Abstract
Meningococcal diseases are serious threats to global health, and new vaccines specifically tailored to meet the age-related needs of various geographical areas are required. This paper focuses on the meningococcal conjugate vaccines developed by GSK Biologicals. Two combined conjugate vaccines were developed to help protect infants and young children in countries where the incidence of meningococcal serogroup C or serogroup C and Y disease is important: Hib-MenC-TT vaccine, which offers protection against Haemophilus influenzae type b and Neisseria meningitidis serogroup C diseases, is approved in several countries; and Hib-MenCY-TT vaccine, which adds N. meningitidis serogroup Y antigen, is currently in the final stages of development. Additionally, a tetravalent conjugate vaccine (MenACWY-TT) designed to help protect against four meningococcal serogroups is presently being evaluated for global use in all age groups. All of these vaccines were shown to be highly immunogenic and to have clinically acceptable safety profiles.
1. Introduction
Invasive diseases caused by Neisseria meningitidis, of which meningitis and septicaemia are the most important, are serious threats to global health [1, 2]. Sporadic as well as endemic cases occur worldwide, and N. meningitidis is the only encapsulated bacterium known to cause large epidemics of bacterial meningitis [3, 4]. Notably, extensive meningococcal disease outbreaks comprising hundreds of thousands of cases occur cyclically in an area of Sub-Saharan Africa, also called the Meningitis Belt [5–8]. Overall, about 500,000 cases of meningococcal disease occur each year causing at least 50,000 deaths [9]. Meningococcal meningitis has a case-fatality rate of 5% to 10% in industrialised countries, which can reach 20% in the developing world [10, 11]. In addition, 12% to 19% of survivors develop long-term neurological sequelae [3, 7, 12–14]. While the highest case-fatality rate is observed among persons older than 65 years and generally decreases with lower age [10], the risk of meningococcal disease is highest in infants and young children with a secondary peak in incidence during adolescence and young adulthood [15].
N. meningitidis is a gram-negative encapsulated diplococcus that colonises the human nasopharynx, where it is usually carried asymptomatically [1]. Meningococci are transmitted through close contact via respiratory droplets [7]. In some cases, bacteria spread from the nasopharynx to nearby epithelial cells causing local invasion of tissue. If the meningococci reach the bloodstream, they may cause meningococcal meningitis or fulminant septicaemia [3, 7, 16]. N. meningitidis is classified into 13 serogroups according to differences in the capsular polysaccharide (PS) antigens. Six of these serogroups (A, B, C, W-135, Y, and more recently X) are responsible for the majority of meningococcal disease cases [3, 17].
Meningococcal incidence and serogroup distribution are highly regional and have a cyclical nature, with peaks typically occurring in a five-to-eight-year pattern [18, 19]. For this reason, meningococcal disease surveillance is required for the assessment of local epidemiology and disease burden, which are key issues for vaccine formulation and prevention strategies [19]. Although many of the surveillance systems for meningococcal disease lack sensitivity and may underestimate disease burden, current meningococcal disease epidemiology can be summarised per region [19]. In Africa and Asia, serogroup A (MenA) remains the cause of most large-scale epidemics, with the highest magnitude in the African Meningitis Belt, while serogroups B and C (MenB and MenC) are associated with sporadic disease [3, 19–21]. In addition, serogroup W-135 (MenW-135) has emerged as a new threat after causing outbreaks in Hajj pilgrims in Saudi Arabia, followed by Burkina Faso and Chad [22, 23]. More recently, various outbreaks due to serogroup X have also been reported in Africa [24–26]. In industrialised countries such as Europe, the United States (USA), Latin America and Australia, MenB and MenC are the most important causes of invasive meningococcal disease [2, 14, 19, 27–29]. In addition, serogroup Y (MenY) accounts for around one-third of meningococcal disease cases in the US and the incidence of this serogroup has also recently increased in Scandinavian countries [10, 14, 17, 30]. In industrialised countries, rates of meningococcal disease are presently very low (0.5–6 per 100,000 population) and this may be explained by a combination of environmental, organism and host factors. Even with this historically low rate, meningococcal disease continues to cause considerable morbidity and mortality among all age groups in these regions and N. meningitidis remains the most common cause of bacterial meningitis in children and young adults [2, 10].
While mass chemoprophylaxis is not recommended to control large outbreaks of meningococcal disease, vaccination is considered to be an effective prevention strategy and the development of effective meningococcal vaccines, which have acceptable safety profiles, is a public health priority [31, 32]. The first vaccines developed were plain PS vaccines that consist of purified capsular PS from specific meningococcal serogroups. GlaxoSmithKline (GSK) Biologicals developed different formulations of plain PS vaccines against serogroups A, C, W-135, and Y (MencevaxAC, MencevaxACW, MencevaxACWY). These vaccines have been used to immunise travellers, Hajj pilgrims, military personnel, and other specific populations at increased risk for invasive meningococcal disease [33, 34]. Meningococcal PS vaccines are available for use in children above two years of age but, aside from the PS from MenA, which is immunogenic in infants as young as three months of age, they are suboptimally immunogenic and therefore not indicated in infants and toddlers [35, 36]. Moreover, PS vaccines induce T-cell-independent immune responses, which are not longlasting, do not result in immune memory and can induce immunological hyporesponsiveness for serogroups A, C, W-135, and Y. For these reasons, PS vaccines are not appropriate when sustained protection is needed [37–40].
To overcome these limitations, conjugate vaccines were developed, in which capsular PS are covalently coupled to carrier proteins that contain T-cell epitopes. These vaccines have been shown to be immunogenic in infants, T-cell dependent, induce immune memory, and induce higher bactericidal titres, which potentially could translate into longer antibody persistence [37, 38, 41]. The most common carrier proteins used in conjugate meningococcal vaccines are tetanus toxoid (TT), diphtheria toxoid (DT), and a nontoxic cross-reacting mutant of DT (CRM197) [38]. Conjugate vaccines against MenC were introduced in 1999 in the United Kingdom (UK) as part of a vaccination programme that included routine infant immunisation and a large-scale catch-up campaign from infants through children 18 years of age, which was later extended to 24 years of age. This programme had a tremendous impact on the incidence of the disease and induced herd immunity [42–44]. MenC vaccination programmes have been successfully implemented in other countries as well, including Canada, Spain, and the Netherlands [45–47]. Presently, two tetravalent conjugate vaccines against serogroups A, C, W-135, and Y, using DT or CRM197 as carrier proteins, have been licensed in various countries.
Unlike other meningococcal serogroups (A, C, W-135, and Y), capsular PS from MenB is poorly immunogenic in humans. Furthermore, the capsular structure of MenB mimics sialylated structures in human neural tissue posing potential safety concerns for a vaccine formulation containing these polymers. Therefore, most of the MenB vaccine research has been focused on the outer membrane proteins (OMPs) [48]. MenB vaccines have been developed using alternative strategies and they are available in Cuba, New Zealand, the Netherlands, and France [29]. However, these vaccines are strain specific and therefore their use is limited [49]. A protein-based investigational MenB vaccine intended for global use has been developed by Novartis, and a Marketing Authorization Application was submitted to the European Medicines Agency (EMA) at the end of 2010. A second investigational protein-based MenB vaccine being developed by Pfizer is currently in Phase II of clinical development.
In this paper, we will focus on vaccines, developed by GSK Biologicals, against the most common serogroups for which PS conjugate vaccine technology can be used (MenA, MenC, MenW-135, and MenY). The vaccine development strategy was driven by the populations at highest risk: first the developing world with a focus on Africa, then more industrialised countries with a focus on the highest risk populations (infants and young children, followed by adolescents). These vaccines have been studied in randomised, controlled clinical trials evaluating the immunogenicity, antibody persistence, induction of immune memory, reactogenicity, and safety of the different vaccines. Results of the studies that are discussed here have been previously presented in peer-reviewed publications (Table 1).
Table 1.
Published clinical vaccination trials of GSK's conjugate meningococcal vaccines.
| Vaccine | Study | Phase | Country | Target group | Schedule | N | Publication |
|---|---|---|---|---|---|---|---|
| NCT00317174 | Phase II | Philippines | Infants | 6, 10, 14 weeks PS challenge at 10 months |
524 217 |
Gatchalian et al., 2008 [59]* | |
| DTPw-HBV/Hib-MenAC | ISRCTN35754083 | Phase II | Ghana | Infants | 6, 10, 14 weeks PS challenge at 12 months |
280 260 |
Hodgson et al., 2008 [60] |
| NCT00317161 and NCT00317187 | Phase III | Philippines, Thailand | Infants | 2, 4, 6 months | 1780 | Kerdpanich et al., 2008 [61] | |
|
| |||||||
| NCT00135486 and NCT00135564 | Phase II | Germany | Infants | 2, 3, 4 months PS challenge at 12–15 months |
520 | Schmitt et al., 2007 [70] | |
| NCT00323050 and NCT00322335 | Phase III | Spain | Infants | 2, 4, 6 months 13-14 months |
237 358 |
Tejedor et al., 2007 [62] Tejedor et al., 2008 [74] |
|
| Hib-MenC-TT | NCT00263653 | Phase III | Spain | Toddlers | 13-14 months | 297 | Carmona et al., 2010 [75] |
| NCT00258700 | Phase III | UK, Poland | Infants | 2, 3, 4 months 12 months |
500 476 |
Pace et al., 2007 [69] Pace et al., 2008 [73] Khatami et al., 2011 [76] |
|
|
NCT00334334 NCT00463437 |
Phase III | Germany, Poland, Spain | Infants | 2, 4, 6 months 11–18 months |
1548 1437 |
Wysocki et al., 2009 [80] Knuf et al., 2009 [71] |
|
| NCT00326118 | Phase III | Australia | Toddlers | 12–18 months | 433 | Booy et al., 2011 [72] | |
| ISRCTN72858898 | Phase IV | UK | Children | 6–12 years | 249 | Perrett et al., 2010 [77] | |
|
| |||||||
| NCT00127855 | Phase II | Australia | Infants | 2, 4, 6 months PS challenge at 11–14 months |
407 394 |
Nolan et al., 2007 [81] | |
| NCT00129116 | Phase II | Belgium, Germany | Infants | 2, 3, 4 months 12–18 months |
388 221 |
Habermehl et al., 2010 [68] | |
| Hib-MenCY-TT | NCT00129129 | Phase II | US | Infants | 2, 4, 6 months 12–15 months |
756 498 |
Marchant et al., 2010 [82] Marshall et al., 2010 [83] |
| NCT00134719 | Phase II | Australia | Infants | 2, 4, 6 months 12–15 months |
1103 1037 |
Nolan et al., 2011 [84] | |
| NCT00289783 | Phase III | US, Mexico, Australia | Infants | 2, 4, 6 months 12–15 months |
4180 | Bryant et al., 2011 [85] | |
|
| |||||||
|
NCT00196950 NCT00126945 |
Phase II | Belgium, Denmark | Adolescents Adults |
15–25 years | 175 | Østergaard et al., 2009 [86] | |
| NCT00126984 | Phase II | Germany, Austria | Toddlers Children |
12–14 months or 3–5 years |
508 | Knuf et al., 2010 [87] | |
| MenACWY-TT |
NCT00464815 NCT00453986 |
Phase III | Philippines, India, Taiwan + Philippines, Lebanon | Adolescents Adults |
11–17 years and 18–55 years |
2272† | Bermal et al., 2011 [88] |
| NCT00454909 | Phase II | US | Adolescents Adults |
11–25 years | 872 | Baxter et al., 2011 [89] | |
| NCT00474266 | Phase III | Finland | Toddlers | 12–23 months | 1000 | Vesikari et al., 2011 [90] | |
| NCT00508261 | Phase III | Austria, Germany, Greece | Toddlers | 12–23 months | 793 | Knuf et al., 2011 [91] | |
N: Total vaccinated cohort including subjects who received the study vaccine and control vaccines, NCT: Clinical Trial Registry Numbers, ISRCTN: International Standard Randomized Controlled Trial Number, *Number of the publication in the reference list, †1025 subjects from study NCT00464815 (Philippines, India, Taiwan) and 1247 from study NCT00453986 (Philippines, Lebanon), For study NCT00453986 only safety data are summarised here.
2. Conjugate Meningococcal Vaccines Developed by GSK Biologicals
The effectiveness of meningococcal vaccines is challenging to evaluate in prospective, randomised efficacy studies because the incidence of endemic meningococcal disease is low, and epidemics are difficult to predict. Therefore, efficacy is inferred from immunogenicity data via laboratory markers that can reliably predict clinical protection [50]. The primary human defence to meningococcus is the activation of the complement system by antigen-specific antibodies resulting in bacterial lysis. Bactericidal assays measuring interactions of antibodies and human complement at the bacterial surface (hSBA) can be used to measure the functional activity of a tested serum against a given strain of meningococcus. Data generated in US military recruits suggested that an hSBA titre ≥1 : 4 correlated with protection against meningococcal serogroup C disease [51]. After introduction of MenC conjugate vaccines in the UK another serological correlate of protection based on a serum bactericidal assay using rabbit complement (rSBA titre ≥1 : 8) became available using both prelicensure age-stratified seroprevalence and disease incidence data, and postlicensure efficacy estimates [52–54]. The seroprotective thresholds for MenC have been extended to serogroups A, W-135, and Y. Both assays are accepted by the WHO for the assessment of the immunogenicity of meningococcal vaccines [55] and both have been used in GSK's clinical development depending on regional health authority requirements.
2.1. DTPw-HBV/Hib-MenAC Combined Vaccine for African Infants
In Africa, the world's highest risk population, PS vaccines against meningococcal disease are available but they do not offer broad serogroup protection to infants and in older subjects they only protect for 3–5 years, leaving the population vulnerable to future epidemics [56–58]. Moreover, the hyporesponsiveness that may be induced by repeated injections of PS vaccines compromises their use in the future because the population remains at life-long risk for invasive meningococcal disease [37].
In 2000, the WHO and representatives from eight African countries called for the development of new conjugate vaccines, which could be used in routine vaccination campaigns to control invasive meningococcal disease in Africa. In response to this request, GSK Biologicals initiated the development of a new heptavalent diphtheria, tetanus, whole cell pertussis, hepatitis B, Haemophilus influenzae type b, N. meningitidis serogroups A, and C- tetanus toxoid conjugate combined vaccine (DTPw-HBV/Hib-MenAC-TT) intended for vaccination of infants [59–61]. Indeed, MenA and to a lesser extent MenC contribute to endemic disease and periodic outbreaks in Africa and at that time, MenW-135 was not yet recognised as an important serogroup in the African Meningitis Belt [22, 23]. The addition of antigens from MenA and MenC to antigens of vaccines routinely administered in paediatric vaccination programmes was intended to promote rapid uptake and high coverage of these components without the need for further vaccination visits or injections, while minimising costs [62–64].
Three-dose primary vaccination with DTPw-HBV/Hib-MenAC-TT was shown to be noninferior to DTPw-HBV/Hib for the five common antigens and induced antibodies against MenA and MenC in the majority of subjects when given to African infants at 6, 10, and 14 weeks of age [60]. The vaccine induced a good persistence of antibodies and immune memory [59]. As for other conjugate vaccines used in infancy [52, 65, 66], a booster dose was required in the second year of life to sustain protection through early childhood [60]. The DTPw-HBV/Hib-MenAC-TT vaccine had a clinically acceptable safety profile and no specific safety issues were raised during the Phase II and Phase III studies [59–61]. No increase in reactogenicity during the primary vaccination course was observed when compared with the licensed DTPw-HBV/Hib vaccines [59].
Following development of this new vaccine, the WHO communicated that they were prioritising mass vaccination in older individuals and for MenA only, since this is currently the predominant serogroup in the African Meningitis Belt. As DTPw-HBV/Hib-MenAC-TT was a combination vaccine designed to be incorporated into the routine vaccination schedule for younger children, final development of the vaccine was stopped following consultation with the WHO. Recently, a new MenA conjugate vaccine (MenAfriVac, the Serum Institute of India) was licensed and prequalified by the WHO. However, this new vaccine will only provide protection against MenA and there may be need in the future for conjugate vaccines directed against other serogroups.
2.2. Hib-MenC-TT Combined Vaccine for Infants: Menitorix
The next high-risk population targeted by GSK Biologicals for the development of conjugate vaccines were infants and young children in countries where MenC is the predominant serogroup, since these age groups are at the highest risk for invasive disease in most countries worldwide [2, 10, 19]. As the list of recommended vaccines to be included in childhood immunisation programmes increases, combination vaccines have been recognised as a means of simplifying immunisation schedules [62–64]. Therefore, GSK Biologicals initiated the development of a combined vaccine (Hib-MenC-TT) intended to provide protection to infants and toddlers against two major pathogens responsible for bacterial meningitis (H. influenzae type b [Hib] and N. meningitidis serogroup C [MenC]). The Hib-MenC-TT vaccine offers an alternative vaccination schedule to the acellular pertussis (DTPa)-Hib combinations coadministered with standalone MenC conjugate vaccines [67].
Hib-MenC-TT contains 5 μg polyribosylribitol phosphate (PRP) from Hib and 5 μg PS from MenC, each conjugated to TT. Phase II and Phase III studies have shown that the Hib-MenC-TT vaccine is immunogenic when administered as three-dose primary series in infants at 2, 3, and 4 months of age [68–70] or at 2, 4, and 6 months of age [62, 71] (Table 2). At least 98.8–100% of subjects had anti-PRP ≥0.15 μg/mL (data not shown) and 99.2–100% had rSBA-MenC titres ≥1 : 8 (Table 2) after completion of the primary vaccination schedule. The Hib-MenC-TT combined vaccine induced MenC and Hib responses comparable to those induced by licensed monovalent vaccines and all primary hypotheses regarding the noninferiority of Hib-MenC-TT to licensed Hib and monovalent MenC conjugate were met [62, 68, 70, 72]. Consistently high anti-PRP GMCs were observed across the studies, suggesting that Hib-MenC-TT can overcome the challenges seen with reduced Hib immunogenicity when Hib conjugate vaccines are given in combination with acellular pertussis compared with separate injections [67].
Table 2.
rSBA-MenC antibody response after Hib-MenC-TT vaccination.
| Reference | Primary vaccination | Booster vaccination | Timepoint | N | rSBA-MenC ≥1 : 8 | rSBA-MenC ≥1 : 128 | GMT | |||
|---|---|---|---|---|---|---|---|---|---|---|
| % | [95% CI] | % | [95% CI] | Value | [95% CI] | |||||
| Schmitt et al., 2007 [70] 2, 3, 4 + 12–15 months of age | Hib-MenC-TT + DTPa-HBV-IPV | Men-PS + DTPa-HBV-IPV/Hib | PD3 | 95 | 100 | [96.2–100] | — | — | 944.2 | [779.4–1143.8] |
| Pre-Ch | 90 | 87.1 | [78.0–93.4] | — | — | 159.3 | [107.9–235.3] | |||
| Post-Ch | 83 | 100 | [95.5–100] | — | — | 5385.4 | [4425.0–5554.2] | |||
| MenC-CRM197 + DTPa-HBV-IPV/Hib | Men-PS + DTPa-HBV-IPV/Hib | PD3 | 105 | 100 | [96.5–100] | — | — | 1400.7 | [1165.4–1683.5] | |
| Pre-Ch | 93 | 80.2 | [70.6–87.8] | — | — | 104.0 | [68.4–158.3] | |||
| Post-Ch | 86 | 96.5 | [90.0–99.3] | — | — | 1552.6 | [1044.4–2307.9] | |||
|
| ||||||||||
| Pace et al., 2007 & 2008 [69, 73] Khatami et al., 2011 [76] 2, 3, 4 + 12–15 months of age | Hib-MenC-TT + DTPa-IPV | Hib-MenC-TT + MMR | PD3 | 354 | 99.2 | [97.5–99.8] | 92.9 | [89.8–95.4] | 581.1 | [514.7–656.2] |
| Pre-B | 346 | 78.0 | [73.3–82.3] | 43.9 | [38.6–49.3] | 61.3 | [50.9–73.7] | |||
| Post-B | 347 | 99.1 | [97.5–99.8] | 97.7 | [95.5–99.0] | 2193.7 | [1881.1–2558.1] | |||
| Post-B (Y1) | 200 | 89.0 | [83.8–93.0] | 54.5 | [47.3–61.5] | 123.0 | [98.9–153.0] | |||
| Post-B (Y2) | 219 | 67.1 | [60.5–73.3] | 39.3 | [32.8–46.1] | 48.0 | [36.8–62.6] | |||
| MenC-CRM197 + DTPa-IPV/Hib | Hib-MenC-TT + MMR | PD3 | 117 | 100 | [96.9–100] | 91.1 | [95.3–100] | 1002.6 | [833.8–1205.6] | |
| Pre-B | 109 | 67.9 | [58.3–76.5] | 33.0 | [24.3–42.7] | 38.6 | [27.5–54.2] | |||
| Post-B | 114 | 95.6 | [90.1–98.6] | 86.0 | [78.2–91.8] | 477.9 | [357.3–639.2] | |||
| Post-B (Y1) | 59 | 69.5 | [56.1–80.8] | 28.8 | [17.8–42.1] | 35.7 | [23.4–54.5] | |||
| Post-B (Y2) | 74 | 40.5 | [29.3–52.6] | 13.5 | [6.7–23.5] | 14.4 | [9.7–21.6] | |||
|
| ||||||||||
| Tejedor et al., 2007 & 2008 [62, 74] 2, 4, 6 + 13–14 months of age | Hib-MenC-TT + DTPa-HBV-IPV | Hib-MenC-TT | PD3 | 111 | 100 | [96.7–100] | 99.1 | 95.1–100 | 2467.1 | [2045.7–2975.3] |
| Pre-B | 81 | 96.3 | [89.6–99.2] | 84.0 | 74.1–91.2 | 366.1 | [270.1–496.2] | |||
| Post-B | 81 | 100 | [95.5–100] | 100 | [95.5–100] | 5266.2 | [4265.6–6501.3] | |||
| Post-B (Y1) | 56 | 87.5 | [75.9– 94.8] | — | — | 94.0 | [59.8–147.9] | |||
| MenC-TT + DTPa/Hib-containing | Hib-MenC-TT | PD2 | 107 | 100 | [96.6–100] | 98.1 | [93.4–99.8] | 1542.9 | [1282.2–1856.5] | |
| Pre-B | 169 | 90.5 | [85.1–94.5] | 59.8 | [52.0–67.2] | 131.0 | [103.5–165.6] | |||
| Post-B | 167 | 99.4 | [96.7–100] | 99.4 | [96.7–100] | 11710.0 | [9441.5–14524.8] | |||
| Post-B (Y1) | 121 | 95.0 | [89.5–98.2] | — | — | 293.8 | [212.8–405.7] | |||
| MenC-CRM197 + DTPa-HBV-IPV/Hib | DTPa-HBV-IPV/Hib | PD3 | 114 | 99.1 | [95.2–100] | 98.2 | 93.8–99.8 | 1833.7 | [1493.7–2251.0] | |
| Pre-B | 82 | 85.4 | [75.8–92.2] | 56.1 | 44.7–67.0 | 120.5 | [80.2–180.9] | |||
| Post-B | 84 | 77.4 | [67.0–85.8] | 56.0 | [44.7–66.8] | 94.1 | [59.6–148.7] | |||
|
| ||||||||||
| Carmona et al., 2010 [75] 2, 4, 6 + 13–14 months of age | MenC-CRM197 + DTPa/Hib-containing | Hib-MenC-TT + MMR | Pre-B* | 93 | 88.2 | [79.8–93.9] | 46.2 | [35.8–56.9] | 103.8 | [74.2–145.1] |
| Post-B | 95 | 98.9 | [94.3–100] | 89.5 | [81.5–94.8] | 670.2 | [497.9–902.1] | |||
| MenC-CRM197 + DTPa/Hib-containing | Hib-MenC-TT | Pre-B* | 94 | 85.1 | [76.3–91.6] | 56.4 | [45.8–66.6] | 107.1 | [74.1–154.7] | |
| Post-B | 95 | 98.9 | [94.3–100] | 92.6 | [85.4–97.0] | 685.0 | [527.0–890.4] | |||
|
| ||||||||||
| Habermehl et al., 2010 [68] 2, 3, 4 + 12–18 months | Hib-MenC-TT + DTPa-HBV-IPV | Hib-MenC-TT + DTPa-HBV-IPV | PD3 | 74 | 100 | [95.1–100] | 95.9 | [88.6–99.2] | 871.0 | [677.3–1120.0] |
| Pre-B* | 41 | 95.1 | [83.5–99.4] | 65.9 | [49.4–79.9] | 150.6 | [96.7–234.5] | |||
| Post-B | 41 | 100 | [91.4–100] | 97.6 | [87.1–99.9] | 3226.2 | [2294.7–4535.8] | |||
| MenC-CRM197 + DTPa-HBV-IPV/Hib | MenC-CRM197 + DTPa-HBV-IPV/Hib | PD3 | 71 | 100 | [94.9–100] | 100 | [94.9–100] | 3557.6 | [2978.8–4248.8] | |
| Pre-B* | 38 | 94.7 | [82.3–99.4] | 68.4 | [51.3–82.5] | 195.5 | [122.4–312.2] | |||
| Post-B | 39 | 100 | [91.0–100] | 100 | [91.0–100] | 11819.3 | [8458.6–16515.2] | |||
|
| ||||||||||
| Knuf et al., 2009 [71] 2, 4, 6 + 11–18 months of age | Hib-MenC-TT + PHiD-CV + DTPa-HBV-IPV | Hib-MenC-TT + PHiD-CV + DTPa-HBV-IPV | PD3 | 137 | 100 | [97.3–100] | 97.1 | [92.7–99.2] | 1590.9 | [1298.5–1949.1] |
| Post-B | 79 | 100 | [95.4–100] | 100 | [95.4–100] | 5099.2 | [3940.4–6598.9] | |||
| Hib-MenC-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenC-TT + 7vCRM + DTPa-HBV-IPV | PD3 | 120 | 99.2 | [95.4–100] | 95.0 | [89.4–98.1] | 1207.7 | [964.4–1512.3] | |
| Post-B | 76 | 100 | [95.3–100] | 97.4 | [90.8–99.7] | 3269.8 | [2489.7–4294.4] | |||
| MenC-TT + DTPa-HBV-IPV/Hib + PHiD-CV | MenC-TT + DTPa/Hib-containing + PHiD-CV | PD2 | 177 | 100 | [97.9–100] | 97.2 | [93.5–99.1] | 1474.2 | [1263.3–1 720.4] | |
| Post-B | 84 | 100 | [95.7–100] | 100 | [95.7–100] | 4587.8 | [3763.1–5593.2] | |||
| MenC-CRM197 + DTPa-HBV-IPV/Hib + PHiD-CV | MenC-CRM197 + DTPa/Hib-containing + PHiD-CV | PD2 | 165 | 98.8 | [95.7–99.9] | 97.0 | [93.1–99.0] | 1299.8 | [1082.0–1561.5] | |
| Post-B | 89 | 100 | [95.9–100] | 98.9 | [93.9–100] | 2779.6 | [2198.5–3514.2] | |||
|
| ||||||||||
| Booy et al., 2011 [72]12–18 months | 3xDTPa/Hib-TT or2xHib-OMP | Hib-MenC-TT + MMR | Pre-Vac | 255 | 14.5 | [10.4–19.4] | 5.9 | [3.3–9.5] | 6.3 | [5.5–7.3] |
| Post-Vac | 281 | 99.6 | [98.0–100] | 87.9 | [83.5–91.5] | 482.8 | [420.7–554.2] | |||
| Post-Vac (Y1) | 249 | 86.7 | [81.9–90.7] | 47.0 | [40.7–53.4] | 91.7 | [75.6–111.3] | |||
| 3xDTPa/Hib-TT or 2xHib-OMP | Hib-TT + MenC-CRM197 + MMR | Pre-Vac | 83 | 8.4 | [3.5–16.6] | 3.6 | [0.8–10.2] | 5.5 | [4.3–7.2] | |
| Post-Vac | 98 | 100 | [96.3–100] | 90.8 | [83.3–95.7] | 621.0 | [480.3–802.9] | |||
| Post-Vac (Y1) | 89 | 76.4 | [66.2–84.8] | 41.6 | [31.2–52.5] | 63.8 | [43.3–94.1] | |||
N: numbers of subjects with available data, %: percentage of subjects with titre within the specified range, 95% CI: 95% confidence interval, GMT: Geometric Mean Titre, PD2: one month after dose 2 (Primary ATP cohort for immunogenicity), PD3: one month after dose 3 (Primary ATP Cohort for Immunogenicity), Pre-Ch: just prior to meningococcal polysaccharide challenge (ATP Cohort for Persistence), Post-Ch: one month after meningococcal polysaccharide challenge (ATP Cohort for Immunogenicity), Pre-B: just prior to the booster vaccination (ATP Cohort for Persistence), Pre-B*: just prior to the booster vaccination (ATP Cohort for Immunogenicity), Post-B: one month after booster vaccination (Booster ATP Cohort for Immunogenicity), Post-B (Y1): one year after booster vaccination (ATP Cohort for Persistence Year 1), Post-B (Y2): two years after booster vaccination (ATP Cohort for Persistence Year 1), Pre-Vac: just prior to the vaccination (Vaccination ATP cohort for Immunogenicity), Post-Vac: one month after vaccination (Vaccination ATP cohort for Immunogenicity), Post-Vac (Y1): one year after vaccination (ATP cohort for Persistence Year 1), Results of this table were previously published [62, 68–76].
Moreover, primary vaccination with Hib-MenC-TT induced persistent antibodies against both antigens up to the second year of life [68, 70, 73, 74]. The immune response postvaccination and the persistence of anti-PRP antibodies up to the booster vaccination was higher following vaccination with Hib-MenC-TT than following vaccination with DTPa/Hib combined vaccines [73, 74]. Moreover, the persistence of rSBA-MenC antibodies, when measured before booster vaccination, was higher in subjects primed with Hib-MenC-TT than in subjects primed with MenC-CRM197 although lower than in subjects primed with MenC-TT [73, 74].
The booster dose administered in the second year of life resulted in very high seroprotection rates against both Hib and MenC [68, 71, 73–75] (Table 2). After the booster vaccination, the percentages of Hib-MenC-TT primed children with anti-PRP ≥1.0 μg/mL and rSBA-MenC titres ≥1 : 128 were 100% (data not shown) and 97.4–100% (Table 2), respectively. Long-term persistence of the immune response induced by the booster was observed up to two years after vaccination [76]. The totality of the clinical data collected demonstrated that Hib-MenC-TT can be used as booster dose for toddlers previously primed with MenC and Hib in infancy [72–74]. Of note, the noninferiority of Hib-MenC-TT to monovalent Hib and MenC conjugate vaccines was demonstrated when administered as a single dose in Hib-primed but MenC-naïve toddlers [72]. The evaluation of the persistence of the Hib and MenC immunogenicity after different vaccination schedules is ongoing for up to five years postbooster vaccination.
In addition, one booster dose of Hib-MenC-TT, given to children aged 6–12 years who were primed six years before with MenC-CRM197, was shown to be highly effective and rSBA-MenC antibody titres ≥1 : 8 were sustained for at least one year in 99.6% of the vaccines [77]. However, these results were somewhat contradictory with other studies showing reduced postbooster responses and antibody persistence when MenC-CRM197 was used for priming, regardless of whether a CRM197 or a TT conjugate vaccine was used for boosting [78, 79].
Notably, no evidence of negative interference on the immune response to any of the antigens was observed when the Hib-MenC-TT vaccine was coadministered with common childhood vaccines including combined measles, mumps, and rubella vaccine [MMR], combined diphtheria, tetanus, acellular pertussis, and inactivated polio vaccine [DTPa-IPV], a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus vaccine [DTPa-HBV-IPV], a 7-valent pneumococcal conjugate vaccine (7vCRM) and a 10-valent pneumococcal nontypeable H. influenzae protein D-conjugate vaccine [PHiD-CV] [62, 69, 71, 75, 80].
In reported studies, a total of 3127 children less than two years were vaccinated with Hib-MenC-TT combined vaccine. In primary and booster studies, Hib-MenC-TT was comparable to control monovalent Hib and MenC vaccines in term of local reactogenicity (pain, redness, and swelling) and general solicited symptoms (fever, irritability/fussiness, drowsiness, and loss of appetite), with some evidence of lower rates of fever. The clinical studies supported the acceptability of the safety profile of Hib-MenC-TT.
In the UK, Hib-MenC-TT has been used as booster vaccination for the Hib and MenC antigens in the second year of life (after monovalent MenC and DTPa/Hib priming in infants) since September 2006. The vaccine is currently approved in some EU countries, in Brazil, and in New Zealand for three-dose primary vaccination in infants from the age of two months up to 12 months and for booster vaccination in toddlers up to the age of two years. The vaccine is also approved in Australia for one-dose vaccination in toddlers previously primed with Hib but naïve for MenC [72].
In summary, Hib-MenC-TT was shown to be immunogenic with an acceptable safety profile when given to infants and toddlers. Primary vaccination resulted in immune priming and the induction of immune memory as reflected by antibody persistence and response to booster vaccination. The consistent observation of significantly higher anti-PRP GMCs versus control vaccines suggests that placing the Hib antigen in combination with meningococcal antigens can overcome the reduction in Hib immunogenicity noted in combination vaccines including Hib and acellular pertussis antigens [67].
2.3. Investigational Hib-MenCY-TT Combined Vaccine for Infants
Unlike in most other industrialised countries, MenY is also a major cause of meningococcal disease in the USA, where MenC and MenY together account for approximately two-thirds of invasive disease [10, 92]. In addition, this serogroup has more recently also been noticed in other regions, including Europe [30]. For this reason, GSK Biologicals is developing an investigational combination vaccine (Hib-MenCY-TT) designed to protect infants and toddlers against invasive diseases caused by MenC, MenY, and Hib. This vaccine has been investigated according to a four-dose immunisation series at 2, 4, 6, and 12–15 months or 2, 3, 4, and 12–18 months. The 2, 4, 6, and 12–15 months schedule was studied because this is the currently recommended Advisory Committee on Immunization Practices schedule for routine Hib conjugate in the USA. Serogroup Y has been included in the investigational combination vaccine because serogroup Y causes approximately 1/3 of invasive meningococcal disease in the USA [10].
A single dose of Hib-MenCY-TT contains 2.5 μg PRP from Hib and 5 μg each of PS from MenC and MenY, all conjugated to TT. In Phase II studies, Hib-MenCY-TT was shown to be immunogenic for all three antigens after three-dose vaccination at 2, 3, and 4 months [68] or 2, 4, and 6 months [81, 82, 84] of age and after a fourth dose at 12–18 months of age [68, 83, 84] (Tables 3 and 4). All primary hypotheses regarding the noninferiority of Hib-MenCY-TT to licensed Hib and monovalent MenC conjugate vaccines were met, and as with the Hib-MenC-TT vaccine, consistently higher anti-PRP concentrations were observed with Hib-MenCY-TT than with licensed Hib conjugate vaccines [68, 82, 84]. In addition, vaccination of infants with Hib-MenCY-TT induced an immune response against MenC and MenY that was significantly higher than that induced by one injection of a licensed tetravalent polysaccharide vaccine in older children [82]. The antibody persistence of the fourth dose was shown for at least one year [83].
Table 3.
rSBA-MenC, rSBA-MenY antibody responses after Hib-MenCY-TT vaccination.
| Reference | Vaccine dose 1–3 | Vaccine dose 4 | Timepoint | N | rSBA ≥ 1 : 8 | rSBA ≥ 1 : 128 | GMT | |||
|---|---|---|---|---|---|---|---|---|---|---|
| % | [95% CI] | % | [95% CI] | Value | [95% CI] | |||||
| rSBA-MenC | ||||||||||
|
| ||||||||||
| Habermehl et al., 2010 [68] 2, 3, 4 + 12–18 months of age | Hib-MenCY-TT + DTPa-HBV-IPV | Hib-MenCY-TT + DTPa-HBV-IPV | PD3 | 70 | 100 | [94.9–100] | 95.7 | [88.0–99.1] | 1005.8 | [773.5–1308.0] |
| Pre-D4 | 46 | 91.3 | [79.2–97.6] | 63.0 | [47.5–76.8] | 153.1 | [90.4–259.4] | |||
| PD4 | 40 | 100 | [91.2–100] | 100 | [91.2–100] | 4762.6 | [3427.5–6617.7] | |||
| MenC-CRM197 + DTPa-HBV-IPV/Hib | MenC-CRM197 + DTPa-HBV-IPV/Hib | PD3 | 71 | 100 | [94.9–100] | 100 | [94.9–100] | 3557.6 | [2978.8–4248.8] | |
| Pre-D4 | 42 | 95.2 | [83.8–99.4] | 69.0 | [52.9–82.4] | 199.2 | [129.6–306.2] | |||
| PD4 | 39 | 100 | [91.0–100] | 100 | [91.0–100] | 11819.3 | [8458.6–16515.2] | |||
|
| ||||||||||
| Nolan et al., 2007 [81] 2, 4, 6 + 11–14 months of age | Hib-MenCY-TT + DTPa-HBV-IPV | Men-PS Challenge | PD3 | 74* | 100 | [94.8–100] | 98.6 | [92.2–100] | 1293.1 | [1027.7–1627.1] |
| Pre-Ch | 80* | 97.4 | [90.8–99.7] | 78.9 | [68.1–87.5] | 265.5 | [197.8–356.3] | |||
| Post-Ch | 73* | 100 | [94.8–100] | 98.6 | [92.2–100] | 1985.5 | [1542.6–2555.8] | |||
| MenC-CRM197 + DTPa-HBV-IPV/Hib | Men-PS Challenge | PD3 | 77* | 100 | [95.1–100] | 98.6 | [92.7–100] | 1931.9 | [1541.2–2421.6] | |
| Pre-Ch | 81* | 90.8 | [81.9–96.2] | 64.5 | [52.7–75.1] | 176.6 | [117.9–264.4] | |||
| Post-Ch | 79* | 97.5 | [91.2–99.7] | 84.8 | [75.0–91.9] | 774.8 | [536.7–1118.5] | |||
|
| ||||||||||
| Nolan et al., 2011 [84] 2, 4, 6 + 12–15 months of age | Hib-MenCY-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + MMR + VAR | PD3 | 287 | 99.0 | [97.0–99.8] | 94.4 | [91.1–96.8] | 805 | [700–925] |
| Pre-D4 | 495 | 90.7 | [87.8–93.1] | 48.7 | [44.2–53.2] | 103 | [90.1–117] | |||
| PD4 | 496 | 99.6 | [98.6–100] | 97.2 | [95.3–98.4] | 1697 | [1516–1900] | |||
| MenC-CRM197 + Hib-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + MMR + VAR | PD3 | 99 | 100 | [96.3–100] | 96.0 | [90.0–98.9] | 790 | [649–961] | |
| Pre-D4 | 169 | 84.6 | [78.3–89.7] | 29.6 | [22.8–37.1] | 53.7 | [43.0–67.1] | |||
| PD4 | 162 | 95.7 | [91.3–98.2] | 71.0 | [63.3–77.8] | 262 | [204–336] | |||
|
| ||||||||||
| rSBA-MenY | ||||||||||
|
| ||||||||||
| Habermehl et al., 2010 [68] 2, 3, 4 + 12–18 months of age | Hib-MenCY-TT + DTPa-HBV-IPV | Hib-MenCY-TT + DTPa-HBV-IPV | PD3 | 69 | 97.1 | [89.9–99.6] | 92.8 | [83.9–97.6] | 470.7 | [351.1–631.2] |
| Pre-D4 | 45 | 86.7 | [73.2–94.9] | 60.0 | [44.3–74.3] | 103.2 | [64.6–164.9] | |||
| PD4 | 40 | 100 | [91.2–100] | 100 | [91.2–100] | 1708.1 | [1313.3–2221.4] | |||
|
| ||||||||||
|
Nolan et al., 2007 [81] 2, 4, 6 + 11–14 months of age |
Hib-MenCY-TT + DTPa-HBV-IPV | Men-PS Challenge | PD3 | 74* | 98.5 | [92.0–100] | 95.5 | [87.5–99.1] | 843.5 | [640.1–1111.7] |
| Pre-Ch | 80* | 89.2 | [79.8–95.2] | 60.8 | [48.8–72.0] | 114.7 | [80.0–164.4] | |||
| Post-Ch | 73* | 100 | [94.7–100] | 100 | [94.7–100] | 1838.0 | [1427.9–2366.0] | |||
|
| ||||||||||
|
Nolan et al., 2011 [84] 2, 4, 6 + 12–15 months of age |
Hib-MenCY-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + MMR + VAR | PD3 | 288 | 99.7 | [98.1–100] | 91.7 | [87.9–94.6] | 728 | [636–835] |
| Pre-D4 | 514 | 98.4 | [97.0–99.3] | 80.5 | [76.9–83.9] | 264 | [241–290] | |||
| PD4 | 496 | 100 | [99.3–100] | 99.4 | [98.2–99.9] | 1987 | [1826–2161] | |||
N: numbers of subjects with available data, *N: numbers of subjects in the ATP Cohort, %: percentage of subjects with titre within the specified range, 95% CI: 95% confidence interval, GMT: Geometric Mean Titre, PD3: one month after dose 3 (ATP Cohort for Immunogenicity), Pre-D4: just prior dose 4 (ATP Cohort for Persistence), PD4: one month after dose 4 (ATP Cohort for Immunogenicity), Pre-Ch: just prior to meningococcal polysaccharide challenge (ATP Cohort for safety challenge phase), Post-Ch: one month after meningococcal polysaccharide challenge (ATP Cohort for Immunogenicity), Results of this table were previously published [68, 81, 84].
Table 4.
hSBA-MenC, hSBA-MenY antibody responses after Hib-MenCY-TT vaccination.
| Reference | Vaccine dose 1–3 | Vaccine dose 4 | Timepoint | N | hSBA ≥ 1 : 4 | hSBA ≥ 1 : 8 | GMT | |||
|---|---|---|---|---|---|---|---|---|---|---|
| % | [95% CI] | % | [95% CI] | Value | [95% CI] | |||||
| hSBA-MenC | ||||||||||
|
| ||||||||||
| Marchant et al., 2010 [82] & Marshall et al., 2010 [83] 2, 4, 6 + 12–15 months of age | Hib-MenCY-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + 7vCRM | PD3 | 121 | 97.5 | [92.9–99.5] | 95.9 | [90.6–98.6] | 523.0 | [398.8–686.0] |
| Pre-D4 | 63 | 90.5 | [80.4–96.4] | 90.5 | [80.4–96.4] | 71.8 | [48.0–107.5] | |||
| PD4 | 65 | 96.9 | [89.3–99.6] | 96.9 | [89.3–99.6] | 657.1 | [438.4–984.8] | |||
| PD4 (Y1) | 116 | 96.6 | [91.4–99.1] | 96.6 | [91.4–99.1] | 150.1 | [116.5–193.4] | |||
| Hib-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + 7vCRM | PD4 | 35 | 94.3 | [80.8–99.3] | 94.3 | [80.8–99.3] | 72.5 | [46.4–113.3] | |
| PD4 (Y1) | 48 | 70.8 | [55.9–83.0] | 70.8 | [55.9–83.0] | 26.3 | [14.8–46.5] | |||
|
| ||||||||||
| Nolan et al., 2011 [84] 2, 4, 6 + 12–15 months of age | Hib-MenCY-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + MMR + VAR | PD3 | 83 | 100 | [95.7–100] | 100 | [95.7–100] | 379 | [295–489] |
| Pre-D4 | 134 | 93.3 | [87.6–96.9] | 93.3 | [87.6–96.9] | 97.7 | [74.0–129] | |||
| PD4 | 132 | 99.2 | [95.9–100] | 99.2 | [95.9–100] | 1808 | [1398–2337] | |||
| MenC-CRM197 + Hib-TT + 7vCRM DTPa-HBV-IPV |
Hib-MenCY-TT + MMR + VAR | PD3 | 28 | 100 | [87.7–100] | 100 | [87.7–100] | 254 | [193–335] | |
| Pre-D4 | 54 | 85.2 | [72.9–93.4] | 85.2 | [72.9–93.4] | 34.6 | [22.8–52.6] | |||
| PD4 | 43 | 95.3 | [84.2–99.4] | 95.3 | [84.2–99.4] | 172 | [94.7–311] | |||
|
| ||||||||||
| Bryant et al., 2011 [85] 2, 4, 6 + 12–15 months of age | Hib-MenCY-TT + DTPa-HBV-IPV + 7vCRM (Mexico) | Hib-MenCY-TT + MMR + VAR | PD3 | 134 | 99.3 | [95.9–100] | 99.3 | [95.9–100] | 3172.6 | [2657.9–3786.8] |
| PD4 | 39 | 100 | [91.0–100] | 100 | [91.0–100] | 10132.9 | [8008.0–12821.7] | |||
| Hib-MenCY-TT + DTPa-HBV-IPV + 7vCRM (US) | Hib-MenCY-TT + MMR + VAR | PD3 | 491 | 98.8 | [97.4–99.6] | 98.8 | [97.4–99.6] | 967.6 | [864.0–1083.5] | |
| Pre-D4 | 420 | 96.0 | [93.6–97.6] | 96.0 | [93.6–97.6] | 176.5 | [153.9–202.4] | |||
| PD4 | 331 | 98.5 | [96.5– 99.5] | 98.5 | [96.5– 99.5] | 2039.8 | [1746.3–2382.6] | |||
|
| ||||||||||
| hSBA-MenY | ||||||||||
|
| ||||||||||
| Marchant et al., 2010 [82] & Marshall et al., 2010 [83] 2, 4, 6 + 12–15 months of age | Hib-MenCY-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + 7vCRM | PD3 | 141 | 91.5 | [85.6–95.5] | 89.4 | [83.1–93.9] | 139.8 | [102.6–190.5] |
| Pre-D4 | 66 | 54.5 | [41.8–66.9] | 50.0 | [37.4–62.6] | 12.2 | [7.7–19.1] | |||
| PD4 | 65 | 95.4 | [87.1–99.0] | 95.4 | [87.1–99.0] | 246.6 | [168.0–362.1] | |||
| PD4 (Y1) | 105 | 84.8 | [76.4–91.0] | 83.8 | [75.3–90.3] | 128.8 | [86.2–192.5] | |||
| Hib-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + 7vCRM | PD4 | 35 | 60.0 | [42.1–76.1] | 57.1 | [39.4–73.7] | 11.1 | [6.5–19.0] | |
| PD4 (Y1) | 47 | 66.0 | [50.7–79.1] | 66.0 | [50.7–79.1] | 41.1 | [20.4–82.9] | |||
|
| ||||||||||
| Nolan et al., 2011 [84] 2, 4, 6 + 12–15 months of age | Hib-MenCY-TT + 7vCRM + DTPa-HBV-IPV | Hib-MenCY-TT + MMR + VAR | PD3 | 85 | 100 | [95.8–100] | 100 | [95.8–100] | 86.4 | [68.9–108.3] |
| Pre-D4 | 114 | 91.2 | [84.5–95.7] | 90.4 | [83.4–95.1] | 81.2 | [58.6–112.6] | |||
| PD4 | 122 | 97.5 | [93.0–99.5] | 97.5 | [93.0–99.5] | 1002 | [741–1356] | |||
|
| ||||||||||
| Bryant et al., 2011 [85] 2, 4, 6 + 12–15 months of age | Hib-MenCY-TT + DTPa-HBV-IPV + 7vCRM (Mexico) | Hib-MenCY-TT + MMR + VAR | PD3 | 135 | 99.3 | [95.9–100] | 99.3 | [95.9–100] | 837.2 | [696.4–1006.3] |
| PD4 | 40 | 100 | [91.2–100] | 100 | [91.2–100] | 5775.8 | [4488.9–7431.7] | |||
| Hib-MenCY-TT + DTPa-HBV-IPV + 7vCRM (US) | Hib-MenCY-TT + MMR + VAR | PD3 | 481 | 96.3 | [94.2–97.8] | 95.8 | [93.7–97.4] | 236.6 | [205.7–272.1] | |
| Pre-D4 | 419 | 93.6 | [90.8–95.7] | 92.8 | [89.9–95.1] | 117.5 | [101.3–136.2] | |||
| PD4 | 342 | 98.8 | [97.0–99.7] | 98.8 | [97.0–99.7] | 1389.5 | [1205.0–1602.2] | |||
N: numbers of subjects with available data, 95% CI: 95% confidence interval, GMT: Geometric Mean Titre, %: percentage of subjects with titre within the specified range, PD3: one month after dose 3 (ATP Cohort for Immunogenicity), Pre-D4: just prior dose 4 (ATP Cohort for Persistence), PD4: one month after dose 4 (ATP Cohort for Immunogenicity), PD4 (Y1): one year after dose 4 (ATP cohort for Persistence Year 1), Results of this table were previously published [82–85].
More recently, a Phase III study conducted in the USA, Mexico, and Australia confirmed that Hib-MenCY-TT was highly immunogenic to the meningococcal antigens and that the immune response induced by this vaccine in terms of anti-PRP concentrations was noninferior to that induced by licensed Hib vaccines when used according to the 2, 4, 6, and 12–15 months schedule [85].
Clinical data for the investigational Hib-MenCY-TT vaccine did not indicate interference with common childhood vaccines including DTPa-HBV-IPV, 7vCRM, MMR, and a varicella vaccine [81, 82]. The lack of immune interference with these antigens that are established in paediatric vaccination schedules is critical for the successful incorporation of the novel antigens.
The reactogenicity and safety profile of Hib-MenCY-TT was shown to be clinically acceptable in infants and toddlers who received four consecutive doses of this vaccine coadministered with routinely recommended paediatric vaccines. The reactogenicity profile of Hib-MenCY-TT was similar to that of Hib-TT, Hib-OMP and MenC-CRM197 and the incidence of grade 3 symptoms was low in all the studies [68, 81–85].
In summary, Hib-MenCY-TT was shown to be immunogenic to the Hib and the meningococcal serogroup C and Y components when given to infants and toddlers as four-dose immunisation series. Hib-MenCY-TT was noninferior to licensed monovalent control vaccines and demonstrated a clinically acceptable safety profile. An application for licensure of this vaccine was submitted to the US Food and Drug Administration (FDA) on August 12, 2009 and is currently under review. Hib-MenCY-TT is not currently licensed in any country.
2.4. Investigational MenACWY-TT Conjugate Vaccine for All Age Groups
Besides infants and young children, adolescents are the second key age group at increased risk for meningococcal disease. Accordingly, GSK Biologicals is developing a new investigational tetravalent meningococcal conjugate vaccine with PS from serogroups A, C, W-135, and Y conjugated to TT (MenACWY-TT) to complement the two other tetravalent conjugate vaccines that are currently available. Indeed, as previously discussed, primary vaccination with a MenC-TT vaccine has been demonstrated to result in higher rSBA-MenC titres as compared to priming with either of the two monovalent MenC conjugate vaccines that use CRM197 as carrier protein, translating into higher persistence up to one year after booster, regardless of the booster vaccine used [78, 79, 93]. Unlike Hib-MenC-TT and Hib-MenCY-TT, MenACWY-TT is not only intended for vaccination of infants and toddlers and therefore Hib was not included as a component of the vaccine. Although the MenACWY-TT vaccine was developed with a focus on adolescents, the investigational vaccine is being evaluated in various age groups including infants (NCT01144663).
Phase II and phase III studies showed that a single dose of MenACWY-TT was highly immunogenic in toddlers, children, adolescents and adults at one month following vaccination (Tables 5, 6 and 7). The observed rSBA and hSBA GMTs against MenC were significantly higher following MenACWY-TT vaccination in toddlers in the second year of life when compared to vaccination with a licensed serogroup C conjugate vaccine (MenC-CRM197) [87, 90, 91]. Recent studies have shown that MenACWY-TT did not interfere with DTPa-HBV-IPV/Hib or a combined MMR and varicella vaccine (MMRV) in toddlers, nor did it affect the safety profile of these vaccines [90, 91]. In children [87], adolescents [88], and young adults [86], exploratory analyses showed that MenACWY-TT induced statistically significantly higher rSBA GMTs for all serogroups compared to a licensed tetravalent plain PS vaccine. In the study conducted in subjects aged 11–17 years, noninferiority of MenACWY-TT to the licensed tetravalent PS vaccine was demonstrated in terms of percentage of subjects with a vaccine response [88]. In addition, a recent study showed that the proportion of subjects with a vaccine response and the hSBA GMTs were higher after vaccination with MenACWY-TT than with a licensed tetravalent diphtheria toxoid conjugate vaccine (MenACWY-DT) (Figure 1) [89].
Table 5.
rSBA-MenA, rSBA-MenC, rSBA-MenW-135, and rSBA-MenY antibody response one month [87, 91] or 42 days [90] after one dose of MenACWY-TT in toddlers (ATP immunogenicity cohort).
| Reference | Age group | Vaccine | N | %≥1 : 8 [95% CI] | %≥1 : 128 [95% CI] | GMT [95% CI] |
|---|---|---|---|---|---|---|
| rSBA-MenA | ||||||
|
| ||||||
| Knuf et al., 2010 [87] | 12–14 months | MenACWY-TT | 42 | 100 [91.6–100] | 100 [91.6–100] | 5665.2 [4086.0–7854.9] |
| Vesikari et al., 2011 [90] | 12–23 months | MenACWY-TT | 354 | 99.7 [98.4–100] | 99.7 [98.4–100] | 2205.0 [2007.8–2421.6] |
| MenACWY-TT + MMRV | 360 | 100 [99.0–100] | 99.7 [98.5–100] | 2085.9 [1905.3–2283.6] | ||
| Knuf et al., 2011 [91] | 12–23 months | MenACWY-TT | 183 | 98.4 [95.3–99.7] | 97.8 [94.5–99.4] | 3169.9 [2577.2–3898.8] |
| MenACWY-TT* | 178 | 100 [97.9–100] | 100 [97.9–100] | 1938.3 [1699.1–2211.2] | ||
| MenACWY-TT + DTPa-HBV-IPV/Hib | 193 | 100 [98.1–100] | 100 [98.1–100] | 3152.9 [2752.5–3611.4] | ||
|
| ||||||
| rSBA-MenC | ||||||
|
| ||||||
| Knuf et al., 2010 [87] | 12–14 months | MenACWY-TT | 42 | 100 [91.6–100] | 95.2 [83.8–99.4] | 983.5 [718.7–1345.9] |
| MenC-CRM197 | 46 | 97.8 [88.5–100] | 87.0 [73.7–95.1] | 372.5 [257.7–538.4] | ||
| Vesikari et al., 2011 [90] | 12–23 months | MenACWY-TT | 354 | 99.7 [98.4–100] | 95.8 [93.1–97.6] | 477.6 [437.3–521.6] |
| MenACWY-TT + MMRV | 357 | 100 [99.0–100] | 94.4 [91.5–96.5] | 519.0 [470.9–571.9] | ||
| MenC-CRM197 | 121 | 97.5 [92.9–99.5] | 70.2 [61.3–78.2] | 212.3 [170.0–265.2] | ||
| Knuf et al., 2011 [91] | 12–23 months | MenACWY-TT | 183 | 97.3 [93.7–99.1] | 94.0 [89.5–97.0] | 828.7 [672.4–1021.4] |
| MenACWY-TT* | 178 | 100 [97.9–100] | 88.2 [82.5–92.5] | 386.0 [333.9–446.2] | ||
| MenACWY-TT + DTPa-HBV-IPV/Hib | 191 | 100 [98.1–100] | 99.0 [96.3–99.9] | 879.7 [763.1–1014.0] | ||
| MenC-CRM197 | 114 | 98.2 [93.8–99.8] | 89.5 [82.3–94.4] | 691.4 [520.8–917.9] | ||
|
| ||||||
| rSBA-MenW-135 | ||||||
|
| ||||||
| Knuf et al., 2010 [87] | 12–14 months | MenACWY-TT | 43 | 100 [91.8–100] | 100 [91.8–100] | 3975.2 [3065.7–5154.5] |
| Vesikari et al., 2011 [90] | 12–23 months | MenACWY-TT | 354 | 100 [99.0–100] | 99.4 [98.0–99.9] | 2681.7 [2453.1–2931.6] |
| MenACWY-TT + MMRV | 360 | 100 [99.0–100] | 100 [99.0–100] | 2055.8 [1871.0–2258.9] | ||
| Knuf et al., 2011 [91] | 12–23 months | MenACWY-TT | 186 | 98.4 [95.4–99.7] | 96.8 [93.1–98.8] | 4022.3 [3269.2–4948.8] |
| MenACWY-TT* | 179 | 100 [98.0–100] | 99.4 [96.9–100] | 2466.4 [2175.4–2796.4] | ||
| MenACWY-TT + DTPa-HBV-IPV/Hib | 193 | 100 [98.1–100] | 100 [98.1–100] | 4147.0 [3670.1–4685.8] | ||
|
| ||||||
| rSBA-MenY | ||||||
|
| ||||||
| Knuf et al., 2010 [87] | 12–14 months | MenACWY-TT | 42 | 100 [91.6–100] | 100 [91.6–100] | 2295.1 [1701.5–3095.8] |
| Vesikari et al., 2011 [90] | 12–23 months | MenACWY-TT | 354 | 100 [99.0–100] | 99.7 [98.4–100] | 2729.4 [2472.7–3012.8] |
| MenACWY-TT + MMRV | 359 | 100 [99.0–100] | 99.7 [98.5–100] | 2282.4 [2051.3–2539.5] | ||
| Knuf et al., 2011 [91] | 12–23 months | MenACWY-TT | 185 | 97.3 [93.8–99.1] | 96.2 [92.4–98.5] | 3167.7 [2521.9–3978.9] |
| MenACWY-TT* | 179 | 99.4 [96.9–100] | 99.4 [96.9–100] | 2446.9 [2088.5–2866.8] | ||
| MenACWY-TT + DTPa-HBV-IPV/Hib | 192 | 100 [98.1–100] | 100 [98.1–100] | 3461.8 [2990.1–4007.9] | ||
Table 6.
rSBA-MenA, rSBA-MenC, rSBA-MenW-135, and rSBA-MenY antibody response one month after MenACWY-TT vaccination in children and adolescents (ATP immunogenicity cohort).
| Reference | Age group | Vaccine | N | VRR | [95% CI] | N | GMT | [95% CI] |
|---|---|---|---|---|---|---|---|---|
| rSBA-MenA | ||||||||
|
| ||||||||
| Østergaard et al., 2009 [86] | 15–19 years | MenACWY-TT | 23 | 87.0 | [66.4–97.2] | 24 | 9264 | [6342–13532] |
| ACWY-PS | 23 | 78.3 | [56.3–92.5] | 25 | 8284 | [6035–11370] | ||
| Knuf et al., 2010 [87] | 3–5 years | MenACWY-TT | 40 | 92.5 | [79.6–98.4] | 49 | 8299.4 | [6734.2–10228.4] |
| ACWY-PS | 31 | 80.6 | [62.5–92.5] | 33 | 3798.4 | [2888.9–4994.2] | ||
| Bermal et al., 2011 [88] | 11–17 years | MenACWY-TT | 615 | 85.4 | [82.3–88.1] | 752 | 6106.8 | [5739.5–6497.6] |
| ACWY-PS | 215 | 79.5 | [73.5–84.7] | 252 | 3203.0 | [2854.1–3594.6] | ||
|
| ||||||||
| rSBA-MenC | ||||||||
|
| ||||||||
| Østergaard et al., 2009 [86] | 15–19 years | MenACWY-TT | 24 | 95.8 | [78.9–99.9] | 24 | 4329 | [2404–7795] |
| ACWY-PS | 24 | 91.7 | [73.0–99.0] | 25 | 1567 | [757–3245] | ||
| Knuf et al., 2010 [87] | 3–5 years | MenACWY-TT | 47 | 93.6 | [82.5–98.7] | 49 | 1577.8 | [1123.5–2215.9] |
| ACWY-PS | 32 | 81.3 | [63.6–92.8] | 34 | 445.4 | [263.3–753.3] | ||
| Bermal et al., 2011 [88] | 11–17 years | MenACWY-TT | 719 | 97.1 | [95.6–98.2] | 754 | 12645.5 | [11531.8–13866.7] |
| ACWY-PS | 237 | 96.6 | [93.5–98.5] | 252 | 8271.6 | [6937.3–9862.4] | ||
|
| ||||||||
| rSBA-MenW-135 | ||||||||
|
| ||||||||
| Østergaard et al., 2009 [86] | 15–19 years | MenACWY-TT | 23 | 91.3 | [72.0–98.9] | 24 | 4422 | [2939–6653] |
| ACWY-PS | 25 | 96.0 | [79.6–99.9] | 25 | 3486 | [2447–4967] | ||
| Knuf et al., 2010 [87] | 3–5 years | MenACWY-TT | 47 | 100 | [92.5–100] | 49 | 5987.2 | [4846.9–7395.7] |
| ACWY-PS | 32 | 96.9 | [83.8–99.9] | 34 | 1811.1 | [1233.3–2659.5] | ||
| Bermal et al., 2011 [88] | 11–17 years | MenACWY-TT | 717 | 96.5 | [94.9–97.7] | 759 | 8390.1 | [7777.8–9050.7] |
| CWY-PS | 242 | 88.0 | [83.2–91.8] | 252 | 2679.3 | [2363.7–3037.2] | ||
|
| ||||||||
| rSBA-MenY | ||||||||
|
| ||||||||
| Østergaard et al., 2009 [86] | 15–19 years | MenACWY-TT | 24 | 79.2 | [57.8–92.9] | 24 | 2756 | [2023–3755] |
| ACWY-PS | 25 | 88.0 | [68.8–97.5] | 25 | 3056 | [1892–4937] | ||
| Knuf et al., 2010 [87] | 3–5 years | MenACWY-TT | 48 | 100 | [92.6–100] | 49 | 6433.0 | [4921.0–8409.6] |
| ACWY-PS | 34 | 79.4 | [62.1–91.3] | 34 | 1435.5 | [972.6–2118.7] | ||
| Bermal et al., 2011 [88] | 11–17 years | MenACWY-TT | 737 | 93.1 | [91.0–94.8] | 758 | 13865.2 | [12968.1–14824.4] |
| ACWY-PS | 246 | 78.0 | [72.3–83.1] | 252 | 5245.3 | [4644.2–5924.1] | ||
N: numbers of subjects with available data, 95% CI: 95% confidence interval, GMT: geometric mean titre, VRR: vaccine response rate, Vaccine response defined as: (1) for initially seronegative subjects, antibody titres ≥1 : 32 at one month after vaccination, (2) for initially seropositive subjects, antibody titres at one month after vaccination ≥4-fold the pre-vaccination antibody titre, Results of this table were previously published [86–88].
Table 7.
Percentage of subjects with hSBA titres ≥1 : 8 and hSBA GMTs against serogroups A, C, W-135, and Y at 42 days after one dose of MenACWY-TT in toddlers (ATP immunogenicity cohort).
| Vaccine | N | % ≥ 1 : 8 [95% CI] | GMT [95% CI] |
|---|---|---|---|
| hSBA-MenA | |||
|
| |||
| MenACWY-TT | 338 | 77.2 [72.4–81.6] | 19.0 [16.4–22.1] |
| MenACWY-TT + MMRV | 348 | 83.9 [79.6–87.6] | 33.7 [28.9–39.2] |
|
| |||
| hSBA-MenC | |||
|
| |||
| MenACWY-TT | 341 | 98.5 [96.6–99.5] | 196.0 [175.4–219.0] |
| MenACWY-TT + MMRV | 346 | 98.0 [95.9–99.2] | 209.1 [183.8–238.0] |
| MenC-CRM197 | 116 | 81.9 [73.7–88.4] | 40.3 [29.5–55.1] |
|
| |||
| hSBA-MenW-135 | |||
|
| |||
| MenACWY-TT | 336 | 87.5 [83.5–90.8] | 48.9 [41.2–58.0] |
| MenACWY-TT + MMRV | 337 | 82.8 [78.3–86.7] | 57.3 [47.0–69.9] |
|
| |||
| hSBA-MenY | |||
|
| |||
| MenACWY-TT | 329 | 79.3 [74.5–83.6] | 30.9 [25.8–37.1] |
| MenACWY-TT + MMRV | 333 | 81.4 [76.8–85.4] | 38.7 [32.2–46.7] |
N: numbers of subjects with available data, %: percentage of subjects with titre within the specified range, 95% CI: 95% confidence interval, GMT: Geometric Mean Titre, Results of this table were previously published [90].
Figure 1.
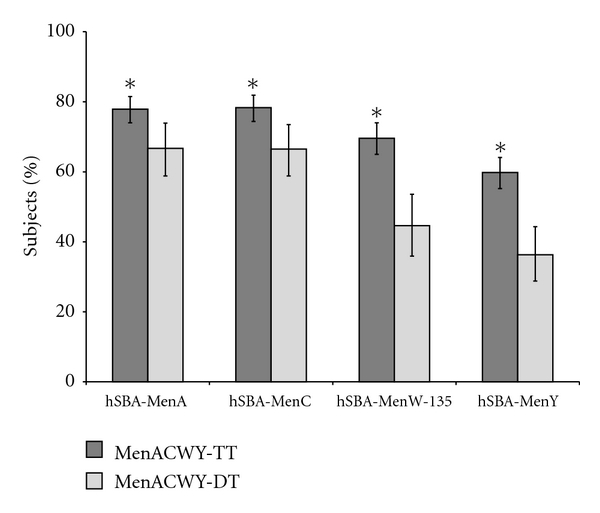
Vaccine response for hSBA antibodies one month after vaccination with MenACWY-TT or with a control vaccine in subjects aged 11–25 years (ATP cohort for immunogenicity). (MenACWY-TT: subjects vaccinated with MenACWY-TT, MenACWY-DT: subjects vaccinated with MenACWY-DT, vaccine response defined as (1) for initially seronegative subjects, antibody titres ≥1 : 16 at one month after vaccination, (2) for initially seropositive subjects, antibody titres at one month after vaccination ≥4-fold the prevaccination antibody titre. Error bars represent the 95% confidence interval. *Statistically significantly higher value in the ACWY-TT group compared to the ACWY-DT group (exploratory analysis). Results of this figure are currently in publication [89].)
Overall, MenACWY-TT was shown to have increased reactogenicity as compared to plain PS vaccines in children, adolescents, and adults and to have a similar reactogenicity profile compared to conjugate vaccines: MenC-CRM197 in toddlers younger than two years and MenACWY-DT in adolescents [86–91]. These results were expected as conjugate vaccines are known to be more reactogenic than PS vaccines and similar findings were observed with a PS vaccine compared to MenACWY-DT and MenACWY-CRM197 [94, 95]. Given the relatively infrequent reports of grade 3 symptoms (≤9.3% of subjects [87]), the MenACWY-TT vaccine was considered to have an acceptable safety profile.
In summary, a single dose of MenACWY-TT was shown to be highly immunogenic for serogroups A, C, W-135, and Y in toddlers, children, adolescents, and young adults and had a clinically acceptable safety profile. A Marketing Authorization Application was submitted to the European Medicines Agency (EMA) on March 4, 2011 and is currently under review. MenACWY-TT is not currently licensed in any country.
3. Conclusions and Perspectives
Because of the severity of invasive meningococcal disease, the rapidity with which it develops, and the high frequency of long-term sequelae, development of effective meningococcal vaccines with clinically acceptable safety profiles is a public health priority. The complete portfolio of conjugate meningococcal vaccines developed by GSK Biologicals is designed to respond to the global need with prioritisation in infants and young children, followed by adolescents, the age groups at highest risk. Two vaccines, using TT as carrier protein, were designed to help protect infants and young children against meningococcal serogroup(s) C (and Y) and Hib diseases in countries where these serogroups are major contributors to meningococcal epidemiology. The first vaccine (Hib-MenC-TT) is presently approved in various countries worldwide for use as three-dose primary vaccination in infants and booster vaccination in toddlers or as one-dose vaccination in toddlers previously primed with Hib but naïve for MenC. Primary vaccination resulted in immune priming and induced immune memory as reflected by antibody persistence and response to booster vaccination. The second vaccine (Hib-MenCY-TT) adds serogroup Y antigen and an application for licensure of this vaccine as four-dose immunisation series in infants and toddlers is currently under review by the US FDA. All primary hypotheses regarding the noninferiority of both vaccines to licensed Hib (DTPa/Hib combinations or Hib standalone vaccines) and monovalent MenC conjugate vaccines were met. On the other hand, GSK Biologicals has more recently evaluated an investigational tetravalent conjugate meningococcal vaccine (MenACWY-TT) designed to offer protection against four of the six most common serogroups worldwide for use in all age groups. This vaccine was shown to be highly immunogenic in toddlers, children, adolescents, and adults. All these vaccines were shown to have an acceptable safety profile, which was comparable to licensed conjugate vaccines. As demonstrated by the broad portfolio of meningococcal conjugate vaccines, GSK remains committed to the development of meningococcal vaccines, targeting the populations at greatest risk.
Conflict of Interests
J. Miller, N. Mesaros, M. V. D. Wielen, and Y. Baine are employees of GlaxoSmithKline Biologicals. Drs M. Miller, N. Mesaros, M. V. D. Wielen, and Y. Baine report ownership of stock options.
Acknowledgments
The authors are grateful to the primary investigators of the clinical studies, as well as the study volunteers and their parents, and the contributing clinicians, nurses, and laboratory technicians. GSK Biologicals was the funding source and took responsibility for all costs associated with the development and publication of the present paper. The authors are grateful to all teams of GSK Biologicals for their contribution to this paper, especially Dr. Dominique Boutriau and Dr. Leonard Friedland for expert clinical input, Dr. Jan Poolman for expert input in meningococcal disease, Dr. Emmanuel Aris, Veronique Bianco, Magalie Caubet, and Laurence Fissette for performing the statistical analyses, and Dr. Pascal Lestrate for the conduct of the immunological assays. They thank Claire Verbelen (XPE Pharma & Science) who provided medical writing services and Nancy Van Driessche and Stephanie Harbers (XPE Pharma & Science) for editorial assistance and paper coordination. Mencevax and Menitorix are trademarks of the GlaxoSmithKline Group of Companies. MenAfriVac is a trademark of the Serum Institute of India, Limited (SIIL). All studies were funded by GlaxoSmithKline Biologicals.
References
- 1.Stephens DS. Conquering the meningococcus. FEMS Microbiology Reviews. 2007;31(1):3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 2.Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Review of Vaccines. 2010;9(3):285–298. doi: 10.1586/erv.10.3. [DOI] [PubMed] [Google Scholar]
- 3.Girard MP, Preziosi MP, Aguado MT, Kieny MP. A review of vaccine research and development: meningococcal disease. Vaccine. 2006;24(22):4692–4700. doi: 10.1016/j.vaccine.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Brooks R, Woods CW, Benjamin DK, Rosenstein NE. Increased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994-2002. Clinical Infectious Diseases. 2006;43(1):49–54. doi: 10.1086/504804. [DOI] [PubMed] [Google Scholar]
- 5.Leake JAD, Kone ML, Yada AA, et al. Early detection and response to meningococcal disease epidemics in sub-Saharan Africa: appraisal of the WHO strategy. Bulletin of the World Health Organization. 2002;80(5):342–349. [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Control of Epidemic Meningococcal Disease. WHO Practical Guidelines. 2nd edition. WHO/EMC/BAC/98.3; 1998. [Google Scholar]
- 7.Al-Tawfiq JA, Clark TA, Memish ZA. Meningococcal disease: the organism, clinical presentation, and worldwide epidemiology. Journal of Travel Medicine. 2010;17, supplement 3–8 doi: 10.1111/j.1708-8305.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- 8.Memish ZA. Meningococcal disease and travel. Clinical Infectious Diseases. 2002;34(1):84–90. doi: 10.1086/323403. [DOI] [PubMed] [Google Scholar]
- 9.Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. Anais da Academia Brasileira de Ciencias. 2005;77(2):293–324. doi: 10.1590/s0001-37652005000200009. [DOI] [PubMed] [Google Scholar]
- 10.Cohn AC, MacNeil JR, Harrison LH, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clinical Infectious Diseases. 2010;50(2):184–191. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 11.Healy CM, Butler KM, Smith EOB, et al. Influence of serogroup on the presentation, course, and outcome of invasive meningococcal disease in children in the Republic of Ireland, 1995–2000. Clinical Infectious Diseases. 2002;34(10):1323–1330. doi: 10.1086/340050. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992–1996. Journal of Infectious Diseases. 1999;180(6):1894–1901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 13.Edwards MS, Baker CJ. Complications and sequelae of meningococcal infections in children. Journal of Pediatrics. 1981;99(4):540–545. doi: 10.1016/s0022-3476(81)80250-8. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. New England Journal of Medicine. 2001;344(18):1378–1388. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 15.Harrison LH, Pass MA, Mendelsohn AB, et al. Invasive meningococcal disease in adolescents and young adults. Journal of the American Medical Association. 2001;286(6):694–699. doi: 10.1001/jama.286.6.694. [DOI] [PubMed] [Google Scholar]
- 16.van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clinical Microbiology Reviews. 2000;13(1):144–166. doi: 10.1128/cmr.13.1.144-166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clinical Infectious Diseases. 2010;50(2):S37–S44. doi: 10.1086/648963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausdorff WP, Hajjeh R, Al-Mazrou A, Shibl A, Soriano-Gabarro M. The epidemiology of pneumococcal, meningococcal, and Haemophilus disease in the Middle East and North Africa (MENA) region–current status and needs. Vaccine. 2007;25(11):1935–1944. doi: 10.1016/j.vaccine.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27, supplement 2:B51–B63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 20.Greenwood B. Manson lecture. Meningococcal meningitis in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93(4):341–353. doi: 10.1016/s0035-9203(99)90106-2. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood B. Editorial: 100 Years of epidemic meningitis in West Africa—has anything changed? Tropical Medicine and International Health. 2006;11(6):773–780. doi: 10.1111/j.1365-3156.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 22.Lingappa JR, Al-Rabeah AM, Hajjeh R, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerging Infectious Diseases. 2003;9(6):665–671. doi: 10.3201/eid0906.020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traoré Y, Njanpop-Lafourcade BM, Adjogble KLS, et al. The rise and fall of epidemic Neisseria meningitidis serogroup W135 meningitis in Burkina Faso, 2002-2005. Clinical Infectious Diseases. 2006;43(7):817–822. doi: 10.1086/507339. [DOI] [PubMed] [Google Scholar]
- 24.Boisier P, Nicolas P, Djibo S, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clinical Infectious Diseases. 2007;44(5):657–663. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 25.Gagneux SP, Hodgson A, Smith TA, et al. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. Journal of Infectious Diseases. 2002;185(5):618–626. doi: 10.1086/339010. [DOI] [PubMed] [Google Scholar]
- 26.Mutonga DM, Pimentel G, Muindi J, et al. Epidemiology and risk factors for serogroup x meningococcal meningitis during an outbreak in western Kenya, 2005-2006. American Journal of Tropical Medicine and Hygiene. 2009;80(4):619–624. [PubMed] [Google Scholar]
- 27.Connolly M, Noah N. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993-6. European meningitis surveillance group. Epidemiol Infect. 1999;122:41–49. doi: 10.1017/s0950268898001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jódar L, Feavers IM, Salisbury D, Granoff DM. Development of vaccines against meningococcal disease. Lancet. 2002;359(9316):1499–1508. doi: 10.1016/S0140-6736(02)08416-7. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Meningococcal disease. 2010. http://www.ecdc.europa.eu/en/activities/surveillance/EU_IBD/Pages/index.aspx.
- 30.World Health Organization. Geneva, Switzerland: WHO Press; The immunological basis for immunization series, module 15: meningococcal disease. Tech. Rep. [Google Scholar]
- 31.Peltola H. Meningococcal vaccines. Current status and future possibilities. Drugs. 1998;55(3):347–366. doi: 10.2165/00003495-199855030-00003. [DOI] [PubMed] [Google Scholar]
- 32.Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease: recommendations of the advisory committee on immunization practices (ACIP) Morbidity and Mortality Weekly Report. Recommendations and Reports. 2005;54(7):1–21. [PubMed] [Google Scholar]
- 33.Shao PL, Chang LY, Hsieh SM, et al. Safety and immunogenicity of a tetravalent polysaccharide vaccine against meningococcal disease. Journal of the Formosan Medical Association. 2009;108(7):539–547. doi: 10.1016/S0929-6646(09)60371-5. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Meningococcal vaccines: polysaccharide and polysaccharide conjugate vaccines. Wkly Epidemiol Rec. 2002;77(7):331–339. [PubMed] [Google Scholar]
- 35.Gold R, Lepow ML, Goldschneider I, Draper TL, Gotschlich EC. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. Journal of Clinical Investigation. 1975;56(6):1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelton SI, Gilmet GP. Expanding prevention of invasive meningococcal disease. Expert Review of Vaccines. 2009;8(6):717–727. doi: 10.1586/erv.09.37. [DOI] [PubMed] [Google Scholar]
- 37.Richmond P, Kaczmarski ED, Borrow R, et al. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. Journal of Infectious Diseases. 2000;181(2):761–764. doi: 10.1086/315284. [DOI] [PubMed] [Google Scholar]
- 38.Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clinical Microbiology Reviews. 2006;19(1):142–164. doi: 10.1128/CMR.19.1.142-164.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granoff DM, Gupta RK, Belshe RB, Andersen EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. Journal of Infectious Diseases. 1998;178(3):870–874. doi: 10.1086/515346. [DOI] [PubMed] [Google Scholar]
- 40.Bröker M, Veitch K. Quadrivalent meningococcal vaccines: hyporesponsiveness as an important consideration when choosing between the use of conjugate vaccine or polysaccharide vaccine. Travel Medicine and Infectious Disease. 2010;8(1):47–50. doi: 10.1016/j.tmaid.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Lesinski GB, Westerink MAJ. Novel vaccine strategies to T-independent antigens. Journal of Microbiological Methods. 2001;47(2):135–149. doi: 10.1016/s0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 42.Maiden MCJ, Ibarz-Pavón AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. Journal of Infectious Diseases. 2008;197(5):737–743. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20, supplement 1:S58–S67. doi: 10.1016/s0264-410x(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 44.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. British Medical Journal. 2003;326(7385):365–366. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Voer RM, Mollema L, Schepp RM, et al. Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal C conjugate vaccine. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012144. Article ID e12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Wals P, Nguyen VH, Erickson LJ, Guay M, Drapeau J, St-Laurent J. Cost-effectiveness of immunization strategies for the control of serogroup C meningococcal disease. Vaccine. 2004;22(9-10):1233–1240. doi: 10.1016/j.vaccine.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Larrauri A, Cano R, García M, Mateo S. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23(32):4097–4100. doi: 10.1016/j.vaccine.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 48.Bai X, Borrow R. Genetic shifts of Neisseria meningitidis serogroup B antigens and the quest for a broadly cross-protective vaccine. Expert Review of Vaccines. 2010;9:1203–1217. doi: 10.1586/erv.10.116. [DOI] [PubMed] [Google Scholar]
- 49.Granoff DM. Review of meningococcal group B vaccines. Clinical Infectious Diseases. 2010;50, supplement 2:S54–S65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jodar L, Cartwright K, Feavers IM. Standardisation and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A and C vaccines. Biologicals. 2000;28(3):193–197. doi: 10.1006/biol.2000.0253. [DOI] [PubMed] [Google Scholar]
- 51.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. Journal of Experimental Medicine. 1969;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364(9431):365–367. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 53.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clinical and Diagnostic Laboratory Immunology. 2003;10(5):780–786. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maslanka SE, Tappero JW, Plikaytis BD, et al. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from montana immunized with a licensed polysaccharide vaccine. Infection and Immunity. 1998;66(6):2453–2459. doi: 10.1128/iai.66.6.2453-2459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization. The Immunological Basis for Immunization Series. Module 15: Meningococcal Disease. Immunization, Vaccines and Biologicals; 2010. [Google Scholar]
- 56.World Health Organization. Enhanced surveillance of epidemic meningococcal meningitis in Africa: a three year experience. Wkly Epidemiol Rec. 2005;80:313–320. [PubMed] [Google Scholar]
- 57.Vergnano S, Heath P. Neisseria meningitidis serogroup A vaccines: an overview. Expert Review of Vaccines. 2003;2(4):571–582. doi: 10.1586/14760584.2.4.571. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. Group A and C meningococcal vaccines. Wkly Epidemiol Rec. 1999;74:297–304. [PubMed] [Google Scholar]
- 59.Gatchalian S, Palestroque E, De Vleeschauwer I, et al. The development of a new heptavalent diphtheria-tetanus-whole cell pertussis-hepatitis B-Haemophilus influenzae type b-Neisseria meningitidis serogroups A and C vaccine: a randomized dose-ranging trial of the conjugate vaccine components. International Journal of Infectious Diseases. 2008;12(3):278–288. doi: 10.1016/j.ijid.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Hodgson A, Forgor AA, Chandramohan D, et al. A phase II, randomized study on an investigational DTPw-HBV/Hib-MenAC conjugate vaccine administered to infants in Northern Ghana. PLoS One. 2008;3(5) doi: 10.1371/journal.pone.0002159. Article ID e2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kerdpanich A, Warachit B, Kosuwon P, et al. Primary vaccination with a new heptavalent DTPw-HBV/Hib-Neisseria meningitidis serogroups A and C combined vaccine is well tolerated. International Journal of Infectious Diseases. 2008;12(1):88–97. doi: 10.1016/j.ijid.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Tejedor JC, Moro M, Ruiz-Contreras J, et al. Immunogenicity and reactogenicity of primary immunization with a novel combined Haemophilus influenzae type b and Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine coadministered with a diphtheria-tetanus- acellular pertussis-hepatitis B-inactivated poliovirus vaccine at 2, 4 and 6 months. Pediatric Infectious Disease Journal. 2007;26(1):1–7. doi: 10.1097/01.inf.0000247070.60063.09. [DOI] [PubMed] [Google Scholar]
- 63.Luman ET, Barker LE, McCauley MM, Drews-Botsch C. Timeliness of childhood immunizations: a state-specific analysis. American Journal of Public Health. 2005;95(8):1367–1374. doi: 10.2105/AJPH.2004.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marshall GS, Happe LE, Lunacsek OE, et al. Use of combination vaccines is associated with improved coverage rates. Pediatric Infectious Disease Journal. 2007;26(6):496–500. doi: 10.1097/INF.0b013e31805d7f17. [DOI] [PubMed] [Google Scholar]
- 65.Auckland C, Gray S, Borrow R, et al. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. Journal of Infectious Diseases. 2006;194(12):1745–1752. doi: 10.1086/509619. [DOI] [PubMed] [Google Scholar]
- 66.Ramsay ME, McVernon J, Andrews NJ, Heath PT, Slack MP. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. Journal of Infectious Diseases. 2003;188(4):481–485. doi: 10.1086/376997. [DOI] [PubMed] [Google Scholar]
- 67.Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist CA. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet. 1999;354(9195):2063–2068. doi: 10.1016/S0140-6736(99)04377-9. [DOI] [PubMed] [Google Scholar]
- 68.Habermehl P, Leroux-Roels G, Sanger R, Machler G, Boutriau D. Combined Haemophilus influenzae type b and Neisseria meningitidis serogroup C (HibMenC) or serogroup C and Y-tetanus toxoid conjugate (and HibMenCY) vaccines are well-tolerated and immunogenic when administered according to the 2, 3, 4 months schedule with a fourth dose at 12-18 months of age. Human Vaccines. 2010;6(8):1–13. doi: 10.4161/hv.6.8.12154. [DOI] [PubMed] [Google Scholar]
- 69.Pace D, Snape M, Westcar S, et al. A new combination Haemophilus influenzae type B and Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine for primary immunization of infants. Pediatric Infectious Disease Journal. 2007;26(11):1057–1059. doi: 10.1097/INF.0b013e31813429fa. [DOI] [PubMed] [Google Scholar]
- 70.Schmitt HJ, Maechler G, Habermehl P, et al. Immunogenicity, reactogenicity, and immune memory after primary vaccination with a novel Haemophilus influenzae-Neisseria meningitidis serogroup C conjugate vaccine. Clinical and Diagnostic Laboratory Immunology. 2007;14(4):426–434. doi: 10.1128/CVI.00377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knuf M, Szenborn L, Moro M, et al. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) Pediatric Infectious Disease Journal. 2009;28(4):S97–S108. doi: 10.1097/INF.0b013e318199f61b. [DOI] [PubMed] [Google Scholar]
- 72.Booy R, Richmond P, Nolan T, et al. Immediate and longer term immunogenicity of a single dose of the combined Haemophilus influenzae type b-Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in primed toddlers 12 to 18 months of age. Pediatric Infectious Disease Journal. 2011;30(4):340–342. doi: 10.1097/INF.0b013e31820013d2. [DOI] [PubMed] [Google Scholar]
- 73.Pace D, Snape M, Westcar S, et al. A novel combined Hib-MenC-TT glycoconjugate vaccine as a booster dose for toddlers: a phase 3 open randomised controlled trial. Archives of Disease in Childhood. 2008;93(11):963–970. doi: 10.1136/adc.2007.136036. [DOI] [PubMed] [Google Scholar]
- 74.Tejedor JC, Moro M, Merino JM, et al. Immunogenicity and reactogenicity of a booster dose of a novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine given to toddlers of 13-14 months of age with antibody persistence Up to 31 months of age. Pediatric Infectious Disease Journal. 2008;27(7):579–588. doi: 10.1097/INF.0b013e31816b4561. [DOI] [PubMed] [Google Scholar]
- 75.Carmona A, Miranda M, Barrio F, et al. Reactogenicity and immunogenicity of combined Haemophilus influenzae type B-meningococcal serogroup C conjugate vaccine booster dose coadministered with measles, mumps, and rubella vaccine. Pediatric Infectious Disease Journal. 2010;29(3):269–271. doi: 10.1097/INF.0b013e3181c15977. [DOI] [PubMed] [Google Scholar]
- 76.Khatami A, Snape MD, John T, et al. Persistence of immunity following a booster dose of Haemophilus influenzae type B-meningococcal serogroup C glycoconjugate vaccine: follow-up of a randomized controlled trial. Pediatric Infectious Disease Journal. 2011;30(3):197–202. doi: 10.1097/INF.0b013e3181f728fd. [DOI] [PubMed] [Google Scholar]
- 77.Perrett KP, Winter AP, Kibwana E, et al. Antibody persistence after serogroup C meningococcal conjugate immunization of United Kingdom primary-school children in 1999-2000 and response to a booster: a phase 4 clinical trial. Clinical Infectious Diseases. 2010;50(12):1601–1610. doi: 10.1086/652765. [DOI] [PubMed] [Google Scholar]
- 78.Borrow R, Andrews N, Findlow H, et al. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and Haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clinical and Vaccine Immunology. 2010;17(1):154–159. doi: 10.1128/CVI.00384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Díez-Domingo J, Planelles Cantarino MV, Baldó Torrentí JM, et al. A randomized, multicenter, open-label clinical trial to assess the immunogenicity of a meningococcal C vaccine booster dose administered to children aged 14 to 18 months. Pediatric Infectious Disease Journal. 2010;29(2):148–152. doi: 10.1097/INF.0b013e3181b9a831. [DOI] [PubMed] [Google Scholar]
- 80.Wysocki J, Tejedor JC, Grunert D, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different Neisseria meningitidis serogroup C conjugate vaccines. Pediatric Infectious Disease Journal. 2009;28(4):S77–S88. doi: 10.1097/INF.0b013e318199f609. [DOI] [PubMed] [Google Scholar]
- 81.Nolan T, Lambert S, Roberton D, et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine. 2007;25(51):8487–8499. doi: 10.1016/j.vaccine.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 82.Marchant CD, Miller JM, Marshall GS, et al. Randomized trial to assess immunogenicity and safety of Haemophilus influenzae type B and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in infants. Pediatric Infectious Disease Journal. 2010;29(1):48–52. doi: 10.1097/INF.0b013e3181c3ce88. [DOI] [PubMed] [Google Scholar]
- 83.Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (hibmency) at 12 to 15 months of age. Pediatric Infectious Disease Journal. 2010;29(5):469–471. doi: 10.1097/INF.0b013e3181cdd379. [DOI] [PubMed] [Google Scholar]
- 84.Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y- tetanus toxoid conjugate vaccine. Pediatric Infectious Disease Journal. 2011;30:190–196. doi: 10.1097/INF.0b013e3181fcb2bf. [DOI] [PubMed] [Google Scholar]
- 85.Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H. influenzae type b-N. meningitidis C/Y conjugate vaccine in infants. Pediatrics. 2011;127(6):1375–1385. doi: 10.1542/peds.2009-2992. [DOI] [PubMed] [Google Scholar]
- 86.Østergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15-25 years. Vaccine. 2009;27(1):161–168. doi: 10.1016/j.vaccine.2008.08.075. [DOI] [PubMed] [Google Scholar]
- 87.Knuf M, Kieninger-Baum D, Habermehl P, et al. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine. 2010;28(3):744–753. doi: 10.1016/j.vaccine.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 88.Bermal N, Huang L-M, Dubey AP, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Human vaccines. 2011;7(2):239–247. doi: 10.4161/hv.7.2.14068. [DOI] [PubMed] [Google Scholar]
- 89.Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10-25 years of age. Pediatric Infectious Disease Journal. 2011;30:e41–e48. doi: 10.1097/INF.0b013e3182054ab9. [DOI] [PubMed] [Google Scholar]
- 90.Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: an open, randomised controlled trial. Vaccine. 2011;29(25):4274–4284. doi: 10.1016/j.vaccine.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 91.Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix Tm hexa is immunogenic, with an acceptable safety profile in 12-23 month old children. Vaccine. 2011;29(25):4264–4273. doi: 10.1016/j.vaccine.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Lingappa JR, Rosenstein N, Zell ER, Shutt KA, Schuchat A, Perkins BA. Surveillance for meningococcal disease and strategies for use of conjugate meningococcal vaccines in the United States. Vaccine. 2001;19(31):4566–4575. doi: 10.1016/s0264-410x(01)00209-2. [DOI] [PubMed] [Google Scholar]
- 93.Diez-Domingo J, Planelles Cantarino MV, Úbeda Sansano MV, et al. Immune response and antibody persistence data after a conjugate MenC booster vaccination. In: Proceedings of the 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; June 2009; Brussels, Belgium. [Google Scholar]
- 94.Keyserling H, Papa T, Koranyi K, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Archives of Pediatrics and Adolescent Medicine. 2005;159(10):907–913. doi: 10.1001/archpedi.159.10.907. [DOI] [PubMed] [Google Scholar]
- 95.Black S, Klein NP, Shah J, Bedell L, Karsten A, Dull PM. Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in children 2-10 years of age. Vaccine. 2010;28(3):657–663. doi: 10.1016/j.vaccine.2009.10.104. [DOI] [PubMed] [Google Scholar]


