Summary
Angioproliferative tumors induced by the Kaposi’s Sarcoma associated herpesvirus (KSHV) have been successfully treated with rapamycin, which provided direct evidence of the clinical activity of mTOR inhibitors in human malignancies. However, prolonged mTOR inhibition may raise concerns in immunocompromised patients, including AIDS-KS. Here, we explored whether KSHV-oncogenes deploy cell-type specific signaling pathways activating mTOR, which could be exploited to halt KS development while minimizing immune suppressive effects. We found that PI3Kγ, a PI3K isoform exhibiting restricted tissue distribution, is strictly required for signaling from the KSHV-encoded vGPCR oncogene to Akt/mTOR. Indeed, by using an endothelial-specific gene delivery system modeling KS development, we provide genetic and pharmacological evidence that PI3Kγ may represent a suitable molecular target for therapeutic intervention in KS.
Introduction
The activation of the PI3K/Akt/mTOR signaling axis may represent one of the most frequent events in cancer (Bunney and Katan, 2010; Manning and Cantley, 2007; Sabatini, 2006), hence prompting the development of molecular inhibitors of this biochemical route as promising anticancer agents, many of which are already in advanced stages of clinical evaluation (Bunney and Katan, 2010; Dancey, 2010; Guertin and Sabatini, 2007). In this regard, the effectiveness of an mTOR inhibitor, rapamycin, as an anti-tumoral agent was first demonstrated for the treatment of an angioproliferative disease known as Kaposi’s sarcoma (KS) arising in immunosuppressed renal transplanted patients (Stallone et al., 2005). Rapamycin and its analogs (rapalogs) are currently under intense investigation for their clinical efficacy in numerous cancer types (Dancey, 2010). Four clinical manifestations of KS differ in their aggressiveness and epidemiology (Ganem, 2010). The mildest form, classic KS, affects elder men of the Mediterranean area. Endemic KS is much more aggressive and is now the leading cancer affecting children and young men in subequatorial Africa. Iatrogenic and AIDS-associated KS are the most aggressive, and related to immunosuppression due to organ transplantation or HIV infection, respectively (Ganem, 2010; Mesri et al., 2010).
The Kaposi sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8) is the etiologic agent of KS (Chang et al., 1994; Ganem, 2010; Mesri et al., 2010). KSHV encodes 84 genes and 12 miRNAs, many of which harbor oncogenic potential (Ganem, 2010). Among them, several lines of evidence support the role for a constitutively active, virally-encoded G protein coupled receptor (vGPCR) in KS initiation and progression (Bais et al., 2003; Mesri et al., 2010; Montaner et al., 2003; Yang et al., 2000). vGPCR activates an intricate network of molecular signaling events, driving both the aberrant growth of endothelial cells and the paracrine transformation of endothelial-derived cells expressing KSHV latent genes (Mesri et al., 2010). The PI3K/Akt/mTOR pathway represents one of the most prominent oncogenic mechanisms deployed by vGPCR as revealed by genetically-defined animal models for KS (Sodhi et al., 2006; Sodhi et al., 2004). These findings provided the foundation for the evaluation and clinical success of the use of mTOR inhibitors for iatrogenic KS lesions, achieving a rapid and complete remission in all the patients involved in the initial study (Stallone et al., 2005). The effectiveness of rapamycin in iatrogenic KS may result from a rare convergence of antitumoral and desirable therapeutic immunosuppressive properties, hence rapamycin is now becoming the standard of care for renal transplanted patients developing KS (Stallone et al., 2008). However, prolonged use of mTOR inhibitors may raise concerns for those affected by the endemic form of this disease and for AIDS patients that are already immunocompromised due to their elevated viral load. In this regard, the possibility still exists that KSHV-oncogenes may deploy cell-type specific signaling events leading to mTOR activation that can be exploited to halt KS development while minimizing immune suppressive effects. Thus, we set up to investigate the molecular mechanism by which vGPCR initiates the oncogenic activation of mTOR in endothelial cells, aimed at identifying alternative molecular targets for the treatment of angioproliferative diseases.
Results
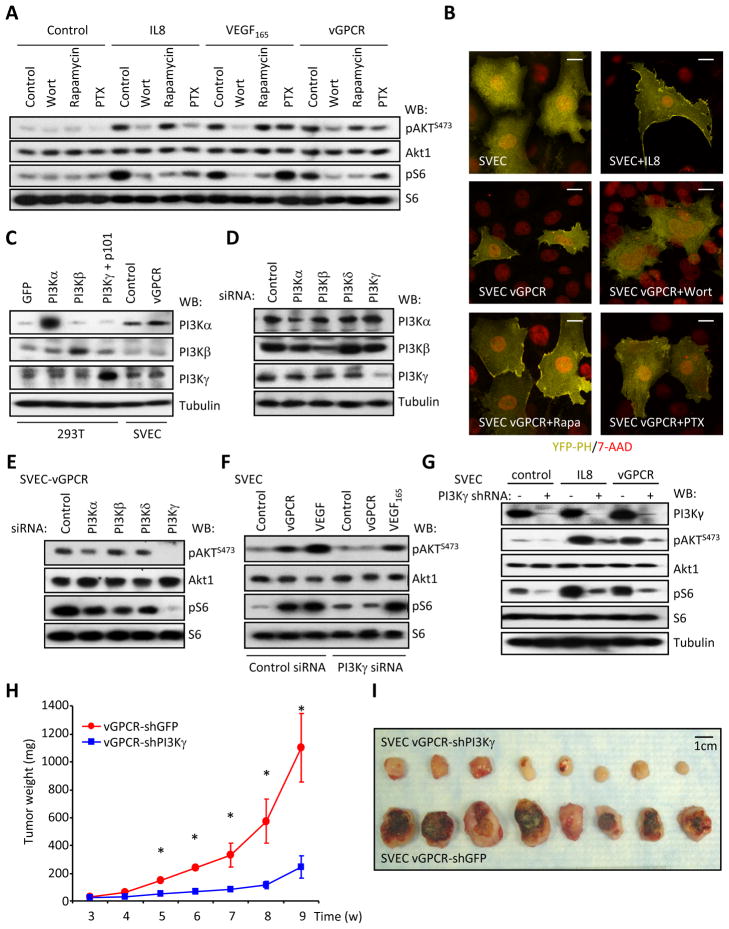
Expression of vGPCR in immortalized murine (SVECs) and human (HMEC-1) endothelial cells stimulates Akt and mTOR potently, as judged by the accumulation of phosphorylated Akt (pAktS473) and S6 (pS6), the latter a downstream target of mTOR (Figures 1A, S1A). This was accompanied by morphological changes (Figure S1B), increased cell size (Figure S1C), and increased survival upon growth factor deprivation (Figure S1D). Activation of Akt and mTOR required PI3K, as it is sensitive to the PI3K inhibitor wortmannin, while pS6 accumulation was blocked by rapamycin that inhibits mTOR. Inhibition of heterotrimeric G proteins of the Gi family by pertussis toxin (PTX) partially prevented the activation of Akt and mTOR by vGPCR. The CXC chemokine IL8, that acts on endogenous Gi-coupled CXCR2 receptors and VEGF that stimulates its cognate tyrosine kinase receptors were used as positive and negative controls, respectively. These results were further confirmed using the pleckstrin homology (PH) domain from Akt fused to YFP, which can be used to monitor the presence of phosphatidylinositol 3,4,5-trisphosphate (PIP3) at the plasma membrane. IL8 treatment or the expression of vGPCR induced the relocalization of the PH-YFP construct from the cytosol to the plasma membrane (Figures 1B, S1E), that was sensitive to wortmannin and PTX, but not to rapamycin. The residual activity after PTX treatment may reflect the more limited ability of vGPCR to signal to Akt by other PTX-insensitive G proteins. We can conclude that a Gi-dependent PI3K activity is largely required for the activation of the Akt/mTOR pathway by vGPCR.
Figure 1. vGPCR induces the PI3Kγ-dependent activation of mTOR.
A, Serum starved murine immortal endothelial (SVEC) cells were pretreated with inhibitors and stimulated with IL8 or VEGF165 (see Supplemental Experimental Procedures for details). vGPCR: SVEC cells stably expressing vGPCR. B, SVEC vGPCR cells expressing YFP-PH (see Supplemental Experimental Procedures) were treated as indicated. See quantification in Figure S1. Bar=20μm. C, Analysis of the expression of the PI3K α, β and γ isoforms in SVEC cells. Expression of the different PI3K isoforms was analyzed by Western blotting. Overexpressing 293T cells were used as controls. D, siRNA-mediated knockdown of different PI3K isoforms in SVEC cells. SVEC cells were transfected with siRNAs as depicted (supplemental experimental procedures). Exponentially growing cultures were analyzed by Western blot after five days. E, PI3Kγ mediates the activation of Akt and mTOR downstream of vGPCR. SVEC vGPCR cells transfected as in panel D were analyzed by Western blotting as indicated after 5 days. F, PI3Kγ knockdown selectively impairs the activation of Akt and mTOR downstream of vGPCR but not VEGF165. SVEC (Control, VEGF) and SVEC vGPCR (vGPCR) cells were treated as depicted and analyzed by Western blotting. G, shRNA-mediated knockdown of PI3Kγ reduces the activation of Akt and mTOR induced by IL8 and vGPCR expression. SVEC and SVEC vGPCR cells transfected with PI3Kγ shRNA expression vectors for 72h were treated as described in material and methods. A Western blot analysis of a representative experiment is shown. H, SVEC vGPCR cells stably expressing a PI3Kγ shRNA show reduced tumorigenesis in nude mice. One million SVEC vGPCR PI3Kγ or GFP shRNA cells (see Supplemental Experimental Procedures) were injected subcutaneously into the flanks of nude mice and allowed to form tumors. Graph represents the mean tumor weight (n=8) ± SEM. (*, p≤0.001) at each time point. I, Gross morphology of the tumor xenografts from panel H. See also Figure S1.
Four different PI3K catalytic subunits have been described in mammalian cells, which display divergent regulation and patterns of expression. PI3K , and are regulated by tyrosine kinase receptors through interaction with their regulatory subunits, with PI3Kα and β being expressed ubiquitously and PI3K expressed mainly by leukocytes (Engelman et al., 2006). PI3K exhibits restricted tissue distribution and is activated by GPCRs by the interaction of its catalytic (p110γ) and regulatory subunit (p101) with Gβγ subunits (Lopez-Ilasaca et al., 1997). PI3K , and isoforms were readily detectable in endothelial cells (Figures 1C, S1A), as reported (Morello et al., 2009), while PI3K was undetectable by Western blotting and qPCR (not shown). PI3Kγ knockdown with specific siRNAs resulted in a dramatic decrease in the activation of Akt/mTOR in endothelial cells expressing vGPCR (Figures 1D and 1E), while knockdown of PI3Kα or PI3Kβ had only a limited effect. In contrast, PI3Kγ siRNA did not interfere with the ability to stimulate Akt/mTOR upon VEGF165 treatment (Figure 1F). As PI3Kγ is expressed in human KS lesions (Figure S1F), we hypothesized that PI3Kγ may represent an attractive candidate to transduce the signal initiated by vGPCR to Akt/mTOR in endothelial derived tumor cells. Based on these findings, we analyzed the impact of PI3Kγ on vGPCR induced tumorigenesis in endothelial xenograft models (Montaner et al., 2003). We first identified PI3Kγ short hairpin RNAs (shRNA) whose stable expression efficiently knockdown the expression of PI3Kγ (Figure 1G), and reduced the activation of Akt and mTOR in response to either IL8 treatment or vGPCR cotransfection. Remarkably, knockdown of PI3Kγ greatly impaired the ability of vGPCR to form tumors in nude mice when compared to shRNA expressing control cells (Figures 1H and 1I). Together, these results suggest PI3Kγ may play an essential role in vGPCR-induced activation of Akt/mTOR and in vGPCR-initiated sarcomagenesis.
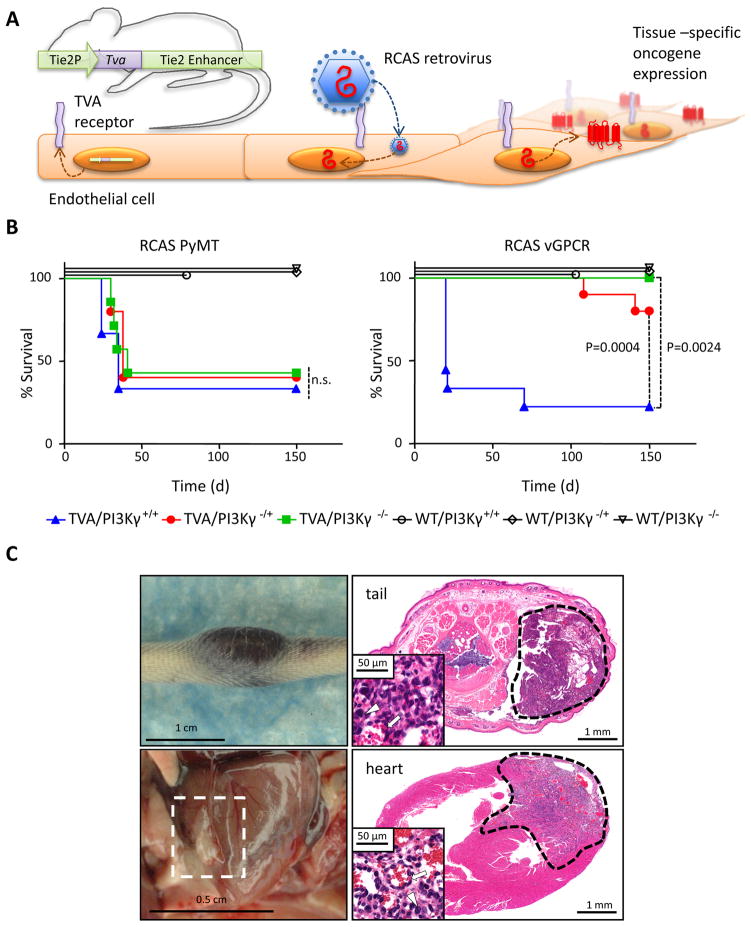
We next sought to challenge these observations in a genetically defined in vivo KS model. In particular, we have previously generated genetically engineered animals expressing the receptor for the avian leukosis virus (ALV) Tva under the control of the Tie2 endothelial specific promoter (Figure 2A) (Montaner et al., 2003). In this system, infection of Tie2-Tva mice with ALV-derived RCAS retroviruses encoding vGPCR or the potent oncogene Polyoma Middle T antigen (PyMT) induce the Tva-dependent death of mice within only few weeks (Montaner et al., 2003). We backcrossed this animal line into a PI3Kγ knockout background (Hirsch et al., 2000) generating mice bearing none (+/+), hemizygous (−/+) and homozygous (−/−) deletion of this gene. Of note, PI3Kγ knockout mice are viable and display only reduced inflammatory responses and impaired neutrophil and monocyte migration in vivo (Ruckle et al., 2006). Infection with RCAS PyMT resulted in the deaths of approximately 60% of the TVA positive mice within 50 days post infection regardless of their genotype for PI3Kγ (Figure 2B), in agreement with the mechanism of action of this viral oncogene, involving PI3Kα and PI3Kβ (Dilworth, 2002). However, when RCAS vGPCR retroviruses were injected, 77.7% of the TVA(+)/PI3K+/+ mice died within 70 days (median survival 20 days) (n=9), but only 20% of the TVA(+)/PI3Kγ+/− died after 150 days (n=10, p=0.0024 with respect to PI3K+/+ mice) while all of the TVA(+)/PI3Kγ−/− mice survived (n=7, p=0.0004 with respect to PI3K+/+ mice) without showing signs of disease even after 9 months of observation. The cause of death was attributed to the presence of multiple internal angioproliferative lesions. Those TVA(+)/PI3Kγ+/+ animals that did not die within the initial 70 day period (22.3%) invariably started developing external and internal KS-like lesions after six months, in agreement with previous reports (Montaner et al., 2003), but no PI3K−/+ and PI3K−/− mice developed lesions even after prolonged observation (Figure 2C).
Figure 2. A genetic model unveils the requirement of PI3Kγ for vGPCR-induced sarcomagenesis.
A, Schematic overview of the RCAS system for somatic gene transfer. Tissue-restricted expression of the avian leukosis virus (ALV) receptor TVA is achieved by transgenic technology. The Tie2 promoter and enhancer sequences target the expression of TVA to endothelial tissues. RCAS viruses encoding a gene of interest are injected into mice resulting in tissue-specific targeted infection and expression of the viral payload. B, Left upper and lower panels, gross appearance of representative KS lesions appearing in TIE2-TVA mice injected with RCAS vGPCR that survived longer than 150 days. Right panels, H&E stained section of the lesions found in the tail and heart. Insets, high power magnification of the same field is shown. Several other tissues, including lung and kidneys displayed similar lesions. C, Effect of the hemi- and homozygotic loss of expression of PI3Kγ on animal survival after infection with RCAS virus on TIE2-TVA animals. Five-days old mice born from TIE2-TVA/PI3Kγ−/+ PI3Kγ-/+ breeding pairs were injected with RCAS-PyMT virus (5.0 107 i.u.), right panel, or RCAS vGPCR (5.0 107 i.u.), left panel. Animals’ deaths occurred naturally and were recorded and correlated to the presence of multiple internal angioproliferative lesions during the necropsy.
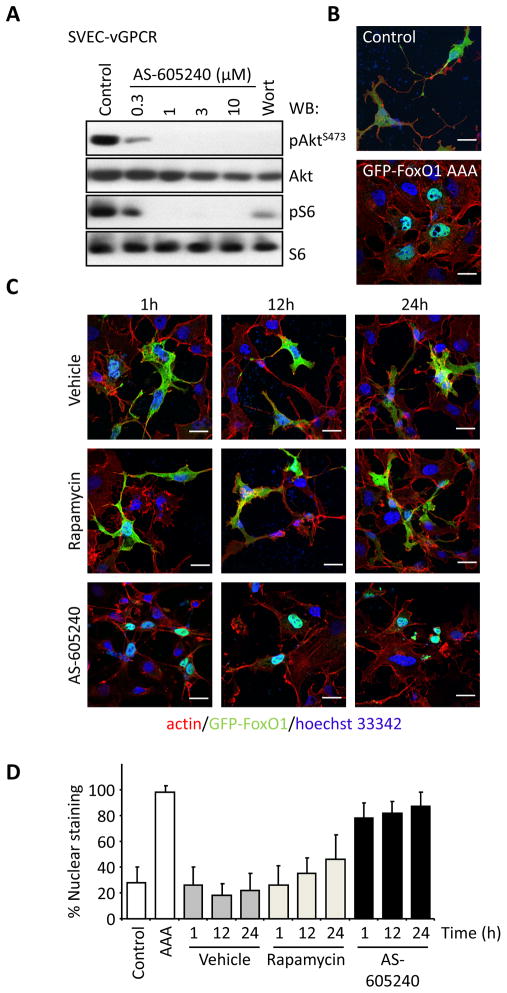
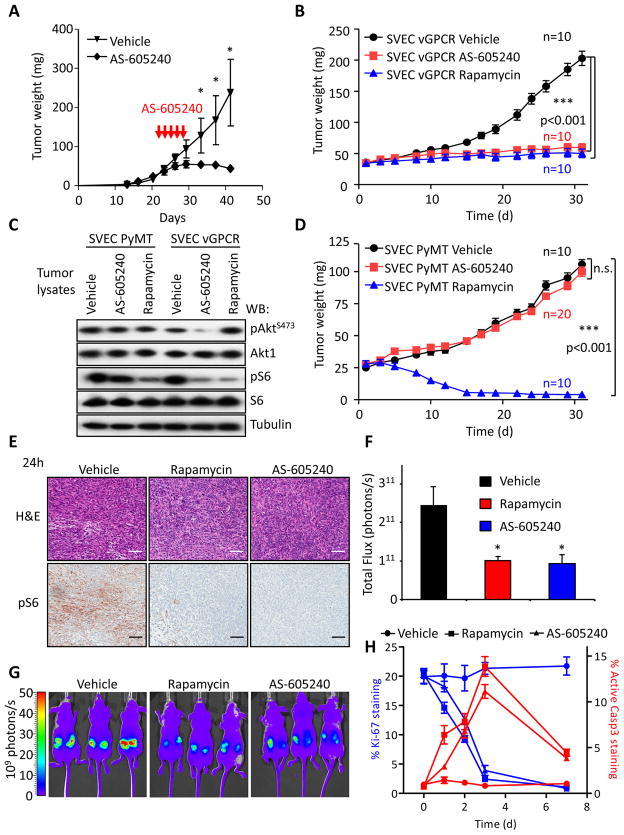
These findings provided a strong rationale for exploring whether pharmacological inhibition of PI3Kγ represents a therapeutic strategy to treat KS. As a proof of principle, we assessed whether AS-605240, a widely used PI3Kγ inhibitor can prevent vGPCR-induced sarcomagenesis. AS-605240 induced a remarkable and dose dependent inhibition of Akt and mTOR activation in endothelial cells expressing vGPCR (Figures 3A, S2A). We further examined the function of this PI3Kγ-inhibitor by analyzing the subcellular localization of GFP-FoxO1, which is excluded from the nucleus upon phosphorylation by Akt (Brunet et al., 2004). As shown in Figures 3B and 3C, AS-605240 inhibited Akt downstream of vGPCR as depicted by the nuclear localization of GFP-FoxO1, using as a control a nuclear-localized FoxO1 mutant (GFP-FoxO1 AAA) that is resistant to Akt-mediated inactivation. Treatment with rapamycin led to only a subtle decrease in Akt activity (Figure 3D), probably reflecting a delayed inhibition of the mTOR complex 2 by rapamycin (Laplante and Sabatini, 2009). Furthermore, treatment of vGPCR expressing cells with AS-6052540 resulted in a dose-dependent inhibition of proliferation and decreased viability (Figure S2B). In addition to promote the direct transformation of endothelial cells, vGPCR expression can promote the tumoral growth of endothelial cells including those expressing latent KSHV transcripts in a paracrine fashion (Mesri et al., 2010; Montaner et al., 2003). This observation prompted us to explore the potential role of PI3Kγ in vGPCR-initiated paracrine signaling. For this analysis, we took advantage of prior observations that vGPCR-expressing cells promote the proliferation and activation of NFκB of endothelial cells in a paracrine fashion. As shown in Figures S2C and S2D, inhibition of PI3Kγ in vGPCR-expressing endothelial cells by the use of specific shRNAs or by the use of PI3Kγ inhibitors nearly abolished the ability of vGPCR-expressing cells to release pro-proliferative (Figures S2C, S2D) and NFκB-activating (Figure S2E) and soluble factors. To test the potential therapeutic properties of PI3Kγ inhibition, nude mice bearing endothelial-vGPCR tumors were treated with AS-605240 showing a marked reduction in tumor growth (Figure 4A) with minimal signs of toxicity (2.11±0.9% body weight loss after five days). The impact of PI3Kγ inhibition was found to be highly specific under these conditions (Figure 4B), since AS-605240 did not inhibit AktS473 or S6 phosphorylation caused by PyMT expression (Figure 4C) nor affected the growth of SVEC-PyMT tumor xenografts, (Figure 4D). Inhibition of the mTOR pathway as judged by pS6 immunostaining was complete 24h after the initiation of the treatment (Figure 4E), to an extent similar to that seen with rapamycin. We also generated endothelial cells expressing vGPCR and the red fluorescent mCherry protein, which allowed visualizing the tumor growth in real time. Treatment with both rapamycin and AS-605240 greatly reduced both the size and fluorescence of the tumors to the same extent (Figures 4F and 4G). Further analysis of tumor biopsies revealed that both treatments induced a marked reduction of cell proliferation as measured by Ki-67 staining and increased the number of apoptotic cells, as determined by active caspase 3 (Figure 4H). Taken together, these results indicate that PI3Kγ inhibition with AS-605240 is at least as effective as rapamycin at reducing the activity of mTOR, resulting in reduced cell proliferation and the apoptotic demise of cancer cells, and the consequent tumor regression.
Figure 3. Pharmacological inhibition of PI3Kγ reduces the activity of the Akt/mTOR pathway by vGPCR in vitro.
A, Serum starved SVEC-vGPCR cells were treated with increasing concentrations of AS-605240, a PI3Kγ specific inhibitor or wortmannin (Wort) for 1h. Protein lysates were analyzed by Western blotting. A representative blot is shown. B, A GFP-FoxO1 construct was used as reporter for Akt activity. COS-7 cells were co-transfected with a vGPCR-encoding plasmid and GFP-FoxO1 (Control) or a GFP-FoxO1 in which the 3 residues phosphorylated by Akt responsible for its nuclear export are mutated to alanine (GFP-FoxO1 AAA). Serum-starved cells were fixed and stained with phalloidin-Texas-red and the nuclear staining Hoechst 33342. Upper panel, Control cells display typical GFP cytosolic localization. Lower panel, cells expressing the AAA mutant show a strong nuclear localization of this construct. Bar=25μm. C, Inactivation of Akt induced by treatment with AS-605240. COS-7 cells expressing vGPCR and GFP-FoxO1 as in panel B, were treated as indicated. AS-605240 induced a strong and sustained inhibition of Akt as reflected by the nuclear relocalization of GFP-FoxO1 while rapamycin only inhibited Akt modestly after 24h. Bar=25μm. D, Quantification of the percentage of cells displaying nuclear translocation of the GFP-FoxO1 constructs from panels B and C. Bars represent the mean percentage ±SD from three different samples in which at least 200 transfected cells were evaluated. See also Figure S3.
Figure 4. PI3Kγ inhibition halts tumor growth induced by vGPCR.
A, SVEC vGPCR xenografts were prepared as in Figure 1H. Tumors were allowed to grow for 3 weeks (average tumor weight 42.3±2.3 mg) and mice were treated with vehicle or 25 mg/kg AS-605240 twice daily intraperitoneally for five consecutive days. Animal weight was monitored for signs of toxicity. Data points represent mean tumor weight ±SEM (n=8). *, p≤0.001. B, Parallel tumor bearing mice received one treatment with vehicle, rapamycin (5 mg/kg, i.p.), or AS-605240 (25 mg/kg, i.p.) and sacrificed 24 h after. Tumors were excised and processed for H&E staining and pS6 immunohistochemistry. A representative field is shown from 4 tumor samples with similar results. Bar=150μm. C, Tumor xenografts were generated as in panel A using SVEC vGPCR cells expressing the red fluorescent protein mCherry. Tumor growth can be monitored in real time using in vivo fluorescence imaging. Tumors were allowed to grow for three weeks and then treated daily with vehicle, rapamycin (5mg/kg) or AS-605240 (25 mg/kg), intraperitoneally. Photometric analysis of the tumors red fluorescence after one month of treatment shows marked reduction in size in the rapamycin and AS-605240 treated groups. Three representative animals of each group (n=5) are shown. D, Quantification of the tumors from the experiment showed in panel C. Bars represent the average Total Flux (n=10 tumors) ±SEM. E, Tumors generated in parallel as in panel B were excised and processed for immunostainings for the proliferation marker Ki-67 and the apoptotic marker cleaved Caspase3 (Active Casp3) after the indicated days of treatment. Representative areas of every preparation were automatically quantified by software analysis (Supplemental Experimental Procedures). Data points represent the mean percentage ±SEM of positive cells in each preparation. F, Western blot analysis of pAktS473 and pS6 in SVEC PyMT or SVEC vGPCR tumor tissues collected 4h after i.p. injection of the indicated treatments, as described below. G and H, Tumor xenografts of SVEC vGPCR or SVEC PyMT were established as described in Experimental Procedures. Tumors were treated with vehicle, AS-605240 (25 mg/kg/day i.p.) or rapamycin (5 mg/kg/day i.p.), as indicated. Tumor size was measured three times a week. Values represent mean tumor weight ± SEM.
Collectively, in vitro and in vivo experiments using endothelial cells, tumor xenografts, and endothelial-specific gene delivery systems in genetically-defined animal support the key role of the vGPCR-PI3Kγ signaling axis in KS initiation and progression, hence representing a candidate molecular target for pharmacological intervention in KS.
Discussion
The dissection of the dysregulated signaling networks leading to tumor initiation and malignant progression has recently afforded the opportunity of developing molecular targeted options for cancer prevention and treatment. Recent studies have highlighted the central role of the PI3K/Akt/mTOR pathway in some of the most prevalent human neoplasias (Bunney and Katan, 2010). In this regard, each PI3K isoform may perform distinct functions (Engelman et al., 2006; Vanhaesebroeck et al., 2005), thus suggesting that their selective inhibition may provide therapeutic opportunities in specific disease conditions while limiting their side effects. For example, PI3Kδ, which is expressed in few immune-derived cells, is now being evaluated as specific target for multiple hematologic malignancies (Ameriks and Venable, 2009). Here, we provide genetic evidence that PI3Kγ, a PI3K isoform exhibiting a restricted tissue distribution and regulated by a distinct mechanism from other Class I PI3Ks (Morello et al., 2009), is strictly required for the growth of endothelial-derived tumors and the development of KS-like lesions. Furthermore, we show that PI3Kγ specific inhibitors promote the rapid decrease in Akt and mTOR activation caused by expression of a KSHV oncogene, vGPCR, in endothelial cells thereby causing their apoptotic death and tumor regression, probably due to impaired vGPCR-initiated direct and paracrine transforming mechanisms.
Extensive studies in mice lacking PI3Kγ have demonstrated that this protein is not required for normal development, life span or basic immune responses, unless under stress conditions, and suggest that PI3Kγ may represent a suitable therapeutic target in a variety of inflammatory and cardiovascular diseases (Ghigo et al., 2010; Ruckle et al., 2006). Interestingly, even the deletion of a single PI3Kγ allele in heterozygous mice was sufficient to prevent the formation of KS-like lesions, and protected the majority of the mice from KSHV vGPCR-caused death highlighting the exquisite sensitivity of endothelial-derived tumors to PI3Kγ inhibition. Taken together, these findings raise the possibility that PI3Kγ inhibitors may represent a suitable therapeutic option to prevent or treat already established KS lesions, likely avoiding the potential toxicities associated with mTOR, Akt, or pan-PI3K inhibition.
KS remains the most prevalent cancer among children and adolescents in Africa, and while the use of highly active antiretroviral therapy (HAART) has dramatically reduced the number of AIDS-KS cases in the last decade, KS is still the most prevalent AIDS-associated malignancy (Ganem, 2010). Failure to adhere to HAART regime, aging, chronic inflammation and the possibility of the emergence of HAART-resistant HIV all pose a risk of KS reemergence that we cannot afford to ignore. In this regard, maintaining a normal immunocompetence appears to be the most effective way to control KS. Paradoxically, the efficacy of the immunosuppressant rapamycin at treating iatrogenic KS highlights the sensitivity to targeted-molecular intervention in this angioproliferative disease despite a depressed immune status (Stallone et al., 2008). This, in turn, may facilitate the development of effective mechanism-based therapies for KS in patients in which the risk of complications derived from immunosuppression outweighs the advantages of direct mTOR inhibition. Indeed, we now show that the distinct coupling ability of a KSHV oncogene to an endothelial expressed PI3K isoform and the strict requirement of a functional PI3Kγ for KS development can be exploited for the development of molecular-targeted treatment options and preventive strategies for this viral-associated malignancy. This emerging role of PI3Kγ in pathological endothelial cell functions and malignant conversion suggest that this PI3K isoform may also represent an attractive candidate for other angioproliferative diseases.
Experimental Procedures
Cell lines, tissue culture, DNAs, transfections, and reagents
SV40-immortalized murine endothelial cells (SVEC4-10) where purchased from ATCC (Manassas, VA). SVEC and COS-7 cells were cultured in DMEM 10% fetal bovine serum supplemented with antibiotics, 5% CO2 at 37°C. SVEC vGPCR cells were generated by stable transfection of pCEFL AU5-vGPCR as reported (Montaner et al., 2003). For stable knockdown, SVEC vGPCR cells were stably transfected with both shRNAS by cotransfection at 1:10 ratio with a Hygromycin resistant vector (pBABE hygro) and then selected in150 μg/ml Hygromycin for two weeks.
Animal work
All animal studies were carried out according to NIH-approved protocols, in compliance with the Guide for the Care and Use of Laboratory Animals.
Establishment of tumor xenografts in athymic nu/nu mice
SVEC cells lines were used to induce endothelial tumor xenografts in athymic mice as described previously (Montaner et al., 2003). TIE2-Tva transgenic animals (Montaner et al 2003) were backcrossed into a PI3Kγ knockout background to generate the six possible combinations of Tva and PI3Kγ gene dosage. Preparation and in vivo infection with RCAS viruses encoding PyMT and AU5 tagged vGPCR was performed as previously described (Montaner et al., 2003).
In vivo fluorescence analysis
SVEC vGPCR cells were infected with a lentivirus encoding an EF-1α-driven red fluorescent protein (pLESIP mCherry) and selected by FACS. Xenografts arising from these cells were quantified with a Xenogen IVIS-100 system (Caliper, Hopkinton, MA) using the built-in Ds-Red filter set and 1 second exposure time. Measurements were performed using Caliper’s Living Image suite.
Statistical Analysis
Data analysis was performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA). ANOVA followed by the Tukey t test was used to analyze the differences between experimental groups. Comparison of survival curves was performed by a Log-rank Mantel-Cox test.
Supplementary Material
Significance.
Activation of the PI3K-Akt pathway resulting in mTOR stimulation is one of the most frequent events in human cancer; hence small molecule inhibitors of this biochemical route represent attractive anticancer agents. However, key immune and metabolic functions of mTOR may limit the clinical benefits of its specific inhibitors for cancer treatment. The activation of a tissue restricted PI3K isoform, PI3Kγ, by the KSHV-encoded vGPCR oncogene enabled inhibiting an upstream event contributing to oncogenic signaling to mTOR, while sparing the normal physiological functions of mTOR. These findings indicate that PI3Kγ may represent a suitable therapeutic target for KS and for a myriad of angioproliferative diseases, including tumor-induced angiogenesis, thereby avoiding the potential toxicities associated with mTOR, Akt, or pan PI3K inhibition.
Highlights.
PI3Kγ is co-opted by the KSHV vGPCR oncogene to activate the Akt/mTOR pathway
Genetic evidence supports that PI3Kγ is strictly required for KS development
PI3Kγ inhibitors may provide effective therapeutic options for KS
PI3Kγ represents a relevant molecular target in angioproliferative diseases
Acknowledgments
This research was supported by a National Institutes of Health Intramural AIDS Targeted Antiviral Program and the National Institute of Dental and Craniofacial Research. We thank M. Simaan and P. Amornphimoltham for their help and expert advice with the fluorescence microscopy studies. We apologize to colleagues whose primary research papers may not have been cited due to space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameriks MK, Venable JD. Small Molecule Inhibitors of Phosphoinositide 3-Kinase (Pi3k) Delta and Gamma. Curr Top Med Chem. 2009;9:738–753. doi: 10.2174/156802609789044434. [DOI] [PubMed] [Google Scholar]
- Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, Dias S, Silverstein RL, Rafii S, Mesri EA. Kaposi's Sarcoma Associated Herpesvirus G Protein-Coupled Receptor Immortalizes Human Endothelial Cells by Activation of the Vegf Receptor-2/ Kdr. Cancer Cell. 2003;3:131–143. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-Dependent Regulation of Foxo Transcription Factors by the Sirt1 Deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M. Phosphoinositide Signalling in Cancer: Beyond Pi3k and Pten. Nat Rev Cancer. 2010;10:342–352. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of Herpesvirus-Like DNA Sequences in Aids-Associated Kaposi's Sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Dancey J. Mtor Signaling and Drug Development in Cancer. Nat Rev Clin Oncol. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- Dilworth SM. Polyoma Virus Middle T Antigen and Its Role in Identifying Cancer-Related Molecules. Nat Rev Cancer. 2002;2:951–956. doi: 10.1038/nrc946. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Ganem D. Kshv and the Pathogenesis of Kaposi Sarcoma: Listening to Human Biology and Medicine. J Clin Invest. 2010;120:939–949. doi: 10.1172/JCI40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo A, Damilano F, Braccini L, Hirsch E. Pi3k Inhibition in Inflammation: Toward Tailored Therapies for Specific Diseases. Bioessays. 2010;32:185–196. doi: 10.1002/bies.200900150. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the Role of Mtor in Cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central Role for G Protein-Coupled Phosphoinositide 3-Kinase Gamma in Inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. Mtor Signaling at a Glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G Protein-Coupled Receptors to the Mapk Signaling Pathway through Pi 3-Kinase Gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Akt/Pkb Signaling: Navigating Downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Boshoff C. Kaposi's Sarcoma and Its Associated Herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. Endothelial Infection with Kshv Genes in Vivo Reveals That Vgpcr Initiates Kaposi's Sarcomagenesis and Can Promote the Tumorigenic Potential of Viral Latent Genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- Morello F, Perino A, Hirsch E. Phosphoinositide 3-Kinase Signalling in the Vascular System. Cardiovasc Res. 2009;82:261–271. doi: 10.1093/cvr/cvn325. [DOI] [PubMed] [Google Scholar]
- Ruckle T, Schwarz MK, Rommel C. Pi3kgamma Inhibition: Towards an 'Aspirin of the 21st Century'? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. Mtor and Cancer: Insights into a Complex Relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, Sawai ET, Molinolo A, Gutkind JS, Montaner S. The Tsc2/Mtor Pathway Drives Endothelial Cell Transformation Induced by the Kaposi's Sarcoma-Associated Herpesvirus G Protein-Coupled Receptor. Cancer Cell. 2006;10:133–143. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA, Sawai ET, Gutkind JS. Akt Plays a Central Role in Sarcomagenesis Induced by Kaposi's Sarcoma Herpesvirus-Encoded G Protein-Coupled Receptor. Proc Natl Acad Sci U S A. 2004;101:4821–4826. doi: 10.1073/pnas.0400835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallone G, Infante B, Grandaliano G, Schena FP, Gesualdo L. Kaposi's Sarcoma and Mtor: A Crossroad between Viral Infection Neoangiogenesis and Immunosuppression. Transpl Int. 2008;21:825–832. doi: 10.1111/j.1432-2277.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's Sarcoma in Renal-Transplant Recipients. N Engl J Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by Pi3k Isoforms: Insights from Gene-Targeted Mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, Lira SA. Transgenic Expression of the Chemokine Receptor Encoded by Human Herpesvirus 8 Induces an Angioproliferative Disease Resembling Kaposi's Sarcoma. J Exp Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.