Abstract
Myopodin is a tumor suppressor gene that suppresses growth of prostate and urothelial carcinomas. However, the mechanism of myopodin tumor suppressor activity or signaling that leads to activation of myopodin remains unclear. In this report, we showed that the N-terminus of myopodin binds integrin-linked kinase (ILK) both in vivo and in vitro. An ILK interaction motif of 78 amino acids (amino acids 82–157) was identified in the N-terminus region of myopodin. Induction of ILK dependent kinase activity by integrin α7 led to phosphorylation of myopodin both in vivo and in vitro. Knocking down ILK dramatically reduced the inhibition of cell growth and motility mediated by myopodin. A mutant of myopodin lacking the ILK interaction motif is inactive in suppressing the growth and motility of PC3 cells. As a result, this study showed a novel and critical signaling pathway that leads to activation of myopodin.
Introduction
Myopodin was initially identified as a gene frequently deleted in invasive prostate cancer (Lin et al., 2001). Complete inactivation of myopodin expression is predictive of metastasis of prostate cancer (Yu et al., 2006). Higher mortality of prostate cancer is observed in patients with myopodin deletion. Loss of nuclear expression in urothelial carcinoma is associated with higher level of relapse and metastasis (Sanchez-Carbayo et al., 2003). Overexpression of myopodin expression in prostate cancer cell lines PC3 and DU145 suppresses cell growth and invasiveness. SCID mice xenografted with PC3 or DU145 cells over-expressing myopodin had developed tumor with smaller tumor size, fewer metastasis and longer survival time (Jing et al., 2004). Our previous study showed that myopodin regulates cell motility through complexing with zyxin (Yu and Luo, 2006). Deletion of zyxin binding motif significantly hampers myopodin's ability to inhibit tumor cell migration (Yu and Luo, 2006). However, the mechanism that leads to the activation of myopodin is still unclear.
Integrin-linked kinase (ILK) was originally identified as an integrin β1 binding protein, and plays a critical role of signal transducer for integrin family members (Hannigan et al., 1996). It involves in diverse cellular processes, including adhesion, migration and growth regulation (Apte et al., 2009; Donthamsetty et al., 2009; Gkretsi et al., 2008; Hannigan et al., 1996; Legate et al., 2006; Wu, 2004). Depletion or dysregulation of ILK leads to abnormality of cytoskeleton structure, cell adhesion, or growth defects (Sakai et al., 2003). Even though many targets of ILK have been identified, few are known to play major role in regulating tumor suppressor activity. In this report, we showed that ILK bound with myopodin and induced the phosphorylation of its N-terminus. The tumor suppressor activity of myopodin was dramatically enhanced through its interaction with ILK. Mutant myopodin that lacks the binding motif with ILK contained minimal growth and motility suppression activity.
Results
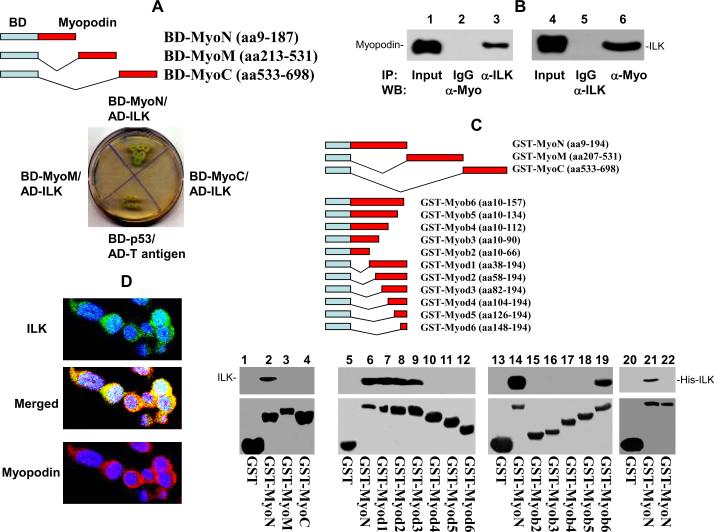
In order to investigate the signaling pathway of myopodin, we ligated the cDNA coding sequences for N-terminus of myopodin into pGBKT7 vector to generate pBD-myoN, of which expresses the fusion protein containing the DNA binding domain of GAL4 and coding region of myopodin. The construct was subsequently transformed into yeast with a prostate pACT2-cDNA library to identify the target proteins that interact with myopodin. Thirty-six clones were isolated after three rounds of nutrient selection with pBD-myoN screening. Plasmid DNA from these clones was subsequently extracted and transformed into E. coli and the colonies (pACT2-target cDNA) grown on the ampicillin plate were selected. After removal of several redundant clones, 24 of these clones were sequenced using an automated sequencer. In our previous analysis, we found that the interaction of C- terminus of myopodin with zyxin, a cell motility and migration regulatory protein, was a crutial contact that controlled the cell motility (Yu and Luo, 2006). In this study, one of the sequenced clones was found to contain cDNA encoding ILK interacting with the N-terminus region of myopodin. As shown in figure 1A, The Yeast AH109 cells were co-transfected with pBD-MyoN and pACT2-ILK. The co-transfectants were able to grow in the high stringency nutrient selective agar lacking leucine, tryptophan, histidine and adenine (figure 1A). These colonies were positive for α-galactosidase activity, confirming that there was an interaction of two human proteins that brought the DNA binding domain and the activation domain of GAL4 together. While co-transfection of pACT2-ILK with pBD-myoM or pBD-myoC into AH109 cells produced no viable colony on nutrient selective agar, indicating the N-terminus, but not middle- and C-termimus of myopodin, is the domain ILK interacts with.
Figure 1. Interaction of N-terminus of myopodin with ILK.
(A) Constructs of N- terminus (BD-myoN), M-segment (BD-myoM) and C-terminus (BD-myoC) of myopodin with bait domain in yeast two hybrid analysis. Co-transformant of pBD-myoN, pBD-myoM, and pBD-myoC with pACT2-ILK on SD agar palte with high strigent nutrient selection (SD-leu-Trp-His-Ade). (B) Co-immunoprecipitation of ILK or myopodin using antibodies specific for myopodin (α-Myo) or ILK (α-ILK). The immunoprecipitates were blotted with the indicated antibodies. (C) Mapping binding motif of ILK on myopodin. Top: Constructs of series of myopodin deletion mutant with GST expression vectors. Bottom: Binding assays on GST or GST-myopodin deletion mutants with ILK from PC3 cells (lanes 1–19) or HisTag-ILK (lanes 20–21) or pET28a lysate (lane 22). The bound ILK was blotted with ILK antibodies. (D) Immunofluorescence staining of I4 cells with antibody against myopodin bound by rhodamin conjugated secondary antibody for rabbit, and antibody specifically against ILK recognized by FITC conjugated secondary antibody for goat.
To investigate whether myopodin interacts with ILK in human cells, co-immunoprecipitations of ILK and myopodin were performed on PC3 cells transformed with myopodin expression vector pCMV-myopodin using anti-myopodin or anti-ILK antibodies. The co-immunoprecipitation analysis showed ILK and myopodin being complexed together, confirming that the interaction between myopodin and ILK occurs in PC-3 cells (figure 1B). To investigate the interaction of myopodin and ILK in vitro, we subsequently constructed the N-terminus, C-terminus and mid-segment of myopodin with GST respectively, and expressed GST-myopodin fusion proteins in bacteria. Binding assays were performed using the purified GST-myoN, GST-myoM and GST-myoN with myopodin from PC3 cell lysate. As shown in figure 1C, ILK was detected in only GST-myoN binding analysis, but not GST-myoM and GST-myoC analysis. To rule out a bridge protein being required for the binding of myopodin and ILK, we constructed ILK coding sequence into pET-28 to create a Histag-ILK fusion protein expressed in bacteria, and performed binding assay of GST-MyoN with HisTag-ILK. HisTag-ILK was detected in the GST-MyoN pull down. The results clearly indicate that the binding between ILK and myopodin N-terminus is direct and independent of any other accessory proteins (figure 1C). To define the ILK binding motif in myopodin, a series of deletion mutants of myoN were made and ligated into pGEX-5X. The binding assays of these constructs with ILK were shown in figure 1C, and the ILK binding motif appears to locate in amino acid 82–157, since fusion proteins deleted of this sequence were negative in binding assays, while constructs that contain this sequence were positive.
Interaction between myopodin and ILK implicates the co-localization of these two proteins in the same cell compartment. We performed double immunofluorescence staining with antibodies against myopodin (with rhodamine labeled secondary antibodies) and antibodies against ILK (with FITC labeled secondary antibodies) on I4 cells (pCMV-myopodin transformed PC3 cells) which express myopodin. A dominant cytoplasmic distribution of both ILK and myopodin in these cells was observed under confocal microscope. Overlapping of ILK and myopodin localizations was extensive (figure 1D).
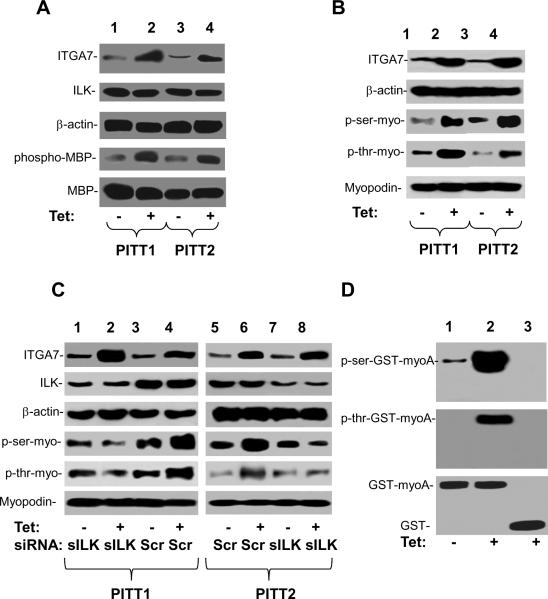
ILK is one of the pivotal molecules that mediate the signaling of several integrins (Legate et al., 2006; Wu, 2004). To investigate whether ILK-myopodin interaction leads to phosphorylation of myopodin, we performed an analysis to examine whether ILK-dependent phosphorylation of myopodin in cells was stimulated by integrin α7 (ITGA7). ILK was first purified by immunoprecipitation from cell lysates of PITT1 and PITT2 cells (clones of pCDNA4-ITGA7/pCDNA6 transformed PC3 cells, ITGA7 inducible by tetracycline), then subjected to the kinase activity analysis on basic myelin protein. As shown in figure 2A, induction of ITGA7 expression in PITT1 and PITT2 cells increased the phosphorylation activity of immunoprecipitated ILK on basic myelin protein. Subsequently, an immunoblot analysis was performed on phosphorylated myopodin of PITT1 and PITT2 cells induced with or without tetracycline. Myopodin was purified by immunoprecipitation and resolved on 8% SDS-PAGE and was analyzed with antiserum against phosphoserine or phosphothreonine in western blot. As shown in figure 2B, induction of ITGA7 expression significantly increased both serine and threonine phosphorylation of myopodin protein. To investigate whether ILK is required for ITGA7-stimulated phosphorylation of myopodin, PITT1 and PITT2 cells were treated with siRNA specific for ILK and induced with tetracycline. A dramatic reduction of myopodin phosphorylation was identified at both serine and threonine residues (figure 2C). To investigate whether ILK phosphorylates myopodin in vitro, kinase assays were performed using ILK immunoprecipitates from PC3 cells incubated with bacterial generated GST-myoN, the recombinant fusion protein of N-terminus of myopodin and GST. In vitro phosphorylation of myopodin N-terminus was observed when immunopurified ILK was incubated with column purified GST-myoN fusion proteins in a kinase reaction cocktail, while control purified GST showed negative of phosphorylation even with ITGA7 induction (figure 2D).
Figure 2. Phosphorylation of myopodin by ILK.
(A) ILK kinase activity induced by integrin α7. ILk was immunopurified from PITT1 and PITT2 cells induced with or without tetracycline. ILK was then subjected to the MBP phosphorylation analysis. Top 3 panels: immunoblots of lysates of PITT1 and PITT2 cells. Bottom 2 panels: immunoblots of kinase assay using myelin basic protein as substrate on ILK immunoprecipitates. (B) Immunopurified myopodin from PITT1 and PITT2 cells induced with or without tetracycline were blotted with antibody against phosphorylated serine or phosphorylated threonine. Top 2 panels: immunoblots of lysates of PITT1 and PITT2 cells. Bottom three panels: immunoblots of myopodin immunoprecipitates. (C) Immunopurified myopodin from PITT1 and PITT2 cells, induced with or without tetracycline and transfected with scramble siRNA or siRNA against ILK, were blotted with antibody against phosphorylated serine or phosphorylated threonine. Top 2 panels: immunoblots of lysates of PITT1 and PITT2 cells. Bottom three panels: immunoblots of myopodin immunoprecipitates. (D) ILK dependent phosphorylation assays on GST-myoN (N-terminus of myopodin) purified through Glutathione sepherose 4B. ILK immunopurified from PITT 1 cells induced with (Tet +) or without (Tet −) tetracycline were incubated with purified GST-MyoN in a kinase reaction. The results were analyzed with antibody specific for phospho-serine or phosphothreonine. GST was used as negative control (lane 3).
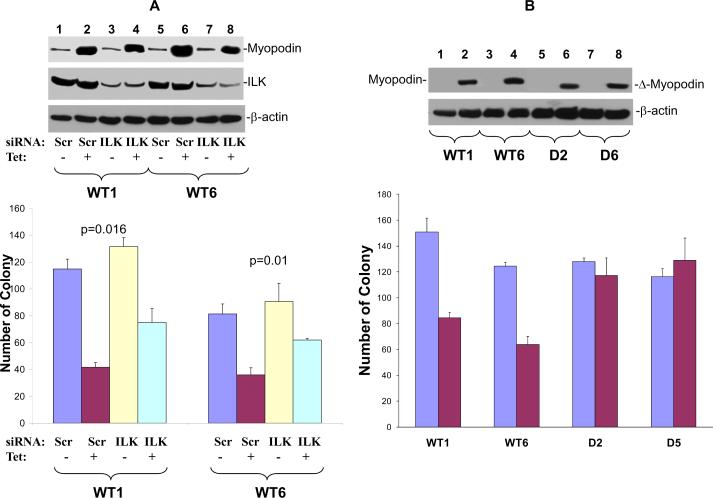
Myopodin is a tumor suppressor gene suppressing cell growth and motility. To investigate the functional significance of ILK-myopodin interaction on myopodin tumor suppressor activity, we performed colony formation assays on clones of tetracycline inducible myopodin expressing PC3 cells (WT1 and WT6). As shown in figure 3A, induction of myopodin expression in WT1 and WT6 produced 62% and 50% decrease of colony numbers, respectively. Introduction of siRNA specific for ILK into these cells significantly negated the inhibition effect of myopodin on colony formation, by reversing 34% (p=0.016) and 43% (p=0.01) of the inhibition of the colony formation in comparison with the scramble controls for WT1 and WT6, respectively. To investigate the effect of ILK-myopodin interaction on the tumor suppressor activity of myopodin, a myopodin mutant with a deletion of 75 amino acids of ILK interaction motif (amino acids 82–157) was constructed into pCDNA4 to create a pCDNA4-Δmyopodin. This vector was subsequently transfected into PC3 cells. Two clones with tetracycline inducible expressing mutant myopodin were selected for further analyses. As shown in figure 3B, expression of mutant myopodin did not suppress the colony formation, supporting that ILK/myopodin interaction is necessary for myopodin mediated suppression of cell growth.
Figure 3. Down regulation of ILK and deletion of ILK binding motif abrogates myopodin cell growth suppression activity.
(A) Colony formation assays for PC3 cell clones expressing wild type (WT1, WT6) treated with tetracycline and transfected with shRNA against ILK (ILK) or scramble control (Scr). Each condition was in triplicates. Top panel: Immunoblots of myopodin, ILK and β-actin of representative samples. Bottom panel: Colony formation assays. (B) Colony formation assays for PC3 cell clones expressing wild type (WT1, WT6) or ILK motif deletion mutants (D2 and D5) of myopodin treated with tetracycline. One thousand cells of wt or mutant myopodin were plated to each well of 6 well plates and colony formation was evaluated. Cells were treated with or without tetracycline. Each condition was in triplicates. Top panel: Immunoblots of myopodin and β-actin of representative samples. Bottom panel: Colony formation assays.
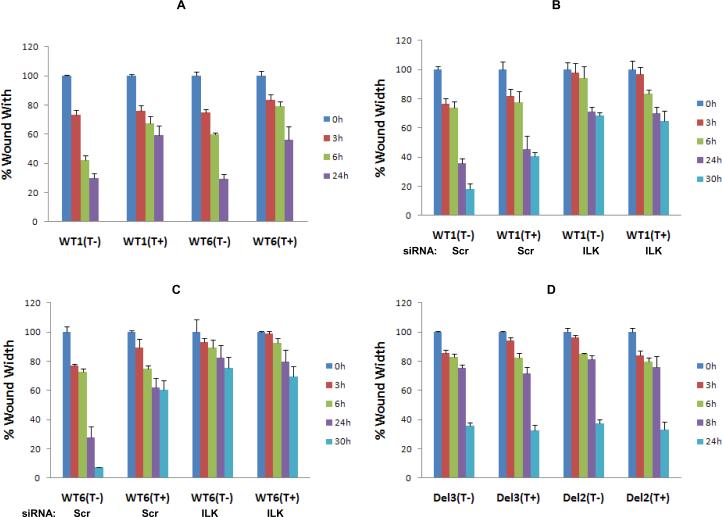
Myopodin is known for its regulatory role in cell motility (5–8). Induction of wild type myopodin reduced the motility in time dependent manner for both WT1 and WT6 cells (figure 4A). To investigate the role of ILK in myopodin mediated suppression of cell motility, siRNA specific for ILK was transfected into WT1 and WT6 cells. As shown in figure 4B and C, knocking down of ILK mimicked the myopodin suppression effect by retarding cell migration of WT1 cells by 3.8 fold (p=0.007) and WT6 by 9.9 fold (p=0.004) even without the induction of myopodin. Induction of myopodin produced no further inhibition of cell motility, suggesting that ILK is critical for myopodin to regulate cell motility. One potential interpretation for motility suppression by ILK knocking down is that ILK regulates the cell motility through both myopodin and other non-myopodin signaling. The interaction of myopodin and ILK is required for myopodin motility inhibition activity, an additional fine control of cell mobility by ILK. Once ILK is eliminated from these signaling, these motility signaling pathways become dysfunctional and cell motility is blocked. We then examined the role of ILK/myopodin interaction in cell motility by performing wound healing assays on D2 and D5 cells that had been transformed with mutant myopodin with deletion of the ILK interacting sequence. As shown in figure 4D, the migration retarding effect by myopodin was completely eliminated in wound healing assays when mutant myopodin that lacks the ILK interaction site was expressed (8.7% faster than uninduced controls for D2, p=0.6; 13.5% faster for D3, p=0.3). To corroborate the wound healing assays, matrigel traverse analyses were performed to assess whether myopodin/ILK interaction impacts on transmigration of prostate cancer cells (Table 2). Our analysis showed that the expression of myopodin decreased transmigration of WT1 cells by 81% (p<0.001) and WT6 cells by 57% (p=0.003). In contrast, myopodin mutant clones D2 and D5 did not inhibit PC3 cell migration. These results clearly demonstrated that ILK/myopodin interaction is crucial for myopodin mediated suppression of cell motility.
Figure 4. Down-regulation of ILK and deletion of ILK binding motif abrogates myopodin cell motility suppression activity.
(A) Wound healing assays for PC3 cell clones expressing wild type (WT1, WT6) treated with or without tetracycline for 3, 6 and 24 hours (h). (B) Wound healing assays for WT1 cells treated with or without tetracycline and transfected with shRNA specific for ILK for 3, 6, 24 and 30 hours (h). (C) Wound healing assays for WT6 cells treated with or without tetracycline and transfected with shRNA specific for ILK for 3, 6, 24 and 30 hours (h). (D) Wound healing assays of PC3 cells transfected with mutant (D2 and D5) myopodin. The wound was measured at 0h, 6h, 8h and 24h. Triplicates were prepared for each cell condition.
Table 2.
Myopodin/ILK interaction is required for myopodin mediated suppression of transmigration of PC3 cells
| Cells | Myopodin induction | Control membrane* | Gel* | Invasion Index** |
|---|---|---|---|---|
| WT1 | No | 447 ±31 | 246 ±25 | 0.55 |
| WT1 | Yes | 94 ±12 | 9 ±4 | 0.10 |
| WT6 | No | 126 ±28 | 106 ±30 | 0.84 |
| WT6 | Yes | 70 ±12 | 26 ±8 | 0.36 |
| D5 | No | 147 ±25 | 70 ±11 | 0.47 |
| D5 | Yes | 144 ±18 | 89 ±12 | 0.62 |
| D2 | No | 395 ±40 | 213 ±12 | 0.54 |
| D2 | Yes | 352 ±57 | 240 ±19 | 0.68 |
Average of five high power fields and three independent experiments.
Number of cells in gel/number of cells/control membrane.
Discussion
Myopodin has been shown to contain tumor suppressor activity in both prostate cancer and urothelial carcinoma of the urinary bladder (Jing et al., 2004; Lin et al., 2001; Sanchez-Carbayo et al., 2003). Genome deletion or methylation of myopodin have been found in subsets of human carcinomas (Cebrian et al., 2008; Lin et al., 2001). Previous study showed that myopodin forms binding complex with zyxin to regulate cell motility (Yu and Luo, 2006). However, the signaling pathway of myopodin tumor suppression remains unclear. To our knowledge, this is the first report indicating that the interaction between ILK and myopodin is critical for myopodin tumor suppressor activity. Several lines of evidences support that ILK interacts with myopodin. First, the N-terminus of myopodin binds ILK both in the yeast two hybrid system and cell free in vitro binding assays. Second, both ILK and myopodin are readily co-immunoprecipitated by antibodies either against ILK or myopodin. Third, myopodin and ILK is co-localized in the cytoplasm of prostate cancer cells. Fourth, ILK dependent phosphorylation of myopodin is found both in vivo and in vitro.
ILK is a versatile molecule that interacts with several molecules, including integrin β1 (Hannigan et al., 1996), myosin light-chain kinase (Deng et al., 2001), β-parvin (Yamaji et al., 2001), Akt (Persad et al., 2001) and MCM7 (Han et al., 2010) and plays critical roles in several integrin related signaling processes. Knocking-out of ILK in the liver produces higher level of cell proliferation and organomegaly (Acconcia et al., 2007; Apte et al., 2009; Donthamsetty et al., 2009; Gkretsi et al., 2008). Interaction of myopodin with ILK is an intriguing finding. Our results suggested that ILK binding and ILK dependent phosphorylation is essential for myopodin to inhibit cell growth and motility since knocking down of ILK or deletion of ILK interaction motif in myopodin abrogated myopodin's tumor suppressor activity. The ILK dependent activation of myopodin provides a novel link between extracellular matrix-integrin-ILK signaling and myopodin tumor suppression. Activation of ILK by integrins-extracellular matrix interaction may enhance the tumor suppressor activity of myopodin. Alternatively, these results may be interpreted as myopodin serving as one of the mediators for integrin-ILK signaling and zyxin or other unknown molecules in cells. The latter interpretation is supported by that some members of integrins, such as integrin α7, exhibit tumor suppressor activity (Ren et al., 2007). As a result, the ILK-myopodin link is probably one of the critical cell growth and motility suppression mechanisms of integrins.
The physiological role of myopodin in prostate epithelial cells appears quite different from the one in skeletal muscles. Myopodin is abundantly expressed in skeletal muscle but only moderately expressed in prostate epithelial cells (Lin et al., 2001). Acinar cells appear to have higher level of myopodin than that of basal cells. Interestingly, this expression pattern overlaps with that of ITGA7. Since both myopodin and ITGA7 inhibit cell growth and cell motility, it suggests that these two tumor suppressors co-operate in prostate epithelial cells to suppress excessive growth of epithelial cells, and limits these cells' motility and random migration. Zyxin appears a good candidate to serve as a motility-suppression effector for myopodin, since elimination of zyxin drastically abrogated the effect of myopodin on cell motility. Quite possibly, signals from ILK that impact on myopodin will be related to zyxin. Zyxin, however, does not account for all the activities of myopodin. Other important effectors or partners for myopodin remain to be discovered. Our study, nonetheless, discovers a novel link that is required for the activation of myopodin, and suggests that myopodin is forming a multi-protein signaling complex to regulate cell growth and motility. Since the interaction of C-terminus of myopodin and zyxin had been shown to inhibit cell motility but not cell growth inhibition. We speculate that additional molecules might be required in cell growth inhibition by myopodin. It would be of interest to identify such molecules for myopodin, that regulate growth and possibly other critical functions of the cells in the future study.
Materials and Methods
Prostate cancer cell line and cell culture
Prostate cancer cell line, PC3 was purchased from ATCC (Manassas, VA) and was cultured in medium F12K (InVitrogen, Carlsbad, CA ) containing 10% FBS with or without selective reagents zeocine (Invitrogen) and blasticidin (Invitrogen) in 5% CO2 at 37°C. The medium was changed every 3–4 days.
Cloning
Constructions for pBD-myopodin fusion proteins
To generate three fragments of myopodin protein fused with BD domain of pGBKT7 vector, three mutagenic primer sets, TAAGAAGCGACGTCATATGGCCAGGAAATACACCCTAGTT / CGTTCTATTCATGTC GACCAT CCTCTG GATGTT TGCTG; CTCG CCAACCCGACATATGACGAGTCCCATT GC GACTTT/CT CACCGTGTCTGAGTCGACCACATACTTCTCCATTCTC; T GTGGCC TATAATCATATGCACTCGCCGTCTTACCCACTG / TTCATTTCAAGCAAAGTCGACAC TCAGCTTCAGCTACAAG were designed to create two restriction sites (Nde1 and Sal1) at the N- and C- terminus of PCR products of each segment of myopodin. The PCR reaction (Invitrogen, Carlsbad, CA) was performed on the template of a donor prostate cDNA, at 94°C for 1 min followed by 35 cycles of 94°C for 30 s, 68°C for 3 min and a final 10-min extension step at 68°C. The PCR product was ligated to TA cloning vector, pCR 2.1, and further restricted with Nde1 and Sal1, gel purified and ligated into a similarly restricted pGBKT7 vector. The construct was then transformed into One Shot™ competent cells (Invitrogen, Carlsbad, CA). Plasmid DNA was extracted from selected transformed cells and digested with Nde1 and Sal1 to detect the presence of the insert. The construction and coding frame were confirmed by sequencing.
Construction for glutathione-s-transferase (GST)-myopodinA fusion protein
A mutagenic primer set (TTAAGAAGCGACGTCGGATCCCCAGGAAATACA/CGTTCTATTCATGTC GACCAT CCTCTGGATGTTTGCTG) was designed to create BamH1 and SalI restriction sites in N-and C- ends of segment encoding 233 amino acids at N- terminal of myopodin. The PCR reaction in the presence of prostate donor cDNA and the primer pair was heated at 94°C for 1 min followed by 35 cycles of 94°C for 30 s, 68°C for 3 min and a final 10-min extension step at 68°C. The PCR-amplified myopodin segment was cloned into pCR2.1 TA cloning vector; the plasmid was purified from the selected colony and restricted with BamH1 and Sal1, and ligated into a similarly cleaved pGEX-5T vector. A serial constructs with different length of deletions, including N- or C- deletions of GST-myoN were performed using the primer sets listed in table 1 (also see figure 1 for aa sequences in the constructs). The procedures for generating these mutants were similar to those described for pGST-myoN. The pGST-myoN and its truncation mutants were transformed into E. coli BL21 cells for recombinant protein expression.
Table 1.
Primers for construction of myopodin deletion mutants
| Primer pairs | Sequence |
|---|---|
| MyoN_D1 | AAGCGACGTCGGATCCCCAGGAAATACGAGGATACATGTGAAGTAGA/ GAGATACATGTGAAGTAGCATTTCTTGGTGCAAGCGAATCAGAGGTGGATGAAGAGT TATTGTCTGACGTT |
| MyoN_D2 | AAGCGACGTCGGATCCCCAGGAAATACGACGACAACACACAAGTTGTG/ GAGGATACATGTGAAGTAGCATTTCTTGGTGCAAGCGAATCAGAGGTGGATGAAGAG TTATTGTCTGACGTT |
| MyoN_D3 | AAGCGACGTCGGATCCCCAGGAAATACGGGGACAAGATGGAGATGTTA/ GAGGATACATGTGAAGTAGCATTTCTTGGTGCAAGCGAATCAGAGGTGGATGAAGAG TTATTGTCTGACGTT |
| MyoN_D4 | AAGCGACGTCGGATCCCCAGGAAATACGAGAGAATGGATCAGATCACA/ GAGGATACATGTGAAGTAGCATTTCTTGGTGCAAGCGAATCAGAGGTGGATGAAGAG TTATTGTCTGACGTT |
| MyoN_D5 | AAGCGACGTCGGATCCCCAGGAAATACCAAGATGCTGCCCAGACCGAT/ GAGGATACATGTGAAGTAGCATTTCTTGGTGCAAGCGAATCAGAGGTGGATGAAGAG TTATTGTCTGACGTT |
| MyoN_D6 | AAGCGACGTCGGATCCCCAGGAAATACGTAAGAACGCAGAGCTCTGTG/ GAGGATACATGTGAAGTAGCATTTCTTGGTGCAAGCGAATCAGAGGTGGATGAAGAG TTATTGTCTGACGTT |
| MyoN_B1 | TTAAGAAGCGACGTCGGATCCCCAGGAAATACA/ CGTTCTATTCATGTCGACCATCCTAAATGCTACTTCACATGTATCCTC |
| MyoN_B2 | TTAAGAAGCGACGTCGGATCCCCAGGAAATACA/ CGTTCTATTCATGTCGACCATCCTGTTCACAACTTGTGTGTTGTCGTC |
| MyoN_B3 | TT AAGAAGCGACGTCGGATCCCCAGGAAATACA/ CGTTCTATTCATGTCGACCATCCTTGGTAACATCTCCATCTTGTCCCC |
| MyoN_B4 | TTAAGAAGCGACGTCGGATCCCCAGGAAATACA/ CGTTCTATTCATGTCGACCATCCTGGCTGTGATCTGATCCATTCTCTC |
| MyoN_B5 | TT AAGAAGCGACGTCGGATCCCCAGGAAATACA/ CGTTCTATTCATGTCGACCATCCTGCCATCGGTCTGGGCAGCATCTTG |
| MyoN_B6 | TT AAGAAGCGACGTCGGATCCCCAGGAAATACA/ CGTTCTATTCATGTCGACCATCCTGCTCACAGAGCTCTGCGTTCTTAC |
Construction for ILK expression in prokaryotes
Full length of ILK cDNA was obtained by amplification of prostate donor cDNA with the primer pair as GGCGCCGGGAGAATTCATG GACGACATTTTC /AGGACCTTCCAGTCCTCGAGGTCCTGCATCTT. The PCR reaction was performed by using bluemix DNA polymerase with 30 heat cycles of 94°C for 30 sec and 68°C for 3 min following a 94°C heat for 1 min. The purified PCR products was restricted with BamH1 and Xho1, and then ligated to pET-28a cloning vector which was linearized with the same restriction enzymes.
Constructions for inducible myopodin expression and motif deleted myopodin expression clone
To create the wild type myopodin and ILK binding motif deletion mutant of mypodin, a pair of primer, GGCACAGAGCAGGGAGAGGATCCACGCTCGGAAAAAGA/ ACAAGATTACTCTTCCACTCTAGATGGTTTCCACACCTGAAT, was designed to amplify the full length of myopodin cDNA and a pair of forward and reverse strand primers encompassed the same coding region but excluding deletion segment in the middle, TACCTTGTCCTCTTC TTTTTGCTTGTCACCTTCTTCCTCCTC/ GAGGAGGAAGAAGGTGACAAGCAAAAA GAAGAGGACAAGGTA were designed to generate the motif deletion mutant. The full length of wild type or motif deleted myopodin was amplified by using expand long PCR DNA polymerase (Applied Biosystem, Carlsbad, California) with a first heat step at 94°C for 1 minute followed by 30 cycles of 94°C for 30 s, 62°C for 1 min, and 65°C for 2 min. A final 10-min extension step at 68°C was applied to the end of the cycle. The PCR products were gel purified, cloned into TOPO TA cloning vector pCR2.1, then excised from purified myo-pCR2.1 by BamH1 and Sal1 and ligated to the similarly restricted pCDNA4-his-a cloning vector (InVitrogen, Carlsbad, CA). Once the sequences and frames of the construct were confirmed by sequencing analysis, wild type or deletion mutant of myopodin constructed with pCDNA4 were cotransfected with pCDNA6 (that confers tetracycline inducible expression in pCNDA4 transfected cells) into PC3 cells by electropolation (GenePulser Xcell System, Bio Rad). The clones were selected with blasticidin and zeocin and the expression of the constructs were verified by western blot analysis of myopodin-c-Myc tag expression upon induction of tetracycline.
Yeast transformation and library screening
The Yeast competent cell preparation was described previously (Yu and Luo, 2006). One hundred microliters of freshly prepared competent AH109 cells were mixed with plasmid DNA (0.25 –0.50 μg) plus 0.5 μg DNA from prostate Yeast Two Hybrid cDNA library constructed in pACT2 in 0.5 ml of PEG/LiAc, incubated at 30°C for 30 min. Following this initial incubation with plasmid DNA, the cell solution was combined with 20 μl of dimethyl sulfoxide (DMSO) and subjected to 15-min incubation at 42°C. The cells were pelleted, re-suspended in 1 ml YPD medium and shaken at 30°C for 40 min. The transformed cells were then pelleted, re-suspended in 0.5 ml 0.9% NaCl and plated onto the appropriate SD agar plate. The transformants were first plated on low and medium stringency plates of SD-Leu/-Trp and SD-Leu/-Trp/-His, respectively. The grown colonies were subjected to the α-galactosidase assay as described previously (Yu and Luo, 2006) and allowed to grow further in the high stringency plate (SD-Ade/-His/-Leu/-Trp).
Validation of protein interactions in AH109
Plasmid DNA from positive clones were isolated from yeast, transformed into E. coli., and selected with ampicillin (100 μg/ml) to obtain genes interacting with the bait-domain fusion protein. These clones were then identified through sequencing. For validation, pACT2-ILK, one of the isolated clones was cotransformed with pGBKT7-MyoN, MyoM, MyoC into AH109 yeast cells and plated on a SD-Ade/-His/-Leu/-Trp high stringency medium, respectively. X-α-gal was used as an additional indicator for protein-protein interaction. Vector pCL was used as positive control for the transformation and X-α-gal. The cotransformation of pGADT7T and pGBKT7-53 was the positive control, and pGADT7T and pGBKT7-Lam the negative control for the interaction of BD and AD.
Immunoprecipitation
Antibody against myopodin or Ilk (Santa Cruz biontech Inc) was incubated with ExactaCruz TM A, or B IP matrix (Santa Cruz Biotech. Inc.), respectively, for 1 hour at 4°C. The IP matrix was pelleted, washed with PBS and further incubated with precleared cell lysates of myopodin expression clone for 16 hours at 4°C. IP matrix was then pelleted and washed four times with RIPA buffer. The bound proteins were eluted with SDS-PAGE sample buffer. The bound ILK or myopodin was electrophoresed in 8% SDS-PAGE and immunoblotted with anti-ILK or myopodin antibodies.
GST fusion proteins pull down to examine ILK/myopodin binding
The cells with GST-myopodin construct were grown in 10ml of LB medium supplemented with ampicillin (100 μg/ml) overnight at 37 °C and continuously shaked at 37 °C upon 10x dilution with LB. Once the cell density of 0.5 reached, IPTG was added to the cells for a final concentration of 1 mM for additional 4 hour incubation. The cells were then pelleted, re-suspended in 4 ml of PBS, and sonicated for 2 min. The proteins were solubilized in 1% triton X-100 and collected after centrifugation at 15,000 g for 5 min. The GST and GST fusion proteins were purified through the Glutathione Sepharose 4B column (GE Bioscience, Piscataway, NJ). The pre-cleared cell lysates were then incubated with GST fusion protein packed on Glutathione Sepharose 4B at 4°C for 2 h. The column was spun at 3000 g at room temperature for 1 min, and further washed twice with PBS. The proteins were eluted from the column with 40 μl of SDS-PAGE gel sample loading dye. SDS-PAGE protein gel and Western blot analyses were subsequently conducted.
Immunofluorescence staining
The myopodin-over-expression cells were cultured on chamber slides for 24 h, then washed with PBS twice, fixed with 4% paraformaldehyde for 1 h at room temperature (Ren et al., 2007), again washed twice with PBS and blocked with PBS containing 10% donkey serum and 0.4% Triton X-100 for 30 min. The cells were then incubated with rabbit antibody against myopodin (MyoC Ab) and goat antisera against ILK (Santa Cruz Inc, CA) at 4°C for 16 h. Washed twice with PBS, the cells were then incubated with two secondary antibodies (rodamin conjugated donkey anti-rabbit and FITC conjugated donkey ant-goat second antibodies) for 1 h at room temperature. The slides were then washed with PBS twice before addition of 4'-6-Diamidino-2-phenylindole (DAPI) for 5 min incubation. With additional washes with PBS, slides were mounted with immuno-mounting medium. Immunofluorescence staining was examined under confocal microscope.
MBP kinase assay
Cell lysates from PITT1 and PITT2 cells were prepared after they were induced by 5 µg/ml of tetracycline for 3 days. Immuno-purified ILK was obtained by incubation of PITT1 and PITT2 cell lysates with ILK goat antibody (Santa Cruz Biotech. Inc.) at 4°C, overnight, and continuous 3 hours after adding 25 μl of protein A/G PLUS agarose. The colum was pelleted by centrifugation at 3000 rpm for 5 minutes and further washed with 1 ml of RIPA buffer for 4 times. immune-purified ILK 10 μl was then applied to MBP kinase assay (Upstate, NY). The procedure was as shown in the manual of the manufacturer. Briefly, the wells containing MBP were wetted with 1×PBS 200 μl for 15 minutes. After removing PBS, kinase assay cocktail containing ATP/Mg2+ 10 μl, 1× dilution buffer 30 μl and purified ILK were added to the wells and were incubated at 30°C for 20 minutes. Following 3 times washing with PBS and 30 minute blocking in the blocking buffer, anti-phospho-MBP antibody conjugated with HRP was added and incubated at 4C, overnight. After removed the antibody and washed 5 times with wash buffer and PBS, LumiGlo chemiluminescent subrate A and B were added to the wells and the luminescence were read in Luminometer (GENio). The wells containing the reacts without ILK antibody in the IP was used as negative control in the assay.
Phosphorylation and kinase assay
ILK was purified by immunoprecipitation of ILK from cell lysate by using ILK antibody and protein G agarose, and GST-myoN fusion protein was purified by incubating GST-myoN bacterial cell lysate with sepherose 4B. After washing with PBS buffer, GST-myoN packed beads were treated with Shrimp phosphostase (New England biolab) at 30°C for 15 minutes, then washed with PBS for three times. The combination of purifed ILK and dephosphorylated GST-myoN were incubated in equal volume of 2× kinase assay cocktail (20 mM Tris-HCl pH 7.4, 10 mM MgCl2, 2mM MnCl2,100nM ATP) by rocking at 30°C for 1 hour. The pellet (beads) was then collected by centrifugation at 3000 rpm for 1 minute and GST-myoN was eluted through sepharose 4B with reduced glutathione. The elute was resolved by electrophoresis on 7% SDS-PAGE polyacrylamide gel. Phosphoserine and phosphothreonine antibodies (upstate, NY) were blotted on the membrane. The elute of dephosphorylated GST-myoN without kinase reaction was used as a negative control.
Colony Formation assay
The cells were induced with tetracycline 5μg/ml for 3 days and transfected with siRNA by lipofectamine™ 2000 (Invitrogen). Three days later, the cells were harvested and the colony formation assay was similar to those previously described(Ren et al., 2007). One thousand cells were plated in each well of 6-well plate, and triplicate experiments were performed for each cell condition. Medium was changed every 4 days. On the 14th day, the plates were stained with 0.1% crystal violet, and colonies with diameter of more than 2 mm were counted.
Wound Healing assays
For the wound healing assay (Yu and Luo, 2006), tetracycline induced or uninduced cells were cultured in 6-well culture plates in the medium described above, and transfected with siRNA for 72 hours. At the surface of confluenced culture plate, a plastic pipette tip was drawn across the center of the well to produce a clean crevice that was 1 mm wide. Microscopic images of the “wounds” were taken in five different areas for each experiment (at an original magnification of X10 with an Olympus inverted system microscope IX) at 0h, 3h, 6h, 24h, 30h, or 66h after wound produced. The images of original locations were taken again, and recovered areas (i.e., the bare area into which cells migrated) was measured as a percentage of the original wound.
Matrigel transmigration analysis
Cells from each clone were suspended in F12K medium containing 0.1% bovine serum albumin added to the upper chamber at 1 x 105 cells/insert. A conditioned medium obtained by incubating NIH 3T3 cells for 24 hours in serum-free DMEM in the presence of 50 μg/ml ascorbic acid were placed in the lower compartment of the invasion chambers as chemoattractants. After 24 hours of culture, the upper surfaces of the inserts were wiped with cotton swabs, and the inserts will be stained with hematoxylin and eosin (H&E). Each experiment was performed twice with each sample in triplicate. The cells that migrate through the Matrigel and the filter pores to the lower surface were counted under a light microscope with five random high-power fields per insert.
Acknowledgement
This work was supported by grants from National Cancer Institute (R56 CA098249 and RO1 CA098249 to JHL) and American Cancer Society (RSG-08-137-01-CNE to YPY).
Footnotes
Conflict of interest. The authors declare no conflict of interest.
References
- Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci U S A. 2007;104:6782–7. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50:844–51. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian V, Alvarez M, Aleman A, Palou J, Bellmunt J, Gonzalez-Peramato P, et al. Discovery of myopodin methylation in bladder cancer. J Pathol. 2008;216:111–9. doi: 10.1002/path.2390. [DOI] [PubMed] [Google Scholar]
- Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase. J Biol Chem. 2001;276:16365–73. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- Donthamsetty S, Bowen W, Mars W, Bhave V, Luo JH, Wu C, et al. Liver Specific Ablation of Integrin Linked Kinase (ILK) in Mice Results in Enhanced and Prolonged Cell Proliferation and Hepatomegaly after Phenobarbital Administration. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008;48:1932–41. doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YC, Yu YP, Nelson J, Wu C, Wang H, Michalopoulos GK, et al. Interaction of integrin-linked kinase and miniature chromosome maintenance 7-mediating integrin {alpha}7 induced cell growth suppression. Cancer Res. 2010;70:4375–84. doi: 10.1158/0008-5472.CAN-09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–6. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Jing L, Liu L, Yu YP, Dhir R, Acquafondada M, Landsittel D, et al. Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol. 2004;164:1799–806. doi: 10.1016/S0002-9440(10)63738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Lin F, Yu YP, Woods J, Cieply K, Gooding B, Finkelstein P, et al. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. American Journal of Pathology. 2001;159:1603–12. doi: 10.1016/S0002-9440(10)63006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, et al. Regulation of protein kinase B/Aktserine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–9. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- Ren B, Yu YP, Tseng GC, Wu C, Chen K, Rao UN, et al. Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst. 2007;99:868–80. doi: 10.1093/jnci/djk199. [DOI] [PubMed] [Google Scholar]
- Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–40. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P. Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene. 2003;22:5298–305. doi: 10.1038/sj.onc.1206616. [DOI] [PubMed] [Google Scholar]
- Wu C. The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H, et al. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol. 2001;153:1251–64. doi: 10.1083/jcb.153.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YP, Luo JH. Myopodin-mediated suppression of prostate cancer cell migration involves interaction with zyxin. Cancer Research. 2006;66:7414–9. doi: 10.1158/0008-5472.CAN-06-0227. [DOI] [PubMed] [Google Scholar]
- Yu YP, Tseng GC, Luo JH. Inactivation of myopodin expression associated with prostate cancer relapse. Urology. 2006;68:578–82. doi: 10.1016/j.urology.2006.03.027. [DOI] [PubMed] [Google Scholar]