Abstract
Influenza infection can affect cardiac function. The recent pandemic of H1N1 influenza A provided an opportunity to study echocardiographic findings in critically ill infected patients.
We hypothesized that critically ill patients with H1N1 infection would have a higher incidence of right and left heart failure than is seen in unselected populations of patients with septic shock and/or Acute Respiratory Distress Syndrome (ARDS).
We retrospectively studied all patients admitted to four ICUs at three hospitals in Salt Lake County, Utah, USA with laboratory-confirmed H1N1 infection in whom a clinical echocardiogram was available.
Twenty-three of 48 patients had qualifying echocardiograms. Right ventricular (RV) dilatation (50–80%) and at least moderate systolic impairment (23%) were common, higher than the range described in general populations with ARDS. Left ventricular systolic dysfunction was present in 17% of patients. No single echocardiographic parameter was associated with 28-day mortality or ventilator-free days to 28 days.
Critically ill patients with H1N1 infection frequently exhibit right heart dilatation and failure. RV basal dilatation was extremely common. These patients have less left heart failure than expected on the basis of prior descriptions of influenza myopericarditis or of general populations of septic patients.
Key-words (MeSH subjects): Acute Respiratory Distress Syndrome, Echocardiography, Heart Failure, Influenza A Virus, Pulmonary Heart Disease
Background
A pandemic of novel H1N1 Influenza A virus (H1N1) recently affected most countries of the world.[1–6] In spring and summer 2009, Salt Lake County, Utah experienced a dramatic surge in intensive care unit admissions for acute cardiopulmonary failure resulting from H1N1 infection.[7] An evolving literature in critical care has begun to describe patterns of cardiac dysfunction in patients with undifferentiated acute respiratory distress syndrome (ARDS) and septic shock. Most of these studies have emphasized relative diastolic dysfunction of both ventricles in patients with septic shock, with a significant minority of patients also demonstrating left ventricular (LV) systolic dysfunction.[8–14] Studies in ARDS have suggested right ventricular (RV) systolic dysfunction and dilatation in a significant minority of patients.[15, 16] In distinction to this critical care literature, the large majority of studies on cardiac dysfunction during influenza infection emphasize myopericarditis. Despite the relative wealth of data on myopericarditis in acute influenza,[17–27] there are no published studies in English of RV findings in life-threatening influenza infection. We reviewed clinical echocardiographic results in our patients to characterize the incidence and extent of right and left heart failure in patients with life-threatening H1N1 infection.
Methods
Study patients were admitted to any of four intensive care units (ICUs) in three academic hospitals in Salt Lake City, Utah, USA. Any patients presenting with symptoms typical of influenza-like illness during the outbreak period (May-June 2009) to participating ICUs were considered to have suspected H1N1. Suspect cases were then confirmed using PCR results of respiratory tract samples, with specific confirmation of the pandemic H1N1 strain at the Utah State laboratory and/or the Centers for Disease Control and Prevention.[28] Of patients with confirmed H1N1, we included those who underwent echocardiography for clinical indications. We excluded patients with known preexisting cardiomyopathy or current pregnancy.
ARDS and acute lung injury (ALI) were defined according to consensus criteria,[29] as was septic shock.[30] Mortality was defined as in-hospital mortality. Secondary endpoints included ventilator-free and ICU-free days at 28 days after admission.[31] Levels of mechanical ventilatory support at the time the echocardiogram was performed were determined from the electronic medical record, which is prospectively updated by respiratory therapists. We also measured receipt of vasopressor and/or inotropic therapy, body mass index (BMI), age, sex, serum troponin I levels nearest in time to the echocardiogram, and admission APACHE II scores.[32] Elevation of troponin I (≥ 0.4 ng/ml) was defined according to the assay employed at study hospitals.
Echocardiographic studies were obtained at the discretion of the clinical teams caring for the patients. Images were obtained using either a Philips SONOS 5500 or iE-33 (Philips Medical Systems, Bothell, WA) bedside transthoracic echocardiographic imaging system and digitally stored as per routine. Images were reviewed by a research sonographer (KH), with subsequent review by the senior author (CG), a Testamur of the National Board of Echocardiography and Level-II critical care echocardiographer. At the time of review, these two authors were blinded to clinical status and patient outcome.
RV fractional area change (RVFAC) was measured in the apical four chamber view, as per consensus guidelines.[33] The tricuspid annular plane systolic excursion (TAPSE), another validated measure of RV systolic function, was also defined as per consensus guidelines and measured using M-mode through the anterior tricuspid annulus in the apical 4-chamber view.[34–36] When an M-mode tracing was unavailable, TAPSE was measured from 2-D images. LV ejection fraction was defined using the Simpson’s method of stacked disks.[37] The ratio of RV to LV areas, a standard measure of relative RV dilatation, and the RV basal and mid-chamber diameters were measured as per consensus guidelines.[37] LV diastolic function was defined on the basis of pulse-wave Doppler of the mitral inflow (in patients in sinus rhythm and without mitral stenosis) and tissue Doppler of the medial (septal) mitral annulus according to consensus guidelines, which stratify diastolic filling into normal, impaired relaxation, pseudonormal, or restrictive.[38] Pulmonary venous inflow into the left atrium was measured using pulse-wave Doppler generally at the right superior pulmonary vein in the apical four chamber view, and was classified as systolic (normal in most adults by middle age), diastolic, or codominant.[38]
Statistical Methods
Descriptive statistics and confidence intervals are provided in Tables 1 and 2. Percentages and Fisher exact 95% confidence intervals are reported for binary measures; means and normal 95% confidence intervals for normally distributed continuous measures; and medians and bootstrap 95% confidence intervals are provided for non-normally distributed continuous measures. Normality was determined by examination and using the Shapiro-Wilk test. To evaluate correlation between continuous variables, we fitted linear regression models. To compare mortality for different measures of RV failure, we employed univariate logistic regression models. A two-tailed p <0.05 was considered statistically significant. All analyses were performed with the R statistical package, version 2.10.1.[39]
Table 1.
Clinical characteristics of patient cohort
| Characteristic | Entire Population (N=23) | Patients with ARDS (N=19) | Patients without echo (N=23) |
|---|---|---|---|
| Point estimate (95% CI) | Point estimate (95% CI) | Point estimate (95% CI) | |
| Age (years) | 41 (36–47) | 42 (36–48) | 30 (24–36) |
| Female sex (%) | 70 (49–90) | 68 (45–91) | 48 (26–70) |
| BMI (kg/m2) | 39 (34–44) | 39 (35–43) | 34 (30–38) |
| ALI or ARDS (%) | 91 (79–100) | NA | 65 (44–86) |
| ARDS (%) | 83 (66–99) | NA | 48 (26–70) |
| Required mechanical ventilation (%) | 87 (72–100) | 95 (84–100) | 35 (14–56) |
| Maximum PEEP (cm H2O) | 21 (17–24) | 21 (17–24) | 16 (8–24) |
| Minimum PaO2:FiO2 ratio | 59 (50–98) | 58 (50–72) | 146 (73–240) |
| Ventilator Days | 7.3 (2.8–13) | 7.8 (3.4–16) | 11 (3–19) |
| Septic shock (%) | 61 (39–82) | 74 (52–95) | 17 (1–34) |
| Minimum ScvO2 (%) | 62 (55–70) | 63 (54–68) | 51 (24–78) |
| APACHE II | 26 (22–30) | 26 (22–36) | 16 (12–20) |
| ICU length-of-stay (days) | 6.4 (3.1–13) | 10 (6.5–14) | 5 (2–8) |
| Troponin I nearest in time to echo (ng/ml) | 0.03 (0.01–0.2) | 0.08 (0.02–0.26) | NA |
| Elevated troponin (%) | 8.7 (0–21) | 11 (0–26) | 0 |
| Inpatient mortality (%) | 30 (10–51) | 37 (13–61) | 4 (0–13) |
Point estimate is mean for normally distributed data, median otherwise.
BMI: Body Mass Index; ALI: Acute Lung Injury; ARDS: Acute Respiratory Distress Syndrome; PEEP: positive end expiratory pressure; PaO2:FiO2: ratio of arterial oxygen pressure to fraction of inspired oxygen; ScvO2: oxygen saturation in superior vena cava; APACHE II: Acute Physiology And Chronic Health Evaluation score
Table 2.
Echocardiographic Characteristics
| Characteristic | Entire cohort: Point estimate (95% CI) | N for parameter | ARDS: Point estimate (95% CI) | N for parameter |
|---|---|---|---|---|
| Depressed LVEF (%) | 17 (5–37) | 23 | 21 (1–41) | 19 |
| Mitral E Velocity (cm/s) | 88 (76–99) | 19 | 87 (76–99) | 17 |
| E:A Ratio | 1.3 (1.0–1.5) | 16 | 1.3 (1.1–1.5) | 15 |
| Low TAPSE (%) | 36 (28–42) | 22 | 34 (27–40) | 18 |
| Low RV FAC (%) | 43 (20–66) | 21 | 47 (21–74) | 17 |
| RA area (cm2) | 14 (11–16) | 17 | 14 (12–17) | 14 |
| Em velocity (cm/s) | 9.5 (7.1–12) | 15 | 10 (7–13) | 12 |
| E:Em Ratio | 9.2 (7.4–12) | 13 | 8.7 (7.3–13) | 12 |
| Abnormal PVI (%) | 69 (40–98) | 13 | 64 (30–98) | 11 |
| High RV:LV ratio (%) | 83 (66–100) | 23 | 90 (74–100) | 19 |
| Dilated RV—base (%) | 90 (77–100) | 21 | 90 (74–100) | 17 |
| Dilated RV—mid (%) | 32 (11–53) | 22 | 33 (9–57) | 18 |
| Septal bowing (%) | 39 (18–61) | 23 | 37 (13–61) | 19 |
| Dilated RV + septal bowing (%) | 30 (10–51) | 23 | 32 (9–55) | 19 |
| High RV area (%) | 19 (1–37) | 21 | 18 (0–38) | 17 |
| TR gradient (mmHg) | 28 (22–34) | 13 | 29 (22–36) | 11 |
Point estimate is mean for normally distributed data, median otherwise.
RV: right ventricle; LV: left ventricle; LVEF: left ventricular ejection fraction; TAPSE: tricuspid annular plane systolic excursion; RV FAC: right ventricular fractional area change; E: early diastolic mitral inflow; A: atrial mitral inflow; Em: early diastolic septal annular velocity; PVI: pulmonary venous inflow; TR: tricuspid regurgitation; RV:LV Ratio: ratio of right ventricular to left ventricular end-diastolic area.
The Institutional Review Boards at Intermountain Healthcare and the University of Utah approved this study with waiver of the requirement for informed consent.
Results
Twenty-five of 48 patients treated in participating ICUs with H1N1 had echocardiograms available within 48 hours of ICU admission. One patient had premorbid cardiomyopathy and was excluded from analysis, as was the single pregnant patient. Of the remainder, all 23 were of sufficient quality to analyze most if not all parameters of RV and LV function. Clinical characteristics of the 23 patients are presented in Table 1.
Almost all patients (91%) met criteria for ALI (83% met criteria for ARDS) with a mean nadir PaO2:FiO2 (P/F) ratio of 88 (median 59) requiring a mean positive end-expiratory pressure (PEEP) among those ventilated of 21 (median 24) cm H2O. Nearly two-thirds were in shock, with a mean nadir ScvO2 of 62%. Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score was 26; seven (30%) of the 23 patients died. Patients who underwent echocardiography were more severely ill than patients who did not undergo echocardiography: mean APACHE II score was 16 in patients who did not undergo echocardiography (p<0.01 for comparison), and mean PEEP was 16 in patients who did not undergo echocardiography (p=0.2 for comparison), while shock was present in 17% of patients who did not undergo echocardiography (p<0.01 for comparison). Venous thrombosis was rare (three patients had thrombosis in a leg vein), while pulmonary embolism was not observed. Ten patients were screened by ultrasound, one by computed tomography. No patients with thrombosis died. Three patients had bacterial superinfection, one of whom had shock. Only the superinfected patient with shock died.
Echocardiographic parameters are presented in Table 2.
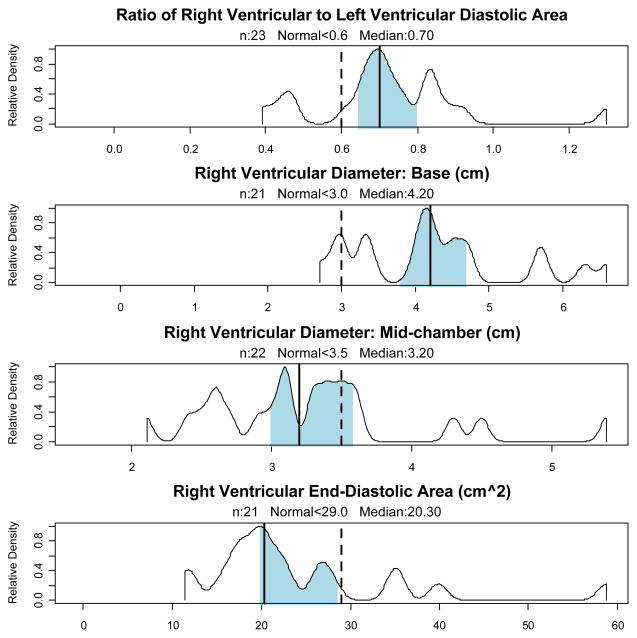
The most common finding was dilatation of the RV, whether measured by the basal RV diameter, mid-RV diameter or RV diastolic area. Using the published range for normals, 90% of patients had an RV basal diameter consistent with dilatation, while 32% met criteria for dilatation on measurement at mid-chamber, and 19% met criteria for at least moderate RV dilatation by diastolic area. Using the proposed ratio of RV to LV end-diastolic areas (>0.6) employed in the critical care echocardiography literature,[40] 83% of patients had RV dilatation. Septal flattening was a less common finding, although 30% of patients had both RV dilatation and septal flattening. Mean RV basal diameter was 4.2 cm, mean RV diastolic area was 23 cm2, and the mean ratio of RV end diastolic area to LV end diastolic area was 0.72, where < 0.6 is considered normal. Density distributions of measures of RV dilatation are presented in Figure 1.
Figure 1. Measures of Right Ventricular Dilatation.
Observed density distributions of measures of systolic function. Median indicated by vertical solid line, with shading indicating 95% bootstrap confidence intervals. The vertical dotted line indicates the upper limit of normal from consensus documents and normal populations.
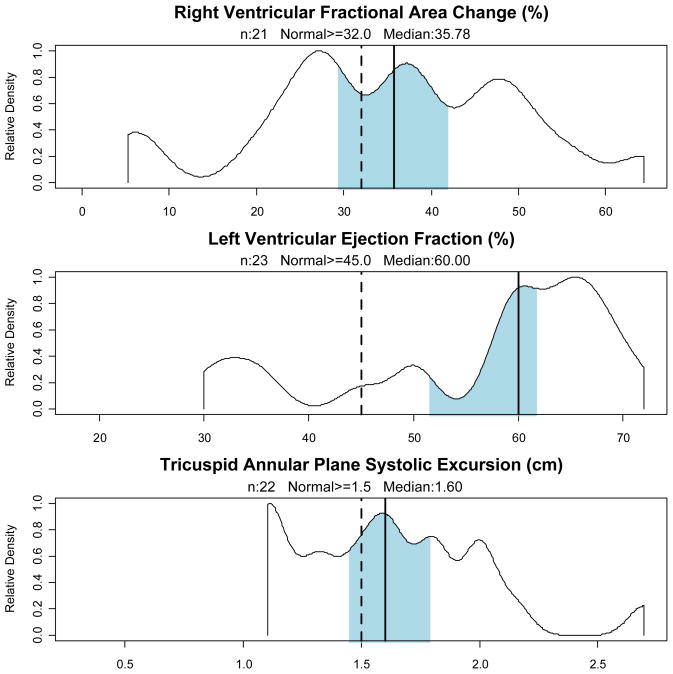
The two main measures of RV systolic function, TAPSE and RVFAC, demonstrated a similarly high rate of RV dysfunction, as demonstrated in Figure 2.
Figure 2. Measures of Ventricular Systolic Function.
Observed density distributions of measures of systolic function. Median indicated by vertical solid line, with shading indicating 95% bootstrap confidence intervals. The vertical dotted line indicates the lower limit of normal from consensus documents and normal populations.
TAPSE was ≤ 1.5 cm in 36% of patients, while RVFAC was below normal (< 32%) in 43% of patients. (Mean RVFAC was 35%; mean TAPSE was 1.6 cm.) These two measures of RV systolic dysfunction were concordant in 16 patients and discordant in 4. If the presence of either finding was considered evidence of RV failure, then 48% of patients exhibited RV systolic failure. In those patients (n=13) who had a tricuspid regurgitation jet velocity measured, the mean tricuspid pressure gradient was 28 mm Hg. Restricting the analysis to the 19 patients with ARDS did not significantly alter these findings.
LV diastolic function was generally evaluable: mitral inflow was fused or indeterminate in only 4 of 23 patients. A normal pattern of mitral inflow was documented in nine patients (39%). Mean mitral E velocity was 88 cm/s, mean A velocity was 69 cm/s, and the mean E:A ratio was 1.25. Mitral septal tissue Doppler velocities were obtained in 15 patients, with mean early velocity (Em) of 9.5 cm/s and a median E/Em ratio of 9.2. In the 13 patients in whom reliable pulmonary venous inflows could be determined, 69% were diastolic or codominant.
Pericardial effusion was an uncommon finding—two patients had a trivial effusion, while one other patient had a small effusion. None had evidence of tamponade physiology. Troponin I was frankly elevated in two (9%) patients, neither of whom had evidence of depressed LV systolic function. Four patients (17%) had depressed LV systolic function, one of whom had a borderline elevation of troponin I (0.38 ng/ml); mean LVEF for the entire cohort was 57%, while for patients with depressed LV systolic function, mean LVEF was 33%. Depressed LV systolic function was only observed in patients with shock, affecting four of 14 patients with septic shock (29%). In patients who survived, LV systolic dysfunction resolved on follow-up echocardiograms.
In this group of critically ill patients, no association between echocardiographic parameters and fatal outcome reached statistical significance on univariate logistic regression. Linear regressions of echocardiographic parameters and ventilator-free days also failed to achieve statistical significance.
Although there was no strong linear correlation between BMI and various measures of RV dilatation, obese patients (BMI ≥ 30) had a higher RV diastolic area (25.5 vs. 16 cm2, p<0.01) and mid-chamber diameter (3.4 vs. 2.6 cm, p<0.01) than non-obese patients (no non-obese patients met criteria for RV dilatation by diastolic area or mid-chamber diameter). Measures of RV systolic function did not correlate with obesity. End-expiratory, peak, or plateau pressures on the mechanical ventilator at the time of echocardiography were not significantly associated with RV dilatation or systolic dysfunction. The ratio of PaO2 to FiO2 was also not significantly associated with RV dilatation or systolic dysfunction.
Discussion
Severe H1N1 infection, in this retrospective cohort of 23 critically ill patients, is frequently associated with RV dilatation and systolic dysfunction, at rates that somewhat exceed those in unselected populations of patients with ARDS. Moderate or severe RV dilatation and/or systolic dysfunction was present in more than half of 23 patients with H1N1 requiring ICU admission for whom echocardiograms were available. This degree of acute pulmonary heart failure (also called acute cor pulmonale) complicating H1N1 appears higher than prior estimates (10–25%) of the prevalence of pulmonary heart disease in a general population of patients with ARDS.[15, 41] Our sample appears similar, however, to patients with sepsis. In one cohort of 67 patients with septic shock, the mean RVFAC was 35% [14] essentially identical to the mean (31%) in patients with septic shock in our cohort.
The prevalence of LV systolic dysfunction in patients with septic shock in our cohort (29%) is somewhat lower than prior estimates (38%) for septic shock.[14] LV systolic dysfunction occurred exclusively in patients with septic shock, suggesting that influenza-specific myopericarditis was not a major factor in the cardiac insufficiency associated with life-threatening H1N1. Compatible with our findings, recent studies in ambulatory patients with influenza suggest that prior estimates of 10% incidence of myopericarditis are significant overestimates.[42–44] Contrary to older reports about the nature of heart failure in life-threatening influenza, the cardiac insufficiency in this group of patients with H1N1 appears to be largely related to lung injury and mechanical ventilation with secondary cor pulmonale and/or septic cardiomyopathy rather than influenza-associated myopericarditis. The recent study by To and colleagues describing findings in a similar-sized cohort of H1N1-infected patients with ARDS in Hong Kong[45] reported a higher incidence (22%) of myopericarditis than in our cohort, though their definition of myopericarditis was not explicit and did not clearly distinguish influenza-specific phenomena from the general effects of sepsis. Whether low-grade influenza myopericarditis occurs, our study suggests that such myopericarditis does not play a major role clinically.
ARDS is a reasonably well-characterized cause of acute cor pulmonale, with evidence for microvascular disruption in the pulmonary circulation, high intrathoracic pressure gradients, and endothelial cell edema all playing an important role.[46–48] Whether H1N1 induces disproportionate pulmonary vascular disease is not known, though preliminary autopsy results may be compatible with this finding.[49]
The thin-walled right heart is particularly susceptible to ischemia and failure in the face of acute increases in afterload. Right heart dysfunction has direct effects on LV diastolic and systolic function.[50, 51] Mechanical ventilation increases the afterload on the RV and impairs ventricular preload. The patients in our cohort had high PEEP levels (mean 21 cm H2O) but were uniformly ventilated using low tidal volumes;[52] patients ventilated with higher tidal volumes would likely exhibit more RV dilatation and failure.[41]
The degree of right heart dilatation in this cohort may be related to the high prevalence of obesity—the mean BMI in this cohort was 39 kg/m2, and at least four of our patients suffered from premorbid obstructive sleep apnea. Whether these patients had RV dilatation before onset of H1N1 is not certain, although there was not a clear relationship between BMI and RV dilatation.
An important limitation of our study is its retrospective nature. The fact that echocardiograms were ordered for clinical purposes means that our results describe the most severely ill patients with H1N1 infection. Our observations may not apply to patients with less severe cardiopulmonary dysfunction during H1N1 infection. Patients not requiring mechanical ventilation may have different echocardiographic findings.
Some authors have suggested, extrapolating from the use of echocardiography during and immediately after surgery, that certain echocardiographic findings should influence ventilation strategies, use of inotropes and vasopressors, and the determination of optimal fluid loading conditions in patients with respiratory and/or circulatory failure.[53–57] In this cohort indices of RV impairment were not significantly associated with mortality or ventilator-free days. While a variety of techniques to unload the RV have been tested in ARDS without clear benefit, and some authors have used extracorporeal membrane oxygenation (ECMO) for ARDS caused by H1N1,[5, 49] these techniques are not yet well-validated. The fact that findings of RV impairment were not clearly associated with inpatient mortality in this cohort may reflect small sample size.
The variability of classification of RV dilatation and impaired systolic function in this population is also an important finding. Different measures of impairment yield different estimates of the prevalence of cor pulmonale in this patient cohort: no single measure is obviously superior to others. Cutoffs for classification of chamber dimensions and function are generally drawn from healthy volunteers and may not be as useful in critically ill patients with acute cor pulmonale. Larger cohorts in general ARDS and septic shock populations should strive to define which parameters at which thresholds most usefully identify clinically relevant distinctions.
This report highlights the high prevalence of right ventricular dilatation and dysfunction in patients with ARDS and septic shock complicating H1N1 infection and the comparatively low incidence of LV systolic dysfunction. Further research should investigate the reproducibility and therapeutic implications of these findings.
Acknowledgments
This study was supported by a grant from the Easton Family Fund of the Deseret Research Foundation.
References
- 1.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernandez M, Stewart TE, Fowler RA. Critically Ill Patients With 2009 Influenza A(H1N1) in Mexico. JAMA. 2009:2009.1536. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April–June 2009. N Engl J Med. 2009 doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler R for the Canadian Critical Care Trials Group H1N1 Collaborative. Critically Ill Patients With 2009 Influenza A(H1N1) Infection in Canada. JAMA. 2009:2009.1496. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 6.Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del Nogal-Saez F, Alvarez-Lerma F, Martinez S, Ferrer M, Avellanas M, Granada R, Maravi-Poma E, Albert P, Sierra R, Vidaur L, Ortiz P, Prieto Del Portillo I, Galvan B, Leon-Gil C, HnSWG T. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13(5):R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller RR, 3rd, Markewitz BA, Rolfs RT, Brown SM, Dascomb KK, Grissom CK, Friedrichs MD, Mayer J, Hirshberg EL, Conklin J, Paine R, 3rd, Dean NC. Clinical findings and demographic factors associated with intensive care unit admission in Utah due to 2009 novel influenza A (H1N1) infection. Chest. 2009 doi: 10.1378/chest.09-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etchecopar-Chevreuil C, Francois B, Clavel M, Pichon N, Gastinne H, Vignon P. Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med. 2008;34(2):250–256. doi: 10.1007/s00134-007-0929-z. [DOI] [PubMed] [Google Scholar]
- 9.Jardin F, Fourme T, Page B, Loubieres Y, Vieillard-Baron A, Beauchet A, Bourdarias JP. Persistent preload defect in severe sepsis despite fluid loading: A longitudinal echocardiographic study in patients with septic shock. Chest. 1999;116(5):1354–1359. doi: 10.1378/chest.116.5.1354. [DOI] [PubMed] [Google Scholar]
- 10.Poelaert J, Declerck C, Vogelaers D, Colardyn F, Visser CA. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997;23(5):553–560. doi: 10.1007/s001340050372. [DOI] [PubMed] [Google Scholar]
- 11.Thierry S, Giroux Leprieur E, Lecuyer L, Brocas E, Van de Louw A. Echocardiographic features, mortality, and adrenal function in patients with cirrhosis and septic shock. Acta Anaesthesiol Scand. 2008;52(1):45–51. doi: 10.1111/j.1399-6576.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 12.Vieillard Baron A, Schmitt JM, Beauchet A, Augarde R, Prin S, Page B, Jardin F. Early preload adaptation in septic shock? A transesophageal echocardiographic study. Anesthesiology. 2001;94(3):400–406. doi: 10.1097/00000542-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Du B, Long Y, Zhao C, Hou B. Right ventricular function of patients with septic shock: clinical significance. Zhonghua Wai Ke Za Zhi. 2000;38(7):488–492. [PubMed] [Google Scholar]
- 14.Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36(6):1701–1706. doi: 10.1097/CCM.0b013e318174db05. [DOI] [PubMed] [Google Scholar]
- 15.Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001;29(8):1551–1555. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt JM, Vieillard-Baron A, Augarde R, Prin S, Page B, Jardin F. Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: impact on right ventricular outflow impedance evaluated by pulmonary artery Doppler flow velocity measurements. Crit Care Med. 2001;29(6):1154–1158. doi: 10.1097/00003246-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Montcriol A, Wiramus S, Ribeiri A, Attard N, Nait-Saidi L, Kerbaul F, Chiche L. Successful management of Influenza A associated fulminant myocarditis: mobile circulatory support in intensive care unit: a case report. Cases J. 2008;1(1):46. doi: 10.1186/1757-1626-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurila JJ, Ala-Kokko TI, Tuokko H, Syrjala H. Cardiac tamponade and septic shock caused by viral infection in a previously healthy woman. Acta Anaesthesiol Scand. 2005;49(9):1384–1386. doi: 10.1111/j.1399-6576.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- 19.Mamas MA, Nair S, Fraser D. Cardiac tamponade and heart failure as a presentation of influenza. Exp Clin Cardiol. 2007;12(4):214–216. [PMC free article] [PubMed] [Google Scholar]
- 20.Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887–892. [PubMed] [Google Scholar]
- 21.Gibson TC, Arnold J, Craige E, Curnen EC. Electrocardiographic studies in Asian influenza. Am Heart J. 1959;57(5):661–668. doi: 10.1016/0002-8703(59)90175-9. [DOI] [PubMed] [Google Scholar]
- 22.Walsh J, Burch GE, White A, Mogabgab W, Dietlein L. A study of the effects of type A (Asian strain) influenza on the cardiovascular system of man. Ann Intern Med. 1958;49(3):502–528. doi: 10.7326/0003-4819-49-3-502. [DOI] [PubMed] [Google Scholar]
- 23.Karjalainen J, Nieminen MS, Heikkila J. Influenza A1 myocarditis in conscripts. Acta Med Scand. 1980;207(1–2):27–30. doi: 10.1111/j.0954-6820.1980.tb09670.x. [DOI] [PubMed] [Google Scholar]
- 24.Verel D, Warrack AJ, Potter CW, Ward C, Rickards DF. Observations on the A2 England influenza epidemic: a clinicopathological study. Am Heart J. 1976;92(3):290–296. doi: 10.1016/s0002-8703(76)80109-3. [DOI] [PubMed] [Google Scholar]
- 25.Nolte KB, Alakija P, Oty G, Shaw MW, Subbarao K, Guarner J, Shieh WJ, Dawson JE, Morken T, Cox NJ, Zaki SR. Influenza A virus infection complicated by fatal myocarditis. Am J Forensic Med Pathol. 2000;21(4):375–379. doi: 10.1097/00000433-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Engblom E, Ekfors TO, Meurman OH, Toivanen A, Nikoskelainen J. Fatal influenza A myocarditis with isolation of virus from the myocardium. Acta Med Scand. 1983;213(1):75–78. doi: 10.1111/j.0954-6820.1983.tb03693.x. [DOI] [PubMed] [Google Scholar]
- 27.Drescher J, Zink P, Verhagen W, Flik J, Milbradt H. Recent influenza virus A infections in forensic cases of sudden unexplained death. Arch Virol. 1987;92(1–2):63–76. doi: 10.1007/BF01310063. [DOI] [PubMed] [Google Scholar]
- 28.CDC protocol of realtime RTPCR for influenza A (H1N1) Geneva: World Health Organization; Apr, 2009. [cited July 10, 2009]; Available from: http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf. [Google Scholar]
- 29.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 30.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 31.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 33.Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD. Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography. 2007;24(5):452–456. doi: 10.1111/j.1540-8175.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 34.Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22(7):776–792. doi: 10.1016/j.echo.2009.04.027. quiz 861–772. [DOI] [PubMed] [Google Scholar]
- 35.Samad BA, Alam M, Jensen-Urstad K. Prognostic impact of right ventricular involvement as assessed by tricuspid annular motion in patients with acute myocardial infarction. Am J Cardiol. 2002;90(7):778–781. doi: 10.1016/s0002-9149(02)02612-7. [DOI] [PubMed] [Google Scholar]
- 36.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107(3):526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 37.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Team RDC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
- 40.Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, Oropello J, Vieillard-Baron A, Axler O, Lichtenstein D, Maury E, Slama M, Vignon P. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050–1060. doi: 10.1378/chest.08-2305. [DOI] [PubMed] [Google Scholar]
- 41.Osman D, Monnet X, Castelain V, Anguel N, Warszawski J, Teboul JL, Richard C. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35(1):69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 42.Greaves K, Oxford JS, Price CP, Clarke GH, Crake T. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch Intern Med. 2003;163(2):165–168. doi: 10.1001/archinte.163.2.165. [DOI] [PubMed] [Google Scholar]
- 43.Connolly AM, Salmon RL, Lervy B, Williams DH. What are the complications of influenza and can they be prevented? Experience from the 1989 epidemic of H3N2 influenza A in general practice. BMJ. 1993;306(6890):1452–1454. doi: 10.1136/bmj.306.6890.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ison MG, Campbell V, Rembold C, Dent J, Hayden FG. Cardiac findings during uncomplicated acute influenza in ambulatory adults. Clin Infect Dis. 2005;40(3):415–422. doi: 10.1086/427282. [DOI] [PubMed] [Google Scholar]
- 45.To KK, Hung IF, Li IW, Lee KL, Koo CK, Yan WW, Liu R, Ho KY, Chu KH, Watt CL, Luk WK, Lai KY, Chow FL, Mok T, Buckley T, Chan JF, Wong SS, Zheng B, Chen H, Lau CC, Tse H, Cheng VC, Chan KH, Yuen KY. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50(6):850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296(9):476–480. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 47.Dhainaut JF, Brunet F. Right ventricular performance in adult respiratory distress syndrome. Eur Respir J Suppl. 1990;11:490s–495s. [PubMed] [Google Scholar]
- 48.Brunet F, Dhainaut JF, Devaux JY, Huyghebaert MF, Villemant D, Monsallier JF. Right ventricular performance in patients with acute respiratory failure. Intensive Care Med. 1988;14 (Suppl 2):474–477. doi: 10.1007/BF00256963. [DOI] [PubMed] [Google Scholar]
- 49.Intensive-care patients with severe novel influenza A (H1N1) virus infection -Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009;58(27):749–752. [PubMed] [Google Scholar]
- 50.Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981;63(1):87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- 51.Brinker JA, Weiss JL, Lappe DL, Rabson JL, Summer WR, Permutt S, Weisfeldt ML. Leftward septal displacement during right ventricular loading in man. Circulation. 1980;61(3):626–633. doi: 10.1161/01.cir.61.3.626. [DOI] [PubMed] [Google Scholar]
- 52.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 53.Vieillard-Baron A, Slama M, Cholley B, Janvier G, Vignon P. Echocardiography in the intensive care unit: from evolution to revolution? Intensive Care Med. 2008;34(2):243–249. doi: 10.1007/s00134-007-0923-5. [DOI] [PubMed] [Google Scholar]
- 54.Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12(3):249–254. doi: 10.1097/01.ccx.0000224870.24324.cc. [DOI] [PubMed] [Google Scholar]
- 55.Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30(9):1734–1739. doi: 10.1007/s00134-004-2361-y. [DOI] [PubMed] [Google Scholar]
- 56.Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F. Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med. 2003;168(11):1270–1276. doi: 10.1164/rccm.200306-816CC. [DOI] [PubMed] [Google Scholar]
- 57.Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest. 2007;132(5):1440–1446. doi: 10.1378/chest.07-1013. [DOI] [PubMed] [Google Scholar]