Abstract
Background
Deficits in incentive motivation, the energizing of behavior in pursuit of a goal, occur in many psychiatric disorders including schizophrenia. We previously reported deficits in both cognition and incentive motivation in a transgenic mouse model of increased striatal-specific dopamine D2 receptor density (D2R-OE mice (1)). This molecular alteration is observed in patients with schizophrenia, making D2R-OE mice a suitable system to study of the cellular and molecular mechanisms of motivation and avolition, as well as a tool for testing potential therapies against motivational deficits.
Methods
Behavioral studies using operant conditioning methods were performed both to further characterize the incentive motivation deficit in D2R-OE mice, and test a novel pharmacological treatment target which arose from an unbiased expression study performed using gene chips and was validated by quantitative RT-PCR, in situ hybridization and immunohistochemistry.
Results
The D2R-OE mice’s reluctance to work is due neither to intolerance for low rates of reward, decreased reactivity to reward, nor increased sensitivity to satiety or fatigue, but to a difference in willingness to work for reward. As in patients with schizophrenia, this deficit was not ameliorated by D2R blockade, suggesting that reversal of the motivational deficit by switching off the transgene results from molecular changes downstream of D2R overexpression. We observed a reversible increase in 5-HT2C receptor expression in D2R-OE mice. Systemic injection of a 5-HT2C antagonist increased incentive motivation in D2R-OE and control mice.
Conclusions
We propose that targeting 5-HT2C receptors may be a useful approach to modulate incentive motivation in psychiatric illness.
Keywords: Motivation, D2 receptor, Animal model, Schizophrenia, Serotonin receptor 5HT2C, Operant conditioning
Introduction
The negative symptoms of schizophrenia include reduced emotional reactivity (blunted affect), poverty of speech (alogia) and decline in motivation (avolition). These chronic and debilitating symptoms dramatically reduce patients’ quality of life and are relatively insensitive to current antipsychotic treatment. A significant overlap exists between these negative symptoms of schizophrenia and those of affective disorders, including schizoaffective disorder and depression (2). However, recent studies suggest that unlike patients with mood disorders, individuals with schizophrenia do not usually suffer from general anhedonia, but rather a specific defect in the ability to anticipate future positive experiences (3), a likely contributor to patient’s poor ability to plan and work toward long term goals. Two recent reviews of reward processing in people with schizophrenia highlight three specific impairments: the representation of reward value, positive reward-related learning and incentive motivation. By contrast, reactivity to the reward itself is unimpaired (4, 5). The study of these reward-related processes is tractable in animal models, and importantly, each specific aspect of the reward related process can be individually tested. For example, rat studies have determined that the components of the mesocorticolimbic circuits that underlie hedonia or “liking” are distinct from those that mediate volition or “wanting” (6). Here, we focus on anticipatory motivation in a genetic mouse model of dopamine dysfunction.
To model the increase in density of striatal D2 receptors (D2Rs) observed in patients with schizophrenia, we previously generated transgenic mice in which D2Rs are selectively overexpressed in the striatum in a temporally controllable manner (D2R-OE mice) (1). To achieve this we generated double-transgenic mice using the tetracycline transactivator (tTA) system. We crossed transgenic mice that express D2R under the tetracycline response element (tetO) to mice expressing the tetracycline transactivator, tTA, under the CamKIIa promoter (7). As previously shown (1), the combination of lines used results in transgene expression restricted to the striatum and olfactory tubercle. Application of doxycycline (40 mg/kg food) for 5 days switches off transgenic D2R mRNA expression by preventing tTA from binding to the tetO promoter. D2R-OE mice display behavioral phenotypes relating to both the cognitive and negative symptoms of schizophrenia. Specifically, we found that striatal D2R overexpression during development results in persistent impairments in prefrontal function, including defects in working memory, behavioral flexibility and conditional associative learning (1, 8, 9). D2R-OE mice also exhibit avolition, measured as a deficit in incentive motivation (8, 10). Unlike the observed cognitive deficits, however, this motivational deficit can be rescued when D2R expression is normalized by switching off the transgene in adulthood. The D2R-OE mouse model thus provides evidence that the cognitive and negative symptoms of schizophrenia may share some common underlying etiologic factors, though they may be upstream of the acute and developmental mechanisms which result in the deficits. This is consistent with the clinical observation that the severity of cognitive symptoms correlates more highly with the negative symptoms than the positive symptoms (11, 12). Furthermore, D2R-OE mice provide a system in which to study the cellular and molecular mechanisms of motivation and avolition, as well as an ideal tool for testing possible therapeutic drugs.
Here we use the D2R-OE mice to explore the mechanisms by which striatal D2R overexpression leads to reduced motivation. We find that although concurrent overexpression of D2Rs is required for the motivational deficit, reversal is not achieved by D2R blockade. Antagonism of the 5HT-2C receptor, which we found to be upregulated in the striatum of D2R-OE mice, significantly improved incentive motivation, suggesting a possible therapeutic approach to ameliorate the lack of motivation suffered by patients with schizophrenia and affective disorders.
Materials and Methods
Mice
The generation of D2R-OE mice, temporal regulation of the transgene and food restriction protocol have been previously described (1) and are detailed in the Supplement.
Behavior testing
Behavioral testing was essentially carried out as previously described for progressive ratio (8, 10), with some adjustments. For a detailed description of the apparatus and procedures used please see outline and text in the Supplement.
Drug treatments
The 5-HT2c antagonist SB24280 (Sigma Aldrich) was dissolved in 0.9% saline and injected, i.p. 20 minutes before behavioral testing. We administered 0.75mg/kg because results from a pilot study suggested that it would not suppress rate of responding (Figure S3 in Supplement 1). Haloperidol was administered chronically via mini-osmotic pumps (Alzet model 2004) to provide a steady delivery rate. After the mice were trained to lever press, the pumps filled with either saline or haloperidol dissolved in saline in order to release either 0.1mg/kg per day or 0.25 mg/kg per day were implanted subcutaneously in the interscapular region under isoflurane anesthesia. One week after the surgery the mice were trained on the FI procedure, then tested on the PRx2 schedule which was completed within 4 weeks from the date of pump implant, the maximum duration of pump release.
Gene expression analysis
Details of the RNA isolation, gene chip analysis, quantitative real time RT-PCR and Oligo in situ Hybridization are provided in the Supplement.
Immunofluorescence
IHC was carried out as previous described (13), sections were imaged using a laser scanning confocal microscope and analyzed using ImageJ software. Details are provided in the Supplement.
Statistical Analysis of Behavioral Data
The progressive ratio data was analyzed as previously described [9]. Survival functions were generated by plotting as a function of session time the percentage of cases (a case was one subject’s performance in one session) in which the subject was still responding. The survival functions were analyzed using the Mantel-Cox logrank test. Other variables, including lever press rates, reinforcers earned, latency to collect rewards and rewards earned but not consumed were analyzed by averaging each animals performance over the number of sessions tested and using 2 tailed students t-tests without assuming equal variance. Or, where more appropriate, repeated measure ANOVA.
Statistical Analysis of Molecular Data
For the genechip, realtime RT-PCR and IHC experiments we used 2-tailed Students t-tests without assuming equal variance.
Results
Selective overexpression of dopamine D2 receptors in striatum results in a reversible impairment in incentive motivation across a range of work requirements, and is not due to a difference in sensitivity to satiety or fatigue
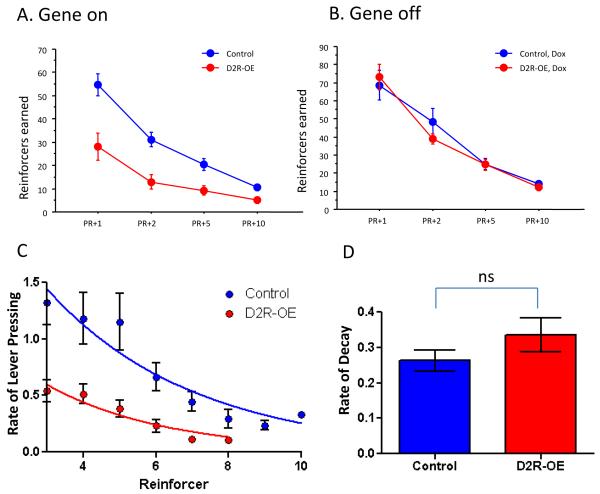
We previously determined that mice with striatal specific overexpression of D2Rs display a reversible impairment in incentive motivation (8, 10). Using an operant progressive ratio (PR) schedule in which the number of lever presses required to earn a reward is doubled after each reward (PRx2), we found that D2R-OE mice earned significantly fewer rewards than control littermates. This phenotypic difference was reversible; it was eliminated by doxycycline, which switched off the expression of the transgene. To determine if this genotypic difference was consistent for less onerous ratios and also across different work requirements, we tested the mice on 4 different schedules, PR+10, PR+5, PR+2 and PR+1. Figure 1A depicts the number of reinforcers earned on each schedule and shows that while performance of both D2R-OE and control mice is sensitive to work requirement, D2R-OE mice work less on all schedules compared to control littermates. Figure 1B shows that the deficit for each schedule is rescued when the transgene is switched off. Because the genotypic difference in willingness to work for food is independent of work requirement and therefore independent of both total number of lever presses made as well as total number of reinforcers earned, D2R-OE mice do not simply drop out earlier than control mice because they are satiated or fatigued earlier. For example, off dox, D2R-OE mice stop working after a mean of 233 lever presses and earning a mean of 5 reinforcers on PR+10, yet work more, making a mean of 522 lever presses and earning a mean of 28 reinforcers on PR+1. Further evidence to suggest that the D2R-OE mice do not quit the task sooner because they more quickly satiate comes from a comparison of the number of reinforcers earned but not consumed: control mice actually missed more earned rewards than D2R-OE mice, the mean for all subjects tested without dox across all PR conditions, Control = 1.0 +/− 0.18, D2R-OE mean 0.35 +/− 0.11, t-test p = 0.0066, repeated measure ANOVA for all ratios p = 0.0023. To further rule out that the poor performance was due to changes in consummatory behavior we used a naïve group of mice on an ad libitum diet and recorded the amount of chow consumed daily, as well as body weight on the ad lib diet. We found no differences in either of these measures between D2R-OE mice and controls (Figure S2 in the Supplement). Therefore, the lack of motivation in the D2R-OE mice observed across all schedules is not due to lack of interest in consuming the reinforcer.
Figure 1.
1A Performance of both D2R-OE and Control mice is sensitive to work requirement (repeated measure ANOVA p < 0.0001). Control mice earned more reinforcers compared to control littermates across all schedules tested (repeated measure ANOVA p= 0.0013, Control n=8, D2R-OE n=7). 1B An independent cohort of mice tested 2 weeks after starting doxycycline diet earned the same number of reinforcers across all four schedules (repeated measure ANOVA p= 0.382. Control n=9, D2R-OE n=6). 1C mean rate of lever pressing during a PRX2 schedule plotted as a function of reinforcer earned. 1D The mean rate of decay of lever pressing rate for each group is not significantly different (Control = 0.263 +/− 0.029, D2R-OE = 0.335 +/− .047 t-test p=0.199. Control n=8, D2R-OE n=7).
Additional evidence to suggest that the D2R-OE mice do not quit the task sooner because they fatigue more quickly comes from analyzing rate of lever pressing as a function of reinforcer earned when tested on a PRx2 schedule, the most demanding schedule tested. We fit the response rate data for each individual subject with negative exponential functions of the form Y=(a*exp(−b*x)) where a is the y intercept of the function and b is the rate of decay. Figure 1C shows that after the third reinforcer earned (the reinforcer earned with the highest response rate by each group,) D2R-OE mice decrease their rate of pressing after each subsequent reinforcer at a similar rate to that of control mice. Figure 1D shows that there is no difference in the rate of decay of lever pressing rate between D2R-OE and control mice, which would not be the case if the D2R-OE mice tired from lever pressing more quickly than controls. Therefore poor performance of the D2R-OE mice on the progressive ratio schedules appears due to a reduced willingness to work for the food reward. To rule out the possibility that any general health or gross motor defects impact the performance of D2R OE mice in the progressive ratio task we performed a thorough physical and neurological examination which did not reveal any differences between mutant and control mice (see Supplement).
The incentive motivation deficit in D2R-OE mice is not due to a decrease in tolerance for decreasing rates of reward or a difference in reactivity to the reward itself
Poor performance on a progressive ratio task may be due to a decrease in willingness to work for reward, or alternatively, a decrease in tolerance for the increasing time between each successive reward. We therefore tested the mice on a progressive interval schedule in which the mice received a reward for a single lever press but the time between rewards increased in a manner similar to that experienced on the PRX2 schedule by doubling with each reward (2s, 4s, 8s, 16s, 32s etc). As with the progressive ratio schedule, trial sessions continued until a response had not been made for 3 minutes, or 2 hours had elapsed. We found no significant difference in either the number of reinforcers earned in each session or the duration of the session (Figure S2 in the Supplement). In addition, there was no difference in the latency to retrieve the rewards, again indicating that the two groups of mice show an equivalent interest in consuming the reward.
Chronic D2 receptor blockade does not reverse the motivational deficit in D2R-OE mice
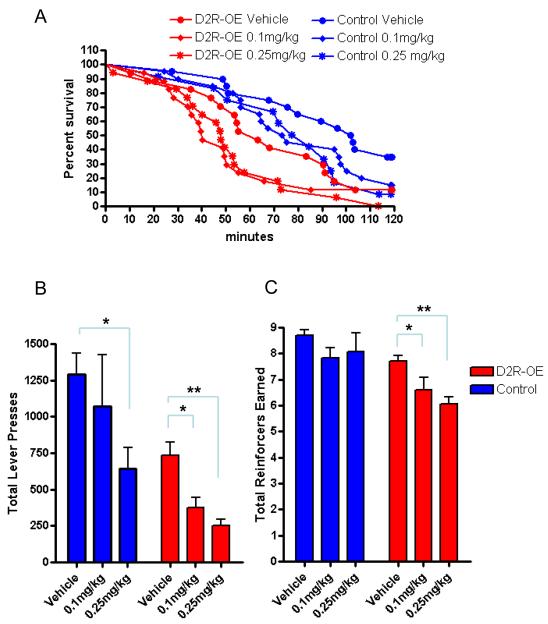
Clinical data suggests that the motivational deficits observed in patients with schizophrenia are not significantly ameliorated by treatment with D2R antagonists (16). This is in contrast to the significant rescue of motivation we observed when we switched off excess transgenic D2Rs. To determine if antagonism of the excess D2Rs was sufficient to ameliorate the motivation deficit in D2R-OE mice, we treated the mice with the antipsychotic haloperidol, administered via mini-osmotic pumps which were implanted subcutaneously and provided a daily dose of (0.25 mg/kg). This dose has previously been shown to result in 60-80% receptor blockade in rodents, which corresponds to the most clinically efficacious dose used in patients with schizophrenia (17, 18). Also, a separate group of mice received a lower daily dose of (0.1 mg/kg), which will block fewer receptors, in an attempt to target mainly the additional D2Rs. Previous studies have shown haloperidol to be active when delivered via minipump for a similar duration (19, 20). Chronic treatment with either dose of haloperidol did not improve motivation, but actually worsened the deficit. Figure 2A shows cumulative survival, the percentage of mice still working for food rewards as a function of session duration in the doubling progressive ratio schedule (where the session is terminated if a lever press has not been made for 3 minutes or after 2 hours have elapsed). A Mantel-Cox logrank test revealed no significant effect of either dose of haloperidol on D2R-OE or control mice. Collapsing across genotype, we found no effect of 0.1mg/kg, but 0.25mg/kg significantly reduced survival. Also, a two way ANOVA (genotype × drug treatment) revealed an overall effect of drug treatment on total number of lever presses (Figure 2B), as well as the total number of reinforcers earned (Figure 2C). While it is possible that haloperidol has a sedating affect, if this occurs, it is likely to be equal in both groups of mice because using 2- way ANOVA we did not find a significant interaction between treatment and genotype for total presses made (figure 2B), reinforcers earned (figure 2C) or rate of lever pressing (p=0.14).
Figure 2.
2A Cumulative survival curves for D2R-OE and control mice show that chronic haloperidol treatment does not reverse the motivation deficit. A Mantel-Cox logrank test for D2R-OE mice (p = 0.185 for 0/1mg/kg, p=0.088 for 0.25mg/kg) controls (p = 0.118 for 0.01mg/kg, p= 0.056 for 0.25mg/kg). Collapsing across genotype revealed no significant effect of 0.1mg/kg (p=0.090), but 0.25mg/kg significantly reduced survival (p=0.005). 2B The total number of lever presses made were significantly reduced by haloperidol treatment, Control vehicle mean =1291 +/− 147, 0.1mg/kg mean = 1072 +/− 355 (ttest p=0.58), 0.25mg/kg mean = 641 +/−125 (t-test p=0.0114). D2R-OE vehicle mean =737 +/− 87, 0.1mg/kg mean = 376 +/− 166 (t-test p=0.009), 0.25mg/kg mean = 253 +/−45 (t-test p=0.001). A significant overall effect of drug treatment on the total number of lever presses made was revealed by two way ANOVA (genotype × drug treatment), figure (p= 0.025, f =4.19). No significant interaction between treatment and genotype was observed (p = 0.738, f=0.31). 2C The total number of reinforcers earned were also significantly reduced by haloperidol treatment, D2R-OE vehicle mean =7.61+/− 0.22, 0.1mg/kg mean = 6.66+/− 1.33 (t-test p=0.079), 0.25mg/kg mean = 6.01 +/−0.33 (t-test p=0.0006).Two way ANOVA (genotype × drug treatment) also revealed a significant effect (p=0.01, f=5.03) while there was no significant interaction between treatment and genotype. (p=0.40, f=0.93).
Number of subjects: Controls: Vehicle n=8, 0.1mg/kg n=6, 0.25mg/kg n=5. D2R-OEs: Vehicle n=5, 0.1mg/kg n=7, 0.25mg/kg n=6. * p< 0.05, ** p< 0.005.
These data correspond with the clinical observation that neither typical nor atypical neuroleptics significantly improves motivation in patients (21, 22). Our findings also support data from others demonstrating that D2R blockade can decrease effort related choice in rats, for review see (23). Most importantly, this result indicates that removing excess striatal D2Rs by turning the transgene off impacts behavior in a way that blocking D2Rs ubiquitously does not and implies that there is a distinct functional difference between receptor number and receptor mediated activity.
D2R-OE mice show reversible up-regulation of the serotonin 5-HT2C receptor
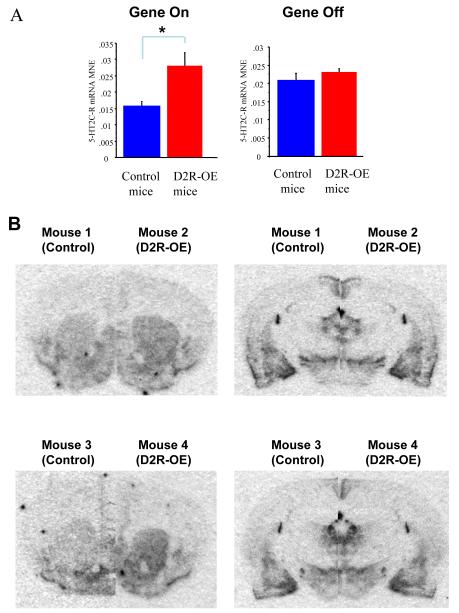
We performed an unbiased gene chip expression profiling experiment to identify possible changes in signaling pathways that may be affected by altered dopamine signaling in the striatum of D2R-OE mice. Because the motivational deficit was reversed by switching off excess striatal D2R expression, we specifically looked for molecular alterations in the striatum that are also reversed by doxycycline. We identified an increase in expression of the 5-hydroxytryptamine (serotonin) receptor 2C gene (fold increase 1.38, p=0.02), that was normalized by doxycycline treatment (fold increase 1.16, p=0.57). No other serotonin receptor subtype was found to be affected in this way (Table S2 in the Supplement). Because the 5-HT2A receptor was not represented on the chip used, we performed quantitative real time RT-PCR using the striatal mRNA used in the gene chip experiments and found no significant difference in expression (Table S2 legend in the Supplement). To determine if the upregulation of 5HT2C receptor expression was restricted to the striatum, we compared the gene expression profiles of frontal cortical tissue from D2R-OE and control mice. We found no significant differences in the expression of any serotonin receptors in the frontal cortex (Table S3 in the Supplement). Performing quantitative real time RT-PCR using the mRNA samples used in the gene chip experiments confirmed that the 5-HT2C gene is reversibly upregulated in D2R-OE mice, figure 3A. To visualize the spatial distribution of increased 5HT-2C mRNA expression we performed in-situ hybridization using a radiolabeled oligoprobe complementary to the 5-HT2C receptor mRNA and confirmed an increase in expression in the dorsal and ventral striatum (Figure 3B), with no obvious increase in the other areas of the brain.
Figure 3.
5-HT2C Receptor mRNA Expression in D2R-OE Mice. 3A Real time RT-PCR confirmed the observed increase in 5-HT2C expression, Control/D2R-OE off Dox MNE = 0.016+−0.001/0.028+−0.004, t-test p<0.05; Control/D2R-OE on Dox MNE = 0.036+−0.016/0.036+−0.016, t-test p=0.67, 3B in situ hybridization using a radiolabeled oligoprobe complementary to the 5-HT2C receptor mRNA in two pairs of mice (1vs 2 and 3 vs 4) confirmed an increase in expression in the dorsal and ventral striatum. No obvious increase was observed in other structures.
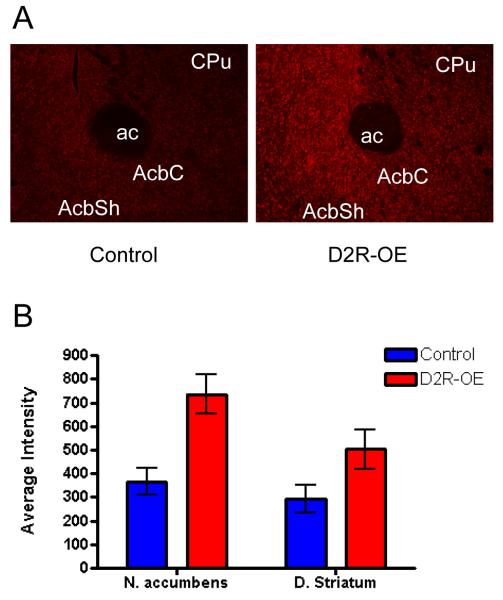
Increased striatal 5-HT2C receptor mRNA expression resulted in increased 5-HT2C receptor protein expression. Figure 4A shows immunofluorescent confocal microscopy images of the striatum using an anti-5HT2C receptor antibody. We quantified the average staining intensity from the dorsal and ventral striatum of D2R-OE and control mice and determined a significant increase in the intensity of 5HT2C staining in D2R-OE mice (Figure 4B).
Figure 4.
5-HT2C Receptor Protein Expression in the Striatum of D2R-OE Mice. 4A Representative confocal microscopic images using a 10X objective from one D2R-OE and one Control mouse. Abbreviations: ac = anterior commissure, AcbC = accumbens nucleus core, AcbSh = accumbens nucleus shell, CPu = Caudate Putamen. 4B Quantitative analysis revealed that the level of 5HT2C protein was increased in striatum of D2R-OE mice, Two-Way ANOVA p= 0.0017. t-tests for specific sub regions of the striatum revealed a significant increase in the ventral striatum (Mean +/− SEM for Control =366.3 ± 55.92, D2R-OE= 736.4 ± 83.65 P= 0.0121) and a trend for an increase in the ventral striatum (Mean+/− SEM control = 292.6 ± 58.39, D2R-OE 504.7 ± 83.22 p= 0.0829 ). Average intensity was derived from 3-5 slices per mouse, n = 5 mice per genotype.
The 5-HT2C selective antagonist SB242084 improves incentive motivation
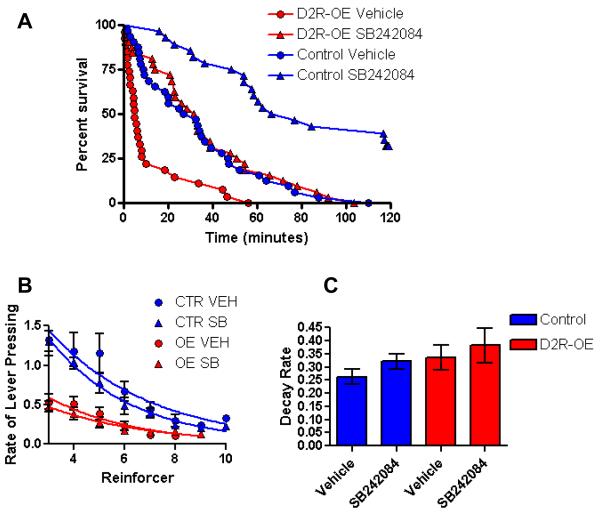
To determine if 5-HT2C receptor upregulation was responsible for the motivational deficit, we acutely treated the mice with 0.75mg/kg of the 5-HT2C selective antagonist SB242084 twenty minutes before testing. This significantly improved performance of the D2R-OE mice as determined by an increase in cumulative survival (Figure 5A), as well as an increase in total number of reinforcers earned (Table 1), However, it is possible that the treatment effect is not a function of reversing the functional consequences of the excess 2C receptors in the striatum of D2R-OE mice because performance of the control mice was also improved by SB242084 (figure 5A and table 1). This suggests that 5-HT2C antagonism robustly enhances appetitive incentive motivation in both a model of motivational impairment and unaffected individuals. While SB242084 increased session times, the total number of lever presses made and the total number of rewards earned, it did not alter lever press rates, the mean latency to collect earned rewards or the number of rewards that were earned but not consumed (Table 1). Additionally, the rate of decay of lever pressing over the course of the session was not affected by the drug (figure 5B and C). These data indicate that the drug-enhanced performance on the task is not due to an increase in appetite or an enhancement in motor function.
Figure 5.
The 5-HT2C Selective Antagonist SB242084 Improves Incentive motivation. 5A Acute injection of 0.75mg/kg SB242084 significantly increased the survival curves of D2R-OE and Control mice, logrank test D2R-OE, p =0.0044, Control p = 0.0017. 5B The rate of lever pressing as a function of number of reinforcers earned. 5C decay rates calculated from each individuals data reveal no significant effect of drug on the rate of decrease of lever pressing rate (Lever presses per minute) for either genotype, Control Vehicle = 0.263 +/− 0.029 , Control SB242084 = 0.322 +/− 0.029 t-test P= 0.172. D2R-OE Vehicle = 0.335 +/− 0.047, D2R-OE SB242084 = 0.383 +/− 0.066 t-test P= 0.581. Number of subjects; Control vehicle = 8, control SB242084 = 8, D2R-OE vehicle = 7, D2R-OE SB242084= 8.
Table 1.
Effects of The 5-HT2C Selective Antagonist SB242084 on performance in the Progressive ratio schedule.
| CONTROL VEHICLE Mean (SEM) |
CONTROL SB242084 Mean (SEM) |
t-test P value |
D2R-OE VEHICLE Mean (SEM) |
D2R-OE SB242084 Mean (SEM) |
t-test P value |
|
|---|---|---|---|---|---|---|
| Session duration (minutes) | 33.2 (7.24) | 83.6 (7.59 | 0.0003 * | 12.1(2.99) | 38.1(8.15) | 0.015 * |
| Total number of lever presses | 502.9 (132.87) | 1016.5 (144.73) | 0.020 * | 113.0 (28.5) | 249.9 (69.52) | 0.100 |
| Total number of rewards earned | 6.7 (0.45) | 8.0 (0.26) | 0.035 * | 3.9 (0.63) | 5.6 (0.40) | 0.044 * |
| Pressing Rate (presses/minute) | 15.7 (1.84) | 12.1 (1.46) | 0.152 | 12.1 (2.02) | 7.9 (1.34) | 0.112 |
| Mean latency to collect reward | 0.7 (0.04) | 0.6 (0.06) | 0.623 | 0.7 (0.05) | 0.8 (0.05) | 0.359 |
| Number of rewards not Consumed | 0.1 (0.07) | 0.3 (0.14) | 0.229 | 0.2 (0.05) | 0.1 (0.09) | 0.562 |
Number of subjects; Control vehicle = 8, control SB242084 = 8, D2R-OE vehicle = 7, D2R-OE SB242084= 8.
= P < 0.05.
Discussion
Two characteristic features of schizophrenia are deficits in incentive motivation and in cognition, each of which negatively impacts the other (24). Both of these features are evident in D2R-OE mice. We find that the transient motivational deficit observed in the D2R-OE mice is due to a reluctance to work for food, rather than a decrease in satiation threshold, a decreased tolerance for low rates of reward or a difference in reactivity to reward, itself. Systemic pharmacological blockade of D2Rs did not improve motivation in these mice, whereas shutting off transgene expression did. The failure of haloperidol to improve motivation may be because systemic administration of the drug results not only in blockade of D2Rs on mediums spiny neurons (MSNs) but also on striatal cholinergic interneurons, presynaptic autoreceptors and extrastriatal receptors. These effects may counteract any positive effect of blocking specifically the transgenic D2Rs expressed on MSNs. Our result with haloperidol mimics the clinical observation that systemic D2R antagonists do not significantly ameliorate the negative symptoms of schizophrenia, including avolition.
There are several possible ways in which striatal D2R-OE expression may impact motivation. Within the striatum MSNs can be separated into two cellular populations, D1R and D2R expressing cells. The degree of overlap between these populations has long been a matter of debate (25, 26) . Some data suggests that the percentage of cells that express both receptors at high levels is around 5-7% in the dorsal striatum and NAc core and around 17% in the NAc shell (27, 28). Using double in situ hybridization we found that transgenic D2Rs are also expressed in D1-positive cells and that the degree of overlap in the dorsal striatum increases from 7% in control mice to 22% in D2R-OE mice (unpublished data). Therefore, it is possible that increased D2R expression in D1R cells contributes to the motivational deficit. Alternatively, the impact of extra D2Rs on motivation may not be the direct result of altered D2 signaling but rather arise from longterm changes in dopaminergic or other molecular pathways.
Striatal D2R overexpression affects striatal 5-HT2C receptor expression
One downstream effect of striatal D2R overexpression is altered serotonin signaling. We identified a reversible increase in 5-HT2C receptor expression, suggesting a specific interaction between the dopamine and the serotonin system within the striatum. The increase in 5-HT2C mRNA and protein expression was verified by four independent methods; gene chip analysis, quantitative RT-PCR, in situ hybridization and quantitative immunofluorescence.
Acute treatment with the 5-HT2C antagonist SB242084 enhanced performance on the progressive ratio task, and we believe this is likely due to an increase in willingness to work for reward, rather than an increase in appetite or motor function. In vitro, some atypical antipsychotics, including clozapine, can act as inverse agonists on 5-HT2C receptors (29), and it has been suggested that this influences the weight gain associated with these drugs (30). However, in our experiments SB242084 did not change latency to retrieve rewards, nor did it decrease the number of rewards earned but not consumed. In line with this, SB242084 administration has previously been found not to affect feeding behavior (31). Additionally, SB242084 did not affect rate of lever pressing, suggesting that its action is not on motor performance.
That 5-HT2C receptor antagonism rescued the motivational deficit in D2R-OE mice is concordant with the result that switching off transgenic D2Rs both normalized 5-HT2C receptor expression levels and rescued the motivation deficit. Because chronic D2R blockade did not rescue the motivation deficit we tested if the treatment affected 5-HT2C expression levels. We performed real time RT-PCR using striatal RNA from the mice that were treated with haloperidol for the behavioral experiment and determined that neither dose of haloperidol used had anaffect on 5-HT2C receptor expression level. There was no overall effect of haloperidol treatment on mean normalized expression of 5-HT2C for either genotype, (2-way ANOVA p=0.614) and no interaction between genotype and treatment (p=0.239), data not shown. This finding shows that removal of the striatal specific transgene has a downstream molecular effect distinct from chronic systemic D2R blockade.
One possible mechanism by which SB242084improves motivation in D2R-OE mice could be through modulation of striatal MSN activity by counteracting the upregulated 5-HT2C receptors located on those cells. However, since control littermates also show improved performance in the progressive ratio task, it seems clear that 5-HT2C receptor antagonism is capable of being efficacious even in the presence of normal endogenous levels of receptors. Because we injected the drug systemically, the site of action could be in the striatum where we see the increase in gene expression, or at a different location, such as the ventral tegmental area (VTA) in which GABAergic interneurons express 5-HT2C receptors. Agonism and antagonism of these receptors has been shown to reduce and enhance, respectively, the firing of mesolimbic dopamine neurons and terminal dopamine release (32). Recently, work using 5HT2C receptor null mice suggests that nigrostriatal dopaminergic activity is also regulated by these receptors (33).
5-HT2C Receptors in Psychiatric Disorders
There are multiple lines of evidence to suggest that the 5-HT2C receptor is involved in the pathophysiology of several psychiatric disorders including schizophrenia, depression, anxiety, sleep disorders, drug addiction and obesity. In the case of schizophrenia and depression, association between the 5-HT2C receptor and disease susceptibility is proposed to be the result of alternative RNA editing, which may affect the signaling characteristics of the receptor (34). The efficiency of RNA editing may be altered in the frontal cortex of patients with schizophrenia (35), potentially resulting in higher 5-HT2C receptor activity in the cortex, although this association has not been replicated in other post mortem studies (36, 37). Our finding of increased 5-HT2C receptors in D2R-OE mice suggests that the striatal D2R hyperactivity observed in patients with schizophrenia results in increased 5-HT2C receptor function which could be responsible for some of the negative symptoms.
We here demonstrate for the first time that 5HT-2C antagonism robustly enhances an appetitive operant measure of incentive motivation. This enhancement behaviorally rescues a mouse model of decreased motivation, therefore suggesting that 2C antagonism may be helpful in targeting disorders of motivation. There have been previous suggestions that 5-HT2C receptor ligands may have therapeutic potential in several diseases including depression and schizophrenia (38, 39), but there has not yet been, to our knowledge, any demonstration of improved motivation from 2C antagonism in any model organisms.
If antagonism of 5-HT2C receptors proves to be a useful pan-disorder treatment for deficits in incentive motivation, 5-HT2C receptor agonism may ameliorate disorders of pathological excessive motivation, such as substance abuse disorders and obsessive-compulsive disorders. Indeed, injections of the 5-HT2C receptor agonist Ro60-0175 reduced cocaine induced locomotor activity and cocaine self administration in rats (40) and the concept of 5-HT2C receptor pharmacotherapy for psychostimulant abuse has recently been reviewed (41). These studies, together with our present finding, lead us to believe that 5-HT2C receptors are a promising target for a number of psychiatric disorders.
Supplementary Material
Acknowledgements
We are indebted to Tessa Hirschfeld-Stoler and Iram Haq for maintaining the transgenic mouse colony and Kathleen Taylor for assistance with the data extraction. This work was supported by the Lieber Center for Schizophrenia Research, National Institute of Mental Health (NIMH) Grant 5R01MH068073 (P.D.B.), the NIMH Silvio O. Conte Center for Schizophrenia Research MH 66171 (E.H.S., C.K. E.R.K.), a Narsad young investigator award to CK and a generous gift from Harold and Shari Levy for schizophrenia research (E.R.K., E.H.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49(4):603–615. doi: 10.1016/j.neuron.2006.01.023. [see comment] [DOI] [PubMed] [Google Scholar]
- 2.Association AP . Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- 3.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia research. 2007 Jul;93(1-3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia bulletin. 2008 Sep;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziauddeen H, Murray GK. The relevance of reward pathways for schizophrenia. Current opinion in psychiatry. Mar;23(2):91–96. doi: 10.1097/YCO.0b013e328336661b. [DOI] [PubMed] [Google Scholar]
- 6.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007 Feb 14;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274(5293):1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 8.Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, et al. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behavioral neuroscience. 2009 Aug;123(4):720–730. doi: 10.1037/a0016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proceedings of the National Academy of Sciences of the United States of America. 2008 Oct 14;105(41):16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007 Jul 18;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophrenia research. 1991 Sep;5(2):123–134. doi: 10.1016/0920-9964(91)90039-t. [DOI] [PubMed] [Google Scholar]
- 12.Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophrenia research. 1997 May 3;25(1):1–10. doi: 10.1016/S0920-9964(96)00098-9. [DOI] [PubMed] [Google Scholar]
- 13.Bubar MJ, Seitz PK, Thomas ML, Cunningham KA. Validation of a selective serotonin 5-HT(2C) receptor antibody for utilization in fluorescence immunohistochemistry studies. Brain research. 2005 Nov 30;1063(2):105–113. doi: 10.1016/j.brainres.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 14.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain research. 1999 Jul 17;835(1):18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 15.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Hormones and behavior. 1997 Jun;31(3):197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 16.Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta psychiatrica Scandinavica. 2007 Jan;115(1):4–11. doi: 10.1111/j.1600-0447.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 17.Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. The Journal of pharmacology and experimental therapeutics. 2003 May;305(2):625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- 18.Naiker DV, Catts SV, Catts VS, Bedi KS, Bryan-Lluka LJ. Dose determination of haloperidol, risperidone and olanzapine using an in vivo dopamine D2-receptor occupancy method in the rat. European journal of pharmacology. 2006 Jul 1;540(1-3):87–90. doi: 10.1016/j.ejphar.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 19.Karl T, Duffy L, O’Brien E, Matsumoto I, Dedova I. Behavioural effects of chronic haloperidol and risperidone treatment in rats. Behavioural brain research. 2006 Aug 10;171(2):286–294. doi: 10.1016/j.bbr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Turrone P, Remington G, Kapur S, Nobrega JN. Differential effects of within-day continuous vs. transient dopamine D2 receptor occupancy in the development of vacuous chewing movements (VCMs) in rats. Neuropsychopharmacology. 2003 Aug;28(8):1433–1439. doi: 10.1038/sj.npp.1300233. [DOI] [PubMed] [Google Scholar]
- 21.Manschreck TC, Boshes RA. The CATIE schizophrenia trial: results, impact, controversy. Harvard review of psychiatry. 2007 Sep-Oct;15(5):245–258. doi: 10.1080/10673220701679838. [DOI] [PubMed] [Google Scholar]
- 22.Mortimer AM. Cognitive function in schizophrenia--do neuroleptics make a difference? Pharmacology, biochemistry, and behavior. 1997 Apr;56(4):789–795. doi: 10.1016/s0091-3057(96)00425-x. [DOI] [PubMed] [Google Scholar]
- 23.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007 Apr;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 24.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 25.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends in neurosciences. 2007 May;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Surmeier DJ, Reiner A, Levine MS, Ariano MA. Are neostriatal dopamine receptors co-localized? Trends in neurosciences. 1993 Aug;16(8):299–305. doi: 10.1016/0166-2236(93)90103-s. [DOI] [PubMed] [Google Scholar]
- 27.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008 May 28;28(22):5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2006 Feb 28;103(9):3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrick-Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. The Journal of pharmacology and experimental therapeutics. 2000 Oct;295(1):226–232. [PubMed] [Google Scholar]
- 30.Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Progress in brain research. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology. 2009 Sep;57(3):259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends in pharmacological sciences. 2001 May;22(5):229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- 33.Abdallah L, Bonasera SJ, Hopf FW, O’Dell L, Giorgetti M, Jongsma M, et al. Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci. 2009 Jun 24;29(25):8156–8165. doi: 10.1523/JNEUROSCI.3905-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RB, Clarke WP. RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. British journal of pharmacology. 2001 Sep;134(2):386–392. doi: 10.1038/sj.bjp.0704255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Molecular psychiatry. 2001 Jul;6(4):373–379. doi: 10.1038/sj.mp.4000920. [DOI] [PubMed] [Google Scholar]
- 36.Dracheva S, Elhakem SL, Marcus SM, Siever LJ, McGurk SR, Haroutunian V. RNA editing and alternative splicing of human serotonin 2C receptor in schizophrenia. Journal of neurochemistry. 2003 Dec;87(6):1402–1412. doi: 10.1046/j.1471-4159.2003.02115.x. [DOI] [PubMed] [Google Scholar]
- 37.Dracheva S, Patel N, Woo DA, Marcus SM, Siever LJ, Haroutunian V. Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Molecular psychiatry. 2008 Nov;13(11):1001–1010. doi: 10.1038/sj.mp.4002081. [DOI] [PubMed] [Google Scholar]
- 38.Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005 Sep-Oct;60(5):441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- 39.Rosenzweig-Lipson S, Dunlop J, Marquis KL. 5-HT2C receptor agonists as an innovative approach for psychiatric disorders. Drug news & perspectives. 2007 Nov;20(9):565–571. doi: 10.1358/dnp.2007.20.9.1162244. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004 Feb;29(2):308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- 41.Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Progress in brain research. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.