Abstract
Chronic itch represents a burdensome clinical problem that can originate from a variety of etiologies. Pruriceptive itch originates following the activation of peripheral sensory nerve endings following damage or exposure to inflammatory mediators and ascends to the brain through the spinal thalamic tract. Much insight has been gained into the understanding of the mechanisms underlying pruriceptive itch through studies using humans and experimental animals. More than one sensory nerve subtype is thought to subserve pruriceptive itch which includes both unmyelinated C-fibers and thinly myelinated Aδ nerve fibers. There are a myriad of mediators capable of stimulating these afferent nerves leading to itch, including biogenic amines, proteases, cytokines, and peptides. Some of these mediators can also evoke sensations of pain and the sensory processing underlying both sensations overlaps in complex ways. Studies have demonstrated that both peripheral and central sensitization to pruritogenic stimuli occur during chronic itch.
Introduction
Itch, or pruritus, can be defined as an unpleasant sensation that evokes the desire to scratch. Although itch may be experienced acutely, chronic itch originates from many different etiologies and is a burdensome condition with serious unmet clinical need. Broadly, subtypes of chronic itch have been delineated and termed pruriceptive, neuropathic, neurogenic, and psychogenic itch [1–3]. Each of these terms provides some information on the causative nature of itch. Pruriceptive itch is a sensation of itch that originates following activation of primary afferent nerve terminals. This type of itch is associated with insect bites or intradermal injection of pruritic substances, and is a very common symptom of inflammatory disorders of the skin [1]. Neuropathic itch is a chronic itch condition that results from nerve injury. Examples of neuropathic itch include itch following varicella zoster infection or nerve trauma [4]. Neurogenic itch refers to itch resulting from central nervous system activation without necessary activation of sensory nerve fibers and is thought to occur in a variety of visceral disease states such as renal disease and kidney failure [5]. Although neurogenic itch is associated with visceral diseases, the mechanisms underlying the pruritus are often complex and can also involve pruriceptive itch. For example, uremic patients often experience changes in skin physiology such as xerosis (dry skin) which would undoubtedly involve activation of sensory nerve fibers [6]. Psychogenic itch results from underlying mental illness and it often occurs with somatization and delirium [7]. The underlying factors mediating these subtypes of chronic itch are complex and can also involve components among the subtypes. There are virtually no drugs that can claim impressive efficacy at combating chronic or acute itch irrespective of their various etiologies. As with our ancestors, the most effective treatment seems to be found in the nail of one’s fingers. As biomedical scientists learn more about the mechanisms underlying itch, the paucity of effective therapies for itch will likely improve. The overall focus of this article is to review the neurophysiology of pruriceptive itch by emphasizing current understandings from studies of both humans and experimental animals. Where applicable, studies pertaining to pruritic skin diseases such as atoptic dermatitis and prurigo nodularis will be emphasized.
Neural Mechanisms of Itch
Primary Afferent Nerves
Two broad theories have been postulated to explain pruriceptive sensory processing in the nervous system and they are named the specificity and pattern theories [8–11]. The specificity theory of itch states that there are specific subtypes of sensory nerve fibers and spinal cord neurons that are responsible for transmitting itch-specific sensory information to the central nervous system. The pattern theory states that the sensation of itch is encoded across the activation of many sensory receptors and spinal cord neurons and the collective pattern of neuronal activity determines the ultimate sensation experienced. Thus, the pattern of activation determines whether itch is experienced rather than pain or non-noxious touch. Evidence has been slowly accumulating and currently favors the specificity theory over the pattern theory, though there remains much to be learned.
Pruriceptive itch originates when specific sensory nerve terminals, generally located in the skin, are activated. Pruriceptive itch can also original from certain mucosal surfaces; however, a majority of research on itch has focused on sensory nerve fibers from skin [12]. The excitation of sensory nerve fibers in the skin leading to pruriceptive itch occurs upon exposure of certain sensory nerve terminals to a pruritic substance and frequently follows skin damage or inflammation. Sensory nerve fibers in the skin originate from the distal processes of primary afferent dorsal root ganglion neurons. Sensory nerve fibers in the skin are broadly classified according their condition of velocity and the sensory modalities that excite them. Fast conducting myelinated nerve fibers (Aβ) respond to non-noxious mechanical stimulation of the skin, while slow conducting myelinated (Aδ) and unmyelinated (C) nerves fibers respond to noxious stimulation and temperature changes of the skin [13]. Although there are many subtypes of primary afferent nerves innervating the skin, it is intriguing to consider that a single nerve subtype or “itch-receptor” is responsible for transducing pruriceptive stimuli. As is often the case with simple scenarios, this idea has not withstood critical scientific inquiry.
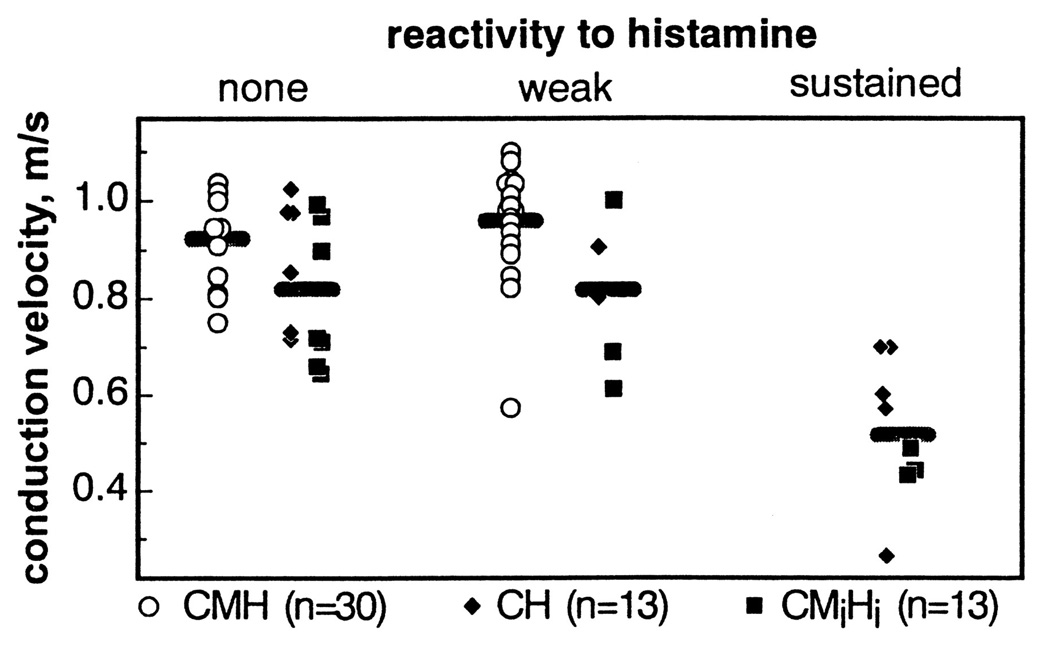
Studies in humans and non-human primates have demonstrated that slowly conducting unmyelinated nerve fibers (i.e. C-fibers) detect and signal pruriceptive information to the central nervous system. C-fibers are classified according to the stimuli that excite them. The most commonly used pruritogen, or itch producing stimulus, employed in experimental studies of itch is histamine. Microneurography experiments in humans have been conducted to delineate the type of sensory nerve that responds immediately to a local application of histamine. Schmelz and colleagues noted that histamine activated mainly C-fibers [14, 15]. Moreover, the time-course of histamine-evoked responses (evoked action potentials) nicely matched the duration of the itch sensation. These histamine-sensitive C-fibers had conduction velocities slower than most C-fibers and were not stimulated by heat or mechanical forces and were classified as a subset of heat- and mechano-insensitive C-fibers, Figure 1. [14, 15].
Figure 1.
Characterization of a subset of human cutaneous C-fibers that respond to histamine. CMH = C-fibers that are mechanically and heat responsive, CH = C-fibers that are heat responsive, CMiHi = C-fibers that are relatively unresponsive to mechanical force or heat. Note that the fibers which respond strongest to histamine are a subset of very slow conducting C-fibers (~0.6 m/s) that are relatively insensitive to heat or mechanical stimulation. Figure used with permission from [14].
Further evidence demonstrating that slow conducting C-fibers are involved in itch transduction has been obtained by using capsaicin, the pungent ingredient in chili pepper that evokes burning pain. A subpopulation of C-fibers express the capsaicin-receptor TRPV1 (transient receptor potential, vanilloid 1) and capsaicin not only activates these nerves, but upon repeated application can desensitize these fibers. Capsaicin-evoked desensitization not only affects the burning pain evoked by capsaicin, it also prevents the itch sensation evoked by histamine administration [16]. Clinically, capsaicin-induced desensitization has also been found to improve pathological itch associated with pruritic psoriasis [17] and itch associated with hemodialysis [18].
Another pruritogen used to study sensory nerve fibers in the skin are the spicules from the peapod of Mucuna pruriens or cowhage. By contrast to histamine-induced itch, the itch evoked following application of cowhage to human skin is not inhibited by a histamine H1 receptor antagonist [19]. As with histamine, cowhage was found to effectively stimulate cutaneous C-fibers. Unlike histamine, however, the subtype of C-fiber from human skin that was excited by cowhage was a mechanically-sensitive subtype of C-fiber [20]. Similarly, electrophysiological recordings made from non-human primate cutaneous nerve fibers also demonstrated preferential excitation of mechanically-sensitive C-fibers by cowhage [21]. In fact, mechano-insensitive C-fibers (that are often histamine sensitive) were not excited by cowhage [21]. Both histamine and cowhage evoke sensations of itch, but they stimulate different subtypes of C-fibers indicating more than one C-fiber subtype mediates the sensation of itch.
Histamine injection into the skin of mice evokes scratching behaviour which is quantified and used as an experimental measure of itch. Investigators have used histamine along with another pruritogen, chloroquine, in an attempt to define the afferent nerve phenotype responsible for itch transduction in mice. Chloroquine is an anti-malarial drug known to evoked pruritus as one potential side-effect in humans [22]. Administration of chloroquine to mice also evokes scratching behavior [23]. The mechanism by which chloroquine evokes itch in mice was found to be secondary to an interaction with a G-protein coupled receptor termed MrgA3 [24]. An evaluation of the sensory nerves that expressed MrgA3 revealed that they were also likely C-fibers. MrgA3-expressing DRG neurons also expressed receptors for histamine (H1) and capsaicin (TRPV1), but they only accounted for a very small subset of TRPV1-expressing DRG neurons. Studies have also been carried out implicating gastrin releasing peptide (GRP) as an important neurotransmitter in the transduction of itch found in DRG neurons [25]. Consistent with this is the observation, a small subset of chloroquine sensitive (MrgA3 expressing) C-fiber neurons also express GRP [24]. Thus, a small subset of TRPV1-, MrgA3- expressing DRG neurons appear to mediate the transduction of itch in mice. Whether cowhage stimulates a subset of C-fibers in the mouse distinct from the histamine or chloroquine sensitive nerves is not known. Currently, it is also not known if other DRG neuron subtypes mediate itch in mice.
The role of myelinated (A-fiber) nerve fibers in mediating pruriceptive itch has been less extensively explored. Electrophysiological recordings made from non-human primate cutaneous nerve fibers found that some slowly conducting mechanically-insensitive A fibers (Aδ fibers) were excited by histamine, but not cowhage [26]. By contrast, mechanically-sensitive Aδ fibers were the predominant fiber type excited by cowhage [26]. This activation profile is similar to the aforementioned findings with C-fibers.
The complexity regarding the specific subtypes of sensory nerves transducing pruriceptive itch exists because more than one nerve subtype is activated by substances that cause itch. Based on their activation profile, both histamine and cowhage-sensitive fibers can also be classified as nociceptors (i.e. they respond to noxious or potentially damaging stimuli). Specifically, cutaneous C-fibers that respond to histamine also respond to capsaicin [20, 27]. Cowhage-responsive cutaneous C-fibers also respond to noxious mechanical and thermal stimuli [20]. Similarly, histamine- and chloroquine-sensitive nerve fibers in the mouse express receptors for capsaicin (TRPV1). Sensory spinal cord neurons that respond to histamine or cowhage also respond to noxious stimuli including noxious thermal and mechanical stimuli as well as capsaicin administration [28, 29].
Since sensory nerve fibers and spinal cord neurons that respond to itch-producing stimuli also respond to noxious or potentially damaging stimuli, how the nervous system processing itch versus noxious or pain-producing stimuli remains puzzling. Theories on how the nervous system processes itch and pain have been discussed by several excellent reviews [8, 30, 31]. A recent study in mice demonstrated that genetic ablation of a glutamate transporter (VGLUT2) in nociceptors resulted in a decreased response to thermal pain, but a substantial increase in scratching behavior to certain pruritic substances like histamine [32]. Whether the interplay between itch and pain occurs at the level of the spinal cord, (i.e. glutamatergic transmission in the spinal cord regulate inhibitory input to secondary “itch neurons”), or at higher levels of processing (or both) has not been clearly worked out.
Spinal Cord
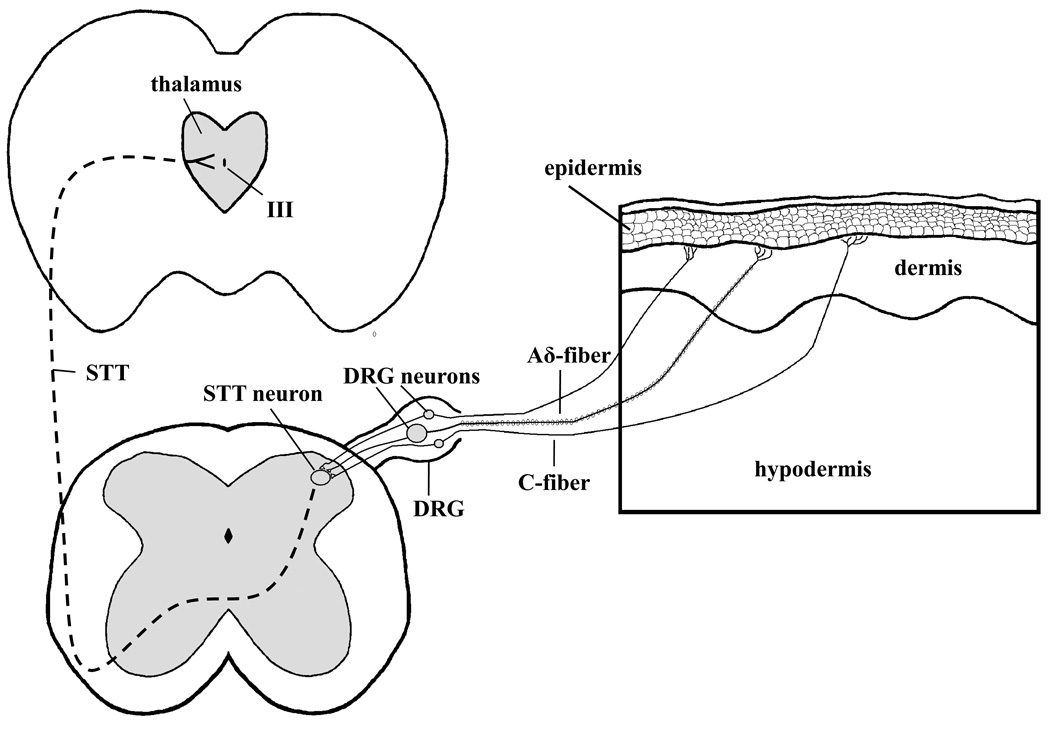
Following activation of peripheral sensory nerve endings of DRG neurons by itch producing stimuli, the central nerve terminals of these neurons form synapses on second-order neurons located in the dorsal horn of the spinal cord. Lesions in the anteriolataeral quadrant of the spinal cord of humans ablate itch sensation contralaterally below the level of lesion [33–35]. The loss of itch sensation indicates that, like pain and temperature sensation, pruriception ascends to the brain via the anteriolateral quadrant of the spinal cord in the spinal thalamic tract (STT). A diagram of the STT can be seen in Figure 2. Electrophysiological studies of non-human primates have also demonstrated that itch information ascends via the STT [28, 29, 36]. Electrophysiological studies of STT neurons from non-human primates reveal that they are activated in response to both histamine and cowhage applied to the skin. In other words, although histamine and cowhage stimulate nerve endings of distinct subtypes of primary afferent nerves, both subtypes form synapses on STT neurons. Interesting, histamine-and cowhage-sensitive afferent nerves do not seem to converge on the same STT neurons, since a given STT neuron responds to either histamine or cowhage, but not both [29].
Figure 2.
Diagram of input to the spinal thalamic tract (STT) from cutaneous sensory nerve fibers. Cross-sections of the brain, spinal cord, and skin indicate the origin of sensory input from the skin to the spinal cord. The spinal thalamic tract is indicated by the hatched line projecting from the dorsal horn of the spinal cord to the thalamus in the brain. Studies in both humans and experimental animals have demonstrated that both C- and Aδ-fibers transduce itch sensation (see text). DRG = dorsal root ganglion, STT = spinal thalamic tract, III = third ventricle.
Brain
Pruriceptive STT neurons were found to project to the posterior and ventral posterior regions of the thalamus in non-human primates and cats [29, 36]. Prior studies in humans have suggested that itch producing stimuli is processed in somatosensory areas of the brain. Imaging studies in humans have demonstrated activation of primary sensory cortex, primary motor cortex, supplementary motor area, and premotor cortex following administration of histamine into the skin [37–39].
Biological Mediators of Itch
Many substances and inflammatory mediators have been demonstrated to evoke itch experimentally in humans and animals such as mice rats, and non-human primates. Itch sensation in humans is quantified during psychophysical experiments were participants rate the intensity of itch sensations. In animals, scratching behavior is quantified and used as an experimental measure of itch. Based on data obtained from these methods in a large number of studies, it is clear that the basic neurophysiology of itch is remarkable conserved among species. Rarely does a substance that evokes itch in humans, not evoke scratching behavior in experimental animals. The use of experimental animals in the study of itch has allowed for in depth analysis of the molecular mechanisms underlying the sensation of itch. The mechanisms underlying how pruritogenic mediators affect sensory neuron excitability will be highlighted below in reference to both humans and experimental animals.
Histamine
Histamine is the most commonly studied pruritogen. Intradermal injection of histamine causes intense itching sensations, but not painful sensations. Histamine in the skin originates largely from dermal mast cells and is intimately linked to symptoms of allergy. It follows that the itch associated with acute allergen exposure is a consequence largely due to histamine release from mast cells, presumably interacting with pruriceptive C-fibers.
Histamine binds to G-protein coupled receptors (GPCR) and to date four histamine receptors have been isolated and cloned which are named as H1-4 [40]. Prior studies have localized H1, H3, and H4 receptors on dorsal root ganglion (DRG) neurons [41–43]. The primary histamine receptor subtype responsible for evoking itch in response to histamine has been shown to be H1 receptors, while H2 receptors contribute a minor role in humans [44]. H1 receptor antagonist also inhibit itch associated with conjunctiva and nasal allergen exposure [45]. Studies have implicated H1 and H4 receptors in mediating experimental pruritus in mice [46–49]. H3 receptor activation in mice is associated with decreases in pruritogen-evoked scratching behavior [50], while antagonism of H3 receptors evoked scratching behavior [51, 52].
H1 receptor activation on DRG neurons leads to activation of Gαq G-proteins and increases in intracellular calcium through phospholipase C [53–55]. GPCR activation must couple in some fashion with a membrane ion channel to depolarize a nerve terminal. The inward (depolarizing) ionic current in DRG neurons secondary to H1 receptor activation (and other Gαq coupled receptors) has been shown to involve opening TRPV1 receptors [56]. Consistent with this observation, histamine-evoked scratching behavior is attenuated by pre-treatment with TRPV1 receptor antagonists and in TRPV1 receptor knock-out mice implicating TRPV1 receptors in histamine-evoked scratching behavior [55]. These observations that TRPV1 receptors are involved in histamine receptor signaling supports findings in humans and non-human primates demonstrating that mechanically-insensitive C-fibers that preferentially respond to histamine are also responsive to capsaicin [15].
Although histamine-induced itch depended on TRPV1 activation, the sensations induced by histamine (itch) are not mimicked by TRPV1 agonists. TRPV1 receptors are activated by a variety of nociceptive stimuli including capsaicin, noxious heat, and acid [57, 58]. Generally, substances that activate TRPV1 evoke sensations of burning pain rather than itch and one possible reason for this is that H1 receptors are only expressed on a subset of TRPV1 expressing DRG neurons. As alluded to earlier, it is possible that although capsaicin stimulates pruriceptive fibers, it also strongly activates pain fibers, and the pain sensation could potentially mask the sensation of itch. Studies in humans have found capsaicin injection into human skin inhibited the itch sensation evoked by histamine [16].
5-hydoxytrptamne (5-HT, Serotonin)
5-HT is another endogenous biogenic amine that has been shown to evoke itch in humans. Consistent with this, 5-HT stimulates action potential discharge in a subset of human cutaneous C-fibers [15]. The itch sensation evoked by 5-HT was weaker than the sensation of itch evoked by histamine [15, 59, 60]. Although serotonin is a relatively weak pruritogen in normal non-lesioned skin, it should be kept in mind that it is a much stronger pruritogen when administered to lesioned skin from patients with atopic dermatitis [60]. 5-HT also evokes scratching behavior in experimental animals and in rats, the scratching behavior to 5-HT is associated with activation of C-fibers, but not faster conducting myelinated nerve fibers [61]. Based on studies with selective 5-HT receptor subtype agonists and antagonists it appears that in mice the 5-HT2 receptor subtype is responsible for 5-HT-induced itch [62]. The receptor subtype involved with 5-HT-induced itch in humans has not been worked out. Nevertheless, it is noteworthy that the itch associated with polycythemia vera may involve 5-HT [63] and may be inhibited with pizotigen, an antagonist of 5-HT2 receptors [64].
Acetylcholine
Intradermal injection of acetylcholine evokes itch in humans, but like 5-HT, it is less pruritogenic than histamine [15]. Acetylcholine can also evoke pain sensations [15]. The relative sensation of pain and itch may be influenced by underlying pathology. For example, in normal healthy participants, injection of acetylcholine consistently evoked sensations of pain, while acetylcholine injection into the skin of subjects with atopic dermatitis resulted in the sensation of itch [65]. Acetylcholine evokes scratching behavior in mice which is blocked by muscarinic M3 receptor antagonists [66]. Interesting, patients with atopic dermatitis have a greater than 10-fold concentration of acetylcholine in their skin compared to normal healthy controls [66]. The source of the acetylcholine is not clear, but choline acetyltransferase (the requisite enzyme for acetylcholine synthesis) can be found in keratinocytes and endothelial cells within skin [67].
Substance P
Substance P (SP) is a neuropeptide found largely in cutaneous nociceptive nerve terminals which can be released and influence vascular and immune responses leading to processes that are collectively is termed neurogenic inflammation [68]. Particularly relevant to allergic inflammation, SP is also found outside the nervous system and has been localized to human skin mast cells [69]. Cutaneous administration of SP in humans evoked itch in normal healthy individuals and in patients with atopic dermatitis [60, 70, 71]. Moreover, patients with prurigo nodularis and atopic dermatitis have an increase density of SP-positive cutaneous nerve fibers [72–74].
The mechanism underlying SP-evoked itch in humans is thought to involve, in large part, the release of histamine from mast cells [60, 63, 70]. SP stimulates histamine release from human skin mast cells and the pruritus evoked by administration SP into healthy and lesioned skin is inhibited by histamine H1 receptor antagonists [60, 70, 75]. SP preferentially activates neurokinin one (NK1) receptors with nanomolar potency. The SP-induced histamine from human skin mast cells, however, generally requires concentrations in excess of 1 µM. SP-induced histamine release from human skin has been partially antagonized by the NK1 receptor L732, 138; however, the concentration required was far in excess of the drug’s affinity constant for the receptor [76]. It seems likely that NK1 receptor mechanisms may be involved in SP-induced mast cell degranulation, but other mechanisms may be involved. It has recently been found that SP stimulates MrgX2 receptors with an EC50 of about 10 µM. MrgX2 receptors are G-protein coupled receptors that are expressed in human mast cells and several other MrgX2 agonists (including SP) evoke histamine release [77]. SP also evokes scratching behaviour in mice, but in contrast to humans, the itch is not inhibited by histamine receptor antagonists [78, 79]. Pharmacological studies demonstrated that SP-evoked scratching behavior was secondary to leukotriene B4 (LTB4) production [80].
Aprepitant, a selective NK1 receptor antagonist, given orally for one week, attenuated itch severity in patients with chronic pruritus [81]. Aprepitant was also found to be effective in treating malignancy-related pruritus [82, 83]. Consistent with these observations with aprepitant in humans, studies using NG/Nga mice, a murine model of atopic dermatitis, demonstrated that administration of NK1 receptor antagonist reduced scratching behaviour [84]. The mechanisms underlying the antipruritic effect of NK1 receptor antagonism is not currently known.
Leukotrienes
Intradermal injection of cysteinyl-leukotrienes and LTB4 cause local inflammatory reactions in human skin which was accompanied by itch in a subset of individuals [85]. Intradermal injections of LTB4 evoked scratching behavior in mice which was inhibited by LTB4 receptor antagonists [79]. SP-evoked scratching behavior in mice is inhibited by 5-lipoxygenase inhibitors and LTB4 receptor antagonism [80]. It is not known if LTB4-induced itch or scratching behavior is due to a direct effect on sensory nerve endings or secondary to other inflammatory effects, but LTB4 has been found to directly sensitize rat cutaneous C-fibers [86].
Bradykinin
In humans, the subjective response to intradermal injection of bradykinin is an intense burning pain [87, 88]. Bradykinin injection also led to mild to moderate itch in about 60% of participants, but the itching was only noticed after cessation of the burning pain [87]. The preference for pain over itch may change in atopic dermatitis where administration of bradykinin in lesional skin causes less pain and a robust itch sensation [60].
Proteases
Endogenous serine proteases such as trypsin and tryptase evoke itch in humans [89–92]. Similarly, injection of trypsin, tryptase, and other proteases in experimental animals evokes scratching behaviour [93–95]. Endogenous proteases are upregulated in human skin with atopic dermatitis [91, 96]. Although proteases can produce a variety of biological effects, one mechanism through which they can evoke itch is through the activation of protease-activated receptors (PARs).
A total of 4 PARs have been isolated, named PAR1-4. All are G-protein couple receptors and the ligands for PARs are found on the extracellular N-terminus which is liberated following cleavage by a protease [97]. Although studies have demonstrated that scratching-evoked behavior can be elicited by activation of PAR1 and PAR4 activation, a majority of studies have focused on the role of PAR2 in pruritus [95]. Administration of selective PAR2 agonists in humans and experimental animals evokes itch and scratching behavior, respectively [92, 98]. Many endogenous and exogenous proteases have been shown to act as PAR2 agonists and evoke itch. Although trypsin can activate all 4 PARs, tryptase has been shown to selectively activate PAR2 [99]. This is relevant to allergic itch in that mast cells are a rich source of tryptase [100]. Indeed, pruritus is a common clinical manifestation of mastocytsis, a condition marked by elevations in serum tryptase [101]. Cathepsin S is another endogenous protease that can evoke itch in humans and activate PAR2 and PAR4 [102]. Kallikrein-related peptidases (KLKs), specifically KLK5 and KLK14, can also activate PAR2 and are upregulated in lesional skin of humans with atopic dermatitis [103].
Exogenous proteases have also been shown to evoke itch and activate PARs. As discussed above, cowhage evokes itch and is commonly used in pruritus research. The pruritogenic component of cowhage is mucunain which is a protease capable stimulating PAR2 and PAR4 receptors [104]. Administration of the plant-derived cysteine protease papin into skin evokes itch and activates PAR2 and PAR4 receptors [105]. Many allergens contain proteases capable of stimulating PARs such as proteases from dust mites and cockroaches which have been found to activate PAR2 [106, 107].
PARs are expressed by numerous cell types, but relevant to the present discussion, they are richly expressed by DRG neurons [108, 109]. PAR2 receptors have been localized on cutaneous sensory nerve in humans and experimental animals [92, 110–112]. PAR2 expression is increased on cutaneous sensory nerves in humans with atopic dermatitis [92, 103]. DRG neurons that express PAR2 also express neuropeptides consistent with a C-fiber nociceptive phenotype. Activation of PAR2 on DRG neurons results in intracellular calcium responses, neuropeptide release, and sensitization of TRPV1 receptor responses [113, 114]. As with other GPCRs, PAR activation may lead to nerve stimulation by gating the TRPV1 ion channel. Trypsin-evoked scratching behavior in mice is inhibited by TRPV1 receptor antagonists or by genetic knockdown of the TRPV1 gene expression [94]. Scratching behavior to PAR2 agonists is also inhibited by bradykinin 2 antagonism implicating a potential indirect mechanism as well [115]. The expression of PAR2 receptors on non-neuronal cells suggests that the itch evoked may also involve indirect actions following PAR2 activation. In mice, PAR2 activation on keratinocytes can also modulate scratching evoked behavior through the production of LTB4 [79, 116].
Interleukin 31
Another potential mediator involved in pruriceptive itch is the Th2 cytokine interleukin 31 (IL-31). Prior studies in humans found that IL-31 is highly upregulated in the skin of patients with pruritic skin diseases such as atopic dermatitis and prurigo nodularis [117, 118]. IL-31 belongs to the interleukin 6 family of cytokines and it binds to heterodimeric receptors consisting of IL-31 receptor α and oncostatin M receptor β [119]. In NC/Nga mice, a murine model of atopic dermatitis, IL31 is increased in skin and transgenic overexpression of IL-31 in mice results in persistent scratching behavior [120]. Interesting, blocking IL-31 via anti-IL-31 antibodies decreased scratching behavior in NC/Nga mice [121]. Receptors for IL-31 have been localized on murine DRG ganglion neurons with nociceptive phenotypes [122, 123]. Although IL-31 can act on many different cell types in the skin, the studies detailed above indicate that IL-31 could potentially act directly on sensory nerve terminals to evoke itch.
Lysophosphatidic Acid and Autotaxin
Lysophosphatidic acid (LPA) is phospholipid-derived mediator that evokes scratching behaviour in mice [124, 125]. Although the mechanism underlying LPA-evoked scratching behaviour is not currently known, H1 receptor antagonism and capsaicin pretreatment attenuated the scratching behaviour [125]. Serum LPA and autotaxin, the enzyme that converts lysophosphatidylcholine into LPA, are strongly unregulated in patients with cholestatic pruritus [124]. Collectively, these studies implicate the role of LPA and autotaxin in cholestatic pruritus.
Toll-Like Receptor 7
Recently toll-like receptor 7 (TLR7) has been implicated in mediating scratching-evoked behavior in mice [126]. Although TLR7 is used by the innate immune system to recognize single-stranded RNA from viruses, genetic knock out of TLR7 attenuated scratching behavior to variety of pruritogens. Scratching behaviour evoked by cholorquine, PAR2 agonists, and serotonin were all attenuated in TLR7 knockout mice. Application of TLR7 agonists directly excited DRG neurons and TLR7 receptors were localized on DRG neurons and cutaneous sensory nerves with nociceptive phenotypes indicating they are likely present on C-fibers. Collectively, these results indicated that TLR7 is involved in the signaling of a variety of unrelated pruritogens, but the mechanism underlying this effect is unknown but may involve some general sensitizing mechanism (see below).
Sensitization of Itch
In addition to changes in the inflammatory environment of the skin, the mechanisms underlying chronic pruriceptive itch may also involve altered processing of itch by the nervous system. Studies have demonstrated that nociceptive stimuli that normally evoke pain in healthy individuals evoke itch when applied to lesional skin of patients with atopic dermatitis [127, 128]. The nociceptive stimuli that evoked itch included mechanical stimulation, heating, and acidic buffer administration into the skin [127, 128]. Bradykinin, a known algesic substance, also evoked itch when administered into lesional skin of patients with atopic dermatitis, whereas the major sensation in normal skin was pain [60].
Sensitization of itch can occur peripherally or centrally. Peripheral sensitization is most often studied in nociceptors and is characterized by a decreased threshold for activation, increased responsiveness, and the presence of ongoing activity [129–131]. Following exposure to inflammatory mediators or injury, the excitability of sensory nerve endings in the skin or other tissue that detect pruritic substances could be enhanced. Electrophysiological recordings made from a patient suffering from chronic pruriceptive itch due to prurigo nodularis demonstrated that mechano-insensitive C-fibers exhibited aberrant firing behaviour indicative of sensitization [27]. Results from psychophysical experiments have demonstrated enhanced responses to pruritogenic stimuli possibly indicating peripheral sensitization. Itch sensation can be evoked in humans by transcutaneous electrical stimulation and the threshold for electrically-evoked itch was lower in skin from patients with atopic dermatitis compared to normal healthy control skin [127, 132, 133]. The dose of histamine required to evoke itch in lesional skin of patients with atopic dermatitis was lower than in normal healthy skin [134]. Overall, it is likely that sensory nerve fiber mediating itch become sensitized during chronic itch.
Sensitization can also occur following changes in the activity CNS neurons which is termed central sensitization. During central sensitization, the excitability of CNS neurons is altered in such a way where non-pruritic stimuli become coupled to pruritic sensations [8, 127, 135]. Although the mechanisms underlying central sensitization to itch are largely unknown, neuroimaging studies have demonstrated altered activity in certain brain regions in patients with atopic dermatitis compared to normal healthy controls [136–138].
Increased nerve fiber density could also potentially mediate chronic pruriceptive itch. Prior studies have implicated neurotrophins in the pathophysiological mechanisms of atopic dermatitis and prurigo nodularis. Neurotrophins are a class of growth factors including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4/5 (NT-4/5). Neurotrophins bind to three different subtypes of tropomyosin-receptor-kinase (Trk) receptors named TrkA, TrkB, and TrkC. All of the neurotrophins and their receptors have been localized in skin among various cell types [139, 140]. Studies in human subjects have demonstrated that skin from patients with atopic dermatitis have increased levels of NGF in keratinocytes and infiltrating leukocytes which are related to disease severity [141]. Skin from patients with prurtigo nodularis also have increased presence of NGF which is primarily found in infiltrating leukocytes [142]. Patients with atopic dermatitis also have increased NT-4 expression in the skin which is primarily localized to keratinocytes [143]. Although neurotrophins are not pruritogens, they can potentially enhance sensory nerve growth and sprouting. Prior studies have demonstrated that cutaneous nerve fiber density is increased in patients with prurtigo nodularis and atopic dermatitis [72, 73, 144, 145].
Conclusion
Effective treatment for chronic itch is a serious unmet medical need. Although certain drugs can offer some relief, there is no evidence to support any given treatment is generally efficacious in the treatment of chronic pruritus. There are numerous inflammatory mediators associated with pathological itch which can also evoke itch when injected into the skin. Beyond histamine, there have been relatively few rigorous clinical studies that have evaluated the effect of antagonizing a given mediator in pathologic itch.
Logically, it may be more fruitful to target pruriceptive itch through mechanisms where several mediators converge. For example, many irritants as well as GPCR agonists stimulate C-fibers by interacting with TRPV1. Even TRPV1 antagonists, however, will only target itch evoked by a subset of mediators. A more general target may the so-called “itch receptors” themselves. As more knowledge is gained about these sensory Aδ and C-fibers and the mediators that excite them, future strategies may avail themselves that will selectively inhibit their excitability and sensitization.
References
- 1.Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690–694. doi: 10.1016/S0140-6736(03)12570-6. [DOI] [PubMed] [Google Scholar]
- 2.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 3.Greaves MW, Khalifa N. Itch: more than skin deep. Int Arch Allergy Immunol. 2004;135:166–172. doi: 10.1159/000080898. [DOI] [PubMed] [Google Scholar]
- 4.Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329–337. doi: 10.1038/ncpneuro0806. [DOI] [PubMed] [Google Scholar]
- 5.Galatian A, Stearns G, Grau R. Pruritus in connective tissue and other common systemic disease states. Cutis. 2009;84:207–214. [PubMed] [Google Scholar]
- 6.Kuypers DR. Skin problems in chronic kidney disease. Nat Clin Pract Nephrol. 2009;5:157–170. doi: 10.1038/ncpneph1040. [DOI] [PubMed] [Google Scholar]
- 7.Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008;21:32–41. doi: 10.1111/j.1529-8019.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmelz M. Itch and pain. Neurosci Biobehav Rev. 2010;34:171–176. doi: 10.1016/j.neubiorev.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Frey Mv. Zur Physiologie der Juckempfindung. Arch Neerl Physiol. 1922;7:142–145. [Google Scholar]
- 10.Greaves MW, Wall PD. Pathophysiology of itching. Lancet. 1997;349:133. doi: 10.1016/s0140-6736(05)60916-6. [DOI] [PubMed] [Google Scholar]
- 11.McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- 12.Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18:292–303. doi: 10.1111/j.1529-8019.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- 13.McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev. 2010;34:148–159. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 16.Toth-Kasa I, Jancso G, Bognar A, Husz S, Obal F., Jr Capsaicin prevents histamine-induced itching. Int J Clin Pharmacol Res. 1986;6:163–169. [PubMed] [Google Scholar]
- 17.Ellis CN, Berberian B, Sulica VI, Dodd WA, Jarratt MT, Katz HI, Prawer S, Krueger G, Rex IH, Jr, Wolf JE. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29:438–442. doi: 10.1016/0190-9622(93)70208-b. [DOI] [PubMed] [Google Scholar]
- 18.Breneman DL, Cardone JS, Blumsack RF, Lather RM, Searle EA, Pollack VE. Topical capsaicin for treatment of hemodialysis-related pruritus. J Am Acad Dermatol. 1992;26:91–94. doi: 10.1016/0190-9622(92)70013-6. [DOI] [PubMed] [Google Scholar]
- 19.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghahowa SE, Obianwu HO, Isah AO, Arhewoh IM. Chloroquine-induced Pruritus. Indian J Pharm Sci. 2010;72:283–289. doi: 10.4103/0250-474X.70471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 26.Schepers RJJL, Hartke TV, Shim B, Borzan J, Meyer RA, Ringkamp M. A subpopulation of Adelta nociceptors in monkey is vigorously activated by cowhage spiculesSociety for Neuroscience. San Diego, CA: 2008. [Google Scholar]
- 27.Schmelz M, Hilliges M, Schmidt R, Orstavik K, Vahlquist C, Weidner C, Handwerker HO, Torebjork HE. Active "itch fibers" in chronic pruritus. Neurology. 2003;61:564–566. doi: 10.1212/01.wnl.0000078193.64949.08. [DOI] [PubMed] [Google Scholar]
- 28.Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- 29.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel KN, Dong X. An itch to be scratched. Neuron. 2010;68:334–339. doi: 10.1016/j.neuron.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyndman ORWJ. Anterior cordotomy: further observations on physiologic results and optimum manner of performance. Arch Neurol Psychiatry. 1943;50:129–148. [Google Scholar]
- 34.White JC, Sweet WH, Hawkins R, Nilges RG. Anterolateral cordotomy: results, complications and causes of failure. Brain. 1950;73:346–367. doi: 10.1093/brain/73.3.346. [DOI] [PubMed] [Google Scholar]
- 35.Nathan PW. Touch and surgical division of the anterior quadrant of the spinal cord. J Neurol Neurosurg Psychiatry. 1990;53:935–939. doi: 10.1136/jnnp.53.11.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 37.Darsow U, Drzezga A, Frisch M, Munz F, Weilke F, Bartenstein P, Schwaiger M, Ring J. Processing of histamine-induced itch in the human cerebral cortex: a correlation analysis with dermal reactions. J Invest Dermatol. 2000;115:1029–1033. doi: 10.1046/j.1523-1747.2000.00193.x. [DOI] [PubMed] [Google Scholar]
- 38.Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 39.Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001;92:295–305. doi: 10.1016/s0304-3959(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 40.Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ninkovic M, Hunt SP. Opiate and histamine H1 receptors are present on some substance P-containing dorsal root ganglion cells. Neurosci Lett. 1985;53:133–137. doi: 10.1016/0304-3940(85)90109-0. [DOI] [PubMed] [Google Scholar]
- 42.Cannon KE, Chazot PL, Hann V, Shenton F, Hough LB, Rice FL. Immunohistochemical localization of histamine H3 receptors in rodent skin, dorsal root ganglia, superior cervical ganglia, and spinal cord: potential antinociceptive targets. Pain. 2007;129:76–92. doi: 10.1016/j.pain.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD, Bitner RS. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res. 2009;1250:41–48. doi: 10.1016/j.brainres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Davies MG, Greaves MW. Sensory responses of human skin to synthetic histamine analogues and histamine. Br J Clin Pharmacol. 1980;9:461–465. [PMC free article] [PubMed] [Google Scholar]
- 45.Rimmer SJ, Church MK. The pharmacology and mechanisms of action of histamine H1-antagonists. Clin Exp Allergy. 1990;20 Suppl 2:3–17. doi: 10.1111/j.1365-2222.1990.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 46.Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Yamaura K, Oda M, Suwa E, Suzuki M, Sato H, Ueno K. Expression of histamine H4 receptor in human epidermal tissues and attenuation of experimental pruritus using H4 receptor antagonist. J Toxicol Sci. 2009;34:427–431. doi: 10.2131/jts.34.427. [DOI] [PubMed] [Google Scholar]
- 49.Cowden JM, Zhang M, Dunford PJ, Thurmond RL. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J Invest Dermatol. 2010;130:1023–1033. doi: 10.1038/jid.2009.358. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto Y, Iba Y, Nakamura Y, Kayasuga R, Kamei C. Pruritus-associated response mediated by cutaneous histamine H3 receptors. Clin Exp Allergy. 2004;34:456–459. doi: 10.1111/j.1365-2222.2004.01876.x. [DOI] [PubMed] [Google Scholar]
- 51.Hossen MA, Sugimoto Y, Kayasuga R, Kamei C. Involvement of histamine H3 receptors in scratching behaviour in mast cell-deficient mice. Br J Dermatol. 2003;149:17–22. doi: 10.1046/j.1365-2133.2003.05341.x. [DOI] [PubMed] [Google Scholar]
- 52.Hossen MA, Inoue T, Shinmei Y, Fujii Y, Watanabe T, Kamei C. Role of substance P on histamine H(3) antagonist-induced scratching behavior in mice. J Pharmacol Sci. 2006;100:297–302. doi: 10.1254/jphs.fpj05028x. [DOI] [PubMed] [Google Scholar]
- 53.Nicolson TA, Bevan S, Richards CD. Characterisation of the calcium responses to histamine in capsaicin-sensitive and capsaicin-insensitive sensory neurones. Neuroscience. 2002;110:329–338. doi: 10.1016/s0306-4522(01)00561-9. [DOI] [PubMed] [Google Scholar]
- 54.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 55.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 58.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 59.Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res. 1997;46:412–416. doi: 10.1007/s000110050213. [DOI] [PubMed] [Google Scholar]
- 60.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Hachisuka J, Furue H, Furue M, Yoshimura M. Responsiveness of C neurons in rat dorsal root ganglion to 5-hydroxytryptamine-induced pruritic stimuli in vivo. J Neurophysiol. 2010;104:271–279. doi: 10.1152/jn.00938.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 63.Fjellner B, Hagermark O. Studies on pruritogenic and histamine-releasing effects of some putative peptide neurotransmitters. Acta Derm Venereol. 1981;61:245–250. [PubMed] [Google Scholar]
- 64.Fitzsimons EJ, Dagg JH, McAllister EJ. Pruritus of polycythaemia vera: a place for pizotifen? Br Med J (Clin Res Ed) 1981;283:277. doi: 10.1136/bmj.283.6286.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogelsang M, Heyer G, Hornstein OP. Acetylcholine induces different cutaneous sensations in atopic and non-atopic subjects. Acta Derm Venereol. 1995;75:434–436. doi: 10.2340/0001555575434436. [DOI] [PubMed] [Google Scholar]
- 66.Miyamoto T, Nojima H, Kuraishi Y. Intradermal cholinergic agonists induce itch-associated response via M3 muscarinic acetylcholine receptors in mice. Jpn J Pharmacol. 2002;88:351–354. doi: 10.1254/jjp.88.351. [DOI] [PubMed] [Google Scholar]
- 67.Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- 68.Peters EM, Ericson ME, Hosoi J, Seiffert K, Hordinsky MK, Ansel JC, Paus R, Scholzen TE. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol. 2006;126:1937–1947. doi: 10.1038/sj.jid.5700429. [DOI] [PubMed] [Google Scholar]
- 69.Toyoda M, Makino T, Kagoura M, Morohashi M. Immunolocalization of substance P in human skin mast cells. Arch Dermatol Res. 2000;292:418–421. doi: 10.1007/s004030000149. [DOI] [PubMed] [Google Scholar]
- 70.Hagermark O, Hokfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- 71.Thomsen JS, Sonne M, Benfeldt E, Jensen SB, Serup J, Menne T. Experimental itch in sodium lauryl sulphate-inflamed and normal skin in humans: a randomized, double-blind, placebocontrolled study of histamine and other inducers of itch. Br J Dermatol. 2002;146:792–800. doi: 10.1046/j.1365-2133.2002.04722.x. [DOI] [PubMed] [Google Scholar]
- 72.Abadia Molina F, Burrows NP, Jones RR, Terenghi G, Polak JM. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344–351. doi: 10.1111/j.1365-2133.1992.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 73.Sugiura H, Omoto M, Hirota Y, Danno K, Uehara M. Density and fine structure of peripheral nerves in various skin lesions of atopic dermatitis. Arch Dermatol Res. 1997;289:125–131. doi: 10.1007/s004030050167. [DOI] [PubMed] [Google Scholar]
- 74.Haas S, Capellino S, Phan NQ, Bohm M, Luger TA, Straub RH, Stander S. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58:193–197. doi: 10.1016/j.jdermsci.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 75.Lawrence ID, Warner JA, Cohan VL, Hubbard WC, Kagey-Sobotka A, Lichtenstein LM. Purification and characterization of human skin mast cells. Evidence for human mast cell heterogeneity. J Immunol. 1987;139:3062–3069. [PubMed] [Google Scholar]
- 76.Guhl S, Lee HH, Babina M, Henz BM, Zuberbier T. Evidence for a restricted rather than generalized stimulatory response of skin-derived human mast cells to substance P. J Neuroimmunol. 2005;163:92–101. doi: 10.1016/j.jneuroim.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 77.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, Naito T. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 78.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 79.Andoh T, Kuraishi Y. Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. Eur J Pharmacol. 1998;353:93–96. doi: 10.1016/s0014-2999(98)00440-3. [DOI] [PubMed] [Google Scholar]
- 80.Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol. 2001;117:1621–1626. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- 81.Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5:e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Booken N, Heck M, Nicolay JP, Klemke CD, Goerdt S, Utikal J. Oral aprepitant in the therapy of refractory pruritus in erythrodermic cutaneous T-cell lymphoma. Br J Dermatol. 2010 doi: 10.1111/j.1365-2133.2010.10108.x. [DOI] [PubMed] [Google Scholar]
- 83.Vincenzi B, Fratto ME, Santini D, Tonini G. Aprepitant against pruritus in patients with solid tumours. Support Care Cancer. 2010;18:1229–1230. doi: 10.1007/s00520-010-0895-9. [DOI] [PubMed] [Google Scholar]
- 84.Ohmura T, Hayashi T, Satoh Y, Konomi A, Jung B, Satoh H. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur J Pharmacol. 2004;491:191–194. doi: 10.1016/j.ejphar.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 85.Camp RD, Coutts AA, Greaves MW, Kay AB, Walport MJ. Responses of human skin to intradermal injection of leukotrienes C4, D4 and B4. Br J Pharmacol. 1983;80:497–502. doi: 10.1111/j.1476-5381.1983.tb10721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin HA. Leukotriene B4 induced decrease in mechanical and thermal thresholds of C-fiber mechanonociceptors in rat hairy skin. Brain Res. 1990;509:273–279. doi: 10.1016/0006-8993(90)90552-m. [DOI] [PubMed] [Google Scholar]
- 87.Cormia FE, Dougherty JW. Proteolytic activity in development of pain and itching. Cutaneous reactions to bradykinin and kallikrein. J Invest Dermatol. 1960;35:21–26. doi: 10.1038/jid.1960.78. [DOI] [PubMed] [Google Scholar]
- 88.Manning DC, Raja SN, Meyer RA, Campbell JN. Pain and hyperalgesia after intradermal injection of bradykinin in humans. Clin Pharmacol Ther. 1991;50:721–729. doi: 10.1038/clpt.1991.212. [DOI] [PubMed] [Google Scholar]
- 89.Rajka G. Itch duration in the involved skin of atopic dermatitis (prurigo Besnier) Acta Derm Venereol. 1967;47:154–157. [PubMed] [Google Scholar]
- 90.Rajka G. Itch duration in the uninvolved skin of atopic dermatitis (prurigo Besnier) Acta Derm Venereol. 1968;48:320–321. [PubMed] [Google Scholar]
- 91.Stander S, Steinhoff M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp Dermatol. 2002;11:12–24. doi: 10.1034/j.1600-0625.2002.110102.x. [DOI] [PubMed] [Google Scholar]
- 92.Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 94.Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, Quintao NL, Juliano L, Brain SD, Calixto JB. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154:1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsujii K, Andoh T, Lee JB, Kuraishi Y. Activation of proteinase-activated receptors induces itch-associated response through histamine-dependent and -independent pathways in mice. J Pharmacol Sci. 2008;108:385–388. doi: 10.1254/jphs.08200sc. [DOI] [PubMed] [Google Scholar]
- 96.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J. 2010;51:808–822. doi: 10.3349/ymj.2010.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol. 2010;160:191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Barry GD, Le GT, Fairlie DP. Agonists and antagonists of protease activated receptors (PARs) Curr Med Chem. 2006;13:243–265. doi: 10.2174/092986706775476070. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz LB. Tryptase a mediator of human mast cells. J Allergy Clin Immunol. 1990;86:594–598. doi: 10.1016/s0091-6749(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 101.Pettigrew HD, Teuber SS, Kong JS, Gershwin ME. Contemporary challenges in mastocytosis. Clin Rev Allergy Immunol. 2010;38:125–134. doi: 10.1007/s12016-009-8164-8. [DOI] [PubMed] [Google Scholar]
- 102.Reddy VB, Shimada SG, Sikand P, Lamotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol. 2010;130:1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, Egelrud T. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008;128:18–25. doi: 10.1038/sj.jid.5700965. [DOI] [PubMed] [Google Scholar]
- 104.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reddy VB, Lerner EA. Plant cysteine proteases that evoke itch activate protease-activated receptors. Br J Dermatol. 2010;163:532–535. doi: 10.1111/j.1365-2133.2010.09862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. J Allergy Clin Immunol. 2003;112:1112–1118. doi: 10.1016/j.jaci.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 107.Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, Ishii A, Ikeda S, Okumura K, Ogawa H. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy. 2009;64:1366–1374. doi: 10.1111/j.1398-9995.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 108.Zhu WJ, Yamanaka H, Obata K, Dai Y, Kobayashi K, Kozai T, Tokunaga A, Noguchi K. Expression of mRNA for four subtypes of the proteinase-activated receptor in rat dorsal root ganglia. Brain Res. 2005;1041:205–211. doi: 10.1016/j.brainres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 109.Vellani V, Kinsey AM, Prandini M, Hechtfischer SC, Reeh P, Magherini PC, Giacomoni C, McNaughton PA. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Mol Pain. 2010;6:61. doi: 10.1186/1744-8069-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, Ansel JC, Bunnett NW. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 111.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 112.Hoogerwerf WA, Zou L, Shenoy M, Sun D, Micci MA, Lee-Hellmich H, Xiao SY, Winston JH, Pasricha PJ. The proteinase-activated receptor 2 is involved in nociception. J Neurosci. 2001;21:9036–9042. doi: 10.1523/JNEUROSCI.21-22-09036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 114.Dale C, Vergnolle N. Protease signaling to G protein-coupled receptors: implications for inflammation and pain. J Recept Signal Transduct Res. 2008;28:29–37. doi: 10.1080/10799890801941913. [DOI] [PubMed] [Google Scholar]
- 115.Costa R, Manjavachi MN, Motta EM, Marotta DM, Juliano L, Torres HA, Pesquero JB, Calixto JB. The role of kinin B1 and B2 receptors in the scratching behaviour induced by proteinase-activated receptor-2 agonists in mice. Br J Pharmacol. 2010;159:888–897. doi: 10.1111/j.1476-5381.2009.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu Y, Wang XR, Peng C, Xu JG, Liu YX, Wu L, Zhu QG, Liu JY, Li FQ, Pan YH, You BM, Hu JH. Induction of leukotriene B(4) and prostaglandin E(2) release from keratinocytes by protease-activated receptor-2-activating peptide in ICR mice. Int Immunopharmacol. 2009;9:1332–1336. doi: 10.1016/j.intimp.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 117.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 118.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, Krieg T, Stanzel S, Heinrich PC, Merk HF, Bosio A, Baron JM, Hermanns HM. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–937. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347–356. doi: 10.1016/j.cytogfr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 121.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Gronhoj-Larsen C, Matsushima K. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 122.Morikawa Y, Tamura S, Minehata K, Donovan PJ, Miyajima A, Senba E. Essential function of oncostatin m in nociceptive neurons of dorsal root ganglia. J Neurosci. 2004;24:1941–1947. doi: 10.1523/JNEUROSCI.4975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006;142:1263–1271. doi: 10.1016/j.neuroscience.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 124.Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J, Prieto J, Rust C, Geenes VL, Williamson C, Moolenaar WH, Beuers U, Oude Elferink RP. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018. doi: 10.1053/j.gastro.2010.05.009. 18 e1. [DOI] [PubMed] [Google Scholar]
- 125.Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72:51–56. doi: 10.1159/000078632. [DOI] [PubMed] [Google Scholar]
- 126.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- 128.van Laarhoven AI, Kraaimaat FW, Wilder-Smith OH, van de Kerkhof PC, Cats H, van Riel PL, Evers AW. Generalized and symptom-specific sensitization of chronic itch and pain. J Eur Acad Dermatol Venereol. 2007;21:1187–1192. doi: 10.1111/j.1468-3083.2007.02215.x. [DOI] [PubMed] [Google Scholar]
- 129.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 130.Raja SN, Meyer RA, Campbell JN. Peripheral mechanisms of somatic pain. Anesthesiology. 1988;68:571–590. doi: 10.1097/00000542-198804000-00016. [DOI] [PubMed] [Google Scholar]
- 131.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 132.Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–154. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 133.Ozawa M, Tsuchiyama K, Gomi R, Kurosaki F, Kawamoto Y, Aiba S. Neuroselective transcutaneous electrical stimulation reveals neuronal sensitization in atopic dermatitis. J Am Acad Dermatol. 2009;60:609–614. doi: 10.1016/j.jaad.2008.11.900. [DOI] [PubMed] [Google Scholar]
- 134.Ikoma A, Rukwied R, Stander S, Steinhoff M, Miyachi Y, Schmelz M. Neuronal sensitization for histamine-induced itch in lesional skin of patients with atopic dermatitis. Arch Dermatol. 2003;139:1455–1458. doi: 10.1001/archderm.139.11.1455. [DOI] [PubMed] [Google Scholar]
- 135.Stander S, Schmelz M. Chronic itch and pain--similarities and differences. Eur J Pain. 2006;10:473–478. doi: 10.1016/j.ejpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 136.Schneider G, Stander S, Burgmer M, Driesch G, Heuft G, Weckesser M. Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. Eur J Pain. 2008;12:834–841. doi: 10.1016/j.ejpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 137.Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol. 2009;161:1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pfab F, Valet M, Sprenger T, Huss-Marp J, Athanasiadis GI, Baurecht HJ, Konstantinow A, Zimmer C, Behrendt H, Ring J, Tolle TR, Darsow U. Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema - a combined psychophysical and neuroimaging study. Allergy. 2010;65:84–94. doi: 10.1111/j.1398-9995.2009.02163.x. [DOI] [PubMed] [Google Scholar]
- 139.Peters EM, Raap U, Welker P, Tanaka A, Matsuda H, Pavlovic-Masnicosa S, Hendrix S, Pincelli C. Neurotrophins act as neuroendocrine regulators of skin homeostasis in health and disease. Horm Metab Res. 2007;39:110–124. doi: 10.1055/s-2007-961812. [DOI] [PubMed] [Google Scholar]
- 140.Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009;53:48–54. doi: 10.1016/j.jdermsci.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 141.Dou YC, Hagstromer L, Emtestam L, Johansson O. Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch Dermatol Res. 2006;298:31–37. doi: 10.1007/s00403-006-0657-1. [DOI] [PubMed] [Google Scholar]
- 142.Johansson O, Liang Y, Emtestam L. Increased nerve growth factor- and tyrosine kinase A-like immunoreactivities in prurigo nodularis skin -- an exploration of the cause of neurohyperplasia. Arch Dermatol Res. 2002;293:614–619. doi: 10.1007/s00403-001-0285-8. [DOI] [PubMed] [Google Scholar]
- 143.Grewe M, Vogelsang K, Ruzicka T, Stege H, Krutmann J. Neurotrophin-4 production by human epidermal keratinocytes: increased expression in atopic dermatitis. J Invest Dermatol. 2000;114:1108–1112. doi: 10.1046/j.1523-1747.2000.00974.x. [DOI] [PubMed] [Google Scholar]
- 144.Urashima R, Mihara M. Cutaneous nerves in atopic dermatitis. A histological, immunohistochemical and electron microscopic study. Virchows Arch. 1998;432:363–370. doi: 10.1007/s004280050179. [DOI] [PubMed] [Google Scholar]
- 145.Kinkelin I, Motzing S, Koltenzenburg M, Brocker EB. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–37. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]