Abstract
After fruit ripening, many fruit-tree species undergo massive natural fruit abscission. Olive (Olea europaea L.) is a stone-fruit with cultivars such as Picual (PIC) and Arbequina (ARB) which differ in mature fruit abscission potential. Ethylene (ET) is associated with abscission, but its role during mature fruit abscission remains largely uncharacterized. The present study investigates the possible roles of ET and polyamine (PA) during mature fruit abscission by modulating genes involved in the ET signalling and biosynthesis pathways in the abscission zone (AZ) of both cultivars. Five ET-related genes (OeACS2, OeACO2, OeCTR1, OeERS1, and OeEIL2) were isolated in the AZ and adjacent cells (AZ–AC), and their expression in various olive organs and during mature fruit abscission, in relation to interactions between ET and PA and the expression induction of these genes, was determined. OeACS2, OeACO2, and OeEIL2 were found to be the only genes that were up-regulated in association with mature fruit abscission. Using the inhibition of ET and PA biosynthesis, it is demonstrated that OeACS2 and OeEIL2 expression are under the negative control of PA while ET induces their expression in AZ–AC. Furthermore, mature fruit abscission depressed nitric oxide (NO) production present mainly in the epidermal cells and xylem of the AZ. Also, NO production was differentially responsive to ET, PA, and different inhibitors. Taken together, the results indicate that PA-dependent ET signalling and biosynthesis pathways participate, at least partially, during mature fruit abscission, and that endogenous NO and 1-aminocyclopropane-1-carboxylic acid maintain an inverse correlation, suggesting an antagonistic action of NO and ET in abscission signalling.
Keywords: Abscission zone, ethylene signalling and biosynthesis, mature fruit abscission, nitric oxide, polyamine
Introduction
Abscission is a highly regulated process that involves structural, biochemical, and molecular changes resulting in the detachment of plant organs (Roberts et al., 2002; Lewis et al., 2006). The two best-studied organs that undergo abscission are flowers and leaves (Patterson, 2001; Cai and Lashbrook, 2008; Agustí et al., 2008; Cho et al., 2008; Sakamoto et al., 2008; Meir et al., 2010). In general, major fruit species have developed a physiological drop of immature fruit (fruitlet) as a self-regulatory mechanism. This process is, at least in part, a consequence of the competition among fruits as well as between fruits and shoots for carbon assimilates. However, other species also display massive natural fruit abscission at maturity. This characteristic is highly desirable for mechanically harvested fruit. In contrast to immature fruit abscission, the abscission of mature fruit is a genetically programmed process that is controlled by two duplicated independent loci (Périn et al., 2002), but it remains unclear how this process is initiated and regulated in these species. Neither of the two loci match the known genes of the ethylene (ET) biosynthetic or signalling pathways (Périn et al., 2002). These data suggest that the character of mature fruit abscission is under complex regulation. The presence of ET-dependent and -independent events that are under the control of ET-regulated and ET-independent genes may take part in this process, as well as in the fruit ripening process (Pech et al., 2008).
Abscission occurs in an anatomically distinct cell layer known as the abscission zone (AZ) (Patterson, 2001). The positionally differentiated and functionally specialized cells that comprise this AZ represent good examples of hormone target cells in higher plants (Osborne and McManus, 2005). Extensive studies have demonstrated in several species and systems that ET enhances abscission, once it begins, and that the interplay between ET and other plant growth regulators is critical in regulating the progression of organ abscission (Patterson and Bleecker, 2004; Lewis et al., 2006). The application of ethephon, an ET-releasing compound, effectively promotes mature fruit abscission and ripening in many species (Brown, 1997; Perin et al., 2002; Pech et al., 2008), whereas inhibitors of ET biosynthesis or action reduce ET production in fruit and delay ripening as well as mature fruit abscission (Brown, 1997; Yuan and Carbaugh, 2007; Li and Yuan, 2008; Pech et al., 2008).
In fruit abscission, ET signalling and biosynthesis have been investigated largely in inmature fruit such as peach (Rasori et al., 2002; Ruperti et al., 2002), persimmon (Nakano et al., 2003), and apple (Dal Cin et al., 2005; Li and Yuan, 2008). However, much less is known about these pathways in mature fruit, and the molecular mechanism whereby ET affects mature fruit abscission inside the AZ remains unclear. Moreover, the involvement of ET signalling in immature fruit abscission has been reported mainly at the ERS, ETR, and CTR level in AZ tissue (Rasori et al., 2002; Dal Cin et al., 2005; Li and Yuan, 2008), and the regulation of EIN3 function in the fruit AZ remains unknown.
In olive (Olea europaea L), one of the most economically important fruit trees worldwide, where applications of ethephon and 1-aminocyclopropane-1-carboxylate (ACC) also promote fruit abscission (Barranco et al., 2004; Burns et al., 2008), it was recently found that PA metabolism is altered in the fruit AZ during mature fruit abscission (Gomez-Jimenez et al., 2010a). Because ET and PA [spermidine (Spd) and spermine (Spm)] share a common precursor, S-adenosyl-L-methionine (SAM), the biosynthetic relationship between those molecules is most often considered in terms of a competitive demand (Pandey et al., 2000). In the olive AZ, the endogenous concentrations of the diamine putrescine (Put) increase, while SAM decarboxylase (SAMDC) activity is inhibited during mature fruit abscission, probably to the benefit of ET biosynthesis, thus providing evidence of a possible antagonism between PA and ET biosynthesis inside the AZ (Gomez-Jimenez et al., 2010a).
Nitric oxide (NO) functions as a ubiquitous signalling molecule and helps control of plant growth and developmental processes, from germination to flowering, fruit ripening, and plant organ senescence (Leshem, 2001; Pagnussat et al., 2004; Manjunatha et al., 2010). The cross-talk of NO with hormones such as auxins, cytokinins, gibberellins, and abscisic acid (ABA) has also been discussed (Leshem, 2001; Pagnussat et al., 2004; Correa-Aragunde et al., 2006). Despite the fact that the interplay between NO and ET in the regulation of the fruit ripening process has already been shown (Leshem, 2001; Manjunatha et al., 2010), no data are available concerning the relationship between NO and ET during mature fruit abscission. NO effectively prevents the autocatalytic ET biosynthesis in climacteric peach fruit, through the binding of NO to 1-aminocyclopropane-1-carboxylic acid oxidase (ACO), forming a binary ACO–NO complex, which is then chelated by ACC to produce a stable ACC–ACO–NO complex. This ternary complex, in turn, decreases ET production. An irreversible transformation of ACC to malonyl-ACC (MACC) has also been reported in NO-treated fruit (Zhu et al., 2006). Yet another mode is that the NO and/or peroxynitriles formed during interaction of NO with H2O2 can also inhibit the activities of key enzymes of the ET pathway, ACC synthase (ACS) and ACO, by oxidative inactivation of cofactors, resulting in an overall reduction in the turnover of ET (Zhu et al., 2006). Similarly, the endogenously generated NO also suppresses ET formation in situ, impairing the progression of apple fruit ripening (Rudell and Mattheis, 2006). On the other hand, PA can stimulate the release of NO from Arabidopsis thaliana seedlings (Tun et al., 2006), suggesting a potential interplay between NO and PA. Treatment with Put significantly inhibits the softening of banana fruit with concomitant increases in endogenously formed NO as well as Put, where the mechanism involved is as yet to be established (Manjunatha et al., 2010). However, functional cross-talk between these molecules during abscission, to the best of our knowledge, has not been demonstrated.
The purpose of this study was first to investigate the possible implications of ET and PA during mature fruit abscission, and the interactions between ET, PA, and NO in the fruit AZ. Two agronomically important olive cultivars, Arbequina (ARB) and Picual (PIC), differ in mature fruit abscission potential (Gomez-Jimenez et al., 2010a). After ripening under natural conditions, ARB displays ∼17% abscission of mature fruit while PIC reaches 92% (Gomez-Jimenez et al., 2010a). In the present work, the hormonal regulation of the fruit detachment force (FDF), ACC/MACC content, and expression profiling of genes encoding the enzymes in the ET signalling and biosynthesis pathways in the AZ of ARB and PIC fruit was examined. For this, two ET biosynthesis genes (ACS and ACO), one ERS-like gene, one CTR1-like gene, and one EIN3-like gene were cloned from olive fruit AZ tissue. The transcript abundance of the five genes was estimated in different tissues as well as during mature fruit abscission in ARB and PIC cultivars. Treatments with ET, PA, and different inhibitors of biosynthesis in planta were used to investigate the interactions between ET and PA, and the induction of expression of these genes in the fruit AZ. In addition, to elucidate whether decreasing levels of NO and mature fruit abscission induction were related events, NO was precisely located in the fruit AZ and endogenous production in relation to the timing of mature fruit abscission following such treatments was examined.
Materials and methods
Plant material and treatments
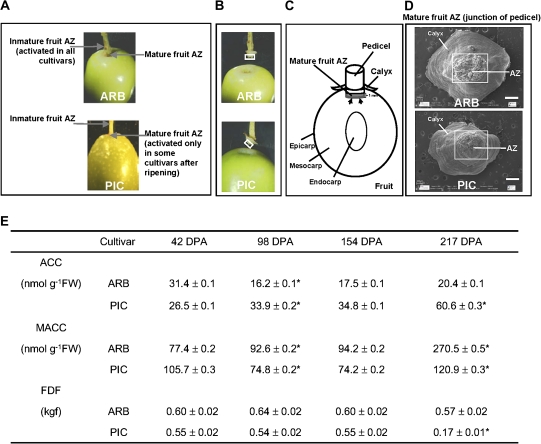
Twenty-year-old olive trees (O. europaea L.) grown under drip irrigation and fertirrigation (irrigation with suitable fertilizers in the solution) in an orchard near Badajoz (Spain) were studied. Two olive cultivars ARB and PIC were chosen according to their mature fruit abscission potential, low and high, respectively. The PIC olive cultivar exhibits massive natural fruit abscission at maturity (Gomez-Jimenez et al., 2010a). In this cultivar, abscission of fully black-ripe fruits occurred at the pedicel–fruit AZ (Fig. 1A–C). The olive mature fruit AZs were collected by cutting 1 mm at the proximal side (junction of the pedicel) of the abscission fracture plane (Fig. 1C). For this, pedicels were manually separated from olive fruits. The olive AZ tissues, containing a few layers of AZ cells at the proximal side of the separation line and adjacent cells (AZ–AC), were dissected from the pedicel samples with a razor blade into pieces of ∼1 mm3 (Fig. 1C, D). Fruit AZ wings containing mesocarp or pedicel/calyx-like tissues were discarded. The olive AZ–AC samples were collected from fruits at four developmental stages [42, 98, 154 (the onset of fruit ripening), and 217 (fruit ripening and mature fruit abscission) days post-anthesis (DPA)] from both olive cultivars, using 300 fruits for each developmental stage, during the 2008–2009 growing seasons. Freshly excised AZ samples were immediately frozen in liquid nitrogen and stored at –80 °C for ACC/MACC determination, RNA isolation, and NO production.
Fig. 1.
(A) Positions of the AZs of olive inmature and mature fruit. Arrows point to the fruit AZ of ARB and PIC olive cultivars. The appearance of the mature fruit AZ, located between the pedicel and fruit pericarp, of ARB and PIC cultivars before (A) and after (B) fruit removal. PIC mature fruits abscise naturally at 217 days post-anthesis (DPA). In this cultivar, abscission of fully black-ripe fruits occurred at the pedicel–fruit AZ. A white box indicates the position of the proximal fracture plane of the mature fruit AZ after fruit removal. (C) Schematic diagram showing a longitudinal section of olive fruit. The grey box indicates the position of the tissue sample of the olive AZ for ACC/MACC determination, RNA extraction, and NO production. Mature fruit AZs (AZ–AC) were manually dissected with a razor blade and separated by cutting 1 mm at the proximal side of the abscission fracture plane. Arrows indicates the position of the abscission fracture plane. (D) Cross-sections of positions corresponding to the white box in (B) and the grey box in (C). Scanning electron micrographs (SEMs) of the proximal (junction of pedicel) fracture plane of the AZ after fruit detachment of ARB and PIC cultivars. Scale bars: 400 μm. (E) Comparison of ACC and MACC content in olive fruit AZ–AC of both cultivars, and fruit detachment force (FDF) measurements of olive fruit during development and abscission. Fruit abscission was carefully observed by forcible removal or natural abscission of the olive fruit at different stages of development (42, 98, 154, and 217 DPA) of both cultivars. Data are the mean ±SD (n = 80). *Values that were determined by the t-test to be significantly different (P <0.05) from the preceding point. (This figure is available in colour at JXB online.)
Fruit AZ treatments were performed in planta. Fruit AZ treatments were performed on olive trees (20 per treatment) of ARB and PIC cultivars. Four branches per tree (total 80) were selected for experiments for uniform size and fruit load. For each treatment, 20 branches (1 branch per tree), including controls (application of water), were sprayed with solutions of ET (10 mM ethephon, Sigma-Aldrich, Spain), PAs (1 mM Put or 1 mM Spd, Sigma-Aldrich, Spain), inhibitors of ET synthesis [10 mM aminooxyacetic acid (AOA) or 10 mM cobalt chloride (CoCl2), Sigma-Aldrich, Spain], or inhibitors of PA synthesis [1 mM methylglycoxalbisguanylhydrazone (MGBG) or 10 mM cyclohexylamine (CHA), Sigma-Aldrich, Spain]. All treatments were applied as an aqueous solution (150 ml per branch) at the onset of fruit ripening (i.e. 154 DPA). To avoid contamination during spraying, at least one guard tree was used to separate each of the test trees, and the trees were sprayed with the solutions only when there was a weak or no wind. For the purposes of this study, olive fruit AZ–AC samples of each cultivar were collected from each tree at 7 d and 15 d after treatments. Fifteen fruit explants were collected from each tree for each treatment and time point, and were immediately separated into fruit pericarp and AZ. Freshly excised AZ–ACs (300 segments of <1 mm thickness for each time point, with <0.5 mm of the visible AZ excised) of treated fruits from both olive cultivars were immediately frozen in liquid nitrogen and stored at –80 °C until ACC/MACC determination, RNA isolation, and NO production.
For the analysis of other tissues, leaves, shoots, flowers, and mature fruits (mesocarp) of the two olive cultivars were collected from the same trees. All tissue samples were also immediately frozen in liquid nitrogen and subsequently stored at –80 °C for further use.
FDF and scanning electron microscopy of the fruit AZ
The FDF or fruit breakstrength, defined as the kg force (kgf) necessary to separate the fruit from the parent plant at the AZ site, was measured in 80 randomly assigned fruits/trees using a dynamometer (Correx, Switzerland) for each olive cultivar as described by Gomez-Jimenez et al. (2010a). The FDF was determined at 42, 98, 154, and 217 DPA for ARB and PIC cultivars, using 80 fruits for each developmental stage, and 7 d and 15 d after treatments in control and treated fruits.
To examine the proximal (junction of pedicel) fracture plane of the olive mature fruit AZ by scanning electron microscopy (SEM), the pedicel of the fruit was separated from the fruit pericarp of ARB and PIC cultivars prior to fixation. Following critical-point drying, tissues were mounted onto steel stubs, coated with gold–palladium, and observed using a LEO 1430VP scanning electron microscope (Gomez-Jimenez et al., 2010a).
Determination of ACC and MACC contents
ACC and MACC contents were analysed as previously described by Martin-Remesal et al. (2000). These analyses were performed in triplicate in three independent experiments, during the 2008–2009 growing seasons.
RNA isolation
Total RNA was extracted from various tissues as described by Gomez-Jimenez et al. (2010b). RNA concentration and purity were determined by scanning UV spectroscopy. This RNA was used in real-time PCR analysis.
Gene cloning and amino acid sequence analysis
Total RNA isolated from the fruit AZ–AC of the PIC cultivar (154 DPA) was used to synthesize single-stranded cDNA using M-MLV reverse transcriptase (RNase H Minus, Promega GmbH, Mannheim, Germany) and an oligo(dT) primer according to the manufacturer's instructions. The degenerate primers for isolation of ACO, ACS, ETR, CTR, and EIL were as follows: paco1, 5′-CGCGGATCCGCNTGYSARAANTGGGGNTT-3′, paco2, 5′-AAACTGCAGNGGYTCYTTNGCYTGRAAYTT-3′; pacs1, 5′-GCTGATCCTGGYGATGCWTT-3′; pacs2, 5′-ACYCKAAATCCTGGWAAMCCT-3′; pers1, 5′-GCTNGTNCAYATHATHCCTG-3′; pers2, 5′-CTCATYTCATGRTTCATNAC-3′; pctr1, 5′;-ATGGARCARGAYTTYCAYGCNGA-3′; pctr2, 5′-GANGGNGKYTTCCANGGYTC-3′; and peil1, 5′-TKGAGARGAGGATGTGGAGRGAC-3′; peil2, 5′-ATAATRGCAAGCCADGTWGCAC-3′, designed according to known gene sequences of other plants from the National Center for Biotechnology Information (NCBI) database. The isolated fragments were cloned using the pGEM-T easy vector (Promega GmbH, Mannheim, Germany), sequenced, and compared with database sequences using the BLAST program (Altschul et al., 1997). The phylogenetic tree was computed using the Clustal-W program (Thompson et al., 1994) employing standard parameters.
Real-time PCR analysis
The PCR amplification was performed with gene-specific primers. Primer sequences were 5′-GATCCTGGTGATGCTTTGCT-3′ (forward) and 5′-TCAGCTTCGTTGTATGCTGC-3′ (reverse) for OeACS2; 5′-CGGATCCGCATGTCAGAAAT-3′ (forward) and 5′-TACTTTCCCAGTCTAAATCA-3′ (reverse) for OeACO2; 5′-AAGTGGAGGGACCATGAGCTT-3′ (forward) and 5′-TCTTTCAAGAAGTTCATGACG-3′ (reverse) for OeERS1; 5′-GAGCAGGATTTTCATGCTGA-3′ (forward) and 5′-ACGAGGTAGTTTGTATAGACT-3′ (reverse) for OeCTR1; and 5′-AGATGATGGAAGTTTGCAAAGCTC-3′ (forward) and 5′-GTGGAACTCCTTTCTCTAATGGAA-3′ (reverse) for OeEIL2. Quantitative RT-PCR product size ranged between 120 bp and 200 bp. The OeUB gene was used as an internal control to normalize small differences in template amounts with the forward primer 5′-ATGCAGATCTTTGTGAAGAC-3′ and the reverse primer 5′-ACCACCACGAAGACGGAG-3′ (Gomez-Jimenez et al., 2010b). The cDNA was amplified using SYBR-Green® PCR Master kit (Applied Biosystems, Foster City, CA, USA) containing an AmpliTaq Gold polymerase on a iCycler (BioRad Munich, Germany), following the protocol provided by the supplier. Samples were subjected to thermal cycling conditions of DNA polymerase activation at 94 °C, 45 s at 52 °C, 45 s at 72 °C, and 45 s at 80 °C; a final elongation step of 7 min at 72 °C was performed. The melting curve was designed to increase 0.5 °C every 10 s from 62 °C. Real-time PCR analysis was performed with two different cDNAs from the same time point (from two different RNAs), and each was made in triplicate. The amplicon was analysed by electrophoresis and sequenced once for identity confirmation. Real-time PCR efficiency was estimated via a calibration dilution curve and slope calculation. Expression levels were determined as the number of cycles needed for the amplification to reach a threshold fixed in the exponential phase of the PCR (CT). The DDCT method was used to analyse data (Pfaffl, 2001). Relative expression values were normalized to the lowest expression value taken as 1.
NO assays
NO staining was performed following the method of Ahlfors et al. (2009). The proximal (junction of pedicel) fracture plane of fresh fruit AZs from olive fruits at 217 DPA were stained with 15 μM DAF-FM-DA (4-amino-5-methylamino-2',7'-difluorofluorescein diacetate; Molecular Probes, now part of Invitrogen, http://www.invitrogen.com) in loading buffer [5 mM MES/KOH, pH 5.7, 0.25 mM KCl, and 1 mM CaCl2]. Parallel sets of samples were treated the same, although they were previously incubated for 1 h with the NO-scavenger cPTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; Sigma-Aldrich, Spain] at a concentration of 400 μM. Negative controls were treated with MES-KCl buffer only instead of DAF-FM-DA. Fluorescent signals were detected using a FV1000confocal laser scanning microscope (Olympus, Hamburg, Germany). The dye was excited at 488 nm, and was collected in the emission range of 515–560 nm. To visualize all cells, chlorophyll fluorescence was collected using a second channel at 600–650 nm.
For fruit AZ–AC, fluorescence measurements were performed according to Ahlfors et al. (2009). Fluorescence emission at 535 nm was measured with a Cary Eclipse Fluorescence Spectrophotometer (Varian, Palo Alto, CA, USA), and results were normalized by weight.
Results
Changes of ACC and MACC contents in olive fruit AZ tissue
In olive, fruit abscission is dependent on the activation of several AZs (Fig. 1A), and only one AZ at a time is selectively activated at a specific developmental stage (Bartolini et al., 1993; Gomez-Jimenez et al., 2010a). Abscission of mature fruit was studied in the pedicel–fruit AZ (Fig. 1A–C). The olive fruit AZ–AC tissues, containing a few layers of AZ cells at the proximal side of the separation line and adjacent cells, were manually dissected from the pedicel samples with a razor blade into pieces of ∼1 mm3 (Fig. 1C, D).
A detailed analysis was made of ACC, an ET precursor, and of MACC contents in fruit AZ–AC tissue of two olive cultivars, ARB and PIC. Fruits of defined developmental stages and at certain time points during the day were harvested, since the aim was to determine the changes of ACC and MACC concentrations in the AZ during activation and abscission of olive mature fruit. In addition, the entire experiment was independently replicated to cover the impact of biological variability caused by environmental factors. Each sample comprised fruit AZ tissue from 80 individual fruits. A significant difference in the time course of ACC and MACC levels between ARB and PIC was detected up to 217 DPA (Fig. 1E). In PIC fruit, the FDF significantly decreased during the late stage of fruit development (217 DPA, fruit ripening), and this coincided with a peak of ACC content (Fig. 1E) in the PIC fruit AZ. In contrast, ARB fruit did not show a decrease in FDF over time and did not display any peak of ACC content, while MACC accumulated to higher levels in the later stage of ARB fruit development (Fig. 1E).
Tissue specificity and ET-related gene expression patterns during fruit AZ activation and abscission
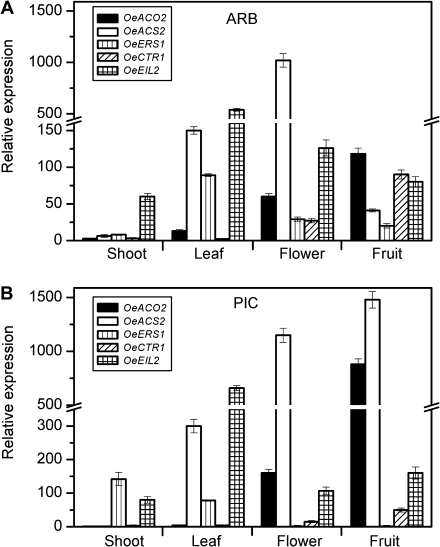
To evaluate the effect of mature fruit abscission on the expression of genes involved in ET synthesis and signalling, homologues of ACS, ACO, ERS, CTR1, and EIN3 were isolated from olive fruit AZ–AC. These homologues were cloned by means of the primer strategy described in the Materials and methods. In total, five partial cDNAs, designated as OeACS2 (HQ008854), OeACO2 (HQ008853), OeERS1 (HQ008857), OeCTR1 (HQ008855), and OeEIL2 (HQ008856), were isolated and their phylogenetic relationships to the known genes are shown in Supplementary Figs. S1–S5 available at JXB online. Real-time PCR was used to test the tissue specificity and expression profiles of the five genes during fruit development and abscission in the ARB and PIC cultivars. To establish the tissue expression pattern of OeACS2, OeACO2, OeERS1, OeCTR1, and OeEIL2, total RNA was extracted from olive leaves, shoots, flowers, and mature fruits. The results showed that none of the five genes was fruit AZ specific and their expression patterns were significantly different in leaves, shoots, flowers, and fruits. Also, OeACS2 was expressed at a low level in shoot tissue in both cultivars. It was found at the highest level in leaf and flower tissue in ARB (Fig. 2A), and in leaf, flower, and fruit tissue in PIC (Fig. 2B). OeACO2 and OeCTR1 transcripts were more abundant in flower and fruit tissue, while OeEIL2 showed a high expression level in leaf tissue of both cultivars (Fig. 2). OeERS1 expression varied with different tissues and cultivars. In ARB, OeERS1 expression was more abundant in leaf, flower, and fruit tissue, while its transcript level was much more abundant in shoot tissue of the PIC cultivar (Fig. 2).
Fig. 2.
Expression of different components of the ET synthesis and signalling pathways in various olive tissues. Real-time PCR was used to analyse the expression patterns of OeACS2, OeACO2, OeERS1, OeCTR1, and OeEIL2. Each value represents the means ±SE of three replicates. Relative expression values were normalized to the lowest expression value taken as 1.
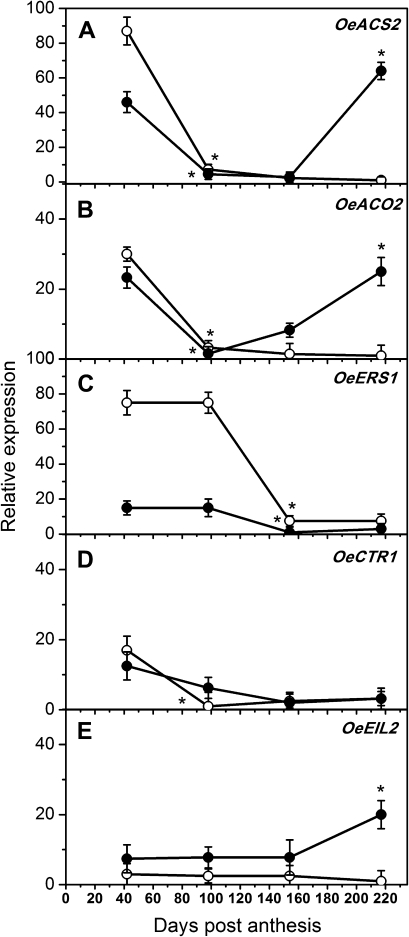
All of these genes were expressed differentially within and between fruit AZ–ACs of the two cultivars (Fig. 3). From 42 DPA through fruit maturity and abscission (217 DPA), OeACS2 and OeACO2 expression levels were similar, with a relatively high abundance in fruit AZ–AC at the early stage of fruit development (42 DPA) and lower expression at 96 DPA in both cultivars, followed by a rise in the expression levels up to mature fruit abscission (217 DPA) in PIC fruit AZ–AC, but OeACO2 increased earlier than did OeACS2 (Fig. 3A, B). The expression pattern of OeERS1 throughout fruit development in PIC AZ–AC had the same trend as in ARB AZ–AC, but its transcript levels were far lower (Fig. 3C). OeCTR1 showed little change over the course of fruit development of both cultivars (Fig. 3D). Meanwhile, OeEIL2 was the most constantly expressed throughout fruit development in both cultivars. However, in the PIC cultivar, OeEIL2 showed marked up-regulation in the last stage at 217 DPA, whereas this did not occur in ARB fruit AZ–AC (Fig. 3E). Therefore, PIC fruit AZ–AC exhibited a dramatic rise in the transcript levels of the OeACS2, OeACO2, and OeEIL2 mRNAs in association with mature fruit abscission (217 DPA), when the two cultivars were compared (Fig. 3).
Fig. 3.
Expression of different components of the ET synthesis and signalling pathways in olive fruit AZ–AC of ARB (open circles) and PIC (solid circles) cultivars during development and abscission. Real-time PCR was used to analyse the expression patterns of OeACS2 (A), OeACO2 (B), OeERS1 (C), OeCTR1 (D), and OeEIL2 (E). Each value represents the mean of three independent experiments ±SE. *Values that were determined by the t-test to be significantly different (P <0.05) from the preceding point.
Interactions between ET and the expression of ET-related genes in mature fruit AZ tissue
The FDF, ACC/MACC content, and expression profiles of the five genes were studied further in the fruit AZ from the two olive cultivars under treatments with ethephon (exogenous ET) and ET biosynthesis inhibitors. Fruit AZ treatments were performed in planta. Changes in FDF as induced by exogenous ET and inhibitors of ET biosynthesis were monitored 7 d and 14 d following treatment at the onset of fruit ripening (154 DPA).
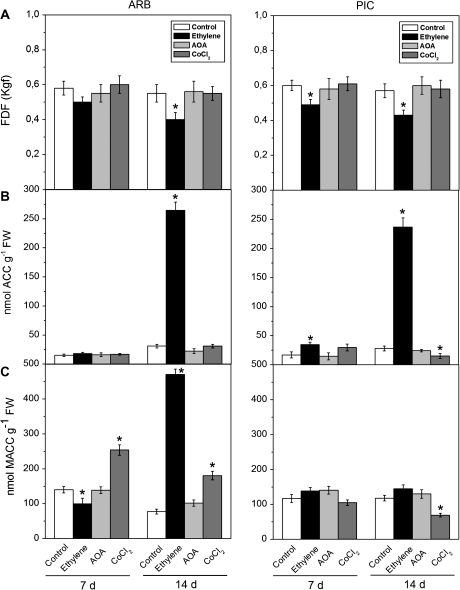
The application of AOA and CoCl2, specific inhibitors of ACS and ACO, respectively, had no effect on fruit abscission in the treated fruits compared with the control fruits in both cultivars (Fig. 4A). In contrast, when 10 mM ethephon was applied to olive trees, the FDF declined slightly in both cultivars but, after 14 d of treatments, the FDF was lower in the non-abscising cultivar ARB than in the abscising cultivar PIC (Fig. 4A). Thus, the impact of these treatments on mature fruit abscission depends on the cultivar genotypes.
Fig. 4.
Changes in FDF measurements (A), ACC (B), and MACC (C) contents in olive fruit AZ–AC of ARB and PIC cultivars treated with water (control), 10 mM ethephon (exogenous ethylene), 10 mM aminooxyacetic acid (AOA), or 10 mM cobalt chloride(CoCl2) at 7 d and 14 d after treatment. Error bars indicate SEs from three replicates. Asterisks above the bars indicate values that were determined by the t-test to be significantly different (P <0.05) from control.
ACC and MACC contents in fruit AZ–AC were also examined following chemical treatments/applications. In both cultivars, the ACC content in fruit AZ–AC was increased by ethephon treatment on day 14 (Fig. 4B), while it displayed minimal or no effect on the ACC content on day 7. In ARB, ethephon-induced differences in MACC content were more marked and became maximal on day 14 compared with controls, whereas slight increases were detected in MACC content in PIC throughout the period following ethephon application (Fig. 4C). In AOA-treated fruit AZ–AC, no significant changes in ACC and MACC contents were detected relative to untreated fruit (Fig. 4B, C). In contrast, the CoCl2 treatment had a different effect on MACC and ACC contents in both cultivars. In ARB, exogenous CoCl2 led to a significant rise in MACC content, whereas in PIC this treatment significantly lowered MACC and ACC contents compared with controls on day 14.
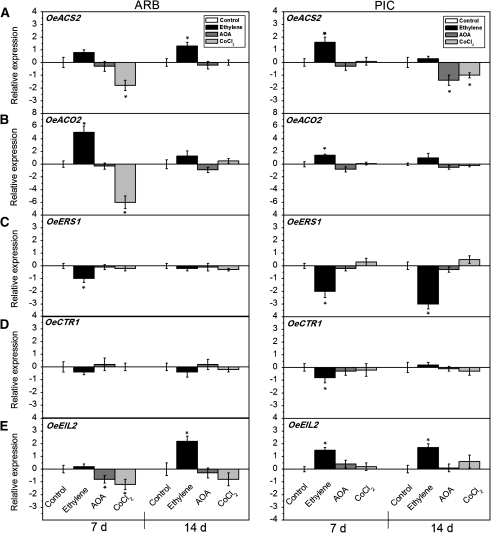
Since most of the gene transcripts were induced in parallel with the mature fruit abscission, the interactions between ET and ET-related gene expression were also studied following the application of ET or inhibitors of ET biosynthesis. Figure 5 shows the expression patterns of OeACS2, OeACO2, OeERS1, OeCTR1, and OeEIL2 in response to exogenous ET (ethephon), AOA, and CoCl2 in fruit AZ–AC of the two cultivars. After 7 d of treatment, OeACS2 was up-regulated by exogenous ET and was not affected by AOA in either cultivar, but it exhibited different expression patterns with the CoCl2 treatment (Fig. 5A). In ARB fruit AZ–AC, the expression levels of OeACS2 declined sharply while maintaining a constant level in PIC fruit AZ–AC with the CoCl2 treatment. Nevertheless, a divergent expression pattern of OeACS2 was found in the two cultivars after 14 d with exogenous ET, AOA, and CoCl2 treatments (Fig. 5A). In ARB fruit AZ–AC, OeACS2 expression was induced by ET and was not affected by the AOA and CoCl2 treatments, whereas in PIC fruit AZ–AC its expression was not affected by ET but was decreased by the AOA and CoCl2 treatments. The OeACO2 expression was not significantly altered by the AOA treatment but was augmented by exogenous ET in both cultivars, especially in ET-treated ARB fruit AZ–AC on day 7, when it was decreased by CoCl2 treatment (Fig. 5B).
Fig. 5.
Expression of OeACS2 (A), OeACO2 (B), OeERS1 (C), OeCTR1 (D), and OeEIL2 (E) in olive fruit AZ–AC of ARB and PIC cultivars treated with water (control), 10 mM ethephon (exogenous ethylene), 10 mM aminooxyacetic acid (AOA), or 10 mM cobalt chloride (CoCl2) at 7 d and 14 d after treatment. The histograms represent the average data from three replications using real-time PCR analysis, with the control set at 0. Error bars indicate SEs from three replicates. Asterisks above the bars indicate values that were determined by the t-test to be significantly different (P <0.05) from control.
The expression of OeERS1 and OeCTR1 was down-regulated when the fruit AZ were treated with ET, while no significantly different expression between control and treatments with AOA and CoCl2 was detected after 7 d and 14 d in either cultivar (Fig. 5C, D). OeEIL2 showed different expression patterns after 7 d of treatments. In PIC fruit AZ–AC, the expression level of OeEIL2 was raised by ET but maintained constant with AOA and CoCl2 treatments, whereas in ARB fruit AZ–AC, levels remained constant under ET treatment but declined with AOA and CoCl2 treatments (Fig. 5E). On the other hand, OeEIL2 expression was very similar in both cultivars after 14 d of treatments. Transcript levels of OeEIL2 displayed no significant changes after treatment with exogenous AOA and CoCl2, but expression was strongly induced by ET after 14 d in ARB and PIC fruit AZ–AC (Fig. 5E).
Interactions between PA and the expression of ET-related genes in mature fruit AZ tissue
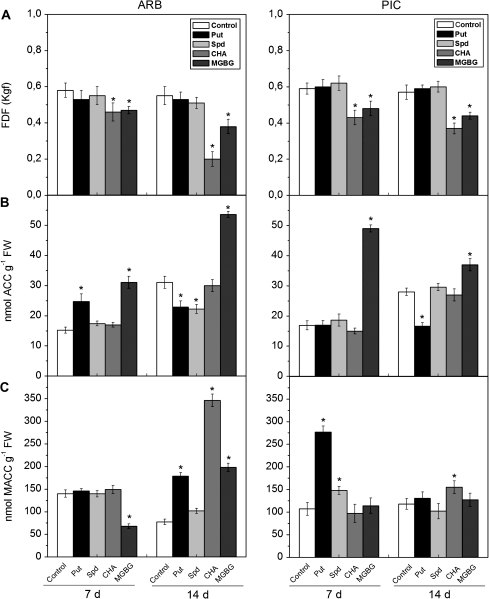
To investigate the interrelationship between ET and PA in the olive AZ during mature fruit abscission, treatments with exogenous PA and inhibitors of PA biosynthesis in planta were performed at the onset of ripening in the ARB and PIC cultivars. Exogenous Put and Spd application had no effect on mature fruit abscission in the treated fruits compared with the control fruits (Fig. 6A). However, the application of MGBG and CHA, SAMDC and spermidine synthase (SPDS) inhibitors, respectively, caused a significant decrease in the FDF in both cultivars after 14 d of treatment, although the CHA application was more effective in controlling mature fruit abscission. In addition, the amplitude of the response depended on the cultivars. The FDF of MGBG-treated and CHA-treated fruits reached 71% and 38%, respectively, of that of the control values in ARB, and 77% and 64%, respectively, of control in PIC after 14 d of treatment (Fig. 6A).
Fig. 6.
Changes of FDF measurements (A), and ACC (B) and MACC (C) contents in olive fruit AZ–AC of ARB and PIC cultivars treated with water (control), 1 mM putrescine (Put), 1 mM spermidine (Spd), 10 mM cyclohexylamine (CHA), or 1 mM methylglycoxalbisguanylhydrazone (MGBG) at 7 d and 14 d after treatment. Error bars indicate SEs from three replicates. Asterisks above the bars indicate values that were determined by the t-test to be significantly different (P <0.05) from control.
With Put treatment, the ACC content significantly increased after 7 d only in ARB fruit AZ–AC, while it decreased by 30% and 43% in ARB and PIC fruit AZ–AC after 14 d, respectively, compared with the control (Fig. 6B). With Spd treatment after 7 d and 14 d, no significant changes in ACC content were found in PIC fruit AZ–AC, while a 30% reduction was found after 14 d in Spd-treated ARB fruit AZ–AC compared with the control ARB fruit AZ–AC (Fig. 6B). CHA did not affect the ACC content in either cultivar after 7 d and 14 d of treatment. In contrast, MGBG treatment significantly raised the ACC content in both cultivars (Fig. 6B). The effects of treatments on MACC concentration varied within and between the two cultivars. In contrast to the PIC cultivar, the Put, Spd, and CHA treatments did not affect the MACC content after 7 d in the ARB cultivar. After 14 d of treatment, the MACC content was boosted by the treatments in ARB but did not respond significantly to treatments in PIC (Fig. 6C).
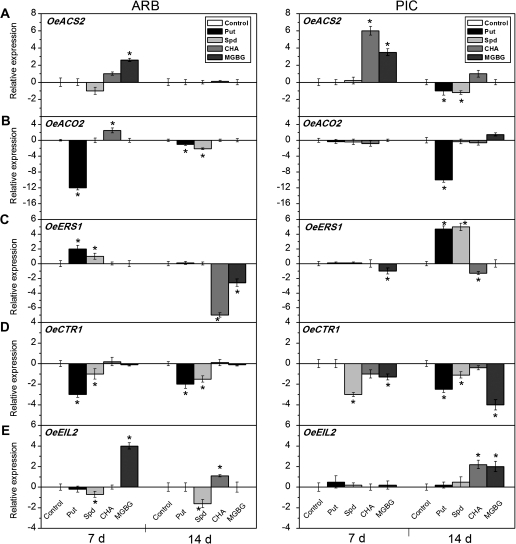
The analysis of gene expression following Put and Spd application at the onset of ripening (Fig. 7) demonstrated that, when the two cultivars were compared, only the OeACS2 transcription level was negatively regulated by Put treatment in PIC fruit AZ–AC, while it did not significantly respond to Put treatment in ARB fruit AZ–AC. Also, only OeACO2 was negatively regulated by Spd treatment in ARB fruit AZ–AC, whereas it did not significantly respond in PIC fruit AZ–AC to Spd treatment (Fig. 7A, B). In contrast, the effects of Put and Spd application on OeERS1, OeCTR1, and OeEIL2 expression were similar in both cultivars. In comparison with the control, Put and Spd treatments increased OeERS1 expression and decreased OeCTR1 expression, but had little effect on the expression of OeEIL2 in either cultivar (Fig. 7C–E).
Fig. 7.
Expression of OeACS2 (A), OeACO2 (B), OeERS1 (C), OeCTR1 (D), and OeEIL2 (E) in olive fruit AZ–AC of ARB and PIC cultivars treated with water (control), 1 mM putrescine (Put), 1 mM spermidine (Spd), 10 mM cyclohexylamine (CHA), or 1 mM methylglycoxalbis-guanylhydrazone (MGBG) at 7 d and 14 d after treatment. The histograms represent the average data from three replications using real-time PCR analysis, with the control set at 0. Error bars indicate SEs from three replicates.
Asterisks above the bars indicate values that were determined by the t-test to be significantly different (P <0.05) from control.
CHA and MGBG treatments induced the expression of OeACS2 and OeEIL2 in both cultivars, suggesting that at this stage of development these genes were under the negative control of Spd in olive fruit AZ–AC (Fig. 7A, E). The effects of these treatments on the expression of OeERS1 in both cultivars were consistent with the results of Spd treatment; OeERS1 expression was inhibited by CHA and MGBG application after 14 d (Fig. 7C). Divergent expression patterns of OeCTR1 and OeACO2 were detected after CHA and MGBG treatments in the two cultivars. Only in PIC fruit AZ–AC was OeCTR1 expression negatively regulated by these treatments after 7 d and 14 d, but no significant difference was noted in OeCTR1 expression in ARB fruit AZ–AC after such treatments (Fig. 7D). However, OeACO2 also showed different expression patterns between CHA and MGBG treatments; OeACO2 was up-regulated only in ARB fruit AZ–AC by CHA treatment and in PIC fruit AZ–AC by MGBG treatment (Fig. 7B).
Relationship between ET and PA in the mature fruit AZ during abscission
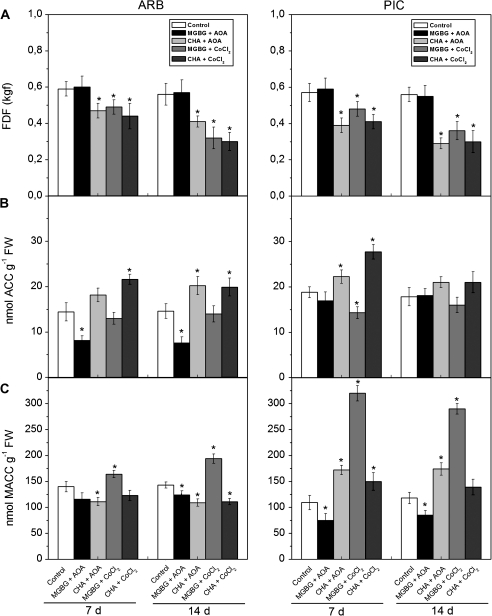
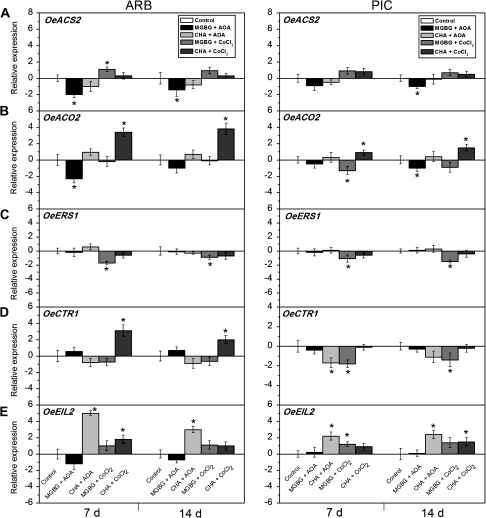
To determine whether PA is linked to ET biosynthesis during abscission, the effect of ET biosynthesis inhibitors on MGBG- or CHA-induced abscission of olive mature fruit was tested. MGBG treatment of the mature fruit AZ accelerated abscission (Fig. 6A), while this abscission was suppressed by the addition of AOA, a specific inhibitor of ACS (Fig. 8A), lowering the ACC content in ARB fruit AZ–AC (Fig. 8B) and the MACC content in PIC fruit AZ–AC (Fig. 8C). In contrast, the specific inhibitor of ACO, CoCl2, in combination with MGBG did not reverse the decline in FDF (Fig. 8A), but modulated the MACC content (Fig. 8C). Importantly, the FDF in the presence of CHA in both cultivars proved quite minor, and was unaffected by the addition of ET inhibitors (AOA or CoCl2).
Fig. 8.
Changes of FDF measurements (A), and ACC (B) and MACC (C) contents in olive fruit AZ–AC of ARB and PIC cultivars treated with water (control), 10 mM aminooxyacetic acid (AOA) and 1 mM methylglycoxalbis-guanylhydrazone (MGBG), 10 mM AOA and 10 mM cyclohexylamine (CHA), 10 mM cobalt chloride (CoCl2) and 1 mM MGBG, or 10 mM CoCl2 and 10 mM CHA at 7 d and 14 d after treatment. Error bars indicate SE0s from three replicates. Asterisks above the bars indicate values that were determined by the t-test to be significantly different (P <0.05) from control.
Next, the effects of ET and PA biosynthesis inhibitors on the expression of ET-related genes during mature fruit abscission were examined (Fig. 9). Mature fruit abscission was accelerated by ET or MGBG, and was suppressed by the addition of an inhibitor of ET (AOA) in combination with MGBG. OeACS2, OeACO2, and OeEIL2 expression clearly increased during mature fruit abscission, without any chemical treatment (Fig. 3), suggesting an active response to endogenous ET in olive fruit AZ–AC. The expression of OeACS2 and OeEIL2 was accelerated by MGBG (Fig. 7), and was inhibited in combination with AOA (Fig. 9). These data imply that PA-dependent ET production participates, at least partially, in abscission signalling in the mature fruit AZ.
Fig. 9.
Expression of OeACS2 (A), OeACO2 (B), OeERS1 (C), OeCTR1 (D), and OeEIL2 (E) in olive fruit AZ–AC of ARB and PIC cultivars treated with water (control), 10 mM aminooxyacetic acid (AOA) and 1 mM ethylglycoxalbis-guanylhydrazone (MGBG), 10 mM AOA and 10 mM cyclohexylamine (CHA), 10 mM cobalt chloride (CoCl2) and 1 mM MGBG, or 10 mM CoCl2 and 10 mM CHA at 7 d and 14 d after treatment. The histograms represent the average data from three replications using real-time PCR analysis, with the control set at 0. Error bars indicate SEs from three replicates. Asterisks above the bars indicate values that were determined by the t-test to be significantly different (P <0.05) from control.
NO production and localization during mature fruit abscission
To improve understanding of the role of NO in plant physiology, NO production in AZ–AC was measured fluorometrically at several stages of fruit development using DAF-FM-DA dye. During fruit development, ARB and PIC fruit AZ–AC underwent different NO production changes (Table 1). Both cultivars produced higher quantities of NO at 42 DPA with respect to other time points measured, and afterwards NO production gradually declined at the later stages of fruit development (Table 1). This decline was very pronounced in the case of the abscising cultivar PIC, when NO production declined by 46% for the fruit AZ–AC during mature fruit abscission (Table 1).
Table 1.
Production of NO in the fruit AZ–AC of two olive cultivars during development and abscission
| Cultivar | 42 DPA | 98 DPA | 154 DPA | 217 DPA |
| ARB | 1378±189 | 1139±178 | 376±49* | 256±45* |
| PIC | 1707±195 | 749±133* | 196±37* | 90±12* |
NO content was measured as relative fluorescence units (RFU) using DAF-FM-DA dye and a fluorometer (n=5, ±SE). Asterisks indicate values that were determined by the t-test to be significantly different (P <0.05) from the preceding point. The experiment was repeated three times with similar results.
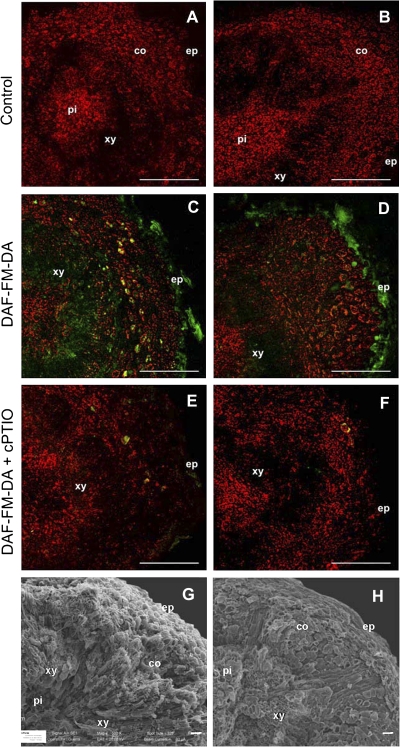
The cellular localization of endogenous NO was analysed in the proximal (junction of pedicel) fracture plane of the AZs, located between the pedicel and fruit pericarp, from olive mature fruits at 217DPA, using DAF-FM-DA dye and confocal microscopy (Fig. 10). This revealed slight differences between the olive cultivars. At 217 DPA, as was also observed in the fluorometric assay (Table 1), the ARB fruit AZ appeared to emit more DAF-FM-DA fluorescence than did the PIC fruit AZ (Fig. 10). NO was localized mainly in the epidermal and vascular tissues (xylem) of the proximal fracture plane from both cultivars (Fig. 10C, D), but fluorescence was more intense in the xylem of ARB than in that of PIC (see Supplementary Figs S6 and S7 at JXB online; Fig. 10C, D). When the NO-specific scavenger (cPTIO) was included in the incubation media, the signal was evidently reduced in the samples studied (Fig. 10E, F).
Fig. 10.
Fluorescence microscopic imaging of NO in the proximal (junction of pedicel) fracture plane of the AZ, located between the pedicel and fruit pericarp, from ARB (A, C, E) and PIC (B, D, F) olive fruit at 217 DPA were incubated in MES-KCl buffer alone (A, B; control), with DAF-FM-DA as fluorescent probe (C, D), or in combination with cPTIO (E, F), and examined by confocal laser scanning microscopy. The presence of NO is shown by green fluorescence, which is distinguishable from autofluorescence, showed here in red. Co-localization of both fluorescence sources results in yellow colour. Experiments were repeated at least five times with similar results. Scanning electron micrographs (SEMs) of the proximal fracture plane of the olive fruit AZ from an ARB (G) and PIC (H) cultivar. co, cortex; ep, epidermis. pi, pith; xy, xylem. Scale bars: 250 μm in A–F; 20 μm in G and H.
Effect of exogenous ET, PA, and different inhibitors on endogenous NO production in mature fruit AZ tissue
For further investigation of the relationship between NO, ET, and PA in the olive mature fruit AZ, NO production was measured fluorometrically in ARB and PIC fruit AZ–AC after 7 d and 14 d of treatment with ethephon (exogenous ET), Put, Spd, AOA, CoCl2, CHA, and MGBG. The accumulation of NO followed the pattern of FDF (Table 2; Figs 4A, 6A). Exogenous ET, CHA, and MGBG induced a marked decrease in NO production mainly after 14 d in both cultivars (Table 2). Meanwhile, AOA treatment boosted NO production in both cultivars after 7 d and 14 d. However, no significant changes in NO production were found by Put, Spd, or CoCl2 treatments in ARB after 7 d and 14 d, whereas NO production increased sharply in PIC after 14 d of such treatments (Table 2). Thus, the NO production was also differentially responsive to ET, PA, and different inhibitors in olive fruit AZ–AC, confirming the decline in the presence of ET and inhibitors of PA biosynthesis when mature fruit abscission actively occurred, but the impact of these treatments on NO production depended on the cultivars and the inhibitor applied.
Table 2.
Relative levels of NO in the olive fruit AZ–AC after treatments
| Cultivar | Control | ET | Put | Spd | AOA | CoCl2 | CHA | MGBG | |
| ARB | |||||||||
| 7 d | 1.0 | 0.5* | 1.0 | 1.0 | 1.2 | 1.0 | 0.4* | 1.0 | |
| 14 d | 1.0 | 0.5* | 1.1 | 1.0 | 1.2 | 1.0 | 0.5* | 0.8 | |
| PIC | |||||||||
| 7 d | 1.0 | 0.1* | 1.2 | 1.0 | 1.4* | 1.0 | 0.8 | 1.0 | |
| 14 d | 1.0 | 0.1* | 1.2 | 2.3* | 1.1 | 3.8* | 0.1* | 0.1* |
Olive trees (ARB and PIC cultivars) were sprayed with water (control), 10 mM ethephon (ET), 1 mM putrescine (Put), 1 mM spermidine (Spd), 10 mM aminooxyacetic acid (AOA), 10 mM cobalt chloride (CoCl2), 10 mM cyclohexylamine (CHA), or 1 mM methylglycoxalbis-guanylhydrazone (MGBG), and the fruit AZ–AC were then harvested 7 d and 14 d after spraying. NO content was measured as relative fluorescence units (RFU) using DAF-FM-DA dye and a fluorometer (n=5, ±SE), and the fold ratio is calculated with the corresponding control sample (water-treated sample). The experiment was repeated three times with similar results, and data for one representative experiment are shown. Asterisks indicate values that were determined by the t-test to be significantly different (P <0.05) from control.
Discussion
Hormonal regulation of ET biosynthesis and signalling during mature fruit abscission remains somewhat obscure. Moreover, the ET cross-talk with other signals also needs to be clarified, in particular the interplay between PA and NO in the fruit AZ during abscission. Olive provides a good model for investigating ET biosynthesis/signalling and the ET cross-talk with PA in mature fruit abscission, since olive cultivars vary naturally in mature fruit abscission, and exogenous ET promotes fruit abscission (Barranco et al., 2004; Burns et al., 2008) and PA metabolism in the AZ during mature fruit abscission (Gomez-Jimenez et al., 2010a). In the present study, treatments that resulted in the differential abscission of olive mature fruit had differential effects on ACC and MACC accumulation, the expression of ET-related genes, and NO production in olive AZ–AC tissue.
Expression patterns of ET-related genes and their possible involvement in mature fruit abscission
Five genes from different levels of the ET signalling and biosynthesis pathways were isolated from olive fruit AZ–AC. Because of the multiple functions of ET in higher plants, the ET-related genes are not expressed exclusively in one tissue (El-Sharkawy et al., 2007; Yin et al., 2008). None of the olive AZ ET-related genes was AZ specific. Among the olive fruit AZ ET-related genes, only OeACO2 and OeCTR1 are expressed in reproductive tissues at their highest levels, while OeEIL2 is expressed at its highest levels in the vegetative tissue of both cultivars. However, OeACS2 and OeERS1 expression also varied between olive cultivars. These data fit with those in fruit tissue from other species such as plum, banana, and loquat, where PsETR1, PsERS1, PsCTR1, and PsERF, MaACS1 and MaACO1, and EjERS1a, EjERS1b, EjETR1, EjCTR1, and EjEIL1, respectively, were also expressed differentially within and between fruit of different cultivars (El-Sharkawy et al., 2007; Choudhury et al., 2008; Wang et al., 2010). The marked expression differences between the two olive cultivars suggest that the two genotypes studied may differ in their mean levels of ACC/MACC and ET sensitivity, suggesting a difference in roles of OeACS2 and OeERS1 in these cultivars.
The expression patterns of ET-related genes differed during olive fruit AZ activation. OeACO2 and OeACS2 showed a strong association with the ACC accumulation during abscission. These results suggest that OeACO2 and OeACS2 in fruit AZ–AC are related to mature fruit abscission. In contrast, in citrus, the expression of ACS1 and ACO, but not that of ACS2, increased in mature fruit, and the leaf AZ was associated with ethephon-induced abscission (Yuan et al., 2005).
The high ET emission level in fruit (mesocarp and epicarp tissues) and pedicel tissues in the PIC cultivar during ripening may be sufficient to trigger the activation of AZ cells, because genes of ET biosynthesis exhibit a higher level of expression in reproductive tissues than in vegetative tissues. In addition, there is a significant difference between cultivars; the expression level is higher in the PIC cultivar which has high mature fruit abscission potential.
The expression of OeERS1 and OeCTR1 did not notably change in the AZ during mature fruit abscission. However, these results differ significantly from those of other AZs during fruitlet abscission; an accumulation of ERS1 and CTR1 expression has been found in apple (Dal Cin et al., 2005) and in peach (Rasori et al., 2002) during fruitlet abscission. Moreover, Dal Cin et al. (2005) hypothesized that the ERS:ETR ratio plays a crucial role in apple fruitlet abscission by regulating sensitivity to the ET. In contrast, Li and Yuan (2008) reported a marked decrease in MdERS1 and MdCTR1 expression during fruitlet abscission in the apple AZ. This discrepancy could be due to a differential apple cultivar response. To date, OeEIL2 is the first EIN3-like gene that has been cloned from fruit AZ–AC, and showed 94–96% identity at the deduced amino acid level with EILs from tomato fruit. One of the most informative results from the present analysis was the up-regulation of OeEIL2 in olive AZ during mature fruit abscission. Similar results have been shown for fruit mesocarp tissue during ripening in climacteric melon (Périn et al., 2002), banana (Mbéguié-A-Mbéguié et al., 2008), and apple (Tacken et al., 2010) where EIL2 is a ripening and ET-inducible gene, unique within the EIL gene family. Recently, the expression of these EILs was examined during fruit development of kiwi, where AdEIL2 and AdEIL3 activated transcription of the ripening-related genes AdACO1 and AdXET5 (Yin et al., 2010).
Interactions between ET and ET-related gene expression
ET and phosphate derived from the decomposition of ethephon are thought to accelerate abscission by promoting ethylene biosynthesis in many higher plants, including olive (Banno et al., 1993; Goren et al., 1998; Barranco et al., 2004). The present study has demonstrated a strong induction of OeACS2 and OeACO2 in response to ET with a concomitant increase in ACC content in both cultivars. On the other hand, the expression of OeACS2 and OeACO2 in olive AZ was reduced by CoCl2 in the non-abscising cultivar, while CoCl2 had little or no effect on the abscising cultivar. In the latter, the repression of OeACS2 expression did not coincide with the changes of ACC content, perhaps because of post-transcriptional or translation regulation on ACS in olive fruit AZ as seen in other tissues of other species (Gomez-Jimenez and Matilla, 2006). The analysis of gene expression following application of ET biosynthesis inhibitors at the onset of fruit ripening demonstrated that the expression of OeERS1 is negatively regulated by ET at this stage in olive fruit AZ–AC tissue. However, a subset of receptor genes is induced by ET in ripening fruit (El-Sharkawy et al., 2007). On the other hand, ET appears to modulate EIN3/EIL at the protein level, whereas treatment with ACC results in the accumulation of EIN3 protein in Arabidopsis (Yanagisawa et al., 2003). In olive fruit AZ–AC, OeEIL2 is an ET-inducible gene, while no other information about AZ tissue of fruits is available.
Interactions between PA and ET-related gene expression
ET and PA pathways are considered to be competitive (Kumar et al., 1996; Pandey et al., 2000; Quin and Lan, 2004; Pang et al., 2006), but the interrelationship between PA and ET may vary with the species, type of tissue, and experimental system used (Wang et al., 1993; Gomez-Jimenez et al., 2001; Quinet et al., 2010). It has recently been shown that the free and soluble conjugated Put content increased in olive AZ during mature fruit abscission, while SAMDC activity was inhibited, thus providing strong evidence that SAMDC plays a key role in regulating PA levels (Gomez-Jimenez et al., 2010a), together with a increase in ACC content. Previous data have also demonstrated that PA levels are linked to a differential accumulation of specific gene transcripts that encompass a wide variety of cellular processes, such as ET biosynthesis (Gomez-Jimenez et al., 2001; Srivastava et al., 2007). In the present study, the gene expression data indicate a role for PA in modulating ET-related gene repression during mature fruit abscission, and thus higher levels of PA or lower levels of PA in the olive fruit AZ could shift the balance of ACC content towards mature fruit abscission. Exogenous PA, Put, and Spd at the onset of ripening did not affect abscission but rather altered ET biosynthesis gene expression in olive fruit AZ–AC. In contrast, the blocking of endogenous Spd and Spm contents with MGBG and CHA stimulated the olive mature fruit abscission and the expression of OeACS2 and OeEIL2 in both cultivars, suggesting that at this stage of development, these genes are under the negative control of Spd in the olive fruit AZ, and that there is a direct antagonism between PA and ET pathways in olive fruit AZ. In addition, MGBG-induced abscission was suppressed by combination with an inhibitor of ET biosynthesis (AOA), and the expression of ET-related genes was affected by both ET and ET inhibitors.
The present work suggests that the impact of PA treatment may vary depending on the cultivar. When the two cultivars were compared, only the OeACS2 transcription level was negatively regulated by Put treatment in PIC fruit AZ–AC, while no significant response to Put treatment was detected in ARB fruit AZ–AC. Only OeACO2 was negatively regulated by Spd treatment in ARB fruit AZ–AC but did not significantly respond to Spd treatment in PIC fruit AZ–AC. Similar results have been reported during tomato fruit ripening, when ACS gene expression showed a negative correlation with endogenous Put levels and ACO was negatively correlated with endogenous levels of Spd and Spm (Handa and Mattoo, 2010). These data therefore suggest that the two olive cultivars may differ in the ET biosynthesis regulation in fruit AZ–AC tissue. On the other hand, the gene transcript abundance that was negatively correlated with endogenous Put levels was found to be positively correlated with endogenous levels of Spd and Spm during tomato fruit ripening (Handa and Mattoo, 2010). Nevertheless, the genes such as OeACS2, down-regulated by Put in olive fruit AZ–AC, were not up-regulated by Spd, while OeACO2, down-regulated by Spd in olive fruit AZ–AC, was not up-regulated by Put.
ET plays a negative role in NO production in the olive fruit AZ during abscission
The present study is the first to report the presence and distribution of NO in AZ tissue during activation and abscission. It bears noting that higher NO production was detected in AZ–AC at the early stages of olive fruit development and then declined until 217 DPA in both cultivars. However, this decline was abrupt in the case of the abscising cultivar PIC in antagonizing ACC content in fruit AZ–AC and following the pattern of FDF. This is consistent with previous studies reporting that NO is formed mainly in actively growing tissue and that the levels decrease in mature and senescent organs (Leshem, 2001).
Synergistic and antagonistic actions between NO and ET have been reported, depending on the plant species, tissue types, developmental stage, and environmental stimuli (Leshem, 2001; Ederli et al., 2006; Rudell and Mattheis, 2006; Zhu et al., 2006; Palavan-Unsal and Arisan, 2009). NO production decreased in olive fruit AZ–AC treated with exogenous ET when mature fruit abscission was actively produced. Furthermore, in the present investigation, it was found that treatment with AOA, the inhibitor of ACS, slightly induced NO production by decreasing the ACC content in the AZ–AC of both cultivars, whereas treatment with the ACO inhibitor, CoCl2, sharply stimulated NO production only in AZ–AC of the abscising cultivar PIC at 14 d where the ACC content decreased. These results indicate that NO production is regulated by ET chiefly by suppressing ACS rather than ACO in ARB, the non-abscising cultivar. Meanwhile, the ET pathway regulation by NO through the suppression of ACO was found in the case of PIC, as reported during the ripening of tomato fruit (Eum et al., 2009). NO strongly induced the response of a gene that coded for alternative oxidase, and the latter in turn counteracted the cytochrome c-dependent respiration as well as ET biosynthesis (Ederli et al., 2006; Manjunatha et al., 2010). Also, NO may mediate the modification of the active ferrous site in ACO, thus leading to the inhibition of the ACO activity. In Arabidopsis, NO markedly reduces ET by down-regulating methionine adenosyl transferase1 (MAT1) activity through post-translational S-nitrosylation regulation, accounting for the reduction of the pool size of the chief ET precursor, SAM, thus affecting the overall turnover of ET biosynthesis (Lindermayr et al., 2006). However, in other species such as tobacco, tomato, and banana, NO alters ET production through changes in the expression levels of homologues of ACO or/and ACS genes (Ederli et al., 2006; Eum et al., 2009). On the basis of these findings, it is concluded that NO generation depends on ACS activity in both cultivars, whereas the inhibition of the ACO activity does not induce NO emission in either cultivar, and that the effect of ET on NO production appears to depend on the cultivar. NO production in ARB non-treated fruit AZ–AC was much higher than in PIC. A slight decline was detected in ARB, but a far sharper decrease was noted in PIC with the exogenous ET treatment. Taking these results together, it was found that endogenous NO and ACC maintain an inverse correlation in olive AZ–AC, suggesting an antagonistic action of NO and ET during mature fruit abscission, as demonstrated during fruit ripening (Leshem, 2001; Manjunatha et al., 2010). However, ET-induced abscission depressed NO production in olive fruit AZ tissues, whereas the abscission (FDF), ACC content, and expression of ET-related genes remained unaffected by the addition of an NO scavenger, cPTIO (data not shown). Treatment with 400 μM cPTIO caused an ∼30% fall in the relative levels of NO in olive AZ–AC (data not shown). This was mainly because of the difficulty of effectively treating the intact AZ with pharmacological reagents. Nevertheless, the differences found in the relative levels of NO between the two olive cultivars indicate that NO production is subject to variation between olive cultivars. Also, in this sense, it cannot be ruled out that NO plays, at least in part, a role in mature fruit abscission signalling and NO may act downstream of ET in the olive AZ.
Interaction between PA and NO in the olive AZ during mature fruit abscission
PA is related to NO through arginine, a common precursor in their biosinthetic pathways, in a similar way as in animals (Yamasaki and Cohen, 2006; Palavan-Unsal and Arisan, 2009). Previous reports present evidence that PA induces the production of NO (Tun et al., 2006; Groppa et al., 2008; Arasimowicz-Jelonek et al., 2009). In A. thaliana, Spd and Spm stimulate NO production whereas Put has little effect (Tun et al., 2006). In Araucaria angustifolia, Spd and Spm inhibited NO biosynthesis in both embryonic and suspensor cells, while Put induced NO biosynthesis in embryonic cells (Silveira et al., 2006). In the present study, treatment with CHA and MGBG, inhibitors of SPDS and SAMDC activity, respectively, significantly induced abscission of olive mature fruit by depressing FDF while concomitantly decreasing endogenously formed NO. In contrast, PA treatments caused no significant changes in FDF compared with those of the control, but induced NO production in olive fruit AZ. Additionally, NO varied depending on the nature of the PA applied and the cultivar treated. PA induced higher NO production in PIC compared with ARB, and the Spd treatment stimulated higher NO production in PIC compared with the Put treatment. These results demonstrate that PA has a regulatory effect on NO production during mature fruit abscission, suggesting the presence of an unknown enzyme that converts PA directly to NO and other products, in agreement with previous data reported (Tun et al., 2006; Yamasaki and Cohen, 2006; Arasimowicz-Jelonek et al., 2009). The way in which PA regulates NO-generating enzymes remains to be clarified. Thus, it is suggested that PA plays a positive role in NO production in olive fruit AZ–AC, indicating putatively PA-dependent NO biosynthesis as a potential link of NO and abscission.
NO is present in the epidermis cells and xylem of the olive fruit AZ
In the present work, NO has been localized in epidermis cells and the vascular tissue (xylem) of the proximal fracture plane of the mature fruit AZ, and NO is commonly found in such cells during fruit development (data not shown). As NO easily forms iron–nitrosyl complexes with haem iron, it is not surprising that NO affects the functioning of plant peroxidases participating in wall lignification, as revealed elsewhere (Ferrer and Ros Barcelo, 1999; Polverari et al., 2003). The present results show that the presence of NO in the xylem of the proximal fracture plane of the olive AZ could be indicative of the involvement of NO in xylem cell wall lignification and differentiation in this tissue. This is consistent with previous reports in stem sections of Zinnia elegans and pea seedlings (Gabaldon et al., 2005; Corpas et al., 2006). However, on the basis of the present data, it was not possible to conclude whether the cells with intense NO fluorescence produced their own NO, or whether it was transported from the neighbouring cells.
In conclusion, a relationship between ET and PA during mature fruit abscission was indicated in the olive AZ: (i) treatments with ET and inhibitors of PA biosynthesis were efficient means of inducing mature fruit abscission; (ii) a direct antagonism between ET and PA pathways acts in stimulating ET-related gene expression to modulate mature fruit abscission; (iii) the expression of the ET biosynthetic (OeACO2 and OeACS2) and signalling (OeEIL2) genes is related to mature fruit abscission, while the expression of OeACS2 and OeEIL2 is under the negative control of PA (Spd); (iv) MGBG-induced abscission was suppressed by an inhibitor of ET biosynthesis (AOA), and the expression of ET-related genes was affected both by ET and ET inhibitors; (v) the present study is the first to report the presence and distribution of NO in AZ tissue during abscission, and endogenous NO and ACC maintain an inverse correlation, suggesting an antagonistic action of NO and ET during mature fruit abscission; and (vi) PA plays a positive role in NO production, indicating putatively PA-dependent NO biosynthesis as a potential link of NO and abscission. Although further experiments are necessary to clarify the precise function of NO in abscission signalling, it is possible that NO inhibits the downstream abscission signalling cooperatively with unidentified signal component(s), including those under the regulation of ET and/or PA.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Phylogenetic analysis of OeACS and other ACS genes. The gene accession number is shown in parentheses for each gene. The phylogenetic tree was computed using the Clustal-W program (Thompson et al., 1994) employing standard parameters.
Figure S2. Phylogenetic analysis of OeACO and other ACO genes. The gene accession number is shown in parentheses for each gene. The phylogenetic tree was computed using the Clustal-W program (Thompson et al., 1994) employing standard parameters.
Figure S3. Phylogenetic analysis of OeERS and other ERS genes. The gene accession number is shown in parentheses for each gene. The phylogenetic tree was computed using the Clustal-W program (Thompson et al., 1994) employing standard parameters.
Figure S4. Phylogenetic analysis of OeCTR and other CTR genes. The gene accession number is shown in parentheses for each gene. The phylogenetic tree was computed using the Clustal-W program (Thompson et al., 1994) employing standard parameters.
Figure S5. Phylogenetic analysis of OeEIL and other EIL genes. The gene accession number is shown in parentheses for each gene. The phylogenetic tree was computed using the Clustal-W program (Thompson et al., 1994) employing standard parameters.
Figure S6. z-Animated 3-D reconstruction of CLSM detection of NO in the ARB fruit AZ at 217 DPA with DAF-FM-DA.
Figure S7. z-Animated 3-D reconstruction of CLSM detection of NO in the PIC fruit AZ at 217 DPA with DAF-FM-DA.
Acknowledgments
The authors thank Miguel A. Paredes for assistance with sample preparation and treatments, and Mercedes Gallardo for ACC/MACC data. They are grateful to Jose Luis Grosson for free access to the plant material. This research was supported by grants from the ‘Ministerio de Ciencia e Innovacion’, Spain (BFU2007-62566 and BFU2010-18116).
Glossary
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylic acid
- ACO
1-aminocyclopropane-1-carboxylic acid oxidase
- ACS
1-aminocyclopropane-1-carboxylic acid synthase
- AOA
aminooxyacetic acid
- ARB
Arbequina cultivar
- AZ
abscission zone
- AZ–AC
abscission zone and adjacent cells
- CHA
cyclohexylamine
- CoCl2
cobalt chloride
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF-FM-DA
4-amino-5-methylamino-2',7'-difluorofluorescein diacetate
- ET
ethylene
- FDF
fruit detachment force
- MACC
(malonyl)-ACC
- MGBG
methylglycoxalbis(guanylhydrazone)
- NO
nitric oxide
- PA
polyamine
- PIC
Picual cultivar
- Put
putrescine
- SAM
S-adenosyl-methionine
- SAMDC
SAM decarboxylase
- Spd
spermidine
References
- Agustí J, Merelo P, Cercós M, Tadeo FR, Talón M. Ethylene-induced differential gene expression during abscission of citrus leaves. Journal of Experimental Botany. 2008;59:2717–2733. doi: 10.1093/jxb/ern138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlfors R, Brosché M, Kollist H, Kangasjärvi J. Nitric oxide modulates ozone-induced cell death, hormone biosynthesis and gene expression in Arabidopsis thaliana. The Plant Journal. 2009;58:1–12. doi: 10.1111/j.1365-313X.2008.03756.x. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubis J. Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. Journal of Plant Growth Regulation. 2009;28:177–186. [Google Scholar]
- Banno K, Martin GC, Carlson RM. The role of phosphorus as an abscission-inducing agent for olive leaves and fruit. Journal of the American Society for Horticultural Science. 1993;118:599–604. [Google Scholar]
- Barranco D, Arquero O, Navarro C, Rapoport HF. Monopotassium phosphate for olive fruit abscission. Hortscience. 2004;39:1313–1314. [Google Scholar]
- Bartolini S, Cantini C, Vitagliano C. Olive fruit abscission: anatomical observations following application of ethylene-releasing compound. Acta Horticulturae. 1993;329:249–251. [Google Scholar]
- Brown KM. Ethylene and abscission. Physiologia Plantarum. 1997;100:567–576. [Google Scholar]
- Burns J, Ferguson L, Glozer K, Krueger WH, Rosecrance RC. Screening fruit loosening agents for black ripe processed table olives. Hortscience. 2008;43:1449–1453. [Google Scholar]
- Cai S, Lashbrook CC. Stamen abscission zone transcriptome profiling reveals new candidates for abscission control: enhanced retention of floral organs in transgenic plants overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiology. 2008;146:1305–1321. doi: 10.1104/pp.107.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SK, Larue CT, Chevalier D, Wang H, Jinn T-L, Zhang S, Walker JC. Regulation of floral organ abscission in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2008;105:15629–15634. doi: 10.1073/pnas.0805539105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Roy S, Saha PP, Singh SK, Sengupta DN. Characterization of differential ripening pattern in association with ethylene biosynthesis in the fruits of five naturally occurring banana cultivars and detection of a GCC-box-specific DNA-binding protein. Plant Cell Reports. 2008;27:1235–1249. doi: 10.1007/s00299-008-0547-4. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Rio LA. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta. 2006;224:246–254. doi: 10.1007/s00425-005-0205-9. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde MN, Lanteri ML, García-Mata C, ten HA, Laxalt AM, Graziano M, Lamattina L. Nitric oxide functions as intermediate in auxin, abscisic acid and lipid signaling pathways. In: Lamattina L, Polacco JC, editors. Nitric oxide in plant growth, development and stress physiology. Series: Plant Cell Monographs. Vol. 6. Berlin: Springer; 2007. pp. 113–130. [Google Scholar]
- Dal Cin V, Danesin M, Boschetti A, Dorigoni A, Ramina A. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borck) Journal of Experimental Botany. 2005;56:2995–3005. doi: 10.1093/jxb/eri296. [DOI] [PubMed] [Google Scholar]
- Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, Ferranti F, Tosti N, Pasqualini S. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiology. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy I, Kim WS, El-Kereamy A, Jayasankar S, Svircev AM, Brown DCW. Isolation and characterization of four ethylene signal transduction elements in plums (Prunus salicina L.) Journal of Experimental Botany. 2007;58:3631–3643. doi: 10.1093/jxb/erm213. [DOI] [PubMed] [Google Scholar]
- Eum HL, Kim HB, Choi SB, Lee SK. Regulation of ethylene biosynthesis by nitric oxide in tomato (Solanum lycopersicum L.) fruit harvested at different ripening stages. European Food Research and Technology. 2009;228:331–338. [Google Scholar]
- Ferrer MA, Ros Barcelo A. Differential effects of nitric oxide on peroxidase and H2O2 production by the xylem of Zinnia elegans. Plant, Cell and Environment. 1999;22:891–897. [Google Scholar]
- Gabaldon C, Gomez Ros LV, Pedreño MA, Ros Barcelo A. Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytologist. 2005;165:121–130. doi: 10.1111/j.1469-8137.2004.01230.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Jimenez MC, Matilla AJ. Cloning of a cDNA-encoding ACC synthase and its mRNA expression during zygotic embryogenesis of chick-pea (Cicer arietinum L.) seeds. Plant Growth Regulation. 2006;50:101–110. [Google Scholar]
- Gomez-Jimenez MC, Paredes MA, Gallardo M, Fernandez-Garcia N, Olmos E, Sanchez-Calle IM. Tissue-specific expression of olive S-adenosyl methionine decarboxylase and spermidine synthase genes and polyamine metabolism during flower opening and early fruit development. Planta. 2010b;232:629–647. doi: 10.1007/s00425-010-1198-6. [DOI] [PubMed] [Google Scholar]
- Gomez-Jimenez MC, Paredes MA, Gallardo M, Sanchez-Calle IM. Mature fruit abscission is associated with up-regulation of polyamine metabolism in the olive abscission zone. Journal of Plant Physiology. 2010a;167:1432–1441. doi: 10.1016/j.jplph.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Gomez-Jimenez MD, García-Olivares E, Matilla AJ. 1-Aminocyclopropane-1-carboxylate oxidase from embryonic axes of germinating chick-pea (Cicer arietinum L.) seeds: cellular immunolocalization and alterations in its expression by indole-3-acetic acid, abscisic acid and spermine. Seed Science Research. 2001;11:243–253. [Google Scholar]
- Goren R, Huberman M, Martin GC. Phosphorus-induced leaf abscission in detached shoots of olive and citrus. Journal of the American Society for Horticultural Science. 1998;123:545–549. [Google Scholar]
- Groppa MD, Rosales EP, Iannone MF, Benavides MP. Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochemistry. 2008;69:2609–2615. doi: 10.1016/j.phytochem.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Handa AK, Mattoo AK. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiology and Biochemistry. 2010;48:540–546. doi: 10.1016/j.plaphy.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Kumar A, Taylor MA, Mad Arif SA, Davies H. Potato plants expressing antisense and sense S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. The Plant Journal. 1996;9:147–158. [Google Scholar]
- Leshem YY. Nitric oxide in plants. 2001. Kluwer Academic, London Publishers. [Google Scholar]
- Lewis MW, Leslie ME, Liljegren SJ. Plant separation: 50 ways to leave your mother. Current Opinion in Plant Biology. 2006;9:59–65. doi: 10.1016/j.pbi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Li J, Yuan R. NAA and ethylene regulate expression of genes related to ethylene biosynthesis, perception, and cell wall degradation during fruit abscission and ripening in ‘Delicious’ apples. Journal of Plant Growth Regulation. 2008;27:283–295. [Google Scholar]
- Lindermayr C, Saalbach G, Bahnweg G, Durner J. Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. Journal of Biological Chemistry. 2006;281:4285–4291. doi: 10.1074/jbc.M511635200. [DOI] [PubMed] [Google Scholar]
- Manjunatha G, Lokesh V, Neelwarne B. Nitric oxide in fruit ripening: trends and opportunities. Biotechnology Advances. 2010;28:489–499. doi: 10.1016/j.biotechadv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Martin-Remesal C, Gomez-Jimenez MC, Matilla AJ. ACC-N-Malonyltransferase activity during zygotic embryogenesis and germination of chick-pea seeds. Seed Science Research. 2000;10:71–76. [Google Scholar]
- Mbéguié-A-Mbéguié D, Hubert O, Fils-Lycaon B, Chillet M, Baurens FC. EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande Naine) Physiologia Plantarum. 2008;133:435–448. doi: 10.1111/j.1399-3054.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- Meir S, Philosoph-Hadas S, Sundaresan S, Selvaraj KSV, Burd S, Ophir R, Kochanek B, Reid MS, Jiang CZ, Lers A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiology. 2010;154:1929–1956. doi: 10.1104/pp.110.160697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano R, Ogura E, Kubo Y, Inaba A. Ethylene biosynthesis in detached young persimmon fruit is initiated in calyx and modulated by water loss from the fruit. Plant Physiology. 2003;131:276–286. doi: 10.1104/pp.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne DJ, McManus MT. Hormones, signals and target cells in plant development. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the índole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiology. 2004;135:279–286. doi: 10.1104/pp.103.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palavan-Unsal N, Arisan D. Nitric oxide signalling in plants. Botanical Review. 2009;75:203–229. [Google Scholar]
- Pandey S, Ranade SA, Nagar PK, Kumar N. Role of polyamines and ethylene as modulators of plant senescence. Journal of Biosciences. 2000;25:291–299. doi: 10.1007/BF02703938. [DOI] [PubMed] [Google Scholar]
- Pang XM, Nada K, Liu JH, Kitashiba H, Honda C, Yamashita H, Tatsuki M, Moriguchi T. Interrelationship between polyamine and ethylene in 1-methylcyclopropene treated apple fruits after harvest. Physiologia Plantarum. 2006;128:351–359. [Google Scholar]
- Patterson SE. Cutting loose, abscission and dehiscence in Arabidopsis. Plant Physiology. 2001;126:494–500. doi: 10.1104/pp.126.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SE, Bleecker AB. Ethylene-dependent and -independent process associated with floral organ abscission in Arabidopsis. Plant Physiology. 2004;134:194–203. doi: 10.1104/pp.103.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech JC, Bouzayen M, Latché A. Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Science. 2008;175:114–120. [Google Scholar]
- Perin C, Gomez-Jimenez MC, Hagen L, Dogimont C, Pech JC, Latche A, Pitrat M, Lelievre JM. Molecular and genetic characterization of a non-climacteric phenotype in melon reveals two loci conferring altered ethylene response in fruit. Plant Physiology. 2002;129:300–309. doi: 10.1104/pp.010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverari A, Molesini B, Pezzotti M, Buonaurio R, Marte M, Delledonne M. Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Molecular Plant-Microbe Interactions. 2003;16:1094–1105. doi: 10.1094/MPMI.2003.16.12.1094. [DOI] [PubMed] [Google Scholar]
- Quin WM, Lan WZ. Fungal elicitor-induced cell death in Taxus chinensis suspension cells is mediated by ethylene and polyamines. Plant Science. 2004;166:989–995. [Google Scholar]
- Quinet M, Ndayiragije A, Lefèvre I, Lambillotte B, Dupont-Gillain CC, Lutts S. Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. Journal of Experimental Botany. 2010;61:2719–2733. doi: 10.1093/jxb/erq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasori A, Ruperti B, Bonghi C, Tonutti P, Ramina A. Characterization of two putative ethylene receptor genes expressed during peach fruit development and abscission. Journal of Experimental Botany. 2002;53:2333–2339. doi: 10.1093/jxb/erf097. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Elliott KA, Gonzalez-Carranza ZH. Abscission, dehiscence, and other cell separation processes. Annual Review of Plant Biology. 2002;53:131–158. doi: 10.1146/annurev.arplant.53.092701.180236. [DOI] [PubMed] [Google Scholar]
- Rudell DR, Mattheis JP. Nitric oxide and nitrite treatments reduce ethylene evolution from apple fruit disks. HortScience. 2006;41:1462–1465. [Google Scholar]
- Ruperti B, Cattivelli L, Pagni S, Ramina A. Ethylene responsive genes are differentially regulated during abscission, organ senescence and wounding in peach (Prunus persica) Journal of Experimental Botany. 2002;53:429–437. doi: 10.1093/jexbot/53.368.429. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Munemura I, Tomita R, Kobayashi K. Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. The Plant Journal. 2008;56:13–27. doi: 10.1111/j.1365-313X.2008.03577.x. [DOI] [PubMed] [Google Scholar]
- Silveira V, Santa-Catarina C, Tun NN, Scherer FE, Handro W, Guerra MP, Floh IS. Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araukaria angustifolia (Bert.) O. Ktze. Plant Science. 2006;171:91–98. [Google Scholar]
- Srivastava A, Chung SH, Fatima T, Datsenka T, Handa AK, Mattoo AK. Polyamines as anabolic growth regulators revealed by transcriptome analysis and metabolite profiles of tomato fruits engineered to accumulate spermidine and spermine. Plant Biotechnology. 2007;24:57–70. [Google Scholar]
- Tacken E, Ireland H, Gunaseelan K, et al. The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiology. 2010;153:294–305. doi: 10.1104/pp.109.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Higgins D, Gibson T. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh IS, Scherer FE. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant and Cell Physiology. 2006;47:346–354. doi: 10.1093/pcp/pci252. [DOI] [PubMed] [Google Scholar]
- Wang CY, Conway WS, Abbott JA, Kramer GF, Sams CE. Postharvest infiltration of polyamines and calcium influences ethylene production and texture changes in ‘Golden Delicious’ apples. Journal of the American Society for Horticultural Science. 1993;118:801–806. [Google Scholar]
- Wang P, Zhang B, Li X, Xu C, Yin X, Shan L, Ferguson I, Chen K. Ethylene signal transduction elements involved in chilling injury in non-climacteric loquat fruit. Journal of Experimental Botany. 2010;61:179–190. doi: 10.1093/jxb/erp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Cohen MF. NO signal at the crossroads: polyamine-induced nitric oxide synthesis in plants? Trends in Plant Science. 2006;11:522–524. doi: 10.1016/j.tplants.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature. 2003;425:521–525. doi: 10.1038/nature01984. [DOI] [PubMed] [Google Scholar]
- Yin X, Allan AC, Chen KS, Ferguson IB. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiology. 2010;153:1280–1292. doi: 10.1104/pp.110.157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XR, Chen KS, Allan AC, Wu RM, Zhang B, Lallu N, Ferguson IB. Ethylene-induced modulation of genes associated with the ethylene signaling pathway in ripening kiwifruit. Journal of Experimental Botany. 2008;59:2097–2108. doi: 10.1093/jxb/ern067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Carbaugh DH. Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’ and ‘Golden Delicious’ apples. HortScience. 2007;42:101–105. [Google Scholar]
- Yuan R, Wu Z, Kostenyuk IA, Burns JK. G-protein-coupled α2A-adrenoreceptor agonists differentially alter citrus leaf and fruit abscission by affecting expression of ACC synthase and ACC oxidase. Journal of Experimental Botany. 2005;56:1867–1875. doi: 10.1093/jxb/eri176. [DOI] [PubMed] [Google Scholar]
- Zhu S, Liu M, Zhou J. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biology and Technology. 2006;42:41–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.