Abstract
S-nitrosoglutathione reductase (GSNOR) reduces the nitric oxide (NO) adduct S-nitrosoglutathione (GSNO), an essential reservoir for NO bioactivity. In plants, GSNOR has been found to be important in resistance to bacterial and fungal pathogens, but whether it is also involved in plant–herbivore interactions was not known. Using a virus-induced gene silencing (VIGS) system, the activity of GSNOR in a wild tobacco species, Nicotiana attenuata, was knocked down and the function of GSNOR in defence against the insect herbivore Manduca sexta was examined. Silencing GSNOR decreased the herbivory-induced accumulation of jasmonic acid (JA) and ethylene, two important phytohormones regulating plant defence levels, without compromising the activity of two mitogen-activated protein kinases (MAPKs), salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK). Decreased activity of trypsin proteinase inhibitors (TPIs) were detected in GSNOR-silenced plants after simulated M. sexta feeding and bioassays indicated that GSNOR-silenced plants have elevated susceptibility to M. sexta attack. Furthermore, GSNOR is required for methyl jasmonate (MeJA)-induced accumulation of defence-related secondary metabolites (TPI, caffeoylputrescine, and diterpene glycosides) but is not needed for the transcriptional regulation of JAZ3 (jasmonate ZIM-domain 3) and TD (threonine deaminase), indicating that GSNOR mediates certain but not all jasmonate-inducible responses. This work highlights the important role of GSNOR in plant resistance to herbivory and jasmonate signalling and suggests the potential involvement of NO in plant–herbivore interactions. Our data also suggest that GSNOR could be a target of genetic modification for improving crop resistance to herbivores.
Keywords: Defence, ethylene, insect herbivore, jasmonic acid, jasmonate signalling, Manduca sexta, Nicotiana attenuata, S-nitrosoglutathione reductase (GSNOR), secondary metabolites, trypsin proteinase inhibitor
Introduction
Plants are constantly challenged by various environmental stresses, such as herbivore attacks, pathogen infections, unfavourable temperatures, drought, and UV-B radiation. Accordingly, plants have evolved to cope with these stresses using sophisticated defence systems, which include receptors and sensors, highly complex regulatory networks, compounds and proteins that directly or indirectly protect plants from these unfavourable conditions (Mittler, 2006; Chen, 2008; Dodds and Rathjen, 2010; Wu and Baldwin, 2010).
Herbivores, especially insects, pose a great challenge for plant survival. Accordingly, plants have developed herbivory-specific defence systems to perceive herbivore attacks and deploy defence responses to optimize their fitness (Heil and Baldwin, 2002; Howe and Jander, 2008; Wu and Baldwin, 2010). Herbivory-induced defence responses have been intensively studied in Nicotiana attenuata, a native annual plant of the semi-arid deserts which ranges from northwest Mexico, east to the Great Basin and north to southern Canada (Baldwin, 2001; Kessler and Baldwin, 2002). Feeding of Manduca sexta, a specialist herbivore for N. attenuata, or the application of M. sexta larval oral secretions (OS) on wounded leaves activates signalling cascades that involve the activation of the mitogen-activated protein kinases (MAPKs), salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK), and bursts of jasmonic acid (JA), JA-isoleucine conjugate (JA-Ile), salicylic acid (SA), and ethylene (Kang et al., 2006; Von Dahl et al., 2007; Wu et al., 2007).
Many studies in Arabidopsis, tomato, and N. attenuata have demonstrated the critical roles of JA biosynthesis and signalling for herbivory-induced defences (McConn et al., 1997; Halitschke and Baldwin, 2003; Li et al., 2004, 2005; Paschold et al., 2007). Importantly, JA-Ile, but not JA, activates most of the JA-induced responses (Staswick and Tiryaki, 2004). JAZs (jasmonate ZIM-domain proteins) form complexes with MYC2, the major activator of JA-induced transcriptional responses, and thus inhibit the activity of MYC2. Binding of JA-Ile to the COI1 (coronatine insensitive1) receptor facilitates the degradation of the JAZs by the SCF(COI1) ubiquitin ligase-mediated pathway and, in turn, releases MYC2 which activates downstream responses (Chini et al., 2007; Thines et al., 2007). In N. attenuata, several compounds have been identified to be important for direct defence against herbivores. These include trypsin proteinase inhibitors (NaTPIs) (Zavala and Baldwin, 2004; Zavala et al., 2004), nicotine (Steppuhn et al., 2004), diterpene glycosides (DTGs) (Jassbi et al., 2008; Heiling et al., 2010), and the phenylpropanoid–polyamine conjugate caffeoylputrescine (CP) (Kaur et al., 2010). Silencing the JA-Ile receptor COI1 greatly impairs the accumulation of these metabolites and dramatically attenuates N. attenuata’s resistance against M. sexta attack in the greenhouse and in nature (Paschold et al., 2007). The function of SA in resistance to chewing insects remains largely elusive (Wu and Baldwin, 2010), although in some plant–herbivore interactions, SA appears to suppress JA accumulation (Diezel et al., 2009). Compared with JA, the gaseous hormone ethylene seems to play a minor role (Wu and Baldwin, 2010): ethylene potentiates JA-inducible proteinase inhibitors in tomato (O'Donnell et al., 1996) and reduces M. sexta herbivory-induced nicotine accumulation in N. attenuata (Kahl et al., 2000; Von Dahl et al., 2007).
Emerging evidence has revealed other small molecules in the regulatory networks in plant–herbivore interactions (Wu and Baldwin, 2009, 2010). In tomato, reactive oxygen species (ROS) are important for the transcript accumulation of several herbivore-resistant genes (Orozco-Cardenas et al., 2001; Sagi et al., 2004). Moreover, nitric oxide (NO), one of the reactive nitrogen species (RNS), seems to be also involved in herbivore defences. Wounding induces NO production in marine macroalgae (Ross et al., 2006) and in Arabidopsis epidermal cells (Huang et al., 2004). NO negatively regulates proteinase inhibitor transcript levels after wounding, systemin, oligosaccharides, and JA treatment (Orozco-Cardenas and Ryan, 2002). NO is highly diffusible and reactive and it readily nitrosylates cysteine (S-nitrosylation) and tyrosine (tyrosine nitration) residues in various proteins (Lindermayr et al., 2005; Besson-Bard et al., 2008). Importantly, S-nitrosylation has been considered to be an important prototypic, redox-based, post-translational protein modification (Stamler et al., 2001; Wang et al., 2006). However, how NO regulates plant resistance to biotic stresses is still unknown, and very likely protein S-nitrosylation by NO plays a critical role (Feechan et al., 2005; Lindermayr et al., 2005; Grennan, 2007).
Although a bona fide NO synthase has yet to been identified in higher plants, at least three genes are associated with NO levels: NOA1 (nitric oxide associated1), NR (nitrate reductase), and GSNOR (S-nitrosoglutathione reductase) (Besson-Bard et al., 2008; Wilson et al., 2008). Unlike NOA and NR, which are positively associated with NO levels in plants (Yamasaki and Sakihama, 2000; Guo et al., 2003), GSNOR is located in a NO removal pathway: NO rapidly reacts with glutathione and forms S-nitrosylated glutathione (GSNO), and GSNO is further metabolized into the oxidized glutathione disulphide (GSSG) and NH3 by GSNOR (Wilson et al., 2008). Consistent with the biochemical property of GSNOR, the Arabidopsis gsnor mutant exhibits elevated NO levels, stunted growth, impaired flower development, and compromised thermotolerance (Lee et al., 2008). Apart from its role in plant development and interaction with abiotic environmental factors, GSNOR also positively controls plant immunity to Pseudomonas syringae pv. tomato DC3000, Blumeria graminis (powdery mildew), and Hyaloperonospora parasitica (downy mildew) (Feechan et al., 2005). By contrast, compared with the wild type, Arabidopsis antisense GSNOR plants are less susceptible to Peronospora parasitica Noco2 (oomycete) (Rusterucci et al., 2007).
Although the function of GSNOR in plant–pathogen interactions has been explored, its role in plant defence against herbivores was unknown. A reverse genetic approach was used here to investigate the function of GSNOR in N. attenuata’s inducible defence against the specialist herbivore M. sexta. Virus-induced gene silencing (VIGS) was used to knock down the transcripts of NaGSNOR, and traits important in herbivore resistance were examined. It was found that silencing NaGSNOR attenuates wounding- and simulated herbivory-induced levels of phytohormones that regulate plant resistance levels and, accordingly, decreased accumulation of the defensive compound NaTPI was detected in NaGSNOR-silenced plants. Moreover, many, but not all jasmonate-inducible responses are compromised in NaGSNOR-silenced plants, indicating the involvement of NaGSNOR in transducing certain jasmonate-induced responses. Taken together, our data highlight the important role of NaGSNOR in plant defence against herbivores.
Materials and methods
Plant growth, plant treatment, and herbivore performance assay
Seeds of N. attenuata Torr. Ex Watts were from a line that had been inbred for 30 generations. Germination and plant cultivation followed Krügel et al. (2002). Plants were transferred into 1.0 l pots 20 d after germination on Petri dishes, and were grown in a climate chamber at 22 °C and under 65% humidity. Light (16 h d−1) was provided by Philips Sun-T Agro 400 sodium lights (Philips, Turnhout, Belgium). Herbivory was simulated by wounding the rosette sink–source transition leaves of N. attenuata with a pattern wheel and immediately applying 20 μl of 1/5 diluted oral secretions (OS) (W+OS) from M. sexta to the puncture wounds; plants whose puncture wounds were treated with 20 μl of water (W+W) were used for comparison. For treatment with methyl jasmonate (MJ), MJ was dissolved in heat-liquefied lanolin (5 mg m−1) and 20 μl of MJ-lanolin paste was applied to the basal part of a leaf; leaves treated with 20 μl of pure lanolin served as controls. All samples were immediately frozen in liquid nitrogen after harvesting and stored at –80 °C until analyses. Neonate M. sexta larvae from laboratory colonies were placed on plants (one larvae per plant, 30 replicated plants), and the larval masses were measured on days 4, 9, and 14.
Cloning of NaGSNOR, virus-induced gene-silencing, and Southern blotting analysis
No GSNOR sequences from Nicotiana spp. were deposited in the GenBank; therefore an Arabidopsis AtGSNOR (At5g43940) sequence was used to blast against the TIGR Plant Transcript Assemblies (http://plantta.jcvi.org/). A 1.48 kb tobacco NtGSNOR sequence was found (Plant TA Accession: TA13797_4097). The partial sequence of NaGSNOR was amplified from N. attenuata cDNA by PCR with primer pair NaGNSOR-1 (5′-GAACCCAACAAGCCTCTGGT-3′) and NaGSNOR-2 (5′-CATCCACCTTGATTTCCTTCT-3′), which were designed according to the sequence of NtGSNOR. The amplified fragment was cloned into the pJET1.2 vector (Fermentas, St Leon-Rot, Germany) and sequenced.
A 326 bp fragment of NaGSNOR was cloned into the pTV00 vector to generate the pTV–NaGSNOR construct, which was then transformed into Agrobacterium tumefaciens (Ratcliff et al., 2001). Virus-induced gene silencing was done according to Saedler and Baldwin (2004). The initiation of silencing was visually monitored using phytoene desaturase (NaPDS)-silenced plants, which showed a photo-bleaching phenotype about 2 weeks after inoculation with A. tumefaciens carrying pTV-NaPDS (Saedler and Baldwin, 2004).
The restriction enzymes EcoRI, HindIII, EcoRV, and XbaI were used to digest DNA of N. attenuata. Five micrograms of digested DNA were separated on a 1% agarose gel and then were further blotted on to a nylon membrane. Hybridization was performed according to Wu et al. (2006) using a probe prepared by PCR amplification of a partial NaGSNOR sequence with the primer pair NaGSNOR-F1 (5′-CCTCTGGTGATCGAGGATGT-3′) and NaGSNOR-R1 (5′-TCTCCTGGCTGAACCTCAGT-3′).
Quantitative real-time PCR (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract RNA. cDNA samples were synthesized from 500 ng of total RNA using the Superscript II reverse transcriptase (Invitrogen). qRT-PCR analyses were performed on a Stratagene MX3005P (Agilent Technologies, Santa Clara, CA, USA) using qPCR SYBR Green kits (Eurogentec, Seraing, Belgium). An N. attenuata actin gene NaActin was used to normalize the variation of cDNA concentrations. All qRT-PCR experiments were performed using five biological replicates. The sequences of primer pairs are listed in Supplementary Table S1 at JXB online.
GSNOR activity assay
GSNOR activity was measured spectrophotometrically at 340 nm using a modified method as described in Sakamoto et al. (2002). In brief, approximately 30 mg of ground leaf tissue were extracted with 300 μl of 50 mM HEPES buffer (pH 8) containing 20% glycerol, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM benzamidine, and 1 mM ϵ-aminocaproic acid. The samples were centrifuged at 4 °C, 16 000 g for 15 min and the supernatants were further desalted using protein desalting spin columns (Thermo Fischer Scientific, Rockford, IL, USA). Protein concentrations were determined and 30 μl of desalted protein samples containing about 70–120 μg of proteins were added to 300 μl of assay mix [20 mM TRIS-HCl (pH 8), 0.2 mM NADH, and 0.5 mM EDTA]. The NADH decomposition without GSNO was observed for 75 s. The enzymatic reaction was started by adding 10 μl of a GSNO solution into the assay mix to achieve a final GSNO concentration of 400 μM. The resulting GSNOR activity was expressed as nmol NADH degraded min−1 mg−1 protein.
In-gel kinase activity assay
Each protein sample was extracted from pooled leaves from five replicated plants. About 100 mg of leaf tissue were resuspended in 300 μl of extraction buffer [100 mM HEPES pH 7.5, 5 mM EDTA, 5 mM EGTA, 10 mM Na3VO4, 10 mM NaF, 50 mM β-glycerolphosphate, 1 mM phenylmethylsulphonyl fluoride, 10% glycerol, one proteinase inhibitor cocktail tablet per 10 ml extraction buffer (Roche, Mannheim, Germany)]. Samples were centrifuged at 4 °C, 13 000 g for 20 min and the supernatants were transferred to fresh tubes. Protein concentrations were measured using the Bio-Rad Protein Assay Dye Reagent (Bio-Rad, Hercules, CA, USA) with BSA (Sigma-Aldrich, Hamburg, Germany) as a standard. Ten micrograms of total protein from each sample were used for in-gel kinase activity assay according to a procedure described by Zhang and Klessig (1997). The image of in-gel kinase activity assays were obtained on a phosphorimager (FLA-3000 phosphor imager system, Fuji Photo Film, Stamford, CT, USA). The same amount of each sample was run on a duplicated gel without the kinase substrate myelin basic protein and the gel was stained with the GelCode Blue Safe Stain reagent (Thermo Fisher Scientific).
Quantification of JA, JA-Ile, SA, ethylene, and direct defence metabolites
Five biological replicates were used for quantification of JA, JA-Ile, and ethylene. For JA and JA-Ile analysis, about 100 mg of frozen and briefly crushed leaf tissue were added to 2 ml Eppendorf tubes containing 1 g of ceramic beads (MP Biomedicals, Illkirch, France). After adding 1 ml of ethyl acetate which contained 200 ng of JA[D2], 40 ng of JA-[13C6]Ile, and 40 ng of SA[D4] as internal standards, the tissue was homogenized on a Geno/Grinder 2000 at 1700 strokes min−1 for 2 min (SPEX CertiPrep, Metuchen, New Jersey, USA). After 10 min centrifugation at 4 °C and 13 000 g, the supernatants were transferred to fresh tubes and completely dried on a vacuum dryer (Eppendorf, Hamburg, Germany). The pellets were extracted with 500 μl of 70% (v/v) methanol, and samples were cleared with another centrifugation step. An HPLC-MS/MS (Varian, Palo Alto, CA, USA) was used to analyse the concentration of JA and JA-Ile in the supernatants. For ethylene quantification, five leaves were untreated as controls or were treated with W+OS and, after recording their fresh mass, they were immediately sealed in a 250 ml three-neck round bottom flask for 4 h under light. The ethylene contents in the flasks were measured on a photoacoustic laser spectrometer (INVIVO, Sankt Augustin, Germany) by comparing sample ethylene peak areas with peak areas generated by an ethylene standard (Von Dahl et al., 2007). Five replicates were done for ethylene measurements.
For analyses of TPI activity, leaves were ground in liquid nitrogen and ∼200 mg of leaf tissue were used for protein extraction and quantification of TPI activity (Jongsma et al., 1994). Contents of nicotine, diterpene glycosides, and caffeoylputrescine were analysed on an HPLC as described in Keinanen et al. (2001).
Results
Herbivory but not wounding transiently reduces the activity of NaGSNOR
A fragment of NaGSNOR [GenBank: HQ830156] with 967 bp was cloned from the N. attenuata cDNA pool. The deduced NaGSNOR partial protein sequence showed 98% and 92% similarity to tomato (Solanum lycopersicum) SlGSNOR [GenBank: ADB43258] and Arabidopsis AtGSNOR1 [GenBank: NP_199207] (Martínez et al., 1996), respectively (see Supplementary Fig. S1 at JXB online). In the Arabidopsis genome, AtGSNOR1 is a single gene (Martínez et al., 1996). Similarly, Southern blotting analysis indicated that NaGSNOR has only one copy in N. attenuata (see Supplementary Fig. S2 at JXB online).
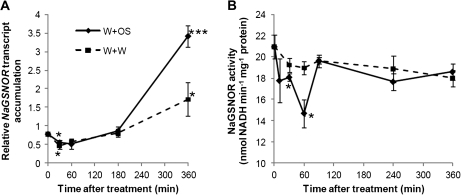
Wounding and chemical components such as fatty-acid amino-acid conjugates (FACs) in the OS of herbivores, which are introduced into wounds during feeding, induce a myriad of reactions on transcriptomic, proteomic, and metabolomic levels (Howe and Jander, 2008; Wu and Baldwin, 2010). The transcript and protein levels of AtGSNOR in Arabidopsis are down-regulated after wounding (Diaz et al., 2003). To examine whether M. sexta herbivory leads to altered NaGSNOR transcript accumulation and NaGSNOR activity in N. attenuata, rosette leaves of N. attenuata were wounded with a pattern wheel and 20 μl of M. sexta larval oral secretions (OS) were immediately applied to wounds (W+OS); this treatment effectively mimics herbivory of M. sexta (Halitschke et al., 2001). For comparison, mechanical wounding was done by applying 20 μl of water to wounds (W+W). Initially, NaGSNOR transcripts were slightly reduced 30 min after both treatments (W+W, W+OS), but regained the levels seen in non-treated plants by 3 h (Fig. 1A). However, 6 h after W+W and W+OS treatment, NaGSNOR transcript levels increased 2.2-fold and 4.3-fold compared with those in non-treated plants. It was next examined whether the activity of NaGSNOR is regulated by wounding and simulated herbivory. After W+W treatment, no obvious changes of NaGSNOR activity were found (Fig. 1B). W+OS treatment suppressed up to 30% of the NaGSNOR activity by 1 h; however, the activity regained the levels found in non-treated plants by 1.5 h and showed no changes even after 6 h (Fig. 1B), suggesting that herbivory (probably the OS of M. sexta) but not wounding, specifically and transiently reduces the activity of NaGSNOR. In addition, transcript levels of NaGSNOR after wounding and herbivory do not correlate with the activity levels of NaGSNOR.
Fig. 1.
NaGSNOR transcript accumulation and enzyme activity after wounding and simulated herbivory. Transition leaves of N. attenuata rosette plants were wounded with a pattern wheel, and were subsequently treated with 20 μl of water (W+W) or 20 μl of M. sexta oral secretions (W+OS). Samples were harvested after the indicated times. (A) Transcript levels (mean ±SE) of NaGSNOR were measured with qPCR. (B) Activity (mean ±SE) of NaGSNOR. Stars indicate significantly different levels between treated and non-treated samples (Student's t test; *P ≤0.05; ***P ≤0.001; n=5).
Silencing NaGSNOR impairs herbivory-induced accumulation of JA and ethylene
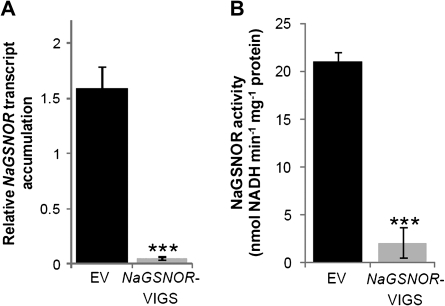
RNAi-based gene silencing was first used to generate plants stably silenced in NaGSNOR. However, all plants of the T1 generation that were well silenced in NaGSNOR showed highly stunted growth, reduced apical dominance, epinastic leaves, and finally aborted all flower buds. Thus, a virus-induced gene silencing (VIGS) approach was used to determine the role of NaGSNOR in the response of N. attenuata to wounding and M. sexta feeding. A pTV-NaGSNOR construct was prepared by inserting a partial NaGSNOR coding sequence into the pTV00 vector (Ratcliff et al., 2001; Saedler and Baldwin, 2004). N. attenuata plants inoculated with Agrobacterium carrying pTV-NaGSNOR and empty vector (pTV00) formed NaGSNOR-VIGS and EV plants respectively. VIGS efficiently reduced the transcript levels of NaGSNOR in NaGSNOR-VIGS to about 3% of those in EV (Fig. 2A). Furthermore, the activity of NaGSNOR was 90% reduced in these plants (Fig. 2B). Consistent with the growth phenotype of Arabidopsis gsnor mutant (Lee et al., 2008), the rosette sizes of NaGSNOR-VIGS were slightly smaller than those of EV plants (see Supplementary Fig. S3 at JXB online) and in the elongated stage, NaGSNOR-VIGS plants exhibited stunted stalks, a reduced number of flower buds, and epinastic leaves. All experiments were done at the rosette stage.
Fig. 2.
NaGSNOR-VIGS plants have highly diminished transcript levels of NaGSNOR and strongly reduced GSNOR activity. N. attenuata plants were infiltrated with Agrobacterium carrying pTV00 or a pTV-NaGSNOR to generate EV and NaGSNOR-VIGS plants, respectively. (A) Transcript levels (mean ±SE) of NaGSNOR and (B) GSNOR activity (mean ±SE) were determined in EV and NaGSNOR-VIGS plants. Stars indicate significantly different levels between EV and NaGSNOR-VIGS plants (Student's t test; ***P ≤0.001; n=5).
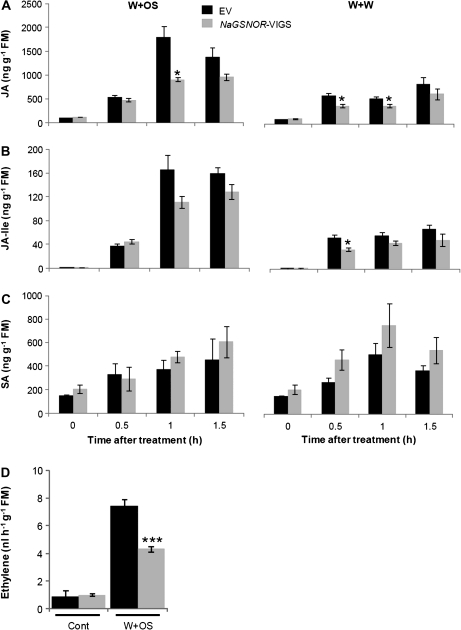
Given the central roles of phytohormones in regulating plant resistance to herbivores, it was determined whether NaGSNOR modulates wounding- and simulated herbivory-induced levels of JA/JA-Ile and ethylene. EV and NaGSNOR-VIGS plants were treated either with W+W or W+OS and JA contents were analysed in samples collected 30, 60, and 90 min after treatments. In EV plants, compared with W+W, W+OS elicited 2-fold higher levels of JA by 1 h, indicating that N. attenuata recognized herbivore elicitors, FACs, in M. sexta OS and accumulated high contents of JA; by contrast, JA contents in NaGSNOR-VIGS plants were about half those found in EV plants (Fig. 3A). Similarly, NaGSNOR-VIGS plants challenged with W+W also showed a reduced JA accumulation (Fig. 3A). The JA-Ile levels also showed a tendency to be decreased in NaGSNOR-VIGS plants after W+W and W+OS treatment (Fig. 3B). Due to the antagonistic nature between the JA and salicylic acid (SA) signalling pathway, it is possible that the suppressed JA levels in NaGSNOR-VIGS resulted from high SA contents in these plants (Pieterse et al., 2009). When untreated, statistically no significantly different levels of SA were detected between EV and NaGSNOR-VIGS (P=0.16), although NaGSNOR-silenced plants tended to have 35% more SA levels than did EV (Fig. 3C). After W+W and W+OS treatment, compared with EV, NaGSNOR-VIGS also exhibited a tendency of having maximally 50% and 30% higher SA levels (P >0.09 and 0.16, respectively) (Fig. 3C). Wounding does not increase ethylene emission from N. attenuata (Von Dahl et al., 2007), hence ethylene emissions were measured in control and W+OS-treated plants. After W+OS, NaGSNOR-VIGS exhibited about 43% reduced ethylene emission compared to EV (Fig. 3D).
Fig. 3.
Wounding- and simulated herbivory-induced levels of phytohormones in EV and NaGSNOR-VIGS plants. EV and NaGSNOR-VIGS plants were wounded with a pattern wheel and were subsequently treated with 20 μl of water (W+W) or 20 μl of M. sexta oral secretions (OS) (W+OS). (A) JA, (B) JA-Ile, and (C) SA contents (mean ±SE) were measured on a HPLC-MS/MS. (D) Ethylene (mean ±SE) emitted from non-treated (Cont) and W+OS-treated EV and NaGSNOR-VIGS plants. Stars indicate significantly different levels between EV and NaGSNOR-VIGS plants (Student's t test; *P ≤0.05; ***P ≤0.001; n=5).
Thus, it was inferred that NaGSNOR is required for wounding- and herbivory-induced accumulation of JA and herbivory-elicited biosynthesis of ethylene in N. attenuata.
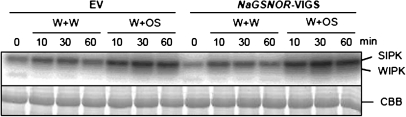
NaGSNOR-VIGS plants do not have altered activity of SIPK and WIPK
In N. attenuata, SIPK and WIPK are required for wounding- and herbivory-induced JA and ethylene biosynthesis (Wu et al., 2007). Using an in-gel kinase activity assay, SIPK and WIPK activity was determined in EV and NaGSNOR-VIGS plants 0, 10, 30, and 60 min after W+W and W+OS treatment (Fig. 4). In EV plants, W+W and W+OS rapidly activated SIPK and compared with W+W, W+OS elicited higher levels of SIPK activity. Low WIPK activity was only detected in W+OS-induced samples. Importantly, EV and NaGSNOR-VIGS plants showed similar levels of SIPK and WIPK activity at all times (Fig. 4). Therefore, the decreased JA and ethylene levels in wounding- and herbivory-induced NaGSNOR-VIGS were not due to impaired MAPK activation.
Fig. 4.
Silencing NaGSNOR does not impair wounding- and simulated herbivory-induced MAPK activity in N. attenuata. EV and NaGSNOR-VIGS plants were wounded with a pattern wheel and were subsequently applied with 20 μl of water (W+W) or 20 μl of M. sexta oral secretions (OS) (W+OS). Samples were harvested after the indicated times. An in-gel kinase activity assay (upper panel) was performed to detect the activity of SIPK and WIPK. Replicated samples were run on a SDS-PAGE gel, and this gel was thereafter stained with Coomassie Brilliant Blue (CBB) for visualization of equal loading (lower panel).
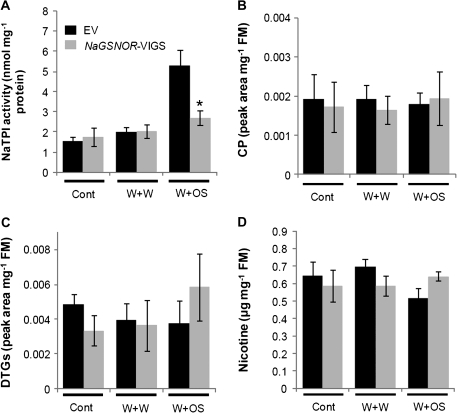
Wounding- and herbivory-induced NaTPI activity levels are compromised in NaGSNOR-VIGS plants
TPIs are important anti-herbivore compounds in solanaceous plants, including N. attenuata (Ryan, 1989; Haq et al., 2004; Zavala et al., 2004). To determine the function of NaGSNOR in regulating the response to wounding and herbivory, defence metabolites were determined in EV and NaGSNOR-VIGS plants 3 d after W+W or W+OS. NaTPI activity was not inducible after W+W and W+OS treatment in NaGSNOR-VIGS, whereas W+OS treatment elicited a 3.3-fold increase in EV plants (Fig. 5A). VIGS requires growing plants under reduced temperatures, which significantly influences secondary metabolism and can selectively alter the amount of particular secondary metabolites in plant tissue (Kaplan et al., 2004; Shohael et al., 2006). This might be the reason why the concentrations of other known JA-inducible secondary metabolites (CP, DTGs, and nicotine) did not increase after wounding and simulated herbivory treatment, even in EV plants (Fig. 5B, C, D).
Fig. 5.
Accumulation of herbivore defense-related secondary metabolites in EV and NaGSNOR-VIGS plants. Leaves of EV and NaGSNOR-VIGS plants were wounded with a pattern wheel, and were thereafter applied with 20 μl of water (W+W) or 20 μl of M. sexta oral secretions (W+OS). The activity of NaTPI (A), contents of caffeoylputrescine (CP) (B), diterpene glycosides (DTGs) (C), and nicotine (D) (mean ±SE) were determined in EV and NaGSNOR-VIGS plants 3 d after treatments; non-treated plants served as controls (Cont). Star indicates significantly different levels between EV and NaGSNOR-VIGS plants (Student's t test; *P ≤0.05; n=5).
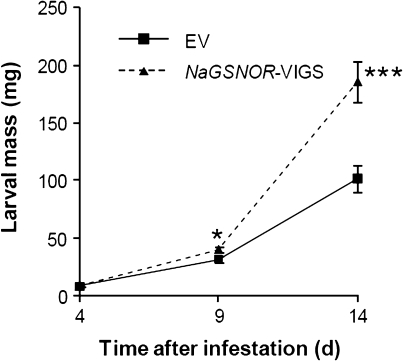
To evaluate the resistance levels of NaGSNOR-silenced plants against M. sexta attack, bioassays were performed. Neonate M. sexta larvae were grown for 14 d on EV and NaGSNOR-VIGS plants and their masses were recorded on days 4, 9, and 14. Average final larval mass on EV plants (102 mg) was only 54% of the mean mass of those reared on NaGSNOR-VIGS plants (186 mg) (Fig. 6), indicating that NaGSNOR is required for N. attenuata’s defence against M. sexta.
Fig. 6.
Silencing NaGSNOR in N. attenuata compromises plant resistance to insect herbivore, M. sexta. Neonate M. sexta larvae were placed on rosette-staged EV and NaGSNOR-VIGS plants and larval masses (mean ±SE) were measured after 4, 9, and 14 d. Stars indicate significantly different larval masses between those fed on EV and on NaGSNOR-VIGS plants (Student's t test; *, P ≤0.05; ***P ≤0.001; n=30).
NaGSNOR-VIGS plants have altered methyl jasmonate-induced responses
Changing NO levels by supplying NO donors to tomato leaves strongly suppresses transcript levels and activity of proteinase inhibitors, whereas levels of several other JA-inducible transcripts are not altered (Orozco-Cardenas and Ryan, 2002). Therefore, it was determined if silencing NaGSNOR also compromises the accumulation of NaTPI transcript levels, and other JA-inducible genes and secondary metabolites.
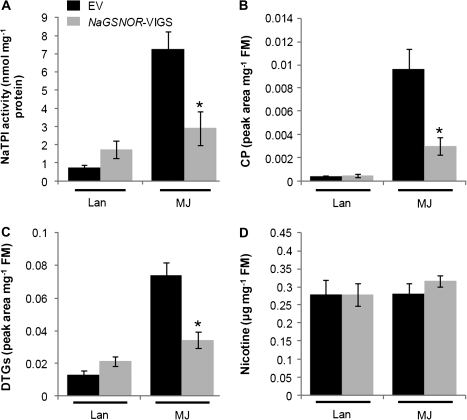
Methyl jasmonate (MJ) in 20 μl of lanolin (5 μg μl−1) was applied to plants, and plants treated with 20 μl of pure lanolin were used as controls. Defence metabolites (NaTPI, CP, and DTG) were measured 3 d after these treatments. When treated with lanolin, NaGSNOR-VIGS plants exhibited 1-fold higher levels of NaTPI activity than did EV plants (Fig. 7A). After MJ application, NaTPI activity levels increased 9.5-fold in EV plants, while only 1.7-fold in NaGSNOR-VIGS (Fig. 7A). Similarly, MJ application highly increased the levels of CP and DTGs in EV, but NaGSNOR-VIGS plants had only about 30% and 50% of the CP and DTG contents found in EV plants (Fig. 7B, C). Probably due to the relatively low growing temperatures, neither MJ treatment nor silencing NaGSNOR altered the levels of nicotine in any plants (Fig. 7D).
Fig. 7.
Herbivore defence-related secondary metabolites in EV and NaGSNOR-VIGS plants after methyl jasmonate treatment. EV and NaGSNOR-VIGS plants were applied with lanolin pastes (20 μl) containing 5 mg ml−1 methyl jasmonate (MJ) or pastes of pure lanolin (Lan) (20 μl) for comparisons. The activity of NaTPI (A), contents of caffeoylputrescine (CP) (B), diterpene glycosides (DTGs) (C), and nicotine (D) (mean ±SE) were determined in EV and NaGSNOR-VIGS plants 3 d after treatments. Stars indicate significantly different levels between EV and NaGSNOR-VIGS plants (Student's t test; *P ≤0.05; n=5).
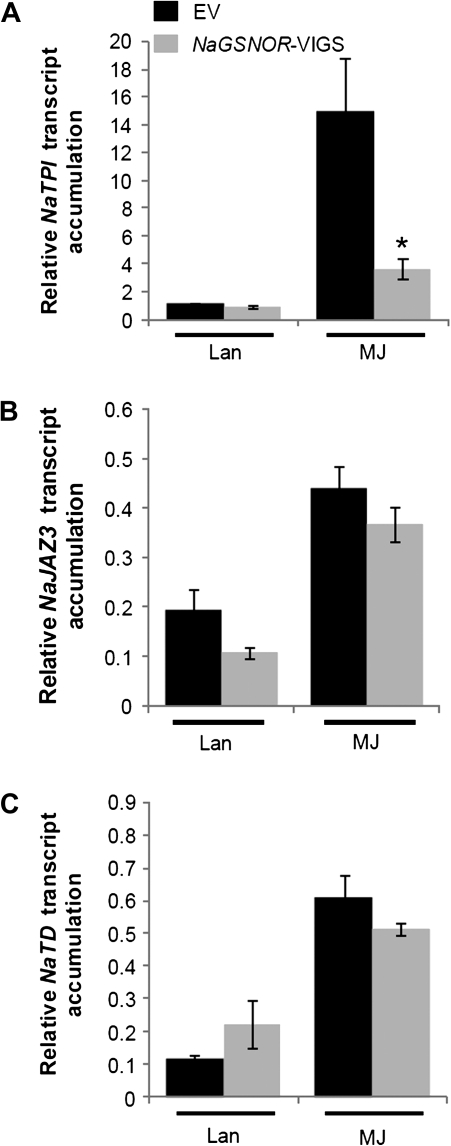
In addition, the transcript levels of several JA-inducible genes were examined. Consistent with the attenuated NaTPI activity in NaGSNOR-silenced plants, MJ treatment induced 4-fold higher NaTPI transcript levels in EV plants than in NaGSNOR-VIGS plants (Fig. 8A). Although compared with those in EV plants, somewhat lower and higher transcript levels of NaJAZ3 (jasmonate ZIM-domain 3) and NaTD (threonine deaminase) were found in control plants, after MJ treatment, transcript levels of NaJAZ3 and NaTD were the same in NaGSNOR-VIGS and EV plants (Fig. 8B, C).
Fig. 8.
Transcript levels of NaTPI, NaJAZ3, and NaTD in methyl jasmonate-treated EV and NaGSNOR-VIGS plants. EV and NaGSNOR-VIGS plants were applied with lanolin pastes (20 μl) containing 5 mg ml−1 methyl jasmonate (MJ), or pastes of pure lanolin (Lan) (20 μl) for comparison. The transcript levels of NaTPI (A), NaJAZ3 (B), and NaTD (C) (mean ±SE) were determined in EV and NaGSNOR-VIGS plants 8 h after treatments. Stars indicate significantly different levels between EV and NaGSNOR-VIGS plants (Student's t test; *P ≤0.05; n=5).
Therefore, it was inferred that NaGSNOR is required for certain, but not all, JA-induced responses in N. attenuata.
Discussion
GSNORs have structural features that are highly conserved in bacteria, animals, and plants (Martínez et al., 1996; Fliegmann and Sandermann, 1997; Liu et al., 2001). In mice, silencing GSNOR leads to increased damage in the lymphatic and liver tissue after being challenged with bacterial endotoxin (Liu et al., 2004). Arabidopsis AtGSNOR1 is a positive regulator of plant immunity against phytopathogens (Feechan et al., 2005). It is shown here that in N. attenuata, NaGSNOR plays an essential role in wounding responses and plant defence against the specialist insect herbivore, M. sexta.
NO rapidly reacts with glutathione and forms GSNO; in addition, it modifies cysteine and tyrosine residues in proteins and therefore forms nitrosylated cysteine and tyrosine. Consistent with the biochemical function of GSNOR, GSNOR–/– mutant mice have high S-nitrosothiol (SNO) haemoglobin levels in red blood cells, which is probably associated with increased NO levels (Liu et al., 2004). Similarly, the Arabidopsis gsnor mutant also exhibits greatly elevated levels of NO, nitrate, SNO, and N-nitroso species (Feechan et al., 2005; Lee et al., 2008). Many proteins, especially those involved in signal transduction, are targets of nitrosylation (Lindermayr et al., 2005; Grennan, 2007; Besson-Bard et al., 2008). In agreement with this, NaGSNOR is required for wounding- and simulated herbivory-induced accumulation of phytohormones (JA/JA-Ile and ethylene) and NaGSNOR is also important for certain responses induced by JA, including the accumulation of defence-related secondary metabolites, suggesting its role in transducing certain aspects of JA signalling.
In plants, JA plays a central role in defence against herbivore stress (Kessler et al., 2004; Howe and Jander, 2008; Wu and Baldwin, 2010). Although almost all the enzymes involved in JA biosynthesis have been identified in various plant species (Wasternack, 2007), little is known about how JA biosynthesis is regulated. Our data indicated that NaGSNOR is positively associated with the levels of wounding- and herbivory-induced JA in N. attenuata. However, how NaGSNOR is involved in the regulation of JA homeostasis remains elusive. It is possible that NaGSNOR-silenced plants over-accumulate GSNO (a source of NO) which may nitrosylate certain JA biosynthetic enzymes and thus decrease their activity. At least one enzyme in the oxylipin pathway for JA biosynthesis, allene oxide cyclase (AOC), has been identified to be a nitrosylation target (Romero-Puertas et al., 2008). Studies in many plant species demonstrated that SA suppresses the accumulation of JA (Spoel et al., 2003; Diezel et al., 2009; Pieterse et al., 2009), and that NPR1 (non-expresser of PR genes1) is important for the suppression effect of SA on JA accumulation and signalling (Spoel et al., 2003). Importantly, NPR1 is also nitrosylated in planta and nitrosylation is important for the homeostasis of NPR1 (Tada et al., 2008). Recently, Lindermayr et al. (2010) demonstrated that GSNO nitrosylates both NPR1 and TGA1, an important transcription factor that activates transcription of PR (pathogenesis-related) genes after binding of NPR1; furthermore, translocation of NPR1 to the nucleus, which is required for the activation of NPR1-induced responses, requires NO. Therefore, in addition to the tendency of increased SA levels in NaGSNOR-silenced plants, which may have some effect on the suppression of JA production (Pieterse et al., 2009), there was speculation that the likely elevated levels of GSNO may increase nitrosylation of NPR1 and thereby enhance NPR1 activity, which, in turn, promotes the suppression of JA accumulation by SA. This hypothesis needs to be examined further.
Compared with JA biosynthesis, ethylene production requires fewer enzymes. Methionine is converted to S-adenosylmethionine (S-AdoMet) by S-AdoMet synthases (SAMSs), and the conversion of S-AdoMet to 1-aminocyclopropane-1-carboxylic acid (ACC) is mediated by ACC synthases (ACSs). ACOs (ACC oxidases) further catalyse the oxidation of ACC to form ethylene (Wang et al., 2002). Among these key enzymes, SAMSs (also methionine adenosyltransferases, MATs) are targets of nitrosylation (Lindermayr et al., 2005), and an in vitro assay suggested that nitrosylation of certain SAMS inhibits its activity (Lindermayr et al., 2006). Consistent with this scenario, in NaGSNOR-silenced plants, herbivory-induced ethylene emissions are greatly compromised. Whether silencing NaGSNOR alters the activity of other ethylene biosynthetic enzymes (ACSs and ACOs) also requires further study.
In N. attenuata, SIPK and WIPK are regulators of wounding- and herbivory-induced biosynthesis of JA (Wu et al., 2007). Moreover, activation of SIPK in N. attenuata and its homologue (AtMPK6) in Arabidopsis is required for 50% of the ethylene emitted after herbivory and pathogen elicitor (flagellin) elicitation (Liu and Zhang, 2004; Wu et al., 2007). However, kinase activity assays revealed either that NaGSNOR modulates the levels of JA and ethylene in a MAPK-independent manner or that NaGSNOR functions downstream of MAPKs.
Supplying excised tomato leaves with NO donors strongly inhibits JA-induced proteinase inhibitor expression and activity; however, JA-induced transcript levels of several signalling pathway-related genes are not altered (Orozco-Cardenas and Ryan, 2002). Similarly, NaGSNOR appears to be important for some but not all JA-induced responses: after MJ treatment, NaGSNOR activity is required for sufficient up-regulation of the genes that are involved in the biosynthesis of NaTPI, CP, and DTGs, but is not important for the transcriptional regulation of NaJAZ3 and NaTD. It is very unlikely that silencing NaGSNOR compromises the activity of the JA-Ile receptor, COI1, or the activity of the SCF(COI1) complex, given that at least two JA-inducible genes, NaJAZ3 and NaTD, have similar levels of transcripts in EV and NaGSNOR-VIGS plants after MJ induction. This also ruled out the possibility that NaGSNOR-VIGS plants have decreased activity of MJ esterase, which releases JA from the inactive MJ (JA is further converted to JA-Ile and therefore activate jasmonate-induced responses) (Wu et al., 2008). In addition to its function in suppression of JA accumulation, NPR1 also plays a critical role in mediating the antagonism between SA and JA signalling (Pieterse et al., 2009). Whether NaGSNOR-deficient plants have enhanced NPR1 activity and therefore have elevated inhibition of certain JA-induced responses by SA needs to be examined.
After wounding, Arabidopsis GSNOR exhibits reduced abundance of both transcripts and protein (Diaz et al., 2003), and this is congruent with increased NO levels induced by wounding (Huang et al., 2004). Recently, wounding was also found to attenuate the activity of GSNOR in sunflower seedlings (Chaki et al., 2011). Although wounding does not alter the activity of NaGSNOR in N. attenuata, simulated herbivory induces a transient decline. These data suggest that compared with mechanical wounding, herbivory not only specifically modifies transcript levels of various genes, the abundance of proteins and secondary metabolites, but also the status of protein posttranslational modification (e.g. nitrosylation and phosphorylation) in plant cells (Foyer and Noctor, 2005; Moreau et al., 2010). Given that diminishing the activity of NaGSNOR using gene silencing compromises plant resistance to M. sexta, the rapid reduction and subsequent regaining of NaGSNOR activity after herbivory implies that a transient decrease of NaGSNOR activity is required for the optimum induction of herbivory-specific defence reactions, which involves a reconfiguration of the protein nitrosylation status. Given the positive association between GSNOR activity and plant defence levels, it is proposed that GSNOR could, potentially, be a target of genetic modification for improving insect resistance in crops.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Alignment of protein sequences of GSNOR in Nicotiana attenuata, Solanum lycopersicum, and Arabidopsis thaliana.
Supplementary Fig. S2. Southern blotting analysis of NaGSNOR in N. attenuata.
Supplementary Fig. S3. Morphology of EV and NaGSNOR-VIGS plants.
Supplementary Table S1. Primer pairs used for qRT-PCR.
Acknowledgments
We thank Dr Klaus Gase, Thomas Hahn, Susan Kutschbach, and Antje Wissgott for sequencing and preparation of the VIGS construct, Dr Tamara Krügel, Andreas Weber, and Andreas Schünzel for their help with plant cultivation, Dahai Yang, Christian Hettenhausen, Stefan Meldau, Maria Heinrich, and Christina Bartnitzek for technical assistance and discussions, and the Max Planck Society for funding.
References
- Baldwin IT. An ecologically motivated analysis of plant–herbivore interactions in native tobacco. Plant Physiology. 2001;127:1449–1458. [PMC free article] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annual Review of Plant Biology. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- Chaki M, Valderrama R, Fernández-Ocaña AM, et al. Mechanical wounding induces a nitrosative stress by down-regulation of GSNO reductase and an increase in S-nitrosothiols in sunflower (Helianthus annuus) seedlings. Journal of Experimental Botany. 2011;62:1803–1813. doi: 10.1093/jxb/erq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-S. Inducible direct plant defense against insect herbivores: a review. Insect Science. 2008;15:101–114. doi: 10.1111/1744-7917.12436. [DOI] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernandez G, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- Diaz M, Achkor H, Titarenko E, MartInez MC. The gene encoding glutathione-dependent formaldehyde dehydrogenase/gsno reductase is responsive to wounding, jasmonic acid and salicylic acid. FEBS Letters. 2003;543:136–139. doi: 10.1016/s0014-5793(03)00426-5. [DOI] [PubMed] [Google Scholar]
- Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiology. 2009;150:1576–1586. doi: 10.1104/pp.109.139550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant–pathogen interactions. Nature Reviews Genetics. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- Feechan A, Kwon E, Yun B-W, Wang Y, Pallas JA, Loake GJ. A central role for S-nitrosothiols in plant disease resistance. Proceedings of the National Academy of Sciences, USA. 2005;102:8054–8059. doi: 10.1073/pnas.0501456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegmann J, Sandermann H. Maize glutathione-dependent formaldehyde dehydrogenase cdna: a novel plant gene of detoxification. Plant Molecular Biology. 1997;34:843–854. doi: 10.1023/a:1005872222490. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grennan AK. Protein S-nitrosylation: potential targets and roles in signal transduction. Plant Physiology. 2007;144:1237–1239. doi: 10.1104/pp.104.900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F-Q, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Baldwin IT. Antisense lox expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. The Plant Journal. 2003;36:794–807. doi: 10.1046/j.1365-313x.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiology. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq SK, Atif SM, Khan RH. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection. Archives of Biochemistry and Biophysics. 2004;431:145–159. doi: 10.1016/j.abb.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Heil M, Baldwin IT. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends in Plant Science. 2002;7:61–67. doi: 10.1016/s1360-1385(01)02186-0. [DOI] [PubMed] [Google Scholar]
- Heiling S, Schuman MC, Schoettner M, Mukerjee P, Berger B, Schneider B, Jassbi AR, Baldwin IT. Jasmonate and pphsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. The Plant Cell. 2010;22:273–292. doi: 10.1105/tpc.109.071449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Huang X, Stettmaier K, Michel C, Hutzler P, Mueller M, Durner J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta. 2004;218:938–946. doi: 10.1007/s00425-003-1178-1. [DOI] [PubMed] [Google Scholar]
- Jassbi AR, Gase K, Hettenhausen C, Schmidt A, Baldwin IT. Silencing geranylgeranyl diphosphate synthase in Nicotiana attenuata dramatically impairs resistance to tobacco hornworm. Plant Physiology. 2008;146:974–986. doi: 10.1104/pp.107.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma MA, Bakker PL, Visser B, Stiekema WJ. Trypsin inhibitor activity in mature tobacco and tomato plants is mainly induced locally in response to insect attack, wounding and virus infection. Planta. 1994;195:29–35. [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gabler R, Kuhnemann F, Preston CA, Baldwin IT. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta. 2000;210:336–342. doi: 10.1007/PL00008142. [DOI] [PubMed] [Google Scholar]
- Kang J-H, Wang L, Giri A, Baldwin IT. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. The Plant Cell. 2006;18:3303–3320. doi: 10.1105/tpc.106.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology. 2004;136:4159–4168. doi: 10.1104/pp.104.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Heinzel N, Schoettner M, Baldwin IT, Galis I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid–polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiology. 2010;152:1731–1747. doi: 10.1104/pp.109.151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinanen M, Oldham NJ, Baldwin IT. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. Journal of Agricultural and Food Chemistry. 2001;49:3553–3558. doi: 10.1021/jf010200+. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Annual Review of Plant Biology. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: induced plant defenses and insect populations. Science. 2004;305:665–668. doi: 10.1126/science.1096931. [DOI] [PubMed] [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12:177–183. [Google Scholar]
- Lee U, Wie C, Fernandez BO, Feelisch M, Vierling E. Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. The Plant Cell. 2008;20:786–802. doi: 10.1105/tpc.107.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Schilmiller AL, Liu G, et al. Role of {beta}-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. The Plant Cell. 2005;17:971–986. doi: 10.1105/tpc.104.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. The tomato homolog of coronatine-insensitive1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindermayr C, Saalbach G, Bahnweg G, Durner J. Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. Journal of Biological Chemistry. 2006;281:4285–4291. doi: 10.1074/jbc.M511635200. [DOI] [PubMed] [Google Scholar]
- Lindermayr C, Saalbach G, Durner J. Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiology. 2005;137:921–930. doi: 10.1104/pp.104.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindermayr C, Sell S, Muller B, Leister D, Durner J. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. The Plant Cell. 2010;22:2894–2907. doi: 10.1105/tpc.109.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- Liu L, Yan Y, Zeng M, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. The Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez MC, Achkor H, Persson B, Fernández MR, Shafqat J, Farrés J, Jörnvall H, Parés X. Arabidopsis formaldehyde dehydrogenase. Molecular properties of plant class III alcohol dehydrogenase provide further insights into the origins, structure and function of plant class P and liver class I alcohol dehydrogenases. European Journal of Biochemistry. 1996;241:849–857. doi: 10.1111/j.1432-1033.1996.00849.x. [DOI] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Abiotic stress, the field environment and stress combination. Trends in Plant Science. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Moreau M, Lindermayr C, Durner J, Klessig DF. No synthesis and signaling in plants: where do we stand? Physiologia Plantarum. 2010;138:372–383. doi: 10.1111/j.1399-3054.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. The Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Ryan CA. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiology. 2002;130:487–493. doi: 10.1104/pp.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. The Plant Journal. 2007;51:79–91. doi: 10.1111/j.1365-313X.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC. Technical advance: tobacco rattle virus as a vector for analysis of gene function by silencing. The Plant Journal. 2001;25:237–245. doi: 10.1046/j.0960-7412.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Campostrini N, Mattè A, Righetti PG, Perazzolli M, Zolla L, Roepstorff P, Delledonne M. Proteomic analysis of S-nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Protomics. 2008;8:1459–1469. doi: 10.1002/pmic.200700536. [DOI] [PubMed] [Google Scholar]
- Ross C, Küpper FC, Jacobs RS. Involvement of reactive oxygen species and reactive nitrogen species in the wound response of Dasycladus vermicularis. Chemistry and Biology. 2006;13:353–364. doi: 10.1016/j.chembiol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Rusterucci C, Espunya MC, Diaz M, Chabannes M, Martinez MC. S-nitrosoglutathione reductase affords protection against pathogens in arabidopsis, both locally and systemically. Plant Physiology. 2007;143:1282–1292. doi: 10.1104/pp.106.091686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA. Proteinase inhibitor gene families: strategies for transformation to improve plant defenses against herbivores. BioEssays. 1989;10:20–24. doi: 10.1002/bies.950100106. [DOI] [PubMed] [Google Scholar]
- Saedler R, Baldwin IT. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. Journal of Experimental Botany. 2004;55:151–157. doi: 10.1093/jxb/erh004. [DOI] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. The Plant Cell. 2004;16:616–628. doi: 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Ueda M, Morikawa H. Arabidopsis glutathione-dependent formaldehyde dehydrogenase is an S-nitrosoglutathione reductase. FEBS Letters. 2002;515:20–24. doi: 10.1016/s0014-5793(02)02414-6. [DOI] [PubMed] [Google Scholar]
- Shohael A, Ali M, Yu K-W, Hahn E-J, Paek K- Y. Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell, Tissue and Organ Culture. 2006;85:219–228. [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant Cell. 2003;15:760–770. [Google Scholar]
- Stamler JS, Lamas S, Fang FC. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. The Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine's defensive function in nature. PLoS Biology. 2004;2:e217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321:952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. JAZ repressor proteins are targets of the scfcoi1 complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- Von Dahl CC, Winz RA, Halitschke R, Kühnemann F, Gase K, Baldwin IT. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. The Plant Journal. 2007;51:293–307. doi: 10.1111/j.1365-313X.2007.03142.x. [DOI] [PubMed] [Google Scholar]
- Wang KL-C, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. The Plant Cell. 2002;14:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yun BW, Kwon E, Hong JK, Yoon J, Loake GJ. S-nitrosylation: an emerging redox-based post-translational modification in plants. Journal of Experimental Botany. 2006;57:1777–1784. doi: 10.1093/jxb/erj211. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: aAn update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ID, Neill SJ, Hancock JT. Nitric oxide synthesis and signalling in plants. Plant, Cell and Environment. 2008;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. Herbivory-induced signalling in plants: perception and action. Plant, Cell and Environment. 2009;32:1161–1174. doi: 10.1111/j.1365-3040.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Baldwin I. Evolution of proteinase inhibitor defenses in North American allopolyploid species of Nicotiana. Planta. 2006;224:750–760. doi: 10.1007/s00425-006-0256-6. [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. The Plant Cell. 2007;19:1096–1122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang L, Baldwin I. Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA? Planta. 2008;227:1161–1168. doi: 10.1007/s00425-008-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Letters. 2000;468:89–92. doi: 10.1016/s0014-5793(00)01203-5. [DOI] [PubMed] [Google Scholar]
- Zavala J, Baldwin IT. Fitness benefits of trypsin proteinase inhibitor expression in Nicotiana attenuata are greater than their costs when plants are attacked. BMC Ecology. 2004;4 doi: 10.1186/1472-6785-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui D, Baldwin IT. Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiology. 2004;134:1181–1190. doi: 10.1104/pp.103.035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. Salicylic acid activates a 48-kD MAP kinase in tobacco. The Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.