Abstract
In eukaryotes, the DNA replication checkpoint prevents entry into mitosis when DNA replication is incomplete and is crucial for maintaining genomic integrity. Much less is known about equivalent controls that operate during meiosis. Here, we show that a DNA replication checkpoint control operates during meiosis in fission yeast. The mitotic checkpoint Rad genes and the Cds1 protein kinase are required for the DNA replication checkpoint during meiosis, with Cds1 playing a more prominent role than it does during mitosis. When DNA replication is blocked, the checkpoint maintains Cdc2 tyrosine 15 phosphorylation keeping Cdc2 protein kinase activity low and preventing onset of meiosis I. Additionally, there is a second checkpoint acting during meiosis that is revealed if cells are prevented from maintaining Cdc2 tyrosine 15 phosphorylation when DNA replication is blocked. Such cells arrest with high Cdc2 protein kinase activity and separated spindle pole bodies, an arrest state similar to that observed in mitotic budding yeast cells when DNA replication is incomplete. This second checkpoint is meiosis specific and may reflect processes occurring only during meiosis such as increased recombination rates, an extended duration of nuclear division, or homolog chromosome pairing.
Keywords: Checkpoint, meiosis, cdc2, tyrosine phosphorylation, Schizosaccharomyces pombe
Faithful replication of DNA in S phase and its accurate segregation at mitosis result in each daughter cell receiving a full complement of genetic information. Checkpoint mechanisms operate during the mitotic cell cycle and ensure that if DNA replication is incomplete or if DNA is damaged, then the subsequent mitosis is blocked (Hartwell and Weinert, 1989). In the fission yeast Schizosaccharomyces pombe, the checkpoint Rad genes (rad1+, 3+, 9+, 17+, 26+, and hus1+) are required for both the DNA replication and DNA damage checkpoints (Al-Khodairy and Carr 1992; Enoch et al. 1992; Jimenez et al. 1992; Rowley et al. 1992; Al-Khodairy et al. 1994). Strains with these mutations are checkpoint defective, allowing entry into mitosis when DNA is damaged or its replication is incomplete. A number of these genes are conserved in budding yeast and metazoan cells and are thought to be involved in the checkpoint controls operating in these organisms (Rhind and Russell 1998a; Weinert 1998b).
Two fission yeast protein kinases, Cds1 and Chk1, act downstream of the checkpoint Rad proteins and are required to communicate checkpoint signals to the mechanisms that control mitosis (Walworth et al. 1993; Murakami and Okayama 1995; Walworth and Bernards 1996; Lindsay et al. 1998). The Cds1 kinase appears to be required for proper recovery from a DNA replication checkpoint block, but is not required for the DNA damage checkpoint control (Murakami and Okayama 1995), whereas the Chk1 kinase is primarily involved in the DNA damage checkpoint control (Walworth et al. 1993). The protein kinase activity of Cds1 and the phosphorylation of Chk1 are dependent on the checkpoint Rad proteins (Walworth and Bernards 1996; Lindsay et al. 1998), and homologs of Cds1 and Chk1 have been found in other organisms (Fogarty et al. 1997; Peng et al. 1997; Sanchez et al. 1997; Sibon et al. 1997; Kumagai et al. 1998; Matsuoka et al. 1998; Blasina et al. 1999). In addition, Crb2/Rhp9, which is structurally related to budding yeast Rad9, has a very similar role to Chk1 (Saka et al. 1997; Willson et al. 1997). Both the DNA replication and DNA damage checkpoint controls block mitosis via the cyclin-dependent kinase (CDK) Cdc2 complexed with the B-type cyclin Cdc13 (Enoch and Nurse 1990; Rhind et al. 1997). The Cdc2–Cdc13 protein kinase is fully activated at the onset of mitosis by dephosphorylation of Cdc2 tyrosine 15 (MacNeill and Nurse 1997). Phosphorylation of Cdc2 on tyrosine 15 is catalyzed by both the Wee1 and Mik1 tyrosine kinases, and dephosphorylation is carried out by the Cdc25 tyrosine phosphatase and to a lesser extent by the Pyp3 phosphatase (MacNeill and Nurse 1997). The DNA replication checkpoint can be abolished by (1) a semi-dominant Cdc2 (Enoch and Nurse 1990), (2) overexpressing cdc25+ to reduce the level of phosphorylation of Cdc2 on tyrosine 15 (Enoch and Nurse 1990), (3) inactivating the Wee1 and Mik1 kinases (Lundgren et al. 1991), or (4) use of a Cdc2-Y15F mutant (Enoch et al. 1991; Rhind and Russell 1998b). These studies strongly suggest that the DNA replication checkpoint acts through tyrosine 15 phosphorylation of Cdc2, although this conclusion has been controversial (Knudsen et al. 1996; Rhind and Russell 1998b). The linkage between the checkpoint Rad genes and the cell cycle machinery is likely to be provided by both Cds1 and Chk1. Inhibition of DNA replication activates Cds1, which subsequently acts through Wee1 and Cdc25 to stop cell cycle progression (Boddy et al. 1998; Zeng et al. 1998), whereas Chk1 is physically associated with Cdc25 and appears to phosphorylate Cdc25 (Furnari et al. 1997) and Wee1 (Nurse 1997; O'Connell et al. 1997).
Our understanding of meiotic checkpoint controls is much less well developed than mitotic checkpoint controls. In budding yeast, Mec1, a counterpart of S. pombe Rad3, is involved in meiotic recombination (Kato and Ogawa 1994) and is required for the meiotic DNA replication checkpoint (Stuart and Wittenberg 1998). Budding yeast mutants defective in the completion of meiotic recombination arrest in prophase, and this arrest is dependent on certain mitotic DNA damage checkpoint genes including Mec1, suggesting the existence of a meiotic recombination checkpoint (Lydall et al. 1996). A number of gene products that operate in mitotic checkpoint controls have been found to be localized in various metazoan cells undergoing meiosis. For example, the human homolog of S. pombe Rad1 is found on both synapsed and unsynapsed chromosomes during meiotic prophase (Freire et al. 1998), whereas the Rad3-related ATM protein kinase is located along paired meiotic chromosomes, and the ATR protein kinase is found distributed along unpaired meiotic chromosomes (Keegan et al. 1996; Barlow et al. 1998). ATM-deficient mice are infertile because meiotic cells arrest in prophase I during spermatogenesis or oogenesis (Xu et al. 1996; Barlow et al. 1998). A human homolog of Chk1 co-localizes with ATR along meiotic chromosomes, and its protein level and localization are dependent on the ATM protein (Flaggs et al. 1997). In Drosophila, MEI-41, a homolog of S.pombe Rad3, is required for normal levels of meiotic recombination in oocytes (Hari et al. 1995).
In this report we carry out a systematic study of the DNA replication checkpoint control during meiosis in fission yeast. We establish that checkpoint Rad and Cds1 proteins are required for the meiotic DNA replication checkpoint, and also that phosphorylation of Cdc2 tyrosine 15 is maintained during a block over DNA replication, keeping Cdc2 protein kinase activity low. However, if Cdc2 is artificially dephosphorylated on tyrosine leading to a rise in Cdc2 protein kinase activity, progression through meiotic nuclear division still remains blocked, suggesting that there is a second control operative in meiotic cells. Therefore, we propose that the replication checkpoint signal pathway in meiosis has two targets; one is phosphorylation of Cdc2 tyrosine 15, and the other is an unidentified downstream target independent of Cdc2 phosphotyrosine regulation.
Results
A DNA replication checkpoint operates in meiosis
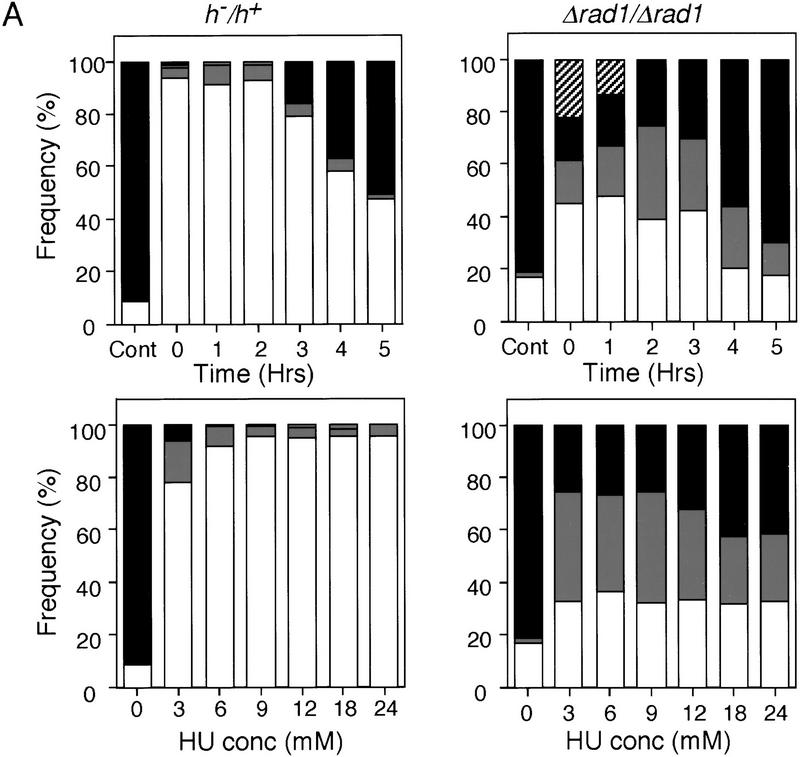
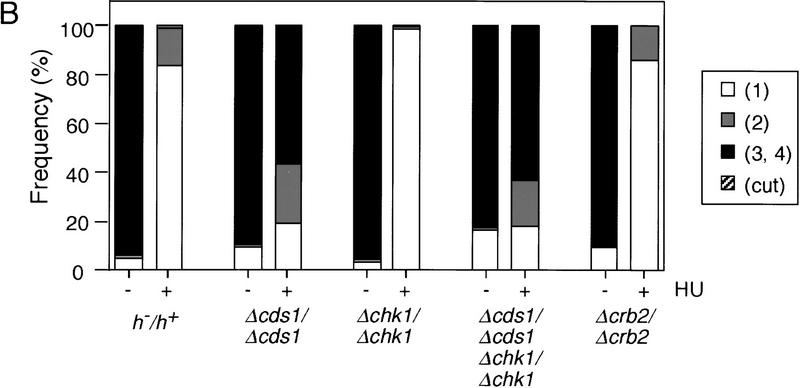
When the completion of DNA replication is prevented in mitotically dividing fission yeast cells with the inhibitor hydroxyurea (HU), the onset of the subsequent mitosis is blocked. To test whether a similar DNA replication checkpoint control is operative during meiosis, fission yeast diploid cells heterozygous for mating-type genes (h−/h+) were induced to undergo meiosis by shifting them from late log phase to nitrogen-free medium (−N). HU was added at different concentrations at various times after the medium change (Fig. 1A, left). Premeiotic DNA replication began ∼2 hr after the medium change as judged by FACS (data not shown). Completion of meiosis was monitored by counting the number of nuclei in each ascus after 17 hr, a time by which meiosis and sporulation is largely complete. Blocks over meiotic nuclear division prevent the formation of multinucleate cells. Cells with only one nucleus were observed in concentrations of HU above 6 mm added within 2 hr of the medium change (Fig. 1A left). This suggests that a DNA replication checkpoint is operative in fission yeast meiotic cells. The effects of various mutants known to be defective in the DNA replication or DNA damage checkpoints during the mitotic cell cycle were then tested. In the absence of HU, the checkpoint-defective strains spourlated efficiently, generating good spore viability of at least 68%. Over 60% of Δradl/Δrad1 diploid cells underwent meiosis when 12 mm HU was added 2 hr after the medium change (Fig. 1A, right), demonstrating that rad1+ is essential for the meiotic checkpoint. Δcds1/Δcds1 also underwent meiosis in the presence of HU, but Δchk1/Δchk1 and Δcrb2/Δcrb2 did not (Fig. 1B). chk1+ and cds1+ appear to have overlapping functions in the mitotic DNA replication checkpoint, yet during meiosis, the double mutant Δcds1/Δcds1 Δchk1/Δchk1 had no further defect in the DNA replication checkpoint than the single Δcds1/Δcds1 mutant (Fig. 1B). In the presence of HU, the checkpoint-defective strains attempted to form spores, but the spores produced were defective and only partially formed, as judged by cellular morphology and colony iodine staining. We conclude that there is a DNA replication checkpoint operative during meiosis, and that the genes rad1+ and cds1+ have a role in this checkpoint, whereas the DNA damage checkpoint genes chk1+ and crb2+ do not.
Figure 1.
Rad1 and Cds1 are required for the meiotic DNA replication checkpoint. (A) h−/h+ or Δrad1/Δrad1 diploid cells heterozygous for the mating-type genes were grown to late log phase, washed, and transferred to −N medium at time 0. (Top) Sporulation was induced in the absence (Cont) or in the presence of 12 mm HU added to the medium at the indicated times after the medium change. (Bottom) Sporulation was induced for 2 hr and then the indicated concentrations of HU were added to the medium. Samples were collected after 17 hr in the sporulation medium. The proportions of cells containing either one nucleus (white), two (gray), three or four nuclei (black),and the cut (hatched) cells were determined by fluorescence microscopy of DAPI-stained cells. The phenotype cut is the result of cells proceeding through mitosis when the DNA replication checkpoint is defective. (B) Δcds1/Δcds1, Δchk1/Δchk1, Δcds1/Δcds1 Δchk1/Δchk1, or Δcrb2/Δcrb2 diploid cells were induced to sporulate at time 0. HU (12 mm) was added to the culture 2 hr after the medium change. The number of nuclei was counted and shown as in A.
Characterization of the meiotic DNA replication checkpoint genes
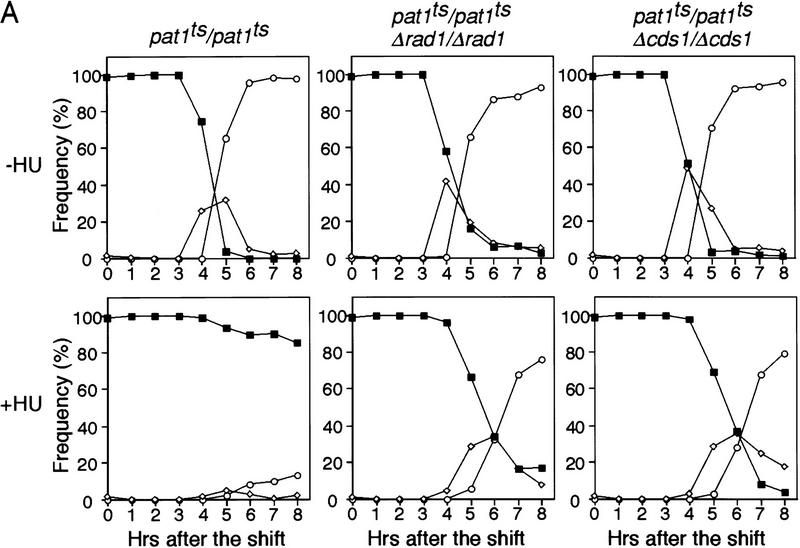
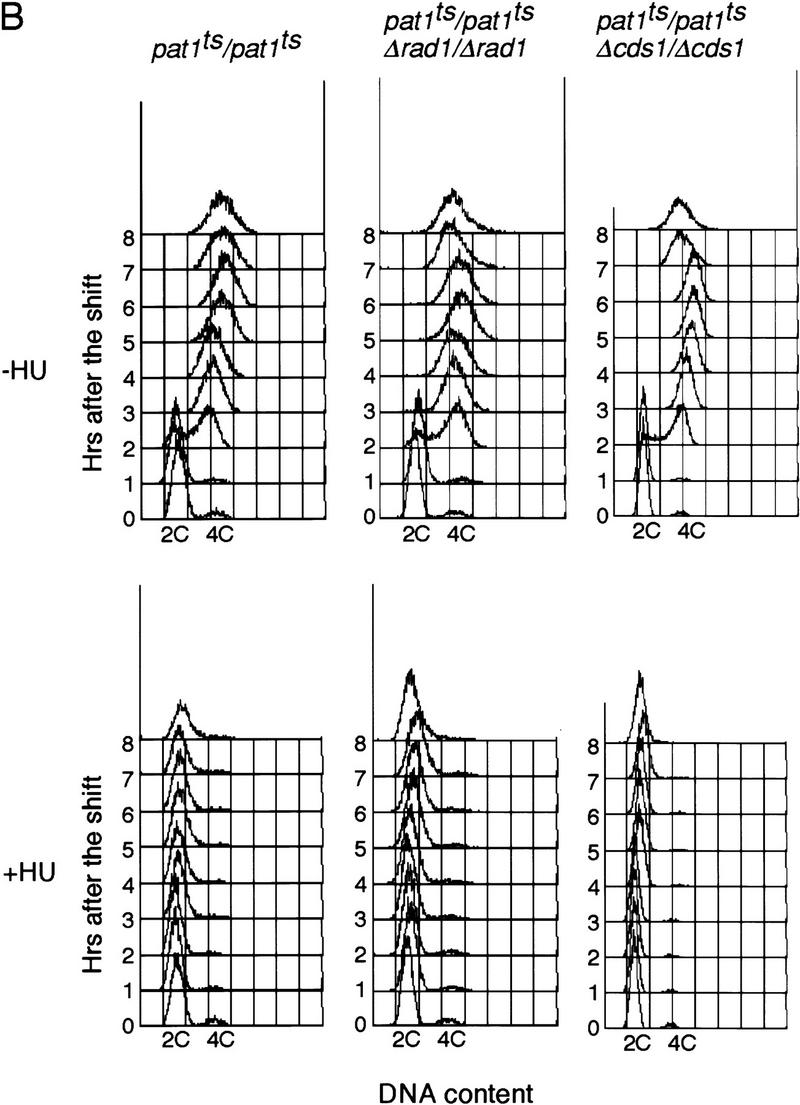
The above experiments were carried out by shifting h−/h+ cells to −N medium, which results in a rather asynchronous entry into meiosis. To investigate the meiotic DNA replication checkpoint further, we used a more synchronous meiotic system on the basis of shifting a pat1ts strain (temperature sensitive for the meiotic negative regulator Pat1) to its restrictive temperature, 34°C (Iino and Yamamoto 1985; Nurse 1985; Bähler et al. 1991). This pat1ts-induced meiosis is indistinguishable from normal meiosis (Y. Watanabe, pers. comm.). The diploid pat1ts/pat1ts strain was grown to late log phase, transferred to −N medium that arrests cells in G1, and then shifted to the restrictive temperature 34°C to induce meiosis. More than 80% of pat1ts/pat1ts cells failed to undergo nuclear division (Fig. 2A) when DNA replication was blocked by HU, as confirmed by FACS (Fig. 2B). In contrast, pat1ts/pat1ts Δrad1/Δrad1 cells continued to undergo nuclear division after addition of HU, although entry into meiosis I was delayed by ∼1 hr. Other checkpoint Rad genes, rad3+ (see below), rad9+, rad17+, and hus1+ were also examined to determine whether they are involved in the meiotic DNA replication checkpoint. We found that mutants of all of these genes underwent meiotic nuclear division in the presence of HU with almost identical kinetics to those observed with the pat1ts/pat1ts Δrad1/Δrad1 strain, indicating that they were defective in the meiotic DNA replication checkpoint.
Figure 2.
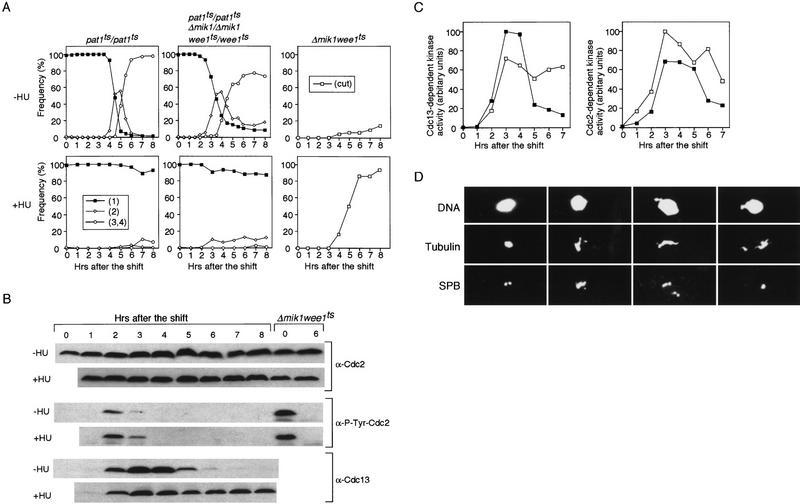
Rad1 and Cds1 are required for the meiotic DNA replication checkpoint in pat1-induced meiosis. (A) pat1ts/pat1ts, pat1ts/pat1ts Δrad1/Δrad1, and pat1ts/pat1ts Δcds1/Δcds1 diploid cells homozygous for the mating-type genes were grown to late log phase, washed, and transferred to −N medium for 14 hr at 25°C. The sporulation was induced by shifting the temperauture to 34°C at time 0. Samples were taken after the temperature shift in the absence (top) or presence of HU (bottom). The proportions of the cells containing one nucleus (█), two (⋄), and three or four nuclei (○) were determined. These results were representative of five independent experiments. The maximum difference of the time when half of the cells has two or more nuclei was 30 min in these experiments. (B) DNA content of the cells over the time course of sporulation in the absence (top) or the presence of HU (bottom) was determined by FACS analysis.
pat1ts/pat1ts Δcds1/Δcds1 cells also entered meiosis in the presence of HU, with similar kinetics to the pat1ts/pat1ts Δrad1/Δrad1 cells (Fig. 2A). This was unexpected because entry into mitosis in the presence of HU is much delayed in mitotic Δcds1 cells (Murakami and Okayama 1995), suggesting that cds1+ plays a more prominent role in the replication checkpoint during meiosis than it does during mitosis. Neither the pat1ts/pat1ts Δchk1/Δchk1 nor the pat1ts/pat1ts Δcrb2/Δcrb2 cells entered meiosis when treated with HU (data not shown), confirming that neither chk1+ nor crb2+ are involved in the meiotic DNA replication checkpoint.
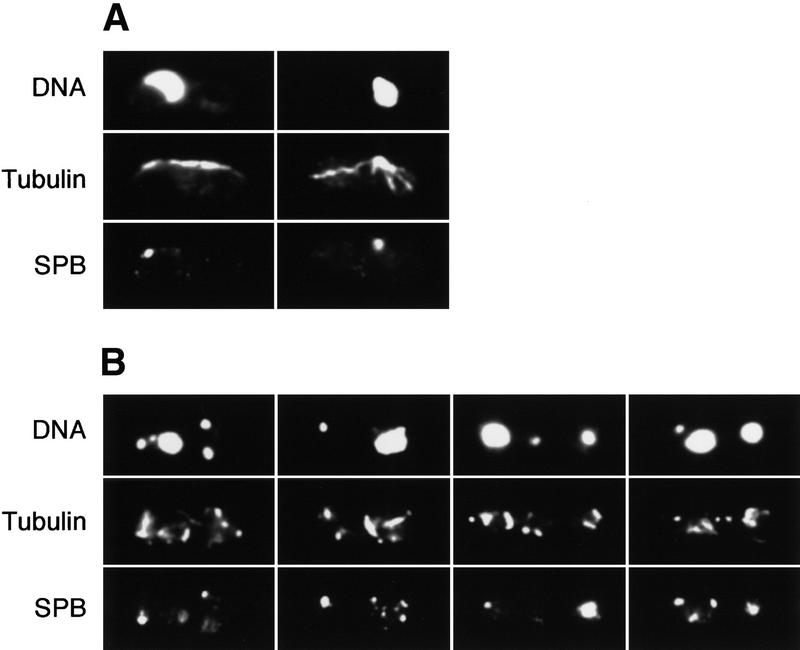
Next, we studied the morphology of meiotic nuclei during these experiments (Fig. 3). It has been shown that at prophase the meiotic nucleus moves backward and forward in a manner described as horse-tail movements (Robinow 1977; Bähler et al. 1993; Chikashige et al. 1994). The horse-tail morphologies of the nuclei at this stage of meiosis in pat1ts/pat1ts cells were similar to those observed in normal diploid cells. When pat1ts/pat1ts Δrad1/Δrad1, pat1ts/pat1ts Δcds1/Δcds1, pat1ts/pat1ts Δchk1/Δchk1, pat1ts/pat1ts Δcrb2/Δcrb2 cells were induced to undergo meiosis, their nuclear morphologies were indistinguishable from pat1ts/pat1ts cells (data not shown). When HU was added, pat1ts/pat1ts cells arrested with nuclei exhibiting horse-tail morphologies (Fig. 3A), whereas pat1ts/pat1ts Δrad1/Δrad1 or pat1ts/pat1ts Δcds1/Δcds1 cells underwent nuclear divisions although an uneven distribution of chromosomes was evident (Fig. 3B; data not shown). Like pat1ts/pat1ts cells, pat1ts/pat1ts Δchk1/Δchk1 or pat1ts/pat1ts Δcrb2/Δcrb2 cells arrested in prophase with nuclei having horse-tail morphology (data not shown).
Figure 3.
The meiotic DNA replication checkpoint arrests cells in prophase. The pat1ts/pat1ts (A) and pat1ts/pat1ts rad3/rad3 cells (B) were induced to undergo meiosis as in Fig. 2 for 6 hr at 34°C in HU, processed for immunofluorescence and stained with anti-TAT1 and anti-Sad1 antibodies. Typical examples of images of a field of the cells are shown. (Top) Position and morphology of chromatin DNA stained with DAPI; (middle) anti-tubulin staining probed with anti-TAT1 antibody; (bottom) anti-Sad1 staining for a component of the SPB.
During normal meiotic prophase, the cytoplasmic microtubules are organized exclusively from the spindle pole body (SPB) of a horse-tail nucleus (Chikashige et al. 1994; Hagan and Yanagida 1995; Svoboda et al. 1995; Hagan 1998), and during metaphase the SPBs separate and intranuclear spindles form as cytoplasmic microtubules disappear (Chikashige et al. 1994). Following an HU block, >70% of the cells showed a typical prophase configuration with most microtubules organized from a single SPB (Fig. 3A). We conclude that cells unable to complete premeiotic S phase become arrested in prophase, although it is not clear whether the arrest point is in early or late prophase or whether linear elements have been formed or not. Linear elements function as a glue between sister chromatids and normally form concomitantly with the onset of premeiotic DNA replication (Bähler et al. 1993). In contrast, pat1ts/pat1ts rad3/rad3 cells treated with HU undergo meiotic nuclear divisions, which, however, were defective (Fig. 3B). Intranuclear spindles were formed but were disorganized, SPBs were duplicated and separated, and the nucleus disintegrated into several fragments. The results indicate that pat1ts/pat1ts rad3/rad3 cells that have failed to complete premeiotic S phase attempt to undergo meiotic nuclear divisions.
The meiotic DNA replication checkpoint inhibits Cdc2 kinase activity through Y15 phosphorylation
Cdc2 tyrosine 15 phosphorylation plays a key role in blocking onset of mitosis when DNA replication is incomplete. To check whether a similar biochemical mechanism is involved in the meiotic checkpoint, we monitored Cdc2 tyrosine phosphorylation and protein kinase activity in pat1ts/pat1ts cells undergoing meiosis and sporulation. The level of Cdc2 protein was almost constant during this experiment, both with and without HU (Fig. 4A). The level of the tyrosine-phosphorylated form of Cdc2 gradually increased during premeiotic S phase and decreased during meiotic division (Fig. 4A), but after HU was added, tyrosine 15 phosphorylation was maintained at a high level. Cdc13 B-cyclin protein gradually accumulated during S phase, peaked around meiotic division, and then decreased, but after HU was added, the level of Cdc13 remained high. The high level of tyrosine 15 phosphorylation observed in HU suggests that Cdc2 protein kinase activity is inhibited when premeiotic S phase is incomplete. To confirm this, we measured Cdc13- and Cdc2-dependent histone H1 kinase activity both with and without HU after immunoprecipitation with anti-Cdc13 and anti-Cdc2 antibodies. The Cdc13-associated H1 kinase activity rose to a peak during the meiotic nuclear divisions (Fig. 4B), consistent with observations made with Suc1 beads (Daya Makin et al. 1992). In contrast, cells in HU had a low Cdc13-associated kinase activity. Similarly, Cdc2 kinase activity peaked during meiotic nuclear division, whereas this activity remained low after addition of HU (Fig. 4B). These results indicate that the meiotic DNA replication checkpoint pathway inhibits Cdc2 kinase activity through Y15 phosphorylation.
Figure 4.
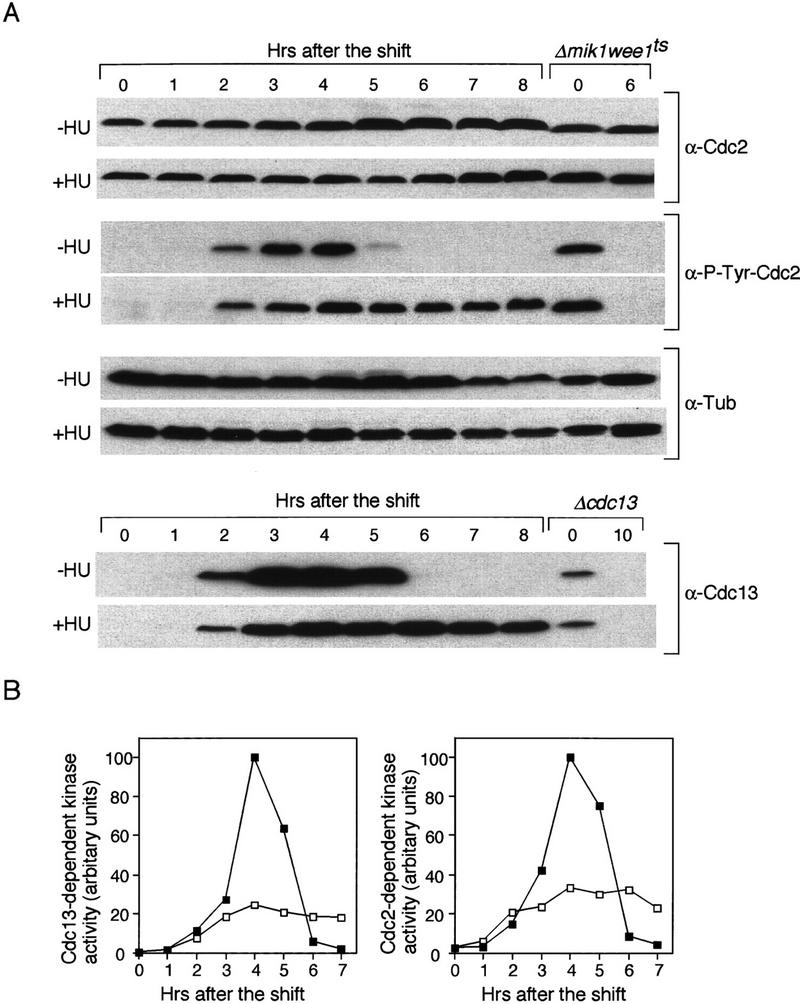
The meiotic DNA replication checkpoint inhibits Cdc2 kinase activity through Y15 phosphorylation. (A) Western blot analysis of the amount of tyrosine 15 phosphorylated Cdc2, and the abundance of Cdc2 and Cdc13 proteins. pat1ts/pat1ts cells were induced to undergo meiosis as in Fig. 2. The samples were taken after the temperature shift at the indicated times in the absence (−HU) or in the presence (+HU) of HU. The levels of tyrosine 15 phosphorylated Cdc2 in the samples were monitored by Western blotting with an antibody specific to tyrosine 15 phosphorylated Cdc2 (α-P-Tyr-Cdc2). As a control for Y15 phosphorylated Cdc2, Δmik1 weelts strain was grown to mid log phase, shifted to 36°C at time 0 and incubated for 6 hr. The blots were reprobed with a monoclonal antibody specific to Cdc2 (α-Cdc2). As a loading control, the amount of α-tubulin was analyzed α-Tub). Similarly, the amount of Cdc13 was analyzed (α-Cdc13). As a control for Cdc13 protein, Δcdc13 strain in which cdc13+ expression is repressible by thiamine was grown in mid log phase. Thiamine was added to shut off the expression of cdc13+ at time 0 and incubated for 10 hr. (B) pat1ts/pat1ts cells were grown and processed as above. Timing of entry into meiosis was delayed for ∼0.5 hr compared with that in A. Kinase activity associated with Cdc13 and Cdc2 was analyzed by immunoprecipitation with anti-Cdc13 and anti-Cdc2 antibodies from the samples in the absence (█) or presence (□) of HU. Immune complexes were washed, assayed for kinase activity with histone H1 as a substrate, and quantified by PhosphorImager analysis.
Next, we examined the level of phosphorylation of Cdc2 tyrosine 15 in pat1ts/pat1ts rad3/rad3 and pat1ts/pat1ts Δcds1/Δcds1 cells with or without HU (Fig. 5A). Like pat1ts/pat1ts cells, the tyrosine phosphorylation level of Cdc2 increased during premeiotic S phase and decreased during meiotic division. When HU was added, tyrosine dephosphorylation still occurred, although it was delayed by ∼1 hr, as was the disappearance of Cdc 13. Thus, tyrosine 15 phosphorylation of Cdc2 cannot be maintained in meiotic cells lacking Rad3 or Cds1. Cdc13- and Cdc2-dependent kinase activities became elevated after the addition of HU to these cells, consistent with the observed tyrosine 15 dephosphorylation (Fig. 5B). The level of Cdc13 was a little higher and persists longer in pat1ts/pat1ts rad3/rad3 cells, suggesting that they may have a slight defect in degrading Cdc13. The peak of kinase activity in pat1ts/pat1ts rad3/rad3 was lower in HU, probably because these cells entered meiosis less synchronously. Thus, when the DNA replication checkpoint control is defective, tyrosine 15 phosphorylation cannot be maintained and Cdc2 protein kinase activity increases. Because the checkpoint response is correlated with tyrosine 15 phosphorylation of Cdc2, we conclude that the checkpoint is mediated, at least in part, through this phosphorylation.
Figure 5.
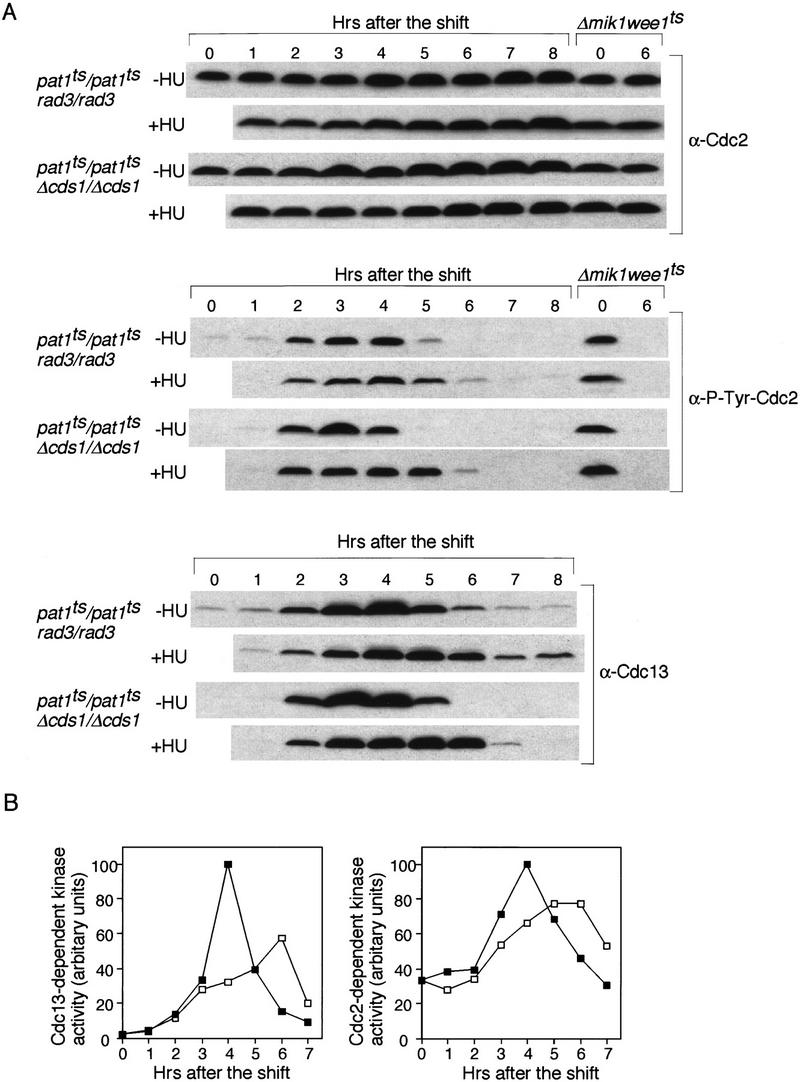
Rad3 and Cds1 act upstream of Cdc2 in the meiotic DNA replication checkpoint (A) pat1ts/pat1ts rad3/rad3 and pat1ts/pat1ts Δcds1/Δcds1 cells were induced to undergo meiosis in the absence (−HU) or in the presence of (+HU) of HU and processed as in Fig. 4. The amount of Cdc2 (α-Cdc2), the phosphorylation of Cdc2 tyrosine 15 (α-P-Tyr-Cdc2), and Cdc13 (α-Cdc13) was analyzed by Western blotting as in Fig. 4. (B)pat1ts/pat1ts rad3/rad3 cells were grown and processed as above. Kinase activity associated with Cdc13 and Cdc2 from the samples in the absence (█) or presence (□) of HU was analyzed by immunoprecipitation with anti-Cdc13 and anti-Cdc2 antibodies and processed as in Fig. 4.
Tyrosine 15 dephosphorylation of Cdc2 is not sufficient to bring about nuclear division in an HU block
If the meiotic DNA replication checkpoint works through Cdc2 tyrosine 15 phosphorylation, then preventing this phosphorylation should allow meiotic nuclear divisions to proceed even during an HU block. Wee1 and Mik1 are the tyrosine kinases responsible for phosphorylating Cdc2 tyrosine 15 (Featherstone and Russell 1991; Lundgren et al 1991). We constructed a pat1ts strain in which mik1+ is deleted and wee1+ is temperature sensitive (pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts). Growing cells were transferred to −N medium and shifted to 34°C. They entered S phase with similar kinetics as the control pat1ts/pat1ts strain (data not shown) but were advanced by ∼1 hr into meiosis I (Fig. 6A). However, in contrast to mitotic cells treated with HU, nuclear divisions were blocked in meiotic cells treated with HU. Because a temperature of 34°C was used to induce efficient sporulation, it was possible that wee1+ was not fully inactivated at this temperature. However, Δmik1 wee1ts cells could proceed through mitosis in HU at 34°C (Fig. 6A). Even at 25°C, the permissive temperature for wee1ts, mitotic cells were unable to block nuclear division after addition of HU (data not shown), suggesting that wee1+ function is compromised enough even at 25°C to abrogate the mitotic checkpoint function when combined with Δmik1.
Figure 6.
The meiotic DNA replication checkpoint arrests cells in metaphase-like stage with high Cdc2 kinase activity when Cdc2 tyrosine 15 in dephosphorylated. (A) pat1ts/pat1ts, pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts diploid strains and Δmik1wee1ts haploid strain were grown, washed, and transferred to −N medium at 25°C. The cultures were then shifted to 34°C at time 0. The samples were taken after the temperature shift at the indicated times in the absence (top) or in the presence (bottom) of HU. The proportions of the cells having one nucleus (█), two (⋄), and three or four neclei (○) for diploid strains and cut (□) for the haploid strain were determined. These results were representative of three independent experiments. The maximum difference of the time when one-half of the cells have two or more nuclei was 20 min in these experiments. (B) The samples were taken after the temperature shift at the indicated times in the absence (−HU) or in the presence (+HU) of HU. The amount of Cdc2 (α-Cdc2), the phosphorylation of Cdc2 tyrosine 15 (α-P-Tyr-Cdc2), and Cdc13 (α-Cdc13) was analyzed by Western blotting as in Fig. 4. (C) Kinase activity associated with Cdc13 and Cdc2 from the samples in the absence (█) or presence (□) of HU was analyzed by immunoprecipitation with anti-Cdc13 and anti-Cdc2 antibodies and processed as in Fig. 4. (D) pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts cells were induced to undergo meiosis for 6 hr in HU. The samples were processed as in Fig. 3 and stained with anti-TAT1 and anti-Sad1 antibodies. Typical examples of images of a field of the cells are shown. (Top) Position and morphology of chromatin DNA stained with DAPI, (middle) anti-tubulin staining probed with anti-TAT1 antibody, (bottom) anti-Sad1 staining for a component of the SPB.
We constructed a diploid strain expressing Cdc2–Y15F or a pat1ts strain expressing Cdc2–Y15F. However, these strains failed to undergo meiosis even without HU. The reason for this failure of Cdc–2Y15F to undergo meiosis is not understood. We also constructed a pat1ts strain either with cdc2-3w, wee1, or mik1 mutation. These strains arrested before meiotic nuclear division in the presence of HU, suggesting that either cdc2-3w, weel, or mik1 mutation is insufficient to override a checkpoint block by HU (data not shown).
We further explored the possibility that HU-induced arrest is dependent on the DNA damage checkpoint using a pat1tsΔmik1wee1tsΔchk1 strain. Nuclear division was also blocked in this strain, suggesting that arrest is not dependent on the Chk1-dependent DNA damage checkpoint (data not shown). We next constructed a pat1tsΔmik1wee1tsrad3 strain to test whether these cells would undergo nuclear division in the presence of HU. This strain was induced to enter meiosis in the presence or absence of HU. In the absence of HU, 60% of the cells had entered meiosis within 8 hr after the temperature shift, as shown by the presence of cells with two or more nuclei. This slow entry into meiosis is probably a consequence of the slow growth rate of this strain. In the presence of HU, 50% of the cells underwent an aberrant meiosis by this time point (data not shown). Similar results were obtained with a pat1ts Δmik1 wee1ts Δrad1 strain. These results clearly show that these strains bypass the checkpoint arrest and undergo meiotic nuclear divisions in HU.
To test whether a third meiosis-specific tyrosine kinase might phosphorylate Cdc2 on tyrosine 15, we monitored the level of Cdc2-phosphorylated tyrosine 15 in pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts cells during meiosis, both with and without HU (Fig. 6B). The Cdc2-phosphorylated tyrosine 15 level transiently increased 2-3 hr after the shift, and then decreased. This was probably because wee1+ was not fully inactive at this temperature. However, tyrosine phosphorylation was completely absent within 4 hr of the shift both in the presence and absence of HU. These results indicate that there is not a third meiosis-specific tyrosine kinase that phosphorylates Cdc2 tyrosine 15 and prevents meiotic nuclear divisions. These cells were also found to have high Cdc2- and Cdc13-associated protein kinase activity both in the presence and absence of HU (Fig. 6C). We conclude that when such cells are treated with HU, progression through meiosis is blocked by a mechanism that is independent of Cdc2 protein kinase inhibition.
When pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts cells were induced to undergo meiosis in the presence of HU, more than half of the cells showed short intranuclear spindles with a condensed nucleus and separated SPBs (Fig. 6D). In addition, a variety of spindle defects and cells with more than two SPBs were observed. These observations establish that pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts cells treated with HU do not arrest in prophase, but arrest later during metaphase of meiosis I.
Discussion
Our investigations into the meiotic DNA replication checkpoint control in fission yeast have revealed the following. (1) A DNA replication checkpoint control operates that blocks onset of meiosis I when DNA replication is incomplete. (2) This checkpoint requires the Cds1, Rad1, 3, 9, 17, and Hus1 gene products. (3) When DNA replication is incomplete, the checkpoint maintains Cdc2 tyrosine 15 phosphorylation, and keeps Cdc2 protein kinase activity low. (4) A second checkpoint exists that blocks meiotic progression if the first checkpoint is inoperative. (5) This checkpoint blocks cells at metaphase of meiosis I with high Cdc2 protein kinase activity.
Meiotic nuclear division has been shown to be inhibited in fission yeast in response to inhibitors and mutations that block DNA replication (Grallert and Sipiczki 1991; Iino et al. 1995). We show here that this is a checkpoint-dependent process that requires all of the checkpoint genes identified previously as being necessary for the mitotic DNA replication checkpoint control. Additionally, the Cds1 kinase is absolutely required for the meiotic checkpoint. During the mitotic cell cycle, cells lacking Cds1 are initially delayed from entering mitosis when DNA replication is blocked by HU, although after some hours, mitosis does occur (Murakami and Okayama 1995). This contrasts with the situation in meiotic cells in which cells lacking Cds1 are completely checkpoint defective, undergoing meiosis I after a delay of only 1 hr when DNA replication is incomplete. Unlike the checkpoint Rad products, Cds1 is not required in the mitotic DNA damage checkpoint, and it is possible that the delay in mitotic onset seen in Δcds1 cells may be a consequence of HU-induced DNA damage, which activates the DNA damage checkpoint and blocks mitosis. This mitotic checkpoint can operate independently of Cds1 but is dependent on the checkpoint Rad gene products. The different situation seen during meiosis may be because HU does not induce DNA damage, or because the damage induced may be more effectively repaired and so the DNA damage checkpoint is not invoked. As a consequence, Δcds1 cells can proceed into meiosis I with the same kinetics as Δrad1 cells. If this interpretation is correct, then Cds1 may be specifically involved in monitoring incomplete or stalled DNA replication in both mitotic and meiotic cells. Alternatively, Cds1 may act redundantly with Chk1 during the mitotic cell cycle (Boddy et al. 1998; Lindsay et al. 19987; Zeng et al. 1998), with Chk1 function suppressed during the meiotic cell cycle, explaining why Δcds1 cells are completely defective in activating the meiotic DNA replication checkpoint.
The checkpoint Rad proteins and Cds1 act upstream of Cdc2 in the meiotic DNA replication checkpoint. When DNA replication is blocked with HU, Cdc2 and the B-cyclin Cdc13 persist and phosphorylation of Cdc2 tyrosine 15 is maintained. Cdc2 protein kinase activity is kept at a low level because of this phosphorylation and the onset of meiosis I is blocked. Similar behavior of Cdc2 is observed when DNA replication is blocked in mitotic cells (Rhind and Russell 1998b). In checkpoint-deficient cells lacking the checkpoint Rad gene functions, Cdc2 tyrosine 15 phosphorylation is not maintained and Cdc2 protein kinase rises resulting in an abortive meiosis I and II.
Unexpectedly, blocking DNA replication with HU in cells that lack tyrosine 15 phosphorylated Cdc2 did not result in cells undergoing abortive nuclear divisions. This experiment was carried out with a pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts mutant that lacks the known Cdc2 tyrosine 15 protein kinases, resulting in undetectable levels of tyrosine phosphorylation and high levels of Cdc2 protein kinase at the time of arrest. Despite high levels of the kinase, the cells do not proceed through abortive nuclear divisions, but arrest around metaphase of meiosis I with a short spindle extending between separated SPBs within an undivided nucleus. The fact that in the absence of the checkpoint Rad genes such cells proceeded into abortive nuclear divisions suggests that the checkpoint control must have two downstream pathways that can potentially block meiotic progression (Fig. 7). The first operates through Cdc2 tyrosine 15 phosphorylation, which is similar to the mitotic DNA replication checkpoint, whereas the second, which is specific for meiosis, must operate through an alternative mechanism downstream of Cdc2 activation.
Figure 7.
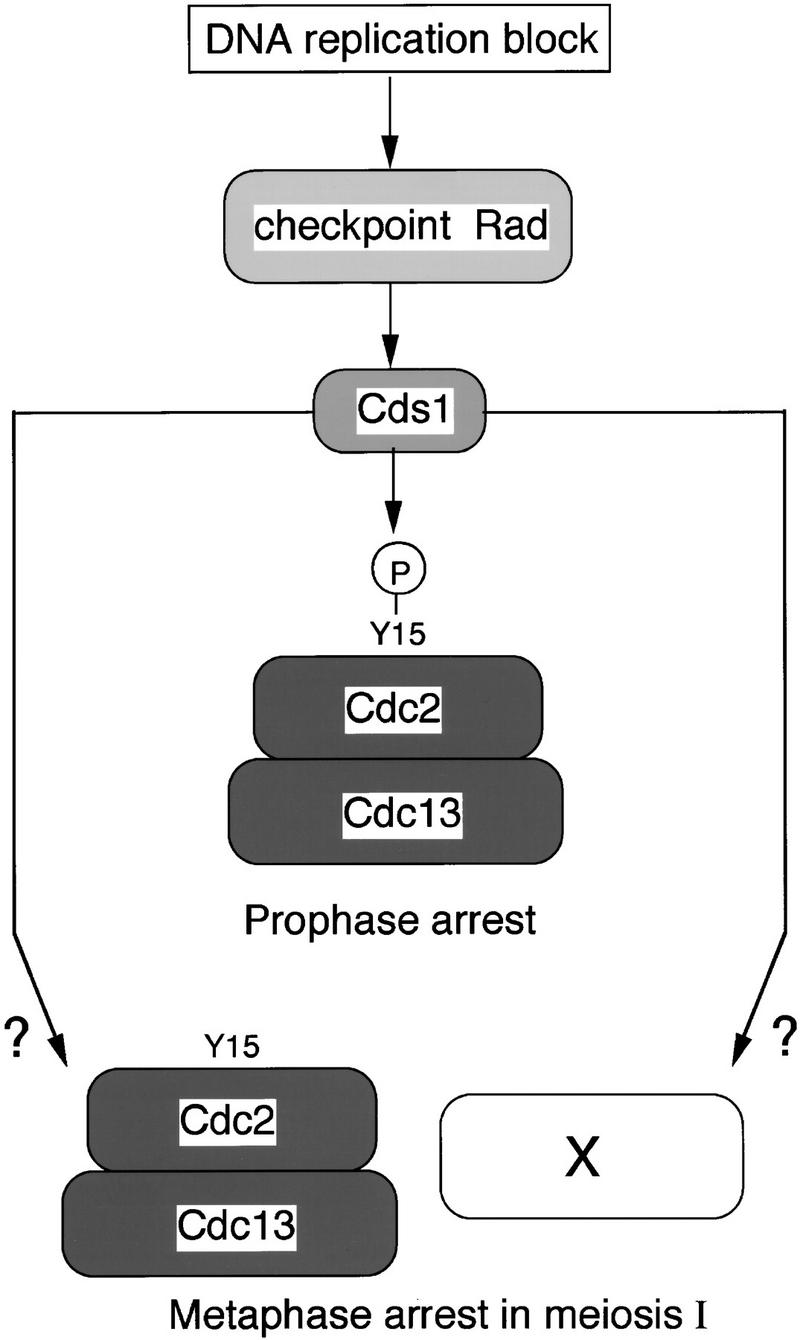
A model for the meiotic DNA replication checkpoint control. The signal of DNA replication block is sensed by the checkpoint Rad proteins and is transmitted to Cds1. The signal from Cds1 is branched in two ways; one is to maintain the tyrosine 15 phosphorylation of Cdc2 in prophase and another is to act on an unknown factor (x) or to stabilize Cdc2 activity in metaphase.
This result leads to two questions: What is the molecular basis of this second mechanism and why should this be operative only in meiotic cells? There have been several reports of two independent mechanisms being involved in mitotic DNA replication checkpoint controls. In Aspergillus nidulans, cells containing nontyrosine-phosphorylated Cdc2 still arrest before mitosis with high Cdc2 protein kinase activity (Ye et al. 1996). However, at this arrest point no spindle has formed, a block at an earlier stage than that observed with the second mechanism operating during fission yeast meiosis. A BimE mutation, defective in an APC subunit in A. nidulans, can overcome this block (Ye et al. 1996). In both Xenopus egg extracts and human cells, tyrosine phosphorylation of Cdc2 is an important but not the sole mechanism operating in the mitotic DNA replication checkpoint (Kumagai and Dunphy 1995; Jin et al. 1996; Blasina et al. 1997). In budding yeast, tyrosine phosphorylation of the Cdc2 homolog Cdc28 does not operate in the mitotic DNA replication checkpoint control, and cells arrest with high Cdc28 protein kinase activity, short microtubule spindles, and separated SPBs (Amon et al. 1992; Sorger and Murray 1992; Stueland et al. 1993). The molecular mechanism underlying this checkpoint is not known (Weinert 1998a,b). These reports suggest that there are likely to be at least two mechanisms that can block completion of mitosis when DNA replication is incomplete, one of which involves Cdc2 tyrosine phosphorylation. During mitosis in fission yeast, only the Cdc2 tyrosine phosphorylation mechanism is operative, but in meiosis, a second mechanism also comes into play, which could be equivalent to the budding yeast mitotic checkpoint control because the block is at a similar stage with a short spindle and separated SPBs. The molecular target of this second mechanism is not known, but two possibilities can be envisaged (Fig. 7). The control might operate through regulation of APC activity, preventing the fall in Cdc2–Cdc13 kinase activity and, as a consequence, blocking further meiotic progression (Hwang et al. 1998), or could regulate an unknown factor X required at a late stage of meiosis I (Fig. 7).
The second question is why should there be an extra checkpoint control that operates during meiosis in fission yeast but not during mitosis? Discussion of this question centers on the differences between the meiotic and mitotic cell cycles (Roeder 1997). One difference is that the levels of recombination are much higher. Possibly, a second checkpoint control exists to monitor whether recombination is complete and blocking DNA replication mimics in some way the production of recombination intermediates and so additionally activates this checkpoint. A recombination checkpoint has been proposed for budding yeast meiosis (Lydall et al. 1996). Another difference may be that meiosis I takes longer to complete than mitosis, and there needs to be a second late checkpoint that can come into play if there is DNA damage after activation of the Cdc2 protein kinase and the onset of meiosis. If a block in DNA replication with HU either generates DNA damage or mimics the structures produced by DNA damage, then this late checkpoint may come into play after Cdc2 protein kinase activation and would arrest cells with a short spindle. A third difference between meiosis and mitosis is that the chromosome arrangements are not identical (Scherthan et al. 1994; Roeder 1997). During meiosis there is chromosome homolog pairing that does not occur during mitosis. A checkpoint control blocking meiotic progression may need to operate that detects whether chromosome homolog pairing has not been completed (Rockmill and Roeder 1998). If DNA replication is incomplete, there cannot be correct homolog pairing and so this checkpoint may be activated. Given that the cells arrest with high Cdc2 protein kinase activity, such a checkpoint must operate after Cdc2 protein kinase activation and may overlap with spindle checkpoint controls.
This work has shown that there is a Cdc2 tyrosine 15 phosphorylation DNA replication checkpoint controlling Cdc2 protein kinase activation, which operates during fission yeast meiosis. This is the first demonstration of the molecular level of a DNA replication checkpoint control during meiosis. In addition, there is a second checkpoint control that does not operate during mitosis (Fig. 7). The molecular mechanism underlying this control is not understood, but it must respond to a situation that only operates during meiosis, such as differences in recombination, the duration of meiotic nuclear division, or chromosomal homolog pairing. It will be important to establish whether similar controls operate during meiosis in multicellular eukaryotes. Although several genes such as ATR, ATM, and Chk1 have been suggested as being involved in the meiotic checkpoint in mouse and human cells (Keegan et al. 1996; Xu et al. 1996; Flaggs et al. 1997; Barlow et al. 1998), the mechanism remains quite uncharacterized. Work in the fission yeast on this control should help direct similar studies in these more complex cells.
Materials and methods
Fission yeast strains and methods
All strains used were constructed by standard procedures (Moreno et al. 1991) and are shown in Table 1; pat1ts/pat1ts cells used in Figure 2 were strain 2158 and in Figures 3, 4, and 6 were strain 2159. The diploid strains homozygous for the mating-type genes were constructed by cell fusion. Complete medium (YES) and minimal medium (EMM) were described previously (Moreno et al. 1991).
Table 1.
S. pombe strains used in this study
| 827 | h− mik1∷LEU2 wee1-50 leu1-32 (Δmik1 wee1ts) |
| 1312 | h− cdc13∷ura4 int REP41∷cdc13 ura4-D18 leu1-32 ade6 (Δcdc13) |
| 2158 | h−/h− pat1-114/pat1-114 leu1-32/leu1-32 ade6-M210/ade6-M216 (pat1ts/pat1ts) |
| 2159 | h+/h+ pat1-114/pat1-114 ade6-M210/ade6-M216 (pat1ts/pat1ts) |
| 2160 | h−/h− pat1-114/pat1-114 rad3-136/rad3-136 ade6-M210/ade6-M216 (pat1ts/pat1ts rad3/rad3) |
| 2161 | h−/h− pat1-114/pat1-114 mik1∷LEU2/mik1∷LEU2 wee1-50/wee1-50 leu1-32/leu1-32 ade6-M210/ade6-M216 (pat1ts/pat1ts Δmik1/Δmik1 wee1ts/wee1ts) |
| 2194 | h−/h− pat1-114/pat1-114 rad1∷ura4/rad1∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (pat1ts/pat1ts Δrad1/Δrad1) |
| 2195 | h−/h−pat1-114/pat1-114 cds1∷ura4/cds1∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (pat1ts/pat1ts Δcds1/Δcds1) |
| 2304 | h−/h+ leu1-32/leu1-32 ade6-M210/ade6-M216 (h−/h+) |
| 2305 | h−/h+ rad1∷ura4/rad1∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (Δrad1/Δrad1) |
| 2306 | h−/h+ cds1∷ura4/cds1∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (Δcds1/Δcds1) |
| 2307 | h−/h+ chk1∷ura4/chk1∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (Δchk1/Δchk1) |
| 2332 | h−/h+ cds1∷ura4/cds1∷ura4 chk1∷ura4/chkl∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216(Δcds1/Δcds1 Δchk1/Δchk1) |
| 2334 | h−/h+ crb2∷ura4/crb2∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (Δcrb2/Δcrb2) |
| 2337 | h−/h− pat1-114/pat1-114 cds1∷ura4/cds1∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216(pat1ts/pat1ts Δcds1/Δcds1) |
| 2338 | h−/h− pat1-114/pat1-114 chk1∷ura4/chkl∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (pat1ts/pat1ts Δchk1/Δchk1) |
| 2339 | h−/h− pat1-114/pat1-114 crb2∷ura4/crb2∷ura4 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 (pat1ts/pat1ts Δcrb2/Δcrb2) |
Strains 827 and 1312 were from our laboratory stock and the others were constructed in this study.
To obtain meiotic cultures for the diploid strains heterozygous for the mating-type genes, single colonies were grown in YES to stationary phase. The cells were diluted in EMM supplemented with 100μg/ml leucine and grown at 30°C with shaking to 1–1.5 × 107 cells/ml. For nitrogen starvation experiments, the cultures were filtrated through a Millipore membrane, washed with −N medium and resuspended in −N medium containing 50 μg/ml leucine and 0.5% glucose. Sporulation was induced by vigorous shaking (200 rpm) at 30°C.
For the diploid strains homozygous for the mating-type genes, single colonies were grown in YES to stationary phase. The cells were diluted in EMM supplemented with 100 μg/ml leucine and grown at 25°C with shaking to 1–2 × 107 cells/ml. The cultures were filtrated through a Millipore membrane, washed with −N medium and resuspended in −N medium containing 50 μg/ml leucine. The concentrations of the cells were adjusted to 4 × 106 to 6 × 106/ml and incubated at 25°C for 13–15 hr. NH4Cl and leucine were added to the cultures at 500 μg/ml and 50μg/ml, respectively, just before the cultures were shifted to 34°C in the presence or absence of 24 mm HU.
DAPI staining
For staining of the cells with DAPI, 1 ml of cell culture (1–2 × 107 cells) was centrifuged briefly, fixed with 70% ethanol and stored. Fifty microliters of fixed cells (∼5 × 105 cells) were then added to 1 ml of water, centrifuged, and resuspended with 100 μl of water. A total of 7 μl was spotted onto slides and dried by heating. The number of nuclei was counted under microscopy with DAPI solutions (5 μg/ml). At each time point, >200 cells were counted.
Criteria for meiotic progression
In meiosis, cells undergo DNA replication, separation of SPBs, and spindle formation, followed by two successive nuclear divisions. In this study, we have used both the number of nuclei by DAPI staining and formation of a spindle by immunostaining as criteria for progression into meiosis.
Flow cytometric analysis
A total of 5 × 105 cells were fixed in 70% ethanol, washed in 1 ml of 10 mm EDTA (pH 8.0), resuspended in 1 ml of 10 mm EDTA solution containing 0.05 mg/ml of RNase A and incubated overnight at 37°C. A total of 2 μg/ml propidium iodide was added to the solution and sonicated. We then followed the previously published protocol for flow cytometry (Sazer and Sherwood 1990), with a Becton-Dickinson FACScan.
Antibodies
The following antibodies were used: phospho-Cdc2 (Tyr15) polyclonal rabbit antibodies (New England Biolabs); Cdc2 monoclonal antibodies y63MAb (ICRF); rabbit polyclonal Cdc2 antibodies C2 (this laboratory); Cdc13 monoclonal antibodies 6F11/2 MAb (this laboratory); rabbit polyclonal Cdc13 antibodies SP4 (this laboratory); TAT1 monoclonal antibodies [gift of K. Gull (Woods et al. 1989)]; Sad1 polyclonal rabbit antibodies [gift of I. Hagan (Hagan and Yanagida 1995)]; anti-α-tubulin monoclonal antibody (Sigma T5168).
Protein preparation and Western blotting
Total boiled protein extracts were prepared from 1–4 × 108 cells harvested by centrifugation, washed in STOP buffer (150 mm NaCl, 50 mm NaF, 10 mm EDTA, 1 mm NaN3 at pH 8.0) and resuspended in 100 μl of HB buffer as described previously (Moreno et al. 1991; Yamaguchi et al. 1997). Cells were boiled for 5 min and broken with glass beads. After the cell breakage, the crude extracts were recovered by washing the glass beads with 200 μl of HB buffer. The protein concentrations of the samples were determined by the BCA assay kit (Sigma). Cell extracts were reboiled in 4× sample buffer (200 mm Tris-Cl at pH6.8, 0.4 m DTT, 8% SDS, 0.4% BPB, 40% glycerol). A total of 40 μg protein from each sample was run on a 12% SDS–polyacrylamide gel. For Western blots, the protein was blotted to Immobilon-P membrane (Millipore) and detected by ECL (Amersham). Dilutions of the antibodies were 1:750 for anti-phospho-Cdc2 (Tyr15), 1:3000 for anti-Cdc2 mAb, 1:750 for anti-Cdc 13 mAb, and 1:50000 for anti-α-tubulin mAb.
Immunoprecipitation and kinase assays
Extracts were prepared from frozen cells with glass beads in HB buffer previously (Moreno et al 1991). Soluble extracts were obtained by centrifugation at 13,000 rpm for 10 min at 4°C. Then, 2 μl of rabbit polyclonal Cdc2 antibody C2 or 5 μl of rabbit polyclonal Cdc 13 antibody SP4 were added to 500 μg of protein and incubated for 45 min on ice. Pre-equilibrated protein A-Sepharose beads (Pharmacia Biotech) were added and the mixture agitated for 30 min at 4°C. Beads were washed three times and then resuspended in 20 μl of reaction buffer, containing 1 mg/ml histone H1 (Calbiochem), 200 mm ATP and 40 μCi/ml [γ-32P] ATP (Amersham). Extracts were incubated at 30°C for 20 min, stopped by boiling for 5 min after the addition of 4× SDS sample buffer and run on a 12% SDS-polyacrylamide gel. Phosphorylated histone H1 was detected by autoradiography and quantitated with a PhosphorImager.
Immunofluorescence
For immunofluorescence, cells were harvested, washed with H2O, and fixed with methanol at −20°C. Cells were further processed for immunofluorescence as described (Sawin and Nurse 1998). Dilutions of the antibodies were 1:15 for TAT1 monoclonal anti-tubulin antibody and 1:25 for anti-Sad1 antibody, which recognizes a component of the SPB. Color images were produced by image capture with a Hamamatsu SIT Camera and C2400 processor for capturing the information. All images were subsequently processed with Adobe Photoshop.
Acknowledgments
We are grateful to Mitsuhiro Yanagida for strains and to Keith Gull and Iain Hagan for antibodies. We thank Yoshinori Watanabe and Jürg Bähler for introductions to meiosis and helpful discussions. We thank Chris Lehane, Dominic Griffiths, Gerald Smith, Jacky Hayles, José Ayté, Jürg Bähler, Satoko Yamaguchi, and Takashi Toda for critical reading of the manuscript. We also thank Jacky Hayles and Damian Brunner for assistance with kinase assays and immunofluorescence. We thank all members of the Nurse laboratory for their help. H.M. was supported by JSPS.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL murakami@icrf.icnet.uk; FAX 0171-269-3610.
References
- Al-Khodairy F, Carr AM. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJF, Lehman AR, Carr AM. Identification and characterisation of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992;355:368–371. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- Bähler J, Schuchert P, Grimm C, Kohli J. Synchronized meiosis and recombination in fission yeast: Observations with pat1-114 diploid cells. Curr Genet. 1991;19:445–451. doi: 10.1007/BF00312735. [DOI] [PubMed] [Google Scholar]
- Bähler J, Wyler T, Loidl J, Kohli J. Unusual nuclear structures in meiotic prophase of fission yeast: A cytological analysis. J Cell Biol. 1993;121:241–256. doi: 10.1083/jcb.121.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C, Liyanage M, Moens PB, Tarsounas M, Nagashima K, Brown K, Rottinghaus S, Jackson SP, Tagle D, Ried T, Wynshaw-Boris A. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development. 1998;125:4007–4017. doi: 10.1242/dev.125.20.4007. [DOI] [PubMed] [Google Scholar]
- Blasina A, Paegle ES, McGowan CH. The role of inhibitory phosphorylation of CDC2 following DNA replication block and radiation-induced damage in human cells. Mol Biol Cell. 1997;8:1013–1023. doi: 10.1091/mbc.8.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasina A, de Weyer IV, Laus MC, Luyten W, Parker AE, McGowan CH. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Ding DQ, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, Hiraoka Y. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994;264:270–273. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- Daya Makin M, Szankasi P, Tang L, MacRae D, Pelech SL. Regulation of p105wee1 and p34cdc2 during meiosis in Schizosaccharomyces pombe. Biochem Cell Biol. 1992;70:1088–1096. doi: 10.1139/o92-154. [DOI] [PubMed] [Google Scholar]
- Enoch T, Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- Enoch T, Gould K, Nurse P. Mitotic checkpoint control in fission yeast. Cold Spri Harb Symp Quant Biol. 1991;56:409–416. doi: 10.1101/sqb.1991.056.01.048. [DOI] [PubMed] [Google Scholar]
- Enoch T, Carr A, Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes & Dev. 1992;6:2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Flaggs G, Plug AW, Dunks KM, Mundt KE, Ford JC, Quiggle MR, Taylor EM, Westphal CH, Ashley T, Hoekstra MF, Carr AM. Atm-dependent interactions of a mammalian chk1 homolog with meiotic chromosomes. Curr Biol. 1997;7:977–986. doi: 10.1016/s0960-9822(06)00417-9. [DOI] [PubMed] [Google Scholar]
- Fogarty P, Campbell SD, Abu-Shumays R, Phalle BS, Yu KR, Uy GL, Goldberg ML, Sullivan W. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- Freire R, Murguia JR, Tarsounas M, Lowndes NF, Moens PB, Jackson SP. Human and mouse homologs of Schizosaccharomyces pombe rad1(+) and Saccharomyces cerevisiae RAD17: Linkage to checkpoint control and mammalian meiosis. Genes & Dev. 1998;12:2560–2573. doi: 10.1101/gad.12.16.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Grallert B, Sipiczki M. Common genes and pathways in the regulation of the mitotic and meiotic cell cycles of Schizosaccharomyces pombe. Curr Genet. 1991;20:199–204. doi: 10.1007/BF00326233. [DOI] [PubMed] [Google Scholar]
- Hagan IM. The fission yeast microtubule cytoskeleton. J Cell Sci. 1998;111:1603–1612. doi: 10.1242/jcs.111.12.1603. [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari KL, Santerre A, Sekelsky JJ, McKim KS, Boyd JB, Hawley RS. The mei-41 gene of D. melanogaster is a structural and functional homolog of the human ataxia telangiectasia gene. Cell. 1995;82:815–821. doi: 10.1016/0092-8674(95)90478-6. [DOI] [PubMed] [Google Scholar]
- Hartwell L, Weinert T. Checkpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW. Budding yeast Cdc20: A target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- Iino Y, Yamamoto M. Mutants of Schizosaccharomyces pombe which sporulate in the haploid state. Mol & Gen Genet. 1985;82:416–421. doi: 10.1007/BF00332932. [DOI] [PubMed] [Google Scholar]
- Iino Y, Hiramine Y, Yamamoto M. The role of cdc2 and other genes in meiosis in Schizosaccharomyces pombe. Genetics. 1995;140:1235–1245. doi: 10.1093/genetics/140.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez G, Yucel J, Rowley R, Subramani S. The rad3+ gene of Schizosaccharomyces pombe is involved in multiple checkpoint functions and in DNA repair. Proc Natl Acad Sci. 1992;89:4952–4956. doi: 10.1073/pnas.89.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Gu Y, Morgan DO. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R, Ogawa H. An essential gene, ESR1, is required for mitotic cell growth, DNA repair and meiotic recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 1994;22:3104–3112. doi: 10.1093/nar/22.15.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan KS, Holtzman DA, Plug AW, Christenson ER, Brainerd EE, Flaggs G, Bentley NJ, Taylor EM, Meyn MS, Moss SB, et al. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes & Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Knudsen ES, Wang JY, Subramani S. p34cdc2 kinase activity is maintained upon activation of the replication checkpoint in Schizosaccharomyces pombe. Proc Natl Acad Sci. 1996;93:8278–8283. doi: 10.1073/pnas.93.16.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes & Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Lydall D, Nikolsky Y, Bishop DK, Weinert T. A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature. 1996;383:840–843. doi: 10.1038/383840a0. [DOI] [PubMed] [Google Scholar]
- MacNeill SA, Nurse P. Cell Cycle Control in Fission Yeast, Schizosaccharomyces pombe. In: Pringle JR, Broach JR, Jones EW, editors. The molecular and cellular biology of the yeast Saccharomyces: Life cycle and cell biology. Cold Spring HarborNew York, NY: Cold Spring Harbor Laboratory; 1997. pp. 697–763. [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Mutants of the fission yeast Schizosaccharomyces pombe which alter the shift between cell proliferation and sporulation. Mol & Gen Genet. 1985;198:497–502. [Google Scholar]
- ————— Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- O'Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Rhind N, Russell P. Mitotic DNA damage and replication checkpoints in yeast. Curr Opin Cell Biol. 1998a;10:749–758. doi: 10.1016/s0955-0674(98)80118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Tyrosine phosphorylation of cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol Cell Biol. 1998b;18:3782–3787. doi: 10.1128/mcb.18.7.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes & Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- Robinow CF. The number of chromosomes in S. pombe: Light microscopy of stained preparations. Genetics. 1977;87:491–497. doi: 10.1093/genetics/87.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockmill B, Roeder GS. Telomere-mediated chromosome pairing during meiosis in budding yeast. Genes & Dev. 1998;12:2574–2586. doi: 10.1101/gad.12.16.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder GS. Meiotic chromosomes: It takes two to tango. Genes & Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- Rowley R, Subramani S, Young PG. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 1992;11:1335–1342. doi: 10.1002/j.1460-2075.1992.tb05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. Damage and replication checkpoint control in fission yeast is ensured by interactions of Crb2, a protein with BRCT motif, with Cut5 and Chk1. Genes & Dev. 1997;11:3387–3400. doi: 10.1101/gad.11.24.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: Linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Nurse P. Regulation of cell polarity by microtubules in fission yeast. J Cell Biol. 1998;142:457–471. doi: 10.1083/jcb.142.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer S, Sherwood SW. Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J Cell Sci. 1990;97:509–516. doi: 10.1242/jcs.97.3.509. [DOI] [PubMed] [Google Scholar]
- Scherthan H, Bahler J, Kohli J. Dynamics of chromosome organization and pairing during meiotic prophase in fission yeast. J Cell Biol. 1994;127:273–285. doi: 10.1083/jcb.127.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- Sorger PK, Murray AW. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34CDC28. Nature. 1992;355:365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- Stuart D, Wittenberg C. CLB5 and CLB6 are required for premeiotic DNA replication and activation of the meiotic S/M checkpoint. Genes & Dev. 1998;12:2698–2710. doi: 10.1101/gad.12.17.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueland CS, Lew DJ, Cismowski MJ, Reed SI. Full activation of p34CDC28 Histone H1 kinase activity is unable to promote entry into mitosis in checkpoint arrested cells of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:3744–3755. doi: 10.1128/mcb.13.6.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda A, Bähler J, Kohli J. Microtubule-driven nuclear movements and linear elements as meiosis- specific characteristics of the fission yeasts Schizosaccharomyces versatilis and Schizosaccharomyces pombe. Chromosoma. 1995;104:203–214. doi: 10.1007/BF00352185. [DOI] [PubMed] [Google Scholar]
- Walworth NC, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- Weinert T. DNA damage and checkpoint pathways: Molecular anatomy and interactions with repair. Cell. 1998a;94:555–558. doi: 10.1016/s0092-8674(00)81597-4. [DOI] [PubMed] [Google Scholar]
- ————— DNA damage checkpoints update: Getting molecular. Curr Opin Genet Dev. 1998b;8:185–193. doi: 10.1016/s0959-437x(98)80140-8. [DOI] [PubMed] [Google Scholar]
- Willson J, Wilson S, Warr N, Watts FZ. Isolation and characterization of the Schizosaccharomyces pombe rhp9 gene: A gene required for the DNA damage checkpoint but not the replication checkpoint. Nucleic Acids Res. 1997;25:2138–2146. doi: 10.1093/nar/25.11.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, McRae T, Baines A, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes & Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Murakami H, Okayama H. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol Biol Cell. 1997;8:2475–2486. doi: 10.1091/mbc.8.12.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XS, Fincher RR, Tang A, O'Donnell K, Osmani SA. Two S-phase checkpoint systems, one involving the function of both BIME and Tyr15 phosphorylation of p34cdc2, inhibit NIMA and prevent premature mitosis. EMBO J. 1996;15:3599–3610. [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. .. [DOI] [PubMed] [Google Scholar]