Abstract
Dialectical Behavior Therapy for Binge Eating Disorder (DBT-BED) aims to reduce binge eating by improving adaptive emotion-regulation skills. Preliminary findings have been promising but have only compared DBT-BED to a wait-list. To control for the hypothesized specific effects of DBT-BED, the present study compared DBT-BED to an active comparison group therapy (ACGT). Men and women (n = 101) meeting DSM-IV BED research criteria were randomly assigned to 20 group sessions of DBT-BED (n = 50) or ACGT (n = 51). DBT-BED had a significantly lower dropout rate (4%) than ACGT (33.3%). Linear Mixed Models revealed that posttreatment binge abstinence and reductions in binge frequency were achieved more quickly for DBT-BED than for ACGT (posttreatment abstinence rate = 64% for DBT-BED vs. 36% for ACGT) though differences did not persist over the 3-, 6-, and 12-month follow-up assessments (e.g., 12-month follow-up abstinence rate = 64% for DBT-BED vs. 56% for ACGT). Secondary outcome measures revealed no sustained impact on emotion regulation. Although both DBT-BED and ACGT reduced binge eating, DBT-BED showed significantly fewer dropouts and greater initial efficacy (e.g., at posttreatment) than ACGT. The lack of differential findings over follow-up suggests that the hypothesized specific effects of DBT-BED do not show long-term impact beyond those attributable to nonspecific common therapeutic factors.
The most studied treatments for binge eating disorder (BED) to date include cognitive behavior therapy (CBT), interpersonal psychotherapy (IPT), and behavioral weight loss (BWL). Such treatments are efficacious, with overall abstinence rates from binge eating ranging from 41% to 79% (e.g., Munsch et al., 2007; Wilfley et al., 2002). Despite the efficacy of these treatments, none focus on the role of dysregulated emotions in the etiology and/or maintenance of binge eating. Because of the sizeable number of patients who remain symptomatic after treatment, there has been interest in developing and researching other theoretical conceptualizations and treatment models for BED.
Dialectical Behavior Therapy for BED (DBT-BED), with its grounding in affect regulation and direct focus on the link between dysregulated emotions and dysregulated eating behaviors, is one such model. Drawing upon an extensive literature linking negative affect and disordered eating (Abraham & Beumont, 1982; Arnow, Kenardy, & Agras, 1992; Arnow, Kenardy, & Agras, 1995; Polivy & Herman, 1993), the affect-regulation model conceptualizes binge eating as a behavioral attempt to influence, change, or control painful emotional states (Linehan & Chen, 2005; Wiser & Telch, 1999; Wisniewski & Kelly, 2003).
DBT was originally developed by Linehan (1993a, 1993b) as a treatment for borderline personality disorder and is currently the most comprehensive and empirically supported affect-regulation treatment for borderline personality disorder (American Psychiatric Association [APA], 2001). Researchers recognized that DBT’s conceptualization of self-injury as a functional (albeit maladaptive) affect-regulation behavior in patients with borderline personality disorder might provide a helpful model for understanding the function of binge eating as an emotion-regulation behavior in patients with disordered eating (Linehan & Chen, 2005; Wiser & Telch, 1999; Wisniewski & Kelly, 2003; Waller, 2003).
To date, preliminary studies investigating the adaptation of DBT to target disordered eating have been promising but limited to single case reports (Safer, Telch, & Agras, 2001a; Telch, 1997a), an uncontrolled case series (Palmer et al., 2003), uncontrolled trials (Salbach et al., 2007; Telch, Agras, & Linehan, 2000), and two randomized controlled trials, each against wait-list controls (DBT-BED: Telch, Agras, & Linehan, 2001; DBT-Bulimia Nervosa: Safer, Telch, & Agras, 2001b).
The existing randomized trial of DBT-BED (Telch et al., 2001) was relatively small, including 44 participants assigned to 20 two-hour weekly group sessions of DBT-BED (n = 22) or a wait-list control (n = 22). Intent-to-Treat (ITT) analyses yielded a significantly higher posttreatment abstinence rate of 73% (16/22) for DBT-BED versus 9% (2/22) for those in the wait-list condition.1 At the final (6 month) follow-up, the ITT abstinence rate for DBT-BED was 54.5% (12/22). No follow-up was conducted for the wait-list participants.
A recommended methodological approach for establishing the efficacy of a newly developed therapy is (1) to compare against a wait-list condition to control for the effects of time and assessments, (2) to compare against an active placebo to control for the nonspecific effects of psychotherapy (e.g., therapeutic alliance, treatment expectations, etc.), and (3) to compare against another established active treatment to investigate outcome (e.g., Chambless & Hollon, 1998). Given DBT-BED’s initial promising findings against a wait-list comparison (Telch et al., 2001), the logical next step before engaging in the expense of a sufficiently powered head-to-head comparison of DBT-BED against another active treatment (e.g., CBT or IPT) is to compare DBT-BED to an “active placebo” or credible control group. Indeed, as noted by Critelli and Neumann (1984), “the placebo control group strategy provides the most direct and unambiguous answer to the question of whether a treatment shows a level of effectiveness beyond that of its placebo effects.”
The aim of the present randomized controlled research study was to evaluate the specific effects of DBT-BED (e.g., the explicit focus on linking emotion dysregulation and binge eating) relative to the general common therapeutic and supportive influences that characterize meaningful group therapy by comparing DBT-BED to an active comparison group therapy (ACGT) at posttreatment and over a 12-month follow-up period.
Method
PARTICIPANTS
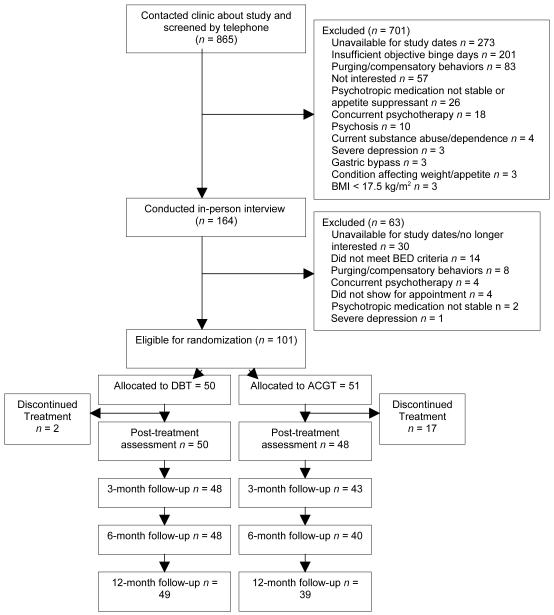
The study was reviewed and approved by the Institutional Review Board of Stanford University Medical Center. Participants were recruited through newspaper advertisements, flyers, and clinic referrals for “treatment for binge eating.” Eligibility was assessed via an initial telephone screen followed by an in-person clinical interview, during which potential participants provided informed written consent. Men and women aged 18 and older who met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA, 1994) research criteria for BED and lived or worked within commuting distance to the clinic were included. Exclusion criteria were: (1) body mass index (BMI) less than 17.5 kg/m2; (2) concurrent psychotherapy treatment; (3) unstable dosage of psychotropic medications over the 3 months prior to initial assessment; (4) regular use of purging or other compensatory behaviors over the past 6 months; (5) psychosis; (6) current alcohol/drug abuse or dependence; (7) severe depression with recent (e.g., within past month) suicidality; (8) current use of weight-altering medications (e.g., phentermine); (9) severe medical condition affecting weight or appetite (e.g., insulin-dependent diabetes, cancer requiring active chemotherapy); (10) current pregnancy or breast feeding; (11) imminently planning or undergoing gastric bypass surgery; and (12) lack of availability for times of group meetings and/or duration of study. A total of 865 individuals expressed initial interest. Based on phone screening, 701 were excluded. An additional 63 were excluded based on in-person interviews (see Figure 1).
Figure 1.
Summary of participant flow in Dialectical Behavior Therapy for BED (DBT-BED) and Active Comparison Group Therapy (ACGT).
TREATMENT
The study involved 101 men and women who were randomly assigned to 20 sessions of group therapy: DBT-BED (n = 50) or ACGT (n = 51). The study was conducted in five temporally sequential phases, with 18 to 22 participants recruited per phase for allocation to the two groups, and with 9 to 11 individuals constituting each group. Both treatments were manual-based and consisted of a pretreatment orientation to the treatment model followed by 20 2-hour weekly group sessions held over 21 weeks (with Sessions 19 and 20 spaced 2 weeks apart).
Two co-therapists were utilized, a senior (Ph.D. or M.D.) therapist and a doctoral candidate co-therapist. Neither were investigators or authors of the current study. As discussed in greater detail by Safer and Hugo (2006), the decision as to whether to keep the therapists constant in a treatment study comparing two (or more) treatments is one with no ideal answer. In this study, the decision was made for the same therapists to participate in both conditions, primarily because doing so minimizes variability due to therapist differences.
DIALECTICAL BEHAVIOR THERAPY FOR BINGE EATING DISORDER (DBT-BED)
The DBT-BED manual was based on Linehan’s DBT for borderline personality disorder (1993a, 1993b) that was previously adapted for BED by Telch (Telch, 1997b). Briefly (for greater detail see Telch et al., 2001; Wiser & Telch, 1999), the 20-session treatment consists of two introductory sessions presenting the DBT-BED rationale and orientation and commitment to treatment, 16 sessions teaching adaptive emotion-regulation skills over three modules, and two final sessions devoted to review and relapse prevention. The three modules included Mindfulness (Sessions 3–5), Emotion Regulation (Sessions 6–12), and Distress Tolerance (Sessions 13–18). Mindfulness skills teach nonjudgmental observation and how to describe moment-to-moment emotional experiences, thoughts, and action urges. Emotion regulation skills encourage understanding of how emotions function, decreasing vulnerability to negative emotions, increasing positive emotions, and changing specific emotional states (e.g., fear and anxiety) by acting opposite to one’s current emotion. Distress-tolerance skills teach adaptive and effective means for tolerating life’s unavoidable stresses and pain without turning to binge eating, and they facilitate acceptance of the current moment’s realities.
ACTIVE COMPARISON GROUP THERAPY (ACGT)
ACGT was developed with the goal of creating a comparison therapy whose rationale and procedures would be credible enough to generate therapeutic factors in common with DBT-BED (i.e., therapeutic alliance, treatment expectations, therapeutic optimism) while lacking the specific elements of DBT-BED and other BED treatments. Interested readers are referred to Safer and Hugo (2006) for a detailed discussion of ACGT’s design. The ACGT manual was modeled after Markowitz and Sacks’ (2002) manual of supportive therapy for chronic depression and subsequently modified to address binge eating for the current study. The manual instructs therapists to follow a Rogerian approach (Rogers, 1951). Self-esteem and self-efficacy are bolstered by highlighting patients’ strengths (i.e., bolstering self-esteem to enhance the ability to stop binge eating). The therapy encourages patients to find answers within themselves instead of providing patients with specific techniques or skills. ACGT’s ingredients (e.g., bolstering self-esteem) were intended to be indistinguishable from those evoked by the common factors of therapeutic alliance and development of therapeutic optimism.
TREATMENT INTEGRITY AND ADHERENCE
To monitor treatment integrity and adherence, DBT-BED and ACGT treatment session audiotapes were reviewed weekly and therapists were given feedback via weekly meetings for the duration of the study. DBT-BED audiotapes were reviewed by a DBT expert who had received advanced training in DBT coding and was independent from the study. Adherence was monitored by assessing standard areas of DBT-BED (e.g., problem assessment strategies, validation strategies, dialectical strategies, etc.) based on an instrument developed by the University of Washington Behavioral Research and Therapy group. Emphasis was on adherence to the adapted DBT-BED manual (Telch, 1997b) used in the earlier randomized controlled trial of DBT-BED (Telch et al., 2001).
ACGT treatment session audiotapes were reviewed by an eating disorder expert who was also independent from the clinical trial. An integrity checklist was used to ensure that ACGT therapists avoided content or procedural overlap with DBT-BED (e.g., systematically linking dysregulated emotional states and binge eating, teaching DBT-BED skills, etc.), IPT (e.g., actively pursuing specific interpersonal change strategies, systematically linking binge eating and interpersonal disputes, grief, etc.), CBT (e.g., focusing on cognitions that identify irrational negative thoughts associated with binge eating, behaviorally focusing on reducing dietary restraint and normalizing eating), or behavioral weight loss (e.g., providing specific weight loss strategies and goals, etc.).
Therapists were also provided with clinical supervision. Luborsky et al. (1999) suggest that therapists be supervised by those who represent expertise for the corresponding treatment mode. Supervision for DBT-BED was provided by a DBT expert independent from the study. As the ACGT treatment was especially designed for the current study, there were no supervisors who represented expertise in this mode. Therefore, a key member of the supervision team with expertise in eating disorders but with no allegiance to DBT-BED provided supervision.
ASSESSMENTS
Except for demographic and diagnostic information (collected at baseline only) and questions regarding treatment suitability (assessed after the pretreatment orientation), all assessment instruments were administered at baseline, posttreatment, and 3, 6, and 12 months following treatment.
General Psychopathology
The Structured Clinical Interview for DSM-IV (SCID-I; First, Spitzer, Gibbon, & Williams, 1995) assesses current and lifetime Axis I disorders consistent with DSM-IV. For the purposes of this study, the SCID-I was only used to determine the presence of past or current major depressive episodes. Due to limited assessor time and the desire to decrease participant burden, the entire SCID I, including assessment of anxiety disorders, was not administered, despite the fact that such disorders are frequent in this population (e.g., Javaras et al., 2008) and might affect outcome. Rates of all Axis I disorders in a similar sample (e.g., BED participants engaged in a DBT-BED treatment study) were gathered by Telch and colleagues (2001), to which the interested reader is referred. As noted, participants with active substance abuse or dependence were excluded in this study. The Structured Clinical Interview for DSM-IV Axis II disorders self-report (SCID II; First, Gibbon, Spitzer, Williams, & Benjamin, 1997) was used to determine the presence of personality disorders in participants. The reliability and validity of the SCID I and SCID II have been well documented (O’Boyle & Self, 1990; Renneberg, Chambless, Dowdall, Fauerbauch, & Gracely, 1993; Segal, Hersen, & Van Hasselt, 1994).
The Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) assesses the degree of depressive symptoms (including somatic, affective, cognitive, and behavioral dimensions), with lower scores reflecting lower levels of depression. The BDI has good test-retest reliability, internal consistency, and convergent validity (Beck, Steer, & Garbin, 1988).
The Rosenberg Self-Esteem Scale (RSE; Rosenberg, 1979) is a brief, 10-item questionnaire measuring beliefs and attitudes regarding general self-worth (e.g., “On the whole, I am satisfied with myself”) based on a 4-point Likert-type scale, with higher scores reflecting higher self-esteem. The RSE has been shown to have satisfactory internal consistency, test-retest reliability, and convergent validity (Demo, 1985).
Eating Disorder Pathology
The Eating Disorder Examination (EDE; Fairburn & Cooper, 1993) is a widely used semistructured interview that assesses primary behavioral and attitudinal eating disorder features. The EDE was used to determine DSM-IV research criteria for BED. Other key variables derived from the EDE included objective binge day frequency, abstinence (defined as the absence of objectively large binge episodes for the 28 days prior to assessment), and the EDE’s four subscales (Restraint, Weight Concerns, Shape Concerns, and Eating Concerns), with lower scores reflecting lower eating-related pathology. All EDE interviewers received extensive training, with the lead assessors trained by Dr. Christopher Fairburn, who developed the measure. All EDE interviews were audiotaped, and consistency of examiners’ interviewing techniques was checked by an independent rater who reviewed randomly selected audiotapes. Interrater agreement for the EDE has been shown to be above .90 for all subscales and behavior items, and test-retest agreement above .70, except for the item on subjective bulimic episodes (.40) (Rizvi, Peterson, Crow, & Agras, 2000).
Emotion Regulation
The Negative Mood Regulation Scale (NMR; Catanzaro & Mearns, 1990) is a 30-item questionnaire that measures the participant’s expectancy that a behavior or cognition will alleviate a negative mood state (e.g., “When I’m upset, I believe that I can usually find a way to cheer myself up”) with higher scores reflecting higher expectancies. The NMR has demonstrated adequate internal consistency, discriminant validity, and temporal stability (Catanzaro & Mearns).
The Emotional Eating Scale (EES; Arnow et al., 1995) measures, on a 5-point Likert-type scale, the extent to which 25 different emotions (e.g., sad, irritated, guilty, uneasy) lead one to feel an urge to eat. Three separate subscales comprise the EES: Anger/Frustration, Anxiety, and Depression. Studies of the scale’s psychometric properties have indicated that it is internally consistent and demonstrates adequate temporal stability (Arnow et al.). Lower scores reflect lower emotion-driven urges to eat.
The Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) has 20 self-report items in which participants report the extent to which they experienced 20 different emotions over the previous week. Emotions are scored on separate subscales, one scale for positive emotions (e.g., enthusiastic, proud) and one for negative emotions (e.g., distressed, ashamed). Higher scores on the positive subscale reflect higher degrees of positive emotions and lower scores on the negative subscale reflect fewer negative emotions.
The Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004) is a 36-item self-report measure that examines difficulties in the ability to regulate emotions. Participants rate how often statements such as “I feel at ease with my emotions” apply to them using a 5-point Likert-type scale, with higher scores reflecting greater difficulties with emotion regulation. A global score can be derived, as well as six subscale scores. To minimize the number of secondary outcome variables, this study utilized the global score. Research suggests the DERS has high internal consistency, good test-retest reliability, and adequate construct and predictive validity (Gratz & Roemer).
Weight, Body Mass Index
Body weight was assessed on a balance beam scale by a trained research assistant, with the participant in lightweight clothing and shoes removed. Height was measured with a stadiometer. For both variables, the average of two measurements was used. Body Mass Index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters).
Eating Disorder History
The Questionnaire on Eating and Weight Patterns (QEWP; Spitzer et al., 1992) is a self-report instrument that asks retrospective questions about the onset of binge eating, dieting, and obesity (e.g., “At what age did you first begin to binge eat?”, “How old were you when you first started dieting?”, and “At what age did you first weigh more than people thought you should?”).
Suitability of Treatment
Suitability of treatment was first assessed postrandomization, by which point, at the conclusion of the pretreatment orientation, participants had been given written and verbal information regarding the treatment rationales for both DBT and ACGT, received their random group assignment, and were oriented in greater detail to the treatment model matching their group assignment). Suitability was assessed again after Session 1 (for all participants, n = 83, except those in Phase 1 of the study, n = 18) and at the posttreatment assessment (for all participants). Using a 10-point visual analogue scale, participants were asked to rate: “How suitable do you think this treatment is for your problems?” from 1 (“This treatment makes much less sense”) to 10 (“This treatment makes much more sense”), with 5 as the midpoint (“This treatment seems equal to other treatments”).
Primary and Secondary Outcomes
The primary outcome variables were abstinence from binge eating (defined as no objectively large binge days over the prior 28 days) and days of objective binge eating (over the prior 28 days). There were a number of secondary outcome variables of interest. In terms of eating pathology, these included the EDE subscales (e.g., Restraint, Weight Concerns, Shape Concerns, and Eating Concerns). General psychopathology was measured with the BDI and the RSE. Emotion regulation measures included the NMR, EES, PANAS, and DERS. Body composition measures included body weight and BMI.
ANALYSIS
For the primary outcomes of abstinence from binge eating and days of objective binge eating (both measured over the past 28 days), statistical analyses were based on the Linear Mixed Models approach (Goldstein, 1986; Longford, 1993; McCullagh & Nelder, 1989; Raudenbush & Bryk, 2002; Snijders & Bosker, 1999). Linear Mixed Models provide theoretical and practical benefits over conducting a repeated measures analysis of variance for longitudinal data. Such benefits include the ability to: (a) model multiple data points over time and interpret the overall trend simultaneously and (b) model missing data via maximum likelihood estimation methodology. Using the Linear Mixed Models approach, participants with missing data on some time points remain in the analyses. The Linear Mixed Models approach tests hypotheses about treatment effects, time course, and treatment-by-time interactions. The within-subjects factor is time; the between-subject factors are treatment and the interactions between time components and treatment. Linear Mixed Models for the binary (abstinence) and continuous (binge day) outcomes were estimated using the Mplus program version 5 (Muthén & Muthén, 1998–2008). For both binary and continuous outcome analyses, maximum likelihood estimation via the expectation-maximization (EM) algorithm was used (Little & Rubin, 2002; McCulloch, 1997; McLachlan & Krishnan, 1997).
The secondary outcomes were analyzed using effect sizes based on Cohen’s d and were evaluated by the conventions: small = .20, moderate = .50, and large = .80 (Cohen, 1988). The rationale for using effect sizes rather than p values included: (a) the study was powered to detect differences in only the primary and not secondary outcomes, and (b) testing the numerous secondary outcomes for group differences would increase the risk of a Type I error. Secondary outcomes are presented for all participants using last-observation-carried forward.
Results
SAMPLE CHARACTERISTICS AND BASELINE ANALYSES
Characteristics of the sample at baseline are presented in Table 1. All demographic (e.g., age, gender), clinical (e.g., BMI, concurrent major depressive episode, criteria met for an Axis II disorder), and eating pathology (e.g., binge frequency) characteristics were analyzed to check for any significant differences in those randomized to DBT-BED or ACGT. No differences were found, with the exception of having a current major depressive episode, which was significantly more prevalent in the DBT-BED group (22%, or 11/50) than in the ACGT group (7.8%, 4/51), χ2(1, N = 101) = 4.00, p = .045.
Table 1.
Sample Characteristics
| DBT-BED n = 50 |
ACGT n = 51 |
Total N = 101 |
|
|---|---|---|---|
| Gender (number, %) | |||
| Female | 43 (86) | 43 (84) | 86 (85) |
| Male | 7 (14) | 8 (15) | 15 (15) |
| Age (M, SD) in years | 51.9 (11.6) | 52.35 (9.52) | 52.2 (10.6) |
| Body Mass Index (M, SD) | 35.84 (9.35) | 36.90 (7.89) | 36.38 (8.62) |
| Ethnicity/Race (number, %) | |||
| Caucasian | 40 (80) | 37 (73%) | 77 (76) |
| Latino | 8 (16) | 5 (10%) | 13 (13) |
| Asian | 2 (4) | 3 (6%) | 5 (5) |
| African American | 0 | 3 (6%) | 3 (3) |
| Unknown/Unreported Ethnicity | 0 | 3 (6%) | 3 (3) |
| Marital Status (number, %) | |||
| Married | 32 (64) | 29 (57) | 61 (60) |
| Divorced | 6 (12) | 13 (26) | 19 (19) |
| Single/Never Married | 11 (22) | 6 (12) | 17 (17) |
| Widowed | 1 (2) | 3 (6) | 4 (4) |
| Educational Background (number, %) | |||
| Completed at least one graduate degree | 14 (28) | 16 (31) | 30 (30) |
| Completed some college/2 year degree | 12 (24) | 18 (35) | 30 (30) |
| Graduate from a 4 year college | 16 (32) | 10 (20) | 26 (26) |
| Completed some graduate school | 5 (10) | 4 (8) | 9 (9) |
| Had not completed high school | 2 (4) | 2 (4) | 4 (4) |
| Have high school degrees or equivalent | 1 (2) | 1 (2) | 2 (3) |
| Employment Status (number, %) | |||
| Employed | 29 (58) | 21 (41) | 50 (50) |
| Retired | 7 (14) | 12 (24) | 19 (19) |
| Homemaker | 5 (10) | 9 (18) | 14 (14) |
| Unemployed | 6 (12) | 6 (12) | 12 (12) |
| Student/Other | 3 (6) | 3 (6) | 6 (6) |
| Age (M, SD) when first | |||
| Overweight | 17.2 (11.3) | 17.5 (11.3) | 17.4 (11.3) |
| Began dieting | 17.3 (9.3) | 18.1 (8.8) | 17.7 (9.0) |
| Began binge eating | 19.8 (12.3) | 19.1 (13.2) | 19.4 (12.7) |
| Concurrent Major Depressive Episode (%) | 11 (22) | 4 (7.8) | 15 (14.9) |
| Past History of Depression (%) | 36 (72) | 28 (54.9) | 64 (63.4) |
| Current Use of Antidepressants (%) | 17 (34) | 16 (31.4) | 33 (32.7) |
| Criteria met for any Axis II disorder (%) | 25 (50) | 18 (35.3) | 43 (42.6) |
TREATMENT SUITABILITY
Participants in DBT-BED rated suitability of treatment (9.2 ±1.3) similarly to those in ACGT (8.8 ± 1.7) (t99= −1.19, p = .24) postrandomization at the conclusion of the pretreatment orientation. After Session 1, suitability ratings were 8.6 ± 1.4 for DBT-BED and 8.1 ± 2.2 for ACGT (t79= −1.3, p = .21). At posttreatment, DBT-BED participants rated suitability as 8.9 ± 1.3 compared to 8.2 ± 2.3 for ACGT participants (t61= −1.6, p = .12).
Treatment Dropout Rates and Study Assessment Dropout Rates
Of the 101 patients, 19 (18.8%) dropped from treatment. Eighty-one of the 82 treatment completers (98%) were physically present for at least 75% (15/20) of the treatment sessions or made up missed sessions by listening to audiotape recordings of the corresponding session. All discontinuations were patient initiated; no participants were withdrawn from treatment. Dropout for DBT-BED (4% or 2/50) was significantly lower than for ACGT (33.3% or 17/51), χ2(1, N = 101) = 14.2, p < .001. Reasons cited for dropping out of DBT-BED were family/medical crisis (n = 1) occurring after Week 1 and moving out of the area (n = 1) occurring after Week 8. Reasons cited for dropping out of ACGT were time conflict with work (n = 7), not finding the treatment helpful (n = 5), moving from the area (n = 2), family/medical crisis (n = 2), and unknown (n = 1, did not show for first session and refused further contact). The mean and median week at which ACGT subjects left treatment was Week 8. About one third (n = 6/51, 35%) of patients dropped out within the first month (n = 1 before 1st week, n = 2 after Week 1, n = 1 after Week 2, n = 2 after Week 4). The drop rate for the remaining subjects was evenly distributed over Weeks 5 to 18.
Analyses of participants who dropped from treatment versus those who completed treatment revealed no significant differences on baseline demographic (e.g., gender), clinical (e.g., BMI, meeting criteria for an Axis II disorder), or eating pathology measures.
Dropping from treatment did not necessarily result in dropping from participation in study assessments. The percentages of participants who completed study assessments on at least the primary outcome variables were: 97% (n = 98/101; DBT-BED = 50, ACGT = 48) at posttreatment, 90% (n = 91/101; DBT-BED = 48, ACGT = 43) at 3 month follow-up, 87.1% (n = 88/101; DBT-BED = 48, ACGT = 40) at 6 month follow-up, and 87.1% (n = 88/101; DBT-BED = 49, ACGT = 39) at 12 month follow-up.
PRIMARY OUTCOMES
Abstinence
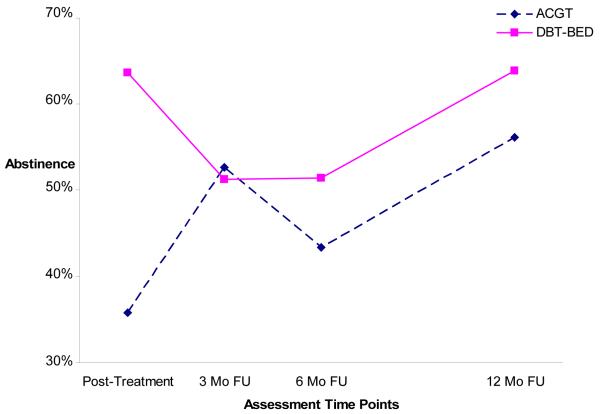
Given that the observed data showed both linear and nonlinear trends, the longitudinal trend of abstinence was modeled using a piecewise growth model. Abstinence status was modeled as quadratic during the first period (e.g., from posttreatment to the 6-month follow-up) and linear during the second period (e.g., from the 6-month to the 12-month follow-up assessment). Figure 2 depicts the abstinence estimated trajectories based on this piecewise growth model. Only estimated curves are presented in Figure 2 because abstinence’s observed and estimated mean trajectories were highly similar. The Linear Mixed Models analyses employed ACGT as the reference group. ACGT results are presented first, followed by presentation of DBT-BED’s results as compared to ACGT.
Figure 2.
Piecewise linear growth model for abstinence (DBT-BED =Dialectical Behavior Therapy for Binge Eating Disorder; ACGT=Active Comparison Group Therapy; Mo=month; FU=follow-up).
Results indicate that during the first period, ACGT abstinence rates increased significantly (p = .026). The growth in abstinence rates for ACGT decelerates after this upturn, but not at a significant rate. DBT-BED’s abstinence rate decreased significantly during the first period and differed significantly from ACGT (p = 0.015). DBT-BED’s abstinence rate increased after the initial decline, but not at a rate significantly different from ACGT. During the second period, both groups’ abstinence rates similarly increased, and there was no significant difference between them. ACGT showed a 36% abstinence rate at posttreatment, 53% at the 3 month follow-up, 43% at the 6 month follow-up, and 56% at the 12 month follow-up. DBT-BED yielded a 64% abstinence rate at posttreatment, 51% at the 3 month follow-up, 52% at the 6 month follow-up, and 64% by the 12 month follow-up.
Intention-to-Treat and Completer Samples for Abstinence
The abstinence percentages reported above were derived from the Linear Mixed Models approach. The pattern of findings is somewhat similar when abstinence rates are calculated based on an ITT sample and a completer sample. For example, posttreatment abstinence rates with an ITT (with last-observation-carried-forward) sample are 64% (32/50) for DBT-BED versus 33.3% (17/51) for ACGT and with a completer sample are 64.6% (31/48) for DBT-BED versus 38.2% (13/34) for ACGT. At the 12-month follow-up, abstinence rates with an ITT sample are 64% (32/50) for DBT-BED versus 49% (25/51) for ACGT [or 62% (31/50) for DBT-BED versus 43% (22/51) for ACGT if the scores of individuals missing 12-month assessments are replaced with pretest values], and with a completer sample are 64.6% (31/48) for DBT-BED versus 54.8% (17/31) for ACGT. Though the Linear Mixed Models employing a maximum likelihood estimation approach is felt to represent the most valid analysis by allowing modeling of missing data via maximum likelihood methodology, the above calculations with ITT and completer samples are offered for the interested reader.
Binge Days
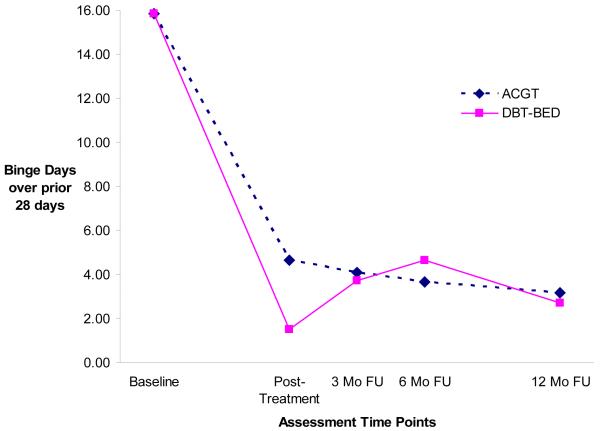
Given that the observed data for objective binge days showed both linear and nonlinear trends, the longitudinal trend for binge days was modeled using a piecewise growth model. Frequencies of objective binge days at baseline and posttreatment (first period) were modeled as having linear growth. Binge days from posttreatment through the 3-, 6-, and 12-month follow-up assessments (second period) were modeled as having quadratic growth. Figure 3 depicts the estimated trajectories of objective binge days based on this piecewise growth model. Since the observed mean trajectories of binge days and the estimated mean trajectories are highly similar, only the estimated curves are presented.
Figure 3.
Piecewise linear growth model for binge days (DBT-BED=Dialectical Behavior Therapy for Binge Eating Disorder; ACGT=Active Comparison Group Therapy; Mo=month; FU=follow-up).
Model estimation results indicate that during the first period, binge days in the ACGT group decreased significantly (p < .001). Compared to ACGT, binge days for DBT-BED decreased significantly more (p = .001). ACGT’s binge days trajectory continued to decline during the second period, but not at the significant rate. DBT-BED’s binge days trajectory increased and then decreased during the second period, resulting in a different trajectory shape from ACGT. In summary, although there was a significant difference in binge day change trajectories between the two groups from the baseline and posttreatment assessment (the first trajectory period), there were no significant group differences over the follow-up period (the second trajectory period).
SECONDARY OUTCOMES
Table 2 presents data from the baseline, posttreatment, and 12-month follow-up assessment for all secondary outcome measures. Data for the 3- and 6-month follow-up assessments did not provide clinically meaningful information different from the 12 month follow-up and are available from the author (D.S.).
Table 2.
Intent-to-treat (N = 101) Secondary Outcome Measures: Means and Standard Deviations at Baseline, Post-treatment and 12 month Follow-up
| DBT-BED Baseline Mean (SD) |
DBT-BED Post- treatment Mean (SD) |
DBT-BED 12 Mo FU Mean (SD) |
ACGT Baseline Mean (SD) |
ACGT Post- treatment Mean (SD) |
ACGT 12 Mo FU Mean (SD) |
Effect Size Post- treatment |
Effect Size 12 Mo FU |
|
|---|---|---|---|---|---|---|---|---|
| EDE- Restraint | 1.73 (1.12) |
1.29 (1.04) |
1.10 (1.09) |
2.00 (1.28) |
1.91 (1.23) |
1.85 (1.42) |
.54 | .59 |
| EDE- Weight concerns | 3.70 (1.02) |
2.53 (1.18) |
2.27 (1.24) |
3.76 (1.05) |
3.00 (1.25) |
2.78 (1.31) |
.39 | .40 |
| EDE- Shape Concerns | 3.91 (1.22) |
2.62 (1.15) |
2.50 (1.39) |
3.97 (0.91) |
3.03 (1.35) |
2.66 (1.30) |
.33 | .12 |
| EDE- Eating Concerns | 2.25 (1.43) |
0.54 (0.71) |
0.88 (1.38) |
2.09 (1.32) |
1.14 (1.39) |
0.66 (0.95) |
.54 | −.19 |
| Beck Depression Inventory |
17.94 (9.37) |
9.10 (9.21) |
10.36 (9.97) |
15.27 (6.83) |
10.84 (6.86) |
10.04 (7.84) |
.21 | −.04 |
| Rosenberg Self-Esteem Scale |
25.72 (6.62) |
30.28 (6.78) |
29.42 (7.66) |
27.31 (5.59) |
29.82 (5.80) |
30.59 (6.15) |
−.07 | .17 |
| NMR | 98.86 (19.24) |
99.54 (16.67) |
108.40 (19.72) |
100.31 (16.26) |
99.71 (13.35) |
110.12 (13.61) |
.01 | .10 |
| EES-Anger | 2.57 (0.95) |
1.83 (0.98) |
1.93 (0.97) |
2.61 (0.73) |
2.06 (1.05) |
1.90 (0.96) |
.23 | −.03 |
| EES -Anxiety | 2.21 (0.86) |
1.51 (0.87) |
1.67 (0.90) |
2.36 (0.78) |
1.81 (0.89) |
1.67 (0.89) |
.34 | 0 |
| EES-Depression | 2.73 (0.90) |
2.06 (0.99) |
2.12 (0.92) |
2.98 (0.64) |
2.43 (0.80) |
2.18 (0.89) |
.41 | .07 |
| DERS | 98.24 (20.80) |
75.58 (23.91) |
77.17 (29.22) |
94.08 (19.05) |
75.94 (21.04) |
71.7 (22.35) |
.02 | −.21 |
| PANAS Positive | 25.04 (8.35) |
30.24 (10.34) |
30.00 (10.36) |
27.16 (6.91) |
30.41 (6.97) |
30.43 (8.48) |
.02 | .05 |
| PANAS Negative | 26.08 (9.45) |
21.26 (8.01) |
22.82 (10.28) |
24.82 (7.94) |
20.45 (6.58) |
19.51 (7.72) |
−.11 | −.36 |
| Weight (pounds) | 216.91 (54.71) |
212.61 (52.60) |
213.23 (52.73) |
224.03 (55.29) |
221.87 (53.19) |
221.61 (54.89) |
.12 | .16 |
| BMI (kg/m2) | 35.84 (9.35) |
35.13 (9.03) |
35.29 (9.07) |
36.90 (7.89) |
36.65 (7.64) |
36.45 (7.53) |
.13 | .14 |
Note. DBT-BED = Dialectical Behavior Therapy for Binge Eating Disorder; ACGT = Active Comparison Group Therapy; Mo = Month; FU = Follow-Up; EDE = Eating Disorder Examination; NMR = Negative Mood Regulation Scale; EES = Emotional Eating Scale; DERS = Disorders of Emotion Regulation Scale; PANAS = Positive and Negative Affect Scale; BMI = Body Mass Index. (+) Effect Size favors DBT; (−) Effect Size favors ACGT.
Of the secondary measures across the posttreatment and 12 month assessments, only three reached a medium effect size according to Cohen (1988). All other effect sizes either met or were less than Cohen’s convention for a small effect (e.g., .20). The three medium effect sizes, all of which favored DBT-BED over ACGT, included the EDE-Restraint subscale at both posttreatment (.54) and the 12-month follow-up assessment (.59), and the EDE-Eating Concerns subscale at posttreatment (.54).
At posttreatment, small effect sizes favoring DBT-BED over ACGT were found for EDE-Weight Concerns subscale (.39), EDE-Shape Concerns subscale (.33), the BDI (.21), and the three EES subscales [Anger (.23), Anxiety (.34), Depression (.41)]. At the 12-month follow-up, a small effect size continued to favor DBT-BED over ACGT for the EDE-Weight Concerns subscale (.40). Unlike at posttreatment, at the 12-month follow-up, ACGT showed small effect sizes over DBT-BED for the DERS (−.21) and the PANAS-negative (−.36). No other effect sizes met the minimum criteria for a small effect.
Though the effect sizes for weight were minimal, mean weight loss is reported for the two groups due to the clinical importance of obesity in BED. For DBT-BED participants, mean weight loss from baseline was 4.3 (±13.5) lbs at posttreatment and 3.7 (±30.2) lbs from baseline at the 12-month follow-up. For ACGT participants, mean weight loss from baseline was 2.2 (±9.6) lbs at posttreatment and 2.4 (±15.3) at the 12-month follow-up. DBT-BED participants who were abstinent at the 12-month follow-up (n = 32) had mean weight losses from baseline of 6.4 lbs (±35.3) lbs. Among ACGT participants abstinent at the 12-month follow-up (n = 25), mean weight losses from baseline were 3.5 (±16.7) lbs.
Discussion
DBT-BED, a recently developed treatment based on the affect-regulation model of binge eating, has heretofore been tested in only one randomized trial (Telch et al., 2001), which showed that DBT-BED was better than no treatment in reducing binge eating. Because there was no comparison with an active control, it is possible that the effects of therapy were due to nonspecific therapeutic elements. Therefore, the current study compared DBT-BED to a structurally equivalent nonspecific psychotherapy treatment to examine whether the hypothesized active ingredients of DBT-BED would show a level of effectiveness beyond that due to shared common therapeutic effects (e.g., therapeutic optimism).
Participants in both the DBT-BED and active comparison group therapy (ACGT) improved substantially in achieving this study’s primary outcomes, abstinence and reducing binge frequency. Posttreatment abstinence rates were 64% for DBT-BED and 36% for ACGT, and 12-month follow-up abstinence rates were 64% and 56% for DBT-BED and ACGT, respectively. Although the DBT-BED group achieved abstinence and reductions in binge frequency more quickly (e.g., at posttreatment), there were no significant differences between the groups at any time during the follow-up period.
The posttreatment abstinence rate in the ACGT group was similar to abstinence rates found in the control arms of other BED treatment outcomes studies. For example, ACGT’s posttreatment abstinence rate is comparable to the mean placebo response rate of 33% reported in a review of psychopharmacological trials for BED by Carter and colleagues (2003) and a 42% abstinence rate (at 24 weeks posttreatment) in the placebo arm of a recent pharmacotherapy randomized controlled study for BED (Wilfley et al., 2008). In addition, such rates are similar to the mean placebo rates (from meta-analyses) of other psychiatric illnesses including major depression (Walsh, Seidman, Sysko, & Gould, 2002) and bipolar mania (Sysko & Walsh, 2007).
The improvement (i.e., increase in abstinence rates) from posttreatment (36%) through the 12-month follow-up assessment (56%) is striking. One interpretation for this improvement is that it is attributable to the delayed treatment effects of ACGT. In this sense, ACGT may be similar to IPT for bulimia nervosa, which initially results in significantly lower postabstinence rates than CBT but which is statistically indistinguishable at 1 year after treatment (Agras, Walsh, Fairburn, Wilson, & Kraemer, 2000). Another possibility is that the improvement is due to the high rate of spontaneous remission seen over time in BED, which could result in the lessening of observed posttreatment differences. As studies on the temporal course of binge eating disorder (e.g., Fairburn, Cooper, Doll, Norman, & O’Connor, 2000; Fichter & Quadflieg, 2007) reveal, binge eating patterns can be unstable over time. Without a wait-list control, it is difficult to distinguish between these competing hypotheses. Indeed, even with such a control it is difficult, as the anticipation of future treatment (often offered to patients after the wait-list period) may diminish this rate of spontaneous improvement, particularly in the BED population.
In terms of the secondary measures, the greatest impact was demonstrated via three medium effect sizes, each of which favored DBT-BED over ACGT: the Eating Disorder Examination (EDE)-Restraint subscale at both posttreatment and 12-month follow-up, and the EDE-Eating Concerns subscale at posttreatment.
Small effect sizes favoring DBT-BED over ACGT were found at posttreatment for the three subscales of the EES and the BDI. However, at the 12-month follow-up, none of the emotion regulation-measures demonstrated meaningful changes favoring DBT-BED over ACGT. At the 12-month follow-up, small effect sizes favoring improvement in ACGT over DBT-BED were found for the DERS and the negative subscale of the PANAS.
A significant finding from this study was the very low dropout rate in the DBT-BED group compared the ACGT group. These results, in combination with the 18% dropout rate found by Telch and colleagues (2001), suggest that DBT for BED is an acceptable treatment for many participants, though further comparisons against other than a wait-list or nonspecific control are needed.
This study confirms and extends the earlier investigation of DBT-BED (Telch et al., 2001) by employing the same manualized DBT-BED treatment but in addition, compares DBT-BED to an active control and lengthens the follow-up period to 12 months. Telch et al. (2001) found posttreatment ITT abstinence rates of 73% for DBT-BED and 9% for those in the wait-list condition. At the 6-month follow-up assessment, the ITT abstinence rate for those in DBT-BED was 54.5% (Telch et al.). The current study’s posttreatment abstinent rate of 64% for DBT-BED participants was somewhat lower than that found by Telch and colleagues. This disparity may be accounted, at least in part, by sample differences between the two studies. The inclusion criteria were broadened in the current study to increase the generalizability of the findings. Specifically, individuals on stable doses of psychotropic medications and both men and women were included, whereas the earlier study excluded those on psychotropics and entered women participants only. The inclusion of participants on psychotropics may have resulted in a slightly higher level of depressive symptomatology reported by group members, as reflected in the higher pretreatment BDI mean of 17.9 ± 9.4 in this study compared to 12.8 ± 7.4 in the earlier study. Previous research (Grilo, Masheb, & Wilson, 2001; Stice et al., 2001) clustering BED patients according to affective and restraint measures has shown poorer response to treatment in those with more severe affective (e.g., BDI) scores.
The present study’s lack of evidence for greater improvements on emotion-regulation measures among the DBT-BED versus ACGT groups replicates Telch and colleague’s (2001) lack of significant posttreatment differences on such measures between DBT-BED and a wait-list control. In other words, neither study’s findings resulted in a measurable impact of DBT on emotion regulation. The lack of differentiation between DBT-BED and ACGT is puzzling and may indicate a true absence of a major DBT-BED treatment impact on emotion regulation or it may reflect the lack of consensus within the field on how to conceptualize and measure emotion regulation (Gratz & Roemer, 2004). Of note, the largest effect size favoring DBT-BED over ACGT was in reducing EDE-Restraint subscale scores at posttreatment and at the 12-month follow-up. It is not obvious why DBT-BED would impact EDE-Restraint as DBT-BED, unlike CBT, does not specifically discuss patterns of food intake or rules regarding food. It is possible that through DBT-BED’s emphasis on nonjudgmental acceptance of emotions, emotionally charged rules regarding food may decrease. In other words, DBT’s emphasis on mindfulness may act indirectly to help reduce the restrictive mindset often found in chronic dieters who, after breaking cognitive barriers, tend to eat more (Heatherton & Polivy, 1992; Polivy & Herman, l995).
The study has a number of limitations. Despite the best efforts to design a highly credible and acceptable comparison treatment and to retain participants for study assessments (even if they dropped from treatment), dropout rates from treatment as well as study assessments were higher in the ACGT than DBT-BED group. For example, the drop-out rate for ACGT was 33.3% (17/51). About one third (35%, 6/17) of these 17 dropouts took place within the first month of treatment, whereas the remainder (65%, 11/17) of dropouts were evenly distributed over Weeks 5–18. This suggests that patients dropped both due a lack of credibility of ACGT once it was under way (despite the lack of significant differences in postrandomization ratings of suitability) as well as a sense that, while credible, they did not find the treatment helpful over time.
Despite this differential dropout rate, nearly all patients (97%; 98/101) were willing to complete at least posttreatment assessments on the primary outcome measures of binge days and abstinence. However, this number of study assessment completers decreased over the follow-up period, particularly among those assigned to ACGT. By the 12-month follow-up, 98% (49/50) of the DBT-BED participants completed assessments on at least the primary outcome measures versus only 76.5% (39/51) of the ACGT participants. The maximum likelihood estimation approach employed in this study assumes that dropout may be associated with observed variables such as treatment assignment (i.e., DBT-BED or ACGT) and baseline measures. However, this approach does not take into account group differences that might be associated with unobserved variables (Little & Rubin, 2002). In other words, if dropout rates from assessment are not missing completely at random, statistical estimates of differential outcomes between the groups would be impacted (e.g., Graham, 2009). A related limitation is that treatment credibility was not assessed prerandomization. Grilo and Masheb (2005), in asking participants to rate the extent to which the study’s two treatment options are logical, provides a useful example of such a rating. This current study was under way, however, after the publication of that study. Suitability was measured at postrandomization, however, as well as after Session 1 and at the end of treatment. It was not measured at the 12-month follow-up. Without the combination of baseline as well as postrandomization ratings (taken at various points during the course of treatment and follow-up), the effect of the postrandomization suitability as a potential mediator of treatment outcome or dropout status cannot be determined.
It is important to acknowledge that an adapted version of DBT (DBT-BED) and not standard DBT was implemented. DBT-BED, originally developed by Telch (1997b) for the earlier Telch et al. (2001) study, is based on the principles of standard DBT. However, to make DBT-BED more comparable in terms of format, length, and cost to other existing BED treatments (e.g., CBT and IPT showed efficacy for BED participants using twenty 2-hour group therapy sessions; Wilfley et al., 1993; Wilfley et al., 2002), the format of standard DBT treatment was modified. DBT-BED modifications included using group therapy only (versus individual plus group therapy) and shortening treatment duration to 6 months (versus 12 months), and consequently omitting one of standard DBT’s four modules (Interpersonal Effectiveness). By using the same DBT-BED manual utilized by Telch et al. (2001) for the current study, replication and extension of the former was possible. However, the effect of using an adapted version versus standard DBT cannot be determined from this study. Furthermore, whereas adherence to the adapted version of DBT-BED was assessed, treatment adherence scores to standard DBT, which has a formalized coding standard via the Linehan Behavioral Tech group, were not obtained.
Despite these limitations, this study has a number of important strengths. It is the first to compare the posttreatment and longer-term outcome of DBT-BED to an active, carefully designed comparison therapy to attempt to control for the effect of common therapeutic factors. In doing so, it provides the first confirmation and extension of the earlier Telch et al. (2001) study comparing DBT-BED to a wait-list control. Other strengths include a moderately large sample size, high retention rate for assessments, and follow-up assessments up to 12 months following treatment cessation. This longer follow-up period is especially valuable given mixed findings regarding the fluctuation of binge eating behaviors within BED over time (Fairburn et al., 2000; Fichter & Quadflieg, 2007; Pope et al., 2006).
In conclusion, the data suggest both DBT-BED and ACGT were beneficial in reducing binge eating. Compared to ACGT, DBT-BED appeared more acceptable to patients as indicated by a much lower dropout rate and a higher follow-up assessment completion rate. The overall lack of differential impact with the emotion-regulation measures (as measured by effect sizes) suggests that DBT-BED’s effects were attributable to therapeutic elements shared across both treatments (see also Safer & Hugo, 2006). DBT-BED yielded faster rates of improvement in the primary outcome measures, with higher binge abstinence and lower rates of binge eating at the end of treatment, but this differential effect was lost over follow-up.
Acknowledgments
The authors gratefully acknowledge Brenda Brownlow, Emily Hugo, Rebecca Klein, Wanda Chui, and Amanda Vaught for their involvement in this study.
This research was funded by a grant from the National Institute of Mental Health, K23MH066330 awarded to Debra Safer, MD.
Footnotes
The Telch et al. (2001) paper reports abstinence rates only on participants who completed treatment. However, data from which to calculate the ITT percentages are available. ITT values are reported presently as they represent a more relevant comparison sample to the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham SF, Beumont PJ. How patients describe bulimia or binge eating. Psychological Medicine. 1982;12:625–635. doi: 10.1017/s0033291700055732. [DOI] [PubMed] [Google Scholar]
- Agras WS, Walsh T, Fairburn CG, Wilson GT, Kraemer HC. A multicenter comparison of cognitive-behavioral therapy and interpersonal psychotherapy for bulimia nervosa. Archives of General Psychiatry. 2000;57:459–66. doi: 10.1001/archpsyc.57.5.459. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed Author; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with borderline personality disorders. American Journal of Psychiatry. 2001;168:1–52. [PubMed] [Google Scholar]
- Arnow B, Kenardy J, Agras WS. Binge eating among the obese: A descriptive study. Journal of Behavioral Medicine. 1992;15:155–170. doi: 10.1007/BF00848323. [DOI] [PubMed] [Google Scholar]
- Arnow B, Kenardy J, Agras WS. The Emotional Eating Scale: The development of a measure to assess coping with negative affect by eating. International Journal of Eating Disorders. 1995;18:79–90. doi: 10.1002/1098-108x(199507)18:1<79::aid-eat2260180109>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RM, Garbin M. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock JE, Erbaugh JK. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Carter WP, Hudson JI, Lalonde J, Pindyck L, McElroy SL, Pope HG., Jr. Pharmacologic treatment of binge eating disorder. International Journal of Eating Disorders. 2003;34:S74–S88. doi: 10.1002/eat.10207. [DOI] [PubMed] [Google Scholar]
- Catanzaro SJ, Mearns J. Measuring generalized expectancies for negative mood regulation: Initial scale development and implications. Journal of Personality Assessment. 1990;54:546–563. doi: 10.1080/00223891.1990.9674019. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Hollon SD. Defining empirically supported therapies. Journal of Consulting and Clinical Psychology. 1998;66:7–18. doi: 10.1037//0022-006x.66.1.7. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Critelli JW, Neumann KF. The placebo: Conceptual analysis of a construct in transition. American Psychology. 1984;39:32–39. doi: 10.1037//0003-066x.39.1.32. [DOI] [PubMed] [Google Scholar]
- Demo DH. The measurement of self-esteem: Refining our methods. Journal of Personality and Social Psychology. 1985;48:1490–1502. [Google Scholar]
- Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. 12th ed. Guildford Press; New York: 1993. pp. 317–360. [Google Scholar]
- Fairburn CG, Cooper Z, Doll HA, Norman P, O’Connor M. The natural course of bulimia nervosa and binge-eating disorder in young women. Archives of General Psychiatry. 2000;57:659–665. doi: 10.1001/archpsyc.57.7.659. [DOI] [PubMed] [Google Scholar]
- Fichter MM, Quadflieg N. Long-term stability of eating disorder diagnoses. International Journal of Eating Disorders. 2007;40:S61–66. doi: 10.1002/eat.20443. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for DSM-IV axis II personality disorders self-report. American Psychiatric Association; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I Disorders–Patient edition (SCID-I/P, version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2004;26:41–54. [Google Scholar]
- Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behaviour Research and Therapy. 2005;43:1509–1520. doi: 10.1016/j.brat.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT. Subtyping binge eating disorder. Journal of Consulting and Clinical Psychology. 2001;69:1066–1072. doi: 10.1037//0022-006x.69.6.1066. [DOI] [PubMed] [Google Scholar]
- Goldstein H. Multilevel mixed linear model analysis using iterative generalized least squares. Biometrika. 1986;73:43–56. [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Polivy J. Chronic dieting and eating disorders: A spiral model. In: Crowther JH, Tennenbaum DL, Hobfold SE, Parris MA, editors. The etiology of bulimia nervosa: The individual and familial context. Hemisphere; Washington, DC: 1992. pp. 133–155. [Google Scholar]
- Javaras KN, Pope HG, Lalonde JK, Roberts JL, Nillni YI, et al. Co-occurrence of binge eating disorder with psychiatric and medical disorders. Journal of Clinical Psychiatry. 2008;69:266–273. doi: 10.4088/jcp.v69n0213. [DOI] [PubMed] [Google Scholar]
- Linehan MM. Cognitive behavioral therapy of borderline personality disorder. Guilford Press; New York: 1993a. [Google Scholar]
- Linehan MM. Skills training manual for treating borderline personality disorder. Guilford Press; New York: 1993b. [Google Scholar]
- Linehan MM, Chen EY. Dialectical behavior therapy for eating disorders. In: Freeman A, editor. Encyclopedia of cognitive behavior therapy. Springer Press; New York: 2005. pp. 168–171. [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; New York: 2002. [Google Scholar]
- Longford N. Random coefficient models. Oxford University Press; New York: 1993. [Google Scholar]
- Luborsky L, Diguer L, Seligman D, Rosenthal R, Krause ED, Johnson S, et al. The researcher’s own therapy allegiances: A “wild card” in comparisons of treatment efficacy. Clinical Psychology: Science and Practice. 1999;6:95–106. [Google Scholar]
- Markowitz J, Sacks M. Manual for brief supportive psychotherapy. 2002. Unpublished manuscript. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. Chapman & Hall; London: 1989. [Google Scholar]
- McCulloch CE. Maximum likelihood algorithms for generalized linear mixed models. Journal of the American Statistical Association. 1997;92:162–170. [Google Scholar]
- McLachlan GJ, Krishnan T. The EM algorithm and extensions. Wiley; New York: 1997. [Google Scholar]
- Munsch S, Biedert E, Meyer A, Michael T, Schlup B, Tuch A, Margraf J. A randomized comparison of cognitive behavioral therapy and behavioral weight loss treatment for overweight individuals with binge eating disorder. International Journal of Eating Disorders. 2007;40:102–113. doi: 10.1002/eat.20350. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Muthén & Muthén; Los Angeles: 1998-2008. [Google Scholar]
- O’Boyle M, Self D. A comparison of two interviews for DSM-III-R personality disorders. Psychiatric Research. 1990;32:85–92. doi: 10.1016/0165-1781(90)90138-u. [DOI] [PubMed] [Google Scholar]
- Palmer RL, Birchall H, Damani S, Gatward N, McGrain L, Parker L. A dialectical behavior therapy program for people with an eating disorder and borderline personality disorder: Description and outcome. International Journal of Eating Disorders. 2003;33:281–286. doi: 10.1002/eat.10141. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman C. Etiology of binge eating: Psychological mechanisms. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. Guilford Press; New York: 1993. pp. 173–205. [Google Scholar]
- Polivy J, Herman CP. Dieting and its relation to eating disorders. In: Brownell KD, Fairburn CG, editors. Eating disorders and obesity: A comprehensive handbook. Guilford Press; New York: 1995. pp. 83–86. [Google Scholar]
- Pope HG, Lalonde JK, Pindyck LJ, Walsh T, Bulik CM, Crow SJ, McElroy SL, Rosenthal N, Hudson JI. Binge eating disorder: A stable syndrome. American Journal of Psychiatry. 2006;163:2181–2183. doi: 10.1176/ajp.2006.163.12.2181. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- Renneberg B, Chambless DL, Dowdall DJ, Fauerbach JA, Gracely EJ. The Structured Clinical Interview for DSM-III-R, Axis II, and the Millon Clinical Multiaxial Inventory: A concurrent validity study of personality disorders among anxious outpatients. Journal of Personality Disorders. 1992;6:117–124. [Google Scholar]
- Rizvi SL, Peterson CB, Crow SJ, Agras WS. Test-retest reliability of the Eating Disorder Examination. International Journal of Eating Disorders. 2000;28:311–316. doi: 10.1002/1098-108x(200011)28:3<311::aid-eat8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Rogers CR. Client-centered therapy. Houghton Mifflin; Boston: 1951. [Google Scholar]
- Rosenberg M. Conceiving the self. Basic Books; New York: 1979. [Google Scholar]
- Safer DL, Hugo EM. Designing a control for a behavioral group therapy. Behavior Therapy. 2006;37:120–130. doi: 10.1016/j.beth.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer DL, Telch CF, Agras WS. Dialectical behavior therapy for bulimia nervosa: A case study. International Journal of Eating Disorders. 2001a;30:101–106. doi: 10.1002/eat.1059. [DOI] [PubMed] [Google Scholar]
- Safer DL, Telch CF, Agras WS. Dialectical behavior therapy for bulimia nervosa. American Journal of Psychiatry. 2001b;158:632–634. doi: 10.1176/appi.ajp.158.4.632. [DOI] [PubMed] [Google Scholar]
- Salbach H, Klinkowski N, Pfeiffer E, Lehmkuhl U, Korte A. Dialectical behavior therapy for adolescents with anorexia and bulimia nervosa (DBT-AN/ BN): A pilot study. Prax Kinderpsychol Kinderpsychiatr. 2007;56:91–108. doi: 10.13109/prkk.2007.56.2.91. [DOI] [PubMed] [Google Scholar]
- Segal DL, Hersen M, Van Hasselt VB. Reliability of the Structured Clinical Interview for DSM-III-R: An evaluative review. Comprehensive Psychiatry. 1994;35:316–327. doi: 10.1016/0010-440x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Sage; Thousand Oaks, CA: 1999. [Google Scholar]
- Spitzer RL, Devlin M, Walsh BT, Hasin D, Wing RR, Marcus M, Stunkard A, Wadden T, Yanovski S, Agras WS, Nonas C. Binge eating disorder: A multisite field trial of the diagnostic criteria. International Journal of Eating Disorders. 1992;11:191–203. [Google Scholar]
- Stice E, Agras WS, Telch CF, Halmi KA, Mitchell JE, Wilson T. Subtyping binge eating-disordered women along dieting and negative affect dimensions. International Journal of Eating Disorders. 2001;30:11–27. doi: 10.1002/eat.1050. [DOI] [PubMed] [Google Scholar]
- Sysko R, Walsh BT. A systematic review of placebo response in studies of bipolar mania. Journal of Clinical Psychiatry. 2007;68:1213–1217. doi: 10.4088/jcp.v68n0807. [DOI] [PubMed] [Google Scholar]
- Telch CF. Skills training treatment for adaptive affect regulation in a woman with binge-eating disorder. International Journal of Eating Disorders. 1997a;22:77–81. doi: 10.1002/(sici)1098-108x(199707)22:1<77::aid-eat10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Telch CF. Emotion regulation skills training treatment for binge eating disorder: Therapist manual. 1997b. Unpublished manuscript. [DOI] [PubMed] [Google Scholar]
- Telch CF, Agras WS, Linehan MM. Group dialectical behavior therapy for binge eating disorder: A preliminary, uncontrolled trial. Behavior Therapy. 2000;31:569–582. [Google Scholar]
- Telch CF, Agras WS, Linehan MM. Dialectical behavior therapy for binge eating disorder. Journal of Consulting and Clinical Psychology. 2001;69:1061–1065. doi: 10.1037//0022-006x.69.6.1061. [DOI] [PubMed] [Google Scholar]
- Waller G. The psychology of binge eating. In: Fairburn CG, Brownell KD, editors. Eating disorders and obesity: A comprehensive handbook. Guilford Press; New York: 2003. pp. 98–102. [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in clinical trials of mood disorders. Journal of the American Medical Association. 2002;287:1840–1847. [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Agras WS, Telch CF, Rossiter EM, Schneider JA, Cole AG, Sifford LA, Raeburn SD. Group cognitive-behavioral therapy and group interpersonal psychotherapy for the nonpurging bulimic individual: A controlled comparison. Journal of Consulting and Clinical Psychology. 1993;61:296–305. doi: 10.1037//0022-006x.61.2.296. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Crow SJ, Hudson JI, Mitchell JE, Berkowitz RI, Blakesley V, Walsh BT, The Sibutramine Binge Eating Disorder Research Group Efficacy of sibutramine for the treatment of binge eating disorder: A randomized multicenter placebo-controlled double-blind study. American Journal of Psychiatry. 2008;165:51–58. doi: 10.1176/appi.ajp.2007.06121970. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Welch RR, Stein RI, Spurrell EB, Cohen LR, Saelens BE, Dounchis JZ, Frank MA, Wiseman CV, Matt GE. A randomized comparison of group cognitive-behavioral therapy and group interpersonal psychotherapy for the treatment of overweight individuals with binge eating disorder. Archives of General Psychiatry. 2002;59:713–721. doi: 10.1001/archpsyc.59.8.713. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Wilson GT, Agras WS. The clinical significance of binge eating disorder. International Journal of Eating Disorders. 2003;34:S96–S106. doi: 10.1002/eat.10209. [DOI] [PubMed] [Google Scholar]
- Wiser S, Telch CF. Dialectical behavior therapy for binge eating disorder. Journal of Clinical Psychology. 1999;55:155–768. doi: 10.1002/(sici)1097-4679(199906)55:6<755::aid-jclp8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wisniewski L, Kelly E. The application of dialectical behavior therapy to the treatment of eating disorders. Cognitive and Behavioral Practice. 2003;10:131–138. [Google Scholar]