Abstract
Despite the long history of drug discovery from natural sources, the marine environment, which covers 70% of the Earth’s surface, is still relatively unexplored. Intense competition for limited resources drives the evolution of specific and potent chemical defenses distinct from their terrestrial counterparts. Based on this rationale, we recently began screening extracts derived from marine invertebrate and cyanobacterial samples for BACE-1 inhibitors in a chemiluminescent enzyme-fragment complementation (EFC) assay. The results of this broad screening are presented here, along with our progress towards the development of a secondary LC-MS homogeneous affinity assay. Incubation of the extracts active in the EFC assay with BACE1, subsequent isolation of the enzyme-inhibitor complex and then analysis of the small molecule inhibitor by LC-MS rapidly links a chemical structure to biological activity. This approach enables the rapid target-orientated discovery of BACE-1 inhibitors from marine sources.
Keywords: Marine natural products, BACE, drug discovery
Alzheimer Disease is a progressive neurodegenerative disorder for which there is no cure. It affects approximately five million people in the US alone. Worldwide a new case is diagnosed every 70 seconds [1]. Recent studies have begun to unravel the cellular processes that lead to the development of Alzheimer’s disease, which has provided a wealth of potential therapeutic targets. Despite these advances, there is still a critical need to develop effective therapeutics for the treatment of Alzheimer’s disease. The question is how to find them?
NATURE AS A SOURCE OF DRUG LEADS
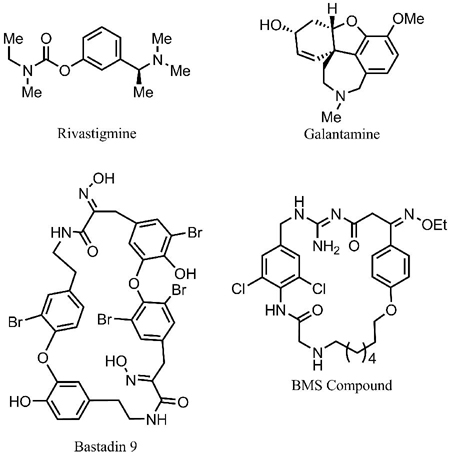
The discovery by Fleming and Florey that microbial metabolites could exert profound biological effects on human pathogens, catalyzed 50 years of intensive research exploring Nature for cures to many human ailments [2]. Modern pharmacology is founded on drugs derived from natural sources and the fundamental biological insights gleaned from their mechanism of action studies. By a recent estimate, 60% of all currently approved antibiotics and over 50% of all anti-cancer agents are derived from microorganisms or plant products [3]. Of the four commonly prescribed drugs for the treatment of Alzheimer’s Disease in the US [Donepezil (Aricept), Rivastigmine (Exelon), Memantine (Namenda) and Galantamine (Razadyne)], two are derived from natural sources. Galantamine is produced by daffodils (Narcissus sp.) and several other plants within the order Amaryllidaceae. Extracts of these plants were widely used in Europe as a traditional medicine before the development of galantamine by Janssen Pharmaceutica as a medication to treat Alzheimer’s disease [4,5]. Rivastigmine, developed by Novartis, is a simplified analogue of physostigmine, which is produced by the West African perennial climbing plant Physostigma venenosum [6]. Despite these years of research, only a small fraction of the Earth’s total biodiversity has been examined. For example, the oceans cover over 70% of the Earth’s surface yet we know comparatively little about the biology, chemistry, and even the identity of its inhabitants. Undoubtedly, a wealth of therapeutics remains to be discovered from the oceans [7].
MODERN DAY NATURAL PRODUCTS LEAD DISCOVERY
Why should any organism produce small molecules to treat human disease? Does a sponge develop Alzheimer’s Disease? The answer to the latter question is clearly, no, but the production of these compounds in the host organism is driven by an evolutionary pressure. While these compounds are most often produced for a defensive purpose, a number of other functions have been ascribed including the promotion of wound healing [8] and the modulation of biological signaling [9,10]. Natural products have been preselected to interact with specific biological targets that often share structural and mechanistic features with macromolecules relevant to maintaining proper human health or to the development of disease states [11]. These similarities in protein structures and biochemical pathways, enabled Nobel Laureate Eric Kandel’s pioneering work with the marine sea slug Aplysia that defined memory functions due to the changes in the form and function of synapses [12]. So while a marine invertebrate would not have specifically evolved a small molecule to slow the development of Alzheimer’s Disease, it is under constant evolutionary pressure to produce a diverse array of biologically active compounds, such as protease inhibitors, which improve the overall fitness of the organism by interacting with targets similar to those involved in human diseases.
Extracellular Aβ is believed to trigger a neurotoxic cascade leading to the observed behavioral effects that are hallmarks of Alzheimer’s disease [13]. Thus, reducing the rate of Aβ formation represents one potential therapeutic strategy. This reduction could be effected by inhibiting the aspartic protease BACE1 [memapsin], as cleavage of its substrate APP is the penultimate step in the formation of Aβ [14]. The development of viable BACE1 inhibitors has been difficult though, and a greater diversity of high quality lead structures is required. Naturally occurring compounds produced by marine organisms are rich sources of potential lead chemicals covering a chemical space complementary to synthetic chemical libraries, and, in many cases, enhanced potency, cell permeability, selectivity, and proteolytic stability have been designed into these natural products through evolutionary pressures [15].
Based on this rationale, we have established a screening protocol involving a BACE1 enzyme fragment complementation assay (referred to as EFC) [16]. In this assay, two genetically engineered fragments of E. coli β-galactosidase recombine (“complement”) in vitro to form an active β-galactosidase enzyme, which subsequently hydrolyses the chemiluminescent substrate. One fragment, the ED, is initially bound within a small cyclic peptide that contains the BACE1 proteolytic cleavage site. When conformationally constrained, the two β-galactosidase fragments have little affinity for one another and consequently, no signal is generated. However, after BACE1 acts on the cleavage site in the cyclized peptide, the resulting linear peptide can complement, form the active β-galactosidase, and generate a chemiluminescent signal. The system has proven easily amenable to high-throughput screening, is ten times more sensitive than a comparable FRET-based assay, and is not plagued by the autofluorescence issues common to FRET-based assays [16]. The assay is reproducible (Z-factor of 0.7) and can reliably distinguish hits from controls (signal window of 4 and a S/N ratio of 10:1). All these factors are acceptable for high throughput screening.
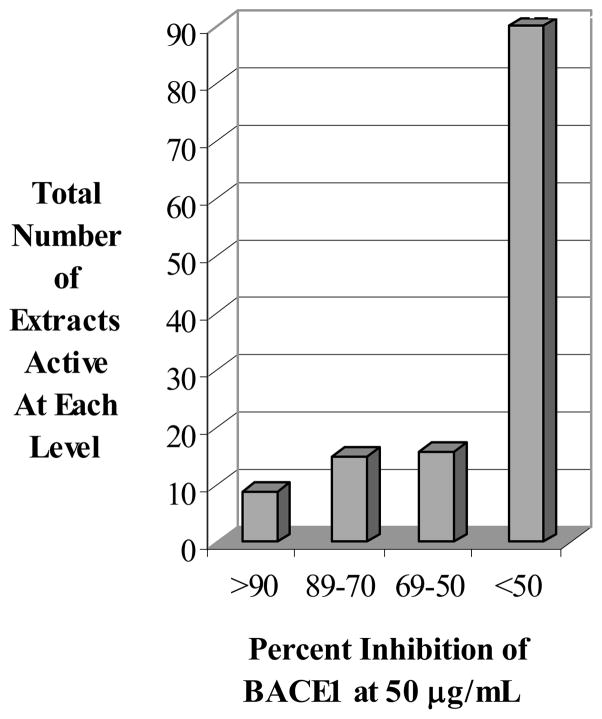
Our initial studies focused on screening 130 prefractionated extracts in this assay. These extracts were primarily derived from marine invertebrates and cyanobacteria collected in the Pacific. Each extract was fractionated into four samples by reversed-phase chromatography prior to screening. Fractionated samples were placed randomly in 96-well plates and screened in triplicate at a fixed concentration of 50 μg/mL. In this initial EFC screen, 7% of those extracts tested inhibited greater than 90% of BACE1 activity, while an additional 11% reduced BACE1 activity between 70–89% (Fig. (1)). Separation of the first extract using a traditional bioassay-guided approach ascribed the observed activity to a class of compounds represented by Bastadin 9. At that time, the bastadins were a new structural class of submicromolar BACE1 inhibitors, as no reports of other oxime (C=NOH) based inhibitors had been disclosed. Interestingly, last year Bristol-Myers Squibb patented a series of oxime-based cyclic nanomolar BACE1 inhibitors that resemble the upper half of bastadins [17], which permeate the blood-brain barrier and display nanomolar potency. These data suggest a critical evaluation of the BACE1 inhibitory effect of the bastadins is warranted and provides additional support for our original hypothesis that screening chemical libraries derived from marine sources for BACE1 inhibitors will lead to new inhibitors. Evaluation of the bastadin class of compounds as BACE1 inhibitors is ongoing.
Fig 1.
BACE1 EFC Screening Results of Marine Invertebrate Extracts
LINKING BIOLOGICAL ACTIVITY TO CHEMICAL STRUCTURE
Over the last two decades though, there has been a reduced emphasis on natural products as a source of pharmaceutical leads. Ultimately, the modern drug discovery timeline of hit to clinic in two years [18] is incompatible with the traditional iterative cycle of natural products lead discovery. The initial biological testing using crude mixtures must be followed by chromatography to separate the constituents and subsequent biological testing. This time-consuming process is often repeated through several cycles before a pure chemical entity is obtained for chemical and biological characterization. Fundamentally this iterative process is necessary to link a specific chemical structure in the mixture to the observed biological activity. Only through the repeated cycles of purification and testing can the activity be linked to any particular compound in the mixture. Several strategies have been developed to accelerate this process. The most popular is automated purification of crude extracts to generate pre-purified libraries before chemical screening [19], although this can also be time-consuming.
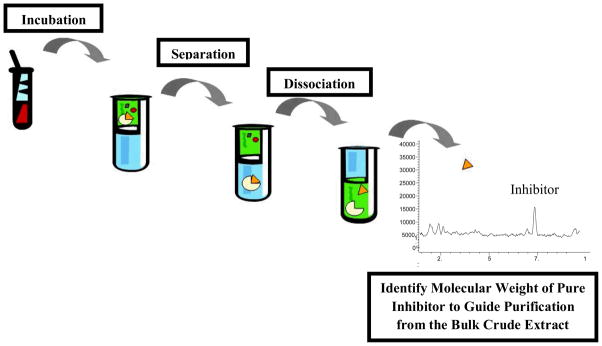
Potentially another complementary method to link biological activity to a specific chemical in a mixture are homogeneous affinity assays coupled to a LC-MS [20]. After initial testing in our primary EFC assay, using this as a secondary assay would allow a rapid targeted isolation of the inhibitor from the mixture using the high resolution molecular weight as an unique identifier. In this vein, our recent efforts have focused on development of an indirect BACE1 homogeneous affinity assay. Our preliminary results indicate the feasibility of this approach. Briefly, samples active in the initial EFC screen are incubated with BACE1 for 24 hours, and then the enzyme-inhibitor complex is separated from the other small molecules. After dissociation of the complex, the small molecule inhibitor is directly analyzed by LC-MS (Fig. (2)). Once optimized and integrated into our discovery platform, this assay has the potential to break the iterative cycle of natural products lead discovery and accelerate the identification of potential novel Alzheimer’s drug leads from marine sources.
Fig 2.
Indirect LC-MS Homogeneous Affinity Assay
Acknowledgments
This work was funded by grants from the Alzheimer’s Drug Discovery Foundation (281204), Victoria S. and Bradley L. Geist Foundation (20070461), the National Science Foundation (OCE04-32479), and the National Institute of Environmental Health Sciences (P50 ES012740), the Alzheimer’s Association (NIRG-08-90880), and the National Institute of Aging (1R21AG032405). Upgrades of the NMR instrumentation used in the work were provided by the CRIF program of the NSF (CH E9974921) and the Elsa Pardee Foundation. The purchase of the Agilent LC-MS was funded by the DOD (W911NF-04-1-0344).
References
- 1.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s and Dementia. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Fenical WH, Jensen PJ. Developing a new resource for drug discovery: marine actinomycete bacteria. Nature Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. Natural Products as Sources of Drugs over the Last 25 Years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 4.Proskurnina NF, Yakovleva AP. Alkaloids of Galanthus woronowi. II Isolation of a new alkaloid. Zhurnal Obshchei Khimii. 1952;22:1899–1902. [Google Scholar]
- 5.Heinrich M, Teoh HL. Glanthamine from snowdrop-the devlopment of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J Ethnopharm. 2004;92:147–162. doi: 10.1016/j.jep.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nature Rev Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 8.Adolph S, Jung V, Rattke J, Pohnert G. Wound closure in the invasive green alga Caulerpa taxifolia by enzymatic activation of a protein cross-linker. Angew Chem, Int Ed. 2005;44:2806–2808. doi: 10.1002/anie.200462276. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura K, Matsunaga S, Fusetani N. Phosphatidylcholine profile-mediated group recognition in catfish. J Exper Biol. 2007;210:1992–1999. doi: 10.1242/jeb.02777. [DOI] [PubMed] [Google Scholar]
- 10.Yambe H, et al. L-Kynurenine, an amino acid identified as a sex pheromone in the urine of ovulated female masu salmon. Proc Nat Acad Sci USA. 2006;103:15370–15374. doi: 10.1073/pnas.0604340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McArdle BM, Quinn RJ. Identification of protein fold topology shared between different folds inhibited by natural products. ChemBioChem. 2007;8:788–798. doi: 10.1002/cbic.200700035. [DOI] [PubMed] [Google Scholar]
- 12.Kandell ER. The Biology of Memory: A Forty-Year Perspective. J Neurosci. 2009;29:12748–12756. doi: 10.1523/JNEUROSCI.3958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy J, Allsop D. Amyloid deposition as the central event in the etiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 14.Citron M. Strategies for disease modification in Alzheimer’s disease. Nature Rev Neurosci. 2004;5:677–685. doi: 10.1038/nrn1495. [DOI] [PubMed] [Google Scholar]
- 15.Clardy J, Walsh C. Lessons from natural molecules. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 16.Eglen RM. Enzyme fragment complementation: a flexible high throughput screening assay technology. Assay Drug Dev Techn. 2002;1:97–104. doi: 10.1089/154065802761001356. [DOI] [PubMed] [Google Scholar]
- 17.Wu YJ, Gerritz S, Shi S, Zhu S, inventors. Bristol-Myers Squibb, assignee. Oxime-containing macrocyclic acyl guanidines as beta-secretase inhibitors and their preparation. 2008139523. United States patent application US. 2008 June 12;
- 18.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 19.Eldridge GR, et al. High-Throughput Method for the Production and Analysis of Large Natural Product Libraries for Drug Discovery. Anal Chem. 2002;74:3963–3971. doi: 10.1021/ac025534s. [DOI] [PubMed] [Google Scholar]
- 20.Vu H, Pham NB, Quinn RJ. Direct screening of natural product extracts using mass spectrometry. J Biomol Screen. 2008;13:265–275. doi: 10.1177/1087057108315739. [DOI] [PubMed] [Google Scholar]