Abstract
Primary lateral sclerosis is a sporadic disorder characterized by slowly progressive corticospinal dysfunction. Primary lateral sclerosis differs from amyotrophic lateral sclerosis by its lack of lower motor neuron signs and long survival. Few pathological studies have been carried out on patients with primary lateral sclerosis, and the relationship between primary lateral sclerosis and amyotrophic lateral sclerosis remains uncertain. To detect in vivo structural differences between the two disorders, diffusion tensor imaging of white matter tracts was carried out in 19 patients with primary lateral sclerosis, 18 patients with amyotrophic lateral sclerosis and 19 age-matched controls. Fibre tracking was used to reconstruct the intracranial portion of the corticospinal tract and three regions of the corpus callosum: the genu, splenium and callosal fibres connecting the motor cortices. Both patient groups had reduced fractional anisotropy, a measure associated with axonal organization, and increased mean diffusivity of the reconstructed corticospinal and callosal motor fibres compared with controls, without changes in the genu or splenium. Voxelwise comparison of the whole brain white matter using tract-based spatial statistics confirmed the differences between patients and controls in the diffusion properties of the corticospinal tracts and motor fibres of the callosum. This analysis further revealed differences in the regional distribution of white matter alterations between the patient groups. In patients with amyotrophic lateral sclerosis, the greatest reduction in fractional anisotropy occurred in the distal portions of the intracranial corticospinal tract, consistent with a distal axonal degeneration. In patients with primary lateral sclerosis, the greatest loss of fractional anisotropy and mean diffusivity occurred in the subcortical white matter underlying the motor cortex, with reduced volume, suggesting tissue loss. Clinical measures of upper motor neuron dysfunction correlated with reductions in fractional anisotropy in the corticospinal tract in patients with amyotrophic lateral sclerosis and increased mean diffusivity and volume loss of the corticospinal tract in patients with primary lateral sclerosis. Changes in the diffusion properties of the motor fibres of the corpus callosum were strongly correlated with changes in corticospinal fibres in patients, but not in controls. These findings indicate that degeneration is not selective for corticospinal neurons, but affects callosal neurons within the motor cortex in motor neuron disorders.
Keywords: diffusion tensor imaging, diffusion tensor tractography, motor neuron disorders, primary lateral sclerosis, corpus callosum
Introduction
Primary lateral sclerosis is a rare form of motor neuron disease characterized by progressive dysfunction of the corticospinal, or upper motor neurons, without clinical signs of lower motor neuron impairment (Pringle et al., 1992; Singer et al., 2007; Floeter and Mills, 2009). The aetiology of primary lateral sclerosis and its relationship to amyotrophic lateral sclerosis (ALS) are uncertain. Primary lateral sclerosis was first described as a clinicopathological entity in patients with progressive spasticity and sclerosis of the lateral columns of the spinal cord on post-mortem evaluation (Erb, 1875, 1902). This pathology, and the frequent clinical pattern of spasticity in the legs ascending over time to the arms and bulbar muscles, suggests a length-dependent, ‘dying-back’ degeneration of corticospinal axons (Fink, 2001; Zhai et al., 2003; Floeter and Mills, 2009). Later studies noted changes in the motor cortex in some patients with primary lateral sclerosis, including atrophy of the precentral gyrus on MRI scans (Pringle et al., 1992) and loss of cortical Betz cells on pathological examination (Hudson et al., 1993). Because of the rarity of primary lateral sclerosis, there have been very few pathological studies comparing ALS and primary lateral sclerosis brains. MRI provides in vivo structural information that may distinguish differences between ALS and primary lateral sclerosis and help to explain how the diseases progress. MRI studies have shown that patients with primary lateral sclerosis have thinning of cortical grey matter in the precentral gyrus (Butman and Floeter, 2007) and generalized atrophy compared with healthy controls (Tartaglia et al., 2009).
Routine MRI sequences do not reliably show abnormalities of the cortical white matter that distinguish patients with primary lateral sclerosis or patients with ALS from healthy individuals. Diffusion tensor imaging, an MRI technique that utilizes the diffusion properties of water molecules, can be used to estimate the overall orientation and integrity of nerve fibres in the white matter. Studies using diffusion tensor imaging in patients with ALS have found reduced fractional anisotropy, indicative of axonal disruption, at various levels of the corticospinal tract (Ellis et al., 1999; Graham et al., 2004; Aoki et al., 2005; Mitsumoto et al., 2007; Iwata et al., 2008). In patients with ALS, reduced fractional anisotropy correlated with differential slowing of central motor conduction in the supra- and infratentorial segments of the corticospinal tract (Iwata et al., 2008). Thus, reduced fractional anisotropy in white matter tracts may be a marker for changes in the physiological properties of axons as well as the structure of white matter tracts.
In the majority of diffusion tensor imaging studies that have been carried out in patients with ALS, diffusion properties of the corticospinal tract were measured within circumscribed regions of interest drawn within anatomical structures known to contain the corticospinal tract at one or a few levels, for example in the posterior limb of the internal capsule. One drawback of visual placement of regions of interest is that the position of the corticospinal fibres varies among individuals (Holodny et al., 2005). The accuracy of positioning a region of interest selectively on the fibres arising from the motor cortex is likely to be further diminished in patients with corticospinal tract degeneration. Diffusion tensor tractography, also known as fibre tracking, allows for the localization of white matter tracts connecting defined regions from diffusion tensor images (Mori et al., 1999) and enables tract-specific measurements of diffusion properties to be made from the reconstructed white matter tracts. We and others have shown that diffusion properties of the corticospinal tract and corpus callosum can be measured reliably and reproducibly in healthy subjects using diffusion tensor tractography (Wakana et al., 2007; Danielian et al., 2010). A disadvantage of diffusion tensor tractography is that it only assesses the selected tracts. Tract-based spatial statistics (TBSS) offers a complementary method for analysing white matter in diffusion tensor images that does not pre-specify tracts or regions of interest. TBSS carries out voxelwise statistical comparisons between co-registered and normalized white matter skeletons of two study populations, providing a non-biased, whole-brain analysis of white matter differences between two groups (Smith et al., 2006).
Although several studies have used diffusion tensor imaging in patients with ALS, there are only a few reports using diffusion tensor imaging in patients with primary lateral sclerosis, and sample sizes were small. A case report (Suh et al., 2006) and a small series with five patients with primary lateral sclerosis (Ulug et al., 2004) found changes in the diffusion properties in regions of the corticospinal tract. Another report compared six patients with primary lateral sclerosis to a group of patients with ALS studied at a separate institution (Ciccarelli et al., 2009). A recent study compared patients with primary lateral sclerosis to patients with hereditary spastic paraplegia and found some differences and similarities in the diffusion properties of several white matter tracts (Unrath et al., 2010). Although these studies provide a starting point to understanding changes in primary lateral sclerosis, a contemporaneous evaluation of patients with primary lateral sclerosis and patients with ALS using the same imaging methods would be valuable to delineate differences between the disorders. To address this point, patients with primary lateral sclerosis, patients with ALS and age-matched controls were prospectively studied with diffusion tensor imaging, using both fibre tracking and TBSS methods to evaluate the white matter tracts. A key question was whether the pattern of changes in white matter tracts would be consistent with a length-dependent axonopathy or with a more diffuse axonal disruption. To address this question, we looked at changes along the length of the corticospinal tract, and differences between tracts of different lengths that originate from the motor cortex. The relationship between diffusion tensor imaging measures and measures of clinical function, and specifically upper motor neuron function, was an additional focus of this study.
Subjects and methods
Subjects
The protocol was approved by the Institutional Review Board, and all subjects gave written informed consent in accord with the Declaration of Helsinki. All eligible patients evaluated by our group between 2006 and 2009 who were willing to participate were enrolled. Eighteen patients with definite or probable ALS by revised El Escorial criteria (Brooks, 1994), 19 patients with primary lateral sclerosis who fulfilled Pringle's criteria (Pringle et al., 1992) and 19 age-matched healthy control subjects participated in the study. Mean age and gender ratios did not differ between groups. All patients underwent a battery of neuropsychological testing and none of the patients had frontotemporal dementia.
Clinical ratings
The ALS Functional Rating Scale-Revised (ALSFRS-R) (Cedarbaum et al., 1999) and an upper motor neuron impairment score were used to quantify clinical severity of motor neuron dysfunction. The upper motor neuron impairment score is a composite score of clinical deficits in three areas—reflexes, tone and movement speed—that occur with upper motor neuron dysfunction, as recommended in a recent consensus report (Turner et al., 2011). The upper motor neuron impairment score primarily consists of published clinical scales for grading reflexes and muscle tone, plus timed measurements of finger tapping, gait and speech. Limb tendon reflexes were scored according to the four-point National Institutes of Health myotatic reflex scale (Litvan et al., 1996), with additional points given for Hoffman's and Babinski's signs; for the cranial region, the reflexes tested were jaw jerk, gag and release signs. Muscle tone was scored using the modified Ashworth Scale (Bohannon and Smith, 1987) for arms and legs; for the cranial region, we substituted a measure of dysarthria. Movement speed was quantified by finger tapping speed, timed gait for a distance of 20 ft and reading time for a standard passage, measures that have been shown to decline over time in primary lateral sclerosis (Floeter and Mills, 2009). Each component was scaled from 0 for normal function to 5 for the maximal degree of upper motor neuron impairment. Each limb and the cranial region were scored and the three components were averaged for each segment. The limb scores on one side and the cranial region were averaged to provide a lateralized score for side-specific comparisons, and a global upper motor neuron impairment score was defined as the mean of all limb and cranial scores. Although this upper motor neuron impairment score has not been broadly validated, it correlated in these patients with a previously reported upper motor neuron scale consisting of a summed pathological reflex score (Iwata et al., 2008). An upper motor neuron rapidity index was calculated by dividing the global upper motor neuron impairment score by the duration of disease in years.
Magnetic resonance imaging data acquisition and image processing
All imaging was performed at 3T (Philips Intera and Philips Achieva MRI) using a receive-only, eight channel SENSE head coil. Multi-slice diffusion weighted imaging was acquired using a single-shot, spin-echo, echo-planar sequence (55 contiguous slices, slice thickness = 2.5 mm, matrix = 96 × 96 reconstructed to 128 × 128, field of view = 240 × 240 mm, echo time = 86 ms, voxel size 1.875 × 1.875 × 2.5 mm). Slices were aligned parallel to the anterior commissure–posterior commissure line. Diffusion weighting was performed along 32 non-collinear directions with a b-value of 1000 s/mm2 and a single volume was collected with no diffusion gradients applied (b0). The diffusion sequence was repeated four times to increase the signal to noise ratio. The scan time for each diffusion sequence was 4 min. An axial T2-weighted image with the same slice positions as the diffusion tensor imaging scans was also collected for anatomical guidance (55 contiguous slices, slice thickness = 2.5 mm, matrix = 128 × 128, field of view = 240 × 240 mm, repetition time/echo time = 5400/100 ms). Image processing was performed using both FSL (University of Oxford, UK) (Smith et al., 2004) and DtiStudio (Johns Hopkins University, Baltimore, MD, USA) (Jiang et al., 2006). Details of the image processing and tensor calculation are reported in a previous study (Danielian et al., 2010).
Diffusion tensor tractography (fibre tracking)
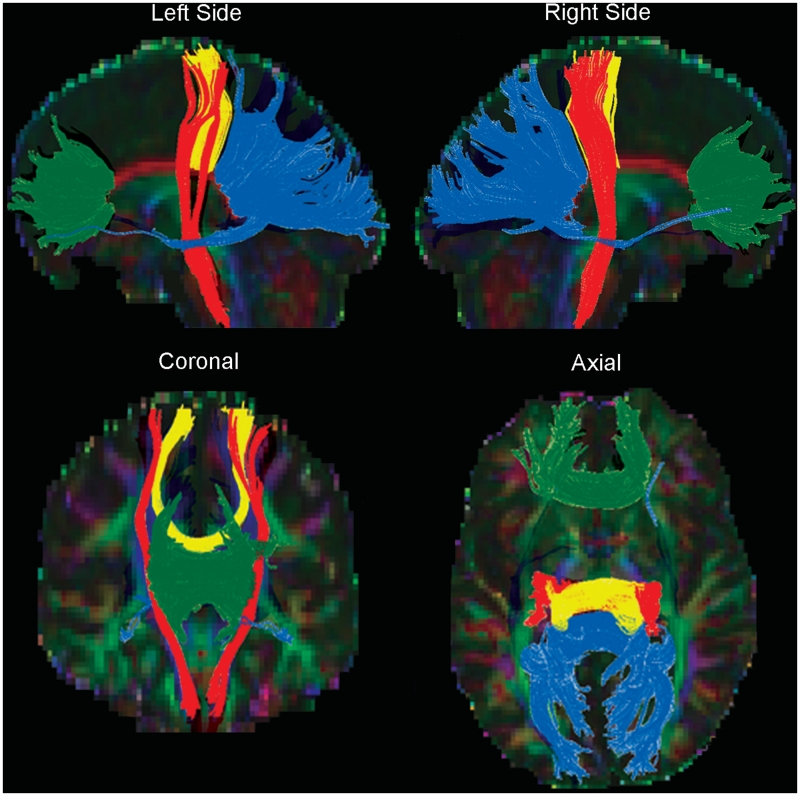
Fibre tracking was performed in DtiStudio using the Fibre Assignment by Continuous Tracking (FACT) method (Mori et al., 1999; Xue et al., 1999) on the right and left side of corticospinal tract and the genu, splenium and motor fibres of the corpus callosum. Tracking was initiated at a fractional anisotropy value of 0.2 and was terminated when fractional anisotropy fell <0.2 or the angle between two adjacent eigenvectors was >40° (Wakana et al., 2007) (Fig. 1). The corticospinal tract was tracked bilaterally using a multiple region of interest approach as outlined by Wakana et al. (2007). The motor fibres of the corpus callosum were isolated using a multiple region of interest approach. First, a large region of interest was drawn around the entire corpus callosum on the mid-sagittal slice. Next, a region of interest was drawn around the entire left side motor cortex, one slice superior to the bifurcation of the motor and sensory fibres at the level of the central sulcus. Fibres that passed between both regions of interest were retained as the motor fibres of the corpus callosum tract. The genu was tracked using a single region of interest on the mid-sagittal slice that encompasses the anterior one sixth of the corpus callosum (Hofer and Frahm, 2006). The splenium was tracked by using a single region of interest on the mid-sagittal slice that encompasses the posterior one fourth of the corpus callosum (Hofer and Frahm, 2006). Whole-track measures of mean fractional anisotropy, mean diffusivity (10−3 mm2/s) and volume of voxels containing fibres were calculated for each localized tract. Profiles of the rostrocaudal extent of corticospinal tract fractional anisotropy, mean diffusivity and cross-sectional area were generated for each subject from axial slices containing tracked corticospinal tract fibres. To adjust for differing brain sizes, slices were binned into 20 equal segments for each subject for slices extending from three slices inferior to the most caudal portion of the middle cerebellar peduncle through the top of the cortex. The means of the right and left side corticospinal tract at each level were averaged to generate a profile for each subject.
Figure 1.
White matter tracts defined by diffusion tensor tractography using the FACT fibre tracking algorithm. The corticospinal tract (red), and the genu (green), motor fibres (yellow), and splenium (blue) of the corpus callosum are shown in one healthy control.
Tract-based spatial statistics
Voxelwise statistical analysis of the fractional anisotropy skeletons was carried out using TBSS (v1.2) (Smith et al., 2006) part of the FMRIB software library analysis package (http://www.fmrib.ox.ac.uk/fsl/) (Smith et al., 2004). Briefly, after skull stripping (Smith, 2002), each subject's fractional anisotropy data were aligned into a common space (Rueckert et al., 1999; Andersson et al., 2007a, b). The most ‘typical’ subject in the study was automatically chosen by TBSS to be the target image for the registration. Using a threshold of 0.25, a fractional anisotropy skeleton that represents the centres of all tracts common to the group was derived from the mean fractional anisotropy map. Each subject's aligned fractional anisotropy data were then projected onto this skeleton and the resulting data used to determine voxelwise cross-subject statistics. The Randomize tool in the FMRIB software library (v2.1, 5000 permutations), which conducts permutation-based inference on t-statistic maps (Nichols and Holmes, 2002), was used to identify clusters of voxels that differed between the healthy controls and each patient group. The threshold for significance was P < 0.05, using the FMRIB software library threshold-free cluster enhancement with corrections for multiple comparisons across space [familywise error rate (FWE)]. Voxels that differed from controls were compared between primary lateral sclerosis and ALS patient groups. The correlation between voxels with differing fractional anisotropy or mean diffusivity and non-lateralized clinical measures (ALSFRS-R, disease duration, and the upper motor neuron rapidity index) were calculated using both hemispheres. A side-specific mask was used for assessing correlations with the lateralized upper motor neuron impairment score (Nichols and Holmes, 2002).
Statistics
Data are reported as means ± standard deviation (SD). For the diffusion tensor tractography, a two-way ANOVA was used to assess whole-track diffusion parameters in the corticospinal tract (diagnosis × side) and the corpus callosum (diagnosis × region of callosum), and regional differences in the corticospinal tract profiles at the level of the peduncle, internal capsule and subcortical white matter (diagnosis × level) with post hoc Fisher's test to identify significant contrasts using a corrected P < 0.05. Correlations between diffusion measures and clinical features were assessed with Pearson's correlation, examining each disease group separately, using P < 0.05 with Bonferroni correction as significant. Pearson's correlation was used to assess side-specific diffusion measurements and the lateralized upper motor neuron impairment score. Statistical analyses on diffusion tensor tractography were performed using Statview (SAS) and SPSS 15.0.
Results
Subject characteristics
As expected, patients had lower ALSFRS-R scores and higher global upper motor neuron impairment scores than healthy controls but these scores did not differ between the ALS and primary lateral sclerosis groups. Patients with primary lateral sclerosis had higher leg upper motor neuron impairment score subscores than patients with ALS. Patients with primary lateral sclerosis also had longer disease durations and a lower upper motor neuron rapidity index than patients with ALS, consistent with the slow progression of the condition (Table 1).
Table 1.
Clinical features of subject groups
| PLS | ALS | Controls | |
|---|---|---|---|
| Male:female (n) | 8:11 | 9:9 | 11:8 |
| Age (years) | 57.6 (7.9) | 53.2 (9.7) | 58.7 (5.9) |
| Disease duration (years) | 12.0a,b (6.5) | 2.4 (1.4) | – |
| ALSFRS-R | 38.3b (4.9) | 35.9b (7.0) | 48.0 (0.0) |
| Global upper motor neuron impairment score | 2.4b (0.9) | 2.0b (0.9) | 0.05 (0.07) |
| Cranial upper motor neuron impairment score | 1.7b (1.5) | 2.1b (1.1) | 0.05 (0.12) |
| Arm upper motor neuron impairment score | 2.2b (1.0) | 2.0b (1.2) | 0.04 (0.09) |
| Leg upper motor neuron impairment score | 3.1a,b (0.7) | 1.9b (1.0) | 0.07 (0.14) |
| Upper motor neuron rapidity index | 0.25b (0.15) | 1.57b (1.60) | – |
Values listed as mean (SD).
a Patient group differed from controls, P < 0.05.
b Patients with primary lateral sclerosis differed from patients with ALS P < 0.05.
PLS = primary lateral sclerosis; Upper motor neuron rapidity index = global upper motor neuron impairment score/disease duration in years.
Diffusion measurements of the corticospinal tract and corpus callosum
The whole-tract diffusion properties of the corticospinal tract differed between ALS and primary lateral sclerosis groups and age-matched controls (ANOVA; fractional anisotropy: F = 29.6, P < 0.0001; mean diffusivity: F = 11, P < 0.0001; volume: F = 4.7, P < 0.01) without significant differences between the two sides (Table 2). Post hoc testing showed reduced corticospinal tract fractional anisotropy and increased mean diffusivity in both patient groups compared with controls. The corticospinal tract fractional anisotropy was significantly lower in patients with ALS compared with patients with primary lateral sclerosis, declining by ∼10% in patients with ALS and 5% in patients with primary lateral sclerosis. Patients with primary lateral sclerosis had significantly reduced volume of the corticospinal tract, with a mean decline of 21% from controls. In the corpus callosum, the fractional anisotropy and mean diffusivity of the corpus callosum motor fibres exhibited differences between patient groups and controls (ANOVA; fractional anisotropy: F = 11.1, P < 0.0001; MD: F = 4.7, P < 0.01; VV: F = 9.3, P < 0.0001). Fractional anisotropy and volume of the motor fibres of the corpus callosum were decreased in primary lateral sclerosis and patients with ALS compared with controls, and mean diffusivity was increased in patients with primary lateral sclerosis (Table 2). The volume of the motor fibres of the corpus callosum volume declined by a mean of 54% in the primary lateral sclerosis group and by 37% in the ALS group. There were no differences in the fractional anisotropy, mean diffusivity or volume of the genu or splenium of the callosum between patients and controls. In patients with primary lateral sclerosis, the fractional anisotropy values of the motor fibres of the corpus callosum fibres were strongly correlated with the fractional anisotropy values of the corticospinal tract (right corticospinal tract: r = 0.78, P < 0.0001; left corticospinal tract: r = 0.76, P < 0.0001). The correlation between motor fibres of the corpus callosum and corticospinal tract fractional anisotropy also reached significance in patients with ALS, although only on one side (right corticospinal tract: r = 0.50; P = 0.0494). In healthy controls, there was no significant correlation between the fractional anisotropy of the corticospinal tract and the motor fibres of the corpus callosum.
Table 2.
Diffusion property measurements of the corticospinal tract and corpus callosum obtained by diffusion tensor tractography fibre tracking
| Fractional anisotropy |
Mean diffusivity (10−3 mm2/s) |
Volume (mm3) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PLS | ALS | HC | PLS | ALS | HC | PLS | ALS | HC | |
| Corticospinal tract | 0.55a (0.03) | 0.52a,b (0.04) | 0.58 (0.03) | 0.75a (0.03) | 0.75a (0.03) | 0.72 (0.03) | 3782a (1403) | 4023 (1586) | 4835 (1627) |
| Corpus callosum motor | 0.47a (0.05) | 0.48a (0.04) | 0.55 (0.03) | 0.86a (0.08) | 0.83 (0.05) | 0.79 (0.06) | 3112a (2015) | 4196a (2496) | 6766 (3153) |
| Corpus callosum genu | 0.50 (0.03) | 0.50 (0.02) | 0.50 (0.03) | 0.85 (0.04) | 0.83 (0.04) | 0.85 (0.05) | 17 423 (5005) | 16 660 (3628) | 16 999 (3558) |
| Corpus callosum splenium | 0.60 (0.02) | 0.60 (0.01) | 0.60 (0.03) | 0.83 (0.05) | 0.83 (0.03) | 0.83 (0.04) | 45 166 (7971) | 42 474 (9712) | 49 358 (10 447) |
Values listed as mean (SD).
a Differs from control group P < 0.05.
b Differs from primary lateral sclerosis group P < 0.05.
HC = healthy controls; PLS = primary lateral sclerosis.
Fibre tracking profiles of the corticospinal tract
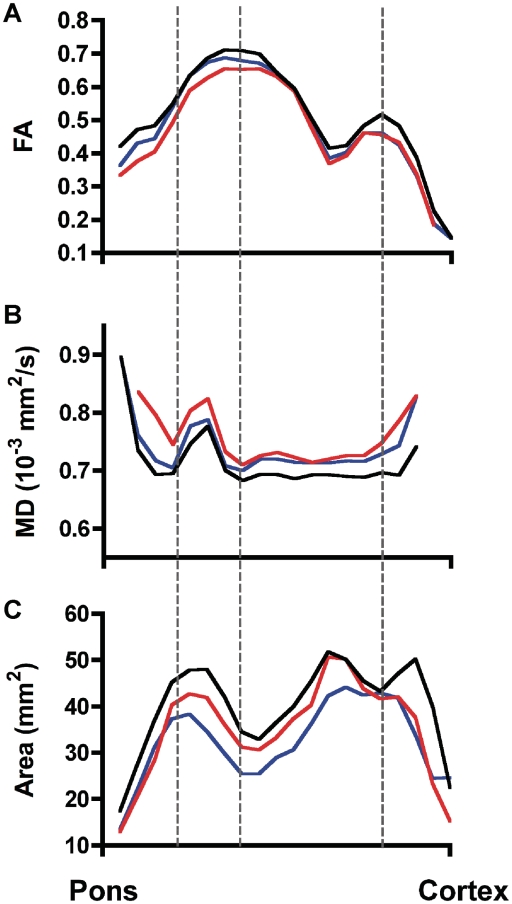
The mean profiles of the fractional anisotropy values (Fig. 2A), mean diffusivity values (Fig. 2B) and the cross-sectional area (Fig. 2C) of the rostrocaudal extent of the corticospinal tract are shown for each group, averaging the right and left sides. The profiles show that fractional anisotropy, mean diffusivity and cross-sectional area differ at multiple levels in the patient groups compared with controls. An ANOVA compared three levels of the profile where corticospinal tract fibres were least variable: the peduncle, internal capsule and white matter below the motor cortex (dashed lines in Fig. 2), with significant differences by diagnosis for fractional anisotropy (P = 0.004), mean diffusivity (P = 0.018) and cross-sectional area (P = 0.049). Post hoc testing indicated that fractional anisotropy and mean diffusivity profiles differed between patients with ALS and controls, and that patients with primary lateral sclerosis had significantly different cross-sectional area profiles from controls. No significant interactions were found between the three levels and diagnosis.
Figure 2.
Profiles of group mean diffusion tensor tractography measures along the rostrocaudal extent of the corticospinal tract, from mid-pons to motor cortex, for controls (black lines), patients with primary lateral sclerosis (blue lines) and patients with ALS (red lines) for (A) fractional anisotropy (FA), (B) mean diffusivity (MD) and (C) cross-sectional area. The right and left corticospinal tract were averaged for each subject. Comparing anatomical levels where the corticospinal tract is least variable (dashed lines from left to right: peduncle, internal capsule, subcortical white matter), corticospinal tract fractional anisotropy of patients with ALS was significantly reduced and mean diffusivity significantly increased from the controls. Corticospinal tract cross-sectional area profiles in patients with primary lateral sclerosis was reduced compared with controls. There were no significant differences between the three levels.
Correlations between diffusion measures and clinical ratings
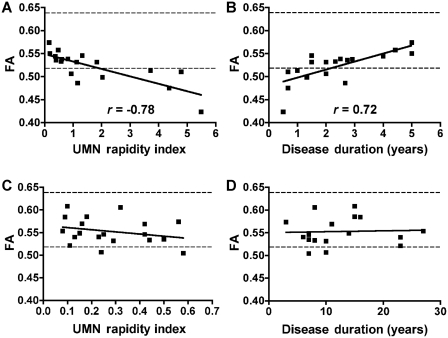
The correlations between diffusion tensor tractography diffusion measures and clinical ratings were assessed separately for ALS and patients with primary lateral sclerosis (Table 3). In patients with ALS, corticospinal tract fractional anisotropy was inversely correlated with the global and lateralized upper motor neuron impairment scores and with the upper motor neuron rapidity index (Fig. 3A), indicating that the more severely affected and more rapidly progressing patients had greater declines in corticospinal tract fractional anisotropy. Paradoxically, however, disease duration was positively correlated with corticospinal tract fractional anisotropy (Fig. 3B), showing that patients with ALS with a longer disease course had less decline in corticospinal tract fractional anisotropy. Corticospinal tract fractional anisotropy was not significantly correlated with the ALSFRS-R score or age in patients with ALS. The upper motor neuron impairment score, disease duration and age were not significantly correlated with the corticospinal tract mean diffusivity or volume. Diffusion measures of the corpus callosum in patients with ALS were not correlated with clinical measures.
Table 3.
Correlations between clinical features and diffusion property measurements obtained using diffusion tensor tractography (fibre tracking)
| Primary lateral sclerosis |
ALS |
|||||
|---|---|---|---|---|---|---|
| Fractional anisotropy | Mean diffusivity | Vol | Fractional anisotropy | Mean diffusivity | Vol | |
| Corticospinal tract | ||||||
| Global upper motor neuron impairment score | −0.36 | 0.53a | −0.44a | −0.60a | 0.28 | 0.10 |
| Lateralized upper motor neuron impairment score | −0.32 | 0.51a | −0.66a | −0.63a | 0.26 | 0.05 |
| ALSFRS-R | 0.26 | −0.42 | 0.21 | 0.18 | −0.46 | −0.10 |
| Age | −0.12 | 0.03 | 0.20 | 0.47 | −0.31 | −0.21 |
| Duration | 0.05 | −0.15 | −0.40 | 0.72a | −0.30 | −0.15 |
| Upper motor neuron rapidity index | −0.27 | 0.41 | 0.22 | −0.78a | 0.35 | 0.34 |
| Corpus callosum motor fibres | ||||||
| Global upper motor neuron impairment score | −0.43 | 0.68a | −0.05 | −0.19 | 0.37 | 0.27 |
| Lateralized upper motor neuron impairment score | −0.32 | 0.59a | 0.07 | −0.33 | 0.35 | 0.44 |
| ALSFRS-R | 0.18 | −0.50 | 0.20 | −0.03 | −0.43 | −0.31 |
| Age | −0.01 | 0.13 | −0.22 | 0.14 | −0.14 | −0.11 |
| Duration | −0.08 | −0.10 | 0.12 | 0.14 | −0.22 | −0.21 |
| Upper motor neuron rapidity index | −0.29 | 0.48 | −0.18 | −0.33 | 0.37 | 0.08 |
a Significant correlations (r-values) with P < 0.05 corrected for multiple comparisons.
Vol = volume of voxels containing fibres in mm3.
Figure 3.
Correlations between corticospinal tract fractional anisotropy (FA) and upper motor neuron (UMN) rapidity index (A and C) and disease duration (B and D) for patients with ALS (A and B) and primary lateral sclerosis (C and D). Each point represents one patient with right and left side corticospinal tract averaged. Significant Pearson's correlation r-values are shown (P < 0.001). Dashed lines represent corticospinal tract fractional anisotropy range of control subjects (mean ± 2 SD). Upper motor neuron rapidity index = global upper motor neuron-impairment score/disease duration in years. Disease duration is counted from symptom onset.
In contrast, in patients with primary lateral sclerosis, the global and lateralized upper motor neuron impairment score was correlated with corticospinal tract mean diffusivity and inversely correlated with corticospinal tract volume. Corticospinal tract fractional anisotropy was not correlated with the upper motor neuron rapidity index (Fig. 3C), with disease duration (Fig. 3D), with the global and lateralized upper motor neuron impairment score or with ALSFRS-R (Table 3). In primary lateral sclerosis, the upper motor neuron impairment score also correlated well with motor fibres of the corpus callosum mean diffusivity.
Tract-based spatial statistics
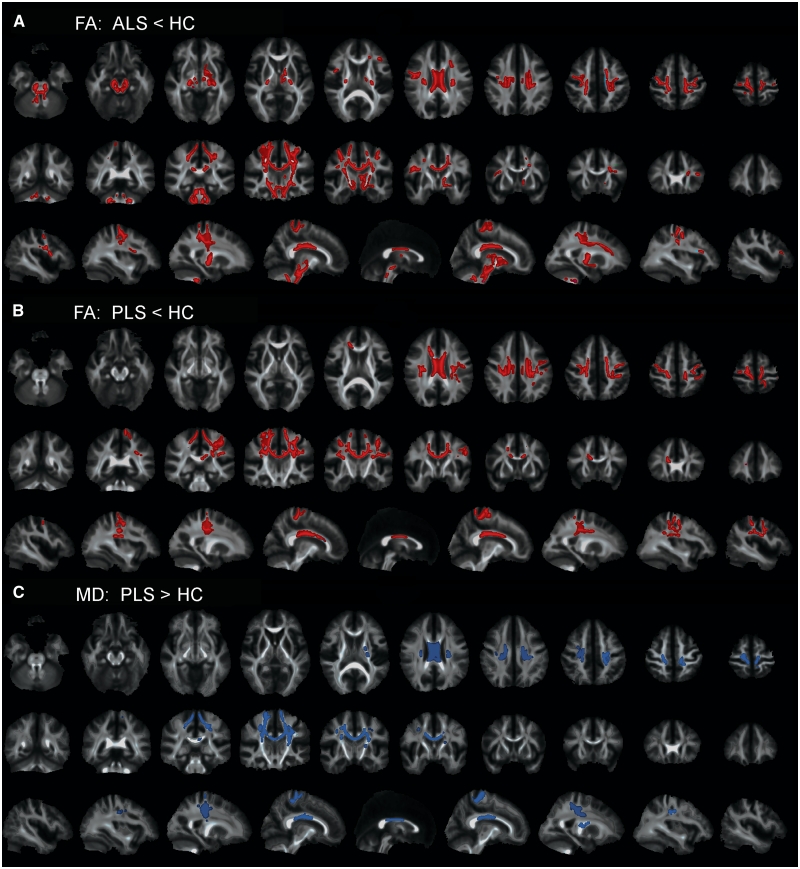
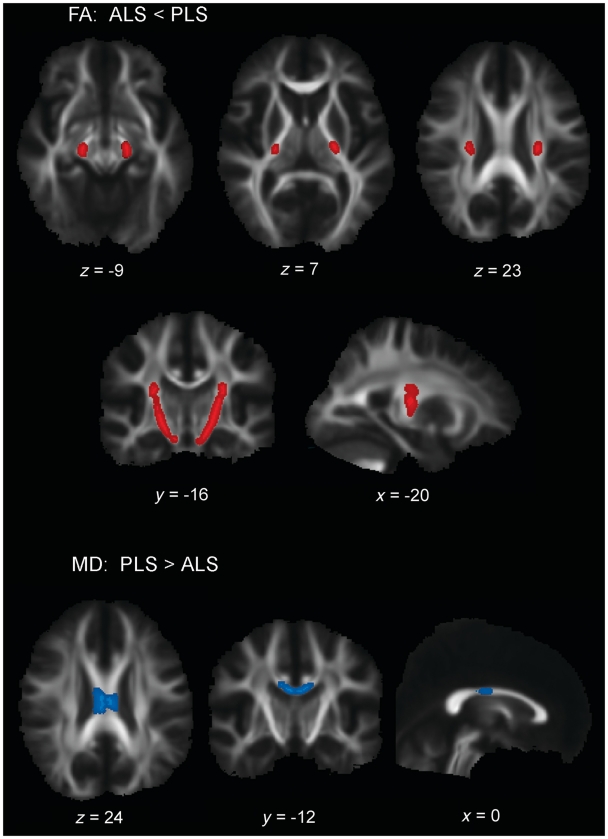
In the first set of contrasts, voxelwise comparisons of total brain white matter were made between each patient group and controls. Patients with ALS had several clusters of voxels with decreased fractional anisotropy that extended the length of the corticospinal tract from the subcortical white matter to the most distal slice (Fig. 4A). Contrasting patients with primary lateral sclerosis to controls, clusters of voxels with reduced fractional anisotropy occurred in the subcortical white matter underlying motor and premotor cortex, but not in more distal portions of the corticospinal tract (Fig. 4B). Both patient groups had reduced fractional anisotropy in the body of the corpus callosum compared with controls (Fig. 4A and B). In contrast to controls, patients with primary lateral sclerosis had increased mean diffusivity in the subcortical white matter, coronal radiata and mid-body of the corpus callosum (Fig. 4C). There were no differences in mean diffusivity in patients with ALS when contrasted with controls. When the two patient groups were contrasted to each other, patients with ALS had reduced fractional anisotropy in the corticospinal tract extending distally from the posterior limb of the internal capsule to the pons (Fig. 5A). Patients with primary lateral sclerosis had increased mean diffusivity in the mid-body of the callosum (Fig. 5B) compared with patients with ALS.
Figure 4.
TBSS analysis showing clusters of voxels with significantly lower fractional anisotropy (FA) (red) in (A) patients with ALS and (B) patients with primary lateral sclerosis (PLS) as compared with healthy controls (HC). Clusters of voxels showing significantly greater mean diffusivity (MD) (blue) in (C) patients with primary lateral sclerosis as compared with healthy controls (P < 0.05), corrected for multiple comparisons across space (FWE) using threshold-free cluster enhancement. Patients with ALS did not have significant differences in mean diffusivity compared with healthy controls (not illustrated). Each panel shows the significant voxel clusters superimposed on the mean fractional anisotropy map in axial (upper row), coronal (middle row) and sagittal (bottom row) slices. Images are displayed in radiological convention (right brain on left side).
Figure 5.
TBSS analysis of clusters of voxels with significantly lower fractional anisotropy (FA) (red) in (A) patients with ALS compared with patients with primary lateral sclerosis (PLS), and significantly greater mean diffusivity (MD) (blue) in (B) patients with primary lateral sclerosis compared with patients with ALS in selected axial, coronal and sagittal sections (P < 0.05), corrected for multiple comparisons across space (FWE) using threshold-free cluster enhancement. MNI coordinates are shown. Images are displayed in radiological convention (right brain on left side).
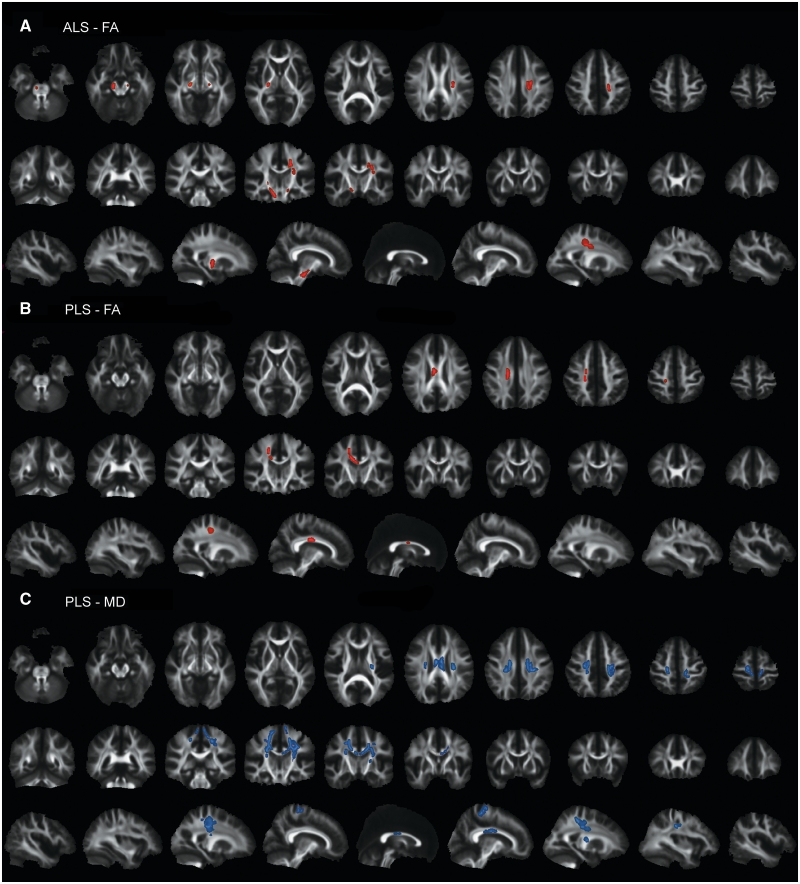
Within each patient group, the voxel clusters that differed from controls were examined for correlations with clinical measures in TBSS. For corticospinal tract fractional anisotropy, findings were similar to those obtained by fibre tracking. In the patients with ALS, reduced fractional anisotropy was correlated with the lateralized upper motor neuron impairment scores in multiple clusters of voxels within the corticospinal tract (Fig. 6A). The location of these clusters along the rostrocaudal extent of the corticospinal tract exhibited some asymmetry, despite the lack of significant side-to-side differences in the upper motor neuron impairment score. As also seen in the diffusion tensor tractography analysis, corticospinal tract fractional anisotropy was positively correlated with disease duration and negatively correlated with the upper motor neuron rapidity index in patients with ALS. No correlations between mean diffusivity and upper motor neuron impairment scores or upper motor neuron rapidity index were identified in the TBSS skeleton for patients with ALS. In patients with primary lateral sclerosis, clusters of voxels with reduced fractional anisotropy that correlated with the lateralized upper motor neuron impairment score were limited to a small region of the subcortical white matter and corpus callosum (Fig. 6B). Also consistent with the diffusion tensor tractography analysis, the upper motor neuron impairment score correlated with increased mean diffusivity in the subcortical white matter and corpus callosum in patients with primary lateral sclerosis (Fig. 6C). Increased mean diffusivity in the corpus callosum also correlated with the ALSFRS-R and upper motor neuron rapidity index in patients with primary lateral sclerosis.
Figure 6.
TBSS analysis showing clusters of voxels with significant correlation between the lateralized upper motor neuron impairment score and fractional anisotropy (FA) (red) in (A) patients with ALS and (B) patients with primary lateral sclerosis (PLS), and between the lateralized upper motor neuron impairment score and mean diffusivity (MD) (blue) in (C) patients with primary lateral sclerosis (P < 0.05, corrected for multiple comparisons across space (FWE) using threshold-free cluster enhancement). Each panel shows the significant voxel clusters superimposed on the mean fractional anisotropy map in axial (upper row), coronal (middle row) and sagittal (bottom row) slices. Images are displayed in radiological convention (right brain on left side). Patients with ALS did not exhibit correlations between the upper motor neuron impairment score and mean diffusivity (not illustrated).
Discussion
Primary lateral sclerosis patients and patients with ALS with a similar severity of upper motor neuron dysfunction had altered diffusion properties of white matter tracts originating from the motor cortex compared with age-matched controls, in agreement with previous literature (Ellis et al., 1999; Ciccarelli et al., 2009; Filippini et al., 2010). The regional distribution of white matter changes differed between primary lateral sclerosis and patients with ALS, suggesting different underlying pathology. Fractional anisotropy was more reduced in the distal portions of the corticospinal tract in patients with ALS compared with patients with primary lateral sclerosis. In patients with primary lateral sclerosis, fractional anisotropy was more reduced in the juxta-cortical white matter. The loss of fractional anisotropy in distal portions of the corticospinal tract would be consistent with the hypothesis of a dying-back degeneration of corticospinal axons in ALS (Wong et al., 2007). This distal predominance was not seen in the patients with primary lateral sclerosis. The greater prominence of altered diffusion properties in the subcortical white matter and corpus callosum in patients with primary lateral sclerosis is in general agreement with two diffusion tensor imaging studies that used tract-based statistical methods of analysis (Ciccarelli et al., 2009; Unrath et al., 2010). In this study, the fibre tracking analysis additionally showed that changes in diffusion properties were accompanied by volume loss and reduced cross-sectional area that spanned the full length of the intracranial corticospinal tract in patients with primary lateral sclerosis. In patients with ALS, the volume of the corticospinal tract overall did not decline, although cross-sectional area was reduced in its most distal portions.
Diffusion properties normally vary along the length of the corticospinal tract, reflecting differences in the compactness and organization of corticospinal axons as they traverse different structures, splaying out, for example, in the corona radiata and becoming tightly packed in the internal capsule (Reich et al., 2006; Wong et al., 2007). The typical rostrocaudal pattern of diffusion properties was preserved in our patients. Regional differences between patients and controls in the corticospinal tract diffusion properties reflect the disease-related alterations in the tissue microstructure. Experimental studies have shown that fractional anisotropy, a measure of the coherence of directional diffusion, is mainly determined by the organized arrangement of axonal membranes, with a smaller contribution from myelin and relatively little from the intra-axonal neurofibrils; measures of mean diffusivity are affected by intra- and extracellular elements that restrict diffusion of water molecules (Beaulieu, 2002). In patients with ALS, the loss of fractional anisotropy distally, detected in the TBSS analysis, indicates disruption of corticospinal axon integrity. The distal gradient of increasing mean diffusivity and decreasing cross-sectional area below the peduncles in the tract profiles, are compatible with an underlying dying back axonopathy. In patients with primary lateral sclerosis, the combination of reduced fractional anisotropy, increased mean diffusivity, and volume loss is more suggestive of tissue loss, with loss of corticospinal axons and expansion of extracellular space.
One interpretation of the differences between primary lateral sclerosis and patients with ALS in the diffusion properties of the corticospinal tract is that these changes represent the early and late stages in a temporal sequence of structural changes that occurs with axonal breakdown and clearance. However, time alone, i.e. disease duration, is not a sufficient explanation. Previous studies in patients with ALS have reported that corticospinal tract fractional anisotropy declined with disease severity, duration or progression (Ellis et al., 1999; Jacob et al., 2003; Cosottini et al., 2005; Iwata et al., 2008; Nickerson et al., 2009; Roccatagliata et al., 2009; Agosta et al., 2010a). In most studies, the ALSFRS-R score was used to quantify disease severity and to calculate disease progression. In this study, the ALSFRS-R was not significantly correlated with diffusion property measurements. Since the ALSFRS-R measures functions that depend on both lower and upper motor neurons, a drop in the ALSFRS-R is not specific for upper motor neuron dysfunction. We confirmed the correlation between reduced corticospinal tract fractional anisotropy and clinical severity with a scale measuring upper motor neuron dysfunction in patients with ALS. But the relationship between corticospinal tract fractional anisotropy and disease duration differed from previous reports: patients with ALS with longer disease durations exhibited less reduction of corticospinal tract fractional anisotropy than patients with ALS with shorter disease durations. This seeming contradiction may be explained by noting that, in this study, the patients with ALS with shorter disease durations also had a higher upper motor neuron rapidity index, and thus a faster rate of progression. Our ALS cohort may have included more patients with slower progression than in other studies, since our focus was on patients with a comparable severity of upper motor neuron dysfunction to patients with primary lateral sclerosis, and patients with ALS with predominantly upper motor neuron signs are known to have a slower rate of progression (Gordon et al., 2009). An extrapolation of the trend for lesser reduction of corticospinal tract fractional anisotropy in patients with ALS with slower progression could lead to similar findings to what was observed in patients with primary lateral sclerosis, whose corticospinal tract fractional anisotropy values were reduced, intermediate between controls and patients with ALS, and were stable with time. Our data support the proposal that the reduction in corticospinal tract fractional anisotropy is a marker for the rate of disease progression, rather than the severity or the duration of disease (Agosta et al., 2010a, b).
Clinical ratings of upper motor neuron dysfunction correlated with reduced corticospinal tract fractional anisotropy in patients with ALS, and with increased corticospinal tract mean diffusivity in patients with primary lateral sclerosis in both diffusion tensor tractography and TBSS analyses. Increased corticospinal tract mean diffusivity in patients with ALS was detected in the fibre tracking analysis, with somewhat more prominence in the distal portions of the corticospinal tract profile. Increased mean diffusivity seems to correspond to regions of white matter areas with more chronic changes, as would be compatible with an evolution of diffusion properties as degeneration progresses. Longitudinal imaging studies and pathological correlation will be critical to refine interpretations of imaging findings in ALS and primary lateral sclerosis. Pathological correlations are needed to determine whether fluctuations in fractional anisotropy correspond to degeneration or loss of axons and crossing fibres, and whether mean diffusivity reflects tissue loss and gliosis.
Changes in the diffusion properties of the corpus callosum were observed in ALS and patients with primary lateral sclerosis by fibre tracking and TBSS analyses. The changes occurred in the mid-posterior portion of the corpus callosum, an area containing fibres originating from the sensory and motor cortex, leaving the genu and splenium unaffected. This localization within the callosum is consistent with images presented in other studies (Bartels et al., 2008; Ciccarelli et al., 2009; Filippini et al., 2010; Unrath et al., 2010), but differs from the callosal regions affected in dementia syndromes (Zhang et al., 2007; Zhuang et al., 2010) and hereditary spastic paraparesis (Unrath et al., 2010). Of note is that none of the patients in this study was demented. The changes in the corpus callosum signify that the degeneration is not selective for corticospinal neurons in ALS and primary lateral sclerosis. Neurons whose axons form the corpus callosum are distinct from those that form the corticospinal tract. Although some corticospinal neurons transiently extend collaterals through the corpus callosum during development, by adulthood, neurons forming the corpus callosum are separate from corticospinal neuron population (Catsman-Berrevoets et al., 1980; Koester and O'Leary, 1993). This differentiation is driven by expression of neuronal subtype specific genes (Molyneaux et al., 2007, 2009). In primates, the pyramidal neurons that form the corpus callosum primarily reside in cortical layer IIIb, whereas corticospinal neurons reside in cortical layer V (Jones et al., 1979). Although callosal neurons are excitatory pyramidal neurons, their major targets are inhibitory interneurons in the contralateral hemisphere (Li and Pleasure, 2011). Callosal activation using transcranial stimulation produces contralateral inhibition of the motor cortex (Avanzino et al., 2007). In ALS, transcallosal inhibition is impaired at an early stage of the disease, before the appearance of upper motor neuron signs (Wittstock et al., 2007). Loss of transcallosal inhibition is likely to underlie the clinical phenomenon of mirror movements observed in patients with ALS, which have been shown to correlate with reduced fractional anisotropy in the corpus callosum in diffusion tensor imaging images (Bartels et al., 2008). Mirror movements were not systematically assessed in this study. Reduction in callosal fractional anisotropy may thus be an early marker for motor neuron disease. Reductions in fractional anisotropy values of the corpus callosum and corticospinal tract were correlated. The concurrent, or earlier, impairment of the shorter callosal axons argues against a strictly length-dependent susceptibility to degeneration. However, concurrent degeneration would be compatible with the hypothesis that degeneration in motor neuron disease begins focally in a region of the brain and spreads to contiguous or homotopic regions (Ravits and La Spada, 2009).
In this study, diffusion tensor tractography, or fibre tracking, was used to define the white matter tracts selectively for quantitation. The major findings from the fibre tracking analysis were confirmed by TBSS, a whole-brain method for analysing white matter tracts. Both methods identified altered diffusion properties of the corticospinal tract and the motor portion of the corpus callosum, as well as correlations of the clinical severity of upper motor neuron impairment with corticospinal tract fractional anisotropy in ALS and with corticospinal tract mean diffusivity in primary lateral sclerosis. However, fibre tracking and TBSS yielded slightly different results. The discrepancies were largely failures of detection, rather than contradictory findings. Diffusion tensor tractography detected increased corticospinal tract mean diffusivity in ALS that was not seen by TBSS. TBSS found correlations between small clusters of voxels with reduced fractional anisotropy in the subcortical white matter and the upper motor neuron impairment score in primary lateral sclerosis that were not detected by fibre tracking. The differing assumptions and computational procedures of the two methods account for these differences. Each method has strengths and weaknesses. Diffusion tensor tractography has the advantage that the white matter tracts visualized are known to connect defined brain regions in individual subjects, whereas the identification of tracts in TBSS is based on images registered to a standardized atlas. Given individual differences in the localization of corticospinal axons, their identification is less precise for TBSS than for diffusion tensor tractography. On the other hand, fibre tracking algorithms only connect contiguous voxels that have fractional anisotropy values above a threshold, creating a bias towards relatively intact axonal tracts. Fibre tracking can truncate prematurely in regions where fibres become less compact or cross fibres with an orthogonal orientation, whereas TBSS is able to detect non-contiguous clusters of voxels within a tract. TBSS is at a disadvantage for evaluating diffusion measures other than fractional anisotropy values. TBSS analyses a thinned fractional anisotropy skeleton that consists of a core of voxels having the highest fractional anisotropy (Smith et al., 2006), thus excluding the full cross-section of tracts that are more variable between individuals. Thus, TBSS will be relatively insensitive to changes in mean diffusivity that occur in voxels outside the core with the highest fractional anisotropy. Fibre tracking and TBSS provide complimentary methods for evaluating white matter, and the limitations of each method should be taken into account for interpretation of differing results.
It is reassuring that the affected white matter tracts identified by diffusion tensor imaging correspond to those that produce the clinical manifestations of motor neuron disease. The differences in diffusion tensor imaging between patients with primary lateral sclerosis and ALS are caused by structural changes in the white matter tracts that reflect underlying pathology. Despite the distinct pathology, it remains to be determined whether the two conditions have a common aetiology. Too little is known about the evolution of imaging changes as degeneration progresses. Nevertheless, the findings of this study have implications for aetiological hypotheses. The concurrent changes in callosal and corticospinal neurons point away from the idea that corticospinal neurons are selectively vulnerable in motor neuron diseases because of the length of their axons and the associated metabolic burden of maintaining a great mass of axoplasm. The pathological pattern of a dying back axonopathy, occurring simultaneously in short and long-axon neurons, would be compatible with a number of the proposed mechanisms for ALS that disrupt normal cellular function, including oxidative stress, accumulation of toxic intracellular aggregates or glutamate excitotoxicity. The diffusion tensor imaging findings also have clinical relevance. Reduced fractional anisotropy in the motor regions of the corpus callosum appears to be a relatively robust finding in this and other studies (Bartels et al., 2008; Ciccarelli et al., 2009; Filippini et al., 2010), and offers a potential biomarker for motor neuron disease. Additionally, the correlation between reduced fractional anisotropy in the corticospinal tract and the rate of progression may lead to future algorithms to predict patients likely to have a more rapid decline.
Funding
Intramural Research Program of the National Institute of Neurological Disorders and Stroke (#NS 002976), National Institutes of Health (ZO1 NS002976).
Acknowledgements
We gratefully acknowledge the assistance Dr John Ostuni with Linux scripting and imaging analysis, and of Michelle Bernal for coordination of patient visits. The protocol is registered on clinicaltrials.gov as NCT00334516.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale-Revised
- TBSS
tract-based spatial statistics
References
- Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor mr imaging tractography study. AJNR Am J Neuroradiol. 2010a;31:1457–61. doi: 10.3174/ajnr.A2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Pagani E, Petrolini M, Sormani MP, Caputo D, Perini M, et al. MRI predictors of long-term evolution in amyotrophic lateral sclerosis. Eur J Neurosci. 2010b;32:1490–6. doi: 10.1111/j.1460-9568.2010.07445.x. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Technical report TR07JA1. 2007a. Available from: www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. FMRIB Technical Report TR07JA2. 2007b. Available from: www.fmrib.ox.ac.uk/analysis/techrep.
- Aoki S, Iwata NK, Masutani Y, Yoshida M, Abe O, Ugawa Y, et al. Quantitative evaluation of the pyramidal tract segmented by diffusion tensor tractography: feasibility study in patients with amyotrophic lateral sclerosis. Radiat Med. 2005;23:195–9. [PubMed] [Google Scholar]
- Avanzino L, Teo JT, Rothwell JC. Intracortical circuits modulate transcallosal inhibition in humans. J Physiol. 2007;583:99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C, Mertens N, Hofer S, Merboldt KD, Dietrich J, Frahm J, et al. Callosal dysfunction in amyotrophic lateral sclerosis correlates with diffusion tensor imaging of the central motor system. Neuromuscul Disord. 2008;18:398–407. doi: 10.1016/j.nmd.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci. 1994;124:96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Butman JA, Floeter MK. Decreased thickness of primary motor cortex in primary lateral sclerosis. AJNR Am J Neuroradiol. 2007;28:87–91. [PMC free article] [PubMed] [Google Scholar]
- Catsman-Berrevoets CE, Lemon RN, Verburgh CA, Bentivoglio M, Kuypers HG. Absence of callosal collaterals derived from rat corticospinal neurons. A study using fluorescent retrograde tracing and electrophysiological techniques. Exp Brain Res. 1980;39:433–40. doi: 10.1007/BF00239308. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, et al. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp. 2009;30:615–24. doi: 10.1002/hbm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosottini M, Giannelli M, Siciliano G, Lazzarotti G, Michelassi MC, Del Corona A, et al. Diffusion-tensor MR imaging of corticospinal tract in amyotrophic lateral sclerosis and progressive muscular atrophy. Radiology. 2005;237:258–64. doi: 10.1148/radiol.2371041506. [DOI] [PubMed] [Google Scholar]
- Danielian LE, Iwata NK, Thomasson DM, Floeter MK. Reliability of fiber tracking measurements in diffusion tensor imaging for longitudinal study. Neuroimage. 2010;49:1572–80. doi: 10.1016/j.neuroimage.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, et al. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology. 1999;53:1051–8. doi: 10.1212/wnl.53.5.1051. [DOI] [PubMed] [Google Scholar]
- Erb WH. Ueber einen wenig bekannten spinalen Symptomencomplex. Berliner Klinische Wochenschrift. 1875;26:357–9. [Google Scholar]
- Erb WH. Spastic and syphilitic spinal paralysis. Lancet. 1902;ii:969–74. doi: 10.1136/bmj.2.2180.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR. Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology. 2010;75:1645–52. doi: 10.1212/WNL.0b013e3181fb84d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JK. Progressive spastic paraparesis: hereditary spastic paraplegia and its relation to primary and amyotrophic lateral sclerosis. Semin Neurol. 2001;21:199–207. doi: 10.1055/s-2001-15265. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Mills R. Progression in primary lateral sclerosis: a prospective analysis. Amyotroph Lateral Scler. 2009;10:339–46. doi: 10.3109/17482960903171136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PH, Cheng B, Katz IB, Mitsumoto H, Rowland LP. Clinical features that distinguish PLS, upper motor neuron-dominant ALS, typical ALS. Neurology. 2009;72:1948–52. doi: 10.1212/WNL.0b013e3181a8269b. [DOI] [PubMed] [Google Scholar]
- Graham JM, Papadakis N, Evans J, Widjaja E, Romanowski CA, Paley MN, et al. Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology. 2004;63:2111–9. doi: 10.1212/01.wnl.0000145766.03057.e7. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Holodny AI, Watts R, Korneinko VN, Pronin IN, Zhukovskiy ME, Gor DM, et al. Diffusion tensor tractography of the motor white matter tracts in man: current controversies and future directions. Ann NY Acad Sci. 2005;1064:88–97. doi: 10.1196/annals.1340.016. [DOI] [PubMed] [Google Scholar]
- Hudson AJ, Kiernan JA, Munoz DG, Pringle CE, Brown WF, Ebers GC. Clinicopathological features of primary lateral sclerosis are different from amyotrophic lateral sclerosis. Brain Res Bull. 1993;30:359–64. doi: 10.1016/0361-9230(93)90265-d. [DOI] [PubMed] [Google Scholar]
- Iwata NK, Aoki S, Okabe S, Arai N, Terao Y, Kwak S, et al. Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology. 2008;70:528–32. doi: 10.1212/01.wnl.0000299186.72374.19. [DOI] [PubMed] [Google Scholar]
- Jacob S, Finsterbusch J, Weishaupt JH, Khorram-Sefat D, Frahm J, Ehrenreich H. Diffusion tensor imaging for long-term follow-up of corticospinal tract degeneration in amyotrophic lateral sclerosis. Neuroradiology. 2003;45:598–600. doi: 10.1007/s00234-003-1014-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–16. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Wise SP. Commissural columns in the sensory-motor cortex of monkeys. J Comp Neurol. 1979;188:113–35. doi: 10.1002/cne.901880110. [DOI] [PubMed] [Google Scholar]
- Koester SE, O'Leary DD. Connectional distinction between callosal and subcortically projecting cortical neurons is determined prior to axon extension. Dev Biol. 1993;160:1–14. doi: 10.1006/dbio.1993.1281. [DOI] [PubMed] [Google Scholar]
- Li G, Pleasure SJ. Exciting information for inhibitory neurons. Neuron. 2011;69:585–7. doi: 10.1016/j.neuron.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Litvan I, Mangone CA, Werden W, Bueri JA, Estol CJ, Garcea DO, et al. Reliability of the NINDS Myotatic Reflex Scale. Neurology. 1996;47:969–72. doi: 10.1212/wnl.47.4.969. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Ulug AM, Pullman SL, Gooch CL, Chan S, Tang MX, et al. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology. 2007;68:1402–10. doi: 10.1212/01.wnl.0000260065.57832.87. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29:12343–54. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–37. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson JP, Koski CJ, Boyer AC, Burbank HN, Tandan R, Filippi CG. Linear longitudinal decline in fractional anisotropy in patients with amyotrophic lateral sclerosis: preliminary results. Klin Neuroradiol. 2009;19:129–34. doi: 10.1007/s00062-009-8040-1. [DOI] [PubMed] [Google Scholar]
- Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain. 1992;115:495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–11. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresi PA, et al. Quantitative characterization of the corticospinal tract at 3T. AJNR Am J Neuroradiol. 2006;27:2168–78. [PMC free article] [PubMed] [Google Scholar]
- Roccatagliata L, Bonzano L, Mancardi G, Canepa C, Caponnetto C. Detection of motor cortex thinning and corticospinal tract involvement by quantitative MRI in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:47–52. doi: 10.1080/17482960802267530. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Singer MA, Statland JM, Wolfe GI, Barohn RJ. Primary lateral sclerosis. Muscle Nerve. 2007;35:291–302. doi: 10.1002/mus.20728. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Suh SI, Song IC, Koh SB. Primary lateral sclerosis with MR diffusion tensor image and tract tracking. Am J Phys Med Rehabil. 2006;85:863–4. doi: 10.1097/01.phm.0000242651.30244.a4. [DOI] [PubMed] [Google Scholar]
- Tartaglia MC, Laluz V, Rowe A, Findlater K, Lee DH, Kennedy K, et al. Brain atrophy in primary lateral sclerosis. Neurology. 2009;72:1236–41. doi: 10.1212/01.wnl.0000345665.75512.f9. [DOI] [PubMed] [Google Scholar]
- Turner MR, Grosskreutz J, Kassubek J, Abrahams S, Agosta F, Benatar M, et al. Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:400–3. doi: 10.1016/S1474-4422(11)70049-7. [DOI] [PubMed] [Google Scholar]
- Ulug AM, Grunewald T, Lin MT, Kamal AK, Filippi CG, Zimmerman RD, et al. Diffusion tensor imaging in the diagnosis of primary lateral sclerosis. J Magn Reson Imaging. 2004;19:34–9. doi: 10.1002/jmri.10433. [DOI] [PubMed] [Google Scholar]
- Unrath A, Muller HP, Riecker A, Ludolph AC, Sperfeld AD, Kassubek J. Whole brain-based analysis of regional white matter tract alterations in rare motor neuron diseases by diffusion tensor imaging. Hum Brain Mapp. 2010;31:1727–40. doi: 10.1002/hbm.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock M, Wolters A, Benecke R. Transcallosal inhibition in amyotrophic lateral sclerosis. Clin Neurophysiol. 2007;118:301–7. doi: 10.1016/j.clinph.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Wong JC, Concha L, Beaulieu C, Johnston W, Allen PS, Kalra S. Spatial profiling of the corticospinal tract in amyotrophic lateral sclerosis using diffusion tensor imaging. J Neuroimaging. 2007;17:234–40. doi: 10.1111/j.1552-6569.2007.00100.x. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42:1123–7. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Zhai P, Pagan F, Statland J, Butman JA, Floeter MK. Primary lateral sclerosis: a heterogeneous disorder composed of different subtypes? Neurology. 2003;60:1258–65. doi: 10.1212/01.wnl.0000058900.02672.d2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. White matter integrity in mild cognitive impairment: A tract-based spatial statistics study. Neurology. 2007;68:13–9. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, et al. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]