Abstract
Specification of the left–right (L-R) axis in the vertebrate embryo requires transfer of positional information from the node to the periphery, resulting in asymmetric gene expression in the lateral plate mesoderm. We show that this activation of L-R lateral asymmetry requires the evolutionarily conserved activity of members of the EGF–CFC family of extracellular factors. Targeted disruption of murine Cryptic results in L-R laterality defects including randomization of abdominal situs, hyposplenia, and pulmonary right isomerism, as well as randomized embryo turning and cardiac looping. Similarly, zebrafish one-eyed pinhead (oep) mutants that have been rescued partially by mRNA injection display heterotaxia, including randomization of heart looping and pancreas location. In both Cryptic and oep mutant embryos, L-R asymmetric expression of Nodal/cyclops, Lefty2/antivin, and Pitx2 does not occur in the lateral plate mesoderm, while in Cryptic mutants Lefty1 expression is absent from the prospective floor plate. Notably, L-R asymmetric expression of Nodal at the lateral edges of the node is still observed in Cryptic mutants, indicating that L-R specification has occurred in the node but not the lateral plate. Combined with the previous finding that oep is required for nodal signaling in zebrafish, we propose that a signaling pathway mediated by Nodal and EGF–CFC activities is essential for transfer of L-R positional information from the node.
Keywords: Left–right asymmetry, isomerism, heterotaxia, node, lateral plate mesoderm, Nodal
Of the three major body axes, the left–right (L-R) axis is the last to be determined during vertebrate embryogenesis. The initial specification of the L-R axis is likely to begin during late stages of gastrulation, but tissue-specific manifestations of morphological L-R asymmetry become apparent much later in development, throughout organogenesis into the late fetal period (for review, see Ramsdell and Yost 1998; Beddington and Robertson 1999). In all vertebrates, the first overt appearance of L-R asymmetry occurs during early somitogenesis, with an initial rightward bending of the linear heart tube that presages the direction of cardiac looping. In the mouse, another early sign of laterality is the direction of embryonic turning that inverts the three primary germ layers of the embryo. Most morphological L-R asymmetry arises at later stages of organogenesis, when unilateral tissues such as the stomach are positioned on one side, or when bilateral paired tissues such as the lung form asymmetrically. Defects in this process of L-R specification can lead to highly pleiotropic effects, including L-R reversals of organ position (inverted situs), mirror image symmetry of bilaterally asymmetric tissues (isomerism), and/or random and independent occurrence of laterality defects in different tissues (heterotaxia).
Recent molecular genetic studies performed in chick, frog, zebrafish, and mouse systems have shown that tissue-specific laterality decisions are mediated by a pathway of regulatory genes that acts during gastrulation and early postgastrulation stages of embryogenesis. These studies have led to a conceptual pathway for L-R axis determination, in which an initial event that breaks L-R symmetry is believed to occur in or around the embryonic node and its derivatives. The resulting L-R positional information is transferred outward to the lateral plate mesoderm, where it is interpreted to generate the situs of individual tissues (for review, see Harvey 1998; Ramsdell and Yost 1998; Beddington and Robertson 1999; King and Brown 1999). Notably, several members of this regulatory pathway are themselves expressed in a L-R asymmetric pattern on the left side of the embryo, in particular the left lateral plate mesoderm.
Several genes in the L-R pathway have roles that appear evolutionarily conserved among vertebrates, including Nodal (Levin et al. 1995; Collignon et al. 1996; Lowe et al. 1996; Lustig et al. 1996; Lohr et al. 1997; Sampath et al. 1997; Rebagliati et al. 1998) and Lefty2 (Meno et al. 1996,1997; Bisgrove et al. 1999; Thisse and Thisse 1999), which encode distant members of the transforming growth factorβ (TGF-β) superfamily, and are asymmetrically expressed in the left lateral plate mesoderm. Another conserved asymmetrically expressed gene is the Pitx2 homeobox gene, which has been proposed to represent a primary regulator of tissue-specific L-R laterality because it is expressed on the left side of many tissues (Logan et al. 1998; Piedra et al. 1998; Ryan et al. 1998; Yoshioka et al. 1998; Campione et al. 1999). In contrast, there are several apparent differences between vertebrate systems that have complicated our understanding of the L-R pathway. For example, many genes that display transient asymmetry of expression in the chick are not asymmetrically expressed in the mouse, including activin βB, activin receptor IIA, and Sonic hedgehog (shh) (Harvey 1998; Ramsdell and Yost 1998; Beddington and Robertson 1999).
We show that L-R axis formation requires the evolutionarily conserved activity of members of the EGF–CFC gene family. The EGF–CFC family is comprised of mammalian Cryptic and Cripto, frog FRL-1, and zebrafish one-eyed pinhead (oep) and encodes extracellular proteins containing a divergent EGF-like motif and a novel cysteine-rich CFC motif (Shen et al. 1997; Zhang et al. 1998). We find that targeted disruption of mouse Cryptic results in L-R laterality defects including randomization of abdominal situs, pulmonary right isomerism, and vascular heterotaxia, as well as randomized embryo turning and cardiac looping. In parallel studies, we show that partial rescue of oep mutant embryos by oep mRNA injection results in randomization of the direction of heart looping and location of the pancreas, revealing that loss of oep function leads to heterotaxia. Notably, in both Cryptic and oep mutant embryos, L-R asymmetric gene expression does not occur in the lateral plate mesoderm. Based on recent studies indicating that EGF–CFC proteins act as essential cofactors for Nodal (Gritsman et al. 1999), we propose that a signaling pathway mediated by Nodal and EGF–CFC proteins is required for activation of L-R asymmetric gene expression in the lateral plate mesoderm.
Results
Targeted disruption of Cryptic
Previous mutational analyses have revealed that oep and Cripto have essential requirements prior to gastrulation (Schier et al. 1997; Ding et al. 1998; Gritsman et al. 1999), but it has been unclear if the later expression of EGF–CFC genes reflects a role in postgastrulation processes. In particular, oep is expressed in the lateral plate mesoderm and forebrain during early somitogenesis (Zhang et al. 1998), and Cryptic is expressed in the lateral plate mesoderm, node, notochordal plate, and prospective floor plate from head-fold stages through approximately the six to eight somite stage (Shen et al. 1997). The expression of oep and Cryptic is symmetric in the lateral plate and precedes the asymmetric expression of genes such as Nodal/cyclops, Lefty2/antivin and Pitx2.
To determine the biological function of Cryptic, we performed targeted gene disruption. The Cryptic targeting construct should result in a null mutation, because it deleted most of the third exon and the entire fourth and fifth exons of the gene, which encode two-thirds of the mature protein including the central EGF and CFC motifs (Fig. 1a–c). In addition, a second targeting construct that deleted the entire Cryptic coding region resulted in the identical homozygous mutant phenotype (Y.-T. Yan, S.M. Price, and M.M. Shen, unpubl.). Homozygosity for the targeted Cryptic mutation resulted in neonatal lethality in the first 2 weeks after birth, apparently because of cardiac defects (see below); to date, only five homozygotes (from >90) have survived past weaning. Our initial indication of a phenotypic defect in L-R laterality was that many newborn Cryptic homozygotes displayed a milk spot (corresponding to the stomach) on their right side, instead of the left (Fig. 1d).
Figure 1.
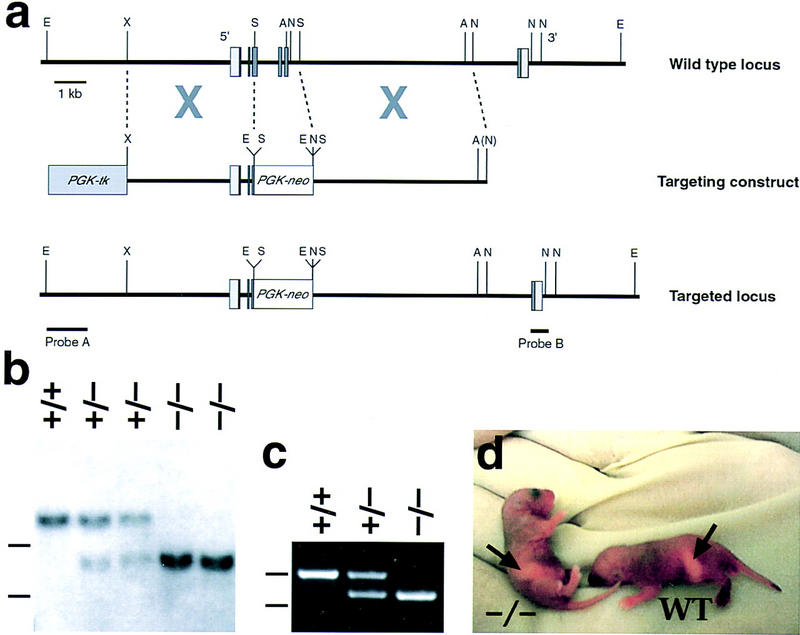
Targeted disruption of Cryptic. (a) Homologous recombination with the targeting vector results in deletion of exons 4 and 5, as well as most of exon 3; exons are shown as boxes, with the coding region in dark gray. (A) AvrII; (E) EcoRI; (N) NheI; (S) SmaI; (X) XbaI. (b) Southern blotting using the 5′-flanking probe. A detects an 18-kb EcoRI fragment from the wild-type genomic locus and an 8.5-kb fragment from the targeted allele in progeny of F1 heterozygous intercrosses; dashes (left) indicate positions of markers at 5 and 10 kb. (c) PCR analysis of visceral yolk sac DNA from 7.5-dpc embryos, showing amplification of an 860-bp band corresponding to Cryptic and a 735-bp fragment corresponding to neo; markers at 615 and 861 bp are indicated as above. (d) Neonatal mice with milk spots on the right side (−/−) and left side (WT).
L-R laterality defects in Cryptic mutant mice
To examine the L-R laterality defects of Cryptic homozygous mutant mice, we analyzed their gross anatomy at 18.5 days post coitum (dpc) and at neonatal stages (P0–P7) (Table 1). We found that Cryptic homozygotes displayed numerous laterality defects, including heterotaxia, randomization of organ situs, and isomerism of bilaterally asymmetric tissues. Thus, within the abdominal cavity, approximately half of the homozygotes (n = 22/49) displayed inverted situs of visceral organs including the stomach, spleen, and pancreas (Fig. 2a,b). In contrast, all homozygous animals displayed asplenia or severe hyposplenia (Fig. 2c–e); a significant proportion of homozygotes also displayed abnormal lobation or midline positioning of the liver (Table 1).
Table 1.
Phenotype of Cryptic homozygous mice
| Phenotype
|
Number of mutant/total (%)
|
|---|---|
| Abdominal tissues | |
| Visceral situs | 22/49 inverted (45%) |
| Spleen | 49/49 asplenic/hyposplenic (100%) |
| Liver | 11/27 abnormal (41%) |
| Abdominal vasculature | |
| Branching of inferior vena cava | 16/25 abnormal (64%) |
| Position of renal veins | 4/19 left anterior (21%) |
| Thoracic tissues | |
| Cardiac apex | 18/50 dextrocardia, 6/50 mesocardia (48%) |
| Cardiac malformation | 14/16 septal defects (88%); 11/11 transposition of great arteries (100%) |
| Atrial shape | 23/34 right isomerism, 1/34 left (71%) |
| Lung bronchi | 50/50 bilateral eparterial (100%) |
| Lung lobes | 50/50 right isomerism (100%) |
| Thoracic vasculature | |
| Aorta relative to pulmonary artery | 44/50 aorta ventral, 5/50 adjacent (98%) |
| Aortic arching | 12/29 right (41%) |
| Position of azygos vein | 5/30 right, 6/30 bilateral (37%) |
| Position of inferior vena cava | 4/33 left, 19/33 bilateral (70%) |
Phenotypes were scored in mice at 18.5 dpc (n = 6) and in neonates at <1 week of age (n = 44). Not all phenotypes were scored in each mouse. Unless indicated otherwise in the text, the occurrences of these phenotypes were not noticeably correlated with each other.
Figure 2.
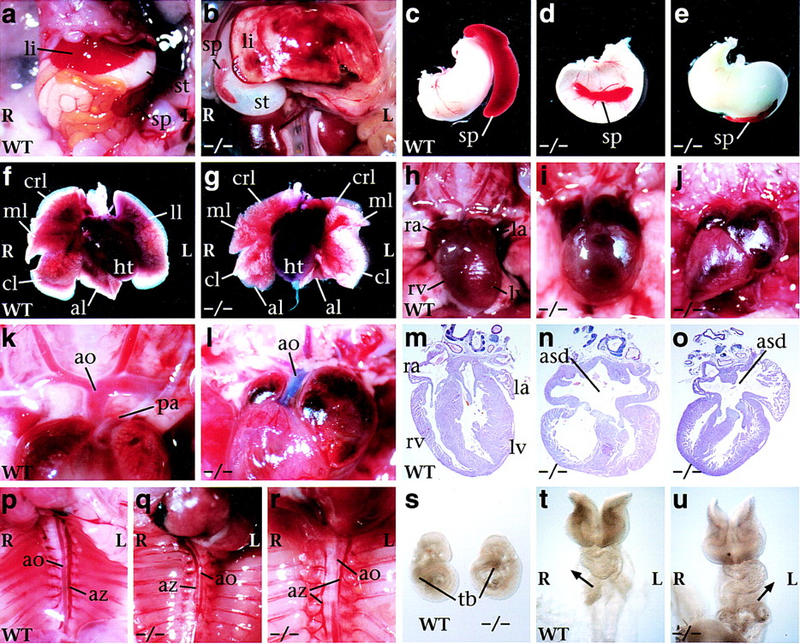
L-R laterality defects in Cryptic null mutants. (a–r) Ventral views of neonatal mice; in all panels, left (L) and right (R) are as indicated. Abdominal cavity of wild type (a) and mutant (b) with inverted situs and hyposplenia. Stomach and spleen from wild type (c), mutant with normal situs (d), and mutant with inverted abdominal situs (e). Heart and lung lobes of wild type (f) and mutant (g) with right pulmonary isomerism. Heart positions of wild type with normal levocardia (h), and mutants with mesocardia (i) and dextrocardia (j); note correlation with altered size of the atrial chambers. High-power view of cardiac arterial relationships. In the wild type (k), the aorta is dorsal to the pulmonary artery and connects to the left ventricle; in the mutant (l), the aorta is ventral and connects to the right ventricle, as shown following injection of blue dye into the right ventricle, consistent with transposition of the great arteries. Sections through hearts of wild type (m), and two mutants (n,o) that show an atrial septal defect. Position of the azygos vein and direction of aortic arching. In the wild type (p), the azygos vein is located on the left, and the aorta arches leftward; in the mutant (q), the azygos vein crosses over to the right and the aorta arches rightward; in the mutant (r), there is a bilateral azygos vein while the aorta arches leftward. (s) Lateral views of 8.5-dpc embryos, showing altered direction of embryo turning in the mutant. (t,u) Ventral views of 10-somite-stage embryos, with arrows indicating direction of cardiac looping in the wild type (t) and Cryptic mutant (u). (al) accessory lobe; (ao) aorta; (asd) atrial septal defect; (az) azygos vein; (cl) caudal lobe; (crl) cranial lobe; (ht) heart; (la) left atrium; (li) liver; (ll) left lung; (lv) left ventricle; (ml) medial lobe; (pa) pulmonary artery; (ra) right atrium; (rv) right ventricle; (sp) spleen; (st) stomach; (tb) tailbud.
In the thoracic cavity, we found that all homozygotes showed right pulmonary isomerism (Fig. 2f,g); this phenotype is correlated frequently with hyposplenia in human patients with laterality defects (Kosaki and Casey 1998). Moreover, approximately half of the homozygotes (n = 24/50) displayed dextrocardia (cardiac apex pointing to the right) or mesocardia (pointing to the middle), as opposed to the normal levocardia (Fig. 2h–j). Regardless of cardiac situs, nearly all homozygotes displayed cardiac abnormalities, most notably transposition of the great arteries (Fig. 2k,l), as well as severe atrial septal defects (Fig. 2m–o). Finally, we observed numerous random and uncorrelated laterality defects within the vasculature, consistent with heterotaxia (Table 1). For example, the azygos vein could be located on the left side (as it is in the wild type), on the right, or bilaterally (Fig. 2p–r).
The L-R laterality defects observed in neonatal Cryptic homozygotes were paralleled by phenotypic defects observed in early embryogenesis. At 8.5–9.5 dpc, Cryptic homozygous embryos were indistinguishable from their wild-type littermates except for randomization of cardiac looping and embryo turning (n = 21/45), with these two phenotypes highly correlated (Fig. 2s–u). Because Cryptic is expressed in the notochordal plate and prospective floor plate, and laterality defects are frequently associated with node and notochord defects (e.g., Danos and Yost 1996; Dufort et al. 1998; King et al. 1998; Melloy et al. 1998), we investigated potential axial midline defects by skeletal staining of homozygous neonates (n = 6), histological sections at 10.5 dpc (n = 3), and in situ hybridization with Shh, followed by sectioning (n = 3). No evidence for axial midline defects was observed (data not shown).
Absence of lateral L-R asymmetric gene expression in Cryptic mutant embryos
To determine the basis for L-R patterning defects in Cryptic mutants, we performed in situ hybridization on gastrulation and early somite-stage embryos (up to 10 somites), using probes for Nodal, Lefty1, Lefty2, and Pitx2, which are asymmetrically expressed at these stages. First, we examined expression of Lefty1 and Lefty2, which are asymmetrically expressed at 2–10 somites in the left prospective floor plate and left lateral plate mesoderm, respectively (Meno et al. 1997, 1998)(Fig. 3a,b,e,f). We found that Cryptic homozygous embryos lacked all expression of Lefty1 (n = 12) and Lefty2 (n = 9) at these stages (Fig. 3c,d,g,h); however, an earlier phase of symmetric Lefty2 expression in newly formed mesoderm during primitive streak stages was unaffected (data not shown). Next, we examined expression of the homeobox gene Pitx2, which is found symmetrically in Rathke's pouch and asymmetrically in the left lateral plate mesoderm and left foregut endoderm from six to eight somites continuing through 9.5 dpc (Ryan et al. 1998; Yoshioka et al. 1998) (Fig. 3i,j). In Cryptic mutants at 8.5 and 9.5 dpc (n = 16), Pitx2 expression was still observed in Rathke's pouch but not in the asymmetric domains (Fig. 3k,l).
Figure 3.
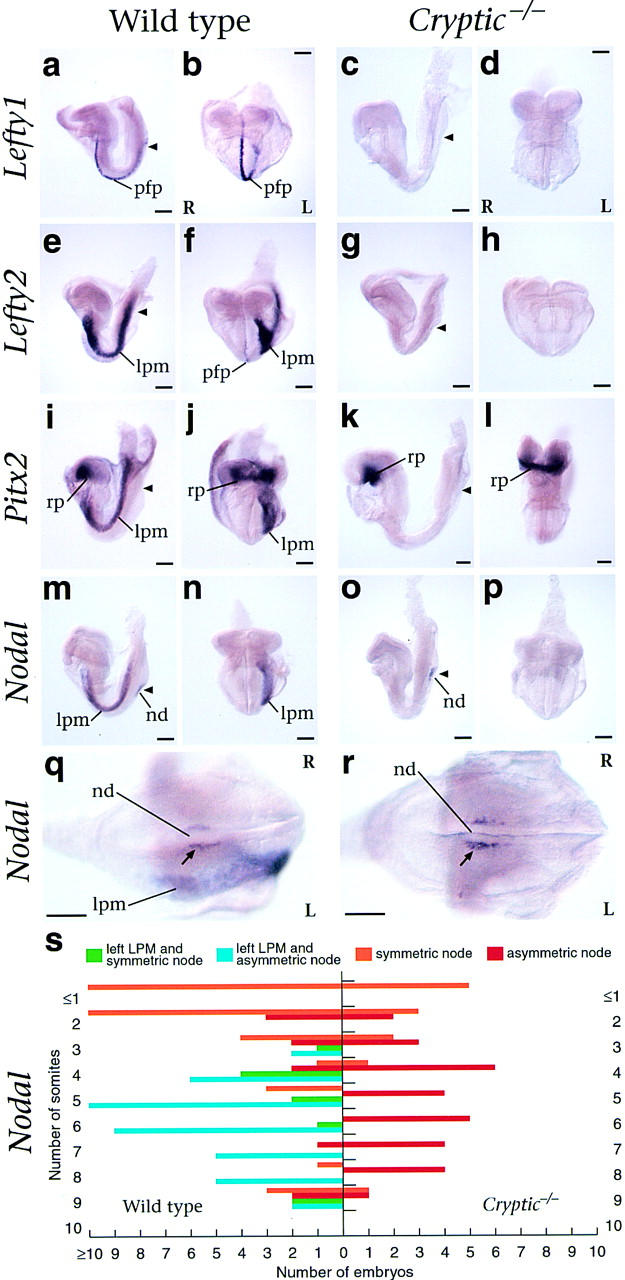
Expression of L-R pathway genes in Cryptic homozygous mutant embryos. (a–p) Lateral and frontal views of early somite stage mouse embryos following whole-mount in situ hybridization. Left (L) and right (R) are as shown, and the position of the node is indicated by an arrowhead. Expression of Lefty1 is detected in the prospective floor plate of wild type (a,b) but not mutant embryos (c,d). Expression of Lefty2 is detected in the left lateral plate mesoderm and weakly in the prospective floor plate of wild type (e,f) but not Cryptic homozygotes (g,h). Pitx2 expression is observed symmetrically in Rathke's pouch in both wild type (I,j) and mutant (k,l) embryos, but asymmetric expression in the left lateral plate mesoderm is observed only in the wild type. Nodal expression is detected in the node of both wild-type (m,n) and mutant (o,p) embryos; left lateral plate expression is observed only in the wild type. High-power caudal views of the node in wild-type (q) and Cryptic homozygote (r) show asymmetric expression of Nodal. (s) Graphical representation of Nodal in situ hybridization results. The numbers of wild-type and Cryptic mutant embryos analyzed with the indicated Nodal expression patterns are graphed according to somite stage. (lpm) lateral plate mesoderm; (nd) node; (pfp) prospective floor plate; (rp) Rathke's pouch.
Finally, we examined expression of Nodal, which is found at the lateral boundaries of the node at head-fold and early somite stages, with a transient phase of L-R asymmetry at four to eight somites, and in the left lateral plate mesoderm at approximately two to eight somites (Collignon et al. 1996; Lowe et al. 1996) (Fig. 3m,n). In Cryptic mutants (n = 41), Nodal expression was observed at the lateral boundaries of the node but was never detected in the lateral plate mesoderm (Fig. 3o,p). Notably, the markedly asymmetric expression of Nodal at the edges of the node at four to eight somites was still observed in the Cryptic mutant embryos (n = 29; Fig. 3q–s). Thus, our in situ hybridization results indicate that L-R laterality has been initiated within the node but not in the lateral plate mesoderm.
Heterotaxia in oep mutant fish
To determine if the function of EGF–CFC genes in L-R determination is conserved in vertebrates, we studied the role of the zebrafish oep gene. Previous studies have shown that oep is required for formation of mesoderm, endoderm, prechordal plate, and ventral neuroectoderm, correlating with the expression of oep in the progenitors of these cell types (Schier et al. 1997; Zhang et al. 1998; Gritsman et al. 1999). During somitogenesis, oep is also expressed in the left and right lateral plate, where progenitors of the heart and other organs are located in wild-type embryos (Serbedzija et al. 1998; Zhang et al. 1998). The potential role of oep in these territories cannot be analyzed in oep mutants, because mutant embryos lack endodermal derivatives and heart (Schier et al. 1997; Gritsman et al. 1999). To circumvent this limitation, we examined the phenotype of maternal–zygotic oep (MZoep) embryos whose early defects were rescued by oep mRNA injection (Fig. 4). Injected mRNA is present throughout gastrulation (data not shown) and is sufficient to completely rescue the formation of endoderm, mesoderm, axial midline, and ventral neuroectoderm (Fig. 4c–f), but is apparently insufficient to complement the loss of oep activity at later stages. Using morphological criteria and marker gene expression, we found that heart and pancreas form, but that the direction of heart looping and the location of the pancreas are randomized with respect to the L-R axis of the embryo (Fig. 4c–f; Table 2). More than 81% (48/59) of oep mutant embryos that display abnormal heart asymmetry during embryogenesis survive to adulthood, demonstrating that mRNA injection rescues the development and function of all essential organs. Notably, there was no correlation between abnormal heart asymmetry and the location of the pancreas, revealing that loss of oep function leads to heterotaxia.
Figure 4.
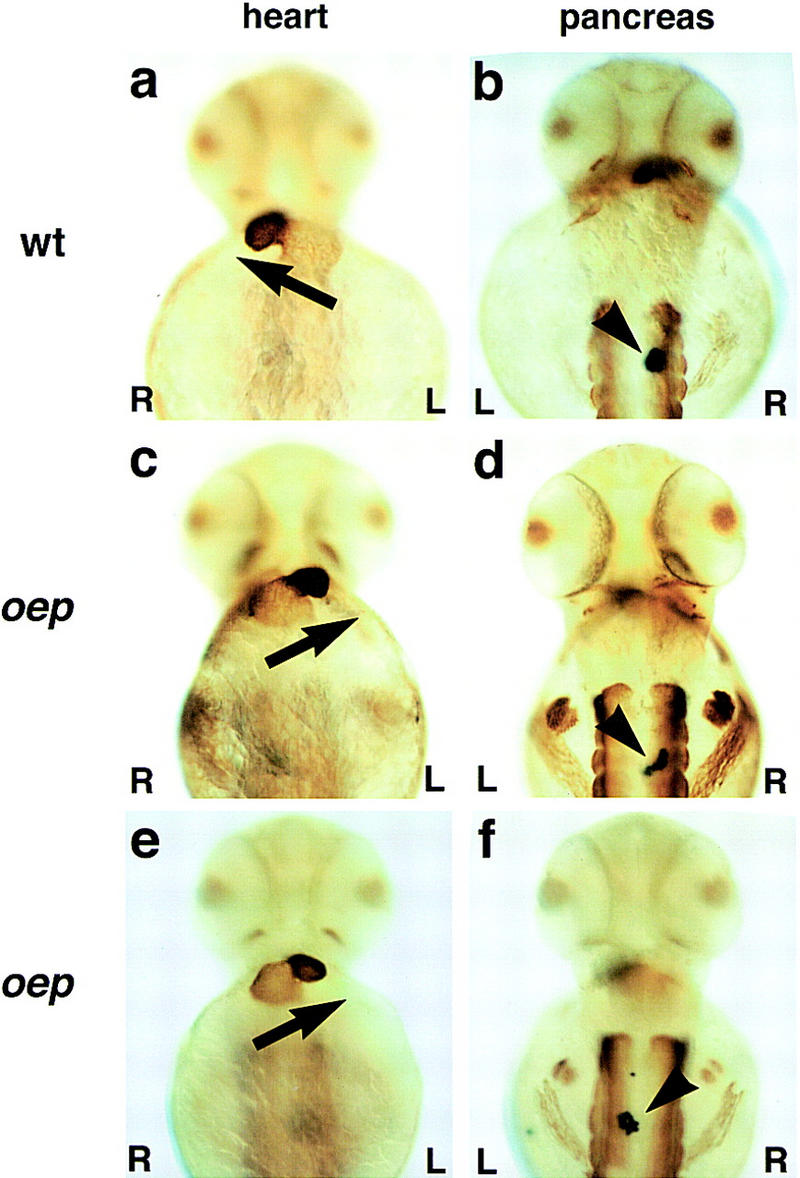
Heart looping and location of the pancreas in oep mutants. Ventral (a,c,e) and dorsal views (b,d,f) of embryos upon immunohistochemistry with MF20 antibody (Bader et al. 1982) and RNA in situ hybridization with insulin probe (Milewski et al. 1998); anterior is up. (a,b) Wild-type embryo; (c,d and e,f) two examples of maternal-zygotic oep mutant embryos rescued by injection of oep mRNA. Note the normal development of eyes and trunk muscle (d,f) in rescued embryos [maternal–zygotic oep mutants show cyclopia and absence of trunk muscle (Gritsman et al. 1999)]. The arrow indicates heart looping from the atrium (weaker staining, posteriorly) to the ventricle (stronger staining, anteriorly); note right looping in a and left looping in c and e. The arrowhead indicates position of pancreas on right (b,d) or left (f) side. We note that the direction of cardiac looping in rescued mutants did not significantly affect their survival to adulthood [50/70 (71%) of right-looping embryos, 46/53 (87%) of left-looping embryos, and 2/6 (33%) of nonlooping embryos survived].
Table 2.
Direction of heart looping and location of pancreas in wild-type and oep mutants
| Genotype: oep/+ (+/+ female × −/− male) | |
| Injected mRNA: lacZ | |
| Total no. embryos: 96 |
| Pancreas (insulin)
|
Heart (MF20)
|
|||
|---|---|---|---|---|
| L
|
M
|
R
|
||
| R | 83 (86.5%) | 2 (2.1%) | 0 | 81 (84.3%) |
| M | 6 (6.2%) | 0 | 4 (4.2%) | 2 (2.1%) |
| L | 7 (7.3%) | 4 (4.2%) | 0 | 3 (3.1%) |
| Genotype: oep/+ (+/+ female × −/− male) |
| Injected mRNA: oep |
| Total no. embryos: 108 |
| Pancreas (insulin)
|
Heart (MF20)
|
|||
|---|---|---|---|---|
| L
|
M
|
R
|
||
| R | 90 (83.3%) | 6 (5.5%) | 3 (2.8%) | 81 (75.0%) |
| M | 4 (3.7%) | 2 (1.9%) | 0 | 2 (1.9%) |
| L | 14 (13.0%) | 9 (8.3%) | 0 | 5 (4.6%) |
| Genotype: oep/oep (−/− female × −/− male) |
| Injected mRNA: oep |
| Total no. embryos: 106 |
| Pancreas (insulin)
|
Heart (MF20)
|
|||
|---|---|---|---|---|
| L
|
M
|
R
|
||
| R | 48 (45.3%) | 20 (18.9%) | 1 (0.9%) | 27 (25.5%) |
| M | 7 (6.6%) | 2 (1.9%) | 1 (0.9%) | 4 (3.8%) |
| L | 51 (48.1%) | 26 (24.5%) | 1 (0.9%) | 24 (22.7%) |
Absence of L-R asymmetric gene expression in oep mutants
To determine the onset of the L-R patterning defect in oep mutants, we performed in situ hybridization on somite-stage embryos using probes for cyclops, antivin (a member of the lefty family), and pitx2, which are all asymmetrically expressed in the lateral plate mesoderm (Rebagliati et al. 1998; Campione et al. 1999; Thisse and Thisse 1999). Analogous to Cryptic mouse mutants, we never detected the normal asymmetric expression of these markers, despite wild-type expression in other regions of the embryo (Fig. 5) . Importantly, asymmetric expression is not initiated, revealing a role for oep in the induction of lateral plate asymmetry.
Figure 5.
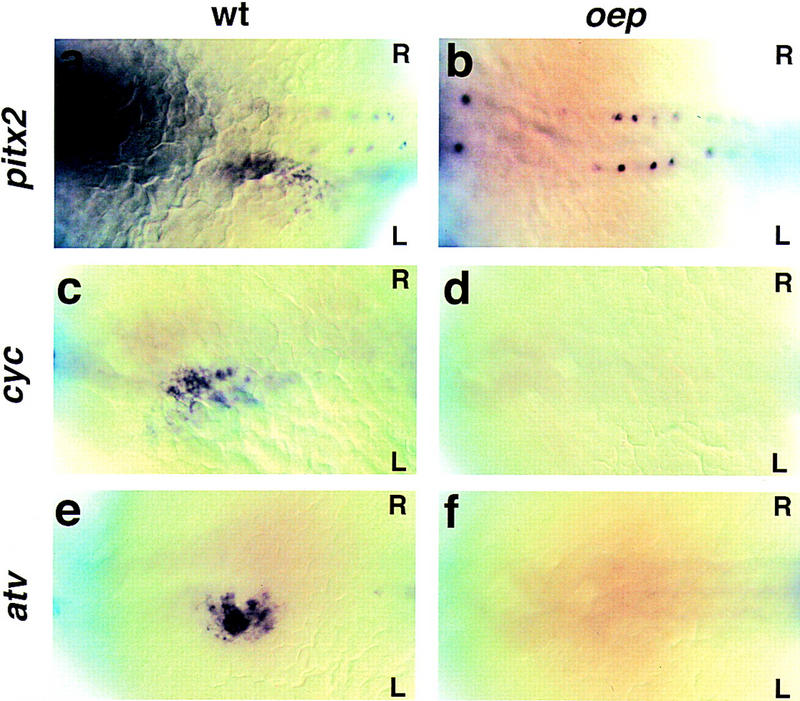
Expression of L-R pathway genes in oep mutant embryos. Dorsal view (anterior to the left) of 22-somite-stage (a–d) and 24-somite-stage (e,f) embryos following whole-mount in situ hybridization with pitx2 (a,b), cyclops (c,d), or antivin (e,f). Normal expression in the left lateral plate is only detected in wild-type (a,c, oep/+ embryo injected with oep mRNA; e, oep/+ embryo injected with lacZ mRNA) but not in maternal–zygotic oep mutants whose early patterning defects were rescued by oep mRNA injection (b, n = 58 embryos analyzed between 14- and 24-somite stages; d, n = 67; f, n = 44). Note the normal expression of pitx2 in the spinal cord (b).
Discussion
Our comparative mutational analyses have shown that homozygous Cryptic null mutant mice and partially rescued oep mutant fish both display highly penetrant L-R heterotaxia defects. Notably, in Cryptic as well as oep mutant embryos, Nodal, Lefty2/antivin, and Pitx2 are not expressed in the lateral plate mesoderm, indicating that EGF–CFC activity is essential for asymmetric gene expression in the lateral mesoderm. Taken together, our findings with oep mutant fish are analogous to those for Cryptic mutant mice, and establish an evolutionarily conserved requirement for EGF–CFC genes in the establishment of L-R asymmetry in vertebrates. Interestingly, this evolutionary conservation of EGF–CFC activity in the L-R pathway markedly contrasts with the apparent non-conserved roles of Fgf8 and Shh in the mouse and chick (Meyers and Martin 1999).
Essential function of EGF–CFC genes in L-R axis specification
Our results can be readily integrated with a general pathway for L-R axis determination in which initial L-R symmetry is broken in or around the node, and subsequent L-R positional information is transferred to the lateral plate mesoderm (Levin et al. 1995; Logan et al. 1998; Pagan-Westphal and Tabin 1998; Beddington and Robertson 1999). Given the requirement of oep activity for nodal signaling in zebrafish and the functional conservation of EGF–CFC proteins (Gritsman et al. 1999), we propose that Cryptic and oep are essential for Nodal signaling in L-R axis specification. Our findings indicate that EGF–CFC activity is required prior to the activation of L-R asymmetric gene expression in the periphery and may be involved in events downstream from an initial process that breaks L-R symmetry.
Specifically, our results are consistent with two possible models for EGF–CFC function in L-R axis formation (Fig. 6). In the first model, Cryptic/oep would be required in the lateral plate mesoderm to mediate the response to an asymmetric ‘left’-determining signal emanating from the node (Fig. 6a,b). This signal might correspond to Nodal itself, as we have shown previously that EGF–CFC proteins are required for cells to respond to Nodal signals (Gritsman et al. 1999). In this scenario, Nodal signaling from the node or its derivatives cannot be received due to the absence of EGF–CFC activity in the lateral plate.
Figure 6.
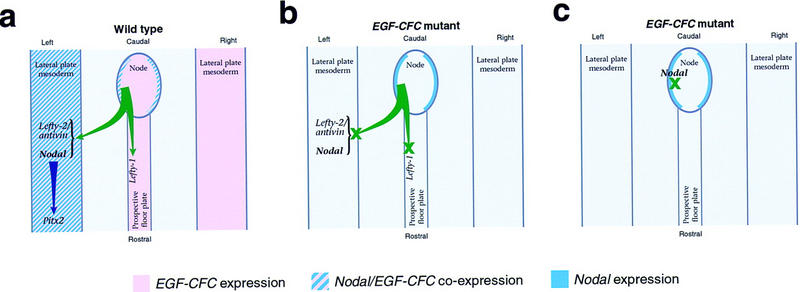
Schematic model for EGF–CFC function in L-R axis formation. (a) In wild-type embryos, a asymmetric signal emanating from the node activates expression of Nodal/cyclops and Lefty2/antivin in the left lateral plate mesoderm, as well as Lefty1 in the left prospective floor plate, leading to subsequent activation of Pitx2 and specification of organ situs. The node-derived signal could correspond to Nodal itself or to a hypothesized factor downstream of Shh signaling that conveys L-R positional information in the chick (Pagan-Westphal and Tabin 1998). (b) In this model, the response to the asymmetric node-derived signal is mediated by Cryptic/oep, which is symmetrically expressed in the lateral plate mesoderm. In the absence of EGF–CFC activity, the lateral plate and prospective floor plate do not respond to the node-derived signal, and asymmetric gene expression fails to occur, resulting in subsequent L-R laterality defects. (c) Here, Cryptic/oep is required to mediate a Nodal activity that is downstream of an initial L-R symmetry-breaking event in or around the node. As a consequence, L-R laterality is specified around the node but fails to be propagated to the lateral plate mesoderm.
In the second model for EGF–CFC function, Cryptic/oep would be required at an earlier stage in the node or its derivatives for the generation or propagation of an asymmetric signal, which could either correspond to Nodal itself or be dependent on Nodal signaling (Fig. 6c). Defects in axial midline structures often result in L-R laterality defects and alterations in asymmetric gene expression, as seen for mouse mutations in no turning, HNF-3β, Brachyury, and SIL (Dufort et al. 1998; King et al. 1998; Melloy et al. 1998; Izraeli et al. 1999) and for zebrafish mutations in no tail or floating head (Danos and Yost 1996; Chen et al. 1997). Although there are no apparent structural defects in the node or its derivatives in Cryptic and partially rescued oep mutants, absence of EGF–CFC activity might result in a block in Nodal signaling in the axial midline, indirectly leading to defects in the lateral plate (Fig. 6c). Of course, these models are not mutually exclusive, and Cryptic/oep may act in both the node and lateral plate. In either case, EGF–CFC activity would play an essential role in transferring L-R positional information from the node to the periphery, resulting in asymmetric Nodal and Lefty2/antivin expression in the lateral plate mesoderm, asymmetric Lefty1 expression in the mouse floor plate, and subsequent asymmetric Pitx2 expression, ultimately leading to specification of individual organ situs.
Cryptic mutants as a model for right isomerism/asplenia syndrome
In humans, the proper L-R situs of the visceral tissues is critical for their morphogenesis and/or physiological function, particularly in the cardiovascular system. In particular, children born with severe heterotaxia generally die shortly after birth, usually due to severe cardiac defects. In many cases, laterality defects in humans that result in heterotaxia can be classified into two primary categories: right isomerism associated with asplenia/hyposplenia and, conversely, left isomerism associated with polysplenia (Goldstein et al. 1998; Kosaki and Casey 1998). Our studies show that Cryptic mutant mice recapitulate many features of the right isomerism/asplenia syndrome, suggesting that the Cryptic mutant mice may represent a model for a major category of human L-R laterality defects.
Interaction of EGF–CFC genes with the Nodal signaling pathway
In contrast to the phenotype reported here for oep, mutations in the zebrafish nodal gene cyclops do not result in a significant incidence of heart looping defects (Chen et al. 1997). These differing laterality phenotypes of oep versus cyclops mutants may be due to redundant functions of zebrafish nodal-related genes in L-R axis determination. Moreover, a direct requirement for mouse Nodal in L-R patterning has also been difficult to establish, due to the early embryonic lethality of Nodal mutants, which precludes analysis of later defects. Nonetheless, the L-R phenotypes of Cryptic and oep mutants, together with the phenotypes of Nodal+/−; HNF-3Β+/− and Nodal+/−; Smad2+/− mutants (Collignon et al. 1996; Nomura and Li 1998), strongly suggest that Nodal signals are essential for L-R axis specification.
Although the Nodal signaling pathway has not been analyzed at the biochemical level, loss- and gain-of-function studies in mouse, frog, and fish suggest that during gastrulation Nodal signals may act via activin-like receptors (Hemmati-Brivanlou and Melton 1992; Armes and Smith 1997; Chang et al. 1997; New et al. 1997; Oh and Li 1997; Gu et al. 1998; Gritsman et al. 1999; Meno et al. 1999) and the transcription factor Smad2 (Baker and Harland 1996; Graff et al. 1996; Nomura and Li 1998; Waldrip et al. 1998; Weinstein et al. 1998). During germ-layer formation, Nodal signaling has also been shown to be dependent on EGF–CFC activity (Gritsman et al. 1999) and to be antagonized by members of the Lefty family (Bisgrove et al. 1999; Meno et al. 1999; Thisse and Thisse 1999). Therefore, it is thought that during gastrulation, Nodal signals are dependent on EGF–CFC proteins to activate activin-like receptors and Smad2, leading to the induction of Lefty genes and the attenuation of Nodal signaling.
Comparison of the L-R phenotypes of Cryptic and oep mutants with the defects found in Lefty1 and ActRIIB mutant mice extends this model to L-R axis determination, raising the possibility that EGF–CFC proteins act universally as essential cofactors for Nodal signaling. First, mice lacking Lefty1 (Meno et al. 1998) frequently display left pulmonary isomerism and bilateral expression of Nodal, Lefty2, and Pitx2. In contrast, Cryptic mutants display right pulmonary isomerism and lack asymmetric gene expression in the lateral plate mesoderm. These opposing phenotypes support the notion that Lefty1 acts by antagonizing EGF–CFC dependent Nodal activity during L-R determination. Secondly, the phenotype of Cryptic mutants superficially resembles that of ActRIIB mutant mice, which display right pulmonary isomerism and severe cardiac defects (Oh and Li 1997). Moreover, although Smad2 homozygotes display early embryonic lethality due to defective specification of the anteroposterior (AP) axis (Nomura and Li 1998; Waldrip et al. 1998; Weinstein et al. 1998), a significant percentage of Nodal+/−; Smad2+/− compound heterozygotes display L-R laterality defects (Nomura and Li 1998), which are similar to those of Cryptic mutants. The greater severity of the laterality defects in Cryptic mice relative to those of ActRIIB mutants may reflect the ability of Nodal in conjunction with EGF–CFC proteins to signal through a type II receptor that is partially redundant with ActRIIB, perhaps ActRIIA (also known as ActRII). In summary, these findings indicate that Nodal signaling during L-R development is mediated by EGF–CFC proteins, activin receptors, and Smad2.
Conservation of EGF–CFC function in embryonic axis formation
The phenotypes of Cripto and Cryptic mutations in mice bear remarkable similarity to those of mutant zebrafish with different timing of oep activity. Specifically, complete removal of both maternal and zygotic oep activity (MZoep mutants) results in loss of head and trunk mesoderm, endoderm, and an incorrectly positioned AP axis (Gritsman et al. 1999), a phenotype similar to that of Cripto mutant mice (Ding et al. 1998). Conversely, restoration of early oep activity to MZoep embryos by oep mRNA injection rescues these defects, but the insufficient persistence of injected mRNA results in a subsequent L-R laterality defect that strongly resembles the phenotype of Cryptic mutants. Taken together, our results indicate that a Nodal and EGF–CFC signaling pathway is essential for both the AP and L-R axes in vertebrates, with the dual role for oep in both processes in fish being divided between the related genes Cripto and Cryptic in mice.
Materials and methods
Gene targeting
A murine Cryptic cDNA was used to screen a λFIXII library constructed from 129Sv/J genomic DNA (Stratagene), resulting in the isolation of a 21-kb genomic clone containing the entire coding region. To construct a targeting vector for Cryptic, a 3.5-kb XbaI–SmaI 5′ flank was subcloned into the XbaI–SmaI sites of pTKLNL (Mortensen 1999), followed by subcloning of a 5.0-kb SmaI–NheI 3′ flank, such that the PGK–neo and PGK–tk cassettes are in the opposite transcriptional orientation to Cryptic. Targeting was performed using TC1 ES cells (Deng et al. 1996), with targeted clones obtained at a frequency of 5% (4/88); ES cell culture and blastocyst injection were performed as described previously (Ding et al. 1998). Chimeric males obtained following blastocyst injection were bred with Black Swiss females (Taconic), and germ-line transmission was obtained from one targeted ES clone; two independent lines were also derived using a different targeting vector (Y.-T. Yan, S.M. Price, and M.M. Shen, unpubl.). These targeted Cryptic mutations have been maintained through backcrossing with outbred Black Swiss mice; the phenotype appears similar in each line. In addition, the homozygous phenotype appears similar in a hybrid 129/SvEvTac-C57BL/6J strain background.
Mouse genotyping and phenotypic analysis
Genotyping was performed by Southern blotting or by PCR using genomic DNA prepared from tails or embryonic visceral yolk sac. Primers for genotyping were as follows: for wild-type Cryptic, 5′GGAGATGGTGCCAGAGAAGTCAGC3′ and 5′AATAGGCAGGGCACACGCAGAAAC3′; for neo, 5′CTGCCGCGCTGTTCTCCTCTTCCT3′ and 5′ACACCCAGCCGGCCACAGTCG3′. The presence of cardiac septal defects and transposition of the great arteries was scored by injection of bromphenol blue dye into the right ventricle (Oh and Li 1997), and ventriculoarterial alignment was confirmed by histological sectioning. Cardiac histology was performed by hematoxylin-eosin staining of paraffin sections, with attention given to L-R orientation of sections. Whole-mount in situ hybridization to mouse embryos was performed as described (Ding et al. 1998), using probes for murine Lefty1 (Meno et al. 1997), Lefty2 (Meno et al. 1997), Nodal (Lowe et al. 1996), and Pitx2 (Lanctèt et al. 1999).
Zebrafish genetics and phenotypic analysis
Homozygous oeptz57/oeptz57 adults were obtained by rescue of homozygous oeptz57/oeptz57 embryos with oep mRNA (Zhang et al. 1998; Gritsman et al. 1999). To rescue the early patterning defects of oep mutants, maternal-zygotic oeptz57/oeptz57 embryos were injected with 25–50 pg of oep mRNA at the one- to four- cell stage. Heart looping was scored in live embryos and by immunohistochemistry using the MF20 antibody (Bader et al. 1982) that recognizes a myosin heavy chain. Embryos were then processed for in situ hybridization using an insulin antisense RNA probe (Milewski et al. 1998). Whole-mount in situ hybridization for cyclops, antivin, and Pitx2 was performed as described (Zhang et al. 1998). Zebrafish pitx2 was cloned by screening of a cDNA library (kindly provided by B. Appel and J. Eisen, University of Oregon, Eugene) with a PCR-amplified pitx2 homeobox probe (R.D. Burdine; A.F. Schier, and W.S. Talbot, GenBank accession nos. AF156905 and AF156906).
Acknowledgments
We thank Anukampa Barth, Jacques Drouin, Hiroshi Hamada, Yoshiyuki Imai, Michael Kuehn, Rick Mortensen, Cliff Tabin, Bernard Thisse, Christine Thisse, and Steve Wilson for gifts of probes and reagents. We also thank Nishita Desai, Rory Feeney, Elizabeth Heckscher, and Magdalena Michalski for technical assistance. We are particularly grateful to Cory Abate-Shen, Robert Cardiff, and Cliff Tabin for helpful discussions and comments on the manuscript. This work was supported by post-doctoral fellowships from the American Heart Association (J.D.) and Damon Runyon–Walter Winchell Cancer Research Fund (R.D.B.), and by grants from the National Science Foundation (M.M.S.), the American Heart Association (M.M.S.), the U.S. Army Breast Cancer Research Program (M.M.S.), and the National Institutes of Health (W.S.T., A.F.S., M.M.S.). A.F.S. is a Scholar of the McKnight Endowment Fund for Neuroscience.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL schier@saturn.med.nyu.edu; FAX (212) 263-7760.
E-MAIL mshen@cabm.rutgers.edu; FAX (732) 235-5318.
References
- Armes NA, Smith JC. The ALK-2 and ALK-4 activin receptors transduce distinct mesoderm-inducing signals during early Xenopus development but do not cooperate to establish thresholds. Development. 1997;124:3797–3804. doi: 10.1242/dev.124.19.3797. [DOI] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JC, Harland RM. A novel mesoderm inducer, Madr2, functions in the activin signal transduction pathway. Genes & Dev. 1996;10:1880–1889. doi: 10.1101/gad.10.15.1880. [DOI] [PubMed] [Google Scholar]
- Beddington RSP, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Essner JJ, Yost HJ. Regulation of midline development by antagonism of lefty and nodal signaling. Development. 1999;126:3253–3262. doi: 10.1242/dev.126.14.3253. [DOI] [PubMed] [Google Scholar]
- Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, Nowotschin S, Viebahn C, Haffter P, Kuehn MR, et al. The homeobox gene Pitx2: Mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 1999;126:1225–1234. doi: 10.1242/dev.126.6.1225. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. Role of notochord in specification of cardiac left-right orientation in zebrafish and Xenopus. Dev Biol. 1996;177:96–103. doi: 10.1006/dbio.1996.0148. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Dufort D, Schwartz L, Harpal K, Rossant J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development. 1998;125:3015–3025. doi: 10.1242/dev.125.16.3015. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Ticho BS, Fishman MC. Patterning the heart's left-right axis: From zebrafish to man. Dev Genet. 1998;22:278–287. doi: 10.1002/(SICI)1520-6408(1998)22:3<278::AID-DVG9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Graff JM, Bansal A, Melton DA. Xenopus Mad proteins transduce distinct subsets of signals for the TGF beta superfamily. Cell. 1996;85:479–487. doi: 10.1016/s0092-8674(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF–CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Gu Z, Nomura M, Simpson BB, Lei H, Feijen A, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes & Dev. 1998;12:844–857. doi: 10.1101/gad.12.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RP. Links in the left/right axial pathway. Cell. 1998;94:273–276. doi: 10.1016/s0092-8674(00)81468-3. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992;359:609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Izraeli S, Lowe LA, Bertness VL, Good DJ, Dorward DW, Kirsch IL, Kuehn MR. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature. 1999;399:691–694. doi: 10.1038/21429. [DOI] [PubMed] [Google Scholar]
- King T, Beddington RSP, Brown NA. The role of the brachyury gene in heart development and left-right axis specification in the mouse. Mech Dev. 1998;79:29–37. doi: 10.1016/s0925-4773(98)00166-x. [DOI] [PubMed] [Google Scholar]
- King T, Brown NA. Embryonic asymmetry: The left side gets all the best genes. Curr Biol. 1999;9:R18–R22. doi: 10.1016/s0960-9822(99)80036-0. [DOI] [PubMed] [Google Scholar]
- Kosaki K, Casey B. Genetics of human left-right axis malformations. Sem Cell Dev Biol. 1998;9:89–99. doi: 10.1006/scdb.1997.0187. [DOI] [PubMed] [Google Scholar]
- Lanctèt C, Moreau A, Chamberland M, Tremblay ML, Drouin J. Hindlimb patterning and mandible development require the Ptx1 gene. Development. 1999;126:1805–1810. doi: 10.1242/dev.126.9.1805. [DOI] [PubMed] [Google Scholar]
- Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- Logan M, Pagan-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 1998;94:307–317. doi: 10.1016/s0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- Lohr JL, Danos MC, Yost HJ. Left-right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development. 1997;124:1465–1472. doi: 10.1242/dev.124.8.1465. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, Overbeek P, Kuehn MR. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature. 1996;381:158–161. doi: 10.1038/381158a0. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Kroll K, Sun E, Ramos R, Elmendorf H, Kirschner MW. A Xenopus nodal-related gene that acts in synergy with noggin to induce complete secondary axis and notochord formation. Development. 1996;122:3275–3282. doi: 10.1242/dev.122.10.3275. [DOI] [PubMed] [Google Scholar]
- Melloy PG, Ewart JL, Cohen MF, Desmond ME, Kuehn MR, Lo CW. No turning, a mouse mutation causing left-right and axial patterning defects. Dev Biol. 1998;193:77–89. doi: 10.1006/dbio.1997.8787. [DOI] [PubMed] [Google Scholar]
- Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H. Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature. 1996;381:151–155. doi: 10.1038/381151a0. [DOI] [PubMed] [Google Scholar]
- Meno C, Ito Y, Saijoh Y, Matsuda Y, Tashiro K, Kuhara S, Hamada H. Two closely-related left-right asymmetrically expressed genes, lefty-1 and lefty-2: Their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Genes Cells. 1997;2:513–524. doi: 10.1046/j.1365-2443.1997.1400338.x. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell. 1998;94:287–297. doi: 10.1016/s0092-8674(00)81472-5. [DOI] [PubMed] [Google Scholar]
- Meno, C., K. Gritsman, S. Ohishi, Y. Ohfuji, E. Heckscher, K. Mochida, A. Shimono, H. Kondoh, W.S. Talbot, E.J. Robertson et al. 1999. Mouse Lefty-2 and zebrafish antivin are feedback inhibitors of Nodal signaling during vertebrate gastrulation. Mol. Cell (in press). [DOI] [PubMed]
- Meyers EN, Martin GR. Differences in left-right axis pathways in mouse and chick: Functions of FGF8 and SHH. Science. 1999;285:403–406. doi: 10.1126/science.285.5426.403. [DOI] [PubMed] [Google Scholar]
- Milewski WM, Duguay SJ, Chan SJ, Steiner DF. Conservation of PDX-1 structure, function, and expression in zebrafish. Endocrinology. 1998;139:1440–1449. doi: 10.1210/endo.139.3.5768. [DOI] [PubMed] [Google Scholar]
- Mortensen R. Gene targeting by homologous recombination. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. New York, NY: John Wiley & Sons; 1999. pp. 9.15.11–19.17.13. [Google Scholar]
- New HV, Kavka AL, Smith JC, Green JB. Differential effects on Xenopus development of interference with type IIA and type IIB activin receptors. Mech Dev. 1997;61:175–186. doi: 10.1016/s0925-4773(96)00639-9. [DOI] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes & Dev. 1997;11:1812–1826. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- Pagan-Westphal SM, Tabin CJ. The transfer of left-right positional information during chick embryogenesis. Cell. 1998;93:25–35. doi: 10.1016/s0092-8674(00)81143-5. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodriguez-Rey JC, Ros MA. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 1998;94:319–324. doi: 10.1016/s0092-8674(00)81475-0. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF, Yost HJ. Molecular mechanisms of vertebrate left-right development. Trends Genet. 1998;14:459–465. doi: 10.1016/s0168-9525(98)01599-6. [DOI] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Fricke C, Haffter P, Dawid IB. Zebrafish nodal-related genes are implicated in axial patterning and establishing left-right asymmetry. Dev Biol. 1998;199:261–272. doi: 10.1006/dbio.1998.8935. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, de la Pena J, Sabbagh W, Greenwald J, Choe S, et al. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- Sampath K, Cheng AM, Frisch A, Wright CV. Functional differences among Xenopus nodal-related genes in left-right axis determination. Development. 1997;124:3293–3302. doi: 10.1242/dev.124.17.3293. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SCF, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Chen JN, Fishman MC. Regulation in the heart field of zebrafish. Development. 1998;125:1095–1101. doi: 10.1242/dev.125.6.1095. [DOI] [PubMed] [Google Scholar]
- Shen MM, Wang H, Leder P. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development. 1997;124:429–442. doi: 10.1242/dev.124.2.429. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng CX. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Natl Acad Sci. 1998;95:9378–9383. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, et al. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell. 1998;94:299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]


