Abstract
The poly(A) signal of the C2 complement gene is unusual in that it possesses an upstream sequence element (USE) required for full activity in vivo. We describe here in vitro experiments demonstrating that this USE enhances both the cleavage and poly(A) addition reactions. We also show that the C2 USE can be cross-linked efficiently to a 55-kD protein that we identify as the polypyrimidine tract-binding protein (PTB), implicated previously in modulation of pre-mRNA splicing. Mutation of the PTB-binding site significantly reduces the efficiency of the C2 poly(A) site both in vivo and in vitro. Furthermore, addition of PTB to reconstituted processing reactions enhances cleavage at the C2 poly(A) site, indicating that PTB has a direct role in recognition of this signal. The C2 USE, however, also increases the affinity of general polyadenylation factors independently for the C2 poly(A) signal as detected by enhanced binding of cleavage-stimulaton factor (CstF). Strikingly, this leads to a novel CstF-dependant enhancement of the poly(A) synthesis phase of the reaction. These studies both emphasize the interconnection between splicing and polyadenylation and indicate an unexpected flexibility in the organization of mammalian poly(A) sites.
Keywords: C2 complement gene, poly(A) signal, upstream sequence element, PTB, cleavage and polyadenylation
Polyadenylation of eukaryotic mRNAs involves the endonucleolytic cleavage of the pre-mRNA followed by the addition of a poly(A) tail to the upstream cleavage product. The signals that dictate the precise site of polyadenylation and determine the efficiency of the process have been studied extensively. These RNA signals are recognized by protein factors that act cooperatively to promote cleavage and poly(A) addition (for recent review, see Colgan and Manley 1997).
The sequence AAUAAA, present 15–30 nucleotides upstream of the poly(A) site of nearly all higher eukaryotic mRNAs (Proudfoot and Brownlee 1976; Wickens 1990), has been shown to have a central role in selection of a poly(A) signal and is recognized by the protein CPSF (cleavage polyadenylation specificity factor). CPSF is essential for both processes and is composed of four polypeptides, one of 160 kD, which interacts directly with the AAUAAA hexamer (Keller et al. 1991; Murthy and Manley 1995), a 100-kD (Jenny et al. 1994), and a 73-kD subunit (Jenny et al. 1996). A 30-kD polypeptide is also part of CPSF (Bienroth et al. 1991; Barabino et al. 1997), although it is not always essential for activity in vitro (Murthy and Manley 1992). A GU- or U-rich sequence is in most cases positioned downstream of the cleavage site and has been shown to enhance the efficiency of polyadenylation (Gil and Proudfoot 1984, 1987; McDevitt et al. 1984, 1986; McLauchlan et al. 1985; Chou et al. 1994). This element is known to bind CstF (cleavage stimulation factor; Weiss et al. 1991; MacDonald et al. 1994; Takagaki and Manley 1997), which in turn stabilizes the binding of CPSF to the RNA and greatly increases the efficiency of the 3′ cleavage reaction (Gilmartin and Nevins 1991; Murthy and Manley 1992). CstF has three subunits of 77, 64, and 50 kD (Takagaki et al. 1990). The 64-kD subunit (CstF-64) has a ribonucleoprotein (RNP)-type RNA-binding domain (Takagaki et al. 1992) and is responsible for the binding of CstF to pre-mRNA (Wilusz and Schenk 1988; Takagaki et al. 1990; Gilmartin and Nevins 1991; Takagaki and Manley 1997). The 77-kD subunit binds to the 160-kD subunit of CPSF (Murthy and Manley 1995), and also bridges the 64-kD and 50-kD subunits of CstF (Takagaki and Manley 1994). The nuclease responsible for the cleavage of the precursor RNA has not been characterized yet, although it is known that CFIm and CFIIm (cleavage factor I and II, mammalian) promote this reaction (Takagaki et al. 1989; Ruegsegger et al. 1996, 1998). Following cleavage, a tail of 200–300 adenylate residues is added to the 3′ end of the pre-mRNA by poly(A) polymerase (PAP; Ryner et al. 1989; Raabe et al. 1991; Wahle et al. 1991). Furthermore, PAP binds to the 160-kD subunit of CPSF and so further stabilizes the CPSF–RNA complex (Murthy and Manley 1992, 1995). Perhaps reflecting this, PAP is also required for cleavage of all poly(A) signals analyzed to date with the exception of SV40 late poly(A) signal (e.g., Takagaki et al. 1988).
USEs (upstream sequence elements) were first identified in viral poly(A) signals where they participate in the selection of poly(A) sites in these complex transcription units—ground squirrel hepatitis virus (GSHV) (Russnak and Ganem 1990; Russnak 1991), adenovirus L1 (DeZazzo and Imperiale 1989), L3 (Prescott and FalckPedersen 1992, 1994), L4 (Sittler et al. 1994), SV40 late (Carswell and Alwine 1989), and HIV-1 (Brown et al. 1991; DeZazzo et al. 1991; Valsamakis et al. 1991; Cherrington and Ganem 1992). In the case of HIV-1, the RNA structure defined by transactivation response (TAR) element has been proposed to be necessary to bring the USE closer to the AAUAAA motif and promote cleavage/polyadenylation (Gilmartin et al. 1992). In two different cases, the binding of a protein to a USE results in the activation of polyadenylation. The USE of the HIV-1 poly(A) signal directly contacts CPSF (Gilmartin et al. 1995), whereas the USE of the SV40 late poly(A) signal interacts with the U1 small nuclear RNP (snRNP) protein A (Lutz and Alwine 1994), which stabilizes CPSF binding (Lutz et al. 1996).
Recently, we have identified USEs in two cellular genes, one encoding the complement factor C2 (Moreira et al. 1995) and the other lamin B2 (Brackenridge et al. 1998). We showed that polyadenylation of transcripts from both genes is activated by their respective USEs in vivo. Extensive mutagenesis of the C2 USE demonstrated that a 53-nucleotide sequence immediately upstream to the AAUAAA is required for full activity. As in the case of the viral and lamin B2 USEs, this element is U-rich (42%). Furthermore, the C2 USE is highly conserved between different mammals (Moreira et al. 1995).
Here we present in vitro data showing that the presence of the C2 USE enhances both cleavage and poly(A) synthesis reactions. Furthermore, a 55-kD protein specifically UV crosslinks to the USE and we identify this protein as the previously characterized polypyrimidine tract-binding protein (PTB). A direct role for PTB in the function of the USE is suggested strongly by experiments demonstrating that mutation of the PTB-binding site significantly reduces the efficiency of the C2 poly(A) signal in vivo and cleavage in vitro. Furthermore we show that recombinant PTB can directly activate C2 RNA cleavage. PTB was originally isolated as an activity that binds to the polypyrimidine tract present near the 3′ splice site of introns (Gil et al. 1991; Patton et al. 1991). It has subsequently been shown to be capable of affecting alternative splice site selection (Lin and Patton 1995; Singh et al. 1995). PTB has also been found associated with an intronic element capable of activating polyadenylation (Lou et al. 1996). Finally, we show that the C2 USE is required for efficient UV crosslinking of CstF-64 to the poly(A) signal, a function attributed previously only to downstream elements. We present additional data that in this context CstF can enhance the second step of the reaction, a previously undocumented activity of this factor. These data therefore indicate that the C2 USE mediates its effect by interaction with both PTB and components of the basal polyadenylation apparatus.
Results
The C2 USE enhances both cleavage and poly(A) addition in vitro
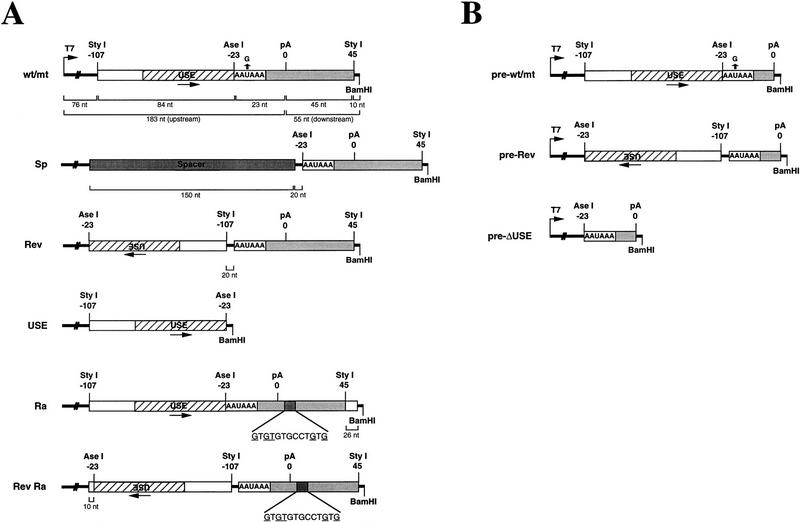
Previous data obtained by analysis of RNA isolated from transfected HeLa cells demonstrated that the USE of the C2 poly(A) signal activates 3′ end-formation (Moreira et al. 1995). To investigate in vitro how the C2 USE functions, we made several pGEM-7 C2 poly(A) signal constructs to allow production of synthetic RNAs. Figure 1 represents schematically these synthetic RNAs, which are divided into those that contain entire poly(A) sites (Fig. 1A) or that end with a restriction site positioned near the site of polyadenylation (Fig. 1B). The wild-type C2 poly(A) signal (wt) is the 152-nucleotide StyI fragment shown previously to be required to promote efficient polyadenylation in vivo (Moreira et al. 1995). This DNA fragment contains 45 bp downstream of the cleavage site and 84 bp upstream of the AAUAAA. The USE is contained within 53 nucleotides immediately 5′ of the AAUAAA. mt has a mutation in the AAUAAA (AAGAAA), Sp has a spacer fragment of 150 nucleotides (isolated from the Escherichia coli lacZ gene) in place of the USE sequence, whereas Rev has the USE in reverse orientation. Although the rabbit C2 poly(A) signal is closely homologous to the human poly(A) signal, the sequence downstream of the cleavage site diverges, as it possesses a GU-rich element, in contrast to the human sequence (Moreira et al. 1995). To create a GU-rich downstream element (DSE) in the wild-type construct, 13 nucleotides downstream of the cleavage site were replaced by the same region from the rabbit sequence (Ra). RevRa has the upstream element inverted in this background. A construct that only contains the upstream element was also made (USE). The RNAs corresponding to these constructs were synthesised in vitro by T7 RNA polymerase in the presence of a radioactive nucleotide, and incubated with either nuclear extracts or partially purified protein fractions to test for mRNA 3′ end formation activity. The sizes of the RNAs and expected cleavage products are shown underneath the wild-type construct.
Figure 1.
C2 poly(A) signal RNAs used in the in vitro 3′ end processing reactions. (A) DNA fragments corresponding to the C2 poly(A) signal were subcloned into pGEM–7Zf(−) to allow RNA synthesis (see Materials and Methods). The AAUAAA is indicated, and the USE is represented by a hatched box. The site of cleavage/poly(A) addition is indicated by 0. The downstream sequence to the AAUAAA is denoted by a gray box. The wild-type RNA has the −107/+45 StyI DNA fragment corresponding to the human C2 poly(A) signal. mt is the same RNA with a point mutation in the AAUAAA as indicated. Sp has a 150-nucleotide spacer sequence in place of USE and Rev has the USE in the reverse orientation to the wild-type RNA. Ra has the sequence downstream of the cleavage site substituted by the corresponding GU-rich sequence from rabbit. RevRa has the USE in reverse orientation to Ra. USE has only RNA sequence from the StyI–AseI DNA fragment. The sizes of the precursor wild-type RNA obtained and the upstream and downstream cleavage products expected after in vitro cleavage are indicated by brackets underneath the wild-type RNA. It should be noted that the precursor size for Ra is 26 nucleotides larger than for wild type because of additional sequence downstream of the C2 sequence (denoted by the unshaded box downstream of the 3′ StyI site; see Materials and Methods). Similarly extra (20 nucleotides) linker sequence is present in Rev, RevRa, and Sp (denoted by thick line). (B) The C2 DNA fragments used to generate constructs for polyadenylation reactions have the sequence downstream of the cleavage site deleted (see Materials and Methods) and were inserted into pGEM–7Zf, to generate pre-wild type pre-mt and pre-Rev as indicated. pre-ΔUSE has the USE sequence deleted.
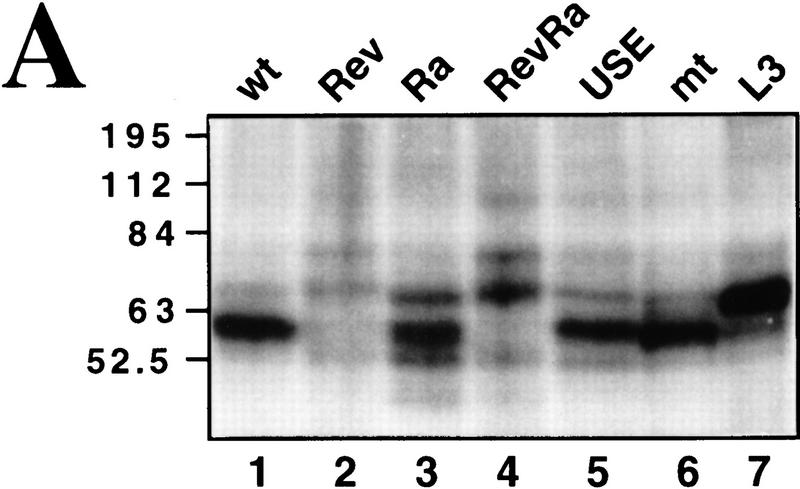
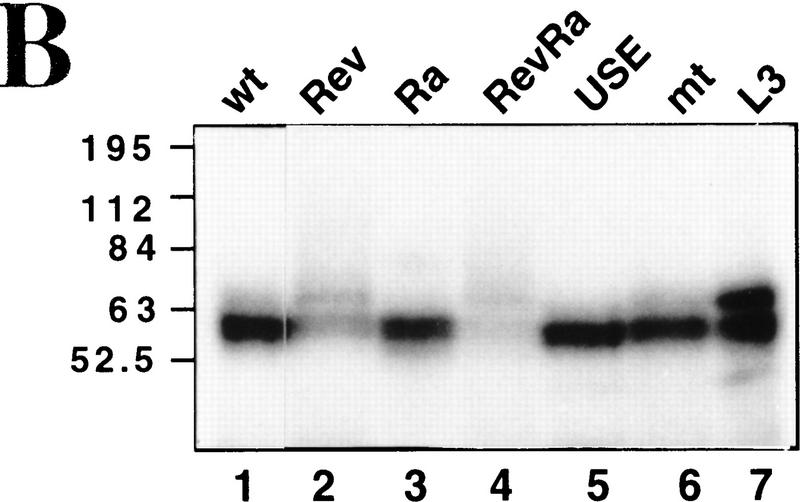
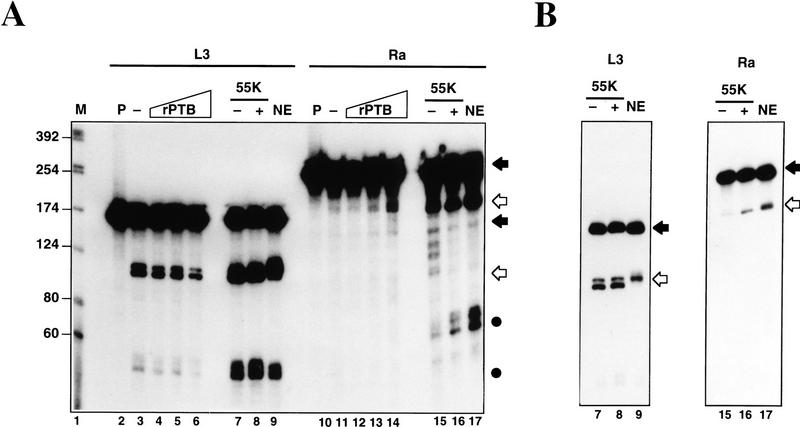
In Figure 2A, cleavage reactions were performed in the presence of a partially purified 3′-processing fraction called CSF (containing CPSF, CstF, CFIm, and CFIIm; Takagaki et al. 1988), with or without PAP, using the C2 substrates described above. EDTA was employed to block polyadenylation. As shown in lanes 2–7 SV40 late and adenovirus L3 poly(A) signals were effectively processed producing the expected upstream (open arrow) and downstream (•) cleavage products. Unlike other poly(A) signals the SV40 late pre-mRNA does not require PAP to be cleaved (Ryner et al. 1989) (Fig. 2A, lanes 3,4). Figure 2A, lanes 8–13, shows similar experiments with C2 substrates. As with the L3 poly(A) signal, the wild-type C2 pre-mRNA was only cleaved at detectable levels in the presence of PAP (Fig. 2A, lane 10). Ra, which contains both the USE and a GU-rich sequence downstream of the AAUAAA, is cleaved more efficiently than wild type (Fig. 2A, lane 13). In particular, the downstream cleavage product of ∼75 nucleotides could be detected. Figure 2B shows cleavage reactions carried out with the various mutated C2 substrates using unfractionated nuclear extracts. 3′ Deoxy-ATP was employed to inhibit poly(A) addition in these experiments. As above, both wild-type and Ra substrates showed cleavage activity over the 60-min time course (Ra gave fourfold more cleavage product than wildtype). Inversion of the USE (Rev and RevRa), however, or its replacement with spacer sequence (Sp) both reduced C2 cleavage at least fivefold.
Figure 2.
The C2 USE activates both in vitro cleavage and polyadenylation. (A) In vitro cleavage reactions (see Materials and Methods), containing radiolabeled SV40 (lanes 2–4), L3 (lanes 5–7), wild type (lanes 8–10), and Ra (lanes 11–13) RNAs and CSF (Takagaki et al. 1988). Cleavage was assayed in the presence (+) or absence (−) of PAP and the cleavage products were analysed on denaturing polyacrylamide gels. Substrate RNAs are indicated by solid arrows, upstream cleavage products by open arrows and downstream cleavage products by dots. Molecular weight markers are denoted by M (lane 1) and input pre-mRNAs by P. (B) Time course of cleavage reactions using wild type, Rev, Sp, Ra, and RevRa RNAs. As before, substrate RNAs are indicated by solid arrows and upstream cleavage products by open arrows. Time in minutes is indicated above each lane. The dependence of the C2 cleavage reaction on the USE sequence is evident. (C) Time course of polyadenylation reactions using pre-wild type, pre-mt, and pre-ΔUSE RNAs over a 60-min period (see Materials and Methods). The appearence of the poly(A) product is evident in pre-wild type (lanes 2–5) and drastically reduced in pre-mt (lanes 6–9) and pre-ΔUSE (lanes 10–13).
Polyadenylation using the precleaved substrates with nuclear extract was also assayed (Fig. 2C). As observed for cleavage, poly(A) synthesis was also greatly enhanced by the presence of the intact USE in the precursor RNA. Therefore, over a 60-min time course, pre-wild type generated significant polyadenylated product, whereas pre-ΔUSE gave very little polyadenylation, at a similar low level to pre-mt, which has the mutated poly(A) sequence AAGAAA. Taken together, these results suggest that the C2 USE significantly enhances both cleavage and poly(A) addition of C2 pre-mRNA in vitro.
PTB cross-links to the USE
To investigate the mechanism of USE-enhanced polyadenylation, we first set out to determine whether proteins that specifically interact with this element could be identified. Protein–RNA interactions can be detected by UV-cross-linking assays where label transfer from a 32P–RNA to the protein is measured. RNA precursors double-labeled with C and U were therefore incubated with nuclear extract or protein fractions to allow cleavage/polyadenylation complexes to form. Following irradiation with UV light, the reaction mixtures were treated with RNase A and the labeled proteins analyzed by SDS-gel electrophoresis. Labeling with A and G gave identical results (data not shown). As show in Figure 3A, using different C2 RNAs incubated with nuclear extract, a protein of ∼55-kD cross-linked to USE-containing pre-mRNAs (lanes 1,3,5,6). Furthermore, Figure 3A, lanes 2 and 4, indicates that when the USE is positioned in the reverse orientation, cross-linking to the 55-kD protein is reduced greatly. The different number of labeled residues present in the sequence of Rev in comparison with wild type (29 and 51 nucleotides, respectively), does not account for the difference observed in the intensity of the bands. Ra and RevRa show similar results to wild type and Rev suggesting that the GU-rich DSE present in these RNAs does not affect 55-kD protein binding (Fig. 3A, cf. lanes 1 and 2 with 3 and 4). USE RNA alone (which corresponds to the 84-nucleotide sequence upstream of the AAUAAA) also cross-linked efficiently to the 55-kD protein, indicating that this sequence alone is sufficient for binding (Fig 3A, lane 5). Finally, cross-linking of the 55-kD protein was not affected by a point mutation in the AAUAAA sequence [AAGAAA mutant (mt) lane 6 of Fig. 3A]. This suggests that the interaction of 55-kD protein with the RNA does not depend on the simultaneous binding of CPSF to the AAUAAA, as CPSF binding requires an intact AAUAAA sequence (e.g., Bardwell et al. 1991)
Figure 3.
The USE of the C2 poly(A) signal cross-links to a 55-kD protein identified as PTB. (A,B) RNA substrates wild type (lane 1), Rev (lane 2), Ra (lane 3), RevRa (lane 4), USE (lane 5), mt (lane 6), and adenovirus-2 L3 (lane 7) were incubated with nuclear extract (A) or a 55-kD protein fraction (B) and the UV-cross-linking assay performed as described in Materials and Methods. (C) UV cross-linking using wild-type RNA and 10- or 100-fold molar excess of recombinant histidine-tagged PTB (lanes 1,2). In lane 3, the 55-kD protein fraction was UV cross-linked to the wild-type pre-mRNA. Immunoprecipitation of cross-linked 55-kD protein was performed as described in Materials and Methods, with anti-PTB serum (lane 4); preimmune serum (lane 5); hybridoma culture supernatant of the mAb anti-64 kD antibody (lane 6); and supernatant of mAb OX1, as an isotype-match control antibody (lane 7). The protein samples were mixed with a reducing protein loading dye and the proteins were separated by SDS-PAGE.
Adenovirus L3 produced only a faint 55-kD protein band on cross-linking (Fig. 3A, lane 7). Although UV-cross-linking assays are nonquantitative, it is interesting to note that a 64-kD protein cross-links efficiently to the L3 RNA and less strongly to the C2 RNAs. It has been shown previously that CstF-64 has a high affinity for the L3 poly(A) signal because of its GU-rich downstream sequence (MacDonald et al. 1994), suggesting that it may be this band. Consistent with this, the 64-kD protein also bound more strongly to the rabbit C2 RNAs, which contain a GU-rich DSE (Fig. 3A, cf. lanes 3 and 4 with 1 and 2). Low levels of cross-linking of a 64-kD protein can be seen in lane 5, where the USE transcript was used. This may suggest a direct and specific interaction of USE and CstF-64, although weak nonspecific binding of CstF to U-rich RNA cannot be ruled out (Takagaki and Manley 1997).
To characterize the 55-kD protein further, a chromatographic fraction enriched in 55-kD cross-linking activity and lacking CSF was used in the UV-cross-linking assay (Fig. 3B; see Materials and Methods). The same USE dependency on the binding of a 55-kD was observed. A band of 55 kD is very strong when the USE is present in the pre-mRNA (Fig. 3B, lanes 1, 3, 5, 6). Increased cross-linking of the 55-kD protein to L3 in this experiment (Fig. 3B, lane 7) is likely to be caused by the higher concentration of the 55-kD protein in this fraction than in the nuclear extract used in Figure 3A. Taken together, these results indicate that a protein of ∼55 kD can be cross-linked to the C2 USE and this process appears to be independent of the AAUAAA and downstream sequences.
PTB is an ∼57-kD protein capable of binding to the polypyrimidine tract present near the 3′ splice site of certain introns (Gil et al. 1991; Patton et al. 1991). Because the C2 USE is pyrimidine-rich and PTB has a similar size to the UV-cross-linking 55-kD protein, we tested whether the 55-kD protein is recognized by PTB-specific antibodies. Immunoprecipitations of the proteins UV-cross-linked to the wild-type C2 RNA were performed, using a polyclonal antibody against PTB. As shown in Figure 3C, the 55-kD protein cross-linked to the wild-type C2 RNA (Fig. 3C, lane 3) was immunoprecipitated with the anti-PTB antibody (lane 4). No band was detected when preimmune serum was used (Fig. 3C, lane 5). Figure 3C, lane 6, corresponds to immunoprecipitation using a monoclonal anti-CstF-64 antibody, and lane 7 to immunoprecipitation with an isotype-matched control antibody, OX1. The absence of any immunoprecipitated proteins in these lanes underlies the specificity of the PTB interaction. The 37-kD protein that also cross-links to the wild-type RNA (Fig. 3C, lane 3) is likely to be heterogeneous nuclear RNP (hnRNP) C, which is known to bind U-rich sequences nonspecifically, as present in this RNA (Wilusz et al. 1990). This has not been tested directly.
To confirm that PTB interacts with the C2 RNA, recombinant PTB was used in the UV-cross-linking assay. As shown in lanes 1 and 2 of Figure 3C, a protein band similar to the one detected with the 55-kD protein fraction is observed when both a smaller or larger amount of recombinant PTB is used. The PTB band detected in lane 4 of Figure 3C is slightly retarded because of excess IgG light chain.
Specific mutation of the PTB-binding site inactivates the C2 poly(A) signal
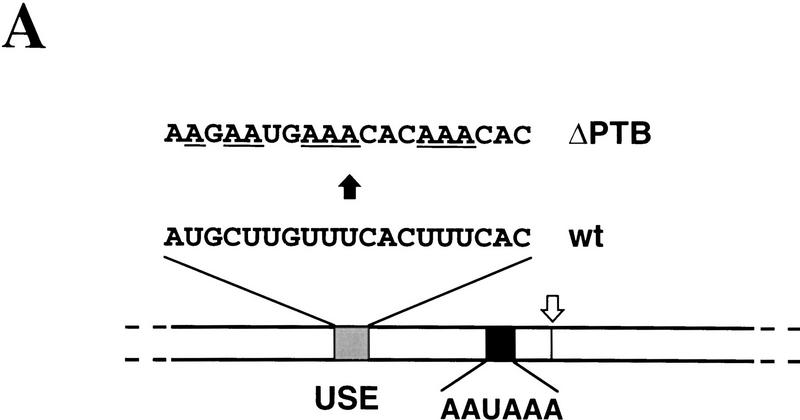
Two studies have defined consensus RNA sequences that bind PTB based on the selection of random sequence by PTB (Singh et al. 1995; Perez et al. 1997). Although neither sequence precisely matches any part of the C2 USE , a central U-rich region has significant homology to these PTB-binding sequences and has been show to directly bind PTB in vitro (data not shown). A specific mutation was therefore generated in this putative PTB-binding site as shown in Figure 4A. Figure 4B shows UV cross-linking of proteins in nuclear extract to the C2 wild-type and ΔPTB RNAs (lanes 1,2). It should be noted that the extract employed in these experiments was prepared differently to that used in Figure 3, and approximately equal cross-linking of CstF-64 and PTB is now seen with the wild-type RNA. The identity of the two major cross-linking species seen in this extract was confirmed by immunoprecipitation using αCstF (Fig. 4B, lane 4) and αPTB (lane 5) antibodies. Strikingly, the ΔPTB mutation results in greatly reduced cross-linking of PTB, even though the signal for CstF-64 is undiminished. This loss of cross-linking signal for the ΔPTB RNA is not simply due to the loss of appropriately positioned label in the mutant RNA, as both labeled U and A residues were incorporated into these RNAs to ensure equivalent labeling over the PTB-binding region. Wild-type C2 RNA labeled only at U residues gives an identical cross-linking pattern to the double-labeled RNA (results not shown). To confirm that the sequence mutated in the ΔPTB RNA does indeed correspond to a PTB binding site, a single-strand DNA oligonucleotide containing this sequence was used as a competitor in crosslinking reactions (Fig. 4B, lanes 7–10). Increasing amounts of this competitor oligonucleotide strongly inhibited the cross-linking of PTB to the wild-type C2 RNA, without affecting the cross-linking of CstF-64. Importantly, a DNA oligonucleotide containing the ΔPTB mutation did not affect the cross-linking of either CstF or PTB (Fig. 4B, lane 11).
Figure 4.
Specific mutation of USE blocks PTB binding. (A) Diagram showing position and sequence of PTB-binding region in the C2 USE with the sequence of the mutated PTB-binding site (ΔPTB) indicated. Altered nucleotides are underscored. Site of cleavage/polyadenylation (open arrow) and the position of the AAUAAA sequence are also shown. (B) UV cross-linking of nuclear extract to wild-type and ΔPTB C2 RNAs. Note that this extract has higher concentrations of CstF than that used in Fig. 3A. Therefore, strong (both CstF-64 and PTB) bands are detected for wild-type RNA (lane 1). Lane 2 shows that ΔPTB RNA no longer binds PTB significantly. Lanes 4 and 5 show immunoprecipitation using anti-CstF-64 and anti-PTB serum of wild-type RNA cross-linked (XL) to nuclear extract as in Fig. 3C. Lanes 6–10 show that the PTB band is competed off by addition of increasing amounts of a DNA oligonucleotide corresponding to the wild-type PTB-binding site (A) but not by the ΔPTB oligonucleotide (lane 11).
Initially we investigated the effect of this ΔPTB mutation on the efficiency of the C2 poly(A) signal, using an in vivo poly(A) site competition assay as described previously (Moreira et al. 1995). As shown in Figure 5A, the C2 wild-type and ΔPTB poly(A) signals were placed downstream of the α2-globin gene in competition with its own poly(A) signal. Following transfection into HeLa cells, the ratio of α2 to C2 poly(A) signal usage was measured by S1 nuclease analysis. As can be seen, the wild-type C2 poly(A) signal is used with approximately the same frequency as the α2 globin signal; in marked contrast, the ΔPTB mutation reduces use of the C2 poly(A) signal ∼10-fold. This suggests that PTB binding to the C2 USE has a critical role in the activity of the C2 poly(A) signal.
Figure 5.


Mutation of PTB-binding site in the C2 USE sequence inhibits in vivo mRNA 3′-end formation and in vitro 3′ cleavage. (A) In vivo poly(A) site competition analysis of C2 wild-type and ΔPTB poly(A) signals placed downstream of the human α2-globin gene in competition with its poly(A) signal. The diagram below shows the 3′ end of the α2-globin/C2 poly(A) site construct with the positions of the poly(A) sites and S1 probe signals indicated (Moreira et al. 1995). The S1 nuclease mapping experiment is shown above. Bands corresponding to usage of either the C2 or α2 poly(A) sites are indicated alongside DNA markers. The ΔPTB mutation results in ∼10-fold inhibition of the C2 poly(A) site (based on PhosphorImager quantitative analysis). (B) Time course of cleavage reactions comparing wild-type and ΔPTB pre-mRNAs. As before, substrate RNAs are indicated by solid arrows, and upstream cleavage products by open arrows. Time in minutes is indicated above each lane. The ΔPTB mutation reduces cleavage activity threefold based on PhosphorImager quantitative analysis. (C) Time course of polyadenylation reactions using pre-wild-type and pre-ΔPTB RNAs over a 60-min period (see Materials and Methods). The appearence of the poly(A) product is evident for both RNAs indicating that the ΔPTB mutation has only a negligible effect on polyadenylation of C2 RNA.
Similar experiments were carried out in vitro to measure the effect of the ΔPTB mutation on either cleavage or poly(A) addition of RNA containing the C2 poly(A) signal. As shown in Figure 5B, the ΔPTB mutation had a significant inhibitory effect (threefold) on the efficiency of wild-type RNA cleavage. In contrast, as shown in Figure 5C, ΔPTB had no significant effect on pre-wild-type RNA poly(A) addition. We conclude from these in vitro experiments that PTB binding to the USE has an activatory effect on cleavage but not polyadenylation. It is possible that the greater effect (10-fold as compared with 3-fold) of this mutation in vivo may result from the ability of PTB to still bind weakly the C2 USE in vitro. Such binding may be excluded in the more tightly regulated in vivo situation.
Recombinant PTB activates cleavage of the C2 poly(A) site
To obtain direct evidence that PTB activates cleavage of the C2 poly(A) site, we tested the effect of adding either recombinant PTB or the 55-kD-enriched fraction to in vitro 3′ processing reactions. Because PTB is already present in unfractionated nuclear extract and to a lesser but significant degree in the CSF fraction used in Figure 2A, we used more highly purified cleavage/polyadenylation fractions. These fractions are less efficient at 3′ processing than nuclear extracts, so cleavage reactions were carried out comparing the more efficient C2 Ra RNA substrate with the L3 control. Figure 6 shows the effect of increasing amounts of PTB on cleavage reactions with L3 and Ra substrates, using a mixture of more extensively purified factors (CPSF, CFIm and IIm, CstF, and PAP). As controls for these experiments, both nuclear extract and CSF were employed and in the case of CSF, the effect of adding the 55-kD fraction was also tested. As shown in Figure 6A, lanes 3–6, it is apparent that PTB has no stimulatory effect on the formation of L3 cleavage products. At the highest concentration of PTB (200 ng, Fig 6A, lane 6), L3 cleavage is inhibited twofold. In contrast, although Ra cleavage is very inefficient with these purified fractions, a low level cleavage product is detectable in Figure 6A, lane 11 which is enhanced twofold with 67 ng (lane 13) and sixfold with 200 ng (lane 14) of added PTB (based on the average of three independent experiments). Increased accumulation of the downstream cleavage product in response to added PTB is also apparent from longer exposures of this gel (data not shown). The fact that high amounts of added PTB actually inhibits cleavage of the L3 template, but activates C2, is strong evidence that the binding of PTB to the C2 USE directly activates cleavage of this RNA substrate. Figure 6, A and B (shorter exposure of the indicated lanes in Fig. 6A), also shows the effect of adding 55-kD fraction to the CSF+PAP-mediated cleavage reactions of L3 and Ra. The 55-kD fraction caused a three fold activation of Ra cleavage (Fig 6, lanes 15, 16), but had no significant effect on L3 processing (Fig. 6, lanes 7,8). This argues against the stimulatory effect of the 55-kD fraction being mediated simply by the presence of general cleavage and polyadenylation factors and confirms the importance of PTB for processing at the C2 poly(A) signal. Note that the downstream products of the cleavage reactions (Fig. 6A, •) were correspondingly increased in the Ra cleavage reaction.
Figure 6.
In vitro cleavage reaction in the presence of recombinant PTB. (A) In vitro cleavage reaction using adenovirus-2 L3 (L3, lanes 2–9) and Ra (lanes 10–17) as RNA substrates. Reactions were performed as described in Materials and Methods. For lanes 3–6 and 11–14 purified fractions were used of CPSF, CFI and CFII, CstF, and PAP plus increasing amounts of recombinant PTB (0, 20, 67, and 200 ng per reaction). For lanes 7, 8, 15, and 16, CSF and PAP + or − a 55-kD fraction was used (see Materials and Methods for details of cleavage/polyadenylation factors employed). Lanes 9 and 17 employed unfractionated nuclear extract. P lanes correspond to the input RNA. (B) Shorter exposure of lanes 7–9 and 15–17, showing that 55-kD fraction enhances cleavage of Ra but not L3 substrates. Note also the enhanced downstream cleavage products (cf. lanes 15 and 16 in A) in the stronger exposure of these lanes (•, A).
We conclude from these experiments and from the analysis of the ΔPTB mutation shown in Figures 4 and 5, that PTB has a direct role in enhancing the cleavage reaction of the C2 pre-mRNA.
USE-dependent activation of poly(A) addition by CstF
Although the C2 USE activates both cleavage and poly(A) synthesis reactions (Fig. 2B,C), PTB only stimulates the cleavage reaction. It is therefore possible that the C2 USE generally increases the affinity of basal polyadenylation factors for the C2 poly(A) signal independently of PTB. To test this possibility, UV-cross-linking experiments were performed with various C2 polyadenylation substrates and a mixture of partially purified CPSF (Takagaki et al. 1989) and highly purified CstF (Takagaki et al. 1990) . As can be seen in Figure 7A, CstF-64 strongly cross-links to both wild-type and pre-wild-type RNAs (Fig. 7, lanes 1 and 4) but more weakly to RNAs with the USE reversed (Fig. 7, lanes 3 and 5). No CstF cross-linking was detected in the absense of CPSF, consistent with the known cooperative effects of these two proteins (data not shown). Because wild-type RNA lacks GU-rich downstream sequence (normally required for CstF binding), whereas pre-wild-type has no downstream sequence, it is likely that CstF-64 interacts with the USE sequence. USE alone retains some binding capacity (Fig. 7, lane 2).
Figure 7.
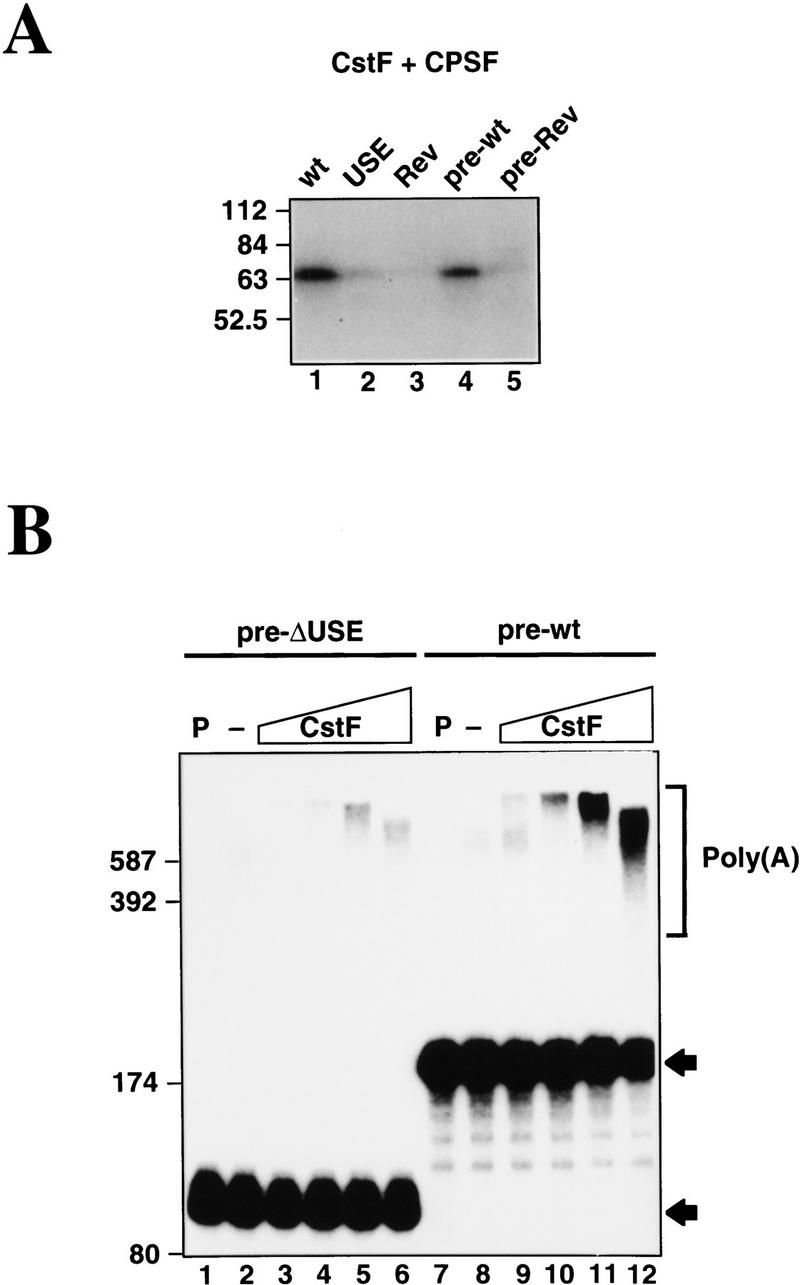
CstF activates USE-dependent polyadenylation of C2 precleaved substrate. (A) UV cross-linking of purified CPSF and CstF to various 32P-labeled C2 RNAs. The cross-linked CstF-64 protein is evident, especially in lane 1 (wt) and lane 4 (pre-wt). (B) Polyadenylation reactions using pre-ΔUSE and pre-wild-type substrates with purified CPSF and PAP together with increasing amounts of purified CstF (0, 5, 15, 50, and 150 ng). Polyadenylated products are indicated. Lanes P correspond to the input RNAs.
The above results raise the possibility that the USE-dependent binding of CstF-64 may be responsible for the PTB-independent effect of the USE on the poly(A) addition step of the reaction. Although CstF has not been observed previously to influence this step, we tested whether increasing concentrations of CstF could enhance poly(A) addition catalyzed by CPSF plus PAP, using as substrates pre-cleaved wild-type C2 RNA (pre-wild-type) and a deletion mutant lacking the USE (pre-ΔUSE). In the absence of CstF, both substrates were polyadenylated very poorly (Fig. 7B, lanes 2,8). Increasing concentrations of CstF stimulated polyadenylation of both the pre-ΔUSE (Fig. 7, lanes 3–6) and pre-wild-type (lanes 9–12) RNAs, most likely by stabilizing the binding of CPSF and PAP to the RNAs. The presence of the USE, however, results in greater stimulation consistent with CstF binding to this sequence. Stable binding of CstF to the pre-wild-type RNA greatly stimulates poly(A) addition, whereas little or no stable interaction of CstF with the pre-ΔUSE RNA diminishes the effect.
We note that poly(A) length shortens at the highest CstF concentration, which also suggests an effect on poly(A) length control. Taken together, the results presented in Figure 7 indicate that CstF is capable of both interacting with upstream sequences and enhancing the second step of the polyadenylation reaction.
Discussion
In this study we show that the USE of the C2 poly(A) signal activates both cleavage and poly(A) addition in vitro (Fig. 2), which agrees well with our previous in vivo data (Moreira et al. 1995). Using both crude extracts and a size-selected fraction, we demonstrate that a protein of ∼55 kD cross-links to this element (Figs. 3 and 4) and further show by immunoprecipitation and UV-cross-linking that it is PTB (Fig. 3). Several lines of evidence implicate PTB in activation of the C2 poly(A) site. First, we identify the binding site for PTB on the USE sequence and then show that mutation of this sequence blocks PTB binding (Fig. 4). Second, this same mutation has a dramatic 10-fold inhibitory effect on the in vivo efficiency of the C2 poly(A) signal. We have previously mutated this region of the C2 poly(A) signal (Moreira et al. 1995) but observed only small, two- to threefold inhibitory effects. Significantly, in this earlier study we either mutated only a part of the PTB-binding site or in one case mutated the whole binding site by replacing Us for Cs and vice versa. In each of these mutations, PTB-binding is still not excluded. We also show in these present studies that the ΔPTB mutation inhibits C2 cleavage, but not poly(A) addition (Fig. 5). Third, we have demonstrated that PTB mediates at least in part the USE-dependent cleavage activation, by reconstituting the effect with recombinant PTB (Fig. 6).
PTB has been shown previously to associate with an additional poly(A) site-enhancing sequence. In this case, an intron enhancer element located 168 nucleotides downstream of the alternatively processed human CT/CGRP exon 4 poly(A) site has been implicated in exon 4 inclusion by activating the upstream poly(A) signal (Lou et al. 1996). This enhancer element has a polypyrimidine tract, a CAG (characteristic of 3′ splice sites), and a 5′ splice site sequence immediately downstream. Importantly, PTB was shown to bind this site, and oligonucleotide competition experiments suggest that this binding is required for poly(A) site activation in vitro. It remains to be established how PTB exerts its stimulatory effect on C2 polyadenylation. One clue may be the fact that the intron enhancer of CT/CGRP and the C2 USE both bind PTB and also enhance the level of the CstF-64 cross-linking to their respective poly(A) sites. Future work will directly address the molecular interaction between PTB and general polyadenylation factors.
A general theme of these different examples of elements that affect the efficiency of nearby poly(A) signals is that there is an overlap between factors associated with poly(A) site selection and splice site selection. PTB was originally isolated as a factor that interacts with the pyrimidine tract adjacent to the pre-mRNA branch site (Gil et al. 1991; Patton et al. 1991). The essential splicing factor U2AF, not PTB, however, was shown to function in recognition of the branch site by interacting with the pyrimidine tract and recruiting U2 snRNP (Zamore and Green 1991). In some cases of alternative splicing, competition between PTB and U2AF may occur such that PTB binds to the pyrimidine tract of an acceptor site, blocking U2AF binding and subsequent splicing. For example, in the case of α-tropomyosin, exons 2 and 3 behave in a mutually exclusive fashion that in part is associated with the binding of PTB to the polypyrimidine tract of the exon 3 acceptor site, which represses the selection of this 3′ splice site (Gooding et al. 1994; Singh et al. 1995). Splice donor sites are also known to regulate the use of poly(A) signals, as it has been demonstrated in the HIV-1 provirus that the major splice donor site inhibits the 5′ long-term repeat (LTR) poly(A) site situated 200 nucleotides upstream (Ashe et al. 1995, 1997), whereas in the case of bovine papillomavirus, a donor site upstream of the late poly(A) site represses its activity (Furth et al. 1994; Gunderson et al. 1998). In both of these examples of donor site poly(A) site inhibition, it is likely that U1 snRNA-binding to the donor site targets snRNA-binding proteins close to the poly(A) site and so blocks polyadenylation by direct protein–protein interactions. Further examples of such regulation of poly(A) site selection are reviewed by Proudfoot (1996).
We have shown in these experiments that the C2 USE not only activates 3′ end cleavage but also has a significant activating effect on poly(A) addition. Although PTB activates the cleavage reaction, it does not affect this second step of the reaction. We therefore reasoned that the USE may have a secondary role of enhancing the affinity of general polyadenylation factors for the C2 poly(A) signal. Consistent with this notion, we found that the USE enhances CPSF-dependent binding of CstF to the C2 poly(A) site, as judged by UV-cross-linking of CstF-64. To our knowledge, this is the first demonstration that CPSF–CstF cooperative binding can be mediated by sequences in the pre-mRNA upstream of AAUAAA. Although CPSF–CstF interactions have been well documented (Wilusz et al. 1990; Weiss et al. 1991; Murthy and Manley 1992, 1995), they have always been shown or presumed to involve downstream sequences of the poly(A) signals. In our previous experiments, the downstream region could be deleted with only minimal effects (Ashfield et al. 1991), whereas the USE was essential (Moreira et al. 1995). We show here that binding of CstF to the USE is functionally significant, by demonstrating that CstF can enhance poly(A) addition in a USE-dependent manner. This is not only the first demonstration that CstF can function in response to upstream sequences, but also the first indication that CstF can enhance the second step of the reaction. As its name implies, CstF was initially identified as an activity that enhances the cleavage reaction (Takagaki et al. 1989; Gilmartin and Nevins 1991), and it has never been observed to affect poly(A) addition. It now seems likely that this reflects the nature of the precleaved RNA substrates used that lack the downstream sequences usually required for CstF function. When binding sites are present upstream, CstF will enhance poly(A) addition. Our data indicate that, as with many transcriptional regulators, CstF can function both upstream and downstream of its target (i.e., CPSF). Although we have not investigated the mechanism by which CstF activates poly(A) synthesis, it likely reflects stabilization of CPSF binding, thereby facilitating interaction between CPSF and PAP (Murthy and Manley 1992, 1995; Bienroth et al. 1993).
It is intriguing to compare the structure and function of the C2 poly(A) site with that emerging as a typical site in Saccharomyces cerevisiae. Although it has been difficult to define clear consensus sequence elements, Guo and Sherman (1996) suggested recently the existence of two sequences upstream of the cleavage site, a 3′ “positioning element” and a 5′ “efficiency element”, that appear to be the principal signals for 3′-end formation. Based on location, sequence and function, the positioning element may be analogous to AAUAAA. If so, then the efficiency element may be related to the GU-rich downstream element found in vertebrate genes, despite their different positions (Manley and Takagaki 1996). It is striking that this organization resembles that of the C2 gene, especially in humans where there appears to be no DSE (Moreira et al. 1995). It is not known which of the characterized yeast polyadenylation factors recognizes the positioning or efficiency elements. If the efficiency element is indeed analogous to the mammalian GU-rich sequence, however, then the yeast factor CFIy is a good candidate to bind it because two of its subunits, RNA14 and RNA15, are the apparent homologs of CstF-77 and CstF-64 (Minvielle-Sebastia et al. 1994; Takagaki and Manley 1994). Interestingly, CFIy is required for both cleavage and poly(A) addition in yeast (Chen and Moore 1992), which had appeared to distinguish it from CstF. Our data demonstrating that CstF can participate in both steps of 3′ end formation removes this apparent difference between the two factors, and further emphasizes the similarities between the proteins required for polyadenylation in yeast and mammals. Finally, it is noteworthy that the distance between the 3′ end of the C2 gene and the 5′ end of the next gene is only 412 nucleotides (Wu et al. 1987) and a very similar gene arrangment exists for the other USE-containing gene Lamin B2 (Brackenridge et al. 1998). Such gene organization is more typical of yeast than mammals. It is intriguing to speculate that this explains the reliance on USEs both in yeast and in closely spaced mammalian genes.
Materials and methods
Constructs used in the in vitro and UV cross-linking assays
The wild-type and mutant (AAUAAA → AAGAAA) full-length C2 poly(A) signal DNA fragments were isolated by StyI digestion of pMLC2.BΔ3 (Ashfield et al. 1991) and pUCC2J plasmid (Moreira et al. 1995), respectively, and blunt-ended with Klenow fragment. The full-length poly(A) signal fragment containing the mutated DSE (Ra) fragment was isolated from StRa/S (Moreira et al. 1995) by digestion with PstI, and blunt-ended with T4 DNA polymerase. It should be noted that compared with the above StyI fragments, the Ra fragment contains an extra G at the 5′ end, and an additional 26 nucleotides of sequence at the 3′ end (both derived from the α2 globin gene 3′-flanking region from StRa/S). These fragments were inserted into pGEM7Zf(−) at the HindIII site in the polylinker, to generate the wild-type, mt, and Ra constructs. Finally, fragments containing the minimal poly(A) signal (i.e., excluding the USE) with the wild-type and Ra DSEs were produced by PCR using primers that anneal at the AAUAAA and the downstream StyI site. These PCR products were cut with HindIII and BamHI and inserted into pGEM7Zf(−) linearized with these enzymes, producing the ΔUSE and ΔUSERa (not used here). The USE fragment was isolated by digestion of wild type with ClaI (pGEM polylinker) and AseI. This fragment was ligated in the anti-sense orientation into at the ClaI blunt ended site of ΔUSE and ΔUSERa to generate Rev and RevRa, and in the sense orientation into HindIII blunt-ended pGEM–7Zf to generate USE. Finally, the Sp construct was produced by inserting an FspI–RsaI fragment from the lacZ gene of pUC19 into the EcoRI site in ΔUSE.
The plasmids used to synthesize pre-cleaved RNA substrates were made by PCR amplification of the fragments StyI–poly(A) site using an antisense oligonucleotide that hybridizes at the poly(A) site and the forward primer −48, with wild-type and Rev as plasmid templates. After PCR amplification, the PCR products were digested with ApaI and blunt-ended with Klenow. The pre-ΔUSE construct was generated by cleavage of the wild-type PCR product with AseI. The precleaved fragments were inserted into the pGEM–7zf polylinker at the HindIII site.
ΔPTB and pre-ΔPTB were made by specific PCR mutagenesis of the wild-type and pre-wild-type pGEM 7Zf(−) plasmids.
The SV40 late and adenovirus L3 poly(A) signal constructs used (SV40 and L3) are described elsewhere as pG3SVL-A and pG3L3-A (Takagaki et al. 1988).
In vitro cleavage and poly(A) addition analysis
In vitro transcription
All of the pGemC2 plasmids were linearized with BamHI, whereas SV40 was linearized with DraI and L3 with BamHI. One microgram of linearized plasmid was transcribed in the presence of 10μCi [α-32P]UTP (800 Ci/mmole) and T7 (for pGemC2 plasmids) or SP6 RNA polymerase (for SV40 and L3). When RNAs for UV-cross-linking experiments were synthesized, two different radionucleotides were used—12.5 μCi [α-32P]UTP and 12.5 μCi [α-32P]CTP. In the experiment shown in Figure 4B, [α-32P]ATP was used in place of CTP to allow efficient labeling of the ΔPTB A-rich mutant sequence.
In vitro RNA 3′-processing reactions
A mix (6.5 μl) containing 40 μg/μl of E. coli tRNA, 1 mm of MgCl2 (for polyadenylation) and either 2 mm EDTA and 1 mm ATP or 1 mm 3′-deoxy-ATP (for cleavage), 20 mm creatine phosphate and 2.5% PVA was incubated with 1 μl of radiolabeled RNA substrate (50 fmoles) and 5 μl of nuclear extract or purified protein fractions in buffer D (3 μl of CSF or CPSF, 1 μl of PAP and 1 μl of the 55-kD protein fraction were used). When a time course cleavage or polyadenylation reaction was performed the final volume of the reaction was 100 μl and aliquots were taken from the tube incubated at 30°C at the time required. Cleavage or poly(A) addition reactions were otherwise allowed to proceed at 30°C for 1.5 hr. The proteins in the reaction were digested with proteinase K, by addition of 112.5 μl of the mixture 20 mm Tris-HCl at pH 7.9, 100 mm NaCl, 10 mm EDTA, 1% SDS, and 0.33 mg/ml proteinase K and incubation at 30°C for 15 min. In time course experiments, 12.5 μl of the reaction were taken from 30°C at the time required, added to a tube with the proteinase K mixture and incubated for 15 min. The cleavage/poly(A) addition RNA products were separated by denaturing (8.3 m urea) polyacrylamide gel electrophoresis.
Nuclear extract and protein fractions
Nuclear extract was prepared according to the method of Dignam et al. (1983) with minor modifications (Takagaki et al. 1988). Figures 2, B and C, 4B, and 5, B and C, all employed nuclear extract made according to Wahle and Keller (1993). CSF is a Superose 6 gel filtration column chromatography protein fraction (Takagaki et al. 1988). CstF and PAP are Mono-S ion exchange column chromatography protein fractions (Ryner et al. 1989; Takagaki et al. 1990) CPSF and CFI+II are Mono-Q ion exchange column chromatography protein fractions (Takagaki et al. 1989). Partially purified 55-kD protein fraction was obtained from a Superose 6 gel filtration column loaded with the 20%–40% ammonium sulfate fraction precipitated from the nuclear extract.
Recombinant histidine-tagged PTB was a kind gift of C. Gooding and C.W.J. Smith, Cambridge University.
UV cross-linking of proteins to RNA
The protocol described by Moore et al. (1988) was used with some modifications. Cleavage reaction mixtures containing 20 fmoles of substrate RNA were incubated at 30°C for 10 min to allow the cleavage/polyadenylation complexes to form on the RNA. In the UV cross-linking competition experiments the labeled RNA substrate was incubated together with the competitor DNA oligonucleotide and the protein fraction. E. coli tRNA was added at final concentration of 0.2 mg/ml to dissociate weakly bound proteins. Proteins were cross-linked to the RNA by exposure to UV light at 254 nm for 10 min at 4°C. The UV light source (Mineralight, UVS-54, 220 V, 50 Hz, 0.12 A, Ultra Violet Products Inc., San Gabriel, CA) was supported 3 cm above the samples. The RNA was digested with 15 μg of RNase A and incubated at 37°C for 30 min. An equal volume of 2× protein gel loading buffer was added to the samples and the proteins were denatured by incubation at 95°C for 5 min. Prestained Sigma and Rainbow molecular weight markers were used. The proteins were separated on a 5% stacking, 10% resolving SDS-polyacrylamide gel. After electrophoresis, the gel was incubated in 10% acetic acid, 2% glycerol for 30 min, washed with water twice for 5 min, and incubated in 1 m salicylic acid for 30 min, to intensify the signal. Autoradiography was performed at −70°C with intensifying screens, after drying the gel.
Immunoprecipitation
Immunoprecipitation of UV cross-linked proteins was performed according to Takagaki et al. (1990), with minor modifications. For immunoprecipitation using polyclonal antibodies, after UV cross-linking, 10 μl of the RNase A-treated samples was added to 100 μl of protein A–Sepharose beads in IP-2 buffer [10% (vol/vol)] and antibody (5 μl of anti-PTB serum or preimmune serum). For immunoprecipitations using the monoclonal antibodies anti-64 kD and OX1, rabbit anti-mouse immunoglobulin was mixed on a vertical wheel with protein A–Sepharose beads (40 μg of RAM/100 μl protein A–Sepharose, 1.5 hr, 4°C). The beads were washed three times, resuspended in 200 μl of hybridoma culture supernatants, and added to the UV cross-linked, RNase A-treated samples. The mixtures were rotated at 4°C for 16 hr. Antibody-antigen complexes formed and bound to protein A–Sepharose beads were resuspended in 20 μl of 1× protein loading buffer and separated on an SDS-polyacrylamide gel as described above.
Antibodies
The rabbit polyclonal anti-PTB antibody was raised against a glutathione-S-transferase (GST)–PTB fusion protein (Kaminski et al. 1995), and was a generous gift from R.J. Jackson (Cambridge University). Rabbit pre-immune serum was also obtained from R.J. Jackson. The anti-64 kD mouse mAb was prepared according to Takagaki et al. (1990). The mouse anti-rat CD45 mAb OX1 (Sunderland et al. 1979), used as a negative control for the immunoprecipitation with the anti-64 kD antibody, was a kind gift from Alexandre Carmo (University of Oxford).
In vivo analysis of wild-type vs. ΔPTB C2 poly(A) signals
The C2 StyI fragment was isolated from ΔPTB or wild-type pGEM plasmids and inserted into the 3′-flanking region PvuII site of α23′PSpSVed (see Moreira et al. 1995). These two plasmids (wild type and ΔPTB) were transfected transiently into HeLa cells and the cytoplasmic RNA isolated from these two transfections was subjected to S1 mapping, all described by Moreira et al. (1995).
Acknowledgments
We are grateful to members of the N.J.P. and J.L.M. laboratories for help and encouragement throughout these studies. We are indebted to Chris Smith and his laboratory for providing us with invaluable reagents. We are also grateful to Masatomo Yonaha for providing nuclear extract. Finally, we thank Alexandre Carmo for help with the immunoprecipitation experiments. A.M. was supported by Porto University and the Junta Nacional de Investigacio Cientifica e Tecnologia of Portugal. These experiments were supported by a program grant from the Wellcome Trust (no. 9622084.3) to N.J.P. and a National Institutes of Health grant (GM 28983) to J.L.M.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL nicholas.proudfoot@pathology.oxford.ac.UK; FAX +44 1865-275556.
References
- Ashe MP, Girffin P, James W, Proudfoot NJ. Poly(A) site selection in the HIV-1 provirus: Inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes & Dev. 1995;9:3008–3025. doi: 10.1101/gad.9.23.3008. [DOI] [PubMed] [Google Scholar]
- Ashe MP, Pearson LH, Proudfoot NJ. The HIV-1 poly(A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. EMBO J. 1997;16:5752–5763. doi: 10.1093/emboj/16.18.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfield R, Enriquez-Harris P, Proudfoot NJ. Transcriptional termination between the closely linked human complement genes C2 and Factor B: Common termination factor for C2 and c-myc? EMBO J. 1991;10:4197–4207. doi: 10.1002/j.1460-2075.1991.tb04998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabino SML, Hubner W, Jenny A, Minvielle-Sebastia L, Keller W. The 30-kD subunit of mammalian cleavage and polyadenylation specificity factor and its yeast homolog are RNA-binding zinc finger proteins. Genes & Dev. 1997;11:1703–1716. doi: 10.1101/gad.11.13.1703. [DOI] [PubMed] [Google Scholar]
- Bardwell VJ, Wickens M, Bienroth S, Keller W, Sproat B, Lamond AI. Site directed ribose methylation identifies 2′OH groups in polyadenylation substrates critical for AAUAAA recognition and poly(A) addition. Cell. 1991;65:4197–4207. doi: 10.1016/0092-8674(91)90414-t. [DOI] [PubMed] [Google Scholar]
- Bienroth S, Wahle E, Suter-Crazzolara C, Keller W. Purification of the cleavage and polyadenylation factor involved in the 3′ processing of messenger RNA precursors. J Biol Chem. 1991;266:19768–19776. [PubMed] [Google Scholar]
- Bienroth S, Keller W, Wahle E. Assembly of a processive mRNA polyadenylation complex. EMBO J. 1993;12:585–594. doi: 10.1002/j.1460-2075.1993.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenridge S, Ashe HL, Giacca M, Proudfoot NJ. Transcription and polyadenylation in a short human intergenic region. Nucleic Acids Res. 1998;25:2326–2335. doi: 10.1093/nar/25.12.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Tiley LS, Cullen BR. Efficient polyadenylation within the human immunodeficiency virus type 1 long terminal repeat requires flanking U3-specific sequences. J Virol. 1991;65:3340–3343. doi: 10.1128/jvi.65.6.3340-3343.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell S, Alwine JC. Efficiency of utilisation of the simian virus 40 late polyadenylation site: Effects of upstream sequences. Mol Cell Biol. 1989;9:4248–4258. doi: 10.1128/mcb.9.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Moore C. Separation of factors required for cleavage and polyadenylation of yeast pre-mRNA. Mol Cell Biol. 1992;12:3470–3481. doi: 10.1128/mcb.12.8.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington J, Ganem D. Regulation of polyadenylation in human immunodeficiency virus (HIV): Contributions of promoter proximity and upstream sequences. EMBO J. 1992;11:1513–1524. doi: 10.1002/j.1460-2075.1992.tb05196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Z-F, Chen F, Wilusz J. Sequence and position requirements for uridylate-rich downstream elements of polyadenylation. Nucleic Acids Res. 1994;22:2525–2531. doi: 10.1093/nar/22.13.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan DF, Manley JL. Mechanism and regulation of mRNA polyadenylation. Genes & Dev. 1997;11:2755–2766. doi: 10.1101/gad.11.21.2755. [DOI] [PubMed] [Google Scholar]
- DeZazzo JD, Imperiale MJ. Sequences upstream of AAUAAA influence poly(A) site selection in a complex transcription unit. Mol Cell Biol. 1989;9:4951–4961. doi: 10.1128/mcb.9.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZazzo JK, Kilpatrick JE, Imperiale MJ. Involvement of a long terminal repeat U3 sequences overlapping the transcription control region in human immunodeficiency virus type 1 mRNA 3′ end formation. Mol Cell Biol. 1991;11:1624–1630. doi: 10.1128/mcb.11.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth PA, Choe W-T, Rex JH, Byrne JC, Baker CC. Sequences homologous to 5′ splice sites are required for the inhibitory activity of papillomavirus late 3′ untranslated regions. Mol Cell Biol. 1994;14:5278–5289. doi: 10.1128/mcb.14.8.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A, Proudfoot NJ. A sequence downstream of AAUAAA is required for rabbit β-globin mRNA 3′ end formation. Nature. 1984;312:473–474. doi: 10.1038/312473a0. [DOI] [PubMed] [Google Scholar]
- ————— Position-dependent sequence elements downstream of AAUAAA are required for efficient mRNA 3′end formation. Cell. 1987;49:399–406. doi: 10.1016/0092-8674(87)90292-3. [DOI] [PubMed] [Google Scholar]
- Gil A, Sharp PA, Jamison SF, Garcia-Blanco MA. Characterization of cDNAs encoding the polypyrimidine tract-binding protein. Genes & Dev. 1991;5:1224–1236. doi: 10.1101/gad.5.7.1224. [DOI] [PubMed] [Google Scholar]
- Gilmartin GM, Nevins JR. Molecular analyses of two poly(A) site processing factors that determine the recognition and efficiency of cleavage of the pre-mRNA. Mol Cell Biol. 1991;11:2432–2438. doi: 10.1128/mcb.11.5.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin GM, Fleming ES, Oetjen J. Activation of HIV-1 3′ processingin vitro requires both an upstream element and TAR. EMBO J. 1992;11:4419–4428. doi: 10.1002/j.1460-2075.1992.tb05542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin GM, Fleming ES, Oetjen J, Graveley BR. CPSF recognition of an HIV-1 mRNA 3′-processing enhancer: Multiple sequence contacts involved in poly(A) site definition. Genes & Dev. 1995;9:72–83. doi: 10.1101/gad.9.1.72. [DOI] [PubMed] [Google Scholar]
- Gooding C, Roberts GC, Moreau G, Nadal-Ginard B, Smith CWJ. Smooth muscle-specific switching of α-tropomyosin mutually exclusive exon selection by specific inhibition of the strong default exon. EMBO J. 1994;13:3861–3872. doi: 10.1002/j.1460-2075.1994.tb06697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson SI, Polycarpou-Schwarz M, Mattaj IW. U1 snRNP inhibits pre-mRNA polyadenylation through a direct interaction between U1 70K and poly(A) polymerase. Mol Cell. 1998;1:255–264. doi: 10.1016/s1097-2765(00)80026-x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Sherman F. 3′ end forming signals in yeast mRNA. Trends in Biochem Sci. 1996;21:477–481. doi: 10.1016/s0968-0004(96)10057-8. [DOI] [PubMed] [Google Scholar]
- Jenny A, Hauri H-P, Keller W. Characterization of cleavage and polyadenylation specificity factor and cloning of its 100-kilodalton subunit. Mol Cell Biol. 1994;14:8183–8190. doi: 10.1128/mcb.14.12.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Minvielle-Sebastia L, Preker PJ, Keller W. Sequence similarity between 73kD protein of CPSF and a subunit of yeast polyadenylation factor 1. Science. 1996;274:1514–1517. doi: 10.1126/science.274.5292.1514. [DOI] [PubMed] [Google Scholar]
- Kaminski A, Hunt SL, Patton JG, Jackson RJ. Direct evidence that polypyrimidine tract binding protein (PTB) is essential for internal initiation of translation of encephalomyocarditis virus RNA. RNA. 1995;1:924–938. [PMC free article] [PubMed] [Google Scholar]
- Keller W, Bienroth S, Lang KM, Christofori G. Cleavage and polyadenylation factor CPF specifically interacts with the pre-mRNA 3′ processing signal AAUAAA. EMBO J. 1991;10:4241–4249. doi: 10.1002/j.1460-2075.1991.tb05002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Patton JG. Regulation of alternative 3′ splice site selection by constitutive splicing factors. RNA. 1995;1:234–245. [PMC free article] [PubMed] [Google Scholar]
- Lou H, Gagel RF, Berget SM. An intron enhancer recognized by splicing factors activates polyadenylation. Genes & Dev. 1996;10:208–219. doi: 10.1101/gad.10.2.208. [DOI] [PubMed] [Google Scholar]
- Lutz CS, Alwine JC. Direct interaction of the U1 snRNP-A protein with the upstream efficiency element of the SV40 polyadenylation signal. Genes & Dev. 1994;8:576–586. doi: 10.1101/gad.8.5.576. [DOI] [PubMed] [Google Scholar]
- Lutz CS, Murthy KGK, Scheck N, O’Connor JP, Manley JL, Alwine JC. Interaction between the U1A snRNP-A protein and the 160-kD subunit of cleavage-polyadenylation specificity factor increases polyadenylation efficiency in vitro. Genes & Dev. 1996;10:325–337. doi: 10.1101/gad.10.3.325. [DOI] [PubMed] [Google Scholar]
- MacDonald CC, Wilusz J, Schenk T. The 64-kilodalton subunit of the CstF polyadenylation factor binds to pre-mRNAs downstream of the cleavage site and influences cleavage site location. Mol Cell Biol. 1994;14:6647–6654. doi: 10.1128/mcb.14.10.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley JL, Takagaki Y. The end of the message-another link between yeast and mammals. Science. 1996;274:1481–1482. doi: 10.1126/science.274.5292.1481. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Imperiale MJ, Ali H, Nevins JR. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984;37:993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Hart RP, Wong WW, Nevins JR. Sequences capable of restoring poly(A) site function define two distinct downstream elements. EMBO J. 1986;5:2907–2913. doi: 10.1002/j.1460-2075.1986.tb04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J, Gaffney JL, Whitton JL, Clements JB. The consensus sequence YGTGTTYY located downstream from the AAUAAA signal is required for efficient formation of mRNA 3′ termini. Nucleic Acids Res. 1985;13:1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minvielle-Sebastia L, Preker PJ, Keller W. RNA 14 and RNA 15 proteins as components of a yeast pre-mRNA 3′ end processing factor. Science. 1994;266:1702–1705. doi: 10.1126/science.7992054. [DOI] [PubMed] [Google Scholar]
- Moreira A, Wollerton M, Monks J, Proudfoot NJ. Upstream sequence elements enhance poly(A) site efficiency of the C2 complement gene and are phylogenetically conserved. EMBO J. 1995;14:3809–3819. doi: 10.1002/j.1460-2075.1995.tb00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Chen J, Whoriskey J. Two proteins crosslinked to RNA containing the adenovirus L3 poly(A) site require the AAUAAA sequence for binding. EMBO J. 1988;7:3159–3169. doi: 10.1002/j.1460-2075.1988.tb03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KG, Manley JL. Characterization of a multisubunit cleavage polyadenylation specificity factor from calf thymus. J Biol Chem. 1992;267:14804–14811. [PubMed] [Google Scholar]
- ————— The 160-kD subunit of human cleavage-polyadenylation specificity factor co-ordinates pre-mRNA 3′end formation. Genes & Dev. 1995;9:2672–2683. doi: 10.1101/gad.9.21.2672. [DOI] [PubMed] [Google Scholar]
- Patton JG, Mayer SA, Tempst P, Nadal-Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: A component of a complex necessary for pre-mRNA splicing. Genes & Dev. 1991;5:1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- Perez I, Lin C-H, McAfee JG, Patton JG. Mutation of PTB binding sites causes misregulation of alternative 3′ splice site selection in vivo. RNA. 1997;3:764–778. [PMC free article] [PubMed] [Google Scholar]
- Prescott JC, Falck-Pedersen E. Varied poly(A) site efficiency in the adenovirus major late transcription unit. J Biol Chem. 1992;267:8175–8181. [PubMed] [Google Scholar]
- ————— Sequence elements upstream of the 3′ cleavage site confer substrate strength to the adenovirus L1 and L3 polyadenylation sites. Mol Cell Biol. 1994;14:4682–4693. doi: 10.1128/mcb.14.7.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot NJ. Ending the message is not so simple. Cell. 1996;87:779–781. doi: 10.1016/s0092-8674(00)81982-0. [DOI] [PubMed] [Google Scholar]
- Proudfoot NJ, Brownlee GG. 3′ non coding region sequences in eukaryotic messenger RNA. Nature. 1976;263:211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Raabe T, Bollum FJ, Manley JL. Primary structure and expression of bovine poly(A) polymerase. Nature. 1991;353:229–234. doi: 10.1038/353229a0. [DOI] [PubMed] [Google Scholar]
- Ruegsegger U, Beyer K, Keller W. Purification and Characterization of human cleavage factor Im involved in the 3′ end processing of messenger RNA precursors. J Biol Chem. 1996;271:6107–6113. doi: 10.1074/jbc.271.11.6107. [DOI] [PubMed] [Google Scholar]
- Ruegsegger U, Blank D, Keller W. Human pre-mRNA cleavage factor Im is related to spliceosomal SR proteins and can be reconstituted in vitro from recombinant subunits. Mol Cell. 1998;1:243–254. doi: 10.1016/s1097-2765(00)80025-8. [DOI] [PubMed] [Google Scholar]
- Russnak R. Regulation of polyadenylation in hepatitis B viruses: Stimulation by the upstream activating signal PS1 is orientation-dependent, distance-dependent, and additive. Nucleic Acids Ress. 1991;19:6449–6456. doi: 10.1093/nar/19.23.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russnak R, Ganem D. Sequences 5′ to the polyadenylation signal mediate differential poly(A) site use in hepatitis B viruses. Genes & Dev. 1990;4:764–776. doi: 10.1101/gad.4.5.764. [DOI] [PubMed] [Google Scholar]
- Ryner L, Takagaki Y, Manley J. Multiple forms of poly(A) polymerases purified from HeLa cells function in specific mRNA 3′-end formation. Mol Cell Biol. 1989;9:4229–4238. doi: 10.1128/mcb.9.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Valcarcel J, Green MR. Distinct binding specificities and functions of higher eurkaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- Sittler A, Gallinaro H, Jacob M. Upstream and downstream cis-acting elements for cleavage at the L4 polyadenylation site of adenovirus-2. Nucleic Acids Res. 1994;22:222–231. doi: 10.1093/nar/22.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland CA, McMaster WR, Williams AF. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979;9:155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, Manley JL. A polyadenylation factor subunit is the human homologue of the Drosophila suppresser of forked protein. Nature. 1994;372:471–474. doi: 10.1038/372471a0. [DOI] [PubMed] [Google Scholar]
- ————— RNA recognition by the human polyadenylation factor CstF. Mol Cell Biol. 1997;17:3907–3914. doi: 10.1128/mcb.17.7.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y, Ryner LC, Manley JL. Separation and Characterization of a poly(A) polymerase and a cleavage/specificity factor required for pre-mRNA polyadenylation. Cell. 1988;52:731–742. doi: 10.1016/0092-8674(88)90411-4. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, Ryner LC, Manley JL. Four factors are required for 3′-end cleavage of pre-mRNAs. Genes & Dev. 1989;3:1711–1724. doi: 10.1101/gad.3.11.1711. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, Manley JL, MacDonald CC, Wilusz J, Shenk T. A multisubunit factor, CstF, is required for polyadenylation of mammalian pre-mRNAs. Genes & Dev. 1990;4:2112–2120. doi: 10.1101/gad.4.12a.2112. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, MacDonald C, Shenk T, Manley JL. The human 64 kDa polyadenylation factor contains a ribonucleoprotein-type RNA binding domain and unusual auxiliary motifs. Proc Natl Acad Sci. 1992;89:1403–1407. doi: 10.1073/pnas.89.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamakis A, Zeichner S, Carswell S, Alwine JC. The human immunodeficiency virus type 1 polyadenylation signal: A 3′ long terminal repeat element upstream of the AAUAAA necessary for efficient polyadenylation. Proc Natl Acad Sci. 1991;88:2108–2112. doi: 10.1073/pnas.88.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E, Martin G, Schiltz E, Keller W. Isolation and expression of cDNA clones encoding mammalian poly(A) polymerase. EMBO J. 1991;10:4251–4257. doi: 10.1002/j.1460-2075.1991.tb05003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E, Keller W. 3′ end-processing of mRNA. In: Higgins SJ, Hames BD, editors. RNA processing, A practical approach. II. Oxford, UK: IRL Press at OUP; 1993. pp. 1–33. [Google Scholar]
- Weiss EA, Gilmartin GM, Nevins JR. Poly(A) site efficiency reflects the stability of complex formation involving the downstream element. EMBO J. 1991;10:215–219. doi: 10.1002/j.1460-2075.1991.tb07938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: Addition of poly(A) in the nucleus. Trends Biochem Sci. 1990;15:271–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Wilusz J, Schenk T. A 64 kDa nuclear protein binds to RNA segments that include the AAUAAA polyadenylation motif. Cell. 1988;52:221–228. doi: 10.1016/0092-8674(88)90510-7. [DOI] [PubMed] [Google Scholar]
- ————— A uridylate tract mediates efficient heterogeneous nuclear ribonucleoprotein C protein-RNA cross-linking and functionally substitutes for the downstream element of the polyadenylation signal. Mol Cell Biol. 1990;10:6397–6407. doi: 10.1128/mcb.10.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J, Shenk T, Takagaki Y, Manley JL. A multicomponent complex is required for the AAUAAA-dependent cross-linking of a 64-kilodalton protein to polyadenylation substrates. Mol Cell Biol. 1990;10:1244–1248. doi: 10.1128/mcb.10.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-C, Morley BJ, Campbell RD. Cell specific expression of the human complement protein factor B gene: Evidence for the role of two distinct 5′ flanking elements. Cell. 1987;48:331–342. doi: 10.1016/0092-8674(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Green MR. Biochemical characterization of U2 snRNP auxiliary factor: An essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991;10:207. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]