Abstract
Behavioural variant frontotemporal dementia is a neurodegenerative disorder with dysfunction and atrophy of the frontal lobes leading to changes in personality, behaviour, empathy, social conduct and insight, with relative preservation of language and memory. As novel treatments begin to emerge, biomarkers of frontotemporal dementia will become increasingly important, including functionally relevant neuroimaging indices of the neurophysiological basis of cognition. We used magnetoencephalography to examine behavioural variant frontotemporal dementia using a semantic decision task that elicits both frontal and temporal activity in healthy people. Twelve patients with behavioural variant frontotemporal dementia (age 50–75) and 16 matched controls made categorical semantic judgements about 400 pictures during continuous magnetoencephalography. Distributed source analysis was used to compare patients and controls. The patients had normal early responses to picture confrontation, indicating intact visual processing. However, a predominantly posterior set of regions including temporoparietal cortex showed reduced source activity 250–310 ms after stimulus onset, in proportion to behavioural measures of semantic association. In contrast, a left frontoparietal network showed reduced source activity at 550–650 ms, proportional to patients’ deficits in attention and orientation. This late deficit probably reflects impairment in the neural substrate of goal-oriented decision making. The results demonstrate behaviourally relevant neural correlates of semantic processing and decision making in behavioural variant frontotemporal dementia, and show for the first time that magnetoencephalography can be used to study cognitive systems in the context of frontotemporal dementia.
Keywords: frontotemporal, dementia, semantic decision, picture categorization, magnetoencephalography
Introduction
Behavioural variant frontotemporal dementia (FTD) is characterized by changes in personality, behaviour and emotion and by impairments of executive function. The underlying pathology is heterogeneous and difficult to determine ante-mortem in the majority of cases that lack a known genetic basis for familial FTD. Neuroimaging studies of behavioural variant FTD have shown marked atrophy of the frontal cortex, and to a lesser extent atrophy of the anterior temporal pole (Rosen et al., 2002; Salmon et al., 2003, 2006; Williams et al., 2005; Perry et al., 2006; Peters et al., 2006; Whitwell et al., 2009). Brain hypometabolism broadly mirrors this pattern of progressive atrophy in behavioural variant FTD (Du et al., 2006; Kipps et al., 2009; Dukart et al., 2010; Hu et al., 2010).
In addition to structural and metabolic neuroimaging biomarkers, it is important to characterize the associated functional changes of behavioural variant FTD neurodegeneration. For example, functional MRI in the resting state is beginning to determine the changes in regional interactions and the relationship to underlying atrophy (Seeley et al., 2009; Zhou et al., 2010). It is also necessary to consider the impact of FTD on networks underlying specific cognitive operations. This approach has been taken with primary progressive aphasia for example (Sonty et al., 2007). The lack of functional neuroimaging studies of behavioural variant FTD, in comparison with other major neurodegenerative diseases, may be in part because complex paradigms and functional MRI are not suitable for many patients with behavioural variant FTD. Nonetheless, such neurophysiological markers of frontotemporal cortical function, in conjunction with neuropsychological assessments, could become important biomarkers for the evaluation of disease-modifying and symptomatic treatments for FTD. These markers would be especially relevant where treatments enhance the efficacy or activity of surviving neurons without affecting established atrophy.
This study used magnetoencephalography to study FTD. Neuroimaging by magnetoencephalography has several potential advantages as a safe, non-invasive imaging technique with which to probe dynamic cognitive processes in health and neurodegenerative disease. Magnetoencephalography has high temporal resolution (milliseconds) while retaining sufficient spatial resolution to detect the regional scale of effects resulting from neurodegenerative diseases, including behavioural variant FTD. In addition, the spatial resolution is superior to electroencephalography, and mapping the sensor data back to brain (the ‘inverse problem’) is more tractable and less sensitive to changes in the boundary between cortex and CSF. Moreover, the sitting posture and lack of distraction by scanner noise are helpful to some patients, in comparison with functional MRI. Despite the infrastructural cost of magnetoencephalography facilities, it is therefore a potentially promising tool to study neurodegenerative disease and response to experimental therapies.
In order to examine frontotemporal deficits in patients, we chose a semantic categorization task, for two main reasons. First, it involves an interaction between frontal and temporal sources in healthy adults. This frontotemporal functional anatomy of the task makes it ideally suited to study the consequences of behavioural variant FTD, providing a model system within which to evaluate magnetoencephalography in the context of behavioural variant FTD. Second, functional imaging requires a task that patients can actually perform, which typically will differ from tasks with maximum sensitivity for a given dementia syndrome (cf. Price and Friston, 1999). The simplicity of instructions and the familiarity of picture stimuli used in this study were intended to enable sustained task engagement.
Semantic categorization tasks require a decision or response-selection based on the properties and associations of an object (e.g. ‘is this a picture of a man-made object?’). Such tasks reliably activate both frontal and temporal cortex in functional MRI (Thompson-Schill et al., 1997), magnetoencephalography and electroencephalography (Vihla et al., 2006) in healthy volunteers. We expected that patients with behavioural variant FTD could engage with a simple semantic categorization task even though behavioural deficits in language, naming and semantics occur in behavioural variant FTD. Although, with the exception of verbal fluency, language impairments are less severe than changes in behaviour, empathy or inhibition, and may be absent (Cotelli et al., 2006; Libon et al., 2007; Torralva et al., 2009; Davis et al., 2010). Using magnetoencephalography, Vihla et al. (2006) reported that semantic and visual picture confrontation tasks consistently activate several cortical sources in sequence. These included bilateral occipital sources with peak activity at ∼120 ms, bilateral parietal sources that peak at 280 ms, left temporal sources that peak after 250 ms and bilateral frontal sources that peak after 400 ms. Semantic categorization, therefore, provides the necessary features with which to evaluate the neurocognitive effects of behavioural variant FTD, using magnetoencephalography.
Similar series of regional early time courses have been observed in picture naming tasks. In a review of visual processes following picture presentation, this set of regions is typically identified over and above activations associated with word generation or overt responses (Indefrey and Levelt, 2004). The time courses between naming and semantic decision making tasks were similar, partly because semantic information can be accessed automatically from the global features of the objects depicted (Levelt et al., 1998; Vihla et al., 2006). Principal cortical sources include occipital cortex, fusiform gyrus, inferior temporal gyrus, inferior frontal gyrus and middle cingulate cortex. The temporal information available from magnetoencephalography and electroencephalography also reveals a characteristic cascade of information processing, which is not accessible from other methods such as PET, functional MRI or behavioural analysis. For example, occipital activity peaks between 100 and 200 ms, parietal cortex at ∼150 ms and between 250 and 300 ms and temporal peaks ∼250 ms and 370 ms (Levelt et al., 1998; Vihla et al., 2006; Liljestrom et al., 2009). Frontal cortical activation is seen later, typically after 400 ms (Levelt et al., 1998; Vihla et al., 2006; Liljestrom et al., 2009).
We predicted that early cortical responses in occipitotemporal cortex would be unaffected by behavioural variant FTD, providing an internal control condition. However, we predicted that later responses related to semantic decision making and response selection would be abnormal: in temporal cortex (250 ms after onset) and frontal cortex (after 400 ms). Further, we predicted that the deficits in temporal and frontal cortex would correlate with disease severity, including measures of semantic knowledge and attention.
Materials and methods
Subjects
Thirteen patients (aged 42–68 years, eight males, mean age 60 years) were recruited from a specialist early dementia clinic, with behavioural variant FTD diagnosed according to the consensus criteria, with additional criteria of abnormal structural or functional brain imaging, and evidence of continuing progression after diagnosis, to exclude those with non-progressive ‘phenocopies’ of behavioural variant FTD (Neary et al., 1998; Rascovsky et al., 2007; Kipps et al., 2010). One patient was excluded for poor magnetoencephalography data quality. Patient details are summarized in Table 1. Sixteen healthy older adults (aged 52–72 years, six males, mean age 61 years) were recruited from the healthy volunteer panel of the Medical Research Council Cognition and Brain Sciences Unit. No subjects in the control group had a history of significant neurological, rheumatological or psychiatric illness, and none had any cognitive complaints. The study was given a favourable opinion by the local Research Ethics Committee.
Table 1.
Details of patients with behavioural variant FTD
| Subject | Age | M/F | Onset age | Diagnosis years | MMSE | ACE-R subscales |
Camel and Cactus (%) | GNT | CBI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Attention | Memory | Fluency | Language | VSp | |||||||||
| 1 | 42 | F | 38 | 3 | 20 | 49 | 16 | 6 | 1 | 18 | 8 | 81.3 | 16 | 120 |
| 2 | 60 | F | 58 | 2 | 30 | 89 | 18 | 20 | 9 | 26 | 16 | 92.2 | 24 | 87 |
| 3 | 62 | F | 58 | 4 | 24 | 70 | 15 | 22 | 2 | 21 | 10 | 75 | 18 | 59 |
| 4 | 63 | F | 61 | 2 | 26 | 84 | 17 | 24 | 4 | 23 | 16 | 65.6 | 18 | 37 |
| 5 | 60 | F | 57 | 3 | 19 | 50 | 13 | 8 | 1 | 18 | 10 | 57.8 | 6 | 61 |
| 6 | 65 | M | 62 | 2 | 27 | 71 | 18 | 19 | 6 | 20 | 8 | x | 19 | 65 |
| 7 | 59 | M | 57 | 2 | 26 | 70 | 15 | 18 | 4 | 23 | 10 | x | x | 156 |
| 8 | 68 | M | 64 | 4 | 21 | 72 | 14 | 16 | 5 | 23 | 14 | x | x | 146 |
| 9 | 57 | M | 61 | 7 | 23 | 69 | 15 | 13 | 8 | 20 | 13 | 87.5 | 19 | 76 |
| 10 | 51 | M | 42 | 8 | 22 | 76 | 13 | 17 | 4 | 26 | 16 | 98.4 | 24 | 212 |
| 11 | 62 | M | 56 | 5 | 14 | 33 | 9 | 0 | 0 | 9 | 15 | 76.6 | 17 | 130 |
| 12 | 68 | M | 61 | 7 | 27 | 85 | 17 | 22 | 11 | 22 | 13 | 92.2 | 14 | 48 |
| Averages | ||||||||||||||
| 59.8 | 56.5 | 4.1 | 23.3 | 68.2 | 15.0 | 15.4 | 4.6 | 20.8 | 12.4 | 80.7 | 17.5 | 99.8 | ||
ACE-R = Addenbrooke's Cognitive Examination (Revised); CBI = Cambridge Behavioural Inventory; GNT = Graded Naming Task; MMSE = Mini-Mental State Examination; VSp = visuospatial subscale; x = score not available.
Behavioural assessments
Patients underwent neuropsychological assessment including the 100-point revised Addenbrooke's Cognitive Examination (Revised) as a general measure of cognitive function, (Mioshi et al., 2006), which includes an attentional subscale and the 30-point Mini-Mental State Examination; the Camel and Cactus task, which provides a measure of semantic association (Bozeat et al., 2000); and the Graded Naming task as a measure of picture naming (McKenna and Warrington, 1980). Three patients were either unable or unwilling to complete the Camel and Cactus and two did not complete the Graded Naming task. Caregivers completed the Cambridge Behavioural Inventory, which provides an assessment of the severity of behavioural symptoms (Wedderburn et al., 2008). Summary details of test scores are provided in Table 1.
Task
The picture categorization task used was adapted from two earlier studies examining semantic decisions (Thompson-Schill et al., 1997; Vihla et al., 2006) and presented using E-prime 1.1 software (www.pstnet.com) in Windows XP (www.microsoft.com). Participants made categorical semantic judgements about line drawing pictures of common objects, deciding if the objects depicted were large or small (with reference to a shoe box size), manmade or natural (Fig. 1). The test pictures were of 200 common objects and selected from the International Picture Naming Projects database, matched for accuracy of responses and frequency (Szekely et al., 2005). The pictures were presented in four successive blocks of 100 and throughout each block the same judgement was made. Each picture was presented twice—once in a small or big judgement block and once in a man-made or natural block. The task was trained with verbal instructions, reinforced by the keywords on screen. During magnetoencephalography, each block started with a written and verbal instruction presented for 2 s (e.g. ‘Are the objects big?’), followed by a sequence of trials.
Figure 1.
Examples of different trial types. A picture was presented for 300 ms, followed by a variable delay of 400–600 ms in which the decision, but not a response, was made. At the end of the delay period, subjects were prompted to make a yes/no response using right-hand buttons. The required judgement was displayed again as a reminder at the response stage and remained constant throughout blocks of 100 stimuli.
In each trial, a picture was presented for 300 ms followed by a variable delay between 400 and 600 ms during which the screen was blank. Then, a response cue was presented for 1500 ms that prompted subjects to answer ‘yes’ or ‘no’, with a reminder of the category type in the centre of the screen (e.g. ‘Yes BIG No’). Subjects responded by pressing a button with either their index finger for ‘yes’ or their middle finger for ‘no’. The variable delay between picture confrontation and response allowed uncoupling of the processes involved in perception and decision making from the motor preparation and response. The categories were counterbalanced across subjects and individual trials were randomized. Training sessions preceded the testing using a separate set of 48 pictures (12 in each category). All experimental trials were included in data analysis, whether the final response was accurate or inaccurate.
Data acquisition and processing
Continuous magnetoencephalography data were collected with a 306-channel Vectorview system (Elekta Neuromag), situated in a magnetically shielded room, with one magnetometer and two orthogonal planar gradiometers located at each of 102 positions within a hemispherical array. Vertical and horizontal eye movements were recorded using electroculography electrodes. Four Head-Position Indicator coils were used to monitor head position at 5 Hz. The 3D locations of these coils and ∼80 ‘head points’ along the scalp, relative to three anatomical fiducials (the nasion and left and right pre-auricular points), were recorded using a 3D digitizer (Fastrak Polhemus Inc.). Data were preprocessed using MaxFilter software (Elekta-Neuromag) and Brain Electrical Source Analysis (BESA 5.3).
Data were high pass filtered to 0.1 Hz, with a notch filter at 50 Hz. The artefact rejection threshold was set to 2500 ft for magnetometers and 900 ft for gradiometers. Eye blinks were corrected using the BESA5.3 adaptive artefact correction. Epochs were time locked to the picture onset, including data from 200 ms before stimulus onset to 1200 ms after onset and baseline corrected to 200 ms before onset. All trial epochs were averaged across each of the four blocks and then across each condition.
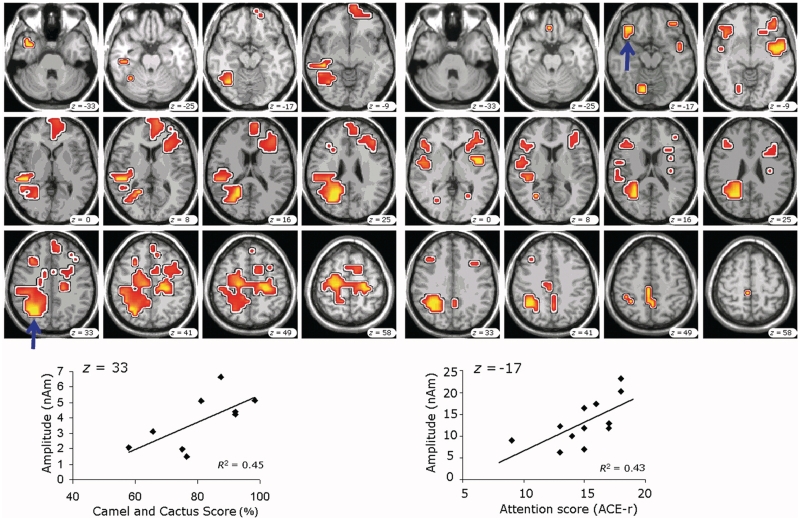
Single subject source analysis used low-resolution brain electromagnetic tomography, LORETA, (Pascual-Marqui et al., 1994), a type of distributed source analysis that computes a current distribution across the entire brain volume. Results are presented for gradiometer magnetoencephalography channels on the averaged data for each individual subject and also on the averaged data of all controls. Statistical comparisons between patients and controls used the Fieldtrip toolbox for between-subjects permutation testing (www.ru.nl/donders/fieldtrip) (Oostenveld et al., 2011). Six time windows were preselected, based on the time course of sources in the closely related task by Vihla et al. (2006). The time windows encompassed early visual analysis: the p50 (30–70 ms) and the N100 (80–120 ms); two mid-stage windows that spanned the parietal source peaks reported at 280 ms (250–310 ms) and the temporal source peaks reported at 370 ms (340–400 ms); and two later decision stage windows between 400–500 and 550–650 ms. Mean source densities (‘activations’) during these epochs were compared across all regions by permutations testing (1000 permutations), with grid size of 7 mm, using initial thresholding P < 0.05, prior to cluster-based statistical threshold to control the family wise error at P < 0.05 (family wise false positive rate under the null hypothesis of no group difference). This addresses the multiple comparisons problem in the presence of spatial non-independence. Two time windows revealed significant differences (Fig. 3), from which we extracted the patients’ source densities in the peak of these temporal and frontal clusters. In two post hoc tests, these frontal and temporal peak source densities were correlated (Pearson's r, Bonferroni corrected for two multiple comparisons) with a clinical measure of attention subscale (Addenbrooke's Cognitive Examination, Mioshi et al., 2006) and semantics, respectively (Camel and Cactus Test, Bozeat et al., 2000; Adlam et al., 2010).
Figure 3.
Significantly reduced amplitudes in patients compared with controls (P < 0.05, permutations tests), masked by activity in the control group. (A) Reduced amplitudes in the 250–310 ms time window. The most reduced source current density in the peak of the left temporoparietal cluster (indicated by the arrow) positively correlated with accuracy on the Camel and Cactus task of semantic knowledge (see plot). (B) Significantly reduced amplitudes in the decision phase, 550–650 ms. The most reduced source current density in the left frontotemporal cluster (indicated by the arrow) correlated with attention scores in the Addenbrooke's Cognitive Examination (Revised) (ACE-r).
Results
Behavioural analysis
In the control group, mean accuracy was 87%. In the patient group, response accuracy was >70% for seven patients, five patients performed near chance (50–60%) and one patient had difficulty pressing the keys with too few responses recorded. The high error rates in at least two of the patients were attributable to motor response perseveration as the same key was pressed repeatedly (>75% of the time the same key was selected). This compares with repetitive button presses in ∼50% of trials in controls, as would be expected from the randomization of trial order.
An ANOVA revealed that patients had significantly lower accuracy than controls [F(1,26) = 44, P < 0.05], and that overall scores on the four semantic decision tasks differed significantly [F(1,26) = 10.4, P < 0.05] with ‘big’ and ‘small’ decisions being less accurate than ‘natural’ and ‘man-made’ decisions. Decision type interacted with group [F(3,78) = 3, P < 0.05], reflecting particular difficulty in decisions by patients for the ‘small’ semantic category. Reaction times to the response cue were slower and more variable for patients than controls [controls mean: 536 ms, standard error 29 ms; patients mean 791 ms, standard error 122 ms; F(1,26) = 5.4, P < 0.05]. There were no significant differences between the four task blocks (big, small, manmade, natural [F(3,75) = 3.2, P = 0.06]) and no interactions between patient and control groups with task block [F(3,78) = 3.5, P = 0.058]. Few subjects made any responses prior to the response cue, although reactions times were shorter on longer latency trials [F(2,52) = 9.8, P < 0.05]. However, there was no interaction between group, trial duration and reaction time (F < 1).
Magnetoencephalography analysis
Separate comparisons of different trial types confirmed that there were no significant magnetoencephalography differences between decision types (big, small, manmade, natural), and subsequent data were collapsed across all trial types. The average time course for the healthy controls over all trials (Fig. 2) showed bilateral occipital source activity extending from 100 to 200 ms, and again ∼400 ms, which follows the termination of the picture. Parietal sources were initially bilateral, but predominantly left sided, emerging from 120 to 200 ms, with further left and more anterior peaks from 240 to 360 and a late sustained left peak after 400 ms up to 650 ms. Temporal source activity was evident bilaterally between 190 and 240 ms, followed by primarily left temporal peaks emerging between 300 and 370 ms. Medial prefrontal sources were sustained throughout the task from ∼200 ms accompanied by early bilateral prefrontal activity, followed by left frontal source activity from 400 to 500 ms. This spatial and temporal pattern is consistent with previous studies of closely related tasks (Thompson-Schill et al., 1997; Vihla et al., 2006).
Figure 2.
Average windows of activity for healthy controls. Clusters are shown for which source current density was significantly >0 (P < 0.05 corrected for multiple comparisons by permutations testing) based on 16 healthy individual subjects’ LORETA images. Times (ms) are with reference to the onset of the picture stimulus.
Differences between patients and controls were analysed across six time windows. There were no significant group differences at 30–70 ms, 80–120 ms, 340–400 ms or 400–500 ms after stimulus presentation. However, at 250–310 ms, left occipitotemporal, occipitoparietal and right prefrontal sources were reduced in patients (Fig. 3A). During the decision phase, there were no differences in the 400–500 ms window, but significant differences emerged between 550 and 650 ms after stimulus presentation. Patients with behavioural variant FTD showed reduced source current densities, predominantly in the left hemisphere in frontotemporal, occipitotemporal and parietal cortex (Fig. 3B).
In the 250–310 ms window, the peak difference was within a temporoparietal cluster, and patients’ source amplitude in the left temporoparietal peak correlated with accuracy on the Camel and Cactus task (R2 = 0.45, P < 0.025 Bonferroni corrected, Fig. 3A). In the 550–650 ms window, the peak difference was within a cluster in the left frontal cortex, and peak source amplitude correlated with the Addenbrooke's Cognitive Examination (Revised) subscale for attention and orientation (R2 = 0.43, P < 0.025, Bonferroni corrected, Fig. 3B).
Discussion
This study confirms that magnetoencephalography can be used to measure the effects of progressive behavioural variant FTD. Key findings were that although neural correlates of early visual processes were intact, there were spatially and temporally defined abnormalities in frontal, temporal and parietal cortex. Furthermore, these spatiotemporally confined abnormalities corresponded to different stages of the semantic decision task and correlated with clinical behavioural measures of cognitive functions.
The data from the healthy controls were in line with previous imaging studies of picture categorization and confrontation tasks. Early responses in occipital and occipitotemporal cortex were followed by parietal responses emerging after 120 ms, and after 240 ms, with peaks at ∼150 and 250 ms, respectively. These parietal sources are congruent with previous electroencephalography and magnetoencephalography reports of early parietal activity at 150 ms, and later parietal activity peaking after 250 ms (Levelt et al., 1998; Wang et al., 2000; Vihla et al., 2006; Liljestrom et al., 2009). Parietal activity after picture confrontation is suggested to be due to working memory, control of visual attention and/or generation of a mental representation of the object (Levelt et al., 1998; Vihla et al., 2006).
Temporal sources were identified after the initial parietal sources, reaching a peak in inferior temporal cortex by 240 ms, with a later more superior peak between 300 and 370 ms. This is also consistent with previous reports of picture confrontation tasks (Levelt et al., 1998; Wang et al., 2000; Vihla et al., 2006; Liljestrom et al., 2009), and is likely to represent successive but interactive processes of object identification and semantic association in the ventral stream (Hauk et al., 2007; Patterson et al., 2007).
The patient group, as predicted, showed deviations from the normal pattern of temporal and parietal cortical activation. Patients had reduced activity in left temporoparietal clusters in the 250–310 ms window compared with the control group, although not in the later 340–400 ms time window when a temporal peak is also observed in health. The reduced temporoparietal cluster activity (250–310 ms) is consistent with impairments in semantic association, even of common objects. However, it might also result from reduced visual attention that would be expected to affect the early cortical responses in occipital and occipitotemporal cortex. Although none of these patients had a primary diagnosis of semantic dementia, deficits in semantic knowledge and association often exist in behavioural variant FTD (Cotelli et al., 2006; Rogers et al., 2006; Libon et al., 2007; Davis et al., 2010) as the two syndromes form part of the spectrum of frontotemporal lobar degeneration and overlap cases with both behavioural and semantic features are common. Moreover, the patients’ peak activity in this temporoparietal cluster correlated with scores on the Camel and Cactus task (Bozeat et al., 2000), which is used to probe semantic knowledge in FTD and was recently shown to correlate with the rostral fusiform region of the temporal lobe in a cohort comprising semantic dementia or mixed semantic and behavioural variant patients (Mion et al., 2010). The Pyramids and Palm trees tests, on which the Camel and Cactus task is based, have also been correlated with grey matter loss throughout the anterior temporal lobes (Mummery et al., 2000; Williams et al., 2005). Taken together, these findings support the hypothesis that diminished temporoparietal sources reflect both temporal neuropathology in behavioural variant FTD and the observed behavioural semantic deficits.
The semantic decision task was also associated with lateral prefrontal cortical activity, bilaterally and most strongly after 500 ms for control subjects (Fig. 1). Prefrontal cortex has been linked with semantic categorization of pictures and visual semantic selection tasks (Thompson-Schill et al., 1997; Vihla et al., 2006), and the left inferior frontal cortex is necessary to mediate the selection of relevant semantic associations (rather than retrieval per se) to guide an appropriate response (Thompson-Schill et al., 1998; Jefferies and Lambon Ralph, 2006). In patients with behavioural variant FTD, the prefrontal cortical sources were diminished as early as 250–310 ms in concert with reductions in temporoparietal sources. However, a more extensive frontal impairment in behavioural variant FTD emerged later in the decision phase, between 550 and 650 ms. The clusters of reduced activity are homologous to the significant activations of the bilateral inferior frontal gyrus in Thompson-Schill et al.'s (1997) semantic classification task. In the current study, the time course of lateral frontal cortical abnormalities is consistent with the principal role of this region in selection among semantic alternatives rather than semantic retrieval.
Patients also had additional, predominantly left-sided, reductions in source activity in the decision phase of the task, 550–650 ms (Fig. 3B). Such late activity has been reported in studies of picture naming, although this may have been confounded by response preparation with reaction times to picture confrontation in younger subjects at ∼500 ms (Levelt et al., 1998). In the current study, we sought to uncouple the activity related to semantic decisions from the response by using a late and variable response cue, jittered between 700 and 900 ms after the stimulus onset.
There are several possible explanations for the reduced frontotemporal source activity in the 550–650 ms window for patients. First, source activity in this period may represent the neural correlate of the semantic decision process itself, with the response selection following retrieval of stimulus associations. The patients’ magnetoencephalography abnormalities would, therefore, indicate directly their impaired semantic decision making.
Alternatively, source activity in this late task period may represent working memory for a prior decision or stimulus. Given that in tasks without a prolonged decision phase, semantic decisions can be made and acted on within ∼500 ms, it is unlikely that the stimulus is passively remembered until the end of the delay period. More likely is that a response is selected early, but remembered and/or prepared until the response cue after 700–900 ms. Evidence for this comes from source activity emerging at the left central sulcus after 600 ms, but shortly before the actual response is made. This central sulcal source is reduced in patients; patients may have failed to remember an earlier decision or chosen response. Working memory for stimuli or response sets is commonly associated with activity of a lateral frontoparietal network (Owen et al., 1998; Levy and Goldman-Rakic, 2000; Postle et al., 2000). Further, the reaction times for controls and patients were similarly affected by the jitter latency, indicating that responses were selected by subjects in advance, in preparation for the cue. Taken together, these factors suggest that the frontotemporal magnetoencephalography abnormalities in the decision phase, 550–650 ms, are not due to a deficit in working memory for the decision.
A third contributor to the reduced frontotemporal source activity in the 550–650 ms window may be invoking cognitive control to manage interference between trials, and in particular to inhibit prior responses to enable the correct current response. The inferior frontal gyri (bilaterally) are critical for response inhibition (Aron et al., 2003; Swick et al., 2008) and are active in tasks requiring high cognitive control as a result of trial to trial interference (Braver et al., 2003). In this study, it is notable therefore that two patients with behavioural variant FTD made very perseverative responses.
There was no evidence that patients had additional sources or greater source activation indicative of compensatory enhancement within less affected cortex, in any region for any of the time windows. In patients and control subjects, we also found no difference between types of categorization, despite previous reports of greater fusiform activity for the classifications of more visual attributes such as size (Thompson-Schill et al., 1997).
There are potential limitations to our study. We included 12 patients with behavioural variant FTD, of varying severity and it is very likely that our group includes different underlying tau and ubiquitin pathologies. These factors may have introduced unmodelled variance that increases the risk of type II error. We did, however, maintain control of type I error, through the cluster-based permutations testing. Although key magnetoencephalography results did correlate with behavioural measures, each subject participated only once, and we do not know how within-subject effects progress over time. While we can infer magnetoencephalography differences at different levels of disease severity in our group, further work is required to know whether magnetoencephalography changes progress within individuals during the course of their disease. This information would enable power calculations if magnetoencephalography were to be planned as a prognostic or therapeutic biomarker in future studies. We note, however, that before magnetoencephalography could be considered as a diagnostic biomarker, sensitivity and specificity across other neuropsychiatric disorders would be required, and our data are not sufficient for this.
The behavioural performance of patients with behavioural variant FTD was lower than controls. This can in general introduce an ambiguity in the interpretation of brain imaging or lesion studies (Price and Friston, 1999) and requires further consideration of the task components. The normality of early visual responses and the reaction times to response probes both indicate continuing task engagement by patients. In other words, the patients were viewing the stimuli, and made appropriately timed responses to cues. The semantic processing may in part be in response to visual cues [cf. Vihla et al. (2006): semantic and visual picture confrontation tasks consistently activate similar cortical sources in sequence], but in the current paradigm it is consistently contextually defined by the decision task and we cannot separate automatic from task-dependent semantic processing. The agreement of categorical judgements between patients and controls was low: the most likely explanation is a causal relationship between frontotemporal neuronal activity and semantic decision making. The magnetoencephalography thus provides additional spatiotemporal information about the physiological basis of this affected cognitive process. However, accuracy of responses can be affected by both poor decision making and response perseveration. At least two subjects perseverated substantially in the scanning session, with a high number of trial pairs in which the same motor response was made to both trials regardless of the accuracy of that response. The motor response may, therefore, not truly reflect the trial-specific semantic decision. A corollary of this type of motor perseveration is that it does at least indicate preservation of working memory for responses over successive trials and argues against a failure of working memory as the basis of late magnetoencephalography abnormalities.
During source analysis, we used a canonical realistic head model, co-registered to fiducial markers and scalp. We did not use subject-specific MRI scans as these were not all available on a standardized sequence or close in time to the magnetoencephalography. It could be argued that group differences in atrophy confound the magnetoencephalography source analysis. We argue that this is not a sufficient explanation for the findings, for several reasons. First, for determining the optimal forward model for magnetoencephalography (not electroencephalography) required for source reconstruction, an individualized cortical mesh is not superior to a canonical model provided that a realistic boundary element head model is used (Henson et al., 2009). Unlike electroencephalography, the forward model in magnetoencephalography is most dependent on the inner skull surface, as the boundary with maximal change in conductivity (Hamalainen and Sarvas, 1989), and this boundary is a priori unlikely to change with behavioural variant FTD. Magnetoencephalography is much less dependent on the boundary between cortex and CSF, which is likely to change with significant cortical atrophy in behavioural variant FTD. Severe atrophy would of course move the ‘true’ cortical source further from the nearest gradiometers. Although magnetic flux density declines rapidly with distance (as a cubic function), with realistic head models the errors in estimation of localization and amplitude of magnetoencephalography sources increase minimally even as sources move up to 2–3 cm deeper from the skull (Tarkiainen et al., 2003). Secondly, we used LORETA distributed source analysis, which constrains the inversion of channel to brain current source densities by a smoothness criterion. Although reducing the anatomical precision of sources, this is robust to minor between-subject differences in anatomy and functional topography. Furthermore, we found behavioural variant FTD abnormalities in posterior parietal and temporal cortex that depend on back projections from frontal cortex, but which can reveal relative preservation of grey matter in behavioural variant FTD by structural imaging (Williams et al., 2005; Pereira et al., 2009; Whitwell et al., 2009; Gordon et al., 2010).
In summary, our data demonstrate the utility of magnetoencephalography to study the pathophysiology of cognitive effects of behavioural variant FTD. Specifically, we have identified the effects of behavioural variant FTD on the neural correlates of a semantic decision task. The spatiotemporal specificity of these changes in frontotemporal and temporoparietal sources, and correlations with cognitive impairments, suggest that the effect of behavioural variant FTD includes contributions from abnormalities of both early semantic processing and later decision making. In particular, the frontotemporal deficits during the decision phase of the task (550–650 ms) are in contrast with normal early visual cortical sources 30–120 ms after presentation of stimuli. Further studies with magnetoencephalography will be needed in behavioural variant FTD and related disorders, both for replication and to assess the diagnostic specificity of the changes we observe. Nonetheless, our results provide clear, if preliminary, evidence of the link between cognitive and neurophysiological dysfunction due to behavioural variant FTD neurodegeneration, and raise the possibility of using magnetoencephalography in conjunction with cognitive assessments as a biomarker of future candidate therapies.
Funding
Wellcome Trust (077029, 088324); Medical Research Council's Cognition and Brain Sciences Unit; NIHR Cambridge Comprehensive Biomedical Research Centre.
Acknowledgements
The authors thank the participants and carers for taking part in this research, and Karalyn Patterson for helpful advice on the article.
Glossary
Abbreviations
- FTD
frontotemporal dementia
References
- Adlam AL, Patterson K, Bozeat S, Hodges JR. The Cambridge Semantic Memory Test Battery: detection of semantic deficits in semantic dementia and Alzheimer's disease. Neurocase. 2010;16:193–207. doi: 10.1080/13554790903405693. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–6. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–26. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Alberici A, Calabria M, Agosti C, et al. Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology. 2006;20:558–65. doi: 10.1037/0894-4105.20.5.558. [DOI] [PubMed] [Google Scholar]
- Davis C, Heidler-Gary J, Gottesman RF, Crinion J, Newhart M, Moghekar A, et al. Action versus animal naming fluency in subcortical dementia, frontal dementias, and Alzheimer's disease. Neurocase. 2010;16:259–66. doi: 10.1080/13554790903456183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Jahng GH, Hayasaka S, Kramer JH, Rosen HJ, Gorno-Tempini ML, et al. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–20. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J, Mueller K, Horstmann A, Vogt B, Frisch S, Barthel H, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010;49:1490–5. doi: 10.1016/j.neuroimage.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Gordon E, Rohrer JD, Kim LG, Omar R, Rossor MN, Fox NC, et al. Measuring disease progression in frontotemporal lobar degeneration: a clinical and MRI study. Neurology. 2010;74:666–73. doi: 10.1212/WNL.0b013e3181d1a879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–71. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Hauk O, Patterson K, Woollams A, Cooper-Pye E, Pulvermuller F, Rogers TT. How the camel lost its hump: the impact of object typicality on event-related potential signals in object decision. J Cogn Neurosci. 2007;19:1338–53. doi: 10.1162/jocn.2007.19.8.1338. [DOI] [PubMed] [Google Scholar]
- Henson RN, Mattout J, Phillips C, Friston KJ. Selecting forward models for MEG source-reconstruction using model-evidence. Neuroimage. 2009;46:168–76. doi: 10.1016/j.neuroimage.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Wang Z, Lee VM, Trojanowski JQ, Detre JA, Grossman M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology. 2010;75:881–8. doi: 10.1212/WNL.0b013e3181f11e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–44. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–47. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Hodges JR, Fryer TD, Nestor PJ. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009;132:2566–78. doi: 10.1093/brain/awp077. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Hodges JR, Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the 'bvFTD phenocopy syndrome'. Curr Opin Neurol. 2010;23:628–32. doi: 10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Praamstra P, Meyer AS, Helenius P, Salmelin R. An MEG study of picture naming. J Cogn Neurosci. 1998;10:553–67. doi: 10.1162/089892998562960. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Liljestrom M, Hulten A, Parkkonen L, Salmelin R. Comparing MEG and fMRI views to naming actions and objects. Hum Brain Mapp. 2009;30:1845–56. doi: 10.1002/hbm.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna P, Warrington EK. Testing for nominal dysphasia. J Neurol Neurosurg Psychiatry. 1980;43:781–8. doi: 10.1136/jnnp.43.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–68. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–85. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci USA. 1998;95:7721–6. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pereira JM, Williams GB, Acosta-Cabronero J, Pengas G, Spillantini MG, Xuereb JH, et al. Atrophy patterns in histologic vs clinical groupings of frontotemporal lobar degeneration. Neurology. 2009;72:1653–60. doi: 10.1212/WNL.0b013e3181a55fa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Graham A, Williams G, Rosen H, Erzinclioglu S, Weiner M, et al. Patterns of frontal lobe atrophy in frontotemporal dementia: a volumetric MRI study. Dement Geriatr Cogn Disord. 2006;22:278–87. doi: 10.1159/000095128. [DOI] [PubMed] [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21:373–9. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Postle BR, Stern CE, Rosen BR, Corkin S. An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. Neuroimage. 2000;11:409–23. doi: 10.1006/nimg.2000.0570. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–8. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord. 2007;21:S14–8. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer's disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology. 2006;20:319–35. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Salmon E, Garraux G, Delbeuck X, Collette F, Kalbe E, Zuendorf G, et al. Predominant ventromedial frontopolar metabolic impairment in frontotemporal dementia. Neuroimage. 2003;20:435–40. doi: 10.1016/s1053-8119(03)00346-x. [DOI] [PubMed] [Google Scholar]
- Salmon E, Kerrouche N, Herholz K, Perani D, Holthoff V, Beuthien-Baumann B, et al. Decomposition of metabolic brain clusters in the frontal variant of frontotemporal dementia. Neuroimage. 2006;30:871–8. doi: 10.1016/j.neuroimage.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci. 2007;27:1334–45. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely A, D'Amico S, Devescovi A, Federmeier K, Herron D, Iyer G, et al. Timed action and object naming. Cortex. 2005;41:7–25. doi: 10.1016/s0010-9452(08)70174-6. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Liljestrom M, Seppa M, Salmelin R. The 3D topography of MEG source localization accuracy: effects of conductor model and noise. Clin Neurophysiol. 2003;114:1977–92. doi: 10.1016/s1388-2457(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–60. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132:1299–309. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- Vihla M, Laine M, Salmelin R. Cortical dynamics of visual/semantic vs. phonological analysis in picture confrontation. Neuroimage. 2006;33:732–8. doi: 10.1016/j.neuroimage.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sakuma K, Kakigi R. The dynamic processes for word and picture encoding in the human brain as revealed by magnetoencephalography. Neurosci Lett. 2000;289:135–8. doi: 10.1016/s0304-3940(00)01298-2. [DOI] [PubMed] [Google Scholar]
- Wedderburn C, Wear H, Brown J, Mason SJ, Barker RA, Hodges J, et al. The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatry. 2008;79:500–3. doi: 10.1136/jnnp.2007.122028. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–46. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–51. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]