Abstract
The effect of mechanical stress on the root apical meristem (RAM) organization of Zea mays was investigated. In the experiment performed, root apices were grown through a narrowing of either circular (variant I) or elliptical (variant II) shape. This caused a mechanical impedance distributed circumferentially or from the opposite sides in variant I and II, respectively. The maximal force exerted by the growing root in response to the impedance reached the value of 0.15 N for variant I and 0.08 N for variant II. Significant morphological and anatomical changes were observed. The changes in morphology depended on the variant and concerned diminishing and/or deformation of the cross-section of the root apex, and buckling and swelling of the root. Anatomical changes, similar in both variants, concerned transformation of the meristem from closed to open, an increase in the number of the cell layers at the pole of the root proper, and atypical oblique divisions of the root cap cells. After leaving the narrowing, a return to both typical cellular organization and morphology of the apex was observed. The results are discussed in terms of three aspects: the morphological response, the RAM reorganization, and mechanical factors. Assuming that the orientation of division walls is affected by directional cues of a tensor nature, the changes mentioned may indicate that a pattern of such cues is modified when the root apex passes through the narrowing, but its primary mode is finally restored.
Keywords: Mechanical stress, root apical meristem organization, tiers of initials, Zea mays
Introduction
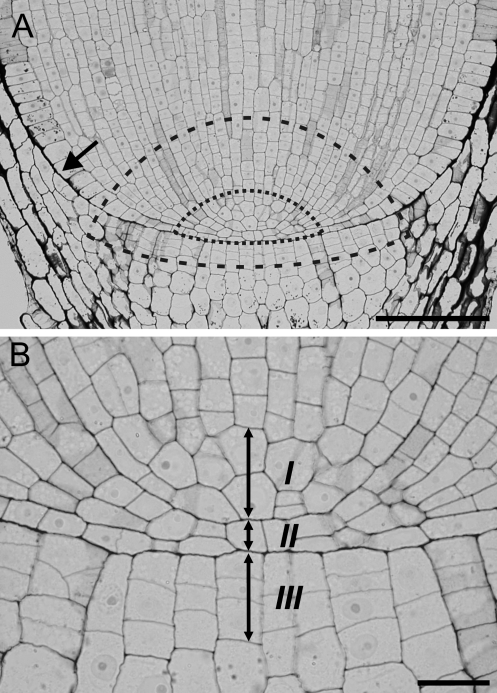
The root apex, which consists of the root proper and the root cap, is an organ responsible for growth and development of the underground part of the plant, but its functioning is crucial to the whole plant body. It comprises the root apical meristem (RAM), where cells grow and divide giving rise to all tissues of the apex (Cutter, 1971). Two types of RAM organization can be distinguished— closed and open (von Guttenberg, 1960). In the closed organization there is a clear root–cap boundary and the cell files can be traced to a few cells at the tip; such a situation occurs in Zea mays (Fig. 1A), which is the subject of the current study. In RAMs with an open organization there is not a sharp boundary between the root cap and the root proper (Clowes, 1976; Jiang and Feldman, 2005). The very central region of the RAM of seed plants is the so-called quiescent centre (QC; Clowes, 1961)—a zone of slowly cycling structural initials (Barlow, 1997). On its proximal face the QC neighbours functional initials (Barlow, 1997; Jiang and Feldman, 2005) from which the cells of the root proper are directly derived (Fig. 1A). According to the description of von Guttenberg (1960), in root apices with a closed organization the most distal cell layers of the QC and adjoining initials of the root cap form the tiers of initial cells. Each tier is specialized in forming particular tissues of the root. For example, stele usually originates from a separate tier while epidermis has a common origin with the cortex or lateral root cap in monocots and dicots, respectively (Rost, 1994). The number of tiers depends on the plant species; in maize there are three such tiers (Fig. 1B). The upper tier (I), formed by a group of cells at the pole of the stele, gives rise to cells of the vascular cylinder, the middle tier (II) consisting of a single cell layer (Barlow and Rathfelder, 1984) gives rise to cells of the cortex and epidermis, and eventually the lower tier (III) comprising up to four cell layers (Clowes, 1980) gives rise to cells of the root cap.
Fig. 1.
Median longitudinal section through a Zea root apex. (A) The root proper–cap boundary (arrow) indicates a closed organization of the root meristem; the approximate regions of functional (dashed line) and structural initials (dotted line) are marked (after Jiang and Feldman, 2005). (B) Magnification of the root pole region, showing tiers of initials (arrows) I, II, and III (after von Guttenberg, 1960). Scale bars: 100 μm (A), 20 μm (B).
The size and shape of the RAM are different in various species (Luxová, 1975; Rost and Baum, 1988). Although the cell arrangement of the mature root apex growing in more or less stable conditions usually remains relatively constant, the RAM organization may undergo natural changes during its lifetime (Seago and Heimsch, 1969; Armstrong and Heimsch, 1976; Clowes and Wadekar, 1989; Baum et al., 2002; Chapman et al., 2003). Steadily growing root apices exposed to physical stimuli (e.g. low temperature, electric field, or X-rays) as well as chemical stimuli [heavy metal treatment, inhibitors of gibberellin biosynthesis, and 2,3,5-triiodobenzoic acid (TIBA) and 1-N-naphthylphthalamic acid (NPA) application] may also show RAM rearrangement (Clowes, 1963; Barlow, 1992; Kerk and Feldman, 1994; Jiang et al., 2003; Kozhevnikova et al., 2007; Wawrecki and Zagórska-Marek, 2007).
The root apices, like other plant organs, grow simplastically (Erickson, 1986); that is, in a continuous and coordinated way. Such growth is of a tensor nature (Silk and Erickson, 1979; Hejnowicz and Romberger, 1984), which is manifested in the property that the field of growth rates of the organ is of the tensor type and, at every point, unless growth is isotropic, three mutually orthogonal principal directions of growth (PDGs) can be recognized. These PDGs are postulated (Hejnowicz, 1989) to affect the orientation of cell divisions. Computer simulations have shown (Nakielski, 2008) that both tensor field of growth rates and the PDGs are needed to control cell growth and cell division at the organ level. Only under such control are new cell walls formed perpendicularly to the PDGs and the cell pattern of the virtual root apex is self-perpetuating, otherwise the pattern changes during development. In this approach any instability in the cell pattern may be considered as resulting from the growth disturbance at the tensor level. A mechanical stress seems to be the most natural factor that may cause such a disturbance.
In natural conditions a growing root needs to push through soil particles, often experiencing mechanical impedance from different directions. In experiments in which such conditions are simulated, various effects have been observed: an increase in the root diameter associated with a change in the sizes of the cortex cells and an increase in the number and diameter of the vessels (Wilson et al., 1977; Bennie, 1996), a decrease in root elongation and/or changes in root osmoregulation (Materechera et al., 1991; Bennie, 1996; Clark et al., 1996, 2001), and enhanced border cell production (Iijima et al., 2000, 2003).
However, little is known about the RAM response to mechanical treatment. In the studies carried out to date on mechanically treated apices the RAM architecture has not been described. As this region plays a crucial role in the functioning of the whole root it seems important to learn the possible influence of mechanical stress on it. The aim of the present research is to determine whether mechanical stimuli affect the root apex organization in Zea. The experiment involved maize root apices being subjected to grow through a narrow gap which caused mechanical impedance. Depending on the shape of the narrowing, both symmetrical and asymmetrical stress distribution within the apex was generated. In both cases a significant deformation of the root apex and a change in the meristem organization from closed to open have been observed. The results are discussed in relation to a possible mechanical stress distribution in the root apex.
Materials and methods
Plant material
Seeds of maize (Z. mays L. cv. Złota Karłowa) were soaked overnight and germinated in rolls of moist filter paper in darkness for 2d. Only the seedlings with straight roots 15–20 mm long were selected for the experiments.
Experiments
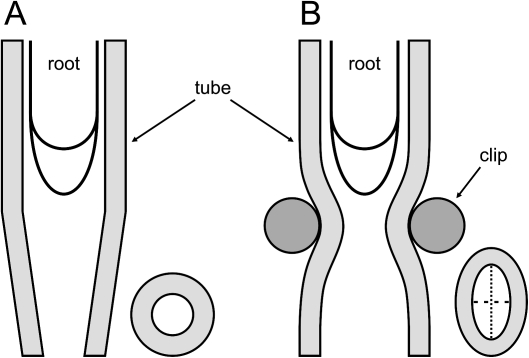
Two variants of the experiment were performed. Variant I involved a conical plastic tube. A root was introduced into a plastic tube with a conical end. The upper part of the tube was a cylinder 35 mm long of internal diameter 1.2 mm, while the length of the conical end was 4.4 mm and its internal diameter at the tip was 0.42±0.01 mm (Fig. 2A). Variant II used a cylindrical igelite tube plus a clip. A root was introduced into an igelite cylindrical tube with an internal diameter of 2 mm. The tube was clipped, which caused a local narrowing, so the cross-sectional shape of the tube changed to elliptical (Fig. 2B). The major diameter of the ellipse indicated the major axial plane of the tube, while the minor diameter of the ellipse indicated the minor axial plane of the tube (see cross-section of the tube in Fig. 2B). In order to avoid complete closure of the tube, a piece of glass ∼2 mm thick was placed between the clip side parts, which made the tube minor internal diameter 0.41±0.06 mm wide. In both variants the tubes were then filled with water and pinned vertically to a polystyrene board placed in a closed, humid chamber. The lower edge of the board stood in the water, but the roots grew above the water surface. The chamber was covered with a glass plate and the culture was kept for 24 h in darkness, at 25 °C and 85% relative humidity. In both variants the internal diameter of the cylindrical parts of the tubes allowed free and straight growth of the root apex. However, when the root apex reached the narrow zone caused by the smaller diameter of the tube (variant I), or by the clip (variant II), it encountered mechanical impedance, because the diameter of the apex was larger than the diameter of the narrowing. The root tip response was a force exerted back to the obstacle. After 24 h of treatment the experiment was ended and all the apices were photographed in their current positions within or outside the tubes. Roots growing in cylindrical tubes without narrowing served as controls.
Fig. 2.
Diagrams of the experimental set-up in which the root apex is growing. (A) A tube with a conical end (variant I) and (B) a locally clipped cylindrical tube (variant II). The right bottom corners show cross-sections of the tubes at their narrowest part; in (B) the major (dotted line) and minor (dashed line) axial planes of the tube are indicated.
Measurement of the force exerted by the root during passing through the narrowing
Material Testing Machine Synergie 100 (MTS Systems Corporation), with a force sensor range of ±10 N and accuracy of load measurement ±0.5% was applied to measure the force of the growing root tip. Measurements were taken in a dark room at 25 °C. The tube with the sample was placed vertically between supporting grips; the upper grip was joined to the force sensor of the machine. Because of the specific conditions of each variant of the experiment water was supplied in two different manners. In variant I the tube ending with the apex was placed in a small water container which might have influenced the unstable experiment conditions in the very initial phase: casual factors, such as water movement, etc., might have not been excluded. So although the apex grew freely a slight increase in the force was observed. After this initial phase the force attained ∼0.10 N, determining the reference value for the measurement in variant I. In variant II, water was delivered to the apex through a plastic tube and this method did not disturb the stability of the conditions. So the reference value for the measurement in variant II was 0 N. The force of the growing root apex exerted on the narrowing was registered by the force sensor continuously for ∼1400 min from the time when the root apex grew freely above the narrowing until the moment when it grew freely again below the narrowing.
Ten measurements of the force were performed for variant I, and 17 for variant II. In order to compare the result of the force measurement with data from the literature for both variants, the mechanical stress of the root tips was calculated as a maximal force divided by the root cross-sectional area (circular in variant I and elliptical in variant II) at ∼500 μm from the root tip passing through the narrowing. Data analysis was performed by the use of the TestWorks 4 Software and Microsoft Excel.
Light microscopy
At the end of the experiment the root tips were excised and fixed in 2.5% glutaraldehyde in 0.05 M sodium phosphate buffer (pH 7.0) for 24 h, washed three times in buffer, dehydrated through an acetone series and propylene oxide, and then embedded in Epon. The samples were sectioned into longitudinal sections (2.5 μm thick) and cross-sections (3.75 μm thick) using a Tesla BS 490A ultramicrotome. In variant II, longitudinal sections were cut in a plane parallel to the minor diameter of the ellipse (see Fig. 2B). The sections were stained by the periodic acid–Schiff (PAS) reaction and counterstained in toluidine blue (O'Brien and McCully, 1981). Specimens were viewed and photographed using an Olympus BX41 light microscope equipped with a CAMEDIA C-3040ZOOM camera. The arrangements of cells in the initial tiers within the root apex meristem (see Fig. 1B) were analysed.
In some of the untreated root apices as well as in those of variant II, root caps were removed in order to observe the cell arrangement at the root pole. In apices of variant I such surgery appeared impossible; any trial resulted in damage to the apex. Apical fragments (1 mm thick) of the root proper of the cap-less apices were excised, and the samples were stained by the PAS reaction and observed under UV light.
Results
Morphology and mechanical stress
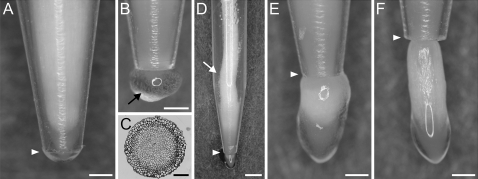
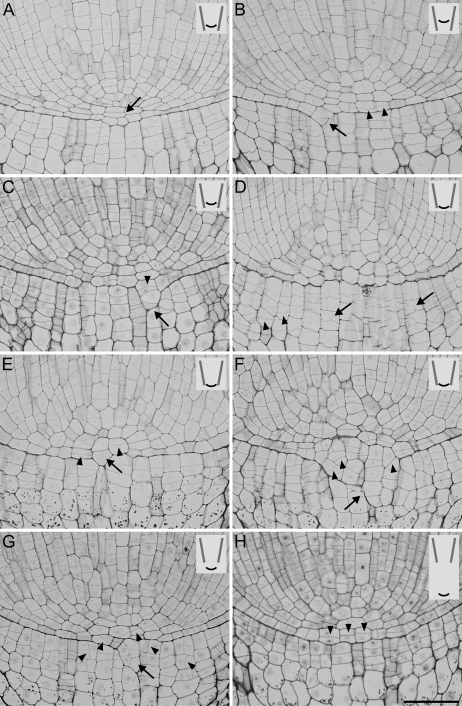
In Fig. 3 roots at different stages of passing through the narrowing in variant I are shown. Before reaching the conical tube ending the roots grow freely within the tube, and subsequently they adjust their geometry to the shape of the conical ending. The apices that have not emerged from the tube are tightly pressed against the interior of the tube (Fig. 3A). Abundant mucilage and released peripheral root cap cells are present on the tube's wall (not shown), as well as on the outside of the tube ending (Fig. 3B). Figure 3C shows the cross-section of the root apex passing through the narrowest part taken at the root–cap boundary level. The shape of the section has not changed; however, the size is smaller in comparison with the control roots. When the root tip reaches the narrowest part, a slight root buckling appears directly above the narrowing, and the buckling becomes stronger when the root tip leaves the tube ending (Fig. 3D). Shortly after leaving the tube (Fig. 3E), the root apex usually changes its shape dramatically, taking on the form of a bulb. A bulb's diameter at its widest part is about the same as the diameter of a control root apex (600 μm on average). Mucilage exudation is still very strong. When the root apex has grown far from the tube ending (Fig. 3F) the root geometry becomes more or less typical again. The only visible change occurs in the part within the tube where a root is still compressed. The mucilage secreted by the roots is not as abundant as previously.
Fig. 3.
Morphology of the root apices in various stages of passing through the narrow zone in variant I. (A, B) Root apex in the tube, (B, arrow) mucilage and loose cap cells outside the tube, (C) cross-section of the treated apex at the root–cap boundary level, (D) buckling of the root body (arrow), (E) bulb-shaped root apex after leaving the tube, (F) root apex far from the tube ending slowly returning to its typical morphology. White arrowheads in A and D–F indicate the tube end. Scale bars: 0.5 mm (A, B, E, F), 0.1 mm (C), 1 mm (D).
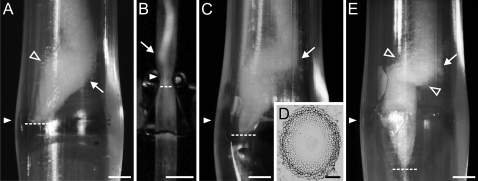
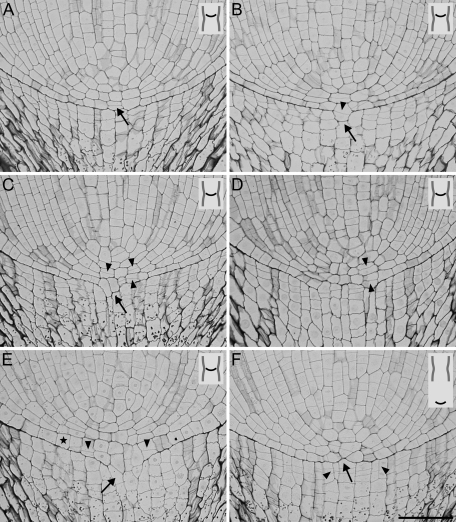
In Fig. 4 the results of variant II of the experiment are shown. Before reaching the narrowing the roots have grown vertically without morphological changes (not shown). When a root tip reaches the narrow zone in a major axial plane of the tube, a root buckling appears above the narrowing and the root diameter increases in the region behind the apex (Fig. 4A). The buckling grows stronger and the root tip begins to move through the narrowest part (Fig. 4B, C), usually near the ending of the major diameter of the ellipse. The cross-section taken at the root–cap boundary level from the root passing through the narrowest part (Fig. 4D) shows how the root apex has adjusted its shape to the elliptical narrowing. Root tips that have passed through the narrow zone regain their typical geometry; however, proximal parts of the roots are still deformed: a flattened region in a place where the root has been compressed as well as the buckling are still visible (Fig. 4E). As in variant I strong mucilage exudation occurs (not shown). Root hairs develop in a shorter distance in comparison with the control. In Fig. 4A and E they resemble cotton fluff forming ∼1.5–2 mm above the root tip.
Fig. 4.
Morphology of the root apices in various stages of passing through the tube in variant II. (A) The root apex has reached the narrowest part; swelling is visible behind the tip. (B, C) The root apex pushing through the narrowest part and (E) after leaving the narrowing. (D) Cross-section through the treated apex at the root–cap boundary level. In all the stages the root undergoes strong buckling (arrows); the view is in the plane of the major (A, C, E) and minor (B) axial plane of the tube. White arrowheads (in A–C and E) indicate the clipped region, dashed lines indicate the position of the root tip, and empty arrowheads (A, E) indicate root hairs formed right above the RAM. Scale bars: 1 mm (A, C, E), 5 mm (B), 0.1 mm (D).
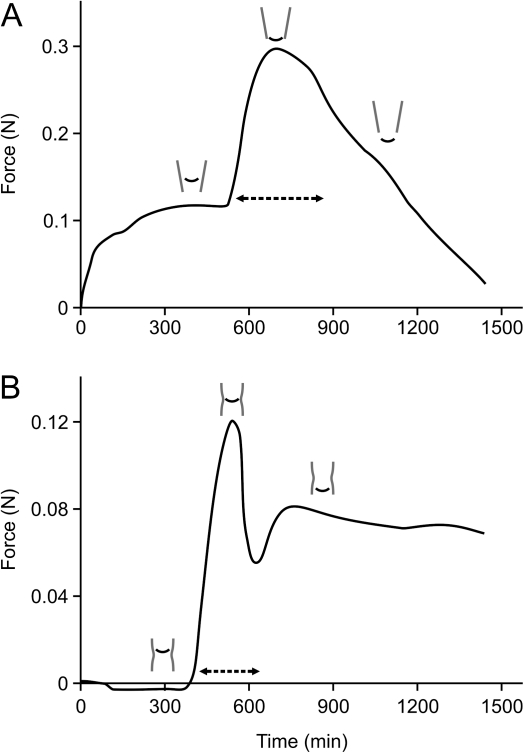
Figure 5 shows a typical time dependence of force exerted by an individual root during passage through the narrow zone. In variant I (Fig. 5A) the apex initially (0–500 min) grows freely within the tube and the observed slight increase in the force leads to stabilization of the experiment (see the Materials and methods). Then the force rapidly increases (500–700 min) to the maximal value of 0.30 N (0.20 N with respect to the reference value for variant I) corresponding to the time when the root tip is passing through the narrowest part of the tube. In the last period (700–1400 min), which refers to the time when the root tip has left the narrowing, the force decreases more or less linearly. In variant II (Fig. 5B) the apex initially also grows freely and does not exert any force (0–350 min). When the root tip meets the narrowing, the force rapidly increases, attaining its maximal value of 0.12 N at 550 min of the measurement. Then a sharp decrease is observed (550–600 min), followed by a short increase (600–700 min), and the force value stabilizes at ∼0.07 N. In both variants the period of passing through the narrowing is rather short; in variant I it takes on average 400 min, while in variant II it takes on average 300 min (see dashed lines in Fig. 5A and B).
Fig. 5.
Root force as a function of time for an individual root in variant I (A) and variant II (B) of the experiment. The time of passing through the narrowing (dashed lines) and the root tip position with reference to the chosen times of the measurement (small schemes above the curves) are shown.
The maximal force exerted by the roots on the narrowing depends on either the individual root or the variant of the experiment. For variant I it ranges from 0.06 N to 0.26 N, with a mean value of 0.15 N (±0.07 N), and for variant II it ranges from 0.04 N to 0.13 N, with a mean value of 0.08 N (±0.03 N). The calculated mechanical stress is 0.65 MPa for variant I and 0.29 MPa for variant II.
The meristem organization
In all treated root apices, a disturbed cell pattern was observed, yet only a few types of changes in the meristem organization can be distinguished. The changes occur in both variants of the experiment as well as in different stages of pushing through the narrowing. The most significant changes in the cell pattern appear within the middle and lower tiers of initials; however, the changes seem to be unique for every sample. In Figs 6 and 7, axial sections of the root apices subjected to mechanical stress in variant I and II of the experiment are shown, respectively.
Fig. 6.
Cell pattern at the pole of root apices in variant I seen in median longitudinal sections; the position of the root apex in the tube is shown in the small insets in the upper right corners of the photographs. (A) Three cell layers (arrow) formed at the pole of the root between the vascular cylinder and the cap. (B, C) Meristem opening starts (arrows) by breaking the root–cap boundary, with periclinal divisions (arrowheads) finally leading to cell expansion towards the cap. (D) Atypical longitudinal (arrows) and oblique (arrowheads) cell divisions in columella and the irregular line of the root-cap junction can be seen. (E) Meristem opening due to strong growth of the cell at the pole on the cap side (arrow), with atypical periclinal divisions (arrowheads) in the neighbouring cells. (F) A greatly disturbed root–cap boundary (arrow), with oblique divisions (arrowheads) in the cells protruding on the cap side. (G) The meristem organization is less disturbed, with cells growing through the root–cap boundary on the cap side (arrow), and atypical oblique and longitudinal divisions (arrowheads) both in the root proper and in the cap. (H) Closed meristem, with atypical periclinal divisions (arrowheads) in the middle tier. Scale bar: 50 μm (A–H).
Fig. 7.
Cell pattern at the pole of root apices in variant II seen in median longitudinal sections; the position of the root apex in the tube is shown in the small insets in the upper right corners of the photographs. (A) Atypical periclinal cell division (arrow) in the middle tier. (B, C) The root–cap boundary is broken (arrows) and the cells of the middle tier grow into the cap. (D) Open meristem; the root–cap boundary is difficult to determine. (E) Meristem opening (arrow); the large anticlinal dimension of epidermis cells (asterisk) and periclinal divisions (B–E, arrowheads) in the middle tier can be seen. (F) Closed meristem with a disturbed root–cap boundary and cell arrangement at the pole of the root proper (arrow), with longitudinal cell divisions in the columella (arrowheads). Scale bar: 50 μm (A–F).
A group of apices shows a closed meristem (Figs 6A, D, H, 7A, F); however, compared with controls a significant rearrangement of the cell pattern is observed. For example, in apices in Figs 6A, H, 7A resulting from periclinal divisions, two or three cell layers appear between the tip of the vascular cylinder and the root cap (in the middle tier of initials), instead of one typically observed in control roots (see Fig. 1B). In these apices the root–cap boundary remains smooth and clear (Figs 6H, 7A) or it becomes irregular (Figs 6D, 7F); sometimes an intrusion of the middle tier cells into the root cap causes stronger deformation of the boundary (Fig. 6A).
In some apices the meristem has opened (Figs 6B, C, E–G, 7B–E), and this happens in any stage of the root passing through the narrow zone and attains different degrees. The opening starts through the axial elongation of two neighbouring undivided cells of the middle tier (Fig. 7C) or the cells divided periclinally (Figs 6C, E, 7B) and their growth continued into the cap side. In roots with clearly open meristems these cells seem to form continuous files together with columella cells (Figs 6B, G, 7D, E). Some of the open meristems, as in the one shown in Fig. 6F, represent cell patterns disturbed to such a degree that it is impossible to distinguish the boundary of the root stele clearly. Moreover, in such apices a group of cells intruding into the cap side undergo oblique divisions. In the group of roots with open meristems (Figs 6B, C, 7C–E) periclinal divisions in the middle tier of initials usually take place in the lateral part of the pole of the root proper, while in closed meristems they most often happen in the central part (Figs 6A, H, 7A).
Another type of change observed in the treated apices concerns the cells of the lower tier; that is, root cap initials. In these cells, longitudinal, atypical divisions are observed leading to a larger number of the cell files in the root cap (Figs 6D, G, 7F). Sometimes the cells of the lateral parts of the root cap undergo unusual oblique divisions (Fig. 6D). Both longitudinal and oblique divisions occur in apices with either an open or closed meristem.
Cell arrangement on the surface of the root pole
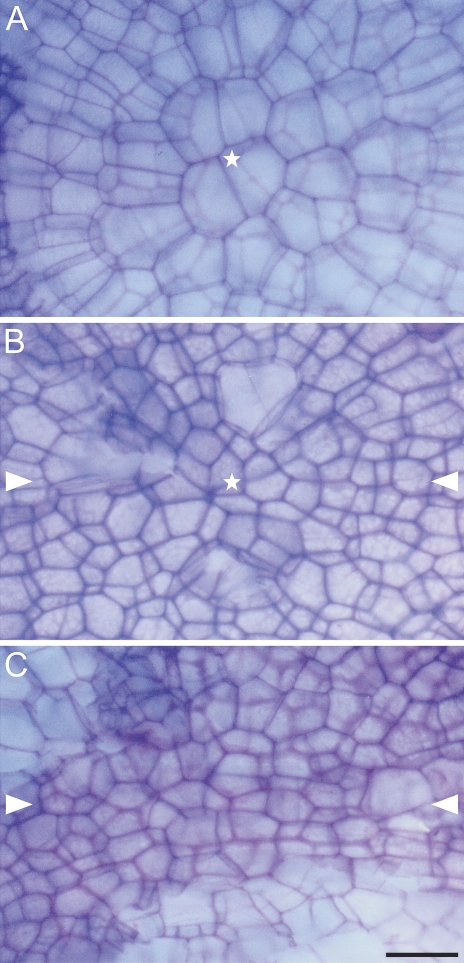
Figure 8 shows the surface at the pole of the root proper of the control (Fig. 8A) and treated (Fig. 8B, C) roots after removal of their caps. The cells in the control roots (Fig. 8A) are larger and in this view they have shapes of regular polygons. A group of cells at the top form a centre in which files of epidermis meet. In the treated roots (Fig. 8B, C) the cells are arranged irregularly and it is hard to discriminate clear files of epidermis. The cells at the top are smaller and they can form a centre-like pattern (Fig. 8B); however, there is no radial cell arrangement typical for the controls. In some root apices central cells have become elongated and formed files in a plane of the major diameter of the narrowing (Fig. 8C).
Fig. 8.
Surface view of the cell pattern at the root pole after cap removal in a typical control root (A) and the treated roots in variant II (B, C). (A) Regular pattern with clearly recognizable distal most cells of the QC (asterisk) and the radial cell arrangement. (B, C) Disturbed patterns with significantly smaller cells, a recognizable centre with numerous irregularly arranged cells (B, asterisk), atypical cell files near the centre (C). The distal most cells are distinguished by the darkest walls. White arrowheads in B and C indicate the major axial plane of the narrowing. Scale bar: 20 μm (A–C). (This figure is available in colour at JXB online.)
Discussion
In the experiments presented here, root apices of Z. mays were exposed to mechanical impedance causing deformation of the organs as well as reorganization of the cell pattern. While the first may be compared with data from the literature (see below), the latter has not been the subject of the studies to date. Thus this is the first case of a description of the RAM organization changes under mechanical stimulus. The results provide evidence of significant influences of mechanical stress on both the morphological and anatomical features of the root apex in maize. Below they are interpreted in terms of three aspects: morphological response, RAM reorganization, and mechanical factors.
Morphological response
During plant growth in natural soil conditions the root is exposed to mechanical stress from various directions. Yet, under laboratory conditions when the influence of mechanical stress on the root apices is studied the external stimulus is usually delivered from one or more chosen directions. For example, in the experiment by Bengough et al. (1994) pea roots grew towards a fixed obstruction and the mechanical pressure reached the apex from the tip. Clark et al. (1996) and Clark and Barraclough (1999) used a special shear beam apparatus to measure a force exerted on a completely impeded pea root fixed in a ceramic cone so the stimulus was also delivered from the side of the tip. A good way to simulate field conditions was to use glass beads as a medium for growing roots (Wilson et al., 1977; Veen, 1982), sand with different levels of compaction (Iijima et al., 2000, 2003), or mesh with pore sizes smaller than the root diameter (Scholefield and Hall, 1985) because the external mechanical stimulus reached the apex from various sides and randomly.
In the studies presented herein, Zea root apices experienced stimuli of a mechanical character delivered from the organ's flanks. This caused significant changes in the apices as shown in the Results. One may ask the question of why two variants of the experiment needed to be performed. There are two main reasons. First, the applied variants were the only ones possible in order to consider stimulus delivery from the flanks. In variant I the apex was stressed circumferentially in the narrowing and the force was uniformly distributed around the organ, while in variant II the root was stressed from two opposite sides. Such an application of the mechanical stimulus had not been used before. It enabled simulation of the soil conditions giving a short-term stress. This allowed the return of the RAM organization to its previous character after the apex had left the narrow zone. Applying another variant, for example growing the root tip into a blocked passage, would give a long-term stimulus and so it would not allow recovery of the cell pattern. Secondly, the two variants resulted in different root apex deformation related to geometry of the narrowing. Namely, in variant I only a change of the size of the circumference occurred (smaller cross-section, see Fig. 3C), while in variant II a change in the cross-section shape to elliptical was observed (Fig. 4D). So in both variants the root diameter was in some way diminished which made unimpeded growth of the root difficult. However, it is variant II which better resembles natural conditions: in soil the roots more often need to push their way through the surrounding particles pressing on it from the sides than to grow into a tiny hole of a regular circular shape.
Except for diminished (in variant I) or changed (in variant II) shape of their cross-sections there were other morphological changes in the treated Zea root apices. The most significant were an increase in root diameter just behind the apex (Fig. 4A, C), buckling (Figs 3D, 4A–C, E) which appeared stronger in variant II because of a larger diameter of the tube (there was more space for the root to buckle), or ectopic root hair formation in the region close to the meristem zone (Fig. 4A, E) where they normally do not occur. Similar changes had been described before, for example swollen regions forming behind the apex in response to high medium strength (compact soil, glass beads, etc.) were observed in barley and lupin roots (Goss and Russell, 1980; Atwell, 1988) and buckling in roots of grasses penetrating wire mesh (Scholefield and Hall, 1985). Ectopic root hair formation was observed in barley roots growing between glass beads (Goss and Drew, 1972; Goss and Russell, 1980) and in roots of Arabidopsis thaliana seedlings grown horizontally on a dialysis membrane-covered agar plate (Okamoto et al., 2008).
RAM reorganization
In both variants of the experiment, thus independently of the manner of deformation, there is a strong rearrangement of the RAM architecture. It begins to be visible as the root tip grows into the narrow zone, then develops while it pushes through the tightest part. Eventually the cell pattern seems to recover when the root apex leaves the narrow zone. The general sequence of events would be the following: the first noticeable change takes place in the middle tier of initials (Figs 6A, 7A) where some cells undergo periclinal divisions which hardly ever occur in this region. Next, most distal cells of the root proper begin to grow into the cap side (Figs 6C, E, 7B) and the root–cap boundary becomes more or less irregular (Fig. 6C, E); in variant II it even remains smooth (Fig. 7B). Finally the border breaks and the typical closed RAM organization turns to open (Figs 6B, G, 7E), which is the most noticeable change in the cell pattern. At the same time periclinal (Figs 6B, 7D, E) and oblique (Figs 6F, G) divisions in cells located between the pole of the stele and the cap initials take place. In some apices oblique divisions also occur in the root cap (Fig. 6D). These atypical divisions may suggest an enhanced axial growth within this area and a change in the stress distribution within the apex (Lynch and Lintilhac, 1997); the latter will be considered below. When only the root apex leaves the narrowing, the RAM organization seems to turn back to its typical closed character. The root–cap border line becomes recognizable, although still irregular (Fig. 7F); longitudinal divisions in root cap cells (Figs 6D, G, 7F) are observed, leading to the enhanced radial growth in this region. The observed changes may suggest that the root apex tries to restore its typical organization. Up to the end of the experiment the root meristem organization had not returned to the typical organization occurring in control roots. However, on the basis of the observed processes it can be postulated that the changes in the RAM organization may be reversible. This hypothesis can be supported by previous studies in which the transformation from an open to a closed meristem took place in Zea during root apex regeneration after removal of the cap and the QC (Feldman, 1976) as well as in roots recovering from low temperature conditions (Kerk and Feldman, 1994).
The above-mentioned changes in meristem organization are in fact the changes in the QC itself, as it is the distal part of the zone that undergoes the most noticeable rearrangement. It concerns diminishing of the mitotically inactive region through divisions of the most distal cells of the QC (Figs 6A, H, 7A, 8B, C) as well as through rupture of the root–cap boundary (Figs 6B, G, 7D, E). Thus what is observed is an activation of the QC cells due to the mechanical stimulus leading to the RAM opening through the root–cap boundary rupture. Such activation of the QC was also observed in response to other kinds of stimuli. For example, both an increase and a decrease in temperature resulted in additional divisions in the distal cells of the QC of maize roots and opening of the RAM (Clowes and Wadekar, 1989; Kerk and Feldman, 1994). Application of NPA to the root of this species caused a decrease in the size of the QC, and affected growth of the QC cells into the root cap and the production of several new layers of cells in the region between the root cap and the tip of the procambial cylinder (Jiang et al., 2003). The zone size may also alter naturally during plant ontogenesis. For example, in maize a 36% reduction in the size of the QC is observed as the root grows from 30 mm to 100 mm long (Clowes and Wadekar, 1989), while in Sinapis alba as the root grows the QC increases in size (Clowes, 1958).
A question arises as to what could be the reason for the rupture of the root–cap boundary after activation of the QC. As shown by Clowes and Steward (1967) and Barlow and Rathfelder (1985), the root cap cells are sensitive to stress of various kinds and the zone activation results from their injury. According to Clowes (1982), in roots of Helianthus annuus and Cucurbita pepo the columella initials become temporarily quiescent which may induce the cell proliferation in the QC. Barlow (2003) suggests that this may not be sufficient for RAM opening, indicating a weakening of the root–cap boundary a condition sine qua non. If the above remarks are put in the context of the present experiments, it can be seen that during passage of the root tip through the narrowing the cap cells are the first to be exposed to the mechanical stimulus; however, neither significant damage nor quiescence of these cells is observed. However, a possible communication between the cap and the QC cells, which may enable the quiescent zone activation, cannot be excluded.
Mechanical factors
Although the manner of the application of the force was different in the two variants of the experiment a character of the time dependence of the force (Fig. 5A, B) is similar in both. Namely, when the root tip reaches the narrow zone the force increases rapidly to attain its maximal value at the moment of passing through the narrowest part. Interestingly, in experiments in which the force exerted by pea roots growing into plastic cones with their bases blocked was measured (Bengough et al., 1994), the graph (Fig. 3 in the cited paper) showing the change in force versus time resembles the present results (Fig. 5A). This shows that although a living tissue is examined a mechanical response of the material appears repeatable, thus the applied method may be regarded as appropriate to studies in the field.
The time of passing through the narrowest part is relatively short in both variants (300–400 min). It is worth emphasizing how such a short-term stimulus appeared sufficient to cause the spectacular changes in the RAM organization described above. The value of the maximal force and consequently the stress exerted by the root tip is greater in variant I which shows that such conditions produced a higher impedance for the growing apices. If the values of mechanical stress obtained in the present experiment (0.65 MPa in variant I and 0.29 MPa in variant II) are compared with the results of other authors, it can be seen that they are similar. For example, a completely impeded maize root exerts the maximum growth pressure of 0.43 MPa (Clark and Barraclough, 1999), while the stress estimated for elongating maize roots is between 0.26 MPa and 0.47 MPa (Bengough and Mullins, 1991). The slight differences may result from differing methods of measurement.
It was mentioned in the Introduction that the plant organ growth as well as the stability of the cell pattern are controlled on the tensor level. Growth can be treated as an irreversible deformation of the cell wall system (Nakielski and Hejnowicz, 2003), and there is a direct relationship between stress and strain (Fung, 1981), so it can be assumed that the field of growth rates is a function of tensile stress in the cell walls. The stress, similarly to the growth rate, is the second rank tensor quantity (Nakielski and Hejnowicz, 2003) that defines their own principal directions. Accordingly, the directional cues included in PDGs may be related to the principal directions of stress. Mechanical experiments (Lynch and Lintilhac, 1997; Zhou et al., 2007) support this view. In the light of the above tensor-based relationship, the present results may be interpreted as follows.
During undisturbed growth in the root apex there is a steady tensor field of growth rates related to unknown mechanical stress distribution, which can be called primary. The primary distribution is probably steady and well adjusted to the root geometry (the cell pattern is typically self-perpetuating). While growing into the narrow zone the apex encounters stronger and stronger impedance. This results in additional stress distribution, which can be called secondary. The secondary stress distribution, of the radial symmetry in variant I and asymmetry in variant II, is unsteady because it depends on the current position of the apex in the narrowing. In spite of the difference in the narrowing shapes (circular and elliptical) the result was the same, namely the boundary between the root proper and the cap was broken. This suggests that such an effect depends mainly on the magnitude of the mechanical impedance. It probably takes place before the moment when the narrowest zone is reached (see insets in Figs 6B, C, 7B), which indicates its correspondence to the increasing part of the plot in Fig. 5. Notice that in the apices where the boundary has been broken the cell pattern near the break is evidently disturbed compared with the control (Fig. 6F), while in the apices where the boundary has remained unbroken there are only a few oblique cell walls in the root cap (Fig. 6D). If the PDGs result from the stress (a possible relationship was suggested by Nakielski, 2008), this disturbance may indicate that the stress distribution changes when the apex passes through the narrowing. The return to the typical cell pattern observed after leaving the narrowing (Figs 6H, 7F) is a good support for this hypothesis—the primary stress distribution has eventually been restored.
A question arises as to whether the described root meristem reorganization is adaptive or a simple consequence of mechanical stress. Too little is known about the stress distribution in roots to give a clear answer to this question. On the one hand, the time period during which the apex passes through the narrowing appeared sufficient to cause disturbances in the root–cap border and to generate oblique cell walls within the RAM. On the other hand, it seems too short to observe significant long-term effects of the adaptation. It is also short enough to let the root geometry and the cell pattern finally become restored. Knowing that the tensile stresses in cell walls of a turgid organ depend on the existing geometry of the organ (Nakielski and Hejnowicz, 2003), the observed reorganization may well be adaptive.
This study shows the effect of the mechanical stimulus on the RAM organization in Z. mays. More results of the same experiment relating to deformation of the root apex as well as of the cell wall system have been obtained; they will be presented in a forthcoming paper.
Acknowledgments
The authors wish to thank Professor Lewis J. Feldman from the Department of Plant and Microbial Biology, UC Berkeley for his critical comments on the manuscript.
Glossary
Abbreviations
- NPA
N-1-naphthylphthalamic acid
- PAS
periodic acid–Schiff
- PDGs
principal directions of growth
- QC
quiescent centre
- RAM
root apical meristem
- TIBA
2,3,5-triiodobenzoic acid
- UV
ultraviolet
References
- Armstrong JE, Heimsch C. Ontogenetic reorganization of the root meristem in the Compositae. American Journal of Botany. 1976;63:212–219. [Google Scholar]
- Atwell BJ. Physiological responses of lupin roots to soil compaction. Plant and Soil. 1988;111:277–281. [Google Scholar]
- Barlow PW. The meristem and quiescent centre in cultured root apices of the gib-1 mutant of tomato (Lycopersicon esculentum Mill.) Annals of Botany. 1992;69:533–543. [Google Scholar]
- Barlow PW. Stem cells and founder zones in plants, particularly their roots. In: Potten CS, editor. Stem cells. London: Academic Press; 1997. pp. 29–57. [Google Scholar]
- Barlow PW. The root cap: cell dynamics, cell differentiation and cap function. Journal of Plant Growth Regulation. 2003;21:261–286. [Google Scholar]
- Barlow PW, Rathfelder EL. Correlations between the dimensions of different zones of grass root apices, and their implications for morphogenesis and differentiation in roots. Annals of Botany. 1984;53:249–260. [Google Scholar]
- Barlow PW, Rathfelder EL. Cell division and regeneration in primary root meristems of Zea mays recovering from cold treatment. Environmental and Experimental Botany. 1985;25:303–314. [Google Scholar]
- Baum SF, Dubrovsky JG, Rost TL. Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. American Journal of Botany. 2002;89:908–920. doi: 10.3732/ajb.89.6.908. [DOI] [PubMed] [Google Scholar]
- Bengough AG, Mackenzie CJ, Elangwe HE. Biophysics of the growth responses of pea roots to changes in penetration resistance. Plant and Soil. 1994;167:135–141. [Google Scholar]
- Bengough AG, Mullins CE. Penetrometer resistance, root penetration resistance and root elongation rate in two sandy loam soils. Plant and Soil. 1991;131:59–66. [Google Scholar]
- Bennie ATP. Growth and mechanical impedance. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York: Marcel Dekker, Inc.; 1996. pp. 453–470. [Google Scholar]
- Chapman K, Groot EP, Nichol SA, Rost TL. Primary root growth and the pattern of root apical meristem organization are coupled. Journal of Plant Growth Regulation. 2003;21:287–295. [Google Scholar]
- Clark LJ, Barraclough PB. Do dicotyledons generate greater maximum axial root growth pressures than monocotyledons? Journal of Experimental Botany. 1999;50:1263–1266. [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB. Partial mechanical impedance can increase the turgor of seedling pea roots. Journal of Experimental Botany. 2001;52:167–171. [PubMed] [Google Scholar]
- Clark LJ, Whalley WR, Dexter AR, Barraclough PB, Leigh RA. Complete mechanical impedance increases the turgor of cells in the apex of pea roots. Plant, Cell and Environment. 1996;19:1099–1102. [Google Scholar]
- Clowes FAL. Development of quiescent centres in root meristems. New Phytologist. 1958;57:85–88. [Google Scholar]
- Clowes FAL. Apical meristems. Oxford: Blackwell Scientific Publications; 1961. [Google Scholar]
- Clowes FAL. X-irradiation of root meristems. Annals of Botany. 1963;27:343–352. [Google Scholar]
- Clowes FAL. The root apex. In: Yeoman MM, editor. Cell division in higher plants. London: Academic Press; 1976. pp. 253–284. [Google Scholar]
- Clowes FAL. Mitosis in the root cap of. Zea mays. New Phytologist. 1980;85:79–87. [Google Scholar]
- Clowes FAL. Changes in cell population kinetics in an open meristem during root growth. New Phytologist. 1982;91:741–748. [Google Scholar]
- Clowes FAL, Steward HE. Recovery from dormancy in roots. New Phytologist. 1967;66:115–123. [Google Scholar]
- Clowes FAL, Wadekar R. Instability in the root meristem of Zea mays L. during growth. New Phytologist. 1989;111:19–24. [Google Scholar]
- Cutter EG. Plant anatomy: experiment and interpretation. Part 2: organs. London: Edward Arnold; 1971. [Google Scholar]
- Erickson RO. Symplastic growth and symplasmic transport. Plant Physiology. 1986;82:1153. doi: 10.1104/pp.82.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman LJ. The de novo origin of the quiescent center regenerating root apices of. Zea mays. Planta. 1976;128:207–212. doi: 10.1007/BF00393230. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics: mechanical properties of living tissues. New York: Springer-Verlag; 1981. [Google Scholar]
- Goss MJ, Drew MC. Effect of mechanical impedance on growth of seedlings. Agricultural Research Council Letcombe Laboratory Annual Report. 1972;1971:35–42. [Google Scholar]
- Goss MJ, Russell RS. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). III. Observations on the mechanism of response. Journal of Experimental Botany. 1980;31:577–588. [Google Scholar]
- Hejnowicz Z. Differential growth resulting in the specification of different types of cellular architecture in root meristems. Environmental and Experimental Botany. 1989;29:85–93. doi: 10.1016/0098-8472(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z, Romberger JA. Growth tensor of plant organs. Journal of Theoretical Biology. 1984;110:93–114. [Google Scholar]
- Iijima M, Barlow PW, Bengough AG. Root cap structure and cell production rates of maize (Zea mays) roots in compacted sand. New Phytologist. 2003;160:127–134. doi: 10.1046/j.1469-8137.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- Iijima M, Griffiths B, Bengough AG. Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytologist. 2000;145:477–482. doi: 10.1046/j.1469-8137.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ. Regulation of root apical meristem development. Annual Review of Cell and Developmental Biology. 2005;21:485–509. doi: 10.1146/annurev.cellbio.21.122303.114753. [DOI] [PubMed] [Google Scholar]
- Jiang K, Meng YL, Feldman LJ. Quiescent center formation in maize roots is associated with an auxin-regulated oxidizing environment. Development. 2003;130:1429–1438. doi: 10.1242/dev.00359. [DOI] [PubMed] [Google Scholar]
- Kerk N, Feldman L. The quiescent center in roots of maize: initiation, maintenance and role in organization of the root apical meristem. Protoplasma. 1994;183:100–106. [Google Scholar]
- Kozhevnikova AD, Seregin IV, Bystrova EI, Ivanov VB. Effects of heavy metals and strontium on division of root cap cells and meristem structural organization. Russian Journal of Plant Physiology. 2007;54:257–266. [Google Scholar]
- Luxová M. Some aspects of the differentiation of primary root tissues. In: Torrey JG, Clarkson DT, editors. The development and function of roots. London: Academic Press; 1975. pp. 73–90. [Google Scholar]
- Lynch TM, Lintilhac PM. Mechanical signals in plant development: a new method for single cell studies. Developmental Biology. 1997;181:246–256. doi: 10.1006/dbio.1996.8462. [DOI] [PubMed] [Google Scholar]
- Materechera SA, Dexter AR, Alston AM. Penetration of very strong soils by seedling roots of different plant species. Plant and Soil. 1991;135:31–41. [Google Scholar]
- Nakielski J. The tensor-based model for growth and cell divisions of the root apex. I. The significance of principal directions. Planta. 2008;228:179–189. doi: 10.1007/s00425-008-0728-y. [DOI] [PubMed] [Google Scholar]
- Nakielski J, Hejnowicz Z. The description of growth of plant organs: a continuous approach based on the growth tensor. In: Nation J, Trofimova I, Rand JD, Sulis W, editors. Formal descriptions of developing systems. Dordrecht: Kluwer Academic Publishers; 2003. pp. 119–136. [Google Scholar]
- O'Brien TP, McCully ME. The study of plant structure: principles and selected methods. Melbourne: Termarcarphi; 1981. [Google Scholar]
- Okamoto T, Tsurumi S, Shibasaki K, Obana Y, Takaji H, Oono Y, Rahman A. Genetic dissection of hormonal responses in the roots of Arabidopsis grown under continuous mechanical impedance. Plant Physiology. 2008;146:1651–1662. doi: 10.1104/pp.107.115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost TL. Root tip organization and the spatial relationships of differentiation events. In: Iqbal M, editor. Growth patterns in vascular plants. Portland: Dioscorides Press; 1994. pp. 59–76. [Google Scholar]
- Rost TL, Baum S. On the correlation of primary root length, meristem size and protoxylem tracheary element position in pea seedlings. American Journal of Botany. 1988;75:414–424. [Google Scholar]
- Scholefield D, Hall DM. Constricted growth of grass roots through rigid pores. Plant and Soil. 1985;85:153–162. [Google Scholar]
- Seago JL, Heimsch C. Apical organization in roots of the Convolvulaceae. American Journal of Botany. 1969;56:131–138. [Google Scholar]
- Silk WK, Erickson RO. Kinematics of plant growth. Journal of Theoretical Biology. 1979;76:481–501. doi: 10.1016/0022-5193(79)90014-6. [DOI] [PubMed] [Google Scholar]
- Veen BW. The influence of mechnical impedance on the growth of maize roots. Plant and Soil. 1982;66:101–109. [Google Scholar]
- von Guttenberg H. Grundzüge der Histogenese höherer Pflanzen. I. Die Angiospermen. Berlin: Gebrüder Borntraeger; 1960. [Google Scholar]
- Wawrecki W, Zagórska-Marek B. Influence of a weak DC electric field on root meristem architecture. Annals of Botany. 2007;100:791–796. doi: 10.1093/aob/mcm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Robards AW, Goss MJ. Effects of mechanical impedance on root growth in barley, Hordeum vulgare L. II. Effects on cell development in seminal roots. Journal of Experimental Botany. 1977;28:1216–1227. [Google Scholar]
- Zhou J, Wang B, Li Y, Wang Y, Zhu L. Responses of chrysanthemum cells to mechanical stimulation require intact microtubules and plasma membrane–cell wall adhesion. Journal of Plant Growth Regulation. 2007;26:55–68. [Google Scholar]