Abstract
The intersubspecific hybrids of autotetraploid rice has many features that increase rice yield, but lower seed set is a major hindrance in its utilization. Pollen sterility is one of the most important factors which cause intersubspecific hybrid sterility. The hybrids with greater variation in seed set were used to study how the F1 pollen sterile loci (S-a, S-b, and S-c) interact with each other and how abnormal chromosome behaviour and allelic interaction of F1 sterility loci affect pollen fertility and seed set of intersubspecific autotetraploid rice hybrids. The results showed that interaction between pollen sterility loci have significant effects on the pollen fertility of autotetraploid hybrids, and pollen fertility further decreased with an increase in the allelic interaction of F1 pollen sterility loci. Abnormal ultra-structure and microtubule distribution patterns during pollen mother cell (PMC) meiosis were found in the hybrids with low pollen fertility in interphase and leptotene, suggesting that the effect-time of pollen sterility loci interaction was very early. There were highly significant differences in the number of quadrivalents and bivalents, and in chromosome configuration among all the hybrids, and quadrivalents decreased with an increase in the seed set of autotetraploid hybrids. Many different kinds of chromosomal abnormalities, such as chromosome straggling, chromosome lagging, asynchrony of chromosome disjunction, and tri-fission were found during the various developmental stages of PMC meiosis. All these abnormalities were significantly higher in sterile hybrids than in fertile hybrids, suggesting that pollen sterility gene interactions tend to increase the chromosomal abnormalities which cause the partial abortion of male gametes and leads to the decline in the seed set of the autotetraploid rice hybrids.
Keywords: Autotetraploid rice, chromosome behaviour, hybrid sterility, microtubule, Oryza sativa, pollen sterility, ultra-structure
Introduction
Polyploidy is a potent source of increasing crop productivity and plays a significant role in plant breeding programmes. Over 70% of all angiosperm species have an increase in ploidy level somewhere in their evolutionary histories. Some polyploids are of recent origin, while others are millions of years old (Levin, 2002). Polyploidy provides genome buffering, increased allelic diversity, permits novel phenotypic variation to be generated, and is often accompanied by a loss of duplicated chromatin and changes in gene expression (Udall and Wendel, 2006). Polyploidy has abundant advantages over diploid crops such as an increase in greenness, lushness, seed index, and biomass yield (Bingham et al., 1994).
Autotetraploid rice has great potential to increase the yield of rice and has more nutrition and is more resistant to insect pests and diseases than diploid rice (Chen et al., 1987; Song and Zhang, 1992). Autotetraploid rice depicted greater genetic variation than diploid rice (Luan et al., 2008) and greater grain length and width than diploid rice (Tu et al., 2007; Shahid et al., 2011). Intersubspecific autotetraploid rice hybrids have more hybrid vigour and are more stable across different environments than diploid hybrids (Shahid et al., 2011). However, lower seed set is the major hindrance in its utilization. Pollen fertility has a significant correlation with seed set and autotetraploid rice has lower pollen fertility than diploid rice (Shahid et al., 2010). Therefore, it is very important to find the reasons for lower pollen fertility in autotetraploid rice.
Asian cultivated rice could be divided into three subspecies, i.e. indica, japonica, and javanica (Chang, 1976). Intersubspecific crosses of Asian cultivated rice exhibits one of the best hybrid combinations in the rice crop, however, the utilization of their hybrid vigour is limited due to the presence of partial to complete sterility (Kato et al., 1928; Zhang et al., 1997; Xiao and Yuan, 2009). Cytological studies revealed that the male gamete abortions (Zhang et al., 1993; Liu et al., 2004a), female gamete abortions (Liu et al., 2004a), and reduced dehiscence of the anthers (Maekawa et al., 1997; Zhang et al., 2006) could cause the hybrid sterility. Pollen sterility is one of the most important reasons of intersubspecific hybrid sterility and contributed equally to the low fertility of intersubspecific hybrids compared with embryo sac sterility (Yan et al., 2003, Song et al., 2005). At least six loci, i.e. S-a, S-b, S-c, S-d, S-e, and S-f have been mapped for pollen sterility in intersubspecific hybrids (Zhang et al., 1993, 1994). The S-a gene has been cloned (Long et al., 2008) and the S-b (Li et al., 2006) and S-c (Yang et al., 2004) genes have been fine mapped. These gene interactions only related to pollen sterility and had no relation with embryo sac sterility and their cytological mechanism is different at different loci (Zhang et al., 2006). Pollen abortion in intersubspecific hybrids was due to allelic interaction of the F1 pollen sterility locus, and the F1 pollen sterility alleles was in accordance with a one-locus sporogametophytic interaction model (Zhang and Lu, 1993). Typical indica and japonica rice carry Si and Sj alleles at F1 pollen sterility loci, respectively. The gametes with genotypes SiSi and SjSj in the hybrids are fertile and the gametes having the Sj allele in the hybrid genotype SiS j are partially aborted (Oka, 1974; Sano, 1990; Zhang et al., 2006). The alleles that show good affinity with indica and japonica varieties and do not interact with alleles Sj (japonica) and Si (indica) are called neutral alleles (Sn), i.e. Sn is compatible with both Si and Sj and no allelic interaction happens between Sn and Sj or Si (Ding et al., 2003; Shi et al., 2009). The neutral alleles Sn-a (Long, 2007) and Sn-b (Shi et al., 2009) have been found in cultivated rice (Guanglu'ai 4) and a wild rice accession (GZW099) of Oryza rufipogon Griff. indigenous to Gaozhou, Guangdong, respectively.
Pollen grains play a vital role in plant fertility through the generation and delivery of the male gametes to the embryo sac for double fertilization in flowering plants (Borg et al., 2009). Chromosome behaviour during meiosis played an important role in normal male gamete formation in the mutants of diploid plants (Kunio and Takeshi, 1983). Plant fertility in the induced autotetraploid of kale depends on the chromosome paring at meiosis (Jenczewski et al., 2002). Chromosome behaviour played a very important role in fertility at the cell level (Srivastava, 1956; Alley, 1957; Wolf et al., 1989; Lavania et al., 1991; Luo et al., 1992; Srivastava et al., 1992). Bremer and Bremer-Reinders (1954) reported that an increase in the fertility of autotetraploid Secale cereale was correlated with an increase in bivalents and a decrease in univalents and multivalents during meiosis. Quadrivalents and bivalents have significant importance in the fertility of polyploids (Jones, 1994). Venkateswarlu and Rao (1976) made a comparative study between the colchicine-induced ‘raw’ autotetraploid Coix lacryma (S0 generation) and the S3 generation (evolved from S0 through selection for vigour and fertility after the selfing-cross of three successive generations). They found a significant decrease in the frequency of quadrivalents and an increase in ring quadrivalents and further suggested that selection for vigour and fertility could increase the number of ring quadrivalents in autotetraploids and that ring quadrivalents are likely to have a more regular disjunction at anaphase I. Our previous studies also showed that abnormal chromosome behaviour resulted in low pollen fertility of intersubspecific autotetraploid rice hybrids (He et al, 2011).
Our previous research revealed that the interaction of S-b might also affect the pollen fertility of intersubspecific autotetraploid rice hybrids (Zhao et al., 2006). However, no detailed information was available about how single or polygene interactions affect the pollen fertility of autotetraploid rice hybrids. Moreover, little is known about the relationship between the interaction of sterile pollen genes and abnormal chromosome behaviour during pollen mother cell meiosis and how they affect the pollen fertility of autotetraploid rice. Some high seed set autotetraploid rice hybrids have been developed, so hybrids with significant variation in seed set were used for this research. The present study was conducted (i) to determine the genotypes of pollen sterility loci (S-a, S-b, and S-c) and their effect on pollen fertility; (ii) to determine the ultra-structural changes in pollen mother cells (PMC) and microtubule organization and chromosome behaviour during meiosis; and (iii) to find a relationship between chromosome behaviour and pollen sterility loci interaction and their effect on seed setting of autotetraploid rice hybrids.
Materials and methods
Plant materials
A total of four autotetraploid rice lines (Guanglu'ai 4-4x, E24-4x, L202-4x, and Jackson-4x) (Table 1) were used to conduct the study and five intersubspecific autotetraploid rice hybrids were developed from these lines (Table 2). Diploid rice varieties (Guanglu'ai 4; E24) and an autotetraploid rice (Guanglu'ai 4-4x) were used as controls. All materials were planted at the farm of the South China Agricultural University (SCAU). Row to row and plant to plant distances were kept as 20 cm and 17 cm, respectively. All the field practices were done according to the recommendation of area.
Table 1.
Parent's genotypes at S-a, S-b, and S-c loci
| Name | S-a(a) | S-b(a) | S-c(a) | Subspecies |
| Guanglu'ai 4-4x | S n-aS n-a S n-aS n-a(b) | S i-bS i-b S i-bS i-b | S i-cS i-c S i-cS i-c | indica |
| L202-4x | S j-aS j-a S j-aS j-a | S j-bS j-b S j-bS j-b | S i-cS i-c S i-cS i-c | javanica |
| Jackson-4x | S j-aS j-a S j-aS j-a | S j-bS j-b S j-bS j-b | S i-cS i-c S i-cS i-c | javanica |
| E24-4x | S i-aS i-a S i-aS i-a | S i-bS i-b S i-bS i-b | S j-cS j-c S j-cS j-c | japonica |
Sn indicates neutral allele; Si indicates indica allele; Sj indicates japonica allele.
Cited from the results of Long (2007).
Table 2.
Hybrid's genotypes at S-a, S-b, and S-c loci and their pollen fertility and seed set
| Hybrids | Type of cross | S-a(a) | S-b(a) | S-c(a) | No. of locus(b) | Pollen fertility (%)±SD | Seed set (%)±SD |
| Guanglu'ai 4-4x×L202-4x | indica×javanica | S n-aS n-a S j-aS j-a | S i-bS i-bS j-bS j-b | S i-cS i-c S i-cS i-c | 1 | 96.0±0.3 | 73.1±6.4 |
| Guanglu'ai 4-4x×Jackson-4x | indica×javanica | S n-aS n-a S j-aS j-a | S i-bS i-bS j-bS j-b | S i-cS i-c S i-cS i-c | 1 | 94.7±3.3 | 70.1±4.0 |
| Guanglu'ai 4-4x×E24-4x | indica×japonica | S n-aS n-a S i-aS i-a | S i-bS i-bS j-bS j-b | S i-cS i-cS j-cS j-c | 2 | 67.4±8.3 | 58.6±1.8 |
| L202-4x×E24-4x | javanica×japonica | S i-aS i-aS j-aS j-a | Si-bSi-bS j-bS j-b | S i-cS i-cS j-cS j-c | 3 | 34.6±5.6 | 11.8±0.1 |
| Jackson-4x×E24-4x | javanica×japonica | Si-aS i-aS j-aS j-a | S i-bS i-bS j-bS j-b | S i-cS i-cS j-cS j-c | 3 | 12.9±4.4 | 24.6±6.4 |
Sn indicates neutral allele; Si indicates indica allele; Sj indicates japonica allele.
Total number of heterozygous genotypes (Si/Sj) in a hybrid at the S-a, S-b, and S-c loci.
Detection of parents and hybrid genotypes at the S-a, S-b and S-c
Marker selection
Polymorphic pairs of primers were selected at the S-a, S-b, and S-c loci. The S-a gene has been cloned and the other two loci have been fine mapped, so closely linked markers to these genes were used. Two SNP markers (G02-76.3 and G02-78.5) were selected at the S-a locus (Long et al., 2008), two markers (A07-55 and A07-130) at the S-b locus (Shi et al., 2009; Wang, 2010), and four markers (P24-85.7, P24-100.7, P24-90.1, and P24-90.3) at the S-c locus (Wang, 2010).
PCR amplification
Young leaves were collected from autotetraploid rice and genomic DNA was extracted according to the SDS simple extraction method of Shi et al. (2009) with some minor modifications. The reaction reagent of 20 μl included 0.15 μmol l−1 SSR primer, 0.2 mmol l−1 dNTP, 1× PCR buffer (50 mmol l−1 KCl, 10 mmol l−1 TRIS-HCl pH 8.3, 1.5 mmol l−1 MgCl2, and 0.01% glutin), 30–50 ng template DNA, and 1 U Taq polymerase. PCR amplification was performed with the PTC-100 PCR machine with the following profile: 5 min at 94 °C to denature; followed by 32 cycles of 1 min at 94 °C, 1 min at 55 °C, and 1 min at 72 °C; and a final extension period of 72 °C for 5 min to complete the PCR reaction. PCR products were separated with 6% polyacrylamide gels and detected according to Li et al. (2002).
Ultra-structural observation on PMC before and during meiosis
Spikelets of 2–3 mm in length were collected and used to prepare the ultrathin sections according to the method of Liu et al. (1997). Ultrathin sections were observed under a TECNAI G2 12 Transmission Electronic Microscope (TEM).
Chromosome behaviour observation
Fixation
Inflorescences were collected from the shoots of rice plants with 1–4 cm between their flag leaf cushion and the second-to-last leaf cushion, and fixed in Carnoy solution (ethanol:acetic acid, 3:1 v/v) for at least 24 h. The samples were then stored in 70% ethanol at 4 °C.
Chromosome staining
Anthers were removed from the floret using forceps and a dissecting needle and placed in a small drop of 1 mg l−1 4', 6-diamidino-2-phenylindole (DAPI) on a glass slide. After 1 min, the glass slide was covered with a slide cover and was examined under a fluorescence microscope (Leica DMRXA). Meiosis stages were classified and explained according to He et al. (2011).
Microtubule observation
Microtubules were observed according to the method of Liu et al. (2004b) with some minor modifications and the details are as follows.
Fixation
Inflorescences were collected from rice shoots of plants with c. 1–4 cm between their flag leaf cushion and their second-to-last leaf cushion. Anthers were dissected out from the panicles, and were fixed in 4% paraformaldehyde in PEMS buffer [containing 10% DMSO, 400 μmol l−1 m-maleimidobenzoic acid N-hydroxysuccinimide ester (MBS), and 1% Triton X-100] for 1–2 h. After fixation, anthers were rinsed three times in PEMS solution (PEMS buffer+10% DMSO+1% Triton X-100), and were transferred to PEM solution (without DMSO, Triton, and MBS) and distilled water for washing (three times each). The anthers were then dehydrated in an alcohol series (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, and 100%).
Infiltration and embedding
After dehydration, the samples were infiltrated in a stepwise graded polyethylene glycol (PEG) mixture:alcohol solution (2:1, 1:1, 1:2 v/v) for 40 min each at 55 °C and then twice in pure PEG mixture for 1 h each at 55 °C. After that, samples were transferred in a tube for 1 h containing a pure PEG mixture. The PEG mixture contained PEG4000:PEG1500=2:3. Finally, the tubes were placed at room temperature until the materials hardened.
Sectioning and indirect immunofluorescence labelling of microtubules
After PEG embedding, sections were cut about 45–50 μm using a Leica CM 1850 freezing microtome. After rinsing 3–5 times in distilled water and three times in phosphate-buffered saline (PBS) solution, sections were incubated in 0.1 mol l−1 NH4Cl for 3 min, washed three times in PBS (5 min each), then in 0.1% Tween for 20 min and in 1% bovine serum albumin (BSA) for 10 min. After that, sections were incubated in mouse immunoglobulin G (IgG) monoclonal anti-tubulin (Sigma, T-9026) diluted 1:100 with PBS for 1 h at 37 °C. The samples were rinsed three times in PBS for 5–10 min each and then incubated in FITC-conjugated goat-anti-mouse IgG (Sigma, F-0257) diluted 1:100 with PBS for 1 h. The samples were rinsed three times in PBS for 5–10 min each and then stained with 1 μg ml−1 PI (propidium iodide in PBS) for 1 min. The samples were again rinsed three times in PBS for 5–10 min each and then mounted in an anti-fade solution. A Leica SP2 laser scanning confocal microscope was used to scan the samples.
Results
Genotypes of parents and hybrids at S-a, S-b and S-c loci and pollen fertility of hybrids
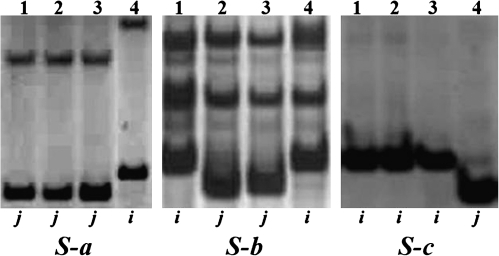
The genotypes of parents and the hybrids at the S-a, S-b, and S-c loci were detected using closely linked molecular makers (Fig. 1) and are presented in Table 1. Parents have different alleles at different loci, so hybrids showed different pollen sterility gene interactions. Pollen fertility, seed set percentage, and alleles at the S-a, S-b, and S-c loci of five hybrids are shown in Table 2. Guanglu'ai 4-4x×L202-4x and Guanglu'ai 4-4x×Jackson-4x have Si and Sj alleles at the S-b locus, Si Si alleles at the S-c locus, and Sn (neutral allele) and Sj alleles at the S-a locus, and their pollen fertility and seed set was high. Guanglu'ai 4-4x×E24-4x has Si and Sj alleles at the S-b and S-c loci, Sn and Sj at the S-a locus and its pollen fertility and seed set was low. L202-4x×E24-4X and Jackson-4x×E24-4x have Si and Sj alleles at these three loci and had very low pollen fertility and seed set. These results indicated that pollen sterility loci interaction affected the pollen fertility of autotetraploid hybrids and pollen fertility further decreased with an increase in pollen sterility gene interactions.
Fig. 1.
Parental genotypes at the S-a, S-b, and S-c loci detected with molecular markers G02-78.5, A07-55, and P24-85.7, respectively. 1–4 indicates parents (Guanglu'ai 4, L-202, Jackson, and E-24), ‘i’ and ‘j’ represents the indica and japonica genotypes, respectively; Guanglu'ai 4 has a neutral allele (Sn) at the S-a locus (Long, 2007).
Ultra-structural changes before and during PMC meiosis in autotetraploid rice hybrids
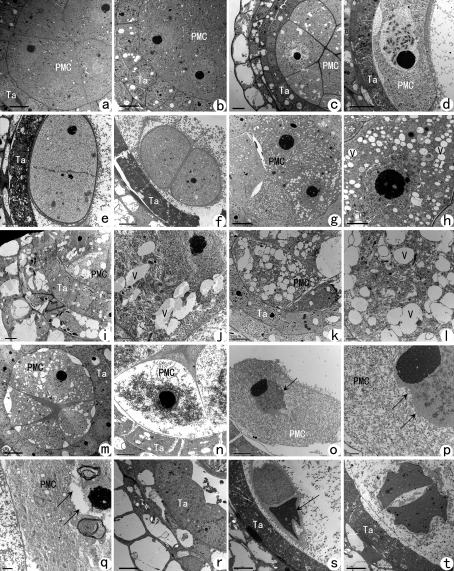
In order to understand the stage-time for pollen sterility gene interaction and its effect on pollen development, ultra-structural changes in PMC before and during meiosis were observed in the hybrids of autotetraploid rice. TEM (Transmission Electronic Microscopy) observation indicated that low seed-setting hybrids (L202-4x×E24-4x and Jackson-4x×E24-4x) displayed irregular ultra-structure, especially abnormalities in the cytoplasm and nucleus at the interphase-stage of PMC. High seed set hybrids produced normal ultra-structure similar to the control, i.e. small vacuolation was less and the structure of the organelles appeared normal in PMCs (Fig. 2a–f). Cytoplasmic abnormality was mainly characterized by excessive vacuolation in some PMCs. Vacuolation in these PMCs was relatively hard and plenty of vacuoles were observed in cells (Fig. 2g–m). The cytoplasm appeared thin, the organelles and the nuclear envelope (karyotheca) degraded gradually, and there was the formation of a compound membrane structure and nuclear vacuoles, the diffusion of karyoplasms, a gradually shrinking of the nucleolus, and, consequently, the degradation of PMCs (Fig. 2n–q). Organelles were pressed and distributed unevenly in PMCs, resulting in the asymmetric division of cytoplasm during meiosis which resulted in abnormal dyads (Fig. 2s) or tetrads (Fig. 2t). An abnormal tapetum was also observed in these PMCs (Fig. 2r). Ultra-structure abnormalities mostly occurred before meiosis and during meiosis prophase I in PMCs of the low-seed-set hybrids but less in high-seed-set hybrids, so it was presumed that these abnormalities were stimulated by pollen sterile gene interactions and was probably not directly related to chromosome behaviour in autotetraploid rice.
Fig. 2.
Ultra-structural changes of a pollen mother cell (PMC) before and during meiosis in the hybrids of autotetraploid rice. (a–d, f) Normal meiosis in diploid rice (CK). (g–t) Abnormal meiosis in the hybrids of autotetraploid rice with a low setting rate; Ta, tapetum; V, vacuole. Bars are 1 μm in (j), (l), (p), and (q), 2 μm in (h),and all other bars are 5 μm. (a) Normal PMC at early interphase; (b) normal PMC at interphase; (c) normal PMC at leptotene; (d) normal PMC at pachytene; (e) normal dyad of Guanglu'ai 4-4x; (f) normal tetrad showing two cells. The other two cells are another section and are not shown. (g) Abnormal PMC at early interphase; vacuoles formed in the PMC; (h) magnification of (g) showing vacuoles in one PMC; (i) abnormal PMC at interphase showing many vacuoles in the PMC; (j) magnification of (i) showing the vacuoles in one PMC; (k) abnormal PMC at leptotene and plenty of vacuoles and abnormal organelles are present in the abnormal PMC; (l) magnification of (k) showing the vacuoles and abnormal organelles in the PMC; (m) leptotene showing the abnormal PMC; (n) zygotene with degraded PMC; (o) pachytene showing the abnormal nucleus of the PMC (arrow); (p) magnification of (o) and the nuclear membrane of PMC has degenerated (arrows); (q) pachytene where the nucleus and cytoplasm are both abnormal at the same time. Arrows indicate the abnormal nucleus of the PMC. (r) Abnormal proliferating tapetum. (s) Abnormal dyad, one has degenerated (arrow). (t) Abnormal tetrad.
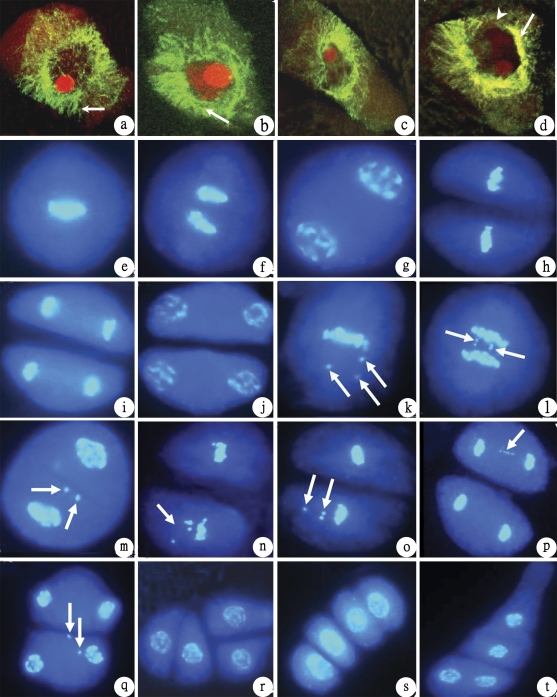
Microtubules were also abnormally distributed at leptotene during meiosis (Fig. 3b, d), at this stage, homologous chromosomes did not pair and, therefore, the abnormality was caused by gene interactions.
Fig. 3.
Changes in the organization pattern of microtubules and chromosome behaviour during PMCs meiosis. (b–d) Microtubules of L202-4x×E24-4x; (e–j) chromosome behaviour of PMC meiosis in diploid rice (normal, control); (k–t) abnormal chromosome behaviour of PMC meiosis in the hybrid of L202-4x×E24-4x (×3000). (a) Leptotene, normal radiating microtubule bundles around the nuclear envelope (arrow) (Guanglu'ai 4-4x×L202-4x, control); (b) leptotene, abnormal radiating microtubules showing the short microtubule bundles (arrow); (c) leptotene, abnormal cell shape and microtubules; (d) leptotene, abnormal cell shape, nuclear envelope breaks down (arrowhead), and abnormal microtubules form an obvious envelope around the nucleus (arrow); (e) metaphase I; (f) anaphase I; (g) telophase I; (h) metaphase II; (i) anaphase II; (j) telophase I; (k) two straggling chromosomes (arrow); (l) lagging chromosome (arrow) at anaphase II; (m) two micronuclei at telophase I (arrows); (n, o) straggling chromosomes at metaphase II; (p) chromosome lagging at anaphase II (arrow); (q) two micronuclei (arrow) at telophase II; (r) abnormal tetrad; (s) tetrad with linear arrangement; (t) abnormal tetrad.
Chromosome behaviours and configuration during PMC meiosis of intersubspecific autotetraploid rice hybrids
Chromosome behaviour and configuration in PMC meiosis
Intersubspecific autotetraploid rice hybrids with genetic interaction between different pollen sterility loci were used to observe PMC meiosis and chromosome behaviour, in order to prove if there is any difference in the chromosome behaviour of PMC meiosis and their relationship with pollen sterility loci. The results showed significant differences in chromosome configuration at diakinesis and metaphase I of the five hybrids (Table 3), though their meiosis processes were similar to that of diploid rice (Figs 3e–j, 4a).
Table 3.
Meiotic chromosome configurations at diakinesis and metaphase I in intersubspecific autotetraploid rice hybrids
| Combinations | Stages | Chromosome configurationa |
| Guanglu'ai 4-4x×L202-4x | Diakinesis | (0.72±1.37)I+(13.00±3.59)II+(0.13±0.37)III+(5.23±1.85)IV |
| Metaphase I | (1.06±1.42)I+(14.13±3.77)II+(0.20±0.41)III+(4.52±1.85)IV | |
| Guanglu'ai 4-4x×Jackson-4x | Diakinesis | (0.23±0.64)I+(12.83±4.03)II+(0.08±0.27)III+(5.47±2.00)IV |
| Metaphase I | (0.57±0.88)I+(14.40±3.87)II+(0.05±0.22)III+(4.62±1.87)IV | |
| Guanglu'ai 4-4x×E24-4x | Diakinesis | (0.42±0.87)I+(10.70±3.81)II+(0.16±0.42)III+(5.30±1.93)IV |
| Metaphase I | (0.92±1.31)I+(12.45±3.40)II+(0.24±0.47)III+(5.36±1.79)IV | |
| L202-4x×E24-4x | Diakinesis | (0.59±1.06)I+(10.55±3.98)II+(0.10±0.31)III+(6.50±2.02)IV |
| Metaphase I | (0.76±1.45)I+(10.89±4.22)II+(0.13±0.34)III+(6.27±2.09)IV | |
| Jackson-4x×E24-4x | Diakinesis | (0.42±0.92)I+(10.26±3.31)II+(0.16±0.45)III+(6.65±1.66)IV |
| Metaphase I | (0.96±1.09)I+(11.83±3.51)II+(0.46±0.62)III+(5.50±1.69)IV |
I, II, III, and IV represent univalent, bivalent, trivalent, and quadrivalent, respectively.
Fig. 4.
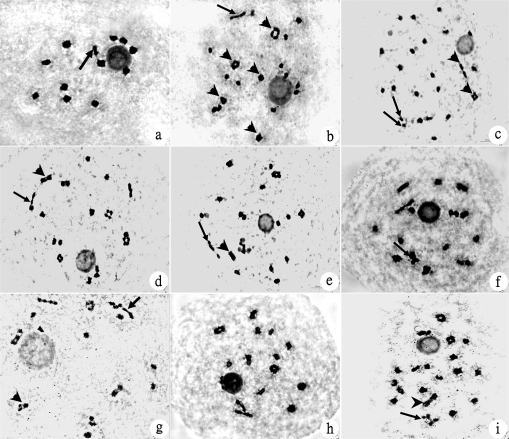
Chromosome configuration at diakinesis in the hybrids of autotetraploid rice (×3000). (a) Chromosome configuration at diakinesis in diploid rice (control), 12II (arrow indicating rod-shaped chromosome); (b) 6IV [5ring shape (arrowhead)+1chain shape (arrow)]+12II (12ring shape); (c) 2IV (arrow head)+19II (17ring shape+2rod shape)+2I (arrow); (d) 7IV [3ring shape+1chain shape (arrow)+1 frying pan shape (arrowhead)+2 double rings shape]+10II (8ring shape+2rod shape);(e) 5IV [4ring shape+1 double rings shape (arrowhead)]+1III (chain shape, arrow)+12II (11ring shape+1rod shape)+1I; (f) 9IV [1 ring shape+2 chain shape+1 OK shape (arrow)+5 double rings shape]+6II (ring shape); (g) 5IV [1ring shape+1chain shape+1double rings shape+2 OK shape (arrowhead)]+1III (chain shape, arrow)+12II (10ring shape+2rod shape)+1I; h. 9IV (5ring shape+1 -0- shape+2 f.pan shape+1chain shape)+6II (5ring shape+1rod shape); (i) 4IV [2 ring shape+1 >-< shape (arrowhead)+1 x shape (arrow)]+16II (14 ring shape+2 rod shape).
Abnormal chromosome configuration, such as quadrivalents, trivalents, and univalents was found in the five hybrids (Fig. 4b–i). There were highly significant differences among the five hybrids (F=8.67**) for quadrivalents and the average number of quadrivalents per cell ranged from 5.23 to 6.65 at diakinesis (Table 3). Hybrids with a low seed-setting rate had a greater number of quadrivalents per cell, whereas hybrids with a high seed-setting rate had a smaller number of quadrivalents per cell. The hybrid, Jackson-4x×E24-4x, had the highest number of quadrivalents per cell (6.65), Guanglu'ai 4-4x×L202-4x had the lowest (5.23). More than six quadrivalents per cell were found in two hybrids with a low seed-setting rate which had genetic interaction of three pollen sterility loci. The main types of quadrivalents were: ring, chain, frying pan, Y shape, double ring shape, OK shape, x shape, -0- shape, and >-< shape during diakinesis (Table 4). Ring and chain shapes were the most frequent configurations of quadrivalents (Table 4). There was a significant difference in the number of ring quadrivalents and a highly significant difference in the number of chain quadrivalents (F=6.06*, F=3.23*) among all the hybrids. Hybrids with the parent E24-4x had genetic interaction of two or three pollen sterility loci, and had more than five quadrivalents per cell.
Table 4.
Frequencies of different chromosome configurations and shapes at diakinesis and metaphase I in intersubspecific autotetraploid rice hybrids
| Materials | Stage | nc | Quadrivalents per cell | Trivalents per cell | Bivalents per cell | Univalents | Chiasma | ||||||||||
| Ring | Chain | F.pan | Y | Double rings | Others | Total | Chain | F.pan | Total | Rod | Ring | Total | per cell | per cell | |||
| Guanglu'ai 4-4x×L202-4x | Diaka | 88 | 4.28 | 0.59 | 0.14 | 0.05 | 0.10 | 0.07 | 5.23 | 0.06 | 0.07 | 0.13 | 3.74 | 9.26 | 13.00 | 0.72 | 42.51 |
| M-Ib | 54 | 3.81 | 0.37 | 0.13 | 0.15 | 0.00 | 0.06 | 4.52 | 0.17 | 0.04 | 0.20 | 4.61 | 9.52 | 14.13 | 1.06 | 41.13 | |
| Guanglu'ai 4-4x×Jackson-4x | Diak | 64 | 4.53 | 0.63 | 0.11 | 0.02 | 0.16 | 0.03 | 5.47 | 0.06 | 0.02 | 0.08 | 3.27 | 9.57 | 12.83 | 0.23 | 43.30 |
| M- I | 58 | 4.24 | 0.16 | 0.07 | 0.00 | 0.12 | 0.04 | 4.62 | 0.05 | 0.00 | 0.05 | 3.12 | 11.27 | 14.40 | 0.57 | 44.03 | |
| Guanglu'ai 4-4x×E24-4x | Diak | 116 | 5.30 | 0.65 | 0.27 | 0.06 | 0.09 | 0.06 | 5.30 | 0.09 | 0.07 | 0.16 | 1.81 | 8.89 | 10.70 | 0.42 | 44.91 |
| M- I | 127 | 5.05 | 0.19 | 0.12 | 0.01 | 0.00 | 0.00 | 5.36 | 0.12 | 0.13 | 0.24 | 3.35 | 9.10 | 12.45 | 0.92 | 43.42 | |
| L202-4x×E24-4x | Diak | 116 | 5.20 | 0.72 | 0.18 | 0.05 | 0.29 | 0.06 | 6.50 | 0.07 | 0.03 | 0.10 | 3.45 | 7.10 | 10.55 | 0.59 | 43.18 |
| M-I | 45 | 5.18 | 0.58 | 0.16 | 0.02 | 0.27 | 0.07 | 6.27 | 0.09 | 0.04 | 0.13 | 3.31 | 7.57 | 10.89 | 0.76 | 43.16 | |
| Jackson-4x×E24-4x | Diak | 31 | 5.68 | 0.45 | 0.19 | 0.10 | 0.23 | 0.00 | 6.65 | 0.06 | 0.10 | 0.16 | 2.45 | 7.81 | 10.26 | 0.42 | 44.48 |
| M-I | 48 | 4.90 | 0.19 | 0.10 | 0.00 | 0.31 | 0.00 | 5.50 | 0.38 | 0.38 | 0.46 | 3.46 | 8.38 | 11.83 | 0.96 | 43.33 | |
| Average | Diak | 5.00 | 0.61 | 0.18 | 0.06 | 0.17 | 0.04 | 5.83 | 0.07 | 0.06 | 0.13 | 2.94 | 8.53 | 11.47 | 0.48 | 43.68 | |
| M-I | 4.64 | 0.30 | 0.12 | 0.04 | 0.14 | 0.03 | 5.25 | 0.16 | 0.12 | 0.22 | 3.57 | 9.17 | 12.74 | 0.85 | 43.01 | ||
Diakinesis.
Metaphase I.
Number of PMCs observed.
Different configurations of quadrivalents with different numbers per cell were also found at metaphase I (Tables 3, 4). The number of quadrivalents clearly decreased in the hybrids with high pollen fertility (Guanglu'ai 4-4x×L202-4x and Guanglu'ai 4-4x×Jackson-4x). However, the hybrids with low pollen fertility, L202-4x×E24-4x and Jackson-4x×E24-4x, still had a high frequency of quadrivalents (Table 4), suggesting that more abnormalities would happen at the later stages with more abnormal pollen produced at maturity.
Guanglu'ai No.4-4x×E24-4x and Jackson-4x×E24-4x had the highest frequency (0.16 per cell) of trivalents, whereas Guanglu'ai No.4-4x×Jackson-4x had the lowest (0.08 per cell) at diakinesis (Table 4). There was insignificant difference for trivalents among the five hybrids.
Bivalents frequency was ranged from 10.26 to 13.00 per cell during diakinesis (Table 4). Hybrids with a low set have a lower frequency of bivalents than the higher seed set hybrids, but the bivalent types showed an insignificant effect on seed set. A higher number of ring bivalents was found than rod bivalents in all hybrids. Guanglu'ai No 4-4x×L202-4x had the highest number of bivalents, while Jackson-4x×E24-4x had the lowest number of bivalents. There was an insignificant difference in the number of univalents per cell at diakinesis among the five hybrids (Table 4).
Abnormal chromosome behaviour in PMC meiosis
The various kinds of chromosomal abnormalities were found in PMC meiosis of low seed-set hybrids (Fig. 3k–t). Hybrids with genetic interaction of three pollen sterility loci showed more irregularities than hybrids with genetic interaction at just one pollen sterility locus. The main types of abnormalities include: chromosome straggling at metaphase I (Fig. 3k), chromosome lagging (Fig. 3l), and a chromosome bridge at anaphase I; micro-nuclei at telophase I (Fig. 3m); chromosome straggling at metaphase II (Fig. 3n, o); abnormal spindle, asynchrony of chromosome disjunction, no separation or incomplete separation of two daughter cells, abnormal cell shape at metaphase II; chromosome lagging at anaphase II (Fig. 3p); two micronuclei at telophase II (Fig. 3q), asynchrony of chromosome separation, tri-fission, incomplete separation or no separation of cytokinesis, abnormal cell shape at telophase II; abnormal tetrad (Fig. 3r–t).
The average frequency of abnormalities at metaphase, anaphase, and telophase I were 24.03%, 27.11%, and 8.33%, respectively (Table 5). Among the five hybrids, the average frequency of abnormalities was higher at metaphase I (11.54%), anaphase I (6.91%), and telophase I (7.57%) in the two hybrids which had low seed-setting rates and genetic interaction of three pollen sterility loci. Hybrid L202-4x×E24-4x had the highest frequency of abnormalities (41.09% at metaphase I, 31.33% at anaphase I, and 18.29% at telophase I). The average frequency of abnormalities in the five hybrids at metaphase II, anaphase II, and telophase II was 30.03%, 24.76%, and 15.63%, respectively. L202-4x×E24-4x had the highest frequency of abnormalities at metaphase II, anaphase II, and telophase II (Table 5).
Table 5.
Frequency of abnormal chromosome behaviors during meiosis of intersubspecific autotetraploid rice hybrids
| Materials | Meiosis I |
Meiosis II |
||||||||||
| Metaphase I |
Anaphase I |
Telophase I |
Metaphase II |
Anaphase II |
Telophase II |
|||||||
| na | Abnormal cells (%) | na | Abnormal cells (%) | na | Abnormal cells (%) | na | Abnormal cells (%) | na | Abnormal cells (%) | na | Abnormal cells (%) | |
| Guanlu’ai 4-4x×L202-4x b | 400 | 16.79 | 343 | 17.82 | 763 | 6.65 | 172 | 24.87 | 86 | 24.89 | 122 | 18.85 |
| Guanglu’ai 4-4x×Jackson-4x | 235 | 17.45 | 91 | 19.78 | 66 | 15.15 | 106 | 17.92 | 158 | 14.56 | 236 | 8.05 |
| Guanlu’ai 4-4x×E24-4x | 348 | 18.68 | 67 | 13.43 | 23 | 4.35 | 236 | 21.19 | 100 | 16.00 | 17 | 5.88 |
| Average | 17.64 | 17.01 | 8.72 | 21.33 | 18.48 | 10.93 | ||||||
| L202-4x×E24-4x b | 183 | 41.09 | 83 | 31.33 | 315 | 18.29 | 256 | 47.38 | 68 | 27.94 | 86 | 33.72 |
| Jackson-4x×E24-4x | 898 | 17.27 | 161 | 17.83 | 101 | 12.73 | 270 | 31.93 | 82 | 20.57 | 87 | 25.29 |
| Average | 29.18 | 24.58 | 15.51 | 39.66 | 24.26 | 29.51 | ||||||
| Total average | 24.03 | 27.11 | 8.33 | 30.03 | 24.76 | 15.63 | ||||||
Number of PMCs observed.
Some data cited from the result of He et al. (2011).
Relationship among chromosome configuration, abnormal chromosome behaviour, seed-setting rate, and pollen fertility
Correlation analysis showed that there was a highly significant and positive correlation between pollen fertility and seed setting rate (r=0.933**) (Table 6). This result showed that pollen fertility is one of the most important factors affecting the seed-setting rate of intersubspecific autotetraploid rice hybrids. Correlation analysis among chromosome configuration, pollen fertility, and seed-setting rate can be seen in Table 6. The total number of quadrivalents showed highly significant negative correlations with pollen fertility and seed setting rate (r= –0.232**, r= –0.197**). Among all kinds of quadrivalent configurations, pollen fertility and seed-setting rate depicted negative correlation with single ring quadrivalents and double ring quadrivalents. Trivalents showed a significant and negative relationship with pollen fertility and seed set during metaphase I. Pollen fertility and seed set were highly significantly and positively correlated with bivalents during diakinesis and metaphase I. Negative and non-significant relationship of seed set and pollen fertility with univalents was also observed.
Table 6.
Correlation analysis among chromosome configurations and chiasma at diakinesis and metaphase I, seed setting rate and pollen fertility of intersubspecific autotetraploid rice hybrids
| Diakinesisa |
Metaphase Ia |
||||||
| Chromosome configuration | Chiasma | Pollen fertility | Seed setting rate | Chiasma | Pollen fertility | Seed setting rate | |
| Quadrivalents | Ring | 0.421** | -0.192** | -0.146** | 0.462** | -0.163** | -0.141* |
| Chain | -0.240** | -0.001 | -0.023 | -0.165** | -0.052 | -0.135* | |
| F.pan | 0.153** | -0.043 | -0.014 | 0.095 | -0.021 | -0.030 | |
| Double rings | 0.186** | -0.132** | -0.160** | 0.196** | -0.278** | -0.313** | |
| Total | 0.350** | -0.232** | -0.197** | 0.434** | -0.216** | -0.230** | |
| Trivalents | Chain | -0.203** | 0.004 | 0.013 | -0.292** | -0.213** | -0.156** |
| F.pan | -0.019 | -0.019 | 0.008 | -0.008 | -0.016 | 0.019 | |
| Total | -0.162** | -0.012 | 0.012 | -0.247** | -0.187** | -0.118* | |
| Bivalents | Ring | 0.174** | 0.232** | 0.261** | -0.788** | 0.239** | 0.253** |
| Rod | -0.784** | 0.056 | -0.048 | 0.191** | 0.038 | 0.045 | |
| Total | -0.278** | 0.235** | 0.203** | -0.299** | 0.238** | 0.248** | |
| Chiasma | — | -0.052 | 0.041 | — | -0.050 | -0.036 | |
| Univalents | -0.483** | -0.020 | -0.040 | -0.563** | -0.034 | -0.006 | |
* Significance at 0.05 probability level; ** significance at 0.01 probability level.
Discussion
Allelic interaction is one of the most important reasons for low pollen fertility
The cytological mechanism of pollen abortion is very complicated in intersubspecific autotetraploid rice hybrids and there are many factors associated with it (Xiao et al., 2005; Zhao et al., 2006; Hu et al., 2009; Luan et al., 2009). Although, it was speculated that irregular meiosis affected the fertility of hybrids along with some genes (Yan and Bao, 1960; Chen et al., 1987), however, there was no direct evidence to prove this speculation. Our previous study indicated that allelic interaction of the F1 sterility loci is the major reason for intersubspecific hybrid sterility in diploid rice and these sterility alleles could also cause intersubspecific hybrid sterility in autotetraploid rice (Zhang et al., 2006; Zhao et al., 2006). In this study, five autotetraploid rice lines with different pollen sterility loci were used and hybrids made with different pollen allelic interaction. The results demonstrated that interaction of the pollen sterile loci may result in partial or complete sterility of the intersubspecific autotetraploid rice hybrids and indicated that, as the number of Si and Sj alleles increased at different loci in the autotetraploid hybrids, their pollen fertility decreased significantly; hybrids with three pairs of Si and Sj alleles had very low pollen fertility and seed set. These results are consistent with the findings of Zhang et al. (2006) in that they also reported the partial abortion of male gametes caused by the interaction between Si and Sj alleles in diploid rice. Moreover, our results also proved that the neutral allele (Sn) did not interact with Si and Sj alleles and autotetraploid hybrids displayed normal pollen fertility with the neutral alleles. Therefore, our results suggest that allelic interaction between Si and Sj alleles caused pollen abortion which, in turn, reduced the seed set of autotetraploid hybrids and, on the other hand, also suggest that neutral alleles may have the potential to overcome hybrid sterility. So far, some new lines or hybrids with normal pollen fertility and seed set have been developed in our laboratory by using materials with the neutral alleles of pollen fertility.
Relationship between pollen sterility interaction of alleles, and abnormal chromosome configuration and their effect on the fertility of intersubspecific autotetraploid hybrids during meiosis
Sterile aneuploid gamete frequency depends on the separation of homologous chromosomes with different associations in autotetraploidy, because quadrivalents are the main chromosome association and its way of separation is the most important factor. The way of quadrivalent separation depends on the chromosome configuration. There were 11 types of quadrivalent configurations found in autotetraploid plants in diakinesis and metaphase I (Kuspira and Bhambhani, 1984), while in autotetraploid rice, three main kinds of quadrivalent configurations could be found, i.e. ring, chain, and frying pan shapes (Luan et al., 2007). A variety of quadrivalents was also found, such as double rings, -0- shape, Y shape, OK shape, x-shape, and >-<shape, along with the three main types, in autotetraploid rice. All these configurations appeared in different ratios. A higher frequency of bivalents and quadrivalents and a smaller number of trivalents and univalent was found. Quadrivalents were the most frequent in all autotetraploid plants (70–100% depending on the plant) and ring-shaped quadrivalents were more common than other shapes (Espinasse et al., 1995). Similarly, ring-shaped quadrivalents and bivalents were found more frequently in the present study compared with other quadrivalent types at diakinesis and Metaphase I of PMCs. Many bivalents and a few trivalents and quadrivalents were observed at diakinesis or Metaphase I of PMCs in tetraploid C. persicum and C. graecum, and ring bivalents were most frequent at Metaphase I of PMCs (Ishizaka, 2003). By contrast, Luan et al. (2009) reported a greater number of rod bivalents than ring bivalents in autotetraploid restorer lines.
There are few reports related to the relationship between chromosome configuration and fertility in autotetraploid plants. Multivalent formation in polyploids is usually negatively correlated with fertility and the tendency of many tetraploids towards low quadrivalent frequencies and high bivalent frequencies has significant importance in biological implications and practical applications (Jones, 1994). A lower frequency of quadrivalents resulted in a higher pollen fertility and seed set in our autotetraploid hybrids. These results are in agreement with previous reports that low quadrivalents resulted in high fertility of autotetraploid rye (Jenkins and Chatterjee, 1994) and tetraploid potato (Watanabe et al., 1994), however, there have been contradictory statements that higher fertility was achieved due to a higher number of quadrivalents in tetraploid Lolium (Crowley and Rees, 1968) and autotetraploid rice (Luan et al., 2009).
Our results showed that there was a significant difference among autotetraploid hybrids, especially, among the hybrids with one pollen sterile gene interaction, which had high pollen fertility, and those with three loci interaction, which had low pollen fertility. As for the reason for the difference in the number of quadrivalents, it is thought that it might be related to pollen sterility loci interaction because more quadrivalent configurations (such as ring and chain shapes) were observed in the hybrids with low pollen fertility than in those with high pollen fertility. It requires further studies to reveal that how pollen sterility loci interaction affect the chromosome configuration.
Relationship between pollen sterility alleles interaction and abnormal chromosome behaviour and their effect on pollen fertility during meiosis in autotetraploid rice
Chromosome differentiation may play an important role in the preferential pairing behaviour of polyploids when homologous partners are contributed by less related parental lines. Competition between preferential chromosome association and potential partner may affect synapsis and chromosome recombination and thus affect the meiotic process and the pollen fertility of colchicine-induced autotetraploid hybrids (Benavente and Orellana, 1991). Reproductive stability and fertility in induced autotetraploids of kale depends on the precise control of chromosome paring at meiosis (Jenczewski et al., 2002). High pollen fertility and normal seed setting are features of normal meiotic chromosome division (Srivastava et al., 1992; Majumdar et al., 2004; Adamowski et al., 2008; He et al., 2011). Pollen viability and plant fertility are directly related to the meiotic abnormalities stimulated by defective chromosome pairing, unequal chromosome distribution or non-disjunction (Luo et al., 1992; Singh, 1992). Similar results were also reported in the present study that chromosome behaviour has a highly significant relationship with pollen fertility and seed set in autotetraploid rice.
In our study, many abnormalchromosome behaviours were observed in the hybrids with low pollen fertility. As the allelic interaction of F1 pollen sterility loci increased, chromosomal abnormalities also increased, which resulted in the low pollen fertility of autotetraploid rice. Some differences were found in pollen fertility, seed-setting rate, and abnormal chromosome behaviour among hybrids derived from the same paternal or maternal parent. The mean frequency of abnormal chromosome behaviour was highest among hybrids whose male parent was E24-4x and the female parent was L202-4x, while a high frequency of abnormal chromosome behaviour was found at metaphase and telophase II in the hybrid whose male parent was Jackson-4x. Therefore, it is supposed that the abnormal chromosome behaviour might be related to the genotype of the parents, i.e. related to the allelic interaction of F1 pollen sterility loci. In other words, pollen sterility loci interaction (S-a, S-b, and S-c) lead to increased chromosomal abnormalities and result in the lower pollen fertility of autotetraploid rice hybrids.
Acknowledgments
The authors thank JinQuan Li, XingJuan Zhao, XueLin Fu, and ShuHong Yu for assistance in conducting these experiments. This work was supported by the National Science Foundation of China (30771328; 30971756).
References
- Adamowski EV, Pagliarini MS, Valle CB. Meiotic behaviour in three interspecific three-way hybrids between Brachiaria ruzizensis and B. brizantha (Poaceae: Paniceae) Journal of Genetics. 2008;87:33–38. doi: 10.1007/s12041-008-0005-7. [DOI] [PubMed] [Google Scholar]
- Alley CJ. Cytogenetics of Vitis. II. Chromosome behaviour and the fertility of some autotetraploid derivatives of Vitis vinifera L. The Journal of Heredity. 1957;48:194–202. [Google Scholar]
- Benavente E, Orellana J. Chromosome differentiation and pairing behaviour of polyploids: an assessment on preferential metaphase I associations in colchicine-induced autotetraploid hybrids within the genus Secale. Genetics. 1991;128:433–442. doi: 10.1093/genetics/128.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham ET, Groose RW, Woodfield DR, Kidwell KK. Complementary gene interaction in alfalfa is greater in autotetraploid than in diploids. Crop Science. 1994;34:823–829. [Google Scholar]
- Borg M, Brownfield L, Twell D. Male gametophyte development: a molecular perspective. Journal of Experimental Botany. 2009;60:1465–1478. doi: 10.1093/jxb/ern355. [DOI] [PubMed] [Google Scholar]
- Bremer G, Bremer-Reinders DE. Breeding of tetraploid rye in the Netherlands. I. Methods and cytological investigations. Euphytica. 1954;3:49–63. [Google Scholar]
- Chang TT. The origin, evolution, cultivation, dissemination and diversification of Asian and African rices. Euphytica. 1976;25:425–441. [Google Scholar]
- Chen ZY, Wu DY, Song WC, Zhang YH, Qin RZ, Bao WK. Research advances in autotetraploid rice breeding. Scientia Agricultura Sinica. 1987;20:20–24. [Google Scholar]
- Crowley JG, Rees H. Fertility and selection in tetraploid. Lolium. Chromosoma. 1968;24:300–308. [Google Scholar]
- Ding XH, Zhang ZM, Zeng RZ, Li WT, Zhang GQ. Genotypic identification of S-b locus in the indica-compatible japonica lines of rice (Oryza sativa) Chinese Journal of Rice Science. 2003;17:297–301. [Google Scholar]
- Espinasse A, Foueillassar J, Kimber G. Cytogenetical analysis of hybrids between sunflower and four wild relatives. Euphytica. 1995;82:65–72. [Google Scholar]
- He JH, Shahid MQ, Chen ZX, Cheng XA, Liu XD, Lu YG. Abnormal PMC microtubule distribution pattern and chromosome behaviour resulted in low pollen fertility of an intersubspecific autotetraploid rice hybrid. Plant Systematics and Evolution. 2011;291:257–265. [Google Scholar]
- Hu CY, Zeng YX, Lu YG, Li JQ, Liu XD. High embryo sac fertility and diversity of abnormal embryo sacs detected in autotetraploid indica/japonica hybrids in rice by whole-mount eosin B-staining confocal laser scanning microscopy. Plant Breeding. 2009;128:187–192. [Google Scholar]
- Ishizaka H. Cytogenetic studies in Cyclamen persicum, C. graecum (Primulaceae) and their hybrids. Plant Systematics and Evolution. 2003;239:1–14. [Google Scholar]
- Jenczewski E, Eber F, Manzanares-Dauleux MJ, Chevre AM. A strict diploid-like pairing regime is associated with tetrasomic segregation in induced autotetraploids of kale. Plant Breeding. 2002;121:177–179. [Google Scholar]
- Jenkins G, Chatterjee R. Chromosome structure and pairing preferences in autotetraploid rye (Secale cereale) Genome. 1994;37:748–793. doi: 10.1139/g94-112. [DOI] [PubMed] [Google Scholar]
- Jones GH. Meiosis in autopolyploid Crepis capilaris. III. Comparison of triploids and tetraploids; evidence for non independence of autonomous pairing sites. Heredity. 1994;73:215–219. [Google Scholar]
- Kato S, Kosaka H, Hara S. On the affinity of rice varieties as shown by the fertility of hybrid plants. Science Bulletin of the Faculty of Agriculture Kyushu University. 1928;3:132–147. [Google Scholar]
- Kuspira J, Bhambhani RN. Cytogenetic analysis of a genome of Triticum monococcum. I. Cytology, breeding behaviour, fertility and morphology of induced autotetraploids. Canadian Journal of Botany. 1984;27:51–63. [Google Scholar]
- Kunio K, Takeshi O. Genetic control of meiosis in rice Oryza sativa L. II. Cytogenetical analyses of desynaptic mutants. Japanese Journal of Genetics. 1983;58:567–577. [Google Scholar]
- Lavania UC, Srivastava S, Sybenga J. Cytogenetics of fertility improvement in artificial autotetraploids of Hyoscyamus niger L. Genome. 1991;34:190–194. [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. New York, NY, USA: Oxford University Press; 2002. [Google Scholar]
- Li WT, Zeng RZ, Zhang ZM, Ding XH, Zhang GQ. Fine mapping of locus S-b for F1 pollen sterility in rice (Oryza sativa L.) Chinese Science Bulletin. 2006;51:404–408. [Google Scholar]
- Li WT, Zeng RZ, Zhang ZM, Zhang GQ. Mapping of S-b locus for F1 pollen sterility in cultivated rice using PCR-based markers. Acta Botanica Sinica. 2002;44:463–467. [Google Scholar]
- Liu HY, Xu CG, Zhang QF. a. Male and female gamete abortions, and reduced affinity between the uniting gametes as the causes for sterility in an indica/ japonica hybrid in rice. Sexual Plant Reproduction. 2004;17:55–62. [Google Scholar]
- Liu XD, Lu YG, Zhu HL, Feng JH, Xu SX. b. Abnormal behaviour of nuclei and microtubule (MT) organizational changes during embryo sac development in the poly-egg mutant, APIV of rice. Acta Botanica Sinica. 2004;46:829–838. [Google Scholar]
- Liu XD, Xu SX, Lu YG. Formation and development of embryo sac wall in rice. Acta Botanica Sinica. 1997;39:895–990. [Google Scholar]
- Long YM. PhD thesis. China: South China Agricultural University; 2007. Cloning and molecular mechanism of interacting genes in the Sa locus for hybrid male sterility in rice (Oryza sativa L) [Google Scholar]
- Long YM, Zhao LF, Niu BX, et al. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proceedings of the National Academy of Sciences, USA. 2008;105:18871–18876. doi: 10.1073/pnas.0810108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan L, Tu SB, Long WB, Wang X, Liu YH, Kong FL, He T, Yan WG, Yu MQ. Cytogenetic studies on two F1 hybrids of autotetraploid rice varieties showing extremely high level of heterosis. Plant Systematics and Evolution. 2007;267:205–213. [Google Scholar]
- Luan L, Wang X, Long WB, Liu YH, Tu SB, Xiao XY, Kong FL. A comparative cytogenetic study of the rice (Oryza sativa L.) autotetraploid restorers and hybrids. Russian Journal of Genetics. 2009;45:1074–1081. [PubMed] [Google Scholar]
- Luan L, Wang X, Long WB, Liu YH, Tu SB, Zhao ZP, Kong FL, Yu MQ. Microsatellite analysis of genetic variation and population genetic differentiation in autotetraploid and diploid rice. Biochemical Genetics. 2008;46:248–266. doi: 10.1007/s10528-008-9156-8. [DOI] [PubMed] [Google Scholar]
- Luo YW, Yen XC, Zhang GY, Liang GH. Agronomic traits and chromosome behaviour of autotetraploid sorghums. Plant Breeding. 1992;109:46–53. [Google Scholar]
- Maekawa M, Inukai T, Shinbashi N. Geneic analysis of hybrid sterility caused by anther indehiscence between distantly related rice varieties. Euphytica. 1997;94:311–318. [Google Scholar]
- Majumdar S, Banerjee S, Kumar K. Meiotic behaviour of chromosomes in PMCs and karyotype of Trifolium repens L. from Darjeeling Himalaya. Acta Biologica Cracoviensia Series Botanica. 2004;46:217–220. [Google Scholar]
- Oka HI. Analysis of genes controlling F1 sterility in rice by the use of isogenic lines. Genetics. 1974;77:521–534. doi: 10.1093/genetics/77.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y. The genic nature of gamete eliminator in rice. Genetics. 1990;125:183–191. doi: 10.1093/genetics/125.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid MQ, Sun JF, Wei CM, Zhang P, Liu XD. Studies on the abnormality of embryo sac and pollen fertility in autotetraploid rice during different growing seasons. Pakistan Journal of Botany. 2010;42:7–19. [Google Scholar]
- Shahid MQ, Liu GF, Li JQ, Naeem M, Liu XD. Heterosis and gene action study of agronomic traits in diploid and autotetraploid rice. Acta Agriculturae Scandinavica Section B-Soil and Plant Science. 2011;61:23–32. [Google Scholar]
- Shi LG, Liu XD, Liu B, Zhao XJ, Wang L, Li JQ, Lu YG. Identifying neutral allele Sb at pollen-sterility loci in cultivated rice with Oryza rufipogon origin. Chinese Science Bulletin. 2009;54:2967–2974. [Google Scholar]
- Singh RN. Chromosomal abnormalities and fertility in induced autotetraploid Helianthus annuus in the C1 and C2 generations. Cytologia. 1992;57:277–281. [Google Scholar]
- Song WC, Zhang YH. Rice tetraploidy and its effect on agronomic traits and nutritional constituents. Acta Agronomica Sinica. 1992;18:137–144. [Google Scholar]
- Song X, Qin SQ, Xu CG, Li XH, Zhang QF. Genetic dissection of embryo sac fertility, pollen fertility, and their contributions to spikelet fertility of intersubspecific hybrids in rice. Theoretical and Applied Genetics. 2005;110:205–211. doi: 10.1007/s00122-004-1798-2. [DOI] [PubMed] [Google Scholar]
- Srivastava RN. Production of fertile autotetraploids in sesame and their breeding behaviour. The Journal of Heredity. 1956;47:241–244. [Google Scholar]
- Srivastava S, Lavania UC, Sybenga J. Genetic variation in meiotic behaviour and fertility in tetraploid Hyoscyamus muticus: correlation with diploid meiosis. Heredity. 1992;68:231–239. [Google Scholar]
- Tu SB, Luan L, Liu YH, Long W, Kong FL, He T, Xu QF, Yan WG, Yu MQ. Production and heterosis analysis of rice autotetraploid hybrids. Crop Science. 2007;47:2356–2363. [Google Scholar]
- Udall JA, Wendel JF. Polyploidy and crop improvement. The Plant Genome. 2006;46:3–14. [Google Scholar]
- Venkateswarlu J, Rao PN. Effect of inbreeding and selection for vigour and fertility on meiotic behaviour in autotetraploid Job's tears, Coix lacryma-jobi L. Theoretical and Applied Genetics. 1976;47:165–169. doi: 10.1007/BF00278374. [DOI] [PubMed] [Google Scholar]
- Wang L. MSc thesis. China: 2010. Construction of single segment substitution lines of Oryza rufipogon Griff. indigenous to Gaozhou of Guangdong province and identify ‘neutral gene’ of pollen sterility at Sa locus South China Agricultural University. [Google Scholar]
- Watanabe KN, Orrillo M, Vega S, Masuelli R, Ishiki K. Potato germplasm enhancement with disomic tetraploid Solanum acaule. I1. Assessment of breeding value of tetraploid F1 hybrids between tetrasomic tetraploid S. tuberosum and S. acaule. Theoretical and Applied Genetics. 1994;88:135–140. doi: 10.1007/BF00225888. [DOI] [PubMed] [Google Scholar]
- Wolf PG, Soltis PS, Soitis DE. Tetrasomics inheritance and chromosome pairing behaviour in the naturally occurring autotetraploid Heuchera grossulariifolia (Saxifragaceae). Genome. 1989;32:655–659. [Google Scholar]
- Xiao B, Liu XD, Lu YG, Li JQ, Zhao XJ. Structure of embryo sac and fertilization in intersubspecific hybrids of autotetraploid rice. Acta Agronomica Sinica. 2005;31:1150–1156. [Google Scholar]
- Xiao GY, Yuan LP. Studies on javanica rice and heterosis of intersubspecific hybrids. Beijing: Science Press; 2009. 87–89. [Google Scholar]
- Yan CJ, Liang GH, Gu SL, Yi CD, Lu JF, Li X, Tang SZ, Gu MH. Molecular marker analysis and genetic basis for sterility of typical indica/japonica hybrids. Acta Genetica Sinica. 2003;30:267–276. [PubMed] [Google Scholar]
- Yan YR, Bao WK. Study on polyploidy breeding methods in cereal crops. I. Tetraploid rice. Chinese Journal of Agriculture. 1960;11:12–14. [Google Scholar]
- Yang CY, Chen ZZ, Zhuang CX, Mei MT, Liu YG. Genetic map and fine physical mapping of the gene S-c for F1 pollen sterility in cultivated rice (Oryza sativa L.) Chinese Science Bulletin. 2004;49:1273–1277. [Google Scholar]
- Zhang GQ, Lu YG. Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa L.). II. A genic model for F1 pollen sterility. Acta Genetica Sinica. 1993;20:222–228. [Google Scholar]
- Zhang GQ, Lu YG, Liu GF, Yang JC, Zhang H. Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). III. Allele differentiation of F1 pollen sterility in different types of varieties. Acta Genetica Sinica. 1993;20:541–551. [Google Scholar]
- Zhang GQ, Lu YG, Zhang H, Yang JC, Liu GF. Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa). IV. Genotypes for F1 pollen sterility. Acta Genetica Sinica. 1994;21:34–41. [Google Scholar]
- Zhang Q, Liu KD, Yang GP, Maroof MAS, Xu CG, Zhou ZQ. Molecular marker diversity and hybrid sterility in indica–japonica rice crosses. Theoretical and Applied Genetics. 1997;95:112–118. [Google Scholar]
- Zhang ZS, Lu YG, Liu XD, Feng JH, Zhang GQ. Cytological mechanism of pollen abortion resulting from allelic interaction of F1 pollen sterility locus in rice (Oryza sativa L.) Genetica. 2006;127:295–302. doi: 10.1007/s10709-005-4848-z. [DOI] [PubMed] [Google Scholar]
- Zhao MH, Liu XD, Lu YG, Li JQ, Guo HB. Chromosome pairing behaviour and reproduction in the hybrid developed by the interaction of different pollen sterile genes in autotetraploid rice. Acta Agronomica Sinica. 2006;32:1472–1478. [Google Scholar]