Abstract
Purpose
This phase I trial evaluated sunitinib combined with radiation therapy (RT) for primary or metastatic central nervous system (CNS) malignancies.
Experimental Design
Eligible patients had CNS malignancies requiring a (minimum) two week course of RT. Sunitinib (37.5 mg) was administered daily for the duration of radiotherapy treatment with optional treatment extension of a month. Urine was collected at 3 time points for correlative biomarker studies. The primary endpoint was acute toxicity defined by the Common Toxicity Criteria version 3.
Results
Fifteen patients were enrolled (12 CNS metastasis; 3 primary tumors). RT doses ranged from 14–70 Gy (1.8–3.5 Gy per fraction). Acute toxicities included hematologic, nausea, hyperglycemia, fatigue, hypocalcemia, and diarrhea. Six patients (40%) developed grade ≤2 toxicities. Grade 3 toxicities occurred in 7 (47%) patients including hematologic, fatigue, deep vein thrombosis, dysphasia, hyperglycemia and hyponatremia. No grade 3–5 hypertensive events or intracerebral hemorrhages occurred. Two Grade 5 adverse events attributed to disease progression occurred. Median follow up was 12.4 months. Two (13%) patients had partial response, 9 (60%) patients had stable disease and 2 (13%) patients had progressive disease. Six-month progression-free survival of brain metastasis patients was 58%. Grade 3 hematologic toxicity correlated with higher VEGF changes between baseline and completion of RT.
Conclusion
Continuous 37.5 mg sunitinib combined with RT in CNS malignancies yields acceptable toxicities and adverse events. Changes in urine VEGF were associated with hematologic toxicity and should be analyzed in a larger cohort. The feasibility, safety, and early response results warrant a Phase II trial.
Keywords: sunitinib, RT, central nervous system, anti-angiogenesis, brain metastases
Purpose
Preclinical studies suggest that the combination of angiogenic blockade and RT can enhance the therapeutic ratio of ionizing radiation via the phenomenon of vascular normalization1, 2. Though counterintuitive and poorly understood, combining ionizing radiation and anti-angiogenic agents may cause aberrant tumor vessels to regress3, 4 thereby increasing tumor oxygen concentration and acting as a potential radiosensitizing agent. Anti-angiogenic agents may also decrease interstitial fluid pressure within tumors allowing improved oxygen delivery to hypoxic tissues5, 6.
Further, anti-angiogenic agents have multiple other effects on the tumor microenvironment which may impact the tumor response to radiation therapy. Agents which disrupt the phosphoinositol-3 kinase (PI3K)-Akt-mTOR signaling pathway may radiosensitize the vascular endothelium7. The ability of endothelial progenitor stem cells which contribute to tumor angiogenesis and growth to mobilize and recruit can be impaired by agents that disrupt the PI3K-Akt-mTOR pathway8–10.
Preclinical studies in glioma11 and meningioma12 suggest anti-angiogenic agents may have an enhanced radiosensitizing effect in central nervous system (CNS) tumors.
SU11248 (sunitinib) is an orally active, indolinone-based, multitargeted receptor tyrosine kinase inhibitor that selectively targets vascular endothelial growth factor receptors (VEGFR1, VEGFR2, VEGFR3), platelet-derived growth factor receptors (PDGFRα, PDGFRβ), stem cell factor (c-KIT), neurotropic factor receptor (RET), FMS-like tyrosine kinase-3 (FLT3), and colony stimulating factor (CSF-1R)13–22. Sunitinib is FDA approved as monotherapy in metastatic renal cell carcinoma and imatinib-refractory gastrointestinal stromal tumor (GIST).
Despite an array of tumor markers currently in use, none serve as general predictors of outcome after therapy. In theory, angiogenic factors can identify patients at risk for recurrent disease, regardless of tumor type since the process of angiogenesis is ubiquitous to cancer. Urine biomarkers provide a non-invasive platform to interrogate disease status and tumor biology. Based on this information we hypothesized that urine VEGF measured at baseline, at the end of radiation, and at one month post-radiation may be predictive of treatment response.
The combination of RT and VEGF pathway targeting anti-angiogenic agents have shown tumor growth delay and tumor cell killing in pre-clinical studies23–27. In addition, preclinical models with sunitinib and ionizing radiation have demonstrated a more than additive effect on tumor growth delay compared with either treatment alone3, 28, 29.
We evaluated the safety and toxicity profile of 37.5 mg of oral sunitinib administered daily to patients undergoing RT for primary or metastatic central nervous system malignancies. In addition, we evaluated correlative urine biomarker results and early response rates for this regimen.
Experimental Design
Patient Selection
This study received approval of the Kimmel Cancer Center Research Review Committee (CCRRC) and Thomas Jefferson University Institutional Review Board (IRB) (IRB #06C.549) prior to patient recruitment and accrual. Patients with histologically confirmed primary or metastatic CNS malignancies whose treatment involved a minimum 2-week course of RT were eligible. Patients with widely metastatic disease with brain metastases did not have biopsy confirmation of the brain metastases in situations where MRI appearance was characteristic of brain metastases. Other eligibility requirements included: age ≥18 years, World Health Organization (WHO) performance status of 0–230, and life expectancy >3 months. Prior surgery, chemotherapy and/or RT (including radiation to the CNS) was allowed provided there was resolution of all acute toxic effects of prior therapies (less than or equal to grade 1 by the National Cancer Institute Common Toxicity Criteria [CTCv3])31. Adequate hematologic, hepatic, and renal function (defined as hemoglobin >9 g/dL, platelets >100,000, absolute neutrophil count >1500/µL, AST & ALT <2.5 times upper limit of normal [ULN], total serum bilirubin <1.5 times ULN, serum calcium <12 mg/dL, and serum creatinine <1.5 time ULN) was required.
Study Design
The study involved a baseline screening assessment period, treatment, and an observation period. During treatment, sunitinib 37.5 mg was given orally starting the first day of RT and was taken daily throughout the RT course including weekends. This regimen was chosen based on multiple phase I and II studies in metastatic renal cell carcinoma32, 33, GIST34, and soft tissue sarcoma35 which demonstrated this schedule was well tolerated with a manageable toxicity profile. In addition, a continuous daily regimen was hypothesized to maximize the potential radiosensitizing effect. Total dose and fractionation of RT varied depending on tumor type, prior RT doses, and physician preference. Dose limiting toxicities (DLT) were defined as any grade 3–5 toxicity attributable to sunitinib, excluding grade 3 hematologic toxicity unless clearly dose limiting. Early termination of the study was stipulated if the DLT rate exceeded 50%. A provision for de-escalation to sunitinib 25 mg using the standard 3+3 phase I trial design (Design 1 described by Simon et al.36) was permitted if more than one third of patients enrolled experienced a DLT. Patients who experienced minimal toxicity were given the option to continue sunitinib for an additional 30 days after completion of RT. All patients had weekly hematology and chemistry panels. Corticosteroid use prior to treatment, during treatment, and at one month followup was documented. Corticosteroids were not required, but were used as necessary at the discretion of the treating physicians.
The primary endpoint of this study was to evaluate the toxicity and safety profile of combining sunitinib and RT. Secondary endpoints included assessment of radiographic tumor response at one month and urine biomarker changes.
Toxicity Grading and Evaluation of Response
All toxicities were documented according to CTCv3 criteria31, and patients were evaluated for potential DLT weekly during treatment and 4 weeks after completion of treatment. Pertinent history, physical exam, hematology and chemistry studies were completed weekly while on treatment and one month after completion of treatment. MRI or CT scan were completed approximately one month after study completion and at regular follow-up appointments thereafter, dependent on the diagnosis, to assess early response. Formal neuro-cognitive functional assessments were not performed. Early imaging response was assessed using the Macdonald criteria37.
Patients, urine collection and processing
Urine was collected from all study patients at baseline, completion of RT, and 1 month post treatment. Urine was stored at −20 °C prior to evaluation, then centrifuged at 3000 rpm for 10 min at 4 ˚C. The supernatant was used for subsequent assays. Urinary creatinine levels were obtained on the Bayer DCA 2000+ Analyzer (Bayer HealthCare, Elkhart, IN) according to the manufacturer’s protocol. All samples were normalized by creatinine level. Urine samples were assayed for VEGF, MET, MMP-2, and MMP-9. These biomarkers were chosen for analysis because of prior published data suggesting an association between these biomarkers and brain tumors38, 39.
Electrochemiluminescence immunoassays
MET, MMP-2, MMP-9, and VEGF were measured in urine specimens via electrochemiluminescent immunoassay (Meso Scale Discovery, Gaithersburg, MD). All samples were thawed at room temperature for 4 hrs. prior to evaluation. MMP-2, MMP-9 and VEGF assays were performed per the manufacturer’s protocol. MET levels were detected according to a previously published protocol40.
Statistical Methods
To assess clinical efficacy, the neurologic progression–free survival (PFS) rate and overall survival (OS) rate were calculated from the time of enrollment using the Kaplan–Meier method41. Median urinary VEGF levels over time were compared using Friedman’s chi square test based on ranks. Median levels of VEGF fold-change were compared based on the development of hematological toxicity using the exact Wilcoxon rank sum test. All statistical tests are 2-sided and p-values less than or equal to 0.05 are considered significant.
Results
Patient characteristics and treatment
Fifteen patients were accrued from April 16, 2007- January 7, 2008. Table 1 displays patient characteristics and total radiation treatment dose and fraction size. Two patients had been previously treated with radiation therapy to the brain. The median total dose was 37.5 Gy (range, 14–70 Gy) including five patients treated to >50 Gy. The median dose per fraction was 2.5 Gy (range, 1.8–3.5 Gy). Thirteen patients completed the prescribed treatment. One patient expired during treatment and another patient chose to discontinue sunitinib secondary to decreased blood counts, vomiting, and fatigue. Seven (47%) patients chose to continue daily sunitinib for 30 days after completion of RT.
Table 1.
Patient and treatment characteristics
| All patients | Metastatic Disease |
Primary Tumor |
|
|---|---|---|---|
| No. Patients | 15 | 12 | 3 |
| Histology | |||
| Melanoma | 4 | 4 | 0 |
| NSCLC | 3 | 3 | 0 |
| Adenoid cystic | 2 | 2 | 0 |
| HNSCC | 1 | 1 | 0 |
| Chordoma | 1 | 1 | 0 |
| Anaplastic meningioma | 1 | 0 | 1 |
| CNS sarcoma | 1 | 0 | 1 |
| WHO grade II astrocytoma | 1 | 0 | 1 |
| Prior RT | 2 | 2 | 0 |
| Age (Median) | 58.7 (31–77) | 54.4 (37–77) | 50.7 (31–76) |
| <45 | 2 | 1 | 1 |
| 45–54 | 3 | 2 | 1 |
| 55–64 | 5 | 5 | 0 |
| 65–74 | 3 | 3 | 0 |
| 75+ | 2 | 1 | 1 |
| Gender | |||
| Male | 12 | 9 | 3 |
| Female | 3 | 3 | 0 |
| Race | |||
| Caucasian | 10 | 9 | 1 |
| African-American | 3 | 3 | 0 |
| Asian-American | 2 | 0 | 2 |
| WHO Performance | |||
| 0 | 3 | 2 | 1 |
| 1 | 12 | 10 | 2 |
| Target | |||
| Whole brain | 9 | 9 | 0 |
| Partial brain | 6 | 3 | 3 |
| RT Dose (median, Gy) | 37.5 (14–70) | 37.5 (14–59.4) | 60.0 (50.4–70) |
| RT dose per fraction | |||
| 1.8 Gy | 2 | 1 | 1 |
| 2.0 Gy | 2 | 0 | 2 |
| 2.5 Gy | 8 | 8 | 0 |
| 3.0 Gy | 1 | 1 | 0 |
| 3.5 Gy | 2 | 2 | 0 |
| Total Dose | |||
| <25 Gy | 1 | 1 | 0 |
| 25–30 Gy | 2 | 2 | 0 |
| 35–45 Gy | 7 | 7 | 0 |
| >45 Gy | 5 | 2 | 3 |
| Median weeks of sunitinib treatment (range) |
5 (0.6–10.8) | 4.7 (0.6–10.8) | 6.4 (2.6–8.8) |
Toxicity Evaluation
Six patients (40%) had grade 1 or 2 toxicities including fatigue, hyperglycemia, nausea, hypocalcemia, and diarrhea. Hematologic toxicities were most common with nearly all patients having at least grade 1 anemia, leukopenia, or thrombocytopenia. Toxicities by grade are listed in Table 2.
Table 2.
Toxicity Profile
| Toxicities | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Any |
|---|---|---|---|---|---|---|
| Hematologic | ||||||
| Leukopenia | 2 | 2 | 2 | 6 | ||
| Thrombocytopenia | 3 | 1 | 1 | 5 | ||
| Anemia | 2 | 2 | 4 | |||
| Lymphopenia | 1 | 1 | ||||
| Neutropenia | 1 | 1 | ||||
| Metabolic | ||||||
| Hyponatremia | 3 | 1 | 4 | |||
| Hyperglycemia | 3 | 1 | 4 | |||
| Hypocalcemia | 1 | 1 | 2 | |||
| Hypercarbia | 1 | 1 | ||||
| Hyperuremia | 1 | 1 | ||||
| Hypokalemia | 1 | 1 | ||||
| Elevated creatinine | 1 | 1 | ||||
| Hepatic | ||||||
| Hyperbilirubinemia | 1 | 1 | 2 | |||
| Hypoproteinemia | 1 | 1 | ||||
| Hypoalbuminemia | 1 | 1 | ||||
| Elevated Alk. Phosphatase | 1 | 1 | ||||
| Elevated ALT | 1 | 1 | ||||
| Elevated AST | 1 | 1 | ||||
| Constitutional | ||||||
| Fatigue | 3 | 3 | 2 | 8 | ||
| Pain | 2 | 2 | ||||
| Dermatitis | 1 | 1 | 2 | |||
| Chest Pain | 1 | 1 | 2 | |||
| Alopecia | 1 | 1 | 2 | |||
| Edema | 1 | 1 | ||||
| Gastrointestinal | ||||||
| Nausea | 3 | 1 | 4 | |||
| Anorexia | 3 | 3 | ||||
| Diarrhea | 1 | 1 | 2 | |||
| Dyspepsia | 1 | 1 | 2 | |||
| Emesis | 1 | 1 | ||||
| Mucositis | 1 | 1 | ||||
| Neurologic | ||||||
| Seizure | 1 | 1 | 2 | |||
| Dysphasia | 1 | 1 | ||||
| Drooling/Difficulty chewing | 1 | 1 | ||||
| Headache | 1 | 1 | ||||
| Motor Neuropathy | 1 | 1 | ||||
| Vascular | ||||||
| DVT | 1 | 1 | 2 | |||
| Pulmonary Embolism | 1 | 1 | ||||
| Dyspnea | 1 | 1 | ||||
| Epistaxis | 1 | 1 | ||||
| Vaginal bleeding | 1 | 1 | ||||
| Hypertension | 0 | 0 | ||||
| All | 43 | 23 | 12 | 0 | 2 | 80 |
Grade 3 toxicities occurred in 7 (47%) patients. Two (13%) grade 3 toxicities met the criteria for DLT. The other grade 3 toxicities were thought not to be attributable to the combination of sunitinib with RT and were not dose limiting. Of the twelve grade 3 toxicities, 7 were thought to be attributed to sunitinib (including neutropenia, leukopenia, thrombocytopenia, hyponatremia, and hyperglycemia), 2 were attributed to RT (dysphasia, difficulty chewing), two were attributed to the combination (fatigue) and one DVT was attributed to the prevalent coagulation abnormalities among patients with brain tumors. No difference in toxicity was observed between patients treated with whole brain RT (WBRT) versus partial brain RT. No difference in toxicity was observed based on radiation therapy fraction size or total dose administered. There was no observed difference in skin toxicity or toxicity of other tissues within the radiation portal. No grade 4 or 5 hypertensive events or intracerebral hemorrhages occurred. Two Grade 5 adverse events (status epilepticus and pulmonary embolism) occurred and were determined to be related to disease progression by the medical monitor. One patient with metastatic melanoma who was retreated with WBRT after prior WBRT and stereotactic radiosurgery died on day 4 of treatment from uncontrolled status epilepticus attributed to multiple brain metastases. A second patient with multiple brain metastases from non-small cell lung cancer died six days after study completion from a presumed pulmonary embolism.
Early Response
Thirteen patients had follow up MRI imaging to assess one-month tumor response. Two (15%) patients demonstrated a partial response, nine (70%) patients had stable disease and 2 (15%) patients had disease progression. All three patients with primary CNS tumors had stable disease. Among patients with metastatic disease, 2 (20%) had partial response, 6 (60%) had stable disease, and 2 (20%) had progressive disease (Table 3). Prior to initiation of treatment, 5 (33%) patients were on high-dose corticosteroids. At 1-month follow up none of the patients required corticosteroids. Three (20%) patients initiated corticosteroid therapy during RT, and all were tapered off corticosteroids within one month after RT.
Table 3.
Early treatment response as determined by gadolinium-enhanced MRI 1 month post treatment and 6-month PFS and median overall survival
| All patients | Metastatic Disease | CNS Primary | |
|---|---|---|---|
| Partial Response | 2 (15%) | 2 (20%) | 0 (0%) |
| Stable Disease | 9 (70%) | 6 (60%) | 3 (100%) |
| Progressive Disease | 2 (15%) | 2 (20%) | 0 (0%) |
| 6-month PFS | 9 (60%) | 8 (67%) | 1 (33%) |
| Median OS | 8.8 months | 7.6 months | Not reached |
One patient initially presented with multiple small parenchymal metastatic brain lesions from adenoid cystic carcinoma, the largest was in the right parietal lobe and measured 14 × 10 mm with an additional 12 × 8 mm lesion in the right frontal lobe. After finishing RT and continuing sunitinib for one month, an MRI revealed an interval decrease in the size of all the previous parenchymal enhancing lesions. Another patient with metastatic renal cell carcinoma presented with a 4.6 cm dural based mass involving the sphenoid sinus, sella, suprasellar cistern, clivus and cribriform plate with residual tumor after transphenoidal resection. One month follow up MRI revealed a significant decrease in the size of the skull base lesion with no enhancement and no metabolic activity on PET.
Survival
With a median follow up of 34.2 months, 6 patients continue to have stable disease and 7 have disease progression. PFS at six months was 60%, with 9 patients having stable disease (Figure 1). Four of the original 15 (27%) patients remain alive. Median survival is 8.8 months (Table 4). Eleven (73%) patients were alive six months after treatment, with a survival range for all patients of 0.1 to 39.9 months. Of the patients with brain metastases 2 of the 12 patients (18%) were alive (range 0.1 to 39.9 months). Seven (58%) of the patients with brain metastases were free of progression for 6 months.
Figure 1.
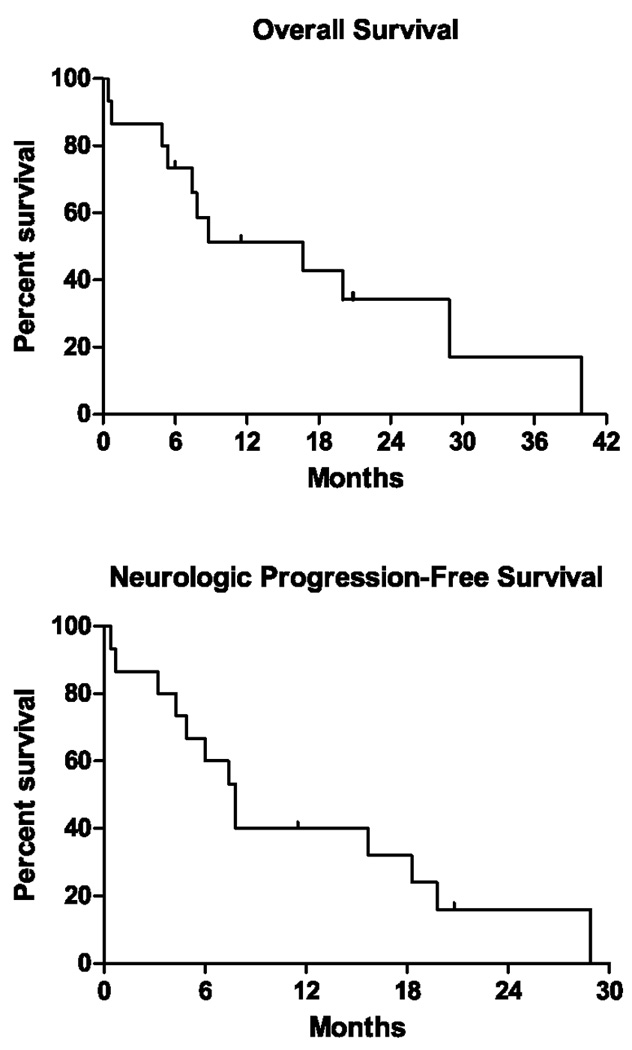
Kaplan-Meier overall survival and neurologic progression-free survival
Table 4.
Histology, response, neurologic progression-free survival and overall survival
| Patient | Histology | Response | Neurologic PFS |
Overall Survival (months) |
|---|---|---|---|---|
| 1 | Adenoid cystic |
PD | 7.4 | 7.4 |
| 2 | Non-small cell lung |
NA | 0.7 | 0.7 |
| 3 | Melanoma | NA | 0.4 | 0.4 |
| 4 | Astrocytoma | SD | 19.8 | 20.9 |
| 5 | Non-small cell lung |
SD | 4.9 | 4.9 |
| 6 | HNSCC | SD | 15.7 | 39.9 |
| 7 | Chordoma | SD | 7.8 | 7.8 |
| 8 | Atypical Meningioma |
SD | 11.5 | 11.5 |
| 9 | Non-small cell lung |
SD | 28.9 | 28.9 |
| 10 | Melanoma | SD | 20.8 | 20.8 |
| 11 | Melanoma | SD | 3.2 | 6.0 |
| 12 | Adenoid cystic |
PR | 6.0 | 8.8 |
| 13 | Melanoma | PD | 4.3 | 5.4 |
| 14 | Renal cell | PR | 18.3 | 20.0 |
| 15 | CNS sarcoma |
SD | 7.8 | 16.7 |
Urine VEGF
Urine samples were collected from 13 of the 15 patients enrolled on this study. Samples at baseline, end of radiation, and one month post-radiation were collected for 12/13, 10/13, and 5/13 patients, respectively. Urine VEGF was detectable in 24/27 (89%) samples. The median VEGF concentration for all time points for patients with detectable levels was 336 pg/mg (range 53–1153 pg/mg). There were no statistically significant differences in VEGF levels between any of the three time points, see Figure 2 (Friedman’s chi square test, p=0.87).
Figure 2.
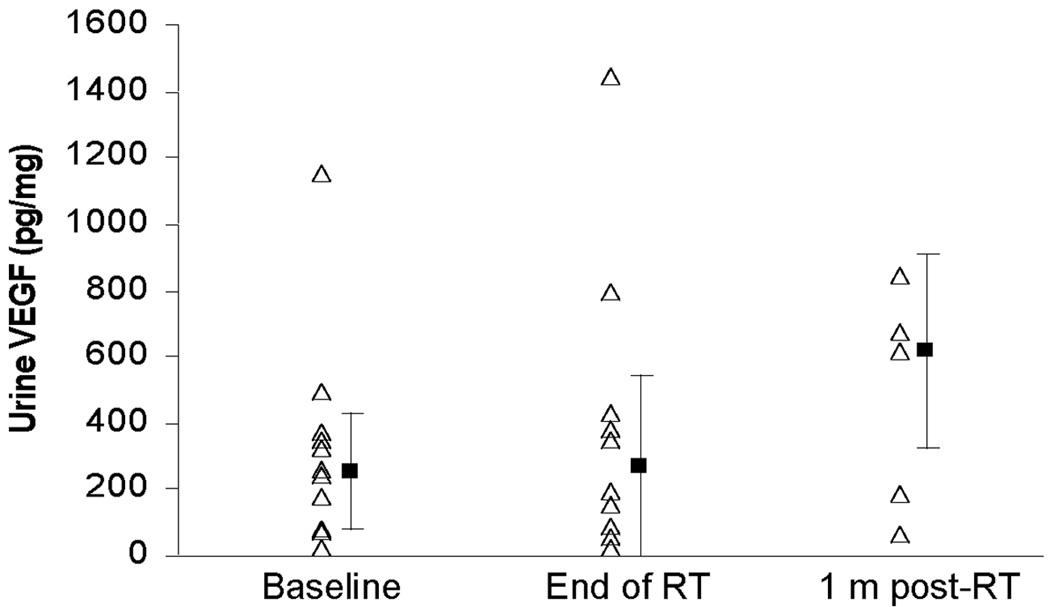
Urinary VEGF levels measured at baseline, end of RT and one month post RT: Urine VEGF levels at baseline, end of radiation (RT) and 1 month (m) post-RT. Patients with undetectable VEGF levels were assumed to have a VEGF value of 24 pg/mg, the lowest detectable level. The ▪ represents the median value and the error bars represent the 95% confidence interval.
Only 10 patients had paired baseline and end of radiation samples. For analysis purposes, patients with an undetectable VEGF level were assumed to have a value of 24 pg/mg, the lowest detectable level of VEGF for our assay. 40% (4/10) of patients had a > 3-fold increase (relative to baseline) in urine VEGF. This is similar to the > 3-fold increase in plasma VEGF seen in 44% of metastatic renal cell cancer patients treated with sunitinib42.
Paired samples between baseline and 1 month post-radiation were available for 4 patients. We were unable to correlate change in urinary VEGF levels and radiation response because of the lack of paired samples. Of the paired samples, 2 of these patients had metastatic melanoma and continued on sunitinib following radiation. One had a > 2 fold increase in VEGF from baseline to 1 month post-radiation and developed progressive disease while the other had a 72% reduction in VEGF between these same time points and had stable disease. We also analyzed changes in VEGF levels between baseline and the end of radiation in patients who did and did not develop grade 3 hematologic toxicities. 3/5 patients with grade 3 hematologic toxicities had paired urine samples between baseline and the end of radiation while 5/5 patients without grade 3 hematologic toxicity had paired samples. The median VEGF fold change (end of radiation/baseline) between those with a grade 3 hematologic toxicity versus those without was 5.0 versus 0.75, (p=0.036) see Figure 3. The median radiation dose delivered to these two groups was not significantly different (p=0.39).
Figure 3.
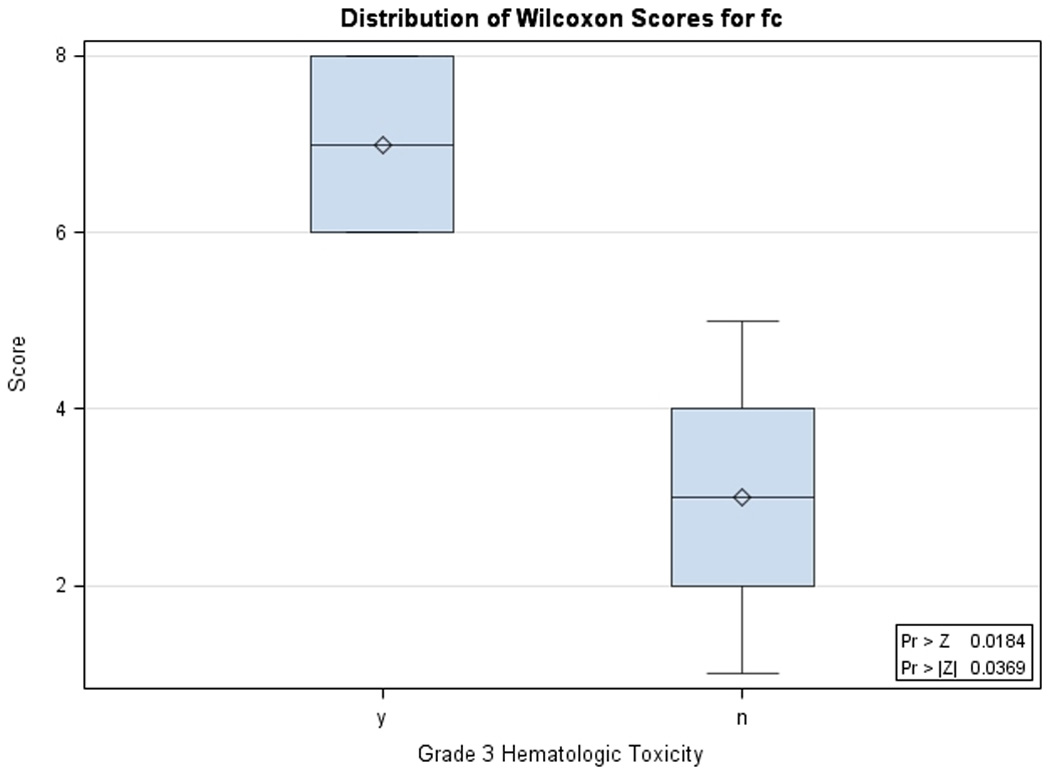
Urinary VEGF fold change in patients with and without grade 3 hematologic toxicity: Urinary VEGF fold change (pg/mg) between baseline and end of radiation for patients who did not (n=5) and did (n=3) develop grade 3 or 4 hematologic toxicity during treatment. Median values for those who did not develop toxicity were significantly different from those who did develop toxicity (0.8 pg/mg range [0.4,2.2] vs. 5.0 pg/mg range [4.1, 17.8], p = 0.037, Wilcoxon Rank Sum exact test). Wilcoxon scores are plotted, which are based on the ranks of the original data.
Urine MMP-9
Urine MMP-9 was detectable in 21/27 (78%) samples. All 6 undetectable samples were from patients with metastatic melanoma and were over various time points (3 baseline, 2 end of RT, 1 one-month post-RT). The median MMP-9 concentration over all three time points for those with detectable levels was 2,095 pg/mg (range 80–804,101 pg/mg) and there was no significant difference between time points. To determine the median fold change for patients with paired samples from baseline and the end of RT samples that were undetectable were assumed to be 72 pg/mg, which is the lowest detectable level of MMP-9 for our assay. There was an increase in MMP-9 from baseline to the end of RT seen in 6/10 patients with a median fold increase of 21 (range 2–227 pg/mg).
Urine MET
Urine MET was detectable in 27/27 (100%) samples. Median MET concentration overall all three time points was 324 pg/mg (range 44–1109 pg/mg) with no significant difference between time points. There was an increase in MET from baseline to the end of RT seen in 5/10 patients with a median fold increase of 1.8 (range 1.7–21 pg/mg).
Urine MMP-2
Urine MMP-2 was detectable in 11/27 (41%) samples. The median MMP-2 concentration over all three time points for those with detectable levels was 878 pg/mg (range 199–10,359 pg/mg). Given the high number of samples with undetectable levels further analysis was not carried out on this biomarker.
Conclusions
This trial sought to assess the safety and tolerability of combining RT to intracranial tumors with continuously dosed sunitinib. Overall, the combination of sunitinib and RT was well tolerated. The majority of the toxicities were not severe, nor did they limit the patient’s ability to complete the combined course. Toxicity was limited to grade 1–2 in 40% of the patients in this study. Forty-seven percent of the patients in this study had grade 3 toxicities, most commonly hematologic and without significant clinical consequences. We were particularly interested in differentiating toxicity that occurred as a result of sunitinib, RT, or the combination to assess the potential for a more than additive toxicity profile. Among the 14 grade 3+ toxicities, 7 hematologic and metabolic toxicities were attributed to sunitinib, 2 were attributed to RT, 2 were attributed to the combination, 1 was attributed to disease progression and 2 venous thromboembolic events occurred which may have been related to sunitinib or to the underlying procoagulant status of patients with brain tumors. Grade 3 toxicity rate did not increase with increasing RT dose.
Comparing the experience from this trial with the known toxicity data of WBRT is possible in a limited manner. Over the past decade 4 prominent phase III trials have used a WBRT alone control arm: Radiation Therapy Oncology Group (RTOG) 011843, RSR-1344, RTOG 950845, and motexafin gadolinium46. When compared to these historic controls, the rate of treatment-related adverse events of continuously dosed sunitinib and intracranial RT may be modestly higher than the toxicity of WBRT alone, see Table 3.
There is only one other study that has evaluated the toxicity of continuously dosed sunitinib and radiation therapy: Kao et al.47 recently published an experience treating 21 patients to 36 non-CNS oligometastatic sites with the combination of sunitinib and hypofractionated, image-guided RT (IGRT). They reported a 57% response rate and 28% rate of stable disease. They described a DLT in 1 of 10 patients when sunitinib 37.5 mg was combined with 10 Gy × 5 fractions and 2 of 5 patients had a DLT with sunitinib 50 mg combined with 10 Gy × 5 fractions. The 1-year local control, PFS, and OS rates were 85%, 44%, and 75%, respectively. They concluded that the combination of sunitinib (25–37.5 mg) to IGRT is tolerable in patients with oligometastatic disease without potentiation of RT-related toxicity. The rate of grade 3–5 toxicities of continuously dosed sunitinib and intracranial RT is similar to the rates seen in other larger studies that use sunitinib 37.5 mg continuous daily dosing alone. However, comparison is difficult because patients on continuously dosed sunitinib trials may have had a greater number of prior therapies and/or a longer duration of exposure to sunitinib.
A debate remains about the optimal timing of anti-angiogenic therapies and radiation therapy. Preclinical studies on inhibition of VEGF2 and the window of vascular normalization and radiosensitization by Winkler et al.48 suggest an open window for maximum radiosensitivity in the range of 4–6 days after initiation of anti-VEGF2 therapies. In the clinical setting of glioblastoma multiforme, Batchelor et al.49 described imaging techniques to detect vascular normalization after anti-angiogenic therapy. They discovered that vascular normalization may begin immediately upon anti-angiogenic therapy and has a window lasting approximately 28 days. The scheme for RTOG 0825, a phase III study testing radiation therapy and temozolomide with or without bevacizumab, starts bevacizumab after 30 Gy of the planned 60 Gy of radiation therapy has been delivered. Future clinic trials in this arena should employ advanced imaging techniques like diffusion-weighted MRI or permeability mapping through dynamic contrast-enhanced MRI to determine that optimal timing of concurrent anti-angiogenic therapy and radiation therapy. Alternately, the development of blood or urine-based biomarkers of vascular normalization could be developed to guide optimal timing of these therapies.
Concern about using antiangiogenic agents in patients with brain metastases has been raised by Pouessel and Culine,50 however no patients from the present study experienced intracranial hemorrhage. Similarly, there was no intracranial hemorrhage in 15 patients with recurrent malignant glioma treated with bevacizumab and RT in a pilot study conducted at Memorial Sloan Kettering51.
When antiangiogenic drugs alter vascular permeability, they may change the apparent size of tumors without affecting the underlying tumor mass compromising our ability to visualize these tumors on MRI. Therefore, response rates as an indication of treatment efficacy should be cautiously interpreted when antiangiogenic agents are used.
Our patients had a median urine VEGF level, over all time points, of 336 pg/mg. This is similar to the mean urine VEGF level of 317 pg/mg seen at baseline for a cohort of patients with prostate, breast, brain, and hematologic malignancies39. However other studies have reported median urine VEGF levels of 753 pg/ml at presentation for a cohort of pediatric and adult brain tumor patients and 101 pg/ml at baseline in patients with advanced soft tissue sarcomas38, 52. These results emphasize the wide range of baseline urine VEGF levels seen for different histologies and stages of disease. It also suggests that looking at trends between different time points is likely to be more fruitful as a predictive marker than absolute value cut offs. However, given the limited number of patients with complete urine sets, we were unable to investigate which comparisons between time points might be most predictive of response.
We found a correlation between grade 3 hematologic toxicity and higher VEGF fold changes between baseline and the end of radiation. Significant changes in white blood cell counts during sunitinib treatment have been reported in patients with GIST53. The largest proportional decrease was seen in monocytes which may reflect the fact that they have higher expression of VEGF receptor 1 relative to other peripheral blood mononuclear cells (PBMC)53. Sunitinib related PBMC changes may also be related to “off-target” inhibition of KIT and FLT-3. Given the small data set, this finding is hypothesis generating and merits evaluation in future studies.
We are encouraged in this limited study by the responses seen. The 58% 6-month PFS of the patients with brain metastases is higher than the historic 6-month PFS of patients with brain metastases54–56. The RTOG reported that brain metastases were the cause of death in 33%–50% of patients that were enrolled on studies 7916, 8528, and 890554. The 13% rate of neurologic death in this study also compares favorably to historic data. We are aware that patient selection can bias small studies, and this improvement will have to be validated by a larger study.
In conclusion, 37.5 mg daily dosing of sunitinib combined with cranial RT yielded acceptable toxicities and adverse events. This unique combination also produced intriguing responses. A phase II study further evaluating the use of sunitinib as a radiosensitizing agent in the setting of brain metastases is being planned by the RTOG.
Table 5.
Comparison of toxicities of trials utilizing whole brain RT compared with toxicity of RT combined with continuously dosed 37.5 mg sunitinib
| WBRT + Sunitinib |
RTOG 950838 | RTOG 011836 | Motexafin Gadolinium39 |
Efaproxiral37 | |
|---|---|---|---|---|---|
| n | 9 | 167 | 93 | 208 | 250 |
| Grade 1–2 acute tox | 40% | 62% | 66% | NR | NR |
| Grade 3 acute tox | 47% | 0% | 27% | NR | NR |
| Grade 4 acute tox | 0% | 0% | 5% | NR | 11% |
| Grade 5 acute tox | 13% | 0% | 2% | NR | NR |
| All grade nausea | 27% | NR | NR | 24% | NR |
| All grade diarrhea | 13% | NR | NR | 2% | NR |
| All grade paresthesia | 13% | NR | NR | 11% | NR |
| All grade pain | 27% | NR | NR | 8% | NR |
| All grade rash | 13% | NR | NR | 14% | NR |
| All grade tachycardia | 0% | NR | NR | 2% | NR |
| All grade peripheral edema | 0% | NR | NR | 11% | NR |
| Hematologic grade 3+ | 20% | NR | NR | NR | NR |
| Neurologic-grade 3+ | 20% | NR | 12% | NR | 3% |
| GI- grade 3+ | 0% | NR | 6% | NR | 6% |
| Nausea grade 3+ | 0% | NR | NR | NR | 2% |
| Vomiting grade 3+ | 0% | NR | NR | NR | 2% |
| Constipation grade 3+ | 0% | NR | NR | NR | 2% |
| Vascular- grade 3+ | 13% | NR | 4% | NR | NR |
| Constitutional-grade 3+ | 13% | NR | 5% | NR | NR |
| Metabolic- grade 3+ | 13% | NR | 3% | NR | NR |
Acknowledgments
This study was supported by the P30 CA056036-08 Kimmel Cancer Center grant and in part by the Intramural Research Program of the NCI, National Institutes of Health
Footnotes
Presented at the 44th Annual Meeting of the American Society of Clinical Oncology June 2008, Chicago, IL.
Conflict of interest statement: Dr. Adam P. Dicker received an unrestricted grant from Pfizer Incorporated in association with this trial. There are no other conflicts of interest for Dr. Dicker or for any of the other authors.
References
- 1.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15637262. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11533692. [DOI] [PubMed] [Google Scholar]
- 3.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9(6):1957–1971. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12796357. [PubMed] [Google Scholar]
- 4.Wachsberger P, Burd R, Dicker AP. Improving tumor response to radiotherapy by targeting angiogenesis signaling pathways. Hematol Oncol Clin North Am. 2004;18(5):1039–1057. doi: 10.1016/j.hoc.2004.06.007. viii. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15474334. [DOI] [PubMed] [Google Scholar]
- 5.Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, et al. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60(19):5565–5570. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11034104. [PubMed] [Google Scholar]
- 6.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–437. doi: 10.1038/nrc2397. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18500244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinohara ET, Cao C, Niermann K, Mu Y, Zeng F, Hallahan DE, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24(35):5414–5422. doi: 10.1038/sj.onc.1208715. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15940265. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, et al. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20(9):1495–1497. doi: 10.1096/fj.05-5137fje. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16754748. [DOI] [PubMed] [Google Scholar]
- 9.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313(5794):1785–1787. doi: 10.1126/science.1127592. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16990548. [DOI] [PubMed] [Google Scholar]
- 10.Su Y, Zheng L, Wang Q, Bao J, Cai Z, Liu A. The PI3K/Akt pathway upregulates Id1 and integrin alpha4 to enhance recruitment of human ovarian cancer endothelial progenitor cells. BMC Cancer. 10:459. doi: 10.1186/1471-2407-10-459. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20796276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9(4):412–423. doi: 10.1215/15228517-2007-024. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17622648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milker-Zabel S, Bois AZ, Ranai G, Trinh T, Unterberg A, Debus J, et al. SU11657 Enhances Radiosensitivity of Human Meningioma Cells. Int J Radiat Oncol Biol Phys. 2008;70(4):1213–1218. doi: 10.1016/j.ijrobp.2007.11.041. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18234428. [DOI] [PubMed] [Google Scholar]
- 13.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2(5):471–478. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12748309. [PubMed] [Google Scholar]
- 14.Abrams TJ, Murray LJ, Pesenti E, Holway VW, Colombo T, Lee LB, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with "standard of care" therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2(10):1011–1021. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14578466. [PubMed] [Google Scholar]
- 15.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–1295. doi: 10.1172/JCI17929. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12727920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter TA, Wodicka LM, Shah NP, Velasco AM, Fabian MA, Treiber DK, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci U S A. 2005;102(31):11011–11016. doi: 10.1073/pnas.0504952102. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16046538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laird AD, Cherrington JM. Small molecule tyrosine kinase inhibitors: clinical development of anticancer agents. Expert Opin Investig Drugs. 2003;12(1):51–64. doi: 10.1517/13543784.12.1.51. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12517254. [DOI] [PubMed] [Google Scholar]
- 18.Marzola P, Degrassi A, Calderan L, Farace P, Nicolato E, Crescimanno C, et al. Early antiangiogenic activity of SU11248 evaluated in vivo by dynamic contrast-enhanced magnetic resonance imaging in an experimental model of colon carcinoma. Clin Cancer Res. 2005;11(16):5827–5832. doi: 10.1158/1078-0432.CCR-04-2655. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16115922. [DOI] [PubMed] [Google Scholar]
- 19.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12538485. [PubMed] [Google Scholar]
- 20.O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–3605. doi: 10.1182/blood-2002-07-2307. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12531805. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Liang C, Shirazian S, Zhou Y, Miller T, Cui J, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem. 2003;46(7):1116–1119. doi: 10.1021/jm0204183. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12646019. [DOI] [PubMed] [Google Scholar]
- 22.Yee KW, Schittenhelm M, O'Farrell AM, Town AR, McGreevey L, Bainbridge T, et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004;104(13):4202–4209. doi: 10.1182/blood-2003-10-3381. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15304385. [DOI] [PubMed] [Google Scholar]
- 23.Geng L, Donnelly E, McMahon G, Lin PC, Sierra-Rivera E, Oshinka H, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61(6):2413–2419. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11289107. [PubMed] [Google Scholar]
- 24.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59(14):3374–3378. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10416597. [PubMed] [Google Scholar]
- 25.Griffin RJ, Williams BW, Wild R, Cherrington JM, Park H, Song CW. Simultaneous inhibition of the receptor kinase activity of vascular endothelial, fibroblast, and platelet-derived growth factors suppresses tumor growth and enhances tumor radiation response. Cancer Res. 2002;62(6):1702–1706. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11912143. [PubMed] [Google Scholar]
- 26.Hess C, Vuong V, Hegyi I, Riesterer O, Wood J, Fabbro D, et al. Effect of VEGF receptor inhibitor PTK787/ZK222584 [correction of ZK222548] combined with ionizing radiation on endothelial cells and tumour growth. Br J Cancer. 2001;85(12):2010–2016. doi: 10.1054/bjoc.2001.2166. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11747347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozin SV, Boucher Y, Hicklin DJ, Bohlen P, Jain RK, Suit HD. Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res. 2001;61(1):39–44. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11196192. [PubMed] [Google Scholar]
- 28.Schueneman AJ, Himmelfarb E, Geng L, Tan J, Donnelly E, Mendel D, et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Res. 2003;63(14):4009–4016. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12873999. [PubMed] [Google Scholar]
- 29.Cuneo KC, Geng L, Fu A, Orton D, Hallahan DE, Chakravarthy AB. SU11248 (sunitinib) sensitizes pancreatic cancer to the cytotoxic effects of ionizing radiation. Int J Radiat Oncol Biol Phys. 2008;71(3):873–879. doi: 10.1016/j.ijrobp.2008.02.062. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18514780. [DOI] [PubMed] [Google Scholar]
- 30.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7165009. [PubMed] [Google Scholar]
- 31.Program CTE. 2006;vol. 2008 http://ctep.cancer.gov/forms/CTCAEv3.pdf. [Google Scholar]
- 32.Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(25):4068–4075. doi: 10.1200/JCO.2008.20.5476. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19652072. [DOI] [PubMed] [Google Scholar]
- 33.Srinivas S, Roigas J, Gillessen S, Harmenberg U, De Mulder PH, Fountzilas G, et al. Continuous daily administration of sunitinib in patients (pts) with cytokine-refractory metastatic renal cell carcinoma (mRCC): Updated results. Proceedings of the 2008 Annual ASCO Meeting. 2007;25(18S):5040. [Abstract] [Google Scholar]
- 34.George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–1968. doi: 10.1016/j.ejca.2009.02.011. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19282169. [DOI] [PubMed] [Google Scholar]
- 35.George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27(19):3154–3160. doi: 10.1200/JCO.2008.20.9890. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19451429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89(15):1138–1147. doi: 10.1093/jnci/89.15.1138. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9262252. [DOI] [PubMed] [Google Scholar]
- 37.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2358840. [DOI] [PubMed] [Google Scholar]
- 38.Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008;14(8):2378–2386. doi: 10.1158/1078-0432.CCR-07-1253. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18413828. [DOI] [PubMed] [Google Scholar]
- 39.Chan LW, Moses MA, Goley E, Sproull M, Muanza T, Coleman CN, et al. Urinary VEGF and MMP levels as predictive markers of 1-year progression-free survival in cancer patients treated with radiation therapy: a longitudinal study of protein kinetics throughout tumor progression and therapy. J Clin Oncol. 2004;22(3):499–506. doi: 10.1200/JCO.2004.07.022. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14752073. [DOI] [PubMed] [Google Scholar]
- 40.Athauda G, Giubellino A, Coleman JA, Horak C, Steeg PS, Lee MJ, et al. c-Met ectodomain shedding rate correlates with malignant potential. Clin Cancer Res. 2006;12(14 Pt 1):4154–4162. doi: 10.1158/1078-0432.CCR-06-0250. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16857786. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53(282):457. [Google Scholar]
- 42.Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17605814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knisely JP, Berkey B, Chakravarti A, Yung AW, Curran WJ, Jr., Robins HI, et al. A phase III study of conventional radiation therapy plus thalidomide versus conventional radiation therapy for multiple brain metastases (RTOG 0118) Int J Radiat Oncol Biol Phys. 2008;71(1):79–86. doi: 10.1016/j.ijrobp.2007.09.016. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18164847. [DOI] [PubMed] [Google Scholar]
- 44.Suh JH, Stea B, Nabid A, Kresl JJ, Fortin A, Mercier JP, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24(1):106–114. doi: 10.1200/JCO.2004.00.1768. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16314619. [DOI] [PubMed] [Google Scholar]
- 45.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15158627. [DOI] [PubMed] [Google Scholar]
- 46.Mehta MP, Rodrigus P, Terhaard CH, Rao A, Suh J, Roa W, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21(13):2529–2536. doi: 10.1200/JCO.2003.12.122. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12829672. [DOI] [PubMed] [Google Scholar]
- 47.Kao J, Packer S, Vu HL, Schwartz ME, Sung MW, Stock RG, et al. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: acute toxicity and preliminary response. Cancer. 2009;115(15):3571–3580. doi: 10.1002/cncr.24412. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19536893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15607960. [DOI] [PubMed] [Google Scholar]
- 49.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17222792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pouessel D, Culine S. High frequency of intracerebral hemorrhage in metastatic renal carcinoma patients with brain metastases treated with tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor. Eur Urol. 2008;53(2):376–381. doi: 10.1016/j.eururo.2007.08.053. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17825982. [DOI] [PubMed] [Google Scholar]
- 51.Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75(1):156–163. doi: 10.1016/j.ijrobp.2008.10.043. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19167838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heymach JV, Desai J, Manola J, Davis DW, McConkey DJ, Harmon D, et al. Phase II study of the antiangiogenic agent SU5416 in patients with advanced soft tissue sarcomas. Clin Cancer Res. 2004;10(17):5732–5740. doi: 10.1158/1078-0432.CCR-04-0157. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15355900. [DOI] [PubMed] [Google Scholar]
- 53.Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13(9):2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17473195. [DOI] [PubMed] [Google Scholar]
- 54.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9128946. [DOI] [PubMed] [Google Scholar]
- 55.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295–1304. doi: 10.1200/JCO.2005.04.6185. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16525185. [DOI] [PubMed] [Google Scholar]
- 56.Sundstrom JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296–299. doi: 10.3109/07853899809005858. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9677016. [DOI] [PubMed] [Google Scholar]


