basis of respiratory carcinogenesis was analyzed and the lung dosimetry of radon and its short-lived progeny was described, it was recognized that alpha-particle emissions from inhaled radon progeny, not from radon itself, cause lung cancer (103). Two of those decay products, polonium-218 and polonium-214, emit alpha particles, which are high-energy and high-mass particles consisting of two protons and two neutrons that cause DNA base mutations and chromosomal strand breaks. The energy of these particles is invariant with concentration of radon progeny so that the potential for passage of alpha particles to damage target cells is the same at high and low concentrations. When the alpha emissions take place within the lung as inhaled and deposited radon progeny decay, the DNA of cells lining the airways is damaged and lung cancer may ultimately result. Animal studies have demonstrated that radon alone through its progeny can induce cancer in the respiratory tract (103).
Elegant experimental studies have documented the occurrence of permanent damage to a cell from just one hit by an alpha particle (103). This experimental finding suggests that assuming a linear non-threshold relationship between exposure and risk at the levels found not only in mines but indoors is biologically appropriate, supporting concern that indoor radon represents a significant public health problem. In this same type of experimental system, a bystander mutagenic effect has been demonstrated; a hit to a cell affects cells adjacent to the cell damaged by a single alpha particle (104). This effect may amplify the risks of radon exposure beyond those anticipated based on the construct that passage of an alpha particle through a cell affects only that cell.
Radon was the first identified environmental cause of lung cancer. As early as the 1920s, the elevated risk of lung cancer in miners in Eastern Europe working in mines with high levels of radon had led to the hypothesis that radon was the causal agent (105). Numerous subsequent epidemiological studies of miners showed a strong association of radon exposure with lung cancer risk (106). These worker groups included several comprised largely of never smokers. In fact, the original case report of respiratory malignancy in underground miners in Schneeberg was published in 1879, long before manufactured cigarettes were available (107). In the United States, Navajo uranium miners, almost all never smokers, experienced a clear excess of lung cancer (108–110). In a cohort study of 757 members of this group 34 deaths from lung cancer were identified when only 10.2 were expected [standardized mortality ratio (SMR) 3.3, 95% confidence interval (CI) 2.3–4.6] (111). In a population-based case-control study of lung cancer in Navajo males from 1969–1982, the majority of the cases were attributable to radon exposure in uranium mines. A cohort study of 516 white miners who have never smoked cigarettes, pipes, or cigars from the Colorado Plateau cohort showed 14 lung cancer deaths with only 1.1 expected, based on comparison to the never smokers in a cohort study of U.S. veterans (112).
The risk of lung cancer in never smoking uranium miners has been quantified in a pooled analysis of data from 11 cohort studies, all having estimates of the exposures of individual miners to radon progeny. A pooled analysis of the 2,798 never smoking miners in the cohorts quantified the risk per working-level month (WLM) as almost three times as high in never smokers as in smokers, consistent with the sub-multiplicative interaction between smoking and radon found with analysis of the full data set (113).
Beginning in the 1970s, there was widespread recognition that radon is present in indoor environments, including homes where people spend the majority of their time (106). At the time, given the already recognized carcinogenicity of radon, concern was raised as to the risk of indoor radon and consideration was given to the most appropriate risk management strategies. Case-control studies of radon and lung cancer risk in the general population were carried out to quantify the risk as a basis for risk management; numerous studies, most involving measurement of radon in the current and previous residences, were initiated. These studies have now been completed, the findings of individual studies reported, and two pooled analyses completed, one of studies in North America and the other of studies in Europe. The results show a significantly increased risk that is comparable in the two analyses (Table 2). The estimated number of lung cancer cases in the United States in never smokers attributable to radon alone is approximately 2,100 to 2,900 annually (103).
Table 2.
Results of pooled analyses of case-control studies evaluating risks of radon for lung cancer among never smokers.
| Study | Design/population | Exposure Group | Reference Group | Risk Estimate (95% CI) | Adjustment Variables | |
|---|---|---|---|---|---|---|
| Krewski et al. (2005) (177) | Meta-analysis: 7 case-control studies with 659 lung cancer cases and 2185 population controls who “never smoked” Study location: North America Publication dates: 1992–2000 |
Per 100 Bq/m3 increase in radon exposure during the 5–30 years before index (diagnosis or death) date | vs. | < 25 Bq/m3 radon exposure during the 5–30 years before index (diagnosis or death) date | 10% increased risk (0.91% – 42%) | Study, gender, age, number of residences, years with a-track measurements |
|
| ||||||
| Darby et al. (2005) (178) | Meta-analysis: 13 case-control studies with 884 lung cancer cases and 5418 controls that were “lifelong non-smokers” Study location: Europe Publication dates: 1992–2005 |
Per 100 Bq/m3 increase in radon exposure during the 5–30 years before index (diagnosis or death) date | vs. | < 25 Bq/m3 radon exposure during the 5–30 years before index (diagnosis or death) date | 10.6% increased risk (0.3% – 28.0 %) | not stated |
|
| ||||||
| Darby et al (2006) (179) | Meta-analysis: 13 case-control studies with 884 lung cancer cases and 5418 controls that were “lifelong non-smokers’ Study location: Europe Publication dates: 1992–2005 |
<25 Bq/m3 cumulative radon exposure during 5–30 years before index (diagnosis or death) date | vs. | 0 Bq/m3 cumulative radon exposure during the 5–30 years before index (diagnosis or death) date | 1.06 (0.78–1.45) | Study, age, gender, residence location |
| 25–49 Bq/m3 cumulative radon exposure during 5–30 years before index (diagnosis or death) date | vs. | 0 Bq/m3 cumulative radon exposure during the 5–30 years before index (diagnosis or death) date | 1.07 (0.90–1.26) | Study, age, gender, residence location | ||
| 50–99 Bq/m3 cumulative radon exposure during 5–30 years before index (diagnosis or death) date | vs. | 0 Bq/m3 cumulative radon exposure during the 5–30 years before index (diagnosis or death) date | 1.02 (0.90–1.16) | Study, age, gender, residence location | ||
| 100–199 Bq/m3 cumulative radon exposure during 5–30 years before index (diagnosis or death) date | vs. | 0 Bq/m3 cumulative radon exposure during the 5–30 years before index (diagnosis or death) date | 1.23 (1.02–1.48) | Study, age, gender, residence location | ||
| 200–399 Bq/m3 cumulative radon exposure during 5–30 years before index (diagnosis or death) date | vs. | 0 Bq/m3 cumulative radon exposure during the 5–30 years before index (diagnosis or death) date | 1.37 (1.00–1.90) | Study, age, gender, residence location | ||
| ≥400 Bq/m3 cumulative radon exposure during 5–30 years before index (diagnosis or death) date | vs. | 0 Bq/m3 cumulative radon exposure during the 5–30 years before index (diagnosis or death) date | 1.72 (1.04–2.88) | Study, age, gender, residence location | ||
Abbreviations:
Bq/m3: becquerels per cubic meter CI: confidence interval
In summary, radon is a well established cause of lung cancer in never smokers. Radon progeny act through a mechanism that predicts risk at any level of exposure, regardless of smoking. Epidemiological studies of miners and of the general population provide strong evidence for a causal association and the risk has been quantified for both groups. Estimates of the burden of lung cancer attributable to radon place indoor radon among the leading causes of lung cancer in never smokers.
Indoor air pollution
Combustion of coal and biomass, and cooking fumes in the household
About half of the world’s population, mostly in low- and medium-resource countries, use solid fuels for cooking or heating, often in poorly ventilated spaces (114). Products of incomplete combustion contain respirable particles and many organic compounds, including carcinogens such as benzo[a]pyrene, formaldehyde, and benzene. Occupational exposure to the combustion products of coal by inhalation is known to cause lung cancer (115), and many studies, mostly from China, now show similar effects from household use of coal. These studies have been recently reviewed by an IARC Working Group that concluded that there is sufficient evidence in humans for the carcinogenicity of indoor emissions from household combustion of coal (116). This evaluation is supported by the results of studies in experimental animals and by mechanistic evidence from humans and animals.
Coal
Although the IARC evaluation was not specific to never smokers, one important feature of the studies of indoor air pollution from coal burning in China (and to a less extent other countries) is that they included a large number of (and often were restricted to) never smoking women. Results of epidemiological studies of indoor combustion of coal and lung cancer risk in never smokers are summarized in Supplemental Table 2. Use of coal for cooking and heating was associated with increased lung cancer risk among never smokers in two of the earliest studies (130, 131). A population-based case-control study found that the risk of lung cancer among women is China is strongly dependent on the type of coal used for home heating and cooking (117). A retrospective cohort study noted a striking correlation between improved ventilation and decreased lung cancer rates in the same population (p < 0.001) (118). Using coal for cooking throughout life compared to using modern cooking fuels (gas, electricity or kerosene) increased the risk of lung cancer (OR: 7.5 (95% CI: 2.2, 25.9) among never smoking males and females in India, after adjustment for age, gender, center, socioeconomic status, and use of non-cigarette tobacco products (119).
Biomass
Worldwide, biomass is much more widely used as fuel than coal but the adverse health effects have been studied less (Supplemental Table 2). In five studies, researchers investigated indoor exposure to smoke from wood, straw, and other solid fuel and lung cancer risk among never smoking women. Three studies, conducted in Japan, China, and Mexico, found an increased risk in lung cancer among never smoking women exposed to smoke produced while cooking with various biomass fuels. However, in two studies conducted in India, ever use of biomass fuels was not associated with an increased risk of lung cancer among never smoking women, nor was long-term use of solid cooking fuel in comparison to modern cooking fuel (gas, electricity, or kerosene). These studies suggest that exposure to smoke from wood combustion is associated with an increased risk of lung cancer, but the results on exposure duration and intensity are difficult to interpret. Furthermore, a study from Central and Eastern Europe provided no supporting evidence that use of solid fuels, including coal and wood, increases the risk of lung cancer among non-smokers (120). The epidemiological evidence of an increased risk of lung cancer for exposure to biomass (mainly wood) combustion emissions was classified by the IARC working group as limited (116): this evaluation, however, was not specific to non-smokers.
Cooking fumes
Stir-frying, deep-frying, and pan-frying, which involve heating oil to high temperatures, are practiced worldwide, especially in China. The epidemiological evidence on cancer from exposure to emissions from high-temperature frying was classified as limited by the IARC Working Group (116). Results from ten case-control studies have investigated the relationship between exposure to cooking fumes and the risk for lung cancer among never smokers (Supplemental Table 3). The majority of the studies found a positive association between lung cancer in never smoking women and various methods of cooking with oil at high temperatures. A study of Chinese women from Singapore, however, did not detect an increased risk for stir-frying (121).
In summary, an increased risk of lung cancer has been consistently shown among never smoking women exposed to indoor biomass smoke and cooking fumes. Less consistent results were found for different types of oils used for cooking and exposure to smoke from coal and risk of lung cancer among never smokers.
Occupational agents
Asbestos
The effect of asbestos exposure on risk of lung cancer among never smokers has been investigated in several cohort and case-control studies (Supplemental Table 4). With a few exceptions, these studies found an increased risk of lung cancer among never smokers who were occupationally exposed to asbestos relative to comparison groups of unexposed never smokers. The relative risks for exposure to asbestos varied between the studies, likely reflecting both the heterogeneity of exposure circumstances (level of exposure, type of fibers) and differences in the definition of asbestos exposure and never smokers. The relative risks of lung cancer for asbestos exposure tended to be higher in never smokers than in smokers (relative asbestos effect [RAE] ranging from 1.5 to 5.4 in most studies). Although the precise nature of the interaction between asbestos and tobacco smoking in lung carcinogenesis remains subject to debate (96), the evidence of a carcinogenic effect of asbestos independent from smoking is very strong.
Arsenic
In a case-control study nested in a cohort of copper smelter workers from Sweden, Pershagen and colleagues (122) reported an OR of 2.6 (95% CI 0.29, 23 [calculated based on raw data]) for exposure to arsenic among non-smokers (defined as subjects who had not smoked daily during more than two years at any time). An expanded analysis of this population confirmed the increased risk of lung cancer (OR 1.4, 5.6 <15 and ≥15 μg/m3/yr arsenic exposure) (123, 124) (Supplemental Table 5). Similar results were reported in a study of Chinese tin mines (125). Additional cohort studies from United States and Japan also reported an increased risk of lung cancer among never (126) and non- smoking (127, 128) miners or smelters, with risk estimates ranging from 2.6 to 5.1, as compared to unexposed workers. Occupational exposure to arsenic was self-reported in a community-based case-control study from Missouri (OR 1.1; 95% CI 0.2, 5.8) (129). In a study from Sweden, residence near a non-ferrous smelter emitting arsenic together with other metals was not associated with increased risk of lung cancer (130).
Silica
Several industry-based studies of workers exposed to silica and of silicotic patients reported an increased risk of lung cancer among never smokers (Supplemental Table 6). In a multicenter case-control study from 7 European countries, in which exposure to 70 agents was assessed by industrial hygienists on the basis of detailed occupational questionnaires, the RR for ever exposure to silica was 1.76 (95% CI 0.97, 3.21), and a positive relationship was suggested with duration of exposure and cumulative exposure (131). Two additional studies reported an increased risk of lung cancer among never and non-smoking workers exposed to silica (132, 133).
At least ten studies analyzed lung cancer risk among never smoking silicosis patients, defined either on the basis of compensation or medical (including necropsy) records. All but three showed an increased RR, in the range 1.6–2.2 (with the exception of a RR of 5.3 with broad confidence interval). In the three studies with point estimates of the RR below unity, the confidence intervals were compatible with an excess risk on the order of 80%. Although a formal meta-analysis is made difficult by the lack of confidence interval of several of the risk estimates, the overall evidence points towards an increased risk of cancer among persons with silicosis in the absence of tobacco smoking.
Exposure to other agents
In the case-control study of lifetime non-smoking women from Missouri, an increased risk of lung cancer was detected for exposure to pesticides (OR 3.1; 95% CI 1.3, 7.5) (129). In the multicentric European study (131), results were reported for 11 agents, in addition to silica: the OR for ever exposure to non-ferrous metal dust and fumes was 1.73 (95% CI 1.02, 2.92), that for ever exposure to organic solvents was 1.46 (95% CI 0.94, 2.24). For these two agents, a duration-response relationship was suggested.
Exposure to any known or suspected occupational lung carcinogens
Three European case-control studies reported results according to employment in job and industries entailing exposure to known (list A) or suspected (list B) occupational lung carcinogens, based on a simplified job exposure matrix developed by Ahrens and Merletti (134) (Supplemental Table 7). In a pooled analysis of 12 European case-control studies of never smoking women, the OR for employment and jobs entailing exposure to suspected carcinogens was 1.69 (95% CI 1.09, 2.63), while the risk estimate for employment in jobs entailing exposure to known carcinogens was imprecise (OR 1.50; 95% CI 0.49, 4.53) (135).
Employment in specific occupations and industries
In a few studies, risk estimates of lung cancer among never smokers were reported according to employment in specific occupational or industry categories (Supplemental Table 8). A systematic analysis of jobs and industries of employment and lung cancer risk among non-smoking US Veterans revealed an increased risk for employment as a baker, agent, farm and home management advisor, therapist or healer, building manager, bookbinder, decorator or window dresser, and painter. The large number of job and industries included in this analysis, however, has likely generated false positive results.
In summary, an increased risk of lung cancer has been consistently shown among workers exposed to asbestos, arsenic and silica. Results on exposure to other known or suspected occupational lung carcinogens among never smokers are sparse. In general, the findings on occupational risk factors in never smokers parallel those in smokers, although the measure of the magnitude of the smoking interaction is complicated by the small number of cases of lung cancer among never smokers included in most studies.
Outdoor air pollution
An increased risk of lung cancer has been reported in populations exposed to high levels of outdoor air pollution. This association, however, might result from confounding by other factors, notably tobacco smoking, rather than from air pollution. Cohort and case-control studies are limited by difficulties in assessing past exposure to the relevant air pollutants. In several studies, exposure to air pollution has been assessed either on the basis of proxy indicators, such as the number of inhabitants in the community, or residence near a major pollution source: which limits the interpretation of the results. In a small number of studies, exposure to outdoor air pollution has been assessed on the basis of data on pollutant level matched to the residence of the study subjects. Two of these studies have reported results for never smokers (Supplemental Table 9): an increased risk of lung cancer for increasing level of exposure to air pollution (measured either as fine particles of nitrogen oxide) has been reported in these studies, although none reached the conventional level of statistical significance. In a third study, which included an equal number of never smokers and long-term quitters, no association was found between exposure to PM10 and lung cancer risk (136).
On the one hand, results on lung cancer risk from outdoor air pollution exposure among never smokers can be biased by residual confounding from occupational exposure to lung carcinogens and other social class-related factors. On the other hand, retrospective exposure to outdoor air pollution may be particularly vulnerable to misclassification, which in prospective studies would likely result in underestimation of the effect. While an increased risk of lung cancer among never smokers exposed to high levels of outdoor air pollution is plausible, the available evidence does not allow an accurate estimate of risk.
Diet
Dietary factors have been noted to be leading preventable causes of cancer (137). The second expert report from the World Cancer Research Fund and the American Institute for Cancer Research comprehensively reviewed the evidence for the association between diet and cancer for both smokers and non-smokers. For lung cancer, the expert panel concluded that fruits and foods containing carotenoids likely protect against lung cancer (138). The expert panel also concluded “[t]here is limited evidence suggesting that non-starchy vegetables protect against lung cancer”. Similarly, for consumption of foods containing selenium, selenium supplements, and foods containing quercetin, they also concluded that the limited evidence was suggestive of protection against lung cancer. The potential protective effect of selenium is the basis of an ongoing randomized clinical study of selenium supplementation vs. placebo in patients with a history of resected stage I lung cancer.
The panel concluded that there is convincing evidence that arsenic in drinking water is a cause of lung cancer, and that there is limited, inconsistent evidence suggesting that high-dose retinol supplements (in smokers), consumption of red meat, processed meats, total fat, and butter are causes of lung cancer (138).
Fruit
In 14 studies, researchers investigated fruit consumption and lung cancer among never smokers. Among these studies, three were cohort studies (139–141) including one multicenter study involving follow-up of 16 cohorts from seven countries over 25 years (139) and one multicenter case-control study with participants from six European countries (142). None of the cohort studies found a significant association between total fruit consumption and lung cancer risk. Of 11 case-control studies (142–152), three found that never smokers who consumed the highest amount of fruit were less likely to have lung cancer when compared to those who consumed the lowest amount (147, 152, 153). The risk reductions observed ranged from 40% to 70% (Supplemental Table 10). Dose-response relationships were identified in two case-control studies. A multi-center case-control study, the study with the largest number of cases found in this literature review, did not find a relationship between total fruit consumption and lung cancer risk (142).
Vegetables
Two cohort studies (139, 140) and eight case-control studies (142, 144, 147, 148, 150–152) investigated consumption of total vegetables and lung cancer among never smokers. The Seven Countries study found a 10% reduction (95% CI 0.67, 1.08) in the risk of lung cancer development among never smokers per 18g increase in total vegetable consumption (139). The Japan Public Health cohort study followed 56,049 participants for seven to ten years; a total of 106 cases of lung cancer developed among never smokers. The authors reported a 40% increased risk (95% CI 0.79, 2.30) of developing lung cancer for those with a high consumption of total vegetables when compared to those with low consumption (140). Of the eight case-control studies that examined total consumption of vegetables, most found a decreased risk for lung cancer among those in the highest category of consumption (Supplemental Table 10).
Most of the studies that considered total vegetable consumption also investigated specific vegetables. Additionally, four studies (one cohort and three case-control studies) were found on the association between specific grouping of vegetables and lung cancer. Results of the few studies in each category are presented in Supplemental Table 10.
Meat and fish
One cohort study and five case-control studies investigated meat consumption and risk of lung cancer among never smokers, mostly women. The cohort study of more than 50,000 Japanese women found those consuming ham and sausages 3 to 4 times or more a week had a two-fold increased risk (95% CI 1.15, 3.53; p trend <0.05) of lung cancer compared to those that ate ham and sausages less than 1–2 times a month, after adjustment of age, and family history of lung cancer. SHS exposure was not a confounder in this study (141).
High fish consumption was found to decrease the risk of lung cancer among never smokers in China. A case-control study of Chinese women found a 60% (95% CI 25%, 84%; p trend <0.05) decreased risk of developing lung cancer among women in the highest tertile of fish consumption compared to women in the lowest tertile, after adjustment for age, number of live births, and education (153).
Miscellaneous
The majority of studies looking at other aspects of diet, including fat consumption, food preparation, and dairy, egg, and soy product consumption (Supplemental Table 10), found no significant association with lung cancer in never smokers.
Micronutrients
One nested case-control study and eight case-control studies investigated dietary carotenoid consumption. A case-control study conducted in Stockholm reported a protective effect and a dose-response with increasing intake of total carotenoids, with adjustment for SHS exposure (146). Candelora et al. conducted a study in Florida, US, among never smoking women and found a decreased risk in lung cancer with increasing consumption of total carotenoids, alpha-and beta-carotene, vitamin A, and vitamin C (152). A few studies also investigated other micronutrients (Supplemental Table 10).
Arsenic in drinking water
In a case-control study from Chile, the risk ratios of lung cancer were 5.9 (95% CI 1.2, 40) and 8.0 (95% CI 1.7, 52) for 50–199 and 200 or more μg arsenic per liter of drinking water, as compared to less than 50 μg/L, after adjustment for age and gender (154). In a similar study from Taiwan, risk ratios were 1.24 (95% CI 0.53, 2.91) and 2.21 (95% CI 0.71, 6.86) for exposure to 10–699 and 700 or more μg/L as compared to less than 10 μg/L, adjustment for age, gender, education, and alcohol consumption (155). The relevance of these data to never smokers specifically has not been addressed.
Other risk factors
Hormone replacement therapy
There is relatively little research in the area of hormone replacement therapy (HRT) and lung cancer risk, although some studies indicate a reduction of lung cancer risk associated with HRT use (Supplemental Table 11). However, although this inverse association has been present in case-control studies that have adjusted for smoking, stratification by smoking status reveals this inverse association exists for current smokers only (156, 157). For example, one study that examined risk among smokers and never smokers who had taken HRT showed that only the current smokers had a significant decrease in lung cancer risk (157). When examining lung cancer risk and hormone replacement therapy among never smokers, there does not appear to be a statistically significant association (158), although very few studies have been conducted that stratified by smoking status. Furthermore, the studies that have examined never smokers include a very small number of patients, so that their results are imprecise. In conclusion, although several studies have demonstrated an inverse association between HRT use and lung cancer risk, it is unclear whether such an association is present among never smokers.
Infections
Human papillomavirus (HPV)
Several studies have examined whether chronic infections can increase lung cancer risk. Human papillomavirus (HPV) infection has been observed in association with lung cancer cases in many studies, particularly in China (Supplemental Table 12). These studies suggest that HPV 6, HPV 16, and HPV 18 are all more prevalent among lung cancer cases than controls. HPV 6, which was examined in only one of these studies, appeared to be associated with smoking status, with male smokers having higher odds of having HPV 6 than male never smokers (OR 7.35, 95% CI 2.11–25.58) (159). Although these studies suggest an association of HPV infection with lung cancer risk, it is still unclear if HPV infection is associated with lung cancer risk among never smokers. These studies were limited to China and it is unknown if similar associations are present in other countries.
Human immunodeficiency virus (HIV)
Human immunodeficiency virus (HIV) has been studied in association with lung cancer risk, although this has not been a large area of study. Results from a retrospective cohort study conducted at a HIV specialty clinic showed that people infected with HIV had approximately a 2-fold significant increase in lung cancer risk, after adjustment for smoking status (160). Kirk et al. conducted a cohort study among injection drug users and reported that people infected with HIV have a significant increase in lung cancer risk (Hazard ratio 3.6, 95% CI 1.6, 7.9), independent of smoking status (161). Although it has been shown that people infected with HIV have an increased lung cancer risk, this has not been stratified by smoking status, so it is unclear of whether the risk is the same or different among never smokers.
Chlamydia pneumoniae
Chlamydia pneumonia has also been investigated in relation to lung cancer risk under the hypothesis that chronic infections may increase risk (162, 163). It has been reported that former smokers that are infected have a larger risk of lung cancer than current smokers, but there were no never smokers included in this study (163). A recent report examining chlamydial immunoglobulin titers in 90 never smokers (defined here as <400 lifetime cigarettes smoked) with lung cancer and 68 never-smoking controls found no evident association between infection and cancer, but the power of this study was clearly suboptimal (164).
History of lung disease prior to lung cancer diagnosis
History of lung disease has been examined in association with lung cancer risk, including tuberculosis, asthma, emphysema, and chronic obstructive pulmonary disease (COPD). Studies have shown that persons with tuberculosis have as much as a 50% increase in lung cancer risk (165), although differences by smoking status has been examined in only a few studies (Table 3). Interestingly, one study that examined lung cancer risk among smokers and never smokers with tuberculosis found that female never smokers with tuberculosis had approximately an 8-fold increase in lung cancer risk, while there was no association among female smokers (166). This study is limited by the small number of never smoking lung cancer patients.
Table 3.
Risk of lung cancer associated with previous lung disease among never smokers
| Study | Design/population | Exposure Group | Reference Group | Risk Estimate (95% CI) | Adjustment Variables | |
|---|---|---|---|---|---|---|
| Hinds et al. (1982) (166) | Case-control: 211 female lung cancer cases (never smokers) 419 female population controls (never smokers) Study location: USA (HI) Study years: 1968–1978 |
History of pulmonary tuberculosis infection | vs. | No history of pulmonary tuberculosis infection | 8.2 (1.3–54.4) | age, birthplace, race |
|
| ||||||
| Zheng et al. (1987) (165) | Case-control: 415 male and female lung cancer cases (never smokers) 714 male and female population controls (never smokers) Study location: China (Shanghai) Study years: 1984–1986 |
Diagnosed with tuberculosis <20 years ago | vs. | Never diagnosed with tuberculosis | 3.5 (1.5–8.0) | age, education, gender |
| Diagnosed with tuberculosis ≥20 years ago | vs. | Never diagnosed with tuberculosis | 1.0 (0.7–1.5) | age, education, gender | ||
|
| ||||||
| Alavanja et al. (1992) (180) | Case-control: 186 female lung cancer cases (never smokers) 234 female population controls (never smokers) Study location: USA (MO) Study years: 1986–1991 |
History of lung lung disease (asthma, chronic bronchitis, emphysema, pluerisy, pneumonia, tuberculosis) | vs. | No history of lung disease | 1.4 | none stated |
| History of asthma | vs. | No history of asthma | 2.7 | none stated | ||
| History of pneumonia | vs. | No history of pneumonia | 1.5 | none stated | ||
| History of tuberculosis | vs. | No history of tuberculosis | “no association” (estimate not stated) | none stated | ||
| History of pleurisy | vs. | No history of pleurisy | “no association” (estimate not stated) | none stated | ||
| History of chronic bronchitis | vs. | No history of chronic bronchitis | “no association” (estimate not stated) | none stated | ||
|
| ||||||
| Lan et al. (1993) (181) | Case-control: 139 female lung cancer cases (nonsmokers) 139 female population controls (nonsmokers) Study location: China (Xuanwei) Study years: 1988–1990 |
History of chronic bronchitis | vs. | No history of chronic bronchitis | 7.04 (1.79–27.77) | age, menstrual cycle length, age of menopause, smoky coal exposure |
|
| ||||||
| Ko et al. (1997) (182) | Case-control: 105 female lung cancer cases (never smokers) 105 female hospital controls (never smokers) Study location: Taiwan Study years: 1992–1993 |
History of pulmonary tuberculosis infection | vs. | No history of pulmonary tuberculosis infection | 5.9 (1.3–25.9) | age, living near industrial district, cooking fuels, fume extractor use, vegetable consumption, SES, residential area, education |
|
| ||||||
| Shen et al. (1998) (183) | Case-control: 70 female adenocarcinoma cases (never smokers) 70 female population controls (never smokers) Study location: China (Nanjing) Study year: 1993 |
History of pulmonary tuberculosis or chronic broncitis | vs. | No history of pulmonary tuberculosis or chronic bronchitis | 3.90 (1.00–20.94) | age, occupation, neighborhood, cooking fumes, family history of cancer |
|
| ||||||
| Santillan et al. (2003) (167) | Meta-analysis: 5 case-control studies 1370 total male and female lung cancer cases Study locations: USA, China Publication dates: 1985–2001 |
History of asthma | vs. | No history of asthma | 1.8 (1.3–2.3) | not stated |
|
| ||||||
| Neuberger et al. (2006) (184) | Case-control: 56 female lung cancer cases (never smokers) 414 female population controls (never smokers) Study location: USA (IA) Study years: 1993–1996 |
Previous lung disease (bronchitis, emphysema, asthma, tuberculosis, silicosis, asbestosis, COPD) | vs. | No previous lung disease | 2.28 (1.24–4.18) | age, education, radon |
|
| ||||||
| Seow et al. (2006) (185) | Case-control: 126 female lung cancer cases (never smokers) 162 female hospital controls (never smokers) Study location: Singapore Study years: 1996–1998 |
History of asthma, allergic rhinitis, or atopic dermatitis (all histological types) | vs. | No history of asthma, allergic rhinitis, or atopic dermatitis (all histological types) | 1.5 (0.8–2.6) | age, fruit and vegetable intake, birthplace, SHS exposure |
| History of asthma, allergic rhinitis, or atopic dermatitis (adenocarcinomas) | vs. | No history of asthma, allergic rhinitis, or atopic dermatitis (adenocarcinomas) | 1.6 (0.9–3.1) | age, fruit and vegetable intake, birthplace, SHS exposure | ||
Abbreviations:
CI: confidence interval
COPD: chronic obstructive pulmonary disease
SES: socioeconomic status
SHS: secondhand smoke
Asthma has also been frequently studied in terms of lung cancer risk. Several studies have examined this potential risk factor, including a meta-analysis consisting of 8 case-control and 10 cohort studies (167). Most of these studies demonstrated an increased risk of lung cancer in never smokers with asthma (Table 3).
Several studies have suggested that patients with idiopathic pulmonary fibrosis or other fibrotic disorders are at increased risk for lung cancer (168–171), but these risk factors have not been clearly defined in never smokers. There has been little research on COPD and lung cancer risk specific to never smokers, as never smokers rarely develop this disease. In a Chinese province in which exposure indoor smoky coal combustion is common, COPD (chronic bronchitis) in never-smoking women has been associated with increased lung cancer risk (172).
Ionizing radiation
Many studies have examined the risk of lung cancer due to ionizing radiation among never smokers. Most of the studies have examined risk following radiation therapy for Hodgkin’s disease or for breast cancer, although some have examined occupational x-ray exposure as well (Table 4). Most studies demonstrate an increased risk of lung cancer due to radiation for treatment of Hodgkin’s disease or breast cancer. However, most of these studies show that this increased risk is higher among smokers, possibly due to the multiplicative effects of cigarette smoking and radiotherapy (173–175). For atomic bomb survivors, the effect of radiation exposure and smoking on lung cancer has been found to be additive (176).
Table 4.
Results of studies on exposure to radiation and risk of lung cancer among never smokers
| Study | Design/population | Exposure Group | Reference Group | Risk Estimate (95% CI) | Adjustment Variables | |
|---|---|---|---|---|---|---|
| Neugut et al. (1994) (173) | Case-control: 16 female lung cancer cases among breast cancer survivors (nonsmokers) 348 female breast cancer survivor controls (nonsmokers) Study location: USA (CT) Study years: 1986–1989 |
Received radiation therapy for breast cancer | vs. | Did not receive radiation therapy for breast cancer | 3.2 (0.6–17.4) | age |
|
| ||||||
| van Leeuwen et al. (1995) (175) | Case-control: From a cohort of 1939 Hodgkin’s disease patients: 8 male and female lung cancer cases (nonsmokers) 33 male and female controls (nonsmokers) Study location: The Netherlands Study years: 1966–1986 |
Received 1–5 Gy radiation therapy for Hodgkin’s disease | vs. | Received <1 Gy radiation therapy for Hodgkin’s disease | 0.99 (0.07–14.7) | age, gender, treatment center, date of Hodgkin’s diagnosis |
| Received ≥5 Gy radiation therapy for Hodgkin’s disease | vs. | Received <1 Gy radiation therapy for Hodgkin’s disease | 2.5 (0.21–29.4) Ptrend = 0.43 |
age, gender, treatment center, date of Hodgkin’s diagnosis | ||
|
| ||||||
| Hu et al. (2002) (144) | Case-control: 161 female lung cancer cases (never smokers) 483 female population controls Study location: Canada Study years: 1994–1997 |
Occupational radiation sources | vs. | No occupational exposure to radiation sources | 2.1 (0.7–6.8) | age, province, education, social class |
|
| ||||||
| Ford et al. (2003) (186) | Case-control: 41 female lung cancer cases among breast cancer survivors (never smokers) 159 female breast cancer survivor controls (never smokers) Study location: USA (TX) Study years: 1960–1997 |
Received radiation therapy for breast cancer | vs. | Did not receive radiation therapy for breast cancer | 0.60 | age, ethnicity, breast cancer histology, type of breast cancer surgery, date of breast cancer diagnosis |
|
| ||||||
| Boffetta et al. (2005) (187) | Case-control: 209 male and female lung cancer cases (never smokers) 976 male and female hospital and population controls (never smokers) Study location: Czech Republic, Hungary, Poland, Romania, Russia, Slovakia Study years: 1998–2002 |
1–30 occupational x-ray examinations | vs. | No occupational x-ray examinations | 1.22 (0.73–2.03) | none stated |
| >30 occupational x-ray examinations | vs. | No occupational x-ray examinations | 2.30 (1.15–4.57) | none stated | ||
|
| ||||||
| Prochazka et al. (2005) (174) | Case series: 82 women diagnosed with breast cancer and then subsequent lung cancer (nonsmokers) Study location: Sweden Study years: 1958–2000 |
Received ipsilateral radiation therapy for breast cancer (breast and lung cancer on same side) | vs. | Contralateral radiation dose to lung ≤15% of the ipsilateral dose (lung on opposite side of breast cancer served as control) | 0.9 (0.37–2.22) | none stated |
Abbreviations:
CI: confidence interval
Gy: gray
SUMMARY
The large numbers of current and former smokers dying of lung cancer have obscured the important problem of lung cancer in never-smokers. Lung cancer in never smokers accounts for 16,000 – 24,000 deaths annually in the US, among the top 10 causes of cancer mortality as a separate entity from smoking-related cancer. Incidence of lung cancer in never smokers increases with age. Current epidemiologic data does not indicate a significant change in risk over time, or a clear gender bias in the risk of lung cancer in never smokers. However, among female never-smokers, large differences in lung cancer risk exist between populations, with strikingly higher risk in several East Asian countries, and in particular China. Factors contributing to these population differences may include both underlying genetic susceptibility as well as exposure to carcinogens including coal smoke, aerosolized cooking oils, and second hand smoke. Studies evaluating gene—environment interactions may provide important insights into carcinogenesis pathways of lung cancer in never smokers. Such studs require not only adequate sample sizes, but also detailed exposure assessments in relevant populations.
Strong evidence from multiple sources supports the causal association of second hand smoke exposure in lung cancer in never smokers. Similarly, exposure to radon, common in indoor environments, is a well established cause of lung cancer in never smokers. In the US, these two factors may account for the majority of cases of lung cancer in never smokers. Indoor air pollution, including combustion of coal or solid fuels for cooking or heating in poorly ventilated spaces, has been clearly associated with increased risk of lung cancer in never smokers, and may be a particularly important factor contributing to the high incidence of lung cancer in never smokers in the East Asia. In addition to radon, other exogenous ionizing radiation exposures have been clearly linked to lung cancer risk. Additional exposures associated with lung cancer in never smokers in multiple studies include asbestos, which has known carcinogenic synergy with tobacco smoke, arsenic, and silica.
Studies of dietary factors contributing to lung cancer have been less consistent, and data specific to never smokers has not been extensively explored. Diets containing relatively high levels of carotenoids, selenium, or quercetin appear to be associated with decreased risk of lung cancer; conversely consumption of meat, fat, and butter may be associated with increased risk of lung cancer.
Viral infections including HPV and HIV have been implicated in lung cancer risk in studies that included both never smokers and ever smokers. It is unclear whether these viruses act synergistically with tobacco, or constitute independent risk factors for lung cancer in never smokers. Finally, chronic lung diseases including tuberculosis, COPD, and asthma have been associated with increased lung cancer risk. Of these, asthma appears to be significantly associated with lung cancer risk in never smokers.
The death rate due to lung cancer in never smokers over several decades has remained relatively constant in the US, and represents a significant ongoing public health problem. Common limitations found in many of the epidemiologic studies reviewed included a failure to clearly stratify analyses by smoking status (never vs. ever; never vs. former vs. active), failure to quantify exposure to second hand smoke and other risk factors, and variable definitions of the terms non-smoker and never smoker. Given the significant impact of lung cancer in never smokers, focused research on genetic and environmental factors associated with this disease, in carefully defined and extensively characterized populations, is warranted.
Supplementary Material
Acknowledgments
Supported by the Flight Attendant Medical Research Institute (FAMRI).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.US Center for Disease Control. Annual smoking-attributable mortality, years of potential life lost, and productivity losses -- United States, 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–8. [PubMed] [Google Scholar]
- 3.Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. Journal of the National Cancer Institute. 2006;98:691–9. doi: 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 4.Polednak AP. Obtaining smoking histories for population-based studies on multiple primary cancers: Connecticut, 2002. International journal of cancer. 2006;119:233–5. doi: 10.1002/ijc.21786. [DOI] [PubMed] [Google Scholar]
- 5.Mayer JL, Boffetta P, Kuroda MM. Comparison of questionnaire-derived and tumour registry-derived smoking histories. Eur J Cancer. 1992;28:116–7. doi: 10.1016/0959-8049(92)90398-l. [DOI] [PubMed] [Google Scholar]
- 6.Rudin CM, Avila-Tang E, Samet JM. Lung cancer in never smokers: a call to action. Clin Cancer Res. 2009:15. doi: 10.1158/1078-0432.CCR-09-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudin CM, Avila-Tang E, Harris CC, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res. 2009:15. doi: 10.1158/1078-0432.CCR-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler I. Primary malignant growths of the lung and bronchi. New York: Longmans, Green and Company; 1912. [Google Scholar]
- 9.Hoffman F. Cancer of the lungs. American Review of Tuberculosis. 1929;19:392. [Google Scholar]
- 10.Doll R. Uncovering the effects of smoking: historical perspective. Statistical methods in medical research. 1998;7:87–117. doi: 10.1177/096228029800700202. [DOI] [PubMed] [Google Scholar]
- 11.Duguid J. The incidence of intrathoracic tumors in Manchester. Lancet. 1927;210:111–6. [Google Scholar]
- 12.US Department of Health, Education, and Welfare. Report of the Advisory Committee to the Surgeon General. Washington, D.C: US Department of Health Education and Welfare (DHEW); 1964. Smoking and Health. Report No.: 1103. [Google Scholar]
- 13.Haenszel W, Taeuber K. Lung-cancer mortality as related to residence and smoking histories. II. White Females. Journal of the National Cancer Institute. 1964;32:803–38. [PubMed] [Google Scholar]
- 14.Samet JM, Wiggins CL, Humble CG, Pathak DR. Cigarette smoking and lung cancer in New Mexico. The American review of respiratory disease. 1988;137:1110–3. doi: 10.1164/ajrccm/137.5.1110. [DOI] [PubMed] [Google Scholar]
- 15.Boffetta P, Jarvholm B, Brennan P, Nyren O. Incidence of lung cancer in a large cohort of non-smoking men from Sweden. International journal of cancer. 2001;94:591–3. doi: 10.1002/ijc.1507. [DOI] [PubMed] [Google Scholar]
- 16.Kahn H. The Dorn study of smoking and mortality among U.S. veterans: report on eith and one-half years of observation. Natl Cancer Inst Monogr. 1966:1–126. [PubMed] [Google Scholar]
- 17.Marugame T, Sobue T, Satoh H, et al. Lung cancer death rates by smoking status: comparison of the Three-Prefecture Cohort study in Japan to the Cancer Prevention Study II in the USA. Cancer science. 2005;96:120–6. doi: 10.1111/j.1349-7006.2005.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda S, Yanai N, Ishikawa S. Flexible bronchofiberscope. Keio J Med. 1968;17:1–16. doi: 10.2302/kjm.17.1. [DOI] [PubMed] [Google Scholar]
- 19.Koss LG. Thin needle aspiration biopsy. Acta cytologica. 1980;24:1–3. [PubMed] [Google Scholar]
- 20.Lundgren R, Bergman F, Angstrom T. Comparison of transbronchial fine needle aspiration biopsy, aspiration of bronchial secretion, bronchial washing, brush biopsy and forceps biopsy in the diagnosis of lung cancer. European journal of respiratory diseases. 1983;64:378–85. [PubMed] [Google Scholar]
- 21.Wittenberg J. Computed tomography of the body. The New England journal of medicine. 1983;309:1224–9. doi: 10.1056/NEJM198311173092006. [DOI] [PubMed] [Google Scholar]
- 22.Henschke CI, Yankelevitz DF, Smith JP, Miettinen OS. Screening for lung cancer: the early lung cancer action approach. Lung cancer (Amsterdam, Netherlands) 2002;35:143–8. doi: 10.1016/s0169-5002(01)00416-0. [DOI] [PubMed] [Google Scholar]
- 23.Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology. 2004;231:164–8. doi: 10.1148/radiol.2311030634. [DOI] [PubMed] [Google Scholar]
- 24.Patel JD, Bach PB, Kris MG. Lung cancer in US women: a contemporary epidemic. JAMA. 2004;291:1763–8. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 25.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene. 2002;21:7307–25. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
- 26.Prescott E, Osler M, Hein HO, et al. Gender and smoking-related risk of lung cancer. The Copenhagen Center for Prospective Population Studies. Epidemiology (Cambridge, Mass. 1998;9:79–83. [PubMed] [Google Scholar]
- 27.Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1184–8. doi: 10.1158/1055-9965.EPI-06-0032. [DOI] [PubMed] [Google Scholar]
- 28.Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. Journal of the National Cancer Institute. 2003;95:470–8. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 29.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–8. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henschke CI, Yankelevitz DF, Libby DM, et al. Early lung cancer action project: annual screening using single-slice helical CT. Annals of the New York Academy of Sciences. 2001;952:124–34. doi: 10.1111/j.1749-6632.2001.tb02733.x. [DOI] [PubMed] [Google Scholar]
- 31.Henschke CI, Miettinen OS. Women’s susceptibility to tobacco carcinogens. Lung cancer (Amsterdam, Netherlands) 2004;43:1–5. doi: 10.1016/j.lungcan.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz AG, Swanson GM. Lung carcinoma in African Americans and whites. A population-based study in metropolitan Detroit, Michigan. Cancer. 1997;79:45–52. doi: 10.1002/(sici)1097-0142(19970101)79:1<45::aid-cncr7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Tobacco control country profiles; The 12th World Conference on Tobacco or Health; Atlanta, GA: American Cancer Society; World Health Organzation; International Union Against Cancer; 2003. [Google Scholar]
- 35.Parkin DM, Muir CS. Comparability and quality of data. IARC scientific publications; 1992. Cancer Incidence in Five Continents; pp. 45–173. [PubMed] [Google Scholar]
- 36.Li J, Committee E. Atlas of Cancer Mortality in teh People’s Republic of China. Shanghai: China Map Press; 1979. [Google Scholar]
- 37.Mumford JL, He XZ, Chapman RS, et al. Lung cancer and indoor air pollution in Xuan Wei, China. Science (New York, NY. 1987;235:217–20. doi: 10.1126/science.3798109. [DOI] [PubMed] [Google Scholar]
- 38.Wu-Williams AH, Dai XD, Blot W, et al. Lung cancer among women in north-east China. British journal of cancer. 1990;62:982–7. doi: 10.1038/bjc.1990.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao YT, Blot WJ, Zheng W, et al. Lung cancer among Chinese women. International journal of cancer. 1987;40:604–9. doi: 10.1002/ijc.2910400505. [DOI] [PubMed] [Google Scholar]
- 40.Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer research. 2006;66:4961–7. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 41.Zhong L, Goldberg MS, Gao YT, Jin F. Lung cancer and indoor air pollution arising from Chinese-style cooking among nonsmoking women living in Shanghai, China. Epidemiology (Cambridge, Mass. 1999;10:488–94. [PubMed] [Google Scholar]
- 42.Metayer C, Wang Z, Kleinerman RA, et al. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung cancer (Amsterdam, Netherlands) 2002;35:111–7. doi: 10.1016/s0169-5002(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 43.Gu D, Wu X, Reynolds K, et al. Cigarette smoking and exposure to environmental tobacco smoke in China: the international collaborative study of cardiovascular disease in Asia. American journal of public health. 2004;94:1972–6. doi: 10.2105/ajph.94.11.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng G, Liang Z, Zhang A, Wu G. On the relationship between smoking and female lung cancer. In: Aoki M, Tominaga S, editors. Smoking and Health. Vol. 1987. Amsterdam: Excerpta Medica; 1988. pp. 483–6. [Google Scholar]
- 45.Lee C, Kang KH, Koh Y, et al. Characteristics of lung cancer in Korea, 1997. Lung cancer (Amsterdam, Netherlands) 2000;30:15–22. doi: 10.1016/s0169-5002(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 46.Enstrom JE. Rising lung cancer mortality among nonsmokers. Journal of the National Cancer Institute. 1979;62:755–60. [PubMed] [Google Scholar]
- 47.Axelson O, Davis DL, Forestiere F, Schneiderman M, Wagener D. Lung cancer not attributable to smoking. Annals of the New York Academy of Sciences. 1990;609:165–76. doi: 10.1111/j.1749-6632.1990.tb32065.x. discussion 76–8. [DOI] [PubMed] [Google Scholar]
- 48.Davis DL. Trends in nonsmoking lung cancer. Epidemiology (Cambridge, Mass. 1993;4:489–92. doi: 10.1097/00001648-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Schneiderman MA, Davis DL, Wagener DK. Lung cancer that is not attributable to smoking. Jama. 1989;261:2635–6. doi: 10.1001/jama.1989.03420180059019. [DOI] [PubMed] [Google Scholar]
- 50.Forastiere F, Perucci CA, Arca M, Axelson O. Indirect estimates of lung cancer death rates in Italy not attributable to active smoking. Epidemiology (Cambridge, Mass. 1993;4:502–10. doi: 10.1097/00001648-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Cybernetic WL Wolfbane Cybernetic Ltd. International classification of diseases. Wolfbane Cybernertic, Ltd; 2007. [Google Scholar]
- 52.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. Jama. 1999;281:61–6. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman FL. Cancer and Smoking Habits. Annals of surgery. 1931;93:50–67. doi: 10.1097/00000658-193101000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ries L, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2004. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 55.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 56.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. International journal of cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 57.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 58.Anton-Culver H, Culver BD, Kurosaki T, Osann KE, Lee JB. Incidence of lung cancer by histological type from a population-based registry. Cancer research. 1988;48:6580–3. [PubMed] [Google Scholar]
- 59.Yun YH, Lim MK, Jung KW, et al. Relative and absolute risks of cigarette smoking on major histologic types of lung cancer in Korean men. Cancer Epidemiol Biomarkers Prev. 2005;14:2125–30. doi: 10.1158/1055-9965.EPI-05-0236. [DOI] [PubMed] [Google Scholar]
- 60.Liam CK, Pang YK, Leow CH, Poosparajah S, Menon A. Changes in the distribution of lung cancer cell types and patient demography in a developing multiracial Asian country: experience of a university teaching hospital. Lung cancer (Amsterdam, Netherlands) 2006;53:23–30. doi: 10.1016/j.lungcan.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. Journal of toxicology and environmental health. 1997;50:307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 62.Hecht SS. Tobacco smoke carcinogens and lung cancer. Journal of the National Cancer Institute. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 63.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. The lancet oncology. 2002;3:461–9. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 64.Hirayama T. Non-smoking wives of heavy smokers have a higher risk of lung cancer: a study from Japan. British medical journal (Clinical research ed. 1981;282:183–5. doi: 10.1136/bmj.282.6259.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trichopoulos D, Kalandidi A, Sparros L, MacMahon B. Lung cancer and passive smoking. International journal of cancer. 1981;27:1–4. doi: 10.1002/ijc.2910270102. [DOI] [PubMed] [Google Scholar]
- 66.US Department of Health and Human Services. The health effects of active smoking: A report of the Surgeon General. Washington, D.C: U.S. Government Printing Office; 2004. [Google Scholar]
- 67.US Department of Health and Human Services; Services UDoHaH. A report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Office on Smoking and Health; 1982. The health consequences of smoking: Cancer. [Google Scholar]
- 68.US Department of Health and Human Services; (USDHHS). UDoHaHS. The health consequences of involuntary smoking: A report of the Surgeon Gneral. Washington, DC: US Government Printing Office; 1986. [Google Scholar]
- 69.International Agency for Research on Cancer. Monograph. 38. Lyon, France: World Health Organization, IARC; 1986. IARC Monographs on the Evaluation fo the Carcinogenic Risk of Chemicals to Humans: Tobacco Smoking. [Google Scholar]
- 70.Lofroth G. Environmental tobacco smoke: overview of chemical composition and genotoxic components. Mutation research. 1989;222:73–80. doi: 10.1016/0165-1218(89)90021-9. [DOI] [PubMed] [Google Scholar]
- 71.Claxton LD, Morin RS, Hughes TJ, Lewtas J. A genotoxic assessment of environmental tobacco smoke using bacterial bioassays. Mutation research. 1989;222:81–99. doi: 10.1016/0165-1218(89)90022-0. [DOI] [PubMed] [Google Scholar]
- 72.Weiss SJ. Tissue destruction by neutrophils. The New England journal of medicine. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 73.Bennett WP, Alavanja MC, Blomeke B, et al. Environmental tobacco smoke, genetic susceptibility, and risk of lung cancer in never-smoking women. Journal of the National Cancer Institute. 1999;91:2009–14. doi: 10.1093/jnci/91.23.2009. [DOI] [PubMed] [Google Scholar]
- 74.Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, Hoffmann D. A tobacco-specific lung carcinogen in the urine of men exposed to cigarette smoke. The New England journal of medicine. 1993;329:1543–6. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- 75.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12:1257–61. [PubMed] [Google Scholar]
- 76.Maclure M, Katz RB, Bryant MS, Skipper PL, Tannenbaum SR. Elevated blood levels of carcinogens in passive smokers. American journal of public health. 1989;79:1381–4. doi: 10.2105/ajph.79.10.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crawford FG, Mayer J, Santella RM, et al. Biomarkers of environmental tobacco smoke in preschool children and their mothers. Journal of the National Cancer Institute. 1994;86:1398–402. doi: 10.1093/jnci/86.18.1398. [DOI] [PubMed] [Google Scholar]
- 78.Mauderly JL, Gigliotti AP, Barr EB, et al. Chronic inhalation exposure to mainstream cigarette smoke increases lung and nasal tumor incidence in rats. Toxicol Sci. 2004;81:280–92. doi: 10.1093/toxsci/kfh203. [DOI] [PubMed] [Google Scholar]
- 79.Trichopoulos D, Mollo F, Tomatis L, et al. Active and passive smoking and pathological indicators of lung cancer risk in an autopsy study. Jama. 1992;268:1697–701. [PubMed] [Google Scholar]
- 80.Brandt A. The Cigarette Centruy: The Rise, Fall, and Deadly Persistence of the Product That Defined America. New York: Basic Books; 2007. [Google Scholar]
- 81.US Department of Health and Human Services; Centers for Disease Control and Prevention (CDC) The health effects of involuntary exposure to tobacco smoke. US Department of Health and Human Services (USDHHS) R; MD: 2006. [Google Scholar]
- 82.Wald NJ, Nanchahal K, Thompson SG, Cuckle HS. Does breathing other people’s tobacco smoke cause lung cancer? British medical journal (Clinical research ed. 1986;293:1217–22. doi: 10.1136/bmj.293.6556.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee P. Misclassification of smoking habits and passive smoking. Berlin: Springer Verlag; 1988. [Google Scholar]
- 84.US Environmental Protection Agency; EPA. Respiratory health effects of passive smoking. Washington, DC: US Government Printing Office; 1992. [Google Scholar]
- 85.Wu AH. Exposure misclassification bias in studies of environmental tobacco smoke and lung cancer. Environmental health perspectives. 1999;107 (Suppl 6):873–7. doi: 10.1289/ehp.99107s6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman GD, Petitti DB, Bawol RD. Prevalence and correlates of passive smoking. American journal of public health. 1983;73:401–5. doi: 10.2105/ajph.73.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of exposure analysis and environmental epidemiology. 2001;11:231–52. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 88.Saracci R, Riboli E. Passive smoking and lung cancer: current evidence and ongoing studies at the International Agency for Research on Cancer. Mutation research. 1989;222:117–27. doi: 10.1016/0165-1218(89)90025-6. [DOI] [PubMed] [Google Scholar]
- 89.Brennan P, Buffler PA, Reynolds P, et al. Secondhand smoke exposure in adulthood and risk of lung cancer among never smokers: a pooled analysis of two large studies. International journal of cancer. 2004;109:125–31. doi: 10.1002/ijc.11682. [DOI] [PubMed] [Google Scholar]
- 90.Thornton A, Lee P, Fry J. Differences between smokers, ex-smokers, passive smokers and non-smokers. Journal of clinical epidemiology. 1994;47:1143–62. doi: 10.1016/0895-4356(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 91.Lee PN. Difficulties in assessing the relationship between passive smoking and lung cancer. Statistical methods in medical research. 1998;7:137–63. doi: 10.1177/096228029800700204. [DOI] [PubMed] [Google Scholar]
- 92.US Department of Health and Human Services; (USDHHS) UDoHaHS. A report of the Surgeon General. Washington, DC: US Government Printing Office; 1989. Reducing the health consequences of smoking. 25 years of progress. [Google Scholar]
- 93.Matanoski G, Kanchanaraksa S, Lantry D, Chang Y. Characteristics of nonsmoking women in NHANES I and NHANES I epidemiologic follow-up study with exposure to spouses who smoke. American journal of epidemiology. 1995;142:149–57. doi: 10.1093/oxfordjournals.aje.a117613. [DOI] [PubMed] [Google Scholar]
- 94.US National Research Council; NRCNaCoP. Smoking. Washington, DC: National Academy Press; 1986. Environmental tobacco smoke: Measuring exposures and assessing health effects. [PubMed] [Google Scholar]
- 95.Californial Environmental Protection Agency; Board CEPACEaAR. Proposed identification of environmental tobacco smoke as a toxic air contaminant. Sacramento, CA: California Environmental Protection Agency; 2005. [Google Scholar]
- 96.IARC Monograph. Vol. 83. France: International Agency for Research on Cancer; 2004. International Agency for Research on Cancer Tobacco smoke and involuntary smoking. [Google Scholar]
- 97.Fontham ET, Correa P, Reynolds P, et al. Environmental tobacco smoke and lung cancer in nonsmoking women. A multicenter study. Jama. 1994;271:1752–9. [PubMed] [Google Scholar]
- 98.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ (Clinical research ed. 1997;315:980–8. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Enstrom JE, Kabat GC. Environmental tobacco smoke and tobacco related mortality in a prospective study of Californians, 1960–98. BMJ (Clinical research ed. 2003;326:1057. doi: 10.1136/bmj.326.7398.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hackshaw A. Passive smoking: paper does not diminish conclusion of previous reports. BMJ. 2003;327:501–2. doi: 10.1136/bmj.327.7413.501-b. Clinical research ed. author reply 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weiss ST. Passive smoking and lung cancer. What is the risk? The American review of respiratory disease. 1986;133:1–3. doi: 10.1164/arrd.1986.133.1.1. [DOI] [PubMed] [Google Scholar]
- 102.Repace JL, Lowrey AH. Risk assessment methodologies for passive smoking-induced lung cancer. Risk Anal. 1990;10:27–37. doi: 10.1111/j.1539-6924.1990.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 103.National Research Council N; (NRC) NRC. Committee on Health Risks of Exposure to Radon, Board on Radiation Effects Research and Commission on Life Sciences Health Effects of Exposure to Radon (BEIR IV) Washington, DC: National Academy Press; 1999. [Google Scholar]
- 104.Hall EJ, Hei TK. Genomic instability and bystander effects induced by high-LET radiation. Oncogene. 2003;22:7034–42. doi: 10.1038/sj.onc.1206900. [DOI] [PubMed] [Google Scholar]
- 105.National Research Council (NRC) CotBEoIR; (NRC) NRC. Health Risks of Radon and Other Internally Deposited Alpha-Emitters: BEIR. Washington, DC: National Academy Press; 1988. [PubMed] [Google Scholar]
- 106.Samet JM. Indoor radon levels may be higher than in uranium mines. Radon and lung cancer: How great is the risk? J Respir Dis. 1989;10:73–83. [Google Scholar]
- 107.Harting F, Hesse W. Der Lungenkrebs, die bergkrankheit in den schneeberger gruben. Viertel Gerichtl Med Oeff Sanitaetswes. 1879;31:102–32. 313–37. [Google Scholar]
- 108.Archer VE, Wagoner JK, Lundin FE. Lung cancer among uranium miners in the United States. Health physics. 1973;25:351–71. doi: 10.1097/00004032-197310000-00001. [DOI] [PubMed] [Google Scholar]
- 109.Gilliland FD, Hunt WC, Archer VE, Saccomanno G. Radon progeny exposure and lung cancer risk among non-smoking uranium miners. Health physics. 2000;79:365–72. doi: 10.1097/00004032-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Samet JM, Kutvirt DM, Waxweiler RJ, Key CR. Uranium mining and lung cancer in Navajo men. The New England journal of medicine. 1984;310:1481–4. doi: 10.1056/NEJM198406073102301. [DOI] [PubMed] [Google Scholar]
- 111.Roscoe RJ, Deddens JA, Salvan A, Schnorr TM. Mortality among Navajo uranium miners. American journal of public health. 1995;85:535–40. doi: 10.2105/ajph.85.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roscoe RJ, Steenland K, Halperin WE, Beaumont JJ, Waxweiler RJ. Lung cancer mortality among nonsmoking uranium miners exposed to radon daughters. Jama. 1989;262:629–33. [PubMed] [Google Scholar]
- 113.Lubin JH, Boice JD, Jr, Edling C, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. Journal of the National Cancer Institute. 1995;87:817–27. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- 114.Rehfuess E, Mehta S, Pruss-Ustun A. Assessing household solid fuel use: multiple implications for the Millennium Development Goals. Environmental health perspectives. 2006;114:373–8. doi: 10.1289/ehp.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans: Supplement 7, overall evaluations of carcinogenecity, and updating of IARC monographs. Lyon, France: International Agency for Research on Cancer; 1987. pp. 1–42. [Google Scholar]
- 116.International Agency for Research on Cancer. Household use of solid fuels and high-temperature frying. Vol. 95. Lyon, France: International Agency for Research on Cancer; 2008. IARC Monographs on the evaluation of carcinogenic risks to humans. [Google Scholar]
- 117.Lan Q, He X, Shen M, et al. Variation in lung cancer risk by smoky coal subtype in Xuanwei, China. International journal of cancer. 2008;123:2164–9. doi: 10.1002/ijc.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lan Q, Chapman RS, Schreinemachers DM, Tian L, He X. Household stove improvement and risk of lung cancer in Xuanwei, China. Journal of the National Cancer Institute. 2002;94:826–35. doi: 10.1093/jnci/94.11.826. [DOI] [PubMed] [Google Scholar]
- 119.Sapkota A, Gajalakshmi V, Jetly DH, et al. Indoor air pollution from solid fuels and risk of hypopharyngeal/laryngeal and lung cancers: a multicentric case-control study from India. International journal of epidemiology. 2008;37:321–8. doi: 10.1093/ije/dym261. [DOI] [PubMed] [Google Scholar]
- 120.Lissowska J, Bardin-Mikolajczak A, Fletcher T, et al. Lung cancer and indoor pollution from heating and cooking with solid fuels: the IARC international multicentre case-control study in Eastern/Central Europe and the United Kingdom. American journal of epidemiology. 2005;162:326–33. doi: 10.1093/aje/kwi204. [DOI] [PubMed] [Google Scholar]
- 121.Seow A, Poh WT, Teh M, et al. Fumes from meat cooking and lung cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2000;9:1215–21. [PubMed] [Google Scholar]
- 122.Pershagen G, Wall S, Taube A, Linnman L. On the interaction between occupational arsenic exposure and smoking and its relationship to lung cancer. Scandinavian journal of work, environment & health. 1981;7:302–9. doi: 10.5271/sjweh.2544. [DOI] [PubMed] [Google Scholar]
- 123.Jarup L, Pershagen G. Arsenic exposure, smoking, and lung cancer in smelter workers--a case-control study. American journal of epidemiology. 1991;134:545–51. doi: 10.1093/oxfordjournals.aje.a116128. [DOI] [PubMed] [Google Scholar]
- 124.Jarup L, Pershagen G. Erratum. American journal of epidemiology. 1992;136:1174. [Google Scholar]
- 125.Hazelton WD, Luebeck EG, Heidenreich WF, Moolgavkar SH. Analysis of a historical cohort of Chinese tin miners with arsenic, radon, cigarette smoke, and pipe smoke exposures using the biologically based two-stage clonal expansion model. Radiation research. 2001;156:78–94. doi: 10.1667/0033-7587(2001)156[0078:aoahco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 126.Pinto SS, Henderson V, Enterline PE. Mortality experience of arsenic-exposed workers. Archives of environmental health. 1978;33:325–31. doi: 10.1080/00039896.1978.10667356. [DOI] [PubMed] [Google Scholar]
- 127.Welch K, Higgins I, Oh M, Burchfiel C. Arsenic exposure, smoking, and respiratory cancer in copper smelter workers. Archives of environmental health. 1982;37:325–35. doi: 10.1080/00039896.1982.10667586. [DOI] [PubMed] [Google Scholar]
- 128.Tsuda T, Nagira T, Yamamoto M, Kume Y. An epidemiological study on cancer in certified arsenic poisoning patients in Toroku. Industrial health. 1990;28:53–62. doi: 10.2486/indhealth.28.53. [DOI] [PubMed] [Google Scholar]
- 129.Brownson RC, Alavanja MC, Chang JC. Occupational risk factors for lung cancer among nonsmoking women: a case-control study in Missouri (United States) Cancer Causes Control. 1993;4:449–54. doi: 10.1007/BF00050864. [DOI] [PubMed] [Google Scholar]
- 130.Besso A, Nyberg F, Pershagen G. Air pollution and lung cancer mortality in the vicinity of a nonferrous metal smelter in Sweden. International journal of cancer. 2003;107:448–52. doi: 10.1002/ijc.11412. [DOI] [PubMed] [Google Scholar]
- 131.Zeka A, Mannetje A, Zaridze D, et al. Lung cancer and occupation in nonsmokers: a multicenter case-control study in Europe. Epidemiology (Cambridge, Mass. 2006;17:615–23. doi: 10.1097/01.ede.0000239582.92495.b5. [DOI] [PubMed] [Google Scholar]
- 132.Forastiere F, Lagorio S, Michelozzi P, et al. Silica, silicosis and lung cancer among ceramic workers: a case-referent study. American journal of industrial medicine. 1986;10:363–70. doi: 10.1002/ajim.4700100404. [DOI] [PubMed] [Google Scholar]
- 133.Siemiatycki J, Gerin M, Dewar R, Lakhani R, Begin D, Richardson L. Silica and cancer associations from a multicancer occupational exposure case-referent study. IARC scientific publications; 1990. pp. 29–42. [PubMed] [Google Scholar]
- 134.Ahrens W, Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. International journal of occupational and environmental health. 1998;4:236–40. doi: 10.1179/oeh.1998.4.4.236. [DOI] [PubMed] [Google Scholar]
- 135.Pohlabeln H, Boffetta P, Ahrens W, et al. Occupational risks for lung cancer among nonsmokers. Epidemiology (Cambridge, Mass. 2000;11:532–8. doi: 10.1097/00001648-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 136.Vineis P, Hoek G, Krzyzanowski M, et al. Air pollution and risk of lung cancer in a prospective study in Europe. International journal of cancer. 2006;119:169–74. doi: 10.1002/ijc.21801. [DOI] [PubMed] [Google Scholar]
- 137.Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360:861–8. doi: 10.1016/S0140-6736(02)09958-0. [DOI] [PubMed] [Google Scholar]
- 138.World Cancer Research Fund International. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 139.Mulder I, Jansen MC, Smit HA, et al. Role of smoking and diet in the cross-cultural variation in lung-cancer mortality: the Seven Countries Study. Seven Countries Study Research Group. International journal of cancer. 2000;88:665–71. doi: 10.1002/1097-0215(20001115)88:4<665::aid-ijc23>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y, Sobue T, Otani T, Tsugane S. Vegetables, fruit consumption and risk of lung cancer among middle-aged Japanese men and women: JPHC study. Cancer Causes Control. 2004;15:349–57. doi: 10.1023/B:CACO.0000027507.22124.20. [DOI] [PubMed] [Google Scholar]
- 141.Ozasa K, Watanabe Y, Ito Y, et al. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC Study) in Japan by sex and smoking habit. Jpn J Cancer Res. 2001;92:1259–69. doi: 10.1111/j.1349-7006.2001.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brennan P, Fortes C, Butler J, et al. A multicenter case-control study of diet and lung cancer among non-smokers. Cancer Causes Control. 2000;11:49–58. doi: 10.1023/a:1008909519435. [DOI] [PubMed] [Google Scholar]
- 143.Kreuzer M, Gerken M, Kreienbrock L, Wellmann J, Wichmann HE. Lung cancer in lifetime nonsmoking men - results of a case-control study in Germany. British journal of cancer. 2001;84:134–40. doi: 10.1054/bjoc.2000.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu J, Mao Y, Dryer D, White K. Risk factors for lung cancer among Canadian women who have never smoked. Cancer detection and prevention. 2002;26:129–38. doi: 10.1016/s0361-090x(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 145.Kreuzer M, Heinrich J, Kreienbrock L, Rosario AS, Gerken M, Wichmann HE. Risk factors for lung cancer among nonsmoking women. International journal of cancer. 2002;100:706–13. doi: 10.1002/ijc.10549. [DOI] [PubMed] [Google Scholar]
- 146.Nyberg F, Agrenius V, Svartengren K, Svensson C, Pershagen G. Dietary factors and risk of lung cancer in never-smokers. International journal of cancer. 1998;78:430–6. doi: 10.1002/(sici)1097-0215(19981109)78:4<430::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 147.Kalandidi A, Katsouyanni K, Voropoulou N, Bastas G, Saracci R, Trichopoulos D. Passive smoking and diet in the etiology of lung cancer among non-smokers. Cancer Causes Control. 1990;1:15–21. doi: 10.1007/BF00053179. [DOI] [PubMed] [Google Scholar]
- 148.Steinmetz KA, Potter JD, Folsom AR. Vegetables, fruit, and lung cancer in the Iowa Women’s Health Study. Cancer research. 1993;53:536–43. [PubMed] [Google Scholar]
- 149.Seow A, Poh WT, Teh M, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. International journal of cancer. 2002;97:365–71. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 150.Galeone C, Negri E, Pelucchi C, La Vecchia C, Bosetti C, Hu J. Dietary intake of fruit and vegetable and lung cancer risk: a case-control study in Harbin, northeast China. Ann Oncol. 2007;18:388–92. doi: 10.1093/annonc/mdl387. [DOI] [PubMed] [Google Scholar]
- 151.Hu J, Johnson KC, Mao Y, et al. A case-control study of diet and lung cancer in northeast China. International journal of cancer. 1997;71:924–31. doi: 10.1002/(sici)1097-0215(19970611)71:6<924::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 152.Candelora EC, Stockwell HG, Armstrong AW, Pinkham PA. Dietary intake and risk of lung cancer in women who never smoked. Nutrition and cancer. 1992;17:263–70. doi: 10.1080/01635589209514195. [DOI] [PubMed] [Google Scholar]
- 153.Koo LC. Dietary habits and lung cancer risk among Chinese females in Hong Kong who never smoked. Nutrition and cancer. 1988;11:155–72. doi: 10.1080/01635588809513983. [DOI] [PubMed] [Google Scholar]
- 154.Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology (Cambridge, Mass. 2000;11:673–9. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 155.Chen CL, Hsu LI, Chiou HY, et al. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. Jama. 2004;292:2984–90. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- 156.Olsson H, Bladstrom A, Ingvar C. Are smoking-associated cancers prevented or postponed in women using hormone replacement therapy? Obstetrics and gynecology. 2003;102:565–70. doi: 10.1016/s0029-7844(03)00564-7. [DOI] [PubMed] [Google Scholar]
- 157.Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10:113–23. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 158.Liu Y, Inoue M, Sobue T, Tsugane S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. International journal of cancer. 2005;117:662–6. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 159.Cheng YW, Chiou HL, Chen JT, et al. Gender difference in human papillomarvirus infection for non-small cell lung cancer in Taiwan. Lung cancer (Amsterdam, Netherlands) 2004;46:165–70. doi: 10.1016/j.lungcan.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 160.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–8. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 161.Kirk GD, Merlo C, POD, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–10. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Laurila AL, Anttila T, Laara E, et al. Serological evidence of an association between Chlamydia pneumoniae infection and lung cancer. International journal of cancer. 1997;74:31–4. doi: 10.1002/(sici)1097-0215(19970220)74:1<31::aid-ijc6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 163.Littman AJ, White E, Jackson LA, et al. Chlamydia pneumoniae infection and risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1624–30. [PubMed] [Google Scholar]
- 164.Smith JS, Kumlin U, Nyberg F, et al. Lack of association between serum antibodies of Chlamydia pneumoniae infection and the risk of lung cancer. Int J Cancer. 2008;123:2469–71. doi: 10.1002/ijc.23814. [DOI] [PubMed] [Google Scholar]
- 165.Zheng W, Blot WJ, Liao ML, et al. Lung cancer and prior tuberculosis infection in Shanghai. British journal of cancer. 1987;56:501–4. doi: 10.1038/bjc.1987.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hinds MW, Cohen HI, Kolonel LN. Tuberculosis and lung cancer risk in nonsmoking women. The American review of respiratory disease. 1982;125:776–8. doi: 10.1164/arrd.1982.125.6.776. [DOI] [PubMed] [Google Scholar]
- 167.Santillan AA, Camargo CA, Jr, Colditz GA. A meta-analysis of asthma and risk of lung cancer (United States) Cancer Causes Control. 2003;14:327–34. doi: 10.1023/a:1023982402137. [DOI] [PubMed] [Google Scholar]
- 168.Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Current opinion in pulmonary medicine. 2005;11:431–7. doi: 10.1097/01.mcp.0000170521.71497.ba. [DOI] [PubMed] [Google Scholar]
- 169.Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respiratory medicine. 2007;101:2534–40. doi: 10.1016/j.rmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 170.Mizushima Y, Kobayashi M. Clinical characteristics of synchronous multiple lung cancer associated with idiopathic pulmonary fibrosis. A review of Japanese cases. Chest. 1995;108:1272–7. doi: 10.1378/chest.108.5.1272. [DOI] [PubMed] [Google Scholar]
- 171.Yousem SA. The pulmonary pathologic manifestations of the CREST syndrome. Human pathology. 1990;21:467–74. doi: 10.1016/0046-8177(90)90002-m. [DOI] [PubMed] [Google Scholar]
- 172.Liu ZY, He XZ, Chapman RS. Smoking and other risk factors for lung cancer in Xuanwei, China. International journal of epidemiology. 1991;20:26–31. doi: 10.1093/ije/20.1.26. [DOI] [PubMed] [Google Scholar]
- 173.Neugut AI, Murray T, Santos J, et al. Increased risk of lung cancer after breast cancer radiation therapy in cigarette smokers. Cancer. 1994;73:1615–20. doi: 10.1002/1097-0142(19940315)73:6<1615::aid-cncr2820730612>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 174.Prochazka M, Hall P, Gagliardi G, et al. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol. 2005;23:7467–74. doi: 10.1200/JCO.2005.01.7335. [DOI] [PubMed] [Google Scholar]
- 175.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiotherapy and smoking in lung cancer following Hodgkin’s disease. Journal of the National Cancer Institute. 1995;87:1530–7. doi: 10.1093/jnci/87.20.1530. [DOI] [PubMed] [Google Scholar]
- 176.Pierce DA, Sharp GB, Mabuchi K. Joint effects of radiation and smoking on lung cancer risk among atomic bomb survivors. Radiation research. 2003;159:511–20. doi: 10.1667/0033-7587(2003)159[0511:jeoras]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 177.Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology (Cambridge, Mass. 2005;16:137–45. doi: 10.1097/01.ede.0000152522.80261.e3. [DOI] [PubMed] [Google Scholar]
- 178.Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ (Clinical research ed. 2005;330:223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Darby S, Hill D, Deo H, et al. Residential radon and lung cancer--detailed results of a collaborative analysis of individual data on 7148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scandinavian journal of work, environment & health. 2006;32 (Suppl 1):1–83. [PubMed] [Google Scholar]
- 180.Alavanja MC, Brownson RC, Boice JD, Jr, Hock E. Preexisting lung disease and lung cancer among nonsmoking women. American journal of epidemiology. 1992;136:623–32. doi: 10.1093/oxfordjournals.aje.a116542. [DOI] [PubMed] [Google Scholar]
- 181.Lan Q, Chen W, Chen H, He XZ. Risk factors for lung cancer in non-smokers in Xuanwei County of China. Biomed Environ Sci. 1993;6:112–8. [PubMed] [Google Scholar]
- 182.Ko YC, Lee CH, Chen MJ, et al. Risk factors for primary lung cancer among non-smoking women in Taiwan. International journal of epidemiology. 1997;26:24–31. doi: 10.1093/ije/26.1.24. [DOI] [PubMed] [Google Scholar]
- 183.Shen XB, Wang GX, Zhou BS. Relation of exposure to environmental tobacco smoke and pulmonary adenocarcinoma in non-smoking women: a case control study in Nanjing. Oncology reports. 1998;5:1221–3. [PubMed] [Google Scholar]
- 184.Neuberger JS, Mahnken JD, Mayo MS, Field RW. Risk factors for lung cancer in Iowa women: implications for prevention. Cancer detection and prevention. 2006;30:158–67. doi: 10.1016/j.cdp.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Seow A, Ng DP, Choo S, et al. Joint effect of asthma/atopy and an IL-6 gene polymorphism on lung cancer risk among lifetime non-smoking Chinese women. Carcinogenesis. 2006;27:1240–4. doi: 10.1093/carcin/bgi309. [DOI] [PubMed] [Google Scholar]
- 186.Ford MB, Sigurdson AJ, Petrulis ES, et al. Effects of smoking and radiotherapy on lung carcinoma in breast carcinoma survivors. Cancer. 2003;98:1457–64. doi: 10.1002/cncr.11669. [DOI] [PubMed] [Google Scholar]
- 187.Boffetta P, Mannetje A, Zaridze D, et al. Occupational X-ray examinations and lung cancer risk. International journal of cancer. 2005;115:263–7. doi: 10.1002/ijc.20854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.