Summary
Cannabinoid agonists regulate NO and cyclic AMP production in N18TG2 neuroblastoma cells, leading to the hypothesis that neuronal cyclic GMP production could be regulated by CB1 cannabinoid receptors. NO (nitric oxide)-sensitive guanylyl cyclase (GC) is a heterodimeric cytosolic protein that mediates the down-stream effects of NO. Genes of proteins in the cyclic GMP pathway (α1, α2, and β1 subunits of NO-sensitive GC, and PKG1 but not PKG2) were expressed in N18TG2 cells, as was the CB1 but not the CB2 cannabinoid receptor. Stimulation of N18TG2 cells by cannabinoid agonists CP55940 and WIN55212-2 increased cyclic GMP levels in an ODQ-sensitive manner. GC-β1 in membrane fractions was increased at 5 or 20 min stimulation, and was significantly depleted in the cytosol by 1 h. The cytosolic pool of GC-β1 was replenished after 48 h of continued cannabinoid drug treatment. Translocation of GC-β1 from the cytosol was blocked by the CB1 antagonist rimonabant (SR141716) and by the Gi/o inactivator pertussis toxin, indicating that the CB1 receptor and Gi/o proteins are required for translocation. Long-term treatment with rimonabant or pertussis toxin reduced the amount of GC-β1 in the cytosolic pool. We conclude that CB1 receptors stimulate cyclic GMP production and that intracellular translocation of GC from cytosol to the membranes is intrinsic to the mechanism and may be a tonically active or endocannabinoid-regulated process.
Keywords: CB1 cannabinoid receptor, CP55940, cyclic GMP, gene expression, G-protein coupled receptors, G-proteins, NO-sensitive guanylyl cyclase, protein translocation, real-time polymerase chain reaction, rimonabant (SR141716), WIN55212-2
1. Introduction
CB1 cannabinoid receptors are found in great abundance in brain and peripheral neuronal systems (for review, see (Howlett et al., 2002a; Pazos et al., 2005)). These G-protein coupled receptors mediate the response to endocannabinoid eicosanoid mediators, particularly anandamide and 2-arachidonoylglycerol, in neurons and other cells throughout the body (Fowler et al., 2005; Bisogno et al., 2005). Agonist drugs for the CB1 receptor include CP55940 and WIN55212-2, and antagonist drugs include rimonabant, also known as SR141716 (Howlett et al., 2002a; Beardsley and Thomas, 2005). CB1 receptors are found predominantly on presynaptic locations, and are important in the regulation of neurotransmitter release (Schlicker and Kathmann, 2001; Diana and Marty, 2004). CB1 receptors block voltage-gated Ca2+ channels leading to reduced probability of transmitter release, and also inhibit cyclic AMP production and thereby decrease protein kinase (PK) A-mediated regulation of K+ channels leading to hyperpolarization (reviewed in (Childers and Deadwyler, 1996; Howlett et al., 2004)).
We determined that stimulation of CB1 receptors increased NO (nitric oxide) production in the neuronal N18TG2 cell model (Mukhopadhyay et al., 2002). One target for NO is the NO-sensitive guanylyl cyclase (GC), also known as “soluble” GC, which exists as a heterodimer of α and β subunits. NO binding to NO-sensitive GC catalyzes the conversion of GTP to the second messenger cyclic GMP, which regulates PKG to phosphorylate target proteins. In cDNA microarray studies, we found that GC-β1 mRNA was expressed in N18TG2 cells and could be increased after 48-h exposure to cannabinoid agonists (Howlett et al., 2002b). In determining the cellular regulation of the protein content, it was discovered that stimulation of the CB1 receptor resulted in a diminution of GC in the soluble fraction. The present studies report the translocation from the soluble fraction to the membranes concurrent with cyclic GMP production, and the requirements for stimulation of the CB1 receptor and Gi/o proteins for translocation and tonic regulation of GC levels.
2. Methods
2.1 Materials
The reagents were purchased from Sigma Chemical Company (St. Louis, MO), unless otherwise stated. CP55940 ((−)-cis-3R-[2-Hydroxy-4-(1, 1-dimethylheptyl)phenyl]-trans-4R-3(3-hydroxypropyl)-1R cyclohexanol) and rimonabant (N-(Piperidin-1yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-H-pyrazole-3-carboxamide) were provided by the National Institute of Drug Abuse drug supply program. WIN55212-2 (R(+)-[2,3-Dihydro-5-methyl-3[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(l-naphthalenyl)methanone mesylate), rolipram and 1-isobutyl-3-methyl-xanthine (IBMX) were from Sigma RBI (St. Louis, MO), and acrylamide, N, N, N′, N-tetramethylethylene diamine, sodium dodecylsulfate (SDS) and polyvinylidine difluoride membranes were from Bio-Rad Laboratories, Inc. (Hercules, CA). 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and GC antibody (catalog # 371712) were from Calbiochem (La Jolla, CA). GC-β1 subunit (soluble) polyclonal antibody (rabbit) (catalog # 160897) was purchased from Cayman Chemical (Ann Arbor, MI). Goat anti-rabbit IgG horseradish peroxidase (catalog # ALI3404) was from Biosource International (Camarillo, CA). Goat serum was from Gibco Life Technologies (Gaithersburg, MD). Rainbow molecular weight markers, Enhanced Chemiluminescence detection kit, and high performance autoradiography film (Hyperfilm) were from Amersham Bioscience (Piscataway, NJ). The RNeasy Mini purification kit was from Qiagen (Valencia, CA). TaqMan Universal PCR Master Mix and Assays-on-Demand Gene Expression Assay Mixes specific for mouse 18S, CB1 and CB2 receptors, α1, α2, β1, and β2 subunits of GC, and PKG1 and PKG2 were from Applied Biosystems, Inc. (Foster City, CA).
2.2 Cell Culture
N18TG2 neuroblastoma cells (passage numbers 25–50) were maintained in 75 cm2 flasks at 37 °C in Dulbecco’s Modified Eagle’s Medium: Ham’s F-12 (1:1) with GlutaMax, sodium bicarbonate, and pyridoxine-HCL, supplemented with penicillin (100 units/ml) and streptomycin (100 μg/ml) (Gibco Life Technologies, Gaithersburg, MD) and 10% heat-inactivated bovine serum (JHR Bioscience, Lenexa, KS). Drug stocks were stored at −20 °C as 10 mM solutions in ethanol. An aliquot was air-dried under sterile conditions in trimethylsilyl-coated glass test-tubes and was taken up in 100-volumes of 5 mg/ml fatty acid-free bovine serum albumin. From this, 100 μl was added to flasks containing 10 ml of media, providing a final concentration of 1 μM CP55940 or WIN55212-2, or a drug-free vehicle. Where indicated, N18TG2 cells were pretreated with the CB1 antagonist rimonabant (1 μM) for 30 min prior to addition of agonists, or at the times indicated before harvesting. Pertussis toxin (100 ng/ml final concentration) (BIOMOL, Plymouth Meeting, PA) was added to flasks containing fresh media 16 h before addition of agonists, or at the times indicated before harvesting.
2.3 Gene expression determinations
Total RNA was extracted and purified from N18TG2 cells according to the instructions provided with the Qiagen RNeasy Mini Kit and purity was determined spectrophotometrically using the 260/280 ratio. Total RNA (1 μg) was reversed transcribed into cDNA using random hexamers according to the methods described in the Applied Biosystems cDNA Archive Kit. Real Time PCR reactions were performed in a 25 μl reaction volume using TaqMan Universal PCR Master Mix and appropriate primer and probes according to the Applied Biosystems, Inc. instructions using a 7500 Real-time PCR System. Ribosomal 18S RNA was the reference standard gene, and relative quantitation was determined using the Delta Delta Ct method (Livak and Schmittgen, 2001).
2.4 Cyclic GMP determination
N18TG2 cells grown to confluence on 96-well culture plates were washed with physiological saline solution (PSS) (150 mM NaCl, 5.4 mM KCl, 1.18 mM MgSO4, 1 mM CaCl2; 6 mM NaHCO3, 5.5 mM D-glucose), and incubated for 5 min with PSS containing 0.1 mM IBMX and 0.1 mM rolipram prior to addition of drugs. When ODQ was included, the inhibitor was added during the 5 min preincubation. Cannabinoid drugs were added for 5 min or 20 min at 37 °C, and the incubation was stopped by aspiration of the media and freezing the plate at −80 °C. Cellular cyclic GMP was determined using the Enzyme-immunoassay Biotrak System Kit (Amersham Biosciences, Piscataway, NJ). Briefly, lysis reagent was added to the wells, and aliquots were acetylated prior to immunoassay using cyclic GMP-horseradish peroxidase conjugate and substrate according to the instructions, and the optical density was read at 450 nm using a VICTOR3 multiplate reader (Perkin Elmer, Wellesley, MA). The standard curve was analyzed and fit, and unknown samples were determined using Prism IV (Graphpad, San Diego, CA).
2.5 Western blot analysis
After treatment, N18TG2 cells were harvested with PBS-EDTA (2.7 mM KCl, 138 mM NaCl, 10.4 mM glucose, 1.5 mM KH2PO4, 8 mM Na2HPO4, 0.625 mM EDTA, pH 7.4). The cells were suspended in cold TME buffer (20 mM TrisCl, pH 7.4, 3 mM MgCl2, 1 mM Na EDTA) with a protease inhibitor cocktail (set III, Cat. No. 59134, Calbiochem (La Jolla, CA) having a broad ability to inhibit aspartic, cysteine, serine, and aminopeptidases. The cells were allowed to swell in the hypotonic solution (15 min), and then were homogenized with a glass-glass homogenizer and sedimented at 1,000 × g at 4 °C for 10 min. The pellet, which contained the nuclear and cellular debris, was discarded and the supernatant was sedimented at 100,000 × g for 30 min at 4 °C, and the membranes were resuspended in 1/20 the cytosol volume. The supernatants and membrane pellets resuspended in cold 50 mM TrisCl buffer pH 7.4 were stored in aliquots at −80 °C. Rat forebrain cytosolic fractions were prepared from frozen whole rat brains purchased from Pel-Freeze (Rogers, AK). The brains were thawed in ice-cold SME solution (320 mM sucrose; 5 mM MgCl2; 2 mM Tris-EDTA). The brain tissue was homogenized in a glass-glass homogenizer in 2 ml of SME per gram of tissue, and sedimented at 1,000 × g at 4 °C for 10 min to remove cellular and nuclear debris. The supernatant was saved and pellet resuspended in 1 ml of SME for a second sedimentation. The supernatants were combined and sedimented at 39,000 × g at 4 °C for 25 min. The cytosolic fractions were stored in aliquots at −80 °C until use. The protein concentrations were determined using the Coomassie dye binding method (Bradford, 1976).
N18TG2 fractions were taken up in Laemmli’s sample buffer with 1 mM dithiothreitol and heated at 60 °C for 10 min. Unless otherwise indicated, equal amounts of protein (7 or 12 μg) were loaded per lane and SDS-10% polyacrylamide gel electrophoresis (PAGE) was run at 50 volts for 30 min and then 120 volts for 80 min. The proteins were transferred to polyvinylidine difluoride membranes in Towbin’s buffer (24 mM Tris Base, 192 mM glycine and 20% methanol, pH 8.3) for 1 h in the cold at 95 volts using a Bio-Rad Trans-Blot Cell with an ice pack. Blots were rinsed three times (5 min each) with Tris-buffered saline (TBS) (20 mM TrisCl, pH 7.4, 137 mM NaCl) and incubated with blocking solution (5% nonfat dry milk, 5% normal goat serum in TBS) at room temperature for 1 h. Blots were then incubated with a NO-sensitive GC antibody or an antibody specific for the GC-β1 subunit (1: 500) in blocking solution for 1 h at room temperature, followed by washing three times with TBS-T (TBS containing 0.1% Tween 20). The blots were incubated with horseradish peroxidase-coupled anti-rabbit IgG (1: 8000) for 1 h at room temperature, followed by five washes with TBS-T and five washes with NANOpure water both at 5 min intervals. Immunoreactive bands were detected using Enhanced Chemiluminescence reagents, and blots were exposed to Hyperfilm at various time intervals to get optimum signals. The blots were developed using a Kodak M35A X-OMAT processor (Rochester, NY). Signals were quantified using an Alpha Imager and AlphaEase software (Alpha Innotech, San Leandro, CA) by measuring the density of the bands (average of pixels per enclosed area after background correction). Density values were normalized to the control samples as 100%. The data were tested for statistically significant differences using one-way ANOVA, and Dunnett’s post-hoc test or Student’s t-test to compare samples to the control (Prism IV software).
3. Results
3.1 The cyclic GMP pathway in N18TG2 cells
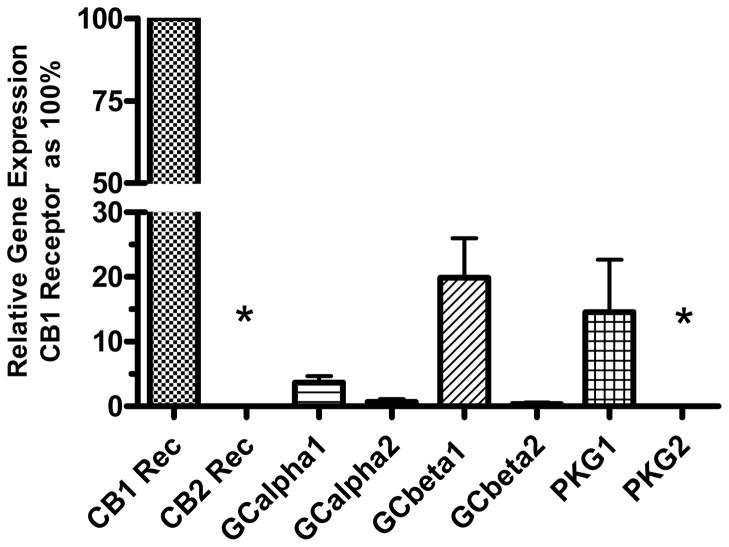
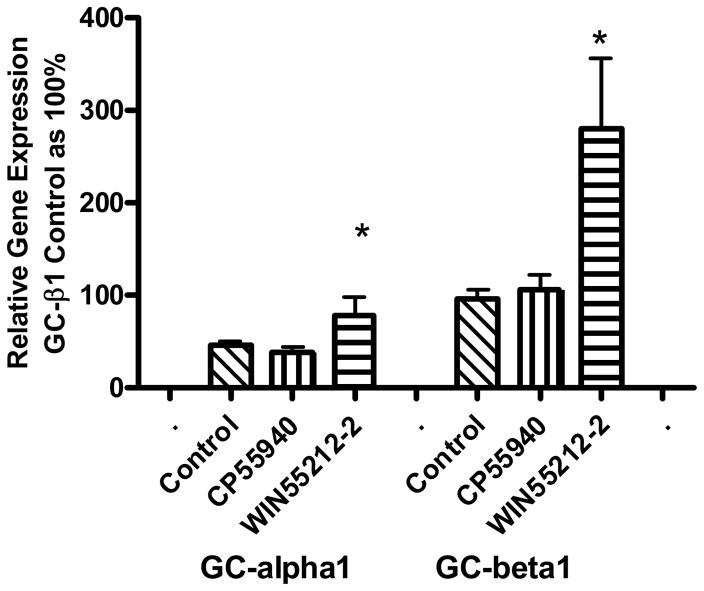
The N18TG2 neuroblastoma cell has been used as a model system to study signal transduction pathways regulated by the CB1 cannabinoid receptor (Mukhopadhyay et al., 2002). Because these cells produce NO in response to cannabinoid agonists, we determined if the soluble form of GC, the “receptor” for NO, was present. N18TG2 cells express the mRNA for CB1 but not CB2 receptors (Fig. 1). Compared to the CB1 receptor expression, mRNA for the subunits of NO-sensitive GC are expressed in relatively lower abundance. Of the GC subunits, GC-β1 is expressed in greatest abundance, and GC-β2 appeared at the lower level of detection of the real-time PCR. mRNA for GC-α1 was expressed at 10% to 20% of the level of GC-β1, whereas mRNA for GC-α2 was expressed at only about 1% of the level of GC-β1. Thus, the predominant heterodimer expressed in N18TG2 is GC-α1β1. PKG1 mRNA but not PKG2 mRNA was expressed in N18TG2 cells. We know that N18TG2 cells express PKG substrates including vasodilator-stimulated protein (VASP) (Jones and Howlett, unpublished observations), a substrate that can be stimulated by NMDA glutamate receptors in cerebellar granule cells (Jurado et al., 2004). N18TG2 cells also express cyclic GMP phosphodiesterase (PDE5) (Giordano et al., 2001). Thus, a complete pathway exists for cyclic GMP-mediated signal transduction regulation in the N18TG2 cell model.
Fig. 1.
Relative expression of genes for cannabinoid receptors, GC subunits and PKG in N18TG2 cells. RNA was extracted and purified, and reverse transcribed to cDNA for real-time PCR determination of relative gene expression normalized to 18S ribosomal RNA. The relative gene expression for each target is reported as a percent of the expression of the CB1 receptor, using the ΔΔCt method. The data are means ± SEM from N= 3 to 5 individual cell pellets, except for PKG1 and PKG2 (mean ± range, N=2). *Indicates data in which the cut-off of 40 cycles was reached before a signal could be detected for these genes.
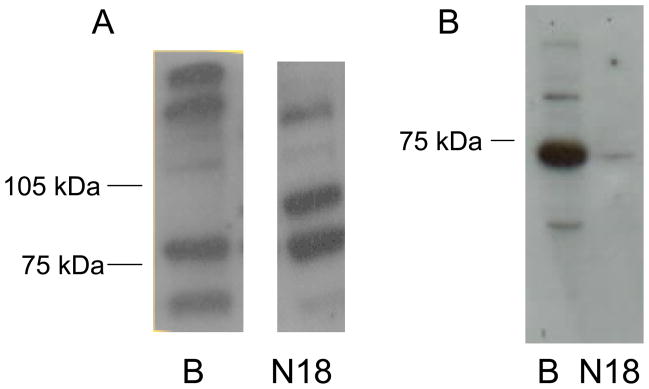
In Western blots to detect proteins, polyclonal antisera that had been made against peptides from both GC-α1 (aa 634–647) and GC-β1 (aa 593–614) (Calbiochem) detected bands from rat brain preparations that appeared at 80–85 kDa for GC-α1 and at 65–70 kDa for GCβ1 (Fig. 2A) (see also (Ibarra et al., 2001; Russwurm et al., 2001)). The 19-mer peptide immunogen recognized by the Calbiochem antisera was 84% identical in GC-α1 and GC-α2 proteins, with two conservative substitutions of cationic residues, and thus, the 80–85 kDa band probably recognizes both isoforms. The Western blot for N18TG2 supernatants identified two bands at around 80–85 kDa and about 100 kDa, consistent with GC-α1 and GC-α2 proteins appearing at two apparent molecular weights (see also multiple bands in (Russwurm et al., 2001)). Using these antisera, a faint GC-β1 band appeared at about 70 kDa in the N18TG2 supernatants. In contrast, the antibody from Cayman Chemical that was selective for the GC-β1 (aa 188–207) with no cross-reactivity with GC-α1, GC-α2 or GC-β2 subunits, detected a strong band in the 65 kDa range (Fig. 2B). There is a comparatively greater amount of GC-β1 in brain compared with N18TG2 cytosols. For the studies described herein, quantitation of GC was performed by immunodetection with the GC-β1-selective antibody.
Fig. 2.
NO-sensitive GC protein subunits in cytosolic fractions of rat brain and N18TG2 neuronal cells. A. Crude cytosolic fraction (39,000 × g) subjected to SDS-PAGE (6.5% polyacrylamide) and stained with antisera (Calbiochem) that recognizes both the GC-α1/α2 and GC-β1 monomers. B. Cytosol (100,000 × G) preparations of rat brain (lane 1 at 7 μg) and N18TG2 (lane 2 at 12 μg) were subjected to SDS-PAGE (10% polyacrylamide) as described in the text, and stained with an antibody (Cayman Chemicals) that recognizes the GCβ1 monomer. Blots are representative of at least three independent experiments.
3.2 Effects of cannabinoid drugs on NO-sensitive GC in N18TG2 cells
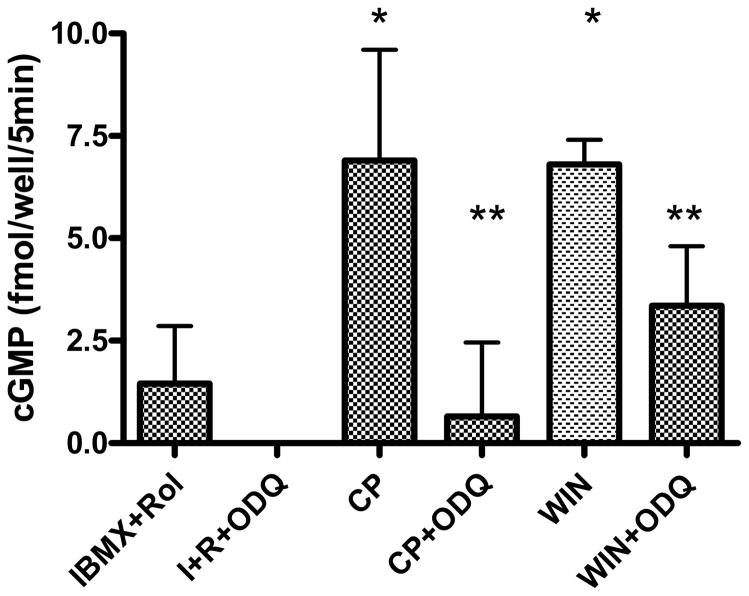
To determine whether cannabinoid compounds regulate NO-sensitive GC, N18TG2 cells were treated with 1 μM CP55940 or WIN55212-2 (Fig. 3). Both agonists increased intracellular cyclic GMP within 5 min (to 20 min, data not shown) in the presence of cyclic nucleotide phosphodiesterase inhibitors IBMX and the phosphodiesterase (PDE) 4 inhibitor rolipram, targeting a prominent isoform in this cell line (Walz et al., 1987). The response to cannabinoid drugs was blocked by a concentration of ODQ that is expected to inhibit NO-dependent GC (Garthwaite et al., 1995), indicating that the detected cyclic GMP was due to enzymatic synthesis by this form of GC.
Fig. 3.
Stimulation of cyclic GMP accumulation by cannabinoid drugs in N18TG2 cells. N18TG2 cells were treated for 5 min with 1 μM CP55940 (CP) or WIN55212-2 (WIN) in the presence of 0.1 mM IBMX plus 0.1 mM rolipram. ODQ was added at 0.3 μM where indicated. Basal values of cyclic GMP (PSS alone) were subtracted, and data are reported as the mean ± SEM from N=3 cell dishes. *Significantly greater than IBMX + rolipram at p<0.05; **significantly lower than drug-stimulated value in the absence of ODQ, but not significantly different from IBMX+rolipram+ODQ at p<0.05 by Student’s t-test. This experiment was representative of four similar experiments having qualitatively similar results.
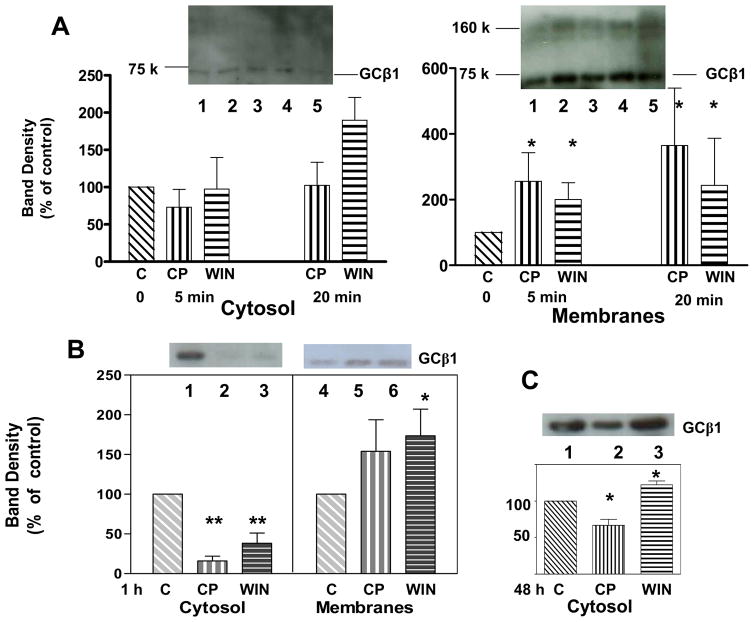
NO-sensitive GC is found predominantly in the cytosol in untreated cells, with 91 ± 6.2 (mean ± SD) of total GC located in the 100,000 × G soluble fraction. Within 5 min of exposure to CP55940 or WIN55212-2, a significant increase in membrane-associated GC-β1 had occurred, doubling by 5 min (Fig. 4A). At these early time points, the amount of GC-β1 in the cytosol was not depleted. However, it is of interest that a high apparent molecular weight band (corresponding with the 160 kDa marker) appeared in the membrane fraction, yet this band was not present in the cytosol. After 1 h treatment with agonists, the membrane GC-β1 was greater than control, and there was a statistically significant decrease in cytosolic GC-β1 by 80% with CP55940 and 60% with WIN55212-2 (Fig. 4B). These data suggest that in response to cannabinoid compounds, GC is able to translocate from the cytosol to a membranous organelle such as the plasma membrane (Zabel et al., 2002) and may form high molecular weight complexes with the same or other proteins.
Fig. 4.
Cannabinoid drug effects on GC-β1 protein in cytosolic or membrane fractions of N18TG2 neuronal cells at A: 5 and 20 min; B: 1 h; and C: 48 h. A. Cells were incubated with vehicle control (lane 1), 1 μM CP55940 (lanes 2, 4) or 1 μM WIN55212-2 (lane 3, 5) for either 5 min (lanes 2, 3) or 20 min (lanes 4, 5). A subcellular fractionation was carried out as described in Methods. Each lane contained 7 μg proteins. B. Cells were incubated with vehicle (lanes 1, 4), 1 μM CP55940 (lanes 2, 5), or 1 μM WIN55212-2 (lane 3, 6), for 1 h, and cytosol and membranes were prepared. C. Cells were incubated with vehicle (lanes 1), 1 μM CP55940 (lanes 2), or 1 μM WIN55212-2 (lane 3), for 48 h, and the cytosol fraction was prepared. Western blot analysis was performed and densities were obtained. The fraction of GC found in the cytosol versus membrane fractions were calculated as the (integrated band density minus background) per μg protein loaded times the total protein in the cytosol or membrane fractions, respectively. Data are presented as treatment values normalized to vehicle control as 100%. Data represent the mean ± SEM of N=3 individual experiments. A one-way ANOVA and Dunnett’s post hoc test was performed (Prism IV, Graphpad, Inc.). Values were significantly different from control * at p < 0.05; ** at p < 0.001.
The cytosolic pool of GC-β1 does not remain depleted upon continued exposure to cannabinoid drugs. Fig. 4C shows that a recovery of cytosolic GC-β1 had occurred within the ensuing 48 h treatment. The CP55940-treated cells showed a 32% decrease rather than the 80% decrease seen at 1 h. The WIN55212-2-treated cells showed a 22% increase above controls (Fig. 4C), which appears to be an overshoot of GC-β1 production levels. The observation of amounts of cytosolic GC-β1 in agonist-treated cells that are approaching or greater than the control would suggest that there is compensation by a mechanism that appears to replenish the cytosolic store of protein. The mRNA gene expression for GC-β1 exhibited an approximately two-fold increase after 24 h incubation with WIN55212-2, although no increase in gene expression appeared with CP55940 at that time point (Fig. 5). A similar pattern of increase was observed for the GC-α1 protein, suggesting linkage in gene expression regulation for the two subunits. Early time points (1 and 4 h) showed no cannabinoid drug-dependent increase in gene expression for GC-β1 or GC-α1; however, the 8 h time point showed a significant increase in gene expression of GC-β1 in response to stimulation by WIN55212-2 but not CP55940 (data not shown).
Fig. 5.
Effect of chronic treatment of N18TG2 cells with cannabinoid drugs on GC-α1 and GC-β1 gene expression. Cells were incubated for 24 h with the vehicle control, 1 μM CP55940, or 1 μM WIN55212-2, and total RNA was extracted as described in the text. Relative gene expression levels were determined by real-time PCR. The relative gene expression for each treatment group is reported as a percent of vehicle control group, using the ΔΔCt method. Data are the mean ± SEM of N=3 replicates, and * indicates significant increase over vehicle at p < 0.05 (Student’s t-test). This experiment was repeated with similar results.
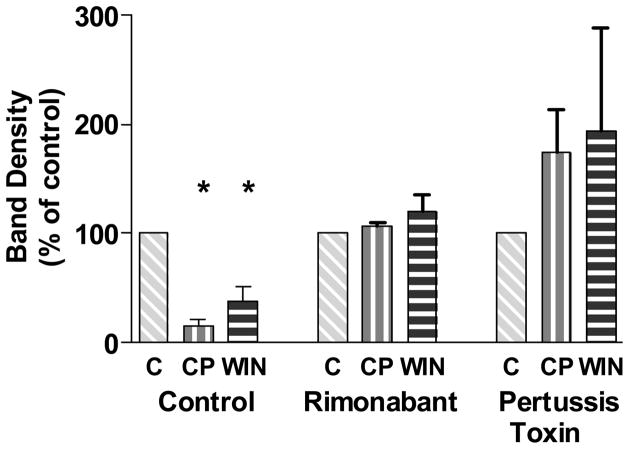
The mechanism for the cannabinoid agonist-mediated GC-β1 translocation response was further studied by determining if the decrease in cytosolic GC-β1 could be blocked by the CB1 receptor-selective antagonist rimonabant. Cells were treated with rimonabant for 30 min, and then either vehicle, CP55940, or WIN55212-2 was added for 1 h. Cells were harvested with protease inhibitors and the cytosol was prepared. Fig. 6 shows that the decrease in cytosolic GC-β1 in response to agonists was precluded by the CB1 receptor antagonist, indicating that the CB1 receptor mediated this response. To test whether Gi/o was required for the translocation response, cells were treated with pertussis toxin for 16 h, and then either vehicle, CP55940, WIN55212-2 was added for 1 h. Blocking the functioning of Gi/o proteins not only inhibited the ability of the cannabinoid agonists to decrease the cytosolic GC-β1, but tended to reverse the action by increasing the amount of cytosolic GC-β1 (Fig. 6).
Fig. 6.
Attenuation of the cannabinoid drug-dependent depletion of GC-β1 from the cytosol by the CB1 antagonist rimonabant or pertussis toxin. N18TG2 cells were treated with 1 μM rimonabant (30 min) or 100 ng/ml pertussis toxin (16 h) followed by incubation with the vehicle control, 1 μM CP55940, or 1 μM WIN55212-2 for 1 h. Cytosolic preparations and Western blotting were performed as described in the Methods. Densitometry was performed, and data were normalized to control as 100 %. For rimonabant treatment groups, data from N=3 individual Western blots are shown as the mean ± SEM, and analyzed by Prism using one-way ANOVA, with no significant differences found between treated and vehicle control samples. For the pertussis toxin treatment, densitometry was performed on N=2 individual Western blots, and data are shown as the range of the values.
3.3 Effects of chronic treatment with a cannabinoid antagonist on cytosolic GC-β1
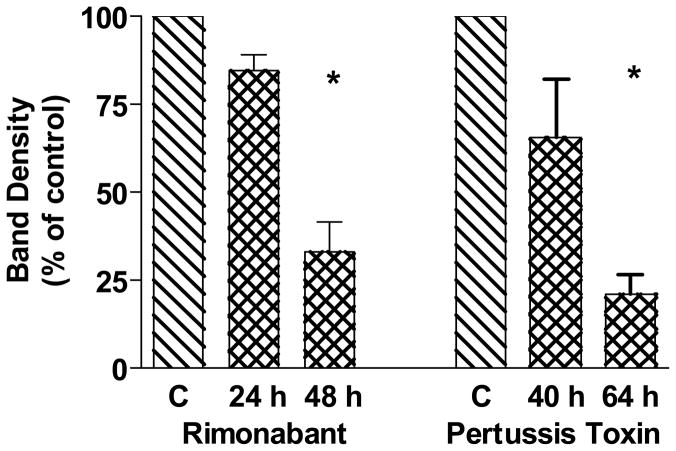
N18TG2 cells are known to produce endocannabinoids including anandamide and 2-arachidonoylglycerol (Di Marzo et al., 1996b; Di Marzo et al., 1996a). In addition, the CB1 receptor appears to exhibit constitutive activity in the absence of exogenous agonists for some signal transduction pathways (Meschler et al., 2000). Thus, if chronic treatment with an agonist such as WIN55212-2 could increase cytosolic GC-β1 over time, then chronic exposure to an antagonist might be predicted to decrease GC-β1. To determine the cytosolic content of N18TG2 cells when receptors were chronically exposed to an antagonist, cells were treated with rimonabant for 24 h and 48 h. The control and rimonabant-treated N18TG2 cells showed a significant decline in GC-β1 over time with the antagonist and this reached significance at 48 h (Fig. 7). A similar pattern was observed after blocking the effects of Gi/o proteins with pertussis toxin (Fig. 7). Over time, a decrement in cytosolic GC-β1 occurred, which reached significance with the 64 h time point.
Fig. 7.
Effects of chronic treatment of N18TG2 cells with the CB1 antagonist rimonabant or pertussis toxin on cytosolic GC-β1 levels. Cells were treated with vehicle or 1 μM rimonabant for 24 h or 48 h as indicated, or with pertussis toxin (100 ng/ml) for 40 h or 64 h as indicated. Cytosolic proteins were subjected to Western blot analysis. Densitometry was performed on N=3 individual Western blots, and data were normalized to control as 100%. Data are shown as mean ± SEM and analyzed by Prism for one-way ANOVA and Dunnett’s post hoc test *Significantly different from control at p <0.001.
4. Discussion
Cannabinoid-stimulated cyclic GMP production in neurons is a novel finding, which contrasts with an earlier report that WIN55212-2 inhibited isoniazid-stimulated cyclic GMP accumulation in rat cerebellum (Rinaldi-Carmona et al., 1995). In the rat heart, an anandamide analog increased cyclic GMP concurrently with decreased contractility (Sterin-Borda et al., 2005), whereas HU210 failed to increase cyclic GMP levels (Maslov et al., 2004). In guinea pig mast cells, CB2 stimulation induced the expression of iNOS, leading to increased NO and cyclic GMP (Vannacci et al., 2004). In endothelial cells, a novel cannabinoid receptor could increase reactive nitrogen species, cyclic GMP, and Ca2+-dependent K+ current in response to abnormal-cannabidiol (Begg et al., 2003; McCollum et al., 2007).
The cannabinoid stimulation of NO-sensitive GC could be governed by a complex regulation that might include Ca2+-dependent and Ca2+-independent mechanisms. Stimulation of N18 cell CB1 receptors inhibits depolarization-dependent N-type Ca2+ channels (Mackie et al., 1993; Sugiura et al., 1997), and we have evidence that cannabinoid agonists fail to elicit intracellular Ca2+ mobilization in N18TG2 cells (data not shown). Stimulation of pituitary cell Gi/o-coupled receptors (ETA endothelin and D2 dopaminergic) inhibited cyclic GMP accumulation by a mechanism consistent with inhibition of cyclic AMP production, stimulation of inwardly rectifying K+ channels, hyperpolarization, and the inactivation of spontaneous Ca2+ currents (Kostic et al., 2002). Therefore, we would not expect to see a CB1 receptor-mediated Ca2+ influx or mobilization that would increase cyclic GMP production. There is precedent for Ca2+-independent mechanisms of stimulation of NO-sensitive GC, including regulation by PKC in a neuronal cell model (Louis et al., 1993). However, cannabinoid drugs have not generally been shown to regulate a Gq-PKC pathway (see (Howlett et al., 2002a) for discussion). A cyclic AMP/PKA-dependent phosphorylation of the α1 isoform led to GC activation in pituitary cells (Kostic et al., 2004; Kostic et al., 2002). However, cannabinoid stimulation of N18TG2 cells fails to elicit cyclic AMP production even after pretreatment with pertussis toxin (Howlett et al., 1986) or co-stimulation of the cells with receptor agonists that might compete for Gi/o proteins (Howlett and Fleming, 1984). CB1 receptor-mediated translocation of NO-sensitive GC to the membrane may serve a “permissive” role to allow spatial and temporal coordination of signaling, rather than direct activation of the enzyme. Co-existence of NO-sensitive GC with nNOS coupled to a Ca2+ influx mechanism would increase the efficiency of synthesis and transfer of NO to its site of action.
The mechanism by which NO-sensitive GC translocates from the cytosol to the membrane in response to cannabinoid agonists is not entirely clear. The GC-α2 subunit localizes to synaptic membranes by binding to post-synaptic density-95 (PSD-95), PSD-93, SAP97 or SAP102 due to its PDZ domain binding capability (Russwurm et al., 2001). In the N18TG2 cells, GC-α2 expression is much less than GC-α1, suggesting that a select subset of GCα2 could be targeted for translocation upon stimulation. In human platelets, activated NO-sensitive GC translocates to the membrane in response to Ca2+ (Zabel et al., 2002). The brain cortex, adrenal gland, skeletal muscle, and colon exhibit membrane-associated NO-sensitive GC (Pyriochou and Papapetropoulos, 2005). In rat lung endothelial cells, the GC-α1β1 heterodimer localizes to membranes in response to a Ca2+ signal in the absence of a PDZ binding domain (Zabel et al., 2002). A mechanism involving Ca2+ fluxes is not likely for the N18TG2 cells, which do not respond to cannabinoid agonists with an increase in intracellular Ca2+ (unpublished observations). Bradykinin stimulation of endothelial cells promoted association of the GC-β1 subunit with Hsp90 in a complex with eNOS (Venema et al., 2003; Papapetropoulos et al., 2005). The endothelial NO-sensitive GC was predominantly particulate, irrespective of the state of activity of Hsp90, and the multi-protein complex was localized to extranuclear membranous organelles (Venema et al., 2003). The role of Hsp90 was most likely structural stabilization and facilitation of multi-protein complex formation, rather than activation or translocation (Venema et al., 2003; Papapetropoulos et al., 2005). On the other hand, NO-sensitive GC and cytosolic Hsp70 formed a complex that co-localized to the plasma membrane in cultured smooth muscle cells (Balashova et al., 2005). Association of NO-sensitive GC with AGAP1, a protein that possesses ‘Arf-GTPase Activating Protein’ activity as well as a pleckstrin homology domain, may facilitate anchoring to polyphosphoinositides in membranes, or function in endosomal membrane trafficking (Meurer et al., 2004). Protein interactions that maintain GC in the cytosol might include chaperonine-containing t-complex polypeptide-eta (CCTη) association with neuronal NO-sensitive GC via the GC-β1 subunit, as exemplified in rat hippocampal sections, where CCTη may participate in a protein folding function (Hanafy et al., 2004).
The GC-α1β1 heterodimer was found in abundance in human cortex, striatum, amygdala, and hippocampus (Ibarra et al., 2001), areas known to express CB1 receptors with high density (Westlake et al., 1994; Mailleux and Vanderhaeghen, 1992). The GC-α2β1 heterodimer was expressed in greater abundance in striatum and cerebellar granule cells (Gibb and Garthwaite, 2001), regions that express nNOS (Blum-Degen et al., 1999), and high densities of CB1 cannabinoid receptors (Herkenham et al., 1991; Tsou et al., 1998). The autocrine function of colocalized nNOS, NO-sensitive GC, and PKG was demonstrated in cerebellar granule cells by NMDA-stimulated phosphorylation of two PKG substrates: VASP and cyclic AMP response element binding protein (CREB) (Jurado et al., 2004). Similarly, NO-cyclic GMP signaling in chromaffin cells suppressed voltage-gated Ca2+ channel opening and catecholamine release (Schwarz et al., 1998). The localization of NO-sensitive GC to presynaptic membranes associated with synaptic puncta containing nNOS in the hippocampus (Burette et al., 2002) could couple cannabinoids to presynaptic regulation of neurotransmitter release (Schlicker and Kathmann, 2001; Diana and Marty, 2004). Phosphorylation of substrates such as VASP, which associates with profilins, zyxin and vinculin, may contribute to actin neurofilament reorganization (Smolenski et al., 1998).
Acknowledgments
This work was supported by NIDA grants R01-DA03690, U24-DA12385, K05-DA00182, NIGMS grant S06-GM008049, and NCMHD grant P20-MD00175. The studies on GC were initiated due to findings using cDNA gene expression microarray analyses by JDJ under the direction of Dr. Kent E. Vrana, Dept. Physiology and Pharmacology, Wake Forest University, performed as part of the Wake Forest University Summer Research Opportunities Program. These studies were in partial fulfillment of the M.S. degree in Biology at NCCU for JDJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Balashova N, Chang FJ, Lamothe M, Sun Q, Beuve A. Characterization of a novel type of endogenous activator of soluble guanylyl cyclase. J Biol Chem. 2005;280:2186–2196. doi: 10.1074/jbc.M411545200. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Thomas BF. Current evidence supporting a role of cannabinoid CB1 receptor (CB1R) antagonists as potential pharmacotherapies for drug abuse disorders. Behav Pharmacol. 2005;16:275–296. doi: 10.1097/00008877-200509000-00003. [DOI] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Heinemann T, Lan J, Pedersen V, Leblhuber F, Paulus W, Riederer P, Gerlach M. Characterization and regional distribution of nitric oxide synthase in the human brain during normal ageing. Brain Res. 1999;834:128–135. doi: 10.1016/s0006-8993(99)01444-4. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burette A, Zabel U, Weinberg RJ, Schmidt HH, Valtschanoff JG. Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J Neurosci. 2002;22:8961–8970. doi: 10.1523/JNEUROSCI.22-20-08961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Deadwyler SA. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem Pharmacol. 1996;52:819–827. doi: 10.1016/0006-2952(96)00419-4. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Sepe N, Buono A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells. Biochem J. 1996a;316:977–984. doi: 10.1042/bj3160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Sugiura T, Waku K. Potential biosynthetic connections between the two cannabimimetic eicosanoids, anandamide and 2-arachidonoyl-glycerol, in mouse neuroblastoma cells. Biochem Biophys Res Commun. 1996b;227:281–288. doi: 10.1006/bbrc.1996.1501. [DOI] [PubMed] [Google Scholar]
- Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br J Pharmacol. 2004;142:9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Holt S, Nilsson O, Jonsson KO, Tiger G, Jacobsson SO. The endocannabinoid signaling system: pharmacological and therapeutic aspects. Pharmacol Biochem Behav. 2005;81:248–262. doi: 10.1016/j.pbb.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Gibb BJ, Garthwaite J. Subunits of the nitric oxide receptor, soluble guanylyl cyclase, expressed in rat brain. Eur J Neurosci. 2001;13:539–544. doi: 10.1046/j.1460-9568.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- Giordano D, De Stefano ME, Citro G, Modica A, Giorgi M. Expression of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in mouse tissues and cell lines using an antibody against the enzyme amino-terminal domain. Biochim Biophys Acta. 2001;1539:16–27. doi: 10.1016/s0167-4889(01)00086-6. [DOI] [PubMed] [Google Scholar]
- Hanafy KA, Martin E, Murad F. CCTeta, a novel soluble guanylyl cyclase-interacting protein. J Biol Chem. 2004;279:46946–46953. doi: 10.1074/jbc.M404134200. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol Rev. 2002a;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Carter P, Jones JD, Vrana KE. Changes in gene expression in response to cannabinoid and aminoalkylindole agonists. Internatl Cannab Res Soc Sympoisum on the Cannabinoids.2002b. [Google Scholar]
- Howlett AC, Fleming RM. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol. 1984;26:532–538. [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–313. [PubMed] [Google Scholar]
- Ibarra C, Nedvetsky PI, Gerlach M, Riederer P, Schmidt HH. Regional and age-dependent expression of the nitric oxide receptor, soluble guanylyl cyclase, in the human brain. Brain Res. 2001;907:54–60. doi: 10.1016/s0006-8993(01)02588-4. [DOI] [PubMed] [Google Scholar]
- Jurado S, Sanchez-Prieto J, Torres M. Elements of the nitric oxide/cGMP pathway expressed in cerebellar granule cells: biochemical and functional characterisation. Neurochem Int. 2004;45:833–843. doi: 10.1016/j.neuint.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Kostic TS, Andric SA, Stojilkovic SS. Receptor-controlled phosphorylation of alpha 1 soluble guanylyl cyclase enhances nitric oxide-dependent cyclic guanosine 5′-monophosphate production in pituitary cells. Mol Endocrinol. 2004;18:458–470. doi: 10.1210/me.2003-0015. [DOI] [PubMed] [Google Scholar]
- Kostic TS, Tomic M, Andric SA, Stojilkovic SS. Calcium-independent and cAMP-dependent modulation of soluble guanylyl cyclase activity by G protein-coupled receptors in pituitary cells. J Biol Chem. 2002;277:16412–16418. doi: 10.1074/jbc.M112439200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Louis JC, Revel MO, Zwiller J. Activation of soluble guanylate cyclase through phosphorylation by protein kinase C in intact PC12 cells. Biochim Biophys Acta. 1993;1177:299–306. doi: 10.1016/0167-4889(93)90126-a. [DOI] [PubMed] [Google Scholar]
- Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Lasukova OV, Krylatov AV, Uzhachenko RV, Pertwee R. Selective cannabinoid receptor agonist HU-210 decreases pump function of isolated perfused heart: role of cAMP and cGMP. Bull Exp Biol Med. 2004;138:550–553. doi: 10.1007/s10517-005-0123-7. [DOI] [PubMed] [Google Scholar]
- McCollum L, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor-independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007;321:930–937. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- Meschler JP, Kraichely DM, Wilken GH, Howlett AC. Inverse agonist properties of N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl (SR141716A) and 1-(2-chlorophenyl)-4-cyano-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxyl ic acid phenylamide (CP-272871) for the CB(1) cannabinoid receptor. Biochem Pharmacol. 2000;60:1315–1323. doi: 10.1016/s0006-2952(00)00447-0. [DOI] [PubMed] [Google Scholar]
- Meurer S, Pioch S, Wagner K, Muller-Esterl W, Gross S. AGAP1, a novel binding partner of nitric oxide-sensitive guanylyl cyclase. J Biol Chem. 2004;279:49346–49354. doi: 10.1074/jbc.M410565200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Shim JY, Assi AA, Norford D, Howlett AC. CB(1) cannabinoid receptor-G protein association: a possible mechanism for differential signaling. Chem Phys Lipids. 2002;121:91–109. doi: 10.1016/s0009-3084(02)00153-6. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Zhou Z, Gerassimou C, Yetik G, Venema RC, Roussos C, Sessa WC, Catravas JD. Interaction between the 90-kDa heat shock protein and soluble guanylyl cyclase: physiological significance and mapping of the domains mediating binding. Mol Pharmacol. 2005;68:1133–1141. doi: 10.1124/mol.105.012682. [DOI] [PubMed] [Google Scholar]
- Pazos MR, Nunez E, Benito C, Tolon RM, Romero J. Functional neuroanatomy of the endocannabinoid system. Pharmacol Biochem Behav. 2005;81:239–247. doi: 10.1016/j.pbb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Pyriochou A, Papapetropoulos A. Soluble guanylyl cyclase: more secrets revealed. Cell Signal. 2005;17:407–413. doi: 10.1016/j.cellsig.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Alonso R, Shire D, Congy C, Soubrie P, Breliere JC, Le Fur G. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Russwurm M, Wittau N, Koesling D. Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J Biol Chem. 2001;276:44647–44652. doi: 10.1074/jbc.M105587200. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Schwarz PM, Rodriguez-Pascual F, Koesling D, Torres M, Forstermann U. Functional coupling of nitric oxide synthase and soluble guanylyl cyclase in controlling catecholamine secretion from bovine chromaffin cells. Neuroscience. 1998;82:255–265. doi: 10.1016/s0306-4522(97)00274-1. [DOI] [PubMed] [Google Scholar]
- Smolenski A, Bachmann C, Reinhard K, Honig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. Analysis and regulation of Vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- Sterin-Borda L, Del Zar CF, Borda E. Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: endogenous signal transduction pathways. Biochem Pharmacol. 2005;69:1705–1713. doi: 10.1016/j.bcp.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kodaka T, Kondo S, Tonegawa T, Nakane S, Kishimoto S, Yamashita A, Waku K. Inhibition by 2-arachidonoylglycerol, a novel type of possible neuromodulator, of the depolarization-induced increase in intracellular free calcium in neuroblastoma x glioma hybrid NG108-15 cells. Biochem Biophys Res Commun. 1997;233:207–210. doi: 10.1006/bbrc.1997.6425. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Vannacci A, Giannini L, Passani MB, Di Felice A, Pierpaoli S, Zagli G, Fantappie O, Mazzanti R, Masini E, Mannaioni PF. The endocannabinoid 2-arachidonylglycerol decreases the immunological activation of Guinea pig mast cells: involvement of nitric oxide and eicosanoids. J Pharmacol Exp Ther. 2004;311:256–264. doi: 10.1124/jpet.104.068635. [DOI] [PubMed] [Google Scholar]
- Venema RC, Venema VJ, Ju H, Harris MB, Snead C, Jilling T, Dimitropoulou C, Maragoudakis ME, Catravas JD. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2003;285:H669–H678. doi: 10.1152/ajpheart.01025.2002. [DOI] [PubMed] [Google Scholar]
- Walz MA, Holt IL, Howlett AC. Cyclic nucleotide phosphodiesterase isozymes in neuroblastoma cells. J Neurosci Res. 1987;17:291–297. doi: 10.1002/jnr.490170314. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Zabel U, Kleinschnitz C, Oh P, Nedvetsky P, Smolenski A, Muller H, Kronich P, Kugler P, Walter U, Schnitzer JE, Schmidt HH. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat Cell Biol. 2002;4:307–311. doi: 10.1038/ncb775. [DOI] [PubMed] [Google Scholar]