Abstract
The Hedgehog (Hh) family of secreted proteins controls many aspects of growth and patterning in animal development. In Drosophila, Hh acts by preventing the formation of a truncated repressor form of Cubitus interruptus (Ci) and stimulating the transcriptional activity of full-length Ci. Here, we provide evidence that Costal2 (Cos2) and Suppressor of Fused [Su(fu)] inhibit Ci by tethering it in the cytoplasm, whereas Hh induces nuclear translocaltion of Ci through Fused (Fu). We have identified a 125 amino acid domain in the C-terminal part of Ci that mediates response to Cos2 inhibition. We show that Cos2 binds Ci, prevents its nuclear import, and inhibits its activity via this domain. We also provide evidence that Su(fu) regulates Ci through two distinct mechanisms: (1) Su(fu) blocks Ci nuclear import through the N-terminal region of Ci , and (2) it inhibits the activity of Ci through a mechanism independent of Ci nuclear translocation. Finally, we show that Cos2 is required for transducing high levels of Hh signaling activity, and it does so by alleviating the blockage of Ci activity imposed by Su(fu).
Keywords: Ci, Cos2, Fu, Su(fu), Hh, nuclear translocation
The Hedgehog family of secreted proteins controls cell growth and patterning in many key developmental processes in both vertebrates and invertebrates (Ingham 1994). In Drosophila wing development, posterior (P) compartment cells express and secrete Hh proteins that diffuse into the anterior (A) compartment and induce neighboring A compartment cells to express decapentaplegic (dpp), which encodes a member of TGFβ/BMP family of secreted proteins (Basler and Struhl 1994; Tabata and Kornberg 1994). Dpp then diffuses into both A and P compartments and functions as a long-range morphogen to control the growth and patterning of cells in the whole wing (Lecuit et al. 1996; Nellen et al. 1996). Although the long-range organizing influence of Hh is mediated by Dpp, Hh functions as a local morphogen to specify patterns near the A/P compartment boundary by activating other genes, including patched (ptc) and engrailed (en), in a concentration dependent manner (Strigini and Cohen 1997). Low levels of Hh signaling activity suffice to activate dpp and ptc, whereas high levels of Hh are required to activate en.
Hh signal is transduced via Cubitus interruptus (Ci), a member of the Gli family of Zn finger transcription factors (Ruiz i Altaba 1997). In imaginal disc development, Ci plays dual roles that are performed by two distinct forms of Ci. In A compartment cells distant from the A/P compartment boundary, Ci is proteolytically cleaved to form a C-terminally truncated form (Ci75) (Aza-Blanc et al. 1997). Ci75 functions as a repressor and blocks the expression of Hh responsive genes such as dpp (Dominguez et al. 1996; Methot and Basler 1999). In A compartment cells near the A/P compartment boundary, Hh signaling prevents the cleavage of Ci and stimulates the activity of accumulated full-length Ci (Aza-Blanc et al. 1997; Methot and Basler 1999). The full-length form of Ci functions as a transcriptional activator and controls the expression of Hh target genes such as ptc and en (Alexandre et al. 1996; Dominguez et al. 1996; Methot and Basler 1999).
Proteolytic processing of Ci requires the activities of at least three components in the Hh pathway: protein kinase A (PKA); Slimb, an F-box/WD40 repeat containing protein; and Costal2 (Cos2), a kinesin-like protein (Jiang and Struhl 1998; Ohlmeyer and Kalderon 1998; Wang and Holmgren 1999). Loss of function in any of these three proteins leads to inhibition of Ci processing and accumulation of full-length Ci. PKA phosphorylates multiple sites in the C-terminal region of Ci, and phosphorylation of these sites is essential for processing Ci as well as for inhibiting the transcriptional activity of full-length Ci (Y. Chen et al. 1999; Price and Kalderon 1999; Wang et al. 1999). Slimb appears to act downstream from PKA to promote Ci processing (Wang et al. 1999). Recent studies suggest that the vertebrate homolog of Slimb, βTRCP, specifies the substrates of the so-called SCF (Skp1, Cdc53, and F-box) ubiquitin ligase complex. This complex targets phosphorylated substrates (such as IκB and β-catenin) for ubiquitination followed by proteasome-mediated proteolysis (Maniatis 1999). By analogy, it has been proposed that Slimb acts downstream from PKA to target phosphorylated Ci for ubiquitin/proteasome-mediated proteolysis (Jiang and Struhl 1998; Maniatis 1999). Consistent with this view, it has been recently shown that proteasome is required for processing Ci (C.H. Chen et al. 1999).
The mechanism by which Cos2 regulates Ci is still poorly understood. Although the constitutive Hh signaling activity in cos2 mutant cells can be attributed, at least in part, to the inhibition of Ci processing (Sisson et al. 1997; Wang and Holmgren 1999), the nearly identical phenotypes caused by loss of Cos2 and PKA function in Drosophila limb development imply that Cos2, like PKA, may also regulate the activity of full-length Ci. Cos2 and Ci form a multiprotein complex that also includes the Ser/Thr kinase Fused (Fu), and possibly, the PEST domain protein Su(fu) (Robbins et al. 1997; Sisson et al. 1997; Monnier et al. 1998). However, the biological significance of this complex formation remains to be determined. For example, it is not clear whether Ci/Cos2 complex formation is required for promoting Ci processing, or inhibiting the activity of full-length Ci, or both. Nor is it known whether Cos2 directly interacts with Ci or forms a complex with Ci through other proteins such as Fu and Su(fu).
To investigate the mechanisms by which Cos2 and Su(fu) regulate Hh signaling, we used both loss-of-function and gain-of-function approaches coupled with structure-functional analysis and cell culture experiments. We provide evidence that Cos2 and Su(fu) inhibit Ci by tethering it in the cytoplasm via complex formation. We identify a 125 amino acid (aa) domain in the C-terminal region of Ci that mediates inhibition of Ci transcriptional activity by Cos2. We show that Cos2 physically interacts with Ci and prevents its nuclear import through this domain. In addition, we provide evidence that Su(fu) also inhibits Ci activity through a mechanism independent of Ci nuclear translocation. Finally, we uncover a novel function for Cos2 in transducing high levels of Hh signal by antagonizing Su(fu).
Results
Cos2 inhibits Ci activity independent of Ci processing
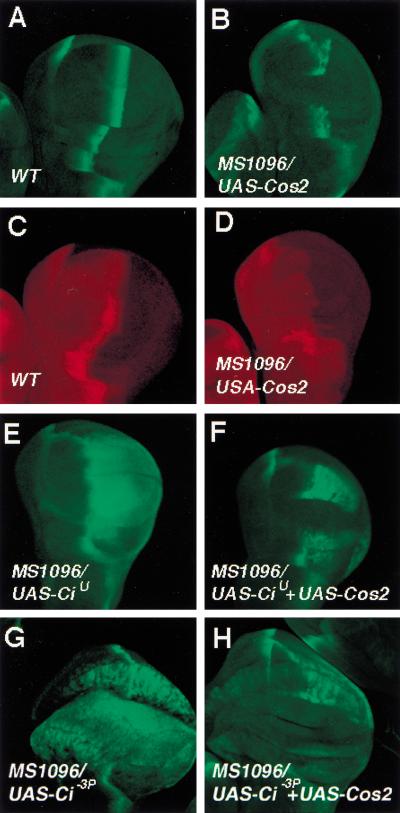
Loss of function mutations in cos2 result in accumulation of high levels of full-length Ci and activation of Hh target genes (Sisson et al. 1997; Wang and Holmgren 1999). However, it has not been possible to uncouple the roles of Cos2 in regulating Ci processing and activity by loss-of-function genetic analysis. To determine if Cos2 regulates Ci activity through a mechanism distinct from Ci processing, we overexpressed several uncleavable forms of Ci in conjunction with Cos2 in developing wing and assessed if the transcriptional activity of these uncleavable forms of Ci could be blocked by coexpressing Cos2. Two uncleavable forms of Ci were used: (1) CiU, which deletes the Ci cleavage site, and (2) Ci-3P, a full-length Ci that has three PKA phosphorylation sites mutated (Methot and Basler 1999; Wang et al. 1999). Both UAS-Cos2 and UAS-Ci transgenes were expressed using a wing disc specific Gal4 driver line MS1096, and the transcriptional activities of the uncleavable forms of Ci were monitored by the expression of a Hh responsive gene ptc-lacZ (Wang et al. 1999).
First, we examined the effect of overexpressing Cos2 on the activity of endogenous Ci. In wild-type wing discs, ptc-lacZ is expressed as a stripe in A compartment cells abutting the A/P compartment boundary in response to Hh (Fig. 1A). In wing discs expressing MS1096/UAS-Cos2, ptc-lacZ expression is disrupted in the wing-pouch region where the Cos2 transgene is expressed (Fig. 1B). In addition, the accumulation of full-length Ci near the A/P boundary is also abolished by overexpressing Cos2 (Fig. 1, cf. D with C), suggesting that excess amounts of Cos2 can override Hh signaling and down-regulate the level and activity of endogenous Ci. We next examined if overexpressing Cos2 could inhibit the activity of overexpressed CiU and Ci-3P. As shown previously, wing discs expressing MS1096/UAS-CiU ectopically express ptc-lacZ in P compartment cells (Fig. 1E; Methot and Basler 1999). However, the ectopic expression of ptc-lacZ induced by CiU is suppressed in the wing-pouch region by coexpressing Cos2 (Fig. 1F). Similarly, the ectopic expression of ptc-lacZ induced by overexpressing Ci-3P is also abolished when Cos2 is coexpressed (Fig. 1, cf. G with H). Unlike endogenous Ci, the levels of overexpressed CiU and Ci-3P are not significantly altered when Cos2 is co-expressed (data not shown). These results indicate that Cos2 inhibits the activity of full-length Ci independent of Ci processing.
Figure 1.
Cos2 inhibits the activity of uncleavable forms of Ci. Wing discs shown in this and following figures were derived from late third-instar larvae and are oriented with anterior toward the left and ventral up. Wing discs in A,B,E–H were stained with anti-βgal antibody to visualize ptc-lacZ expression (green). Wing discs in C and D were stained with anti-Ci antibody (2A1) that recognizes full-length Ci (red). The genotypes for the wing discs are as follows: (A,C) wild type; (B,D) MS1096, UAS-Cos2; (E) MS1096, UAS-CiU; (F) MS1096, UAS-CiU, UAS-Cos2; (G) MS1096, UAS-Ci-3P; (H) MS1096, UAS-Ci-3P, UAS-Cos2.
Cos2 and Hh regulate Ci nuclear translocation
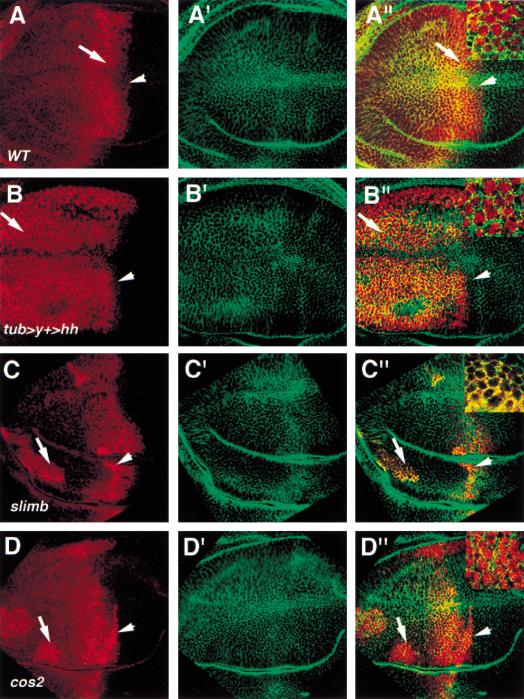
Previous studies revealed that full-length Ci accumulates in the cytoplasm in either cos2 mutant cells or Hh receiving cells during imaginal disc development (Aza-Blanc et al. 1997; Sisson et al. 1997). The lack of nuclear staining of Ci is mostly likely caused by the presence of a robust nuclear export mechanism that keeps the majority of Ci in the cytoplasm and the instability of the activator form of Ci in the nucleus (Ohlmeyer and Kalderon 1998; C.H. Chen et al. 1999). Indeed, cultured cells treated with Leptomycin B (LMB), a drug that blocks CRM1 dependent nuclear export, show low levels of Ci staining in the nucleus (Kudo et al. 1998; C.H. Chen et al. 1999). Therefore, we examined if nuclear Ci could be detected in imaginal discs treated with LMB. As shown in Figure 2A, A compartment cells near the compartment boundary accumulate full-length Ci in the nucleus after LMB treatment, whereas A compartment cells distant from the boundary retain Ci in the cytoplasm. To determine if the LMB dependent nuclear localization of Ci is induced by Hh, we misexpressed Hh indiscriminately throughout wing discs with the tub > y+ > hh transgene (Basler and Struhl 1994). As shown in Figure 2B, LMB-treated wing discs with hh uniformly expressed show nuclear staining of Ci in all A compartment cells. As Hh signaling stabilizes full-length Ci, the LMB-dependent nuclear translocation of Ci induced by ectopic hh expression could be attributed to an increase in the levels of full-length Ci. To rule out this possibility, we examined the subcellular localization of Ci in LMB-treated wing discs carrying slimb mutant clones in which Ci processing is blocked and full-length Ci accumulates to high levels (Jiang and Struhl 1998). As shown in Fig. 2C, slimb mutant cells distant from the boundary accumulate high levels of Ci in the cytoplasm, whereas mutant cells near the boundary accumulate Ci in the nucleus in response to LMB treatment. These results argue that preventing Ci processing is not sufficient to induce Ci nuclear translocation and that Hh stimulates Ci nuclear import in addition to inhibiting Ci processing.
Figure 2.
Hh and Cos2 have opposing effects on nuclear translocation of Ci. Wing discs in all panels were treated with LMB followed by immunostaining with anti-Ci antibody 2A1 (red) and anti-Armadillo (Arm) antibody (green). Arm shows membrane and cytoplasmic staining. Insets in A′′–D′′ show high magnification views of the regions indicated by corresponding arrows. (A, A′,A′′) A wild-type wing disc showing Ci (red in A and A′′) and Arm (green in A′ and A′′) expression. A compartment cells near the compartment boundary accumulate high levels of Ci in the nucleus (arrow in A; insets in A′′) whereas A compartment cells away from the compartment boundary retain Ci in the cytoplasm. Note that A compartment cells abutting the compartment boundary show low levels of Ci in the nucleus (arrowhead). (B, B′, B′′) A wing disc ectopically expressing tub > y+ > hh. The flip-out cassette was removed in most cells at early third instar by severe heat shock to induce high levels of FLP activity. A compartment cells ectopically expressing hh accumulate Ci in the nucleus regardless of their positions. (C, C′, C′′) A wing disc bearing slimb mutant clones, which are recognized by strong Ci staining (arrow and arrowhead). slimb mutant cells near the A/P compartment boundary accumulate Ci in the nucleus (arrowhead) whereas slimb mutant cells away from the boundary accumulate Ci in the cytoplasm (arrow; inset in C′′). (D, D′, D′′) A wing disc bearing cos2 mutant clones, which are indicated by strong Ci staining. cos2 mutant cells in A compartment accumulate Ci in the nucleus regardless of their positions (arrow and arrowhead; inset in D′′). Note that cos2 mutant cells abutting the A/P compartment boundary (arrowhead in D) show higher levels of nuclear Ci than wild-type cells in the same position (arrowhead in A).
We next examined if Cos2 is responsible for the cytoplasmic tethering of Ci in the absence of Hh signaling. If so, one prediction would be that loss of cos2 function should lead to constitutive nuclear import of Ci in response to LMB treatment. To test this, we generated cos2 mutant clones in imaginal discs followed by LMB treatment. As shown in Figure 2D, cos2 mutant cells situated in the A compartment accumulate Ci in the nucleus regardless of their positions relative to the A/P compartment boundary, suggesting that Cos2 prevents LMB dependent nuclear localization of Ci in the absence of Hh.
Mapping the Ci domain that mediates Cos2 inhibition in vivo
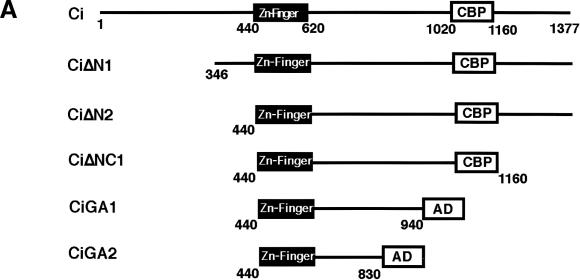
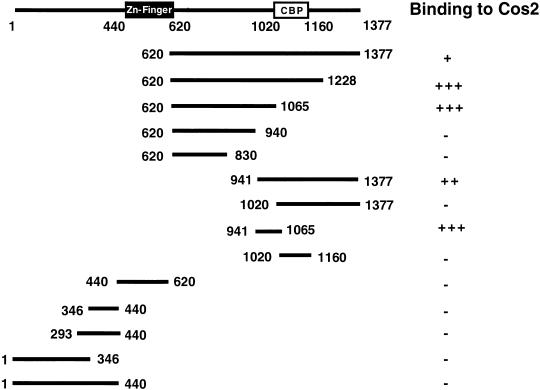
As shown above, the constitutive Hh signaling activity induced by overexpressing Ci can be blocked by coexpressing Cos2 (Fig.1E–H). This in vivo coexpression assay provided us an opportunity to define the Ci domain that mediates Cos2 repression. We reasoned that if Cos2 inhibits Ci by direct binding, then deletion of the putative Ci/Cos2 interacting domain should render the resulting Ci deletion mutants resistant to Cos2 repression. A list of Ci deletion mutants analyzed in this study is shown in Figure 3A.
Figure 3.
Regulation of the activity of Ci deletion mutants by Cos2. (A) A summary of UAS-Ci constructs analyzed by in vivo coexpression assay. Filled boxes indicate the Zn-finger DNA-binding domain (from aa 440 to aa 620), and open boxes indicate dCBP bind domain (CBP) or the Gal4 activation domain (AD). Numbers indicate the positions of amino acid residues where Ci deletion mutants end. Two copies of HA tag (not shown) are fused at the N terminus of each construct to allow monitoring the expression level. (B, B′, C, C′) Wing discs expressing MS1096/UAS-CiΔN2 (B) or MS1096/UAS-CiΔNC1 (C) show ectopic ptc-lacZ expression uniformly in the wing-pouch region. However, the ectopic ptc-lacZ expression is suppressed by coexpressing UAS-Cos2 (B′ and C′). (D, D′,E, E′) Wing discs expressing MS1096/UAS-CiGA1 (D) or MS1096/UAS-CiGA2 (E) show ectopic ptc-lacZ expression with higher levels in P compartment cells than in A compartment cells. The ectopic ptc-lacZ expression induced by CiGA1 and CiGA2 persists when UAS-Cos2 is coexpressed (D, E′).
CiΔN1 is an N-terminally truncated form that removes aa 1–346, a region previously shown to interact with Su(fu) (Monnier et al. 1998), whereas CiΔN2 lacks the entire region N-terminal to the Zn finger DNA-binding domain (Fig. 3A). Wing discs expressing MS1096/UAS-CiΔN1 or UAS-CiΔN2 ectopically express ptc-lacZ in a similar fashion in both A and P compartments (Fig. 3B; data not shown). However, the ectopic expression of ptc-lacZ induced by CiΔN1 or CiΔN2 is suppressed when Cos2 is coexpressed (Fig. 3B′; data not shown), suggesting that the N-terminal sequences are not required for Cos2 regulation. To determine if Cos2 regulates Ci via binding to a C-terminal domain, we next made a series of deletions from the C-terminal end of CiΔN2 (Fig. 3A). It has been previously shown that the Ci fragment containing aa 1020–1160 binds the Ci co-activator dCBP (Akimaru et al. 1997) and is required for Ci to transactivate Hh target genes (G. Wang and J. Jiang, unpubl.). To assess the activities of Ci deletion mutants that lack the dCBP-binding domain, we fused the Gal4 activation domain (GA) to their C-termini, as in the case for CiGA1 and CiGA2 (Fig. 3A). As shown in Figure 3, the ectopic ptc-lacZ expression induced by overexpressing CiΔNC1 is suppressed by coexpressing Cos2 (Fig. 3, cf. C′ with C). In contrast, CiGA1 and CiGA2 are resistant to Cos2 repression as the ectopic ptc-lacZ expression induced by these mutant forms of Ci are not significantly altered by coexpressing Cos2 (Fig. 3, cf. D′,E′ with D,E). These results suggest that the Ci region between aa 941 and aa 1160 mediates Cos2 inhibition of Ci activity.
Cos2 binds to a C-terminal domain of Ci
In parallel with in vivo functional mapping of the Cos2 interaction domain, we used yeast two hybrid to determine if Cos2 interacts directly with Ci and where in Ci it binds. We fused various parts of Ci to the Gal4 activation domain and tested if the resulting fusion proteins bind Cos2 in yeast. A list of CiGal4 fusion constructs are shown in Figure 4, CiGal4 fusion proteins containing Ci fragments aa 620–1377, aa 620–1228, aa 620–1065, aa 941–1377, and aa 941–1065 interact with Cos2, whereas Ci fragments containing aa 620–940, aa 620–830, aa 1020–1377, and aa 1020–1160 fail to bind Cos2 in yeast. The Zn finger DNA-binding domain of Ci (aa 440–620), as well as several N-terminal fragments including aa 1–346, aa 293–440, aa 346–440, and aa 1–440, all fail to interact with Cos2 in the yeast two hybrid assay. Thus, the C-terminal region of Ci from aa 941 to aa 1065 defines a Cos2 binding domain, which is deleted in Ci mutants that are insensitive to Cos2 inhibition (Fig. 3).
Figure 4.
Binding of Ci fragments to Cos2. Various Ci fragments were fused to the Gal4 activation domain and the fusion constructs were cotransformed into yeast with a bait construct containing full-length Cos2 fused to the LexA DNA-binding domain. Relative strength of binding was measured by a liquid β-galactosidase assay.
Cos2 inhibits Ci nuclear import via the C-terminal domain of Ci
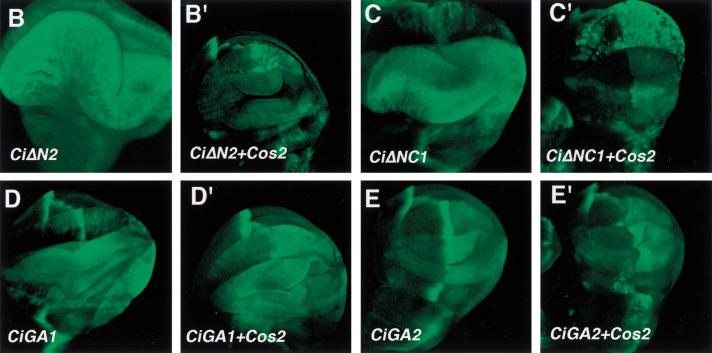
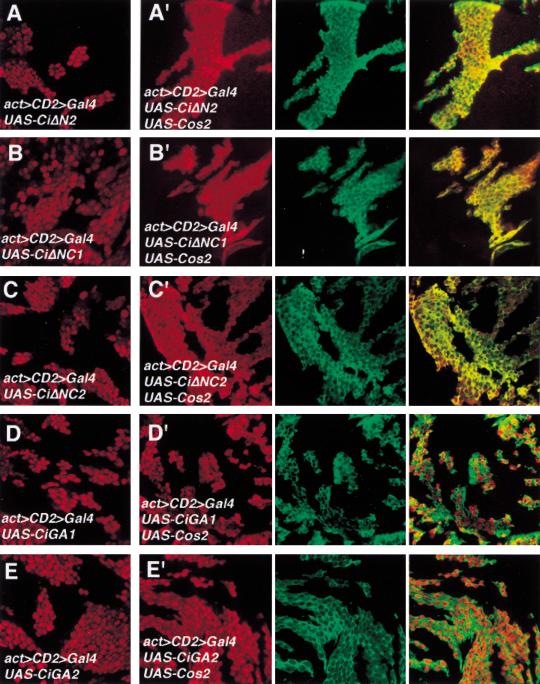
To determine if the Cos2 interacting domain defined above mediate cytoplasmic retention by Cos2, we overexpressed various Ci deletion mutants in imaginal discs and examined if their nuclear translocation was blocked by coexpressing Cos2. We used the actin > CD2 > Gal4 driver line to generate clones of cell expressing Ci deletion mutants either alone or in conjunction with Cos2 (Pignoni et al. 1997). We tested two truncated forms of Ci (CiΔN2 and CiΔNC1) whose activity can be suppressed by Cos2 and two forms (CiGA1 and CiGA2) that are resistant to Cos2 inhibition. As shown in Figure 5, CiΔN2 and CiΔNC1 are localized predominantly in the nucleus after LMB treatment (Fig. 5A,B). However, these Ci mutants are retained significantly in the cytoplasm when Cos2 is coexpressed (Fig. 5A′,B′). In contrast, CiGA1 and CiGA2 are localized largely in the nucleus regardless of whether Cos2 is coexpressed or not (Fig. 5D,D′,E,E′). We tested an additional Ci deletion mutant, CiΔNC2, that retains Ci sequence from aa 440 to aa 1065. In the absence of Cos2 overexpression, CiΔNC2 translocates into the nucleus on LMB treatment (Fig. 5C). However, when Cos2 is coexpressed, CiΔNC2 is sequestered largely in the cytoplasm (Fig. 5C′). We conclude from these results that the Ci sequence from aa 941 to aa 1065, which corresponds to the minimal Ci domain that binds Cos2, mediates cytoplasmic retention by Cos2. We refer to this 125 amino acid domain as CORD (Cos2 responsive domain).
Figure 5.
Regulating nuclear translocation of Ci deletion mutants by Cos2. Wing discs carrying clones of cells expressing Ci deletion mutants either alone (A–E) or in conjunction with UAS-Cos2 (A′–E′) were treated with LMB followed by immunostaining with anti-Ci antibody (red) and anti-Flag antibody (green). The Cos2 transgene is tagged by Flag epitope. Ci and Cos2 transgenes were expressed using the actin > CD2 > Gal4 driver line. All Ci deletion mutants accumulate in the nucleus after LMB treatment (A–E). However, CiΔN2, CiΔNC1, CiΔNC2 are retained significantly in the cytoplasm (A′–C′) whereas CiGA1 and CiGA2 remain primarily in the nucleus when the UAS-Cos2 transgene is coexpressed in the same cells (D′–E′).
CORD is sufficient to mediate cytoplasmic retention by Cos2
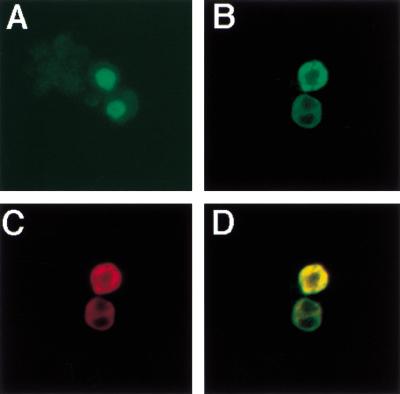
To determine if CORD is sufficient to render a heterologous protein under the control of Cos2, we fused aa 941–1065 of Ci in frame with a nuclear form of GFP (GFPNLS) to generate GFPNLS-CORD. cl-8 cells were used to express GFPNLS-CORD either alone or in conjunction with Cos2 by transfection. cl-8 cells transfected with GFPNLS-CORD expressing construct alone accumulate GFPNLS-CORD in the nucleus (Fig. 6A). However, cl-8 cells transfected with both GFPNLS-CORD and Cos2 expressing constructs retain Ci in the cytoplasm (Fig. 6B–D).
Figure 6.
CORD is sufficient to mediate cytoplasmic retention by Cos2. cl-8 cells were transfected with GFPNLS-CORD expressing construct either alone (A) or in conjunction with HA-Cos2 expressing construct (B–D). GFPNLS-CORD expression is shown in green (A,B,D) and HA-Cos2 in red (C,D). In the absence of HA-Cos2, GFPNLS-CORD accumulates in the nucleus (A). When HA-Cos2 is coexpressed, GFPNLS-CORD is sequestered in the cytoplasm and is colocalized with HA-Cos2 (B–D).
Su(fu) inhibits nuclear translocation of Ci
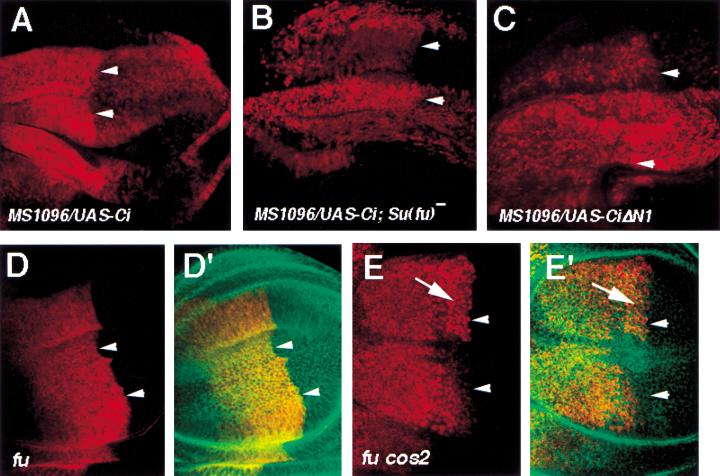
Genetic studies have suggested that Su(fu) inhibits Hh signaling activity by blocking the maturation of Ci into a labile nuclear activator (Ohlmeyer and Kalderon 1998). However, it is not clear whether Su(fu) inhibits Ci by blocking its nuclear import or by affecting its activity once in the nucleus. It has not been possible to assess the effect of Su(fu) mutation on the nuclear import of endogenous Ci because Ci is unstable in Su(fu) mutant background (Ohlmeyer and Kalderon 1998). Although the mechanism by which Ci is degraded in Su(fu) mutant cells remains to be determined, we speculate that such a mechanism could be saturated by excess amounts of Ci derived from overexpression. Therefore, we examined the effect of Su(fu) mutation on nuclear import of Ci in wing discs expressing MS1096/UAS-Ci. As shown in Figure 7, LMB-treated wing discs expressing MS1096/UAS-Ci show both cytoplasmic and nuclear staining of Ci, with A compartment cells accumulating Ci largely in the cytoplasm and P compartment cells in the nucleus (Fig. 7A). In contrast, LMB-treated Su(fu) mutant discs expressing the same Ci transgene accumulate Ci predominantly in the nucleus in both A and P compartment cells (Fig. 7B). Thus, the LMB-dependent nuclear translocation of full-length Ci is blocked by endogenous Su(fu) in the A compartment, and this block is alleviated by Hh signaling in the P compartment.
Figure 7.
Regulation of Ci nuclear translocation by Su(fu) and Fu. All discs in this figure were treated with LMB followed by immunostaining with anti-Ci antibody, 2A1(red in A–E), and anti-Arm antibody (green in D′,E′). Arrowheads in each panel indicate the A/P compartment boundary. (A) A wing disc expressing MS1096/UAS-Ci. A compartment cells contain cytoplasmic Ci at higher levels than nuclear Ci. In contrast, P compartment cells accumulate higher levels of Ci in the nucleus than in the cytoplasm. (B) A Su(fu) mutant wing disc expressing MS1096/UAS-Ci accumulates Ci largely in the nucleus in both A and P compartment cells. (C) A wing disc expressing MS1096/UAS-CiΔN1. CiΔN1 accumulates primarily in the nucleus in both A and P compartments. (D,D′) A fu mutant wing disc. A compartment cells near the A/P compartment boundary retain significant amounts of Ci in the cytoplasm. (E,E′) A wing disc carrying clones of fu cos2 double mutant cells. fu cos2 double mutant cells are recognized by high levels of Ci staining (arrow). fu cos2 mutant cells near the A/P compartment retain little if any Ci in the cytoplasm.
It has been shown previously that Su(fu) binds the N-terminal region of Ci between aa 1 and aa 346 (Monnier et al. 1998). If Su(fu) blocks Ci nuclear import by direct binding, deleting the Su(fu)-binding domain should have the same effect on Ci nuclear import as Su(fu) mutation. To test this, we expressed the Ci deletion mutant, CiΔN1, that lacks the Su(fu)-binding domain in wing discs followed by LMB treatment. In contrast to full-length Ci, CiΔN1 is largely localized in the nucleus in A compartment cells (Fig. 7C). Thus, removal of Su(fu) binding domain from Ci phenocopies Su(fu) mutation.
Fu promotes nuclear translocation of Ci
Fu kinase physically interacts with Cos2 and Su(fu) and positively regulates Hh signal transduction (Robbins et al. 1997; Sisson et al. 1997; Monnier et al. 1998). One possible mechanism by which Fu facilitates Hh signaling is to antagonize Su(fu) and Cos2, thereby permitting Ci nuclear import. To test this, we examined the subcellular distribution of Ci in fu mutant wing discs treated with LMB. As shown in Figure 7D, fu mutant cells situated in the A compartment near the compartment boundary retain significant amounts of Ci in the cytoplasm even though they receive Hh signal (compared with Fig. 2A). To determine if Cos2 is responsible for cytoplasmic retention of Ci in fu mutant cells, we generated fu cos2 double mutant clones in wing discs followed by LMB treatment. As shown in Figure 7E, fu cos2 double mutant cells situated in the A compartment near the compartment boundary accumulate Ci predominantly in the nucleus, as in the case for cos2 singly mutant cells (compared with Fig. 2D).
Cos2 is required for transducing high levels of Hh signaling activity
In the course of analyzing cos2 mutant phenotypes, we noticed that cos2 mutant cells abutting the A/P compartment boundary accumulate Ci at levels much higher than wild-type cells at the same location (Fig. 8B), a phenotype also observed for fu mutation (Ohlmeyer and Kalderon 1998). To assess if cos2 mutation attenuates Hh signaling activity in these cells, we examined en expression, which is activated by high levels of Hh (Strigini and Cohen 1997). As shown in Figure 8B, cos2 mutant cells abutting the A/P compartment boundary express diminished levels of en, indicating that these cells fail to transduce high levels of Hh signaling activity. Interestingly, we find that cos2 mutant clones derived from the A compartment often cross the compartment boundary (Fig. 8C). As the property of A compartment cells to respect the A/P compartment restriction is induced by Hh signaling (Rodriguez and Basler 1997; Dahmann and Basler 2000), the failure of cos2 mutant clones to obey the A/P compartment restriction further supports that Hh signaling activity is compromised in cos2 mutant cells.
Figure 8.
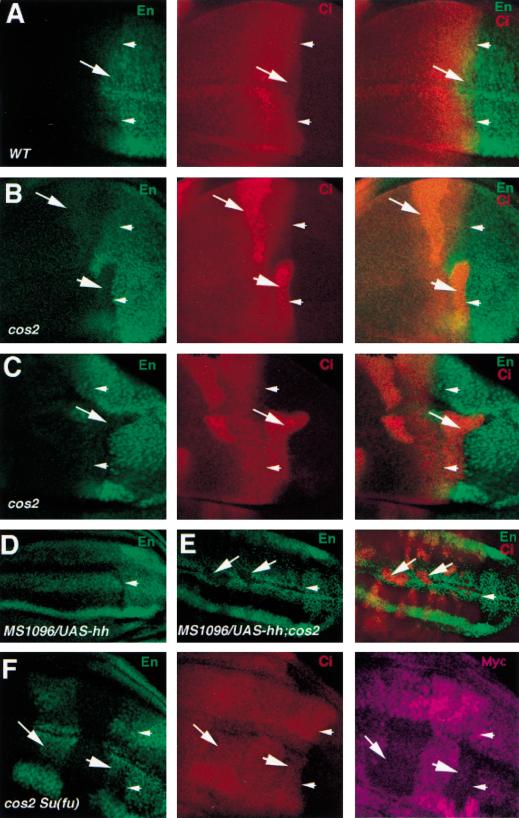
Cos2 transduces high levels of Hh signaling activity by antagonizing Su(fu). All discs were immunostained with anti-En (green) and/or anti-Ci (red) antibodies. Arrowheads in each panel indicate the A/P compartment boundary. (A) A wild-type wing disc. A compartment cells immediately adjacent to the compartment boundary express En and show low levels of Ci staining (arrow). (B) A wing disc carrying cos2 mutant clones. cos2 mutant cells immediately adjacent to the A/P compartment boundary accumulate high levels of Ci and show diminished levels of En expression (big arrowhead). (C) A clone of cos2 mutant cells derived from the A compartment invade into the P compartment (arrow). The cos2 clone is originated from the A compartment because it expresses Ci but not En. (D) A wing disc expressing MS1096/UAS-hh shows uniform En expression in the wing pouch region of the A compartment. (E) A wing disc expressing MS1096/UAS-hh and carrying cos2 mutant clones. cos2 mutant cells situated in the A compartment accumulate high levels of Ci and show diminished levels of En expression (arrows). (F) A wing disc carrying clones of cos2 Su(fu) double mutant cells and triply stained with anti-En (green), anti-Ci (red) and anti-Myc (purple) antibodies. cos2 Su(fu) double mutant cells are recognized by the lack of Myc expression. cos2 Su(fu) double mutant cells immediately adjacent to the A/P compartment boundary (big arrowhead) or distant from the compartment boundary (arrow) express En and show low levels of Ci. Note that the apparent non-uniform En staining along the dorsoventral axis in D and F is because the mutant wing discs are folded and some of the En expressing cells are out of focus.
To determine if the requirement of Cos2 for transducing high levels of Hh signaling activity is a general property of Cos2 that applies to all A compartment cells, we generated cos2 mutant clones in wing discs that ectopically express high levels of hh. As shown in Figure 8D, in wing discs expressing MS1096/UAS-hh, en is uniformly expressed in all A compartment cells in the wing pouch region; however, the ectopic en expression is blocked in cos2 mutant cells, regardless of where the mutant clones are located in the A compartment (Fig. 8E).
Cos2 transduces high levels of Hh signal by antagonizing Su(fu)
The decrease in Hh signaling activity in cos2 mutant cells correlates with an increase in the level of full-length Ci (Fig. 8B), suggesting that Cos2 may be required for Hh to convert Ci into the short-lived nuclear activator form by antagonizing Su(fu). If so, removal of Su(fu) activity from cos2 mutant cells should rescue cos2 mutant phenotypes at the A/P compartment boundary. To test this, we generated cos2 mutant clones in Su(fu) mutant discs and examined en expression and Ci accumulation. As shown in Figure 8F, cos2 Su(fu) double mutant cells situated in the A compartment abutting the compartment boundary accumulate low levels of Ci and restore normal levels of en expression. Moreover, cos2 Su(fu) double mutant cells distant from the compartment boundary ectopically express en at levels comparable to those near the A/P compartment boundary. These observations argue that Su(fu) is responsible for attenuating Ci activity in cos2 mutant cells and that Cos2 is normally required for Hh to antagonize Su(fu) and convert Ci into the short-lived active form.
Discussion
In this study, we show that Cos2 inhibits Ci activity by tethering it in the cytoplasm via a 125 amino acid domain in the C-terminal region of Ci, whereas Su(fu) inhibits Ci nuclear import through the N-terminal region of Ci. In addition, we provide genetic evidence that Su(fu) inhibits Ci activity by a mechanism independent of Ci nuclear import. Surprisingly, we find Cos2 is required for alleviating such an inhibitory mechanism to transduce high levels of Hh signaling activity.
Cos2 and Hh have antagonistic influences on Ci nuclear import
A long-standing puzzle in the Hh signaling pathway is that full-length Ci, which transduces Hh signal into the nucleus to activate Hh target genes, can only be detected in the cytoplasm in Hh receiving cells. A recent study using cultured cells showed that Hh stimulates nuclear import of Ci in cl-8 cells (C.H. Chen et al. 1999). Moreover, this study revealed that Ci is sequestered in the cytoplasm by a nuclear export mechanism that can be inhibited by LMB. However, nuclear staining of Ci in either Hh-stimulated or LMB-treated cl-8 cells is not visually convincing, and the detection of nuclear Ci is largely based on careful fractionation to separate nuclear and cytoplasmic proteins (C.H. Chen et al. 1999). Here, we applied LMB to in vitro cultured imaginal discs and assessed the nuclear localization of Ci in various genetic backgrounds. We find that wild-type wing discs accumulate Ci in the nucleus in Hh receiving cells after LMB treatment (Fig. 2A). Furthermore, ectopic hh expression in A compartment cells away from the compartment boundary induces LMB-dependent nuclear translocation of Ci in these cells (Fig. 2B). The stimulation of LMB-dependent nuclear import by Hh appears to be much more efficient in the developing imaginal discs than in cl-8 cells. One possible explanation is that cl-8 cells might not fully recapitulate all the Hh signaling properties. The ability of LMB to block nuclear export of Ci in cultured imaginal discs provided us an opportunity to address the roles of Cos2 and other Hh signaling components in regulating Ci nuclear import. We find that cos2 mutation results in constitutive nuclear translocation of Ci independent of Hh signaling (Fig. 2D). In contrast, fu mutation attenuates Ci nuclear translocation induced by Hh (Fig. 7). Similar results have recently been obtained by Wang and Holmgren (2000). Taken together, these experiments show that Cos2 and Hh have opposing influences on Ci nuclear import: Cos2 exerts a block on Ci nuclear translocation, whereas Hh stimulates Ci nuclear translocation through Fu.
Cos2 tethers Ci in the cytoplasm through binding to CORD
Using deletion analysis coupled with in vivo coexpression assays, we have succeeded in identifying a 125 amino acid domain in the C-terminal part of Ci (aa 961–1065) that mediates transcriptional repression and cytoplasmic retention by Cos2. We named this domain CORD for Cos2 responsive domain. Ci deletion mutants that lack CORD are insensitive to Cos2 repression and are no longer sequestered in the cytoplasm by Cos2 in our assays (Figs. 3D,E, 5D,E). Moreover, CORD is sufficient to mediate Cos2-dependent cytoplasmic retention when fused to a heterologous protein (Fig. 6). In yeast two hybrid assay, we find that CORD is the only region of Ci that binds Cos2 (Fig. 4A). Taken together, these data provide strong evidence that Cos2 inhibits Ci activity by tethering it in the cytoplasm via directly binding to CORD.
Previous studies have identified a Ci region from aa 703 to aa 850 that can act to sequester heterologous proteins in the cytoplasm (Aza-Blanc et al. 1997; Wang and Holmgren 1999). However, we find that this region does not mediate cytoplasmic retention by Cos2 because Ci deletion mutants that retain it fail to be sequestered by Cos2 and are resistant to Cos2 inhibition in our in vivo assay. Moreover, Ci fragments containing this region fail to bind Cos2 in yeast. Rather, this Ci domain appears to mediate Ci nuclear export as its effect on nuclear localization is abolished by LMB treatment (C.H. Chen et al. 1999).
Su(fu) regulates Ci via two distinct mechanisms
The mechanism by which Su(fu) inhibits Hh signaling has remained controversial. Overexpression studies using mammalian cultured cells have shown that Su(fu) can sequester Gli1 in the cytoplasm (Kogerman et al. 1999; Stone et al. 1999). However, a different result was obtained from overexpression study using Drosophila cultured cells. For example, overexpressing Su(fu) in Drosophila cl-8 cells failed to block LMB-induced nuclear accumulation of Ci (C.H. Chen et al. 1999). In addition, several studies have revealed that Su(fu) can interact with Gli1 on DNA, raising the possibility that Su(fu) might affect Gli activity in the nucleus (Kogerman et al. 1999; Pearse et al. 1999). Here we provide genetic evidence that Su(fu) regulates Ci/Gli by both blocking its nuclear import and affecting its activity after nuclear translocation. To overcome the problem of Ci instability in Su(fu) mutant cells, we analyzed the effect of Su(fu) mutation on nuclear translocation of overexpressed Ci that appears to saturate the mechanism responsible for degrading Ci in the absence of Su(fu). We find that overexpressed Ci is significantly retained in the cytoplasm in A compartment cells of wild-type wing discs but is largely accumulated in the nucleus in Su(fu) mutant wing discs (Fig. 7A,B). Removal of Su(fu) binding domain has a similar effect on Ci nuclear translocation to Su(fu) mutation (Fig. 7C), suggesting that Su(fu) sequesters Ci in the cytoplasm by directly binding to the N-terminal region of Ci. The ability of Su(fu) to sequester Ci in the cytoplasm appears to depend on Cos2, as Su(fu) does not prevent LMB-dependent Ci nuclear import in cos2 mutant cells (Fig. 2D).
Evidence arguing that Su(fu) affects Ci transcriptional activity in the nucleus came from our analysis of cos2 mutant phenotypes. In wild-type wing discs, A compartment cells abutting the A/P compartment boundary transduce high levels of Hh signaling activity, which convert Ci into a labile transcription activator by antagonizing the inhibitory role of Su(fu). As a consequence, these cells activate en and show low levels of Ci staining (Ohlmeyer and Kalderon 1998). We find that cos2 mutant cells abutting the compartment boundary accumulate high levels of Ci and show low levels of Hh signaling activity as they fail to activate en (Figs. 2D, 8B). Thus, it appears that the majority of Ci in cos2 mutant cells remains in a latent stable form, likely in a complex with Su(fu). In support of this view, we show that removal of Su(fu) from cos2 mutant cells restores high levels of Hh signaling activity and simultaneously decreases the concentration of Ci in these cells (Fig. 8F). Because Hh induction of Ci nuclear import is not affected by Su(fu) in cos2 mutant cells near the A/P compartment boundary (Fig. 2D), we conclude that Su(fu) inhibits Ci activity at a step after it translocates into the nucleus. A possible mechanism by which Su(fu) inhibits Ci activity in the nucleus is to prevent it from forming an active transcriptional complex, as it has been shown that Su(fu) can interact with Gli on DNA (Kogerman et al. 1999; Pearse et al. 1999).
Dual roles of Cos2 in blocking and transducing Hh signal
cos2 was identified as a negative component in the hh pathway by previous genetic studies (Forbes et al. 1993; Sisson et al. 1997). Here we uncovered a novel, positive role for Cos2 in the hh pathway. We found that, in addition to blocking Hh signal transduction in A compartment cells away from the compartment boundary, Cos2 is required for transducing high levels of Hh signaling activity by antagonizing Su(fu) in A compartment cells near the A/P compartment boundary. In addition, we show that this requirement is a general property of Cos2 that applies to all A compartment cells (Fig. 8E). Thus, our results underscore an unusual relationship between Cos2 and Su(fu): in the absence of Hh signaling, Cos2 acts cooperatively with Su(fu) to block Ci nuclear import by forming a complex with Ci; in cells receiving high dose of Hh signal, Cos2 is required to alleviate the block on Ci transcriptional activity imposed by Su(fu).
A model for regulating Ci by complex formation with Cos2, Fu, and Su(fu)
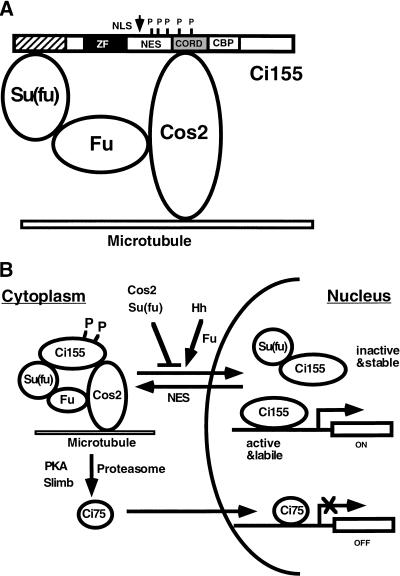
Based on the evidence presented here and elsewhere, we propose a working model for how Cos2, Fu and Su(fu) regulate the nuclear translocation and activity of Ci (Fig. 9). Su(fu) and Cos2 bind Ci via the N- and C-terminal domains, respectively, and the complex binds microtubules through Cos2 and retains Ci in the cytoplasm. In addition, Cos2 promotes the proteolysis of Ci to generate a truncated repressor form (Ci75), a process that also requires the activities of PKA, Slimb, and proteasome. Hh stimulates Ci nuclear translocation through Fu kinase and inhibits Ci processing possibly through dephosphorylating Ci. The transcriptional activity of full-length Ci is attenuated in the nucleus by Su(fu), which also stabilizes the latent form of Ci. High levels of Hh signaling activity convert Ci into a labile and active form, possibly by dissociating it from Su(fu), and this process requires the activities of Fu and Cos2.
Figure 9.
Model for regulating Ci nuclear import and activity by complex formation with Cos2, Fu, and Su(fu). (A) A drawing of Ci/Cos2/Fu/Su(fu) tetrameric complex bound to microtubules, with full-length Ci (Ci155) represented by a bar. Several functional motifs of Ci are indicated. The stippled box indicates the Cos2 binding domain (CORD); the hatched box indicates the Su(fu) binding domain; the filled box represents the Zn-finger DNA-binding domain (ZF) ; and the open box depicts the dCBP binding domain (CBP). The short vertical lines mark the positions of PKA phosphorylation sites and the arrow indicates the cleavage site. NLS: nuclear localization signal; NES: nuclear export signal; P: PKA phosphorylation sites. (B) Regulation of Ci processing, nuclear import and transcriptional activity. Cos2 and Su(fu) prevent Ci nuclear translocation by tethering it in the cytoplasm whereas Hh stimulates Ci nuclear import through Fu. Su(fu) also affects the activity of full-length Ci after it enters the nucleus. Cos2 promotes proteolytic processing of Ci in conjunction with PKA, Slimb, and proteasome to generate a C-terminally truncated form of Ci (Ci75), which translocates into the nucleus to repress Hh target gene expression. See text for details.
Several important issues regarding this model need to be addressed. For example, how Hh antagonizes Cos2 and Su(fu) to promote Ci nuclear translocation remains an important unsolved problem. It is likely that Hh stimulates Ci nuclear import by dissociating Ci complex from microtubules and, further, by releasing Ci from the complex. In support of this view, it has been shown that Hh can induce dissociation of Cos2 from microtubules (Robbins et al. 1997). Moreover, it has also been implicated that dissociation of Ci tetrameric complex might proceed the nuclear translocation of Ci (Stegman et al. 2000). However, no biochemical evidence has been obtained indicating that Hh induces dissociation of Ci from Cos2 and Su(fu).
Fu kinase appears to be required for Hh to stimulate Ci nuclear translocation, as Ci is retained significantly in the cytoplasm in fu mutant cells that receive Hh signal (Fig. 7). The substrate for Fu kinase still remains a mystery. One attractive candidate is Su(fu), which binds Fu and whose function is antagonized by Fu (Preat 1992; Monnier et al. 1998; Ohlmeyer and Kalderon 1998). As Su(fu) is a PEST domain protein, phosphorylation of Su(fu) might cause its degradation and subsequent disassembly of Ci complex. Another good candidate for a Fu substrate is Cos2, which also interacts with Fu. It has been shown that Hh induces phosphorylation of Cos2 (Robbins et al. 1997); however, the kinase responsible for Hh-dependent phosphorylation of Cos2 has not been identified. It remains to be determined if Fu contributes to Cos2 phosphorylation. Although the biological significance of Cos2 phosphorylation has not been shown yet, it is conceivable that such phosphorylation could cause dissociation of Cos2 from microtubules or from Ci, leading to Ci nuclear translocation. In support of this view, we find that the effect of fu mutation on Ci nuclear translocation can be suppressed by removal of Cos2 (Fig. 7E), arguing that Fu promotes Ci nuclear import by antagonizing Cos2.
Finally, how Cos2 positively regulates Hh signaling activity remains to be determined. Our finding that Cos2 is required for Hh to antagonize Su(fu) could be explained by the observation that Cos2 forms a complex with Fu and Su(fu). One scenario is that Cos2 might simply play a structural role in which it antagonizes Su(fu) by recruiting Fu. Alternatively, Cos2 might play a more active role in which it recruits other positive components in close proximity to Fu and regulate its activity in response to Hh signaling. Structure and functional analysis of Cos2 and identifying other Cos2 interacting proteins may help to resolve this important issue.
Material and methods
Transgenes and mutant stocks
UAS-Ci constructs were generated by placing cDNA encoding various portions of Ci between KpnI and XbaI sites in the pUAST vector (Brand and Perrimon 1993). A double HA-epitope tag was fused in frame to the N terminus of each Ci deletion mutant (Wang et al. 1999). UAS-Cos2 constructs were made by inserting the full-length Cos2 coding sequence into the pUAST vector. A double HA-epitope tag or single copy of Flag-epitope tag was fused in frame to the N terminus of Cos2. HA-Cos2 and Flag-Cos2 behave similarly in vivo. UAS-hh and tubulin > y+ CD2 > hh were generated by Basler and Struhl (Basler and Struhl 1994). P[fu+] is a transgene that rescues fu mutants (Ohlmeyer and Kalderon 1998). Gal4 driver lines used in this study were: MS1096 (Wang et al. 1999) and actin < CD2 < Gal4 (Pignoni et al. 1997).
slimb1 is a hypomorphic allele that specifically affects the hh pathway (Jiang and Struhl 1998). cos2[2] is a strong allele of cos2 (Grau and Simpson 1987). Su(fu)LP is a genetically null allele of Su(fu), and Df(3R)kar-Sz21 deletes the chromosomal region from 87C7 to 87C9 that contains the Su(fu) locus (Preat 1992). fumH63 is a strong hypomorphic class I allele (Preat 1992).
Generating clones of marked cells
Clones of mutant cells were generated by FLP/FRT-mediated mitotic recombination (Xu and Rubin 1993). Genotypes for generating clones are as follows: cos2 mutant clones: y hsp-flp.1/y or Y; FRT42D hsp-Myc-GFP, w+ (or hsp-CD2, y+)/FRT42D cos2[2]; slimb mutant clones: y hsp-flp.1/y or Y; FRT82E slimb1/FRT82E hsp-CD2, y+; cos2 mutant clones in wing discs ectopically expressing hh: y hsp-flp.1/MS1096; FRT42D hsp-Myc-GFP (or hsp-CD2)/FRT42D cos2[2]; UAS-hh; cos2 Su(fu) double mutant clones: y hsp-flp.1/y or Y; FRT42D hsp-Myc-GFP/FRT42D cos2[2]; Su(fu)LP/Df(3R)kar-Sz21; cos2 fu double mutant clones: y hsp-flp.1 fumH63/Y; FRT42B P[fu+]/FRT42B cos2[2]; and expressing UAS-Ci in Su(fu) mutants: y MS1096/y or Y; UAS-Ci; Su(fu)LP/Df(3R)kar-Sz21
Imaginal disc staining, LMB treatment and cell culture experiments
Standard protocols for immunofluorescence staining of imaginal discs were used (Jiang and Struhl 1998). Primary antibodies used for this study are monoclonal rat anti-Ci antibody, 2A1(Motzny and Holmgren 1995); mouse anti-HA antibody (Santa Cruz); Rabbit anti-βgal (Cappel); mouse anti-Myc (Santa Cruz); monoclonal mouse anti-Flag, M5 (Sigma), monoclonal mouse anti-En (DSHB, University of Iowa). In experiments involving LMB treatment, late third instar larvae were dissected and submerged in LMB containing cl-8 cell medium for 4 h followed by immunostaining. LMB was used at a concentration of 100–200 nM.
cl-8 cells were cultured as described (van Leeuwen et al. 1994) and transfected with DNA constructs using cellfectin reagents (GIBCO BRL). GFPNLS, GFPNLS-CORD, and HA-Cos2 were expressed in cl-8 cells using the Gal4/UAS system with an actin-gal4 expressing construct (Aza-Blanc et al. 1997).
Yeast two hybrid assay
pEG202 was used to fuse Cos2 to the DNA-binding domain of LexA, and pACT2 was used to generate prey proteins with various portions of Ci fused in frame with the GAL4 activation domain. The yeast strain L-40 was transformed with plasmids encoding the bait and prey proteins sequentially, followed by liquid β-galactosidase as described previously (Tall et al. 1999).
Acknowledgments
We thank Drs. D. Kalderon, K. Basler, G. Struhl, and J. Treisman and the Bloomington and Umea stock centers for providing fly stocks; Drs. M. Scott, S. Carroll, and R. Holmgren and DSHB for antibodies; Dr. M. Yoshida for LMB; Dr. Y. Chen for Cos2 cDNA and cl-8 cells; John Peters for nuclear form of GFP construct; and C. Cowan for advice in yeast two hybrid and cell culture experiments. We are grateful to Drs. C. Chen, D.J. Pan, and L. Parada for critically reading the manuscript. This work was supported by a NIH grant to J.J. (GM61269–01). J.J. is a Searle Scholar supported by the Chicago Community Trust, a Eugene McDermott Endowed Scholar in Biomedical Research, and a Leukemia and Lymphoma Society Special Fellow.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jiang05@utsw.swmed.edu; FAX (214) 648-1960.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.843900.
References
- Akimaru H, Chen Y, Dai P, Hou DX, Nonaka M, Smolik SM, Armstrong S, Goodman RH, Ishii S. Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signalling. Nature. 1997;386:735–738. doi: 10.1038/386735a0. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of Hedgehog target genes in Drosophila is mediated directly by the Cubitus interruptus protein, a member of the GLI family of the zinc finger DNA-binding proteins. Genes & Dev. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber F, Laget M, Schwartz C, Kornberg T. Proteolysis that is inhibited by Hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Chen CH, von Kessler DP, Park W, Wang B, Ma Y, Beachy PA. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell. 1999;98:305–316. doi: 10.1016/s0092-8674(00)81960-1. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cardinaux JR, Goodman RH, Smolik SM. Mutants of cubitus interruptus that are independent of PKA regulation are independent of hedgehog signaling. Development. 1999;126:3607–3616. doi: 10.1242/dev.126.16.3607. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Basler K. Opposing transcriptional outputs of Hedgehog signaling and engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell. 2000;100:411–422. doi: 10.1016/s0092-8674(00)80677-7. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Brunner M, Hafen E, Basler K. Sending and receiving the Hedgehog signal: Control by the Drosophila Gli protein Cubitus interruptus. Science. 1996;272:1621–1625. doi: 10.1126/science.272.5268.1621. [DOI] [PubMed] [Google Scholar]
- Forbes, A.J., Nakano, Y., Taylor, A.M., and Ingham, P.W. 1993. Genetic analysis of hedgehog signalling in the Drosophila embryo. Dev. Suppl. 115–124. [PubMed]
- Grau Y, Simpson P. The segment polarity gene costal-2 in Drosophila. I. The organization of both primary and secondary embryonic fields may be affected. Dev Biol. 1987;122:186–200. doi: 10.1016/0012-1606(87)90344-7. [DOI] [PubMed] [Google Scholar]
- Ingham PW. Hedgehog points the way. Curr Biol. 1994;4:347–350. doi: 10.1016/s0960-9822(00)00076-2. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, Toftgard R, Zaphiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Brook WJ, Ng M, Callega M, Sun H, Cohen SM. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381:387–393. doi: 10.1038/381387a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T. A ubiquitin ligase complex essential for the NF-κB, Wnt/Wingless, and Hedgehog signaling pathways. Genes & Dev. 1999;13:505–510. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell. 1999;96:819–831. doi: 10.1016/s0092-8674(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Monnier V, Dussillol F, Alves G, Lamour-Isnard C, Plessis A. Suppressor of fused links fused and Cubitus interruptus on the hedgehog signalling pathway. Curr Biol. 1998;8:583–586. doi: 10.1016/s0960-9822(98)70227-1. [DOI] [PubMed] [Google Scholar]
- Motzny CK, Holmgren R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech Dev. 1995;52:137–150. doi: 10.1016/0925-4773(95)00397-j. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and Long-Range Action of a DPP Morphogen Gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Ohlmeyer JT, Kalderon D. Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature. 1998;396:749–753. doi: 10.1038/25533. [DOI] [PubMed] [Google Scholar]
- Pearse RV, II, Collier LS, Scott MP, Tabin CJ. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol. 1999;212:323–336. doi: 10.1006/dbio.1999.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni, F., Hu, B., Zavitz, K.H., Xiao, J., Garrity, P.A., and Zipursky, S.L. 1997. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development Cell 91: 881–891. [DOI] [PubMed]
- Preat T. Characterization of Suppressor of fused, a complete suppressor of the fused segment polarity gene of Drosophila melanogaster. Genetics. 1992;132:725–736. doi: 10.1093/genetics/132.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development. 1999;126:4331–4339. doi: 10.1242/dev.126.19.4331. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Basler K. Control of compartmental affinity boundaries by hedgehog. Nature. 1997;389:614–618. doi: 10.1038/39343. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Catching a Gli-mpse of Hedgehog. Cell. 1997;90:193–196. doi: 10.1016/s0092-8674(00)80325-6. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Stegman MA, Vallance JE, Elangovan G, Sosinski J, Cheng Y, Robbins DJ. Identification of a tetrameric hedgehog signaling complex. J Biol Chem. 2000;275:21809–21812. doi: 10.1074/jbc.C000043200. [DOI] [PubMed] [Google Scholar]
- Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, Phillips H, Brush J, Goddard A, de Sauvage FJ, Rosenthal A. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. 1999;112:4437–4448. doi: 10.1242/jcs.112.23.4437. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development. 1997;124:4697–4705. doi: 10.1242/dev.124.22.4697. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Tall GG, Hama H, DeWald DB, Horazdovsky BF. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell. 1999;10:1873–1889. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen F, Harryman Samos C, Nusse R. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature. 1994;368:342–344. doi: 10.1038/368342a0. [DOI] [PubMed] [Google Scholar]
- Wang G, Wang B, Jiang J. Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes & Dev. 1999;13:2828–2837. doi: 10.1101/gad.13.21.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA. The subcellular localization and activity of Drosophila cubitus interruptus are regulated at multiple levels. Development. 1999;126:5097–5106. doi: 10.1242/dev.126.22.5097. [DOI] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA. Nuclear import of cubitus interruptus is regulated by hedgehog via a mechanism distinct from Ci stabilization and Ci activation. Development. 2000;127:3131–3139. doi: 10.1242/dev.127.14.3131. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]