Abstract
Major depressive disorder (MDD) is a common psychiatric illness affecting nearly 20% of adults in the United States at least once during their lifetime. MDD is frequently diagnosed and treated in the primary care setting. Management of the disease may be complicated by patients and family members feeling stigmatized by the diagnosis and not understanding that depression is a treatable medical illness, which, in turn, fosters low rates of adherence to medication schedules. Incomplete or delayed response to treatment, adverse events associated with antidepressants, and medical or psychiatric comorbidities also interfere with optimal depression management. This paper presents an overview of diagnostic and treatment guidelines for MDD and focuses on challenges encountered by primary care physicians. The role of antidepressant medications, psychotherapy, and nonpharmacologic interventions for the treatment of patients with MDD is described, and factors influencing treatment selection, such as adverse event profiles and patient characteristics are examined.
Keywords: Major depressive disorder, depression, antidepressant treatment, collaborative care, somatic interventions
INTRODUCTION
Major depressive disorder (MDD) is a common psychiatric illness that affects nearly 15 million adults in the United States annually1,2 and approximately 1 in 5 adults at some time in their lives.3 Patients with MDD present with “core” emotional and physical symptoms (e.g., sadness, anhedonia, disturbed sleep)4 and impaired psychosocial functioning.3 The presence of continuing subthreshold dysphoric symptoms and psychosocial impairments further increases the risk of episodic recurrence.5,6 While the physiologic factors that influence the development, progression, and chronicity of MDD are poorly elucidated, preclinical and clinical studies have shown that altered monoamine neurotransmitter (e.g., norepinephrine, dopamine, serotonin) availability, as well as differences in receptor regulation and sensitivity are associated with the disease.7–11 Psychosocial factors, such as social isolation, severely stressful life circumstances,12 and lack of supportive/confiding relationships also increase the risk for MDD.13
MDD is underdiagnosed and undertreated because many patients do not report depressive symptoms to their primary care physicians (PCPs), who initially diagnose and treat the majority of patients with MDD.14 Additionally, many physicians do not screen for or recognize MDD in their patients.15 This is especially important as patients with MDD and other affective disorders account for nearly half of all suicides.16 Additionally, MDD increases the morbidity and mortality of a number of other medical conditions. Patients with recurrent MDD and cardiovascular disease (CVD) have twice the rate of morbidity and mortality seen in patients with CVD alone.17,18 MDD also reduces survival rates for patients with other serious illnesses, including end-stage renal disease19 and cancer.20
While antidepressant prescribing has increased during the past decade, this trend does not necessarily correlate with improved outcomes for patients with MDD.21–23 Complete remission of depressive symptoms has long been recognized as the primary goal of treatment.24 Long-term outcomes are significantly better among patients with MDD who fulfill the criteria for full symptom remission (Table 1)15 compared with patients who report only symptomatic improvement.5,25 Studies have shown that approximately 40% of patients with MDD will achieve full remission with first- or even second-line treatment,21,25 and only a few socioethnodemographic or clinical features have been identified that will reliably predict who will respond optimally to a given antidepressant therapy.22 Rather, the presence of particular medical and psychiatric comorbidities and the occurrence of certain adverse events (AEs) may be more important considerations in the selection of a specific therapy.22 This paper presents an overview of the diagnosis and treatment of patients with MDD using pharmacologic and/or nonpharmacologic therapies with a focus on the management challenges encountered by PCPs. The relationship between MDD and other comorbidities is also reviewed.
TABLE 1.
PHQ-9-based definitions of MDD symptom response, remission, and recovery
| Therapeutic Status | PHQ-9 Criteria |
|---|---|
| Response | PHQ-9 score improvement of 50% from baseline |
| Full Remission | PHQ-9 score ≤4 for ≥1 month |
| Recovery | PHQ-9 score ≤4 for ≥6 months |
Abbreviations: MDD, major depressive disorder; PHQ-9, Patient Health Questionnaire, 9-item. Adapted, with permission, from the Department of Veterans Affairs, Department of Defense.15
MDD DIAGNOSIS AND INITIAL MANAGEMENT
Criteria for a major depressive episode according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) are listed in Table 2.4 The core symptoms of depressed mood and loss of interest or pleasure must be accompanied by at least 4 other depressive symptoms and must persist for at least 2 weeks for the diagnosis of MDD to be made. Further, the affected individual’s condition cannot be better accounted for by another psychiatric condition, and the individual cannot have a history of manic, mixed, or hypomanic episodes unless these episodes were substance- or treatment-induced or directly caused by a medical condition. The number and combination of presenting symptoms may vary considerably among patients.15 In the primary care setting patients may initially complain of nonspecific symptoms, including insomnia, fatigue, and headache, with some older patients exhibiting a global decline in functionality.15 Thus, physicians need to be vigilant as to the variable presentations of MDD.
TABLE 2.
DSM-IV-TR criteria for the diagnosis of a major depressive episode
| MDD diagnosis requires the presence of symptom 1, 2, or both; and at least 5 of 9 total symptoms, which must persist for at least 2 weeks |
|
Abbreviations: DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; MDD, major depressive disorder. Adapted, with permission, from the American Psychiatric Association.4
To assist in the screening, diagnosis, and treatment of patients with MDD, a number of questionnaires, guidelines, and algorithms have been developed. It is recommended that patients in the primary care setting undergo annual MDD screening with standardized questionnaires to improve rates of detection and to initiate treatment as soon as possible, to reduce the morbidity and mortality associated with MDD.15 Brief questionnaires and scales (e.g., Beck Depression Inventory, Inventory for Depressive Symptomatology–Self Rated, Patient Health Questionnaire, 2- and 9-item [PHQ-9] versions, Quick Inventory of Depression Self-Report, Hospital Anxiety and Depression Scale) can be completed by patients in the office prior to their seeing the physician, but these patient-reported questionnaires do not replace diagnoses based on in-depth, physician-completed interviews.15,26–31 The Mini International Neuropsychiatric Interview (MINI) is an efficient structured interview (lasting approximately 15 minutes) that can assist in the definitive diagnosis of MDD.A study of validity as compared to the Composite International Diagnostic Interview for major depressive disorder/dysthymia showed sensitivity of 0.96/0.67, specificity of 0.88/0.99, positive predictive value of 0.87/0.45 and negative predictive value of 0.97/0.99. It is highly reliable with test retest of 0.87 for major depressive disorder.32 It is also important in establishing the diagnosis to assess the patient’s risk for suicide and/or homicide and determine the most appropriate treatment.24 Patients with MDD who report active suicidal or homicidal ideation should be immediately referred to emergency services and mental health specialty care.15,24
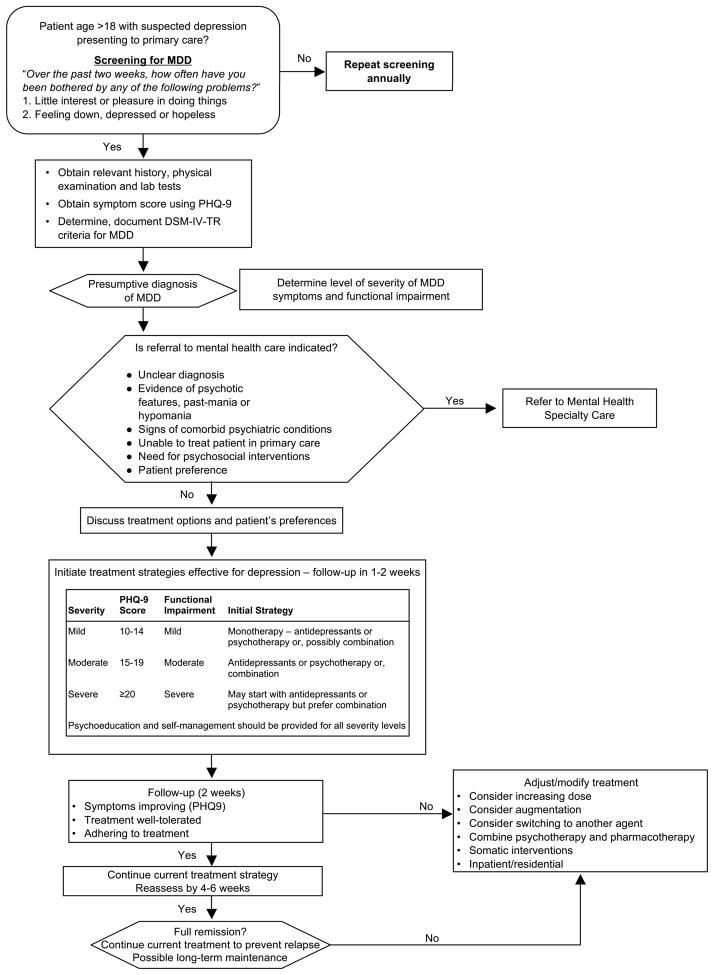
A recently published algorithm for the management of MDD in the primary care setting is shown in Figure 1.15 While this United States Departments of Veterans Affairs and Defense (VA/DoD) algorithm is similar to the one published by the American Psychiatric Association (APA),24 the VA/DoD algorithm contains information for PCPs with respect to patient assessments, treatment selection, ongoing management, and referral to specialty care. The VA/DoD algorithm recommends that the acute phase of outpatient therapy (during which the patient receives medication, psychotherapy, or a combination of these modalities) should last for 8 to 12 weeks. It further recommends that patients with MDD should receive appropriate psychoeducation and self-management instruction, including information on monitoring for depressive symptoms, treatment of AEs, and the importance of getting adequate sleep, regular exercise, and reducing or eliminating tobacco and caffeine.15 Similarly, the APA algorithm recommends once-weekly office visits during the acute treatment phase to monitor for therapeutic response, treatment adherence, AEs, and suicidality.24 Patients who have reached successful symptom remission at the end of the acute treatment phase should continue to receive the acute treatment regimen for an additional 6 to 12 months to prevent a potential recurrence of MDD. Patients with a history of 2 or more prior MDD episodes or who are considered at high risk for recurrence should be considered for long-term (2-years to lifelong) maintenance treatment.15,24
FIGURE 1.
Algorithm for the diagnosis and treatment of adult patients with MDD, abbreviated.a Adapted, with permission, from the Department of Veterans Affairs, Department of Defense.15
aThis is an abbreviated algorithm adapted from the Department of Veterans Affairs, Department of Defense and does not contain all details regarding diagnosis, assessment, and treatment steps for patients with presumptive MDD. For patients who do not achieve remission following adequate treatment trials of 3 different antidepressants and/or psychotherapies, including augmentation, diagnosis should be reevaluated and referral to specialty mental health care should be considered.
Abbreviations: MDD, major depressive disorder; PHQ-9, Patient Health Questionnaire, 9-item.
Once depression treatment with psychotherapy and/or antidepressant therapy has been initiated the PHQ-9, which consists of 9 items with each scored on a scale from 0 to 3, can be used to monitor disease progression (Figure 1). A shift of at least 5 points is considered to reflect a clinically meaningful change in depressive symptoms,33 and a PHQ-9 total score of 4 or less maintained for at least 1 month is indicative of full remission.15 Data from an analysis of 434 older adults with MDD (mean age, 71 years) enrolled in the Improving Mood–Promoting Access to Collaborative Treatment (IMPACT) trial showed that the PHQ-9 is sensitive to change in depressive symptoms during treatment.33 Patients considered by interviewers to be in full remission showed the largest improvements in PHQ-9 scores (−13.0 points) while patients considered unchanged showed the smallest improvements (−4.4 points).33
DEPRESSION CARE MANAGEMENT
The inclusion of a depression care manager (DCM) on the medical team for patients with MDD is considered a key determinant of successful outcomes in primary care by several health services research investigations.33 The DCM can provide several crucial interventions: (1) education about depression as a treatable illness, (2) facilitation of choice of therapies (i.e., psychotherapy versus pharmacotherapy), (3) behavioral activation planning, and (4) monitoring of AEs and depressive symptom changes. These interventions support the PCP’s involvement with the patient to make adjustments in treatment to achieve MDD remission.
ANTIDEPRESSANT PHARMACOTHERAPIES
The selection of pharmacotherapy for patients with MDD should be guided by the patient’s medication history and comorbidities, the efficacy and safety profile of the medication, and the prescribing physician’s familiarity with the particular drug or drugs. Pharmacotherapeutic “success” is dependent on appropriate dosing and a clearly defined duration of treatment. Once antidepressant therapy has been initiated, full therapeutic effects may take up to 8 weeks to achieve. Patients who show a partial response after 4 to 6 weeks of treatment should be maintained on their initial medication for an additional 4 to 6 weeks. Patients who show little or no response may require second-step therapy, which could include an increase in the dosage of their current medication, a switch to a different medication, or the addition of another drug to their current therapeutic regimen.15 The importance of using adequate doses of antidepressant medications has been illustrated by data from a long-term observational study conducted over 20 years, which demonstrated that patients who received higher doses of antidepressant medications were nearly twice as likely to recover from recurrent affective episodes as patients who were not administered these somatic treatments (P = 0.002).34
Table 3 lists commonly available antidepressant medications, categorized by drug class.21,23,24,35 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are considered first-line pharmacotherapeutic options for patients with MDD. SSRIs constitute the antidepressant class most commonly prescribed by PCPs due, in part, to their reduced need for dose titration and their relatively low potential for AEs.15,21,23,24,35 Older, less commonly prescribed antidepressant classes include the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), which may be prescribed for patients with depressive symptoms who have not responded to the first-line therapies. The use of these older drugs is limited by their treatment-limiting AEs, greater lethality in the event of overdose, higher potential for drug-drug interactions, and the need for dietary restrictions in the case of the MAOIs. The newer, transdermal formulation of selegiline, an MAO type B inhibitor, does not require dietary restrictions. Other individual antidepressant agents that are considered first line include bupropion, which affects dopaminergic and noradrenergic, but not serotonergic function, and mirtazapine, which increases the release of norepinephrine and serotonin.
TABLE 3.
Commonly available antidepressantsa
| SSRIs | SNRIs | |||||||
|---|---|---|---|---|---|---|---|---|
| Chemical Name (Brand) | Citalopram (Celexa®) | Escitalopram (Lexapro®) | Fluoxetine (Prozac®) | Paroxetine (Paxil®) | Sertraline (Zoloft®) | Desvenlafaxine (Pristiq®) | Duloxetine (Cymbalta®) | Venlafaxine (Effexor®) |
| Notes | Slower onset of action (up to 8 weeks), sexual AEs, may promote suicidality in children, discontinuation syndrome, cytochrome P450 interactions, nausea, weight gain | Similar to SSRIs, potentially increased BP | ||||||
| TCAs | ||||||||
| Chemical Name (Brand) | Amitriptyline (Elavil®, Endep®) | Clomipramine (Anafranil®) | Desipramine (Norpramine®, Pertofrane®) | Doxepin (Sinequan®) | Imipramine (Tofranil®) | Nortriptyline (Pamelor®) | Protriptyline (Vivactil®) | Trimipramine (Surmontil®) |
| Notes | Antimuscarinic actions (ie, dry mouth, urinary retention, flushing), weight gain, hypotension, arrhythmias, cytochrome P450 interactions, suicide risk, toxicity in overdose problematic, clomipramine may increase seizure risk | |||||||
| MAOIs | ||||||||
| Chemical Name (Brand) | Isocarboxazid (Marplan®) | Phenelzine (Nardil®) | Selegiline (Eldepryl®, Zelapar®, EMSAM®) | Tranylcypromine (Parnate®) | ||||
| Notes | Tyramine/hypertensive crisis, suicide risk | |||||||
| SDRIs | Other | |||||||
| Chemical Name (Brand) | Bupropion hydrobromide (Aplenzin™) | Bupropion hydrochloride (Wellbutrin®) | Buspirone (BuSpar®) | Mirtazapine (Remeron®) | Trazodone (Desyrel®) | |||
| SR | XL | |||||||
| Notes | Minimal drug interactions | Reduced seizure threshold | Slower onset of action | Tetracyclic antidepressant (similar to TCAs), sedating | Discontinuation syndrome | |||
These medications are available as generics (except Aplenzin™).
Abbreviations: AE, adverse event; BP, blood pressure; MAOI, monoamine oxidase inhibitor; SDRI, serotonin-dopamine reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; SR, sustained release; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; XL, extended release.
First-line antidepressant therapies are considered similarly effective and, therefore, medication selection is often based upon tolerability and safety profiles, as none of the antidepressant medications is free of AEs (Table 3).15,21,23,24,35 SSRIs have been associated with nausea, insomnia, weight gain, sexual dysfunction, and decreased libido.36,37 Because the SSRIs do not all have the same cytochrome P450 metabolism and pharmacokinetic characteristics, their differences in potential for drug-drug interactions (DDIs) may influence treatment selections. Paroxetine and fluoxetine pose the greatest risk for DDIs, particularly when they are co-prescribed with certain β-blockers (e.g., metoprolol), antiarrhythmics (e.g., propafenone, flecainide), or atypical antipsychotics (e.g., risperidone).15 The safety and tolerability profiles of the SNRIs are similar to those of the SSRIs. Nausea and vomiting may be more frequent with the SNRIs, and venlafaxine and duloxetine present risks for DDIs.15,23 Additionally, as SNRIs have been associated with increased blood pressure, blood pressure monitoring is recommended for patients treated with these agents.38,39 Also, a number of agents, including bupropion hydrochloride, clomipramine, amoxapine, and maprotiline may increase the risk of seizures, especially in patients with a history of seizures or brain injury.37,40 The US Food and Drug Administration (FDA) has applied a warning to all antidepressant medications regarding an increased risk of suicidality, especially in pediatric patients and young adults less than 24 years of that may indicate age.35,41,42 When beginning antidepressant therapy, physicians should educate patients and their family members about signs of increasing risk for suicide, such as agitation, increase psychomotor activity or marked worsening or sadness or hopelessness. Family members must report these symptoms to the physician promptly.
There are a number of new and reformulated antidepressant agents and adjunctive therapies. A novel formulation of bupropion (which has been available to treat MDD for 2 decades), conjugated with a bromide salt instead of a chloride salt, has been developed and approved for use by the FDA. The hydrobromide salt of bupropion is formulated for once-daily dosing and has shown a low potential for interactions with other drugs, but there is a risk of bromism developing in patients treated with high doses of any bromide-containing pharmacotherapy.43, 44 Data from a preclinical study showed that bupropion hydrobromide had a lower risk for inducing seizures in mice than similar doses of bupropion hydrochloride, but studies are needed to determine whether these findings would apply to humans.43 Studies are currently ongoing to evaluate the transdermal formulation of selegiline as a potential first-line treatment.15,24,35
As some patients with MDD will require a second- or third-step treatment to achieve remission, there has been increased investigation of combination pharmacotherapy. Lithium, thyroid hormone, buspirone, and stimulants are known to be effective adjunctive therapies.45 The atypical antipsychotic aripiprazole has been approved as adjunctive therapy for patients with treatment-resistant MDD, and data have corroborated the efficacy and safety of this agent.46 Olanzapine-fluoxetine combination therapy has received FDA approval for the treatment of patients with resistant MDD.47 Other atypical antipsychotics are also being evaluated as possible augmentative therapies.45 Data from randomized placebo- and active-controlled trials with alternative therapies suggest that St. John’s wort has beneficial effects in patients with MDD.48,49 This may possibly be related to its modulation of monoamine oxidase, reuptake of serotonin, norepinephrine, and dopamine, and binding at central benzodiazepine receptors.48 On the down side, St. John’s wort has been shown to interact with, and reduce the efficacy of frequently prescribed medications, including oral contraceptives, digoxin, and cyclosporine.35
NONPHARMACOLOGIC TREATMENT OPTIONS FOR MDD
Mental health specialists are more likely than PCPs to use psychotherapy and other nonpharmacologic treatments, including electroconvulsive therapy (ECT), phototherapy, vagal nerve stimulation (VNS), seizure therapy, and transcranial magnetic stimulation (TMS).15 Further, PCPs may best be served by referring patients with MDD, who desire nonpharmacologic treatment or who may require intensive care, to a mental health specialist.15,24 ECT is used when a rapid therapeutic response is needed, when other treatments are not tolerated or have failed, when patients have severe symptoms or psychotic features, or when patients become pregnant.24,50,51 ECT is very effective but has been associated with postictal confusion and impaired memory, which usually resolve upon cessation of treatment.24,52 Phototherapy has been shown to improve both seasonal and nonseasonal depression symptoms and to augment the effects of concurrent antidepressant medications, but it has been associated with headache, eye strain, insomnia, and irritability.24,53,54 Vagal nerve stimulation (VNS), approved in 2005 for the treatment of patients with refractory depression, has a response rate of up to 30%.52 Magnetic seizure therapy, focal electrically administered seizure therapy, and deep-brain stimulation are currently under investigation.35,52,55,56 Transcranial magnetic stimulation (TMS) has been approved for patients with treatment-resistant MDD in the United States and Canada, but more studies are needed to corroborate its efficacy.52,57,58 AEs commonly associated with TMS include transient headache and scalp pain. Cognitive behavioral therapy and interpersonal therapy are among the oldest, most empirically tested psychotherapeutic treatments for patients with unipolar depressive disorders.15,24 Although psychotherapies are also recommended as first-line treatment for patients with moderate-to-severe MDD, results from a decision analysis of systematic literature reviews (>5000 citations) showed that early initiation of combination psychotherapy and pharmacotherapy may be superior to either approach alone and is likely to be beneficial in inducing remission and preventing relapse in patients with moderate-to-severe disease.59 Depressive episodes in which symptoms are mild-to-moderate are equally responsive to psychotherapeutic interventions or pharmacotherapy.24,35
OTHER MDD TREATMENT CONSIDERATIONS
The selection of antidepressant treatment has an important impact on patient outcomes. Consideration of individual patient characteristics (including age; gender; medical comorbidities; and financial issues) can guide treatment selection and improve the chance of therapeutic success.22 Special considerations need to be taken into account with respect to metabolism, efficacy, and safety in older patients.21 For example, bupropion may be a good therapeutic option in older patients because of its lower incidence of somnolence, diarrhea, and constipation, and minimal DDIs in this population.60 The IMPACT trial showed that depressed individuals ≥60 years of age in the primary care setting receive shorter-than-recommended courses of pharmacotherapy (by ~2 months).61 On the other hand, older patients who received intensive and collaborative antidepressant treatment (with ancillary counselors) for more than 6 months demonstrated significantly higher rates of remission than patients who did not receive intensive and collaborative antidepressant treatment (P <0.001).61
Hormonal fluctuations unique to pregnant, premenopausal, and postmenopausal women affect the development, progression, and treatment of MDD.62,63 Data have shown that ~10% of gravid women fulfill the criteria for MDD, and almost 20% demonstrate depressive symptomatology during pregnancy.62 Further, women transitioning into menopause have an increased risk for developing MDD compared with premenopausal or postmenopausal women.63 Intensive and specialized MDD interventions for women with breast cancer, including depression care management provided by trained nurses, improve depression symptoms more than care without these interventions.64 Further study of this treatment approach is ongoing.65 Finally, the cost of antidepressant treatment and patient health insurance coverage has a significant impact on treatment selection. Because treatment regimens are recommended to continue for more than 9 months, healthcare providers must remain aware of the available treatment options that cover the many cost levels.23
CONCLUSION
PCPs are often the first clinicians to diagnose and treat patients with MDD. While patients in the primary care setting may initially present with non-localized physical complaints, brief screening questionnaires can help to identify patients who may require additional, in-depth evaluation. Many of the currently available guidelines and treatment algorithms, have been developed for use by PCPs. These treatment guidelines and algorithms should help to improve outcomes in patients with MDD and reduce morbidity and mortality. The most effective treatment option for patients with MDD incorporates a patient-centered and team-based approach to care in which the PCP works with a nurse or social worker who serves as a Depression Care Manager. Mild MDD can be equally and successfully treated with pharmacologic or nonpharmacologic therapies, whereas severe MDD requires a combination of these therapies to achieve optimal outcomes.
Acknowledgments
The authors thank Melissa LoSchiavo for editorial and administrative assistance in the development of this manuscript.
Footnotes
Sources of Support/Disclaimers: Dr Weihs has nothing to disclose.
Dr Wert discloses conflicts of interest with sanofi-aventis.
References
- 1.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Mental Health. [Accessed April 26, 2010];The numbers count: mental disorders in America. Available at http://www.nimh.nih.gov/health/publications/the-numbers-count-mental-disorders-in-america/index.shtml. Last updated August 10, 2009.
- 3.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- 5.Simon GE. Long-term prognosis of depression in primary care. Bull World Health Organ. 2000;78:439–45. [PMC free article] [PubMed] [Google Scholar]
- 6.Vittengl JR, Clark LA, Jarrett RB. Deterioration in psychosocial functioning predicts relapse/recurrence after cognitive therapy for depression. J Affect Disord. 2009;112:135–43. doi: 10.1016/j.jad.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Kornum BR, Weikop P, Moller A, et al. Serotonin depletion results in a decrease of the neuronal activation caused by rivastigmine in the rat hippocampus. Brain Res. 2006;1073–1074:262–8. doi: 10.1016/j.brainres.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Kuśmider M, Faron-Górecka A, Dziedzicka-Wasylewska M. Delayed effects of antidepressant drugs in rats. Behav Pharmacol. 2006;17:641–9. doi: 10.1097/FBP.0b013e3280116ea2. [DOI] [PubMed] [Google Scholar]
- 10.Moreno FA, Heninger GR, McGahuey CA, Delgado PL. Tryptophan depletion and risk of depression relapse: a prospective study of tryptophan depletion as a potential predictor of depressive episodes. Biol Psychiatry. 2000;48:327–9. doi: 10.1016/s0006-3223(00)00893-3. [DOI] [PubMed] [Google Scholar]
- 11.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 13.Moran PM, Bifulco A, Ball C, Campbell C. Predicting onset of depression: the Vulnerability to Depression Questionnaire. Br J Clin Psychol. 2001;40:411–27. doi: 10.1348/014466501163896. [DOI] [PubMed] [Google Scholar]
- 14.Mark TL, Levit KR, Buck JA. Psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009;60:1167. doi: 10.1176/ps.2009.60.9.1167. [DOI] [PubMed] [Google Scholar]
- 15.Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of major depressive disorder (MDD) Washington (DC): Department of Veterans Affairs, Department of Defense; 2009. [Accessed April 26, 2010]. Available at http://www.healthquality.va.gov/mdd/mdd_full09_c.pdf. Last updated May 2009. [Google Scholar]
- 16.Arsenault-Lapierre G, Kim C, Turecki G. Diagnoses in 3275 suicides: a meta-analysis. BMC Psychiatry. 2004;4:37–48. doi: 10.1186/1471-244X-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 18.Vaccarino V, McClure C, Johnson BD, et al. Depression, the metabolic syndrome and cardiovascular risk. Psychosom Med. 2008;70:40–48. doi: 10.1097/PSY.0b013e31815c1b85. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel PL, Peterson RA, Weihs KL, et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int. 2000;57:2093–8. doi: 10.1046/j.1523-1755.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 20.Mykletun A, Bjerkeset O, Dewey M, et al. Anxiety, depression, and cause-specific mortality: the HUNT study. Psychosom Med. 2007;69:323–31. doi: 10.1097/PSY.0b013e31803cb862. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. [Accessed April 26, 2010];Comparative effectiveness of second-generation antidepressants in the pharmacologic treatment of adult depression. Available at http://effectivehealthcare.ahrq.gov/healthInfo.cfm?infotype=rr&ProcessID=7%20&DocID=61. Last updated January 24, 2007. [PubMed]
- 22.Rush AJ, Wisniewski SR, Warden D, et al. Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. Arch Gen Psychiatry. 2008;65:870–80. doi: 10.1001/archpsyc.65.8.870. [DOI] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality. [Accessed April 26, 2010];Choosing antidepressants for adults: clinician’s guide. Available at http://effectivehealthcare.ahrq.gov/healthInfo.cfm?infotype=sg&DocID=9&ProcessID=7. Last updated August 15, 2007. [PubMed]
- 24.Karasu TB, Gelenberg A, Merriam A, Wang P for the American Psychiatric Association. [Accessed April 26, 2010];Practice guidelines for the treatment of patients with major depressive disorder. (2). Available at http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx. Last updated 2000.
- 25.Rost K, Nutting P, Smith JL, et al. Managing depression as a chronic disease: a randomised trial of ongoing treatment in primary care. BMJ. 2002;325:934–9. doi: 10.1136/bmj.325.7370.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 27.Dodd S, Williams LJ, Jacka FN, et al. Reliability of the Mood Disorder Questionnaire: comparison with the Structured Clinical Interview for the DSM-IV-TR in a population sample. Aust N Z J Psychiatry. 2009;43:526–30. doi: 10.1080/00048670902873706. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rush AJ, Giles DE, Schlesser MA, et al. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 30.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 31.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (suppl 20):22–33. [PubMed] [Google Scholar]
- 33.Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42:1194–201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Leon AC, Solomon DA, Mueller TI, et al. A 20-year longitudinal observational study of somatic antidepressant treatment effectiveness. Am J Psychiatry. 2003;160:727–33. doi: 10.1176/appi.ajp.160.4.727. [DOI] [PubMed] [Google Scholar]
- 35.Fochtmann LJ, Gelenberg AG. [Accessed April 26, 2010];Guideline Watch: Practice guideline for the treatment of patients with major depressive disorder. (2). Available at http://www.psychiatryonline.com/content.aspx?aid=148217. Last updated September 2005.
- 36.Demyttenaere K, Jaspers L. Bupropion and SSRI-induced side effects. J Psychopharmacol. 2008;22:792–804. doi: 10.1177/0269881107083798. [DOI] [PubMed] [Google Scholar]
- 37.Gartlehner G, Gaynes BN, Hansen RA, et al. Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann Intern Med. 2008;149:734–50. doi: 10.7326/0003-4819-149-10-200811180-00008. [DOI] [PubMed] [Google Scholar]
- 38.Eli Lilly and Company. [Accessed April 26, 2010];Cymbalta® (duloxetine hydrochloride) full prescribing information. Available at http://www.cymbalta.com/index.jsp. Last updated January 2010.
- 39.Wyeth Pharmaceuticals Inc. [Accessed April 26, 2010];Pritiq® (desvenlafaxine) full prescribing information. Available at http://www.wyeth.com/content/showlabeling.asp?id=497. Last updated February 2010.
- 40.Jefferson JW, Pradko JF, Muir KT. Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther. 2005;27:1685–95. doi: 10.1016/j.clinthera.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 41.US Food and Drug Administration. [Accessed April 26, 2010];FDA launches a multi-pronged strategy to strengthen safeguards for children treated with antidepressant medications. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108363.htm. Last updated October 15, 2004.
- 42.US Food and Drug Administration. [Accessed April 26, 2010];FDA proposes new warnings about suicidal thinking, behavior in young adults who take antidepressant medications. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108905.htm. Last updated May 2, 2007.
- 43.Henshall DC, Dürmüller N, White HS, et al. Electroencephalographic and behavioral convulsant effects of hydrobromide and hydrochloride salts of bupropion in conscious rodents. Neuropsychiatr Dis Treat. 2009;5:189–206. doi: 10.2147/ndt.s4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James LP, Farrar HC, Griebel ML, Bates SR. Bromism: intoxication from a rare anticonvulsant therapy. Pediatr Emerg Care. 1997;13:268–70. [PubMed] [Google Scholar]
- 45.Fleurence R, Williamson R, Jing Y, et al. A systematic review of augmentation strategies for patients with major depressive disorder. Psychopharmacol Bull. 2009;42:57–90. [PubMed] [Google Scholar]
- 46.Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:843–53. doi: 10.4088/jcp.v68n0604. [DOI] [PubMed] [Google Scholar]
- 47.Eli Lilly and Company. [Accessed April 26, 2010];Symbyax® (olanzapine and fluoxetine hydrochloride) full prescribing information. Available at http://pi.lilly.com/us/symbyax-pi.pdf. Last updated January 27, 2010.
- 48.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:118–27. doi: 10.1016/j.pnpbp.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database of Systematic Reviews. 2008;4:Art. No: CD000448. doi: 10.1002/14651858.CD000448.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:235–42. doi: 10.1097/PSY.0b013e318190d7ca. [DOI] [PubMed] [Google Scholar]
- 51.UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 52.Kennedy SH, Milev R, Giacobbe P, et al. for the Canadian Network for Mood and Anxiety Treatments. Canadian Network for Mood and Anxiety Treatments (CANMAT). Clinical guidelines for the management of major depressive disorder in adults: IV. Neurostimulation therapies. J Affect Disord. 2009;117(suppl 1):S44–S53. doi: 10.1016/j.jad.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 53.Even C, Schröder CM, Friedman S, Rouillon F. Efficacy of light therapy in nonseasonal depression: a systematic review. Affect Disord. 2008;108:11–23. doi: 10.1016/j.jad.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Levitt AJ, Joffe RT, Kennedy SH. Bright light augmentation in antidepressant nonresponders. J Clin Psychiatry. 1991;52:336–7. [PubMed] [Google Scholar]
- 55.Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology. 2009;34:2002–10. doi: 10.1038/npp.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward HE, Hwynn N, Okun MS. Update on deep brain stimulation for neuropsychiatric disorders. Neurobiol Dis. 2010;38:346–53. doi: 10.1016/j.nbd.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Bares M, Kopecek M, Novak T, et al. Low frequency (1-Hz), right prefrontal repetitive transcranial magnetic stimulation (rTMS) compared with venlafaxine ER in the treatment of resistant depression: a double-blind, single-centre, randomized study. J Affect Disord. 2009;118:94–100. doi: 10.1016/j.jad.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Dumitriu D, Collins K, Alterman R, Mathew SJ. Neurostimulatory therapeutics in management of treatment-resistant depression with focus on deep brain stimulation. Mt Sinai J Med. 2008;75:263–275. doi: 10.1002/msj.20044. [DOI] [PubMed] [Google Scholar]
- 59.Simon J, Pilling S, Burbeck R, Goldberg D. Treatment options in moderate and severe depression: decision analysis supporting a clinical guideline. Br J Psychiatry. 2006;189:494–501. doi: 10.1192/bjp.bp.105.014571. [DOI] [PubMed] [Google Scholar]
- 60.Weihs KL, Settle EC, Jr, Batey SR, et al. Bupropion sustained release versus paroxetine for the treatment of depression in the elderly. J Clin Psychiatry. 2000;61:196–202. doi: 10.4088/jcp.v61n0309. [DOI] [PubMed] [Google Scholar]
- 61.Unützer J, Katon W, Callahan CM, et al. for the IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 62.Marcus SM. Depression during pregnancy: rates, risks and consequences. Can J Clin Pharmacol. 2009;16:e15–e22. [PubMed] [Google Scholar]
- 63.Soares CN. Practical strategies for diagnosing and treating depression in women: menopausal transition. J Clin Psychiatry. 2008;69:e30. doi: 10.4088/jcp.1008e30. [DOI] [PubMed] [Google Scholar]
- 64.Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372:40–48. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 65.Walker J, Cassidy J, Sharpe M for the SMaRT Oncology-2 Trialists. The second Symptom Management Research Trial in Oncology (SMaRT Oncology-2): a randomised trial to determine the effectiveness and cost-effectiveness of adding a complex intervention for major depressive disorder to usual care for cancer patients. Trials. 2009;10:18. doi: 10.1186/1745-6215-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]