Abstract
Objective
To examine the pinocytotic pathways mediating native low-density lipoprotein (LDL) uptake by human macrophage colony-stimulating factor–differentiated macrophages (the predominant macrophage phenotype in human atherosclerotic plaques).
Methods and Results
We identified the kinase inhibitor SU6656 and the Rho GTPase inhibitor toxin B as inhibitors of macrophage fluid-phase pinocytosis of LDL. Assessment of macropinocytosis by time-lapse microscopy revealed that both drugs almost completely inhibited macropinocytosis, although LDL uptake and cholesterol accumulation by macrophages were only partially inhibited (approximately 40%) by these agents. Therefore, we investigated the role of micropinocytosis in mediating LDL uptake in macrophages and identified bafilomycin A1 as an additional partial inhibitor (approximately 40%) of macrophage LDL uptake that targeted micropinocytosis. When macrophages were incubated with both bafilomycin A1 and SU6656, inhibition of LDL uptake was additive (reaching 80%), showing that these inhibitors target different pathways. Microscopic analysis of fluid-phase uptake pathways in these macrophages confirmed that LDL uptake occurs through both macropinocytosis and micropinocytosis.
Conclusion
Our findings show that human macrophage colony-stimulating factor–differentiated macrophages take up native LDL by macropinocytosis and micropinocytosis, underscoring the importance of both pathways in mediating LDL uptake by these cells.
Keywords: atherosclerosis, macrophages, LDL, macropinocytosis, micropinocytosis
Atherosclerosis is characterized by cholesterol accumulation within medium and large elastic arteries. Macrophages are present at all stages of the disease and facilitate cholesterol accumulation and inflammation that can lead to infarction.1 Thus, the uptake of LDL by macrophages is a critical process in the development of atherosclerosis. Modification of LDL is believed to be necessary for the uptake of LDL by macrophages.2 However, previous studies show that human monocyte–derived macrophages take up physiological levels of native LDL found unbound in the interstitial fluid of the vessel wall (0.7 to 2.7 mg/mL)3–5 by fluid-phase pinocytosis, causing the formation of foam cells, a hallmark of atherosclerosis.6,7 Fluid-phase pinocytosis also occurs in macrophages within atherosclerotic lesions of apolipoprotein E–knockout mice, indicating that macrophage fluid-phase pinocytosis of LDL is an important mechanism of foam cell formation in vivo.8
Fluid-phase pinocytosis is a cellular mechanism of receptor-independent solute uptake that occurs by the formation of large vacuoles (>0.5 µm) through macropinocytosis and/or small vesicles (<0.2 µm) through micropinocytosis.9,10 Macropinosomes form by actin-rich plasma membrane protrusions extending from the cell and subsequently fusing with the plasma membrane, resulting in the engulfment of large volumes of fluid. In contrast, micropinosomes form by membrane invagination and scission to form small vesicles that, because of their size, engulf much smaller amounts of fluid than macropinosomes. By these mechanisms of uptake, solute present within fluid is taken up by cells at levels that are directly proportional to the concentration of solute present within the fluid. In contrast to receptor-mediated endocytosis, solute uptake by fluid-phase pinocytosis does not require receptors and, therefore, does not show receptor saturation. Consequently, when concentrations of solute are high, similar to LDL present within the vessel wall, substantial cellular uptake of solute occurs.
Atherosclerotic lesions contain cytokines that promote monocyte differentiation into macrophages. In particular, the cytokine macrophage colony-stimulating factor (M-CSF) is critical for the proliferation and survival of macrophages and is necessary for the development of atherosclerosis in mice.11,12 Human monocytes cultured in the presence of M-CSF differentiate into macrophages with an elongated and heavily vacuolated phenotype.7 This macrophage phenotype, as assessed by surface marker expression and morphology, is the predominant macrophage phenotype found within human atherosclerotic lesions.13 Macrophages of this phenotype constitutively take up native LDL through fluid-phase pinocytosis and form foam cells when incubated with concentrations of LDL found within the human vessel wall7; however, the pinocytotic pathways that mediate uptake of LDL have not been thoroughly examined. In this study, we assessed these pathways and found that human M-CSF–differentiated macrophages take up substantial amounts of LDL by both macropinocytosis and micropinocytosis.
Methods
Detailed methods can be found in the supplemental material (available online at http://atvb.ahajournals.org).
Culture of Human Monocyte–Derived Macrophages
Human monocytes were purified by counterflow centrifugal elutriation of mononuclear cells from healthy human donors. Cells were plated at a density of 2 × 105 monocytes per square centimeter in RPMI medium 1640 (MediaTech, Washington, DC) containing 10% FBS (GIBCO, Carlsbad, Calif) in 6-well Cellbind plates (Corning, Corning, NY). After a 2-hour incubation at 37°C with 5% CO2 plus 95% air, cells were rinsed 3 times with RPMI medium 1640 and cultured for 5 days with complete medium: RPMI medium 1640 containing 10% FBS, 50 ng/mL M-CSF (PeproTech, Rocky Hill, NJ), and 25 ng/mL interleukin 10 (PeproTech).14 Cells were then rinsed 3 times with RPMI medium 1640 and cultured further with complete medium. After 7 days in culture, experiments were performed with serum-free RPMI medium 1640, 50-ng/mL M-CSF, 25-ng/mL interleukin 10, and the indicated addition.
Analysis of Iodine 125-LDL Cell Association and Degradation
Macrophage cell association and degradation of iodine 125 (125I)-LDL was determined according to the method of Goldstein et al.15 Culture media samples were centrifuged at 15 000g for 10 minutes, and trichloroacetic acid–soluble organic iodide radioactivity was measured to quantify lipoprotein degradation.
To measure cell-associated 125I-LDL, macrophages were rinsed 3 times with Dulbecco PBS containing Ca2+, mg2+, and 0.2% BSA; followed by 3 rinses with Dulbecco PBS containing Ca2+ and Mg2+, all at 4°C. Macrophages were dissolved overnight in 0.1 N sodium hydroxide at 37°C and then assayed for 125I radioactivity with a γ counter. 125I radioactivity values for wells incubated with 125I-LDL without macrophages were subtracted from 125I radioactivity values obtained for macrophages incubated with 125I-LDL. Values were less than 1% of cell-associated values for 125I-LDL. For protein quantification, a small aliquot of cell lysate was measured using the method of Lowry et al16 with a BSA standard. 125I-LDL uptake is presented as the sum of cell-associated 125I-LDL and degraded 125I-LDL.
Quantification of Macrophage Cholesterol
Cells were rinsed 3 times in Dulbecco PBS containing Mg2+ and Ca2+, lysed with 1 mL of ultrapure water per well, and then detached with a cell scraper. Lipid was isolated using the method of Folch et al,17 and cholesterol was quantified as previously described by Gamble et al.18 For protein quantification, a small aliquot of cell lysate was measured using the method of Lowry et al16 with a BSA standard.
Microscopic Analysis of Fluid-Phase Pinocytosis Pathways
Macrophage cultures were pretreated with complete medium containing the indicated agent for 4.5 hours. Medium was then replaced with DMEM containing 10 mmol/L Hepes (Sigma, St. Louis, Mo) and 2 mg/mL mannan (to saturate the mannose receptor)19,20 (Sigma), with or without inhibitor, and then incubated for 30 minutes. Next, macrophages were incubated for 10 minutes with the fluid-phase tracer horseradish peroxidase (HRP), 1 mg/mL (Sigma), in the same medium. Subsequently, macrophages were thoroughly rinsed and then fixed with 2.5% glutaraldehyde overnight at 4°C. After fixation, macrophages were rinsed and incubated for 30 minutes with 2 mg/mL diaminobenzidine (Sigma) and then incubated for 60 minutes with 2 mg/mL diaminobenzidine containing 0.01% hydrogen peroxide. Then, macrophages were rinsed and removed from culture dishes by scraping. Macrophages were pelleted (1500g for 5 minutes at 4°C) and then fixed for 1 hour with 2% osmium tetroxide (OsO4). After each subsequent step (up to embedding), macrophages were pelleted by centrifugation. Macrophages were then progressively dehydrated with 70%, 95%, and 100% ethanol. Samples were infiltrated and then embedded and polymerized at 70°C. Thick and thin sections without a counterstain were analyzed using a light microsope (Zeiss Axiophot) or an electron microscope (Joel 1200EX), respectively.
To directly visualize LDL entering macrophages by fluid-phase pinocytosis, macrophages were incubated with 5 mg/mL LDL, fixed, embedded in LR white resin, and prepared for immunogold labeling of LDL as previously described,21 except that 1% dry skim milk instead of 1% BSA was used to block nonspecific staining. All sections were counterstained with lead and analyzed with an electron microscope (Joel 1200EX).
Statistical Analysis
Data are presented as the mean±SEM. Means were representative of 3 replicate wells. A t test was used for statistical comparisons, with P<0.05 considered significant.
Results
Macropinocytosis Partially Mediates LDL Uptake by Macrophages
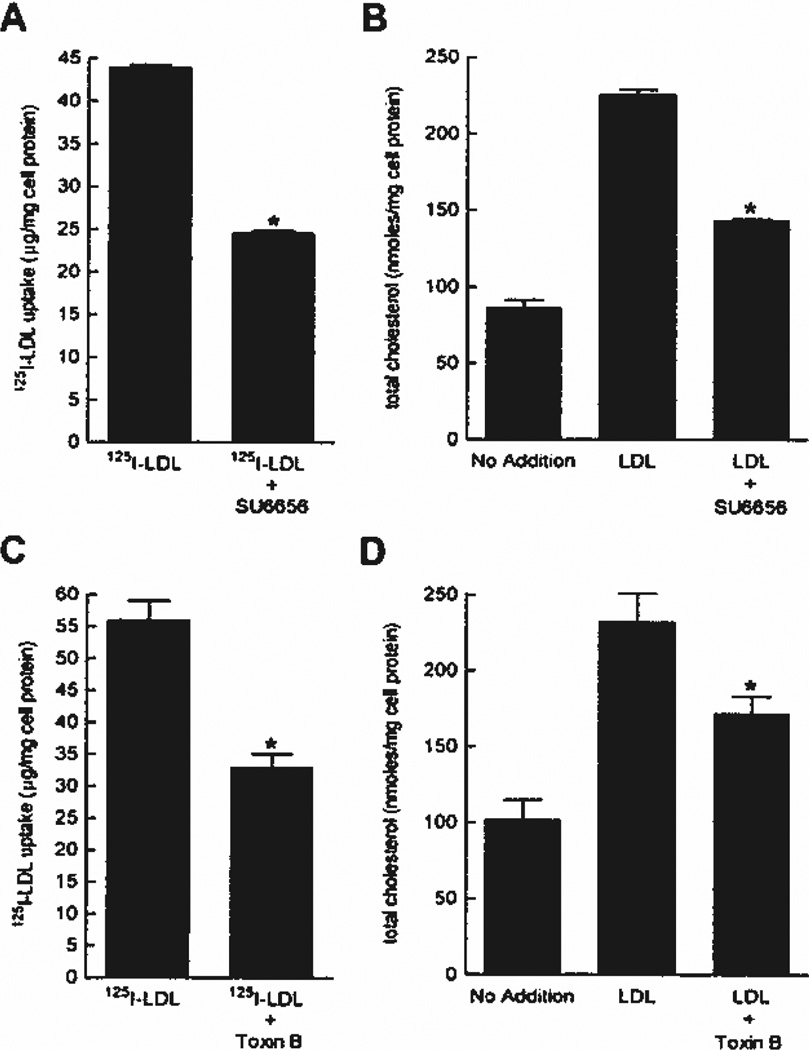
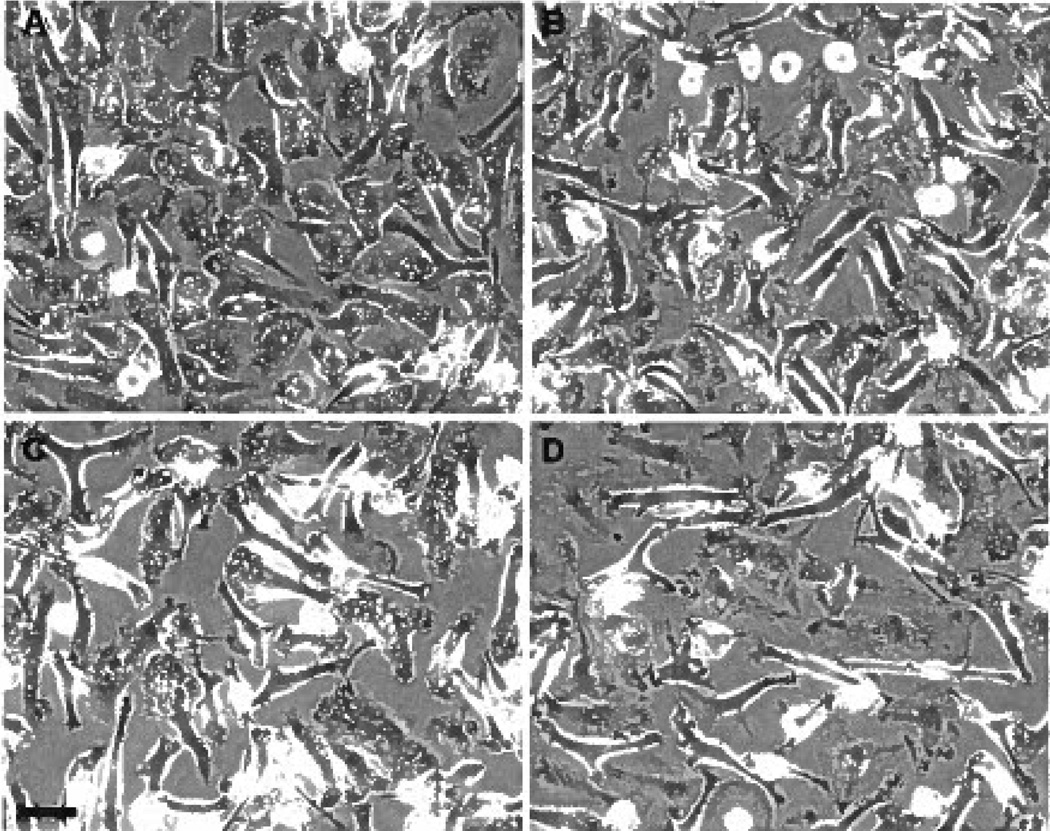
Previously, it was reported that fluid-phase pinocytosis of native LDL by M-CSF–differentiated human macrophages accounts for all native LDL uptake by these macrophages and promotes the formation of foam cells.7 First, to identify inhibitors of native LDL uptake by M-CSF–differentiated human macrophages, we screened a panel of drugs and found that the kinase inhibitor SU6656 decreased LDL uptake and cholesterol accumulation by approximately 40% compared with vehicle-treated macrophages (Figure 1A and B). Assessment of cell morphology by phase-contrast microscopy showed abundant phase-bright vacuoles in untreated cells, whereas SU6656-treated macrophages showed a near complete loss of vacuoles (Figure 2A and B). Because M-CSF–differentiated human macrophages form large vacuoles (ie, macropinosomes) by macropinocytosis (supplemental Figure I),7 we assessed the effect of SU6656 on macropinocytosis by time-lapse microscopy. Macrophage cultures without drug addition showed vigorous macropinocytosis, whereas macropinocytosis was almost completely inhibited in SU6656-treated macrophages (supplemental Figure II), thus explaining the near absence of macropinosome vacuoles in the treated macrophages.
Figure 1.
A through D, SU6656 or toxin B only partially inhibits macrophage LDL uptake and cholesterol accumulation. Macrophages were incubated for 24 hours with 250 µg/mL 125I-LDL and the indicated addition (A and C) or 1 mg/mL LDL and the indicated addition (B and D). 125I-LDL uptake is the sum of cell-associated and degraded 125I-LDL. To determine basal macrophage cholesterol levels, macrophages were also incubated for 24 hours without LDL addition. *P< 0.05 for LDL vs LDL plus added inhibitor.
Figure 2.
A through D, SU6656 or toxin B almost completely inhibits macrophage macropinosome formation. Macrophages were treated for 5 hours without SU6656 (A) or with 20 µmol/L SU6656 (B) or for 5 hours without toxin B (C) or with 100 ng/mL toxin B (D) and imaged by phase-contrast microscopy. The macropinosome vacuoles were substantially decreased in cultures with added inhibitor. The bar indicates 50 µm.
SU6656 was designed as a specific inhibitor of Src family kinases,22 suggesting a role for these kinases in mediating macrophage macropinocytosis. We examined 2 additional Src family kinase inhibitors, PP1 and PP2, to determine whether the effect of SU6656 on macropinocytosis of LDL is the result of inhibition of Src family kinases. Macrophage uptake of LDL was unaffected by PP1 or PP2 compared with untreated macrophages (supplemental Figure III). We considered the possibility that PP1 and PP2 may not be effective inhibitors of the Src family kinases expressed in macrophages. To examine this possibility, we assessed M-CSF–differentiated human macrophage expression of Src family kinases by Western blot analysis and determined that M-CSF–differentiated human macrophages express Hck, Fyn, Fgr, Lyn, and Blk (supplemental Figure IV). All of these Src family kinases expressed in M-CSF–differentiated human macrophages were previously effectively inhibited by PP1 and/or PP2 in cell-based assays,23–25 suggesting that a non-Src family kinase mediates macropinocytosis in M-CSF–differentiated macrophages. Because previous studies26,27 showed that the non-Src family kinase Syk mediates murine macrophage macropinocytosis in response to minimally modified LDL, we assessed macrophage macropinocytosis by time-lapse microscopy in the presence or absence of Syk inhibitor (supplemental Figure V). Although inhibition of Syk altered macrophage morphology, there was no effect on macropinocytosis compared with untreated cells. Collectively, these results strongly suggest that M-CSF–differentiated human macrophage macropinocytosis is not mediated by an Src family kinase or Syk.
Rho GTPases are molecules known to mediate macropinocytosis in certain cell types.28–32 Therefore, we examined the effect the Rho GTPase inhibitor toxin B on LDL uptake and cholesterol accumulation by M-CSF–differentiated macrophages. In the presence of toxin B, macrophage phase-bright vacuoles were almost completely absent compared with control (Figure 2C and D). LDL uptake and cholesterol accumulation by toxin B–treated macrophages was inhibited approximately 40% compared with vehicle-treated macrophages (Figure 1C and D). Similar to the effect of SU6656, macrophage macropinocytosis was nearly completely inhibited by toxin B (compare C and D in Figure 2 with supplemental Figure VI). Because macrophage macropinocytosis was almost completely inhibited by 2 different macropinocytosis inhibitors, but LDL uptake was only partially inhibited, these data suggest that fluid-phase micropinocytosis of LDL accounts for the macrophage LDL uptake that remains after inhibition of macropinocytosis.
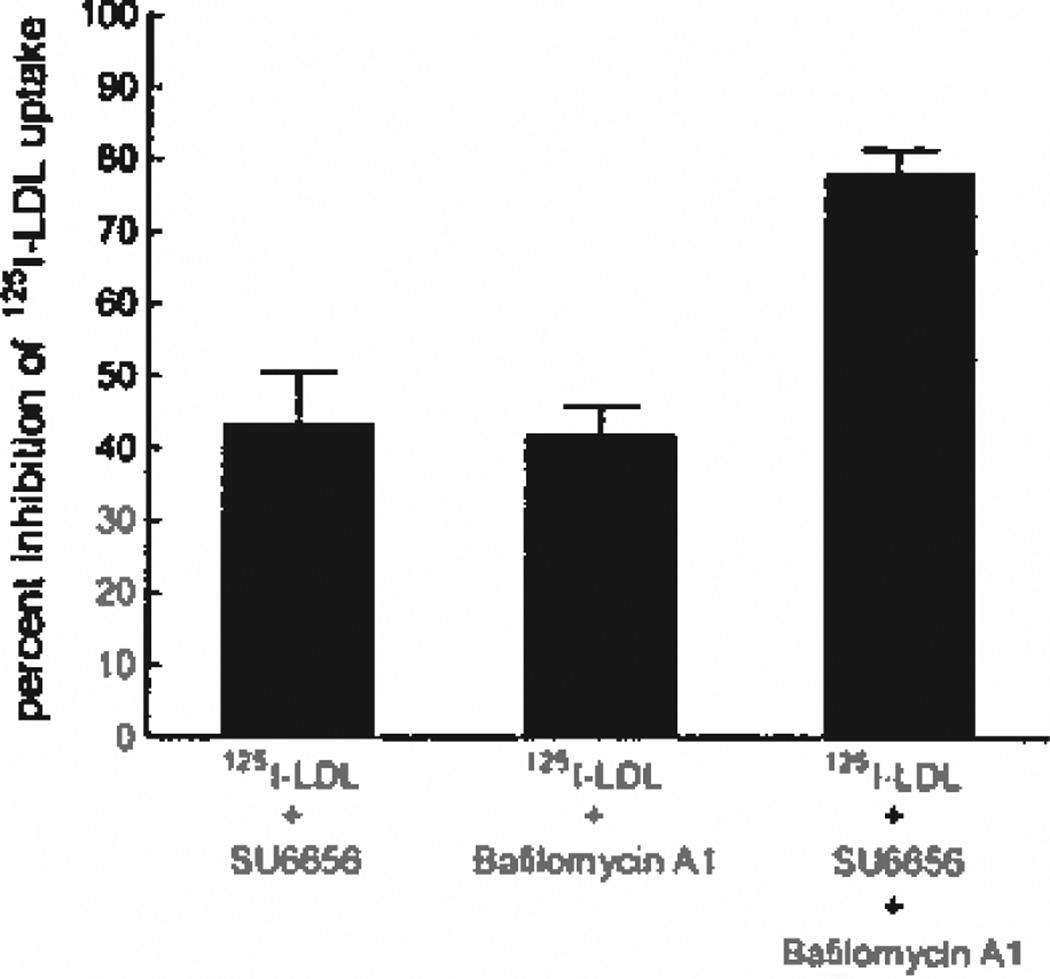
Efficient Inhibition of LDL Uptake by Macrophages Requires Targeting Macropinocytosis and Micropinocytosis
Because the macropinocytosis inhibitors SU6656 and toxin B only partially inhibited LDL uptake by macrophages, we postulated that efficient inhibition of macrophage LDL uptake would require multiple inhibitors targeting both macropinocytosis and micropinocytosis. Therefore, we sought to identify an inhibitor of micropinocytosis. We investigated the effect of bafilomycin A1 on macrophage uptake of LDL. Macrophage cultures were incubated with 125I-LDL in the presence of bafilomycin A1 or SU6656 (Figure 3). Compared with vehicle-treated cells, uptake of 125I-LDL by macrophages was inhibited by 42% for bafilomycin-treated macrophages and by 44% for SU6656-treated macrophages. To determine whether these 2 inhibitors target different pinocytotic pathways, SU6656 and bafilomycin A1 were combined during the incubation of macrophages with 125I-LDL. Macrophage uptake of 125I-LDL was inhibited additively by the 2 drugs at 79% compared with vehicle-treated macrophages, showing that these 2 drugs target different pathways (Figure 3). Because SU6656 almost completely inhibited macrophage macropinocytosis and bafilomycin A1 showed additive inhibition of LDL uptake, these data indicate that bafilomycin A1 inhibits micropinocytosis, not macropinocytosis. To verify that macropinocytosis was unaffected by bafilomycin A1, we assessed macropinocytosis by time-lapse microscopy and found that macropinocytosis was unaffected by the presence of bafilomycin A1 (supplemental Figure VII).
Figure 3.
LDL uptake by macrophages is efficiently inhibited by a combination of macropinocytosis and micropinocytosis inhibitors. Macrophages were incubated for 5 hours in medium containing 250 µg/mL 125I-LDL and the indicated inhibitor. The percentage inhibition of uptake compared with control is shown. 125I-LDL uptake for untreated macrophages was 10 µg/mg cell protein.
Macropinocytosis and Micropinocytosis Mediate Solute Uptake by Macrophages
Next, we examined macrophage pinocytotic pathways by microscopy to gain morphological insight about the pathways mediating fluid-phase solute uptake by macrophages. To determine the initial pinocytotic events mediating macrophage uptake of solute, cell cultures were briefly incubated with the fluid-phase pinocytosis tracer HRP and the macropinocytosis inhibitor, SU6656, the micropinocytosis inhibitor, bafilomycin A1, or vehicle (Figure 4). Pinocytotic vesicles containing HRP show a peripheral rim of the HRP-generated reaction product, precipitated diaminobenzidine (DAB), that is typical for this method.33 The precipitated DAB appears brown by light microscopy. Bright-field light microscopic analysis of macrophages incubated without drug showed DAB-positive small vesicles and large vacuoles (Figure 4B). SU6656-treated macrophages showed only small vesicles positive for DAB (Figure 4C), consistent with time-lapse microscopy showing that SU6656 inhibits macropinocytosis and its generation of large vacuoles (similar results were obtained with toxin B [supplemental Figure VIII]). In contrast, bafilomycin A1–treated macrophages showed predominantly large vacuoles containing DAB with only rare small vesicles (Figure 4D), further supporting that bafilomycin A1 inhibits micropinocytosis but does not inhibit macropinocytosis. As a control, macrophages were incubated without HRP and, as expected, showed no DAB (Figure 4A). In agreement with the macrophage LDL uptake data, these results show that pinocytosis of solute occurs through both macropinocytosis and micropinocytosis.
Figure 4.
A through D, Macrophages take up the fluid-phase pinocytosis tracer HRP within both macropinosomes and micropinosomes. Macrophages were incubated for 10 minutes without HRP (A) or with 1 mg/mL HRP (B) without inhibitor addition, with 10 µmol/L SU6656 (C), or with 500 nmol/L bafilomycin A1 (D). SU6656 inhibited the formation of macropinosomes but not micropinosomes, whereas bafilomycin A1 inhibited the formation of micropinosomes but not macropinosomes. The bar indicates 10 µm.
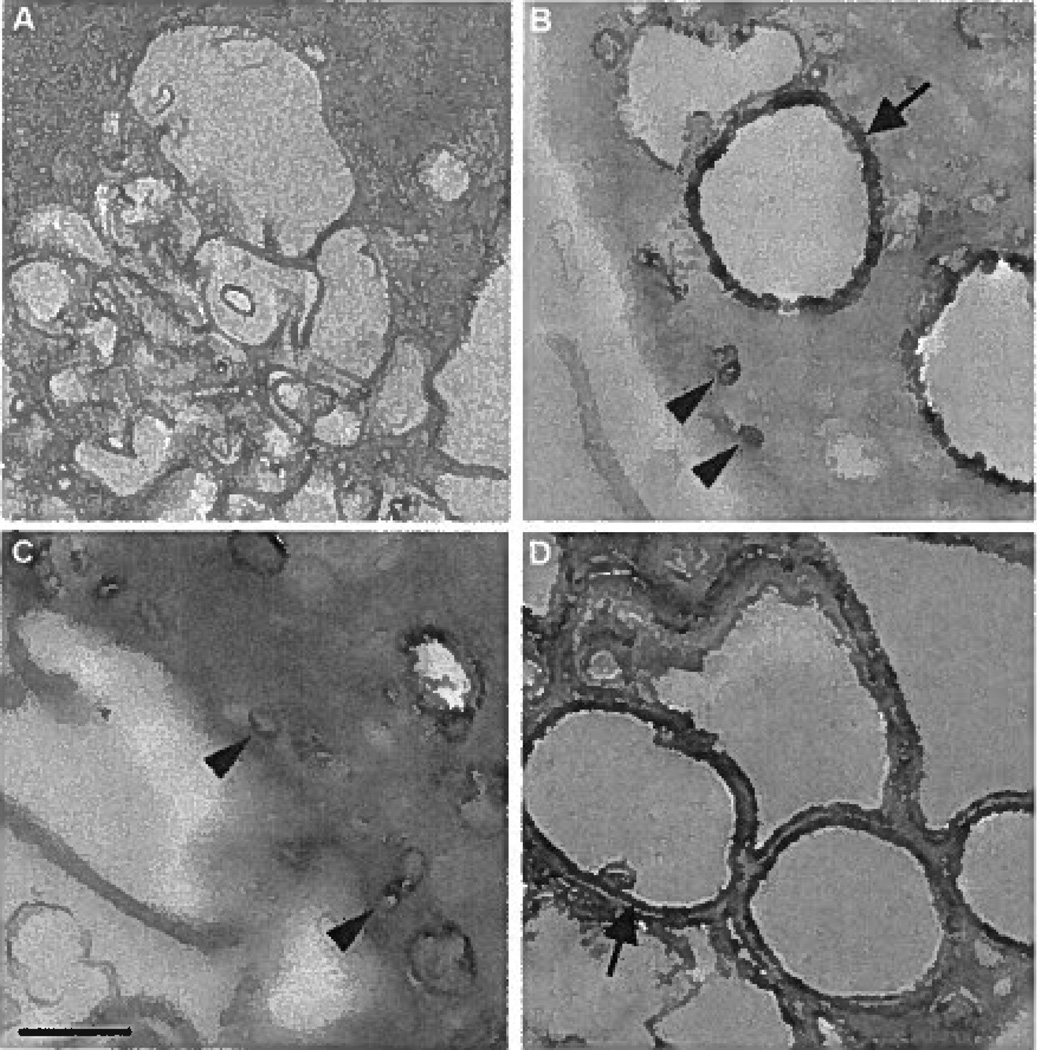
We further assessed macrophage pinocytotic pathways at high magnification by electron microscopy to gain ultrastructural insight into the pathways mediating solute uptake. The HRP-generated reaction product DAB appears black by electron microscopy. Macrophages without drug addition showed 2 distinct DAB reaction product–positive endocytic compartments: small micropinosomes (<0.2 µm) and large vacuoles (>0.5 µm) (Figure 5B and supplemental Figure IX). Treatment of macrophages with the macropinocytosis inhibitor, SU6656, showed mostly small DAB-positive micropinosomes (<0.2 µm) (Figure 5C), whereas treatment of macrophages with the micropinocytosis inhibitor bafilomycin A1 showed almost exclusively large DAB-positive macropinosomes (>0.5 µm) (Figure 5D). Macrophages incubated without HRP showed no DAB, as expected (Figure 5A).
Figure 5.
A through D, Electron microscopy of macrophage uptake of the fluid-phase pinocytosis tracer HRP within macropinosomes and micropinosomes. Macrophages were incubated for 10 minutes without HRP as a control (A) or with 1 mg/mL HRP (B) without inhibitor addition, with 10 µmol/L SU6656 (C), or with 500 nmol/L bafilomycin A1 (D). Arrows and arrowheads indicate macropinosomes and micropinosomes, respectively. The bar indicates 0.5 µm.
To more directly assess bafilomycin A1 inhibition of micropinocytosis, macrophages were incubated with the micropinocytosis tracer cholera toxin subunit B (supplemental Figure X). Although cholera toxin binds to cell surface receptors, it is endocytosed by small vesicles, thus serving as a tracer of micropinocytosis.34 Compared with control, macrophage uptake of cholera toxin was almost completely inhibited by bafilomycin A1, whereas SU6656, as expected, had no effect on cholera toxin uptake. We confirmed that cholera toxin is a tracer of micropinocytosis by incubating macrophages with HRP-conjugated cholera toxin and assessing uptake by electron microscopy (supplemental Figure XI). Macrophages incubated with the micropinocytosis tracer HRP-conjugated cholera toxin showed uptake only within DAB-positive micropinosomes (<0.2 µm). In contrast, macrophage uptake of the indiscriminate fluid-phase pinocytosis tracer HRP showed uptake in both DAB-positive micropinosomes (<0.2 µm) and macropinosomes (>0.5 µm), as expected. To confirm that bafilomycin A1 inhibits micropinocytosis, macrophages were incubated with HRP-conjugated cholera toxin in the presence or absence of bafilomycin A1 and uptake was assessed by electron microscopy (supplemental Figure XII). In the presence of bafilomycin A1, macrophages showed no uptake of HRP-conjugated cholera toxin, consistent with bafilomycin A1 inhibiting micropinocytosis. In contrast, the incubation of macrophages with HRP-conjugated cholera toxin and SU6656 showed DAB-positive micropinosomes, similar to untreated macrophages, further supporting that SU6656 inhibits macropinocytosis but not micropinocytosis. These results show that cholera toxin can be used as a selective tracer for micropinocytosis in these macrophages and confirm that bafilomycin A1 inhibits macrophage micropinocytosis.
Macropinocytosis and Micropinocytosis Mediate LDL Uptake by Macrophages
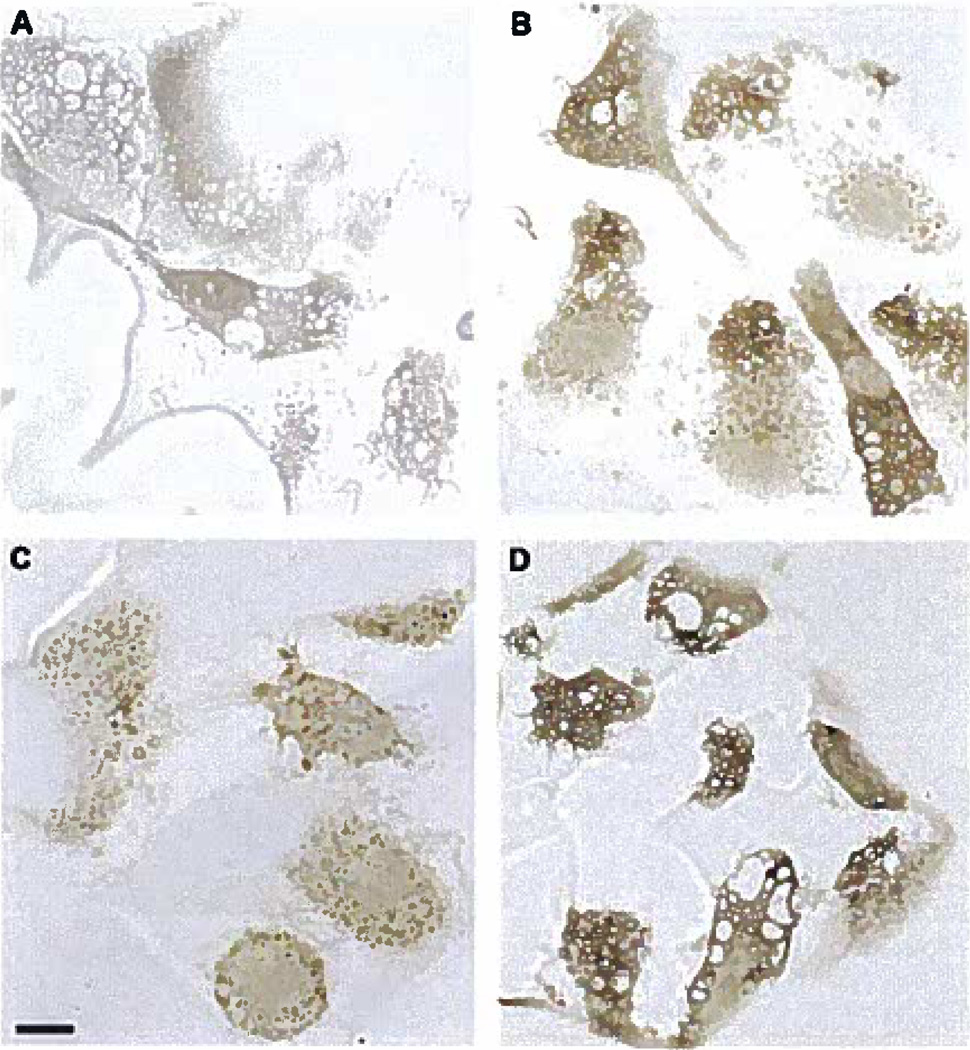
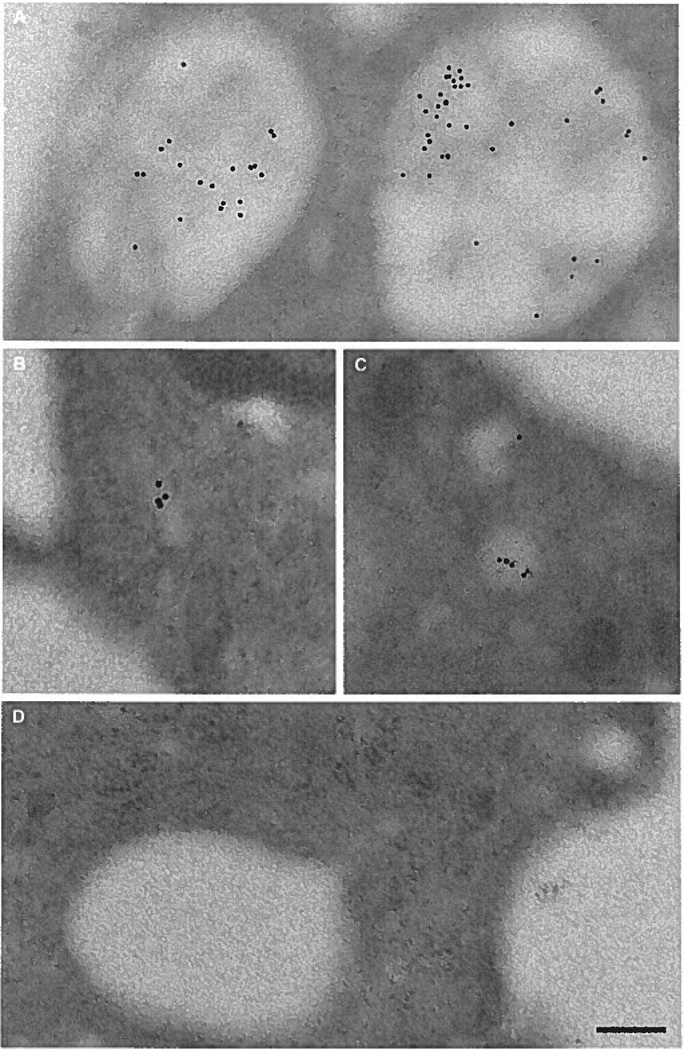
Macrophage uptake of the fluid-phase pinocytosis tracer HRP into both macropinosomes and micropinosomes shows that receptor-independent solute uptake occurs by both pinocytotic pathways. To confirm that LDL is taken up by macrophages via macropinosomes and micropinosomes, macrophages were incubated with LDL and assessed by electron microscopy (Figure 6). LDL, identified by immunogold labeling, was contained in both micropinosomes (<0.2 µm) (Figure 6B and C) and macropinosomes (>0.5 µm) (Figure 6A), consistent with macrophage uptake of the fluid-phase tracer HRP. Macrophages incubated without LDL showed no immunogold labeling (data not shown) nor did macrophages treated with LDL and immunogold labeled with a control antibody to green fluorescent protein (Figure 6D).
Figure 6.
A through D, Macrophages take up LDL within macropinosomes and micropinosomes. Macrophages were incubated for 10 minutes with 5 mg/mL LDL. LDL detected with anti-LDL immunogold labeling was observed in both macropinosomes (A) and micropinosomes (B and C). As a control, samples were treated with anti–green fluorescent protein immunogold labeling and this showed no labeling (D). The bar indicates 0.2 µm.
Discussion
The uptake of cholesterol-rich LDL by macrophages is thought to be critical in mediating the development of atherosclerotic plaques. Therefore, understanding and targeting the pathways mediating LDL uptake by macrophages is an important goal. In this study, by using inhibitors of LDL uptake by M-CSF–differentiated human macrophages, we quantitatively and morphologically demonstrate that native LDL uptake by macrophages occurs by both macropinocytosis and micropinocytosis. Targeting both pinocytotic pathways is necessary for efficient inhibition of LDL uptake by M-CSF–differentiated macrophages because inhibition of either macropinocytosis or micropinocytosis only results in an approximate 40% decrease in LDL uptake, whereas inhibiting both pathways results in an 80% decrease in LDL uptake.
Fluid-phase pinocytosis is a receptor-independent mechanism of solute uptake by cells that occurs by macropinocytosis and/or micropinocytosis.35 Because it was previously noted that fluid-phase pinocytosis of native LDL by M-CSF–differentiated macrophages accounts for all native LDL uptake by these macrophages,7 fluid-phase macropinocytosis and fluid-phase micropinocytosis must account for all macrophage uptake of LDL in this study.
We found that the kinase inhibitor SU6656 inhibited macropinocytosis. SU6656 was identified as a specific small-molecule inhibitor of Src family kinases.22 Previous studies36–38 showed that overexpression of Src or other nonpalmitoylated Src family kinases promotes macropinocytosis in certain cell lines, suggesting that Src family kinases may mediate macropinocytosis by M-CSF–differentiated macrophages. However, when M-CSF–differentiated macrophages were treated with other Src family kinase inhibitors, PP1 or PP2, there was no effect on LDL uptake, suggesting that macropinocytosis may be mediated by a non-Src family kinase. Although SU6656 was initially identified as a “specific” Src family kinase inhibitor,22 non-Src family kinases can be efficiently inhibited by this drug.39 Previous studies26,27 showed that minimally modified LDL stimulates murine macrophage macropinocytosis by activating the non-Src family kinase Syk. We observed no role for Syk-mediating macrophage macropinocytosis. Because we did not stimulate macrophages with minimally modified LDL, it is not surprising that Syk inhibition did not affect macrophage macropinocytosis in this study. Although beyond the scope of this study, future studies will determine the kinase mediating macropinocytosis in M-CSF–differentiated macrophages.
We also found that the Rho GTPase inhibitor toxin B inhibited macropinocytosis, in agreement with previous studies28–32 showing that Rho GTPases mediate macropinocytosis. However, similar to SU6656, although toxin B substantially inhibited macropinocytosis, this agent only partially inhibited macrophage LDL uptake and cholesterol accumulation, further supporting that micropinocytosis contributes substantially to LDL uptake and cholesterol accumulation.
The inhibition of both macropinocytosis with SU6656 and micropinocytosis with bafilomycin A1 resulted in an 80% decrease in LDL uptake by macrophages, showing that inhibition of at least 1 pathway is incomplete. In the presence of bafilomycin A1, we found a nearly complete absence of micropinosomes containing the fluid-phase tracer HRP by electron microscopy, suggesting that inhibition of macropinocytosis is incomplete. Also, electron microscopic analysis showed occasional macropinosomes present in SU6656-treated macrophages; and SU6656-treated macrophages showed residual macropinocytosis, as observed by time-lapse microscopy (supplemental Figure II). Therefore, the remaining 20% of macrophage LDL uptake in the presence of SU6656 and bafilomycin A1 is most likely the result of incomplete inhibition of macropinocytosis by SU6656. Insufficient dosage of SU6656 was not the reason for incomplete inhibition of macrophage macropinocytosis because the doses used in this study maximally inhibited macropinocytosis and LDL uptake (data not shown), suggesting that at least 1 SU6656-insensitive macropinocytotic pathway exists. Attributing 20% of macrophage LDL uptake to SU6656-insensitive macropinocytosis and 40% to SU6656-sensitive macropinocytosis results in 60% of LDL uptake mediated by macropinocytosis. Consequently, 40% of LDL uptake can be attributed to micropinocytosis.
Previous studies40,41 show that murine macrophages take up fluid by micropinocytosis and macropinocytosis. We extend this finding, relating it to macrophage pinocytosis of LDL. M-CSF–differentiated human macrophages were generally found to contain macropinosomes (>0.5 µm) and micropinosomes (<0.2 µm). These pinosome sizes are consistent with previous observations for murine M-CSF–differentiated macrophages.42 In murine M-CSF–differentiated macrophages, most macropinosomes are 0.5 to 1.0 µm,42 similar to what we observed with human M-CSF–differentiated macrophages in this study. Macropinosomes, because of their size, contain substantially more fluid than micropinosomes. For example, assuming a spherical macropinosome, we calculate that 524 aL of fluid is contained within a single 1 µm-diameter macropinosome. In contrast, a spherical 0.1 µm-diameter micropinosome (the approximate diameter of a clathrin-coated vesicle) only contains 0.5 aL of fluid. Because the fluid volume within macropinosomes is much greater than within micropinosomes, the amount of LDL taken up by an individual macropinosome must be substantially greater (> 1000-fold) than that of a micropinosome. Therefore, extensive micropinocytosis by macrophages must occur for 40% of LDL uptake to be attributed to micropinocytosis. Assessment of HRP-treated macrophages by electron microscopy showed more micropinosomes than macropinosomes containing HRP reaction product (supplemental Figure IX), albeit much less than the greater than 1000-fold difference calculated. However, static microscopy does not provide an indication of rates of formation and turnover of vesicles and vacuoles. Thus, the relative numbers of pinocytotic vesicles and vacuoles observed in electron micrographs does not provide an accurate evaluation of the flux of solute carried by micropinosomes and macropinosomes.
Murine macrophages cultured without M-CSF deliver 0.43% of the cell volume per minute by fluid-phase pinocytosis mediated by micropinocytosis.33 We calculate that human macrophages take up 0.89% of the cell volume per minute by fluid-phase pinocytosis, of which 0.36% can be attributed to micropinocytosis, roughly the amount of fluid pinocytosed through micropinocytosis in murine macrophages cultured without M-CSF. Because it was later found that M-CSF stimulation of murine macrophages induces macropinocytosis and increases fluid-phase pinocytosis approximately 2-fold,41 it would be expected that 0.86% of the cell volume per minute would be pinocytosed for murine macrophages in the presence of M-CSF, similar to the 0.89% we calculate for human macrophages. Thus, these calculations show that the relative amount of fluid taken up through micropinocytosis and macropinocytosis in murine and human macrophages is similar. In contrast, an important difference is that M-CSF must be present for macropinocytosis in M-CSF–differentiated murine macrophages41 but is not required for macropinocytosis in M-CSF–differentiated human macrophages, which show constitutive macropinocytosis.7
Because of multiple micropinocytotic subpathways, targeting 1 subpathway may not result in substantial inhibition of solute uptake by cells. Furthermore, inhibition of certain pinocytotic pathways may upregulate other pinocytotic pathways. For example, the molecule dynamin is essential for clathrin-dependent and some clathrin-independent endocytic processes. The expression of nonfunctional mutant dynamin in human cell line cells results in upregulation of other fluid-phase pinocytotic pathways, completely compensating for the loss of fluid uptake by dynamin-dependent endocytosis.43 We found that inhibition of dynamin using the inhibitor dynasore increased rather than decreased LDL uptake by macrophages (JJA, HSK, unpublished observation, 2008), showing that upregulation of other endocytic pathways can also occur in macrophages. Because of the potential for upregulation of pinocytotic pathways, we cannot exclude the possibility that the macropinocytosis inhibitors used in this study upregulate micropinocytosis. Therefore, the percentage of LDL macropinocytosed by untreated macrophages may be greater than macrophages treated with macropinocytosis inhibitors. This possibility of secondary upregulation of micropinocytosis pathways may preclude substantial inhibition of macrophage LDL uptake and cholesterol accumulation with macropinocytosis inhibitors alone.
Clathrin- and caveolin-dependent micropinocytotic pathways can be inhibited by depletion of cellular cholesterol.44–47 Previously, it was reported that cholesterol depletion of M-CSF–differentiated macrophages with the cholesterol sequesterant methyl-β-cyclodextrin had no effect on macrophage uptake of the fluid-phase pinocytosis tracer 125I-BSA,48 showing that micropinocytotic pathways of M-CSF–differentiated macrophages do not depend on cellular cholesterol. These results suggest that clathrin- and caveolin-dependent micropinocytotic pathways do not contribute substantially to fluid uptake by M-CSF–differentiated macrophages. In a separate study, it was also determined that greater than 95% of LDL uptake by M-CSF–differentiated macrophages is dependent on actin polymerization.7 Because micropinocytosis has previously been reported to be actin independent,49,50 these data suggested that nearly all macrophage uptake of LDL occurs by macropinocytosis, a process requiring actin polymerization. In contrast, we clearly demonstrate in this study that micropinocytosis contributes substantially to LDL uptake by macrophages, showing that almost all micropinocytotic uptake of LDL occurs in an actin-dependent manner. Although micropinocytosis is generally actin independent, certain cell types show actin-dependent micropinocytosis.51,52 Therefore, our results show that almost all micropinocytosis by M-CSF–differentiated macrophages is actin dependent and cholesterol independent. These data emphasize that the properties of endocytic processes can vary between cell types and warrant caution in generalizing findings to all cell types.
Macropinocytosis only occurs in some cell types, whereas micropinocytosis is common to all cells, suggesting that other cell types may accumulate cholesterol by this pathway. Fibroblasts have been shown to take up LDL by fluid-phase pinocytosis.53,54 Because macropinocytosis is not known to occur in fibroblasts, the uptake of LDL by fibroblasts most likely occurs by micropinocytosis; however, cholesterol accumulation in these cells does not occur because all the LDL cholesterol taken up is excreted by the fibroblasts.54 In contrast, micropinocytosis of LDL by M-CSF–differentiated macrophages leads to cholesterol accumulation because LDL uptake and cholesterol accumulation by macrophages occurred in the presence of macropinocytosis inhibitors.
Previously, it was shown that human serum-differentiated macrophages stimulated with phorbol 12-myristate 13-acetate (PMA) take up LDL and accumulate cholesterol by macropinocytosis.6 Nearly all LDL uptake and cholesterol accumulation by PMA-stimulated human serum–differentiated macrophages were attributed to macropinocytosis because inhibitors of macropinocytosis almost completely inhibited LDL uptake and cholesterol accumulation. Although not directly investigated in that study, a small amount of LDL uptake by human serum–differentiated macrophages can be attributed to micropinocytosis because, in the absence of macropinocytosis (ie, without PMA stimulation), LDL uptake occurred. The amount of LDL micropinocytosed by unstimulated human serum–differentiated macrophages was approximately 20% to 30% of the total uptake of PMA-stimulated human serum–differentiated macrophages and only led to a small increase in cellular cholesterol (approximately 10% of PMA-stimulated cellular cholesterol). In contrast, micropinocytosis of LDL by M-CSF–differentiated macrophages accounts for at least 40% of LDL and cholesterol accumulation. It remains to be determined if micropinocytosis of LDL contributes substantially to cholesterol accumulation in other cell types or is a unique feature of M-CSF–differentiated macrophages.
One commonly known inhibitory function of bafilomycin A1 is disruption of lysosomal function.55 This occurs by inhibition of the vacuolar type H(+)-ATPase, causing an increase in lysosomal pH, thereby preventing enzymatic degradation of endocytosed cargo. Consistent with this, we found that bafilomycin A1 substantially inhibited macrophage degradation of LDL compared with untreated cells (26% and 76% of pinocytosed LDL was degraded, respectively). Bafilomycin A1 can also inhibit other cellular functions. For example, bafilomycin A1 slows endosomal recycling to the plasma membrane and prevents the formation of multivesicular bodies.56 Furthermore, bafilomycin A1 may have other undiscovered inhibitory properties. Although it has not been previously described that bafilomycin A1 inhibits pinocytosis, we found that bafilomycin A1 inhibited micropinocytosis in M-CSF–differentiated human macrophages. Previously, bafilomycin A1 was used to determine whether phorbol ester–stimulated human serum–differentiated macrophages degrade LDL using lysosomes.6 Although not investigated in that study, bafilomycin A1 was also found to inhibit fluid phase–mediated LDL uptake by phorbol ester–stimulated human serum–differentiated macrophages, supporting the inhibitory effect on pinocytosis by bafilomycin A1. Further supporting our finding that bafilomycin A1 can inhibit pinocytosis, a previous study57 showed that bafilomycin A1 inhibits receptor-mediated endocytosis of albumin by mouse tubular cells. In that study, the vacuolar type H(+)-ATPase was reported to act as a sensor of endosomal pH, with increases in endosomal pH inhibiting endocytosis. Additional studies have shown that the vacuolar type H(+)-ATPase is found in the plasma membrane of kidney epithelial cells58 and macrophages59–61 and can form a vesicle coat, suggesting a potential role for the vacuolar type H(+)-ATPase in mediating vesicle trafficking. Taken together, these studies substantiate that the vacuolar type H(+)-ATPase inhibitor bafilomycin A1 can inhibit micropinocytosis.
In summary, we find that fluid-phase macropinocytosis and fluid-phase micropinocytosis each contribute substantially to LDL uptake by human M-CSF–differentiated macrophages, with both pathways promoting macrophage cholesterol accumulation. This study shows that targeting macrophage uptake of LDL should not only consider macropinocytosis as a mechanism of macrophage LDL uptake but also micropinocytosis and that both uptake pathways can facilitate substantial cholesterol accumulation in macrophages.
Supplementary Material
Acknowledgments
We thank the Department of Transfusion Medicine, Clinical Center, National Institutes of Health, for providing elutriated monocytes; and the Electron Microscopy Core Facility, National Heart, Lung, and Blood Institute, National Institutes of Health, for providing assistance with electron microscopy.
Sources of Funding
This study was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J Lipid Res. 2009;50 suppl:S376–S381. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoff HF, Gaubatz JW, Gotto AM., Jr Apo B concentration in the normal human aorta. Biochem Biophys Res Commun. 1978;85:1424–1430. doi: 10.1016/0006-291x(78)91162-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith EB. Transport, interactions and retention of plasma proteins in the intima: the barrier function of the internal elastic lamina. Eur Heart J. 1990;11 suppl E:72–81. doi: 10.1093/eurheartj/11.suppl_e.72. [DOI] [PubMed] [Google Scholar]
- 5.Smith EB, Ashall C. Low-density lipoprotein concentration in interstitial fluid from human atherosclerotic lesions: relation to theories of endothelial damage and lipoprotein binding. Biochim Biophys Acta. 1983;754:249–257. doi: 10.1016/0005-2760(83)90139-x. [DOI] [PubMed] [Google Scholar]
- 6.Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, Combs CA, Malide D, Zhang WY. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Li Y, Buono C, Waldo SW, Jones NL, Mori M, Kruth HS. Constitutive receptor-independent low density lipoprotein uptake and cholesterol accumulation by macrophages differentiated from human monocytes with macrophage-colony-stimulating factor (M-CSF) J Biol Chem. 2006;281:15757–15762. doi: 10.1074/jbc.M510714200. [DOI] [PubMed] [Google Scholar]
- 8.Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nano-particles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J Clin Invest. 2009;119:1373–1381. doi: 10.1172/JCI35548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 10.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 11.Clinton SK, Underwood R, Hayes L, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, Lusis AJ, Rajavashisth TB. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteo-petrolic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 13.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akagawa KS. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int J Hematol. 2002;76:27–34. doi: 10.1007/BF02982715. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Gamble W, Vaughan M, Kruth HS, Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- 19.Lang T, de Chastellier C. Fluid phase and mannose receptor-mediated uptake of horseradish peroxidase in mouse bone marrow-derived macrophages: biochemical and ultrastructural study. Biol Cell. 1985;53:149–154. doi: 10.1111/j.1768-322x.1985.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Dalle-Molle E, Hardison WG. Hepatocyte horseradish peroxidase uptake is saturable and inhibited by mannose-terminal glyco-proteins. Am J Physiol. 1993;264:G880–G885. doi: 10.1152/ajpgi.1993.264.5.G880. [DOI] [PubMed] [Google Scholar]
- 21.Kruth HS, Skarlatos SI, Lilly K, Chang J, Ifrim I. Sequestration of acetylated LDL and cholesterol crystals by human monocyte-derived macrophages. J Cell Biol. 1995;129:133–145. doi: 10.1083/jcb.129.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656. a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo W, Castaigne JG, Mooney N, Charron D, Al-Daccak R. Signaling through HLA-DR induces PKC beta-dependent B cell death outside rafts. Eur J Immunol. 2003;33:928–938. doi: 10.1002/eji.200323351. [DOI] [PubMed] [Google Scholar]
- 24.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 25.Yan SR, Novak MJ. Src-family kinase-p53/Lyn p56 plays an important role in TNF-alpha-stimulated production of O2- by human neutrophils adherent to fibrinogen. Inflammation. 1999;23:167–178. doi: 10.1023/a:1020245129632. [DOI] [PubMed] [Google Scholar]
- 26.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218. 221. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radhakrishna H, Klausner RD, Donaldson JG. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J Cell Biol. 1996;134:935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 30.West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10:839–848. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 31.Sun P, Yamamoto H, Suetsugu S, Miki H, Takenawa T, Endo T. Small GTPase Rah/Rab34 is associated with membrane ruffles and macropinosomes and promotes macropinosome formation. J Biol Chem. 2003;278:4063–4071. doi: 10.1074/jbc.M208699200. [DOI] [PubMed] [Google Scholar]
- 32.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 33.Steinman RM, Brodie SE, Cohn ZA. Membrane flow during pinocytosis: a stereologic analysis. J Cell Biol. 1976;68:665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinnapen DJ, Chinnapen H, Saslowsky D, Lencer WI. Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol Lett. 2007;266:129–137. doi: 10.1111/j.1574-6968.2006.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 36.Kasahara K, Nakayama Y, Sato I, Ikeda K, Hoshino M, Endo T, Yamaguchi N. Role of Src-family kinases in formation and trafficking of macropinosomes. J Cell Physiol. 2007;211:220–232. doi: 10.1002/jcp.20931. [DOI] [PubMed] [Google Scholar]
- 37.Mettlen M, Platek A, Van Der Smissen P, Carpentier S, Amyere M, Lanzetti L, de Diesbach P, Tyteca D, Courtoy PJ. Src triggers circular ruffling and macropinocytosis al the apical surface of polarized MDCK cells. Traffic. 2006;7:589–603. doi: 10.1111/j.1600-0854.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 38.Veithen A, Amyere M, Van Der Smissen P, Cupers P, Courtoy PJ. Regulation of macropinocytosis in v-Src-transformed fibroblasts: cyclic AMP selectively promotes regurgitation of macropinosomes. J Cell Sci. 1998;111(pt 16):2329–2335. doi: 10.1242/jcs.111.16.2329. [DOI] [PubMed] [Google Scholar]
- 39.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevemic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102(pt 4):867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- 42.Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94(pt 1):135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- 43.Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci U S A. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 48.Buono C, Li Y, Waldo SW, Kruth HS. Liver X receptors inhibit human monocyte-derived macrophage foam cell formation by inhibiting fluid-phase pinocytosis of LDL. J Lipid Res. 2007;48:2411–2418. doi: 10.1194/jlr.M700170-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Pratten MK, Lloyd JB. Effects of temperature, metabolic inhibitors and some other factors on fluid-phase and adsorptive pinocytosis by rat peritoneal macrophages. Biochem J. 1979;180:567–571. doi: 10.1042/bj1800567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies P, Allison AC. Effects of cytochalasin B on endocytosis and exocytosis. From Biol. 1978;46:143–160. [PubMed] [Google Scholar]
- 51.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 52.Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts: comparison of cells from a normal subject and from a patient with homozygous familial hypercho-lesterolemia. J Biol Chem. 1974;249:5153–5162. [PubMed] [Google Scholar]
- 54.Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science. 1976;191:150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin Al, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 56.Presley JF, Mayor S, McGraw TE, Dunn KW, Maxfield FR. Bafilomycin Al treatment retards transferrin receptor recycling more than bulk membrane recycling. J Biol Chem. 1997;272:13929–13936. doi: 10.1074/jbc.272.21.13929. [DOI] [PubMed] [Google Scholar]
- 57.Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8:124–136. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- 58.Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol. 2009;212:1762–1772. doi: 10.1242/jeb.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bidani A, Heming TA. Effects of bafilomycin A1 on functional capabilities of LPS-activated alveolar macrophages. J Leukoc Biol. 1995;57:275–281. doi: 10.1002/jlb.57.2.275. [DOI] [PubMed] [Google Scholar]
- 60.Swallow CJ, Grinstein S, Rotstein OD. A vacuolar type H(+)-ATPase regulates cytoplasmic pH in murine macrophages. J Biol Chem. 1990;265:7645–7654. [PubMed] [Google Scholar]
- 61.Tapper H, Sundler R. Cytosolic pH regulation in mouse macrophages: proton extrusion by plasma-membrane-localized H(+)-ATPase. Biochem J. 1992;281(pt l):245–250. doi: 10.1042/bj2810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.