Abstract
Background
Ethanol consumption during pregnancy can lead to FetalAlcohol Spectrum Disorder (FASD), which consists of the complete spectrum of developmental deficits including neurological dysfunction. FASD is associated with a variety of neurobehavioral disturbances dependent on the age and duration of exposure. Ethanol exposure in neonatal rodents can alsoinduce widespread apoptotic neurodegeneration and long-lasting behavioral abnormalities similar to FASD. The developmental stage of neonatal rodent brains that are at the peak of synaptogenesis is equivalent to the third trimester of human gestation.
Methods
Male and female C57BL/6By mice were injected with ethanol (20%, 2.5g/kg, two s.c. injections) or an equal volume of saline (controls) on postnatal day 7 (P7). Animals were allowed to mature and at 3 months were tested on an olfactory habituation task known to be dependent on piriform cortex function, a hippocampal-dependent object place memory task, and used for electrophysiological testing of spontaneous and odor-evoked local field potential (LFP) activity in the olfactory bulb, piriform cortex and dorsal hippocampus.
Results
P7 ethanol induced widespread cell death within 1 day of exposure, with highest levels in the neocortex, intermediate levels in the dorsal hippocampus and relatively low levels in the primary olfactory system.No impairment of odor investigation or odor habituation was detected in P7 ethanol exposed 3 months old mice compared to saline controls. However, hippocampal-dependent object place memory was significantly impaired in the P7 ethanol treated adult mice. Odor-evoked LFP activity was enhanced throughout the olfacto-hippocampal pathway, primarily within the theta frequency band, although the hippocampus also showed elevated evoked delta frequency activity. In addition, functional coherence between the piriform cortex and olfactory bulb and between the piriform cortex and dorsal hippocampus was enhanced in the beta frequency range in P7 ethanol treated adult mice compared to controls.
Conclusions
P7 ethanol induces an immediate wave of regionally selective cell death followed by long-lasting, changes in local circuit and regional network function that are accompanied by changes in neurobehavioral performance. The results suggest that both the activity of local neural circuits within a brain region and the flow of information between brain regions can be modified by early alcohol exposure which may contribute to long-lasting behavioral abnormalities known to rely on those circuits.
Keywords: Fetal Alcohol Spectrum Disorders, Olfactory bulb, Piriform cortex, Hippocampus, Local field potentials, Memory, Alcohol
Introduction
Fetal alcohol spectrum disorder (FASD) affects as many as 1 out of 100 children born in the United States, while the more narrowly defined fetal alcohol syndrome affects as many 1 out of 1000(May and Gossage, 2001; May et al., 2009). Ethanol induces toxicity in the developing brain by disrupting proliferation, differentiation, migration, and survival of neural cells (Bonthius and West, 1990; Gil-Mohapel et al., 2010; Ikonomidou et al., 2000; Klintsova et al., 2007; West et al., 1990). FASD is associated with cognitive, behavioral, memory and sensory impairments, as well as heightened susceptibility to seizures (Bell et al., 2010; Berman and Hannigan, 2000; Carr et al., 2010; Mattson et al., 2010; Morasch and Hunt, 2009; Riley and McGee, 2005; West et al., 1990). The specific set of symptoms is dependent on the age, duration and intensity of the ethanol exposure (Riley and McGee, 2005). A variety of animal models of FASD exist which also vary in age, duration and intensity of exposure. These models have proven useful for understanding neural consequences of early ethanol exposure, and both the underlying cell biology of neural damage and how the neural changes contribute to behavioral outcomes. Identifying specific mechanisms at the cellular, synaptic and system levels may contribute to treatments to reverse or alleviate the behavioral consequences.
In addition to neuroanatomical changes (Gil-Mohapel et al., 2010; Godin et al., 2010; Jones and Smith, 1973; Olney et al., 2002b; Saito et al., 2010a), FASD and ethanol exposure during brain development are associated with abnormal electroencephalographic (EEG) activity and sensory evoked potentials in both humans and animals models (Criado and Ehlers, 2010; Kaneko et al., 1993; Loffe et al., 1984; Slawecki et al., 2004). In general, sensory evoked potentials, reflecting synaptic transmission and neural activation through specific central circuits, show modified latency and amplitudes in individuals exposed to ethanol during development (Criado and Ehlers, 2010; Kaneko et al., 1993), suggesting a long-lasting change in local or regional circuit function. EEG recordings also suggest long-lasting changes in abnormal synchronicity, such as enhanced activity in the slow delta (1–4 Hz) and theta (5–15 Hz) bands in adults exposed to alcohol during adolescence (Criado and Ehlers, 2010). Changes in EEG-monitored sleep structure are also reported in adults following ethanol exposure during development (Ehlers and Criado, 2010).
Despite the emerging evidence of long-lasting circuit dysfunction following developmental ethanol exposure, the findings are often difficult to interpret given the complexity of neocortical circuits and multi-synaptic sensory pathways. The present study took advantage of the relative simplicity of the olfactory sensory pathway and its strong connection with the hippocampal formation – a region known to be particularly vulnerable to early ethanol exposure (Gil-Mohapel et al., 2010; Kaneko et al., 1993) – to examine the long-lasting effects of postnatal ethanol exposure on local and regional neural circuit function in adults. Olfaction begins when odors are inhaled through the nose and activate a large population of olfactory sensory neurons, which send axons directly into an extension of the forebrain called the olfactory bulb. Second order neurons within the olfactory bulb, mitral and tufted cells, receive direct glutamatergic input from the sensory neurons and in turn send axons via the lateral olfactory tract to the olfactory cortex. The largest subregion of the olfactory cortex is the piriform cortex which is a trilaminar cortex containing pyramidal neurons, onto which the glutamatergic mitral/tufted cells synapse. Both the piriform cortex and a small subset of mitral cells project to the lateral entorhinal cortex, which serves as the primary input to the hippocampal formation. Thus, within four synapses, odor-evoked activity travels from the nose to the hippocampal formation, where it can induce both single-unit and local field potential responses (Eichenbaum et al., 1987; Heale and Vanderwolf, 1994). In rodents, the glutamatergic principle neurons of this circuit, olfactory sensory neurons, mitral cells, and piriform cortical pyramidal cells all develop very early during prenatal ontogeny (Bayer, 1983; Bayer, 1986; Brunjes and Frazier, 1986; Schwob and Price, 1978), allowing rudimentary olfactory guided behavior even in utero(Smotherman et al., 1990). However, extensive postnatal neurogenesis and synaptogenesis also occur, most notably in olfactory bulb inhibitory granule cell interneurons, whose neurogenesis occurs entirely postnatally and extends throughout life (Bayer, 1983; Carleton et al., 2003).
Simple olfactory behaviors, such as investigation, discrimination and habituation are mediated by computations within the olfactory bulb – piriform cortex circuitry. Manipulations of the olfactory bulb and/or piriform cortex have been shown to modulate odor discrimination and long- and short-term odor habituation (Best et al., 2005; Mandairon et al., 2006; McNamara et al., 2008; Yadon and Wilson, 2005). For example, short-term odor habituation is mediated by a metabotropic glutamate receptor dependent synaptic depression of mitral cell input to piriform cortex pyramidal cells (Best et al., 2005; Best and Wilson, 2004). Furthermore, olfactory perceptual learning is associated with changes within both the olfactory bulb (Fletcher and Wilson, 2003; Mandairon et al., 2006) and piriform cortex (Kadohisa and Wilson, 2006; Li et al., 2008; Wilson, 2003). Other forms of odor memory involve interactions between the olfactory system and regions such as the hippocampus, orbitofrontal cortex and amygdala (Chapuis et al., 2009; Cohen et al., 2008; Kay and Beshel, 2010; Martin et al., 2004; Moriceau and Sullivan, 2004). Thus, understanding how ethanol exposure in the developing brain affects information flow through the olfactory-hippocampal network may provide insight into the concomitant behavioral consequences.
Early ethanol exposure is known to affect the olfactory system, though this work has primarily involved repeated or prolonged pre- or postnatal exposure. Here, we examined the effects of ethanol exposure at postnatal day 7 (P7) on spontaneous and odor-evoked activity within the olfactory bulb, piriform cortex and dorsal hippocampus of adult mice. P7 ethanol exposure models third trimester maternal binge alcohol consumption, and is well characterized at the cellular and anatomical levels (Olney et al., 2002a; Olney et al., 2002b; Saito et al., 2010a). We also examined piriform cortical-dependent odor-evoked behavior (Yadon and Wilson, 2005) in the same mice, as well as hippocampal-dependent object place memory (Ennaceur et al., 1997). The results suggest long-lasting changes in information flow through this simple circuit following brief ethanol exposure in the neonatal brain.
Methods
Subjects
Male and female C57BL/6By mice were used as subjects. Mice were bred and born in the Nathan Kline Institute animal facility and mothers maintained on ad lib food and water at all times. All procedures were approved by the Nathan Kline Institute IACUC and were in accordance with NIH guidelines for the proper treatment of animals. Mothers and their litters were housed individually in standard mouse cages. On postnatal day 7 (P7) pups were given ethanol exposure through subcutaneous injections of ethanol (20 % solution in saline) at 2.5 g/kg or saline injections. Two injections were given with a 2h inter-injection interval. After injections, pups were returned to the litter. Weaning occurred at P22–25. Animals were tested when 3 months old.
Behavioral assays
Mice were tested in either an olfactory or spatial memory task. The olfactory memory assay (Wesson olfactory task) involved presentation of a scented cotton applicator to individually housed mice and quantifying investigation time (Wesson et al., 2010). Applicators had 200 µL of one of 4 molecularly and perceptually diverse odorants (acetic acid, eugenol, heptanal or nonane; Sigma) infused and the applicator end was inserted into a plastic pipette tip to prevent the mouse from coming in physical contact with the odorant. The scented end of the applicator was inserted into the cage for 20 sec and the duration of time the mouse spent sniffing within 1 cm of the tip was recorded. The applicator was then removed for 30 sec and the same odorant then presented again. Each odorant was presented for 4 trials with a 30 sec inter-trial interval. Thirty sec after 4 trials with one odorant, the next was presented and the process repeated for each odor.
The object placement task is a hippocampal dependent task (Ennaceur et al., 1997), and involved quantifying investigation of three visually and potentially olfactory distinct objects (a plastic toy mouse, a polypropylene bottle cap and a glass vial, all of approximately equal size, 3–5 cm diameter) placed in an arena (20cm W × 44 cm L × 20 cm H) lined with corncob bedding. On day 1, mice were allowed to explore the arena freely for two 5 min sessions (20 min inter-trial interval [ITI]) with no objects present. On day 2, the objects were placed in specific locations and the mice allowed to explore for three, 5 min sessions with a 5 min ITI. The last session was videotaped and the duration of investigation of each object determined. Finally, on day 3, one object was moved from its original location to a new location and the mice allowed to explore for a single 5 min session. This session was also videotaped and the duration of investigation of each object determined by two independent observers (inter-rater correlation of investigation times = 0.71). Final data were derived by taking the mean investigation times of the two independent observers. The ethanol injections often left a small white patch of fur at the injection site which was visible in these mature mice, thus observations truly blind to the drug condition were not possible.
Electrophysiology
Mice tested in the olfactory behavioral assays were subsequently used for electrophysiological analyses (n= 6 saline and 6 ethanol). They were anesthetized with urethane (1.5 g/kg) and placed in a stereotaxic apparatus. Respiration was monitored with a piezoelectric plethysmograph strapped to the animal’s chest. Monopolar tungsten electrodes were placed in the olfactory bulb (granule cell layer), anterior piriform cortex (Layer III), and dorsal hippocampus (primarily CA1). One animal in each group was not used for olfactory bulb recordings. Signals were amplified and filtered (0.5–300Hz), digitized at 10kHz, and stored and analyzed with Spike2 software (Cambridge Electronic Design, Inc). Spontaneous and odor-evoked local field potentials (LFPs) were analyzed in all three regions with fast-fourier transforms (FFT; 2.49Hz bins). For display purposes, pseudocolor displays of time-FFT LFP analyses were created with the event-related spectral perturbation (ERSP) analysis in the EEGLAB toolbox for MatLab(Delorme and Makeig, 2004). The odorant for all tests was a 2 sec pulse of saturated isoamyl acetate (Sigma). The odor stimulus was repeated four times and mean baseline (3 sec prior to odor onset) and odor (3 sec starting at odor onset) evoked activity were used in the analyses. In addition to FFT analyses, coherence of spontaneous LFPs in the piriform cortex and dorsal hippocampus was determined using the ‘cohere’ script function within Spike2, with 5 Hz resolution. Additional details of these analyses can be found in (Wilson and Yan, 2010). Finally, following the LFP recordings, the olfactory bulb electrode was used as a stimulating electrode to evoke monosynaptic potentials in the piriform cortex for paired-pulse assessment of local circuit function and feedback inhibition (Ketchum and Haberly, 1993; Neville and Haberly, 2004). Two constant current pulses (500 µA, 0.1 mA) were delivered at varying inter-stimulus intervals (20, 50, 100, 300 ms) with a 10 sec inter-pair interval. The slope of the initial positive evoked potential was used as a measure of response amplitude and the slope of the response to the second, or test pulse was expressed as a percentage of the response to the first, or conditioning pulse. Comparisons were made between treatment groups using ANOVA’s and post-hoc Fisher tests.
Histology
Following electrophysiological recordings, brains were removed, fixed in 10% formalin, and subsequently sectioned and stained with cresyl violet to determine electrode placements. In addition, in order to confirm the effects of P7 ethanol on apoptotic neurodegeneration, separate animals were sacrificed at 8 hr post P7 ethanol treatment for cleaved (active) caspase-3 immunostaining (Olney et al., 2002) to identify cells undergoing apoptosis (D'Mello et al., 2000), or at 19 hr for Fluoro-Jade staining of degenerating cells (Schmued et al., 1997) using the Fluoro-Jade C staining solution (Millipore).
The extent of neurodegeneration detected by Fluoro-Jade C staining was quantified in the retrosplenial cortex (RS), hippocampal CA (CA), anterior and posterior piriform cortex (aPCX and pPCX), and olfactory bulb (OB). The number of stained cells in each area of interest (AOI) and the area of AOI were measured using the Image-Pro software version 4.5 (MediaCybernetics, Silver Spring, MD, USA). The boundary of these regions was defined in accordance with the atlas of the developing mouse brain (Paxinos et al., 2007). The Fluoro-Jade C appeared to stain not only irregular-shaped degenerating cell bodies but also other structures such as fragmented axons and dendrites. For quantification, only the degenerating cell bodies were counted. Automatic cell counting after correction (splitting adjacent cells and excluding artifacts) gave similar results to those of manual counting. The extent of neurodegeneration was expressed as the number of Fluoro-Jade C-stained cells per square millimeter. For each brain, data from four to five sections (50 µm thick) were averaged, and four brains per each treatment group were analyzed. The sections analyzed for RS corresponded to those between 4.35 and 5.19 mm from the most rostral part of P6 brain in accordance with the atlas of Paxinos et al.(2007). Likewise, between 4.35 and 5.19 mm, between 2.79 and 3.51 mm, between 4.35 and 5.19 mm, and between 0.99 and 1.35 mm were used for CA, aPCX, pPCX, and OB, respectively.
Results
Neurodegeneration
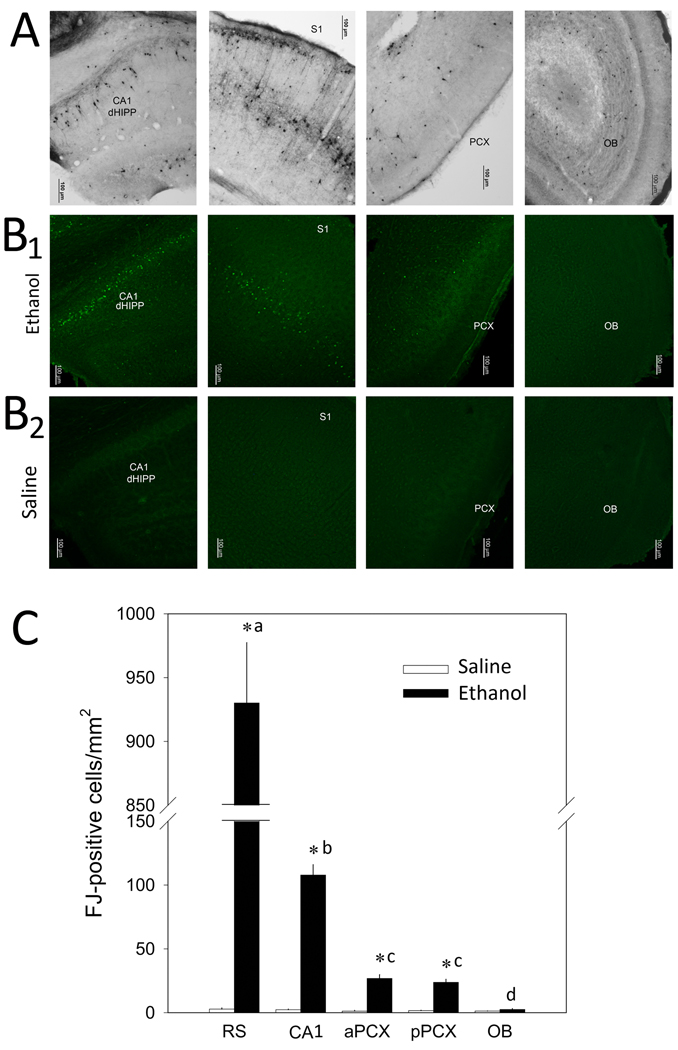
As has been reported previously (Olney et al., 2002a; Olney et al., 2002b; Saito et al., 2010a; Saito et al., 2007), P7 ethanol induced widespread caspase-3 activation and neurodegeneration within the first day after injection (Figs 1 and 2A). Interestingly, the piriform cortex and olfactory bulb appear relatively spared, with only minor cleaved caspase-3 and Fluoro-Jade (Fig. 2B) positive cells compared to neocortical and hippocampal regions. Quantification of Fluoro-Jade labeled cells (Fig. 2C) showed significantly greater neocortical cell death 19 hr post P7 ethanol exposure than hippocampal cell death, and significantly greater hippocampal cell death than olfactory system (olfactory bulb or piriform cortex)cell death. A drug treatment X brain region ANOVA revealed a significant main effect of drug treatment (F(1,71) = 620.5 p < 0.001) and a significant drug treatment X brain region interaction (F(4,71) = 424.2, p < 0.001). Post-hoc tests revealed a significant difference in Fluoro-Jade positive cells between ethanol and saline treated mice in all areas except the olfactory bulb (p < 0.01). In addition, the ethanol treated neocortex (retrosplenial cortex) had significantly more positive cells than all other areas, and the hippocampus (CA1) had more positive cells than the piriform cortex and olfactory bulb (p < 0.01). The anterior and posterior piriform cortex also had significantly more positive cells than the olfactory bulb (p < 0.05).
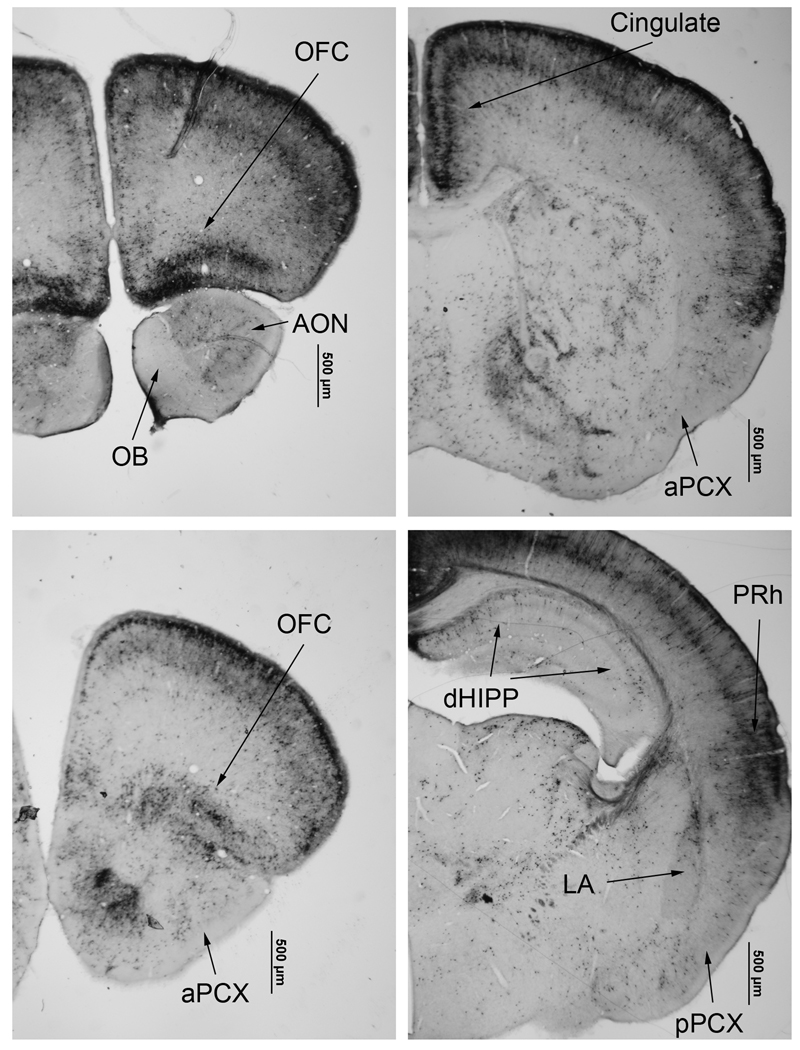
Figure 1.
Active caspase-3 immunostaining of tissue acquired 8 hr after P7 ethanol treatment. Widespread active caspase staining is observed throughout the forebrain, though with apparently reduced staining in olfactory regions such as the olfactory bulb (OB), anterior olfactory nucleus (AON) and anterior (aPCX) and posterior piriform cortex (pPCX). Abbreviations, OFC = orbitofrontal cortex, LA = lateral amygdala, PRh = perirhinal cortex, dHIPP = dorsal hippocampus.
Figure 2.
(A) Active caspase-3 immunostaining at 8 hr after P7 ethanol from regions of the field CA1 of the dorsal hippocampus (dHIPP), somatosensory neocortex (S1), piriform cortex (PCX) and olfactory bulb (OB). (B) Fluoro-Jade staining at 19 hrin the same regions in ethanol (B1) and saline control (B2) mice. (C) Quantification of Fluoro-Jade positive cells in neocortex (retrosplenial cortex, RS), hippocampus (CA1), anterior and posterior piriform cortex (aPCX and pPCX) and olfactory bulb (OB). Asterisks signify significant difference between ethanol and control within brain regions. Different letter markings denote ethanol-treated brain regions that are significantly different from each other.
Behavior
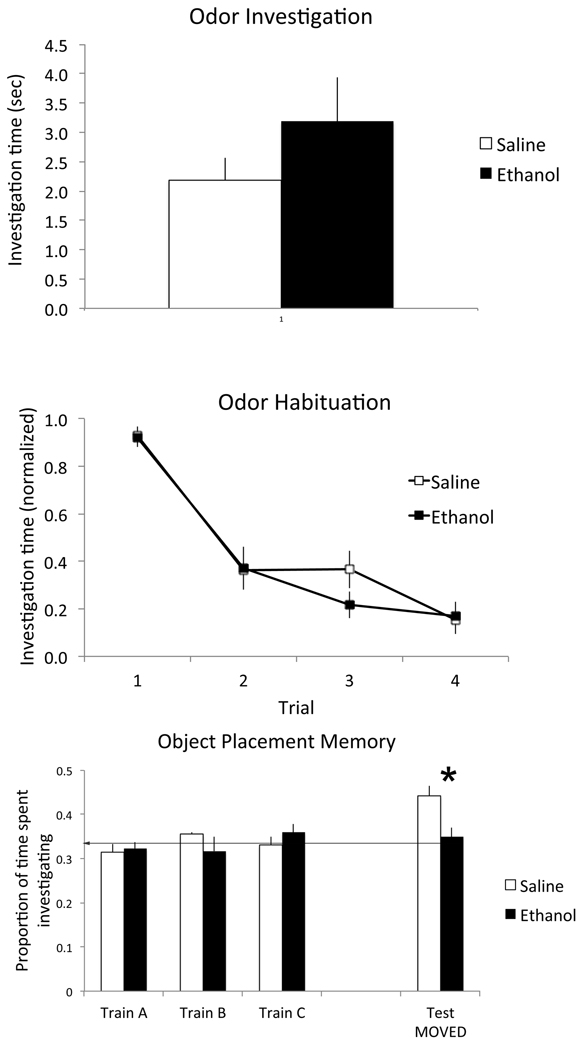
As shown in Figure 3, simple odor investigation and short-term habituation in 3 month old mice were unimpaired by P7 ethanol exposure. Initial investigation of an odorant introduced into the animals cage was similar in both P7 ethanol (n=8) and saline (n=8) treated mice (t-test, t(14) = 1.16, N.S.). Furthermore, both groups (treatment × trial repeated measures ANOVA; main effect of trial, F(3,42) = 59.59, p < 0.01) showed piriform cortex dependent short-term habituation of the investigation response (Yadon and Wilson, 2005) which did not differ between groups (main effect of treatment, F(1,42) = 0.19, N.S.).
Figure 3.
Behavioral performance on the Wesson olfactory task. Adult mice exposed to ethanol at P7 showed no deficits in odor investigation (top) or piriform cortex-dependent odor habituation (middle), but were significantly impaired in hippocampal-dependent object placement memory (bottom). In the object placement task, all objects were investigated equally during the training phase, but in controls the moved object was investigated significantly more than in P7 ethanol treated mice.
In contrast hippocampal-dependent object place memory was significantly impaired in adult mice following P7 ethanol treatment (Fig. 3). Investigation of the objects during training trials did not differ, either between treatment groups (saline (n=4) mean total investigation of all objects = 34.7 s, ethanol (n=5) = 36.1 s, t(7) = 0.14, N.S.) or between objects (ANOVA, treatment X object, main effect of object, F(2,21) = 0.15, N.S.; main effect of treatment F(1,21) = 0.00, N.S.). Thus, each object was investigated equally during the final training trial in both P7 saline and ethanol treated mice. However, the P7 saline treated mice recognized which object was moved on the test trial by investigating it more, while the ethanol treated mice did not (t(7) = 3.32, p < 0.05), suggesting impaired hippocampal-dependent memory following P7 ethanol treatment.
Electrophysiology
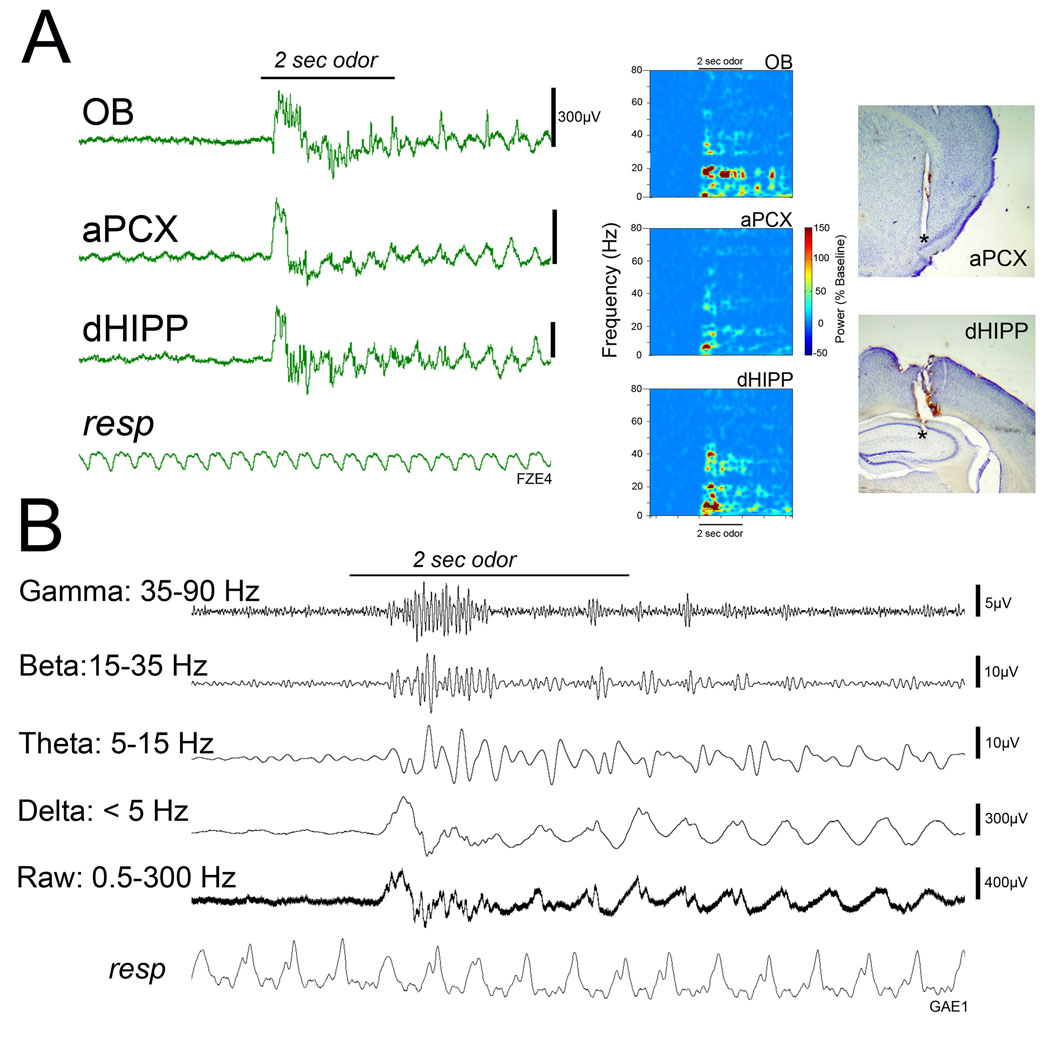
Spontaneous and odor-evoked local field potential activity were examined in the olfactory bulb, anterior piriform cortex and dorsal hippocampus in urethane anesthetized adult mice. As shown in Figure 4, odor stimulation evoked oscillatory responses in all three regions and included activity in the delta (0.5–5 Hz), theta (5–15 Hz), beta (15–35 Hz) and gamma (35–90 Hz) bands. In the olfactory bulb and piriform cortex, spontaneous activity generally included a strong low frequency entrainment to respiration.
Figure 4.
(A) Representative local field potentials (Left) and power spectral analyses (Right) of responses to odors recorded simultaneously in the olfactory bulb (OB), anterior piriform cortex (aPCX) and dorsal hippocampus (dHIPP) of an adult mouse exposed to ethanol at P7. Odor evoked activity is conveyed throughout these three regions in the theta, beta and gamma frequency bands. Vertical bar represents 300 µV in each trace. (B) Odor-evoked LFP activity in a different mouse filtered for each of the frequency bands investigated here.
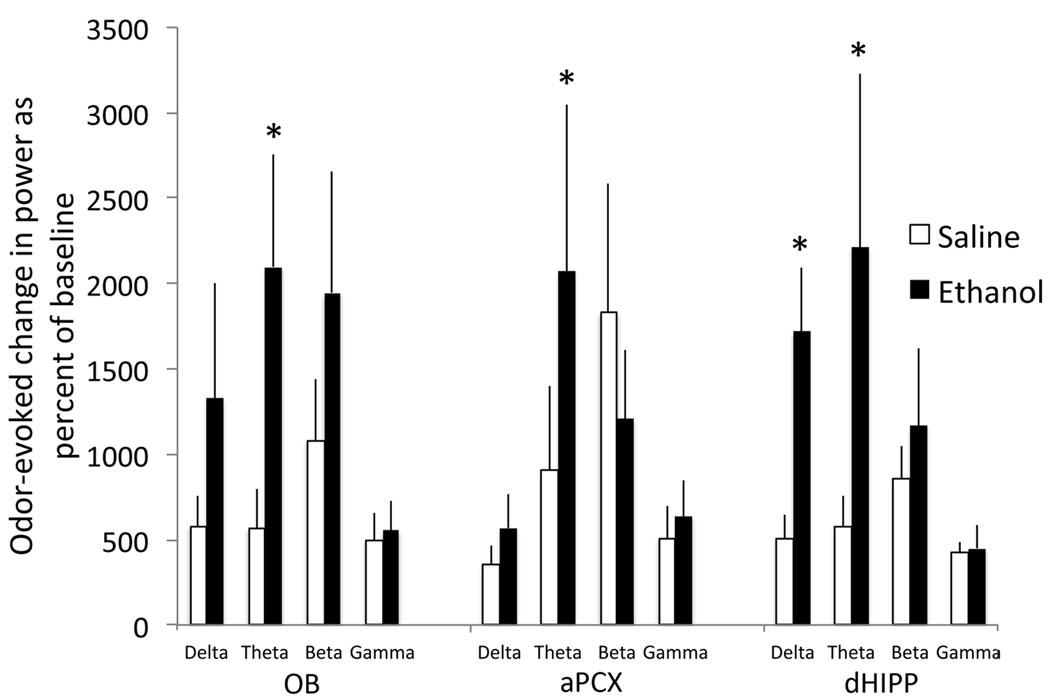
P7 ethanol treatment had no significant effect on spontaneous activity in the adult olfactory bulb-piriform-hippocampal circuit. A 3-way ANOVA (treatment X region X frequency band) on spontaneous activity revealed a significant main effect of frequency band (F(3,64) = 7.65, p < 0.01), but no significant effect of drug treatment or any interactions. In contrast to spontaneous activity, P7 ethanol significantly enhanced odor-evoked activity. This effect was most robust in the dorsal hippocampus but was also seen in the olfactory bulb and piriform cortex (Fig. 5). A 3-way ANOVA (treatment X region X frequency band) on odor-evoked activity revealed a significant main effect of frequency band (F(3,64) = 3.89, p < 0.05) and a main effect of drug treatment (F(1,64) = 6.47, p < 0.05). There were no significant interactions. Post-hoc Fisher tests revealed that odor-evoked theta band power was enhanced in all three regions (p < 0.05). In addition in the dorsal hippocampus, odor-evoked delta band activity was also significantly enhanced (p < 0.05). These results suggest a long-lasting robust and potentially regionally specific change in the profile of odor-evoked activity in the olfacto-hippocampal circuit induced by early ethanol exposure.
Figure 5.
Mean odor-evoked LFP activity in each brain region expressed as a percent of pre-odor spontaneous activity within each frequency band. A 3-way ANOVA revealed a significant main effect of both frequency band and of drug treatment. Odor-evoked activity was enhanced across all three regions in the theta band. The dorsal hippocampus also showed a selective, strong enhancement in the delta band that was not observed in the other regions. Asterisks signify significant differences from saline control, p < 0.05.
As a measure of functional connectivity between the regions of the olfacto-hippocampal pathway, coherence of spontaneous local field potentials between the olfactory bulb and piriform cortex and between the piriform cortex and dorsal hippocampus were examined. At least 50 sec of continuous spontaneous activity was measured to allow assessment of tonic state of coherence rather than transient fluctuations. As shown in Figure 6, coherence between the olfactory bulb and piriform cortex was enhanced in P7 ethanol treated mice compared to saline controls. A 2-way ANOVA (treatment X frequency band) revealed a significant main effect of treatment group (F(1,136) = 27.28, p < 0.01. Post-hoc Fisher tests revealed significant enhancement in coherence between groups at 0.5, 15, 20, 25 and 30 Hz). Furthermore, coherence between the piriform cortex and dorsal hippocampus was enhanced in P7 ethanol treated mice compared to controls, particularly in the beta frequency band (ANOVA, treatment X frequency, main effect of treatment, F(1,170) = 9.32, p < 0.01. Post-hoc Fisher tests revealed significant differences between ethanol and saline at 15, 20, 25 and 30 Hz).
Figure 6.
Coherence analysis of anterior piriform cortex = olfactory bulb (A and B) and anterior piriform cortex – dorsal hippocampus (C and D) functional connectivity during spontaneous activity in adult mice exposed to ethanol and saline at P7. Coherence was significantly enhanced in the beta frequency range (15–35 Hz) in P7 ethanol exposed mice compared to controls in both pathways and also the delta frequency band (0.5 Hz) in the piriform cortex - olfactory bulb pathway. Asterisks signify significant differences from control, p < 0.05.
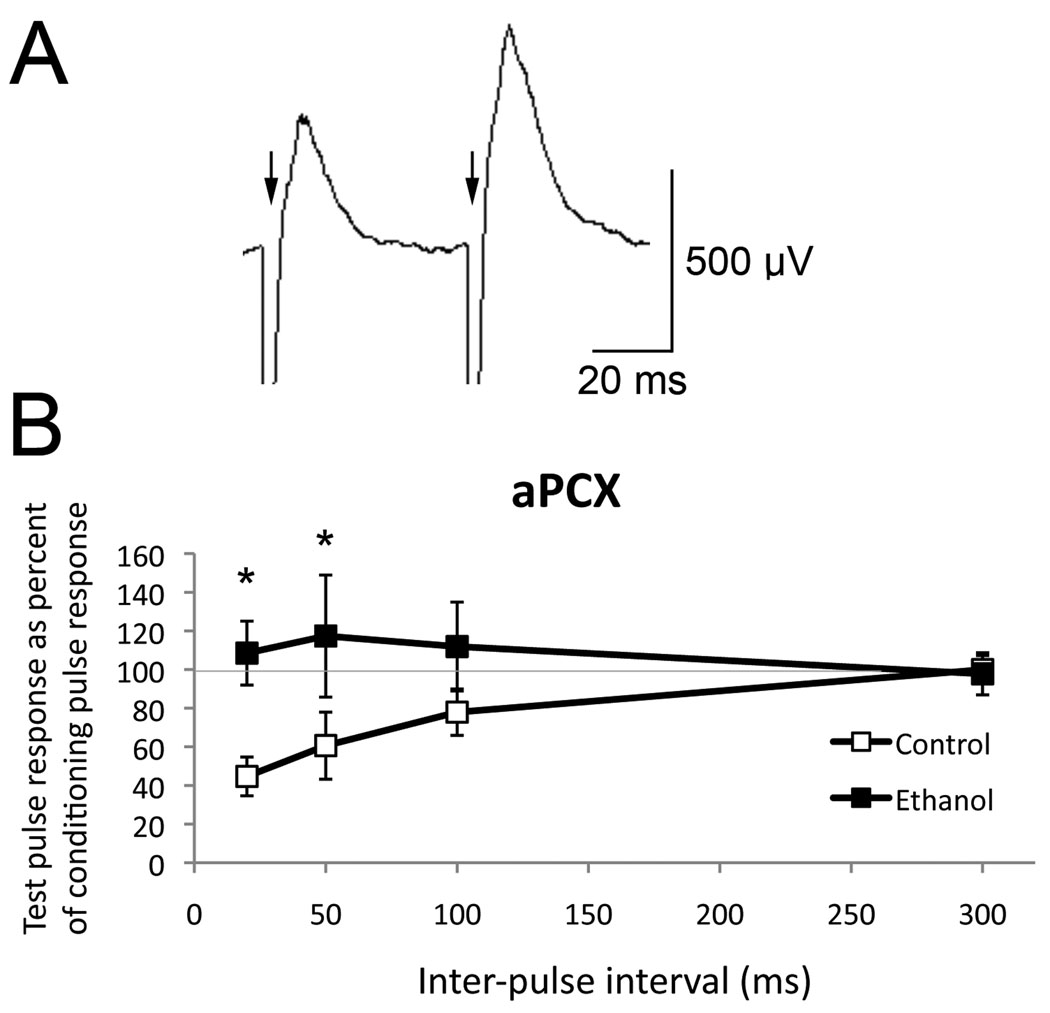
Finally, as an additional assay of local circuit function within the piriform cortex, paired-pulse testing was performed by electrical stimulation of the olfactory bulb and examination of the monosynaptic evoked potentials in the piriform cortex (Fig. 7). Paired-pulse testing can be used to indicate the functioning of feedback inhibition.For example, in saline treated mice, a conditioning pulse induced depression of the response to the test pulse delivered at 20 or 50 ms later which decayed by 300 ms, suggesting activation of local feedback synaptic inhibition (Ketchum and Haberly, 1993; Neville and Haberly, 2004). In contrast, in P7 ethanol treated mice, the same stimulation induced no inhibition. P7 ethanol and saline treated mice were significantly different at 20 and 50 ms inter-pulse intervals (repeated measures ANOVA, treatment X inter-pulse interval interaction, F(3,24) = 3.11, p < 0.05. Post-hoc Fisher tests revealed a significant difference between P7 ethanol and saline at 20 ms and 50 ms, p < 0.05; one animal in each group was not included due to no acquired data).
Figure 7.
Paired-pulse analysis of the monosynaptic response in anterior piriform cortex to olfactory bulb input. (A) Representative evoked potentials in a P7 ethanol treated mouse. Arrows mark the stimulus artifact. (B) Paired-pulse depression was significantly reduced in the anterior piriform cortex of adult mice exposed to ethanol at P7 compared to controls, suggesting enhanced excitability and/or reduced synaptic inhibition in the P7 ethanol exposed mice. Asterisks signify significant difference from control, p < 0.05.
Discussion
The present results suggest a long-lasting modification of local circuit oscillatory function and corresponding behavior following a single day of ethanol exposure during early postnatal development. Furthermore, in addition to changes in local oscillatory activity within regions, ethanol exposure during the synaptogenic period modified functional connectivity between brain regions in the adult. Thus, both local and regional circuit function were disturbed by P7 ethanol exposure in the mouse. More specifically, the olfactory system was relatively spared of the initial wave of cell death after P7 ethanol, as was simple olfactory behavior. In contrast in the hippocampus, which showed significantly greater cell death after P7 ethanol exposure than the olfactory system, hippocampal-dependent behavior was disrupted. In addition to these neuroanatomical and behavioral effects, P7 ethanol induced hyper-excitability/hyper-synchrony in response to odorstimulation throughout the olfacto-hippocampal pathway in the theta frequency band, with additional changes observed in the delta frequency range selectively within the hippocampus. These changes in sensory evoked activity are similar to hyper-excitability previously reported after early ethanol exposure(Cortese et al., 1997; Slawecki et al., 2004), and may contribute to the impaired behavioral function. Together, these results suggest that P7 ethanol exposure induces long-lastingchanges in local circuit and regional network function that are associated with system-specific changes in behavioral performance. Our data suggest that both the activity of local neural circuits within a brain region and the flow of information between brain regions can be modified by developmental alcohol exposure, which may contribute to behavioral abnormalities known to rely on those circuits.
The effects of developmental ethanol exposure are heavily dependent on the brain growth stage and duration of exposure. It is unclear if the effects observed here are specific to P7 exposure, or would also be observed at other ages. For example, P7 ethanol appeared to leave the olfactory cortex relatively spared of caspase-3 activation and cell death compared to other forebrain regions including the hippocampus. In contrast, early gestational ethanol exposure (e.g., gestational day 8) can result in nearly complete failure of olfactory bulb development (Godin et al., 2010; Parnell et al., 2009). Furthermore, gestational and/or prolonged early postnatal ethanol exposure can modify olfactory perception (Youngentob and Glendinning, 2009; Youngentob et al., 2007a; Youngentob et al., 2007b) and odor memory (Abate et al., 2002; Hunt and Morasch, 2004; Morasch and Hunt, 2009) in rodents, and odor preferences in humans (Mennella and Beauchamp, 1991). The principle neurons of the primary olfactory pathway mature early in development (Bayer, 1986; Mair et al., 1982; Schwob and Price, 1978; Schwob and Price, 1984), and odor-guided behavior expressed at birth is critical for survival (Sullivan and Holman, 2010). In contrast, extensive neurogenesis occurs postnatally in the olfactory bulb, and in fact continues throughout life (Bayer, 1983; Carleton et al., 2003). Understanding this apparent differential sensitivity to developmental ethanol exposure across brain regions will require further analyses. Nonetheless, the relative sparing of the olfactory bulb and cortex corresponded well with the relatively reduced impact P7 ethanol on olfactory behavior observed here.
Although P7 ethanol-evoked cell death was highly region dependent (Fig. 1), the observed physiological effects were more widespread. For example, despite no detectable difference in spontaneous activity, odor stimulation evoked enhanced LFP oscillations in the olfactory bulb, piriform cortex and dorsal hippocampus compared to saline controls. These enhanced responses were present in the theta frequency band throughout the olfacto-hippocampal circuit, as well as the delta frequency band in the hippocampus. In awake animals hippocampal theta activity occurs primarily during active exploration and sniffing, and facilitates synaptic plasticity(Buzsaki, 2006; Larson et al., 1986).In the olfactory system of awake animals, theta may also correspond to active sniffing, though in the anesthetized animal odor-evoked theta can occur without a change in respiratory activity (e.g., Fig. 4). The non-respiratory mechanism of theta activity in the olfactory system are unknown, although theta rhythm may facilitate information flow through the olfactory bulb (Margrie and Schaefer, 2003). Thus, the changein sensory evoked theta in the ethanol exposed mice may alter both normal sensory processing and memory. As shown in Fig. 4, delta frequencies in these anesthetized mice largely correspond to the respiratory cycle, and thus may be driven primarily by simple sensory afferent input. The role of delta frequency activity in the hippocampus, and how changes in this activity may affect hippocampal function, are unknown.
In piriform cortex, the observed changes in sensory evoked LFP oscillations may reflect, in part, a change in local synaptic inhibition. Paired-pulse analysis showed a significant decrease in synaptic inhibition in piriform cortex of P7 ethanol exposed mice. A change in synaptic inhibition could enhance excitability or synchrony of neural ensembles and contribute to the enhanced sensory-evoked activity. Work is ongoing to examine the differential susceptibility of pyramidal and interneurons within the olfacto-hippocampal circuit to early ethanol exposure.
Finally, in addition to the local circuit changes observed here, there was enhanced coherence of spontaneous activity between the piriform cortex and both the olfactory bulb and hippocampus. This enhanced coherence was expressed primarily in the beta frequency range and suggests greater functional connectivity between these regions. In the olfacto-hippocampal pathway, beta frequency oscillations are believed to reflect activity in reciprocal connections over larger anatomical scales, such as between the piriform cortex and olfactory bulb (Neville and Haberly, 2003) or between the hippocampus/entorhinal cortex and piriform(Gourevitch et al., 2010; Kay and Beshel, 2010). Activity in this frequency range is strongly affected by olfactory learning and task demands, and thus disruption of normal beta frequency coherence may be expected to impact these functions. Furthermore, enhanced regional functional connectivity combined with the observed hyperexcitability/hypersynchronycould be relevant to understanding the enhanced seizure susceptibility following developmental ethanol exposure (Bell et al., 2010; Mattson et al., 2010; Riley and McGee, 2005). The reduced inhibition observed in the piriform cortex could also contribute to seizure susceptibility.
Using behavioral assays with known underlying neural substrates combined with electrophysiological recordings from those substrates has allowed us to begin understand how long-term traces of developmental ethanol exposure can differentially and selectively modify behavior. Further work with more sensitive olfactory behavioral assays will be required to fully understand the selectivity and age-dependence of these effects. Tying these system level functional disturbances to underlying cell biological cascades induced by the ethanol exposure (Gil-Mohapel et al., 2010; Olney et al., 2002a; Saito et al., 2010b; Saito et al., 2007) may help identify potential treatments or therapies for addressing neurobehavioral abnormalities associated with FASD.
Acknowledgments
This work was supported by an NIH/NIAAA grants R01 AA015355 to M.S. and R01 AA019443 to B.S.B., and an NIH/NIDCD grant R01 DC003906 to D.A.W.
References
- Abate P, Varlinskaya EI, Cheslock SJ, Spear NE, Molina JC. Neonatal activation of alcohol-related prenatal memories: impact on the first suckling response. Alcohol Clin Exp Res. 2002;26(10):1512–1522. doi: 10.1097/01.ALC.0000034668.93601.8F. [DOI] [PubMed] [Google Scholar]
- Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50(2–3):329–340. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Neurogenesis in the rat primary olfactory cortex. Int J Dev Neurosci. 1986;4(3):251–271. doi: 10.1016/0736-5748(86)90063-8. [DOI] [PubMed] [Google Scholar]
- Bell SH, Stade B, Reynolds JN, Rasmussen C, Andrew G, Hwang PA, Carlen PL. The remarkably high prevalence of epilepsy and seizure history in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34(6):1084–1089. doi: 10.1111/j.1530-0277.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Best AR, Thompson JV, Fletcher ML, Wilson DA. Cortical metabotropic glutamate receptors contribute to habituation of a simple odor-evoked behavior. J Neurosci. 2005;25(10):2513–2517. doi: 10.1523/JNEUROSCI.5298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci. 2004;24(3):652–660. doi: 10.1523/JNEUROSCI.4220-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14(1):107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Frazier LL. Maturation and plasticity in the olfactory system of vertebrates. Brain Res. 1986;396(1):1–45. doi: 10.1016/s0006-8993(86)80188-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6(5):507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Carr JL, Agnihotri S, Keightley M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol Clin Exp Res. 2010;34(6):1022–1032. doi: 10.1111/j.1530-0277.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Garcia S, Messaoudi B, Thevenet M, Ferreira G, Gervais R, Ravel N. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J Neurosci. 2009;29(33):10287–10298. doi: 10.1523/JNEUROSCI.0505-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci. 2008;28(26):6664–6669. doi: 10.1523/JNEUROSCI.0178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Krahl SE, Berman RF, Hannigan JH. Effects of prenatal ethanol exposure on hippocampal theta activity in the rat. Alcohol. 1997;14(3):231–235. doi: 10.1016/s0741-8329(96)00147-4. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav Brain Res. 2010;210(2):164–170. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello SR, Kuan CY, Flavell RA, Rakic P. Caspase-3 is required for apoptosis-associated DNA fragmentation but not for cell death in neurons deprived of potassium. J Neurosci Res. 2000;59(1):24–31. [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44(1):27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci. 1987;7(3):716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113(3):509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Olfactory bulb mitral-tufted cell plasticity: odorant-specific tuning reflects previous odorant exposure. J Neurosci. 2003;23(17):6946–6955. doi: 10.1523/JNEUROSCI.23-17-06946.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Godin EA, O'Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcohol Clin Exp Res. 2010;34(1):98–111. doi: 10.1111/j.1530-0277.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourevitch B, Kay LM, Martin C. Directional coupling from the olfactory bulb to the hippocampus during a go/no-go odor discrimination task. J Neurophysiol. 2010;103(5):2633–2641. doi: 10.1152/jn.01075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heale VR, Vanderwolf CH. Dentate gyrus and olfactory bulb responses to olfactory and noxious stimulation in urethane anaesthetized rats. Brain Res. 1994;652(2):235–242. doi: 10.1016/0006-8993(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Morasch KC. Modality-specific impairments in response habituation following postnatal binge ethanol. Neurotoxicol Teratol. 2004;26(3):451–459. doi: 10.1016/j.ntt.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci U S A. 2006;103(41):15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko WM, Riley EP, Ehlers CL. Electrophysiological and behavioral findings in rats prenatally exposed to alcohol. Alcohol. 1993;10(2):169–178. doi: 10.1016/0741-8329(93)90099-a. [DOI] [PubMed] [Google Scholar]
- Kay LM, Beshel J. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task. J Neurophysiol. 2010;104(2):829–839. doi: 10.1152/jn.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum KL, Haberly LB. Membrane currents evoked by afferent fiber stimulation in rat piriform cortex. I. Current source-density analysis. J Neurophysiol. 1993;69(1):248–260. doi: 10.1152/jn.1993.69.1.248. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31(12):2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368(2):347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319(5871):1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffe S, Childiaeva R, Chernick V. Prolonged effects of maternal alcohol ingestion on the neonatal electroencephalogram. Pediatrics. 1984;74(3):330–335. [PubMed] [Google Scholar]
- Mair RG, Gellman RL, Gesteland RC. Postnatal proliferation and maturation of olfactory bulb neurons in the rat. Neuroscience. 1982;7:3105–3116. doi: 10.1016/0306-4522(82)90233-0. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci U S A. 2006;103(36):13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Schaefer AT. Theta oscillation coupled spike latencies yield computational vigour in a mammalian sensory system. J Physiol. 2003;546(Pt 2):363–374. doi: 10.1113/jphysiol.2002.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Gervais R, Chabaud P, Messaoudi B, Ravel N. Learning-induced modulation of oscillatory activities in the mammalian olfactory system: the role of the centrifugal fibres. J Physiol Paris. 2004;98(4–6):467–478. doi: 10.1016/j.jphysparis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34(9):1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25(3):159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15(3):117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Beauchamp GK. The transfer of alcohol to human milk. Effects on flavor and the infant's behavior. N Engl J Med. 1991;325(14):981–985. doi: 10.1056/NEJM199110033251401. [DOI] [PubMed] [Google Scholar]
- Morasch KC, Hunt PS. Persistent deficits in heart rate response habituation following neonatal binge ethanol exposure. Alcohol Clin Exp Res. 2009;33(9):1596–1604. doi: 10.1111/j.1530-0277.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24(5):1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville KR, Haberly L. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. 5th ed. New York: Oxford University Press; 2004. pp. 415–454. ed^eds. [Google Scholar]
- Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol. 2003;90(6):3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D'Sa C, Roth KA. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002a;9(2):205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2002b;133(2):115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Parnell SE, O'Leary-Moore SK, Godin EA, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 8. Alcohol Clin Exp Res. 2009;33(6):1001–1011. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang HQ. Atlas of the developing mouse brain at E17.5, P0 and P6. Burlington, MA: Academic Press; 2007. [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Hegde M, Ohsie J, Paik SM, Vadasz C, Saito M. Involvement of ceramide in ethanol-induced apoptotic neurodegeneration in the neonatal mouse brain. J Neurochem. 2010a;115(1):168–177. doi: 10.1111/j.1471-4159.2010.06913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Mao RF, Paik SM, Vadasz C, Saito M. Tau phosphorylation and cleavage in ethanol-induced neurodegeneration in the developing mouse brain. Neurochem Res. 2010b;35(4):651–659. doi: 10.1007/s11064-009-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Mao RF, Wang R, Vadasz C, Saito M. Effects of gangliosides on ethanol-induced neurodegeneration in the developing mouse brain. Alcohol Clin Exp Res. 2007;31(4):665–674. doi: 10.1111/j.1530-0277.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751(1):37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The cortical projection of the olfactory bulb: development in fetal and neonatal rats correlated with quantitative variations in adult rats. Brain Research. 1978;151:369–374. doi: 10.1016/0006-8993(78)90891-0. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of axonal connections in the central olfactory system of rats. J Comp Neurol. 1984;223(2):177–202. doi: 10.1002/cne.902230204. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thomas JD, Riley EP, Ehlers CL. Neurophysiologic consequences of neonatal ethanol exposure in the rat. Alcohol. 2004;34(2–3):187–196. doi: 10.1016/j.alcohol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR, Ronca AE, Alberts JR, Hepper PG. Heart rate response of the rat fetus and neonate to a chemosensory stimulus. Physiology & Behavior. 1990;50(1):47–52. doi: 10.1016/0031-9384(91)90496-b. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci Biobehav Rev. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer's disease mouse model. J Neurosci. 2010;30(2):505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin Exp Res. 1990;14(6):813–818. doi: 10.1111/j.1530-0277.1990.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90(1):65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Yan X. Sleep-like states modulate functional connectivity in the rat olfactory system. J Neurophysiol. 2010;104(6):3231–3239. doi: 10.1152/jn.00711.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadon CA, Wilson DA. The role of metabotropic glutamate receptors and cortical adaptation in habituation of odor-guided behavior. Learn Mem. 2005;12(6):601–605. doi: 10.1101/lm.41405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Glendinning JI. Fetal ethanol exposure increases ethanol intake by making it smell and taste better. Proc Natl Acad Sci U S A. 2009;106(13):5359–5364. doi: 10.1073/pnas.0809804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Molina JC, Spear NE, Youngentob LM. Experience-induced fetal plasticity: the effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behav Neurosci. 2007a;121(6):1293–1305. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Molina JC, Spear NE, Youngentob LM. The effect of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behav Neurosci. 2007b;121(6):1306–1315. doi: 10.1037/0735-7044.121.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]