Abstract
Tetraarsenic hexaoxide (As4O6) has been used in Korean folk remedy for the treatment of cancer since the late 1980s, and arsenic trioxide (As2O3) is currently used as a chemotherapeutic agent. However, evidence suggests that As4O6-induced cell death pathway was different from that of As2O3. Besides, the anticancer effects and mechanisms of As4O6 are not fully understood. Therefore, we investigated the anticancer activities of As4O6 on apoptosis and autophagy in U937 human leukemic cells. The growth of U937 cells was inhibited by As4O6 treatment in a dose- and a time-dependent manner, and IC50 for As4O6 was less than 2 μM. As4O6 induced caspase-dependent apoptosis and Beclin-1-induced autophagy, both of which were significantly attenuated by Bcl-2 augmentation and N-acetylcysteine (NAC) treatment. This study suggests that As4O6 should induce Beclin-1-induced autophagic cell death as well as caspase-dependent apoptosis and that it might be a promising agent for the treatment of leukemia.
1. Introduction
Arsenic trioxide (As2O3), a component of Chinese medicine, has been successfully employed for the treatment of acute promyelocytic leukemia (APL) [1, 2] and it has recently been shown to have some efficacy against a certain type of solid cancers [3, 4]. It is taken parenterally via an IV drip. With regard to anticancer effects of As2O3, many studies have shown that As2O3 is capable of inducing programmed cell death. There are two types of programmed cell death reported. One is apoptosis, type I programmed cell death which is characterized by a highly stereotypical series of morphological and biological changes, such as cytoplasmic shrinkage, blebbing of the plasma membrane, chromatin condensation, and DNA degradation [5]. Another is autophagy, type II programmed cell death [6]. Autophagy is originally named as a process of protein recycling. It begins with sequestering cytoplasmic organelles in a membrane vacuole called autophagosome, which are double-membrane cytoplasmic vesicles to engulf various cellular constituents, and to fuse with lysosomes, where the sequestered cellular constituents are degraded and recycled.
Tetraarsenic hexoxide (As4O6) has been used as a Korean folk remedy for the management of cancer since the late 1980s because its toxicities were minimal compared to conventional cytotoxic chemotherapy. However, the anticancer effects of As4O6 have not been investigated much although the anticancer effects of arsenic trioxide (As2O3) have been investigated in many leukemic cells [7–9]. A comparison study of the anticancer effects between As2O3 and As4O6 demonstrated that As4O6 was more effective in suppressing human cancer cells in vitro and in vivo, and that As4O6-induced cell death pathway was different from that of As2O3 [10]. Upregulation of p53 and v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (ERBB2) was noted in As4O6-induced cell, but not in As4O6-induced cell death. In addition, As4O6 has been used orally, whereas As2O3 has been used as a parenteral drug. Oral agents are more convenient to take than parenteral agents. Hence identifying the molecular mechanisms involved in its anticancer effects would allow us to contribute to developing a new oral agent. Here, we investigated the mechanisms of anticancer effects of As4O6 in U937 human leukemic cells.
2. Materials and Methods
2.1. Cells and Reagents
U937 human leukemic cells from the American type culture collection (Rockville, MD, USA) were cultured in RPMI 1640 medium (Invitrogen Corp, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (GIBCO BRL, Grand Island, NY, USA), 1 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2. The Bcl-2 overexpressing U937 cells were a generous gift from Dr T.K. Kwon (Department of Immunology, Keimyung University School of Medicine, Taegu, Republic of Korea) and were maintained in a medium containing 0.7 μg/mL geneticin (G418 sulfate). As4O6 was obtained from Chonjisan institute (Seoul, Republic of Korea). Antibodies against Bcl-2, Bax, Bad, Bcl-xL, XIAP, procaspase 3, procaspase 8, and procaspase 9 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against poly (ADP-ribose) polymerase (PARP), PLCγ-1, LC3, and Beclin-1 were purchased from PharMingen (San Diego, CA, U.S.A.). Antibody against β-actin was from Sigma (Beverly, MA). Peroxidase-labeled donkey anti-rabbit and sheep anti-mouse immunoglobulin and an enhanced chemiluminescence (ECL) kit were purchased from Amersham (Arlington Heights, IL). Caspase activity assay kits were purchased from R&D systems (Minneapolis, MN, USA.). All other chemicals not specifically cited here were purchased from Sigma Chemical Co. (St. Louis, MO). All these solutions were stored at −20°C. Stock solutions of DAPI (100 μg/mL) and propidium iodide (PI, 1 mg/mL) were prepared in phosphate-buffered saline (PBS).
2.2. Cell Viability Assays
For the cell viability assay, the cells were seeded onto 24-well plates at a concentration of 5 × 105 cells/mL and then treated with the indicated concentration of As4O6 for 24 h. MTT (0.5 mg/mL) was subsequently added to each well. After 3 h of additional incubation, 100 μL of a solution containing 10% SDS (pH 4.8) plus 0.01 N HCl was added to dissolve the crystals. The absorption values at 570 nm were determined with an ELISA plate reader.
2.3. Nuclear Staining
After treatment with the indicated concentration of As4O6, the cells were harvested, washed with phosphate-buffered saline (PBS), and fixed with 3.7% paraformaldehyde in PBS for 10 minutes at room temperature. Fixed cells were washed with PBS and stained with 2.5 μg/mL 4,6-diamidino-2-phenylindole (DAPI) solution for 10 min at room temperature. The cells were washed two times with PBS and analyzed by a fluorescent microscope.
2.4. Flow Cytometry Assay
The cells were plated at a concentration of 2 × 105 cells/well in six-well plates. Reduced (sub-G1) DNA content was measured by PI staining. The DNA content in each cell nucleus was determined with a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, U.S.A.). Two independent experiments were performed [11].
2.5. Western Blotting
The cells were harvested and lysed, and protein concentrations were quantified using the BioRad protein assay (BioRad Lab., Hercules, CA, U.S.A.). The proteins of the extracts were resolved by electrophoresis, electrotransferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), and then the membrane was incubated with the primary antibodies followed by a conjugated secondary antibody to peroxidase. Blots were developed with an ECL detection system.
2.6. Caspase Activity Assay
Caspase activity was determined by a colorimetric assay according to the manufacturer's protocol in a kit for caspase activity. In brief, the cells were lysed in the supplied lysis buffer. The supernatants were collected and incubated with the supplied reaction buffer containing dithiothreitol and substrates at 37°C. The reaction was measured by determining the change in absorbance at 405 nm using the microplate reader [12].
2.7. Quantification of Acidic Vesicular Organelles (AVOs) with Acridine Orange Staining
In acridine orange-stained cells, the cytoplasm and nucleolus fluoresce bright green and dim red, whereas acidic compartments fluoresce bright red. Therefore, we stained the cells with acridine orange for 17 min. Green (510–530 nm) and red (650 nm) fluorescence emission from 1 × 104 cells illuminated with blue (488 nm) excitation light was measured with a a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, U.S.A.). Three independent experiments were performed.
2.8. Statistics
Each experiment was performed in triplicate. The results were expressed as means ± SD. Significant differences were determined using the one-way analysis of variance (ANOVA) with post-test Neuman-Keuls in the cases at least three treatment groups and Student's t-test for two group comparison. Statistical significance was defined as P < 0.05.
3. Results
3.1. Responses of U937 Human Leukemic Cells to As4O6
To investigate the antitumor activity of As4O6, U937 cells were treated with various concentrations of As4O6 for 24 h. The cell growth was assessed by MTT assay. The MTT assay revealed that the growth of U937 cells was inhibited by As4O6 treatment in a dose- and time-dependent manner, and the 50% inhibition of cell growth (IC50) was less than 2 μM (Figures 1(a) and 1(b)). The efficacy of As4O6 was superior to that of As2O3 in terms of growth inhibition (Figure 1(a)).
Figure 1.
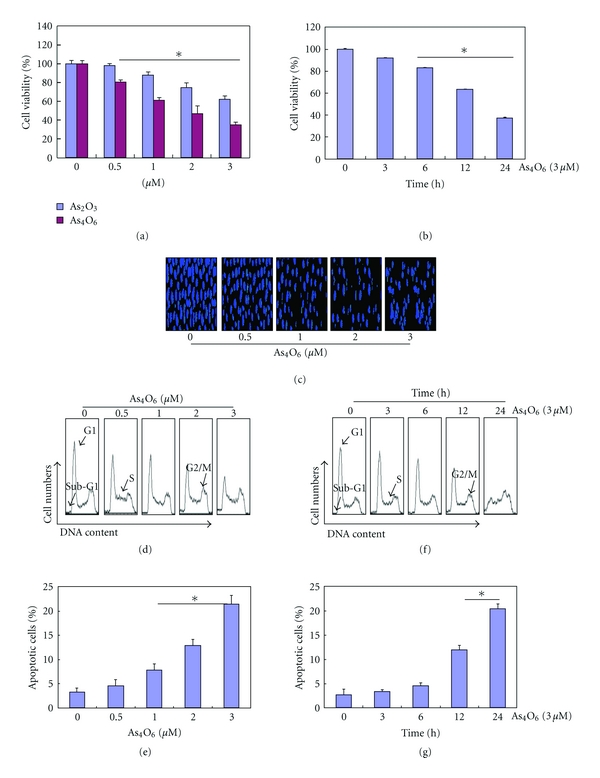
Inhibition of cell growth and induction of apoptosis by As4O6 in U937 cells. The growth inhibition and cytotoxicity As4O6 are a dose- and time-dependent manner. The efficacy of As4O6 is superior to that of As2O3. The cells were seeded at the density of 5 × 104 cells per mL. The inhibition of cell growth was measured by MTT assay. (a) and (c) The cells were treated with the indicated concentrations of As4O6 and As2O3 for 24 hours. (b) and (f) The cells were treated with 3 μM of As4O6 for the indicated times. The growth inhibition and cytotoxicity As4O6 are exhibited in a time-dependent manner. (c) After fixation, the cells were stained with DAPI solution to observe apoptotic bodies, which were more frequently seen in higher doses. Stained nuclei were then observed under fluorescent microscope using a blue filter (Magnification, X 400). (d)–(g) To quantify the extent of As4O6-induced apoptosis, sub-G1 DNA content, which represents the fractions undergoing apoptotic DNA degradation, was analyzed by flow cytometry. The data are shown as means ± SD of three independent experiments. *P < 0.05 between the treated and the untreated control groups.
3.2. Effects of As4O6 on Apoptosis
To determine whether the decrease in viability of U937 cells was caused by the induction of apoptosis, we assessed the changes in nuclear morphology of As4O6-treated cells by DAPI staining. The DAPI staining revealed the condensed and fragmented nuclei at a concentration of 2 μM or higher. This is usually witnessed in apoptosis (Figure 1(c)). To estimate the population of the cell death, we measured cells with sub-G1 DNA content by flow cytometry. A significant accumulation of cells with sub-G1 DNA content was noted in a dose-dependent (Figures 1(d) and 1(e)) and time-dependent manner (Figures 1(f) and 1(g)).
3.3. Caspases Activation and Subsequent Cleavage of Their Substrates by As4O6
We then assessed the effects of As4O6 on caspases and their substrates (PARP and PLCγ-1). As4O6 decreased the expression levels of procaspase-3, procaspase-8, and procaspase-9 in a dose- and time-dependent manner. With the decrease of procaspases, the cleavages of PARP and PLCγ-1, the substrates of caspases, were found to be progressed in a dose- and time-dependent manner (Figures 2(a) and 2(b)). These findings suggest that As4O6 may induce apoptosis through caspase activation. To confirm and quantify the proteolytic activation of caspases, we assessed their activities using colorimetric assay kits. The caspase activity assay also showed that As4O6 increased proteolytic activities of caspases in a dose- and time-dependent manner (Figures 2(c) and 2(d)).
Figure 2.
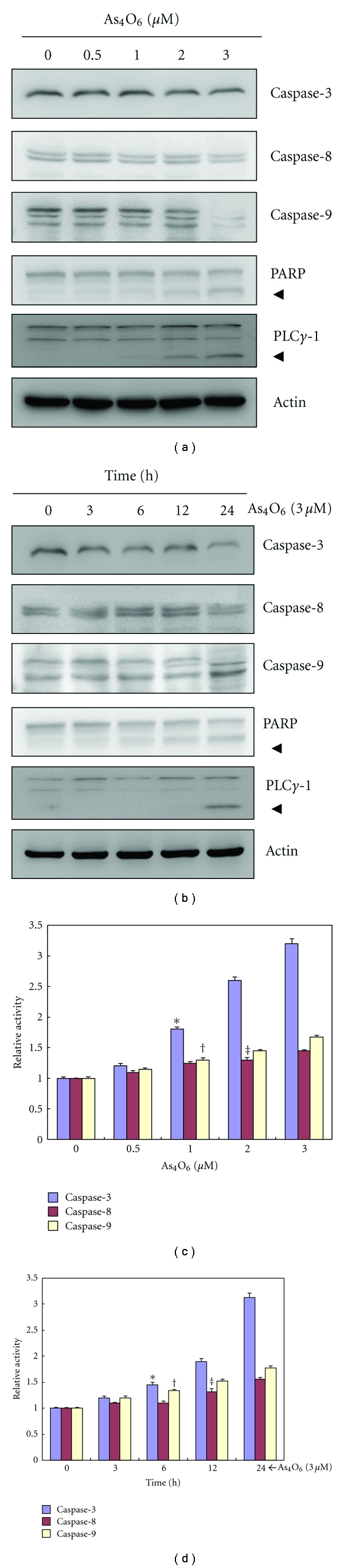
Activation of caspases and cleavage of PARP during the As4O6-induced apoptosis in U937 cells. The activation of caspases and cleavage of PARP by As4O6 are a dose- and time-dependent. (a) and (c) The cells were incubated at the indicated concentrations of As4O6 for 24 h. (b) and (d) The cells were treated with 3 μM of As4O6 for the indicated times. (a) and (b) Total cell lysates were resolved by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the anticaspase-3, anticaspase-8, anticaspase-9, and anti-PARP antibodies. The proteins were visualized using an ECL detection system. β-Actin was used as an internal control. (c) and (d) The cell lysates from the cells treated with As4O6 were assayed for in vitro caspase-3, caspase-8, and caspase-9 activity using DEVD-pNA, IETD-pNA, and LEHD-pNA, respectively, as substrates. The released fluorescent products were measured. Each bar graph represents mean ± SD of three independent experiments. *P < 0.05 between the treated and the untreated control groups.
3.4. Effects of As4O6 on Bcl-2 Family Members and X-Linked Inhibitor of Apoptosis (XIAP)
To elucidate further underlying mechanisms of As4O6-induced apoptosis, we assessed the levels of Bax, Bcl-2, Bad, Bcl-xL, and XIAP, which play a crucial role in apoptosis. Western blotting revealed that As4O6 induced an increase in the expressions of Bax (proapoptotic protein) in a dose- and time-dependent manner whereas the expression of Bcl-2, Bad, Bcl-xL, and XIAP (antiapoptotic proteins) remained unchanged or slightly reduced (Figure 3(a)). The induction of Bax expression began to clearly be observed at 12 hours after the treatment (Figure 3(b)). This finding suggested the possibility that the mechanism of Bax induction was related to the transcriptional activity. These findings suggested that upregulation of Bax protein and increased Bax/Bcl-2 ratio should be an important mechanism of As4O6-induced apoptosis in U937 cells.
Figure 3.
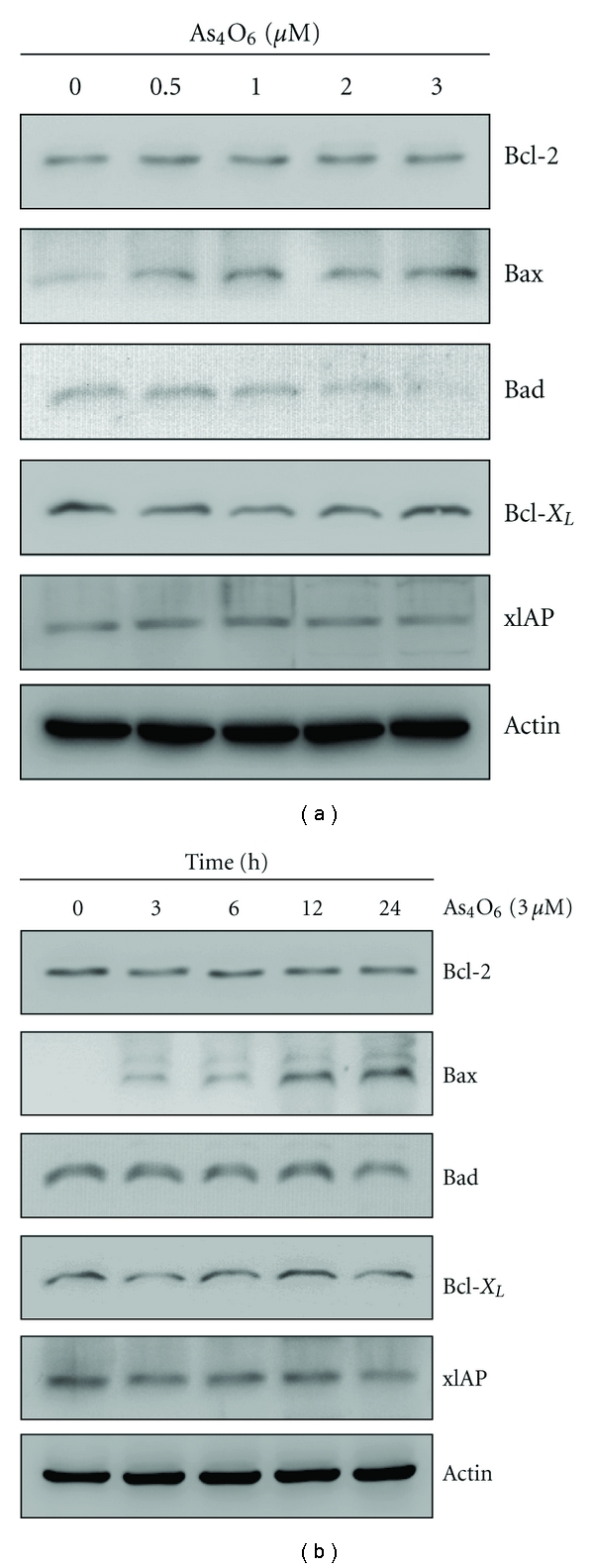
Regulation of Bcl-2 and IAP family proteins during As4O6-induced apoptosis. As4O6 increases the expressions of Bax in a dose- and time-dependent manner whereas the expressions of Bcl-2, Bad, Bcl-xL, and XIAP remain unchanged or slightly reduced. (a) The cells were treated with the indicated concentrations of As4O6 for 24 h. (b) The cells were treated with 3 μM of As4O6 for the indicated times. The cells treated with As4O6 were lysed and equal amounts of proteins were then separated by SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were probed with the indicated antibodies and detected by an ECL detection system. To confirm equal loading, the blot was stripped of the bound antibody and reprobed with the anti β-Actin antibody. The results are from one representative experiment of at least two independent experiments that showed similar patterns.
3.5. Effects of As4O6 on Autophagy
Many studies have demonstrated that As2O3 can induce cell death through autophagy [13]. During autophagy, LC3-1 is converted to membrane-bound LC3-II that correlates with the extent of autophagosome formation which characterizes autophagy. For the autophagosome formation, Beclin-1 is important in mammalian cells. Hence, we assessed the expression of LC-3 (a marker for autophagy) and beclin-1 to check whether As4O6-induced cell death is involved in type II programmed cell death, autophgy. Western blotting revealed that As4O6 induced LC3 conversion (increase in the ratio of LC3-II/LC3-I) and increased the expressions of beclin-1 in a dose- and time-dependent manner (Figures 4(a) and 4(b)). The level of autophagosome formation corresponds with the ratio of LC3-II/LC3-I. Moreover, we also obtained evidence for As4O6-induced autophagy by measuring AVO formation through acridine orange staining. As shown in Figures 4(c) and 4(d), As4O6 induced the accumulation of AVO in a dose- and time-dependent manner.
Figure 4.
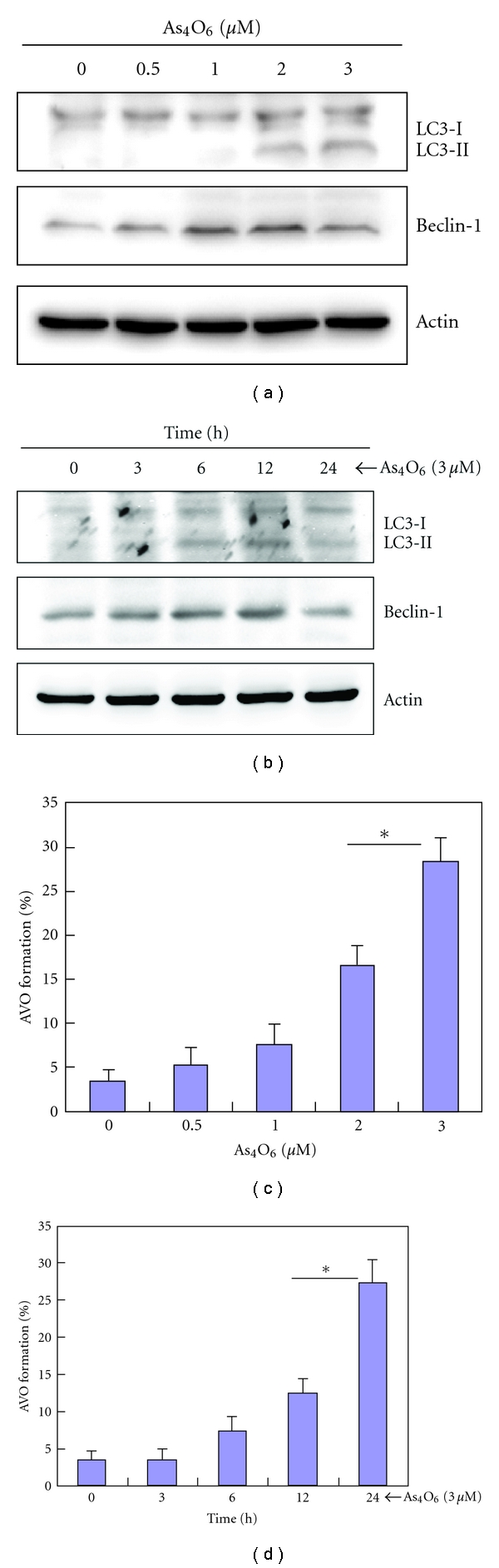
Effects of As4O6 on the autophagy in U937 cells. AVO formation by As4O6 is dose- and time-dependent. (a) and (c) The cells were treated with the indicated concentrations of As4O6 for 24 h. (b) and (d) The cells were treated with 3 μM of As4O6 for the indicated times. (a) and (c) The cells treated with As4O6 were lysed and equal amounts of proteins were then separated by SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were probed with the indicated antibodies and detected by an ECL detection system. To confirm equal loading, the blot was stripped of the bound antibody and reprobed with the anti β-Actin antibody. (b) and (d) The cells treated with As4O6 were stained with 5 μg/mL acridine orange for 17 min and collected in phenol red-free growth medium. Green (510–530 nm) and red (650 nm) fluorescence emission illuminated with blue (488 nm) excitation light was measured with a FACSCalibur (Becton Dickinson).
3.6. Effects of Bcl-2 on As4O6-Induced Autophagy and Apoptosis
From the above, we found that As4O6 induced not only apoptosis through Bax induction but also autophgy through Beclin-1 induction. It has been suggested that the autophagy can be induced by apoptotic insults through up-regulation of Beclin-1. Bcl-2 is a well-known antiapoptotic molecule, and the interaction between Bcl-2 and Beclin-1 is important in the induction of autophgy. Therefore, we assessed Beclin-1 response to Bcl-2 overexpression and the effects of Bcl-2 overexpression on As4O6-induced autophgy and apoptosis by comparing those between U937/vector and U937/Bcl-2 cells that constitutively express high levels of Bcl-2. As shown in Figure 5(a), Bcl-2 overexpression led to significantly suppress the apoptosis induced by As4O6. We assessed the changes in nuclear morphology of As4O6-treated cells by DAPI staining. The DAPI staining showed that Bcl-2 overexpression reduced the frequency of condensed and fragmented nuclei in the As4O6-treated U937 cells which indicate apoptosis (Figure 5(b)). We also assessed the effects of Bcl-2 overexpression on As4O6-induced autophagosome formation. It reduced the As4O6-As4O6-induced AVO formation (Figure 5(c)). To confirm this finding at the molecular level, we performed western blotting for the molecules involved in As4O6-induced apoptosis and autophagy. It was observed on Western blotting that the overexpression of Bcl-2 suppressed the induction of Beclin-1 and LC3 conversion in response to As4O6, with the suppression of As4O6-induced caspase-3 activation and PARP cleavages (Figures 5(d) and 5(e)). These findings suggested that the increased Bcl-2 should significantly influence the antitumor effects of As4O6 through suppressing autophagy as well as apoptosis, and that Beclin-1 induction by As4O6 might be related to apoptosis induction.
Figure 5.
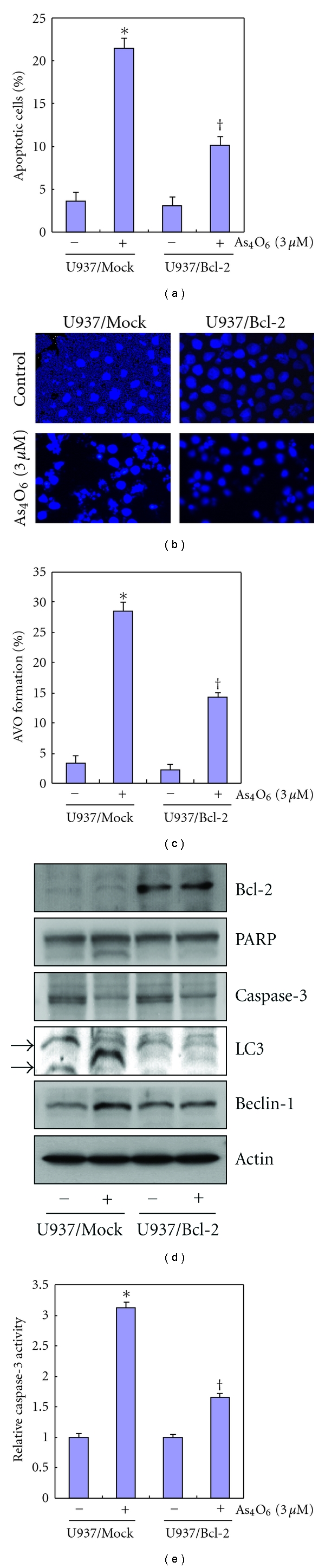
Effects of Bcl-2 overexpression in U937 cells on the apoptosis and autophagy induced by As4O6. Overexpression of Bcl-2 suppresses the induction of Beclin-1 and LC3 conversion in response to As4O6 as well as As4O6-induced caspase-3 activation and PARP cleavages (a) U937/vector or U937/Bcl-2 cells were treated with 3 μM of As4O6 for 24 h. Sub-G1 DNA content was analyzed by flow cytometry. (b) To confirm apoptosis, the cells were stained with DAPI solution after fixation. Stained nuclei were then observed under fluorescent microscope using a blue filter (Magnification, X 400). (c) The cells treated with As4O6 were stained with 5 μg/mL acridine orange for 17 min, and collected in phenol red-free growth medium. Green (510–530 nm) and red (650 nm) fluorescence emission illuminated with blue (488 nm) excitation light was measured with a FACSCalibur (Becton Dickinson). (d) The cells were lysed and equal amounts of proteins were then separated by SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were probed with the indicated antibodies and detected by an ECL detection system. (e) The cell lysates from the cells treated with As4O6 were assayed for in vitro caspase-3activity using DEVD-pNA. The released fluorescent products were measured. The data are shown as means ± SD of three independent experiments. *P < 0.05 between the groups treated with and without As4O6, † P < 0.05 between the U937/vector and U937/Bcl-2 cells.
3.7. Inhibition of As4O6-Induced Apoptosis and Autophagy in U937 Cells by N-Acetylcysteine (NAC)
A previous study showed that As4O6 induced reactive oxygen species (ROS) leading to loss of mitochondrial potential (MMP, ΔΨm) [14]. In addition, As2O3 induced apoptosis in leukemic cell lines via modulation of the glutathione (GSH) redox system [15]. NAC is an antioxidant that functions by donating a cysteine to the de novo synthesis of GSH. To assess the effects of NAC on As4O6-induced autophgy and apoptosis, we analyzed the cells with sub-G1 DNA content and AVOs using flow cytometry after As4O6 treatment and observed changes in nuclear morphology of As4O6-treated cells by DAPI staining. We found that NAC reduced the As4O6-induced autophagosome formation as well as As4O6-induced cell death (Figures 6(a) and 6(b)). The DAPI staining revealed that NAC reduced the frequency of condensed and fragmented nuclei in the As4O6-treated U937 cells (Figure 6(c)).
Figure 6.
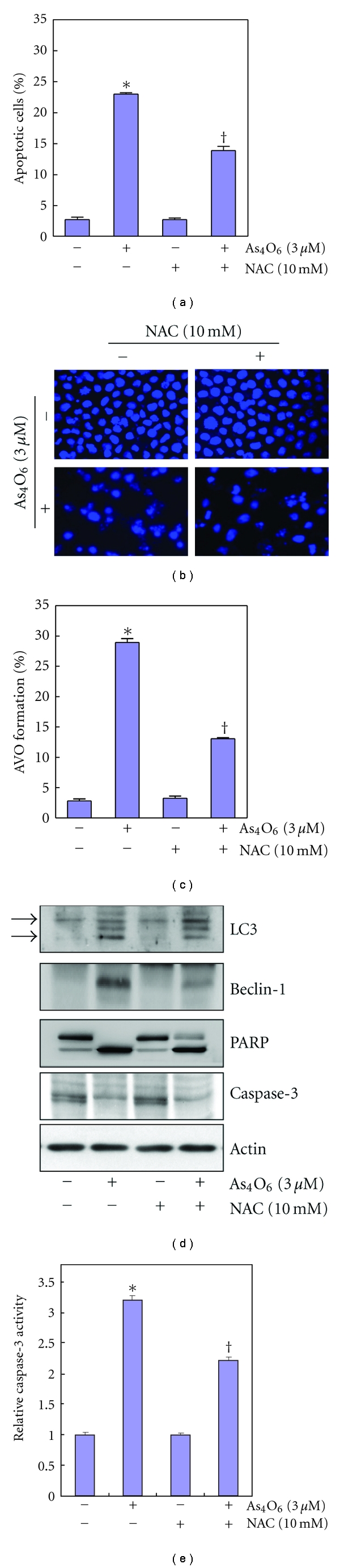
Inhibition of As4O6-induced apoptosis and autophagy in U937 cells by N-acetylcysteine (NAC). NAC reduces the As4O6-induced autophagosome formation as well as As4O6-induced cell death. (a) U937 cells were treated with NAC (10 mM) 30 min before As4O6 (3 μM) for 24 h. The cells treated with As4O6 were stained with 5 μg/mL acridine orange for 17 min and collected in phenol red-free growth medium. Green (510–530 nm) and red (650 nm) fluorescence emission illuminated with blue (488 nm) excitation light was measured with a FACSCalibur (Becton Dickinson). (b) Sub-G1 DNA content was analyzed by flow cytometry. (c) To confirm apoptosis, the cells were stained with DAPI solution after fixation. Stained nuclei were then observed under fluorescent microscope using a blue filter (Magnification, X 400). (d) The cells were lysed and equal amount of the lysate was separated by SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were probed with the indicated antibodies and detected by an ECL detection system. To confirm equal loading, the blot was stripped of the bound antibody and reprobed with the anti β-Actin antibody. (e) The cell lysates from the cells treated with As4O6 were assayed for in vitro caspase-3activity using DEVD-pNA. The released fluorescent products were measured. The data are shown as means ± SD of three independent experiments. *P < 0.05 between the groups treated with and without As4O6, † P < 0.05 between the groups treated with and without NAC.
To confirm this finding at the molecular level and determine whether the Beclin-1-induction is associated with ROS production, we performed western blotting for the molecules involved in As4O6-induced apoptosis and autophagy. Western blotting revealed that NAC suppressed As4O6-induced Beclin-1 induction and LC3 conversion and As4O6-induced caspase-3 activation and PARP cleavages (Figures 6(d) and 6(e)). These findings suggested that the As4O6-induced autophagy as well as apopotosis should be related to ROS production. These findings suggested that ROS production by As4O6 should be related to Beclin-1-induced autophagy as well as apoptosis.
4. Discussion
This study was designed to determine whether As4O6 has anticancer properties in human leukemic cells and further to investigate the underlying mechanisms as compared to that of the anticancer effects of As2O3. Regarding the As4O6-induced cell death, it has not been reported that autophagic cell death is a critical mechanism for the effects. To gain insights into the mechanisms for As4O6-induced cell death, we investigated the both apoptosis and autophagy. Here, we found that As4O6 did not only induce caspase-dependent apoptotic cell death but also induce autophagic cell death. Arsenic trioxide (As2O3) is well known to have anticancer properties against leukemic cells as well as other cancer cells. The reported mechanisms of As2O3-induced cell death vary depending on the cell lines: caspase-dependent apoptosis [16, 17], caspase-independent [18], and autophagic cell death [13, 19]. Even in the studies on As2O3-induced cell death of U937 cells, some studies reported that caspase-dependent apoptosis is a major mechanism for the cell death [20] and other studies suggested that autophagic cell death is a critical mechanism for the antileukemic effects [13]. In other leukemic cell lines, arsenic trioxide did not only induce apoptosis but also induced autophagic cell death in leukemia cell lines via upregulation of Beclin-1 [21]. The mechanism for As2O3-induced cell death appears similar to that of As4O6 although there is a report showing a significant difference between As2O3- and As4O6-induced cell death [10].
Apoptosis is the process of programmed cell death that can be executed through extrinsic pathway and intrinsic pathway. Either pathway is involved in mitochondrial outer membrane permeabilization which is a critical event in apoptosis [22]. The mitochondrial outer membrane permeabilization is controlled by several factors, such as the Bcl-2 and IAP protein family. The Bcl-2 family consists of proapoptotic factors (e.g., Bax, Bad, etc.) and antiapoptotic factors (e.g., Bcl-2, Bcl-xL, etc.). The Bax/Bcl-2 ratio is known as a key factor in triggering the apoptotic process. We found that caspase-dependent apoptosis was one of mechanisms for the antileukemic effects of As4O6 through the induction of Bax protein. At first we were puzzled at this result (Bax induction by As4O6) in p53-deficient U937 cells because tumor suppressor p53 plays the central role in regulating Bax protein, a proapoptotic protein. However, the previous report that Bax protein can be induced in U937 cells through the transaction of p73 gene can explain our results [23].
This study also suggested that the Beclin-1-induced autophagic cell death could be another mechanism for As4O6-induced cell death. This finding showing As4O6-induced autophagy in As4O6-induced cell death is also similar to that in As2O3-induced leukemic cell death [13, 21]. Recently it has been reported that arsenic trioxide induces a Beclin-1-independent autophagic cell death in ovarian cancer cells [24]. This finding suggested that mechanisms of As2O3-induced cell death should vary depending on the cell lines; so it is not unknown whether our results are applicable to other cancer cells. Therefore, we are going to investigate the mechanism for As4O6-induced cell death in other solid cancer cells. Our results were derived from a single leukemic cell line; so it is difficult to generalize this finding to all leukemic cells. However, those indicated that As4O6-induced Beclin-1 induction which led to autophagy can be another mechanism for its antileukemic effects on U937 cells.
Another limitation is that we have not verified yet whether Beclin-1-induced autophagy is a critical mechanism for As4O6-induced cell death or a mechanism to rescue cancer cells from toxic damage. Now that the autophagic cell death is mainly a morphologic definition (i.e., cell death associated with autophagosomes/autolysosomes), there is still no definite evidence that a specific mechanism for autophagic death actually exists. Nonetheless, it is quite conceivable that the autophagy induced by As4O6 could eventually destroy a cell because it has been reported that autophagic cell death is a major mechanism for the anticancer activities of radiation [25] and temozolomide [26] as well as arsenic compounds [13, 21].
Unlike As2O3-induced cell death in U937 cells, As4O6 did not suppress Bcl-2 expression in this study, but we tested the effects of augmented Bcl-2 on apoptosis and autophagy as well as apoptosis induced by As4O6. We observed that augmented Bcl-2 significantly suppressed the autophagic cell death as well as apoptotic cell death induced by As4O6. This finding is consistent with the previous study [27–29].
In aerobic organisms ROS is produced in the mitochondria via the electron transport chain during energy production. Under normal circumstances, reductive enzymes such as catalase and superoxide dismutase can defend cells from the ROS damage, but if ROS is produced high enough to cause severe cellular damage, a cell may undergo programmed cell death [20, 30]. We observed that NAC suppressed As4O6-induced autophagy as well as As4O6-induced apoptosis. This finding suggested that ROS production should be greatly involved in As4O6-induced autophagy as well as As4O6-induced apoptosis. Although the possibility that Beclin-1-induced autophagy can be a process to rescue cancer cells from As4O6-induced apoptosis could not be excluded, our finding suggested that ROS induced by As4O6 should lead to Beclin-1-induced autophagy.
In conclusion, we have demonstrated that As4O6-induced cell death is carried on through Beclin-1-induced autophagic cell death as well as caspase-dependent apoptosis, and that the ROS production by As4O6 plays important roles in triggering both Beclin-1-induced autophagic cell death and caspase-dependent apoptosis. This study provides evidence that As4O6-induced cell death is related to Beclin-1-induced autophagy as well as caspase-dependent apoptosis and As4O6 might be an effective agent for the treatment of leukemia similar to As2O3.
Acknowledgments
This study was supported by grants from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0820050), and from Basic Science Research Program through the National Research Foundation of Republic of Korea (NRF), the Ministry of Education, Science, and Technology (2010–0001730). The authors thank Professor Hicks Timothy R. for helping the revision and proofreading of this manuscript.
References
- 1.Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–3360. [PubMed] [Google Scholar]
- 2.Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324. [PubMed] [Google Scholar]
- 3.Munshi NC, Tricot G, Desikan R, et al. Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia. 2002;16(9):1835–1837. doi: 10.1038/sj.leu.2402599. [DOI] [PubMed] [Google Scholar]
- 4.Lin YC, Li DR, Lin W. Relationship between radiotherapy enhancing effect of arsenic trioxide and the proliferation and apoptosis of related protein in nasopharyngeal carcinoma patients. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27(8):704–707. [PubMed] [Google Scholar]
- 5.Walker PR, Sikorska M. New aspects of the mechanism of DNA fragmentation in apoptosis. Biochemistry and Cell Biology. 1997;75(4):287–299. [PubMed] [Google Scholar]
- 6.Schwartz LM, Smith SW, Jones MEE, Osborne BA. Do all programmed cell deaths occur via apoptosis? Proceedings of the National Academy of Sciences of the United States of America. 1993;90(3):980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen GQ, Zhu J, Shi XG, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML- RARα/PML proteins. Blood. 1996;88(3):1052–1061. [PubMed] [Google Scholar]
- 8.Zhu XH, Shen YL, Jing YK, et al. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. Journal of the National Cancer Institute. 1999;91(9):772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 9.Look AT. Arsenic and apoptosis in the treatment of acute promyelocytic leukemia. Journal of the National Cancer Institute. 1998;90(2):86–88. doi: 10.1093/jnci/90.2.86. [DOI] [PubMed] [Google Scholar]
- 10.Chang HS, Bae SM, Kim YW, et al. Comparison of diarsenic oxide and tetraarsenic oxide on anticancer effects: relation to the apoptosis molecular pathway. International Journal of Oncology. 2007;30(5):1129–1135. [PubMed] [Google Scholar]
- 11.Cui ZG, Hong NY, Guan J, et al. AMP antagonizes ERK-dependent antiapoptotic action of insulin. Biochemistry and Molecular Biology Reports. 1997;44(3):205–210. doi: 10.5483/BMBRep.2011.44.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Cho SY, Lee JH, Bae HD, et al. Transglutaminase 2 inhibits apoptosis induced by calcium- overload through down-regulation of Bax. Experimental and Molecular Medicine. 2010;42(9):639–650. doi: 10.3858/emm.2010.42.9.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goussetis DJ, Altman JK, Glaser H, McNeer JL, Tallman MS, Platanias LC. Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. Journal of Biological Chemistry. 2010;285(39):29989–29997. doi: 10.1074/jbc.M109.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park IC, Park MJ, Woo SH, et al. Tetraarsenic oxide induces apoptosis in U937 leukemic cells through a reactive oxygen species-dependent pathway. International Journal of Oncology. 2003;23(4):943–948. [PubMed] [Google Scholar]
- 15.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93(1):268–277. [PubMed] [Google Scholar]
- 16.Ai Z, Lu W, Qin X. Arsenic trioxide induces gallbladder carcinoma cell apoptosis via downregulation of Bcl-2. Biochemical and Biophysical Research Communications. 2006;348(3):1075–1081. doi: 10.1016/j.bbrc.2006.07.181. [DOI] [PubMed] [Google Scholar]
- 17.Nutt LK, Gogvadze V, Uthaisang W, Mirnikjoo B, McConkey DJ, Orrenius S. Indirect effects of Bax and Bak initiate the mitochondrial alterations that lead to cytochrome c release during arsenic trioxide-induced apoptosis. Cancer Biology and Therapy. 2005;4(4):459–467. doi: 10.4161/cbt.4.4.1652. [DOI] [PubMed] [Google Scholar]
- 18.Kang YH, Yi MJ, Kim MJ, et al. Caspase-independent cell death by arsenic trioxide in human cervical cancer cells: reactive oxygen species-mediated poly(ADP-ribose) polymerase-1 activation signals apoptosis-inducing factor release from mitochondria. Cancer Research. 2004;64(24):8960–8967. doi: 10.1158/0008-5472.CAN-04-1830. [DOI] [PubMed] [Google Scholar]
- 19.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Research. 2003;63(9):2103–2108. [PubMed] [Google Scholar]
- 20.Sanchez Y, Calle C, de Blas E, Aller P. Modulation of arsenic trioxide-induced apoptosis by genistein and functionally related agents in U937 human leukaemia cells. Regulation by ROS and mitogen-activated protein kinases. Chemico-Biological Interactions. 2009;182(1):37–44. doi: 10.1016/j.cbi.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leukemia Research. 2007;31(3):329–339. doi: 10.1016/j.leukres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Borutaite V. Mitochondria as decision-makers in cell death. Environmental and Molecular Mutagenesis. 2010;51(5):406–416. doi: 10.1002/em.20564. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty J, Banerjee S, Ray P, et al. Gain of cellular adaptation due to prolonged p53 impairment leads to functional switchover from p53 to p73 during DNA damage in acute myeloid leukemia cells. Journal of Biological Chemistry. 2010;285(43):33104–33112. doi: 10.1074/jbc.M110.122705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death and Differentiation. 2010;17(12):1867–1881. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang W, Qin Z, Liang Z. The role of autophagy in sensitizing malignant glioma cells to radiation therapy. Acta Biochimica et Biophysica Sinica. 2009;41(5):341–351. doi: 10.1093/abbs/gmp028. [DOI] [PubMed] [Google Scholar]
- 26.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death and Differentiation. 2004;11(4):448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 27.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Ciechomska IA, Goemans CG, Tolkovsky AM. Why doesn’t Beclin 1, a BH3-only protein, suppress the anti-apoptotic function of Bcl-2? Autophagy. 2009;5(6):881–882. doi: 10.4161/auto.9096. [DOI] [PubMed] [Google Scholar]
- 29.Ciechomska IA, Goemans GC, Skepper JN, Tolkovsky AM. Bcl-2 complexed with Beclin-1 maintains full anti-apoptotic function. Oncogene. 2009;28(21):2128–2141. doi: 10.1038/onc.2009.60. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Wan F, Dutta S, et al. Autophagic programmed cell death by selective catalase degradation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]


