Abstract
A subset of serotonin pathway polymorphisms has been shown to confer risk for psychological dysfunction, particularly in individuals who experience early adversity. Understanding the developmental processes underlying these gene × environment interactions will strengthen the search for risk factors for behavioral dysfunction. We investigated the combined influence of two serotonin pathway polymorphisms and species-atypical, and possibly adverse, rearing (nursery-rearing, NR) on two dimensions of behavioral stress response in infant rhesus macaques. We hypothesized that the experience of NR and possession of both “high risk” genotypes (genotypes that are thought to confer low 5-HT function) would predict the greatest behavioral stress response to maternal/social separation. Using a matched-pair design, the impact of early experience and the serotonin transporter [rh5-HTTLPR] and monoamine oxidase A [rhMAO-A-LPR] promoter polymorphisms on behavioral reactivity of 136 infant rhesus macaques (90–120 days of age) during a 25-hour social separation/relocation procedure was assessed. Each pair included one infant reared with mother in a large, outdoor field enclosure (FR) and one infant reared in a nursery (NR). Pairs were matched for putative gene activity of each polymorphism, sex, age and weight at testing. Behavioral responses in a “Human Intruder” test were recorded, and Activity and Emotional Reactivity composites were created to detect different aspects of psychological adaptation to stress. Our hypothesis that high-risk groups would be the most reactive to stress was not entirely borne out. Rh5-HTTLPR × rhMAOA-LPR interactions predicted Emotional Reactivity and tended to predict Behavioral Activity scores. However, this interaction was exacerbated by the experience of nursery rearing. We conclude that serotonin pathway multigene-environment interactions influence behavioral development in rhesus macaques.
Keywords: rh5-HTTLPR, rhMAOA-LPR, Epistasis, Gene × environment interaction, development, rhesus macaque
Early life experiences exert a profound influence on behavioral development (Nemeroff, 2004; Charney and Manji, 2004). Yet the effects of early experience may be buffered or exacerbated by genetic factors, suggesting that gene × environment (G × E) interactions contribute to complex behavioral traits, although consistent replication has proved challenging (Caspi, McClay, Moffitt, Mill, Martin et al., 2002; Caspi, Sugden, Moffitt, Taylor, Craig, 2003; Suomi, 2005; Barr, Newman, Shannon, Parker, Dvoskin, Becker et al., 2004; Zalsman Huang, Oquendo, Burke, Hu, Brent et al., 2006; Kaufman, Yang, Douglas-Palumberi, Houshyar, Lipschitz, Krystal et al., 2004; Cicchetti, Rogosch, & Sturge-Apple., 2007; Stein, Schork, and Gelernter, 2008; Nilsson, Sjöberg, Wargelius, Leppert, Lindström, Oreland, 2007; Foley, Eaves, Wormley, Silberg, Maes, Kuhn et al., 2004; Kim-Cohen, Caspi, Taylor, Williams, Newcombe, Craig et al., 2006; Vanyukov, Maher, Devlin, Kirillova, Kirisci, Yu, et al., 2007; Newman, Syagailo, Barr, Wendland, Champoux, Graessle et al., 2005; Karere, Kinnally, Sanchez, Famula, Lyons, and Capitanio, 2008; although see Munafo, Durrant, Lewis and Flint, 2008; Surtees, Wainwright, Willis-Owen, Luben, Day, and Flint, 2006; Haberstick, Lessem, Hopfer, Smolen, Ehringer, Timberlake et al., 2005). This irregularity may result from competing gene × gene (G × G) or G × E interactions (Gottlieb, 2007) that influence behavioral outcomes. Indeed, the two best-characterized functional polymorphisms in the 5-HT pathway have repeatedly been shown to differ in their association with psychological outcomes based the physical, psychological, and genetic environment of the individual; the serotonin transporter promoter polymorphism (5-HTTLPR) and the monoamine oxidase A-untranslated variable nucleotide tandem repeat polymorphism (MAOA-uVNTR).
The 5-HTTLPR (rh5-HTTLPR in rhesus macaques) polymorphism is a 44- bp insertion/deletion region (21-bp ortholog in rhesus macaques) located in the serotonin transporter gene promoter region. The presence of the insertion region characterizes the long allele, the absence of the insertion characterizes the short allele. The short, “low activity” allele is so-named because it is associated with lower transcriptional efficiency of the transporter gene, the protein product of which regulates 5-HT reuptake from the synaptic cleft (Lesch, Bengel, Heils, Sabol, Greenberg, Petri et al., 1996; Hranilovic, Stefulj, Schwab, Borrmann-Hassenbach, Albus et al., 2004; Bradley, Dodelzon, Sandhu, and Philibert, 2005). Possession of a short 5-HTTLPR allele has been associated with lower levels of 5-HIAA (5-hydroxyindoleacetic acid), a 5-HT metabolite in cerebrospinal fluid (CSF) in humans (Williams, Marchuk, Gadde, Barefoot, Grichnik et al., 2003), and with a higher risk of anxiety-related traits (Lesch et al., 1996, Gunthert, Conner, Armeli, Tennen, Covault, and Kranzler, 2007; although see Middeldorp, de Geus, Beem, Lakenberg, Hottenga, Slagboom, Boomsma, 2007). The experience of early stress exacerbates the influence of the low activity genotype on behavioral outcomes and may partly explain failures to replicate 5-HTTLPR-behavior associations. For example, individuals with low activity 5-HTTLPR genotypes who experienced early life stressors exhibit the highest depression scores (Caspi et al., 2003; Kaufman, Yang, Douglas-Palumberi, Houshyar, Lipschitz et al., 2004; Cicchetti et al., 2007; Eley, Sugden, Corsico, Gregory, Sham, McGuffin, et al., 2004; although see Surtees et al., 2006), higher rates of suicidality (Gibb, McGeary, Beevers, and Miller, 2008), greater alcohol use (Kaufman, Yang, Douglas-Palumberi, Crouse-Artus, Lipschitz, Krystal et al., 2007) and higher anxiety (Stein et al., 2008) compared with other groups.
The MAOA-uVNTR polymorphism (rhMAOA-LPR in rhesus macaques) is located on the X chromosome and consists of an upstream variable number of tandem repeats (uVNTR) including 2, 3, 3.5, 4 or 5 30-bp repeats in humans (Sabol and Hu, 1998) or 5, 6, or 7 18-bp repeats in rhesus macaques (Newman et al., 2005). The number of repeats in this promoter region impacts the production of monoamine oxidase A (MAOA), an enzyme responsible for the oxidation/inactivation of the monoamines norepinephrine and 5-HT (Sabol and Hu, 1998). “High activity” MAOA-LPR alleles (3.5- and 4- repeats in humans; 5- and 6-repeats in rhesus macaques) are so-named because they confer greater transcriptional efficiency (Sabol and Hu, 1998; Newman et al., 2005). Low activity MAOA-uVNTR alleles are associated with lower levels of CSF 5-HIAA in humans (Williams et al., 2003), and a greater risk for aggressive and impulsive behavior in humans (Manuck, Flory, McCaffery, Matthews, Mann et al., 1998). Genotypic influences are exacerbated by early experiences, as men and women who had a low activity MAOA-uVNTR genotype and who had been abused as children showed an increased risk for antisocial behavior (Taylor and Kim-Cohen, 2007; Kim-Cohen, Caspi, Taylor, Williams, Newcombe et al., 2006; Caspi et al., 2002; Vanyukov et al., 2007; Nilsson et al., 2007; Foley et al., 2004; although see Haberstick et al., 2005) impulsivity (Huang, Cate, Battistuzzi, Oquendo, Brent, and Mann, 2004) and aggression (Frazzetto, Di Lorenzo, Carola, Proietti, Sokolowska, Siracusano et al., 2007).
The cumulative effects of early adversity, 5-HTTLPR and MAOA-uVNTR genotype on psychiatric outcomes have also been described in humans. As might be expected, the inheritance of the two low activity genotypes, when combined with adverse early experience, conferred the highest risk for violent behavior and depressive symptoms of all groups (Reif, Rösler, Freitag, Schneider, Eujen 2007; Cicchetti et al., 2007). Just as the “low risk” alleles of serotonin pathway genes are thought to buffer individuals from the effects of early stress, these studies suggest that, even in the presence of one “high risk” genotype, the presence of other “low risk” gene variants may buffer individuals from adverse outcomes.
Rhesus macaques are invaluable models for understanding the influence of experience and serotonin pathway genes on the development of complex behavioral traits. First, functional rh5-HTTLPR and rhMAOA-LPR orthologs are present in rhesus macaques. Additionally, experimental manipulation of individual early experiences in rhesus macaque models allows for the discrimination of true serotonin pathway gene-environment interactions, as opposed to the gene-environment correlations that can confound human studies (Jaffee and Price, 2007). Finally, it has been suggested that the impact of serotonin pathway gene × environment interactions on adult behavior may be rooted in the developmental timing of the early stressor (Ansorge, Zhou, Lira, Hen, and Gingrich, 2004); animal studies allow for the assessment of the developmental timing of these interactions influencing behavioral or psychiatric outcomes. For example, following a well-established maternal separation procedure for rhesus macaques, Champoux and colleagues (2002) showed that infants who are removed from their mother on the first day of life and reared in a nursery (NR), and which also possess the low activity rh5-HTTLPR genotype, show lower orientation scores (a predictor of impulsivity later in life) at 1 month of age compared with infants that were mother-reared regardless of their rh5-HTTLPR genotype, or with other NR infants that had a high activity rh5-HTTLPR genotype. Similarly, Spinelli and colleagues (2007) showed that these maternally deprived short-allele carrying infants showed a higher risk for behavioral pathology (high emotionality and reduced exploration) at 6 months of age. Our group has previously shown that infant rhesus macaques that carry the low activity rhMAOA-LPR genotype and are reared in small social groups are at higher risk for expression of aggressive behaviors during a stressful situation while those reared by mothers alone in a restricted environment exhibit greater rates of behavior thought to reflect anxiety (Karere et al., 2008). While these experimental paradigms for macaques are not perfect approximations of early trauma experienced by humans, these studies support the notion that serotonin pathway gene × environment interactions influence behavioral response to stress early in life in a rhesus macaque model.
The developmental sequelae by which genes confer risk following early life experiences are not well-known. Evidence that 5-HTTLPR × MAOA-uVNTR × environment interactions influence behavioral response during or immediately following an early life stressor will lend insight to the ontogeny of risk for behavioral dysfunction. In the present study, we investigated the combined effects of rh5-HTTLPR and rhMAOA-LPR genotypes and early environment on the quality of behavioral response to stress in infant rhesus macaques. First, we assessed behavioral response to social/maternal separation and relocation at weaning (90 – 120 days) in male and female infants. Behavioral Activity and Emotional Reactivity are distinct aspects of infant stress response in humans (Bell, 1975). Analysis of infant behavioral stress response revealed similar dimensions in rhesus macaques (Golub, Hogrefe, Widaman, and Capitanio, 2008). Composites reflecting each of these dimensions were thus generated for the present analysis. Infants were genotyped for rh5-HTTLPR and rhMAOA-LPR polymorphisms and were grouped based on putative gene activity. We selected for analysis 68 infant pairs matched for rearing history, genotype, sex, age at testing, and weight at testing. We hypothesized that “high risk” Low activity rh5-HTTLPR and rhMAOA-LPR alleles would be associated with the greatest reactivity in response to stress in infants that experienced early stress.
Method
Subjects
Sixty-eight matched pairs of male (n = 18 pairs) and female (n = 50 pairs) infant rhesus macaques (Macaca mulatta) were selected from a large cohort of animals that were subjects in a biobehavioral assessment project conducted at the California National Primate Research Center (CNPRC). Subjects were matched for sex, age at testing (90–122 days, mean=108.1 days), weight at testing (0.77 kg to 1.39 kg, mean=1.07 kg), and genotype (see next paragraph). Maximum differences between members of any pair were 6 days in age (mean=1.0 day), and 0.30 kg in weight (mean=0.07 kg). One member of each pair was raised with its mother in half-acre outdoor field cages at the CNPRC, a group referred to here as FR (field cage-reared) animals. Each enclosure contained a large social group comprising at least 6 genetically distinct matrilines with extended kin networks. FR animals were drawn from 15 different enclosures. The other member of each pair was nursery-reared (NR). These animals were separated from their mothers on the first day of birth and reared in an incubator for one month, after which they were housed in indoor individual cages (.46 × .61 × .69 m) with intermittent or continuous access to a sex-matched, same-aged pair-mate.
Each pair included one FR individual and one NR individual that were matched based on rh5-HTTLPR/rhMAO-A-LPR genotype activity. Individuals belonged to one of six possible genotype activity groups: 1) High rh5-HTTLPR/High rhMAO-A-LPR (H/H) (n= 15 pairs), 2) High rh5-HTTLPR/Heterozygote rhMAO-A-LPR (H/Het) (n= 9 pairs), 3) High rh5-HTTLPR/Low rhMAO-A-LPR (H/L) (n= 16 pairs), 4) Low rh5-HTTLPR/High rhMAO-A-LPR (L/H) (n= 11 pairs), 5) Low rh5-HTTLPR/Heterozygote rhMAO-A-LPR (L/Het) (n= 7 pairs), and 6) Low rh5-HTTLPR/Low rhMAO-A-LPR (L/L) (n= 10 pairs). (See Genotyping Methods for definitions of “High”, “Heterozygote”, and “Low” Activity rh5-HTTLPR and rhMAOA-LPR.) Pairs were also selected for low (<12.5%) pairwise relatedness, sex, age at testing and body weight at testing. Only two genotype groups included individuals that were more than 12.5 % related. Within the H/Het group, two NR individuals were 25% related. Within the H/L group, two individuals (one FR and one NR) were 18 % related. Removal of these pairs from the analysis did not change the significance or pattern of results and were therefore included. The mean relatedness of all 136 subjects was < 0.001%.
Biobehavioral Assessment
FR infants were separated from mothers in the field cage setting and NR infant pairs were separated from each other. Animals were then transported to an unfamiliar testing suite, and were housed for the next 25-hrs in individual cages (.81 m × .61 m × .66 m) in a temperature-controlled room under a 12:12 hr light/dark cycle. Infants experienced a variety of standardized procedures over the 25-hr period designed to assess behavioral and physiological reactivity (see Capitanio et al., 2006). In the present study, we utilized data from one of these assessments, the Human Intruder Test.
Human Intruder Test
Each animal was removed from the temporary home cage and placed in a testing cage measuring 38.7 cm × 52.0 cm × 39.0 cm, which had a clear plastic front between the subject and the human intruder. Each animal was tested in 4 one-minute trials in fixed order, and behavioral responses were videotaped, with the camera approximately 1 m from the testing cage. The human intruder was always female and between 1.7 m and 2 m in height. For the first trial (profile far) the intruder positioned herself 1 m in front of the testing cage and presented her left profile to the animal for one minute. At the end of the minute, the intruder moved sideways toward the animal’s cage, positioning herself 0.33 – 0.5 m from the front of the testing cage, still holding the profile position (profile near). One minute later, the intruder moved back to 1 m distance and made (and maintained) direct eye contact with the animal (stare far). After one minute, the intruder moved to the near position while maintaining eye contact for one minute (stare near). Behavioral responses were recorded from the videotapes by an experienced technician who had been trained by an expert in primate behavior and had demonstrated 85% or better agreement on the occurrence of the behaviors (see Table 1). Annually, the technician reestablished reliability at the 85% or better level either with herself (intra-observer reliability, demonstrated by coding a tape that had been coded years earlier and comparing the codings) and/or with another technician using the same ethogram (see Ethogram, Table 1).
Table 1.
Human Intruder Ethogram
| Locomote | Directed movement from one location to another. |
| Crouch | Ventral surface close to floor; head at or below the level of the shoulders. |
| Hang | Holding onto ceiling or front mesh; all 4 limbs off of floor. |
| Active | Whole body movement; step, jump. |
| Scratch | Common usage. |
| Coo-vocalization | Medium-pitched, moderately intense, clear call. |
| Bark-vocalization | Gruff, abrupt, low-pitched vocalization. |
| Lipsmack | Rapid lip movement usually with pursed lips, accompanied by a smacking sound. |
| Threat | Scored with at least two or more of the following: open mouth stare, head bob, ear flaps, bark vocalizations. |
| Fear grimace | Exaggerated grin with teeth showing. |
| Tooth grind | Loud gnashing of teeth. |
| Environmental Explore | Discrete manipulation by hand or mouth with the physical environment or objects in the cage. |
| Eat | Common usage. |
| Drink | Common usage. |
| Yawn | Common usage |
Blood Sampling and DNA Extraction
Blood for genotyping was collected at a separate time from behavioral assessment, during routine health checks while the animal resided in their natal cages (field cage or nursery) at the CNPRC. Blood was collected via femoral venipuncture, decanted to EDTA-coated tubes, and stored at 4 °C until separation, usually within 24 hours. Blood was centrifuged at 1500 × g for approximately 30 minutes at 4 °C. Plasma was removed and white blood cell layer removed to a fresh tube. Genomic DNA was extracted from white blood cells according to manufacturer’s instructions using a Qiagen DNeasy Tissue kit. Briefly, cells were lysed, and DNA precipitated using 100% ethanol. The supernatant was applied to a spin column and serially washed with a buffer containing ethanol. DNA was then eluted with an elution buffer and stored at 4 °C until use.
Genotyping
Rh5-HTTLPR
Primers were designed from the published sequence of the rh5-HTTLPR (Genbank accession number AJ544234). Each 25 ul PCR reaction contained 100ng DNA, 10 pmol of each primer, 1.5 mM MgCl (Promega, Madison, WI), enhancer buffer with betaine, 10 X amplification buffer (Promega, Madison, WI), 2.0 uM dNTP and 0.5 U Taq Polymerase (Promega, Madison, WI). Primers were as following: F1 5′GGCGTTGCCGCTCTGAATGC 3′; R1 5′ GAGGGACTGAGCTGGACAACCAC 3′. PCR cycling parameters were; 1) initial denaturation for 5 min at 95° C, 2) 35 cycles at 95° C for 1 min, 52° C for 1 min and 74° C for 1 min, 3) a final primer extension at 74° C for 10 min. Following amplification, rh5-HTTLPR products were cleaved using restriction enzyme PST I for at least 1.5 hours at 37° C. Samples were electrophoresed on a 3% agarose gel with ethidium bromide for DNA fluorescence. Long and short alleles of this polymorphism have been characterized in macaques (Lesch et al., 1997). Animals with two long rh-5HTTLPR alleles yielded bands at 41, 45, 50, 87, 151 and 302 bp. Animals with two short alleles yielded bands at 45, 50, 87, 172 and 302 bp. Finally, animals with one of each long and short allele yielded bands at 41, 45, 50, 87, 151, 172 and 302 bp. Gels were visualized using ultraviolet light, and representative genotypes were confirmed via sequencing. Genotypes were grouped for analysis according to putative activity based on previous reports of gene expression associated with promoter variants (Lesch et al., 1996): High (long homozygosity, l/l) or Low (short homozygosity, s/s or long/short heterozygosity, l/s).
RhMAOA-LPR
Primers were designed from the published sequence of the rhMAO-A-LPR (Genbank accession number AJ544234). The forward primer was fluorescently labeled at the 5′ end with FAM. Each 20ul reaction contained 200ng of DNA, 10pmol of each primer, 1X PCR Buffer, 1.25 mM MgCl2, 2.0 mM dNTP and 0.5 U of Taq DNA polymerase (Applied Biosystems). PCR reactions were performed on an ABI 9700 with the following profile; 1) initial denaturation for 3 min at 95°C, 2) 35 cycles of 95°C at 1min, 57°C at 1min and 72°C at 1 min, 3) and a final primer extension at 72oC for 10 min. The PCR products were electrophoresed on an ABI 377 DNA analyzer and analyzed using STRand software (UCDavis Veterinary Genetics Laboratory). Samples were also run on a 3% agarose gel for confirmation. Sequencing of rhMAOA-LPR was carried out using an ABI 377 DNA sequencer on a 6% polyacrylamide gel using manufacturer’s instructions. 5-, 6- and 7- repeat alleles have been characterized in macaques. The location of MAOA is on the X chromosome; thus, males are hemizygous and females are either homozygous or heterozygous. Genotypes were grouped according to putative activity based on previous reports of gene expression associated with promoter variants (Newman et al., 2005): High (5-, 6-repeat hemizygosity, homozygosity, or heterozygosity); High Activity/Low Activity Heterozygote (5/7 or 6/7 repeat heterozygosity); or Low (7-repeat hemizygosity or homozygosity) for analyses. There is little in vivo or in vitro evidence regarding the impact of high/low heterozygosity for rhMAOA-LPR on measures of serotonin function or behavior, so heterozygotes were grouped separately.
Statistics
Composites for behavioral response to a human intruder were created based on previous work suggesting that emotional reactivity and activity are common responses of both human and nonhuman primate infants challenging circumstances involving social separation and exposure to a novel environment (Bell, 1975; Golub et al., 2008; Capitanio et al., 2006). Means for each recorded behavior exhibited (see Ethogram, Table 1) across all four conditions (profile far, profile near, stare far, stare near) was computed for each animal in this sample. Z-scores were then calculated for each behavior to normalize the data. We used Cronbach’s alpha tests to establish internal consistency reliability among behaviors hypothesized to reflect Emotional Reactivity and Behavioral Activity. For the Behavioral Activity composite, the highest reliability (Cronbach’s alpha = .87) was found when the z-scores of the following behaviors were combined: mean proportion of time spent active during all four conditions and mean rate of environmental exploration during all four conditions. For the Emotional Reactivity composite, the highest reliability (Cronbach’s alpha = .73) was found when the z-scores of the following behaviors were combined: mean rates of coo vocalization, bark vocalization, lip smacks, fear grimaces, threats and tooth grinds during all four conditions. Composite scores were log transformed for analysis. Behavioral Activity and Emotional Reactivity composites were positively correlated (r = .31, p < .001).
Analyses of variance were conducted using SPSS 15.0 statistical software. A preliminary analysis revealed no sex differences in our measures, so all analyses combined males and females. Separate three-way analyses of variance were conducted for Activity and Emotional Reactivity, which included rearing (FR and NR), rh5-HTTLPR genotype activity (High and Low), and rhMAO-A-LPR genotype activity (High, Heterozygote and Low). T-tests were used for all post-hoc analyses. The variance accounted for by each statistically significant variable or interaction is reported as partial η2.
Results
Behavioral Activity
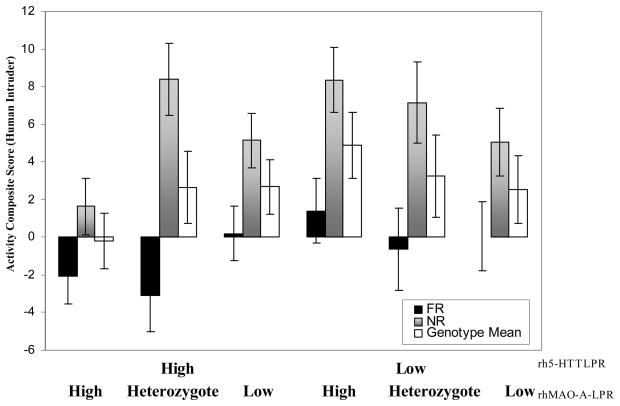
Main effects of either genotype were not detected, nor were single genotype × rearing interactions (p > .05). A main effect of rearing history and a trend level interaction between rh5-HTTLPR and rhMAOA-LPR genotypes influenced Behavioral Activity scores in the Human Intruder Test. Overall, NR infants exhibited higher Behavioral Activity compared to FR infants (F (1, 124) = 41.85, p < .001, partial η2 = .25, see Figure 1). The interaction between rh5-HTTLPR and rhMAOA-LPR approached significance (F (1, 124) = 2.82, p = .059, partial η2 = .05). Post hoc tests revealed that H/H infants were less Active than both L/H (t = 3.0, p < .01) and L/Het (t = 2.03, p < .05) infants, regardless of rearing history.
Figure 1.
Activity composite scores in response to human intruder. NR (grayscale bars) infants were more active than FR infants (black bars). H/H infants were significantly less active than L/H and H/Het individuals, across rearing groups (white bars). Means are presented ± standard error of the mean.
Emotional Reactivity
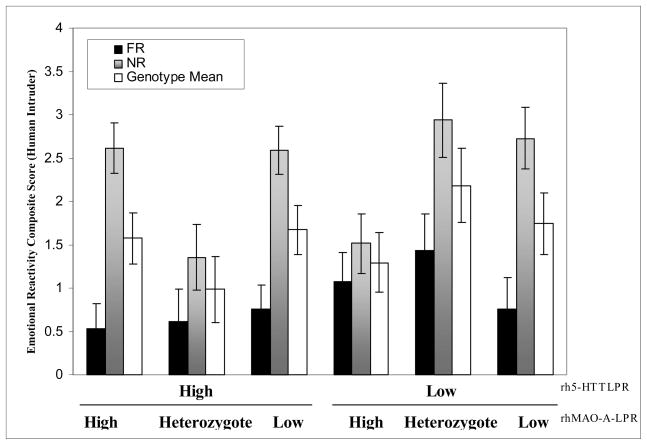
Main effects of either genotype were not detected, nor were single genotype × rearing interactions (p > .05). A main effect of rearing history, an interaction between rh5-HTTLPR and rhMAOA-LPR genotypes, and a three-way interaction among rearing history, rh5-HTTLPR and rhMAOA-LPR genotypes significantly impacted infant Emotional Reactivity during the Human Intruder Test. Overall, NR infants exhibited higher Emotional Reactivity scores than FR infants (F (1,124) = 50.01, p < .001, partial η2 = .287; see Figure 2). A significant interaction between rh5-HTTLPR and rhMAOA-LPR genotypes was found (F (1, 124) = 4.27, p < .05, partial η2 = .06), and post-hoc analysis showed that H/Het and L/H infants were less Emotionally Reactive than did other genotype groups (t-tests, p < .05). A significant three-way interaction, however, indicated that this gene × gene interaction differed between rearing groups (F (1, 124) = 3.31, p < .05; partial η2 = .05). Specifically, only among NR monkeys were H/Het and L/H infants less Emotionally Reactive than all other genotype groups (multiple t-tests, all p < .05). Among FR infants, genotype groups did not differ from each other (p > .05).
Figure 2.
Emotional Reactivity composite scores in response to human intruder. NR H/Het and L/H infants displayed significantly less Emotional Reactivity than all other NR genotype groups (p < .05), Means are presented ± standard error of the mean.
Discussion
Our findings provide one example of the complexity of genetic and environmental interactions that contribute to behavioral development in infant rhesus macaques. Our results suggest that gene × gene (G × G) interactions between two well-characterized serotonin pathway polymorphisms influenced two different aspects of response to a stressful situation in infant rhesus macaques. Infants with two high activity genotypes tended to be less Behaviorally Active than other infants, while infants with the L/H or H/Het genotype were less Emotionally Reactive than other groups. These epistatic interactions (Falconer and Mackay, 1997) were particularly potent in infants who experienced species atypical, and possibly “adverse”, rearing. Previous studies have indicated that the effects of early experience are modulated by single polymorphisms in the 5-HT pathway (Karere et al., 2009; Champoux et al., 2002). We extend these earlier findings to illustrate that environmental influences on behavior are moderated by the cumulative effects of multiple loci. These results point to the exciting possibility that G × G or G × E interactions that predispose infants toward particular behavior patterns can be augmented or attenuated by genetic or environmental factors.
Though replication of our results is required, our data demonstrate the association of G × G interactions with two distinct behavioral dimensions. The present results indicate that L/H and H/Het groups showed lower Emotional Reactivity scores compared with other genotype groups. In contrast, H/H infants showed lower Behavioral Activity compared with L/H and L/Het infants. While the results pertaining to the individual responses are important, understanding the integrated patterns of response to a stressful situation may be more informative. In humans and macaque infants, the low activity 5-HTTLPR allele has often been associated with “anxiety”, often operationalized as enhanced affective response and/or reduced motor activity (Bethea et al., 2004; Auerbach et al., 1999; Champoux et al., 2002; Lakatos et al., 2003; Kraemer et al., 2008). In contrast, in the present study, H/H animals showed relatively high levels of Emotional Reactivity and low Behavioral Activity. Our results are consistent with one previous G × E study in rhesus macaques which showed that in the presence of peer-rearing (similar to our nursery rearing paradigm), the high activity rh5-HTTLPR genotype was associated with enhanced emotionality and reduced behavioral activity response to stress in 6-month old macaques (Spinelli et al., 2007). We suggest that only High activity rh5-HTTLPR infants that also possess high activity rhMAOA-LPR show this pattern of behavior. The opposite pattern of behavior (high Behavioral Activity and low Emotional Reactivity) exhibited by L/H and H/Het infants, in contrast, resembles a pattern that has been associated with later impulsivity in adult humans (Olson et al., 2002). If true, it may be that H/H infants exhibit the greatest anxiety in response to a human intruder, whereas L/H and H/Het infants exhibit the least behavioral inhibition in response to a stressful situation. Future studies should investigate this possibility using additional measures of infant temperament.
Notably, the joint influences of rh5-HTTLPR and rhMAOA-LPR on our Emotional Reactivity measure were most apparent in NR infants. Many studies have shown that genotypic effects are exaggerated in individuals with adverse early experiences (Champoux et al., 2002; Caspi et al., 2002; Reif et al., 2007; Manuck et al., 2004; Spinelli et al., 2007; Barr et al., 2003; Barr et al., 2004; Bennett et al., 2002). We expected that NR infants with “high risk” genotypes would be more Emotionally Reactive than other groups, but instead found that this group was only one of four groups that exhibited comparable and high Emotional Reactivity. We did observe, however, that with the exception of the H/L group, the possession of either one or two “low risk” alleles, or advantageous early rearing, was associated with diminished behavioral response in at least one dimension. If we assume that a reduction in either Behavioral Activity or Emotional Reactivity is a risk-avoidance strategy, it may be that such a reduction reflects better adaptation in response to stress. Non-specific and extensive reactivity that characterized “high risk” (NR individuals with two low activity genotypes) infants, in contrast, may be considered a maladaptive response to a stressful situation.
Previous studies have described genotype-based differences in NR, but not FR, infant rhesus macaques (Champoux et al., 2002; Spinelli et al., 2007; but see Newman et al., 2005, Karere et al., 2008). Our results align with these previous findings. Why should genetic susceptibility to behavioral responsiveness differ between NR and FR infants? We suggest one experimental, one developmental, and one evolutionary reason why this may be the case, and recognize that they are not mutually exclusive. First, because elements of the testing conditions (e.g., being without mother, human proximity, indoor housing) may have been more similar to conditions experienced regularly by indoor-housed NR infants, they may have been less stressed by the situation, allowing true individual differences in behavioral traits emerge. A second possibility is that genotype-behavior associations emerge earlier in development in NR infants than in FR infants, due to the absence of a mother. A third explanation for our findings is that natural selection has maintained in the population genotypes that cause infants to react more strongly to challenges presented by atypical environments (Wendland, Hampe, Newman, Syagailo, Meyer et al., 2006). Genotypes that promote behavioral reactivity to unfavorable, but not to favorable, conditions may confer an adaptive advantage within species-atypical or disadvantageous environments.
The mechanism(s) by which multiple genotypes and experience interact to influence behavior are unknown. It is possible that the combined influence of these genetic variants and experience may have altered 5-HT function, with consequences for behavior. Human and non-human primate studies indicate that adverse early experience, as well as low activity 5-HTTLPR and MAOA-LPR alleles are associated with lower CSF 5-HIAA (Higley and Linnoila, 1992; Manuck et al., 2004; Bennett et al., 2002). In the present study, NR L/L infants may have had the lowest 5-HT function. These infants also exhibited high rates of both Behavioral Activity and Emotional Reactivity during a stressful social separation. This is consistent with some studies showing that low CSF 5-HIAA is associated with hyperactivity and aggression early in life in humans (Clarke et al., 1999). However, similar behavior patterns were observed in infants with different genotypes, and potentially distinct 5-HT function. The mechanisms of gene—environment-behavior associations in these infants therefore remain to be explained. Developmental events may play an important role in our findings: it is possible that genotypes exert specific and yet-uncharacterized influences on brain development early in life that may predict adult 5-HT function or the function of other neural systems. In support of this notion, we have demonstrated, along with others (Champoux et al., 2002; Spinelli et al., 2007), that behavioral effects of G × G and G × E interactions are measurable early in life.
We have presented evidence that G × E interactions influence behavior in both male and female infants. Sex is an important factor in MAOA-association studies, as the MAOA gene resides on the X chromosome. As a result, at least two issues must be considered while interpreting MAOA – association studies. First, males are hemizygous (ie, have one copy of the gene) and females either homozygous or heterozygous (ie, have two copies of the gene) for the MAOA polymorphism. This means that in females, one of the two X chromosomes in any given cell is inactivated, and the patterns of X inactivation are unknown. In the present study, we addressed this issue by categorizing females with two versions of the high activity allele as “high activity”; any cell, regardless of which X chromosome was inactivated, would have an active high activity allele. In parallel, females with two low activity alleles were categorized as low activity. In the case where females possessed two alleles with different activity (high/low activity “heterozyotes”), we created separate groups for analysis. The second issue is more complex, as it is possible that incomplete X chromosome inactivation (Carrell and Willard, 2005; but see Nordquist and Oreland, 2006) may lead to sex differences in MAOA genotype potency and corresponding MAOA activity. Previous studies have indicated that brain MAOA activity does not differ based on sex (Meyer et al., 2006), however. Additionally, we did not find that genotype was differentially associated with behavior between male and female infants. In genotype groups that included males and females (i.e., H/H, L/H, H/L and L/L), G × G and G × E interactions influencing behavior were identical between sexes (data not shown); further, there were no sex differences in behavioral stress response to the human intruder. It appears that at this early stage in development, G × G and G × E interactions influence infant rhesus macaque behavior similarly between sexes.
Replications of published studies illustrating G × E interactions influencing behavior have been somewhat inconsistent, posing challenges to the notion that gene-environment interactions are ubiquitous in their influence on behavioral development (Gottlieb, 2007). Our data suggest that G × E interactions influencing behavior may be either offset or intensified by related G × G or G × E interactions at an early stage in development. While the present findings must be replicated, we propose that consideration of the developmental timing of compensatory or competing G × G or G × E interactions will strengthen the search for risk factors for behavioral dysfunction and disease.
Table 2.
Means and Confidence Intervals of Rearing and Genotype Groups
| Activity | Emotional Reactivity | |
|---|---|---|
| Mother-Reared | ||
| H/H | −2.05 (−4.98–.89) | 0.58(−0.05–1.11) |
| H/HET | −3.09(−6.88–0.70) | 0.62(−0.13–1.37) |
| H/L | 0.20(−2.64–3.05) | 0.75(0.19–1.31) |
| L/H | 1.40(−2.03–4.83) | 1.07(0.40–1.75) |
| L/HET | −0.64(−4.94–3.66) | −1.43(0.58–2.28) |
| L/L | 0.05(−3.55–3.64) | 0.76(0.05–1.47) |
| Nursery-Reared | ||
| H/H | 1.64 (−1.30–4.57) | 2.62(2.04–3.20) |
| H/HET | 8.36 (4.57–12.15) | 1.35(0.61–2.10) |
| H/L | 5.14 (2.30–7.99) | 2.59(2.03–3.15) |
| L/H | 8.36(4.93–11.79) | 1.52(0.84–2.19) |
| L/HET | 7.15(2.85–11.44) | 2.94(2.09–3.78) |
| L/L | 5.05(1.46–8.65) | 2.73(2.02–3.44) |
Note. High rh5-HTTLPR/high rhMAO-A-LPR (H/H), high rh5-HTTLPR/heterozygote rhMAO-A-LPR (H/Het), high rh5-HTTLPR/low rhMAO-A-LPR (H/L), low rh5-HTTLPR/high rhMAO-A-LPR (L/H), low rh5-HTTLPR/heterozygote rhMAO-A-LPR(L/Het), low rh5-HTTLPR/low rhMAO-A-LPR (L/L).
Acknowledgments
We gratefully acknowledge the contributions of Dr. Thomas Famula, Dr. Robert Grahn, Elizabeth Milano and Laura Del Rosso to this work. We also thank several anonymous reviewers for their useful comments. This work was funded by RR000169 to CNPRC, and RR019970 to JPC.
References
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306(5697):879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Auerbach J, Geller V, Lezer S, Shinwell E, Belmaker RH, Levine J, et al. Dopamine D4 receptor (D4DR) and serotonin transporter promoter (5-HTTLPR) polymorphisms in the determination of temperament in 2-month-old infants. Molecular Psychiatry. 1999;3(3):369–373. doi: 10.1038/sj.mp.4000531. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, et al. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2(6):336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bell RQ. A congenital contribution to emotional response in early infancy and the preschool period. Ciba Foundation Symposium. 1975;33:201–212. doi: 10.1002/9780470720158.ch12. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry. 2002;7(1):118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2005;136(1):58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, Del Rosso LA, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. New York: Springer; 2006. [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, SJS Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7(10):1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and Psychopathology. 2007;19(4):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Clarke R, Murphy DL, JNC Serotonin and externalizing behavior in young children. Psychiatry Research. 1999;86(1):29–40. doi: 10.1016/s0165-1781(99)00022-0. [DOI] [PubMed] [Google Scholar]
- Colder C, Mott JA, ASB The interactive effects of infant activity level and fear on growth trajectories of early childhood behavior problems. Development and Psychopathology. 2002;14(1):1–23. doi: 10.1017/s0954579402001013. [DOI] [PubMed] [Google Scholar]
- Ebstein R, Levine J, Geller V, Auerbach J, Gritsenko I, RHB Dopamine D4 receptor and serotonin transporter promoter in the determination of neonatal temperament. Molecular Psychiatry. 1999;3(3):238–246. doi: 10.1038/sj.mp.4000363. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9(10):908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Falconer DS, MacKay TFC. Quantitative Genetics. Harlow, England: Pearson; 1996. [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61(7):738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, et al. Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype. PLos ONE. 2007;2(5):e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb B, McGeary J, Beevers C, Miller IW. Serotonin transporter (5-HTTLPR) genotype, childhood abuse, and suicide attempts in adult psychiatric inpatients. Suicide and life-threatening behavior. 2006;36(6):687–693. doi: 10.1521/suli.2006.36.6.687. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Developmental Psychobiology. 2008;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Probabilistic epigenesis. Developmental Science. 2007;10:1–11. doi: 10.1111/j.1467-7687.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Gunthert K, Conner T, Armeli S, Tennen H, Covault J, Kranzler H. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: a daily process approach to gene-environment interaction. Psychosomatic Medicine. 2007;69(8):762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Lessem J, Hopfer C, Smolen A, Ehringer M, Timberlake D, et al. Monoamine oxidase A (MAOA) and antisocial behaviors in the presence of childhood and adolescent maltreatment. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2005;135B(1):59–64. doi: 10.1002/ajmg.b.30176. [DOI] [PubMed] [Google Scholar]
- Herman A, Kaiss KM, Ma R, Philbeck JW, Hasan A, Dasti H, et al. Serotonin transporter promoter polymorphism and monoamine oxidase type A VNTR allelic variants together influence alcohol binge drinking risk in young women. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;133(1):74–78. doi: 10.1002/ajmg.b.30135. [DOI] [PubMed] [Google Scholar]
- Higley J, ML Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Annals of the New York Academy of Sciences. 1997;836:39–56. doi: 10.1111/j.1749-6632.1997.tb52354.x. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, et al. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol Psychiatry. 2004;55(11):1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29(8):1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry. 2007;12(5):483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jaffee S, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, NS Early childhood predictors of adult anxiety disorders. Biological Psychiatry. 1999;46(11):1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “Adverse” Environment? Interactions of Rearing Experiences and MAOA Genotype in Rhesus Monkeys. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.11.004. Published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal J, et al. Genetic and environmental predictors of early alcohol use. Biological Psychiatry. 2007;61(11):1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11(10):903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kinnally E, Lyons LA, Abel K, Mendoza S, JPC Effects of early experience and genotype on serotonin transporter regulation in infant rhesus macaques. Genes Brain and Behavior. 2008 doi: 10.1111/j.1601-183X.2007.00383.x. Published online Jan 16. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flügge G, Hinney A, Hebebrand J, et al. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Journal of Neural Transmission. 1997;104(11–12):1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19(4):287–299. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Manuck S, Flory JD, Ferrell RE, MFM Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;29(5):651–658. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Manuck S, Flory JD, Ferrell RE, Mann JJ, MFM A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Research. 2000;95(1):9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene x Environment Interactions at the Serotonin Transporter Locus. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.06.009. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- Newman T, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biological Psychiatry. 2005;57(2):167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjöberg RL, Wargelius HL, Leppert J, Lindström L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Olson S, Bates JE, Sandy JM, EMS Early developmental precursors of impulsive and inattentive behavior: from infancy to middle childhood. Journal of Child Psychology and Psychiatry. 2002;43(4):435–447. doi: 10.1111/1469-7610.00035. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Papp AC, Sadee W. Allelic mRNA expression of X-linked monoamine oxidase a (MAOA) in human brain: dissection of epigenetic and genetic factors. Human Molecular Genetics. 2006;15(1):2636–2649. doi: 10.1093/hmg/ddl192. [DOI] [PubMed] [Google Scholar]
- Reif A, Rösler M, Freitag CM, Schneider M, Eujen A, Kissling C, et al. Nature and nurture predispose to violent behavior: serotonergic genes and adverse childhood environment. Neuropsychopharmacology. 2007;32(11):2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- Rende R. Longitudinal relations between temperament traits and behavioral syndromes in middle childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(2):287–290. doi: 10.1097/00004583-199303000-00008. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shelton S, Shelledy W, Garcia R, Kalin N. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain and Behavior. 2008;7(4):463–469. doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol S, Hu S, DH A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103(3):273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Hajduk A, Samochowiec A, Horodnicki J, Stepie G, Grzywacz A, et al. Association studies of MAO-A, COMT, and 5-HTT genes polymorphisms in patients with anxiety disorders of the phobic spectrum. Psychiatry Research. 2004;128(1):21–26. doi: 10.1016/j.psychres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ, et al. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Development and Psychopathology. 2007;19:977–987. doi: 10.1017/S095457940700048X. [DOI] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33:312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Suomi S. Risk, resilience, and gene x environment interactions in rhesus monkeys. Annals of the New York Academy of Sciences. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biological Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Taylor A, Kim-Cohen J. Meta-analysis of gene-environment interactions in developmental psychopathology. Development and Psychopathology. 2007;19(4):1029–1037. doi: 10.1017/S095457940700051X. [DOI] [PubMed] [Google Scholar]
- Urwin R, KPN Epistatic interaction between the monoamine oxidase A and serotonin transporter genes in anorexia nervosa. European Journal of Human Genetics. 2005;13(3):370–375. doi: 10.1038/sj.ejhg.5201328. [DOI] [PubMed] [Google Scholar]
- Vanyukov M, Maher B, Devlin B, Kirillova G, Kirisci L, Yu L, et al. The MAOA promoter polymorphism, disruptive behavior disorders, and early onset substance use disorder: gene-environment interaction. Psychiatric Genetics. 2007;17(6):323–332. doi: 10.1097/YPG.0b013e32811f6691. [DOI] [PubMed] [Google Scholar]
- Wendland J, Hampe M, Newman TK, Syagailo Y, Meyer J, Schempp W, et al. Structural variation of the monoamine oxidase A gene promoter repeat polymorphism in nonhuman primates. Genes Brain and Behavior. 2006;5(1):40–45. doi: 10.1111/j.1601-183X.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- Williams R, Marchuk D, Gadde K, Barefoot J, Grichnik K, Helms M, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28(3):533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. American Journal of Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]