Abstract
Myelin-associated inhibitors of axon growth, including Nogo, MAG and OMgp, have been the subject of intense research. A myriad of experimental approaches have been applied to investigate the potential of targeting these molecules to promote axonal repair after spinal cord injury. However, there are still conflicting results on their role in axon regeneration and therefore a lack of a cohesive mechanism on how these molecules can be targeted to promote axon repair. One major reason may be the lack of a clear definition of axon regeneration in the first place. Nevertheless, recent data from genetic studies in mice indicate that the roles of these molecules in CNS axon repair may be more intricate than previously envisioned.
Introduction
Spinal cord injury induces both anatomical as well as synaptic changes along the entire neuroaxis (Darian-Smith, 2009; Kokotilo, et al., 2009; Lane, et al., 2009). However, frank (or true) regeneration of lesioned axons normally does not occur in the adult mammalian central nervous system (CNS). One prevailing theory is the presence of inhibitory molecules that prevent injured axons from regenerating beyond the injury site. Berry (1982) was the first to propose that CNS myelin may be responsible for the failure of axon regeneration. Through a series of seminal studies, Schwab and colleagues demonstrated that CNS myelin and mature oligodendrocytes are non-permissive substrates for neurite outgrowth (Caroni and Schwab, 1988b; Savio and Schwab, 1989), which led to the development of the IN-1 antibody that can neutralize, at least partially, the non-permissive nature of CNS myelin in vitro (Caroni and Schwab, 1988a) and can promote corticospinal tract (CST) axon regeneration in vivo (Bregman, et al., 1995; Schnell and Schwab, 1990). Characterization of an antigen for IN-1 (Spillmann, et al., 1998) led to the identification of a novel protein called Nogo (or Rtn4) (Chen, et al., 2000; GrandPre, et al., 2000; Prinjha, et al., 2000).
Ever since the early encouraging results from the IN-1 antibody, myelin-mediated inhibition of axon growth has been an intense area of research in the field of CNS injury and repair. If the regenerative potential of CNS axons can be simply unleashed by neutralizing one (or few) inhibitors, this would provide a rather attractive therapeutic strategy. Apart from Nogo, MAG (myelin associated glycoprotein) was in fact the first protein identified as a myelin-associated inhibitor of axon growth (McKerracher, et al., 1994; Mukhopadhyay, et al., 1994); OMgp (oligodendrocyte myelin glycoprotein), the third in the trio, was later recognized as a myelin inhibitor (Kottis, et al., 2002; Wang, et al., 2002b). The link between the three molecules was further strengthened by the intriguing discovery that despite sharing no structural similarity, all three bind to common receptors including NgR1 and PirB (Atwal, et al., 2008; Domeniconi, et al., 2002; Fournier, et al., 2001; Liu, et al., 2002; Wang, et al., 2002b). The molecular identification of the inhibitory ligands and receptors made it possible to determine the contribution of individual molecules as well as that of myelin inhibition in general in axon regeneration using specific molecular perturbations. These studies produced both consistent and discrepant results.
Here we discuss the role of myelin inhibitors in CNS axon repair in the context of these mixed findings. We argue that different definitions of axon sprouting vs. regeneration, the technical difficulty in distinguishing sprouting and regenerating axons and variable degrees of axon sparing in different studies are at least partly responsible for some of the discrepant findings. Proper understanding of the role of myelin-associated inhibitors (or any other molecules for that matter) in axonal repair therefore requires a thorough understanding of the particular injury models used and the type of axon growth elicited by targeting these molecules. Although there are at least three other myelin-associated inhibitors: Netrin (Low, et al., 2008), Sema4D (Moreau-Fauvarque, et al., 2003), and Ephrin-B3 (Benson, et al., 2005), the in vivo role of these molecules as endogenous myelin-associated inhibitors has not been evaluated. Thus, we will focus our discussion on Nogo, MAG and OMgp, often referred to as the prototypical myelin-associated axon growth inhibitors.
Overview of Nogo, MAG and OMgp
Although Nogo was initially discovered as a myelin-associated inhibitor of axon growth (Chen, et al., 2000; GrandPre, et al., 2000; Prinjha, et al., 2000), its physiological role as a member of the Reticulon family member seems to be maintaining the tubular shape of the endoplasmic reticulum (Voeltz, et al., 2006). Nogo is also expressed on the adaxonal (innermost) and abaxonal (outermost) membranes of myelin (Buss and Schwab, 2003; Wang, et al., 2002c). Nogo’s expression both on the inner and outer surface of myelin sheaths is consistent with its stabilizing role to reduce axonal growth after CNS injury. There are three isoforms of Nogo (A, B and C) generated by alternative splicing and differential promoter usage. The two main inhibitory domains of Nogo are the N-terminus region and a 66 amino acid loop located between two transmembrane domains (GrandPre, et al., 2000). The N-terminus region is specific to Nogo-A while the 66 amino acid region (termed Nogo-66) is common to all three isoforms.
MAG is a transmembrane glycoprotein expressed in the adaxonal membrane of oligodendrocytes between axons and the innermost myelin sheath to function in the maintenance of myelinated axons in the adult nervous system (for review see Quarles, 2007; Schnaar, 2010). However, upon the discovery that MAG can inhibit neurite outgrowth in vitro (McKerracher, et al., 1994; Mukhopadhyay, et al., 1994), the focus on MAG’s role in adult CNS has shifted to axon regeneration, with MAG serving as a model inhibitor to understand the signaling mechanism of myelin inhibition of axon regeneration (for review see Domeniconi and Filbin, 2005; Filbin, 2003). Interestingly, MAG is known to have a dual role in axon growth depending on the developmental stage of the neurons studied: growth promoting on younger neurons while growth inhibitory on older neurons, with this transition occurring at or soon after birth (Mukhopadhyay, et al., 1994). However, it is much less appreciated that MAG may also have multiple roles in the axonal responses to injury in the adult CNS (Mehta, et al., 2010; Nguyen, et al., 2009 and see below).
OMgp is a glycoprotein that was originally discovered as a peanut agglutinin-binding protein found in CNS myelin preparations (Mikol and Stefansson, 1988; for review see Vourc’h and Andres, 2004). We now know that OMgp is also expressed prominently in neurons (Habib, et al., 1998; Hunt, et al., 2003; Lee, et al., 2009a). We have only recently started to get a glimpse of the physiological role of OMgp. In OMgp knockout mice, the thalamocortical axons in the barrel field overshoot their layer IV target and ectopically invade layers II–III (Gil, et al., 2010). Since the OMgp gene is located in an intron of the neurofibromatosis type 1 gene (or NF1) (Viskochil, et al., 1991), it was initially thought that OMgp may play a role in the development of neurofibromatosis. However, a clear link has never been established and to our knowledge tumorigenesis in OMgp knockout mice has yet to be reported. Huang et al (2005) initially reported an interesting physiological role of OMgp, which was to inhibit aberrant collateral sprouting from the nodes of Ranvier through its expression in oligodendroglia-like cells that ensheath the nodes during development. However, a more recent study disputes this phenotype and attributes the reported node-ensheathing OMgp immunoreactivity to cross reactivity of the antibodies used with versican V2 (Chang, et al., 2010). Thus, the physiological role of OMgp in developmental axon sprouting remains to be fully understood.
Receptors for myelin-associated inhibitors
Nogo receptor 1 (NgR1), a GPI-linked leucine rich repeat protein, was identified by Strittmatter and colleagues in a screen for Nogo-66 interacting protein (Fournier, et al., 2001). It was later found to also bind MAG (Domeniconi, et al., 2002; Liu, et al., 2002) and OMgp (Wang, et al., 2002b) despite the fact that the three ligands do not share structural similarity. Two other homologues exist: NgR2 and NgR3. NgR2 also binds to MAG, apparently with a higher affinity than NgR1 (Venkatesh, et al., 2005). The current working model is that NgR1 complexes with co-receptors, such as p75 (or TROY) and LINGO-1 to mediate the inhibitory signals (Park, et al., 2005; Shao, et al., 2005; Wang, et al., 2002a; Wong, et al., 2002). MAG can also signal through ganglioside receptors, which is the signaling mechanism that has been described for MAG’s role in myelination (for review see Quarles, 2002). In addition, integrin receptors may also mediate the inhibitory properties of MAG (Goh, et al., 2008) as well as the amino-terminal Nogo (Hu and Strittmatter, 2008). More recently, PirB (paired-immunoglobulin-like receptor B, also known as LILRB) was discovered as another promiscuous receptor for Nogo, MAG and OMgp, and in combination with NgR1, has been proposed to mediate nearly all myelin-associated axon growth inhibition (Atwal, et al., 2008).
Recently, there have been other reported binding partners for NgR1 such as BLyS (B lymphocyte stimulator, Zhang, et al., 2009), and FGF1, 2 (fibroblast growth factor, Lee, et al., 2008). PirB is well characterized as a receptor for MHCI (major histocompatibility complex class I) (Takai, 2005). The fact that both NgR1 and PirB receptors bind to proteins other than myelin-associated axon growth inhibitors indicates that the effect of blocking these receptors (either genetically or pharmacologically) for axon regeneration studies must be interpreted with caution.
Axon regeneration vs. sprouting
Before we discuss experimental evidence examining the role of myelin inhibitors in CNS axon repair, it is important to consider the types of axonal growth possible after injury. The traditional model of identifying regenerating axons was to lesion a certain axonal tract in the spinal cord and then detecting these axons, if any, beyond the injury site after the experimental manipulation. The idea was that since the axons were cut, their presence beyond the injury site would be due to regeneration. Any enhanced growth detected proximal to the lesion site was classified as sprouting. However, if the lesions are not performed carefully, uncut spared axons can be detected beyond the injury site, which can be easily misconstrued as regenerated axons (Steward, et al., 2003). In practice, the complexity of the axonal tracts (Bareyre, et al., 2005; Steward, et al., 2004) and the inherent variability in spinal cord injury models often make it difficult to consistently eliminate all axons of a tract in a partial injury model (a complete transection is more assuring but often is too harsh a lesion to allow for any regeneration).
Axon sparing may complicate the analysis of axon regeneration in experimental spinal cord injury. One could argue that since axon sparing should be present more or less equally (but still variable on an individual basis) in both the experimental and control animals, any effect detected with an experimental manipulation could still be valid. Thus, in theory, spared axons should not be a major problem. A more complex situation arises when the experimental treatment has a selective effect on promoting the growth of spared but not lesioned axons. Under this situation, similar amount of axon sparing (whether advertently or inadvertently) in experimental and control groups could lead to much more axons detected beyond the injury site in the experimental group. Not knowing that some axons have been spared, this would lead to the conclusion that the experimental treatment promoted axon regeneration. However, had the surgical lesion not spared any of the targeted axons, the experimental treatment would not have led to more axons detected beyond the injury site, and would have been considered ineffective in promoting axon regeneration. Both experiments could have faithfully reported their findings, but came to very different conclusions depending on whether there was axon sparing in the first place. Further complicating the matter is the possibility for a genetic or pharmacological manipulation to effect neuroprotection in a partial injury model, where enhanced survival of spared but compromised axons gives the appearance of enhanced axon regeneration.
To avoid such confusion, we propose to define regeneration as the growth of injured axons (or axonal branches) and sprouting as the growth of uninjured axons (or axonal branches), as has been applied in our recent study of Nogo, MAG and OMgp knockout mice (Lee, et al., 2010b). We consider both regeneration and sprouting a form of injury-induced axonal growth. Both may contribute to functional recovery. The distinction between the two is based upon whether or not the axon (or axonal branch) of interest has been severed in the first place regardless of the distance grown. An interesting scenario arises when in response to an injury to one axonal branch, another axonal branch from the same neuron grows, such as in collateral sprouting from an injured axon proximal to the injury site. This would still fall into the category of axon sprouting. However, sprouting of uninjured axonal branches from an injured neuron may share molecular determinants with both the regeneration of injured axons and axon sprouting from an uninjured neuron. It is important to note these subtleties as they pertain to the molecular mechanisms of injury-induced axonal growth.
Strittmatter and colleagues proposed three criteria for their classification: the initiating perturbation, anatomical distance of axon growth and time required for axon growth, with distance and time being the most distinguishing parameters (Cafferty, et al., 2008). Under these criteria, a regenerating axon would be an axotomized axon that has grown over a long distance (millimeters) over a long period of time (weeks), whereas a sprouting axon would be an injured or uninjured axon that has grown over a short distance (micrometers) over a shorter period of time (days). Therefore, under these definitions, regeneration and sprouting are related concepts with overlapping domains in a continuum, and hence the distinction between the two is more quantitative than qualitative.
Schwab and colleagues recognized early on the effect of the IN-1 antibody on axon sprouting (Bregman, et al., 1995; Thallmair, et al., 1998; Z’Graggen, et al., 1998). In fact, they often used the two terms interchangeably (Fouad, et al., 2004; Schnell and Schwab, 1993). In some studies, regeneration, along with sprouting, was discussed using an injury model where some axons were knowingly spared (Simonen, et al., 2003). In other studies, a more cautious term, regenerative sprouting, was used to reflect the uncertain nature of the axon growth observed (Fouad, et al., 2004; Liebscher, et al., 2005). Together, these studies illustrated the difficulty in distinguishing regenerating and sprouting axons.
Therefore, it is important to consider these differences in defining axonal growth after injury in different studies in order to consider the apparent discrepancies from different studies and the implications on the role of myelin inhibitors on axonal repair. For the purpose of this review, the conclusions of sprouting and/or regeneration will be stated as reported in the original study (summarized in Table 1), while noting key issues when necessary.
Table 1.
Summary of axon regeneration and/or sprouting studies targeting myelin-associated inhibitors.
| Regeneration
|
No regeneration
|
Sprouting
|
||||||
|---|---|---|---|---|---|---|---|---|
| Author | Method | Injury model | Author | Method | Injury model | Author | Method | Injury model |
| Nogo | Nogo | Nogo | ||||||
| Schnell, 1990 | IN-1 antibody-producing hybridoma | DH for CST | Zheng, 2003 | Nogo-A,B,C KO, Nogo-A,B KO | DH for CST | Thallmair, 1998 | IN-1 antibody | PYRX for CST and corticofugal |
| Bregman, 1995 | IN-1 antibody-producing hybridoma | Over-hemisect for CST | Lee, 2009b | Nogo null, Nogo-A,B GT | DH for CST | Z’Graggen, 1998 | IN-1 antibody | PYRX for corticofugal |
| Sicotte, 2003 | Nogo-66 and MAG immunization | DH for CST | Lee, 2010b | Nogo/MAG/OMgp null | DH for CST; TX for 5HT | Blochlinger, 2001 | IN-1 antibody | PYRX for corticofugal |
| Kim, 2003 | Nogo-A,B GT | DH for CST | MAG | Freund, 2006, 2007 | Nogo antibodies (11C7, hNogo-A) | unilateral lesion of DL funiculus for CST | ||
| Simonen, 2003 | Nogo-A KO | DH for CST | Bartsch, 1995 | MAG KO | DH for CST | Cafferty, 2006 | Nogo-A,B GT | PYRX for CST |
| Liebscher, 2005 | Nogo antibodies (11C7, 7B12) | T-shaped lesion for CST | Lee, 2010b | Nogo/MAG/OMgp null | DH for CST; TX for 5HT | Mullner, 2008 | Nogo antibodies (11C7, 7B12) | T-shaped lesion for 5HT |
| Dimou, 2006 | Nogo-A KO | DH for CST | Cafferty, 2010 | OMgp/MAG KO | DH for CST and 5HT | Maier, 2009 | Nogo antibody (11C7) | T-shaped lesion for 5HT |
| Maier, 2009 | Nogo antibody (11C7) | T-shaped lesion for CST | OMgp | Lee, 2010b | Nogo null | PYRX for CST | ||
| Cafferty, 2010 | Nogo-A,B/MAG/OMgp GT/KO, Nogo-A,B GT | DH for CST and 5HT | Ji, 2008 | OMgp KO | DH for CST | MAG | ||
| MAG | Lee, 2010b | Nogo/MAG/OMgp null | DH for CST; TX for 5HT | Lee, 2010b | MAG KO | LH for 5HT | ||
| Sicotte, 2003 | Nogo-66 and MAG immunization | DH for CST | Cafferty, 2010 | OMgp/MAG KO | DH for CST and 5HT | Mountney, 2010 | sialidase treatment | Contusion for 5HT |
| OMgp | NgR1/p75/LINGO-1/PirB | OMgp | ||||||
| Ji, 2008 | OMgp KO | TX for 5HT, sensory | Song, 2004 | p75 KO | DH for CST | Ji, 2008 | OMgp KO | TX for 5HT |
| NgR1/p75/LINGO-1/PirB | Kim, 2004 | NgR1 KO | DH, TX for CST | Lee, 2010b | OMgp KO | LH for 5HT | ||
| GrandPre, 2002 | NgR1 antagonist (NEP1-40) | DH for CST | Zheng, 2005 | p75 KO, NgR1 KO | DH for CST | NgR1/p75/LINGO-1/PirB | ||
| Kim, 2004 | NgR1 KO | TX for 5HT, RST | Steward, 2008 | NgR1 antagonist (NEP1-40) | DH for CST | Li, 2004 | NgR1 ectodomain protein | DH for CST and 5HT |
| Li, 2003 | NgR1 antagonist (NEP1-40) | DH for CST | Lee, 2010a | NgR1/MAG/Nogo-A,B KO, NgR1 KO | TX for 5HT | MacDermid, 2004 | NgR1 ectodomain protein | Rhizotomy for 5HT |
| Nakamura, 2011 | PirB KO with NgR1 antagonist (NEP1-40) | DH for CST | Li, 2005 | NgR1 ectodomain transgenic mouse | DH for CST and 5HT | |||
| Ji, 2005 | NgR1 ectodomain protein | DH for CST | ||||||
| Cafferty, 2006 | NgR1 KO | PYRX for CST | ||||||
| Ji, 2006 | LINGO-1 antagonist | DH for CST; LH for RST | ||||||
| Wang, 2006 | NgR1 ectodomain protein | contusion for CST and 5HT | ||||||
| Cao, 2008 | NgR1 antagonist (NEP1-40) | LH for 5HT, CGRP, RST | ||||||
Abbreviations: KO, targeted knockout; GT, gene trap mutant; DH (dorsal hemisection); DL (dorsolateral); CST (corticospinal tract axons); RST (rubrospinal tract axons); TX (transection); 5HT (serotonergic axons); PYRX (pyramidotomy); LH (lateral hemisection); CGRP (calcitonin gene related peptide); OMgp (oligodendrocyte myelin glycoprotein); MAG (myelin associated glycoprotein); NgR1 (Nogo Receptor 1). Nogo null refers to the fully viable Nogo mutation that is null for all Nogo isoforms (Nogo-A,B,C) to distinguish it from other mutants which either still express some Nogo isoform(s) or are not fully viable.
Regeneration after spinal cord injury
As summarized above, there is a high level of potential redundancy between myelin-associated inhibitors at both the ligand and receptor levels. This raised the possibility that several pathways would have to be targeted simultaneously in order to promote significant regeneration. This hypothesis is underscored when considering previous studies that have targeted single molecules. MAG knockout mice failed to show enhanced axon regeneration in either the optic nerve or the CST (Bartsch, et al., 1995). Two different OMgp knockout mice failed to show significant corticospinal axon regeneration after spinal cord injury (Ji, et al., 2008; Lee, et al., 2010b). While Ji et al. reported enhanced growth of serotonergic (5-HT) and dorsal column sensory axons in OMgp knockout mice, it was not clear whether the enhanced growth was regeneration or sprouting, as the two terms were used interchangeably in this study. Before using Nogo knockout mice, its role in axon regeneration was tested using the IN-1 antibody primarily by Schwab and colleagues. Administration of this antibody after spinal cord injury enhanced axonal regeneration, sprouting and plasticity, and promoted behavioral recovery, which led to the current clinical trial with anti-Nogo antibodies in Europe (for reviews see Buchli and Schwab, 2005; Fouad, et al., 2001). Even though more specific antibodies against Nogo-A have yielded less regenerative profiles (Freund, et al., 2006; Freund, et al., 2007; Liebscher, et al., 2005; Maier, et al., 2009), it is consistent with Nogo’s role in inhibiting axon regeneration.
Therefore, it came as a surprise when genetic analysis using Nogo knockout mice produced three different outcomes ranging from robust (Kim, et al., 2003), suggestive (Simonen, et al., 2003), to no regeneration (Zheng, et al., 2003), which have led to a major controversy regarding Nogo’s role in axon regeneration (Filbin, 2003; Zheng, et al., 2006). These discrepancies have been attributed to an axon labeling artifact, genetic background effect, different effects on the Nogo isoforms in different Nogo mutants, and differential developmental compensation (Cafferty and Strittmatter, 2006; Dimou, et al., 2006; Simonen, et al., 2003; Steward, et al., 2007). One possible explanation for the lack of robust regeneration is that among all the fully viable Nogo mutant lines analyzed at the time, all still express Nogo-C, which contains the Nogo-66 inhibitory domain. To address this, we generated and analyzed a fully viable Nogo null mutant that lacks all known Nogo isoforms (Lee, et al., 2009b). However, the corticospinal axons in this mutant still failed to display enhanced regeneration. In addition to analyzing the Nogo null mutant, we also re-analyzed the Nogo-A,B gene trap mutant, but could not reproduce the robust axon regeneration reported previously (Kim, et al., 2003; Lee, et al., 2009b).
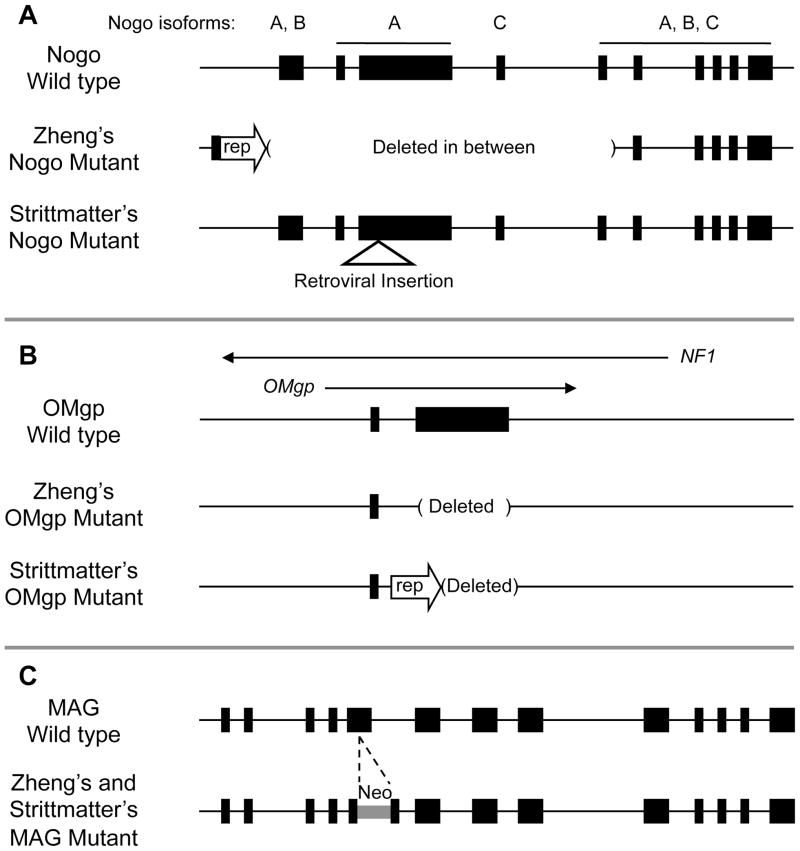
The results of the exhaustive analysis of Nogo mutant mice as well as of MAG and OMgp mutant mice question whether any of these three inhibitors by themselves is a significant inhibitor of axon regeneration. Therefore an attractive hypothesis was that all three molecules may need to be blocked in order to promote significant axon regeneration. To test this, both the Strittmatter group and our group generated triple knockout mice that are deficient in Nogo, MAG and OMgp, however, with some potentially important differences between the two triple knockout lines (Cafferty, et al., 2010; Lee, et al., 2010b) (Figure 1). Both triple knockouts contain the same MAG mutation (Li, et al., 1994) (available from the Jackson Laboratory), but differ in the Nogo and OMgp mutations. Strittmatter’s Nogo mutant allele is their previously characterized Nogo-A,B gene trap mutant, where a 309 amino acid N-terminal fragment of Nogo-A may still be expressed (Kim, et al., 2003). Our Nogo mutant allele is the more recently described fully viable Nogo null mutant that lacks the expression of all known Nogo isoforms (Lee, et al., 2009b). Strittmatter’s OMgp mutant allele is a previously described OMgp mutant by Ji et al (2008) where a fusion eGFP-Neo reporter/selection cassette is inserted at the initiating ATG codon. The OMgp mutant used in our study also has all the coding sequence deleted, with the difference that no reporter/selection cassette was left behind in order to minimize any effect on the expression of the NF1 gene, in which OMgp resides. Finally, while Strittmatter’s triple mutant was backcrossed into a much more pure C57BL/6 background, our triple mutant was analyzed in a mixed background containing C57BL/6 and 129S7 strains.
Figure 1.
Comparisons of Nogo, MAG and OMgp mutant alleles used in the two triple knockout studies. A) Our Nogo targeting strategy utilized Cre-loxP technology to delete a region between the N- and C-terminus, resulting in a null allele that lacks all three Nogo isoforms. Strittmatter’s Nogo mutant allele was the result of a gene trap strategy using a retroviral insertion targeting the Nogo-A isoform, which also affected Nogo-B expression to result in a Nogo-A,B knockout mouse. B) Our OMgp targeting strategy utilized loxP and frt sites to delete both the entire coding sequence as well as the reporter/selection cassette. Strittmatter’s OMgp mutant allele similarly has the coding sequence deleted, but retaining the reporter gene (rep). C) Both labs used the same MAG mutation generated by Roder and colleagues where a neomycin coding sequence is inserted into the fifth exon to disrupt MAG expression.
Using Nogo-A,B gene trap mutants as a control group, Strittmatter and colleagues again reported significant corticospinal axon regeneration after a dorsal hemisection model of spinal cord injury (as illustrated in Figure 2A) (Cafferty, et al., 2010). When combined with MAG and OMgp knockouts, the corticospinal axon regeneration as well as locomotor recovery observed in the Nogo mutant was enhanced even further, indicating a synergistic effect of deleting all three inhibitors. Meanwhile, MAG and OMgp double knockout mice did not display significant axon regeneration or locomotor improvements, indicating that of the three inhibitory molecules, Nogo is the primary inhibitor.
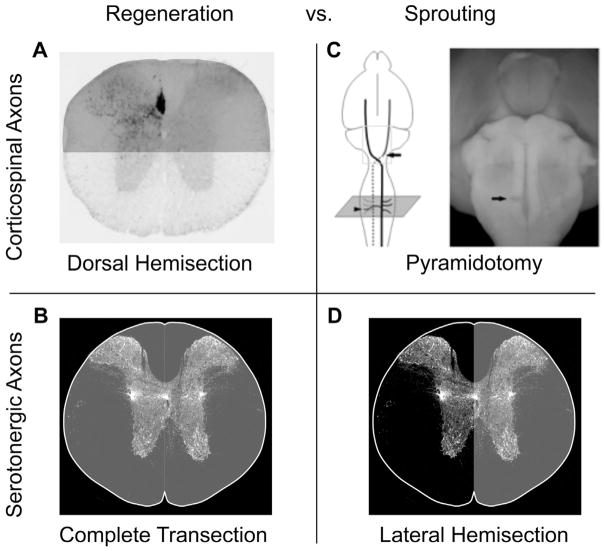
Figure 2.
Different injury models to assess axon regeneration and sprouting. A) To investigate regeneration of corticospinal tract (CST) axons, we use a mid-thoracic dorsal hemisection model of spinal cord injury that disrupts virtually all CST input into the caudal spinal cord. B) To investigate regeneration of serotonergic axon, we used a mid-thoracic complete transection model, which is necessary to cut serotonergic axons that are located throughout the spinal cord. C) To investigate sprouting of CST axons, we used a pyramidotomy model where descending CST axons are lesioned at the brainstem level before the decussation. The contralateral intact CST axons are traced and then detected at the level of the cervical spinal cord to assess sprouting across the midline. D) To investigate serotonergic axon sprouting, we used a mid-thoracic lateral hemisection, which lesions one side of the spinal cord. Serotonergic axon sprouting from the intact side is then detected immunohistochemically at the level of the lumbar spinal cord (i.e. below the level of injury). Areas shaded in gray in (A, B, D) were injured.
In contrast, using a similar dorsal hemisection model of spinal cord injury, we did not find any significant enhancement in corticospinal axon regeneration or locomotor improvement in either the triple mutant or any of the single mutant mice (Lee, et al., 2010b and unpublished results). Although the two studies agreed that both MAG and OMgp deletions are insufficient to promote axon regeneration, the effect of deleting Nogo and its synergistic relationship with MAG and OMgp in axon regeneration are the main points of disagreement. At the moment, the discrepancy on the CST regeneration phenotype in the Nogo-A,B gene tap mutants is most likely attributable to differences in the experimental manipulations and analyses since the same mutant gave different results when analyzed by different groups (Kim, et al., 2003; Lee, et al., 2009b). Strittmatter and colleagues also reported enhanced serotonergic axon regeneration in the triple mutant mice, where they used the same dorsal hemisection model to assess both corticospinal and serotonergic axon regeneration. Unlike corticospinal axons, however, serotonergic axons are not restricted to specific descending pathways, and a dorsal hemisection will inevitably lead to significant sparing of these axons. Thus, with our definition of regeneration vs. sprouting discussed above, it would have been impossible to distinguish regeneration from sprouting using this experimental paradigm.
Instead, we used a complete transection (Figure 2B) to assess serotonergic axon regeneration since this was the only way to ensure the complete severing of all serotonergic axons. This analysis indicated that Nogo/MAG/OMgp triple knockout mice do not display enhanced regeneration of serotonergic axons through a complete transection, a model where there is no axon sparing when performed properly. We did, however, analyze serotonergic axon growth in a partial injury model, using lateral hemisection instead of dorsal hemisection. Because this model intentionally spared serotonergic axons from one side of the cord, we reasoned that any enhanced axon growth caudal to the injury site could best be characterized as sprouting but not regeneration. Therefore, the overall observations from the two studies were similar: there was an increased amount of serotonergic axons detected caudal to the injury in a partial injury model that spares a significant portion of serotonergic axons in the triple mutants (see below). The main difference lies in the interpretation of the data: we characterized this increased growth as sprouting and the other group as regeneration. Details regarding which specific inhibitor(s) contributes more also differ (see below).
An alternative strategy to blocking Nogo, MAG and OMgp simultaneously is to target their common promiscuous receptor. Before the discovery of other NgR1 homologues and PirB, targeting NgR1 seemed the most attractive way of blocking myelin inhibition. Strittmatter and colleagues have taken multiple approaches to test this hypothesis. Administration of a peptide antagonist blocking Nogo/NgR1 interaction, NEP1-40, promoted CST regeneration (GrandPre, et al., 2002; Li and Strittmatter, 2003). However, it was not clear if this robust effect is reproducible (Nakamura, et al., 2011; Steward, et al., 2008). Furthermore, both the Strittmatter group and our group observed a lack of CST regeneration in NgR1 null mice (Kim, et al., 2004; Zheng, et al., 2005). Similarly, PirB knockout mice do not display enhanced CST axon regeneration (Nakamura, et al., 2011). In fact, even administering NEP1-40 in PirB knockout mice failed to promote CST regeneration (Nakamura, et al., 2011), which indicates that targeting both NgR1 and PirB is insufficient to promote axon regeneration. In addition, mice deficient in p75NTR, an NgR1 co-receptor, also do not exhibit enhanced CST regeneration (Song, et al., 2004; Zheng, et al., 2005).
Regarding serotonergic axons, Strittmatter group reported enhanced regeneration through a complete transection in their mutant (Kim, et al., 2004), while we failed to detect enhanced serotonergic axon regeneration in our NgR1 mutant using the same lesion model (Lee, et al., 2010a). Even when NgR1 was deleted along with MAG and Nogo-A,B, we did not detect enhanced serotonergic axon regeneration through a complete transection injury (Lee, et al., 2010a). While the lack of CST regeneration in NgR1 mutants may reflect the presence of other receptors such as NgR2 and PirB, the discrepant results on serotonergic axon regeneration in NgR1 mutants (along with the discrepant results on CST regeneration in the Nogo-A,B gene trap mutant, see above) indicate that experimental manipulations and analyses account for at least some of these discrepancies. Future studies are required to address these discrepancies.
In addition to genetic and pharmacological approaches, others have utilized an immunological approach to investigate myelin-associated inhibitors. David and colleagues used spinal cord homogenate, purified myelin or Nogo-66/MAG to immunize mice and induce endogenous production of antibodies against proteins present in myelin and reported corticospinal axon regeneration after spinal cord injury (Huang, et al., 1999; Sicotte, et al., 2003). Interestingly, others have shown that immunization against a Nogo-A derived peptide elicits a neuroprotective effect through a T cell-mediated response (Hauben, et al., 2001). However, immunization with a peptide derived from MBP, not considered a myelin inhibitor, was at least as effective as the Nogo-A derived peptide in eliciting this neuroprotective effect (Hauben, et al., 2001). Because increased neuroprotection can give the appearance of increased axon regeneration if it enhances the survival of spared axons, future experiments need to distinguish between possible effects on regeneration vs. neuroprotection. It will also be interesting to see if immunization with peptides derived from non-inhibitory myelin protein may also promote axon regeneration or sprouting.
Sprouting after spinal cord injury
Here we define sprouting as the growth of uninjured axons in response to an injury elsewhere in the CNS. After spinal cord injury, compensatory sprouting occurs spontaneously in response to synaptic loss as a result of injury to axons that normally innervate the region of interest. For example, after a mid-thoracic spinal cord injury, spared descending axons such as serotonergic axons as well as local sensory afferents can sprout in the denervated distal lumbar region (Ondarza, et al., 2003; Saruhashi, et al., 1996). This is in contrast to axon regeneration that refers to re-growth of axons that have been actually injured. Although the difference between the two terms seem very clear and logical, they are often difficult to distinguish using a single experimental injury paradigm. For example, while a dorsal hemisection of the spinal cord is commonly used to assess corticospinal axon regeneration, it may not be the best model to detect sprouting of corticospinal axons.
Therefore, in order to distinguish between axon sprouting and axon regeneration, we used different injury models to assess the role of myelin-associated inhibitors in these different axon growth responses to an injury (Lee, et al., 2010b). As described above, the dorsal hemisection and complete transection models used to study corticospinal and serotonergic axon regeneration respectively did not reveal any significant regeneration in the triple or single knockout mice. However, the pyramidotomy and the lateral hemisection models used to study sprouting of corticospinal axons and serotonergic axons respectively revealed a more complex picture (Figure 2).
After a unilateral pyramidotomy (Figure 2C) in the Nogo knockout mice, the contralateral uninjured corticospinal axons showed enhanced sprouting across the midline in the cervical spinal cord (Lee, et al., 2010b). This was consistent with previous reports by Strittmatter and colleagues using a similar pyramidotomy model to show enhanced corticospinal axon sprouting in Nogo-A,B as well as NgR1 mutant mice (Cafferty and Strittmatter, 2006). In fact, Schwab and colleagues reported a while ago enhanced corticofugal and corticospinal axon sprouting after pyramidotomy with IN-1 treatment (Blochlinger, et al., 2001; Thallmair, et al., 1998; Z’Graggen, et al., 1998). However, because regeneration is a much more sought-after goal, these data have not received the same attention as the data reporting regeneration.
In our genetic study, while OMgp mutants did not show enhanced corticospinal axon sprouting, MAG mutants unexpectedly displayed less sprouting than wild-type controls (Lee, et al., 2010b). The triple knockout mice did not show enhanced corticospinal axon sprouting, consistent with the opposing effects of deleting MAG and Nogo (Lee, et al., 2010b). Since this effect of MAG was not attributed to any developmental defects, it suggests that MAG may be required for compensatory sprouting of corticospinal axons. Although the mechanism is unclear, it may be related to MAG’s role in axon stabilization and/or its recently reported role in axon protection (Lopez, et al., 2011; Mehta, et al., 2010; Nguyen, et al., 2009). An alternative explanation is that MAG may have a dual role on axon growth in the adult just as for developing axons. Indeed, a previous in vitro study suggests that MAG (as well as Nogo) can be growth inhibitory or promoting depending on the particular intracellular signaling pathways elicited (Hasegawa, et al., 2004). Whether this is true in vivo remains to be explored. Regardless, the complex roles of MAG cautions against a therapeutic strategy that indiscriminately targets all myelin-associated inhibitors and further underscore the importance of a thorough understanding of different myelin-associated inhibitors.
In order to investigate sprouting of serotonergic fibers, we employed a mid-thoracic lateral hemisection (Figure 2D) that severs one side of the spinal cord to observe sprouting of intact serotonergic axons across the midline in the caudal lumbar spinal cord (Lee, et al., 2010b). After a lateral hemisection, MAG and OMgp single knockouts displayed enhanced sprouting of serotonergic axons, while Nogo single knockouts displayed an increasing trend that was not significant. Triple mutants showed enhanced sprouting that was similar in degree to MAG or OMgp single mutants, potentially reflecting a ceiling effect of modulating the extrinsic factors in eliciting sprouting. Interestingly, following a contusion injury in rats, Mountney et al (2010) demonstrated enhanced serotonergic axon sprouting after treatment with sialidase, an enzyme that hydrolyzes sialic acids and interferes with MAG-sialoglycan binding. As discussed above, Strittmatter and colleagues used a dorsal hemisection model to investigate a similar type of axon growth from spared serotonergic axons (although they used it as a model of regeneration). Furthermore, the contribution of individual molecules in their study differs from our results: they reported enhanced growth in Nogo knockout mice, but not in MAG/OMgp double knockout mice (Cafferty, et al., 2010). Using a more specific anti-Nogo-A antibody, Schwab and colleagues also reported sprouting of serotonergic axons after spinal cord injury (Maier, et al., 2009; Mullner, et al., 2008). Therefore, although there seems to be a general agreement that myelin-associated inhibitors can affect sprouting of serotonergic axons, more work is needed to clarify the contribution of individual molecules in this process.
Sprouting of corticospinal and serotonergic axons has also been previously reported using other experimental approaches to block myelin-associated inhibition. Using a soluble function-blocking NgR1 ectodomain that binds to Nogo, MAG and OMgp (Ji, et al., 2005; Li, et al., 2005; Li, et al., 2004; MacDermid, et al., 2004; Wang, et al., 2006), previous studies have demonstrated sprouting of various axonal tracts after spinal cord injury. Both positive (Cao, et al., 2008; GrandPre, et al., 2002; Li and Strittmatter, 2003) and negative (Nakamura, et al., 2011; Steward, et al., 2008) results were reported using the NgR1 competitive antagonist peptide NEP1-40. In addition to NgR1, targeting its co-receptor LINGO-1 by administration of a LINGO-1 antagonist (soluable LINGO-1-Fc) promoted sprouting of both rubrospinal and corticospinal axons (Ji, et al., 2006).
Myelin-associated inhibitors and synaptic plasticity
An intriguing clue on the physiological function of NgR1 and PirB came from studies of ocular dominance plasticity. In both NgR1 knockout mice (McGee, et al., 2005) and PirB knockout mice (Syken, et al., 2006), ocular dominance plasticity is extended into adulthood or enhanced at all ages. These results indicate a role for these two receptors in restricting experience-dependent plasticity, which may well be relevant to their proposed role in restricting axon growth after injury to the adult CNS. Perhaps not coincidentally, chondroitin sulfate proteoglycans (CSPGs), another class of glia-derived axon growth inhibitors, also appear to restrict ocular dominance plasticity (Pizzorusso, et al., 2002). In fact, the similarity between the myelin inhibitor system and CSPGs does not stop here. In addition to axon regeneration, axon sprouting has also been discussed as a major mechanism by which neutralizing the CSPGs may lead to improved functional recovery (Bradbury and McMahon, 2006; Bradbury, et al., 2002). Genetically deleting one receptor for CSPGs, PTPσ, has limited effect on axon regeneration after spinal cord injury (Shen, et al., 2009) (for detailed discussions on CSPGs, see other review articles by Fawcett, Bradbury, Silver and their colleagues in this issue).
Recent studies by Giger and others suggest that Nogo and OMgp may also be involved in synaptic plasticity. Neutralizing Nogo or NgR1 pharmacologically or genetically enhances long-term potentiation (Delekate, et al., 2011; Lee, et al., 2008) and attenuates long-term depression (Lee, et al., 2008) without changes in basal synaptic transmission. When Nogo-66 or OMgp is added to acute hippocampal slices, it suppresses LTP in an NgR1-dependent manner (Raiker, et al., 2010). Therefore, activation of NgR1 seems to reduce synaptic strength, while loss of NgR1 seems to have the opposite effect. Together with a role of NgR1 and PirB in ocular dominance plasticity discussed above (McGee, et al., 2005; Syken, et al., 2006), these results indicate that myelin inhibition may have a role in both injury-induced axonal growth and activity-dependent synaptic plasticity.
If the effect of NgR1 on synaptic strength is common to other types of synapses, it raises interesting possibilities about previous studies targeting NgR1 after spinal cord injury. Enhanced synaptic strength due to loss of NgR1 may facilitate behavioral recovery due to either affecting the actual neural circuit of the behavior or affecting the learning and/or extinction of the behavior (Jorntell and Hansel, 2006). Therefore, improved behavioral recovery by blocking NgR1 may be a result of enhanced synaptic strength rather than axonal growth. Future studies will need to take this into consideration when targeting NgR1 after spinal cord injury.
Concluding remarks
Different laboratories have utilized various approaches to test whether blocking myelin-associated inhibitors can promote axon regeneration. There seems to be a general consensus that myelin-associated inhibitors, namely Nogo, MAG and OMgp, do in fact inhibit axonal growth. The real division seems to occur when classifying the type of axonal growth that is affected by the experimental manipulations (i.e. axon regeneration vs. sprouting). A major part of this reason could be due to how different laboratories define axon regeneration and sprouting as discussed above. In this regard, it is important to consider what injury models are used to investigate regeneration vs. sprouting.
While no single model is perfect, some are better than others to detect regeneration or sprouting. For example, when assessing CST axon regeneration, the dorsal hemisection lesion model is often used where both the main dorsal CST and the minor dorsolateral CST are severed. As long as these tracts are completely lesioned, the model works well in mice because the ventral corticospinal axons are rarely labeled using traditional anterograde tracing methods. However, it becomes more of a problem in rats where similar tracing techniques often robustly label the ventral fibers that would be spared in the dorsal hemisection model. Therefore, this must be taken into consideration for proper interpretation of the results. In the end, the choice of the model is likely to be a compromise between severing as many axons as reasonably possible in a given axonal tract/population and leaving enough tissue bridge for axons to regenerate through. In this regard, complete transection may be used to sever all axons in the spinal cord, but it also creates an environment that is minimally conducive to any axon regeneration. Final proof of axon regeneration may have to come from studies with in vivo imaging (Davalos, et al., 2008; Dray, et al., 2009; Kerschensteiner, et al., 2005).
Until there is a clear consensus on the definition of axon regeneration, it will be difficult to reach a consensus whether or not blocking Nogo, for example, can promote axon regeneration. One could make an argument that whether it is axon regeneration or sprouting, what is really important is that the experimental treatment results in any axonal growth that leads to behavioral recovery. This would certainly be the case for patients looking to get out of a wheelchair and walk on their own. However, if certain treatments were more likely to promote sprouting (growth of uninjured axons or injured axons over short distance) rather than regeneration (growth of injured axons over a long distance), it would be more appropriate to use it as a treatment for patients with incomplete spinal cord injury. Treating patients with complete spinal cord injury with a treatment that is likely to only promote sprouting may result in false-negatives that could delay potential treatments for patients with incomplete spinal cord injury. In other words, understanding the effect of targeting these molecules on mechanistically different forms of axonal growth has important bearing on the development of therapeutic strategies.
In addition to the clinical importance of distinguishing between sprouting and regeneration, there is also a biological importance in these two mechanistically different forms of axonal growth. In order for axons to grow, they must first detect the perturbation. In the case of an injured axon, the injury itself may be the signal that is needed to initiate the growth process. However, this detection system must be inherently different in uninjured axons that remain mostly unperturbed but yet must be able to detect an injury elsewhere and initiate the growth cascade. In our genetic study using different injury models to study sprouting vs. regeneration, we observed that deleting Nogo, MAG and/or OMgp may promote the growth of uninjured axons (i.e. sprouting) but not the growth of injured axons (i.e. regeneration). This indicates that, unintuitively, the growth response of uninjured axons to a CNS injury is more sensitive to the deletion of myelin inhibitors than that of injured axons. Why would injured and uninjured axons respond differentially to reduced growth inhibition in the CNS environment? Early studies using retinal ganglion neurons indicated that CNS neurons undergo a developmental downregulation of neuron-intrinsic axon growth ability (Goldberg, et al., 2002). He and colleagues showed that upon injury, axotomized mature neurons lose their intrinsic ability to grow that is already reduced compared to developing neurons, and overcoming this loss through PTEN (phosphatase and tensin homolog) deletion can promote axon regeneration in both the optic nerve and the CST (Liu, et al., 2010; Park, et al., 2008). Perhaps uninjured neurons still possess a baseline level of intrinsic growth ability while injured neurons have almost completely lost their intrinsic growth ability, and thus the former but not the latter can still respond to reduced growth inhibition. Based on these studies, it would be interesting to investigate whether maintaining or enhancing the intrinsic growth capacity of injured neurons after spinal cord injury could unmask the full potential of removing myelin-associated inhibitors. Interestingly, a previous study on optic nerve regeneration indicates that expressing a dominant negative form of NgR1 has a synergistic effect with activating an intrinsic growth program in the retinal ganglion neurons (Fischer, et al., 2004).
With these and any other types of regeneration-promoting manipulations, it is important to also consider the possibility of neuroprotection. Surgical lesions as well as contusion injuries will undoubtedly lead to secondary damage of surrounding tissue and it is possible that treatments intended to promote axon growth may (in addition or instead) have neuroprotective effects that results in increased preservation of surrounding tissue. This neuroprotection can be misinterpreted as axon regeneration. A simple but effective solution is to choose an earlier time point and demonstrate a similar phenotype between the control and experimental groups. This time course study will also prove that axon sparing is not contributing to any potential axon regeneration.
In summary, there is an agreement that Nogo, MAG and OMgp are inhibitory molecules that can modulate axonal growth after an injury in the adult mammalian CNS. However, the type of axonal growth that can be elicited when targeting these molecules, the degree of such axonal growth in comparison with manipulating intrinsic and other extrinsic factors, the exact contribution of these molecules individually or synergistically to specific axonal populations remains to be better understood. Inducible gene deletion using conditional knockout technology will be an extremely valuable approach in further dissecting the role of these molecules in axonal plasticity in the adult CNS. Not only is such understanding important to understanding the biology of limited axonal growth after injury, it is essential for the design of rational and effective therapeutic strategy to treat spinal cord injury and related neurological conditions.
Acknowledgments
Supported by grants from the Roman Reed Research Fund, International Spinal Research Trust, Christopher and Dana Reeve Foundation, the Dana Foundation and NIH/NINDS (R01NS054734) to B.Z. Our work on Nogo and OMgp knockout mice were initiated in Marc Tessier-Lavigne’s lab. J.K.L. was supported by an NRSA Postdoctoral Fellowship (F32NS056697).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. Post-injury myelin-breakdown products inhibit axonal growth: an hypothesis to explain the failure of axonal regeneration in the mammalian central nervous system. Bibl Anat. 1982:1–11. [PubMed] [Google Scholar]
- Blochlinger S, Weinmann O, Schwab ME, Thallmair M. Neuronal plasticity and formation of new synaptic contacts follow pyramidal lesions and neutralization of Nogo-A: a light and electron microscopic study in the pontine nuclei of adult rats. J Comp Neurol. 2001;433:426–436. doi: 10.1002/cne.1150. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Buss A, Schwab ME. Sequential loss of myelin proteins during Wallerian degeneration in the rat spinal cord. Glia. 2003;42:424–432. doi: 10.1002/glia.10220. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Shumsky JS, Sabol MA, Kushner RA, Strittmatter S, Hamers FP, Lee DH, Rabacchi SA, Murray M. Nogo-66 receptor antagonist peptide (NEP1–40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil Neural Repair. 2008;22:262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988a;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988b;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KJ, Susuki K, Dours-Zimmermann MT, Zimmermann DR, Rasband MN. Oligodendrocyte myelin glycoprotein does not influence node of ranvier structure or assembly. J Neurosci. 2010;30:14476–14481. doi: 10.1523/JNEUROSCI.1698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Lee JK, Smith WB, Brinkman B, Ellisman MH, Zheng B, Akassoglou K. Stable in vivo imaging of densely populated glia, axons and blood vessels in the mouse spinal cord using two-photon microscopy. J Neurosci Methods. 2008;169:1–7. doi: 10.1016/j.jneumeth.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delekate A, Zagrebelsky M, Kramer S, Schwab ME, Korte M. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc Natl Acad Sci U S A. 2011;108:2569–2574. doi: 10.1073/pnas.1013322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Schnell L, Montani L, Duncan C, Simonen M, Schneider R, Liebscher T, Gullo M, Schwab ME. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Dray C, Rougon G, Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci U S A. 2009;106:9459–9464. doi: 10.1073/pnas.0900222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Dietz V, Schwab ME. Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res Brain Res Rev. 2001;36:204–212. doi: 10.1016/s0165-0173(01)00096-0. [DOI] [PubMed] [Google Scholar]
- Fouad K, Klusman I, Schwab ME. Regenerating corticospinal fibers in the Marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur J Neurosci. 2004;20:2479–2482. doi: 10.1111/j.1460-9568.2004.03716.x. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- Freund P, Wannier T, Schmidlin E, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J Comp Neurol. 2007;502:644–659. doi: 10.1002/cne.21321. [DOI] [PubMed] [Google Scholar]
- Gil V, Bichler Z, Lee JK, Seira O, Llorens F, Bribian A, Morales R, Claverol-Tinture E, Soriano E, Sumoy L, Zheng B, Del Rio JA. Developmental expression of the oligodendrocyte myelin glycoprotein in the mouse telencephalon. Cereb Cortex. 2010;20:1769–1779. doi: 10.1093/cercor/bhp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh EL, Young JK, Kuwako K, Tessier-Lavigne M, He Z, Griffin JW, Ming GL. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain. 2008;1:10. doi: 10.1186/1756-6606-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Habib AA, Marton LS, Allwardt B, Gulcher JR, Mikol DD, Hognason T, Chattopadhyay N, Stefansson K. Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J Neurochem. 1998;70:1704–1711. doi: 10.1046/j.1471-4159.1998.70041704.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Fujitani M, Hata K, Tohyama M, Yamagishi S, Yamashita T. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci. 2004;24:6826–6832. doi: 10.1523/JNEUROSCI.1856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Ibarra A, Mizrahi T, Barouch R, Agranov E, Schwartz M. Vaccination with a Nogo-A-derived peptide after incomplete spinal-cord injury promotes recovery via a T-cell-mediated neuroprotective response: comparison with other myelin antigens. Proc Natl Acad Sci U S A. 2001;98:15173–15178. doi: 10.1073/pnas.011585298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28:1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, McKerracher L, Braun PE, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, 3rd, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Prinjha RK, Campbell G, Anderson PN. Nogo-A expression in the intact and injured nervous system. Mol Cell Neurosci. 2003;24:1083–1102. doi: 10.1016/j.mcn.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Ji B, Case LC, Liu K, Shao Z, Lee X, Yang Z, Wang J, Tian T, Shulga-Morskaya S, Scott M, He Z, Relton JK, Mi S. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol Cell Neurosci. 2008;39:258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Li M, Budel S, Pepinsky RB, Walus L, Engber TM, Strittmatter SM, Relton JK. Effect of combined treatment with methylprednisolone and soluble Nogo-66 receptor after rat spinal cord injury. Eur J Neurosci. 2005;22:587–594. doi: 10.1111/j.1460-9568.2005.04241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Li M, Wu WT, Yick LW, Lee X, Shao Z, Wang J, So KF, McCoy JM, Pepinsky RB, Mi S, Relton JK. LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol Cell Neurosci. 2006;33:311–320. doi: 10.1016/j.mcn.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kokotilo KJ, Eng JJ, Curt A. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma. 2009;26:2113–2126. doi: 10.1089/neu.2008.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, Braun PE. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Case LC, Chan AF, Zhu Y, Tessier-Lavigne M, Zheng B. Generation of an OMgp allelic series in mice. Genesis. 2009a;47:751–756. doi: 10.1002/dvg.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Chan AF, Luu SM, Zhu Y, Ho C, Tessier-Lavigne M, Zheng B. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci. 2009b;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Chow R, Xie F, Chow SY, Tolentino KE, Zheng B. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J Neurosci. 2010a;30:10899–10904. doi: 10.1523/JNEUROSCI.2269-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010b;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature. 1994;369:747–750. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- Li S, Kim JE, Budel S, Hampton TG, Strittmatter SM. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, Worley D, Sah DW, Pepinsky B, Lee D, Relton J, Strittmatter SM. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PH, Ahmad AS, Mehta NR, Toner M, Rowland EA, Zhang J, Dore S, Schnaar RL. Myelin-associated glycoprotein protects neurons from excitotoxicity. J Neurochem. 2011;116:900–908. doi: 10.1111/j.1471-4159.2010.07069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermid VE, McPhail LT, Tsang B, Rosenthal A, Davies A, Ramer MS. A soluble Nogo receptor differentially affects plasticity of spinally projecting axons. Eur J Neurosci. 2004;20:2567–2579. doi: 10.1111/j.1460-9568.2004.03715.x. [DOI] [PubMed] [Google Scholar]
- Maier IC, Ichiyama RM, Courtine G, Schnell L, Lavrov I, Edgerton VR, Schwab ME. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132:1426–1440. doi: 10.1093/brain/awp085. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Mehta NR, Nguyen T, Bullen JW, Griffin JW, Schnaar RL. Myelin-associated glycoprotein (MAG) protects neurons from acute toxicity using a ganglioside-dependent mechanism. ACS Chem Neurosci. 2010;1:215–222. doi: 10.1021/cn900029p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol DD, Stefansson K. A phosphatidylinositol-linked peanut agglutinin-binding glycoprotein in central nervous system myelin and on oligodendrocytes. J Cell Biol. 1988;106:1273–1279. doi: 10.1083/jcb.106.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, Dusart I, Chedotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci U S A. 2010;107:11561–11566. doi: 10.1073/pnas.1006683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Mullner A, Gonzenbach RR, Weinmann O, Schnell L, Liebscher T, Schwab ME. Lamina-specific restoration of serotonergic projections after Nogo-A antibody treatment of spinal cord injury in rats. Eur J Neurosci. 2008;27:326–333. doi: 10.1111/j.1460-9568.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Fujita Y, Ueno M, Takai T, Yamashita T. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J Biol Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Mehta NR, Conant K, Kim KJ, Jones M, Calabresi PA, Melli G, Hoke A, Schnaar RL, Ming GL, Song H, Keswani SC, Griffin JW. Axonal protective effects of the myelin-associated glycoprotein. J Neurosci. 2009;29:630–637. doi: 10.1523/JNEUROSCI.5204-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Quarles RH. Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cell Mol Life Sci. 2002;59:1851–1871. doi: 10.1007/PL00012510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhashi Y, Young W, Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol. 1996;139:203–213. doi: 10.1006/exnr.1996.0094. [DOI] [PubMed] [Google Scholar]
- Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL. Brain gangliosides in axon-myelin stability and axon regeneration. FEBS Lett. 2010;584:1741–1747. doi: 10.1016/j.febslet.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci. 1993;5:1156–1171. doi: 10.1111/j.1460-9568.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicotte M, Tsatas O, Jeong SY, Cai CQ, He Z, David S. Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol Cell Neurosci. 2003;23:251–263. doi: 10.1016/s1044-7431(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Song XY, Zhong JH, Wang X, Zhou XF. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci. 2004;24:542–546. doi: 10.1523/JNEUROSCI.4281-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillmann AA, Bandtlow CE, Lottspeich F, Keller F, Schwab ME. Identification and characterization of a bovine neurite growth inhibitor (bNI-220) J Biol Chem. 1998;273:19283–19293. doi: 10.1074/jbc.273.30.19283. [DOI] [PubMed] [Google Scholar]
- Steward O, Sharp K, Yee KM, Hofstadter M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp Neurol. 2008;209:446–468. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Zheng B, Banos K, Yee KM. Response to: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187–199. Neuron. 2007;54:191–195. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Ho C, Anderson K, Tessier-Lavigne M. The dorsolateral corticospinal tract in mice: an alternative route for corticospinal input to caudal segments following dorsal column lesions. J Comp Neurol. 2004;472:463–477. doi: 10.1002/cne.20090. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology. 2005;115:433–440. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thallmair M, Metz GA, Z’Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskochil D, Cawthon R, O’Connell P, Xu GF, Stevens J, Culver M, Carey J, White R. The gene encoding the oligodendrocyte-myelin glycoprotein is embedded within the neurofibromatosis type 1 gene. Mol Cell Biol. 1991;11:906–912. doi: 10.1128/mcb.11.2.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Vourc’h P, Andres C. Oligodendrocyte myelin glycoprotein (OMgp): evolution, structure and function. Brain Res Brain Res Rev. 2004;45:115–124. doi: 10.1016/j.brainresrev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002c;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Z’Graggen WJ, Metz GA, Kartje GL, Thallmair M, Schwab ME. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zheng S, Wu H, Wu Y, Liu S, Fan M, Zhang J. Identification of BLyS (B lymphocyte stimulator), a non-myelin-associated protein, as a functional ligand for Nogo-66 receptor. J Neurosci. 2009;29:6348–6352. doi: 10.1523/JNEUROSCI.5040-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]